Abstract
Study Objectives:
The gold standard for diagnosis of pediatric obstructive sleep apnea (OSA) is level 1 polysomnography (PSG). At our centre, some children are selected for unattended level 2 home sleep apnea testing (HSAT) with telehealth support, and we sought to review this home service.
Methods:
A retrospective audit was conducted from 2013 to 2020. All level 2 HSAT reports in children aged 5–18 years referred for suspected OSA were analyzed. American Academy of Sleep Medicine–compliant portable PSG acquisition equipment with electroencephalogram was used. The primary outcome was the proportion of technically successful tests achieved, and of these, the percentage with potential underestimation of diagnostic category. Secondary outcomes included sleep quality and parental acceptance by nonvalidated service-specific questionnaire. Data were analyzed using descriptive and inferential statistics. χ2 tests were used for categorical variables.
Results:
There were 233 (139 male, 59.6%) patients studied between 2013 and 2020 (7 years). The mean age was 10.8 (standard deviation 3.6) years. Sixty-seven patients (28.8%) had comorbidities. Technically successful studies were obtained in almost 90% (209/233) and failed studies occurred in just over 10% (24/233). One failed study still achieved a diagnosis. There was no significant difference between failed studies set up by hospital-in-the-home nurses compared with sleep scientists (P = .2). Overall, an accurate diagnosis was made in 80% (167/209) of patients, with potential for underestimation in 20% (42/209). Six hours or more of sleep was obtained in 89.5%. Parental questionnaires revealed 89.3% perceived high-level care, 91% perceived increased convenience, and 76% perceived good/excellent telehealth support.
Conclusions:
Telehealth-supported pediatric HSAT achieves technical success in almost 90% of patients investigated for OSA, with 89.5% achieving ≥ 6 hours sleep duration and excellent family acceptability.
Citation:
Griffiths A, Mukushi A, Adams A-M. Telehealth-supported level 2 pediatric home polysomnography. J Clin Sleep Med. 2022;18(7):1815–1821.
Keywords: pediatric obstructive sleep apnea, home sleep study, polysomnography, telehealth
BRIEF SUMMARY
Current Knowledge/Study Rationale: Home sleep apnea testing (HSAT) in children lacks a substantial evidence base despite the significant advantages to children and families. As physicians around the world convert medical services to telehealth in the home to keep children safe during the COVID-19 pandemic, HSAT should be re-considered.
Study Impact: This study demonstrates feasibility, safety, and accuracy of level 2 HSAT with telehealth support in normal and complex children who present with obstructive sleep apnea. Children were demonstrated to sleep very well in their own bed, and family acceptance of the procedure was excellent.
INTRODUCTION
Snoring affects 10–20% of children, although only 1–5% of all children have obstructive sleep apnea (OSA).1 The gold standard for diagnosis of pediatric OSA is in-laboratory attended polysomnography (PSG),2 also known as “level 1” PSG.3 This is a multichannel test complete with video, audio, and attendance by a trained sleep scientist. However, level 1 PSG is time and labor intensive, expensive, not always available, and inconvenient to parents.4 In adults, unattended home PSG (home sleep apnea testing [HSAT]) is the most frequently used diagnostic test for OSA and has excellent reliability in those without comorbidities and with high pretest probability of OSA.5 Pediatric HSAT, subgroup “level 2” (with electroencephalogram [EEG]) is feasible in school-aged children6,7; however, it has been technically limited due to the potential for children to pull off electrodes and sensors. This displacement is either not recognized or not adequately replaced, leading to insufficient information, and reduced diagnostic capacity. Safety issues have also been raised relating to the potential for entanglement in the wires. Pediatric HSAT is currently not recommended by the American Academy of Sleep Medicine (AASM) due to insufficient evidence.8 However, pediatric HSAT is advantageous for children and families for many reasons, even more so in the coronavirus disease 2019 (COVID-19) pandemic due to increased risk of viral acquisition in the hospital setting. Children sleep better in the comfort and familiarity of their own bedrooms, better sleep consolidation leads to the potential for improved diagnostic yield, and parents with other children and busy lives enjoy the convenience of not needing to attend a sleep laboratory. Regional and remote patients may also be disadvantaged when it comes to attending in-laboratory PSG. Last, in Australia, type 2 pediatric HSAT costs only 50% of type 1 polysomnography.9
The Royal Children’s Hospital has well-developed telehealth services for patient consultations using specific user-friendly software (https://about.healthdirect.gov.au/video-call). Telehealth had been used daily for consultations with regional and remote patients by all hospital departments well prior to the COVID-19 pandemic, originally commencing in 2013. Many medical subspecialties are now incorporating telehealth into their diagnostic capacities (eg, telehealth spirometry10). This is the first report of using telehealth for pediatric HSAT to improve diagnostic capacity by technical accuracy.
METHODS
The service
This is a retrospective audit of our clinical pediatric HSAT service between December 2013 and December 2020. Acceptability criteria for selection into the program were children aged 5–18 years with a history suggestive of OSA recruited from outpatients at our hospital. Exclusion criteria were severe autism, attention-deficit/hyperactivity disorder, psychiatric disorder, or medical comorbidity interfering with sleep or cooperation.
The family were contacted 1 week prior to the study for a Telehealth software pretest. This was essentially to ensure technical accuracy for the Telehealth component of the study and confirm the details of the test.
The patients referred for pediatric HSAT were either set up at the Royal Children’s Hospital by a trained sleep nurse, then admitted to hospital-in-the-home (HITH), or they were set up in their home by sleep-trained HITH nurses following admission to this service. Our HITH service allows patients to have medical management at home by sending out trained nurses or allied health staff in fleet cars within a distinct geographical region (60-km radius) of the hospital.
Patients had the following channels recorded during the study using the AASM-compliant Compumedics Somté PSG acquisition device (version 2; Compumedics Limited, Australia): electrocardiogram, electro-oculogram, electromyogram (chin, right and left leg), EEG (O1, O2, C3, C4, M1, M2, F3, F4, Reference), thermistor, nasal pressure, oximetry, abdominal and thoracic effort (respiratory inductance plethysmography bands), and body position. There was no surrogate measurement of carbon dioxide (CO2) available. Of note, this device is the most extensively used in Australia for HSAT and, given the EEG leads, is far superior to level 3 devices (cardiorespiratory monitors), significantly reducing the chance that OSA could be missed.
A telehealth consultation with the sleep nurse was scheduled just prior to usual bedtime in the patient’s home. The parent was guided through a checklist of all technical aspects of the portable PSG equipment, including signal assessments, and then assisted to reattach any loosened sensors or electrodes. Parents were encouraged to sleep in the same room as the child and any safety-related issues were documented. The parents were asked to check the electrodes and sensors overnight, and to use a provided checklist to ensure optimal signal quality if the child woke overnight. Telehealth assistance via the hospital sleep laboratory night-duty scientist was available if needed overnight, and a direct phone number was provided. HITH nurses visited families the next morning to remove electrodes and return the portable sleep equipment to the hospital for the data card to be downloaded, analyzed, and reported. Staging and scoring were completed by trained sleep scientists and reporting performed by sleep physicians using the standard criteria defined by the AASM.11
The questionnaire
Patients completed a nonvalidated service-specific questionnaire designed to evaluate their experience of the service. The questionnaire has 10 questions with a rating score of 1–5, with 1 being excellent and 5 being poor.
Technical assessment
Technical accuracy of each study was assessed by the authors (AG and A-MA) in relation to adequacy of individual signals. If signal loss was present, it was classified as follows:
Minor—intermittent loss of 1 or more signals for less than 20% of total sleep time (TST), not dissimilar to that seen during level 1 (in-lab) PSG
Intermediate—prolonged periods of loss of airflow, oximetry, or EEG signals, but still enabling at least 4 hours of data interpretation and signals acceptable for at least 75% of TST6
Major—where study failed to record or signal loss was greater than 25% of TST or less than 4 hours of interpretable data were obtained; a repeat study was deemed necessary.
The diagnosis in each study was assessed by the authors, based on the classification of signal loss, if present, as follows:
Satisfactory—no reason for underestimation based on signal quality
Potentially underestimated—intermediate signal loss
The following sleep variables were recorded as a measure of sleep quality: TST (hours:minutes) and sleep efficiency (%).11 The OSA diagnosis severity was also reported in accordance with AASM criteria for pediatric OSA.11
Statistical analysis
Data exploration was performed using descriptive statistical analysis and inferential statistics. Data are shown in frequencies or percentages, means and standard deviation (SD), medians and ranges, and graphics. The 95% confidence intervals (CIs) have been calculated for the differences in percentages and medians. To examine categorical characteristics, χ2 tests were used. All analyses were conducted using Stata version 15.1 (StataCorp, College Station, TX, USA).
RESULTS
A total of 233 (139 male, 59.6%) patients underwent telehealth-supported pediatric HSAT between December 2013 and December 2020, with a mean age of 10.8 (SD 3.6) years (median 10.2, range 3.1 to 18.4 years). Sixty-seven patients (28.8%) had comorbidities, which included asthma, epilepsy, obesity, and Pierre-Robin sequence ± cleft palate. All patients had OSA as the indication for HSAT. There were no adverse patient events.
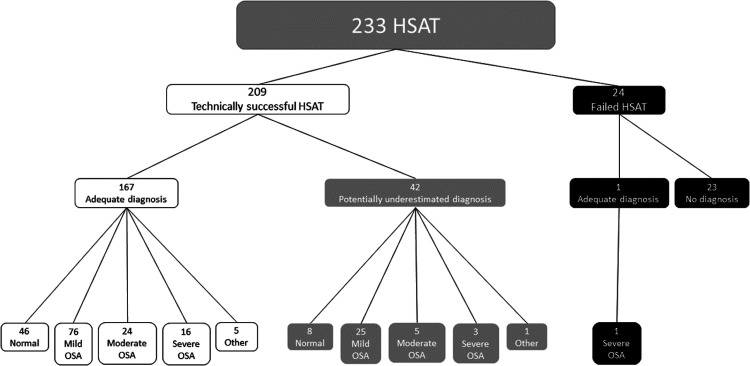
Technically successful pediatric HSATs were obtained from 89.7% (209/233) of patients who were able to achieve a diagnosis, and technically failed studies occurred in 10.3% (24/233) of patients (Figure 1).
Figure 1. HSAT distribution.
HSAT = home sleep apnea testing, OSA = obstructive sleep apnea.
Of the 24 technically failed studies, a satisfactory diagnosis was still able to be obtained in 1 study. This additional diagnosis was a study where the extent of the signal loss was significant enough for it to be deemed an overall failed study for technical reasons; however, enough signals remained for the study to lead to a diagnosis. The remaining oximetry, respiratory effort, and flow signals were present for a period of 4 hours (equivalent to a level 3 home study) and severe OSA was able to be diagnosed. There was no diagnosis made in the remaining 23/24 of failed studies. Twenty of the 24 patients with failed studies were offered “in-laboratory” level 1 PSG and successful results were obtained. Three (3/25) patients who had failed HPSG studies were not offered repeat in-laboratory studies due to intolerance of the setup and they were deemed unsuitable for further PSG of any kind. Three of the failed studies occurred when the patients were set up by the sleep team (3/24 = 12%) and 21/24 (88%) when they were set up by the HITH team.
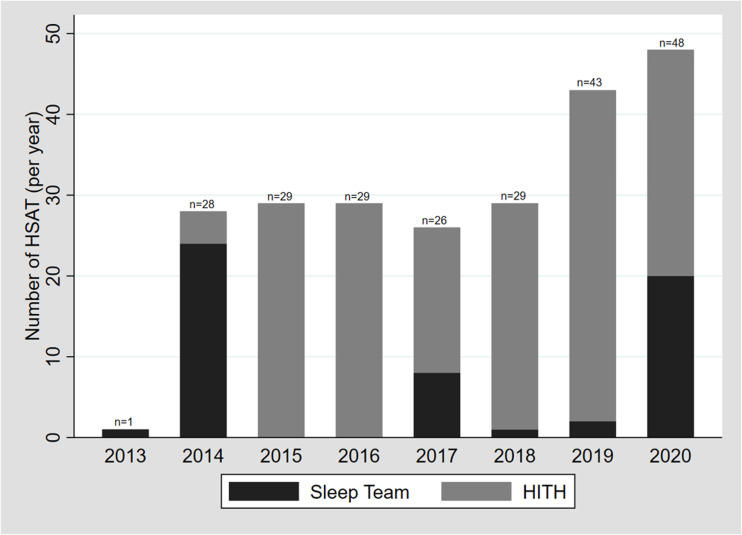
Although the studies set up by the sleep team had proportionately more with no/minor issues and less with intermediate or major technical issues when compared with those setups by the HITH team, the differences were not significant [χ2(3) = 6.7; P = .1]. Figure 2 shows the set-up team frequencies per annum, noting that, during the COVID-19 pandemic, the sleep team stepped in to assist more often than previously.
Figure 2. Set-up team frequency per annum.
HITH = hospital in the home, HSAT = home sleep apnea testing.
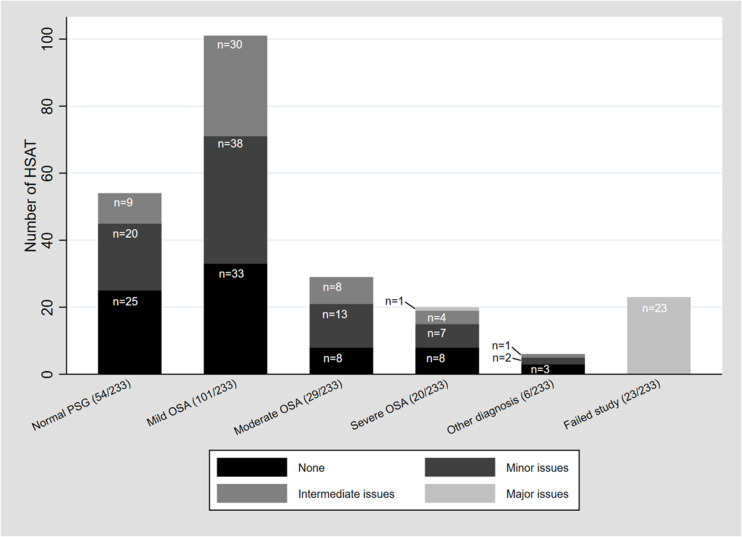
Overall, the diagnosis was deemed satisfactory (no reason for underestimation) in 80% of the technically successful studies (167/209), which was 71.57% of the study population in total (167/233). The diagnosis was potentially underestimated in 20% of those able to achieve a diagnosis (42/209) and this group made up 18.0% of the total study population (42/233). Of the 167 patients with a satisfactory diagnosis, most had no technical issues (77/167, 46%) or minor technical issues (80/167, 48%) (see Figure 3).
Figure 3. HSAT study diagnoses in relation to technical issues.
HSAT = home sleep apnea testing, OSA = obstructive sleep apnea, PSG = polysomnography.
The questionnaires were completed by 51.9% (121/233) of patients. One hundred and eight (89.3%) noted that they received a high level of care, 9.0% noted an average level of care, and only 1.7% noted that they received a low level of care. One-hundred and two (91.0%) found that the home study was more convenient than coming into the hospital. Seventy-six percent (65/86) of those who responded noted that the Telehealth support was very good or excellent; 7 noted that it was average but with some problems. Fourteen patients (15.7%) felt that the telehealth service was not good enough or a complete failure. There was no significant difference over the years the service has been running in the level of telehealth service [χ2(1) = 26.0, P = .2].
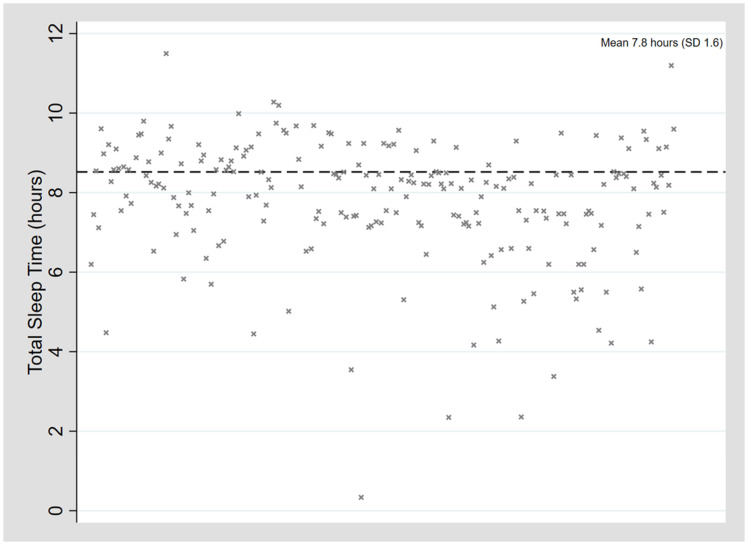
The mean TST 7.8 (SD 1.6) hours (median 8.2, range 0.3–11.3 hours) (see Figure 4). Approximately 89.5% (187/209) of patients satisfied the hospital in-laboratory PSG minimum TST of 6 hours; 10.9% (22/209) had a TST < 6 hours. The mean sleep efficiency was 78.3% (SD 12.1%) (median 81.0%, range 34–97.1%).
Figure 4. Total sleep time.
SD = standard deviation.
DISCUSSION
In 2017, the AASM commissioned a taskforce of pediatric sleep medicine experts to write a position paper on the use of HSAT in children based on literature available and clinical expertise.8 They concluded that the available evidence does not support the use of HSATs for the diagnosis of OSA in children, due mostly to a lack of sufficient validation in the home and insufficient monitoring available in most devices used to conduct HSAT. However, there was no distinction made between the various levels of devices. Evidence for level 3 devices with far fewer channels and no EEG was included with evidence for level 2 devices (with far greater channels and multiple EEG leads). Since that time, additional feasibility12 and validation studies have emerged in this area.9,13 Furthermore, the AASM paper was published prior to the COVID-19 pandemic and, for practical reasons in terms of risk minimization and infection control, performing pediatric HSAT is now an ideal solution for selected children.
Our retrospective audit of telehealth-supported level 2 pediatric HSATs between December 2013 and December 2020 again demonstrates that this kind of service is feasible with almost a 90% success rate for technically acceptable studies. These figures are in line with previous authors of level 2 HSAT studies reporting on feasibility in children. Goodwin et al14 first reported technically acceptable studies on the first night in 91% of children aged 5–12 years in 2001, with airflow signals being the major challenge. Marcus et al6 later reported 91% accuracy in a large multisite level 2 study of children aged 5–12 years in 2014 with setup by a trained technologist. More recently, in 2020, Ioan et al12 studied 57 children aged 3–6 years, including 8 with developmental delay, and they achieved 81% technical acceptability. Even in more limited channel (level 3) studies, Brockmann et al7 documented 93% technical success.
The study failure rate of 10% was due to various equipment-related issues, such as battery failure and a broken oximeter probe as well as major signal loss. As a result of this service, lessons have been learned and issues relating to equipment failures have been addressed. This has included the use of new longer-lasting alkaline batteries and spare electrodes and sensors being supplied in the HSAT equipment bag (eg, spare oximeter probes). Our parental education via the telehealth platform has improved with time and enabled parents to replace leads lost and improve data collection. We also changed to setting up patients in their own home after the first 25 patients, with less potential for signal loss than during a hospital setup (given they do not need to travel home). With the impact of the COVID-19 pandemic, we are currently setting up an interactive online training module for staff that replaces current face-to-face training and should improve study failures related to high HITH staff turnover. While there was a trend toward more failures in those set up by HITH vs sleep team staff, this was not statistically significant (P = .2). Given the small sample size, we acknowledge this may related. However, both staff types are trained, and we believe this is important given previous authors’ report of increased failure rates in relation to caregiver setups vs trained setups.13,15 There are no previous studies that compare usual sleep staff with other trained groups, such as HITH nurses.
Studies on validation of level 2 pediatric HSAT against the gold-standard level 1 PSG have been scarce, as noted by authors commenting on progress in the area in 2015.16 Until then, only Goodwin14 and Marcus et al6 had validated small numbers of studies, and reported similar respiratory parameters in 5 and 4 children, respectively, who underwent both level 1 and level 2 studies. In 2017, a pilot validation study performed by Scalzitti et al13 in children aged 2–17 years showed that there was no significant difference in apnea-hypopnea index and SpO2 nadir for children above 6 years who underwent level 3 home studies compared with level 1 PSG. Until then, previous authors had demonstrated underestimation of the apnea-hypopnea index and variability when level 3 studies were compared with level 1 studies.17,18 Withers et al9 recently published a validation study of level 2 vs level 1 PSG in 81 children aged 6–18 years simultaneously in the sleep laboratory, and a further 47 children aged 5–16 years who had in-laboratory level 1 and home level 2 studies. The simultaneous hospital group showed excellent correlation in arousals, respiratory disturbance index, and sleep stages, while the home level 2 vs level 1 hospital group showed a false-positive rate of 6.6% and a false-negative rate of 3% for a diagnosis of OSA. Our study is not a validation study, which is a notable limitation, and this is an area worthy of further exploration.
Clinical accuracy is addressed in our study indirectly by defining adequacy of technical signals. We used the same criteria as Marcus et al6 to define a technically acceptable study in the context of intermediate signal loss—namely, at least 4 hours of data per signal, with signal present for at least 75% of TST. Twenty percent of our studies were potentially underestimated due to minor or intermediate signal loss, which has possible implications for clinical management. This is perhaps more significant in the normal/primary snoring group who may have progressed to adeno-tonsillectomy if they had more significant OSA detected. From our study, it is therefore not possible to exclude OSA in this group. This is a limitation of level 2 sleep studies in their current form in children, and improvement in this area is the subject of our ongoing research.
While most of the patients in our study were healthy children with symptoms of OSA, we did study 69 patients (29.4%) with minor comorbidities. These included asthma, mild epilepsy, mild autism, obesity, Pierre-Robin sequence ± cleft palate, Treacher-Collins syndrome, neurofibromatosis, and multiple sclerosis. All of these patients were studied with an indication of OSA. Other authors have performed HSAT in pediatric patients with comorbidities, such as Down syndrome,19 Treacher-Collins syndrome,20 and mucopolysaccharidoses,21 but these did not focus on feasibility, which may potentially be more difficult in this group. This paper thus contributes to the feasibility data of HSAT in children with comorbidities.
Family satisfaction with the procedure was excellent according to our questionnaires, with 91% of families reporting the (level 2) HSAT more convenient than in-laboratory (level 1) PSG. Marcus et al6 also reported high family acceptance of HSAT on the Likert scale. In those families where the telehealth component was reported as suboptimal (16%), the usual reason was failed Wi-Fi with interruption to the appointment and switching to a phone call. There were a few families where connection was not possible due to other reasons (lack of computer, computer skills, or language barriers). We found that children slept at least 6 hours in 90% of cases, with a mean TST of 8 hours and a median sleep efficiency of 81%. This shows that children slept well in their beds at home, which is a likely contributor to high family acceptance. This finding is also in agreement with Withers et al,9 where children undergoing level 2 HSAT showed more rapid eye movement (REM) sleep and better sleep efficiency than level 1 P-HPSG.
One of the concerns raised by the AASM taskforce was the inability of pediatric HSAT to measure transcutaneous carbon dioxide,8 although it was clarified that, at present, the incidence of isolated obstructive hypoventilation in children is unknown. It is theoretically possible to address this by attaching a transcutaneous monitor to a level 2 device such as ours, and although this was not done in the current analysis, we have gone on to do this subsequently in select patients with success. There is no literature describing this in children to date.
CONCLUSIONS
Telehealth-supported level 2 pediatric HSAT at our center is feasible in children aged 5–18 years both with and without minor comorbidities, it is safe when parents sleep next to their child, and is technically accurate, achieving a diagnosis in 90%. Families accept HSAT as it is more convenient and children sleep well in their own beds. The device we use is commercially available and hence the service is scalable. Pediatric HSAT should be reconsidered in the context of the COVID-19 pandemic with level 1 PSG posing a potential risk of increased virus exposure. Future research is currently being directed at improving the limitations of level 2 pediatric HSAT.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Royal Children’s Hospital, Sleep Unit, Department of Respiratory and Sleep Medicine. Financial support was via a Telehealth program grant from the Royal Children’s Hospital for our Sleep Unit to start the home sleep study service. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the HITH administrative staff, nurses, and Director (Penelope Bryant); and Telehealth program manager (Susan Jury), with thanks for the $AUD5000 grant provided by the Royal Children’s Hospital Telehealth program.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- COVID-19
coronavirus 2019 disease
- EEG
electro-encephalogram
- HITH
hospital in the home
- HSAT
home sleep apnea testing
- OSA
obstructive sleep apnea
- PSG
polysomnography
- SD
standard deviation
- SE
sleep efficiency
- TST
total sleep time
REFERENCES
- 1. Fitzgerald NM , Fitzgerald DA . Managing snoring and obstructive sleep apnoea in childhood . J Paediatr Child Health. 2013. ; 49 ( 10 ): 800 – 806 . [DOI] [PubMed] [Google Scholar]
- 2. Marcus CL , Brooks LJ , Draper KA , et al. ; American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome . Pediatrics. 2012. ; 130 ( 3 ): 576 – 584 . [DOI] [PubMed] [Google Scholar]
- 3. Pamula Y , Nixon GM , Edwards E , et al . Australasian Sleep Association clinical practice guidelines for performing sleep studies in children . Sleep Med. 2017. ; 36 ( Suppl 1 ): S23 – S42 . [DOI] [PubMed] [Google Scholar]
- 4. Tan HL , Kheirandish-Gozal L , Gozal D . Pediatric home sleep apnea testing: slowly getting there! Chest. 2015. ; 148 ( 6 ): 1382 – 1395 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lipatov K , Hayek A , Ghamande S , Boethel C , Chen W , Jones S . Predictors of obstructive sleep apnea on a home sleep apnea test after a negative attended polysomnography . J Clin Sleep Med. 2018. ; 14 ( 11 ): 1889 – 1894 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marcus CL , Traylor J , Biggs SN , et al . Feasibility of comprehensive, unattended ambulatory polysomnography in school-aged children . J Clin Sleep Med. 2014. ; 10 ( 8 ): 913 – 918 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brockmann PE , Perez JL , Moya A . Feasibility of unattended home polysomnography in children with sleep-disordered breathing . Int J Pediatr Otorhinolaryngol. 2013. ; 77 ( 12 ): 1960 – 1964 . [DOI] [PubMed] [Google Scholar]
- 8. Kirk V , Baughn J , D’Andrea L , et al . American Academy of Sleep Medicine Position Paper for the use of a home sleep apnea test for the diagnosis of OSA in children . J Clin Sleep Med. 2017. ; 13 ( 10 ): 1199 – 1203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Withers A , Maul J , Rosenheim E , O’Donnell A , Wilson A , Stick S . Comparison of home ambulatory type 2 polysomnography with a portable monitoring device and in-laboratory type 1 polysomnography for the diagnosis of obstructive sleep apnea in children . J Clin Sleep Med. 2022. ; 18 ( 2 ): 393 – 402 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Logie K , Welsh L , Ranganathan SC . Telehealth spirometry for children with cystic fibrosis . Arch Dis Child. 2020. ; 105 ( 12 ): 1203 – 1205 . [DOI] [PubMed] [Google Scholar]
- 11. Berry RB , Gamaldo CE , Harding SM , et al . AASM Scoring Manual Version 2.2 updates: new chapters for scoring infant sleep staging and home sleep apnea testing . J Clin Sleep Med. 2015. ; 11 ( 11 ): 1253 – 1254 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ioan I , Weick D , Schweitzer C , Guyon A , Coutier L , Franco P . Feasibility of parent-attended ambulatory polysomnography in children with suspected obstructive sleep apnea . J Clin Sleep Med. 2020. ; 16 ( 7 ): 1013 – 1019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scalzitti N , Hansen S , Maturo S , Lospinoso J , O’Connor P . Comparison of home sleep apnea testing versus laboratory polysomnography for the diagnosis of obstructive sleep apnea in children . Int J Pediatr Otorhinolaryngol. 2017. ; 100 : 44 – 51 . [DOI] [PubMed] [Google Scholar]
- 14. Goodwin JL , Enright PL , Kaemingk KL , et al . Feasibility of using unattended polysomnography in children for research—report of the Tucson Children’s Assessment of Sleep Apnea study (TuCASA) . Sleep. 2001. ; 24 ( 8 ): 937 – 944 . [DOI] [PubMed] [Google Scholar]
- 15. Poels PJ , Schilder AG , van den Berg S , Hoes AW , Joosten KF . Evaluation of a new device for home cardiorespiratory recording in children . Arch Otolaryngol Head Neck Surg. 2003. ; 129 ( 12 ): 1281 – 1284 . [DOI] [PubMed] [Google Scholar]
- 16. Gozal D , Kheirandish-Gozal L , Kaditis AG . Home sleep testing for the diagnosis of pediatric obstructive sleep apnea: the times they are a changing...! Curr Opin Pulm Med. 2015. ; 21 ( 6 ): 563 – 568 . [DOI] [PubMed] [Google Scholar]
- 17. Tan HL , Gozal D , Ramirez HM , Bandla HP , Kheirandish-Gozal L . Overnight polysomnography versus respiratory polygraphy in the diagnosis of pediatric obstructive sleep apnea . Sleep. 2014. ; 37 ( 2 ): 255 – 260 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zucconi M , Calori G , Castronovo V , Ferini-Strambi L . Respiratory monitoring by means of an unattended device in children with suspected uncomplicated obstructive sleep apnea: a validation study . Chest. 2003. ; 124 ( 2 ): 602 – 607 . [DOI] [PubMed] [Google Scholar]
- 19. Breslin J , Spanò G , Bootzin R , Anand P , Nadel L , Edgin J . Obstructive sleep apnea syndrome and cognition in Down syndrome . Dev Med Child Neurol. 2014. ; 56 ( 7 ): 657 – 664 . [DOI] [PubMed] [Google Scholar]
- 20. Plomp RG , Bredero-Boelhouwer HH , Joosten KF , et al . Obstructive sleep apnoea in Treacher Collins syndrome: prevalence, severity and cause . Int J Oral Maxillofac Surg. 2012. ; 41 ( 6 ): 696 – 701 . [DOI] [PubMed] [Google Scholar]
- 21. Kasapkara CS , Tümer L , Aslan AT , et al . Home sleep study characteristics in patients with mucopolysaccharidosis . Sleep Breath. 2014. ; 18 ( 1 ): 143 – 149 . [DOI] [PubMed] [Google Scholar]