Abstract
Study Objectives:
Craniofacial malformations with micrognathia cause high grades of obstructive sleep apnea (OSA) measured by polysomnography (PSG). Mandibular distraction osteogenesis is a novel procedure for upper airway obstruction relief. Our primary objective was to describe the utilization of PSGs to improve obstruction in patients undergoing mandibular distraction.
Methods:
This is a retrospective study. Patients with micrognathia and severe upper airway obstruction, presenting with severe OSA diagnosed by PSG, were included from a single tertiary care center between 2015 and 2019. PSGs were done (1) prior to surgery, (2) once the cosmetic goal was achieved (Post-Op 1), and (3) if residual moderate-to-severe OSA was seen, every 2 nights until mild or no OSA was achieved (Post-Op 2).
Results:
Thirteen patients were included. The median age at surgery was 1.1 months (10 days–3 months). All 13 patients had baseline severe OSA, with a median obstructive apnea-hypopnea index of 33 events/h and a median O2 nadir of 73%. Post-Op 1 PSG was done at a median of 6 days after surgery. Median first postoperative obstructive apnea-hypopnea index in all 13 patients was 6.8 events/h, with a median O2 nadir of 87%. A median additional distraction of 3 mm was needed beyond the traditionally recommended advancement. Long-term follow-up studies at or after 1 year were done in 5 patients, all showing persistent nonsevere OSA.
Conclusions:
This is the first case series utilizing PSGs as a guide for mandibular distraction osteogenesis in patients with micrognathia showing the need for jaw overcorrection to achieve resolution of OSA.
Citation:
Kochhar R, Modi V, de Silva N, et al. Polysomnography-guided mandibular distraction osteogenesis in Pierre Robin sequence patients. J Clin Sleep Med. 2022;18(7):1749–1755.
Keywords: apnea, sleep, polysomnography, mandibular distraction osteogenesis
BRIEF SUMMARY
Current Knowledge/Study Rationale: Congenital craniofacial malformations associated with micrognathia, such as Pierre Robin sequence, can cause obstructive sleep apnea due to upper airway obstruction. The gold standard for obstructive sleep apnea diagnosis is polysomnography. A newer procedure, such as mandibular distraction osteogenesis, done at an early age is an effective treatment option for severe upper airway obstruction and can avoid the morbidity and mortality associated with tracheostomy, the traditional treatment. However, the endpoint for advancement to relieve upper airway obstruction is not clear.
Study Impact: This is the first case series using polysomnography during mandibular distraction as a guide for jaw advancement in patients undergoing mandibular distraction osteogenesis. Our findings suggest that polysomnography can be useful in determining the adequacy of mandibular distraction osteogenesis, particularly in the neonatal period.
INTRODUCTION
Infants with craniofacial dysmorphologies, such as micrognathia and Pierre Robin sequence, are at risk of sleep-disordered breathing, with obstructive sleep apnea (OSA) being the most common.1 The prevalence of OSA in Pierre Robin sequence is as high as 46–100% depending on the criteria used.2–5 Children with OSA are at high risk of failure to thrive, poor neurocognitive outcomes, and cardiovascular dysfunction, which occur secondary to intermittent chronic hypoxemia and sleep fragmentation.6–9 Therefore, timely and appropriate resolution of OSA can prevent these complications and allow for normal growth and development. Overnight polysomnography (PSG) is the gold standard to diagnose OSA.10 Prior to a recorded PSG, physicians would estimate the severity of obstruction qualitatively using techniques such as airway endoscopy and respiratory monitoring. However, both the diagnosis and the post-treatment improvement tend to be ambiguous due to the lack of a defined measuring technique.
Mandibular distraction osteogenesis (MDO), also known as mandibular advancement surgery, is a relatively new surgical procedure. It allows advancement and elongation of the jaw as a result of initial osteotomy, and a progression of the tongue to reduce both tongue base and supraglottic obstruction.11 Reports have supported the safety and efficacy of MDO in alleviating upper airway obstruction (UAO) in these patients, but there is a paucity of studies that characterize the degree of airway obstruction and the improvement after surgery.12 Currently, there is no protocol or objective measure determining the amount of mandibular advancement needed in order to resolve OSA. Many studies use self-reported aesthetic measures or improvement in oxygen saturation to evaluate mandibular advancement, due to a lack of standard criteria to stop or continue mandibular advancement. Mudd et al13 reported that distraction was continued until light prognathia was evident or soft tissue envelope extent was reached. Sesenna et al14 reported the endpoint of distraction was when symptoms and signs of airway obstruction were resolved and maxillomandibular relationship was improved. To our knowledge, this is the first case series using PSG to guide distraction in infants undergoing MDO.
We hypothesized that PSG-guided MDO can provide a better indication of airway patency and advancement length compared with traditional MDO. The primary objective of our study was to describe the utilization of PSGs to improve obstruction in infants undergoing mandibular distraction.
METHODS
This was a retrospective study. All participants were treated at a tertiary care hospital located in an urban environment (New York Presbyterian–Weill Cornell Medicine, Komansky Children’s Hospital) between January 2015 and September 2019. Included were infants younger than or equal to 1 year of age at initial presentation, who presented with severe UAO.2 The cause for UAO was micrognathia. Severe UAO was determined by PSG showing severe OSA with an obstructive apnea-hypopnea index (OAHI) ≥ 10 events/h and nadir oxygen saturations < 90%.Participants were identified using the International Classification of Diseases and Related Health Problems, Tenth Revision, codes for micrognathia, severe OSA, aged 0–1 year, and the Current Procedural Terminology (CPT) code for mandibular advancement surgery. Subject inclusion occurred through outpatient offices (craniofacial clinic, pulmonary, or otolaryngology [ENT] offices), or when admitted to the hospital. The craniofacial team included ENT, neurosurgery, plastic surgery, oral and maxillofacial surgery, and sleep physicians as well as speech and feeding therapists. Evaluations included laryngoscopy, feeding study, and PSG.
If severe UAO was identified, participants underwent preoperative maxillofacial computed tomography (CT) scan for Virtual Surgical Planning (VSP; 3D Systems, Inc, Rock Hill, SC) with planned advancement of prognathic profile.
Outcome measures
Outcome measures were (1) degree of UAO, reflected by the initial PSG results (preoperative [Pre-Op] PSG), post-MDO when optimal malocclusion asymmetry was corrected (postoperative [Post-Op] PSG 1); (2) resolution of UAO determined by PSG showing mild (OAHI < 5 events/h) or no OSA (Post-Op PSG 2); and (3) follow-up assessment of airway patency 1 year after surgery, including PSG and growth and development milestones, determined by the managing pediatrician.
Polysomnography
Included patients underwent standard level I PSG using a Natus system (Natus Medical Incorporation, San Carlos, CA, USA). The study was attended by a registered polysomnographic technologist. The PSGs were scored using the American Academy of Sleep Medicine (AASM)–recommended rules by a registered polysomnographic technologist with the entire study reviewed and interpreted by a single board-certified sleep physician (H.V.). Scoring of obstructive, central, and mixed apneas and hypopneas was based on accepted AASM criteria.15 Mild OSA was defined as OAHI ≥ 1 and < 5 events/h of sleep, moderate OSA as OAHI ≥ 5 and < 10 events/h of sleep, and severe OSA as OAHI ≥ 10 events/h of sleep. A significant central sleep apnea (CSA) was defined as a central apnea index of ≥ 5 events/h of sleep. The oxygen desaturation index is calculated by the number of oxygen desaturations ≥ 3% per hour of sleep. Initial PSG was done either in the sleep laboratory as an outpatient procedure or at the bedside in the hospital. All postoperative PSGs were done at the bedside, using the same portable Natus system. The studies were performed with the patient in the supine position. All follow-up studies were done as outpatient procedures at least 1 year later.
Surgery
All patients underwent a preoperative maxillofacial CT imaging for virtual surgical planning, and the creation of a 3-dimensional prefabricated guide to plan for screw placements. Patients underwent bilateral mandibular distraction under general anesthesia with nasotracheal intubation. All surgeries were performed by the same surgeon (J.M.N.). All patients had 2-mm advancement intraoperatively before the closure of the incision sites and a further 1 mm every 12 hours. All patients obtained postoperative CT to assess airway changes and mandibular advancement. Patients were treated with antibiotics as long as distractors were in place.
Statistical analysis
Demographic and clinical data are presented as number (n), percentage (%), mean, median, and range. PSG parameters including OAHI values between Pre-Op and Post-Op 1, and between Post-Op 1 and Post-Op 2 PSGs, were compared using paired Wilcoxon signed-rank test. Oxygen nadir values were compared in the same manner. Data for these are shown as box plots; the bottom of the box represents the 25th percentile, the middle bar the median, and the top of the box the 75th percentile and significance were determined by paired Wilcoxon signed-rank test. Pearson’s correlation coefficients were used to assess the relationships between length of total distraction and OAHI values. All P values were 2-sided, with statistical significance evaluated at the 0.05 alpha level. Analyses were performed in R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). The study was approved by Weill Cornell Medicine’s Institutions Review Board.
RESULTS
Demographics
We reviewed data in 13 children, including 9 males. All infants underwent preoperative PSGs. Of these, 10 children had syndromic Pierre Robin sequence with cleft palate, 1 patient was later diagnosed with Kabuki syndrome, and 3 had isolated micrognathia and hypotonia. Nine of the 13 infants were born full term. The median age at surgery was 1.1 months (range: 10 days–3 months). The most common presenting symptoms were noisy breathing including snoring (n = 10), biphasic stridor (n = 5), feeding intolerance, and poor weight gain (n = 4) (Table 1).
Table 1.
Patient demographics and clinical characteristics.
| Patient Demographics | Overall (n = 13), n (%) |
|---|---|
| Birth | |
| Full term | 9 (69.3%) |
| Preterm | 4 (30.7%) |
| Sex | |
| Female | 4 (30.7%) |
| Male | 9 (69.3%) |
| Age at surgery (months) | |
| Mean (SD) | 1.47 (1.09) |
| Median [min, max] | 1.1 [0.3, 3] |
Defined as numbers (n) and percentages (%), for categorical variables and mean (with SD) and median [minimum and maximum], for continuous variables. max = maximum, min = minimum, SD = standard deviation.
Upper airway evaluation with ENT
Six patients underwent preoperative upper airway evaluation with the otolaryngologist. These included the infants outside the neonatal age group. In all 6 patients, examination demonstrated glossoptosis, micrognathia, and some airway edema, and led to the involvement of the oral and maxillofacial surgery team and a 3-dimensional CT scan preoperatively. One patient had mild laryngomalacia and shortened aryepiglottic folds in addition to the above findings. The remaining 7 patients were diagnosed with OSA in the neonatal intensive care unit or in the craniofacial clinic and directly underwent a 3-dimensional CT scan preoperatively when deemed suitable for the procedure.
PSG results
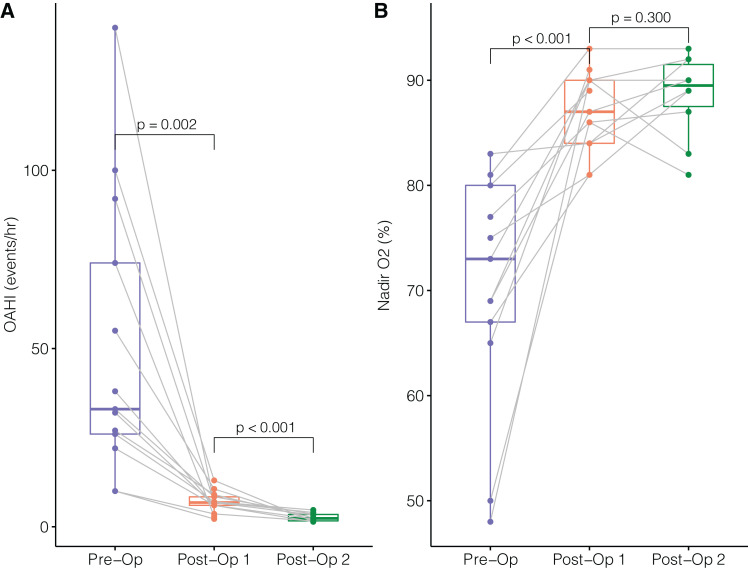
Preoperative PSG (Pre-Op) was done in 13 patients (Figure 1). Five PSGs (38%) were done in the sleep laboratory; the rest were performed in the intensive care unit (either neonatal or pediatric units). The median OAHI was 33 events/h (range: 10–140 events/h) with a median O2 nadir of 73% (range: 48–83%) (Table 2). The median minimum end-tidal CO2 value was 37.6 torr (range: 29–46.2 torr), and the median maximal end-tidal CO2 was 54 torr (range: 47–69.7 torr). The median central apnea index in these 13 patients was 1.3 events/h (range: 0.1–19.8 events/h). The median time between initial PSG to surgery was 7 days (range: 6 days–4 weeks).
Figure 1. Changes in airway obstruction evaluated by PSG following MDO.
(A) Obstructive apnea-hypopnea indices. (B) Nadir oxygen saturations. MDO = mandibular distraction osteogenesis, OAHI = obstructive apnea-hypopnea index, Post-Op = postoperative, Pre-Op = preoperative, PSG = polysomnography.
Table 2.
PSG characteristics: pre- and post-distraction.
| PSG Characteristics | Pre-Op (n = 13) | Post-Op 1 (n = 13) | Post-Op 2 (n = 10) |
|---|---|---|---|
| OAHI (events/h) | |||
| Median [min, max] | 33.0 [10.0, 140] | 6.8 [2.2, 13.0] | 2.35 [1.4, 4.7] |
| O2 nadir (% saturation) | |||
| Median [min, max] | 73.0 [48, 83] | 87.0 [81, 93] | 90.0 [81, 93] |
| Sleep efficiency (%) | |||
| Median [min, max] | 74.5 [45.8, 77.9] | 65 [24.7, 82.2] | 65.2 [57.5, 84.7] |
| ODI (events/h) | |||
| Median [min, max] | 36.5 [4.6, 119.7] | 9.7 [1.3, 41.2] | 4.25 [0.5, 19.4] |
| AI (events/h) | |||
| Median [min, max] | 37.5 [6.9, 123.1] | 32 [18.2, 67.4] | 33 [15.2, 47.3] |
| OSA severity (% of n) | |||
| Mild | 0 | 0 | 0 |
| Moderate | 0 | 23 | 100 |
| Severe | 100 | 77 | 0 |
AI = arousal index, max = maximum, min = minimum, OAHI = obstructive apnea-hypopnea index, ODI = oxygen desaturation index, OSA = obstructive sleep apnea, Post-Op = postoperative, Pre-Op = preoperative, PSG = polysomnography.
First postoperative PSG (Post-Op 1) was done once the prognathic profile with mandibular alveolar crest 2 mm anterior to the maxillary alveolar crest was achieved. The first postoperative PSG was done at a median duration of 6 days (range: 5–13 days) postsurgery in all 13 patients. The median first postoperative OAHI in 13 patients was 6.8 events/h (range: 2.2–13 events/h), with a median O2 nadir of 87% (range: 81–93%) (Table 2).
Advancement continued at a rate of 1 mm twice a day for another 2 days, on average, for 10 patients. Three patients could not be distracted further as per the oral and maxillofacial surgery guidelines; hence, the second postoperative PSG results are available for these 10 patients only. The median second postoperative OAHI in 10 patients was 2.35 events/h (range: 1.4–4.7 events/h), with a median O2 nadir of 90% (range: 81–93%) (Table 2). The second postoperative PSG was done at a median duration of 8 days (range: 7–16 days) from the surgery. Two patients had lower O2 nadirs on the second postoperative study compared with the first, attributable to more central apneic events during the duration of the study. All preoperative and postoperative comparative PSG characteristics are noted in Table 2. The changes in OAHIs and oxygen nadirs between preoperative and post-MDO are noted in Figure 1.
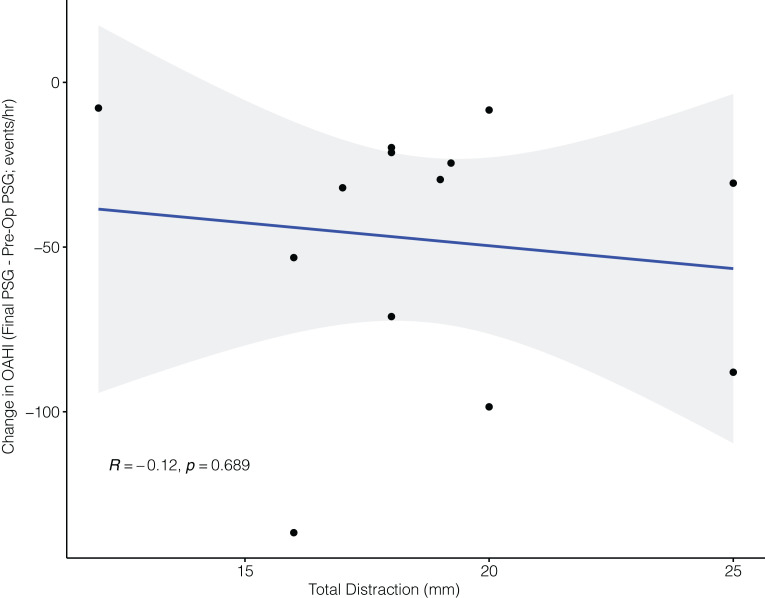
The median initial advancement in the 13 patients was 16 mm (range: 11–20 mm), with a median final advancement of 19 mm (range: 12–25 mm). Three patients could not be advanced further as per the oral and maxillofacial surgery guidelines. A median additional distraction of 3 mm (range: 0–6 mm) was needed beyond the advancement indicated by traditional criteria. Interestingly, the final distraction length was not significantly associated with the change in OAHI (Figure 2).
Figure 2. Changes in PSG obstruction parameters and mandibular distraction length.
OAHI = obstructive apnea-hypopnea index, Pre-Op = preoperative, PSG = polysomnography.
Starting with 100% of infants having severe OSA, on the first postoperative PSG 77% of infants continued to show significant OSA (apnea-hypopnea index [AHI] > 5 events/h). These infants continued to be distracted, and on the second postoperative PSG, all infants achieved a nonsevere OSA, as determined by AHI (Table 2).
Follow-up
Long-term follow-up studies at ≥ 1 year were done in 5 patients, showing that the improvements in AHI were maintained with persistence of nonsevere OSA. All of them were growing well and on an average at the 68th percentile for their weights-for-length (range: 40th–99th percentiles).
DISCUSSION
To our knowledge, this is the first study looking at patients with micrognathia causing severe UAO using PSG to guide MDO. Looby et al16 had previously compared preoperative and postoperative PSGs and maxillofacial CT scans, and similarly, Hammoudeh et al17 had shown improved postoperative AHIs on PSGs when compared with the preoperative AHI; however, our center is the first to utilize the PSGs to guide the distraction process to an adequate degree to achieve resultant nonsevere sleep apnea. In our cohort of 13 patients, we show that MDO can provide immediate and complete airway openings in a subset of craniofacial patients including infants with micrognathia. In addition, we demonstrate that the traditional 2-mm mandibular crest anterior to the maxillary alveolar crest advancement is not always sufficient in resolving UAO in these patients.
This study shows that, when using traditional techniques to determine the sufficiency of advancement, 10 out of 13 patients had residual OSA in the moderate to severe range. A median of 3 mm of additional advancement was required to resolve airway obstruction in those patients, demonstrating the superiority of using PSG to assess the severity of UAO in these patients. As MDO gains popularity as a treatment of micrognathia and other craniofacial abnormalities, it is gradually replacing the more traditional treatment options for severe UAO, such as tracheostomy. It is important to note that surgical correction to an acceptable level might not confirm complete resolution of OSA, and different landmarks should be developed to allow for full airway patency.
Other tools to assess UAO in children with craniofacial malformations include direct observation of the airways using laryngoscopy or drug-induced sleep endoscopy (DISE). Laryngoscopy is done during awake time and does not reflect the worsening of obstruction during sleep and in the supine position. DISE can evaluate airway caliber during sleep but carries the risk of sedation, especially when the majority of patients are infants. Therefore, PSG may be a complementary noninvasive and safe tool to assess airway obstruction. This is supported by Reddy12 who presented a comprehensive review of the literature exploring the role of PSG in the evaluation of UAO in infants with Pierre Robin sequence. He concluded that PSG can help in objectively grading the severity of UAO and has the potential to guide the selection of appropriate intervention. Even though the ideal PSG findings and/or endoscopic findings to guide final distraction status remain unclear, they should ideally correlate with one another. We suggest that surgeons may reconsider what might be the end point of advancement, as a median further advancement of 3 mm was needed for UAO relief in our small cohort of patients. Our study was unfortunately not designed to assess long-term follow-up of the MDO procedure, but we will continue to follow our small cohort over the long term to assess how long the results of MDO are sustained and determine when repeat assessments should happen.
Other reports discussing the utility of MDO as a surgical approach for patients with micrognathia and severe UAO18–22 have shown this to be a safe method for UAO relief. Prior work by Goudy et al23 and Lee et al24 have shown longer lengths of stay and higher complications in neonates with underlying cardiac and feeding issues. Moreover, follow-up PSG studies of 5 of the cohort patients, done at a median duration of 1.2 years (range: 3 months–4 years) after initial surgical repair, had shown persistently low OAHI and oxygen desaturation index values. Normal weight and height percentiles were seen as well.
There are a few limitations to this study. Primarily, the small number of patients included does not allow for robust statistical analysis and conclusions. Inclusion of more patients was difficult because of the rarity of the condition, the inclusion of only patients with severe OSA and UAO, and using only a single center. In addition, the skills needed to perform MDO in the neonatal age are not available in many medical centers.
Because there is no clear definition of normal OAHI in infants, declaring the “normalization of OAHI” is difficult. We refer to research published by Guilleminault and colleagues25 reporting term infants have an obstructive apnea rate of 0.6 events/h at 3 weeks, 1.1 event/h at 6 weeks, 0.4 events/h at 3 months, and 0.2 events/h at 6 months. Similarly, Hoppenbrouwers and colleagues26 reported an obstructive apnea rate in term infants of 0.7 events/h at 1 month, 0.6 events/h at 3 months, and 0.2 events/h at 6 months. However, the fact that, with further advancements OAHI decreased significantly, speaks to the need for further advancement.
We had desired to study these patients yearly after surgery with a PSG; however, there was some limitation to follow-up. In addition, the study was a retrospective one, and even though the same group of physicians was involved in the care of the patients—the same surgeon performed the surgery and the same sleep specialist interpreted all studies—the ability to better control the differences between patients and in-hospital care was compromised. However, despite these limitations, we believe it is important to emphasize the availability of MDO at a young age and the benefit of using PSG as a tool to reflect airway compromise. As the long-term impact of MDO in early infancy is still unclear, prospective longitudinal follow-up studies combining PSGs and clinical parameters are needed.
In conclusion, we believe that PSG may be a helpful tool to guide the degree of mandibular distraction needed to alleviate obstruction in infants undergoing MDO. More research is needed to establish these recommendations in a larger cohort of patients, as well as long-term evaluation of the outcomes of early MDO and possibly expanding it to a multicenter study.
ACKNOWLEDGMENTS
Authors’ contributions: R.K., V.M., S.G.-N., N.d.S., J.M.N., M.J.W., and H.V. designed and performed the study. R.K., H.V., L.M.G., A.A., and E.M. analyzed and interpreted the data. R.K., V.M., and H.V. wrote, revised, and approved the final manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CT
cmputed tomography
- MDO
mandibular distraction osteogenesis
- OAHI
obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- Post-Op
postoperative
- Pre-Op
preoperative
- PSG
polysomnography
- UAO
upper airway obstruction
DISCLOSURE STATEMENT
The final manuscript was seen, read, and approved by all of the authors. Work for this study was performed at Weill Cornell Medicine, New York. The first author was a Pediatric Pulmonary Fellow at Weill Cornell Medicine, New York at the time of the study. She is currently a clinical Sleep Medicine Fellow at Yale School of Medicine, New Haven, Connecticut. The authors report no conflicts of interest.
REFERENCES
- 1. Carey JC , Fineman RM , Ziter FA . The Robin sequence as a consequence of malformation, dysplasia, and neuromuscular syndromes . J Pediatr. 1982. ; 101 ( 5 ): 858 – 864 . [DOI] [PubMed] [Google Scholar]
- 2. Bravo G , Ysunza A , Arrieta J , Pamplona MC . Videonasopharyngoscopy is useful for identifying children with Pierre Robin sequence and severe obstructive sleep apnea . Int J Pediatr Otorhinolaryngol. 2005. ; 69 ( 1 ): 27 – 33 . [DOI] [PubMed] [Google Scholar]
- 3. Gilhooly JT , Smith JD , Howell LL , Deschaine BL , Richey SL . Bedside polysomnography as an adjunct in the management of infants with Robin sequence . Plast Reconstr Surg. 1993. ; 92 ( 1 ): 23 – 27 . [DOI] [PubMed] [Google Scholar]
- 4. Wilson AC , Moore DJ , Moore MH , Martin AJ , Staugas RE , Kennedy JD . Late presentation of upper airway obstruction in Pierre Robin sequence . Arch Dis Child. 2000. ; 83 ( 5 ): 435 – 438 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daniel M , Bailey S , Walker K , et al . Airway, feeding and growth in infants with Robin sequence and sleep apnoea . Int J Pediatr Otorhinolaryngol. 2013. ; 77 ( 4 ): 499 – 503 . [DOI] [PubMed] [Google Scholar]
- 6. Tal A , Leiberman A , Margulis G , Sofer S . Ventricular dysfunction in children with obstructive sleep apnea: radionuclide assessment . Pediatr Pulmonol. 1988. ; 4 ( 3 ): 139 – 143 . [DOI] [PubMed] [Google Scholar]
- 7. Gozal D . Sleep-disordered breathing and school performance in children . Pediatrics. 1998. ; 102 ( 3 Pt 1 ): 616 – 620 . [DOI] [PubMed] [Google Scholar]
- 8. Amin RS , Kimball TR , Bean JA , et al . Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea . Am J Respir Crit Care Med. 2002. ; 165 ( 10 ): 1395 – 1399 . [DOI] [PubMed] [Google Scholar]
- 9. Bonuck K , Parikh S , Bassila M . Growth failure and sleep disordered breathing: a review of the literature . Int J Pediatr Otorhinolaryngol. 2006. ; 70 ( 5 ): 769 – 778 . [DOI] [PubMed] [Google Scholar]
- 10. Marcus CL , Brooks LJ , Draper KA , et al. ; American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome . Pediatrics. 2012. ; 130 ( 3 ): 576 – 584 . [DOI] [PubMed] [Google Scholar]
- 11. Côté A , Fanous A , Almajed A , Lacroix Y . Pierre Robin sequence: review of diagnostic and treatment challenges . Int J Pediatr Otorhinolaryngol. 2015. ; 79 ( 4 ): 451 – 464 . [DOI] [PubMed] [Google Scholar]
- 12. Reddy VS . Evaluation of upper airway obstruction in infants with Pierre Robin sequence and the role of polysomnography—review of current evidence . Paediatr Respir Rev. 2016. ; 17 : 80 – 87 . [DOI] [PubMed] [Google Scholar]
- 13. Mudd PA , Perkins JN , Harwood JE , Valdez S , Allen GC . Early intervention: distraction osteogenesis of the mandible for severe airway obstruction . Otolaryngol Head Neck Surg. 2012. ; 146 ( 3 ): 467 – 472 . [DOI] [PubMed] [Google Scholar]
- 14. Sesenna E , Magri AS , Magnani C , Brevi BC , Anghinoni ML . Mandibular distraction in neonates: indications, technique, results . Ital J Pediatr. 2012. ; 38 ( 1 ): 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berry RB , Budhiraja R , Gottlieb DJ , et al. ; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events . J Clin Sleep Med. 2012. ; 8 ( 5 ): 597 – 619 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Looby JF, Schendel SA, Lorenz HP, Hopkins EM, Aizenbud D. Airway analysis: with bilateral distraction of the infant mandible . J Craniofac Surg. 2009. ; 20 ( 5 ): 1341 – 1346 . [DOI] [PubMed] [Google Scholar]
- 17. Hammoudeh J , Bindingnavele VK , Davis B , et al . Neonatal and infant mandibular distraction as an alternative to tracheostomy in severe obstructive sleep apnea . Cleft Palate Craniofac J. 2012. ; 49 ( 1 ): 32 – 38 . [DOI] [PubMed] [Google Scholar]
- 18. Rhee ST , Buchman SR . Pediatric mandibular distraction osteogenesis: the present and the future . J Craniofac Surg. 2003. ; 14 ( 5 ): 803 – 808 . [DOI] [PubMed] [Google Scholar]
- 19. Lin SJ , Roy S , Patel PK . Distraction osteogenesis in the pediatric population . Otolaryngol Head Neck Surg. 2007. ; 137 ( 2 ): 233 – 238 . [DOI] [PubMed] [Google Scholar]
- 20. Tibesar RJ , Price DL , Moore EJ . Mandibular distraction osteogenesis to relieve Pierre Robin airway obstruction . Am J Otolaryngol. 2006. ; 27 ( 6 ): 436 – 439 . [DOI] [PubMed] [Google Scholar]
- 21. Denny AD , Talisman R , Hanson PR , Recinos RF . Mandibular distraction osteogenesis in very young patients to correct airway obstruction . Plast Reconstr Surg. 2001. ; 108 ( 2 ): 302 – 311 . [DOI] [PubMed] [Google Scholar]
- 22. Wittenborn W , Panchal J , Marsh JL , Sekar KC , Gurley J . Neonatal distraction surgery for micrognathia reduces obstructive apnea and the need for tracheotomy . J Craniofac Surg. 2004. ; 15 ( 4 ): 623 – 630 . [DOI] [PubMed] [Google Scholar]
- 23.Goudy S, Jiramongolchai P, Chinnadurai S. Logistic regression analysis of Pierre Robin sequence patients requiring surgical intervention . Laryngoscope. 2017. ; 127 ( 4 ): 945 – 949 . [DOI] [PubMed] [Google Scholar]
- 24.Lee KC, Eisig SB, Chuang SK, Perrino MA. Neonatal mandibular distraction does not increase inpatient complications . Cleft Palate Craniofac J. 2020. ; 57 ( 1 ): 99 – 104 . [DOI] [PubMed] [Google Scholar]
- 25. Guilleminault C , Ariagno R , Korobkin R , et al. Mixed and obstructive sleep apnea and near miss for sudden infant death syndrome: 2. Comparison of near miss and normal control infants by age . Pediatrics. 1979. ; 64 ( 6 ): 882 – 891 . [PubMed] [Google Scholar]
- 26. Hoppenbrouwers T , Hodgman JE , Cabal L . Obstructive apnea, associated patterns of movement, heart rate, and oxygenation in infants at low and increased risk for SIDS . Pediatr Pulmonol. 1993. ; 15 ( 1 ): 1 – 12 . [DOI] [PubMed] [Google Scholar]