Abstract
Background
Current recommendations for people with irritable bowel syndrome (IBS) to partake in physical activity are based on low‐level evidence, do not incorporate evidence from all available randomised controlled trials (RCTs) and provide little information regarding potential adverse effects.
Objectives
To assess the benefits and harms of physical activity interventions in adults diagnosed with irritable bowel syndrome and to explore possible effect moderators including type, setting and nature of physical activity interventions.
Search methods
We searched nine electronic databases including CENTRAL, MEDLINE and Embase to 5 November 2021. We handsearched reference lists and sought unpublished studies through trial registries.
Selection criteria
We included RCTs involving adults (aged 18 years or older) diagnosed with IBS and conducted in any setting comparing a physical activity intervention with no intervention, usual care or wait‐list control group or another physical activity intervention group and assessing a validated measure of symptoms, quality of life or bowel movement.
Data collection and analysis
At least two review authors independently selected studies for inclusion, extracted study data, and performed risk of bias and GRADE assessments to assess the certainty of evidence. We pooled studies that evaluated similar outcomes using a random‐effects meta‐analysis, and synthesised data from other studies narratively.
Main results
We included 11 RCTs with data for 622 participants. Most (10/11) were set in high‐ or middle‐ to high‐income countries, with five involving supervised physical activity, three unsupervised activity and three a mix of supervised and unsupervised activity. No trial was at low risk of bias. Four trials specified a minimally important difference for at least one assessed outcome measure. Data for 10 trials were obtained from published journal articles, with data for one obtained from an unpublished Masters degree thesis.
Irritable bowel syndrome symptoms
Six RCTs assessed the effectiveness of a physical activity intervention compared with usual care on global symptoms of IBS. Meta‐analysis of five studies showed an observed improvement in reported symptoms following physical activity (standardised mean difference (SMD) –0.93, 95% confidence interval (CI) –1.44 to –0.42; 185 participants). We rated the certainty of evidence for this outcome as very low due to unclear and high risk of bias, inconsistency and imprecision from sparse data. This means physical activity may improve IBS symptoms but the evidence is very uncertain. The results of the remaining study supported the meta‐analysis but were at unclear risk of bias and sample size was small.
Two studies assessed the effectiveness of a yoga intervention compared with a walking intervention on global IBS symptoms. Meta‐analysis of these two studies found no conclusive evidence of an effect of yoga compared with walking on IBS symptoms (SMD –1.16, 95% CI –3.93 to 1.62; 124 participants). We rated the certainty of evidence as very low, meaning the evidence is very uncertain about the effect of yoga interventions compared with walking interventions on IBS symptoms.
Two studies assessed the effectiveness of a physical activity intervention (yoga) compared with medication. One reported no observed difference in global IBS symptoms, though CIs were wide, suggesting uncertainty in the observed estimates and risk of bias was high (MD –1.20, 95% CI –2.65 to 0.25; 21 participants). We excluded IBS symptom data for the remaining study as it used a non‐validated method.
One study compared a yoga intervention with a dietary intervention and reported an observed improvement in symptoms with both interventions but neither intervention was superior to the other.
Quality of life
Five RCTs assessed the impact of physical activity on self‐reported quality of life compared with usual care. Meta‐analysis of data from four studies found no improvement in quality of life following a physical activity intervention (SMD 1.17, 95% CI –0.30 to 2.64; 134 participants; very low certainty due to risk of bias, inconsistency and imprecision). We rated the certainty of evidence as very low, meaning the evidence is very uncertain about the effect of physical activity interventions on quality‐of‐life outcomes in people with IBS.
One study assessed the impact on quality of life of a yoga intervention compared with walking and observed an improvement in the yoga group (MD 53.45, 95% CI 38.85 to 68.05; 97 participants ).
One study reported no observed difference in quality of life between a yoga and a dietary intervention.
Abdominal pain
Two trials assessed the impact of physical activity compared with usual care on reported abdominal pain. Meta‐analysis found no improvement in abdominal pain with physical activity compared with usual care (SMD 0.01, 95% CI –0.48 to 0.50; 64 participants). We rated the certainty of the evidence as very low due to risk of bias and imprecision, meaning the evidence is very uncertain about the effect of physical activity interventions on abdominal pain in people with IBS.
One study assessing the impact of a yoga intervention compared with walking advice reported no observed differences between groups on abdominal pain.
One study comparing a yoga intervention with a dietary intervention found neither intervention had a more beneficial impact than the other and both interventions did not conclusively reduce abdominal pain.
There was insufficient evidence to adequately assess adverse effects associated with physical activity due to a lack of reporting in trials. One study reported a musculoskeletal injury in a yoga intervention group but this did not result in withdrawal from the study.
Authors' conclusions
Findings from a small body of evidence suggest that physical activity comprising of yoga, treadmill exercise or support to increase physical activity may improve symptoms but not quality of life or abdominal pain in people diagnosed with IBS but we have little confidence in these conclusions due to the very low certainty of evidence.
The numbers of reported adverse events were low and the certainty of these findings was very low for all comparisons, so no conclusions can be drawn.
Discussions with patients considering physical activity as part of symptom management should address the uncertainty in the evidence to ensure fully informed decisions. If deemed sufficiently important to patients and healthcare providers, higher quality research is needed to enable more certain conclusions.
Keywords: Adult, Humans, Abdominal Pain, Exercise, Irritable Bowel Syndrome, Irritable Bowel Syndrome/therapy, Quality of Life, Yoga
Plain language summary
What are the benefits and harms of physical activity for people with irritable bowel syndrome
Key messages
– Physical activity interventions of between six and 24 weeks may improve symptoms in people with irritable bowel syndrome but the evidence is very uncertain.
– There is probably little or no difference between physical activity interventions and usual care for quality of life and abdominal pain.
– There was not enough evidence to assess adverse effects associated with physical activity interventions due to a lack of reporting in trials.
What are physical activity interventions?
Physical activity is defined as any bodily movement produced by your muscles that results in energy expenditure. Examples of physical activity include activity performed as part of daily life (housework, shopping), sport and recreational activity, and activity performed as part of work (e.g. travelling to work, manual labour).
Exercise is a subset of physical activity that is planned, structured and repetitive, and has the aim of improving or maintaining overall fitness. Stretching and activities to improve balance are also considered forms of physical activity and exercise.
The UK Department of Health and Social Care currently recommends that adults participate in a minimum of 30 minutes of daily physical activity at least five days a week.
There is strong evidence that physical activity and exercise interventions are effective in helping people prevent and manage long‐term health conditions including coronary heart disease (narrowing of the blood vessels supplying the heart), diabetes and depression. Whether physical activity helps people diagnosed with irritable bowel syndrome manage their symptoms is not clear.
What is irritable bowel syndrome
Irritable bowel syndrome is a common bowel disorder characterised by symptoms that include episodes of abdominal pain, bloating and changes in bowel habit. About 10% to 20% of adults in Western countries are diagnosed with irritable bowel syndrome. The management of irritable bowel syndrome follows no clear pathway and involves managing individual symptoms including laxatives for constipation, medicines to prevent gut spasms for pain, medicines to slow gut activity for diarrhoea, dietary changes, fluid intake, psychological management, antidepressants for low mood and physical activity.
What did we want to find out?
We wanted to find out whether physical activity intervention improves symptoms, quality of life and abdominal pain in adults diagnosed with irritable bowel syndrome. We searched for all available randomised controlled trials to help answer this question. A randomised controlled trial is a type of study in which participants are assigned randomly to one of two or more treatment groups. This is the best way to ensure that a fair comparison is made between new and existing treatments.
What did we do?
We searched nine electronic databases and trial registries for all randomised controlled trials involving adults (18 years or older) diagnosed with irritable bowel syndrome that compared a physical activity intervention with no physical activity intervention in adults diagnosed with irritable bowel syndrome. We compared and summarised the results of these trials and rated our confidence in the overall evidence, based on factors such as study methods and the amount of information they provided.
What did we find?
We found 11 randomised controlled trials involving 622 people with irritable bowel syndrome. The biggest trial was in 102 people and the smallest was in 20 people. Six trials were conducted in high‐income countries worldwide and two were conducted in a low‐ to middle‐income country. One study included people with irritable bowel syndrome where constipation was the main stool pattern, two included people where diarrhoea was the main stool pattern and five included people with a mixed stool pattern.
Five trials assessed a yoga physical activity intervention, three assessed advice to increase physical activity levels, two assessed treadmill exercise, and one assessed a Qigong (breathing and slow movements) intervention. Seven trials involved a 12‐week intervention period, two involved an eight‐week period and one a six‐week period. The longest trial lasted six months.
Main results
Physical activity interventions may improve IBS symptoms compared to usual care but the evidence is very uncertain. The average improvement in symptom score was approximately 69 points but could be as high as 106 and as low as 31 points. A change in symptoms score of 50 points would be considered meaningful for most people. Our findings suggest physical activity interventions may provide both important and non‐important improvements in IBS symptoms.
Physical activity interventions result in little or no difference in quality of life and abdominal pain.
We could not draw any conclusions about unwanted effects reported by participants because very few trials reported these.
What are the limitations of the evidence?
We have very little confidence in the evidence. Our confidence was lowered mainly because of concerns about how the trials were conducted, which included that many trials did not report all their results or reported new ones.
How up to date is this review?
The evidence is up‐to‐date to 5 November 2021.
Summary of findings
Summary of findings 1. Physical activity compared to usual care for treatment of irritable bowel syndrome.
| Physical activity compared to usual care for treatment of irritable bowel syndrome | |||||
| Patient or population: people with IBS Setting: outpatient Intervention: physical activity Comparison: usual care | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with usual care | Risk difference with physical activity | ||||
|
IBS symptoms
assessed with: self‐report questionnaire (various) Follow‐up: 6–24 weeks |
185 (5 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | — | The mean IBS symptoms was 0 SD | SMD 0.93 SD lower (1.44 lower to 0.42 lower) |
|
Quality of life
assessed with: self‐report questionnaire (various) Follow‐up: 6–12 weeks |
134 (4 RCTs) | ⊕⊝⊝⊝ Very lowd,e,f | — | The mean quality of life was 0 SD | SMD 1.17 SD higher (0.30 lower to 2.64 higher) |
|
Abdominal pain
assessed with: self‐report questionnaire (various) Follow‐up: median 9 weeks |
64 (2 RCTs) | ⊕⊝⊝⊝ Very lowf,g | — | The mean abdominal pain was 0 SD | SMD 0.01 SD higher (0.48 lower to 0.5 higher) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IBS: irritable bowel syndrome; RCT: randomised controlled trial; SD: standard deviation; SMD: standardised mean difference. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to high risk of bias for blinding of assessors in two studies, incomplete outcome data in three studies and selective outcome reporting in two studies. bDowngraded one level due to evidence of inconsistency supported by the presence of substantial heterogeneity (I² = 50% to 90%) and point estimates that differed widely (Chi² P = 0.04). cDowngraded one level due to evidence of imprecision supported by presence of small sample size (fewer than 400, optimal information size not met) and wide 95% confidence interval that included clinically important and non‐important effects. dDowngraded one level due to high risk of bias for allocation concealment in one study, blinding of assessors in two studies, incomplete outcome data in two studies and selective outcome reporting in one study. eDowngraded one level due to evidence of inconsistency supported by the presence of considerable heterogeneity (I² = 75% to 100%) and point estimates that differed widely (Chi² P < 0.00001). fDowngraded two levels due to evidence of imprecision supported by presence of small sample size (fewer than 400, optimal information size not met) and wide 95% confidence interval that included clinical improvement and worsening of quality of life. gDowngraded one level due to high risk of bias for blinding of assessors in one study, incomplete outcome data in two studies and selective outcome reporting in one study.
Summary of findings 2. Yoga compared to walking for treatment of irritable bowel syndrome.
| Yoga compared to walking for treatment of irritable bowel syndrome | |||||
| Patient or population: people with IBS Setting: outpatient Intervention: yoga Comparison: walking | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with walking | Risk difference with yoga | ||||
|
IBS symptoms
assessed with: self‐report questionnaire (various) Follow‐up: 8–12 weeks |
124 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | — | The mean IBS symptoms was 0 SD | SMD 1.16 SD lower (3.93 lower to 1.62 higher) |
|
Quality of life
assessed with: self‐report questionnaire (IBS‐QOL) Follow‐up: median 12 weeks |
97 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d | — | The mean quality of life was 0 SD | MD 53.45 SD higher (38.85 higher to 68.05 higher) |
|
Abdominal pain
assessed with: self‐report questionnaire (NRS) Follow‐up: median 8 weeks |
27 (1 RCT) | ⊕⊝⊝⊝ Very lowe,f | — | The mean abdominal pain was 0 SD | MD 2.3 SD higher (0.79 lower to 5.39 higher) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IBS: irritable bowel syndrome; IBS‐QOL: Irritable Bowel Syndrome Quality Of Life; MD: mean difference; NRS: Numeric Rating Scale; RCT: randomised controlled trial; SD: standard deviation; SMD: standardised mean difference. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded two levels due to evidence of inconsistency supported by the presence of substantial heterogeneity (I² = 75% to 100%) and point estimates and 95% confidence intervals that widely differed (Chi² P < 0.00001). bDowngraded two levels due to evidence of imprecision supported by the presence of small sample size (fewer than 400, optimal information size not met) and wide 95% confidence intervals that included clinically important improvement and worsening of symptoms. cDowngraded one level due to high risk of bias from a lack of blinding of participants/personnel and unclear risk of bias for insufficient outcome data and selective outcome reporting. dDowngraded two levels due to evidence of imprecision supported by the presence of small sample size (fewer than 400, optimal information size not met). eDowngraded one level due to high risk of bias from a lack of blinding of participants/personnel and insufficient outcome data and unclear risk of bias for random sequence generation, allocation concealment and selective outcome reporting. fDowngraded two levels due to evidence of imprecision supported by the presence of small sample size (fewer than 400, optimal information size not met) and wide 95% confidence intervals that included important improvement and worsening of abdominal pain.
Summary of findings 3. Similar physical activity interventions compared to any control for treatment of irritable bowel syndrome.
| Similar physical activity interventions compared to any control for treatment of irritable bowel syndrome | |||||
| Patient or population: people with IBS Setting: outpatient Intervention: similar physical activity interventions Comparison: any control | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with any control | Risk difference with similar physical activity interventions | ||||
|
IBS symptoms – yoga interventions
assessed with: self‐report questionnaire (various) Follow‐up: 6–12 weeks |
218 (5 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | — | The mean IBS symptoms – yoga interventions was 0 SD | SMD 0.75 SD lower (2.01 lower to 0.51 higher) |
|
IBS symptoms – supervised treadmill exercise
assessed with: self‐report questionnaire (IBS‐SSS) Follow‐up: 6–24 weeks |
71 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d | — | The mean IBS symptoms – supervised treadmill exercise was 0 SD | SMD 1.24 SD lower (2.64 lower to 0.15 higher) |
|
IBS symptoms – advice to increase physical activity
assessed with: self‐report questionnaire (various) Follow‐up: median 12 weeks |
93 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,e | — | The mean IBS symptoms – advice to increase activity was 0 SD | SMD 0.72 SD lower (1.61 lower to 0.17 higher) |
|
Quality of life – yoga interventions
assessed with: self‐report questionnaire (various) Follow‐up: 6–12 weeks |
177 (3 RCTs) | ⊕⊝⊝⊝ Very lowf,g,h | — | The mean quality of life – yoga interventions was 0 SD | SMD 0.60 SD higher (0.59 lower to 1.79 higher) |
|
Quality of life – supervised treadmill exercise
assessed with: self‐report questionnaire (IBS‐QOL) Follow‐up: median 6 weeks |
20 (1 RCT) | ⊕⊝⊝⊝ Very lowi,j | — | The mean quality of life – supervised treadmill exercise was 0 SD | SMD 2.39 SD higher (1.18 higher to 3.59 higher) |
|
Quality of life – advice to increase physical activity
assessed with: self‐report questionnaire (IBS‐QOL) Follow‐up: median 12 weeks |
93 (2 RCTs) | ⊕⊝⊝⊝ Very lowe,g,h | — | The mean quality of life – advice to increase physical activity was 0 SD | SMD 1.04 SD higher (1.65 lower to 3.74 higher) |
|
Abdominal pain
assessed with: self‐report questionnaire (NRS) Follow‐up: median 7 weeks |
48 (2 RCTs) | ⊕⊝⊝⊝ Very lowk,l,m | — | The mean abdominal pain was 0 SD | SMD 0.13 SD higher (0.45 lower to 0.72 higher) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IBS: irritable bowel syndrome; IBS‐SSS: Irritable Bowel Syndrome Severity Scoring System; IBS‐QOL: Irritable Bowel Syndrome Quality Of Life; NRS: numerical rating scale; RCT: randomised controlled trial; SD: standard deviation; SMD: standardised mean difference. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to high risk of bias of blinding for participants/personnel in all five studies and low risk of bias for incomplete outcome data and selective outcome reporting in no more than two studies. bDowngraded two levels due to evidence of inconsistency supported by the presence of considerable heterogeneity (I² = 75% to 100%) and point estimates and 95% confidence intervals that widely differed (Chi² P < 0.00001). cDowngraded two levels due to evidence of imprecision supported by the presence of small sample size (fewer than 400, optimal information size not met) and wide 95% confidence intervals that included clinically important improvement and worsening of symptoms. dDowngraded one level due to high risk of bias for blinding of participants/personnel in both studies, blinding of assessors in one study, and incomplete outcome data and selective reporting in the other study. eDowngraded one level due to high risk of bias for allocation concealment in one study, blinding of participants/personnel in both studies, and unclear or high risk of bias for blinding of assessors, incomplete outcome data and selective reporting in both studies. fDowngraded one level due to high risk of bias of blinding for participants/personnel in all three studies and unclear or high risk of bias for incomplete outcome data and selective outcome reporting in at least two studies. gDowngraded two levels due to evidence of inconsistency supported by the presence of considerable heterogeneity (I² = 75% to 100%) and point estimates and 95% confidence intervals that widely differed (Chi² P < 0.0001). hDowngraded two levels due to evidence of imprecision supported by the presence of small sample size (fewer than 400, optimal information size not met) and wide 95% confidence intervals that included clinically important improvement and worsening of quality of life. iDowngraded one level due to high risk of bias for blinding of participants/personnel and blinding of assessors, and for unclear risk of bias for random sequence generation, allocation concealment and selective outcome reporting. jDowngraded two levels due to evidence of imprecision due to small sample size (fewer than 400, optimal information size not met) and wide confidence intervals. kDowngraded one level due to high risk of bias for blinding of participants/personnel and incomplete outcome data in both studies and selective outcome reporting in one study. lDowngraded one level due to evidence of inconsistency supported by the presence of substantial heterogeneity (I² = 50% to 90%) and point estimates and 95% confidence intervals that favoured both intervention and control. mDowngraded one level due to evidence of imprecision supported by the presence of small sample size (fewer than 400, optimal information size not met) and wide 95% confidence intervals that included improvement and worsening of abdominal pain.
Summary of findings 4. Physical activity compared to pharmacological therapy for treatment of irritable bowel syndrome.
| Physical activity compared to pharmacological therapy for treatment of irritable bowel syndrome | |||||
| Patient or population: people with IBS Setting: outpatient Intervention: physical activity Comparison: pharmacological therapy | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with pharmacological therapy | Risk difference with physical activity | ||||
|
IBS symptoms
assessed with: self‐report questionnaire (autonomic score) Follow‐up: median 8 weeks |
21 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — | The mean IBS symptoms was 0 SD | SMD 1.2 SD lower (2.65 lower to 0.25 higher) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IBS: irritable bowel syndrome; RCT: randomised controlled trial; SD: standard deviation; SMD: standardised mean difference. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to high risk of bias for blinding of participants/personnel and unclear risk of bias for random sequence generation, allocation concealment, blinding of assessors and selective outcome reporting. bDowngraded two levels due to evidence of imprecision supported by the presence of small sample size (fewer than 400, optimal information size not met) and wide 95% confidence intervals that included clinically important improvement and worsening of symptoms.
Summary of findings 5. Physical activity compared to dietary interventions for treatment of irritable bowel syndrome.
| Physical activity compared to dietary interventions for treatment of irritable bowel syndrome | |||||
| Patient or population: people with IBS Setting: outpatient Intervention: physical activity Comparison: dietary interventions | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with dietary interventions | Risk difference with physical activity | ||||
| IBS symptoms – 12 weeks assessed with: self‐report questionnaire (IBS‐SSS) | 59 (1 RCT) | ⊕⊕⊝⊝ Lowa | — | The mean IBS symptoms – 12 weeks was 0 | MD 33.31 higher (12.86 lower to 79.48 higher) |
| Quality of life – 12 weeks assessed with: self‐report questionnaire (IBS‐QOL) | 59 (1 RCT) | ⊕⊕⊝⊝ Lowb | — | The mean quality of life – 12 weeks was 0 | MD 1.67 lower (7.62 lower to 4.28 higher) |
| Quality of life – 24 weeks assessed with: self‐report questionnaire (IBS‐QOL) | 59 (1 RCT) | ⊕⊕⊝⊝ Lowb | — | The mean quality of life – 24 weeks was 0 | MD 0.2 higher (4.9 lower to 5.3 higher) |
| Abdominal pain – 12 weeks assessed with: self‐report questionnaire (IBS‐SSS pain subscale) | 59 (1 RCT) | ⊕⊕⊝⊝ Lowc | — | The mean abdominal pain – 12 weeks was 0 | MD 12 higher (4.96 lower to 28.29 higher) |
| Abdominal pain – 24 weeks assessed with: self‐report questionnaire (IBS‐SSS pain subscale) | 59 (1 RCT) | ⊕⊕⊝⊝ Lowc | — | The mean abdominal pain – 24 weeks was 0 | MD 7.89 higher (8.19 lower to 23.97 higher) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IBS: irritable bowel syndrome; IBS‐QOL: Irritable Bowel Syndrome Quality Of Life; IBS‐SSS: Irritable Bowel Syndrome Severity Scoring System; MD: mean difference; RCT: randomised controlled trial; SD: standard deviation. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded two levels due to evidence of imprecision supported by the presence of small sample size (fewer than 400, optimal information size not met) and wide 95% confidence intervals that included clinically important improvement and worsening of symptoms. bDowngraded two levels due to evidence of imprecision supported by the presence of small sample size (fewer than 400, optimal information size not met) and wide 95% confidence intervals that included improvement and worsening of quality of life. cDowngraded two levels due to evidence of imprecision supported by the presence of small sample size (fewer than 400, optimal information size not met) and wide 95% confidence intervals that included improvement and worsening of abdominal pain.
Background
Description of the condition
Irritable bowel syndrome (IBS) is a common chronic functional bowel disorder characterised by symptoms that include episodes of abdominal pain, bloating and changes in bowel habit. Estimates in the general population suggest that IBS has a prevalence of approximately 10% to 20% in Western countries with increasing prevalence in the low‐income countries (Ford 2012; NICE 2008; Spiller 2007a). Women are twice as likely to have IBS as men and this disorder most commonly affects people under the age of 35 years, although older people can also experience IBS.
The diagnosis of IBS is largely one of exclusion and is suspected in the presence of specific symptoms. These symptoms include the presence of any one, or combination of symptoms including abdominal pain or discomfort, bloating, or a change in bowel habit lasting for a period of six months or more in the absence of any suggestion of serious underlying pathology. More formalised diagnostic criteria are used for clinical trials and include the Rome IV and Manning criteria (Drossman 2016; Longstreth 2006; Talley 1990). Currently there is no known diagnostic test to confirm the presence of IBS.
IBS can be categorised as either constipation‐predominant IBS (IBS‐C) or diarrhoea‐predominant IBS (IBS‐D). However patients often describe mixed episodes of diarrhoea or constipation (IBS‐M). In addition to pain, altered bowel habit and bloating, IBS is often associated with systemic symptoms such as lethargy and tiredness, muscle aches, headaches, anxiety or low mood (Spiller 2007a). However, the presence of these symptoms often vary across patients. The management of IBS follows no clear pathway for each patient and involves managing individual symptoms including laxatives for constipation, antispasmodics for pain, antimotility drugs for diarrhoea, dietary changes, fluid intake, psychological management, antidepressants for mood and physical activity (NICE 2008). A growing evidence base of the possible role of the gut microbiome in some people with IBS. Of the specific interventions targeting a potential microbiome‐based mechanism (e.g. probiotics, fecal transplants), the American College of Gastroenterology (ACG) recommends against them due to lack of supportive evidence (ACG 2021). The ACG also state "Future research is needed to better understand the role of the gut microbiome in patients with IBS and to understand the genesis of visceral pain."
The high prevalence and morbidity associated with IBS has a significant impact on healthcare costs (Canavan 2014; Leong 2003). In addition, IBS is estimated to result in between 8.5 and 21.6 days off work per year (Maxion‐Bergemann 2006), and has a significant impact on quality of life with patients, on average, willing to sacrifice between 10 and 15 years of their remaining life expectancy for a permanent and immediate cure (Canavan 2014). Therefore, management options that show a clear benefit can be of great use to people with IBS as well as clinicians and policy makers. Likewise, where good evidence points to no overall benefit, the use of that management option can be limited to reduce patient expectations as well as overall healthcare costs. To that end, despite physical activity being advocated for people with IBS in several guidelines worldwide, the evidence underpinning its potential benefit is based on non‐systematic assessments of low‐level primary studies (Moayyedi 2017; NICE 2008; Quigley 2015; Song 2018; Spiller 2007b).
Description of the intervention
Physical activity is defined as any bodily movement produced by skeletal muscles that results in energy expenditure. Examples include sports, housework or occupational activity. Exercise is a subset of physical activity that is planned, structured and repetitive, and has the objective of improving or maintaining physical fitness (Caspersen 1985). There is strong evidence that physical activity interventions are effective in the prevention and management major conditions (Nunan 2013), including coronary heart disease (Murphy 2003), diabetes (Thomas 2006), and depression (Cooney 2013). The UK Department of Health currently recommends that adults partake a minimum of 30 minutes of moderate intensity physical activity at least five days a week (CMO 2019).
People with IBS appear to have a greater tendency to use alternative medicines and therapies compared to people with organic gastrointestinal (GI) diseases (Camilleri 2001). This may be due to disenchantment with current treatments (Wilson 2004). Therefore, physical activity may be a valuable alternative or adjunct to current treatments.
How the intervention might work
In healthy people, moderate physical activity reduces intestinal gas retention, improves gas transit time and reduces abdominal distension (Dainese 2004). Furthermore, physical activity may improve symptoms in people with other GI conditions such as peptic ulcers, cholelithiasis, and diverticular disease (Peters 2001), and is associated with reduced incidence of inflammatory bowel disease (Chan 2014; Wang 2016). Physical activity reduces colonic transit times, incomplete defecations and hard stools in people with constipation (De Schryver 2005), a common symptom in people with IBS.
However, high performance athletes often complain of adverse GI symptoms such as bloating, belching, abdominal cramps and diarrhoea after strenuous exercise (de Oliveira 2009). Therefore, people with IBS who exercise may develop adverse effects or find their symptoms are exacerbated.
Physical activity may alter GI function through decreased splanchnic blood flow, increased GI motility, enhanced immune function, or mechanical movement and compression of the gut (Peters 2001). As many of these mechanisms alter physiological systems that are thought to be dysfunctional in IBS, physical activity may have a significant effect.
Why it is important to do this review
Several international guidelines advise people with IBS to partake in physical activity (Moayyedi 2017; NICE 2008; Quigley 2015; Song 2018). UK guidelines (NICE 2008) defer to the UK Department of Health recommendations for physical activity (CMO 2019). However, they also state that there is low‐level evidence to support this recommendation, and since publication of these guidelines, new randomised controlled trials (RCTs) have been performed (Daley 2008; Evans 2014; Johannesson 2011). Therefore the objective of this review is to assess the efficacy and safety of physical activity for IBS patients based on data from RCTs, thus providing a potentially higher level of evidence to support future recommendations.
Objectives
To assess the benefits and harms of physical activity interventions in adults diagnosed with irritable bowel syndrome and to explore possible effect moderators including type, setting and nature of physical activity interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs that compared a physical activity intervention with a control group. We excluded studies employing quasi‐ or non‐randomised designs.
Types of participants
Adults (aged 18 years or over) with a diagnosis of IBS based on diagnostic criteria such as Manning, Rome I, Rome II, Rome III, Rome IV or clinical symptoms consistent with IBS were eligible for inclusion, with no restrictions based on gender, race, educational status or duration of IBS. Participants needed to be able to participate in physical activity to be eligible for inclusion.
Types of interventions
We considered studies including the following comparisons:
any type of physical activity, exercise or advice to increase physical activity or exercise plus standard medical care (usual care) versus usual care alone;
any type of physical activity, exercise or advice to increase physical activity or exercise versus normal physical activity or no exercise;
one type of physical activity, exercise or advice to increase physical activity or exercise versus another type of physical activity or exercise.
All forms of physical activity were included, regardless of whether these activities were structured or unstructured and whether occupational or recreational.
Types of outcome measures
We planned to collect data on the following outcomes.
Primary outcomes
Global improvement of symptoms (participant‐reported or clinician‐evaluated or both) as measured by a global IBS symptoms score (e.g. Irritable Bowel Syndrome Severity Scoring System (IBS‐SSS), Adequate Relief Measure, GI Symptom Rating Scale, Functional Bowel Disorder Severity Index or IBS Symptom Questionnaire) or as defined by the included studies.
Although a wide range of instruments are available to measure health‐related outcomes in IBS many of these scales vary in quality and some have not been validated. It has been shown that the use of rating scales that have not been published in a peer‐reviewed journal may be associated with bias (Marshall 2000). Therefore, we excluded studies that reported IBS symptoms using non‐validated scales. Where a study reported IBS symptoms using a non‐validated scale but also reported other outcomes of interest, we did not report on the IBS symptom outcomes.
Secondary outcomes
Quality of life as measured by a validated quality‐of‐life measure e.g. EuroQol 5D (EQ‐5D), 36‐item Short Form (SF‐36).
Improvement in abdominal pain, discomfort or distention.
Stool consistency and frequency.
Bowel transit time.
Adverse events.
Withdrawal due to adverse events related to physical activity such as injury or fatigue, or withdrawal due to other adverse effects.
We also assessed possible moderating effects on the primary and secondary outcomes above, presented in the Subgroup analysis and investigation of heterogeneity section.
Search methods for identification of studies
Electronic searches
We conducted electronic searches of the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, Wiley (Issue 11, 2021).
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) (OvidSP) (1946 to 5 November 2021).
Embase (OvidSP) (1974 to 17 November 2021).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost) (1982 to 5 November 2021).
Physiotherapy Evidence Database (PEDro) database (Physiotherapy Evidence Database via www.pedro.org.au/).
Science Citation Index Expanded (SCI‐EXPANDED) & Conference Proceedings Citation Index‐Science (CPCI‐S) on Thomson Reuters Web of Science (1945 to 5 November 2021).
SPORTDiscus database (EBSCOhost) (inception to 5 November 2021).
We applied the Cochrane highly sensitive search strategy for identifying RCTs in MEDLINE: sensitivity‐maximising version (2008 revision); Ovid format and adaptations of it to the other databases (e.g. Embase (2018 revision; Ovid format)) with the exception of CENTRAL (Lefebvre 2021).
We also searched trial registries for potentially relevant studies that were completed or in progress (November 2021), using ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/).
We applied no language or publication date restrictions to the searches.
We screened the reference lists of included studies to identify potential studies. We also conducted forward citation searches on the Web of Science for papers that cited included studies. Furthermore, we screened the reference lists of the systematic reviews found through a search of the Cochrane Database of Systematic Reviews to identify further potentially relevant studies.
A detailed description of our search strategy is provided in Appendix 1.
Searching other resources
We contacted authors of included studies for missing data and other unpublished or ongoing studies. We contacted the first author of any abstracts or conference proceedings for missing data and information regarding full publication. A related articles search for included studies was also conducted in PubMed.
We used Endnote (X9) bibliographic software and the Covidence online tool to manage the results generated from the above searches including deduplication.
Data collection and analysis
Selection of studies
At least two review authors (DN, JMOM, ETT, AG) independently assessed titles and abstracts for inclusion, resolving any disagreements through discussion and arbitration by a third review author (DN or KM) where necessary. We obtained full‐text articles of potentially eligible trials identified in the first screening phase, and assessed them for inclusion against our predefined criteria using the same procedures as for titles and abstracts.
Data extraction and management
We developed and piloted a data extraction sheet to ensure it enabled reliable and accurate extraction of appropriate data and then at least two review authors (DN, JMOM, ETT, AG) independently extracted data on study and participant characteristics along with outcome data. For two articles written in Chinese language, one author proficient in Chinese (TC) translated and extracted data, which was then verified by a second review author (DN). Any inconsistencies in data extraction were resolved via consensus. When a study reported relevant data from more than one intervention arm, we extracted data separately for each arm of the study. Where data were missing or unclear, we contacted study authors. Two review authors (DN, ETT) entered the data into Review Manager Web (Review Manager Web 2021).
Assessment of risk of bias in included studies
At least two review authors independently assessed the risk of bias for each study using the original Cochrane RoB assessment tool (RoB 1) (Higgins 2021a).
Was allocation sequence randomly generated using an appropriate method (selection bias)?
Was allocation adequately concealed (selection bias)?
Were participants and personnel blinded to knowledge of allocated interventions adequately (performance bias)?
Were outcome assessors blinded to allocated intervention (detection bias)?
Were incomplete outcome data adequately addressed (attrition bias)?
Were reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at risk of bias?
We considered trials to be at an overall low risk of bias when they met all of the above criteria or when there was no more than one unclear criterion (with no criterion assessed as high risk). We judged studies to be at unclear risk of bias if more than one criterion was assessed as unclear risk of bias (with no more than one criterion assessed as high risk) and at high risk of bias if two or more criteria were assessed as high risk.
Measures of treatment effect
We used Review Manager Web to analyse the data (Review Manager Web 2021). Analyses were performed using the intention‐to‐treat (ITT) principle where possible. For continuous outcomes, we calculate the mean difference (MD) and corresponding 95% confidence interval (CI) when studies used the same measurement scale. If studies used different scales, we calculated the standardised mean difference (SMD) with 95% CIs. For dichotomous outcomes, we calculated risk ratios (RR) with 95% CIs.
Unit of analysis issues
Where trials include a control arm, a physical activity or exercise arm and a ‘established treatment’ arm (e.g. antispasmodic, laxative), we extracted data on physical activity or exercise versus established treatment and control. For three‐arm trials (e.g. established treatment compared with physical activity or exercise and established treatments plus physical activity or exercise), we made two comparisons: 1. established treatment plus physical activity or exercise versus established treatment alone, and include this analysis in the meta‐analysis of treatment versus control; and 2. physical activity or exercise versus established treatment. In the case of multiple observations (e.g. multiple time points), each observation was analysed separately against the control. The unit of analysis was the person. For cross‐over trials, we extracted and analysed data for the first period only due to the potential 'carry‐over' effect of physical activity or exercise. For eligible cluster‐randomised trials, we adjusted the data to account for clustering if the study authors had not already done so.
Dealing with missing data
Where possible we included all data in the review using an ITT as well as available case (per‐protocol) analysis for comparison. We contacted corresponding authors where missing data were present. Where studies reported dropouts or withdrawals, we extracted data on the number and reasons for missing data. We considered those who dropped out as treatment failures. When we could not obtain information either from the publication or from authors, we classified the trial as 'not ITT', and used the data from the available cases in the meta‐analysis. When studies failed to report necessary information to calculate the MD or SMD (e.g. standard deviations (SD)), we used the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions to derive the missing data (Higgins 2021b). We considered the potential impact of missing data by performing sensitivity analyses where possible.
Assessment of heterogeneity
We assessed statistical heterogeneity using the Chi² test and the I² statistic. We considered significant heterogeneity when the P value for Chi² was less than 0.010. The ranges for the I² statistic were:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; and
75% to 100%: considerable heterogeneity.
The importance of the observed value for the I² statistic depends on the magnitude of direction of effects and the strength of evidence for heterogeneity (e.g. P value from the Chi² test). Where there was significant heterogeneity for the primary outcome, we explored clinical and methodological reasons for heterogeneity, and where possible, we performed additional analyses as described in the Sensitivity analysis section.
Assessment of reporting biases
We compared protocols and preregistrations, where available, to included reports to assess potential reporting bias. Where protocols were not available, we compared the outcomes listed in the methods section to those reported in the results section. We contacted authors for further information to clarify missing data. We planned to use funnel plots to identify small‐study effects, which in turn, could indicate publication bias.
Data synthesis
We used a random‐effects model for meta‐analyses since we anticipated heterogeneity between included studies due to variations in the composition of physical activity interventions in terms of mode, intensity and duration, and outcome assessment methodology. We combined data from individual trials for meta‐analysis when the participant groups and outcomes were sufficiently similar. We calculated the pooled RR and 95% CI for dichotomous outcomes and the pooled MD and corresponding 95% CI for continuous outcomes. For pooled analyses of the MD, we combined both endpoint data and change data in the analysis in accordance with Deeks 2021. Where continuous outcomes were deemed sufficiently similar but used different scales, we used the SMD to combine data. When studies reported standard errors (SE) instead of SD, we converted the former to SD. Where both SE and SD were missing, we estimated SD from P values or use the mean of the other studies (Furukawa 2006).
In our protocol, we planned not to pool data for if there was a high degree of statistical heterogeneity (I² = 75% or greater) (Nunan 2015). On review, we decided not to use this cut‐off and instead explored clinical and methodological reasons that may have explained the high degree of heterogeneity and whether these justified a decision to pool or not. Where relevant, we also performed sensitivity analyses to identify the impact of clinical and methodological heterogeneity on statistical heterogeneity and the overall pooled estimates (see Differences between protocol and review).
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses to assess the impact of different types and severity of IBS (e.g. IBS‐C or IBS‐D; severity of symptoms at baseline), different types of physical activity interventions (e.g. aerobic or resistance and other types of exercise or advice to exercise) and different comparators (e.g. no treatment, usual care, placebo or other active treatment) if there were sufficient data.
Sensitivity analysis
We planned sensitivity analyses for studies that utilised a per‐protocol (available case) analysis and where possible to explore how much variation between studies was explained by between‐study differences in:
publication type (peer‐reviewed journal, conference abstract or proceedings, doctoral thesis);
random sequence generation and allocation concealment (selection bias);
blinding of participants, researchers, outcome assessors or a combination of these (performance bias); and
inclusion of only trials at low risk of bias.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess the certainty of evidence for the primary outcome and selected secondary outcomes (Schünemann 2021), and reported these assessments in summary of findings tables. Outcomes from RCTs started as higher certainty evidence but were downgraded based on our judgements regarding the five GRADE considerations (i.e. study limitation (overall risk of bias), inconsistency of study effects, imprecision, indirectness and publication bias) where necessary. We reported the reasons for downgrading the certainty of the evidence in the footnotes of the summary of findings tables. We interpreted and described the overall certainty level for each outcome following the definitions provided in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021).
Two review authors (DN, ETT) judged the certainty of the evidence, with disagreements resolved by discussion, and involving a third review author (KM) where needed.
We performed certainty of evidence assessments for the outcomes of IBS symptoms, quality of life and abdominal pain across the following five comparisons.
Any physical activity intervention compared to usual care.
Yoga intervention compared to walking intervention.
Similar types of physical activity intervention (i.e. yoga, treadmill exercise, advice to increase physical activity) compared to any control.
Any physical activity intervention compared with pharmacological therapy.
Any physical activity intervention compared with any dietary intervention.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies tables.
Results of the search
The search identified 2663 records (Figure 1). After deduplication and screening title and abstracts, we identified 30 eligible studies. After applying the exclusion criteria to the full‐text publications of these 30 potentially eligible records, we excluded 18 studies (see Characteristics of excluded studies). Therefore, we included 11 RCTs in this review. Ten trials provided data in published journal articles, one of which was written in Chinese language with an English abstract (Feng 2010). One trial provided data in an unpublished Masters degree thesis also with an abstract in English (Jia 2016). Only three prospectively registered their studies in a trial registry (Evans 2014; Kavuri 2015a; Schumann 2018).
1.
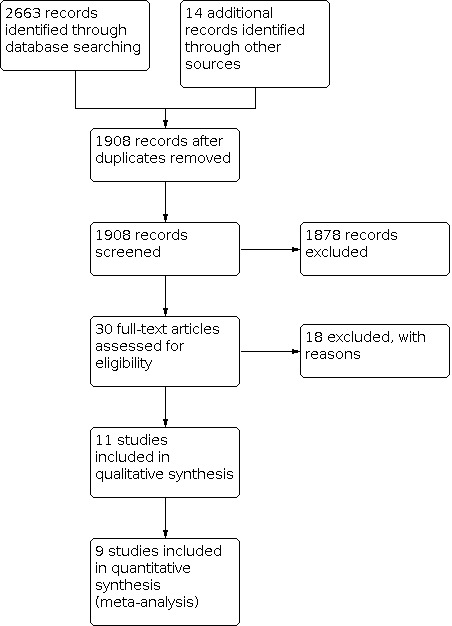
Study flow diagram.
Included studies
See Characteristics of included studies table for full details.
Study design
All studies were RCTs randomised by individual (Daley 2008; Evans 2014; Fani 2019; Feng 2010; Hajizadeh Maleki 2018; Jia 2016; Johannesson 2011; Kavuri 2015a; Schumann 2018; Shahabi 2016; Taneja 2004). There were no cluster RCTs. All RCTs compared an intervention and control arm, except one study that included two intervention arms (Kavuri 2015a). Two studies were labelled as pilot studies (Fani 2019; Shahabi 2016).
Five studies were in high‐income countries (Germany, Schumann 2018; Sweden, Johannesson 2011; UK, Daley 2008; USA, Evans 2014; Shahabi 2016); four in middle‐ to high‐income countries (China, Feng 2010; Jia 2016; Iran, Fani 2019; Hajizadeh Maleki 2018); one in a low‐ to middle‐income country (India, Taneja 2004); and one in both a high‐income and low‐ to middle‐income country (USA and India, Kavuri 2015a).
Participants
The total number of randomised participants was 622. The largest study randomised 102 participants (Johannesson 2011), the smallest 20 participants (Fani 2019), with a median of 59 (interquartile range 46.5) across the 11 studies. Studies recruited people with a diagnosis of IBS according to either Rome II (Daley 2008; Feng 2010; Johannesson 2011; Taneja 2004) or Rome III (Evans 2014; Fani 2019; Hajizadeh Maleki 2018; Jia 2016; Kavuri 2015a; Schumann 2018; Shahabi 2016) criteria for adults. One study recruited men only (Taneja 2004), and two studies recruited women only (Fani 2019; Hajizadeh Maleki 2018), but included studies did not consistently describe full details of study participants (including mean age, sex and ethnicity). One study enrolled people aged 14 to 26 years but performed separate analyses in a group aged 14 to 17 years and a group aged 18 to 26 years (Evans 2014). We included data from the group aged 18 to 26 years only. One study stated that they only included people with IBS‐C (Feng 2010), while two included people with IBS‐D only (Jia 2016; Taneja 2004). Five included people with IBD‐M (Daley 2008; Johannesson 2011; Kavuri 2015a; Schumann 2018; Shahabi 2016), and three studies did not define this IBS characteristic (Evans 2014; Fani 2019; Hajizadeh Maleki 2018).
Interventions and comparisons
Five studies assessed a yoga intervention (Evans 2014; Kavuri 2015a; Schumann 2018; Shahabi 2016; Taneja 2004); one assessed a Qigong intervention (breathing and slow, controlled movement) with follow‐up telephone support (Feng 2010); two a treadmill exercise intervention (Fani 2019; Hajizadeh Maleki 2018). Three studies involved exercise consultations and support to increase physical activity levels, one of these also included the use of a pedometer and mail prompts (Daley 2008), one included regular telephone contact and a cycle test at six weeks (Johannesson 2011), and one included monthly guidance by telephone, texts and emails (Jia 2016). One study evaluated an additional intervention arm that combined a yoga intervention with the continuation of medication as part of usual care (Kavuri 2015a). Data for this arm of the study were combined with the yoga intervention arm as single pair‐wise comparison with usual care control as per recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021c).
Six studies used a usual care comparison group (Daley 2008; Evans 2014; Fani 2019; Hajizadeh Maleki 2018; Jia 2016; Johannesson 2011), and, in two of these, the control group was wait‐list control (Daley 2008; Evans 2014). Two studies specifically described the comparison group as a pharmacological intervention (Feng 2010; Taneja 2004). Two studies used a walking group for comparison, one of which also employed a wait‐list protocol (Kavuri 2015a), while the other did not (Shahabi 2016). One study used a dietary intervention as the comparison group (Schumann 2018).
Settings
Four studies were set in university laboratory or campus settings and involved supervised delivery of the intervention (Evans 2014; Fani 2019; Hajizadeh Maleki 2018; Shahabi 2016). One study was set in a medical centre with supervised intervention delivery (Kavuri 2015a). Two studies were in unsupervised home settings (Johannesson 2011; Taneja 2004). Three studies involved a hospital or university baseline appointment or training session followed by the intervention delivered in a home setting, with the home setting being unsupervised (Daley 2008; Feng 2010; Jia 2016). One study took place in a university department with supervised intervention delivery and a home setting with printed and video tuition (Schumann 2018).
Outcomes
Global irritable bowel syndrome symptoms
Ten studies assessed the effect of physical activity on overall symptoms of IBS using a validated measure. Four studies reported difference in end of intervention mean score of the IBS‐SSS following a six‐week (Fani 2019), 12‐week (Jia 2016; Kavuri 2015a), or 24‐week intervention (and an eight‐week follow‐up) (Hajizadeh Maleki 2018). One study reported the difference in change in mean IBS‐SSS score following a 12‐week intervention and at 24‐week follow‐up (Schumann 2018). Another reported the change in median IBS‐SSS score following a 12‐week intervention (Johannesson 2011). One study reported difference in end of intervention mean score using the Birmingham IBS questionnaire following a 12‐week intervention (Daley 2008). One study reported difference in change scores of the Child Somatization Inventory (CSI‐18) after a 12‐week intervention (and eight‐week follow‐up) (Evans 2014). One study reported an overall GI symptoms score (Numeric Rating Scale (NRS)) following an eight‐week intervention and six‐month follow‐up (Shahabi 2016). The remaining study reported bowel symptoms using Talley's bowel diseases questionnaire following an eight‐week intervention (Taneja 2004).
One study used a non‐validated measure of IBS symptoms and we excluded their data (Feng 2010).
Quality of life
Seven studies reported the effect of physical activity on quality of life. Six studies used the IBS quality of life questionnaire, four reporting difference in end of intervention score at six weeks (Fani 2019) or 12 weeks (Daley 2008; Jia 2016; Kavuri 2015a), one reporting median change from baseline for specific domain scores at 12 weeks (Johannesson 2011), one reporting the same domains but compared change in mean scores at 12 weeks end of intervention and 24‐week follow‐up (Schumann 2018). Three studies measured quality of life using the 36‐item Short‐Form health survey (SF‐36), one reporting mean change from baseline at 12 weeks for the physical function domain only (Evans 2014), one reporting difference in change scores for all domains at 12 weeks end of intervention and 24‐week follow‐up (Schumann 2018), and one reporting median change from baseline for all domains at 12 weeks (Johannesson 2011).
Abdominal pain
Four studies reported the effect of physical activity on abdominal pain. Two used an abdominal pain severity NRS and reported mean change from baseline at eight weeks (Shahabi 2016), or median change from baseline at 12 weeks (Evans 2014). The remaining two studies reported the pain subscale of the IBS‐SSS questionnaire, reporting the mean end of intervention score at 12 weeks (Daley 2008), and at 12 and 24 weeks (Schumann 2018).
Stool consistency and frequency
Two studies reported the effect of physical activity on stool consistency separately. Both studies used the Bristol Stool Form Chart, reporting median change from baseline at 12 weeks (Johannesson 2011), or the proportion of participants in each category before and after a six‐week intervention (Feng 2010). One study reported a measure of stool frequency, reporting median change from baseline at 12 weeks (Johannesson 2011).
Bowel transit time
One study assessed a measure of bowel transit time as oranal transit time, reporting median change from baseline at 12 weeks (Johannesson 2011).
Adverse events
Three studies specifically reported adverse events as a separate outcome (Evans 2014; Kavuri 2015a; Schumann 2018).
Excluded studies
We excluded 18 studies for not meeting one or more of our inclusion criteria:
10 studies had an illegible design (i.e. they were not RCTs);
three studies specifically excluded participants with IBS;
two studies used a non‐validated outcome assessment scale;
two studies did not assess a physical activity intervention;
two studies had a co‐intervention in the intervention arm only;
one study used a control intervention that did not match the review question.
The Characteristics of excluded studies table contains the key studies excluded with reasons.
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
We identified two ongoing studies, details of which are given in the Characteristics of ongoing studies table.
Risk of bias in included studies
An overview of the risk of bias for each study and as a percentage of overall studies is presented in Figure 2 and Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Of the 11 RCTs, four reported an adequate method of both sequence generation and allocation concealment (Daley 2008; Evans 2014; Kavuri 2015a; Schumann 2018). One reported an adequate method of sequence generation but provided no information on the method of allocation concealment (Johannesson 2011). One reported adequate sequence generation but had a risk of bias for their allocation concealment methods (Jia 2016). The remaining five RCTs provided insufficient information to judge the risk of bias for either criterion (Fani 2019; Feng 2010; Hajizadeh Maleki 2018; Shahabi 2016; Taneja 2004).
Blinding
None of the 11 RCTs blinded the study participants. However, in trials of physical activity and exercise versus a non‐exercise control, it is often not possible to blind participants and personnel who delivered the interventions to treatment allocation. Consequently, we judged all studies a high risk of performance bias.
The level of reporting of whether outcome assessment was blinded to group allocation was mixed. Among studies that provided sufficient information, three studies were at low risk of detection bias (Evans 2014; Kavuri 2015a; Schumann 2018), and two were at high risk of bias (Daley 2008; Fani 2019). The remaining six studies provided insufficient information to judge (Feng 2010; Hajizadeh Maleki 2018; Jia 2016; Johannesson 2011; Shahabi 2016; Taneja 2004).
Incomplete outcome data
We judged four studies at low risk of attrition bias due to reporting few dropouts that were similar between study arms (Fani 2019; Feng 2010; Schumann 2018; Taneja 2004). Four were at high risk of bias due to overall dropout rates of greater than 20%, often uneven between intervention and control arms, and lack of ITT analysis (Daley 2008; Evans 2014; Hajizadeh Maleki 2018; Shahabi 2016). One study was at unclear risk of attrition bias due to high (27%) overall but equal between‐group dropouts and reporting both per‐protocol and ITT analyses, though ITT excluded 11 participants (Johannesson 2011). Information was insufficient in two studies judged at unclear risk (Jia 2016; Kavuri 2015a).
Selective reporting
The presence of reporting bias was based primarily on study preregistration. We found trial registration entries for three studies (Evans 2014; Kavuri 2015a; Schumann 2018), and used these to determine a low risk of reporting bias for one (Evans 2014). We judged the risk of selective reporting bias for Schumann 2018 to be high due to a secondary outcome listed in the trial registry being reported as the primary outcome and the introduction of non‐registered outcomes in the published study. We judged the risk of selective reporting bias in Kavuri 2015a as unclear due to registration being performed 15 months after the trial had started and over two years after ethical approval.
Six of the remaining eight studies were at unclear risk of reporting bias due to a lack of trial registration, although outcomes listed in the methods section of all included studies were reported in the results section. The final two studies were judged at high risk of bias; Hajizadeh Maleki 2018 due to reporting data for new outcomes in the results section and further additional outcomes mentioned in the discussion but without any data presented or provided in the results section; Daley 2008 due to reporting that focused on secondary outcomes with statistically significant P values and little mention of a null finding for the primary outcome.
Other potential sources of bias
One study was at low risk of bias for other potential sources of bias (Daley 2008).
Feng 2010, which specifically looked at the efficacy of Qigong, was funded by the Qigong Center Project of the State Sports General Administration. The lead author of another study assessing the efficacy of yoga was an employee of the funding organisation, which was also a yoga research foundation (Kavuri 2015a). We considered these as notable conflicts of interest and important considerations when interpreting their findings, particularly given the high risk of performance bias.
We judged Shahabi 2016 at unclear risk of bias due to uneven group sizes and differences in baseline characteristics including bowel habits and the proportion of participants already exercising.
All remaining studies were at unclear risk of bias, predominantly for a lack of information around baseline characteristics of included participants.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Physical activity compared with usual care
Global irritable bowel syndrome symptoms
Six studies assessed the effect of physical activity on IBS symptoms compared with usual care (Daley 2008; Evans 2014; Fani 2019; Hajizadeh Maleki 2018; Jia 2016; Johannesson 2011). We were able to pool data from five of these studies, which observed an improvement in the group receiving a physical activity intervention (SMD –0.93, 95% CI –1.44 to –0.42; 185 participants; Analysis 1.1). This effect size would be considered large (Cohen 1988). CIs were wide, suggesting uncertainty in the observed estimates, and heterogeneity was moderately high (Chi² = 10.01, P = 0.04; I² = 60%).
1.1. Analysis.

Comparison 1: Physical activity compared with usual care, Outcome 1: IBS symptoms
Using GRADE criteria, we downgraded the certainty of evidence one level due to risk of bias, one level for inconsistency due to high heterogeneity and one level for imprecision (Table 1). Thus, we considered the certainty of evidence to be very low, meaning physical activity may improve IBS symptoms but the evidence is very uncertain.
A sensitivity analysis of studies at low risk of selection bias slightly reduced the proportion of total variability due to between‐study heterogeneity (Chi² = 2.28, P = 0.13; I² = 56%), reducing the observed effect size for physical activity compared with usual care control to moderate, though CIs remained wide and now consistent with both a beneficial and small harmful effect (SMD –0.62, 95% CI –1.44 to 0.21; 64 participants; Analysis 1.2).
1.2. Analysis.

Comparison 1: Physical activity compared with usual care, Outcome 2: IBS symptoms (studies with low risk of selection bias)
We were unable to pool data on IBS symptoms from Johannesson 2011 as values were presented as median and 10th and 90th percentiles. The study reported improved symptoms in the intervention (median change –51 points, 10th percentile –130 and 90th percentile 49) compared with the usual care group (median change –5, 10th percentile –101 and 90th percentile 118) using a per‐protocol analysis (P = 0.003). There was a smaller improvement in the intervention arm (median change –37, 10th percentile –142 and 90th percentile 37) compared with usual care group (median change 0, 10th percentile –97 and 90th percentile 109) using an ITT analysis. The percentile ranges were wide, suggesting uncertainty in the observed estimates. Johannesson 2011 also presented results for IBS symptoms dichotomously according to a predefined clinically significant change in reported symptom severity of greater than 50 points. The proportion of participants reporting a clinically significant decrease (improvement) in symptom severity was greater in the physical activity (43%) compared with control (26%) group, though the 95% CI was compatible with both greater and fewer participants in the physical activity group reporting a decrease in symptom severity (P = 0.07). Compared with the physical activity group (8%), the control group (23%) had a greater proportion of participants reporting a clinically significant increase in symptom severity (P < 0.01). We judged Johannesson 2011 at unclear risk of bias due to poor reporting, meaning and findings should be interpreted with this in mind.
Quality of life
Five RCTs assessed the effect of physical activity on self‐reported quality of life compared with usual care (Daley 2008; Evans 2014; Fani 2019; Jia 2016; Johannesson 2011). Meta‐analysis of data from four of these trials found no conclusive evidence of an improvement in quality of life following a physical activity intervention (SMD 1.17, 95% CI –0.30 to 2.64; 134 participants; Analysis 1.3), and there was large heterogeneity between studies (Chi² = 39.1, P = 0.0001; I² = 92%).
1.3. Analysis.

Comparison 1: Physical activity compared with usual care, Outcome 3: Quality of life
We downgraded the certainty of evidence one level due to risk of bias, two levels for inconsistency due to high heterogeneity and one level for imprecision (Table 1). Thus we considered the certainty of evidence for physical activity on quality of life to be very low, meaning the evidence is very uncertain about the effect of physical activity interventions on quality‐of‐life outcomes in people with IBS.
A sensitivity analysis based on studies with low risk of selection bias reduced the proportion of total variability due to between‐study heterogeneity (Chi² = 1.42, P = 0.23; I² = 30%) and decreased the size and direction of the observed effect estimates, which were compatible with both worsening and improvement in quality of life (SMD –0.11, 95% CI –0.61 to 0.38; Analysis 1.4).
1.4. Analysis.

Comparison 1: Physical activity compared with usual care, Outcome 4: Quality of life (studies with low risk of selection bias)
We were unable to pool data on quality of life from Johannesson 2011 as values were presented as median and 10th and 90th percentiles. Reporting domains separately, the study observed improvement (P = 0.015) in physical function outcome (median post‐treatment change 16, 10th percentile –2 and 90th percentile 35) and physical role outcome (median change 6, 10th percentile –14 and 90th percentile 50) domains in the physical activity group compared with the control group (physical function outcome: median change 0, 10th percentile –27 and 90th percentile 30; physical role outcome: median change 0, 10th percentile –39 and 90th percentile 31). The authors reported similar findings under ITT analysis, although the reported data did not appear to support this (physical activity versus control group for physical function outcome: median change 0, 10th percentile –37 and 90th percentile 20 versus median change 8, 10th percentile –3 and 90th percentile 28; P = 0.01); and physical role outcome (median change 0, 10th percentile –40 and 90th percentile 31 versus median change 0, 10th percentile –14 and 90th percentile 50; P = 0.03). There were no observed between‐group differences reported for the remaining seven domains. The percentile ranges were wide throughout, suggesting uncertainty in the observed estimates.
Abdominal pain
Two trials assessed the impact of physical activity compared with usual care on reported abdominal pain (Daley 2008; Evans 2014). Meta‐analysis of these two studies found no improvement in abdominal pain with physical activity compared with usual care (SMD 0.01, 95% CI –0.48 to 0.50; 64 participants; Analysis 1.5). CIs were wide, suggesting uncertainty in the observed estimates but there was little evidence of statistical heterogeneity (Chi² = 1.04, P = 0.31; I² = 3%).
1.5. Analysis.

Comparison 1: Physical activity compared with usual care, Outcome 5: Abdominal pain
We downgraded the certainty of evidence one level due to risk of bias and two levels for imprecision (Table 1). Thus, we considered the certainty of evidence to be very low, meaning the evidence is very uncertain about the effect of physical activity interventions on abdominal pain in people with IBS.
Stool consistency
Two trials provided data on the effect of physical activity on stool consistency compared with a usual care control (Daley 2008; Johannesson 2011); however, we were unable to pool these data due to differences in data analysis and reporting between studies. In Daley 2008, MD in end of study scores demonstrated no conclusive impact on diarrhoea symptoms in the physical activity group compared with controls (MD 0.5, 95% CI –7.3 to 8.4; 43 participants). Conversely, the physical activity group demonstrated improvement compared with the control group for symptoms of constipation, but CIs were wide, suggesting uncertainty in the observed estimates (MD –10.9, 95% CI –20.1 to –1.6; 43 participants).
Johannesson 2011 reported stool consistency using the Bristol Stool Form Scale. There were no observed differences in stool consistency in the physical activity group (median end of study score 5, 10th percentile 2 and 90th percentile 6) compared with the control group (median end of study score 5, 10th percentile 3 and 90th percentile 6).
Stool frequency
One trial provided data on the effect of physical activity on stool frequency compared with a usual care control (Johannesson 2011). There was no observed difference in median number of weekly bowel movements between the physical activity (median 12, 10th percentile 6 and 90th percentile 26) and control (median 9, 10th percentile 3 and 90th percentile 20) groups.
Bowel transit time
One trial provided data on the effect of physical activity on bowel transit time compared with a usual care control (Johannesson 2011). There was no observed difference in oroanal transit time between physical activity (median 1.2, 10th percentile 0.2 and 90th percentile 2.8) and control groups (median 1.2, 10th percentile 0.3 and 90th percentile 3.3).
Adverse events
One trial reported adverse effects as a separate outcome, noting one adverse event following a yoga intervention where a participant slipped out of a rope while in a headstand position and hit their knee (Evans 2014). The participant went on to complete the yoga intervention. The study authors reported that the event was self‐limited and deemed by the investigators and data safety monitoring board to not be serious.
Withdrawal due to adverse events related to physical activity
No studies assessed the effect of physical activity compared with usual care on withdrawal due to adverse events related to physical activity.
Yoga compared with walking
Global irritable bowel syndrome symptoms
Two trials assessed the effect of a yoga intervention compared with a walking intervention on IBS symptoms (Kavuri 2015a; Shahabi 2016). One of these studies also assessed the effect of a yoga intervention in combination with medication as per usual care compared with walking (Kavuri 2015a). Meta‐analysis of these two studies found no conclusive evidence of an effect of yoga compared with walking on IBS symptoms (SMD –1.16, 95% CI –3.93 to 1.62; 124 participants; Analysis 2.1), but heterogeneity was very high (Chi² =33.08, P < 0.00001; I² = 97%).
2.1. Analysis.

Comparison 2: Yoga compared with walking, Outcome 1: IBS symptoms
We downgraded the certainty of evidence two levels due to imprecision and two levels due to inconsistency (Table 2). Thus we considered the certainty of evidence to be very low, meaning the evidence is very uncertain about the effect of yoga interventions compared with walking interventions on IBS symptoms.
Quality of life
One study assessed the effect of a yoga intervention and walking on quality of life (Kavuri 2015a). The study reported an observed improvement in the yoga compared to walking group (MD 53.45, 95% CI 38.85 to 68.05, 97 participants; Analysis 2.2).
2.2. Analysis.

Comparison 2: Yoga compared with walking, Outcome 2: Quality of life
Abdominal pain
One trial reported no observed difference in change in reported severity of abdominal pain between a yoga intervention (8.1, SD 4.2) and walking (5.8, SD 3.8) groups (MD 2.30, 95% CI –0.79 to 5.39; 27 participants; Analysis 2.3) (Shahabi 2016).
2.3. Analysis.

Comparison 2: Yoga compared with walking, Outcome 3: Abdominal pain
Stool consistency
No studies assessed the effect of a yoga intervention and walking on stool consistency.
Stool frequency
No studies assessed the effect of a yoga intervention and walking on stool frequency.
Bowel transit time
No studies assessed the effect of a yoga intervention and walking on bowel transit time.
Adverse events
In Kavuri 2015a, four participants experienced adverse events that resulted in withdrawal from the study for the following reasons: influenza (group details not given), cataract surgery (group details not given), breast cancer (yoga group), fractured foot (combination group). One participant in the wait‐list control group died following a cardiac arrest. Three participants, two in the yoga group and one in the combination group, experienced lower back pain, though these were not recorded as adverse events and did not result in discontinuation from the study.
Withdrawal due to adverse events related to physical activity
No studies assessed the effect of a yoga intervention compared with walking on withdrawal due to adverse events related to physical activity.
Studies with similar physical activity interventions compared with any control
Irritable bowel syndrome symptoms
Yoga intervention
Five trials assessed the impact of a yoga intervention on IBS symptoms (Evans 2014; Kavuri 2015a; Schumann 2018; Shahabi 2016; Taneja 2004). Meta‐analysis of these studies found no conclusive evidence of an improvement following a yoga compared with a control intervention (SMD –0.75, 95% CI –2.01 to 0.51; 225 participants; Analysis 3.1). CIs were wide, suggesting uncertainty in the observed estimates and heterogeneity was high (Chi² = 62.64, P < 0.00001; I² = 94%).
3.1. Analysis.

Comparison 3: Studies with similar physical activity interventions compared with any control , Outcome 1: IBS symptoms
We downgraded the certainty of evidence one level due to risk of bias, two levels for inconsistency due to high heterogeneity and two levels for imprecision (Table 3). Thus, we considered the certainty of evidence to be very low, meaning the evidence is very uncertain for the effect of yoga interventions on IBS symptoms.
Supervised treadmill exercise
Two trials assessed the impact of supervised treadmill exercise on IBS symptoms (Fani 2019; Hajizadeh Maleki 2018). Meta‐analysis of these studies found no conclusive evidence of an improvement following a supervised treadmill exercise compared with a control intervention (SMD –1.24, 95% CI –2.64 to 0.15; 71 participants; Analysis 3.1). CIs were wide, suggesting uncertainty in the observed estimates and heterogeneity was high (Chi² = 4.99, P < 0.03; I² = 80%).
We downgraded the certainty of evidence one level due to risk of bias and two levels for imprecision (Table 3). Thus, we considered the certainty of evidence to be very low, meaning the evidence is very uncertain about the effect of supervised treadmill exercise interventions on IBS symptoms.
Advice to increase physical activity
Two trials assessed the impact of advice to increase physical activity levels on IBS symptoms (Daley 2008; Jia 2016). Meta‐analysis of these studies found no conclusive evidence of an improvement following advice to increase physical activity compared with a control intervention (SMD –0.72, 95% CI –1.61 to 0.17; 93 participants; Analysis 3.1).
We downgraded the certainty of evidence one level due to risk of bias and two levels for imprecision (Table 3). Thus, we considered the certainty of evidence to be very low, meaning the evidence is very uncertain about the effect of interventions giving advice to increase physical activity on IBS symptoms.
Quality of life
Yoga intervention
Three trials assessed the impact of a yoga intervention on quality of life (Evans 2014; Kavuri 2015a; Schumann 2018). Meta‐analysis of these studies found no conclusive evidence of an improvement in quality of life following a yoga compared with a control intervention (SMD 0.60, 95% CI –0.59 to 1.79; 177 participants; Analysis 3.2).
3.2. Analysis.

Comparison 3: Studies with similar physical activity interventions compared with any control , Outcome 2: Quality of life
We downgraded the certainty of evidence one level due to risk of bias, two levels for inconsistency due to high heterogeneity and two levels for imprecision (Table 3). Thus, we considered the certainty of evidence to be very low, meaning the evidence is very uncertain about the effect of yoga interventions on quality of life.
Supervised treadmill exercise
One trial assessed the impact of supervised treadmill exercise on quality of life (Fani 2019). It reported an observed improvement in quality of life in the intervention group (SMD 2.39, 95% CI 1.18 to 3.59; 20 participants). CIs were wide suggesting uncertainty in the observed estimates and risk of bias was high (Analysis 3.2).
Advice to increase physical activity
Two trials assessed the impact of advice to increase physical activity levels on quality of life (Daley 2008; Jia 2016). Meta‐analysis found no conclusive evidence of an improvement in quality of life following advice to increase physical activity compared with a control intervention (SMD 1.04, 95% CI –1.65 to 3.74; 93 participants; Analysis 3.2).
We downgraded the certainty of evidence one level due to risk of bias, two levels for inconsistency due to high heterogeneity and two levels for imprecision (Table 3). Thus, we considered the certainty of evidence very low, meaning the evidence is very uncertain about the effect of interventions giving advice to increase physical activity on quality of life.
Abdominal pain
Yoga intervention
Two trials assessed the impact of a yoga intervention on abdominal pain (Evans 2014; Shahabi 2016). Meta‐analysis found no conclusive evidence of an improvement in abdominal pain following a yoga compared with a control intervention (SMD 0.13, 95% CI –0.45 to 0.72; 48 participants; Analysis 3.3).
3.3. Analysis.

Comparison 3: Studies with similar physical activity interventions compared with any control , Outcome 3: Abdominal pain
We downgraded the certainty of evidence one level due to risk of bias, one level for inconsistency and one level for imprecision (Table 3). The certainty of evidence was very low, meaning the evidence is very uncertain about the effect of yoga interventions on abdominal pain.
Stool consistency
No studies assessed the effect of physical activity interventions and a control intervention on stool consistency.
Stool frequency
No studies assessed the effect of physical activity interventions and a control intervention on stool frequency.
Bowel transit time
No studies assessed the effect of physical activity interventions and a control intervention on bowel transit time.
Adverse events
No studies reported adverse events for physical activity interventions and a control intervention.
Withdrawal due to adverse events related to physical activity
No studies assessed the effect of similar physical activity interventions compared with a control intervention on withdrawal due to adverse events related to physical activity.
Physical activity compared with pharmacological therapy
Global irritable bowel syndrome symptoms
One study assessed the effect of a yoga intervention compared specifically with medication (loperamide) on IBS symptoms in people with IBS‐D (Taneja 2004). It found no conclusive difference between groups (MD –1.20, 95% –2.65 to 0.25; 21 participants; Analysis 4.1). CIs were wide and compatible with both improvement and worsening of symptoms following a yoga intervention compared with medication.
4.1. Analysis.

Comparison 4: Physical activity compared with pharmacological therapy, Outcome 1: IBS symptoms
We downgraded the certainty of evidence one level due to risk of bias and two levels due to very serious imprecision (Table 4). Thus, the certainty of evidence was very low, meaning the evidence is very uncertain about the effect of a yoga intervention compared with medication alone on IBS symptoms in people with IBS‐D.
Another study assessed IBS symptoms using a non‐validated method and its results were therefore excluded (Feng 2010).
Quality of life
No studies assessed the effect of physical activity and pharmacological therapy on quality of life.
Abdominal pain
No studies assessed the effect of physical activity and pharmacological therapy on abdominal pain.
Stool consistency
One study assessed the effect of a Qigong intervention compared with medication (tegaserod) on stool consistency in people with IBS‐C, reporting the number and proportion of participants in each category of the Bristol Stool Form Scale before and after the intervention (Feng 2010). In the intervention group, 11 (37%) participants reported constipation (grade 1 or 2) at baseline compared with 3 (10%) after the intervention, a reduction of 27%. In the control group, 10 (33%) participants experienced constipation at baseline compared with 8 (27%) after the intervention, a reduction of 6% (reported P < 0.05).
Stool frequency
No studies assessed the effect of physical activity and pharmacological therapy on stool frequency.
Bowel transit time
No studies assessed the effect of physical activity and pharmacological therapy on bowel transit time.
Adverse events
No studies reported adverse events for physical activity and pharmacological therapy.
Withdrawal due to adverse events related to physical activity
No studies assessed the effect of physical activity compared with pharmacological therapy on withdrawal due to adverse events related to physical activity.
Physical activity compared with dietary interventions
One trial assessed the effect of a 12‐week yoga intervention compared with a dietary (FODMAP [fermentable oligosaccharides, disaccharides, monosaccharides and polyols]) intervention on IBS symptoms (IBS‐SSS), quality of life (Irritable Bowel Syndrome Quality Of Life (IBS‐QOL) and SF‐36) and abdominal pain (subdomain of the IBS‐SSS) (Schumann 2018).
Global irritable bowel syndrome symptoms
While both groups experienced an improvement in overall symptoms, the study reported inconclusive improvement in the dietary intervention, but CIs were wide, suggesting uncertainty in the observed estimates (MD 33.31, 95% CI –12.86 to 79.48; 59 participants; Analysis 5.1).
5.1. Analysis.

Comparison 5: Physical activity compared with dietary interventions, Outcome 1: IBS symptoms
We downgraded the certainty of evidence two levels due to evidence of very serious imprecision (Table 5). The certainty of evidence was graded low, meaning the evidence suggests that yoga results in little to no difference in IBS symptoms when compared with a FODMAP dietary intervention.
Similarly there were inconclusive differences at 24‐week follow‐up (MD 33.41, 95% CI –4.21 to 71.04 (as reported in the paper due to no data provided on end of intervention or change score in each group).
Quality of life
There was an inconclusive difference between groups for overall quality of life based on the IBS‐QOL questionnaire at 12 weeks (MD –1.67, 95% CI –7.62 to 4.28; 59 participants; Analysis 5.2) and 24 weeks (MD 0.20, 95% CI –4.90 to 5.30; 59 participants; Analysis 5.2). There was an observed improvement in the yoga group on the physical component summary subscale of the SF‐36 but no other between‐group differences.
5.2. Analysis.

Comparison 5: Physical activity compared with dietary interventions, Outcome 2: Quality of life
We downgraded the certainty of evidence two levels due to evidence of very serious imprecision (Table 5). The certainty of evidence was low, meaning yoga may result in little to no difference in quality‐of‐life outcomes when compared with a FODMAP dietary intervention.
Abdominal pain
There was an inconclusive improvement in severity of abdominal pain in the diet compared with yoga group at both 12 weeks (MD 12.00, 95% CI –4.96 to 28.96; 59 participants; Analysis 5.3) and 24 weeks (MD 7.89, 95% CI –8.19 to 23.97; 59 participants; Analysis 5.3). CIs were wide and included both improvement and worsening of abdominal pain.
5.3. Analysis.

Comparison 5: Physical activity compared with dietary interventions, Outcome 3: Abdominal pain
We downgraded the certainty of evidence two levels due to evidence of very serious imprecision (Table 5). The certainty of evidence was low, meaning the evidence suggests that yoga results in little to no difference in abdominal pain when compared with a FODMAP dietary intervention
Stool consistency
The study did not assess the effect of physical activity and dietary interventions on stool consistency.
Stool frequency
The study did not assess the effect of physical activity and dietary interventions on stool frequency.
Bowel transit time
The study did not assess the effect of physical activity and dietary interventions on bowel transit time.
Adverse events
Three participants in the FODMAP group reported adverse events during the study intervention (Schumann 2018). One participant experienced a serious event related to a major depressive episode, and two participants experienced non‐serious events (one mild self‐reported depressive episode and one unwanted loss of weight). Two participants in the yoga group reported adverse events, with one serious event relating to a newly diagnosed deep leg vein thrombosis and one non‐serious event relating to back pain caused by heavy lifting. The authors judged none of these events were related to the study interventions.
Withdrawal due to adverse events related to physical activity
No studies assessed the effect of physical activity compared with dietary interventions on withdrawal due to adverse events related to physical activity.
Preplanned subgroup and sensitivity analyses
We used the SE to derive the SD for all outcomes in one study (Daley 2008), and for one outcome in one study (Feng 2010) in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021b). Most studies used continuous data, with very few reporting dichotomous data. Therefore, we calculated MDs with 95% CIs for each study when possible. Due to heterogeneity in instruments to assess symptoms and other outcomes, as well as in the way data were reported (e.g. mean or median, difference in end of intervention or change scores), we calculated SMD with 95% CIs for most outcomes. We calculated pooled effect estimates using either an MD or SMD with 95% CIs.
Due to the small number of eligible and included studies, as well as differences in the reporting of findings, we were unable to perform a number of preplanned subgroup analyses. We were able to perform subgroup analyses to explore the comparative effectiveness of different types of physical activity (yoga and walking) as well as physical activity compared with pharmacological and dietary interventions (see Effects of interventions). We also performed a subgroup analysis of similar types of physical interventions compared to any control, which found no observed differences when compared with primary analyses.
None of our meta‐analyses included 10 studies or more. As a result, we did not perform funnel plot analyses based on current recommendations (Page 2021).
Discussion
Global irritable bowel syndrome symptoms
Six trials assessed the impact of a physical activity intervention in comparison with usual care on reported symptoms in people with IBS. The studies employed a variety of interventions including advice to increase physical activity, supervised and unsupervised yoga therapy, and supervised treadmill exercise. Meta‐analysis of five of these trials observed improvement in reported symptoms following a physical activity intervention (SMD –0.93, 95% CI –1.44 to –0.42). A minimal clinically important difference (MCID) of 0.5 SD for bowel symptoms in people with IBS has been suggested (Spiegel 2009). The observed range of estimates are consistent with both clinically important and non‐important improvements in IBS symptoms following a physical activity intervention. When considered on an original item scale, the observed difference approximates to an absolute mean reduction of 69 points (95% CI 31 to 106) for total symptoms of the IBS‐SSS (based on the mean control group baseline SD of 74 from Fani 2019; Hajizadeh Maleki 2018; Jia 2016). The MCID for the total symptoms score of the IBS‐SSS has been defined as a difference of 50 points or more (Francis 1997). The observed range in estimates are consistent with both clinically important and non‐important improvements in symptoms as measured by the IBS‐SSS following a physical activity intervention.
We considered the certainty of the evidence as very low, providing very little confidence in the observed estimates. In a preplanned sensitivity analysis including only the studies deemed at low risk of selection bias, we found that the effect estimate reduced to a moderate size (SMD –0.62, 95% CI –1.44 to 0.21) still in favour of physical activity but estimates were now consistent with both improvement and worsening of symptoms. Larger studies at low risk of bias are needed to increase confidence in the observed estimates.
We considered the remaining study at unclear risk of bias (Johannesson 2011). Its results support the meta‐analysis for observed differences in change scores but found no observed difference between groups in the proportion of participants achieving a clinical improvement in symptom score of 50 points or more.
When assessing data from trials with similar types of physical activity intervention, we observed estimates that were consistent with both improvement and worsening of symptoms for yoga interventions, supervised treadmill exercise and advice to increase physical activity compared with a control intervention. CIs were wide throughout and the certainty of evidence was very low for all three types of intervention, providing little confidence in the evidence.
Two trials assessed the comparative effectiveness of different types of exercise on IBS symptoms and found that yoga was not conclusively more beneficial for symptoms compared with a walking intervention. The certainty of evidence was very low meaning we cannot reliably conclude that one form of exercise provides benefit over the other.
There was a limited amount of available evidence for the comparative effectiveness of physical activity interventions compared with pharmacological therapy for IBS symptoms, with one small study reporting observed estimates that were consistent with both improvement and worsening of symptoms with physical activity.
One study comparing physical activity (yoga) to a dietary intervention also reported inconclusive findings, with estimates again consistent with both improvement and worsening of symptoms with physical activity.
Quality of life
Five trials assessed the impact of a physical activity intervention compared with usual care on quality‐of‐life measures. Meta‐analysis of four trials did not conclusively observe improved quality of life following a physical activity intervention (SMD 1.17, 95% CI –0.30 2.64). We considered the overall certainty of evidence as very low, meaning we have very little confidence in the observed estimates. In a preplanned sensitivity analysis including only the studies deemed at low risk of selection bias, we observed the effect estimate changed to worsening of symptoms and the range in estimates remained consistent with both worsening and improved quality of life following a physical activity intervention. Larger studies at low risk of bias are needed to increase confidence in the observed estimates.
The remaining study was at unclear risk of bias but its results did not support the meta‐analysis, instead reporting an observed improvement in physical function and physical role domains with physical activity compared to controls, but not for the remaining seven domains of the IBS quality of life questionnaire (Johannesson 2011). However, observed estimates were small and CIs were wide throughout.
Assessing trials with similar types of physical activity intervention, we observed estimates that were consistent with both improvement and worsening of quality of life for yoga interventions and advice to increase physical activity compared with a control intervention. CIs were wide throughout and the certainty of evidence was very low for all three types of intervention, providing little confidence in the evidence. One trial reported an observed improvement in quality of life in 10 people undergoing supervised treadmill exercise; however, we have little confidence in this finding due to wide CIs and a high risk of bias (Fani 2019).
One trial assessed the comparative effectiveness of different types of exercise on quality of life and found that yoga was more beneficial for quality of life compared with a walking intervention. The findings were tempered by the small sample size, potential conflicts of interest and a lack of additional supportive studies.
There was no evidence to assess the comparative effectiveness of physical activity interventions compared with pharmacological therapy for quality‐of‐life outcomes.
One study comparing physical activity (yoga) to a dietary intervention also reported inconclusive findings, with estimates again consistent with both improvement and worsening of quality of life with physical activity apart from responses to one subscale item of the SF‐36 questionnaire that favoured the physical activity intervention.
Abdominal pain
Two trials reported abdominal pain outcomes separately, one assessing the impact of advice to increase physical activity and the other assessed a yoga intervention compared with usual care. Meta‐analysis of these studies showed no observed effect of physical activity on abdominal pain, but CIs were wide, suggesting uncertainty in the observed estimates (SMD 0.01, 95% CI –0.48 to 0.50). One study gave an MCID of –1.74 NRS points (Evans 2014). Based on the control baseline, the observed estimates equate to a difference of 0.02 (95% CI –1.03 to 1.08) and are consistent with small, clinically unimportant increased and decreased abdominal pain following a physical activity intervention. We considered the certainty of evidence from these studies very low, and our confidence in the observed estimates is very limited.
We were unable to assess the impact of similar types of physical activity interventions or the comparative effectiveness of physical activity compared with pharmacological therapy, other physical activity interventions or diet.
Potential harms associated with physical activity
Wide CIs meant that the data were compatible with physical activity worsening quality of life and abdominal pain outcomes. Only three trials specifically reported adverse events. Kavuri 2015a reported four participants who experienced adverse events that resulted in withdrawal from the study, which were mainly from events unlikely to be related to the interventions. Three participants, two in a yoga group and one in a combination group (yoga plus medication maintenance) experienced lower back pain, though these were not recorded as adverse events and did not result in discontinuation from the study. In Evans 2014, one participant in the yoga group injured their knee but was able to complete the study. Schumann 2018 reported two serious adverse events, one related to a major depressive episode in one participant in the diet group and a newly diagnosed deep leg vein thrombosis in one participant in the yoga group. Both of these events were unrelated to the study interventions.
Overall completeness and applicability of evidence
We used an extensive search strategy involving a comprehensive range of databases and other sources and, while possible, it is unlikely that we missed relevant references. In addition, we included only studies with validated outcome measures of symptoms and quality of life, increasing the applicability of the evidence to clinical practice. However, the applicability of the evidence was limited by several study characteristics. Five of 11 included studies assessed yoga as the physical activity intervention; two studies assessed a more health systems applicable intervention such as advice and support to increase overall physical activity by any means according to preference. Many studies involved supervised physical activity, with little indication of the resources required. The longest duration of intervention was 24 weeks and the longest period of follow‐up was six months. Therefore, we are uncertain of the effect of longer duration interventions and the impact of short‐term interventions on long‐term outcomes. The extent to which the results are applicable to people with IBS‐C or IBS‐D only is uncertain as most studies included people with IBS‐M. We acknowledge the lack of consistency for IBS symptoms and quality‐of‐life outcomes associated with different measurement instruments is a potential limitation, constraining the extent to which the SD units are comparable across studies.
One of the strengths of this reviews is that we included all types of physical activity, including advice to increase activity, and looked to compare the effectiveness of different types of physical activity compared with one another and with other interventions (e.g. diet) in any setting. Thus, the results represent the current evidence base for physical activity as an intervention that are applicable to healthcare settings.
Quality of the evidence
We assessed the certainty of the evidence included in this review as low or very low using GRADE criteria. These low ratings were largely due to the small number of studies with an unclear or high risk of bias, and inconsistency and imprecision in the observed estimates.
Potential biases in the review process
At least two review authors were independently involved with the selection of studies and in the data extraction and quality assessment processes, thus reducing the potential for review author error and bias. We sought published and unpublished studies in any language, thus reducing the potential for language and publication bias. As with any review, it is possible that we failed to identify all relevant research for inclusion in the review, although we attempted to reduce this possibility by citation searching and, where needed, contacting researchers in the field regarding eligible studies. We also made several changes to the review after writing the protocol and provide rationale for these in Differences between protocol and review section.
Agreements and disagreements with other studies or reviews
We identified three existing systematic reviews that concerned one or more physical activity interventions, one of which assessed only yoga interventions and had similar inclusion criteria as our review in terms of study design and outcomes evaluated (Schumann 2016). Based on a narrative synthesis of six studies, the authors concluded that yoga might be a feasible and safe adjunctive treatment for people with IBS. Conclusions were tempered, however, due to the quality of studies being judged by the review authors as poor. Four of the studies included in that review also met the inclusion criteria for our review. We judged them unclear or poor quality, and the certainty of evidence across outcomes was low to very low. This systematic review differed to our review in that it included participants of any age, including adolescents, only included published studies, excluded other types of physical activity as interventions, and it did not include a meta‐analysis or GRADE assessments. The authors of the review also included one study that we excluded for the use of a non‐validated symptom measurement tool (Madhu 1988).
A more recent systematic review of RCTs followed a similar protocol to ours but with key differences including the inclusion of children and adults, a focus on statistical significance, a lack of sensitivity/subgroup analyses, and no GRADE assessments (Zhou 2019). Based on a narrative synthesis of 14 studies, the authors concluded that exercise might be a feasible and effective treatment approach in people with IBS but that no firm recommendation could be made due to the small size and quality of studies and heterogeneity of included participants, interventions and outcomes. Zhou 2019 included 10 of the studies included in our review, with an additional four that were either excluded from our review or not obtainable. We excluded three due to the use of a non‐validated symptom measurement instrument (Li 2008; Liang 2010; Madhu 1988). We contacted the author of Zhou 2019 regarding studies they included published in Chinese that were not retrieved from our searches. We learned from the lead author that citations given in their published systematic review did not match with the manuscripts they reviewed and provided to us. One of these was a study cited as "Yue Zhao 2013" that we could not find. The authors provided us with a study that we excluded due to it not being an RCT or including people with IBS (Lui 2007). This raises some concerns with the overall quality of the Zhou 2019 review.
A third review assessed self‐management strategies on IBS symptoms and quality of life and included 'exercise' as an eligible intervention (Cong 2018). They included 20 studies, one of which was also included in our review (Shahabi 2016). This systematic review differed to ours in that it included a number of types of study designs that did not meet our eligibility criteria, and it did not include a meta‐analysis or present results of quality assessments of the included studies.
Authors' conclusions
Implications for practice.
The results from a small body of low‐ and very low‐certainty evidence suggests that physical activity comprising of yoga, treadmill exercise or advice and support to increase physical activity may improve symptoms but not quality of life or abdominal pain in people diagnosed with irritable bowel syndrome. Discussions with patients considering physical activity as part of symptom management should address the very uncertain evidence base to ensure a fully informed decision is made.
Implications for research.
The evidence available for this review was limited in quality and quantity. Large, higher‐quality studies are needed to address the low certainty of existing evidence and to address the dearth of evidence on the potential adverse effects of physical activity. The evidence‐base would also be improved by the use of high‐quality randomised controlled trials with evaluations of long‐term effects and clear reporting of methods, especially those relating to risk of bias and the intervention(s) given and could consider the use of specific reporting checklists (e.g. the template for intervention description and replication (TIDieR, Hoffman 2014). Given its probable application to a large number of people and to most settings, high‐quality studies assessing the impact of walking would provide clinically applicable evidence.
Further research would benefit from considering as part of their intervention specific behavioural change theories and methodologies that better reflect the evidence‐to‐practice gap. Patients, healthcare professionals and other relevant consumers should be consulted to ascertain if physical activity interventions are of sufficient importance to warrant further high‐quality trials and, if so, the population, interventions and outcomes of interest that should be assessed.
History
Protocol first published: Issue 1, 2015
Acknowledgements
The authors wish to thank Joanna Boughtflower who supported the development and writing of the original protocol.
Cochrane Gut supported the authors in the development of this systematic review.
The following people conducted the editorial process for this article.
Co‐ordinating Editor/Sign‐off Editor (final editorial decision): Professor Paul Moayyedi, Cochrane Gut Group, McMaster University, Canada.
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Teo Quey, Yuhong Yuan, Cochrane Gut Group, McMaster University, Canada.
Copy Editor (copy‐editing and production): Anne Lawson, Copy Edit Support, Cochrane.
Peer‐reviewers (provided comments and recommended an editorial decision): Dr Rinaldo Pellicano, Gastroenterology Unit, Molinette hospital, Turin, Italy (clinical review); Professor Dan Lucian Dumitraşcu, Iuliu Hatieganu University of Medicine and Pharmacy, Cluj County Clinical Emergency Hospital, Cluj‐Napoca, Romania (clinical review); Dr SS Hoque, lead gastroenterologist, Barts Health NHS Trust, Whipps Cross University Hospital, Queen Mary University of London (clinical review); Dr Kaylan Saginala (clinical review); Dr Yuhong Yuan, McMaster University, Canada (search review).
Funding for the IBD/FBD (Inflammatory Bowel Disease and Functional Bowel Disorders) Review Group (1 September 2010 to 31 August 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON – 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III) and the Ontario Ministry of Health and Long Term Care (HLTC3968FL‐2010‐2235).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Appendices
Appendix 1. Electronic search strategies
CENTRAL (Cochrane Library, Wiley)
IDSearch #1MeSH descriptor: [Irritable Bowel Syndrome] explode all trees #2MeSH descriptor: [Colonic Diseases] this term only #3MeSH descriptor: [Colonic Diseases, Functional] this term only #4((irritable or functional or spastic) next (bowel or colon)):ti,ab,kw (Word variations have been searched) #5((irritable or functional or spastic) near (bloat* or constipat* or diarrh*)):ti,ab,kw (Word variations have been searched) #6ibs:ti,ab,kw (Word variations have been searched) #7#1 or #2 or #3 or #4 or #5 or #6 #8MeSH descriptor: [Exercise] explode all trees #9MeSH descriptor: [Exercise Therapy] explode all trees #10MeSH descriptor: [Physical Therapy Modalities] explode all trees #11MeSH descriptor: [Motor Activity] explode all trees #12MeSH descriptor: [Physical Exertion] explode all trees #13MeSH descriptor: [Physical Fitness] explode all trees #14MeSH descriptor: [Physical Education and Training] explode all trees #15MeSH descriptor: [Sports] explode all trees #16(physical* near (activ* or fitness or fit or train* or endur* or exertion)):ti,ab,kw (Word variations have been searched) #17motor activity:ti,ab,kw (Word variations have been searched) #18(walk or walking or running or jogging or bicycl* or aerobic* or swim* or sport*):ti,ab,kw (Word variations have been searched) #19((cycle or cycling) near (school* or work or workplace or commut* or travel* or leisure* or recreation*)):ti,ab,kw (Word variations have been searched) #20((resistance or weight* or strength*) near train*):ti,ab,kw (Word variations have been searched) #21((lifestyle* or life style* or leisure* or recreation*) near (activ* or physical*)):ti,ab,kw (Word variations have been searched) #22((lifestyle or life style) near (modif* or change*)):ti,ab,kw (Word variations have been searched) #23(outdoor near (lifestyle* or life style* or activit* or recreation*)):ti,ab,kw (Word variations have been searched) #24#8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 #25#7 and #24 #26MeSH descriptor: [Irritable Bowel Syndrome] explode all trees and with qualifier(s): [Rehabilitation ‐ RH] #27MeSH descriptor: [Colonic Diseases, Functional] explode all trees and with qualifier(s): [Rehabilitation ‐ RH] #28#25 or #26 or #27
MEDLINE (OvidSP)
# ▲SearchesResults
1Irritable Bowel Syndrome/6468
2colonic diseases/ or colonic diseases, functional/20586
3((irritable or functional or spastic) adj (bowel or colon)).ti,ab.13006
4((irritable or functional or spastic) adj5 (bloat* or constipat* or diarrh*)).ti,ab.3001
5ibs.ti,ab.7815
61 or 2 or 3 or 4 or 534177
7exp Exercise/174424
8exp Exercise Therapy/44875
9exp Physical Therapy Modalities/142118
10motor activity/99847
11physical exertion/61142
12physical fitness/27544
13exp "Physical Education and Training"/14381
14exp Sports/173862
15exercise*.ti,ab.270146
16(physical* adj3 (activ* or fitness or fit or train* or endur* or exertion)).ti,ab.118333
17(motor adj activity).ti,ab.14758
18(walk or walking or running or jogging or bicycl* or aerobic? or swim? or swimming or sport*).ti,ab.324527
19((cycle or cycling) adj5 (school* or work or workplace or commut* or travel* or leisure* or recreation*)).ti,ab.2560
20((resistance or weight* or strength*) adj3 train*).ti,ab.15714
21((lifestyle* or life style* or leisure* or recreation*) adj5 (activ* or physical*)).ti,ab.21510
22((lifestyle or life style) adj5 (modif* or change*)).ti,ab.19043
23(outdoor adj5 (lifestyle* or life style* or activit* or recreation*)).ti,ab.2048
247 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23907443
256 and 241010
26Irritable Bowel Syndrome/rh [Rehabilitation]17
27Colonic Diseases, Functional/rh [Rehabilitation]7
2825 or 26 or 271031
29randomized controlled trial.pt.505458
30controlled clinical trial.pt.100426
31randomized.ab.442267
32placebo.ab.205474
33drug therapy.fs.2147127
34randomly.ab.305249
35trial.ab.465908
36groups.ab.1885345
3729 or 30 or 31 or 32 or 33 or 34 or 35 or 364448873
38exp animals/ not humans.sh.4743200
3937 not 383847673
4028 and 39366
Embase (OvidSP)
# ▲SearchesResults
1irritable colon/22041
2*colon disease/ or intestine function disorder/7678
3((irritable or functional or spastic) adj (bowel or colon)).ti,ab.19025
4((irritable or functional or spastic) adj5 (bloat* or constipat* or diarrh*)).ti,ab.4752
5ibs.ti,ab.12988
61 or 2 or 3 or 4 or 536646
7exp Exercise/289377
8exp kinesiotherapy/66578
9physiotherapy/76366
10physical activity/123682
11fitness/35916
12training/76294
13exp sport/137125
14exercise*.ti,ab.327899
15(physical* adj3 (activ* or fitness or fit or train* or endur* or exertion)).ti,ab.145946
16(motor adj activity).ti,ab.17132
17(walk or walking or running or jogging or bicycl* or aerobic? or swim? or swimming or sport*).ti,ab.387991
18((cycle or cycling) adj5 (school* or work or workplace or commut* or travel* or leisure* or recreation*)).ti,ab.2857
19((resistance or weight* or strength*) adj3 train*).ti,ab.17774
20((lifestyle* or life style* or leisure* or recreation*) adj5 (activ* or physical*)).ti,ab.26442
21((lifestyle or life style) adj5 (modif* or change*)).ti,ab.26322
22(outdoor adj5 (lifestyle* or life style* or activit* or recreation*)).ti,ab.2462
237 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 221061902
246 and 231360
25randomized controlled trial/483842
26single blind procedure/ or double blind procedure/174315
27crossover procedure/54247
28random*.tw.1265503
29((singl* or doubl*) adj (blind* or mask*)).tw.207876
30(crossover or cross over or factorial* or latin square).tw.125783
31(assign* or allocat* or volunteer*).tw.668286
3225 or 26 or 27 or 28 or 29 or 30 or 311907142
3324 and 32244
CINAHL (EBSCOHost)
#Query
1( ((irritable or functional or spastic) N1 (bowel or colon)) ) OR ( ((irritable or functional or spastic) N5 (bloat* or constipat* or diarrh*)) ) OR ibs
2(MH "Irritable Bowel Syndrome")
3(MH "Colonic Diseases, Functional") OR (MH "Colonic Diseases")
4S1 OR S2 OR S3
5(MH "Exercise+") OR (MH "Therapeutic Exercise+") OR (MH "Physical Therapy+")
6(MH "Physical Endurance") OR (MH "Physical Activity") OR (MH "Physical Performance")
7(MH "Physical Fitness") OR (MH "Physical Education and Training+")
8(MH "Sports+")
9( (physical* N3 (activ* or fitness or fit or train* or endur* or exertion)) ) OR "motor activity" OR ( (walk or walking or running or jogging or bicycl* or aerobic* or swim* or sport*) ) OR ( ((cycle or cycling) N5 (school* or work or workplace or commut* or travel* or leisure* or recreation*)) ) OR ( ((resistance or weight* or strength*) N3 train*) ) OR ( ((lifestyle* or "life style*" or leisure* or recreation*) N3 (activ* or physical*)) ) OR ( ((lifestyle or "life style") N3 (modif* or change*)) ) OR ( (outdoor N3 (lifestyle* or "life style*" or activit* or recreation*)) )
10S5 OR S6 OR S7 OR S8 OR S9
11S4 AND S10
12S4 AND S10 Limiters ‐ Clinical Queries: Therapy ‐ Best Balance
Science Citation Index & Conference Proceedings Citation Index (Web of Science)
# 4322
#3 AND #2 AND #1
# 3249,808,601TS=((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*))
# 21,141,541
TS=((physical* NEAR/3 (activ* or fitness or fit or train* or endur* or exertion))) OR TS=("motor activity") OR TS=(exercise*) OR TS=((walk or walking or running or jogging or bicycl* or aerobic* or swim* or sport*)) OR TS=(((cycle or cycling) NEAR/5 (school* or work or workplace or commut* or travel* or leisure* or recreation*))) OR TS=(((resistance or weight* or strength*) NEAR/3 train*)) OR TS=(((lifestyle* or "life style*" or leisure* or recreation*) NEAR/3 (activ* or physical*))) OR TS=(((lifestyle or "life style") NEAR/3 (modif* or change*))) OR TS=((outdoor NEAR/3 (lifestyle* or "life style*" or activit* or recreation*)))
# 123,806
TS=(((irritable or functional or spastic) NEAR/1 (bowel or colon))) OR TS=(((irritable or functional or spastic) NEAR/5 (bloat* or constipat* or diarrh*))) OR TS=(IBS)
SportDISCUS (EBSCOHost)
#QueryResults
S15S13 AND S1484
S14random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*89,132
S13( ((irritable or functional or spastic) N1 (bowel or colon)) ) OR ( ((irritable or functional or spastic) N5 (bloat* or constipat* or diarrh*)) ) OR ibs320
PEDRo
Search terms:Hits:
"irritable bowel "
"irritable colon"
"spastic bowel"
"spastic colon"
"functional bowel"
"functional colon"
Total: 26
ClinicalTrials.gov
("irritable bowel" OR "irritable colon") AND (exercise OR exercises OR sport OR sports OR walk OR walking OR swim OR swimming OR cycle OR cycling OR bicycle OR bicycling OR "physical activity" OR training)94
("spastic bowel" OR "spastic colon") AND (exercise OR exercises OR sport OR sports OR walk OR walking OR swim OR swimming OR cycle OR cycling OR bicycle OR bicycling OR "physical activity" OR training)94
("functional bowel" OR "functional colon") AND (exercise OR exercises OR sport OR sports OR walk OR walking OR swim OR swimming OR cycle OR cycling OR bicycle OR bicycling OR "physical activity" OR training)4
Total: 192
WHO ICTRP
Irritable bowel AND exercise OR irritable bowel AND exercises OR irritable bowel AND sport OR irritable bowel AND sports OR irritable bowel AND physical activity OR irritable bowel AND walk OR irritable bowel AND walking OR irritable bowel AND run OR irritable bowel AND running OR irritable bowel AND swim OR irritable bowel AND swimming OR irritable bowel AND cycle OR irritable bowel AND cycling OR irritable bowel AND bicycle OR irritable bowel AND bicycling OR irritable bowel AND training36 Irritable colon AND exercise OR irritable colon AND exercises OR irritable colon AND sport OR irritable colon AND sports OR irritable colon AND physical activity OR irritable colon AND walk OR irritable colon AND walking OR irritable colon AND run OR irritable colon AND running OR irritable colon AND swim OR irritable colon AND swimming OR irritable colon AND cycle OR irritable colon AND cycling OR irritable colon AND bicycle OR irritable colon AND bicycling OR irritable colon AND training53 Spastic bowel AND exercise OR spastic bowel AND exercises OR spastic bowel AND sport OR spastic bowel AND sports OR spastic bowel AND physical activity OR spastic bowel AND walk OR spastic bowel AND walking OR spastic bowel AND run OR spastic bowel AND running OR spastic bowel AND swim OR spastic bowel AND swimming OR spastic bowel AND cycle OR spastic bowel AND cycling OR spastic bowel AND bicycle OR spastic bowel AND bicycling OR spastic bowel AND training0 Spastic colon AND exercise OR spastic colon AND exercises OR spastic colon AND sport OR spastic colon AND sports OR spastic colon AND physical activity OR spastic colon AND walk OR spastic colon AND walking OR spastic colon AND run OR spastic colon AND running OR spastic colon AND swim OR spastic colon AND swimming OR spastic colon AND cycle OR spastic colon AND cycling OR spastic colon AND bicycle OR spastic colon AND bicycling OR spastic colon AND training48 Functional bowel AND exercise OR functional bowel AND exercises OR functional bowel AND sport OR functional bowel AND sports OR functional bowel AND physical activity OR functional bowel AND walk OR functional bowel AND walking OR functional bowel AND run OR functional bowel AND running OR functional bowel AND swim OR functional bowel AND swimming OR functional bowel AND cycle OR functional bowel AND cycling OR functional bowel AND bicycle OR functional bowel AND bicycling OR functional bowel AND training2 Functional colon AND exercise OR functional colon AND exercises OR functional colon AND sport OR functional colon AND sports OR functional colon AND physical activity OR functional colon AND walk OR functional colon AND walking OR functional colon AND run OR functional colon AND running OR functional colon AND swim OR functional colon AND swimming OR functional colon AND cycle OR functional colon AND cycling OR functional colon AND bicycle OR functional colon AND bicycling OR functional colon AND training0 Total: 139
Data and analyses
Comparison 1. Physical activity compared with usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 IBS symptoms | 5 | 185 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.44, ‐0.42] |
| 1.2 IBS symptoms (studies with low risk of selection bias) | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.44, 0.21] |
| 1.3 Quality of life | 4 | 134 | Std. Mean Difference (IV, Random, 95% CI) | 1.17 [‐0.30, 2.64] |
| 1.4 Quality of life (studies with low risk of selection bias) | 2 | 64 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.61, 0.38] |
| 1.5 Abdominal pain | 2 | 64 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.48, 0.50] |
Comparison 2. Yoga compared with walking.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 IBS symptoms | 2 | 124 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐3.93, 1.62] |
| 2.2 Quality of life | 1 | 97 | Mean Difference (IV, Random, 95% CI) | 53.45 [38.85, 68.05] |
| 2.3 Abdominal pain | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [‐0.79, 5.39] |
Comparison 3. Studies with similar physical activity interventions compared with any control .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 IBS symptoms | 9 | 389 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.55, ‐0.16] |
| 3.1.1 Yoga intervention | 5 | 225 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐2.01, 0.51] |
| 3.1.2 Supervised treadmill exercise | 2 | 71 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.64, 0.15] |
| 3.1.3 Advice to increase physical activity | 2 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.61, 0.17] |
| 3.2 Quality of life | 6 | 290 | Std. Mean Difference (IV, Random, 95% CI) | 1.00 [0.04, 1.97] |
| 3.2.1 Yoga intervention | 3 | 177 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.59, 1.79] |
| 3.2.2 Supervised treadmill exercise | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 2.39 [1.18, 3.59] |
| 3.2.3 Advice to increase physical activity | 2 | 93 | Std. Mean Difference (IV, Random, 95% CI) | 1.04 [‐1.65, 3.74] |
| 3.3 Abdominal pain | 2 | 48 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.45, 0.72] |
| 3.3.1 Yoga intervention | 2 | 48 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.45, 0.72] |
Comparison 4. Physical activity compared with pharmacological therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 IBS symptoms | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.65, 0.25] |
Comparison 5. Physical activity compared with dietary interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 IBS symptoms | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | 33.31 [‐12.86, 79.48] |
| 5.1.1 12 weeks | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | 33.31 [‐12.86, 79.48] |
| 5.2 Quality of life | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐4.47, 3.28] |
| 5.2.1 12 weeks | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐1.67 [‐7.62, 4.28] |
| 5.2.2 24 weeks | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐4.90, 5.30] |
| 5.3 Abdominal pain | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | 9.83 [‐1.83, 21.50] |
| 5.3.1 12 Weeks | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | 12.00 [‐4.96, 28.96] |
| 5.3.2 24 Weeks | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | 7.89 [‐8.19, 23.97] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Daley 2008.
| Study characteristics | ||
| Methods |
Study design: RCT Funding: not reported Published protocol/trial registration: no |
|
| Participants | Adults aged 18–65 years attending a district general hospital in Sutton Coldfield, UK Number randomised: 56 Age: mean 43.1 (SD 12.4) years % Female: 73% SES and ethnicity: white 93%; in paid employment 66% Inclusion criteria
Exclusion criteria
Recruitment: identified from hospital records Recruitment rate: 18.4% (56/305) Region: Sutton Coldfield, UK |
|
| Interventions |
Number of experimental conditions: 2; exercise intervention and usual care control Number of participants (analysed): intervention: 22; control: 21 Description of intervention: 2 individual person‐centred exercise consultations over 12 weeks. Intervention centred on equipping participants with skills, knowledge and confidence so that they felt able to participate in regular exercise. First consultation centred on uptake of exercise and focused on enhancing motivation, self‐efficacy for exercise, perceived pros and cons of becoming more physically active, overcoming barriers and developing appropriate activity plans. Participants given a pedometer as a motivational tool and to assist in quantifying amount of activity each day/week. Second consultation at week 4 centred on prevention of relapse back to sedentary behaviour or improving maintenance of an active lifestyle (or both). Exercise patterns over previous 4 weeks reviewed. Participants were mailed postcard prompts that encouraged exercise during weeks 3 and 9. Duration: 12 weeks Number of contacts: 2 (40‐minute consultations at baseline and week 4) Setting: hospital and home Modality: face‐to‐face consultations followed by unsupervised (with pedometer and mailed postcard prompts) Interventionist: trial research assistant Integrity: "A review of exercise patterns over the previous four weeks was also included." Date of study: not reported Description of control: participants were asked not to change their current exercise patterns during study and offered an exercise consultation and pedometer at end of study. |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcome relating to reported adverse events: not reported Outcome assessment time points: 12 weeks – end of intervention Preplanned subgroup analyses: IBS subgroups (IBS‐C, IBS‐D and IBS‐M) Unplanned subgroup analyses: – Lost to follow‐up: 13 participants did not return follow‐up questionnaires (intervention: 6; control: 7) Analysis: per‐protocol |
|
| Notes | Minimal/clinically important difference was not considered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list. |
| Allocation concealment (selection bias) | Low risk | Allocation performed remotely via telephone. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Group allocation not blinded to participants, clinicians or researchers. However, it was not possible to blind participants and personnel for this intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Group allocation was not blinded to participants, clinicians or researchers. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Similar number of dropouts in both groups (intervention: 6; control: 7), for same reasons (not returning questionnaire). Stated ITT analysis was performed in methods but number of participants analysed (intervention: 22; control: 21) did not match with numbers randomised (28 each for intervention and control). Overall dropout rate was 23.2%. |
| Selective reporting (reporting bias) | High risk | No protocol or trial registration to determine whether outcomes reported were preplanned. Reporting of outcomes in the results and discussion sections focused on findings of secondary outcomes with statistically significant P values with little mention of a null finding for the primary outcome. |
| Other bias | Low risk | Differences between groups for a number of baseline characteristics (e.g. weight, use of medications), but performed covariate analysis. |
Evans 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Funding: not reported Published protocol/trial registration:trialsjournal.biomedcentral.com/articles/10.1186/1745-6215-12-15; www.clinicaltrials.gov/ct2/show/NCT01107977 |
|
| Participants | Males and females aged 14–26 years with a diagnosis of having either recurrent abdominal pain, or IBS using Rome III adult criteria for 18‐ to 26‐year‐olds. Number randomised: 21 Age: overall: mean 19.0 (SD 3.9) years; mean age not reported for separate age groups. Young adults range 18–26 years % Female: overall: 84.3%; not given for separate age groups SES and ethnicity: Asian 4% overall; African American 6% overall; multiracial 18% overall; white 72% overall. Education status (highest): Masters degree or higher 2% overall; (lowest): some high school 20% overall Inclusion criteria
Exclusion criteria
Recruitment: advertisements in gastroenterology offices and local community bulletin boards, support group newsletters and events, physician referrals of patients, postings in university and local community toilet facilities and online sources (e.g. Craigslist, ClinicalTrials.gov website) Recruitment rate: 58.9% (76/129) Region: University of California, Los Angeles, USA |
|
| Interventions |
Number of experimental conditions: 2; exercise intervention and usual care control Number of participants (analysed): intervention: 11; control: 10 Description of intervention: 6 weeks of classes twice per week. Each class 1.5 hours in duration (total 18 hours). A make up class was available. Maximum 6 students, led by an experienced yoga teacher and assisted by a junior teacher. To standardise delivery, senior teacher developed a working list of poses and was an advisor for study. Range of yoga postures taught to students (reclining postures, standing postures, forward bends, supported inversions, backbends and seated postures) using props. Classes were sequenced, and as students developed skills, more challenging postures were introduced. To ensure access, classes held during a weekday evening and a weekend afternoon. Homework was suggested, but not required, and interested participants were invited to take props home. Duration: 6 weeks Number of contacts: twice per week Setting: UCLA Pediatric Pain Program Yoga Studio and home Modality: supervised Interventionist: yoga teacher, research assistants Integrity: to standardise delivery, a senior teacher developed a working list of poses and served as an advisor to the study. Date of study: October 2009 to May 2013 Description of control: participants continued with usual care under supervision of their primary care physician or gastroenterologist. |
|
| Outcomes |
Primary outcome
Secondary outcome
Outcome relating to reported adverse events
Outcome assessment time points: 6 weeks – end of intervention; 14 weeks – post‐intervention follow‐up (8 weeks) Preplanned subgroup analyses: adolescents vs young adults Unplanned subgroup analyses: – Lost to follow‐up: dropout before end of intervention: intervention: 10; control: 15; dropout before 2‐month follow‐up: intervention: 15; control: 16 (additional) Analysis: per‐protocol |
|
| Notes | An MCID of 50 points for the IBS‐SSS was used to power the study and later referred to in the discussion. This was used because an MCID value was not available for the symptom tool used (CSI). Methods stated clinical significance for global improvement outcome was calculated using percentage of participants reporting global improvement ratings above "slight improvement". Clinical significance for abdominal pain was calculated, with a change of –1.74 points on the NRS considered clinically meaningful for young adults. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Research randomiser programme. |
| Allocation concealment (selection bias) | Low risk | Researcher or principal investigator not involved in study allocated participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Group allocation was not blinded to participants, clinicians or researchers. However, it was not possible to blind participants and personnel for this intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Emailed link to complete questionnaires. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall 33% dropout with unequal numbers of dropouts between groups (intervention: 26%; control: 41%); no ITT analysis performed. |
| Selective reporting (reporting bias) | Low risk | Data reported for all outcomes as prespecified in the trial registry entry. |
| Other bias | Unclear risk | No information on the subgroup characteristics provided. Covariate analysis performed but information on specific covariates not provided. |
Fani 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Funding: Isfahan University of Medical Sciences Published protocol/trial registration: no |
|
| Participants | 20 women with mild and moderate IBS enrolled in a controlled clinical trial performed at the Musculoskeletal Disorders Research Center of the rehabilitation faculty of Isfahan University of Medical Sciences Number randomised: 20 Age: intervention: mean 29.1 (SD 6.8) years; control: mean 32.7 (SD 10.3) years % Female: 100% SES and ethnicity: unmarried 45%; diploma or higher (educational status) 100% Inclusion criteria
Exclusion criteria
Recruitment: referred to the centre by gastroenterologists from across the city Recruitment rate: not reported Region: Isfahan, Iran |
|
| Interventions |
Number of experimental conditions: 2; exercise intervention and usual activities control Number of participants (analysed): intervention: 10; control: 10 Description of intervention: treadmill exercises 3 times per week for 6 weeks. Target and maximum heart rate were calculated (target heart rate = 70% maximum heart rate, maximum heart rate = 220 – age). Treadmill used was able to record heart rate by participant placing her hands on treadmill handles. Participants walked at slow speed for 5 minutes to warm‐up. Then increased speed until they reached the target heart rate and maintained it for 20 minutes. Then walked for 5 minutes at slow speed to cool‐down. Treadmill used with supervision of physiotherapist in all sessions. Duration: 6 weeks Number of contacts: 18 Setting: university Modality: supervised Interventionist: physiotherapist Integrity: – Date of study: not reported Description of control: usual daily activities |
|
| Outcomes |
Primary or secondary not specified Outcome relating to reported adverse events: not reported Outcome assessment time points: 6 weeks – end of intervention Preplanned subgroup analyses: none Unplanned subgroup analyses: – Lost to follow‐up: none Analysis: 0 dropouts, therefore ITT |
|
| Notes | MCID is not specifically given for any measured outcomes but methods stated clinical severity of IBS symptoms was categorised into mild (40%), moderate (35%) and severe (25%) groups based on a score of 75–175 (mild), 175–300 (moderate) and > 300 (severe) from the IBS‐SSS questionnaire. No reference to severity in the results or discussion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Group allocation not blinded to participants, clinicians or researchers. However, it was not possible to blind participants and personnel for this intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Group allocation was not blinded to participants, clinicians or researchers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported dropouts. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration to determine whether outcomes reported were preplanned. |
| Other bias | Unclear risk | Insufficient details about baseline characteristics (e.g. other morbidities impacting on outcomes) and no information on covariate analysis. |
Feng 2010.
| Study characteristics | ||
| Methods |
Study design: RCT Funding: National Sports General Administration Health Qigong Center Fund Project (QG06B005) Published protocol/trial registration: no |
|
| Participants | Adults aged 60–75 years Number randomised: 60 Age: intervention: mean 66.5 (SD 3.3) years; comparator: mean 66.5 (SD 3.2) years % Female: intervention: 43%; comparator: 37% SES and ethnicity: Chinese 100% Inclusion criteria
Exclusion criteria
Recruitment: not reported Recruitment rate: not reported Region: Guangzhou, China |
|
| Interventions |
Number of experimental conditions: 2; exercise intervention + pharmacological therapy and pharmacological therapy comparator Number of participants (analysed): intervention: 30; comparator: 30 Description of intervention: individual sessions using a National (published) Banduajin (Qi‐gong) programme consisting of 2 sets of Qi‐gong exercises with a 2‐minute break between sets for a total of 45 minutes + tegaserod 6 mg Duration: 12 weeks Number of contacts: 2 sessions (morning and evening), 5 days per week Setting: mixed – supervised and home Modality: 2 weeks of supervised training followed by unsupervised home programme Interventionist: Qi‐gong instructor delivered training (not part of research team) Integrity: follow‐up telephone calls at 1, 2, 6 and 12 weeks Date of study: not reported Description of comparator: tegaserod 6 mg only |
|
| Outcomes |
Primary and secondary outcomes not specified Outcome relating to reported adverse events: not reported Outcome assessment time points: 12 weeks – end of intervention Preplanned subgroup analyses: none Unplanned subgroup analyses: not applicable Lost to follow‐up: 0 Analysis: not reported |
|
| Notes | Funded by "Qigong Center Project of the State Sports General Administration (QG06B005)". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Group allocation was not blinded to participants, clinicians or researchers. However, it was not possible to blind participants and personnel for this intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants followed up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration to determine whether outcomes reported were preplanned. |
| Other bias | Unclear risk | Insufficient details about baseline characteristics (e.g. other morbidities impacting on outcomes) and no information on covariate analysis. |
Hajizadeh Maleki 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Funding: not reported Published protocol/trial registration: no |
|
| Participants | Women fulfilling Rome III criteria for IBS from the outpatient clinical of the Department of Internal Medicine of the University Hospital in Urmia, Iran Number randomised: 60 Age: mean 34 (SD 7.5) years % Female: 100% SES and ethnicity: not reported Inclusion criteria
Exclusion criteria
Recruitment: 109 women (aged 18–41 years) from the outpatient clinic of the Department of Internal Medicine of the University Hospital in Urmia, Iran, were screened. Recruitment rate: 109 screened, 60 randomised (55%) Region: Urmia, Iran |
|
| Interventions |
Number of experimental conditions: 2; exercise intervention and usual activities control Number of participants (analysed): intervention: 24; control: 27 Description of intervention: during first 12 weeks of intervention, participants walked or jogged (between 6 a.m. and 8 a.m.) on a treadmill at 45–55% of VO2max (25–30 minutes per day, 3–4 days per week), and then exercised at 56–69% of VO2max (40–45 minutes per day, 4–6 days per week) over the final 12 weeks. Exercise adherence measured using Polar heart rate monitors, and participants were given feedback to modify to the prescribed exercise intensity. Duration: 24 weeks Number of contacts: 3–4 days per week during first 12 weeks; 4–6 days per week during final 12 weeks Setting: university hospital outpatient Modality: supervised Interventionist: not specified Integrity: – Date of study: not reported Description of control: usual physical activity levels |
|
| Outcomes |
Primary and secondary outcomes not specified Outcome relating to reported adverse events: not reported Outcome assessment time points: 24 weeks – end of intervention; 28 weeks – post‐intervention follow‐up; 32 weeks – post‐intervention follow‐up Preplanned subgroup analyses: none Unplanned subgroup analyses: not applicable Lost to follow‐up: overall: 30%; intervention: 6/30 (20%); control: 3/30 (10%) Analysis: per‐protocol |
|
| Notes | MCID not considered for any measured outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Random number generation was used for randomization in which, at the time of randomization, sealed envelope containing group assignment was opened by the study coordinator". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Random number generation was used for randomization in which, at the time of randomization, sealed envelope containing group assignment was opened by the study coordinator". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No reporting of who was blinded in the study methods or discussion but not possible to blind participants and individuals supervising the exercise. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No reporting of who was blinded in the study methods or discussion. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 (20%) dropouts from intervention group, 3 (10%) from control group (30% overall) and ITT was not performed. |
| Selective reporting (reporting bias) | High risk | No protocol or trial registration to determine whether outcomes reported were preplanned. Data were reported for all outcomes described in the methods but not in the order specified in the methods. Data for new outcomes were added in the results and new outcomes were mentioned in the discussion but without any data presented or provided in the results section. |
| Other bias | Unclear risk | No information on covariate analysis and no information as to the conditions under which dietary and medication data were collected (e.g. in person, telephone, mail). |
Jia 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Funding: not reported Published protocol/trial registration: no |
|
| Participants | People with IBS‐D treated in the gastroenterology department outpatient of a top 3 hospital in Yangzhou selected through convenience sampling
Number randomised: 90 Age: mean 41.5 (SD 10.7) years % Female: 49% SES and ethnicity: married: overall: 85%; intervention: 26/30 (87%); control: 18/20 (90%). Education status (highest): college degree: overall: 17%; intervention: 12/30 (40%); control: 8/20 (40%). Employment: employed: overall: 64%; intervention: 22/30 (73%); control: 11/20 (55%); retired: intervention: 4/30 (13%); control: 6/20 (30%) Inclusion criteria
Exclusion criteria
Recruitment: convenience sample; city level hospital; no information on sampling strategy Recruitment rate: not reported Region: Yangzhou, China |
|
| Interventions |
Number of experimental conditions: 3; exercise intervention, pharmacotherapy intervention and usual activities control Number of participants (analysed): exercise intervention: 30; pharmacotherapy intervention: 32; control: 20 Description of exercise intervention: moderate‐intensity aerobic exercises that involved major muscle groups, e.g. jogging, speed‐walking, cycling, swimming. Participants chose form of exercises according to personal conditions and preferences. Moderate intensity defined by heart rate during exercises that was self‐tested and reached 55–65% of individual maximum heart rate (calculated by 220 – age according to the guideline by American College of Sports Medicine). Length of exercise each time 30–60 minutes. Frequency of exercises 3–5 times per week. Participants kept a diary of exercises for 12 weeks. Supervision group consisted of researchers, gastroenterology clinicians and rehabilitation nurses and provided monthly guidance through telephone, texts and emails to ensure participants stuck to the exercise plans and answered questions from participants. Duration: 12 weeks Number of contacts: 3 (for all groups) – monthly telephone and email for exercise group (advice); monthly telephone only for control group (reason for call not given) Setting: home Modality: unsupervised Interventionist: not applicable Integrity: monthly follow‐up and exercise diary. Participants recorded exercises in a diary throughout the 12‐week intervention to ensure participants carried out exercise plans rigorously and provide data. Date of study: only date period for enrolment reported – March 2014 to July 2015 Description of pharmacotherapy intervention: people with anxiety or depression received flupentixol/melitracen (Deanxit). People with abdominal pain received pinaverium bromide (Dicetel). People with diarrhoea received oral live combined bifidobacterium, lactobacillus and enterococcus powder. Every month the researchers contacted the participants by telephone to check their general health. Description of control: participants did not use medications or any other interventions and maintained their own lifestyles and habits. Every month, researchers contacted participants by telephone to check their general health condition. |
|
| Outcomes |
Primary and secondary outcomes not specified Outcome relating to reported adverse events: not reported Outcome assessment time points: 12 weeks – end of intervention Preplanned subgroup analyses: no Unplanned subgroup analyses: no Lost to follow‐up: intervention: 5 (14%); control: 0 Analysis: per‐protocol (quote: "Only the participants who completed the trial were included in the analysis"). |
|
| Notes | Severity categorisation used for IBS‐SSS: maximum score was 500 and scores in each domain were stratified into 4 levels: < 75 was remission, 75–175 was mild symptom, 175–300 was moderate symptom and > 300 was sever symptom. Total score was sum of the 5 domains. At end of intervention, efficacy was classified into 4 grades according to total score: total score < 75 was "cured"; a reduced total score by 2 levels was "substantially improved" symptoms; a reduced total score by 1 level was "improved" symptoms; a total score remaining at the same level or increased was "not improved" symptoms. For IBS‐QOL, a score of > 80 (out of 100) was regarded as no influence on quality of life. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were randomised into 3 groups using a list of random numbers. |
| Allocation concealment (selection bias) | High risk | List of random numbers was an open list available on the site of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Investigators provided monthly guidance on exercises and checked the use of medications in participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Scores were made based on the structured questionnaires filled by participants under the on‐site guidance from trained investigators. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unequal dropout. 5 (14%) participants in the exercise intervention group dropped out due to pregnancy (1), using antibiotics (1) and loss of follow‐up (3). No participants dropped out in control group. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the methods section were reported, though not in matching same order. No protocol or trial registration to determine whether outcomes reported were preplanned. |
| Other bias | Unclear risk | Formula for sample size calculation are given but no indication as to which specific outcome/measure this related to. No information on covariate analysis. |
Johannesson 2011.
| Study characteristics | ||
| Methods |
Study design: RCT Funding: Health and Medical Care Executive Board of the Västra Götaland Region and by the Research and Development Council in Södra Älvsborg, Sweden Published protocol/trial registration: no |
|
| Participants | People with clinical diagnosis of IBS, based on the ROME II criteria referred from gastroenterology units at community hospitals and a university hospital in Västra Götalandsregion, Sweden. Number randomised: 102 Age: median 38 (range 18–65) years % Female: 79.4% (81 participants) SES and ethnicity: intervention: 84% employed, 54% physically demanding work, 86% high school or higher, 30% single; control: 71% employed, 45% physically demanding work, 82% high school or higher, 34% single Inclusion criteria
Exclusion criteria
Recruitment: referred from gastroenterology units at community hospitals and a university hospital in Västra Götalandsregion, Sweden Recruitment rate: 63.0% (102/162) Region: Västra Götalandsregion, Sweden |
|
| Interventions |
Number of experimental conditions: 2; exercise intervention and usual activities control Number of participants (analysed): per‐protocol (primary analysis): intervention: 37; control: 38. ITT (secondary analysis): intervention: 46; control: 45 Description of intervention: telephone contact once or twice per month with a physiotherapist who gave individual advice regarding physical activity for 12 weeks to encourage. Participants completed a training diary and performed a cycle test after 6 weeks. Duration: 12 weeks Number of contacts: 2–4 (1–2 per month) Setting: home Modality: unsupervised (telephone contact at home) with 1 supervised session at 6 weeks Interventionist: physiotherapist Integrity: advice and recommendations based on Swedish National Institute of Public Health publication, Physical Activity in the Prevention and Treatment of Disease (a guide for prescribing physical activity) and American College of Sports Medicine. Date of study: June 2005 to August 2008 Description of control: maintained their lifestyle and had supportive telephone contact with the physiotherapist once per month |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcome relating to reported adverse events: – Outcome assessment time points: 12 weeks – end of intervention Preplanned subgroup analyses: number of participants in each IBS bowel habit subgroup Unplanned subgroup analyses: – Lost to follow‐up: intervention: 13; control: 14 Analysis: per‐protocol and ITT |
|
| Notes | Reduction of 50 points on the IBS‐SSS was considered clinical improvement (cited Francis 1997). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in blocks of 4 participants, in which 2/4 were randomised to the exercise group. |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether envelopes were sealed or opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Group allocation was not blinded to participants, clinicians or researchers. However, it was not possible to blind participants and personnel for this intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information as to how questionnaire was administered or data were analysed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition: overall: 27%; intervention: 30% (16/54); control: 26% (13/50). Per‐protocol and ITT analysis performed but per‐protocol reported as primary. Stated ITT performed but some participants randomised were excluded from ITT analysis (intervention: 4; control: 7). |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration to determine whether outcomes reported were preplanned. |
| Other bias | Unclear risk | Insufficient details about baseline characteristics (e.g. other morbidities impacting on outcomes) and no information on covariate analysis. |
Kavuri 2015a.
| Study characteristics | ||
| Methods |
Study design: RCT Funding: Vivekananda Yoga Research Foundation (VYRF aka VYASA‐LA), Norwalk, California, USA Published protocol/trial registration:www.isrctn.com/ISRCTN42102754 (retrospectively) |
|
| Participants | Men and women aged ≥ 18 years with a diagnosis of IBS using Rome III criteria referred from physicians' clinics in Los Angeles, California Number randomised: 97 (including combination group) Age: mean 44.55 years % Female: 87.5% SES and ethnicity: 59.7% Hispanic (of 52 who completed study) Inclusion criteria
Exclusion criteria
Recruitment: principal recruitment strategy was to publicise the clinical trial to all gastroenterology clinics, primary care physicians and psychiatry clinics around White Memorial Medical Center, Los Angeles, California. Recruitment rate: 53.3% (64/120) Region: University of California, Los Angeles, California, USA |
|
| Interventions |
Number of experimental conditions: 3; exercise intervention, exercise + medication (combined) intervention and control Number of participants (analysed): ITT: exercise intervention: 33; combined intervention: 33; control: 31. Per‐protocol: exercise intervention: 25; combined intervention: 26; control: 27 Description of exercise intervention: yoga for 1 hour 3 days per week for 12 weeks provided under the guidance of certified Yoga instructors. Participants were advised to voluntarily reduce their medications (prescription and supplement use if any) for IBS to 3 days per week. A gastroenterologist was available in case of aggravated symptoms due to reduction in medications. Participants were excluded from analysis but not from attending yoga sessions if they were using medication > 3 times per week. Description of combined intervention: participants continued their medications, if any, under the guidance of their physician, and attended yoga classes 3 times per week for 12 weeks along with the yoga group. Duration: 12 weeks Number of contacts: 3 per week for 12 weeks; assessment at 6, 12 weeks Setting: research centre, White Memorial Medical Center, Los Angeles, California, USA Modality: supervised Interventionist: yoga instructors, principal investigator Integrity: 6 certified yoga instructors were trained, instructed and monitored by the lead yoga instructor and overseen by study principal investigator to ensure that module was followed exactly and there were no differences in the intervention received from different yoga instructors. Date of study: September 2012 to September 2014 Description of control: participants continued their medications if any, under the guidance of their physician, and were suggested to maintain their lifestyle. The research staff had advised these controls to walk for 60 minutes 3 times per week during their waiting period of 12 weeks. They were contacted and reminded 1 week before their next assessment interval at week 6 and 12. After 12 weeks of waiting, they were offered the same yoga intervention. |
|
| Outcomes |
Primary outcome
Secondary outcome
Outcome relating to reported adverse events: self‐reported Outcome assessment time points: 6 weeks – mid intervention; 12 weeks – end of intervention Preplanned subgroup analyses: IBS subgroups (IBS‐C, IBS‐D or IBS‐M) Unplanned subgroup analyses: – Lost to follow‐up: exercise intervention: 8; combined intervention: 7; control: 4 Analysis: per‐protocol and ITT |
|
| Notes | The trial was registered retrospectively approximately 15 months after the first participants were enrolled and approximately 2 years after ethical approval. Reduction of 50 points on the IBS‐SSS was considered clinical improvement (cited development paper Francis 1997). An increment of 14 points (when transformed to 0–100 range) on the IBS‐QOL was considered a clinically significant improvement. Funded by Vivekananda Yoga Research Foundation, which is also the employer of the lead author. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised sequence generated using software. |
| Allocation concealment (selection bias) | Low risk | Randomisation process was concealed from research team collecting data and outcome assessors. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Group allocation was not blinded to participants, clinicians or researchers. However, it was not possible to blind participants and personnel for this intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Questionnaires were scored by assessors blinded to group allocation and not directly involved in the research. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unequal numbers of dropouts (double the number of dropouts in exercise intervention group compared to control group); however, did perform an ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Trial registration was retrospective; therefore, unable to determine whether selective outcome reporting occurred. |
| Other bias | Unclear risk | Insufficient details about baseline characteristics (e.g. other morbidities impacting on outcomes) and no information on covariate analysis. |
Schumann 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Funding: not reported Published protocol/trial registration:clinicaltrials.gov/ct2/show/study/NCT02721836 |
|
| Participants | Men and women aged 18–75 years diagnosed with IBS using Rome III criteria for ≥ 6 months
Number randomised: 59
Age: intervention: 56.33 (SD 8.50) years; comparator: 53.52 (SD 10.47) years % Female: 88.1% SES and ethnicity: education (completed high school or further studies): intervention: 56.7%; comparator: 37.9%; work (in part‐time or full‐time work): intervention: 56.7%; comparator: 63.3% Inclusion criteria
Exclusion criteria
Recruitment: Department of Internal and Integrative Medicine via advertisements online and in the local press in March and April 2016. Recruitment rate: not reported Region: Essen, Germany |
|
| Interventions |
Number of experimental conditions: 2; exercise intervention and dietary intervention comparator Number of participants (analysed): 59 Description of intervention: traditional Hatha yoga group sessions of 75 minutes' duration twice weekly for 12 weeks designed by a certified Hatha yoga instructor, prior to the intervention, and specifically customised for people with IBS. Level, difficulty and intensity increased carefully as programme progressed. Participants were provided with a written manual and 3 × 30‐minute videos and were encouraged to practice their yoga at home every day. Prior to participants' home practice, yoga practice was introduced in class. Duration: 12 weeks Number of contacts: 24 Setting: Department of Internal and Integrative Medicine at the University of Duisburg‐Essen and home Modality: face‐to‐face, home practice Interventionist: certified Hatha yoga Instructor Integrity: intervention provided by same yoga instructor throughout the study. Date of study: recruitment occurred in March–April 2016. Dates of intervention period not reported. Description of comparator: FODMAP diet: participants received 4 × 60‐ to 90‐minute sessions of nutritional counselling; consisting of an educational group lecture, 2 sessions of individual counselling based around a food diary and a group counselling session. After the study’s 12‐week intervention period (elimination phase), participants rechallenged each week a different FODMAP group for 2–3 days during that week to test individual tolerance levels to each of the FODMAP groups (reintroduction phase). All participants completed a 6‐day food diary on 2 occasions; once during the study screening period and once during the last week of the 12‐week study intervention period. A nutritionist scored diaries according to the amount of FODMAP consumption and patient compliance. |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcome relating to reported adverse events: safety: adverse events Outcome assessment time points: 12 weeks – end of intervention; 24 weeks – end of follow‐up Preplanned subgroup analyses: exploratory subgroup analyses performed to control for potential differences within different IBS subtypes Unplanned subgroup analyses: none Lost to follow‐up: loss to week 12: intervention: 3/30 (10%); comparator: 4/29 (14%); loss to week 24: intervention: 2/27 (7%); comparator: 3/25 (12%) Analysis: ITT |
|
| Notes | The outcome of IBS‐SSS at 24 weeks was listed as a secondary outcome in the trial registry entry but reported as the primary outcome in the published paper. Additional outcomes that were not listed in the trial registry entry were reported in the study including assessment of perceived stress (PSQ), BAQ and BRS. Clinically relevant improvements for IBS symptoms were defined as decrease in ≥ 50 points on the IBS‐SSS (cites development paper Francis 1997). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent researcher not involved in recruitment created the randomisation sequence. Block randomisation with stratification by history of depression. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes prepared by an independent researcher. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Group allocation was not blinded to participants, clinicians or researchers. However, it was not possible to blind participants and personnel for this intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study outcomes were assessed at week 12 after randomisation by an outcome assessor blinded to participant's allocation; however, highly possible that blinding could have been broken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20% loss to follow‐up overall, but comparable across arms (intervention: 5/30; control: 7/29) and an ITT analysis was performed. |
| Selective reporting (reporting bias) | High risk | Secondary outcome of IBS‐SSS at 24 weeks switched to primary outcome in published study (originally intended primary outcome of IBS‐SSS at 12 weeks was reported). Non‐registered outcomes newly introduced in published study to assess perceived stress (PSQ), BAQ and BRS. |
| Other bias | Unclear risk | Insufficient details about baseline characteristics (e.g. other morbidities impacting on outcomes) but information on covariate analysis was provided. |
Shahabi 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Funding: Gail and Gerald Oppenheimer Family Foundation and Marcled Foundation Published protocol/trial registration: no |
|
| Participants | Men and women aged 18–65 years who reported their primary medical complaint as chronic abdominal pain or discomfort and associated bowel habit changes consistent with ROME III criteria for IBS. Number randomised: 35 Age: mean 36.3 (SD 12.8) years % Female: 88.9% SES and ethnicity: education 96.2% attained high school diploma; 40.7% married, 48.2% single Inclusion criteria
Exclusion criteria
Recruitment: flyers in the community, Internet announcements and referrals from University of California, Los Angeles and local physicians Recruitment rate: not reported Region: University of California, Los Angeles, USA |
|
| Interventions |
Number of experimental conditions: 2; exercise intervention and walking control Number of participants (analysed): intervention: 17; control: 10 Description of intervention: 16 group sessions of yoga offered on a biweekly basis, led by an Iyengar yoga‐certified instructor. Sessions lasted about 60 minutes. At each session, participants alternated between practicing 2 sequences of Iyengar yoga postures. Postures were first demonstrated by the instructor who also discussed the health benefits of each posture. Participants were encouraged to practice select postures at home between sessions. Pictures of the poses provided to help with home practice, and instructors discussed ways of facilitating home practice (e.g. use of cushion as a bolster). Sequences and home practice postures consisted of seated poses, inversions, backbends, twists and restorative supine poses. Postures selected because they were believed to be therapeutic for abdominal symptoms associated with IBS. Senior Iyengar yoga instructors were consulted to select postures, and sequences were approved by Mr Iyengar, the founder of the Iyengar School of Yoga, in India. Because Iyengar yoga emphasises alignment, props including bolsters, chairs, belts and blocks were used to help achieve postures. Duration: 8 weeks Number of contacts: 16 Setting: university Modality: supervised Interventionist: Iyengar yoga‐certified instructor, physical trainers Integrity: senior Iyengar yoga instructors were consulted to select postures, and sequences were approved by the founder of the Iyengar School of yoga, in India. Date of study: not reported Description of control: 16 group sessions of non‐aerobic, moderate paced, outdoor walking led by physical trainers who set the pace and led discussion during each session. Walking sessions were offered on a biweekly basis and lasted about 60 minutes. Discussion during each walking session focused on health benefits of walking and physical activity. |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome relating to reported adverse events: not reported Outcome assessment time points: 8 weeks – end of intervention; 26 weeks – post‐intervention follow‐up Preplanned subgroup analyses: none Unplanned subgroup analyses: – Lost to follow‐up: 8 (23%) until end of intervention (intervention: 5 (14%); control: 3 (9%)); 15 (43%) at 6‐month follow‐up (intervention: 10 (29%); control: 5 (14%)) Analysis: per‐protocol |
|
| Notes | No specific MCID stated for any of the measured outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Group allocation was not blinded to participants, clinicians or researchers. However, it was not possible to blind participants and personnel for this intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Rate of attrition was 23% at 8 weeks and 42% at 6 months, with uneven rates between groups and ITT analysis was not performed. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration to determine whether outcomes reported were preplanned. |
| Other bias | Unclear risk | Uneven group sizes; differences in baseline characteristics including baseline bowel habits and current exercise habits. |
Taneja 2004.
| Study characteristics | ||
| Methods |
Study design: RCT Funding: Central Council for Research in Yoga and Naturopathy Published protocol/trial registration: no |
|
| Participants | Men with confirmed IBS‐D (Rome II criteria) Number randomised: 22 Age: mean 30.9 (SD 6.79) years % Female: 0% SES and ethnicity: 100% Indian Inclusion criteria
Exclusion criteria
Recruitment: gastroenterology clinics of All India Institute of Medical Sciences and Safdarjung Hospital Recruitment rate: not reported Region: New Delhi, India |
|
| Interventions |
Number of experimental conditions: 2; exercise intervention and pharmacological intervention comparator Number of participants (analysed): intervention: 9; comparator: 12 Description of exercise intervention: participants taught and advised to practice Surya Nadi pranayama (right‐nostril breathing) and a set of 12 asanas (Vajrasana, Shashankasana in 2 variations, Ushtrasana, Marjariasana, Bhujangasana, Padhastasana, Dhanurasana, Trikonasana in 2 variations, Pawanmuktasana, and Paschimottanasana) twice per day, morning and evening for 2 months. Duration: 8 weeks Number of contacts: 2: month 1 and 2 Setting: home Modality: unsupervised Interventionist: not reported Integrity: not reported Date of study: not reported Description of comparator: loperamide 2–6 mg/day |
|
| Outcomes |
Primary and secondary outcomes not specified Outcome relating to reported adverse events: not reported Outcome assessment time points: 8 weeks – end of intervention Preplanned subgroup analyses: not reported Unplanned subgroup analyses: not reported Lost to follow‐up: 1 Analysis: per‐protocol |
|
| Notes | No specific MCID stated for any of the measured outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Group allocation was not blinded to participants, clinicians or researchers. However, it was not possible to blind participants and personnel for this intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information as to how questionnaire was administered or data were analysed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 dropout in the control arm; however, no ITT analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration to determine whether outcomes reported were preplanned. |
| Other bias | Unclear risk | Did not specify baseline characteristics of each group. |
BAQ: Body Awareness Questionnaire; BRS: Body Responsiveness Scale; CPSS: Cohen Perceived Stress Scale; CSI‐18: Child Somatization Inventory‐18; GI: gastrointestinal; HADS: Hospital Anxiety and Depression Scale; IBS: irritable bowel syndrome; IBS‐C: constipation‐predominant irritable bowel syndrome; IBS‐D: diarrhoea‐predominant irritable bowel syndrome; IBS‐M: irritable bowel syndrome with mixed episodes of diarrhoea or constipation; IBS‐QOL: Irritable Bowel Syndrome Quality Of Life; IBS‐SSS: Irritable Bowel Syndrome Severity Scoring System; IL: interleukin; ITT: intention to treat; MCID: minimal clinically important difference; NRS: Numeric Rating Scale; PSQ: Perceived Stress Questionnaire; RCT: randomised controlled trial; SD: standard deviation; SES: socioeconomic status; SF‐36: 36‐item Short Form; TNF: tumour necrosis factor; VO2max: maximal oxygen consumption.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Banerjee 2019 | Yoga‐enhanced CBT: co‐intervention in the intervention group only. |
| D'Silva 2019 | Not an RCT. |
| De Schryver 2009 | Specifically excluded people with IBS. |
| Eriksson 2007 | Not an RCT. |
| Evans 2018 | Not an RCT (follow‐up of Evans 2014 already included). |
| Hamaguchi 2013 | Control intervention did not match review question. |
| Johannesson 2015 | Not an RCT (observational study follow‐up of previous RCT included in review). |
| Kavuri 2015b | Not an RCT (observational study follow‐up of previous RCT included in review). |
| Li 2008 | Use of non‐validated stool assessment scale (unable to contact authors to confirm). |
| Liang 2010 | Not IBS participants and not an RCT. |
| Lui 2007 | Not IBS participants and not an RCT. |
| Madhu 1988 | Use of non‐validated symptom scale (unable to contact authors to confirm). |
| Shah 2020 | Not an RCT. |
| Tavakoli 2019 | Not a physical activity intervention. |
| Villoria 2006 | Not an RCT. |
| Zhao 2019 | CBT + exercise: co‐intervention in the intervention group only. |
| Zhou 2019 | Not an RCT. |
| Zia 2014 | Not physical activity intervention. |
CBT: cognitive behavioural therapy; IBS: irritable bowel syndrome; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800015204.
| Study name | Research on exercise therapy of patients with irritable bowel syndrome based on intelligent monitoring technique |
| Methods | Randomised trial (parallel assignment) |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Exercise therapy based on intelligent monitoring technique |
| Outcomes | Primary outcome: IBS severity (IBS‐SSS) Secondary outcomes: quality of life (IBS‐QOL); anxiety; depression; social support; resilience scale |
| Starting date | April 2018 |
| Contact information | Zhou Changli; +86 18243062519; zhoucl16@mails.jlu.edu.cn |
| Notes |
NCT04315714.
| Study name | Impact of a yoga intervention on chronic abdominal pain, and associations with the metagenome and metabolome in participants with IBS |
| Methods | Randomised trial (cross‐over assignment) |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
10 participants with IBS will be randomised to the 6‐week yoga intervention at beginning of trial, followed by 6‐week control condition (observation/active monitoring).
10 participants with IBS will be randomised to the 6‐week wait‐list control (observation/active monitoring) at beginning of trial, followed by 6‐week yoga intervention.
10 participants serving as HC will be randomised to the 6‐week yoga intervention at beginning of trial, followed by 6‐week control condition (observation/active monitoring).
10 participants serving as HC will be randomised to the 6‐week wait‐list control (observation/active monitoring) at beginning of trial, followed by the 6‐week yoga intervention. |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | March 2021 |
| Contact information | Kristen R Weaver, PhD; 410‐706‐5119; kristen.weaver@umaryland.edu |
| Notes |
HC: healthy control; IBS: irritable bowel syndrome; IBS‐QOL: Irritable Bowel Syndrome Quality of Life; IBS‐SSS: Irritable Bowel Syndrome Symptom Severity Score.
Differences between protocol and review
Due to a lack of information reported in the included studies, we were unable to conduct many of the preplanned subgroup analyses given in the original protocol (Nunan 2015). In our protocol, we stated our meta‐analysis model decision would predominantly be based on the presence of statistical heterogeneity alongside anticipated methodological and clinical heterogeneity. Version 6.1 of the Cochrane Handbook for Systematic Reviews of Interventions (2021) recommends against basing model decisions on a statistical test for heterogeneity (Higgins 2021d). At the time of writing our protocol (2015), we referred to Version 5.1.0 (2011), which did not include this recommendation (Higgins 2011). We subsequently based our model decision solely on the anticipated heterogeneity between included studies including differences in physical activity interventions and outcome assessment methods.
We stated in our protocol that we would not pool data from individual studies if statistical heterogeneity as measured by the I² statistic was greater than 75%. On review, we decided not to use this cut‐off for determining whether we should pool studies or not, instead we chose to explore clinical and methodological reasons that may have explained the high degree of heterogeneity present and whether these justified a decision to pool or not. Where relevant, we also performed sensitivity analysis to identify the impact of clinical and methodological heterogeneity on statistical heterogeneity and the overall pooled estimates.
One author of the protocol (Joanna Boughtflower) did not contribute to the final review. New authors who joined the review team since the protocol was published are: Ting Cai, Antoni Gardner, José M Ordóñez‐Mena and Elizabeth T Thomas
Contributions of authors
Drafted the protocol: Joanna Boughtflower, KM, NR, DN
Developed search strategy: NR, DN
Searched for studies: NR
Obtained copies of studies: TC, AG, JMOM, NR, ETT, DN
Selected which studies to include: TC, AG, KM, JMOM, ETT, DN
Extracted data from studies: TC, AG, JMOM, ETT, DN
Translated and extracted data from articles written in Chinese: TC
Contacted study authors for clarification and missing data: DN
Entered data into Review Manager Web: ETT, DN
Conducted analyses: ETT, DN
Interpreted the analysis: TC, AG, KM, JMOM, ETT, DN
Assessed risk of bias: TC, AG, JMOM, ETT, DN
Assess the certainty of the evidence: ETT, DN
Drafted the final review: TC, AG, KM, JMOM, NR, ETT, DN
Sources of support
Internal sources
-
None, Other
non‐applicable
External sources
-
Royal College of General Practitioners, UK
This study was funded in part by the Royal College of General Practitioners Scientific Foundation Board (SFB).
-
Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529), Canada
Funding for the IBD/FBD Review Group (1 September 2010 to 31 August 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529).
-
CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD)New Source of support, Canada
Funding for the IBD/FBD Review Group (1 September 2010 to 31 August 2015) has been provided by the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD).
-
Funding for the IBD/FBD Review Group (1 September 2010 to 31 August 2015) has been provided by the Ontario Ministry of Health and Long Term Care (HLTC3968FL‐2010‐2235), Canada
Funding for the IBD/FBD Review Group (1 September 2010 to 31 August 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529) Ontario Ministry of Health and Long Term Care (HLTC3968FL‐2010‐2235).
-
Olive Stewart Fund, Canada
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Declarations of interest
TC, AG, KM, JMOM, NR, ETT and DN have no personal, political, academic, financial or other possible conflicts of interest.
New
References
References to studies included in this review
Daley 2008 {published data only}
- Daley AJ, Grimmett C, Roberts L, Wilson S, Fatek M, Roalfe A, et al. The effects of exercise upon symptoms and quality of life in patients diagnosed with irritable bowel syndrome: a randomised controlled trial. International Journal of Sports Medicine 2008;29(9):778-82. [DOI] [PubMed] [Google Scholar]
Evans 2014 {published data only}
- Evans S, Lung KC, Seidman LC, Sternlieb B, Zeltzer LK, Tsao CI. Iyengar yoga for adolescents and young adults with irritable bowel syndrome. Journal of Pediatric Gastroenterology and Nutrition 2014;59(2):244-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fani 2019 {published data only (unpublished sought but not used)}
- Fani M, Mostamand J, Fani M, Chitsaz N, Feizi A. The effect of aerobic exercises among women with mild and moderate irritable bowel syndrome: a pilot study. Journal of Bodywork and Movement Therapies 2019;23(1):161-5. [DOI] [PubMed] [Google Scholar]
Feng 2010 {published data only}
- Feng YC, Bian BG, Pan HS, Chen CJ, Chen CR. Observation of the efficacy of Baduanjin exercise on the constipation-predominant irritable bowel syndrome of the elderly. Sport Science Research 2010;2:89-98. [Google Scholar]
Hajizadeh Maleki 2018 {published data only}
- Hajizadeh Maleki B, Tartibian B, Mooren FC, FitzGerald LZ, Krüger K, Chehrazi M, et al. Low-to-moderate intensity aerobic exercise training modulates irritable bowel syndrome through antioxidative and inflammatory mechanisms in women: results of a randomized controlled trial. Cytokine 2018;102:18-25. [DOI] [PubMed] [Google Scholar]
Jia 2016 {published data only}
- Jia Y. Moderate Intensity Aerobic Exercise for the Treatment of Patients with Diarrhea Predominant Irritable Bowel [Masters thesis]. Yangzhou (China): Yangzhou University, 2016. [Google Scholar]
Johannesson 2011 {published data only}
- Johannesson E, Simrén M, Strid H, Bajor A, Sadik R. Physical activity improves symptoms in irritable bowel syndrome: a randomized controlled trial. American Journal of Gastroenterology 2011;106:915-22. [DOI] [PubMed] [Google Scholar]
Kavuri 2015a {published data only}
- Kavuri V, Selvan P, Malamud A, Raghuram N, Selvan SR. Remedial yoga module remarkably improves symptoms in irritable bowel syndrome patients: a 12-week randomized controlled trial. European Journal of Integrative Medicine 2015;7:595-608. [Google Scholar]
Schumann 2018 {published data only}
- Schumann D, Butto L, Langhorst J, Dobos G, Haller D, Cramer H. Effects of yoga versus the low-FODMAP diet on gastrointestinal symptoms and the microbiota in patients with irritable bowel syndrome – a randomized controlled trial. World Congress Integrative Medicine and Health; 2017 May 3-5; Berlin, Germany.
- Schumann D, Langhorst J, Dobos G, Cramer H. Randomised clinical trial: yoga vs a low-FODMAP diet in patients with irritable bowel syndrome. Alimentary Pharmacology and Therapeutics 2018;47(2):203-11. [DOI] [PubMed] [Google Scholar]
Shahabi 2016 {published data only}
- Shahabi L, Naliboff BD, Shapiro D. Self-regulation evaluation of therapeutic yoga and walking for patients with irritable bowel syndrome: a pilot study. Psychology, Health and Medicine 2016;21(2):176-88. [DOI] [PubMed] [Google Scholar]
Taneja 2004 {published data only}
- Taneja L, Deepak KK, Poojary G, Acharaya IN, Pandey RM, Sharma MP. Yogic versus conventional treatment in diarrhea-predominant irritable bowel syndrome: a randomized control study. Applied Psychophysiology and Biofeedback 2003;29(1):19-33. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Banerjee 2019 {published data only}
- Banerjee D, Bhatt S, Chatterjee S, Sharma R. Yoga-enhanced cognitive behavioural therapy (Y-CBT) versus rifamixin-probiotic sequential treatment for irritable bowel syndrome (IBS): a randomised clinical trial. Gut 2019;68(Suppl 1):A100. [Google Scholar]
D'Silva 2019 {published data only}
- D'Silva A, MacQueen G, Nasser Y, Taylor LM, Vallance JK, Raman M. Yoga as a therapy for irritable bowel syndrome. Digestive Diseases & Sciences 2019;12:2503-14. [DOI] [PubMed] [Google Scholar]
De Schryver 2009 {published data only}
- De Schryver AM, Keuleman YC, Peters HP, Akkermans LM, Smout AJ, De Vries WR, et al. Effects of regular physical activity on defecation pattern in middle-aged patients complaining of chronic constipation. Scandinavian Journal of Gastroenterology 2009;40(4):422-9. [DOI] [PubMed] [Google Scholar]
Eriksson 2007 {published data only}
- Eriksson EM, Moller IE, Soderberg RH, Eriksson HT, Kurlberg GK. Body awareness therapy: a new strategy for relief of symptoms in irritable bowel syndrome patients. World Journal of Gastroenterology 2007;13(23):3206-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2018 {published data only}
- Evans S, Seidman LC, Lung K, Sternlieb B, Zeltzer LK. Yoga for teens with irritable bowel syndrome: results from a mixed-methods pilot study. Holistic Nursing Practice 2018;32(5):253-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamaguchi 2013 {published data only}
- Hamaguchi T, Tayama T, Saigou T, Tomiie T, Kanazawa M, Fukudo, S. Changes in salivary physiological stress markers induced by aromatherapy and physical therapy in patients with IBS. Psychotherapy and Psychosomatics 2013;82:41. [Google Scholar]
Johannesson 2015 {published data only}
- Johannesson E, Ringstrom G, Abrahamsson H, Sadik R. Intervention to increase physical activity in irritable bowel syndrome shows long-term positive effects. World Journal of Gastroenterology 2015;21(2):600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavuri 2015b {published data only}
- Kavuri V, Selvan P, Tabesh A, Raghuram N, Selvan SR. Remedial yoga module improves symptoms of irritable bowel syndrome: replication in the wait-list group and sustained improvements at 6 months. European Journal of Integrative 2015;7(6):609-16. [Google Scholar]
Li 2008 {published data only}
- Li C-l, Feng H-Y, Xiao S-C, Huang H-Y. The influence of Tai Ji Quan on the therapeutic effect of irritable bowel syndrome. Journal of Practical Medical Techniques 2008;15(11):1384-6. [Google Scholar]
Liang 2010 {published data only}
- Liang YS, Zhang Y, Feng Y JJ. Observation of therapeutic effects of shadowboxing (Chinese shadowboxing/Tai Chi) and Acupoint Catgut embedding in the treatment for C-IBS. Hubei Journal of Traditional Chinese Medicine 2010;32:50-1. [Google Scholar]
Lui 2007 {published data only}
- Lui BQ, Qui Y. Effect of mountain climbing on physical quality, psychological quality and psychological health of college students. Journal of Clinical Rehabilitative Tissue Engineering Research 2007;30:6006-9. [Google Scholar]
Madhu 1988 {published data only}
- Madhu SV, Vij KC, Bhatnagar OP, Krishnamurthy N, Anand BS, Chuttani HK. Colonic myoelectrical activity in irritable bowel syndrome before and after treatment. Indian Journal of Gastroenterology 1988;7(1):31-3. [PubMed] [Google Scholar]
Shah 2020 {published data only}
- Shah K, Ramos-Garcia M, Bhavsar J, Lehrer P. Mind-body treatments of irritable bowel syndrome symptoms: an updated meta-analysis. Behaviour Research & Therapy 2020;128:103462. [DOI] [PubMed] [Google Scholar]
Tavakoli 2019 {published data only}
- Tavakoli T, Davoodi N, Jafar Tabatabaee TS, Rostami Z, Mollaei H, Salmani F, et al. Comparison of laughter yoga and anti-anxiety medication on anxiety and gastrointestinal symptoms of patients with irritable bowel syndrome. Middle East Journal of Digestive Diseases 2019;11(4):211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Villoria 2006 {published data only}
- Villoria A, Serra J, Azpiroz F, Malagelada JR. Physical activity and intestinal gas clearance in patients with bloating. American Journal of Gastroenterology 2006;101:2552-7. [DOI] [PubMed] [Google Scholar]
Zhao 2019 {published data only}
- Zhao SR, Ni XM, Zhang XA, Tian H. Effect of cognitive behavior therapy combined with exercise intervention on the cognitive bias and coping styles of diarrhea-predominant irritable bowel syndrome patients. World Journal of Clinical Cases 2019;7(21):3446-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2019 {published data only}
- Zhou C, Zhao E, Li Y, Jia Y, Li F. Exercise therapy of patients with irritable bowel syndrome: a systematic review of randomized controlled trials. Neurogastroenterology & Motility 2019;31(2):e13461. [DOI] [PubMed] [Google Scholar]
Zia 2014 {published data only}
- Zia J, Cain K, Barney P, Jarrett M, Heitkempe M. What strategies do patients with irritable bowel syndrome continue to use following a 9-week comprehensive self-management program? American Journal of Gastroenterology 2014;109:S539. [Google Scholar]
References to ongoing studies
ChiCTR1800015204 {unpublished data only}
- Research on exercise therapy of patients with irritable bowel syndrome based on intelligent monitoring technique. Ongoing study. April 2018. Contact author for more information.
NCT04315714 {unpublished data only}
- Impact of a yoga intervention on chronic abdominal pain, and associations with the metagenome and metabolome in participants with IBS. Ongoing study. March 2021. Contact author for more information.
Additional references
ACG 2021
- Lacy BE, Pimentel M, Brenner D, Chey WD, Keefer LA, Long MD, et al. ACG clinical guideline: management of irritable bowel syndrome. American Journal of Gastroenterology 2021;116(1):17-44. [DOI] [PubMed] [Google Scholar]
Camilleri 2001
- Camilleri M. Management of the irritable bowel syndrome. Gastroenterology 2001;120(3):652-68. [DOI] [PubMed] [Google Scholar]
Canavan 2014
- Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. Alimentary Pharmacology and Therapeutics 2014;40:1023-34. [DOI] [PubMed] [Google Scholar]
Caspersen 1985
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Reports 1985;100(2):126-31. [PMC free article] [PubMed] [Google Scholar]
Chan 2014
- Chan D, Robbins H, Rogers S, Clark S, Poullis A. Inflammatory bowel disease and exercise: results of a Crohn's and Colitis UK survey. Frontline Gastroenterology 2014;5(1):44-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
CMO 2019
- Department of Health and Social Care. UK Chief Medical Officers' physical activity guidelines. Available from assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832868/uk-chief-medical-officers-physical-activity-guidelines.pdf (accessed 14 November 2021).
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioural Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates Inc, 1988. [Google Scholar]
Cong 2018
- Cong X, Perry M, Bernier KM, Young EE, Starkweather A. Effects of self-management interventions in patients with irritable bowel syndrome: systematic review. Western Journal of Nursing Research 2018;40(11):1698-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooney 2013
- Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, et al. Exercise for depression. Cochrane Database of Systematic Reviews 2013, Issue 9. Art. No: CD004366. [DOI: 10.1002/14651858.CD004366.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dainese 2004
- Dainese R, Serra J, Azpiroz F, Malagelada JR. Effects of physical activity on intestinal gas transit and evacuation in healthy subjects. American Journal of Medicine 2004;116(8):536-9. [DOI] [PubMed] [Google Scholar]
Daley 2008
- Daley AJ, Grimmett C, Roberts L, Wilson S, Fatek M, Roalfe A, et al. The effects of exercise upon symptoms and quality of life in patients diagnosed with irritable bowel syndrome: a randomised controlled trial. International Journal of Sports Medicine 2008;29(9):778-82. [DOI] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
de Oliveira 2009
- Oliveira EP, Burini RC. The impact of physical exercise on the gastrointestinal tract. Current Opinion in Clinical Nutrition and Metabolic Care 2009;12(5):533-8. [DOI] [PubMed] [Google Scholar]
De Schryver 2005
- De Schryver AM, Keulemans YC, Peters HP, Akkermans LM, Smout AJ, De Vries WR, et al. Effects of regular physical activity on defecation pattern in middle-aged patients complaining of chronic constipation. Scandinavian Journal Gastroenterology 2005;40(4):422-9. [DOI] [PubMed] [Google Scholar]
Drossman 2016
- Drossman DA, Hasler WL. Rome IV-functional GI disorders: disorders of gut−brain interaction. Gastroenterology 2016;150:1257-61. [DOI] [PubMed] [Google Scholar]
Evans 2014
- Evans S, Lung KC, Seidman LC, Sternlieb B, Zeltzer LK, Tsao JC. Iyengar yoga for adolescents and young adults with irritable bowel syndrome. Journal of Pediatric Gastroenterology and Nutrition 2014;59(2):244-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ford 2012
- Ford AC, Talley NJ. Irritable bowel syndrome. BMJ 2012;345:e5836. [DOI] [PubMed] [Google Scholar]
Francis 1997
- Francis CY, Morris J, Whorwell PJ. The Irritable Bowel Severity Scoring System: a simple method of monitoring irritable bowel syndrome and its progress. Alimentary Pharmacology & Therapeutics 1997;11:395-402. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021a
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2021b
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2021c
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2021d
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Hoffman 2014
- Hoffman TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Leong 2003
- Leong SA, Barghout V, Birnbaum HG, Thibeault CE, Ben-Hamadi R, Frech F, et al. The economic consequences of irritable bowel syndrome: a US employer perspective. Archives of Internal Medicine 2003;163(8):929-35. [DOI] [PubMed] [Google Scholar]
Longstreth 2006
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130(5):1480-91. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Adams C, Bradley C, Joy C, Fenton M. Unpublished rating scales – a major source of bias in randomised controlled trials of treatments for schizophrenia? British Journal of Psychiatry 2000;176:249-52. [DOI] [PubMed] [Google Scholar]
Maxion‐Bergemann 2006
- Maxion-Bergemann S, Thielecke F, Abel F, Bergemann R. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics 2006;24(1):21-37. [DOI] [PubMed] [Google Scholar]
Moayyedi 2017
- Moayyedi P, Mearin F, Azpiroz F, Andresen V, Barbara G, Corsetti M, et al. Irritable bowel syndrome diagnosis and management: a simplified algorithm for clinical practice. United European Gastroenterology Journal 2017;5(6):773-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2003
- Murphy M, Foster C, Nicholas JJ, Pignone M. Cardiovascular disorders. Primary prevention. Clinical Evidence 2003;Dec(10):154-87. [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Care Excellence (NICE). Irritable bowel syndrome: diagnosis and management of irritable bowel syndrome in primary care (last updated 4 April 2017). www.nice.org.uk/guidance/CG61 (accessed 12 January 2021).
Nunan 2013
- Nunan D, Mahtani KR, Roberts N, Heneghan C. Physical activity for the prevention and treatment of major chronic disease: an overview of systematic reviews. Systematic Reviews 2013;2:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2021
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Available from training.cochrane.org/handbook/archive/v6.2.
Peters 2001
- Peters HP, De Vries WR, Vanberge-Henegouwen GP, Akkermans LM. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut 2001;48(3):435-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quigley 2015
Review Manager Web 2021 [Computer program]
- Review Manager Web (RevMan Web). Available at: revman.cochrane.org: The Cochrane Collaboration, 2021.
Schumann 2016
- Schumann D, Anheyer D, Lauche R, Dobos G, Langhorst J, Cramer H. Effect of yoga in the therapy of irritable bowel syndrome: a systematic review. Clinical Gastroenterology and Hepatology 2016;14(12):1720-31. [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Song 2018
- Song KH, Jung HK, Kim HJ, Koo HS, Kwon YH, Shin HD, et al, The Clinical Practice Guidelines Group Under the Korean Society of Neurogastroenterology and Motility. Clinical practice guidelines for irritable bowel syndrome in Korea, 2017 revised edition. Journal of Neurogastroenterology and Motility 2018;24:197-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spiegel 2009
- Spiegel B, Camilleri M, Bolus R, Anderson V, Chey WD, Fehnel S, et al. Psychometric evaluation of patient reported outcomes in IBS randomised controlled trials: a Rome Foundation Working Group report. Gastroenterology 2009;137(6):1944-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spiller 2007a
- Spiller R. Clinical update: irritable bowel syndrome. Lancet 2007;369(9573):1586-8. [DOI] [PubMed] [Google Scholar]
Spiller 2007b
- Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut 2007;56(12):1770-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Talley 1990
- Talley NJ, Phillips SF, Melton LJ, Mulvihill C, Wiltgen C, Zinsmeister AR. Diagnostic value of the Manning criteria in irritable bowel syndrome. Gut 1990;31(1):77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2006
- Thomas D, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD002968. [DOI: 10.1002/14651858.CD002968.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2016
- Wang Q, Xu KQ, Qin XR, Wen-Lu, Yan-Liu, Wang XY. Association between physical activity and inflammatory bowel disease risk: a meta-analysis. Digestive and Liver Disease 2016;48(12):1425-31. [DOI] [PubMed] [Google Scholar]
Wilson 2004
- Wilson S, Roberts L, Roalfe A, Bridge P, Singh S. Prevalence of irritable bowel syndrome: a community survey. British Journal of General Practice 2004;54(504):495-502. [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Nunan 2015
- Nunan D, Boughtflower J, Roberts NW, Mahtani KR. Physical activity for treatment of irritable bowel syndrome. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD011497. [DOI: 10.1002/14651858.CD011497] [DOI] [PMC free article] [PubMed] [Google Scholar]


