Abstract
Human idiopathic hypercalciuria (IH) is the most common cause of calcium oxalate nephrolithiasis with perturbed calcium metabolism with increased bone resorption and decreased renal calcium reabsorption, which can be phenotype-copied in the genetic hypercalciuric stone-forming (GHS) rat model. We previously demonstrated that high VDR expression plays important roles in the development of hypercalciuria in the GHS rats. However, the underlying mechanism through which VDR impact hypercalciuria development remains to be fully understood. Here, we sought to determine how VDR regulated its target genes that are implicated in calcium homeostasis and potentially hypercalciuria. We found that VDR expression in the GHS rats was elevated in the calcium transporting tissues, as well as in the thymus and prostate, but not in lung, brain, heart, liver and spleen, when compared with control SD rats. Snail expression in the GHS rats was significantly downregulated in kidney, intestine, thymus and testis. Intraperitoneal injection of 1,25(OH)2D3 significantly upregulated the expression of renal calcium sensing receptor (CaSR), intestinal calcium transporters transient receptor potential vanilloid type 6 (TRPV6), and VDR in GHS rats, compared with that in control SD rats. ChIP assays revealed that VDR specifically bound to the proximal promoters of target genes, followed by histone H3 hyperacetylation or hypermethylation. Collectively, our results suggest that elevated VDR expression may contribute to the development of hypercalciuria by sensitizing VDR target genes to 1,25(OH)2D3 through histone modifications at their promoter regions in a genetic hypercalciuric stone-forming (GHS) rat model.
Keywords: Acetylation, ChIP, GHS, Methylation, Snail, VDR, VDR target Gene
Introduction
Human idiopathic hypercalciuria (IH), the most common cause of calcium oxalate nephrolithiasis, and GHS rats exhibit similar changes in Ca metabolism, including increased bone resorption and decreased renal calcium reabsorption.1, 2, 3, 4, 5, 6 Our previous studies have found that higher VDR expression levels in the intestine, kidney, and bone play important roles in hypercalciuria in GHS rats.4,5,7,8 The lengthy in vivo half-life of VDR mRNA and protein can partially explain the high VDR expression in GHS rats.7,9 Snail is a transcriptional repressor of the VDR gene.10 Our recent data show that Snail downregulates VDR transcription and expression by binding to multiple E-boxes in the VDR promoter regions and deacetylating histones H3 and H4; more interestingly, Snail inversely correlates with VDR in the intestine and kidney at the mRNA and protein levels.11 These data strongly suggest that Snail plays an important role in the VDR levels being high, as they relate to Ca transport and metabolism in GHS rats.
VDR is an essential nuclear receptor that has been reported to function in Ca homeostasis, the immune system, and cell proliferation and differentiation.12, 13, 14 VDR exerts its biological effects by regulating gene expression. The 1,25(OH)2D3 level in vivo is tightly controlled by several cytochrome P450 (CYP) family enzymes, such as CYP24 and CYP27B1.15,16 CYP24 was found in the kidney, intestine, and bone and can hydroxylate 25-OH-D3 or 1,25(OH)2D3 into 24,25(OH)2D3 and 1,24,25(OH)3D3.15 This process is believed to be the first step in degrading and maintaining appropriate active 1,25(OH)2D3 levels in vivo.16 CYP27B1 is another enzyme that promotes the synthesis of 1,25(OH)2D3, which it does by catalyzing 25-OH D3 into 1,25(OH)2D3.14 1,25(OH)2D3 can modulate CYP24 and CYP27B1 expression, and the detailed regulatory mechanisms responsible for this regulation have been discovered in vitro.16, 17, 18, 19 However, the detailed mechanisms of how 1,25(OH)2D3 up- and downregulates CYP24 and CYP27B1 expression in vivo are not clear, especially in our GHS rat model. Our previous data indicated that VDR gene expression is hypersensitive to 1,25(OH)2D3 administration in GHS rats7; however, whether VDR target genes CYP24 and CYP27B1 are hyperresponsive to 1,25(OH)2D3 remains unknown.
GHS rats are characterized by defects in Ca transport and metabolism.1 High VDR levels are associated with hypercalciuria in GHS rats. Elevated VDR levels mediate the upregulation of Ca transport and reabsorption genes such as 9- and 28-kDa calbindins and CaSR in the small intestine and kidney, contributing to hypercalciuria and stone formation in GHS rats.8,9 CaSR is a VDR target gene and is expressed in various tissues, including the kidney.20,21 VDR can upregulate CaSR expression by binding to its promoter.21 However, how VDR activates CaSR expression in vivo is not completely understood. TRPV6 is an epithelial Ca channel that is mainly expressed in the intestine and is responsive to intestinal Ca absorption.22 TRPV6 knockout mice showed a 60% decrease in intestinal Ca absorption.23 Therefore, the role of TRPV6 in hypercalciuria and how 1,25(OH)2D3 upregulates its expression in GHS rats remain to be explored.
VDR regulates target gene expression through interaction with the retinoid X receptor (RXR) and through recruiting coregulators to target gene promoter regions, modifying histone state and chromatin structure, which results in gene up- or downregulation.24,25 Therefore, this study was designed to test whether VDR modulates target gene expression through promoter binding and histone modification in GHS rats.
Material and methods
Animals models
A GHS rats colony was created by the selective breeding of male and female Sprague–Dawley rats (S-D, Harlan, Inc., Indianapolis, IN) with spontaneous hypercalciuria.2 Beyond the 65th generation, GHS rats have consistently excreted 8–10 times the level of urine calcium that is excreted by wild-type normocalciuric (NC) SD rats. Male and female GHS rats were raised at the University of Rochester and were shipped to the University of Chicago at 6–7 weeks of age and with a body weight of 260–280 g. SD rats purchased from Harlan, Inc. Indianapolis were matched with the GHS rats based on age and body weight. All animal experiments were approved by the University of Chicago Institutional Animal Care and Use Committee.
Western blots
Tissue was homogenized in cold PBS buffer with a protein inhibitor cocktail. The mix was vortexed at full speed for 20s at 5 min intervals and then centrifuged for 15 min at 14,000×g at 4 °C. The supernatant was removed and stored at −80 °C. Protein concentration was determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA). Aliquots of total protein (20 μg) were denatured in 6x SDS sample buffer (7 ml 4x Tris/HCl, SDS [pH 6.8], 3.0 ml of glycerol, 1 g of SDS, 0.93 g of dithiothreitol, and 1.2 mg of bromophenol blue in 10 ml of distilled deionized H2O) and then loaded onto SDS denaturing gels for discontinuous electrophoresis. The proteins were transferred onto polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA) by electroblotting at 90 V for 1 h. The membranes were then blocked with 5% nonfat dried milk in TBS that contained 0.1% Tween-20 (TBS-T). A primary antibody was diluted with 3% nonfat dried milk in TBS-T. Then, the membrane was incubated with the diluted primary antibody at room temperature for one hr. Membranes were washed with TBS-T and incubated with horseradish peroxidase-conjugated anti-rat IgG for one hr at room temperature. Protein bands were developed using an ECL Chemiluminescence System (Amersham Life Science, Buckinghamshire, UK). The blots were quantified with One-Scan 1-D gel Analysis software (Scanalytics Inc., Fairfax, VA).
Real-time PCR
Female GHS rats were IP injected with 200 ng/kg BW 1,25(OH)2D3 or with ethanol (as a control). Twelve hours later, RNA was isolated from the kidneys and intestines from both groups using TRIzol, and cDNA was subsequently synthesized using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to the manufacturer's directions. The primer sequences for rat CYP24, CYP27, VDR, CaSR, TRPV6 and GAPDH were as follows: CYP24 (forward) 5′-GATCCTGGAAGGACAGGAGT-3′, (reverse) 5′-GGAAGTCAGCCAAGACCTCA-3’; CYP27 (forward) 5′-AAAGGTGTCTGTCCAGTCCA-3′, (reverse) 5′-CTCATAGAGTGCCCAGGAGA-3’; VDR (forward) 5′-TGTTCACCTGTCCCTTCAAT-3′, (reverse) 5′-CGCTGTACCTCCTCATCTGT-3’; CaSR (forward) 5′-GTGGAGAGACAGATGCGAGT-3′, (reverse) 5′-TGCAAGAAGTGTGGTTCTCA-3’; TRPV6 (forward) 5′-GATGGCTGTGGTAATCTTGG-3′, (reverse) 5′-ATCGATGATGGTGAGGAAGA-3’; and GAPDH (forward) 5′-GCACAGTCAAGGCTGAGAAT-3′, (reverse) 5′-TGAAGACGCCAGTAGACTCC-3’. Quantification of the PCR products was performed using the comparative cycle threshold (Ct) method, as described previously.26,27 GAPDH was used as an internal control. Statistical analysis was performed using Student's t-tests for unpaired comparisons. Data are presented as the means ± SE, and P < 0.05 was considered significant.
Tissue ChIP assays
For tissue ChIP assays, frozen intestine and kidney tissues from GHS rats injected with either 1,25(OH)2D3 or a vehicle were thawed at room temperature, cut to generate 1 mm slices, and crossed-linked in 1.5% formaldehyde for 15 min. Tissues were then homogenized with Tissuemiser (Fisher Scientific, Pittsburgh, PA), and the cell suspension was centrifuged at 14,000 rpm for 10 min. SDS lysis buffer was added to the pellets, which were then subjected to sonication. Anti-VDR and acetylated H3 antibodies were used to immunoprecipitate the DNA and protein complex, and IgG alone was used as a negative control. After overnight incubation with the antibodies, immunocomplexes were collected using 80 μL of protein A agarose beads in a slurry (Upstate Biotechnology, Temecula, CA). The agarose beads were washed twice with the following buffers (Upstate Biotechnology): low salt wash buffer; high salt wash buffer; LiCl wash buffer; and TE buffer. DNA was eluted with 1% SDS and 0.1 M NaHCO3 elution buffer, and then it was subjected to reverse cross-linking, proteinase digestion, and purification using a commercial kit (Qiagene, Valencia, CA). The primers used to amplify the target gene promoters are listed in Table 1. For quantitative ChIP, real-time PCR was performed using the SYBR Green PCR Master Mix Kit to amplify the immunoprecipitated DNA, and data are shown as the percentage of input as described before.28
Table 1.
Primers for tissue ChIP.
| Primer | Start | Sequence |
|---|---|---|
| CASR-FW1 | −4976 | CCTGATTGTAGCCAGACCAGC |
| CASR-RE1 | −4822 | AGGACTTGGTAGTAACAGAAC |
| CASR-FW2 | −4595 | TTCCTCTTTGAAGAATGCACAC |
| CASR-RE2 | −4447 | ATCCTCTATCCCAGTGTACCC |
| CASR-FW3 | −4090 | CCAGACGTGTGCAACTACGCT |
| CASR-RE3 | −3938 | GCTGTCAGGAAACTCTCTGGG |
| CASR-FW4 | −3850 | TTCGCCCGCCAAATAAGCTCT |
| CASR-RE4 | −3703 | GGAGAGAGATCTTGGCACCTT |
| CASR-FW5 | −3298 | ACCATGTCCTTTCTAGGTACAC |
| CASR-RE5 | −3144 | AGAATCTGGACAGGACTTGCT |
| CASR-FW6 | −2567 | CAACTGCAGGCACTCTGTTAAC |
| CASR-RE6 | −2417 | CTGAGTCTTGGCAACAGAAAG |
| CASR-FW7 | −1810 | TGAGGGAGAGTCAGGCTCTGTT |
| CASR-RE7 | −1663 | ACCAGTTGAAGAAGCTGCGTC |
| CASR-FW8 | −1265 | ACACTATCTCATATCAAGCATC |
| CASR-RE8 | −1115 | TACAGAAAAACAAGGGACTGT |
| CASR-FW9 | −949 | CCCTATTGAGGGAGTCTTAAGT |
| CASR-RE9 | −797 | GCCTCCCAGTTTTCTTAGAAC |
| CASR-FW10 | −502 | TGCTGTGCCAGAGCCCGAGAAC |
| CASR-RE10 | −352 | TCCAGCGTTGTGTCTGCGGTC |
| TRPV6-FW1 | −4893 | GCAGAGTTAGATCAAGGAAGT |
| TRPV6-RE1 | −4748 | TGTCCTATGACTAGTTTGTGC |
| TRPV6-FW2 | −4569 | AAATGTGCACACATGCATCTC |
| TRPV6-RE2 | −4415 | CCCACTCTCTGTTTCTTTTGA |
| TRPV6-FW3 | −4308 | AACAGCAGTTGACAGGAGGAAG |
| TRPV6-RE3 | −4161 | AGAAATAGGAGTCAGGTGCAG |
| TRPV6-FW4 | −3920 | TTTGAACCCAGTGACTGGAAC |
| TRPV6-RE4 | −3769 | CAGGAAAATGTCTGTAGGTGG |
| TRPV6-FW5 | −2438 | GCAGGACTGGAGTGCATGATGG |
| TRPV6-RE5 | −2288 | CAGTTGGGGTGGCTTGGCACT |
| TRPV6-FW6 | −2149 | CTCACTCCACAGCATGATACAAG |
| TRPV6-RE6 | −2000 | AGGACCTTTGTGCCCATTAAG |
| TRPV6-FW7 | −2039 | GCGTCAGACTAACACTTGGCTT |
| TRPV6-RE7 | −1891 | TACAGCCCTGAGCAAACAATG |
| TRPV6-FW8 | −1700 | CTACAGCAACAATACTACACC |
| TRPV6-RE8 | −1579 | TATGTGCTTTCCATGGAACGT |
| TRPV6-FW9 | −1008 | TCTCTTTGCCAATTAGAGTGAG |
| TRPV6-RE9 | −858 | AGAGCCTAGAAGGCAGTTATT |
| TRPV6-FW10 | −385 | AGTTGGTGAGAACTCAGAATCT |
| TRPV6-RE10 | −231 | AGGAGAAGGAAAGCAAGAAAG |
| CYP27B1-FW1 | −5031 | CCATTTCTTGGGACAAGGAGAG |
| CYP27B1-RE1 | −4884 | CAAAGGCCATAGACCCAGAGT |
| CYP27B1-FW2 | −4520 | AGGAGCGTGGTGTGTGAGACTG |
| CYP27B1-RE2 | −4366 | CCACCACCGCCTAGCTTCATT |
| CYP27B1-FW3 | −4150 | CCTGGCTGGTTACTTTATGTTT |
| CYP27B1-RE3 | −3998 | GCCTTTAATCCTAAGAATTGG |
| CYP27B1-FW4 | −3660 | CCTGTCTCCGCAGTAACGCCAT |
| CYP27B1-RE4 | −3513 | GGACAGATGCGCCAACAGGAT |
| CYP27B1-FW5 | −2952 | TCAACTGAGGAAGGAAAGAAAG |
| CYP27B1-RE5 | −2799 | GCTCATCTTTTTCCAGTCACT |
| CYP27B1-FW6 | −1960 | GGGGCCAGAAAAAGGAAGATGGT |
| CYP27B1-RE6 | −1807 | ACTCCTCCAGGTTAGTGACTG |
| CYP27B1-FW7 | −1817 | CCTGGAGGAGTCTGTAACTGAG |
| CYP27B1-RE7 | −1672 | GCCAACCCTCACCTCCTATAC |
| CYP27B1-FW8 | −1669 | AAGTGCACATATGCTCGTACA |
| CYP27B1-RE8 | −1524 | ATGAAGAAATGTCCTAGGGTC |
| CYP27B1-FW9 | −175 | GAGACCCAAAAGCCAGCGAGT |
| CYP27B1-RE9 | −22 | CTTCTGCACTTGCCTGAACCC |
| CYP24A-FW1 | −5294 | GTCTCGCCAAAACTAAACAAAC |
| CYP24A-RE1 | −5147 | TCTAGTGTGGATTTAGAGACC |
| CYP24A-FW2 | −4900 | CAACTTCCAGAGCTTGGATGCC |
| CYP24A-RE2 | −4780 | GTGTGCCTCGGTGTGAGCCTG |
| CYP24A-FW3 | −4481 | ACTTGTTCACAGCCTGTTATTC |
| CYP24A-RE3 | −4327 | CATGTTGACCAGTCACTGTTT |
| CYP24A-FW4 | −3829 | TTTTCTTTGTTTTAATTCACTGT |
| CYP24A-RE4 | −3675 | GTGGAATCCCTAGAGACTTAT |
| CYP24A-FW5 | −3274 | CACCACCTTCTGCTCAAGAAAT |
| CYP24A-RE5 | −3128 | TGTACTGGGAAACACAAAGCC |
| CYP24A-FW6 | −2858 | CATGTCCAGACCTTTCATTAG |
| CYP24A-RE6 | −2713 | CTAGAAGAGTTCAGAACACCA |
| CYP24A-FW7 | −1964 | GTTTGAGCTAGCCTGGTCTATG |
| CYP24A-RE7 | −1810 | GCTTAGTCCATTGTCGCAAAC |
| CYP24A-FW8 | −1718 | CCCCCACGCATATACAACAGAG |
| CYP24A-RE8 | −1572 | ACCATTTCCCCTTCCCTTCTG |
| CYP24A-FW9 | −1182 | CAAATCGGATTGCAGAAGCTTC |
| CYP24A-RE9 | −1029 | TTTCCAATGCAAAGCCACCAG |
| CYP24A-FW10 | −837 | TGGGCGGTGCGCAAGAAGGAAA |
| CYP24A-RE10 | −692 | GGTCTACACAGGTGTGTGTCC |
| CYP24A-FW11 | −267 | ACCCGCTGAACCCTGGGCTCGAC |
| CYP24A-RE11 | −119 | GCGGGTGTGGGAAGAGGATGG |
| VDR-FW1 | −4950 | CATTGTCTGCTGCAGACTTGG |
| VDR-RE1 | −4802 | ACACGGTGTCAGTCAGCATGT |
| VDR-FW2 | −4696 | GCAATGGAGCCAGAAGGATAC |
| VDR-RE2 | −4549 | CTATCCTCCAGAAGCCTATGG |
| VDR-FW3 | −4008 | TTCAGTCCAGGGGCTGGGCTACC |
| VDR-RE3 | −3858 | AGGTGAGGAGGTGGGAGGGCA |
| VDR-FW4 | −3340 | GAGTGCTGTCTGTCTTCCCAT |
| VDR-RE4 | −3187 | TCCTAAAAGCAGATCCTTGTC |
| VDR-FW5 | −2789 | GGATCTAGCAGATGGTTCCTG |
| VDR-RE5 | −2642 | AGGCAGGTGGATCTGTGAGTT |
| VDR-FW6 | −2317 | CTGGAGATGGTGGAAAGGGTT |
| VDR-RE6 | −2170 | GCATACTCTGTGGTTGGTCCA |
| VDR-FW7 | −1882 | AGAGCAGTGTTCTTAATGGTTG |
| VDR-RE7 | −1736 | GACTAACGGGCATCATTTCTT |
| VDR-FW8 | −1650 | GCTCTGTCTTCAAAGATTGACAC |
| VDR-RE8 | −1496 | GGAAGGAAGATAAGCACCAAC |
| VDR-FW9 | −499 | AGCGCCCTGCAGGAGAAAGTC |
| VDR-RE9 | −345 | CGCCGTGGTTCTACCCAGTCT |
| VDR-FW10 | −199 | GTCTCCAAGGCGACAGTGCAG |
| VDR-RE10 | −54 | AGAGACTGCTCAGCACCTGGC |
Results
Snail inversely correlated with VDR expression in GHS rats
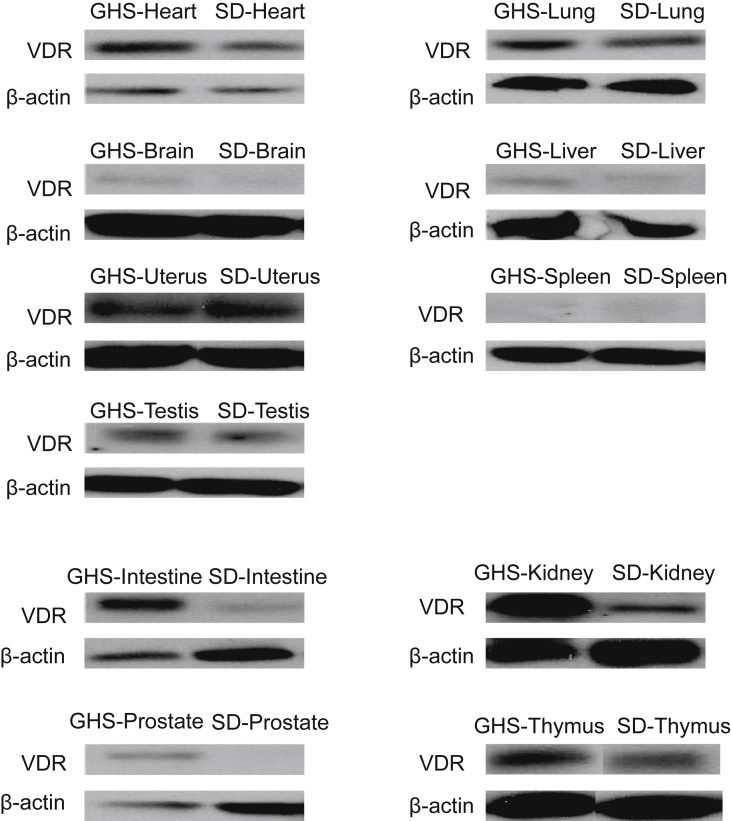
Our previous data show that VDR is highly expressed in the kidneys, intestines, and bones of GHS rats, which accounts for the hypercalciuria in GHS rats.4,5,7,8 In this study, we investigated whether VDR expression was higher in various tissues. Western blot results show that compared to SD control rats, VDR is highly expressed GHS rat tissues with high levels of Ca transport and metabolism, such as the intestine, kidney, thymus, and prostate (Fig. 1). However, for the liver, heart, lung, spleen, brain, and uterus, VDR expression was not different between GHS and SD rats (Fig. 1). Snail is a transcription factor that promotes epithelial to mesenchymal transition (EMT).29 A recent report has shown that Snail is a transcriptional repressor of VDR gene expression and that its levels are inversely correlated with VDR expression in colon cancer cells.10,29 Here, we investigated Snail expression levels in tissues with higher or normal VDR expression (Fig. 2). Our data indicate that Snail is only detectable in the intestine, kidney, thymus, testis, and brain. More interestingly, Snail is downregulated in the presence of higher VDR levels in tissues such as the intestine, kidney, and thymus in GHS rats compared to SD rats. There was no difference in Snail expression between the brain of GHS and SD rats. These results suggest that Snail is inversely correlated with VDR expression in GHS rat tissues that exhibit high VDR expression.
Figure 1.
VDR expression in various tissues of GHS and SD rats. Nuclear proteins were isolated from tissues of three GHS rats and SD rats and then were subjected to Western blots using anti-VDR antibodies. Representative data are shown in Figure 1. The expression pattern of VDR in various tissues; VDR is highly expressed in the intestine, kidney, prostate and thymus in GHS rats compared to SD rats.
Figure 2.
Snail expression in various tissues of GHS and SD rats. Nuclear proteins were isolated from tissues of three GHS rats and SD rats and then were subjected to Western blots using anti- Snail antibodies. Representative data are shown in Figure 2. Snail expression patterns were detected in GHS and SD rat tissues, and Snail expression was low in the intestine, kidney, thymus, and testis of GHS rats compared to SD rats.
1,25(OH)2D3 strongly induces or suppresses target gene transcription in GHS and SD rats
1,25(OH)2D3 exerts its function through up- or downregulation of VDR response gene expression.30 In the current study, the following VDR target genes were investigated: Ca transporters CaSR and TRPV6 and the vitamin D catabolism enzymes CYP24 and CYP27B1 as well as VDR itself. Twelve hours after 1,25(OH)2D3 administration in GHS rats, RNA was isolated from their intestines and kidneys and then was subjected to real-time PCR. 1,25(OH)2D3 increased the transcription of CYP24, CaSR, TRPV6, and VDR by 32.8-, 8.9-, 17- and 4.5-fold in GHS rats and 39.5-, 1.6-, 1.83-, and 2.2-fold in SD rats, respectively (Fig. 3). At the same time, 1,25(OH)2D3 decreased CYP27B1 transcription by 68% in GHS rats and 53% in SD rats (Fig. 3). Our data show that high VDR levels in GHS rats strongly mediated 1,25(OH)2D3 action on target gene expression. CaSR, TRPV6, and VDR are more sensitive to 1,25(OH)2D3 treatment in GHS rats than they are in SD rats.
Figure 3.
VDR target gene expression followed the administration of 1,25(OH)2D3 in SD and GHS rats. Twelve hours after 1,25(OH)2D3 treatment, kidney and intestine RNA was isolated and subjected to real-time PCR using specific primers for CaSR, TRPV6, VDR, CYP24, and CYP27. 1,25(OH)2D3 upregulates CaSR, TRPV6, VDR, and CYP24 transcription and inhibits CYP27 transcription. Data are shown as the mean ± SEM, and the relative expression of target genes of the 1,25(OH)2D3 treatment groups was normalized to the levels of the vehicle treatment groups.
Hyperacetylation or hypermethylation of histone H3 in multiple regions of the target gene promoter after 1,25(OH)2D3 treatment
Histone acetylation and methylation are associated with gene activation and repression.31,32 In this study, we investigated whether VDR regulates target gene expression through histone modification. After GHS rats were treated with 1,25(OH)2D3 or vehicle, their intestines and kidneys were harvested and then subjected to tissue ChIP. We divided the proximal promoters of CaSR, TRPV6, VDR, CYP27B1, and CYP24 into 9 to 11 regions from the TSS or ATG to negative 5000 bp, and we designed the corresponding primers to amplify these regions. For the CaSR promoter regions, after administration of 1,25(OH)2D3, the acetylated histone H3 increased only in region 10 and not in other regions compared to the vehicle treatment group (Fig. 4A). Histone modification within the intestinal TRPV6 promoter regions was strongly enhanced in regions 3, 4, and 6 (Fig. 4B). Furthermore, the modification of histone H3 within the VDR promoter in regions 1 and 7 was strongly enhanced by 1,25(OH)2D3 treatment (Fig. 4C). Although a previous study showed that VDR only binds to two introns within the mouse VDR gene in vitro,31 our data show that 1,25(OH)2D3 modifies histone H3 at several VDR promoter regions. For the CYP27 promoter, since this gene was strongly inhibited after 1,25(OH)2D3 administration (Fig. 4D), we investigated histone k9 methylation. Figure 4D shows that histone H3 methylation slightly increased in regions 1, 2, 3, and 4 in the 1,25(OH)2D3 treatment group. For the CYP24 gene, we found that 1,25(OH)2D3 could change histone 3 acetylation in regions 1, 2, 5 and 8 (Fig. 4E).
Figure 4.
VDR binds to multiple promoter regions of its target genes and modifies histone 3. Four hours after 1,25(OH)2D3 or vehicle treatment, the GHS rat intestines and kidneys were harvested and subjected to quantitative tissue ChIP. The data show real-time PCR results of input and DNA precipitated with anti-VDR or normal IgG using different region-specific primers (Table 1). Each group had at least three GHS rats, data are shown as the mean + SEM, and P < 0.05 was considered to be statistically significant. GHS rats were treated with 1,25(OH)2D3 and vehicle. Twelve hours later, the kidney and intestine tissues were harvested and subjected to ChIP assays. Specific antibodies against acetylated and methylated histone H3 were used to precipitate the DNA. Primers as listed in Table 1 were used to amplify the different regions of target gene promoters. PCR products were run on a 2% agarose gel. (A) shows that VDR binds to CaSR promoter region 10 and modifies histone 3 acetylation in those region. (B) shows that VDR binds to TRPV6 promoter regions 3, 4 and 6 and modifies histone 3 acetylation in those regions. (C) shows that VDR binds to VDR promoter regions 1 and 7 and modifies histone 3 acetylation in those regions. (D) shows that VDR binds to CYP27 promoter regions 1, 2, 3 and 4 and modifies histone 3 K9 methylation in those regions. (E) shows that VDR binds to CYP24 promoter regions 1, 2, 5 and 8 and modifies histone 3 acetylation in those regions.
VDR binds to multiple target gene promoter regions with histone modifications in GHS rats
VDR regulates target gene expression by recruiting coregulators and modifying the histone state.25,30 To determine whether VDR regulates target gene expression through binding to promoter regions with histone modifications, quantitative tissue ChIP was performed on GHS rats that were treated with either a vehicle or 1,25(OH)2D3. For the CaSR promoter, we screened 10 regions for VDR binding. We only found that VDR binds to region 10 when there was a histone modification after 1,25(OH)2D3 injection (Fig. 4A). For the TRPV6 promoter, VDR binds to regions 3, 4, and 6 after 1,25(OH)2D3 treatment (Fig. 4B). VDR accumulated in VDR promoter regions 1 and 7 when there were histone modifications after 1,25(OH)2D3 administration (Fig. 4C). Previous data reported that 1,25(OH)2D3 could automatically upregulate VDR transcription through two enhancers within introns.31 We next explored the localization of VDR at the CYP27B1 promoter after 1,25(OH)2D3 treatment. Our data show that VDR binds to regions 1, 2, 3, 4, and 6. Interestingly, methylated histone H3 was slightly increased after 1,25(OH)2D3 injection in the same regions (Fig. 4D). For the CYP24 promoter, VDR accumulated in regions 1, 2, 5, and 8 following 1,25(OH)2D3 treatment (Fig. 4E). Taken together, our in vivo data strongly show that 1,25(OH)2D3 induced or suppressed gene expression through VDR binding and histone modification.
Discussion
GHS rats resulted from breeding male and female SD rats with spontaneous hypercalciuria.1 The high VDR levels in intestine, kidney, and bone cells can explain the hypercalciuria in GHS rats.4, 5, 6 In this study, we explored whether VDR is highly expressed in all VDR-positive tissues. Our results show that VDR is highly expressed in the intestine and kidney, as previously reported,4,7,8 which indicates that the high VDR in such tissues plays very important roles in defective Ca metabolism in GHS rats (Fig. 1). VDR is an essential nuclear receptor functioning in Ca metabolism, cell proliferation and differentiation in multiple tissues.12, 13, 14 We also found that VDR is highly expressed in the prostate and thymus (Fig. 1), but the function of high VDR levels in such tissues remains unknown. Current studies show that Snail is a transcriptional repressor of VDR expression. Snail downregulates VDR expression through promoter binding, corepressor recruitment, and histone modification.11 More importantly, Snail mRNA and protein levels are inversely correlated with those of VDR in GHS rats.11 These results indicate that Snail is one of the essential regulators of high VDR expression in GHS rats. In this study, we investigated Snail expression patterns in various tissues. Our results show that Snail is detectable in a few tissues in SD rats, such as the intestine, kidney, thymus, and testis, but it is absent or weakly expressed in GHS rats, which means that Snail is downregulated in tissues with high VDR expression in GHS rats (Fig. 2). Snail expression was not different between GHS and SD rat brains (Fig. 2). Interestingly, VDR expression also showed no difference in brains from GHS and SD rats (Fig. 1). Therefore, Snail and VDR are inversely expressed in rats, which may account for the higher VDR expression in GHS rats. The reason behind the low expression of Snail in GHS rats requires further study.
VDR-dependent gene regulation is a complex process involving promoter binding, coregulator recruitment, histone modification, and activation of the RNA pol II complex.24,25 CYP24 and CYP27B1 are two vitamin D metabolizing enzymes and direct target genes of VDR. However, the mechanism by which 1,25(OH)2D3 up- or downregulates CYP24 and CYP27B1 expression in a disease model has never been reported. In this study, 12 h after 1,25(OH)2 D3 treatment of GHS rats, the mRNA levels of CYP24 and CYP27B1 dramatically increased by approximately 40-fold and decreased by approximately 70% (P < 0.01), respectively. Previous studies have found that 1,25(OH)2 D3 levels correlate negatively or positively with kidney CYP24 and CYP27B1 levels.15,33 Therefore, our results indicate that VDR maintains the normal 1,25(OH)2D3 level in GHS rats through up- or downregulation of CYP24 and CYP27B1 gene expression. We also found that VDR upregulates CYP24 expression by binding to promoter regions 1, 2, 5, and 8 (approximately −5 kb, −4.5 kb, −2.5 kb, −1.5 kb and −0.5 kb upstream of the TSS) and subsequently hyperacetylates histone H3 in those regions (Fig. 4E). Previous in vitro data show that there are two VDREs within the proximal rat CYP24 promoter that mediate 1,25(OH)2D3-activated CYP24 gene transcription. The first one is located from −262 to −238 bp, and the second one is located from −154 to −134 bp.19 Furthermore, we found that VDR binds to other proximal and distal promoter regions and changes histone acetylation in those regions. These results suggest that there may be multiple VDR binding sites within the rat CYP24 promoter. Histone methylation is an event associated with repression of gene expression.32, 33, 34 Our in vivo data show that after 1,25(OH)2D3 treatment, VDR binds to multiple regions of CYP27B1, regions 1, 2, 3, 4, and 6 (approximately −5 kb, −4.5 kb, −4 kb, −3 kb and −2.5 kb upstream of ATG, respectively), and it binds to slightly hypermethylated histone H3 at the same regions (Fig. 4D). Previous in vitro studies have indicated that VDR binds to multiple regions of the human CYP27B1 promoter and mediates transcriptional repression.18 Our in vivo ChIP results indicate that VDR binds to rat CYP27B1 promoter regions 1, 2, 3, 4, and 6, which is consistent with Turunen's studies on the human CYP27B1 promoter.18
TRPV6 is an epithelial channel that is mainly expressed in the intestine.22 The targeted distribution of TRPV6 in mice results in a 60% decrease in intestinal absorption, loss of bone mineral density, and lower fertility, which indicates that TRPV6 plays a central role in intestinal Ca absorption and whole-body Ca homeostasis.23 GHS rats exhibit intestinal Ca hyperabsorption, which partially results from high levels of VDR, 8-kDa and 28-kDa calbindin, and CaSR in the intestine and kidney.1,7, 8, 9 In this study, we investigated how elevated VDR upregulates TRPV6 expression in the intestines of GHS rats. After 1,25(OH)2D3 treatment, TRPV6 mRNA increased approximately 15-fold compared to the vehicle group (Fig. 3), indicating that the TRPV6 gene is very sensitive to 1,25(OH)2D3 stimuli. Therefore, the elevated VDR in GHS rats may upregulate TRPV6 expression, which contributes to intestinal Ca hyperabsorption. To date, very few studies have been performed on the mechanism of how 1,25(OH)2D3 regulates rat TRPV6 gene expression in vivo. Our tissue ChIP results show that VDR quickly binds to multiple TRPV6 promoter regions, 3, 4, and 6 (approximately −4 kb, −3 kb, and −2 kb upstream of the TSS, respectively), which is followed by histone acetylation at the same regions and results in remarkable activation of TRPV6 gene transcription (Fig. 4B). Pike's group investigated VDREs in human and mouse TRPV6 promoters in vitro and in vivo.17,35 Their results show that the active VDREs in the human TRPV6 promoter are located at −4.3 and −2.1 kb relative to the TSS, and the mouse VDREs in the TRPV6 promoter are located at −2 and −4 kb relative to the TSS. Collectively, these data, together with our in vivo results, indicate that there may be some VDR binding sites in human, mouse, and rat TRPV6 promoter regions.
In vitro studies have revealed that 1,25(OH)2D3 can directly modulate VDR transcription. VDR binds to two enhancers located in two introns and mediates automatic activation of the mouse VDR gene.31 Our previous in vivo data showed that 1,25(OH)2D3 could dramatically upregulate VDR transcription and expression at the mRNA and protein levels in GHS rats.7 In the present study, we found that VDR binds to distal and proximal rat VDR promoter regions 1 and 7 (approximately −5 kb and −3.5 kb upstream of the TSS, respectively), which is followed by hyperacetylation of histone H3 at the same regions (Fig. 4C). Renal CaSR plays an essential role in renal Ca reabsorption through a CaSR-responsive K-dependent voltage-gated chloride channel.35,36 The activation of CaSR may decrease renal tubule Ca reabsorption and cause hypercalciuria through suppression of Ca-sensitive potassium channel activity.8 Our previous data show that CaSR is highly expressed in GHS rats compared to normal SD rats and contributes to kidney stone-forming and hypercalciuria in GHS rats.8 However, the detailed mechanisms underlying the VDR-mediated regulation of CaSR are not clear. Here, we found that VDR binds to the rat proximal CaSR promoter region 10, which is followed by hyperacetylation of histone H3. Another in vitro study on the human CaSR promoter reported that VDR binds to the same region within the human CaSR promoter.21 Taken together, these results indicate that there may be potential VDREs in the rat CaSR proximal promoter region.
Conclusion
In summary, our studies indicate that the elevation of VDR up- or downregulates target genes associated with vitamin D metabolism and Ca homeostasis by binding to multiple promoter regions and hyperacetylating or hypermethylating histone H3. These in vivo data provide deep insights into how VDR regulates rat target gene expression in vivo.
Conflict of interests
There are no interests conflict.
Acknowledgements
The research is supported by the hospital’s own funds (ynlc201721).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2020.09.001.
Contributor Information
Biao Zhong, Email: biao.zhong@139.com.
Murray J. Favus, Email: mjfavus@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Favus M.J. Hypercalciuria: lessons from studies of genetic hypercalciuric rats. J Am Soc Nephrol. 1994;5:S54–S58. doi: 10.1681/ASN.V55s54. [DOI] [PubMed] [Google Scholar]
- 2.Bushinsky D.A., Favus M.J. Mechanism of hypercalciuria in genetic hypercalciuric rats. Inherited defect in intestinal calcium transport. J Clin Invest. 1988;82:1585–1591. doi: 10.1172/JCI113770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pak C.Y., Oata M., Lawrence E.C., Snyder W. The hypercalciurias. Causes, parathyroid functions, and diagnostic criteria. J Clin Invest. 1974;54:387–400. doi: 10.1172/JCI107774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X.Q., Tembe V., Horwitz G.M., Bushinsky D.A., Favus M.J. Increased intestinal vitamin D receptor in genetic hypercalciuric rats. A cause of intestinal calcium hyperabsorption. J Clin Invest. 1993;91:661–667. doi: 10.1172/JCI116246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krieger N.S., Stathopoulos V.M., Bushinsky D.A. Increased sensitivity to 1,25(OH)2D3 in bone from genetic hypercalciuric rats. Am J Physiol. 1996;271:C130–C135. doi: 10.1152/ajpcell.1996.271.1.C130. [DOI] [PubMed] [Google Scholar]
- 6.Tsuruoka S., Bushinsky D.A., Schwartz G.J. Defective renal calcium reabsorption in genetic hypercalciuric rats. Kidney Int. 1997;51:1540–1547. doi: 10.1038/ki.1997.212. [DOI] [PubMed] [Google Scholar]
- 7.Yao J., Kathpalia P., Bushinsky D.A., Favus M.J. Hyperresponsiveness of vitamin D receptor gene expression to 1,25-dihydroxyvitamin D3. A new characteristic of genetic hypercalciuric stone-forming rats. J Clin Invest. 1998;101:2223–2232. doi: 10.1172/JCI1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao J.J., Bai S., Karnauskas A.J., Bushinsky D.A., Favus M.J. Regulation of renal calcium receptor gene expression by 1,25-dihydroxyvitamin D3 in genetic hypercalciuric stone-forming rats. J Am Soc Nephrol. 2005;16:1300–1308. doi: 10.1681/ASN.2004110991. [DOI] [PubMed] [Google Scholar]
- 9.Karnauskas A.J., Van Leeuwen J.P., Van den Bemd G.J., et al. Mechanism and function of high vitamin D receptor levels in genetic hypercalciuric stone-forming rats. J Bone Miner Res. 2005;20:447–454. doi: 10.1359/JBMR.041120. [DOI] [PubMed] [Google Scholar]
- 10.Palmer H.G., Larriba M.J., Garcia J.M., et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med. 2004;10:917–919. doi: 10.1038/nm1095. [DOI] [PubMed] [Google Scholar]
- 11.Bai S., Wang H., Shen J., Zhou R., Bushinsky D.A., Favus M.J. Elevated vitamin D receptor levels in genetic hypercalciuric stone-forming rats are associated with downregulation of Snail. J Bone Miner Res. 2010;25:830–840. doi: 10.1359/jbmr.091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holick M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 13.Holick M.F. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holick M.F. Calcium plus vitamin D and the risk of colorectal cancer. N Engl J Med. 2006;354:2287–2288. doi: 10.1056/NEJMc060753. [DOI] [PubMed] [Google Scholar]
- 15.Anderson P., May B., Morris H. Vitamin D metabolism: new concepts and clinical implications. Clin Biochem Rev. 2003;24:13–26. [PMC free article] [PubMed] [Google Scholar]
- 16.Zierold C., Darwish H.M., DeLuca H.F. Two vitamin D response elements function in the rat 1,25-dihydroxyvitamin D 24-hydroxylase promoter. J Biol Chem. 1995;270:1675–1678. doi: 10.1074/jbc.270.4.1675. [DOI] [PubMed] [Google Scholar]
- 17.Meyer M.B., Zella L.A., Nerenz R.D., Pike J.W. Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D3 in mouse kidney and intestine in vivo. J Biol Chem. 2007;282:22344–22352. doi: 10.1074/jbc.M703475200. [DOI] [PubMed] [Google Scholar]
- 18.Turunen M.M., Dunlop T.W., Carlberg C., Vaisanen S. Selective use of multiple vitamin D response elements underlies the 1 alpha,25-dihydroxyvitamin D3-mediated negative regulation of the human CYP27B1 gene. Nucleic Acids Res. 2007;35:2734–2747. doi: 10.1093/nar/gkm179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerry D.M., Dwivedi P.P., Hahn C.N., Morris H.A., Omdahl J.L., May B.K. Transcriptional synergism between vitamin D-responsive elements in the rat 25-hydroxyvitamin D3 24-hydroxylase (CYP24) promoter. J Biol Chem. 1996;271:29715–29721. doi: 10.1074/jbc.271.47.29715. [DOI] [PubMed] [Google Scholar]
- 20.Chikatsu N., Fukumoto S., Takeuchi Y., et al. Cloning and characterization of two promoters for the human calcium-sensing receptor (CaSR) and changes of CaSR expression in parathyroid adenomas. J Biol Chem. 2000;275:7553–7557. doi: 10.1074/jbc.275.11.7553. [DOI] [PubMed] [Google Scholar]
- 21.Canaff L., Hendy G.N. Human calcium-sensing receptor gene. Vitamin D response elements in promoters P1 and P2 confer transcriptional responsiveness to 1,25-dihydroxyvitamin D. J Biol Chem. 2002;277:30337–30350. doi: 10.1074/jbc.M201804200. [DOI] [PubMed] [Google Scholar]
- 22.Perez A.V., Picotto G., Carpentieri A.R., Rivoira M.A., Peralta Lopez M.E., De Talamoni N.G.T. Minireview on regulation of intestinal calcium absorption. Emphasis on molecular mechanisms of transcellular pathway. Digestion. 2008;77:22–34. doi: 10.1159/000116623. [DOI] [PubMed] [Google Scholar]
- 23.Bianco S.D., Peng J.B., Takanaga H., et al. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res. 2007;22:274–285. doi: 10.1359/jbmr.061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pike J.W., Meyer M.B., Watanuki M., et al. Perspectives on mechanisms of gene regulation by 1,25-dihydroxyvitamin D3 and its receptor. J Steroid Biochem Mol Biol. 2007;103:389–395. doi: 10.1016/j.jsbmb.2006.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pike J.W., Zella L.A., Meyer M.B., Fretz J.A., Kim S. Molecular actions of 1,25-dihydroxyvitamin D3 on genes involved in calcium homeostasis. J Bone Miner Res. 2007;22:V16–V19. doi: 10.1359/jbmr.07s207. [DOI] [PubMed] [Google Scholar]
- 26.Wang H.W., Muguira M., Liu W.D., et al. Identification of an INSM1-binding site in the insulin promoter: negative regulation of the insulin gene transcription. J Endocrinol. 2008;198:29–39. doi: 10.1677/JOE-08-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Z., Wang C., Wang M., et al. A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J Cell Biol. 2008;182:509–517. doi: 10.1083/jcb.200801079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thuault S., Tan E.J., Peinado H., Cano A., Heldin C.H., Moustakas A. HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition. J Biol Chem. 2008;283:33437–33446. doi: 10.1074/jbc.M802016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dusso A.S. Vitamin D receptor: mechanisms for vitamin D resistance in renal failure. Kidney Int Suppl. 2003;63(S 85):S6–S9. doi: 10.1046/j.1523-1755.63.s85.3.x. [DOI] [PubMed] [Google Scholar]
- 31.Zella L.A., Kim S., Shevde N.K., Pike J.W. Enhancers located within two introns of the vitamin D receptor gene mediate transcriptional autoregulation by 1,25-dihydroxyvitamin D3. Mol Endocrinol. 2006;20:1231–1247. doi: 10.1210/me.2006-0015. [DOI] [PubMed] [Google Scholar]
- 32.Villeneuve L.M., Reddy M.A., Lanting L.L., Wang M., Meng L., Natarajan R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci U S A. 2008;105:9047–9052. doi: 10.1073/pnas.0803623105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panda D.K., Al Kawas S., Seldin M.F., Hendy G.N., Goltzman D. 25-hydroxyvitamin D 1alpha-hydroxylase: structure of the mouse gene, chromosomal assignment, and developmental expression. J Bone Miner Res. 2001;16:46–56. doi: 10.1359/jbmr.2001.16.1.46. [DOI] [PubMed] [Google Scholar]
- 34.Li Y., Reddy M.A., Miao F., et al. Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-kappaB-dependent inflammatory genes. Relevance to diabetes and inflammation. J Biol Chem. 2008;283:26771–26781. doi: 10.1074/jbc.M802800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer M.B., Watanuki M., Kim S., Shevde N.K., Pike J.W. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol. 2006;20:1447–1461. doi: 10.1210/me.2006-0031. [DOI] [PubMed] [Google Scholar]
- 36.Wang W.H., Lu M., Hebert S.C. Cytochrome P-450 metabolites mediate extracellular Ca(2+)-induced inhibition of apical K+ channels in the TAL. Am J Physiol. 1996;271:C103–C111. doi: 10.1152/ajpcell.1996.271.1.C103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.