Abstract
This review provides an updated account on the current methods, principles and mechanism of action of therapies for the detection of molecular markers of therapeutic importance in the prognosis of breast cancer progression and recurrence, which includes estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor2 (HER2). Indeed, hormone-receptors namely, ER, PR, proto-oncogene HER2 are the basic molecular markers that are recognized and established prognostic factors and predictors of response, for therapeutic practice. These markers can be detected by using immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH), which are established, faster and cost effective detection methods. These molecular markers along with clinicopathological prognostic parameters give the best prediction of the prognosis of cancer recurrence and progress. Finally, hormone receptors and HER2 as molecular markers are of prime therapeutic importance and have the capability to take part in future drug development techniques.
Keywords: Breast cancer, Molecular diagnostic, Molecular markers, Predictive markers, Prognostic
Introduction
During 2018 around 9.6 million people were annihilated by cancer as the second-highest fatality, worldwide. Breast-cancer is second to lung cancer with 2.09 million new registered cases.1, 2, 3 In India, for example, 0.1 million new cases of breast cancer and 87,090 deaths were registered during 2018.4, 5, 6, 7 Clinical details, mammography and cytological analysis of molecular markers in the affected breast tissue are the standard operational practices, as the ‘triple-assessment method’ in breast cancer diagnosis with minimum time and limited resources. Indeed, the positive predictive value of ‘triple-assessment method’ exceeded 99.9% for this disease.8
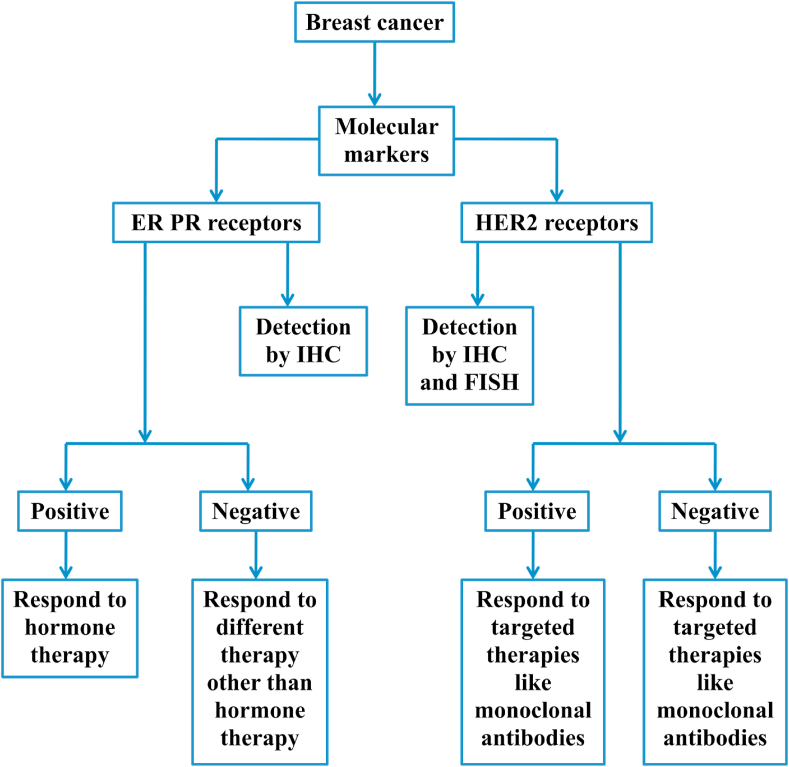
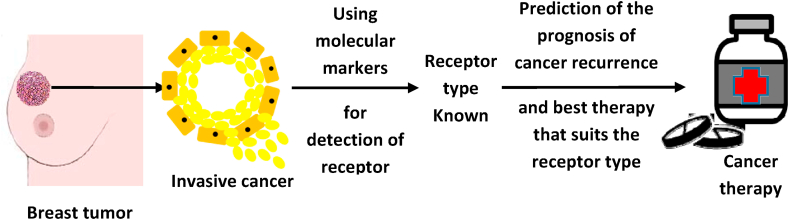
Molecular markers are a type of protein receptors, with the capability of attaching to hormones.9 Those being expressed by cancerous cells are used in determining the response to a specific therapy. Molecular markers used in cancer detection are both proteins and modified sequence of DNA in cancerous tissue.10 Furthermore, specific drugs or other preventives such as, an antibody are used in a suitable targeted therapy (Figure 1, Figure 2) to block the growth and the metastatic spread of doppelgängers of neoplastic cells without destroying healthy cells.11
Figure 1.
Use of molecular markers in therapeutic decision.
Figure 2.
Detection of type of receptor using molecular markers and prediction of prognosis.
Viewed from the trenches of public health, the vast accumulated information on carcinogenesis and molecular marker-status-based treatments have enormously expanded the options of cancer treatment regimens by using of both prognostic and predictive molecular markers to satiate the obsessive quest of the targeted treatment.12 Utilizing molecular markers is an appropriate method for the diagnosis of stage and grade of breast cancer, with the coveted evaluation of therapeutic responses.10 Indeed, prognostic and predictive molecular markers are useful in selecting patients for standard medical treatments, prophecy about the due course of cancer, and the probability of its recurrence after the initial treatment (Figure 1, Figure 2). Concomitantly, the predictive markers predict a particular medical treatment, to get the best therapy suitable for a specific type of breast cancer based on molecular marker status.12
The latest practices and future capabilities in the use of molecular markers for breast cancer had been well dealt.13 The simultaneous estimation of a myriad of parameters could be achieved through high-efficiency means by raising the likelihood that serves as a panel of markers, which will be adequately sensitive and specific to allow prediction of the prognosis of every person. Secondly, molecular targets for therapy are identified by pharmaceutical chemistry; these therapeutics would only be successful if a target is present, necessitating the development of methods to evaluate tumors in general. Furthermore, methods of the use of predictive and prognostic molecular markers were considered to predict one from several options of cancer treatment.12 The utilization of traditional and innovative prognostic molecular markers in identifying specific types of breast cancer episodes/manifests and accessory clinical importance have been considered.14 Moreover, the literature on the utilization of molecular markers of breast cancer in predicting tumor progression has been summarized.10 Indeed, the traditional molecular markers and employing the next-generation sequencing (NGS) technologies could be comparatively more effective prognosis for breast cancer.15
This review considers the imbroglio of current methods and principles for the detection of molecular markers, the mechanism of action of therapies, the prognosis of cancer progression and recurrence. Moreover, the used marker-status helps in suggesting oncologists/physicians for the selection of an appropriate treatment method, notwithstanding the fact that, the traditional markers, namely, hormone receptors and human epidermal growth factor receptor2 (HER2) are the coveted molecular markers of therapeutic prominence in the prognosis of breast cancer. This review provides a unifying narration of molecular markers of therapeutic importance used for detection with the eventual benefits of breast cancer management.
Molecular markers
Protein receptors are used as molecular markers expressed by cancerous cells in cancer diagnosis and determining the response to a specific therapy. Moreover, as gene expression markings, modified sequences of DNA in cancerous tissue are also established as tumor markers.10 The fundamental molecular markers for breast cancer used routinely are ER, PR and HER2. Those markers, along with clinicopathological prognostic parameters, namely, age at diagnosis, lymph-node status, tumor size and histopathological grading, provide the suitable prediction of the prognosis of cancer recurrence after initial remedial treatment, with an indication of eventual future appropriate treatment. Thus, hormone receptors and HER2 as molecular markers are of prime therapeutic importance in the management of breast cancer and those have the capability to take part in the future drug development techniques.
Hormone receptor – Estrogen receptors (ERs) and progesterone receptors (PRs)
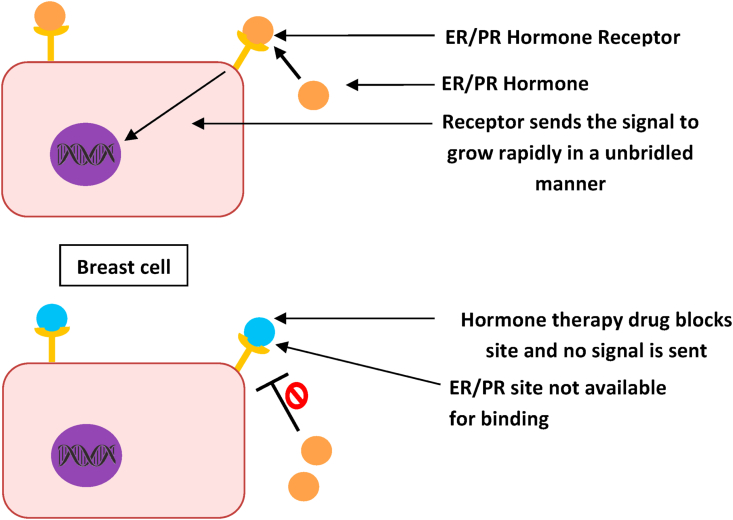
ERs and PRs are proteinaceous molecular markers found on mammary cells, which receive and gets stimulated by the circulated ovarian hormones, estrogen and progesterone.14 Both these hormones are vital for several functional events in females, such as sexual maturation (including breast development), pregnancies, parturition and menopause. Breast cancer cells with these receptors receive signals from related hormones to grow, just like normal cells do, but since those are already cancerous due DNA damage they grow rapidly in an unbridled manner (Fig. 3). The endocrine steroid hormones activate ERs and PRs to proliferate, survive and insidiously spread the neoplastic cells as metastatic invasion to innards.14 Consequently, breast cancer with ERs and PRs grows more slowly and steadily than other receptors such as HER2 with a better prognosis.16
Figure 3.
Mechanism of hormone therapy drug.
Activated and influenced by the sex hormones, estrogen (17βestradiol) and progesterones, ERs and PRs, found in cytoplasm and cell-membrane, respectively. The expressions of high ERs and PRs in tumors are known as ER and PR positive cancer, which grow or spread when fueled by the corresponding hormones.16, 17, 18, 19 Hence, once triggered by estrogen and progesterone, receptors get significantly translocated into the nucleus and, after that, get attached to the specific DNA segment, which starts to control the actions of genes, Esr1, Esr2, and PGR gene responsible for estrogen and progesterone, respectively.20 Alpha (α) and beta(β) ERs are encoded by two genes Esr1 and Esr2, respectively.21, 22, 23 PRs also acknowledged as nuclear receptor subfamily 3, group C, member 3 (NR3C3)24; it is a heterodimeric protein with A and B subunits that vary in the inherent molecular-weight values being encoded by PGR gene.25,26,14
Since, these receptors are expressed on cancerous cells of tumor, the individual status of ER and PR is easily diagnosed by immunohistochemistry (IHC).12,14 When the breast tumor cells express ERs, the tumor is classified as ER-positive breast-cancer tumor; while, the cells express PRs, the tumor is classified as PR-positive breast cancer tumor. When cells do not express either of these two receptors, the tumor is classified as ERPR-negative breast cancer tumor. After erudition of receptor marker-status, whether a tumor is ER and/or PR positive or negative, it helps the oncologists/physicians in suggesting the selection of an appropriate treatment method, such as whether the cancer can be treated with hormone therapy or some other therapy and the future outcome (prognosis) (Fig. 1).10 The ER and/or PR positive breast cancer can be treated with hormone therapy, which blocks these receptors from receiving stimulating signals from related hormones, as a result of the therapy the tumor slows or stops further growth (Fig. 3). A receptor marker-status also helps in prognosis to assess an individual's recurrence risk or complete cure after an initial treatment. As known, ER-positive cancer is more common among postmenopausal women.12,14 Thus, PR positive cancer is generally appraised to have a vigilant outcome than ER-positive cancer due to the slow progression of cancer in women, as the presence of PR is related to hormone dependency and prolonged survival.17
Human epidermal growth factor receptor2 (HER2)
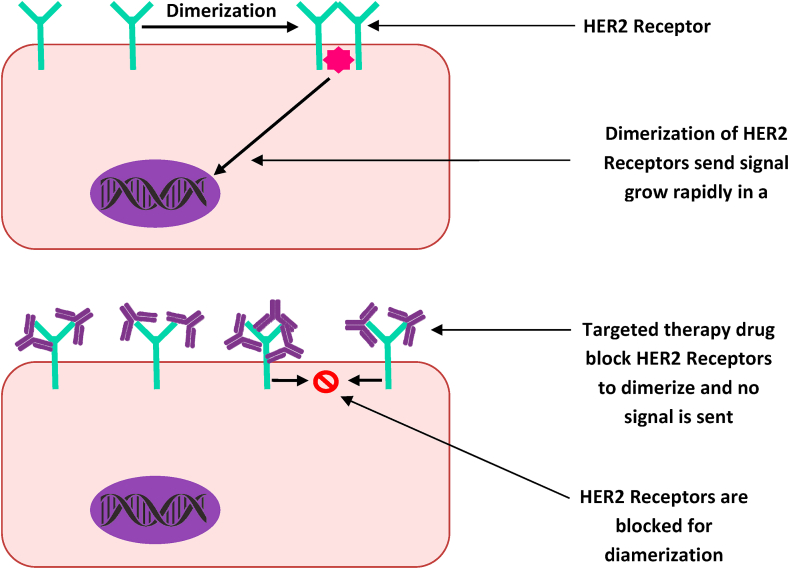
HER2 is a growth-promoting oncoprotein synthesized by HER2 gene (ERBB2 gene in humans), which is also known by the following names, tyrosine kinase, erbB2, CD340 (cluster of differentiation 340), ERBB2 (human), Erbb2 (rodent), NEU, HER2/neu, HER2, NGL, MLN 19 and TKR1.27, 28, 29 HER2 protein receptors found on mammary cells help and control the mammary cells to grow in a healthy way, to divide and repair itself. But sometimes, the HER2 gene does not function appropriately and starts to produce an excessive replica of itself, which is known as HER2 gene amplification. Then, due to these excessive replicas of HER2 genes, the mammary cells start to make an excessive HER2 receptors, which is the HER2 protein overexpression.30 This presage the mammary cells to grow, develop and multiply in an unbridled way (Fig. 4). The HER2 gene and HER2 protein status could be easily identified by IHC and fluorescence in situ hybridization (FISH).10,14 When HER2 protein is overexpressed, it can be detected by IHC, while HER2 gene can be amplified through FISH,12,14 then the breast cancer is called HER2 positive.31,32 When the tumor does not have HER2 gene amplification or the HER2 protein overexpression, it is classified as HER2 negative breast cancer. Indeed, an over-expression of this oncogene plays a crucial function in the growth and advancement of specific types of aggressive breast cancer.33, 34, 35
Figure 4.
Mechanism of targeted therapy drug.
After erudition of receptor marker-status, whether a tumor is HER2 positive or negative, it helps in suggesting oncologists/physicians for the selection of an appropriate treatment method, such as whether the cancer can be treated with targeted therapy or some other therapy. HER2 positive breast cancer can be treated with targeted therapy such as a therapy with monoclonal antibody or a kinase inhibitor, which down-regulate molecular-signaling of HER2 genes and the future outcome (prognosis) (Fig. 4). HER2-negative breast cancer can be hormone positive or negative; if hormone positive, it can be treated with hormone therapy and if it is hormone negative (i.e. ER, PR, HER2 negative or triple negative), it can be treated with immunotherapy alone or in combination with chemotherapy. Lately, this protein has become a significant molecular marker and the target of medical therapy for 30% breast cancer patients, approximately.16,34 It could be concluded that ER, PR and HER2 positive tumors have the better prognosis than the respective negative ones, because it is known that the ovarian sex hormones and HER2 gene amplification influence the growth of positive tumor.
Detection and principle of methods for molecular markers
There is a noble harmony between IHC and FISH techniques, each having respective limitations for concomitant effectiveness. The primary constraints of FISH are the requirement of a dark chamber with a particular type of microscope or an advanced method namely, the chromogenic in situ hybridization (CISH) or/and the dual in situ hybridization (DISH) that are being used for evaluation by a standard light microscope.15
Immunohistochemistry (IHC)
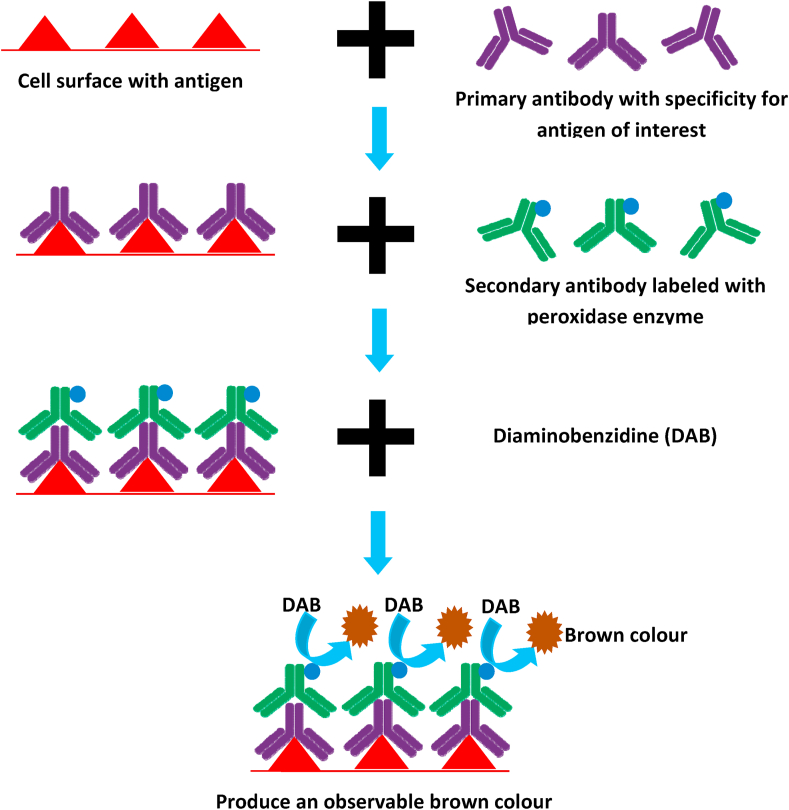
IHC has the major function as appraisal of both prognostic and predictive factors for infiltrating breast cancer.36,37 Presently, three markers used in routine clinical practices are the estrogen receptor alpha (ERɑ), PR, and HER2 oncoprotein. As it is, IHC is the coveted method for evaluating these receptors, and it is a process of marking antigens (e.g., proteins) by using antibodies that bind to specific antigens monitored in the tissue section and this method was executed in 1941.38,39
The principle of IHC is based on the theme that any rigid part of an antibody can act as an epitope to induce another antibody; so, an immunoglobulin (Ig) is able to perform both as an antibody binding to tissue antigen and also as antigen offering epitope to which, a secondary antibody can attach. Antibodies (Ab) are Igs that have a paratope on the concerned tip,40 which has specificity to a particular epitope on an antigen. IgG is the most frequently used Ab for IHC. In IHC, a primary antibody (mouse monoclonal Ab) with specificity against a specific antigen (Ag) in the test is administered to the tissue section and extra antibody is cleaned off (Fig. 5). Thereafter an annotated secondary Ab (the antibody that is conjugated with a peroxidase enzyme), which possess a specificity for the antigenic epitope existing on the primary Ab, is then administered. This helps to annotate sites on tissue localized by primary Ab (Fig. 5). This staining method results in the acute positioning of target constituents of the cells and tissue based on a satisfying signal-to-noise ratio. Magnifying the signal, while decreasing non-specific background staining (noise), is the key plan to achieve a satisfactory and practically useful result.
Figure 5.
Principle of immunohistochemistry.
Fluorescence in situ hybridization (FISH)
The FISH is an approach to pinpoint the position of a specific DNA-sequence in the chromosome. In FISH method, fluorescent probes are utilized to distinguish and pinpoint the location of a particular sequence of DNA on the chromosome.41,42 Confirmatory recognition of predictive or prognostic genetic markers in cancer is obtained by FISH. Moreover, FISH use is to detect the HER2 gene mostly, which encode the HER2 protein.12,43,44 Lately, FISH technologies have been advanced into two forms: chromogenic in situ-hybridization (CISH) and, secondly, silver-enhanced in situ-hybridization (SISH). This newer method uses peroxidase enzyme-labeled probes, whose colour intensity does not perish quickly and eventually allow the tissue section to be observed in various microscopy conditions, particularly bright field. CISH and SISH methods being reported to evaluate the HER2 gene status, less recently.12,45
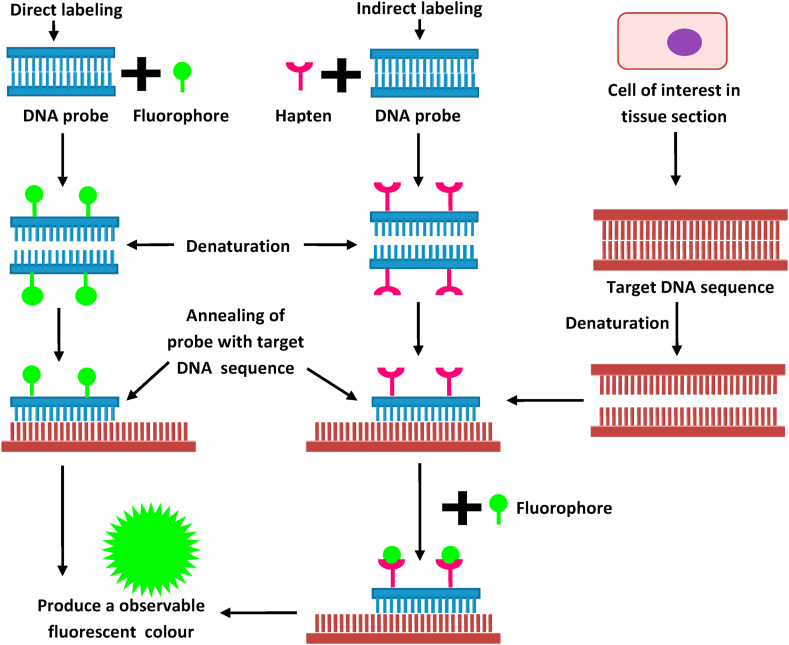
The essential component of FISH is a DNA probe and a target DNA sequence, located by it. Before hybridization, the DNA probe is labeled by several techniques, such as random primed labeling, nick translation and PCR amplification. These following two labeling methods are frequently used: direct labeling and indirect labeling (Fig. 6). For the direct labeling method, nucleotides are used that have been directly altered to contain a fluorophore; whereas, in the indirect labeling, probes are labeled with altered nucleotides that have a hapten. Thereafter, the labeled probe and the target DNA are denatured, and the pairing of denatured probes occurs with the target DNA during the annealing of cDNA sequences. If the probe is labeled indirectly, an additional step would be necessary for observing the nonfluorescent hapten that uses an immunological or an enzymatic detection system. Hence, direct-labeled probes are faster, and alternately, the indirect-labeling provides the adequately signal amplification by using multiple films of antibodies; consequently, that could produce an intensely brighter signal in comparison with background levels (Fig. 6).46 Thus, it can be stated that IHC and FISH are easy, fast and remain economical for the early detection of molecular markers.
Figure 6.
Principle of fluorescence in situ hybridization.
Therapeutic importance of molecular-markers in the prognosis of breast cancer
When a patient is diagnosed clinically having breast cancer, procedures for ER, PR, and HER2 are conducted through IHC and FISH methods. These receptors help to access suitable therapeutic practice, which would stop the hormones and accessory events involved in cancer development. Moreover, ER, PR and HER2 are therapeutically established molecular markers, and those have been in use as prognostic and predictive markers for recognizing a high-risk cancer stage for ensuing targeted therapies.10 The appearance of hormone receptors, ER and PR positive breast tissue is an example of poor prognostic, but remain as the powerful predictive molecular markers (Table 1, Table 2).30 It is clear that although ER-positive tumors have a 10-year prognosis, those are related to a possibility to recur in 10–20 years after the initial diagnosis and treatment.15 For example, when a tumor expresses ER and/or PR, it can be predicted that the person would often get a benefit from hormone therapy, namely, 1. Selective estrogen receptor modulator (e.g., tamoxifen, toremifene), 2. Selective estrogen receptor degrader (fulvestrant), 3. Cyclin-dependent kinases (CDKs) 4/6 inhibitors (palbociclib, ribociclib, and abemaciclib), and 4. Luteinizing hormone-releasing hormone agents (LHRHs) recommended for premenopausal women. Furthermore, aromatase inhibitors namely, letrozole, anastrozole, exemestane, goserelin and leuprolide (Table 3) are recommended for postmenopausal women.15,36
Table 1.
Prognostic molecular markers of breast cancer.
| Prognostic molecular markers | Prognosis | Detection method | Potential medical use | References |
|---|---|---|---|---|
| ER | ER-positive breast cancer Patients have better survival rate than patients with ER-negative cancer. | IHC | Yes | 11,32,52 |
| Her2 | Her2 positive breast cancers are more aggressive and have a poor prognosis compared to Her2/neu-negative tumors. | IHC and FISH | Yes | 8,9,11,32 |
| PR | PR-positive breast cancer Patients have better survival rate than patients with PR-negative cancer. | IHC | Yes | 11,13,32 |
Table 2.
Predictive molecular markers of breast cancer.
| Predictive molecular markers | Targeted treatment | Detection method | Potential medical use | References |
|---|---|---|---|---|
| ER | Higher expression of ER in tumor predicts benefit from hormone therapy such as tamoxifen but ER negative tumor benefits more from chemotherapy since they are not stimulated by ovarian sex hormones | IHC | Yes | 9,11,31,32,52 |
| Her2 | overexpression of Her2 in tumors predicts benefit from targeted therapy like monoclonal antibody (trastuzumab) or by kinase inhibitor (lapatinib) in the metastatic as well as in the adjuvant setting. Her2 negative tumors benefits from other targeted therapy such as everolimus | IHC and FISH | Yes | 8,9,11,14,32 |
| PR | Higher expression of PR in tumor predicts benefit from hormone therapy such as tamoxifen but PR negative tumor benefits more from chemotherapy since they are not stimulated by ovarian sex hormones | IHC | Yes | 1,9,11,13,32,42 |
Table 3.
Molecular markers status and suggestive therapies of breast cancer.
| Status of molecular markers | ||||
|---|---|---|---|---|
| Hormone positive | Hormone negative | HER2 positive | HER2 negative | Triple negative |
Hormone therapy Premenopausal
|
Chemotherapy
|
Targeted therapy Monoclonal antibody
|
Targeted therapy
|
Immunotherapy PD-L1 inhibitors
|
Over-expression of HER2 protein detected by IHC or HER2 gene amplification by FISH is an indicator of plausibility of response to anti-HER2 therapies (Table 1, Table 2). Moreover, for anti-HER2 therapies, for instance, a monoclonal antibody (trastuzumab, pertuzumab and ado-trastuzumabemtansine) therapy or a kinase inhibitor (lapatinib and neratinib) are in use (Table 3).15,36 Trastuzumab is a recombinant humanized IgG1 monoclonal antibody for the proto-oncogene HER2 receptor, which is an epidermal growth factor receptor. When the HER2 is over-expressed in breast cells, it over-amplifies the signal produced by other receptors of the HER family by creating heterodimers with eventual development of tumors. The HER2 receptor is a type of transmembrane tyrosine kinase receptor that comprises of an intracellular or cytoplasmic tyrosine kinase domain, a transmembrane region, and an extracellular ligand-binding domain.47 It is triggered by the fabrication of homodimers or heterodimers with other epidermal growth factor receptor (EGFR) proteins, which causes to dimerization and auto phosphorylation and/or trans phosphorylation of certain tyrosine residues in EGFR intracellular domains. Eventually, downstream molecular-signaling cascades, such as the Raf/Ras/mitogen-activated protein kinase (MAPK), the phospholipase Cγ(PLCγ)/protein kinase C (PKC) and the phosphoinositide 3-kinase/Akt pathways are activated; thus, cell proliferation, escape immunity and cell cycle ameliorated. Due to over-expression of HER2 in cancer cells, hyper-activation of these signaling pathways and abnormal cell-proliferation was noted.47
Over-expression of the oncogene HER2 in breast cancer is an illustration of both prognostic and predictive molecular markers, suggesting a worse prognosis, i.e., a high rate of recurrence. However, it also predicts that one would often benefit from anthracycline and taxane-based chemotherapies. The targeted therapies for HER2 such as trastuzumab would be fruitful, but it would not respond to hormone-based therapies.30 In breast cancer, clinic pathological prognostic parameters, namely, age at diagnosis, lymph node status, tumor size, and histopathological grading, are in statistical terms triumphant at predicting the upshot of breast cancer patients, but these are inadequate in the therapeutic decision-making practice for individual patients.13 Thus, molecular markers could be used for evaluation of the patient in several clinical milieus, including guessing the amount of risk of a particular type of cancer drifting to metastasis, screening for baffling primary cancer types. Discern between benign and malignant tumors remain easy or one type of malignancy from another with molecular markers. These can also be used in detecting prognosis and prediction for a woman, tracking its grade to have a moratorium in the metastatic invasion; indeed, the determination of a particular therapy with her ancillary response for eradication is the coveted goal.48
Mechanism and action of tamoxifen, trastuzumab and other therapies
Chemically similar to estrogen, the non-steroidal drug tamoxifen competitively binds to ER, causing configurational changes in the ER, with an eventual obstruction or vary in the expression of estrogen-dependent genes. Those configurational changes lead to several events namely, lowers DNA polymerase activity, weakened in thymidine consumption, siege of estradiol intake and declined in estrogen response. Tamoxifen co-operates with other co-activators or co-repressors in the tissue and bonds with various estrogen receptors, ER-α or ER-β, generating both estrogenic and anti-estrogenic effects.49,50
Furthermore, selective estrogen receptor degraders obstruct and annihilate ERs; cyclin-dependent kinases (CDKs)4/6 inhibitors block CDK4 and CDK6 proteins; luteinizing hormone-releasing hormone agents (LHRHs) stop the ovaries from making estrogen; likewise, aromatase inhibitors help in reducing estrogen by jamming an enzyme called aromatase and which doesn't allow it to convert androgen into estrogen.35,51 This suggests that only hormone therapy is the best therapeutic intervention for hormone receptor-positive cancer, since those are structurally similar to influencing hormone and block the site on receptors.
Moreover, trastuzumab is an extracellular ligand binding domain and it obstructs the gap of the extracellular domain of HER2 to encourage antibody induced receptor down-regulation; eventually, it inhibits HER2-mediated intracellular signaling cascades.34,50, 51, 52 Inhibition of mitogen-activated protein kinases (MAPKs) and phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathways enhance cell cycle arrest and the repression of cell growth and proliferation. Trastuzumab also arbitrates the actuation of antibody-dependent cell-mediated cytotoxicity (ADCC) by alluring immune cells, such as the natural killer (NK) cells, to neoplastic sites that overexpress HER2.34,47,50, 51, 52
Similarly, lapatinib is a 4-anilinoquinazoline-kinase inhibitor of the intracellular tyrosine-kinase domains of both epidermal growth factor receptor (HER1/EGFR/ERBB1) and human epidermal growth factor receptor type 2 (HER2/ERBB2) with a splitting half-life of ≥300 min. Lapatinib prevents ERBB motivated tumor-cell development in vitro and in vivo.34,47,50, 51, 52 This suggests that targeted therapy is a better therapeutic intervention for HER2 positive cancer since they target and down-regulates the HER2 gene.
Conclusion and expert opinion
There is no doubt that estrogen receptors, progesterone receptors and the epidermal growth factor receptor-2 are the most routinely used basic, prime molecular markers for detection of breast cancer worldwide. These markers provide the suitable prediction of the prognosis of cancer recurrence after an initial remedial treatment, with an indication of eventual future appropriate treatment; and thus, those play a key role in the management of breast cancer. For a better management of breast cancer, the diagnostic process should be fast, so that the oncologist could decide which therapeutic would be the better option for the patient. The above described processes are fast, thus universally accepted, and have a high level of ‘sensitivity and specificity’; but, newer technologies are available that are even quicker by half the time of immunohistochemistry. Such technologies include different immunological assays and PCRs. The RT-PCR technique can detect low concentrations of ER/PR/HER2 mRNA. Relatively low levels of ER/PR/HER2 protein can be detected by enzyme-linked immunosorbent assay (ELISA). Although, an extensive trials for these approaches are yet to be implemented; these techniques are less strenuous as it skips the tissue processing part, proportionately expeditious, cheaper, and has immense possibilities. If a new breast cancer scoring system based on these newer expeditious technologies is established, then grading and management of breast cancer will be easier, enhanced and quicker. Finally, hormone receptors and HER2 as molecular markers are of prime therapeutic importance and have the capability to take part in future drug development techniques.
Conflict of interests
Authors declared no conflict of interest.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Swati Sucharita Mohanty, Email: swatimohany16@gmail.com.
Rabindra Nath Padhy, Email: rnpadhy54@gmail.com.
References
- 1.World Health Organization (WHO) Cancer. News, fact sheets. 2018. https://www.who.int/news-room/fact-sheets/detail/cancer
- 2.National Cancer Institute (NCI) Cancer statistics. About cancer. 2018. https://www.cancer.gov/about-cancer/understanding/statistics
- 3.Mohanty S.S., Mohanty P.K. Obesity as Potential Breast Cancer Risk Factor for Postmenopausal Women. Genes Dis. 2019;8(2):117–123. doi: 10.1016/j.gendis.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Globocan . World Health Organization (WHO); 2018. India Factsheet. Cancer Today. Asia. Global Cancer Observatory (GCO). International Agency for Research on Cancer (IARC)http://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf [Google Scholar]
- 5.India Against Cancer . National Institute of Cancer Prevention and Research (NICPR); 2019. Cancer Statistics.http://cancerindia.org.in/cancer-statistics/ Indian Council of Medical Research (ICMR) [Google Scholar]
- 6.Sahoo C.R., Paidesetty S.K., Padhy R.N. Norharmane as a potential chemical entity for development of anticancer drugs. Eur J Med Chem. 2019;162:752–764. doi: 10.1016/j.ejmech.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Sahoo C.R., Paidesetty S.K., Padhy R.N. Nornostocine congeners as potential anticancer drugs: an overview. Drug Dev Res. 2019;80:878–892. [Google Scholar]
- 8.Williams N.S., Bulstrode C.J.K., O'Connell P.R. 26th ed. CRC Press, Taylor and Francis; UK: 2013. Bailey and Love's Short Practice of Surgery; pp. 798–822. [Google Scholar]
- 9.Cancer. Net . American Society of Clinical Oncology (ASCO); 2019. Biomarkers to Guide Treatment for Metastatic Breast Cancer.https://www.cancer.net/research-and-advocacy/asco-care-and-treatment-recommendations-patients/biomarkers-guide-treatment-metastatic-breast-cancer [Google Scholar]
- 10.Banin Hirata B.K., Oda J.M., Losi Guembarovski R., Ariza C.B., Oliveira C.E., Watanabe M.A. Molecular markers for breast cancer: prediction on tumor behavior. Dis Markers. 2014;2014:513158. doi: 10.1155/2014/513158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute (NCI) Targeted cancer therapies. About Cancer. 2019 [Google Scholar]
- 12.Mehta S., Shelling A., Muthukaruppan A., et al. Predictive and prognostic molecular markers for cancer medicine. Ther Adv Med Oncol. 2010;2(2):125–148. doi: 10.1177/1758834009360519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winokur T., Chhieng D.C., Siegal G.P. Updates in diagnostic pathology- molecular markers in breast cancer current practice and future possibilities. Springer Science & Business Media. 2007 [Google Scholar]
- 14.Taneja P., Maglic D., Kai F., et al. Classical and novel prognostic markers for breast cancer and their clinical significance. Clin Med Insights Oncol. 2010;4:15–34. doi: 10.4137/cmo.s4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gökmen-Polar Y., Badve S. Breast cancer prognostic markers: an overview of a changing menu. MLO Med Lab Obs. 2015;47(10) 8, 10, 12-3; quiz 14. [PubMed] [Google Scholar]
- 16.Kosir M.A. Merck and co., Inc.; Kenilworth, New Jersey: 2017. Breast Cancer: Breast Disorders: Merck Manual Professional. [Google Scholar]
- 17.Law M.L., Kao F.T., Wei Q., et al. The progesterone receptor gene maps to human chromosome band 11q13, the site of the mammary oncogene int-2. Proc Natl Acad Sci Unit States Am. 1987;84(9):2877–2881. doi: 10.1073/pnas.84.9.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Misrahi M., Atger M., d'Aurio L., et al. Complete amino acid sequence of the human progesterone receptor deduced from cloned cDNA. Biochem Biophys Res Commun. 1987;143(2):740–748. doi: 10.1016/0006-291x(87)91416-1. [DOI] [PubMed] [Google Scholar]
- 19.Dahlman W.K., Cavailles V., Fuqua S.A., et al. International union of pharmacology: lxiv: estrogen receptors. Pharmacol Rev. 2006;58(4):773–781. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- 20.Levin E.R. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19(8):1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuiper G.G., Enmark E., Pelto-Huikko M., Nilsson S., GustafssonJA Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen E.V., Jordan V.C. The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 2003;9(6):1980–1989. [PubMed] [Google Scholar]
- 23.Burns K.A., Korach K.S. Estrogen receptors and human disease: an update. Arch Toxicol. 2012;86(10):1491–1504. doi: 10.1007/s00204-012-0868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ensembl.org. Gene: ESR1 (ENSG00000091831) 2019. http://www.ensembl.org
- 25.Gadkar-Sable S., Shah C., Rosario G., Sachdeva G., Puri C. Progesterone receptors: various forms and functions in reproductive tissues. Front Biosci. 2005;10:2118–2130. doi: 10.2741/1685. [DOI] [PubMed] [Google Scholar]
- 26.Speroff L., Fritz M.A. Lippincott Williams and Wilkins; Philadelphia: 2005. Clinical Gynecologic Endocrinology and Infertility; pp. 573–620. [Google Scholar]
- 27.Barh D., Gunduz M. CRC Press, Taylor and Francis Group; New York: 2015. Noninvasive Molecular Markers in Gynecologic Cancers; p. 427. [Google Scholar]
- 28.HUGO Gene Nomenclature Committee . National Institutes of Health; 2019. ERBB2 erbb2 Receptor Tyrosine kinase 2 [Homo sapiens (human)] [Google Scholar]
- 29.Genetics Home Reference ERBB2 Gene. erb-B2 Receptor Tyrosine kinase 2. https://ghr.nlm.nih.gov/gene/ERBB2. 2019 U.S. National Library of Medicine. National Institutes of Health.
- 30.Iqbal B.M., Buch A. Hormone receptor (ER, PR, HER2/neu) status and proliferation index marker (Ki-67) in breast cancers: their onco-pathological correlation, shortcomings and future trends. Med J DY Patil Univ. 2016;9(6):674–679. [Google Scholar]
- 31.Wolff A.C., Hammond M.E., Hicks D.G., et al. American society of clinical oncology and college of American pathologists, Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists. Clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 32.Ulaner G.A., Riedl C.C., Dickler M.N., Jhaveri K., Pandit-Taskar N., Weber W. Molecular imaging of biomarkers in breast cancer. J Nucl Med. 2016;57(Suppl 1):53S–59S. doi: 10.2967/jnumed.115.157909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burstein H.J. The distinctive nature of HER2 positive breast cancers. N Engl J Med. 2005;353(16):1652–1654. doi: 10.1056/NEJMp058197. [DOI] [PubMed] [Google Scholar]
- 34.Mitri Z., Constantine T., O'Regan R. The HER2 receptor in breast cancer: pathophysiology, clinical use, and new advances in therapy. Chemother Res Pract. 2021;2012:743193. doi: 10.1155/2012/743193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Cancer Society (ACS) 2019. Breast Cancer. [Google Scholar]
- 36.Harvey J.M., Clark G.M., Osborne C.K., Allred D.C. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assays for predicting response to adjuvant therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 37.Rampaul R.S., Pinder S.E., Elston C.W., Ellis I.O. Nottingham Breast Team. Prognostic and predictive factors in primary breast cancer and their role in patient management: the Nottingham breast team. Eur J Surg Oncol. 2001;27(3):229–238. doi: 10.1053/ejso.2001.1114. [DOI] [PubMed] [Google Scholar]
- 38.Coons A.H., Creech H.J., Jones R.N. Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med. 1941;47(2):200–202. [Google Scholar]
- 39.Ramos-Vara J.A., Miller M.A. When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry the red, brown, and blue technique. Vet Pathol. 2014;51(1):42–87. doi: 10.1177/0300985813505879. [DOI] [PubMed] [Google Scholar]
- 40.Maverakis E., Kim K., Shimoda M., et al. Glycans in the immune system and the altered glycan theory of autoimmunity: a critical review. J Autoimmun. 2015;57:1–13. doi: 10.1016/j.jaut.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Connor C. Fluorescence in situ hybridization (FISH) Nat Educ. 2008;1:171. [Google Scholar]
- 42.Wright C.M. Elsevier Inc.; 2015. Epigenetic Cancer Therapy. Long Non-coding RNAs and Cancer; pp. 91–114. [Google Scholar]
- 43.Lebeau A., Deimling D., Kaltz C., et al. Her 2/Neu analysis in archival tissue samples of human breast cancer: comparison of immunohistochemistry and fluorescence in situ hybridization. J Clin Oncol. 2001;19(2):354–363. doi: 10.1200/JCO.2001.19.2.354. [DOI] [PubMed] [Google Scholar]
- 44.Ross J.S. Breast cancer biomarkers and Her2 testing after 10 years of anti-Her2 therapy. Drug News Perspect. 2009;22(2):93–106. doi: 10.1358/dnp.2009.22.2.1334452. [DOI] [PubMed] [Google Scholar]
- 45.Penault-Llorca F., Bilous M., Dowsett M., et al. Emerging technologies for assessing HER2 amplification. Am J Clin Pathol. 2009;132(4):539–548. doi: 10.1309/AJCPV2I0HGPMGBSQ. [DOI] [PubMed] [Google Scholar]
- 46.Speicher M.R., Carter N.P. The new cytogenetics: blurring the boundaries with molecular biology. Nat Rev Genet. 2005;6(10):782–792. doi: 10.1038/nrg1692. [DOI] [PubMed] [Google Scholar]
- 47.Hubbard S.R., Miller W.T. Receptor tyrosine kinases: mechanisms of activation and signaling. Curr Opin Cell Biol. 2007;19(2):117–123. doi: 10.1016/j.ceb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henry N.L., Hayes D.F. Cancer biomarkers. Mol Oncol. 2012;6(2):140–146. doi: 10.1016/j.molonc.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodsell D.S. The molecular perspective: tamoxifen and the estrogen receptor. Oncologist. 2002;7(2):163–164. doi: 10.1634/theoncologist.7-2-163. [DOI] [PubMed] [Google Scholar]
- 50.Drugbank Tamoxifen, trastuzumab and lapatinib. 2019. https://www.drugbank.ca
- 51.Breast cancer. 2019. https://www.breastcancer.org
- 52.Murphy C.G., Modi S. HER2 breast cancer therapies: a review. Biologics. 2009;3:289–301. [PMC free article] [PubMed] [Google Scholar]