Abstract
Basement membrane proteins are known to guide cell structures, differentiation, and tissue repair. Although there is a wealth of knowledge on the functions of laminins, perlecan, and type IV collagen in maintaining tissue homeostasis, not much is known about nidogen. As a key molecule in the basement membrane, nidogen contributes to the formation of a delicate microenvironment that proves necessary for stem cell lineage-specific differentiation. In this review, the expression of nidogen is delineated at both cellular and tissue levels from embryonic to adult stages of development; the effect of nidogens is also summarized in the context of musculoskeletal development and regeneration, including but not limited to adipogenesis, angiogenesis, chondrogenesis, myogenesis, and neurogenesis. Furthermore, potential mechanisms underlying the role of nidogens in stem cell-based tissue regeneration are also discussed. This concise review is expected to facilitate our existing understanding and utilization of nidogen in tissue engineering and regeneration.
Keywords: Adipose, Basement membrane, Cartilage, Differentiation, Nerve, Nidogens, Vessel
Introduction
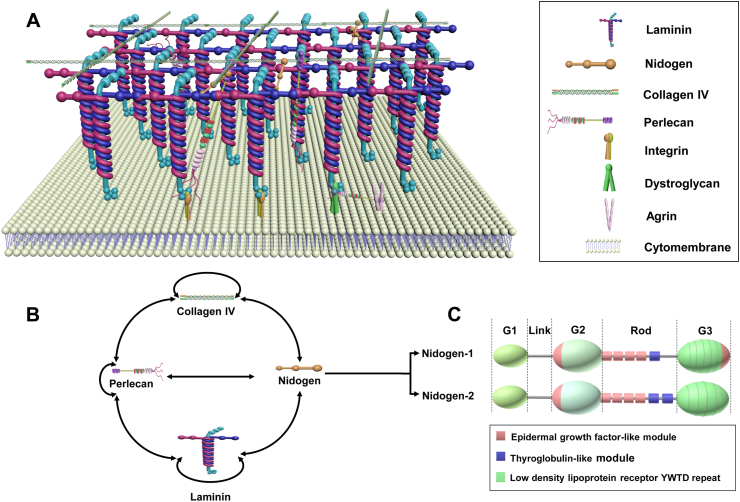
The musculoskeletal system consists of bone, cartilage, fat, muscle, ligaments, nerves, and blood vessels that coordinate under mutual influence and interdependence. Basement membrane, the most ancient and conserved type of specialized extracellular matrix (ECM), basally underlies epithelia and wraps around organs including nerve, adipose, cartilage, and muscle tissue to regulate cell signaling and tissue growth.1 For example, in Drosophila wing discs, the elimination of basement membrane reduces bone morphogenetic protein (BMP)/transforming growth factor β (TGFβ) ligand Decapentaplegic (Dpp) signaling, ultimately resulting in decreased cell compression and reduced wing size.2 Moreover, in the Drosophila embryo, interactions between type IV collagen and Dpp have been shown to promote gradient formation to augment Dpp signaling.3 Functionally, the basement membrane enables cells to be distinct yet interactive with their external environment and provides scaffolding during embryonic development.4,5 Basement membrane is primarily composed of four defined cardinal glycoprotein and proteoglycan components: laminin, type IV collagen, perlecan, and nidogen, all of which are considered widely conserved proteins (Fig. 1).6, 7, 8 Additional components of the basement membrane—for example, thrombospondin-1, matrilin-2/4, and fibronectin—are not expressed ubiquitously, but rather in a tissue-specific manner.9 Following a brief introduction of laminin, type IV collagen, and perlecan, this review will primarily focus on the role of nidogen in musculoskeletal tissue differentiation and regeneration.
Figure 1.
Core basement membrane components and binding interactions. (A) Both type IV collagen and laminin are trimeric proteins that are capable of self-assembly into independent networks. While laminin initiates basement membrane assembly, type IV collagen contributes tensile strength. The link of this interaction is strengthened by nidogens and perlecan, the latter of which regulates the hydration of the basement membrane by providing a negative charge through heparan sulfate sugar chains.1,5,133 (B) The collagen network interacts with the laminin network through binding bridges - perlecan and nidogen, in which perlecan can also interact with itself. The double-headed arrows represent interactions.1 (C) Structural representation of nidogen-1 and -2, each consisting of 3 globular domains—G1, G2, and G3—separated by a link region and a rod domain. The rod domain of nidogen-2 has four EGF (epidermal growth factor-like module) and two TY (thyroglobulin-like module) motifs while nidogen-1 has four EGF and one TY motif. In the globular domain G3, nidogen-1 has one more EGF motif than nidogen-2.134
Laminins are heterotrimeric glycoproteins made up of three distinct polypeptide chains: α-chain, β-chain, and γ-chain.10 All three chains coil together to form the long arm, which is followed by five homologous laminin globular-like (LG) domains at the distal end. While the LG domains activate intracellular signaling pathways by binding to cellular receptors, the three shorter arms interact with each other to form networks.11 Laminin plays a vital role in early embryonic development and basement membrane formation,4 and its deficiency has been shown to result in insufficient basement membrane assembly which leads to a myriad of human diseases that stem from skeletal muscle damage.12,13 For instance, laminin is exclusively present in the pericellular matrix (PCM) of healthy articular cartilage; as such, its absence may indicate the degeneration of normal cartilage and/or dedifferentiation of chondrocytes.14
Composed of six individual α-chains that assemble into three types of heterotrimers, type IV collagen self-assembles into a polygonal network composed of an N-terminal domain (7S), Gly-X-Y triple repeats, and a C-terminal non-collagenous domain (NC1). This structure functions to provide the basement membrane with tensile strength.1,15 Interestingly, in 2004, Poschl and colleagues first demonstrated that while type IV collagen is not critically important during early development, it becomes indispensable for maintaining the integrity of basement membrane-like matrices during the later stages of development.16 Besides contributing to the structural strength and integrity of the basement membrane, type IV collagen networks also provide a scaffolding function to bind various basement membrane proteins.17 As such, type IV collagen deficiency results in basement membrane mechanical instability and inadequate cell-basement membrane interactions.16 Notably, chaperone proteins heat shock protein 47 (Hsp47) and transport and golgi organization protein 1 (Tango1) are required to maintain type IV collagen promoter stability and guide its secretion and assembly into the basement membrane.18
As a proteoglycan, perlecan is not only ubiquitous in basement membrane and vascular tissues, but also in cartilaginous tissues which lack both basement membrane and blood vessels.19, 20, 21 Encoded by the heparan sulfate proteoglycan 2 (HSPG2) gene, perlecan has a pearls-on-a-string-like appearance and is indispensable for musculoskeletal tissue formation.22 Mutations in the HSPG2 gene can lead to disorders of the musculoskeletal system, specifically the autosomal recessive skeletal diseases: Silverman-Handmaker Syndrome and Schwartz-Jampel Syndrome.23 Perlecan also interacts with a diverse range of ECM molecules to maintain ECM stabilization and organization. It has been shown that not only does perlecan function as a component of basement membrane but it can also be used as a chondrogenic marker in prenatal cartilage.24
Ubiquitous in the basement membrane, nidogens are glycoproteins containing three globular-like (G) domains (G1-G3) separated by a link-like and rod-like segment. Nidogen has two isoforms: nidogen-1/entactin-125 and nidogen-2/entactin-226. These isoforms structurally differ in their rod segments and the G3 domains.5 While both nidogen isoforms interact with the laminin 1 short arm chain through their G3 domains,26,27 only nidogen-1 binds to type IV collagen and perlecan through its G2 domain.28, 29, 30, 31
Primarily expressed in mesenchymal cells, nidogen contributes to the epithelial and endothelial basement membranes during development.32 Despite the fact that nidogen-1 and -2 have comparable levels of interaction with perlecan and type I and type IV collagen, only nidogen-1 can bind to fibulins.8,26 Furthermore, compared to nidogen-1's strong-affinity binding on the laminin 1 short arm chain, nidogen-2 only displays moderate-affinity binding.26,27 Recently, nidogen-1 was found to be expressed in bone marrow peri-sinusoidal stromal cells, which constituted the hematopoietic stem cell niche. As loss of nidogen-1 impairs early B cell expansion and differentiation,33 nidogen-1 can be seen to play a vital role in stem cell expansion and differentiation.
Surprisingly, although nidogen-1 and nidogen-2 are present in all basement membranes,34 the basement membrane can indeed be formed without nidogens.35 Genetic deletion of either nidogen-1 or nidogen-2 alone in the mouse model does not cause obvious alterations in tissue and basement membrane architecture.36,37 Nevertheless, nidogen double null mice exhibit perinatal death due to lung and heart abnormalities, directly related to basement membrane defects.38 As such, these findings suggest that both nidogens play crucial roles in basement membrane stabilization during late embryogenesis but are not necessary for basement membrane initial assembly. Furthermore, nidogen-2 expression in nidogen-1 null mice is redistributed and upregulated—suggesting that the two nidogens have compensatory functions.36 Different basement membranes are observed to have different composition requirements for nidogens.
Although nidogens are well characterized and known to be primarily expressed in the basement membrane,39 their spatial/temporal distribution and tissue/organ-specific mechanisms remain unclarified. In this concise review, we investigate the role and importance of nidogens in the musculoskeletal system by summarizing their expression during the embryonic and adult stages of development. We then go on to identify their potential impact in adipogenesis, angiogenesis, chondrogenesis, myogenesis, and neurogenesis as well as potential molecular mechanisms.
Stage-dependent expression of nidogen
First identified from an extracellular basement membrane-like matrix deposited by a mouse endodermal cell line in 1981,40 nidogen-1 was subsequently found in the basement membrane of a murine Engelbreth-Holm-Swarm tumor in 1983.25 Later on, in 1998, human nidogen-2 was cloned for the first time,26 and in the same year, mouse nidogen-2 was isolated from KUSA cells, a murine osteoblast-like cell line.41
Embryonic stage
Invertebrates, like Caenorhabditis elegans (C. elegans), only express a single nidogen gene,42 while mammals possess both isoforms, nidogen-1 and nidogen-2.41
In C. elegans, nidogen-1 appears at the beginning of embryonic morphogenesis, and then subsequently accumulates on pharyngeal, intestinal, and gonad primordial tissue during development.43 In this species, nidogen-1 is nonessential for the normal localization of type IV collagen in basement membrane43 but is required to direct longitudinal nerves dorsoventrally and axons at the midline.44 Moreover, together with type XVIII collagen, nidogen-1 is crucial in C. elegans synapse organization.45
During mouse embryonic and fetal development, nidogen-1 is first detected on the compacted 8- to 16-cell stage morulae.46 At later stages of development and in adult mouse tissues, nidogen-1 and -2 display identical patterns of expression (Fig. 2).26,27,41,47,48 The discovery of nidogen-1 mRNA in the mesoderm, endoderm, and ectoderm indicates its expression in all three germ layers of the mouse embryo.48 Additionally, embryonic basement membranes are only capable of having a fully developed ultrastructural architecture when nidogen-1 is present, indicating that nidogen-1 plays an important role in stabilizing basement membranes in the in vivo embryo.48 This point is further demonstrated when nidogen-1 and laminin binding interactions are disrupted through antibody binding, in which case the basement membrane gets distorted and epithelial morphogenesis of mouse embryonic submandibular glands, lung, and kidney is disturbed.49,50 Moreover, nidogen-1 and -2 have been found to be essential for maintaining the integrity of cardiac tissue and lung tissue during the late stages of development.38 Evidence indicates that basement membranes are stabilized by nidogen-1, expressed by epithelial and mesenchymal cells, during the early stages of mouse embryo development.48 As the functions of the two isoforms are complementary, loss of both results in ectodermal basement membrane breakdown in the limb bud.27,35
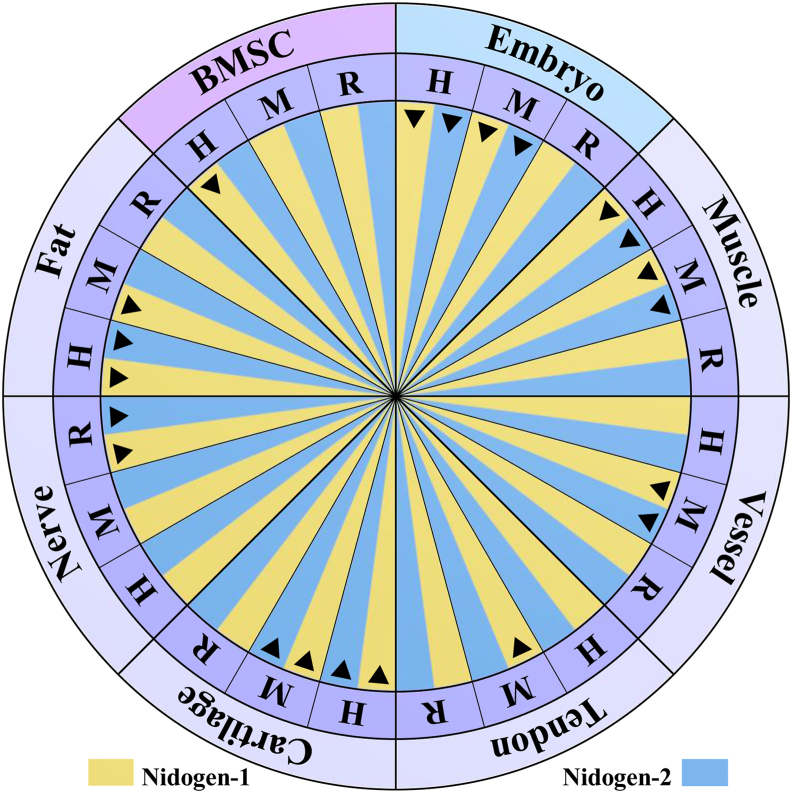
Figure 2.
Proven expression of nidogen isoforms. Nidogen-1 has been detected not only in human bone marrow stromal cells (BMSCs)59,63 but also in human and mouse embryos along with nidogen-2.27,79,85,135 During the adulthood of human, mouse and rat models, nidogen expression has been broadly found in various tissues, such as muscle,26,36, 37, 38,84 vessel,67,75,136 tendon,129 cartilage,78, 79, 80 nerve,92,94 and fat54,62,64 (Table 1 for details). The black triangle represents positive expression of nidogen isoforms in the cells or tissues of human (H), mouse (M) and/or rat (R).
In human embryonic development, nidogen-1 and -2 are ubiquitous in the developing epithelia of most major organ systems (Fig. 2), with the exception of the intestine and the pancreas anlage, in which only nidogen-1 is present.51
Adult stage
In larvae and adults of C. elegans, nidogen-1 is typically enriched around the developing nerve ring and the gonad.43 In mice, nidogen-1 is located in most basement membranes while nidogen-2 accumulation is limited to the endothelial basement membranes of the kidney, skeletal muscle, and heart.36,37,47 Interestingly, in most organs, such as kidney, loss of nidogen-1 has no notable impact on nidogen-2 staining. Exceptions include in striated muscles and in the heart where nidogen-1 deficiency results in increased nidogen-2 expression.36 These findings indicate that, in the absence of nidogen-1, there is either an unmasking of nidogen-2 epitopes or a nidogen-2 redistribution from other extracellular sources. In contrast, in NID2-null mice, nidogen-1 levels in the endothelial basement membrane fail to increase in the same compensatory manner.37
Role of nidogen in lineage-specific differentiation
Nidogens are present pericellularly in adipocytes, endothelial cells, chondrocytes, myocytes, and nerve cells in a laminar arrangement and play significant roles in each of their differentiations (Fig. 2 and Table 1).
Table 1.
Expression of nidogen isoforms in lineage differentiation of musculoskeletal tissues.
| Tissue/cells | Nidogen-1 | Nidogen-2 | Ref. |
|---|---|---|---|
| Cartilage | mouse femoral head (qPCR, IHC) | bovine MCP (IGEM, IHC); mouse femoral head (qPCR, IHC) |
78 |
| mouse TMJ (IHC) | mouse TMJ (IHC) | 80 | |
| mouse embryo (IF) | mouse embryo (IF) | 27 | |
| human embryo metacarpus or rib anlagen (IHC); human healthy and OA knee (IGS, IHC) | human embryo metacarpus or rib anlagen (IHC); human healthy and OA knee (IGS, IHC) | 79 | |
| Fat | human visceral fat (proteomics) | human visceral fat (proteomics) | 54 |
| – | human visceral fat (CILAIR-based secretome analysis) | 62 | |
| mouse epidydimal and subcutaneous fat (IF) | – | 64 | |
| Muscle | mouse embryo limb (IF) | – | 85 |
| mouse diaphragm and limb muscles (IF) | mouse diaphragm and limb muscles (IF) | 84 | |
| mouse cardiac muscles (IF) | mouse cardiac muscles (IF) | 38 | |
| mouse soleus and cardiac muscles (IF, WB) | mouse soleus and cardiac muscles (IF, WB); mouse soleus muscles (IGS) | 36 | |
| mouse skeletal and cardiac muscles (IF, RIA) | mouse skeletal and cardiac muscles (IF, RIA) | 26 | |
| mouse skeletal muscles (IF, NB, RIA, WB) | mouse skeletal muscles (IF, NB, RIA, WB) | 37 | |
| Nerve | C. elegans sublateral nerve and nerve ring (IF) | – | 43 |
| C. elegans nerve cord (IF) | – | 45 | |
| rat DRG (IF) | – | 92 | |
| rat sciatic nerve (IF, ISH, qPCR, WB) | rat sciatic nerve (IF, ISH, qPCR, WB) | 94 | |
| Tendon | mouse (IF) | – | 129 |
| Vessel | mouse embryo radial glia (IF) | – | 135 |
| mouse capillary in muscles (IF) | – | 136 | |
| mouse vessels in RIP-Tag2 pancreatic islet tumors (IF) | – | 67 | |
| mouse retinal capillaries (IF, WB) | mouse retinal capillaries (IF) | 75 | |
| BMSC | expression in human cells following 28-day adipogenic induction (IF) | – | 63 |
| increased expression in human cells following 28-day adipogenic induction (qPCR) | – | 59 | |
| 3T3-L1 | increased expression in supernatants but decreased in mouse cells in 6-day adipogenic induction (IP) | – | 55 |
| – | increased expression in mouse cells in 14-day adipogenesis (microarray) | 56 | |
| peak expression at day 3 in supernatants of mouse cells in 7-day adipogenic induction (SILAC) | peak expression at day 3 in supernatants of mouse cells in 7-day adipogenic induction (SILAC) | 58 | |
| increased expression in supernatants of mouse cells in 9-day adipogenic induction (proteomics) | – | 57 | |
| – | increased in mouse cells in 14-day adipogenic induction (IF, qPCR) | 61 | |
| peak expression in supernatants of mouse cells in the middle stage of 10-day adipogenic induction (MS) | – | 60 |
Abbreviations: BMSC: bone marrow stromal cells; C. elegans: Caenorhabditis elegans; CILAIR: Comparison of Isotope-Labeled Amino acid Incorporation Rates; DRG: dorsal root ganglia; IF: immunofluorescence; IGEM: immunogold electron microscopy; IGS: immunogold histochemistry; IHC: immunohistochemistry; IP: immunoprecipitation; ISH: in situ hybridization; MCP: metacarpophalangeal joint; MS: mass spectrometry; NB: northern blot; OA: osteoarthritis; qPCR: quantitative polymerase chain reaction; RIA: radioimmunoassays; SILAC: stable isotope labeling with amino acids in cell culture; TMJ: temporomandibular joint; WB: western blot.
Adipogenesis
An early feature in adipogenesis is the biogenesis of an extracellular basement membrane.52,53 Nidogen-1 and -2 are found in 3T3-L1 preadipocytes, mouse white adipose tissue, and human visceral adipocytes and bone marrow stromal cells,54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 maintaining adipocyte basal lamina stability through their binding interactions to type IV collagen and laminins.5
The strong nidogen-1 upregulation during the first six-day differentiation period of 3T3-L1 preadipocytes indicates a cellular transition from a fibrillar to a laminar make-up, suggesting that synthesis of nidogen-1 is vital for adipose tissue morphogenesis.55 A proteomic study further confirms this increase in nidogen-1 expression during adipogenic differentiation of 3T3-L1 preadipocytes.57 Moreover, in 3T3-L1 preadipocytes, nidogen-1 secretion is enhanced when adipose conversion is stimulated by ascorbic acid phosphate.65 Similarly, nidogen-2 mRNA is seen to increase during adipogenesis of 3T3-L1 preadipocytes.56,61 Interestingly, the expression of nidogen-1 and -2 during adipogenic differentiation shows a similar temporal pattern—peaking halfway through adipogenesis. Thus, nidogen-1 and -2 are likely associated with the middle stage of adipogenic differentiation.58
Angiogenesis
Basement membrane, located beneath epithelia and endothelia, also mediates angiogenesis. Nidogens can be detected in the basement membranes of blood vessels in various tissues. Over the entire angiogenic course of a wound-healing model, nidogen-1 exhibits high expression.66 Particularly, the basement membrane of tumor blood vessels has attracted growing interest as a significant contributor to angiogenesis. For instance, nidogen-1 has been detected in the blood vessels of both RIP-Tag2 pancreatic tumors of mice67 and epidermal tumors of immunocompromised rats.68 Neovascularization or angiogenesis after traumatic injuries involves changes of the immunoreactivity of nidogens.69 The level of nidogen-1 in brain vessels increases after kainic acid treatment.69 Moreover, nidogen-1 immunoreactivity in the vascular basal lamina of the spinal cord is dramatically increased within 24 h of a dorsal hemisection, and later returns to basal level after one week,70 which may be a representative feature of neovascularization.
The function of nidogen-1 in the angiogenic process has been established in various contexts. In an aortic explant model, angiogenesis-related sprouting is dependent on laminin-nidogen complex concentration.71 In human microvascular endothelial cells (HMEC-1), cleavage of nidogen-1 by matrix metalloproteinase 19 (MMP-19) inhibits the formation of capillary-like structures.72 In nidogen-1 and -2 deficient mice, the vessels are almost completely devoid of pericytes or less-defined perivascular cells.73 In mice, absence of niodgen-2 in the lungs may lead to subtle alternations in the endothelial basement membrane, facilitating lung metastasis.74 Choroidal neovascularization (CNV) resulting from a serious complication in age-related macular degeneration is the culmination of new vessels sprouting from the choroidal vasculature coupled with proteolytic degradation of Bruch's membrane and changes in basement membrane components. Nidogen-1 exists in the retinal capillaries where it helps to stabilize the basement membrane, thus preventing the infiltration of endothelial cells or the sprouting of new vessels.75 Furthermore, locally restricted bleeding within the heart wall has been reported in mutant embryos and appears to be associated with microvasculature leakage and an absence of capillary basement membrane. Therefore, although nidogens do not seem essential for basement membrane formation, they may play a role in maintenance of capillary integrity.76
Chondrogenesis
Nidogen-1 and -2 have been observed in the mouse tissue mesenchyme during limb development.27,77 In mouse developing cartilage, staining for nidogen-1 is very faint while staining for nidogen-2 is distinct, suggesting that the binding activities between nidogen-2 and perlecan hold importance for cartilage development.27 In newborn mouse cartilage, nidogen-1 is present in the territorial and interterritorial matrix.78 In addition to their presence in developing cartilage, both nidogens have been shown to exist in mouse, bovine, and human adult articular cartilage.78, 79, 80 Though nidogen-1 and -2 mRNAs are both expressed in mouse femoral head cartilage, the level of nidogen-2 mRNA is notably higher than that of nidogen-1 mRNA.78 In adult mice, nidogen-1 is located in the narrow pericellular zone around the chondrocytes, but with aging, the zone becomes less distinct, especially in the obvious mechanical attrition areas.78 As seen in bovine articular cartilage, interestingly, nidogen-2 is deposited in a narrow, capsule-like zone surrounding chondrocytes.78
In healthy human articular cartilage, nidogen-1 and -2 exhibit increased expression around elongated chondrocytes in the deep surface fissures of osteoarthritic cartilage.79 Interestingly, Kruegel et al found that healthy cartilage contained two-fold more pericellular nidogen-1 than diseased cartilage affected with osteoarthritic defects, while diseased chondrocytes contained five-fold more pericellular nidogen-2 protein—suggestive of a nidogen-2 substitution for nidogen-1 in osteoarthritic cartilage.79 Notably, this process is similarly observed in skeletal muscle.81 Therefore, it can be concluded that during late-stage osteoarthritis, nidogen activity is an indication of cartilage regeneration.79 Interestingly, chondrogenic progenitor cells express more nidogen-2 mRNA than do healthy or osteoarthritic chondrocytes,82 though this point may need further investigation. In osteoarthritic chondrocytes, nidogen-1 co-localizes with integrin β1 and αv.79 In contrast, nidogen-2 does not co-localize with these integrin subunits, suggesting that the nidogen-2 isoform may bind through other integrin or non-integrin receptors.79
Nidogen-1 and -2 play positive roles in chondrogenesis; accordingly, their disappearance results in functional failure of the ectodermal basement membrane caused by dysregulation of interdigital apoptosis.35 Furthermore, addition of nidogen-2 enhances chondrogenic differentiation as seen in mouse DDR-1 (discoidin domain receptor 1)-deficient chondrocytes from the temporomandibular joint, where nidogen-2 increases chondrogenic differentiation with significantly decreasing RUNX2 (runt-related transcription factor 2) mRNA levels.80 In human osteoarthritic tissue derived chondrogenic progenitor cells, nidogen-2 significantly increased the ACAN (Aggrecan) and SOX9 (SRY-Box 9) mRNA levels.82 Moreover, knockdown of the nidogen-2 gene reduces the level of SOX9 mRNA but upregulates RUNX2 expression via the reduction of SMAD (small mothers against decapentaplegic) signaling.82
Myogenesis
Much evidence has been found to support nidogen protein expression in the developing, adult, and diseased muscles. Not only is nidogen-1 secreted into the ECM around the early developing myotubes,83 nidogen-1 and -2 have also been found to be present in mouse and human muscles.51,81,84,85 Specifically, in adult muscle, though both nidogen-1 and nidogen-2 initially exist throughout the muscle fiber basal lamina, nidogen-2 is later found to be localized to the synapses.84 Interestingly, patients afflicted with Duchenne muscular dystrophy have been shown to express lower levels of nidogen-1.86
The different nidogen-1 and -2 expression levels in myogenic differentiation may be indicative of their distinct functions. In murine C2C12 myoblasts, the level of nidogen-1 strongly decreases 16 h after myogenic induction and becomes barely detectable after 40 h; in contrast, nidogen-2 expression is increased within the first 16 h after induction, reaching the peak around 24 h and dropping to baseline level around 62 h.87
Nidogens play a vital role in myogenesis. In the early stages of myogenic differentiation, Matrigel (a soluble basement membrane containing nidogen-1) has a stimulatory effect on C2C12 myoblasts in vitro.88 Furthermore, nidogen-1 alone is reported to promote long-term maintenance and adhesion of skeletal myotubes.89 Interestingly, laminin and nidogen-1 seem to enhance primary mouse myotube formation that has been previously impaired by interaction with cross-linked type I collagen films in vitro.90 It is worth noting that a high level of nidogen-1 has also been reported to inhibit myogenic differentiation of murine C2C12 myoblasts.87 Nidogen-2 knockdown has an unclear influence on myogenesis, but one report has shown that nidogen-2 knockdown in murine C2C12 myoblasts reduces expression of p21, an anti-proliferative and differentiation-associated gene87 whereas another study found promoted myofibroblast differentiation upon nidogen-2 knockdown in scleroderma fibroblasts.91 Moreover, for nidogen-2 knockout mice, neuromuscular junctions only express topological abnormalities after birth during maturation; as type IV collagen and heparin sulfate proteoglycans both still exist in nidogen-2 knockouts, such an abnormality is likely not a derivative of defects in the synaptic basal lamina integrity.84
Neurogenesis
Nidogens are involved at varied time points and localizations during C. elegans, mouse, and rat neurogenesis. In C. elegans, nidogen-1 accumulates mostly around the nerve ring and on the sublateral nerves which run under muscles in the larvae and adult periods43 and directs longitudinal nerves dorsoventrally and axons at the midline.44 Moreover, nidogen-1 is also concentrated laterally between the muscles and nerve cord.45 In rats, nidogen-1 shows similar expression between the naïve DRG (doral root ganglia) and DRG, but it decreases and sometimes even fully disappears from the surface of the SGCs (satellite glial cells) of primary sensory neurons when it is subjected to nerve compression.92 Both nidogen isoforms are localized in the rat sciatic nerve; following rat sciatic nerve transection, nidogen levels become increased during nerve regeneration.93,94
Nidogen-1 increases Schwann cell formation and protects them from serum-deprivation-induced death, indicating that the protein plays a pro-survival role in Schwann cell development.94 Further studies indicate that nidogen-1 is required for proper peripheral nerve regeneration after injury.95 Wolfstetter and colleagues found that during Drosophila development, although nidogen was not essential for the assembly, it mediated basement membrane stability and ECM-dependent neural plasticity.96 It is interesting that nidogen-1 plays a vital role in neuronal plasticity; upon nidogen-1 ablation, spontaneous epileptiform activity is observed in vitro and epileptic activity is detected in vivo, suggesting that modulatory mechanisms of synaptic excitability and plasticity may reach beyond the confines of classical cellular interactions.39,97
Potential mechanisms underlying the role of nidogen in stem cell differentiation
Contribution of basement membrane to stem cell niche
Over the past few years, it has become clear that stem cell growth and differentiation occur in what is known as “stem cell niches”, supporting structures that contain basement membrane to sustain stem cell development.98 Known as basal lamina, basement membrane is a specialized and essential component of ECM that lies close to stem cells and acts as a dynamic microenvironment for stem cell behavior and differentiation.99 For instance, satellite cells, a population of muscle stem cells, are localized to their niche—the myofiber basal lamina—a structure which assists in the activation of satellite cells in proliferation and terminal differentiation as well as self-renewal upon exposure to external damage or following muscular exercise.100,101 Similarly, neural stem cell maintenance, differentiation, and migration are modulated by adjacent vascular basement membrane.102
Basement membrane manages cellular functions by adjusting local concentrations of growth factors and cytokines as well as regulating cell polarity, adhesion, spreading, and migration. Considering its tissue- and site-specific properties,103 the basement membrane functions as a stem cell niche through a highly selective specialized ECM to modulate stem cell properties in adult tissues.104,105 For example, four cardinal components of basement membrane are found in a narrow, capsule-like PCM of mature cartilage, which is dynamically modulated from dispersed distribution of these components throughout territorial matrix and interterritorial matrix in newborn mice.78 Interestingly, expression of the four cardinal components in PCM is mostly restricted to superficial layer chondrocytes, a unique structure that protects chondrocytes from apoptosis and maintains cell phenotype.78,106
As with other ECMs, the basement membrane influences cell fate through both mechanical and chemical means (Fig. 3). The mechanical (viscoelastic) properties of gel-like polymer in basement membrane are generated through laminin and type IV collagen interactions.107,108 The stem cell niche can then transmit physical cues through these basement membrane components and corresponding integrin receptors, which may directly determine cell fate by influencing nuclear events.109 Stiffness, geometric, and mechanical cues from their environment have been well documented to influence stem cell differentiation.110,111 Notably, cells are able to dynamically remodel their cytoskeletal networks to tune the mechanical properties of basement membrane; these cell-generated forces are also sufficient to direct cell fate.110,112
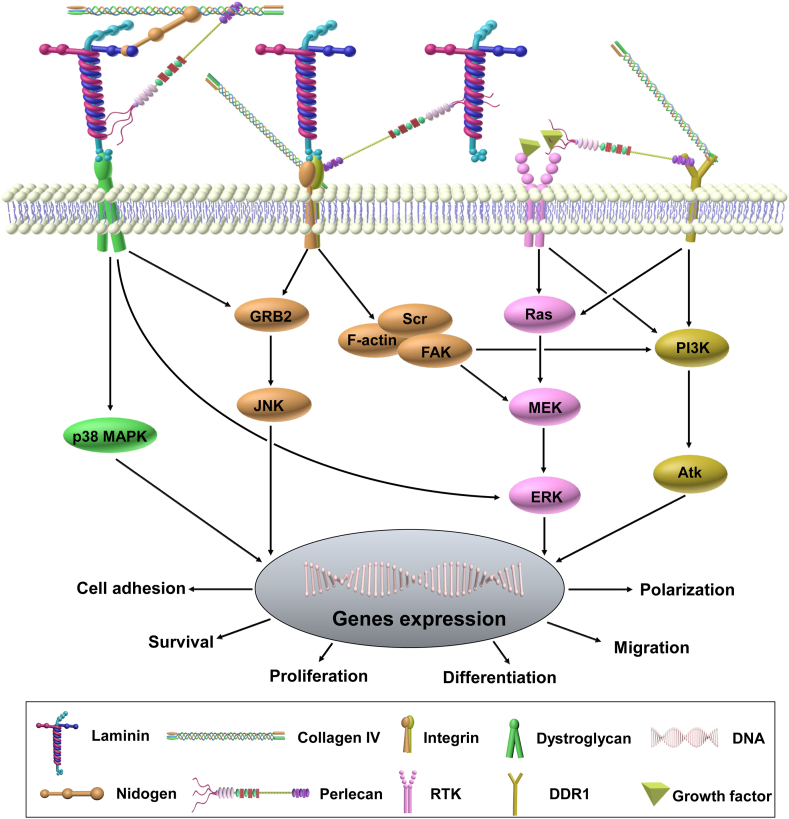
Figure 3.
Basement membrane signaling cascade. Basement membrane core molecules—laminin, type IV collagen, perlecan, and nidogen—regulate cell adhesion, migration, polarization, proliferation, and differentiation via directly or indirectly binding to cell surface receptors such as dystroglycan (DG), integrin, receptor tyrosine kinase (RTK), and discoidin domain receptor 1 (DDR1). For example, laminin-6 plays a role in mechanical signaling transduction by assembling into multi-molecular fibrillary complexes with perlecan via a DG-dependent and integrin-independent manner.137 Laminin binding to DG also triggers the GRB2 (growth factor receptor-bound protein 2)-RAC1-PAK1-JNK (c-Jun N-terminal kinase) pathway that contributes to hypertrophy.138 Evidence shows a direct connection between DG and MAP kinase (MEK) and between DG and extracellular signal-regulated kinase (ERK).139 Type IV collagen plays a role by interacting with the laminin network through perlecan and nidogen followed by binding with integrins and DDR1.76 Perlecan modulates cell survival, migration, and proliferation by tethering growth factors.15,140 By binding to laminin and type IV collagen, nidogen plays a role in maintaining capillary integrity through forming a non-covalent high-affinity stabilizing bridge.76
In contrast, other basement membrane components, such as fibronectin,113 perlecan,114,115 and nidogen,61 can mediate cellular signaling by binding to growth factors and their receptors.116 The spatio-temporal regulation of these basement membrane components during development provides instructive differentiation signals that can ultimately influence cell fate through the creation of morphogenetic gradients.117 Mechanistically, cells migrate and differentiate from areas with lower concentration of basement membrane components to areas with higher concentrations due to an adhesion gradient.118 Moreover, proteases, such as MMPs, can remodel the basement membrane by releasing soluble growth factors, which are important in regulating stem cell differentiation, tissue growth, and development.119
Potential influence of nidogen on stem cell fate
Nidogen is a basement membrane linker molecule that is able to bind to laminin, type IV collagen, perlecan, and fibulin.120 Studies investigating nidogen's role in modulating cellular phenotypes and differentiation are still in the early stages, and knowledge about how nidogen transduces intracellular pathways remains limited. However, as it is known that basement membrane is a regulator of stem cell fate, nidogen may very well contribute to the regulation of cellular differentiation through creating a morphogenetic gradient during development or sending chemical or physical cues through its modulation of basement membrane composition.121,122 In the assembly of basement membrane, nidogen-1 and -2 isoforms act in a compensatory manner as the genetic deletion of either does not result in significant basement membrane alterations.36,37,123 Interestingly, although double mutant mice die shortly after birth from defective basement membrane assembly,35,38 certain tissues (such as kidney and skin) exhibit ultrastructurally normal basement membranes.73 Thus, nidogen requirements to form basement membranes appear to be tissue-dependent. It should be noted, however, that only nidogen-1 null mice have neurological deficits; thus, nidogen proteins must be differentially characterized according to their isoform-specific functions.97,123 Therefore, the regulation of cell differentiation by nidogen is likely complementary and lineage-dependent, which warrants further investigation.
Conclusions and perspectives
As a stem cell niche, the basement membrane is able to control local stem cell fate by modulating its signaling cascade and preserving overall cellular structure, indicating that different components making up the basement membrane may influence stem cell-based tissue regeneration through driving lineage-specific differentiation and maintaining biological stability of such engineered tissue.124, 125, 126, 127, 128 However, there are limited data on nidogen's behavior within tissues like the tendon, ligament, and bone. For instance, existing studies have detected nidogen-1 presence in the basement membrane of postnatal mouse tendon129 and nidogen-1 involvement in human periodontal ligament formation.130 Kimura et al found that mRNA expression of nidogen-1 and -2 decreased with the onset of osteogenic differentiation in KUSA cells41 however, nidogen participation within osteogenic differentiation remains understudied.
As can be seen, our current knowledge of nidogen involvement in lineage-specific differentiation is in its infancy. Foundational studies have indicated that nidogen-2 levels are upregulated in the absence of nidogen-1 in chondrogenesis and myogenesis, suggesting that nidogen-2 may compensate for nidogen-1 activity in these two types of differentiation.79,81 Whether this compensation is involved in other tissue regeneration requires further investigation. Although we have learned that nidogen-1 binds to integrin receptors β1 and αv during cartilage differentiation,79 we still lack knowledge of the nidogen receptor identity in adipose, muscle, and bone tissue as well as potential downstream nidogen signaling in these respective tissues. Moreover, increasing evidence suggests that epithelial-to-mesenchymal transition (EMT) and endothelial-to-mesenchymal transition (EndMT) occur following basement membrane disruption due to tissue injury and inflammation131; it is still unknown whether nidogen is involved in this transdifferentiation through direct or indirect means. Studies further investigating nidogen using the clustered regularly interspaced palindromic repeats and associated protein (CRISPR/Cas) are needed132; ultimately, this information may further clarify the whole picture of nidogen's exact role in tissue injury and regeneration.
Conflict of interests
The authors declare no conflict of interest.
Acknowledgements
We thank Suzanne Danley for editing the manuscript. This work was supported by a Research Grant from the National Institutes of Health, USA (No. 1R01AR067747) to M.P. and Health Commission of Sichuan Province (No. 18PJ008) and Science & Technology Department of Sichuan Province (No. 2019YFS0267) to S.C.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Pozzi A., Yurchenco P.D., Iozzo R.V. The nature and biology of basement membranes. Matrix Biol. 2017;57–58:1–11. doi: 10.1016/j.matbio.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma M., Cao X., Dai J., Pastor-Pareja J.C. Basement membrane manipulation in Drosophila wing discs affects Dpp retention but not growth mechanoregulation. Dev Cell. 2017;42(1):97–106. doi: 10.1016/j.devcel.2017.06.004. e4. [DOI] [PubMed] [Google Scholar]
- 3.Wang X., Harris R.E., Bayston L.J., Ashe H.L. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455(7209):72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- 4.Kruegel J., Miosge N. Basement membrane components are key players in specialized extracellular matrices. Cell Mol Life Sci. 2010;67(17):2879–2895. doi: 10.1007/s00018-010-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yurchenco P.D. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3(2) doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Canc. 2003;3(6):422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 7.Ozbek S., Balasubramanian P.G., Chiquet-Ehrismann R., Tucker R.P., Adams J.C. The evolution of extracellular matrix. Mol Biol Cell. 2010;21(24):4300–4305. doi: 10.1091/mbc.E10-03-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timpl R., Brown J.C. Supramolecular assembly of basement membranes. Bioessays. 1996;18(2):123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- 9.Torricelli A.A., Singh V., Santhiago M.R., Wilson S.E. The corneal epithelial basement membrane: structure, function, and disease. Invest Ophthalmol Vis Sci. 2013;54(9):6390–6400. doi: 10.1167/iovs.13-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hohenester E. Structural biology of laminins. Essays Biochem. 2019;63(3):285–295. doi: 10.1042/EBC20180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hohenester E., Yurchenco P.D. Laminins in basement membrane assembly. Cell Adhes Migrat. 2013;7(1):56–63. doi: 10.4161/cam.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yurchenco P.D., McKee K.K., Reinhard J.R., Rüegg M.A. Laminin-deficient muscular dystrophy: molecular pathogenesis and structural repair strategies. Matrix Biol. 2018;71–72:174–187. doi: 10.1016/j.matbio.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yurchenco P.D. Integrating activities of laminins that drive basement membrane assembly and function. Curr Top Membr. 2015;76:1–30. doi: 10.1016/bs.ctm.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Foldager C.B., Toh W.S., Gomoll A.H., Olsen B.R., Spector M. Distribution of basement membrane molecules, laminin and collagen type IV, in normal and degenerated cartilage tissues. Cartilage. 2014;5(2):123–132. doi: 10.1177/1947603513518217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayadev R., Sherwood D.R. Basement membranes. Curr Biol. 2017;27(6):R207–R211. doi: 10.1016/j.cub.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Poschl E., Schlotzer-Schrehardt U., Brachvogel B., Saito K., Ninomiya Y., Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131(7):1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 17.Brown K.L., Cummings C.F., Vanacore R.M., Hudson B.G. Building collagen IV smart scaffolds on the outside of cells. Protein Sci. 2017;26(11):2151–2161. doi: 10.1002/pro.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chioran A., Duncan S., Catalano A., Brown T.J., Ringuette M.J. Collagen IV trafficking: the inside-out and beyond story. Dev Biol. 2017;431(2):124–133. doi: 10.1016/j.ydbio.2017.09.037. [DOI] [PubMed] [Google Scholar]
- 19.Njoto I., Fatchiyah F., Handono K., Abdurrachman A., Soeatmadji D.W., Kalim H. Modulation of perlecan protein towards chondrocyte secretion factors at the articular cartilage in hyperglycemic animal model. J Pure App Chem Res. 2019;8(1):80–86. [Google Scholar]
- 20.Sanchez-Adams J., Wilusz R.E., Guilak F. Atomic force microscopy reveals regional variations in the micromechanical properties of the pericellular and extracellular matrices of the meniscus. J Orthop Res. 2013;31(8):1218–1225. doi: 10.1002/jor.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shu C.C., Smith S.M., Little C.B., Melrose J. Elevated hypertrophy, growth plate maturation, glycosaminoglycan deposition, and exostosis formation in the Hspg2 exon 3 null mouse intervertebral disc. Biochem J. 2019;476(2):225–243. doi: 10.1042/BCJ20180695. [DOI] [PubMed] [Google Scholar]
- 22.Cohen I.R., Grassel S., Murdoch A.D., Iozzo R.V. Structural characterization of the complete human perlecan gene and its promoter. Proc Natl Acad Sci USA. 1993;90(21):10404–10408. doi: 10.1073/pnas.90.21.10404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arikawa-Hirasawa E., Le A.H., Nishino I., et al. Structural and functional mutations of the perlecan gene cause Schwartz-Jampel syndrome, with myotonic myopathy and chondrodysplasia. Am J Hum Genet. 2002;70(5):1368–1375. doi: 10.1086/340390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith S.M., Shu C., Melrose J. Comparative immunolocalisation of perlecan with collagen II and aggrecan in human foetal, newborn and adult ovine joint tissues demonstrates perlecan as an early developmental chondrogenic marker. Histochem Cell Biol. 2010;134(3):251–263. doi: 10.1007/s00418-010-0730-x. [DOI] [PubMed] [Google Scholar]
- 25.Timpl R., Dziadek M., Fujiwara S., Nowack H., Wick G. Nidogen: a new, self-aggregating basement membrane protein. FEBS J. 1983;137(3):455–465. doi: 10.1111/j.1432-1033.1983.tb07849.x. [DOI] [PubMed] [Google Scholar]
- 26.Kohfeldt E., Sasaki T., Gohring W., Timpl R. Nidogen-2: a new basement membrane protein with diverse binding properties. J Mol Biol. 1998;282(1):99–109. doi: 10.1006/jmbi.1998.2004. [DOI] [PubMed] [Google Scholar]
- 27.Salmivirta K., Talts J.F., Olsson M., Sasaki T., Timpl R., Ekblom P. Binding of mouse nidogen-2 to basement membrane components and cells and its expression in embryonic and adult tissues suggest complementary functions of the two nidogens. Exp Cell Res. 2002;279(2):188–201. doi: 10.1006/excr.2002.5611. [DOI] [PubMed] [Google Scholar]
- 28.Aumailley M., Battaglia C., Mayer U., et al. Nidogen mediates the formation of ternary complexes of basement membrane components. Kidney Int. 1993;43(1):7–12. doi: 10.1038/ki.1993.3. [DOI] [PubMed] [Google Scholar]
- 29.Fox J.W., Mayer U., Nischt R., et al. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 1991;10(11):3137–3146. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pöschl E., Mayer U., Stetefeld J., et al. Site-directed mutagenesis and structural interpretation of the nidogen binding site of the laminin gamma1 chain. EMBO J. 1996;15(19):5154–5159. [PMC free article] [PubMed] [Google Scholar]
- 31.Ries A., Göhring W., Fox J.W., Timpl R., Sasaki T. Recombinant domains of mouse nidogen-1 and their binding to basement membrane proteins and monoclonal antibodies. Eur J Biochem. 2001;268(19):5119–5128. doi: 10.1046/j.0014-2956.2001.02437.x. [DOI] [PubMed] [Google Scholar]
- 32.Dziadek M. Role of laminin-nidogen complexes in basement membrane formation during embryonic development. Experientia. 1995;51(9–10):901–913. doi: 10.1007/BF01921740. [DOI] [PubMed] [Google Scholar]
- 33.Balzano M., De Grandis M., Manh T.P., et al. Nidogen-1 contributes to the interaction network involved in pro-B cell retention in the peri-sinusoidal hematopoietic stem cell niche. Cell Rep. 2019;26(12):3257–3271. doi: 10.1016/j.celrep.2019.02.065. [DOI] [PubMed] [Google Scholar]
- 34.LeBleu V.S., Macdonald B., Kalluri R. Structure and function of basement membranes. Exp Biol Med. 2007;232:1121–1129. doi: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]
- 35.Böse K., Nischt R., Page A., Bader B.L., Paulsson M., Smyth N. Loss of nidogen-1 and -2 results in syndactyly and changes in limb development. J Biol Chem. 2006;281:39620–39629. doi: 10.1074/jbc.M607886200. [DOI] [PubMed] [Google Scholar]
- 36.Murshed M., Smyth N., Miosge N., et al. The absence of nidogen 1 does not affect murine basement membrane formation. Mol Cell Biol. 2000;20(18):7007–7012. doi: 10.1128/mcb.20.18.7007-7012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schymeinsky J., Nedbal S., Miosge N., et al. Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol Cell Biol. 2002;22(19):6820–6830. doi: 10.1128/MCB.22.19.6820-6830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bader B.L., Smyth N., Nedbal S., et al. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol Cell Biol. 2005;25(15):6846–6856. doi: 10.1128/MCB.25.15.6846-6856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Köhling R., Nischt R., Vasudevan A., et al. Nidogen and nidogen-associated basement membrane proteins and neuronal plasticity. Neurodegener Dis. 2006;3(1–2):56–61. doi: 10.1159/000092094. [DOI] [PubMed] [Google Scholar]
- 40.Carlin B., Jaffe R., Bender B., Chung A.E. Entactin, a novel basal lamina-associated sulfated glycoprotein. J Biol Chem. 1981;256(10):5209–5214. [PubMed] [Google Scholar]
- 41.Kimura N., Toyoshima T., Kojima T., Shimane M. Entactin-2: a new member of basement membrane protein with high homology to entactin/nidogen. Exp Cell Res. 1998;241(1):36–45. doi: 10.1006/excr.1998.4016. [DOI] [PubMed] [Google Scholar]
- 42.Hutter H., Vogel B.E., Plenefisch J.D., et al. Conservation and novelty in the evolution of cell adhesion and extracellular matrix genes. Science. 2000;287:989–994. doi: 10.1126/science.287.5455.989. [DOI] [PubMed] [Google Scholar]
- 43.Kang S.H., Kramer J.M. Nidogen is nonessential and not required for normal type IV collagen localization in Caenorhabditis elegans. Mol Biol Cell. 2000;11(11):3911–3923. doi: 10.1091/mbc.11.11.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S., Wadsworth W.G. Positioning of longitudinal nerves in C. elegans by nidogen. Science. 2000;288(5463):150–154. doi: 10.1126/science.288.5463.150. [DOI] [PubMed] [Google Scholar]
- 45.Ackley B.D., Kang S.H., Crew J.R., Suh C., Jin Y., Kramer J.M. The basement membrane components nidogen and type XVIII collagen regulate organization of neuromuscular junctions in Caenorhabditis elegans. J Neurosci. 2003;23:3577–3587. doi: 10.1523/JNEUROSCI.23-09-03577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dziadek M., Timpl R. Expression of nidogen and laminin in basement membranes during mouse embryogenesis and in teratocarcinoma cells. Dev Biol. 1985;111(2):372–382. doi: 10.1016/0012-1606(85)90491-9. [DOI] [PubMed] [Google Scholar]
- 47.Miosge N., Kother F., Heinemann S., Kohfeldt E., Herken R., Timpl R. Ultrastructural colocalization of nidogen-1 and nidogen-2 with laminin-1 in murine kidney basement membranes. Histochem Cell Biol. 2000;113(2):115–124. doi: 10.1007/s004180050014. [DOI] [PubMed] [Google Scholar]
- 48.Miosge N., Quondamatteo F., Klenczar C., Herken R. Nidogen-1. Expression and ultrastructural localization during the onset of mesoderm formation in the early mouse embryo. J Histochem Cytochem. 2000;48(2):229–238. doi: 10.1177/002215540004800208. [DOI] [PubMed] [Google Scholar]
- 49.Ekblom P., Ekblom M., Fecker L., et al. Role of mesenchymal nidogen for epithelial morphogenesis in vitro. Development. 1994;120(7):2003–2014. doi: 10.1242/dev.120.7.2003. [DOI] [PubMed] [Google Scholar]
- 50.Kadoya Y., Salmivirta K., Talts J.F., et al. Importance of nidogen binding to laminin gamma1 for branching epithelial morphogenesis of the submandibular gland. Development. 1997;124(3):683–691. doi: 10.1242/dev.124.3.683. [DOI] [PubMed] [Google Scholar]
- 51.Miosge N., Holzhausen S., Zelent C., Sprysch P., Herken R. Nidogen-1 and nidogen-2 are found in basement membranes during human embryonic development. Histochem J. 2001;33(9–10):523–530. doi: 10.1023/a:1014995523521. [DOI] [PubMed] [Google Scholar]
- 52.Kuri-Harcuch W., Arguello C., Marsch-Moreno M. Extracellular matrix production by mouse 3T3-F442A cells during adipose differentiation in culture. Differentiation. 1984;28(2):173–178. doi: 10.1111/j.1432-0436.1984.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 53.Napolitano L. Cytolysomes in metabolically active cells. J Cell Biol. 1963;18:478–481. doi: 10.1083/jcb.18.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alvarez-Llamas G., Szalowska E., de Vries M.P., et al. Characterization of the human visceral adipose tissue secretome. Mol Cell Proteomics. 2007;6(4):589–600. doi: 10.1074/mcp.M600265-MCP200. [DOI] [PubMed] [Google Scholar]
- 55.Aratani Y., Kitagawa Y. Enhanced synthesis and secretion of type IV collagen and entactin during adipose conversion of 3T3-L1 cells and production of unorthodox laminin complex. J Biol Chem. 1988;263(31):16163–16169. [PubMed] [Google Scholar]
- 56.Hackl H., Burkard T.R., Sturn A., et al. Molecular processes during fat cell development revealed by gene expression profiling and functional annotation. Genome Biol. 2005;6(13):R108. doi: 10.1186/gb-2005-6-13-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kratchmarova I., Kalume D.E., Blagoev B., et al. A proteomic approach for identification of secreted proteins during the differentiation of 3T3-L1 preadipocytes to adipocytes. Mol Cell Proteomics. 2002;1(3):213–222. doi: 10.1074/mcp.m200006-mcp200. [DOI] [PubMed] [Google Scholar]
- 58.Molina H., Yang Y., Ruch T., et al. Temporal profiling of the adipocyte proteome during differentiation using a five-plex SILAC based strategy. J Proteome Res. 2009;8(1):48–58. doi: 10.1021/pr800650r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noro A., Sillat T., Virtanen I., et al. Laminin production and basement membrane deposition by mesenchymal stem cells upon adipogenic differentiation. J Histochem Cytochem. 2013;61(10):719–730. doi: 10.1369/0022155413502055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ojima K., Oe M., Nakajima I., Muroya S., Nishimura T. Dynamics of protein secretion during adipocyte differentiation. FEBS open bio. 2016;6(8):816–826. doi: 10.1002/2211-5463.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patlaka C., Mai H.A., Lang P., Andersson G. The growth factor-like adipokine tartrate-resistant acid phosphatase 5a interacts with the rod G3 domain of adipocyte-produced nidogen-2. Biochem Biophys Res Commun. 2014;454(3):446–452. doi: 10.1016/j.bbrc.2014.10.112. [DOI] [PubMed] [Google Scholar]
- 62.Roca-Rivada A., Bravo S.B., Pérez-Sotelo D., et al. CILAIR-based secretome analysis of obese visceral and subcutaneous adipose tissues reveals distinctive ECM remodeling and inflammation mediators. Sci Rep. 2015;5:12214. doi: 10.1038/srep12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sillat T., Saat R., Pollanen R., Hukkanen M., Takagi M., Konttinen Y.T. Basement membrane collagen type IV expression by human mesenchymal stem cells during adipogenic differentiation. J Cell Mol Med. 2012;16(7):1485–1495. doi: 10.1111/j.1582-4934.2011.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaicik M.K., Kortesmaa J.T., Moverare-Skrtic S., et al. Laminin α4 deficient mice exhibit decreased capacity for adipose tissue expansion and weight gain. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0109854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ono M., Aratani Y., Kitagawa I., Kitagawa Y. Ascorbic acid phosphate stimulates type IV collagen synthesis and accelerates adipose conversion of 3T3-L1 cells. Exp Cell Res. 1990;187(2):309–314. doi: 10.1016/0014-4827(90)90096-s. [DOI] [PubMed] [Google Scholar]
- 66.Sephel G.C., Kennedy R., Kudravi S. Expression of capillary basement membrane components during sequential phases of wound angiogenesis. Matrix Biol. 1996;15(4):263–279. doi: 10.1016/s0945-053x(96)90117-1. [DOI] [PubMed] [Google Scholar]
- 67.Baluk P., Morikawa S., Haskell A., Mancuso M., McDonald D.M. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163(5):1801–1815. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang X., Multhaupt H., Chan E., Schaefer L., Schaefer R.M., Couchman J.R. Essential contribution of tumor-derived perlecan to epidermal tumor growth and angiogenesis. J Histochem Cytochem. 2004;52(12):1575–1590. doi: 10.1369/jhc.4A6353.2004. [DOI] [PubMed] [Google Scholar]
- 69.Niquet J., Represa A. Entactin immunoreactivity in immature and adult rat brain. Brain Res Dev Brain Res. 1996;95(2):227–233. doi: 10.1016/0165-3806(96)00089-2. [DOI] [PubMed] [Google Scholar]
- 70.Ae Seo I., Kyoung Lee H., Mi Park Y., Jin Ahn K., Tae Park H. Acute changes of nidogen immunoreactivity in the basal lamina of the spinal cord vessels following dorsal hemisection without correlative changes of nidogen gene expression. Acta histochemical. 2007;109(6):446–453. doi: 10.1016/j.acthis.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 71.Nicosia R.F., Bonanno E., Smith M., Yurchenco P. Modulation of angiogenesis in vitro by laminin-entactin complex. Dev Biol. 1994;164(1):197–206. doi: 10.1006/dbio.1994.1191. [DOI] [PubMed] [Google Scholar]
- 72.Titz B., Dietrich S., Sadowski T., Beck C., Petersen A., Sedlacek R. Activity of MMP-19 inhibits capillary-like formation due to processing of nidogen-1. Cell Mol Life Sci. 2004;61(14):1826–1833. doi: 10.1007/s00018-004-4105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mokkapati S., Baranowsky A., Mirancea N., Smyth N., Breitkreutz D., Nischt R. Basement membranes in skin are differently affected by lack of nidogen 1 and 2. J Invest Dermatol. 2008;128(9):2259–2267. doi: 10.1038/jid.2008.65. [DOI] [PubMed] [Google Scholar]
- 74.Mokkapati S., Bechtel M., Reibetanz M., Miosge N., Nischt R. Absence of the basement membrane component nidogen 2, but not of nidogen 1, results in increased lung metastasis in mice. J Histochem Cytochem. 2012;60(4):280–289. doi: 10.1369/0022155412436586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Semkova I., Kociok N., Karagiannis D., et al. Anti-angiogenic effect of the basement membrane protein nidogen-1 in a mouse model of choroidal neovascularization. Exp Eye Res. 2014;118:80–88. doi: 10.1016/j.exer.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 76.Marchand M., Monnot C., Muller L., Germain S. Extracellular matrix scaffolding in angiogenesis and capillary homeostasis. Semin Cell Dev Biol. 2019;89:147–156. doi: 10.1016/j.semcdb.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 77.Thomas T., Dziadek M. Genes coding for basement membrane glycoproteins laminin, nidogen, and collagen IV are differentially expressed in the nervous system and by epithelial, endothelial, and mesenchymal cells of the mouse embryo. Exp Cell Res. 1993;208(1):54–67. doi: 10.1006/excr.1993.1222. [DOI] [PubMed] [Google Scholar]
- 78.Kvist A.J., Nystrom A., Hultenby K., Sasaki T., Talts J.F., Aspberg A. The major basement membrane components localize to the chondrocyte pericellular matrix--a cartilage basement membrane equivalent? Matrix Biol. 2008;27(1):22–33. doi: 10.1016/j.matbio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 79.Kruegel J., Sadowski B., Miosge N. Nidogen-1 and nidogen-2 in healthy human cartilage and in late-stage osteoarthritis cartilage. Arthritis Rheum. 2008;58(5):1422–1432. doi: 10.1002/art.23480. [DOI] [PubMed] [Google Scholar]
- 80.Schminke B., Muhammad H., Bode C., et al. A discoidin domain receptor 1 knock-out mouse as a novel model for osteoarthritis of the temporomandibular joint. Cell Mol Life Sci. 2014;71(6):1081–1096. doi: 10.1007/s00018-013-1436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miosge N., Sasaki T., Timpl R. Evidence of nidogen-2 compensation for nidogen-1 deficiency in transgenic mice. Matrix Biol. 2002;21(7):611–621. doi: 10.1016/s0945-053x(02)00070-7. [DOI] [PubMed] [Google Scholar]
- 82.Schminke B., Frese J., Bode C., Goldring M.B., Miosge N. Laminins and nidogens in the pericellular matrix of chondrocytes: their role in osteoarthritis and chondrogenic differentiation. Am J Pathol. 2016;186(2):410–418. doi: 10.1016/j.ajpath.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 83.Robson M.I., Jose I., Czapiewski R., et al. Tissue-specific gene repositioning by muscle nuclear membrane proteins enhances repression of critical developmental genes during myogenesis. Mol Cell. 2016;62(6):834–847. doi: 10.1016/j.molcel.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fox M.A., Ho M.S., Smyth N., Sanes J.R. A synaptic nidogen: developmental regulation and role of nidogen-2 at the neuromuscular junction. Neural Dev. 2008;3:24. doi: 10.1186/1749-8104-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Godfrey E.W., Gradall K.S. Basal lamina molecules are concentrated in myogenic regions of the mouse limb bud. Anat Embryol. 1998;198(6):481–486. doi: 10.1007/s004290050198. [DOI] [PubMed] [Google Scholar]
- 86.Holland A., Dowling P., Zweyer M., et al. Proteomic profiling of cardiomyopathic tissue from the aged mdx model of Duchenne muscular dystrophy reveals a drastic decrease in laminin, nidogen and annexin. Proteomics. 2013;13(15):2312–2323. doi: 10.1002/pmic.201200578. [DOI] [PubMed] [Google Scholar]
- 87.Neu R., Adams S., Munz B. Differential expression of entactin-1/nidogen-1 and entactin-2/nidogen-2 in myogenic differentiation. Differentiation. 2006;74(9–10):573–582. doi: 10.1111/j.1432-0436.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- 88.Langen R.C., Schols A.M., Kelders M.C., Wouters E.F., Janssen-Heininger Y.M. Enhanced myogenic differentiation by extracellular matrix is regulated at the early stages of myogenesis. In Vitro Cell Dev Biol Anim. 2003;39(3–4):163–169. doi: 10.1007/s11626-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 89.Funanage V.L., Smith S.M., Minnich M.A. Entactin promotes adhesion and long-term maintenance of cultured regenerated skeletal myotubes. J Cell Physiol. 1992;150(2):251–257. doi: 10.1002/jcp.1041500205. [DOI] [PubMed] [Google Scholar]
- 90.Grefte S., Adjobo-Hermans M.J.W., Versteeg E.M.M., Koopman W.J.H., Daamen W.F. Impaired primary mouse myotube formation on crosslinked type I collagen films is enhanced by laminin and entactin. Acta Biomater. 2016;30:265–276. doi: 10.1016/j.actbio.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 91.He Y., Tsou P.S., Khanna D., Sawalha A.H. Methyl-CpG-binding protein 2 mediates antifibrotic effects in scleroderma fibroblasts. Ann Rheum Dis. 2018;77(8):1208–1218. doi: 10.1136/annrheumdis-2018-213022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dubovy P., Jancalek R., Klusakova I. A heterogeneous immunofluorescence staining for laminin-1 and related basal lamina molecules in the dorsal root ganglia following constriction nerve injury. Histochem Cell Biol. 2006;125(6):671–680. doi: 10.1007/s00418-005-0115-8. [DOI] [PubMed] [Google Scholar]
- 93.Bryan D.J., Litchfield C.R., Manchio J.V., et al. Spatiotemporal expression profiling of proteins in rat sciatic nerve regeneration using reverse phase protein arrays. Proteome Sci. 2012;10(1):1–6. doi: 10.1186/1477-5956-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee H.K., Seo I.A., Park H.K., et al. Nidogen is a prosurvival and promigratory factor for adult Schwann cells. J Neurochem. 2007;102(3):686–698. doi: 10.1111/j.1471-4159.2007.04580.x. [DOI] [PubMed] [Google Scholar]
- 95.Lee H.K., Seo I.A., Suh D.J., Park H.T. Nidogen plays a role in the regenerative axon growth of adult sensory neurons through Schwann cells. J Kor Med Sci. 2009;24(4):654–659. doi: 10.3346/jkms.2009.24.4.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wolfstetter G., Dahlitz I., Pfeifer K., et al. Characterization of Drosophila Nidogen/entactin reveals roles in basement membrane stability, barrier function and nervous system patterning. Development. 2019;146(2):dev168948. doi: 10.1242/dev.168948. [DOI] [PubMed] [Google Scholar]
- 97.Vasudevan A., Ho M.S., Weiergräber M., et al. Basement membrane protein nidogen-1 shapes hippocampal synaptic plasticity and excitability. Hippocampus. 2010;20(5):608–620. doi: 10.1002/hipo.20660. [DOI] [PubMed] [Google Scholar]
- 98.Moore K.A., Lemischka I.R. Stem cells and their niches. Science. 2006;311(5769):1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 99.Gattazzo F., Urciuolo A., Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840(8):2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rayagiri S.S., Ranaldi D., Raven A., et al. Basal lamina remodeling at the skeletal muscle stem cell niche mediates stem cell self-renewal. Nat Commun. 2018;9(1):1075. doi: 10.1038/s41467-018-03425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanes J.R. The basement membrane/basal lamina of skeletal muscle. J Biol Chem. 2003;278:12601–12604. doi: 10.1074/jbc.R200027200. [DOI] [PubMed] [Google Scholar]
- 102.Barros C.S., Franco S.J., Müller U. Extracellular matrix: functions in the nervous system. Cold Spring Harb Perspect Biol. 2011;3(1) doi: 10.1101/cshperspect.a005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McMillan J.R., Akiyama M., Shimizu H. Epidermal basement membrane zone components: ultrastructural distribution and molecular interactions. J Dermatol Sci. 2003;31(3):169–177. doi: 10.1016/s0923-1811(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 104.Daley W.P., Peters S.B., Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121(Pt 3):255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 105.Spradling A., Drummond-Barbosa D., Kai T. Stem cells find their niche. Nature. 2001;414(6859):98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 106.Thomas C.M., Fuller C.J., Whittles C.E., Sharif M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis Cartilage. 2007;15(1):27–34. doi: 10.1016/j.joca.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 107.Yurchenco P.D., Furthmayr H. Self-assembly of basement membrane collagen. Biochemistry. 1984;23(8):1839–1850. doi: 10.1021/bi00303a040. [DOI] [PubMed] [Google Scholar]
- 108.Yurchenco P.D., Cheng Y.S., Schittny J.C. Heparin modulation of laminin polymerization. J Biol Chem. 1990;265(7):3981–3991. [PubMed] [Google Scholar]
- 109.Wang N., Tytell J.D., Ingber D.E. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10(1):75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 110.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 111.Guilak F., Cohen D.M., Estes B.T., Gimble J.M., Liedtke W., Chen C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5(1):17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McBeath R., Pirone D.M., Nelson C.M., Bhadriraju K., Chen C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 113.Martino M.M., Hubbell J.A. The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. Faseb J. 2010;24(12):4711–4721. doi: 10.1096/fj.09-151282. [DOI] [PubMed] [Google Scholar]
- 114.Iozzo R.V. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol. 2005;6(8):646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- 115.Whitelock J.M., Melrose J., Iozzo R.V. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008;47(43):11174–11183. doi: 10.1021/bi8013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hynes R.O. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Walker C., Mojares E., Del Rio Hernandez A. Role of extracellular matrix in development and cancer progression. Int J Mol Sci. 2018;19(10):3028. doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rozario T., DeSimone D.W. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341(1):126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Theocharis A.D., Gialeli C., Bouris P., et al. Cell–matrix interactions: focus on proteoglycan–proteinase interplay and pharmacological targeting in cancer. FEBS J. 2014;281(22):5023–5042. doi: 10.1111/febs.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ho M.S., Bose K., Mokkapati S., Nischt R., Smyth N. Nidogens-Extracellular matrix linker molecules. Microsc Res Tech. 2008;71(5):387–395. doi: 10.1002/jemt.20567. [DOI] [PubMed] [Google Scholar]
- 121.Chermnykh E., Kalabusheva E., Vorotelyak E. Extracellular matrix as a regulator of epidermal stem cell fate. Int J Mol Sci. 2018;19(4):1003. doi: 10.3390/ijms19041003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kleinman H.K., Graf J., Iwamoto Y., et al. Role of basement membranes in cell differentiation. Ann N Y Acad Sci. 1987;513:134–145. doi: 10.1111/j.1749-6632.1987.tb25004.x. [DOI] [PubMed] [Google Scholar]
- 123.Dong L., Chen Y., Lewis M., et al. Neurologic defects and selective disruption of basement membranes in mice lacking entactin-1/nidogen-1. Lab Invest. 2002;82(12):1617–1630. doi: 10.1097/01.lab.0000042240.52093.0f. [DOI] [PubMed] [Google Scholar]
- 124.Higuchi Y., Shiraki N., Yamane K., et al. Synthesized basement membranes direct the differentiation of mouse embryonic stem cells into pancreatic lineages. J Cell Sci. 2010;123(Pt 16):2733–2742. doi: 10.1242/jcs.066886. [DOI] [PubMed] [Google Scholar]
- 125.Rodin S., Domogatskaya A., Ström S., et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28(6):611–615. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- 126.Sun Y., Wang T.L., Toh W.S., Pei M. The role of laminins in cartilaginous tissues: from development to regeneration. Eur Cell Mater. 2017;34:40–54. doi: 10.22203/eCM.v034a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Von der Mark K., Park J., Bauer S., Schmuki P. Nanoscale engineering of biomimetic surfaces: cues from the extracellular matrix. Cell Tissue Res. 2010;339:131–153. doi: 10.1007/s00441-009-0896-5. [DOI] [PubMed] [Google Scholar]
- 128.Wang Y., Fu Y., Yan Z., Zhang X.B., Pei M. Impact of fibronectin knockout on proliferation and differentiation of human infrapatellar fat pad-derived stem cells. Front Bioeng Biotechnol. 2019;7:321. doi: 10.3389/fbioe.2019.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Taylor S.H., Al-Youha S., Van Agtmael T., et al. Tendon is covered by a basement membrane epithelium that is required for cell retention and the prevention of adhesion formation. PLoS One. 2011;6(1) doi: 10.1371/journal.pone.0016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nishida E., Sasaki T., Ishikawa S.K., et al. Transcriptome database KK-Periome for periodontal ligament development: expression profiles of the extracellular matrix genes. Gene. 2007;404(1–2):70–79. doi: 10.1016/j.gene.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 131.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mohammadinejad R., Biagioni A., Arunkumar G., et al. EMT signaling: potential contribution of CRISPR/Cas gene editing. Cell Mol Life Sci. 2020;77(14):2701–2722. doi: 10.1007/s00018-020-03449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pastor-Pareja J.C. Atypical basement membranes and basement membrane diversity - what is normal anyway? J Cell Sci. 2020;133(8):jcs241794. doi: 10.1242/jcs.241794. [DOI] [PubMed] [Google Scholar]
- 134.Bechtel M., Keller M.V., Bloch W., et al. Different domains in nidogen-1 and nidogen-2 drive basement membrane formation in skin organotypic cocultures. Faseb J. 2012;26(9):3637–3648. doi: 10.1096/fj.11-194597. [DOI] [PubMed] [Google Scholar]
- 135.Li H., Chang Y.W., Mohan K., et al. Activated Notch1 maintains the phenotype of radial glial cells and promotes their adhesion to laminin by upregulating nidogen. Glia. 2008;56(6):646–658. doi: 10.1002/glia.20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thyboll J., Kortesmaa J., Cao R., et al. Deletion of the laminin α4 chain leads to impaired microvessel maturation. Mol Cell Biol. 2002;22(4):1194–1202. doi: 10.1128/MCB.22.4.1194-1202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jones J.C., Lane K., Hopkinson S.B., et al. Laminin-6 assembles into multimolecular fibrillar complexes with perlecan and participates in mechanical-signal transduction via a dystroglycan-dependent, integrin-independent mechanism. J Cell Sci. 2005;118(Pt 12):2557–2566. doi: 10.1242/jcs.02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Berk B.C., Fujiwara K., Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117(3):568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Spence H.J., Dhillon A.S., James M., Winder S.J. Dystroglycan, a scaffold for the ERK-MAP kinase cascade. EMBO Rep. 2004;5(5):484–489. doi: 10.1038/sj.embor.7400140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lord M.S., Chuang C.Y., Melrose J., Davies M.J., Iozzo R.V., Whitelock J.M. The role of vascular-derived perlecan in modulating cell adhesion, proliferation and growth factor signaling. Matrix Biol. 2014;35:112–122. doi: 10.1016/j.matbio.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]