Abstract
Excessive oxidative stress is a major causative factor of endothelial dysfunction in hypertension. As an endogenous pro-oxidant, thioredoxin-interacting protein (TXNIP) contributes to oxidative damage in various tissues. The present study aimed to investigate the role of TXNIP in mediating endothelial dysfunction in hypertension. In vivo, an experimental model of acquired hypertension was established with two-kidney, one-clip (2K1C) surgery. The expression of TXNIP in the vascular endothelial cells of multiple vessels was significantly increased in hypertensive rats compared with sham-operated rats. Resveratrol, a TXNIP inhibitor, suppressed vascular oxidative damage and increased the expression and activity of eNOS in the aorta of hypertensive rats. Notably, impaired endothelium-dependent vasodilation was effectively improved by TXNIP inhibition in hypertensive rats. In vitro, we observed that Ang II increased the expression of TXNIP in primary human aortic endothelial cells (HAECs) and that TXNIP knockdown by RNA interference alleviated cellular oxidative stress damage and mitigated the impaired eNOS activation and intracellular nitric oxide (NO) production observed in Ang II-treated HAECs. However, inhibiting thioredoxin (TRX) with PX-12 completely blunted the protective effect of silencing TXNIP. In addition, TXNIP knockdown facilitated TRX expression and promoted TRX nuclear translocation to further activate AP1 and REF1. TRX overexpression exhibited favorable effects on eNOS/NO homeostasis in Ang II-treated HAECs. Thus, TXNIP contributes to oxidative stress and endothelial dysfunction in hypertension, and these effects are dependent on the antioxidant capacity of TRX, suggesting that targeting TXNIP may be a novel strategy for antihypertensive therapy.
Keywords: Endothelial dysfunction, eNOS, Hypertension, Oxidative stress, Thioredoxin-interacting protein
Introduction
Vascular endothelial cells, which form the inner layer of the blood vessel wall, are essential for maintaining the normal function of the vasculature and hemodynamic stability.1 Endothelial dysfunction prompts vascular remodeling and is recognized as an early feature of the adverse cardiovascular events observed in hypertension.2,3 Accumulated evidence from both clinical trials and experiments indicates that oxidative stress is a major contributing factor in the pathogenesis of endothelial dysfunction in hypertension.4,5 Therefore, antioxidant treatments exert protective effects on endothelial function and potentially alleviate hypertension-induced cardiac remodeling.6,7
Immoderate activation of the renin-angiotensin system (RAS) characterized by high accumulation of angiotensin II (Ang II) in the circulation is not only a hallmark of hypertension but also a trigger that induces vascular oxidative stress by activating NAD(P)H oxidase, thereby augmenting intracellular reactive oxygen species (ROS) generation in vascular endothelial cells.8,9 ROS detrimentally affect endothelial function via several mechanisms, including suppressing endothelial NO synthase (eNOS) activation and promoting eNOS uncoupling, eventually leading to an alteration in nitric oxide (NO) bioavailability.10,11 Although emerging evidence suggests a causal relationship between oxidative stress and endothelial dysfunction in hypertension, the precise mechanisms involved remain unclear.
Thioredoxin-interacting protein (TXNIP) is a ubiquitously expressed α-arrestin that can induce oxidative damage by binding to the catalytic site of thioredoxin (TRX) and thereby inhibiting TRX antioxidant activity.12,13 TXNIP is linked to various physiological processes related to cell growth and survival and is sensitive to high glucose levels, inflammation, ROS and other types of stress.14,15 Abnormally high levels of TXNIP can enhance oxygen consumption and mitochondrial ATP synthesis, resulting in oxidative stress in multiple cells.12 Furthermore, recent studies suggest that TXNIP can potentially suppress eNOS activity and disrupt NO signaling,16,17 which suggests that TXNIP may act as a detrimental factor in endothelial function. However, currently, little is known about the role of TXNIP in mediating endothelial dysfunction in hypertension.
In the present study, we hypothesized that vascular TXNIP expression could be abnormally upregulated by hypertension and contribute to endothelial dysfunction. To test this hypothesis, we established a hypertensive rat model and used human Ang II to induce endothelial injury in primary human aortic endothelial cells (HAECs). The regulatory effects of TXNIP on endothelial function were determined, and the concrete mechanisms involved were also elucidated.
Materials and methods
Animals and experimental protocols
The protocols for the animal experiments were performed in accordance with the guidelines of the Ethics Committee of Chongqing Medical University (Chongqing, China) and complied with the Guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health. All animals were raised in specific pathogen-free conditions and housed in appropriate containers with a 12-h light/dark cycle and free access to standard rodent food and water. The animal experiments were performed in two independent parts, as described below.
Part I: Male Sprague–Dawley rats (weighing 180–220 g) were randomly divided into control, sham, and hypertension groups (n = 10 per group). A rat model of experimental hypertension was established by two-kidney, one-clip (2K1C) surgery. Hemodynamic parameters, including systolic blood pressure (SBP), mean blood pressure (MBP) and heart rate (HR), were monitored weekly in conscious rats using a noninvasive tail-cuff system (Softron Biotechnology, Beijing, China). Four weeks after the surgery, blood samples were collected from the left femoral artery, and the heart, thoracic aorta, and carotid artery were harvested. The heart mass index (presented as the heart weight/body weight) was calculated.
Part II: Male Sprague–Dawley rats were randomly divided into sham, sham + resveratrol (trans-3,40,5-trihydroxystilbene), hypertension and hypertension + resveratrol groups (n = 8 per group). Resveratrol has been identified to significantly suppress TXNIP expression at both the protein and mRNA levels and is widely used as a natural inhibitor of TXNIP.18,19 Resveratrol (Solarbio, Beijing, China) was dissolved in 0.5% sodium carboxymethylcellulose (CMC) and diluted with physiological saline. To diminish the underlying pharmacological action on the cardiovascular system, according to a study published by Vella RK et al,20 a low dose of resveratrol (2 mg/kg/d) or equal volume of vehicle (0.5% CMC) was administered to rats via oral gavage for 4 consecutive weeks. At 4 weeks after treatment, the SBP, MBP, HR and weight were detected. Blood samples were collected from the caudal vein for fasting blood glucose detection using a glucometer (Sinocare, Changsha, China), and the thoracic aorta was harvested for vascular tension detection and other experiments.
Establishment of hypertensive rats
2K1C-induced hypertensive rats have been widely used to assess oxidative stress-related target organ injury in hypertension.21 According to previous methods,22 rats were anesthetized with an intraperitoneal injection of pentobarbitone sodium (50 mg/kg) and placed in the lateral decubitus position. The left kidney was exposed through a retroperitoneal flank incision. After the left renal artery was separated from the adjacent renal vein and connective tissues, a 0.25-mm U-shaped silver clip was placed around the artery to partially block renal blood flow. Sham-operated rats underwent the same procedure without silver clip implantation.
Enzyme-linked immunosorbent assay (ELISA)
Blood samples were centrifuged at 3000 rpm for 10 min at 4 °C, and the supernatant plasma was collected for Ang II detection using a rat Ang II ELISA kit (Meibiao, Yuncheng, China) according to the manufacturer's instructions.
Histological and immunohistochemical assays
Hearts, carotid arteries, and thoracic aortas were immersed in 4% neutral buffered paraformaldehyde and embedded in paraffin wax. After being cut into 5-μm-thick sections, the tissues were stained with hematoxylin and eosin (HE). The HE-stained sections were imaged under a microscope (Leica, Wetzlar, Germany). Myocyte areas (calculated from at least 100 myocytes per section) were used to evaluate cardiac remodeling. Similarly, the medial thicknesses of the carotid artery and thoracic aorta were used to evaluate vascular remodeling; at least 10 independent fields per section were assessed. The medial thickness and myocyte area results were quantified using Image-Pro Plus software (Media Cybernetics, MD, USA).
TXNIP protein in the carotid artery and thoracic aorta was identified and quantified by immunohistochemistry. In brief, after processing similar to that described above, vascular sections were processed for antigen retrieval, blocked, and then incubated with a primary antibody against TXNIP (1:100; Proteintech) in a moist chamber at 4 °C overnight. Then, diaminobenzidine dye was applied to visualize the positive protein staining. Finally, the sections were counterstained with hematoxylin for nuclear staining.
Dihydroethidium (DHE) staining
Thoracic aorta sections were incubated with a DHE (Sigma–Aldrich, MO, USA) solution according to the manufacturer's instructions. The red fluorescent signal was captured using a fluorescence microscope (Leica) and quantified using Image-Pro Plus software.
Endothelium-dependent vascular reactivity experiment
The thoracic aorta was cut into aortic rings (5 mm in length), which were then mounted on the stainless steel pins of the Multi Wire Myograph System (DMT, Hinnerup, Denmark), incubated at 37 °C with a physiolfogical salt solution (PSS; 74.7 mM NaCl, 14.9 mM NaHCO3, 1.17 mM MgSO4, 1.6 mM CaCl2, 1.18 mM KH2PO4 and 5.5 mM glucose) and gassed with 95% O2 and 5% CO2 at a steady flow. The tension of the aortic rings was adjusted to 1 g and equilibrated for 60 min. Then, the aortic rings were stabilized by two successive near-maximum contractions with a KCl (60 mM)-PSS. When stable contraction induced by norepinephrine (NE; 100 nM) was obtained, the concentration-relaxation response of each aortic ring to cumulative concentrations of acetylcholine (ACh) or sodium nitroprusside (SNP) (both ranged from 10−10 to 10−5 M) was recorded and analyzed using LabChart software (AD Instruments, Dunedin, New Zealand).
Cell culture
Primary HAECs were kindly provided as a gift by Professor Xiangjian Chen's laboratory (Research Institute of Cardiovascular Disease affiliated with Nanjing Medical University, Nanjing, China). HAECs were cultured in endothelial cell medium (ECM; ScienCell, CA, USA) supplemented with 5% fetal bovine serum, 1% endothelial cell growth supplement and a 1% penicillin/streptomycin solution in a humidified incubator at 37 °C with 5% CO2. Cells in passages 3 to 6 were used in the experiments.
RNA interference
A specific small interfering RNA (siRNA) targeting TXNIP and a negative control (NC) scrambled RNA were constructed by Hanbio (Shanghai, China). HAECs were transiently transfected with siRNA oligos with Lipofectamine™ 2000 reagent (Invitrogen, CA, USA) according to the manufacturer's instructions. The siRNA-TXNIP sequence was 5′-GGAACAUCCUUCAAAGGAA-3′, and the siRNA-NC sequence was 5′-UUCUCCGAACGUGUCACGU-3′. The transfected cells were cultured for 24 h prior to Ang II treatment and other treatments.
Plasmid transfection
A TRX-overexpression plasmid (GV230-TRX) and scrambled NC plasmid (GV230-NC) were commercially constructed by Jikai Gene Chemical Technology Co., Ltd. (Shanghai, China). The plasmids were transfected into HAECs according to the manufacturer's instructions.
Western blotting
Total protein was extracted from HAECs and vascular tissues derived from rats. Nuclear extracts were isolated from HAECs using a nuclear protein extraction kit (Beyotime, Shanghai, China). Protein samples were separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking with 5% skim milk, the membranes were incubated with the appropriate primary antibodies including anti-TXNIP (1:1000; Proteintech), anti-TRX (1:2000; Proteintech), anti-AP1 (1:1000; Proteintech), anti-REF1 (1:1000; Bimake), anti-GAPDH (1:5000; Proteintech), anti-PCNA (1:2000; Proteintech), anti-NOX4 (1:1000; Proteintech), anti-NOX2 (1:1000; Santa Cruz Biotechnology), anti-SOD2 (1:2000; Santa Cruz Biotechnology), anti-eNOS (1:1000; Cell Signaling Technology), anti-p-eNOS (ser 1177) (1:1000; Cell Signaling Technology), anti-AKT (1:1000; Cell Signaling Technology), and anti-p-AKT (ser 473) (1:500; Santa Cruz Biotechnology) overnight at 4 °C. Then, the membranes were incubated with species-matched secondary antibodies (1:5000; Proteintech) for 1.5 h at room temperature. The blots were developed with a hypersensitive ECL chemiluminescence kit (Beyotime) and examined using a Bio-Rad gel imaging system (Bio-Rad, CA, USA).
Cell immunofluorescence
HAECs were cultured on glass coverslips in a 24-well plate and fixed with 4% paraformaldehyde for 10 min. Then, the cells were permeabilized with 0.25% Triton X-100 and blocked with 1% bovine serum albumin (BSA) for 30 min at room temperature. Subsequently, the cells were incubated with primary antibodies against TXNIP (1:100; Proteintech) and TRX (1:200; Proteintech) overnight at 4 °C. Alexa Fluor 555-labeled and FITC-labeled secondary antibodies (all from Beyotime, Shanghai, China) were used to detect TXNIP (in red) and TRX (in green), respectively. DAPI was then added for nuclear staining. Fluorescence signals were captured under a confocal microscope (Carl Zeiss, Oberkochen, Germany) and quantitatively analyzed using Image-Pro Plus software.
Intracellular ROS assay
Intracellular ROS level were measured with a 2′7′-dichlorodihydrofluorescein diacetate (DCFH-DA) solution (Beyotime, Shanghai, China) according to the manufacturer's instructions. Hydrogen peroxide served as a positive control. Image-Pro Plus software was used to quantitatively detect the intracellular fluorescence indicative of ROS.
Intracellular NO measurement
Intracellular NO levels were detected using the fluorescent dye 3-amino,4-aminomethyl-2’,7’-difluorescein, diacetate (DAF-FM DA; Beyotime, Shanghai, China), which could penetrate the cell membrane and bind to intracellular NO, leading to fluorescence excitation at 495 nm. The fluorescence signal for NO was captured with a fluorescence microscope, and the fluorescence density from at least 10 random fields in each well was quantitatively analyzed with Image-Pro Plus software.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software V.7.0 (GraphPad Software, CA, USA). The data are presented as the mean ± standard deviation (SD). Comparisons of two groups were performed using an unpaired, two-tailed Student's t-test. Comparisons among multiple groups were conducted using one-way analysis of variance (ANOVA) followed by Tukey's test. A P-value < 0.05 was considered statistically significant.
Results
Hypertension promoted the upregulation of TXNIP expression in vascular tissues in vivo
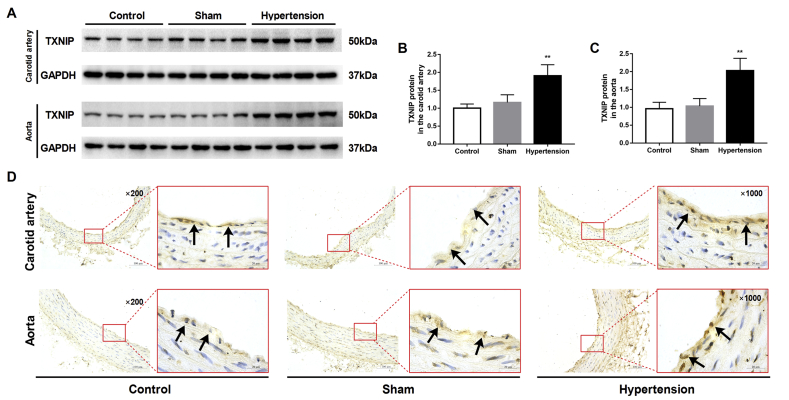
A successful hypertensive rat model was validated by detecting consistent blood pressure elevation and cardiovascular remodeling (Fig. S1). There was no difference in HR values among groups (date not shown). Western blot analysis showed that the expression of TXNIP was significantly increased in the carotid artery and thoracic aorta of hypertensive rats compared with those of sham-operated rats (both P < 0.01; Fig. 1A–C). Moreover, as endothelial cells are crucial for vasodilation, we examined TXNIP expression in vessel endothelial cells with immunohistochemistry. The results showed that the positive staining for TXNIP in endothelial cells was markedly increased in the carotid artery and thoracic aorta of hypertensive rats (Fig. 1D).
Figure 1.
Hypertension promoted the upregulation of TXNIP expression in vascular tissues in vivo. Rats were subjected to 2K1C surgery to establish an animal model of acquired hypertension. At 4 weeks after surgery, the carotid artery and aorta were harvested. (A) Western blot analysis of TXNIP in the carotid artery (upper panel) and aorta (lower panel). (B, C) Quantitative detection of the TXNIP protein in the carotid artery (B) and aorta (C) (n = 8 per group). (D) Immunohistochemical analysis of the TXNIP protein in the carotid artery (upper panel) and thoracic aorta (lower panel). Black arrows indicate positive staining for TXNIP in endothelial cells (original magnifications, 200 × and 1000 × ). ∗∗P < 0.01 vs. the sham group.
Inhibition of TXNIP alleviated vascular oxidative stress in hypertensive rats
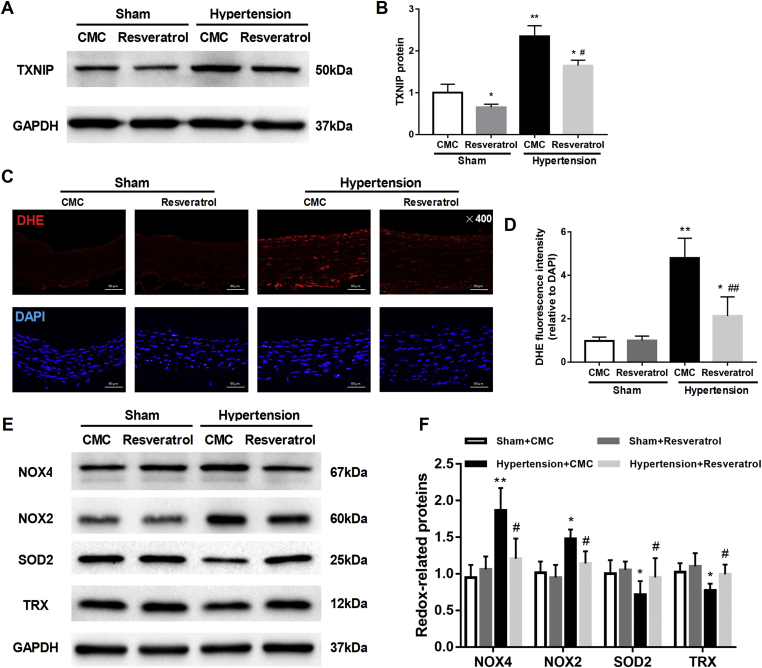
To test the role of TXNIP in hypertension, we used resveratrol to inhibit TXNIP. The inhibitory effect of resveratrol was evidenced by decreased TXNIP expression in the aorta of sham-operated and hypertensive rats (Fig. 2A, B). No differences in body weight, blood glucose levels or the HR were found among the different groups of rats (all P > 0.05). Hypertensive rats exhibited elevations in SBP and MBP, which were partially mitigated by resveratrol treatment (Table S1). DHE staining showed that ROS accumulation was significantly increased in the hypertensive rats compared with the normotensive rats (sham group). Resveratrol significantly reduced the ROS levels in the vessels of hypertensive rats (P < 0.01; Fig. 2C, D). Moreover, the increased NOX4 and NOX2 expression and decreased SOD2 expression in the aorta of hypertensive rats were also partially ameliorated by resveratrol (all P < 0.05; Fig. 2E, F). TRX is a crucial antioxidant protein that binds to TXNIP. We observed that the expression of TRX was significantly decreased in the aorta of hypertensive rats and that this effect was blunted by resveratrol (P < 0.05; Fig. 2E, F). These results further suggested that inhibition of TXNIP alleviated oxidative stress-related damage in hypertensive rats.
Figure 2.
Inhibition of TXNIP alleviated vascular oxidative stress in hypertensive rats. Sham-operated and hypertensive rats were established, followed by the administration of resveratrol (2 mg/kg/d) or CMC for 4 weeks. After 4 weeks of treatment, the aorta was harvested. (A) Western blot analysis of TXNIP in the aorta of rats in each group. (B) Quantitative detection of the TXNIP protein in each group (C) (n = 4 per group). (C) DHE and DAPI staining of the aorta (original magnification, 400 × ). (D) Quantitative detection of the fluorescence intensity of DHE relative to that of DAPI (n = 4 per group). (E) Western blot analysis of several representative redox proteins (NOX4, NOX2, SOD2 and TRX) in the aorta. (F) Quantitative detection of the protein levels of the above named redox proteins in the groups (n = 4 per group). ∗P < 0.05 vs. the sham + CMC group; ∗∗P < 0.01 vs. the sham + CMC group; #P < 0.05 vs. the hypertension + CMC group; ##P < 0.01 vs. the hypertension + CMC group. CMC, sodium carboxymethylcellulose.
Inhibition of TXNIP improved endothelium-dependent vasorelaxation in hypertensive rats
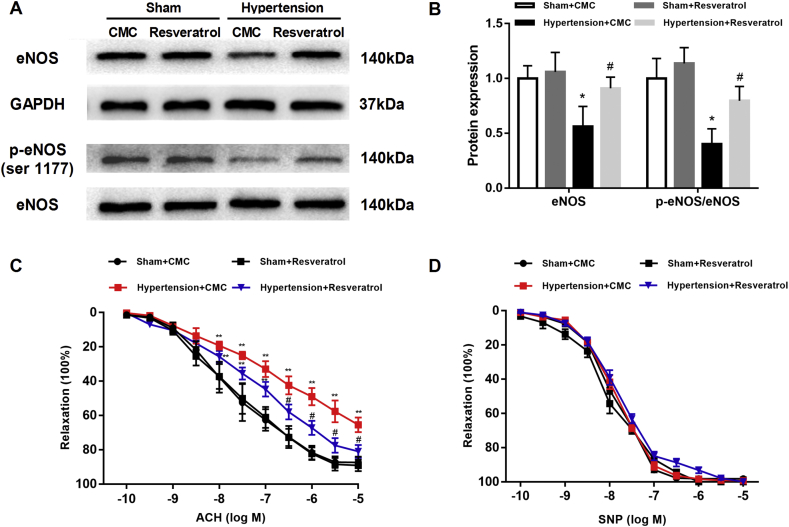
To determine the effect of TXNIP on vasorelaxation in hypertension, we tested eNOS and p-eNOS (ser 1177) in the rat aorta. Compared with that of sham-operated rats, the aorta of hypertensive rats showed decreased expression and active site phosphorylation of eNOS, while TXNIP inhibition significantly restored the protein expression and phosphorylation of eNOS (both P < 0.05; Fig. 3A, B). Vascular tension curves (ACh) displayed an obvious shift to right in the aorta of hypertensive rats compared with that of sham-operated rats, indicating that ACh-induced relaxations were impaired in hypertension. Importantly, TXNIP inhibition markedly improved the ACh-induced relaxations in the aorta of hypertensive rats without affecting those in normotensive rats (Fig. 3C). The quantitative relaxation degrees of the aorta induced by different concentrations of ACh in each group are shown in Table S2. There were no significant differences in SNP-induced vasodilatations of the aorta among all groups (P > 0.05; Fig. 3D).
Figure 3.
Inhibition of TXNIP improved endothelium-dependent vasorelaxation in hypertensive rats. (A) Western blot analysis of eNOS and p-eNOS (ser 1177) in the aorta. (B) Quantitative detection of the eNOS protein and the ratio of p-eNOS/eNOS in each group (n = 4 per group). (C) Vascular tension curves for ACh-induced aortic relaxation. (D) Vascular tension curves for SNP-induced aortic relaxation. (n = 4 per group). ∗P < 0.05 vs. the sham + CMC group; ∗∗P < 0.01 vs. the sham + CMC group; #P < 0.05 vs. the hypertension + CMC group.
Ang II induced oxidative stress via TXNIP in isolated HAECs
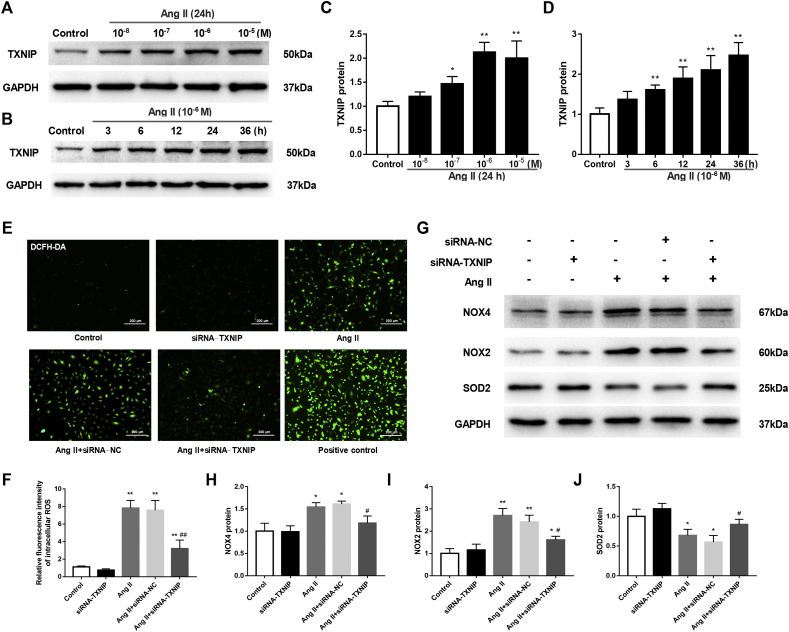
As a crucial trigger of endothelial dysfunction in hypertension, the Ang II level was elevated in the plasma of hypertensive rats (Fig. S1). To mimic endothelial injury in vitro, HAECs were exposed to stimulation with Ang II at various concentrations (ranging from 10−8 to 10−5 M) for 24 h. The results showed that the expression of TXNIP was gradually upregulated as the concentration of Ang II increased and peaked at 10−6 M (P < 0.01; Fig. 4A, C). Subsequently, TXNIP expression also showed an upward trend in a time-dependent manner in Ang II-treated HAECs (Fig. 4B, D). Inhibition of Ang II with losartan (an Ang II type I receptor blocker) almost completely eliminated the increased expression of TXNIP in HAECs, as evidenced by Western blot and immunofluorescence results, suggesting that the increase in the expression of TXNIP was directly mediated by Ang II (Fig. S2).
Figure 4.
Ang II induced oxidative stress via TXNIP in isolated HAECs. HAECs were treated with Ang II at different concentrations (10−8 to 10−5 M) or for different durations (3 h–36 h) to explore the effects of Ang II on TXNIP expression. Then, the HAECs were transfected with TXNIP- or NC-siRNA oligos (50 nM) 24 h prior to Ang II stimulation (10−6 M, 24 h). The fluorescent probe DCFH-DA (10 μM) was used to label intracellular ROS, and hydrogen peroxide (100 μM, 2 h) was used as a positive control. (A, B) Western blot analysis of TXNIP in HAECs treated with Ang II at different concentrations (A) or for different durations (B). (C and D) Quantitative detection of TXNIP protein levels in HAECs treated with Ang II at different concentrations (C) or for different periods (D). (E) DCFH-DA fluorescence staining of ROS in HAECs (original magnification, 100 × ). (F) Quantitative detection of the relative fluorescence intensity of ROS in each group. (G) Western blot analysis of several representative redox proteins (NOX4, NOX2 and SOD2) in HAECs. (H–J) Quantitative detection of the protein levels of NOX4 (H), NOX2 (I) and SOD2 (J) in each group. ∗P < 0.05 vs. the control group; ∗∗P < 0.01 vs. the control group; #P < 0.05 vs. the Ang II + siRNA-NC group; ##P < 0.01 vs. the Ang II + siRNA-NC group.
Ang II also increased ROS levels in HAECs, as shown in Figure 4E and F. To determine whether TXNIP inhibition can protect against Ang II-induced oxidative stress in HAECs, TXNIP expression was knocked down with siRNA. Effective knockdown was confirmed by Western blotting (Fig. S3). Results showed that silencing TXNIP markedly decreased the oxidative stress induced by Ang II (P < 0.01; Fig. 4E, F). In addition, the increases in the expression of NOX4 and NOX2 and decrease in the expression of SOD2 were also blunted by silencing TXNIP in Ang II-treated HAECs (all P < 0.05; Fig. 4G–J).
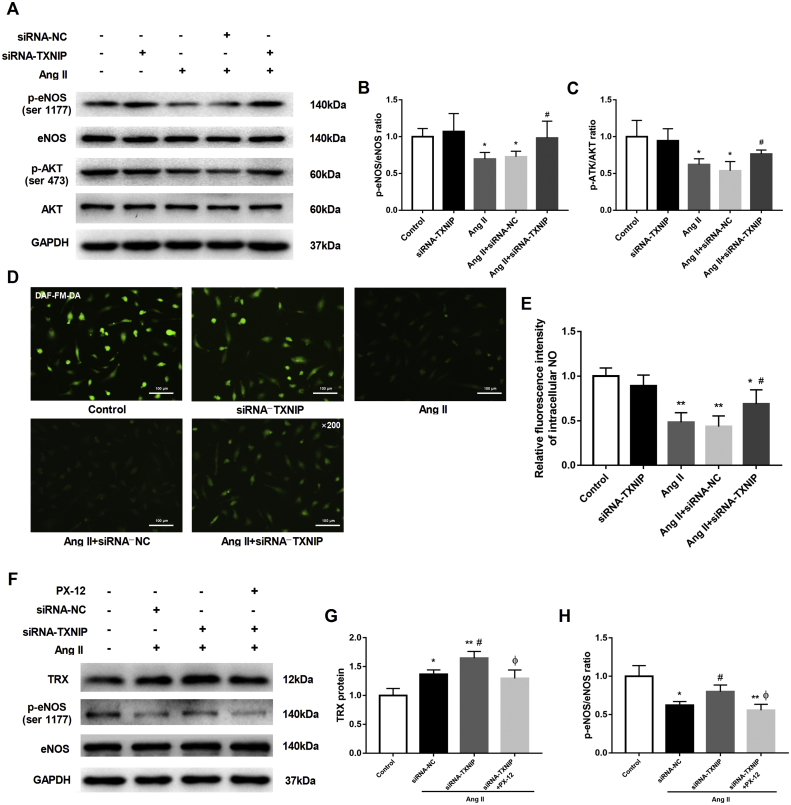
TXNIP knockdown alleviated the impaired eNOS function in HAECs exposed to Ang II
The phosphorylation of eNOS at ser 1177 mediated by AKT signaling maintains eNOS activation and NO synthesis, which serve as important indicators of endothelial function.23 Western blot analysis showed that Ang II substantially downregulated the phosphorylation of eNOS at ser 1177 and that of AKT at ser 473 in HAECs, whereas upon TXNIP knockdown, the abovementioned phosphorylated protein levels were notably upregulated (both P < 0.05, Fig. 5A–C). In accordance with this finding, intracellular NO production was significantly decreased in Ang II-stimulated HAECs compared with the corresponding control HAECs; however, knockdown of TXNIP expression partially restored NO production (P < 0.05, Fig. 5D, E). In addition, the increased TRX expression induced by Ang II was further enhanced by silencing TXNIP (P < 0.05, Fig. 5F, G), and inhibiting TRX with PX-12 (a TRX inhibitor) eliminated the effect of silencing TXNIP on eNOS activation (Fig. 5F, H). These results indicated that TXNIP knockdown protected eNOS activity against Ang II stimulation in HAECs and that this effect might be mediated by TRX.
Figure 5.
TXNIP knockdown alleviated the impaired eNOS function in HAECs stimulated with Ang II. HAECs were transfected with NC–or TXNIP-siRNA, and the fluorescent probe DAF-FM DA (5 μM) was used to label intracellular NO in the HAECs. In addition, the transfected HAECs were pretreated with PX-12 (1 μM) 2 h prior to Ang II stimulation (10−6 M, 24 h). (A) Western blot analysis of the protein levels of eNOS, p-eNOS (ser 1177), AKT and p-AKT (ser 473) in HAECs. (B, C) Quantitative detection of the ratios of p-eNOS/eNOS (B) and p-AKT/AKT (C) in each group. (D) DAF-FM DA fluorescence staining of intracellular NO in HAECs (original magnification 200 × ). (E) Quantitative detection of the relative fluorescence intensity of intracellular NO in each group. (F) Western blot analysis of the protein levels of TRX, eNOS and p-eNOS (ser 1177) in HAECs. (G, H) Quantitative detection of the TRX protein (G) and ratio of p-eNOS/eNOS (H) in each group ∗P < 0.05 vs. the control group; ∗∗P < 0.01 vs. the control group; #P < 0.05 vs. the Ang II + siRNA-NC group; ΦP < 0.05 vs. the Ang II + siRNA-TXNIP group.
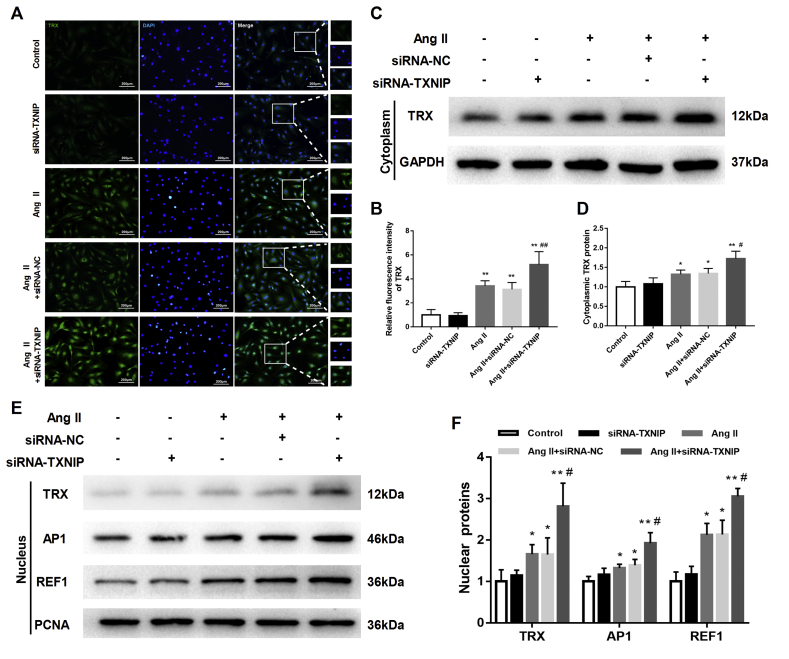
TXNIP suppressed the expression and nuclear translocation of TRX in Ang II-treated HAECs
To explore the intrinsic interactions between TXNIP and TRX in the process of endothelial dysfunction, the expression and subcellular location of TRX were detected. As shown in Fig. 6A, an immunofluorescence assay indicated that Ang II slightly increased intracellular TRX expression in HAECs and that TXNIP knockdown led to a further increase in TRX expression in Ang II-treated HAECs (Fig. 6A, B). More interestingly, we observed that the content of TRX was increased in both the cytoplasm and the nucleus (Fig. 6C–F) by TXNIP knockdown. This effect was also supported by immunofluorescence results (Fig. 6A, B). TRX can translocate into the nucleus and functions as a transcription factor to promote the expression of AP1 and REF1, which contribute to DNA repair during oxidative stress.12 We also found that the nuclear expressions of AP1 and REF1 were markedly elevated by TXNIP knockdown in Ang II-treated HAECs (all P < 0.05; Fig. 6E, F). These data suggested that silencing TXNIP increased the expression and nuclear translocation of TRX in HAECs upon Ang II stimulation.
Figure 6.
TXNIP suppressed the expression and nuclear translocation of TRX in Ang II-treated HAECs. (A) Immunofluorescence detection of the expression and distribution of intracellular TRX (in green) in HAECs (original magnification, 100 × ). (B) Quantitative detection of the relative fluorescence intensity of TRX in each group. (C) Western blot analysis of the protein level of TRX in the cytoplasm of HAECs. (D) Quantitative detection of cytoplasmic TRX in each group. (E) Western blot analysis of the protein levels of TRX, REF1 and AP1 in the nucleus of HAECs. (F) Quantitative detection of nuclear TRX, REF1 and AP1 in each group. ∗P < 0.05 vs. the control group; ∗∗P < 0.01 vs. the control group; #P < 0.05 vs. the Ang II + siRNA-NC group; ##P < 0.01 vs. the Ang II + siRNA-NC group.
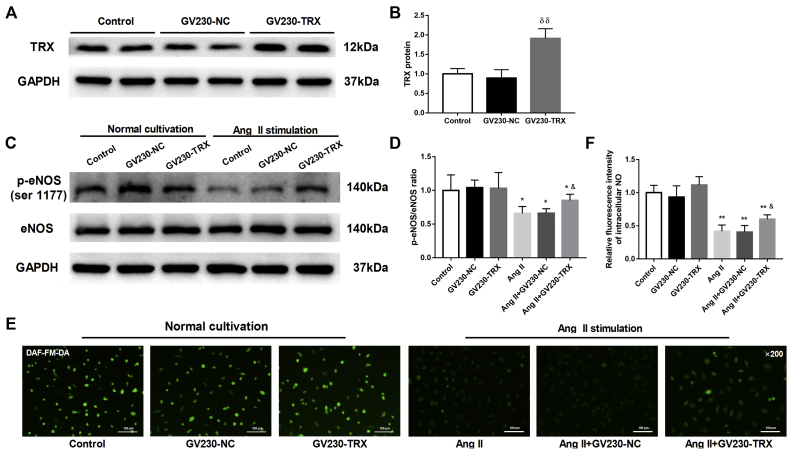
TRX protected eNOS activity against Ang II stimulation in HAECs
To explore the direct biological effects of TRX, which is negatively regulated by TXNIP, on endothelial function in HAECs, a TRX-loaded plasmid was transfected into HAECs to overexpress TRX; an NC plasmid was used as the control. Western blot analysis showed that the TRX-loaded plasmid significantly increased TRX expression in HAECs (P < 0.01; Fig. 7A, B). Under normal culture conditions, overexpression of TRX had no significant effect on the phosphorylation of eNOS at its active site (P > 0.05; Fig. 7C, D). Upon Ang II stimulation, overexpression of TRX significantly upregulated the ratio of p-eNOS (ser 1177) to total eNOS compared with NC expression (P < 0.05; Fig. 7C, D). Furthermore, DAF-FM DA staining revealed that intracellular NO production was significantly elevated in Ang II-treated HAECs transfected with the TRX-overexpression plasmid (P < 0.05; Fig. 7E, F). These data suggested that TRX exerted protective effects on HAECs upon Ang II stimulation.
Figure 7.
TRX protected eNOS activity against Ang II stimulation in HAECs. HAECs were transfected with an NC–or a TRX-loaded GV230 plasmid for 48 h, and then the transfection efficacy was detected by Western blotting. Twenty-four hours after transfection, the HAECs were cultured in the presence or absence of Ang II (10−6 M, 24 h). (A) Western blot analysis of TRX expression in HAECs transfected with the NC or TRX plasmid. (B) Quantitative detection of the TRX protein in each group. (C) Western blot analysis of the protein levels of eNOS and p-eNOS (ser 1177) in HAECs. (D) Quantitative detection of the ratio of p-eNOS/eNOS in each group. (E) DAF-FM DA fluorescence staining of intracellular NO in HAECs (original magnification, 200 × ). (F) Quantitative detection of the relative fluorescence intensity of intracellular NO in each group. δδP <0.01 vs. the GV230-NC group; ∗P < 0.05 vs. the control group; ∗∗P < 0.01 vs. the control group; &P < 0.05 vs. the Ang II + GV230-NC group.
Discussion
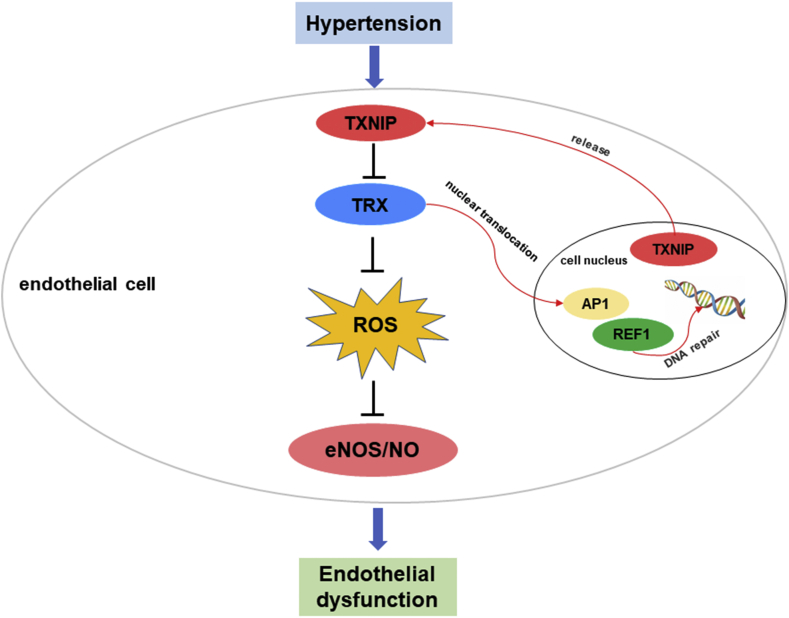
In the present study, for the first time, we found that the expression of TXNIP was significantly upregulated in the vascular endothelial cells of hypertensive rats and in Ang II-treated HAECs. The increased TXNIP expression led to oxidative stress and endothelial dysfunction in hypertension. Inhibition of TXNIP with resveratrol in vivo or siRNA in vitro effectively ameliorated oxidative stress and restored endothelial function. Our study further revealed that TXNIP exerted regulatory effects on endothelial function in a TRX-dependent manner. These results revealed the crucial role of the TXNIP/TRX complex in endothelial function and indicated that targeting TXNIP/TRX might provide a new method to prevent hypertension (Fig. 8).
Figure 8.
Schematic illustration of TXNIP mediating endothelial dysfunction in hypertension.
Endothelial dysfunction is characterized by decreased NO bioavailability,24 which not only impairs vascular tension but also accelerates vascular remodeling in hypertension.25 Clinical studies have demonstrated that endothelial dysfunction is an established marker of cardiovascular risk; thus, antihypertensive therapy that concomitantly improves endothelial function more effectively reduces cardiovascular risk than that does not.26,27 Evidence from both clinical research and basic research supports the conclusion that oxidative stress plays a central role in inducing endothelial dysfunction in hypertension.6 A previous study reported that antioxidant therapy effectively restored endothelium-derived NO production and improved endothelial function in a hypertensive animal model.28 Our study also showed excessive vascular oxidative stress accompanied by impaired endothelium-dependent dilatation in the aorta of hypertensive rats. Of note, the specific molecular mechanisms leading to endothelial dysfunction in hypertension are not fully elucidated.
TXNIP has been identified as an endogenous inhibitor of TRX and linked to oxidative stress, apoptosis and inflammation in multiple diseases.29 Abnormal TXNIP expression has been reported in ischemia/reperfusion injury, diabetes, stroke, etc.15,30, 31, 32 Evidence from gene polymorphism detection suggests that relatively high TXNIP expression in individuals increases susceptibility to chronic metabolic disorders such as diabetes and hypertension.33 Our study revealed that TXNIP expression was significantly increased in the vascular endothelial cells of the carotid artery and aorta in hypertensive rats. Similarly, Ang II induced upregulation of TXNIP expression in HAECs in a time- and dose-dependent manner, suggesting a crucial role for TXNIP in endothelial function. In fact, TXNIP can be activated by various endogenous and exogenous stimuli.15,31 Saxena G et al indicated that TXNIP could be released from the nucleus and shuttle into the mitochondria to disrupt the cellular redox state in response to oxidative stress.34 Therefore, the upregulation of TXNIP expression in endothelial cells may serve as an important mediator for promoting oxidative damage in hypertension.
Current evidence suggests that inhibition of TXNIP may be beneficial in preventing inflammation, neurodegeneration, and diabetes progression.29 In the present study, we provided in vivo and in vitro evidence that TXNIP inhibition effectively alleviated endothelial dysfunction in hypertension. eNOS is one of the most crucial regulatory proteins that maintains endothelial function by catalyzing NO synthesis.2,24 Consistent with previous studies,35,36 we found that eNOS activity was significantly suppressed in the aorta of hypertensive rats. Inhibition of TXNIP enhanced eNOS activity and improved the endothelial relaxation function of the aorta. As a vasoactive substance widely used to mimic endothelial injury in hypertension, Ang II induces endothelial dysfunction largely through a mechanism dependent on the induction of oxidative stress.37,38 In in vitro experiments, silencing TXNIP partially restored the decreased levels of p-eNOS (ser 1177) and p-AKT (ser 473) as well as the NO production in Ang II-treated HAECs. Furthermore, a previous study reported that TXNIP overexpression could downregulate eNOS activity and impair endothelial function.16 This evidence suggests that TXNIP may play a deleterious role in endothelial function in hypertension.
TRX is an indispensable component in the regulation of the effects of TXNIP on endothelial function. Our study revealed that the TRX protein level was slightly increased in Ang II-treated HAECs, which might be a compensatory response to the oxidative stress induced by Ang II. However, TRX expression was decreased in the aorta of hypertensive rats compared with that of normotensive rats, and a similar result was previously found in spontaneously hypertensive rats.39 The discordant TRX expression observed in vivo might be attributable to the long-term duration of hypertension that decompensates the TRX system. Both in vivo and in vitro, we found that TXNIP inhibition positively activated TRX expression. Especially in Ang II-treated HAECs, TXNIP knockdown promoted the nuclear translocation of TRX. It has been reported that TRX can translocate into the nucleus to activate several transcription factors, among which AP1 and REF1 play important roles in protecting cells from oxidative damage and facilitating DNA repair under oxidative stress.40,41 Thus, the alleviation of cellular oxidative damage mediated by TXNIP inhibition may require the antioxidant capability of TRX.
In clinical practice, increases in TRX levels have been detected in plasma and mononuclear cells from hypertensive individuals, and TRX expression dynamically changes with blood pressure fluctuation.42 In addition, a large single-nucleotide polymorphism study indicated that allelic mutation of the TRX gene was significantly associated with blood pressure elevation in the general population.43 In fact, the concrete regulatory mechanisms of TRX in hypertension remain inadequately characterized.44 Previous studies suggest that the favorable effects of TRX on the vasculature may be attributed to improvements in arterial stiffness and the suppression of inflammation in vessels.45,46 On that basis, our study further suggests that inhibition of TXNIP or overexpression of TRX may potentially benefit blood pressure control by regulating endothelial function in hypertension.
One major limitation is that although a low dose of resveratrol, as a natural inhibitor of TXNIP, has shown to significantly inhibit TXNIP expression in vivo, strictly, we still could not entirely exclude the other pharmacological effects of resveratrol on the vasculature. Therefore, RNAi adenovirus or vascular-specific TXNIP-knockout animal model may be essential to further verify the concrete role of TXNIP in the pathological process of endothelial dysfunction in hypertension.
Conclusion
In summary, our study suggests that TXNIP is involved in endothelial dysfunction in hypertension and that inhibition of TXNIP effectively improves endothelial function by suppressing oxidative stress. These findings provide novel insights into the redox state and vascular homeostasis in hypertension, which may be beneficial for exploring new treatments for hypertension.
Ethical approval
All animal protocols were approved by the Animal Research Committee at Chongqing Medical University (animal permit number SYXK2018-0003).
Conflict of interests
None of the authors have any conflicts of interest to declare.
Funding
The work was supported by the National Natural Science Foundation of China (No. 81370440) and the National Science-Technology Support Projects of China (No. 2015BAI01B00).
Acknowledgements
We acknowledge Professor Xiangjian Chen for providing the HAECs used in this study.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2020.08.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Vane J.R., Anggård E.E., Botting R.M. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323(1):27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 2.Dharmashankar K., Widlansky M.E. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep. 2010;12(6):448–455. doi: 10.1007/s11906-010-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvey A., Montezano A.C., Touyz R.M. Vascular biology of ageing-Implications in hypertension. J Mol Cell Cardiol. 2015;83:112–121. doi: 10.1016/j.yjmcc.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pennathur S., Heinecke J.W. Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep. 2007;7(4):257–264. doi: 10.1007/s11892-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 5.Lau Y.S., Ling W.C., Murugan D., Mustafa M.R. Boldine ameliorates vascular oxidative stress and endothelial dysfunction: therapeutic implication for hypertension and diabetes. J Cardiovasc Pharmacol. 2015;65(6):522–531. doi: 10.1097/FJC.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinh Q.N., Drummond G.R., Sobey C.G., Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int. 2014;2014:406960. doi: 10.1155/2014/406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rababa'h A.M., Guillory A.N., Mustafa R., Hijjawi T. Oxidative stress and cardiac remodeling: an updated edge. Curr Cardiol Rev. 2018;14(1):53–59. doi: 10.2174/1573403X14666180111145207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Touyz R.M. Molecular and cellular mechanisms in vascular injury in hypertension: role of angiotensin II. Curr Opin Nephrol Hypertens. 2005;14(2):125–131. doi: 10.1097/00041552-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Wen H., Gwathmey J.K., Xie L.H. Oxidative stress-mediated effects of angiotensin II in the cardiovascular system. World J Hypertens. 2012;2(4):34–44. doi: 10.5494/wjh.v2.i4.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brito R., Castillo G., González J., Valls N., Rodrigo R. Oxidative stress in hypertension: mechanisms and therapeutic opportunities. Exp Clin Endocrinol Diabetes. 2015;123(6):325–335. doi: 10.1055/s-0035-1548765. [DOI] [PubMed] [Google Scholar]
- 11.Karbach S., Wenzel P., Waisman A., Munzel T., Daiber A. eNOS uncoupling in cardiovascular diseases-the role of oxidative stress and inflammation. Curr Pharm Des. 2014;20(22):3579–3594. doi: 10.2174/13816128113196660748. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzawa A. Thioredoxin and redox signaling: roles of the thioredoxin system in control of cell fate. Arch Biochem Biophys. 2017;617:101–105. doi: 10.1016/j.abb.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Spindel O.N., World C., Berk B.C. Thioredoxin interacting protein: redox dependent and independent regulatory mechanisms. Antioxid Redox Signal. 2012;16(6):587–596. doi: 10.1089/ars.2011.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L., Ismael S., Nasoohi S., et al. Thioredoxin-interacting protein (TXNIP) associated NLRP3 inflammasome activation in human Alzheimer's disease brain. J Alzheimers Dis. 2019;68(1):255–265. doi: 10.3233/JAD-180814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong C.R., Chan W.P.A., Nguyen T.H., et al. Thioredoxin-interacting protein: pathophysiology and emerging pharmacotherapeutics in cardiovascular disease and diabetes. Cardiovasc Drugs Ther. 2014;28(4):347–360. doi: 10.1007/s10557-014-6538-5. [DOI] [PubMed] [Google Scholar]
- 16.Tian D., Dong J., Jin S., Teng X., Wu Y. Endogenous hydrogen sulfide-mediated MAPK inhibition preserves endothelial function through TXNIP signaling. Free Radic Biol Med. 2017;110:291–299. doi: 10.1016/j.freeradbiomed.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Schulze P.C., Liu H., Choe E., et al. Nitric oxide-dependent suppression of thioredoxin-interacting protein expression enhances thioredoxin activity. Arterioscler Thromb Vasc Biol. 2006;26(12):2666–2672. doi: 10.1161/01.ATV.0000248914.21018.f1. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Q., Che X., Zhang H., et al. Thioredoxin-interacting protein mediates apoptosis in early brain injury after subarachnoid haemorrhage. Int J Mol Sci. 2017;18(4):E854. doi: 10.3390/ijms18040854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Q., Che X., Zhang H., et al. Thioredoxin-interacting protein links endoplasmic reticulum stress to inflammatory brain injury and apoptosis after subarachnoid haemorrhage. J Neuroinflammation. 2017;14(1):104. doi: 10.1186/s12974-017-0878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vella R.K., Pullen C., Coulson F.R., Fenning A.S. Resveratrol prevents cardiovascular complications in the SHR/STZ rat by reductions in oxidative stress and inflammation. Biomed Res Int. 2015;2015:918123. doi: 10.1155/2015/918123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lerman L.O., Kurtz T.W., Touyz R.M., et al. Animal models of hypertension: a scientific statement from the American heart association. Hypertension. 2019;73(6):e87–e120. doi: 10.1161/HYP.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doggrell S.A., Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res. 1998;39(1):89–105. doi: 10.1016/s0008-6363(98)00076-5. [DOI] [PubMed] [Google Scholar]
- 23.Chen C.A., Druhan L.J., Varadharaj S., Chen Y.R., Zweier J.L. Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. J Biol Chem. 2008;283(40):27038–27047. doi: 10.1074/jbc.M802269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang P.L. Endothelial nitric oxide synthase and endothelial dysfunction. Curr Hypertens Rep. 2003;5(6):473–480. doi: 10.1007/s11906-003-0055-4. [DOI] [PubMed] [Google Scholar]
- 25.Lobato N.S., Filgueira F.P., Akamine E.H., Tostes R.C., Carvalho M.H.C., Fortes Z.B. Mechanisms of endothelial dysfunction in obesity-associated hypertension. Braz J Med Biol Res. 2012;45(5):392–400. doi: 10.1590/S0100-879X2012007500058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vita J.A., Keaney JF Jr Endothelial function: a barometer for cardiovascular risk. Circulation. 2002;106(6):640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 27.Modena M.G., Bonetti L., Coppi F., Bursi F., Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40(3):505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 28.Laursen J.B., Rajagopalan S., Galis Z., Tarpey M., Freeman B.A., Harrison D.G. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95(3):588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 29.Yoshihara E., Masaki S., Matsuo Y., Chen Z., Tian H., Yodoi J. Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases. Front Immunol. 2014;4:514. doi: 10.3389/fimmu.2013.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao C., Wang R., Li B., et al. TXNIP/Redd1 signalling and excessive autophagy: a novel mechanism of myocardial ischaemia/reperfusion injury in mice. Cardiovasc Res. 2020;116(3):645–657. doi: 10.1093/cvr/cvz152. [DOI] [PubMed] [Google Scholar]
- 31.Parikh H., Carlsson E., Chutkow W.A., et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007;4(5):e158. doi: 10.1371/journal.pmed.0040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva-Adaya D., Gonsebatt M.E., Guevara J. Thioredoxin system regulation in the central nervous system: experimental models and clinical evidence. Oxid Med Cell Longev. 2014;2014:590808. doi: 10.1155/2014/590808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira N.E., Omae S., Pereira A., et al. Thioredoxin interacting protein genetic variation is associated with diabetes and hypertension in the Brazilian general population. Atherosclerosis. 2012;221(1):131–136. doi: 10.1016/j.atherosclerosis.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Saxena G., Chen J., Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J Biol Chem. 2010;285(6):3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo W., Wang Y., Yang H., et al. Heme oxygenase-1 ameliorates oxidative stress-induced endothelial senescence via regulating endothelial nitric oxide synthase activation and coupling. Aging (Albany NY) 2018;10(7):1722–1744. doi: 10.18632/aging.101506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kong X., Yang J.R., Guo L.Q., et al. Sesamin improves endothelial dysfunction in renovascular hypertensive rats fed with a high-fat, high-sucrose diet. Eur J Pharmacol. 2009;620(1-3):84–89. doi: 10.1016/j.ejphar.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe T., Barker T.A., Berk B.C. Angiotensin II and the endothelium: diverse signals and effects. Hypertension. 2005;45(2):163–169. doi: 10.1161/01.HYP.0000153321.13792.b9. [DOI] [PubMed] [Google Scholar]
- 38.Masi S., Uliana M., Virdis A. Angiotensin II and vascular damage in hypertension: role of oxidative stress and sympathetic activation. Vascul Pharmacol. 2019;115:13–17. doi: 10.1016/j.vph.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Tanito M., Nakamura H., Kwon Y.W., et al. Enhanced oxidative stress and impaired thioredoxin expression in spontaneously hypertensive rats. Antioxid Redox Signal. 2004;6(1):89–97. doi: 10.1089/152308604771978381. [DOI] [PubMed] [Google Scholar]
- 40.Fritz G., Grösch S., Tomicic M., Kaina B. APE/Ref-1 and the mammalian response to genotoxic stress. Toxicology. 2003;193(1-2):67–78. doi: 10.1016/s0300-483x(03)00290-7. [DOI] [PubMed] [Google Scholar]
- 41.Hirota K., Matsui M., Iwata S., Nishiyama A., Mori K., Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc Natl Acad Sci USA. 1997;94(8):3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mansego M.L., Blesa S., Gonzalez-Albert V., et al. Discordant response of glutathione and thioredoxin systems in human hypertension? Antioxid Redox Signal. 2007;9(4):507–514. doi: 10.1089/ars.2006.1472. [DOI] [PubMed] [Google Scholar]
- 43.Mansego M.L., De Marco Solar G., Alonso M.P., et al. Polymorphisms of antioxidant enzymes, blood pressure and risk of hypertension. J Hypertens. 2011;29(3):492–500. doi: 10.1097/HJH.0b013e328341f1b2. [DOI] [PubMed] [Google Scholar]
- 44.Ebrahimian T., Touyz R.M. Thioredoxin in vascular biology: role in hypertension. Antioxid Redox Signal. 2008;10(6):1127–1136. doi: 10.1089/ars.2007.1985. [DOI] [PubMed] [Google Scholar]
- 45.Hilgers R.H., Kundumani-Sridharan V., Subramani J., et al. Thioredoxin reverses age-related hypertension by chronically improving vascular redox and restoring eNOS function. Sci Transl Med. 2017;9(376):eaaf6094. doi: 10.1126/scitranslmed.aaf6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berk B.C. Novel approaches to treat oxidative stress and cardiovascular diseases. Trans Am Clin Climatol Assoc. 2007;118:209–214. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.