Abstract
Study Objectives:
In most standardized approaches to cognitive behavioral therapy for insomnia, it is commonly the case that total wake time is reduced substantially during sleep restriction, but self-reported total sleep time (TST) is minimally affected. By follow-up, however, TST increases by almost 1 hour on average. A secondary analysis was undertaken to assess what percentage of participants meet or appreciably exceed baseline TST after cognitive behavioral therapy for insomnia.
Methods:
Data were drawn from a randomized controlled trial assessing acute and maintenance therapies for chronic insomnia (n = 80). The present analyses assessed the percentage of participants that 1) reached (≥ 0 minute increase) and 2) appreciably exceeded (≥ 30 minutes increase) baseline TST as assessed via daily sleep diaries at posttreatment and 3, 6, 12, and 24 months following treatment.
Results:
By the end of acute treatment, 45% of participants reached or exceeded baseline TST. By 24 months follow-up, this percentage had increased to 86%. Only 17% of participants achieved a 30-minute increase in TST by the end of acute treatment, and this proportion only increased to 58% over time.
Conclusions:
These findings suggest that cognitive behavioral therapy for insomnia in its current form does not appreciably increase self-reported TST in a significant proportion of patients with insomnia. Whether participants would benefit from further increases in TST warrants investigation. The further titration of sleep opportunity may be useful to accelerate increases in TST, to extend the effect to a larger subset of patients, and/or to increase the magnitude of the TST gain.
Citation:
Scott H, Cheung JMY, Muench A, et al. Does total sleep time substantially increase after cognitive behavioral therapy for insomnia? J Clin Sleep Med. 2022;18(7):1823–1829.
Keywords: insomnia, total sleep time, sleep restriction, sleep opportunity, cognitive behavioral therapy for insomnia
BRIEF SUMMARY
Current Knowledge/Study Rationale: This study is the first to evaluate modal changes in sleep duration following cognitive behavioral therapy for insomnia. This secondary analysis of a previously published investigation aimed to assess the percentage of insomnia patients who met or appreciably exceeded baseline sleep duration after treatment.
Study Impact: While reductions in total wake time are rapid and substantial, increases in sleep duration are slow, variable, and unsubstantial in magnitude in the months following cognitive behavioral therapy for insomnia in a sizeable proportion of patients. Extending cognitive behavioral therapy for insomnia beyond 4–8 weekly sessions focusing on the further upward titration of sleep opportunity may be warranted. While diagnostic criteria for insomnia do not include inadequate sleep duration, increasing sleep duration to meet sleep need may be an important therapeutic target for cognitive behavioral therapy for insomnia.
INTRODUCTION
According to diagnostic criteria, insomnia disorder is defined by difficulties initiating or maintaining sleep and does not require that patients exhibit a short sleep duration.1,2 Presumably, self-reported total sleep time (TST) was not included in the symptom profile because of the difficulties in defining precisely what constitutes pathological short sleep in the general population and what constitutes insufficient sleep specifically for the individual. Since the nosology of insomnia does not include inadequate self-reported sleep duration, it should not come as a surprise that addressing sleep duration is often not a focus of treatment. Yet, short sleep is common in insomnia patients, with the average baseline TST being about 5–5.5 hours in clinical practice.3–5 Such short sleep is considered to be below recommendations from population-level norms6 and may contribute substantially to the reported daytime impairments associated with chronic insomnia. Thus, it is warranted to investigate whether insomnia treatment significantly increases sleep duration.
Sleep restriction therapy (a widely used component of cognitive behavioral therapy for insomnia [CBT-I]) occurs in 2 phases.7,8 Phase 1 is focused on reducing sleep opportunity to match sleep ability (average TST). Phase 2 is focused on the systematic upward titration of time in bed (TIB). The aim of Phase 1 is to reduce the frequency and duration of wake intervals. The aim of phase 2 is to systematically extend TIB to slowly extend sleep duration to meet or exceed baseline levels while maintaining high sleep efficiency. Ultimately, phase 1 produces about a 50% reduction in total wake time (sum of sleep latency, wake after sleep onset, and early morning awakenings [EMA]). This positive outcome is often paralleled by a reduction in TST, with sleep durations at treatment end being lower than those observed at baseline.9 The time required to achieve increased TST varies from patient to patient based upon their adherence to the treatment regimen, the amount of time allowed for sleep extension (phase 2, upward TIB titration), and each individual’s basal sleep need.
Conducting phase 2 with the typical 4- to 8-session treatment model means that TST often remains at or near baseline levels by the end of acute treatment. This is the case because the upward titration of TIB occurs in 15-minute increments (a duration likely adopted by Spielman and colleagues to minimize treatment reversals7), limiting the potential gains in TST that are possible during acute treatment. Several meta-analyses support this conclusion, indicating that self-reported sleep duration is only slightly higher at posttreatment than at baseline on average.10–12 The pretreatment and posttreatment effect sizes for sleep duration after CBT-I are modest (typically approximately10- to 20-minute increases), with effect sizes ranging between d = 0.32 and 0.46.11,13,14 This clinical “gain” is roughly half of what can be expected for sleep latency (SL) and wake after sleep onset (WASO) outcomes. SL within-patient Cohen’s d effect sizes range between 0.67 and 1.05, and WASO effect sizes range between 0.65 and 1.03.11,13,14 The difference in magnitude of these outcomes leads one to wonder if more could and should be done with respect to sleep duration.
Interestingly, when CBT-I has been evaluated for its long-term outcomes, no additional gains are typically made on SL and WASO beyond treatment end, and yet, on average, TST improves in a relatively linear manner without clinical intervention. In the handful of studies that exist, TST appears to improve over the 6-month period following therapy and stabilizes thereafter. More specifically, while SL and WASO are stable, self-reported TST increases by approximately 45 minutes.9,15,16 This change occurs in the absence of continued treatment. Importantly, a 45-minute increase in TST is the average gain: whether this change is common across patients is unknown. Accordingly, the current study was undertaken to evaluate, using archival data from one of the few long-term studies of CBT-I,9,15,16 the percentage of participants who met and/or appreciably exceeded baseline TST at posttreatment, and 3, 6, 12, and 24 months after CBT-I.
METHODS
The present study is a secondary analysis of a previously published investigation on the effects of CBT-I, singly or combined with zolpidem, and the effects of different maintenance strategies on the durability of treatment outcomes.9,15,16 A summary of the methods for the parent study are presented below in addition to the particulars of the current analysis.
Parent study
Participants
Participants > 30 years old who met diagnostic criteria for Insomnia Disorder were recruited for this treatment study. Exclusion criteria included diagnosis of a serious medical condition (eg, cancer, dementia), a history of psychiatric conditions (bipolar disorder, suicide attempts, > 2 major depressive episodes), use of medications that interfere with sleep, substance use disorder within the past 12 months, shift work, or evidence of another occult sleep disorder. Baseline sleep duration was not considered in the eligibility criteria. To assess eligibility, an initial telephone screen was followed by a Structured Clinical Interview based on a combination of criteria from the Diagnostic and Statistical Manual of Mental Disorders, fourth edition,2 and the International Classification of Sleep Disorders, second edition,17 a health examination, and an overnight polysomnography sleep study.
Study protocol
Participants were initially randomized to receive either 6 weekly sessions of CBT-I alone (n = 80) or in combination with 10 mg nightly zolpidem (n = 80). In the CBT-I alone arm, participants received 6 weeks of CBT-I, which consisted of sleep restriction therapy and other standard components (eg, stimulus control and cognitive therapies). Then, participants were randomized again to either a 6-month extended CBT-I condition (6 monthly sessions) or to no additional treatment. The primary focus of the monthly sessions was to consolidate treatment gains through the fine-tuning of the clinical procedures, and to teach strategies for coping with residual symptoms of insomnia and their daytime sequelae. The content of these sessions was more flexible than during acute treatment; as such, they were based on a case-by-case analysis which sought to identify remaining factors that exacerbate or perpetuate sleep disturbances. Generally speaking, the additional sessions were not used for upward titration of TIB, but adjustments were made for those who showed reversals in clinical gains (downward titration). Note that the CBT-I plus medication conditions were not considered in the current analysis.
All participants completed daily sleep diaries during baseline, throughout the treatment phase, postacute treatment, postextended treatment (3 months), and at the 6-, 12-, and 24-month follow-up time points. Derived outcomes included SL, WASO, EMA, TIB, TST, and sleep efficiency.
Present study
Methods
The present analysis focused on the follow-up self-reported TST data from the CBT-I alone arms in the parent study. Both CBT-I only conditions were considered in the current analysis, collapsed together as the study findings were consistent across conditions. Changes in TST were examined at posttreatment and follow-up time points as the percentage of participants who achieved and/or exceeded various thresholds for changes on TST. First, the percentage of participants who achieved an increase in TST (≥ 0 minutes) and/or an appreciable increase (≥ 30, ≥ 60, ≥ 90 minutes) compared to baseline was evaluated at posttreatment and follow-up time points (6, 12, 24 months). Thirty minutes was the chosen threshold as it is a common quantitative threshold for what is considered to be a clinically significant duration for SL, WASO, and EMA. As an exploratory analysis, and because there is no consensus on a threshold for a clinically significant change in TST, the percentage of participants who achieved an increase in TST in 15-minute incremental thresholds (ie, < 0 minutes, 0–14 minutes, 15–29 minutes, 30–44 minutes, etc, up to 90+ minutes) was also evaluated. Fifteen minutes was chosen as it showcased more incremental changes in TST and is the duration by which sleep opportunity should be titrated for sleep restriction therapy. Only self-reported TST was assessed over time in the current study because only this sleep diary variable was found to significantly change after CBT-I.9,15,16
Statistical analyses
Analyses were conducted in IBM SPSS Statistics 25 (IBM Corporation, Armonk, NY). A Friedman test was conducted to test for percentage differences in the TST change categories (< 0, 1–30, and appreciable increases: 31–60, 61–90, and 91+ minutes compared to pretreatment) across the 5 time points after CBT-I treatment (posttreatment and 3-, 6-, 12-, and 24-months follow-up). Since the omnibus test was significant, separate Wilcoxon signed-rank tests were conducted to identify which time points differed. A Bonferroni adjustment for multiple comparisons were applied, resulting in a significance level set at P < .005.
RESULTS
Participant demographics and attrition
While these data have been summarized previously at pretreatment, the data below are presented to characterize the demographic profile across time points. See Table 1 for participants’ sociodemographic characteristics. At pretreatment, participants were typically middle-aged, female, White, employed, and married or common law. The average insomnia duration was 17.5 years, with most participants having a medical comorbidity (> 58%). Further, of the 80 participants randomized to the CBT-I only treatment conditions, 75 completed acute treatment with CBT-I (94% completion rate), 68 completed maintenance CBT-I (85% completion rate), and 57 participants completed the 24-months follow-up assessment (71% completion rate).
Table 1.
Sociodemographic characteristics of participants at each assessment time point.
| Characteristic | Pre-CBT-I (n = 80) | Post-CBT-I (n = 75) | 3 Months (n = 68) | 6 Months (n = 66) | 12 Months (n = 63) | 24 Months (n = 57) |
|---|---|---|---|---|---|---|
| Age, mean (SD), years | 51.7 (10.4) | 51.2 (10.1) | 50.5 (10.1) | 51.2 (10.0) | 51.4 (10.1) | 51.5 (9.9) |
| Sex, n (%) | ||||||
| Female | 50 (62.5) | 49 (65.3) | 44 (64.7) | 43 (65.2) | 43 (68.3) | 38 (66.7) |
| Male | 30 (37.5) | 26 (34.7) | 24 (35.3) | 23 (34.8) | 20 (31.7) | 19 (33.3) |
| Ethnicity, n (%) | ||||||
| White | 79 (99) | 74 (99) | 67 (99) | 65 (99) | 63 (100) | 57 (100) |
| Black | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 0 (0) | 0 (0) |
| Hispanic | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Asian | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Amerindian | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Occupation, n (%) | ||||||
| Employed | 53 (66.3) | 49 (68.1) | 46 (69.7) | 45 (70.3) | 43 (69.4) | 39 (69.6) |
| Retired | 22 (27.5) | 20 (26.7) | 18 (26.5) | 17 (25.8) | 17 (27.0) | 15 (26.3) |
| Homemaker | 2 (2.5) | 2 (2.7) | 1 (1.5) | 1 (1.5) | 1 (1.6) | 1 (1.8) |
| Unemployed | 0 | 0 | 0 | 0 | 0 | 0 |
| Marital status, n (%) | ||||||
| Single | 4 (5.0) | 4 (5.3) | 4 (5.9) | 4 (6.1) | 4 (6.3) | 4 (7.0) |
| Married or common law | 57 (71.2) | 53 (70.7) | 48 (70.6) | 46 (69.7) | 43 (68.3) | 39 (68.4) |
| Divorced or separated | 15 (18.8) | 14 (18.7) | 13 (19.1) | 13 (19.7) | 13 (20.6) | 12 (21.1) |
| Widowed | 4 (5.0) | 4 (5.3) | 3 (4.4) | 3 (4.5) | 3 (4.8) | 2 (3.5) |
| Education duration, mean (SD), years | 14.7 (3.6) | 14.8 (3.6) | 15.2 (3.0) | 15.0 (3.5) | 15.0 (3.6) | 15.0 (3.7) |
| Insomnia duration, mean (SD), years | 17.5 (15.2) | 17.4 (15.0) | 17.1 (15.2) | 17.7 (15.3) | 16.6 (14.6) | 16.4 (14.5) |
| Comorbidity, n (%) | ||||||
| Medical | 48 (60.0) | 45 (60.0) | 40 (58.8) | 40 (60.6) | 38 (60.3) | 35 (61.4) |
| Psychiatric | 11 (13.8) | 11 (14.7) | 8 (11.8) | 10 (15.2) | 10 (15.9) | 10 (17.5) |
CBT-I = cognitive behavioral therapy for insomnia, SD = standard deviation.
Table 2 displays the changes in sleep diary outcomes and the Insomnia Severity Index (ISI) at each time point after CBT-I. As shown in the table, TST on average increased rapidly between posttreatment and 3-months follow-up, and steadily thereafter. Gains in SL, WASO, EMA, sleep efficiency, and the ISI were largely maintained after CBT-I.
Table 2.
Pretreatment and mean changes in sleep diary and ISI outcomes at each assessment time point following CBT-I.
| Outcome | Pre-CBT-I (n = 80) | Post-CBT-I (n = 75) | 3 Months (n = 68) | 6 Months (n = 66) | 12 Months (n = 63) | 24 Months (n = 57) |
|---|---|---|---|---|---|---|
| TST, minutes | 343.97 (75.42) | –6.63 (43.19) | 24.34 (49.46) | 37.54 (56.06) | 39.01 (39.55) | 43.91 (45.86) |
| SL, minutes | 37.18 (37.21) | –20.65 (32.07) | –15.43 (24.40) | –17.14 (29.02) | –16.91 (27.01) | –13.76 (17.17) |
| WASO, minutes | 58.96 (35.45) | –30.67 (30.80) | –21.97 (27.68) | –22.67 (24.34) | –21.39 (28.69) | –20.25 (29.65) |
| EMA, minutes | 57.54 (41.57) | –36.44 (32.29) | –28.70 (28.05) | –29.61 (27.20) | –25.63 (30.23) | –26.70 (24.66) |
| SE, % | 68.99 (14.61) | 14.16 (10.37) | 11.66 (9.70) | 12.76 (9.55) | 11.63 (8.59) | 11.37 (7.39) |
| ISI | 17.26 (4.12) | –8.09 (4.94) | –8.74 (5.35) | –8.03 (5.71) | –8.46 (6.06) | –8.39 (5.66) |
Values are presented as mean (standard deviation). CBT-I = cognitive behavioral therapy for insomnia, EMA = early morning awakenings, ISI = Insomnia Severity Index, SE = sleep efficiency, SL = sleep latency, TST = total sleep time, WASO = wake after sleep onset.
Changes in TST after CBT-I
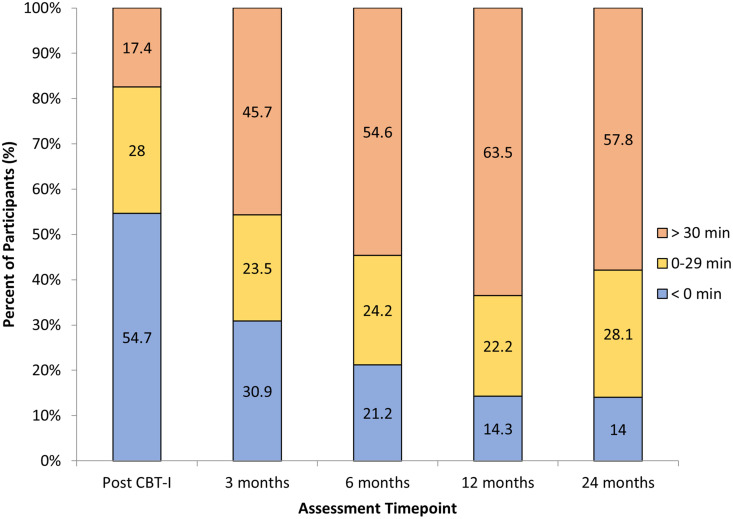
A Friedman test indicated a significant difference in the percentage of patients in the TST change categories (< 0, 1–30, 31–60, 61–90, and 91+ minutes) across the 5 assessment time points after CBT-I, χ2(4) = 60.14, P < .001. Post hoc comparisons across time points were conducted using Wilcoxon signed-rank tests. There were statistically significant differences between the posttreatment and 3-month (Z = –4.89, P < .001), 6-month, (Z = –5.55, P < .001), 12-month (Z = –5.46, P < .001), and 24-month follow-up time points (Z = –5.26, P < .001). At posttreatment, as shown in Figure 1, only 45.4% of participants reached or exceeded baseline TST by the end of the acute CBT-I treatment period. The proportion of the sample that increased TST by between 0 and 29 minutes was 28.0% and only 17.4% increased TST by more than 30 minutes.
Figure 1. Percentage of participants reporting changes in sleep diary total sleep times of less than 0 minutes, between 0 and 29 minutes, or greater than 30 minutes compared with baseline at each assessment time point after treatment.
CBT-I = cognitive behavioral therapy for insomnia.
The percentage of participants who reported increased TST relative to baseline rose from 45.4% at posttreatment to 85.9% by the 24-month follow-up, Z = –2.77, P = .005 (see Figure 1). With Bonferroni correction, there were no significant differences between any other time points, P > .012. Notably, 57.8% of participants reported clinically significant increases in TST of more than 30 minutes at 24-month follow-up. Additionally, between 22.2% and 28.1% of participants consistently reported TST gains of between 0 and 29 minutes across all assessment time points.
Increases in TST were further assessed in 15-minute increments to examine changes in TST more precisely. As shown in Table 3, the majority of participants who increased TST relative to baseline at the posttreatment time points (45% of sample) did so between 0 and 14 and 15 and 29 minutes (28% of sample). By 24-months follow-up, there were more widespread changes in TST across the 15-minute increments. Few patients (14% of sample) reported much greater improvements of > 90 minutes compared to the sample average of approximately 45 minutes, yet 53% of the sample reported changes in TST that were less than the sample average.
Table 3.
Number and percentage of participants reporting changes in sleep diary TST relative to baseline at the posttreatment and follow-up time points in 15-minute intervals.
| Change in TST, minutes | Post-CBT-I (n = 75) | 3 Months (n = 68) | 6 Months (n = 66) | 12 Months (n = 63) | 24 Months (n = 57) |
|---|---|---|---|---|---|
| < 0 | 41 (55) | 21 (31) | 14 (21) | 9 (14) | 8 (14) |
| 0–14 | 12 (16) | 10 (15) | 9 (14) | 6 (10) | 8 (14) |
| 15–29 | 9 (12) | 6 (9) | 7 (11) | 8 (13) | 8 (14) |
| 30–44 | 6 (8) | 7 (10) | 10 (15) | 15 (24) | 6 (11) |
| 45–59 | 2 (3) | 7 (10) | 7 (11) | 8 (13) | 9 (16) |
| 60–74 | 3 (4) | 6 (9) | 4 (6) | 5 (8) | 5 (9) |
| 75–89 | 0 (0) | 6 (9) | 5 (8) | 9 (14) | 5 (9) |
| > 90 | 2 (3) | 5 (7) | 10 (15) | 3 (5) | 8 (14) |
Values are presented as n (%). CBT-I = cognitive behavioral therapy for insomnia, TST = total sleep time.
DISCUSSION
The findings of the current study indicate that the reported average of an approximately 45-minute gain in self-reported TST after CBT-I is not modal, or common, across patients.15 At posttreatment, most participants’ TST was not appreciably higher than baseline levels (83%). While TST increases in a linear fashion in the 24 months following treatment discontinuation, a substantial proportion of participants (> 36.5%) do not appreciably increase their sleep duration (by ≥ 30 minutes). Additionally, participants who eventually increased TST by an appreciable duration did not experience these improvements until 3, 6, and up to 12 months after treatment. Therefore, the original finding that TST increases after 6–8 sessions of CBT-I is more nuanced than first appears.
It is known that large gains in SL and WASO can be achieved with CBT-I,10–12 with an approximately 45-minute increase in TST seeming to naturally emerge by 6 months following treatment.9,15,16 The current study elucidates the latter finding, indicating that a reasonable proportion of patients do not experience substantial gains in TST. Examining the changes in 15-minute increments at each time point indicated that there was considerable variability in TST gains after CBT-I, especially at later time points (6-, 12-, and 24-months follow-up). Patients who appreciably increased their TST after CBT-I appeared to be upwardly titrating their sleep opportunity, since the increases in TST occurred without increases in SL, WASO, and EMA. Notably, the gains with this approach were slow, since appreciable changes in TST did not seem to arise until 3–6 months after CBT-I. In addition, these gains in TST occurred without improvements in the ISI. This may be because the first 3 items of the ISI relate to sleep continuity disturbance (and none of the items assess sleep duration). Thus, changes on the ISI were unlikely to occur when SL, WASO, and EMA remained stable. Regardless, a considerable proportion of patients do not appreciably increase TST above the 5.5–6 hours per night reported at pretreatment.
Given that some patients do not appreciably increase TST and that average TST remained relatively low at each follow-up compared to population-level norms of 7–9 hours per night,6 it may be possible and appropriate to appreciably increase TST through further intervention. Higher dose CBT-I (additional sessions) with a dedicated focus on the upward titration of sleep opportunity may be useful to increase the magnitude of the TST gain and to extend the effect to a larger proportion of patients. The current study included an extended CBT-I arm; however, the additional sessions were not focused on the upward titration of sleep opportunity. Considering that phase 2 of sleep restriction therapy actively aims to increase TST, additional CBT-I sessions focused on upwardly titrating sleep opportunity may be more effective to increase TST. Even for those patients who appreciably increased TST unsupervised in the 3–6 months following treatment, guiding them through sleep opportunity titration may help to accelerate the process. Whether extended CBT-I focused on upwardly titrating sleep opportunity could lead to greater and faster gains in TST for more patients compared to 6–8 sessions of CBT-I warrants further investigation. If effective, these additional sessions may be administered via telehealth and similar modalities, regularly or as needed, and potentially automated via online CBT-I programs, improving the feasibility of extended CBT-I while also potentially greatly benefitting patients.
The caveat to the recommendation of extending CBT-I is whether patients would benefit from additional increases in TST. Given that short sleep duration is associated with negative health outcomes,18,19 it is expected that additional gains in TST would be therapeutic for patients. How large the gains must be to observe clinically meaningful improvements in health and functioning is a question for further exploration. Long sleep is also associated with adverse health and cognitive consequences,20–23 indicating that excessively increasing TST may be harmful, but findings from a recent sleep extension study suggests that beneficial effects may emerge from extending TST in short sleepers.24 Ending CBT-I may be best done not by the adoption of arbitrary doses but by achieving the therapeutic goal of the longest duration of sleep possible while maintaining high sleep efficiency and low daytime sleepiness and fatigue. In extended CBT-I, the goals of therapy would not only be to minimize sleep continuity problems and maximize total sleep time, but also to ensure that optimal sleep is achieved for maximal daytime function. Recognizing this issue, Spielman proposed the Sleep Need Questionnaire to determine when sleep need is being met (assessed indirectly via daytime function) to assist with the determination of when the upward titration of sleep opportunity should be discontinued.25 Sleep need could thus be considered and assessed via questionnaires during extended CBT-I to ensure that optimal sleep duration is reached for maximal daytime functioning. Another approach would be to assess daytime function directly via other measures, such as the PROMIS sleep-related impairment scale or the Glasgow Sleep Impact Index, to assist in the determination of when optimal functioning has been reached.26,27 Future research could examine the efficacy of this approach vs 6–8 sessions of CBT-I to determine whether more patients can achieve larger gains in TST more quickly, and whether this leads to better health and functioning.
The present analysis, while based on a rare and informative dataset, has several limitations. This analysis only included data from treatment completers, and few were lost to attrition (5 lost at postacute treatment from n = 80 at baseline). Such retention is not typical during acute treatment nor during follow-up periods. Thus, this may not be a generalizable group to those found in clinical practice. Additionally, the current study considered follow-ups at standard 3- to 12-month intervals, so the week-to-week trajectory is unknown, as is if/when treatment reversals occurred.
As suggested above, studies are needed to test extended CBT-I with sessions focusing on sleep opportunity titration, to test whether the main findings can be replicated in clinical practice, and to examine individual and group data in shorter incremental follow-ups over time. This research may be facilitated by the use of sleep technology (eg, consumer sleep trackers) to monitor sleep duration for an extended time period. Another important consideration for future research is whether data available at baseline could be used to predict who will achieve appreciable gains in TST at follow-up. One predictor worth analyzing in particular is age, as it is putatively associated with limits on sleep ability and/or sleep need. Sleep diaries and objective sleep assessments (such as polysomnography and actigraphy-based devices) could also be evaluated to elucidate whether 1) objectively derived sleep duration shows a similar pattern to self-reported TST as found in this study and 2) baseline sleep duration predicts changes in TST after CBT-I, as suggested by some studies into short sleepers.28 Future research will consider whether demographic, sleep, and/or health variables can predict treatment effect on TST in this dataset.
CONCLUSIONS
CBT-I is effective at reducing total wake time quickly and substantially, but increases in TST are slow and unsubstantial in magnitude for a sizeable proportion of patients. TST on average increases in a linear trend by 30–40 minutes in the 6 months following treatment, yet a large proportion of participants do not increase TST by 30 minutes or more in this time. Unguided by clinicians, participants had varying success in increasing TST after 4–8 weekly sessions of CBT-I. The current study suggests that extending CBT-I beyond 4–8 weekly sessions to increase sleep duration through sleep opportunity titration is warranted. It is anticipated that with closer supervision of sleep opportunity titration over a longer period and with proxy measures of sleep need, larger and more consistent increases in TST will occur and thus reveal a new therapeutic target: the determination of how much sleep is required for optimal functioning.
ACKNOWLEDGMENTS
While it is unusual to provide an acknowledgment for a coauthor, we would like to acknowledge Dr. Charles Morin’s gracious willingness to share data with us. We also acknowledge the team of researchers who contributed to the parent study.
ABBREVIATIONS
- CBT-I
cognitive behavioral therapy for insomnia
- EMA
early morning awakening(s)
- ISI
Insomnia Severity Index
- SL
sleep latency
- TIB
time in bed
- TST
total sleep time
- WASO
wake after sleep onset
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interest.
REFERENCES
- 1. American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed . Darien, IL: : American Academy of Sleep Medicine; ; 2014. . [Google Scholar]
- 2. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: : American Psychiatric Association; ; 2013. . [Google Scholar]
- 3. Sweetman A , Lovato N , Micic G , et al . Do symptoms of depression, anxiety or stress impair the effectiveness of cognitive behavioural therapy for insomnia? A chart-review of 455 patients with chronic insomnia . Sleep Med. 2020. ; 75 : 401 – 410 . [DOI] [PubMed] [Google Scholar]
- 4. Perlis M , Aloia M , Millikan A , et al . Behavioral treatment of insomnia: a clinical case series study . J Behav Med. 2000. ; 23 ( 2 ): 149 – 161 . [DOI] [PubMed] [Google Scholar]
- 5. van de Laar M , Pevernagie D , van Mierlo P , Overeem S . Psychiatric comorbidity and aspects of cognitive coping negatively predict outcome in cognitive behavioral treatment of psychophysiological insomnia . Behav Sleep Med. 2015. ; 13 ( 2 ): 140 – 156 . [DOI] [PubMed] [Google Scholar]
- 6. Hirshkowitz M , Whiton K , Albert SM , et al . National Sleep Foundation’s updated sleep duration recommendations: final report . Sleep Health. 2015. ; 1 ( 4 ): 233 – 243 . [DOI] [PubMed] [Google Scholar]
- 7. Spielman AJ , Saskin P , Thorpy MJ . Treatment of chronic insomnia by restriction of time in bed . Sleep. 1987. ; 10 ( 1 ): 45 – 56 . [PubMed] [Google Scholar]
- 8. Spielman AJ , Caruso LS , Glovinsky PB . A behavioral perspective on insomnia treatment . Psychiatr Clin North Am. 1987. ; 10 ( 4 ): 541 – 553 . [PubMed] [Google Scholar]
- 9. Morin CM , Beaulieu-Bonneau S , Ivers H , et al . Speed and trajectory of changes of insomnia symptoms during acute treatment with cognitive-behavioral therapy, singly and combined with medication . Sleep Med. 2014. ; 15 ( 6 ): 701 – 707 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trauer JM , Qian MY , Doyle JS , Rajaratnam SM , Cunnington D . Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis . Ann Intern Med. 2015. ; 163 ( 3 ): 191 – 204 . [DOI] [PubMed] [Google Scholar]
- 11. Okajima I , Komada Y , Inoue Y . A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia . Sleep Biol Rhythms. 2011. ; 9 ( 1 ): 24 – 34 . [Google Scholar]
- 12. Miller CB , Espie CA , Epstein DR , et al . The evidence base of sleep restriction therapy for treating insomnia disorder . Sleep Med Rev. 2014. ; 18 ( 5 ): 415 – 424 . [DOI] [PubMed] [Google Scholar]
- 13. Morin CM , Culbert JP , Schwartz SM . Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy . Am J Psychiatry. 1994. ; 151 ( 8 ): 1172 – 1180 . [DOI] [PubMed] [Google Scholar]
- 14. Smith MT , Perlis ML , Park A , et al . Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia . Am J Psychiatry. 2002. ; 159 ( 1 ): 5 – 11 . [DOI] [PubMed] [Google Scholar]
- 15. Beaulieu-Bonneau S , Ivers H , Guay B , Morin CM . Long-term maintenance of therapeutic gains associated with cognitive-behavioral therapy for insomnia delivered alone or combined with Zolpidem . Sleep. 2017. ; 40 ( 3 ): zsx002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morin CM , Vallières A , Guay B , et al . Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial . JAMA. 2009. ; 301 ( 19 ): 2005 – 2015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Academy of Sleep Medicine . International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: : American Academy of Sleep Medicine; ; 2005. . [Google Scholar]
- 18. Meng L , Zheng Y , Hui R . The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies . Hypertens Res. 2013. ; 36 ( 11 ): 985 – 995 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Itani O , Jike M , Watanabe N , Kaneita Y . Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression . Sleep Med. 2017. ; 32 : 246 – 256 . [DOI] [PubMed] [Google Scholar]
- 20. Bin YS , Marshall NS , Glozier N . Sleeping at the limits: the changing prevalence of short and long sleep durations in 10 countries . Am J Epidemiol. 2013. ; 177 ( 8 ): 826 – 833 . [DOI] [PubMed] [Google Scholar]
- 21. Patel SR , Malhotra A , Gottlieb DJ , White DP , Hu FB . Correlates of long sleep duration . Sleep. 2006. ; 29 ( 7 ): 881 – 889 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Low DV , Wu MN , Spira AP . Sleep duration and cognition in a nationally representative sample of U.S. older adults . Am J Geriatr Psychiatry. 2019. ; 27 ( 12 ): 1386 – 1396 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jike M , Itani O , Watanabe N , Buysse DJ , Kaneita Y . Long sleep duration and health outcomes: A systematic review, meta-analysis and meta-regression . Sleep Med Rev. 2018. ; 39 : 25 – 36 . [DOI] [PubMed] [Google Scholar]
- 24. Hartescu I , Stensel DJ , Thackray AE , et al . Sleep extension and metabolic health in male overweight/obese short sleepers: a randomised controlled trial . J Sleep Res. 2021. ; 31 ( 2 ): e13469 . [DOI] [PubMed] [Google Scholar]
- 25.Manber R, Carney C, Edinger J, et al. Dissemination of CBTI to the non-sleep specialist: protocol development and training issues. J Clin Sleep Med. 2012;8(2):209--218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kyle SD , Crawford MR , Morgan K , Spiegelhalder K , Clark AA , Espie CA . The Glasgow Sleep Impact Index (GSII): a novel patient-centred measure for assessing sleep-related quality of life impairment in Insomnia Disorder . Sleep Med. 2013. ; 14 ( 6 ): 493 – 501 . [DOI] [PubMed] [Google Scholar]
- 27. Buysse DJ , Yu L , Moul DE , et al . Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments . Sleep. 2010. ; 33 ( 6 ): 781 – 792 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fernandez-Mendoza J . The insomnia with short sleep duration phenotype: an update on it’s importance for health and prevention . Curr Opin Psychiatry. 2017. ; 30 ( 1 ): 56 – 63 . [DOI] [PubMed] [Google Scholar]