Abstract
Study Objectives:
This review aimed to evaluate the association between craniofacial features in children and adolescents with pediatric obstructive sleep apnea (OSA).
Methods:
Seven databases were searched to fulfill our research objectives. Clinical studies that included participants younger than 18 years with fully diagnosed OSA or without OSA and that evaluated skeletal, soft craniofacial features, or dental arch morphology were considered for this review. The risk of bias and certainty of evidence were assessed. A meta-analysis was performed when low methodological and clinical heterogeneity were detected. This review followed the protocols recommended by the Preferred Reporting Items for a Systematic Review and Meta-analysis (PRISMA-2020) guidelines.
Results:
Nine studies were identified at the end of the selection process, from which 5 did not report differences. Four studies reported differences between craniofacial features when OSA was compared to an asymptomatic control group. Mandibular retrognathia, reduced anteroposterior linear dimensions of the bony nasopharynx (decreased pharyngeal diameters at the levels of the adenoids), longer facial profile, and a narrower intercanine width were described among children with OSA. A meta-analysis was performed considering the studies with a similar methodological approach, and no differences were observed in all the considered cephalometric angles (SNA, SNB, ANB, NSBa, U1-L1, U1-SN). All the included studies were considered at low risk of bias even though some limitations were noted.
Conclusions:
Due to the very low to moderate level of certainty, neither an association nor a lack thereof between craniofacial morphology and pediatric OSA can be supported by these data.
Citation:
Fagundes NCF, Gianoni-Capenakas S, Heo G, Flores-Mir C. Craniofacial features in children with obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med. 2022;18(7):1865–1875.
Keywords: obstructive sleep apnea, child, face, diagnoses
BRIEF SUMMARY
Current Knowledge/Study Rationale: There is controversy regarding the association between pediatric obstructive sleep apnea (OSA) and craniofacial morphology. This systematic review adopted a reliable eligibility criterion to explore the possible link between fully diagnosed pediatric OSA and craniofacial morphology. After retrieving more than 8000 citations, 9 papers were identified. When qualitatively assessed, a specific subgroup of pediatric OSA presented with an increased mandible retrognathia, and/or an extended facial profile compared to children without OSA. However, while the present meta-analysis did not confirm this suggestion from a causality perspective, it supports previous studies that describe specific malocclusion phenotypes as frequent, but not consistently, comorbidities with sleep-disordered breathing and OSA.
Study Impact: Because of low to moderate certainty of the evidence, a clear causal relationship between craniofacial morphology and pediatric OSA cannot be supported at this time.
INTRODUCTION
Obstructive sleep apnea (OSA) is a respiratory sleep disorder resulting in partial or complete airflow obstruction.1 Among children, OSA prevalence has been reported to vary from 1% to 5%.2,3 In the absence of proper management of OSA cases, a typical result of underdiagnoses, several health conditions may arise, including growth impairment,4 behavioral and cognition problems,5,6 and respiratory and cardiac comorbidities.7 From a social perspective, pediatric OSA is related to an increased cost of health care services and unsatisfactory academic progress.8,9
Previous cross-sectional studies suggested a subset of craniofacial features, such as increased facial height, labial incompetency, mandible retrognathia, increased overjet, higher mandible angle, and steeper mandibular plane presented in a higher frequency in children with OSA compared to a non-OSA control group.10,11 The presence of these craniofacial features has been hypothesized as a possible cause or consequence of airway obstruction and OSA development.
A potential benefit of a craniofacial morphology evaluation to identify pediatric OSA is that it is accessible and convenient for routine clinical use in dental practices. The facial analysis can be performed by a clinical examination in the dental office and a craniofacial skeletal screening done by X-rays (ie, cephalometric analysis).
A systematic review and meta-analysis published 8 years ago summarized the differences in skeletal craniofacial features in children with OSA.10 However, there was a paucity of controlled studies with a definitive non-OSA control group (assessed through the nocturnal polysomnography [nPSG]). The nPSG is the standard exam to diagnose OSA in children and adults. Standardizing methodological approaches to analyze OSA patients and associated factors is important for fair comparison among groups. In addition, new studies have been published over the last 5 years, and other craniofacial techniques have been explored among children, such as the assessment of soft facial features, measurements of dental arches, and the evaluation of tooth position.11,12 There is a need to update this literature synthesis.
This systematic review aimed to evaluate the association between craniofacial features in children and adolescents and pediatric OSA. The further investigation of pediatric OSA pathophysiology, specifically the craniofacial morphology role, may improve OSA screening methods and reduce the backlog of nPSG assessments by improving the referral algorithms.
METHODS
Protocol and registration
This systematic review has followed the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)13 and was registered at PROSPERO database (University of York, York, UK) under the code CRD42020203051.
Search strategy and eligibility criteria
The definition of eligibility criteria was guided by a PECO (Population, Exposure, Comparison, and Outcome) question: “In children and adolescents, are specific craniofacial features linked to fully diagnosed pediatric OSA?” The studies focused on children or adolescents (P) in which the craniofacial features were assessed with a positive OSA diagnosis through nPSG (E) compared to those with a negative diagnosis for OSA through nPSG (C), evaluating the differences in mean values of craniofacial variables (O).
Observational studies were included if they evaluated OSA by a whole-night nPSG monitored by a sleep technician. To be considered for the non-OSA group, a participant had to have a negative diagnosis after an nPSG. As exclusion criteria in this review, we did not consider studies with adults (≥ 18 years) and without an nPSG non-OSA control group. We also excluded studies that evaluated only obese patients, children presenting with known craniofacial syndromes, or those who had received orthodontic or orthognathic treatment before craniofacial evaluation. No restrictions were made regarding the type of craniofacial assessment or craniofacial area that was considered. Studies using lateral cephalometrics, photographic analysis, and in vivo clinical evaluation were deemed eligible for this review. Reviews, letters, conference abstracts, and personal opinions were also excluded. No restriction of sex or ethnicity was considered.
Searches were conducted in 7 electronic databases until May 2021: PubMed, MEDLINE via OvidSP, Embase, Web of Science, The Cochrane Library, and LILACS. A narrow gray literature search was also performed in OpenGrey. According to the rules of each database and with the guidance of a health sciences librarian, all searches were conducted using a combination of controlled predefined MeSH (Medical Subject Headings) and free terms related to the topic (Table S1 (142.7KB, pdf) in the supplemental material). The results were imported to a reference manager software (Rayyan software; Qatar Computing Research Institute, Doha, Qatar),14 and duplicate citations were excluded.
Study selection
The selection process was conducted in 2 phases by 2 reviewers (N.C.F.F. and S.G.C.) and checked by a third examiner (C.F.M.) in cases of disagreement. First, the citations were evaluated according to their title and abstract. Second, the selected articles were assessed through their full text. After these 2 steps, additional citations were sought by an analysis of the reference lists of all previously selected articles. Finally, the eligibility criteria, including the specified PECO strategy and study types, were considered the analysis of the articles in both phases.
Data extraction
A table was used to report the country, year of publication, demographic features (age, body mass index, and ethnicity), criteria adopted to define OSA, methods used to assess the craniofacial area, main results, and statistical analysis. This extraction was performed by 2 examiners (N.C.F.F. and S.G.C.). If necessary, in the case of lack of information, attempts to contact the authors were made by email. The contact attempts consisted of weekly emails for up to 3 consecutive weeks.
Outcomes
The main outcome considered was a finding of differences in the craniofacial abnormalities of children and adolescents with and without OSA. Secondary outcomes were the association of these results with demographic features and OSA severity.
Risk of bias among included studies
The risk-of-bias evaluation was performed using the Joanna Briggs Institute Critical Appraisal Checklist for Analytical Cross‐sectional Studies.15 The articles included were judged as high risk (yes score ≥ 49%), moderate risk (yes score = 50%–69%), and low risk (yes score ≥ 70%).16 The evaluation was performed by 2 reviewers (N.C.F.F. and S.G.C.), and disagreements were resolved by a third reviewer (C.F.M.).
Synthesis of results
The difference between craniofacial features of children with and without OSA was assessed using Review Manager software v.5.3 (Cochrane, London UK) when a low methodological and clinical heterogeneity was detected. The statistical heterogeneity significance was evaluated using the I2 index. Thresholds for the interpretation of the I2 statistic adopted from the Cochrane Handbook for Systematic Reviews of Interventions (www.training.cochrane.org/handbook; accessed January 28, 2022): 0%–40%: might not be significant, 30%–60%: may represent moderate heterogeneity, 50%–90%: may represent substantial heterogeneity, 75%–100%: considerable heterogeneity.
Risk of bias across studies
The overall strength of evidence was evaluated using the grading of recommendations, assessment, development, and evaluations tool (GRADE Handbook). 17 Included studies were evaluated according to their study design, risk of bias, inconsistent results, indirect evidence, imprecision, and publication bias.
RESULTS
Study selection
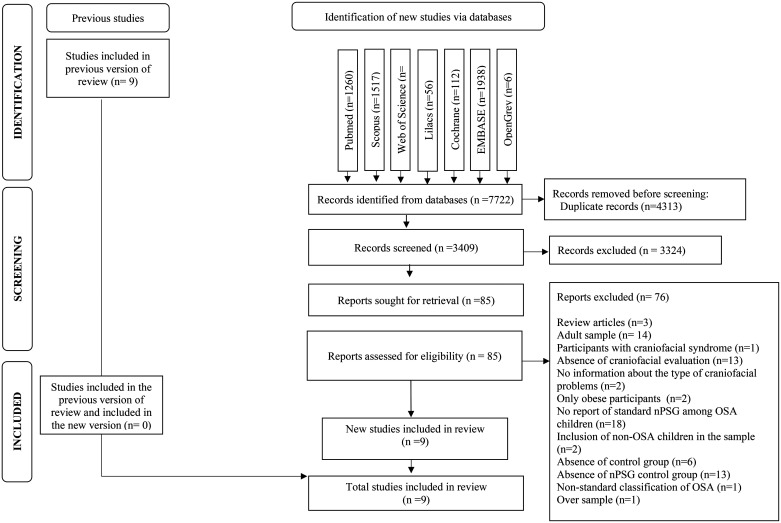
From electronic searches, 8288 citations were identified. After removing duplicate results, 3475 records were assessed by title and abstract, and out of these, 87 were considered for full-text reading. Among these, 76 studies did not meet our eligibility criteria and were excluded (Table S2 (142.7KB, pdf) in the supplemental material). In addition to the electronic searches, the 9 studies included in the previous version of this systematic review10 were also screened in the full-text phase. However, none of these articles met the updated inclusion criteria proposed by the present review. After the selection process, 9 studies fit our criteria and were included11,12,18–24 (Figure 1).
Figure 1. Flowchart according to PRISMA guidelines.
nPSG = nocturnal polysomnography, OSA = obstructive sleep apnea, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study characteristics
Among the 9 included studies, 4 presented a cross-sectional design,11,19,23,24 4 were case-control studies,12,18,21,22 and one was a prospective cohort.20 For the studies that were not cross-sectional, only the relevant information at the initial data gathering point was considered (at that data point cross-sectional in nature).
Six studies evaluated craniofacial skeletal features assessed through lateral cephalometrics.18,19,21–24 Two studies analyzed dental arch dimensions and tooth position through dental models,12,20 and 1 study performed evaluations of facial soft tissue features through 2-dimensional photo analysis11 (Table 1).
Table 1.
Characteristics of the included studies.
| Author/Year/ Study Design | Source of Sample | n | Age | Control Group Features | OSA Index | Ethnicity | BMI | Assessment of Adeno-tonsillar Size | Craniofacial Evaluation | Statistical analysis | Main Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deng 2012/CS 18 | Beijing Children’s Hospital (sleep center) and Department of Orthodontics, Peking University, China | Total: 30 OSA: 15 Control: 15 | 9.5 ± 1.0 | Nonsnoring, first visit patients in Department of Orthodontics | Control group: AHI < 1; OSA: AHI = 6.29 ± 6.48 | Asian | NI | NI | Cephalometric analysis (20 morphologic variables). | Paired t tests, ANOVA | SNB (78.71 ± 2.61 vs75.82 ± 4.30), PG-NB (0.62 ± 0.60 mm vs 1.32 ± 0.84), Na-Me (108.50 ± 6.93 mm vs 13.62 ± 10.0 mm), and ANS-Me (61.51 ± 3.22 mm vs 65.12 ± 5.91 mm) showed statistical difference between control and OSA groups. A more inferior and retrusive hyoid was described in OSA group. |
| Di Francesco 2012/CC 19 | Otolaryngology Department of the University of São Paulo Medical School, Brazil. | Total: 77 OSA: 36 Control: 41 | 3.0–12.0 | Children with OSA symptoms | NI | NI | BMI z-score Male: –0.5 (–1.38, 0); Females: 0 –1.5, 0) | Yes | Cephalometric analysis (6 skeletal craniofacial variables). | Spearman’s rank correlation test | Facial depth (r = –.336), vertical growth tendency (r = –.337) and mandibular plane (r = .486) correlated with AHI in boys, but no correlations were found in girls. |
| Markkanen 2019/P-CH 20 | Tampere University Hospital, Finland | Total: 27 OSA: 9 Control: 18 | 1.9–2.8 | Nonsnoring children | Control group: NI; OSA: OAHI= 1.2–6.3 (median= 1.5) | NI | BMI: OSA group: 15.5–17.0; control: 16.0–17.8 | Yes | Dental and face evaluated by wax bite and profile photograph | Mann-Whitney U test | Children with OSAS (median: 27.0 mm) had narrower intercanine width than nonsnoring children (median: 28.2 mm). |
| Caiza Rennella 2017/CC 23 | Pontificia Universidad Javeriana, Colombia | Total: 43 OSA: 19 Control: 24 | 6.0–13.0 | Children with an indication to nPSG | NI | NI | NI | NI | Cephalometric analysis (12 skeletal craniofacial variables). | Independent t test and Mann-Whitney U test | Children with OSAS had same features as controls. |
| Soares 2020/CC 24 | Centro do Respirador Bucal of the Clinics Hospital Ribeirão Preto Medical School, University de São Paulo, Brazil | Total: 76 OSA: 62 Control: 14 | 7.0–10.0 | Children with respiratory and OSA symptoms | Control group: OAHI = 0.5 ± 0.2; OSA: OAHI = 13.0 ± 8.4 | NI | BMI> 95th percentile patients were excluded. | NI | Cephalometric analysis (9 skeletal craniofacial variables). | Independent t test | There were no differences between the 2 groups for any craniofacial measure. Children with OSA showed a more inferior hyoid position in relation to the mandibular plane (HyMP: control group = 10.9 ± 0.9 and OSA group = 13.1 ± 0.5; 95% CI: 0.08; 4.32). |
| Sutherland 2019/CC 11 | Melbourne Children’s Sleep Centre for PSG, Australia. | Total: 59 OSA: 50 Control: 9 | 7.2 ± 3.4 | Nonsnoring children | Control group: OAHI = 0.2 ± 0.3 for the control group; mild OSA group: OAHI = 2.8 ± 1.3; moderate-severe OSA group: OAHI= 14.5 ± 11.1 | 73.1% were Caucasian | BMI z-score: 0.6 ± 1.3 (− 3.7–3.3) | NI | Craniofacial measurements from 2-dimensional photography. | MANOVA | No association was observed between OSA and facial features. A direct association was observed between OSA severity and the inferior and posterior position of the hyoid bone. |
| Pirilä-Parkkinen 2009/CS 12 | Children referred from primary health care units to the Department of Otorhinolaryngology of Oulu University Hospital, Finland | Total: 123 OSA: 41 Snoring: 41 | 3.8–11.4 | Snoring children | Control group: AHI = 0.1 ± 0.2; OSA: AHI = 3.5 ± 3.60. | NI | NI | Yes | Dimensions of dental arches measured in upper and lower dental casts | ANOVA | Children with OSAS had same features as snoring children. |
| Pirilä-Parkkinen 2010/CS 21 | Children referred from primary health care units to the Department of Otorhinolaryngology of Oulu University Hospital, Finland | Total: 140 OSA: 26 Snoring: 27 Upper airway resistance syndrome: 17 Control: 70 | 4.7 ± 2.1 | Snoring children | Control group: AHI = 0.2 ± 0.1; OSA group: AHI = 2.5 ± 1.2 | NI | Only nonobese children | Yes | Cephalometric analysis (11 morphologic, 10 airway, 3 hyoid bone position, and 5 postural variables). | Paired t tests, ANOVA | Children with OSAS had same features than snoring children. |
| Wang 2012/CS 22 | Qilu Hospital, Shandong University, Jinan, China | Total: 70 OSA: 24 Snoring:12 Control: 34 | 9.6 ± 1.9 | Snoring children | Control group: AHI = 1.7 ± 1.2; OSA group: AHI = 8.5 ± 3.6 | NI | OSA: 14.790 ± 1.125 control: 15.993 ± 1.303 | NI | Cephalometric analysis (16 craniofacial skeletal variables, 7 craniofacial soft tissue variables). | ANOVA | Children with OSAS had same features as snoring children. A reduced anteroposterior linear dimensions of the bony nasopharynx (decreased pharyngeal diameters at the levels of the adenoids) was observed when children with OSA were compared to a non-nPSG group. |
AHI = apnea-hypopnea index, ANOVA = analysis of variance, ANS-Me = anterior nasal spine to menton point, BMI = body mass index, CC = case-control study design, CH = prospective cohort, CS = cross-sectional, HyMP = hyoid position in relation to the mandibular plane, MANOVA = Multivariate Analysis of Variance, Na-Me = nasion-A point to menton line, NI = Not indicated, OAHI = obstructive apnea-hypopnea index, OSA = obstructive sleep apnea, OSAS = OSA syndrome, PG-NB = pogonion to nasion-B point, PSG = polysomnography, SNB = sella-nasion to B point angle.
In the 6 studies that evaluated skeletal craniofacial features, 182 children with OSA and 133 control children were screened. Three studies18,19,21 found differences between children with OSA and the non-OSA control group. For example, children with OSA presented with:
a retrusive mandible (reduced sella-nasion to B point angle [SNB] angle, OSA group = 75.8 ± 4.3 degrees vs control = 78.71 ± 2.6),18
deficient chin (increased pogonion to nasion-B point line [PG-NB], OSA group = 1.3 ± 0.8 mm vs control = 0.62 ± 0.60 mm),18 and
long lower face (increased anterior nasal spine to menton point [ANS-Me], OSA group = 67.4 ± 6.4 mm vs control = 62.2 ± 3.1 mm).18
In addition, among boys, some craniofacial features, including dolichocephaly facial pattern (r = –.33), mandibular plane (r = .48), and facial depth (r = –.33), were correlated to OSA in 1 study.19 The other 3 studies did not report statistical differences in craniofacial skeletal features. A reduced anteroposterior linear dimensions of the bony nasopharynx (decreased pharyngeal diameters at the levels of the adenoids) was observed when children with OSA were compared to a non–polysomnography non-nPSG group:
reduced PNS-ad1 (distance from the posterior nasal spine [the most posterior point of the bony hard palate] to the nearest adenoid tissue measured along the line PNS–Ba), OSA group = 17.3 ± 6.2 mm vs non-nPSG control = 20.9 ± 3.9 mm;
reduced ve1-ve2 (minimal distance from the velum palatine to the posterior pharyngeal wall measured perpendicular to the direction of the airway), OSA group = 4.0 ± 3.0 mm vs non-nPSG control = 7.4 ± 2. 9 mm;
reduced u1-u2 (airway space on a line from the tip of uvula to the posterior pharyngeal wall measured perpendicular to the direction of the airway), OSA group = 5.6 ± 3.3 mm vs non-nPSG control = 9.6 ± 3.4 mm;
reduced rl1-rl2 (minimal distance from the radix linguae [base of the tongue] to the posterior pharyngeal wall measured perpendicular to the direction of the airway), OSA group = 12.7 ± 3.8 mm vs non-nPSG control = 10.1 ± 3.0 mm.19
Two studies analyzed dental arch dimensions and tooth position,12,20 in which 35 children with OSA and 41 non-OSA snoring children were evaluated. Patients from different age groups were included in both studies. Compared to a negative nPSG control group, both studies did not show differences in the variables being assessed. In a group of 2.5-year-old children, a narrower upper intercanine width in the OSA group (median = 27 mm) compared to a nonsnoring group (median = 28.2 mm) was identified (P = .03).20
One study evaluated soft facial features of 59 children with OSA and 9 non-OSA, nonsnoring, control children by analyzing 2-dimensional facial photos. An increase in the obstructive apnea-hypopnea index (OAHI) was associated with an increase in the cervicomental angle (β = 0.18, 95% CI = 0.07, 0.29) and an increase in the ratio of upper to lower–face height (β = –37.16, 95% CI = –65.71, –8.62).11
Eight of the 9 studies included the evaluation of comorbidities associated with pediatric OSA (ethnicity, body mass index/obesity status, and adenotonsillar hypertrophy).
Three studies assessed the size of adenoids and tonsils in their sample without analyzing the interaction between OSA and craniofacial morphology.20,21,25
Regarding the characteristics of the non-OSA control groups, all studies included children with a negative nPSG result (apnea-hypopnea index < 1 or OAHI < 2). In addition, 3 studies included snoring patients,21,22,25 3 studies included children with respiratory or OSA symptoms,19,23,24 and 3 studies had only nonsnoring children in the control group.11,18,20
Risk of bias among included studies
The risk of bias was classified as low in all included studies. Nevertheless, specific problems were identified in some domains. None of the studies considered confounding factors. Rennella et al 201723 presented unclear information regarding how the nPSG diagnosed the OSA. Soares et al 202024 did not report the period of data collection (Table S3 (142.7KB, pdf) in the supplemental material).
Synthesis of results
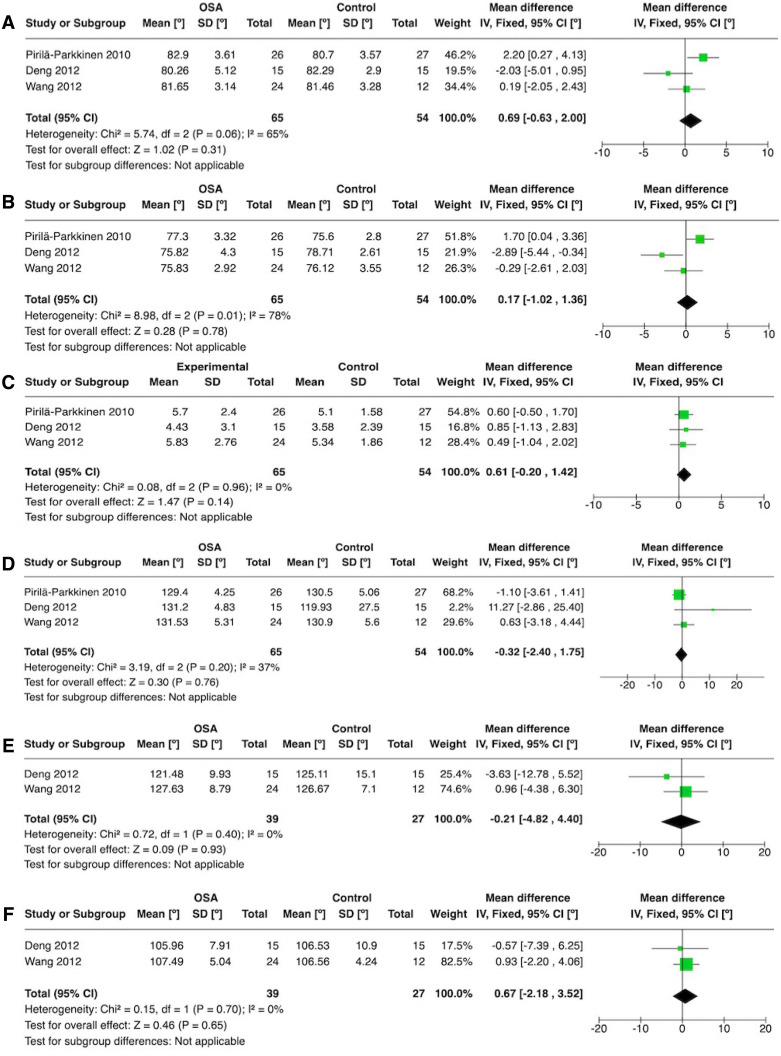
Among the 6 studies which evaluated cephalometric parameters, 3 studies18,21,22 reported a few consistent cephalometric variables and presented methodological and clinical comparable data to justify a quantitative synthesis. Six independent meta-analyses were performed to evaluate the mean differences of sella-nasion to A point angle (SNA) (1), sella-nasion to B point angle SNB (2), A point-nasion-B point angle (ANB) (3), nasion-sella-basion angle (cranial base flexure angle) (NSBa) (4), upper incisor to lower incisor angle (U1-L1) (5), and upper incisor to nasion-sella angle (U1-SN) (6). For the SNA, ANB, and NSBa, all 3 studies18,21,22 were included. For the U1-L1 and U1-SN features, only 2 studies18,22 were compared. The meta-analyses results did not show differences in any of the 6 evaluated features (Figure 2).
Figure 2. Forest plot of meta-analysis.
Mean difference among OSA and control groups for the following skeletal angles: (A) SNA, (B) SNB, (C) ANB, (D) NSBa, (E) U1-L1, (F) U1-SN. ANB = A point-nasion-B point angle, CI = confidence interval, IV = inverse variance, NSBa = nasion-sella-basion angle (cranial base flexure angle), SD = standard deviation, SNA = sella-nasion to A point angle, SNB = sella-nasion to B point angle, U1-L1 = upper incisor to lower incisor angle, U1-SN = upper incisor to nasion-sella angle.
A quantitative evaluation was impossible among the studies that analyzed dental arches and tooth position because the age range in the 2 studies was not comparable. Markkanen et al (2019) included children at 2.5 years old, while Pirilä-Parkkinen (2009) evaluated children from 3–10 years old.12,20
Risk of bias across studies
Two certainty analyses were performed after data collection. Due to the small number of studies included on each outcome (n < 10), publication bias was not considered. In the first analysis, 3 main outcomes were considered: skeletal features, soft facial features, and dental arch morphology. A low to moderate certainty level was observed in which only the skeletal features reported some differences between OSA and non-OSA groups (Table 2).
Table 2.
Certainty assessment (GRADE tool) for the evaluation of skeletal, soft facial features, and dental arched morphology outcomes.
| Outcome; Number of Participants (Studies) | Relative Effect (95% CI) | Certainty | What Happens |
|---|---|---|---|
| Skeletal features; 315 (6 observational studies) | Not estimable | ⊕⊕◯◯ LOWa | Three studies found differences in the cephalometric features of children with OSA compared to a control group. Two studies reported a class II skeletal pattern and a vertical craniofacial growth tendency in the OSA group. One study also reported an inferiorly positioned hyoid in the OSA group. |
| Soft facial features; 59 (1 observational study) | Not estimable | ⊕⊕⊕◯ MODERATEb | OSA probably results in little to no difference in soft facial features. |
| Dental arches morphology; 109 (2 observational studies) | Not estimable | ⊕⊕◯◯ LOWa | Children with OSA may present little to no difference in dental arches morphology. |
aOverlap among CIs was observed across studies. bOnly 1 study was included and presented a wide variation among CIs. CI = confidence interval, GRADE = Grading of Recommendations, Assessment, Development and Evaluations, OSA = obstructive sleep apnea. Very low = The true effect is probably markedly different from the estimated effect, Low = The true effect might be markedly different from the estimated effect, Moderate = The authors believe that the true effect is probably close to the estimated effect, High = The authors have a lot of confidence that the true effect is similar to the estimated effect.
A very low to moderate certainty level was detected among the 6 cephalometrically assessed outcomes following the meta-analyses results: SNA (1), SNB (2), ANB (3), NSBa (4), U1-L1 (5), and U1-SN (6). A serious and very serious inconsistency was observed in SNA and SNB outcomes due to moderate to high statistical heterogeneity. Another pitfall that downgraded the overall certainty was the presence of a serious imprecision in the ANB outcome and a very serious imprecision in the SNA, SNB, NSBa, U1-L1, and U1-SN outcomes (Table 3).
Table 3.
Certainty assessment (GRADE tool) for the quantitative cephalometric variables evaluated.
| Certainty assessment | Summary of findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) Follow up | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence | Study event rates (%) | Relative effect (95% CI) | Anticipated absolute effects | ||
| With control | With OSA | Risk with control | Risk difference with OSA | ||||||||
| SNA | |||||||||||
| 119 (3 observational studies) | not serious | seriousa | not serious | very seriousb | none | ⨁◯◯◯ VERY LOW | 54 | 65 | - | The mean SNA was 81.5º | MD 0.69º higher (0.63 lower to 2 higher) |
| SNB | |||||||||||
| 119 (3 observational studies) | not serious | very seriousc | not serious | very seriousb | none | ⨁◯◯◯ VERY LOW | 54 | 65 | - | The mean SNB was 76.81º | MD 0.17º higher (1.02 lower to 1.36 higher) |
| ANB | |||||||||||
| 119 (3 observational studies) | not serious | not serious | not serious | serious | none | ⨁⨁⨁◯ MODERATE | 54 | 65 | - | The mean ANB was 4.7º | MD 0.61º higher (0.2 lower to 1.42 higher) |
| NSBa | |||||||||||
| 119 (3 observational studies) | not serious | not serious | not serious | very serious | none | ⨁⨁◯◯ LOW | 54 | 65 | - | The mean NSBa was 127.11º | MD 0.32º lower (2.4 lower to 1.75 higher) |
| U1-L1 | |||||||||||
| 66 (2 observational studies) | not serious | not serious | not serious | very serious | none | ⨁⨁◯◯ LOW | 27 | 39 | - | The mean U1-L1 was 125.89º | MD 0.21º lower (4.82 lower to 4.4 higher) |
| U1-SN | |||||||||||
| 66 (2 observational studies) | not serious | not serious | not serious | very seriousb | none | ⨁⨁◯◯ LOW | 27 | 39 | - | The mean U1-SN was 106.54º | MD 0.67º higher (2.18 lower to 3.52 higher) |
aI2 = 65%. bA large variation in 95% CI was detected. cI2 = 78%.
CI: Confidence interval; MD: Mean difference.
DISCUSSION
Previously, craniofacial morphology has been suggested as one of the potential causes of airway collapsibility during sleep. This systematic review screened over 8000 citations and identified 9 studies investigating this relationship. Among those, 5 articles reported no differences in the craniofacial features of OSA and control groups. The other 4 articles suggested that a specific group of children with OSA might present with a set of skeletal and craniofacial features suggestive of a class II tendency and a long facial profile. However, these results were not supported by meta-analyses. In sum, these results indicate that we should not suggest the existence of an association between specific craniofacial features and pediatric OSA. Even though a particular subgroup of pediatric OSA might present with an increased mandibular retrognathia, maxillary transverse deficiency, or a long facial profile, the investigation of associated clinical factors is needed to confirm or refute these features as possibly being causatively or consequentially associated with OSA in children. An important consideration is that this lack of strong association may reflect the methodological approaches. Lately, stronger arguments have arisen that imply that specific clinical phenotypes may have a stronger association with craniofacial features while other phenotypes do not.
The evaluation of the main features of craniofacial morphology included skeletal, soft features, and dental analyses. Regarding dental assessment, a narrower intercanine width was described among children with OSA.20 The reported skeletal differences suggest a class II malocclusion tendency (retruded mandible) and a vertical craniofacial growth tendency (long lower face, dolichocephaly facial pattern).18,21 In concordance with skeletal results, the analysis of the soft features also suggested an increase in lower face height (relative to upper face height) among children with OSA.11 However, when data of this review was quantitatively evaluated, none of the 6 skeletal variables (SNA, SNB, ANB, NSBa, U1-L1, and U1-SN) compared through a meta-analysis showed a difference between OSA and non-OSA groups. These findings indicate the need to further investigate craniofacial morphology as a clinical phenotyping factor in pediatric OSA. Even though some of the included studies reported differences, there is no consensus in the literature.
Our group conducted a previous systematic review on the same topic and only considered skeletal features, and no exclusion criteria were defined for non-PSG control groups.10 Similarly to the results reported in the present review, a vertical direction of growth and a tendency to class II malocclusion were described.
We raise some hypotheses to justify a possible or lack of an association between some craniofacial features and pediatric OSA. One of them is the influence of craniofacial bones and position on airway size and contribution to airway obstruction. On the other hand, reduced mandibular growth might be a consequence of airway obstruction and sleep-disordered breathing (SDB). Children presenting with a mandible retrognathia, resulting in a class II, were associated with a narrower pharyngeal airway.19 However, the association between a class II skeletal pattern and a reduced airway size among healthy children is still controversial.26 Also, other craniofacial features, including the cranial base length, have not presented an association (or the lack of it) with SDB in children.27 In summary, the differences in the craniofacial pattern observed in children with OSA might be linked to other factors not exclusively dependent on these anatomical features.
A vertical craniofacial direction of growth, and an increased lower anterior face height, could also represent a consequence of airway obstruction, as suggested by animal studies.28 This feature was associated with multiple SDB signs and symptoms, including mouth breathing and adenotonsillar hypertrophy. To better understand a possible interaction between the vertical direction of growth and OSA, the causal relationship of this association should be explored in a longitudinal analysis.
The reported differences between craniofacial features of children with OSA and an nPSG-negative control group in only part of the studies included in this review could also be explained by the heterogeneity and multifactorial nature of OSA. Despite the abnormalities on craniofacial bones, other anatomical factors (ie, muscle tone), including obesity, adenotonsillar size, pharyngeal size, and genetic or biomechanical factors (ie, fluid dynamics), as airflow resistance, could be risk factors for pediatric OSA.29
Overall, there is limited knowledge of clinical and physiological phenotypes of OSA, and the majority of evidence is focused on nPSG sleep variables.30 Available evidence suggests that lateral pharyngeal wall thickness and blood pressure are potential OSA phenotypes in children and adolescents.31 There is a need to explore further the clinical phenotypes linked to pediatric OSA to improve the understanding regarding the role of craniofacial morphology in this disease.
Regarding the influence of other pediatric OSA risk factors on the craniofacial assessment, the adenotonsillar size and mouth breathing have been evaluated by the studies included in this systematic review. The adenotonsillar size was assessed in 2 studies. One of them reported no association between this variable and apnea-hypopnea index (AHI) values in a group of 4- to 11-year-old children.12 The other study observed a larger adenoid size and increased mouth breathing among the OSA group in a group of 2.5-year-old children.20 However, the interaction between those factors and craniofacial features was not explored in any of the included articles. In all selected studies, only nonobese or participants with matched body mass index values were included.
The influence of age has not been investigated in the papers included in this review. However, a wide age range has been considered in the studies. Three studies included participants from preschoolers until adolescence,12,19,21 while 1 study included only minors younger than 3 years,20 and the other 5 articles included children older than 6 years.11,18,22–24 None of the included studies investigated the relationship between anatomical craniofacial changes and pediatric OSA over time. Understanding the effect of normal growth among children with and without OSA might explain the role of craniofacial growth in this population.
This systematic review aimed to evaluate the differences in craniofacial features among OSA and non-OSA groups of children. The criteria defined as control was the presence of a negative result in an nPSG evaluation. First, it is important to highlight the controversies associated with identifying a negative nPSG control group. Among the articles reviewed during our selection process, 12 studies reported healthy children without SDB symptoms and without an nPSG exam as a control group.32–43 Among those, different results were also observed. While 5 studies reported no differences between OSA and the control group, the other 7 described differences in craniofacial morphology. Differences in craniofacial features were observed in the mandible, maxilla, facial height, nasopharyngeal airway at the adenoids, and position of the hyoid bone, and narrower intertooth distances for the first and second deciduous molars and the first permanent molars in children with OSA (Table S5 (142.7KB, pdf) in the supplemental material).
Adopting the nPSG-based diagnosis might also have limitations due to the reliance on a single sleep index, the AHI or the OAHI. In the review, all the selected studies used these indices to define an OSA case. The use of these indices alone for diagnostic and management approaches has been questioned.44 Both AHI and OAHI are based only on the number of obstructive events, without further consideration of comorbidities, OSA symptoms, and quality of life. Other studies should explore the pediatric OSA in its multiple clinical features, including associated factors, for a more reliable diagnosis and understanding of the associated clinical and physiological phenotypes.
Collectively, despite myriad published studies over the previous 100 years within medical and dental journals indicating a secular trend toward a comorbid association of specific malocclusion phenotypes and SDB/OSA symptoms, the results of this systematic review indicate that neither an association nor a lack thereof between craniofacial morphology and pediatric OSA can be supported or refuted. Some specific sets of craniofacial features, including mandible retrognathia, smaller cranial base angle, deficient intercanine width, and a long facial profile were more frequent among a specific subgroup of pediatric OSA. However, there is limited evidence of clinical phenotypes that would help understand the nature of this association. In the future, if this link is confirmed to be a reliable indicator of increased SDB/OSA risk, dental professionals may become even more helpful within collaborative efforts aimed at identifying children at high risk of OSA when SDB signs and symptoms are also identified in this group of children.45 Children presenting these characteristics and other SDB signs and symptoms should be monitored by a sleep medicine or ear, nose, and throat specialist when justified.
Limitations
As a limitation of this systematic review, we may highlight the small sample size and the absence of a sample size justification in the included studies. These characteristics likely represent a bias in the interpretation of the results outside the study. One reason that might explain the difficulty of achieving larger sample sizes among children with pediatric OSA are the accessibility barriers to the nPSG exam, including the high cost and long wait lines for public health services.46,47
The eligibility criteria for the control group in this review was a negative nPSG result. However, only 3 of the selected papers reported that the participants from the control group did not present with any signs or symptoms of SDB.11,18,20 The presence of these signs and symptoms may represent a confounding factor for the craniofacial assessment. Some of these features, such as mouth breathing, are associated with increased clockwise rotation of the mandible and increased lower facial height.48
Even though a low risk of bias was identified, some problems were found when confounding and controlling factors were defined in the analysis of the individual studies. That is why the certainty level for the conclusions was downgraded.
OSA has been associated with multiple comorbidities and disorders in children, including respiratory problems, obesity, adenotonsillar hypertrophy, and craniofacial and behavioral syndromes. The majority of the included studies reported excluding or matching participants regarding obesity, craniofacial syndromes, and adenotonsillar size.4 However, none of the studies evaluated the influence of these features in their results. The consideration of other associated OSA risk factors, such as respiratory problems and behavioral conditions, could be included in future investigations to narrow the possible confounding factor for pediatric OSA.
CONCLUSIONS
Some specific craniofacial features, including mandibular retrognathia, reduced anteroposterior linear dimensions of the bony nasopharynx, smaller cranial base angle, deficient intercanine width, and a long facial profile, were more frequent, but not consistently, among a specific subgroup of pediatric OSA patients. However, due to the very low to moderate certainty level, neither an association nor a lack thereof between craniofacial morphology in pediatric OSA cases can be supported at this time.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- nPSG
nocturnal polysomnography
- non-PSG
non-polysomnography
- NSBa
nasion-sella-basion angle (cranial base flexure angle)
- OAHI
obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- SDB
sleep-disordered breathing
- SNA
sella-nasion to A point angle
- SNB
sella-nasion to B point angle
- U1-L1
upper incisor to lower incisor angle
- U1-SN
upper incisor to nasion-sella angle
DISCLOSURE STATEMENT
All authors have read and approved the final manuscript. Work for this study was performed at the University of Alberta–Canada. Nathalia Fernandes Fagundes is sponsored by Capes Foundation (Brazilian Federal Agency for Support and Evaluation of Graduate Education-88881.128202/2016-01). The authors report no conflicts of interest.
REFERENCES
- 1. Guilleminault C , Tilkian A , Dement WC . The sleep apnea syndromes . Annu Rev Med. 1976. ; 27 ( 1 ): 465 – 484 . [DOI] [PubMed] [Google Scholar]
- 2. Bixler EO , Vgontzas AN , Lin H-M , et al . Sleep disordered breathing in children in a general population sample: prevalence and risk factors . Sleep. 2009. ; 32 ( 6 ): 731 – 736 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang SJ , Chae KY . Obstructive sleep apnea syndrome in children: epidemiology, pathophysiology, diagnosis and sequelae . Korean J Pediatr. 2010. ; 53 ( 10 ): 863 – 871 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joosten KF , Larramona H , Miano S , et al . How do we recognize the child with OSAS? Pediatr Pulmonol. 2017. ; 52 ( 2 ): 260 – 271 . [DOI] [PubMed] [Google Scholar]
- 5. Cross N , Lampit A , Pye J , Grunstein RR , Marshall N , Naismith SL . Is obstructive sleep apnoea related to neuropsychological function in healthy older adults? A systematic review and meta-analysis . Neuropsychol Rev. 2017. ; 27 ( 4 ): 389 – 402 . [DOI] [PubMed] [Google Scholar]
- 6. Olaithe M , Bucks RS , Hillman DR , Eastwood PR . Cognitive deficits in obstructive sleep apnea: Insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation . Sleep Med Rev. 2018. ; 38 : 39 – 49 . [DOI] [PubMed] [Google Scholar]
- 7. Lacedonia D , Carpagnano GE , Patricelli G , et al . Prevalence of comorbidities in patients with obstructive sleep apnea syndrome, overlap syndrome and obesity hypoventilation syndrome . Clin Respir J. 2018. ; 12 ( 5 ): 1905 – 1911 . [DOI] [PubMed] [Google Scholar]
- 8. Leger D , Bayon V , Laaban JP , Philip P . Impact of sleep apnea on economics . Sleep Med Rev. 2012. ; 16 ( 5 ): 455 – 462 . [DOI] [PubMed] [Google Scholar]
- 9. Harding R , Haszard JJ , Schaughency E , Drummond B , Galland B . Parent report of children’s sleep disordered breathing symptoms and limited academic progress in reading, writing, and math . Sleep Med. 2020. ; 65 : 105 – 112 . [DOI] [PubMed] [Google Scholar]
- 10. Flores-Mir C , Korayem M , Heo G , Witmans M , Major MP , Major PW . Craniofacial morphological characteristics in children with obstructive sleep apnea syndrome: a systematic review and meta-analysis . J Am Dent Assoc. 2013. ; 144 ( 3 ): 269 – 277 . [DOI] [PubMed] [Google Scholar]
- 11. Sutherland K , Weichard AJ , Davey MJ , Horne RS , Cistulli PA , Nixon GM . Craniofacial photography and association with sleep-disordered breathing severity in children . Sleep Breath. 2020. ; 24 ( 3 ): 1173 – 1179 . [DOI] [PubMed] [Google Scholar]
- 12. Pirilä-Parkkinen K , Pirttiniemi P , Nieminen P , Tolonen U , Pelttari U , Löppönen H . Dental arch morphology in children with sleep-disordered breathing . Eur J Orthod. 2009. ; 31 ( 2 ): 160 – 167 . [DOI] [PubMed] [Google Scholar]
- 13. Page MJ , McKenzie JE , Bossuyt PM , et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews . BMJ. 2021. ; 372 : n71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ouzzani M , Hammady H , Fedorowicz Z , Elmagarmid A . Rayyan-a web and mobile app for systematic reviews . Syst Rev. 2016. ; 5 ( 1 ): 210 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. JBI Critical Appraisal Checklist for Analytical Cross Sectional Studies . Adelaide, Australia: The Joanna Briggs Institute; 2022. . Accessed February 15, 2022.
- 16. Polmann H , Domingos FL , Melo G , et al . Association between sleep bruxism and anxiety symptoms in adults: A systematic review . J Oral Rehabil. 2019. ; 46 ( 5 ): 482 – 491 . [DOI] [PubMed] [Google Scholar]
- 17. Schunemann H , Brożek J , Guyatt G , Oxman A . GRADE Handbook: Introduction to GRADE Handbook. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. . https://gdt.gradepro.org/app/handbook/handbook.html. Accessed January 28, 2022.
- 18. Deng J , Gao X . A case-control study of craniofacial features of children with obstructed sleep apnea . Sleep Breath. 2012. ; 16 ( 4 ): 1219 – 1227 . [DOI] [PubMed] [Google Scholar]
- 19. Di Francesco R , Monteiro R , Paulo ML de M , Buranello F , Imamura R . Craniofacial morphology and sleep apnea in children with obstructed upper airways: differences between genders . Sleep Med. 2012. ; 13 ( 6 ): 616 – 620 . [DOI] [PubMed] [Google Scholar]
- 20. Markkanen S , Niemi P , Rautiainen M , et al . Craniofacial and occlusal development in 2.5-year-old children with obstructive sleep apnoea syndrome . Eur J Orthod. 2019. ; 41 ( 3 ): 316 – 321 . [DOI] [PubMed] [Google Scholar]
- 21. Pirilä-Parkkinen K , Löppönen H , Nieminen P , Tolonen U , Pirttiniemi P . Cephalometric evaluation of children with nocturnal sleep-disordered breathing . Eur J Orthod. 2010. ; 32 ( 6 ): 662 – 671 . [DOI] [PubMed] [Google Scholar]
- 22. Wang W , Wang Y , Wang X . [Cephalometry study of craniofacial growth in mixed dentition with OSAHS children] . Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012. ; 26 ( 24 ): 1127 – 1129 . [PubMed] [Google Scholar]
- 23. Caiza Rennella ADC , Sotomayor Guamán GE , Terreros Peralta AC , López E , Suarez Á , Otero Mendoza L . Morfología craneofacial en niños con apnea obstructiva del Sueño [Craniofacial morphology in children with obstructive sleep apnea] . Univ Odontol. 2017. ; 36 ( 76 ). [Google Scholar]
- 24. Soares MM , Romano FL , Dias FV da S , et al . Association between the intensity of obstructive sleep apnea and skeletal alterations in the face and hyoid bone. Braz J Otorhinolaryngol. Published online July 27, 2020. [DOI] [PMC free article] [PubMed]
- 25. Pirilä-Parkkinen K , Pirttiniemi P , et al . Cervical headgear therapy as a factor in obstructive sleep apnea syndrome . Pediatr Dent. 1999. ; 21 ( 1 ): 39 – 45 . [PubMed] [Google Scholar]
- 26. Chan L , Kaczynski R , Kang H-K . A cross-sectional retrospective study of normal changes in the pharyngeal airway volume in white children with 3 different skeletal patterns from age 9 to 15 years: part 1 . Am J Orthod Dentofacial Orthop. 2020. ; 158 ( 5 ): 710 – 721 . [DOI] [PubMed] [Google Scholar]
- 27. Abtahi S , Phuong A , Major PW , Flores-Mir C . Cranial base length in pediatric populations with sleep disordered breathing: a systematic review . Sleep Med Rev. 2018. ; 39 : 164 – 173 . [DOI] [PubMed] [Google Scholar]
- 28. Baddam P , Biancardi V , Roth DM , et al . Neural crest-specific deletion of Bmp7 leads to midfacial hypoplasia, nasal airway obstruction and disordered breathing, modelling obstructive sleep apnea . Dis Model Mech. 2021. ; 14 ( 2 ): dmm047738 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stark TR , Pozo-Alonso M , Daniels R , Camacho M . Pediatric considerations for dental sleep medicine . Sleep Med Clin. 2018. ; 13 ( 4 ): 531 – 548 . [DOI] [PubMed] [Google Scholar]
- 30. Armoni Domany K , Hossain MM , Nava-Guerra L , et al . Cardioventilatory control in preterm-born children and the risk of obstructive sleep apnea . Am J Respir Crit Care Med. 2018. ; 197 ( 12 ): 1596 – 1603 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Au CT , Chan KC-C , Zhang J , et al . Intermediate phenotypes of childhood obstructive sleep apnea . J Sleep Res. 2021. ; 30 ( 3 ): e13191 . [DOI] [PubMed] [Google Scholar]
- 32. AlHammad NS , Hakeem LA , Salama FS . Orofacial findings associated with obstructive sleep apnea in a group of Saudi Children . Pak J Med Sci. 2015. ; 31 ( 2 ): 388 – 392 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bergamo AZN , Itikawa CE , de Almeida LA , et al . Adenoid hypertrophy, craniofacial morphology in apneic children . Pediatr Dent J. 2014. ; 24 ( 2 ): 71 – 77 . [Google Scholar]
- 34. Perillo L , Cappabianca S , Montemarano M , et al . [Craniofacial morphology and obstructive sleep apnoea-hypopnoea syndrome: a craniometric comparative analysis] . Radiol Med (Torino). 2013. ; 118 ( 4 ): 648 – 659 . [DOI] [PubMed] [Google Scholar]
- 35. Feng G , Gong X , Yu M , Huang X , Gao X . Differences of craniofacial characteristics in oral breathing and pediatric obstructive sleep apnea . J Craniofac Surg. 2021. ; 32 ( 2 ): 564 – 568 . [DOI] [PubMed] [Google Scholar]
- 36. Cappabianca S , Iaselli F , Negro A , et al . Magnetic resonance imaging in the evaluation of anatomical risk factors for pediatric obstructive sleep apnoea-hypopnoea: a pilot study . Int J Pediatr Otorhinolaryngol. 2013. ; 77 ( 1 ): 69 – 75 . [DOI] [PubMed] [Google Scholar]
- 37. Vieira BB , Itikawa CE , de Almeida LA , et al . Cephalometric evaluation of facial pattern and hyoid bone position in children with obstructive sleep apnea syndrome . Int J Pediatr Otorhinolaryngol. 2011. ; 75 ( 3 ): 383 – 386 . [DOI] [PubMed] [Google Scholar]
- 38. Vieira BB , Itikawa CE , de Almeida LA , et al . Facial features and hyoid bone position in preschool children with obstructive sleep apnea syndrome . Eur Arch Otorhinolaryngol. 2014. ; 271 ( 5 ): 1305 – 1309 . [DOI] [PubMed] [Google Scholar]
- 39. Zettergren-Wijk L , Forsberg CM , Linder-Aronson S . Changes in dentofacial morphology after adeno-/tonsillectomy in young children with obstructive sleep apnoea—a 5-year follow-up study . Eur J Orthod. 2006. ; 28 ( 4 ): 319 – 326 . [DOI] [PubMed] [Google Scholar]
- 40. Eimar H , Al-Saleh MAQ , Cortes ARG , Gozal D , Graf D , Flores-Mir C . Sleep-disordered breathing is associated with reduced mandibular cortical width in children . JDR Clin Trans Res. 2019. ; 4 ( 1 ): 58 – 67 . [DOI] [PubMed] [Google Scholar]
- 41. Kawashima S . Sex-dependent differences in the craniofacial morphology of children with a sleep-related breathing disorder . Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002. ; 94 ( 2 ): 167 – 174 . [DOI] [PubMed] [Google Scholar]
- 42. Schiffman PH , Rubin NK , Dominguez T , et al . Mandibular dimensions in children with obstructive sleep apnea syndrome . Sleep. 2004. ; 27 ( 5 ): 959 – 965 . [DOI] [PubMed] [Google Scholar]
- 43. Smith DF , Dalesio NM , Benke JR , et al . Anthropometric and dental measurements in children with obstructive sleep apnea . J Clin Sleep Med. 2016. ; 12 ( 9 ): 1279 – 1284 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Won CHJ . When will we ditch the AHI? J Clin Sleep Med. 2020. ; 16 ( 7 ): 1001 – 1003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fagundes NCF , Flores-Mir C . Pediatric obstructive sleep apnea—Dental professionals can play a crucial role [published online ahead of print, 2021 Jan 26]. Pediatr Pulmonol. [DOI] [PubMed]
- 46. Katz SL , Witmans M , Barrowman N , et al . Paediatric sleep resources in Canada: the scope of the problem . Paediatr Child Health. 2014. ; 19 ( 7 ): 367 – 372 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Markun LC , Sampat A . Clinician-focused overview and developments in polysomnography . Curr Sleep Med Rep. 2020. ; 6 ( 4 ): 309 – 321 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harari D , Redlich M , Miri S , Hamud T , Gross M . The effect of mouth breathing versus nasal breathing on dentofacial and craniofacial development in orthodontic patients . Laryngoscope. 2010. ; 120 ( 10 ): 2089 – 2093 . [DOI] [PubMed] [Google Scholar]