Abstract
Background:
Proton pump inhibitors (PPIs) are widely prescribed and may be associated with harm; hypomagnesemia and reduced effectiveness of calcium carbonate phosphate binders may be important in end-stage kidney disease (ESKD).
Objectives:
Our objectives included (1) discontinuing PPIs and H2 blockers and (2) assessing the impact on serum magnesium and markers of mineral metabolism.
Design:
Prospective cohort.
Setting:
Satellite hemodialysis unit of a tertiary care hospital.
Patients:
Incident and prevalent patients with ESKD treated with hemodialysis.
Measurements:
We assessed the impact of stopping PPI/H2 blockers in patients who did not have an absolute indication as per guidelines in the general population; serum magnesium, calcium, and phosphate were measured before and approximately 8 weeks later. Analysis of variance (ANOVA) test and Kruskal-Wallis was used to describe the population. Wilcoxon signed rank test for the paired change scores (from pre to post)
Methods:
The electronic medical record (EMR) was extensively searched for absolute indications for a PPI. Results were reviewed with the primary nephrology team before approaching patients about stopping the PPI. Basic demographic information and select medications were also collected.
Results:
Electronic medical records were reviewed for 179 patients, 74 had a PPI or H2 antagonist or both on their medication list (43%); 23 (31%) were assessed as appropriate. After primary team and patient review, 29 patients agreed to a trial of PPI withdrawal. Fourteen patients restarted their PPI, most for gastroesophageal reflux disease. Three patients had a GI bleed, 1 fatally. Serum calcium (P = .17) and the dose of phosphate binders (P = .075) did not change but serum phosphate increased (1.55 [0.29] to 1.85 [0.34] mmol/L; P = .0005). Serum magnesium also increased (1.01 [0.16] to 1.06 [0.14] mmol/L; P = .01).
Limitations:
Small patient numbers and observational nature of the study does not establish causation in this population at high risk to experience a gastrointestinal bleed.
Conclusions:
Our results suggest that PPI deprescribing as recommended in the general population may be associated with harm in patients with ESKD and requires further study.
Trial Registration:
Not registered.
Keywords: hemodialysis, quality improvement, proton pump inhibitors, deprescribing, mineral metabolism
Abrégé
Contexte:
Les inhibiteurs de la pompe à protons (IPP) sont largement prescrits et peuvent être associés à une atteinte rénale; l’hypomagnésémie et la réduction de l’efficacité des chélateurs de phosphate à base de carbonate de calcium peuvent devenir significatifs chez les patients avec insuffisance rénale terminale (IRT).
Objectifs:
Nos objectifs comprenaient 1) l’arrêt des IPP et des antagonistes H2 et 2) l’évaluation des conséquences sur le taux de magnésium sérique et les marqueurs du métabolisme minéral.
Conception:
Étude de cohorte prospective.
Cadre:
L’unité d’hémodialyse satellite d’un hôpital de soins tertiaires.
Sujets:
Patients incidents et prévalents atteints d’IRT et traités par hémodialyse.
Mesures:
Nous avons évalué les conséquences de l’arrêt des IPP et antagonistes H2 chez les patients qui n’avaient pas d’indication absolue pour ces médicaments, conformément aux directives pour la population générale. Les taux sériques de magnésium, de calcium et de phosphate ont été mesurés avant l’arrêt et environ huit semaines plus tard. Les tests ANOVA et Kruskal-Wallis ont été utilisés pour décrire la population, et le test de rang de Wilcoxon pour les scores de changement appariés (de pré à post-intervention)
Méthodologie:
Les dossiers médicaux électroniques (DMÉ) ont été consultés rigoureusement à la recherche d’une indication absolue pour un IPP. Les résultats ont été revus avec l’équipe de néphrologie primaire avant d’approcher les patients quant à un arrêt des IPP. Les données démographiques initiales et les prescriptions pour certains médicaments ont également été recueillies.
Résultats:
Les DMÉ de 179 patients ont été consultés, révélant que 74 (43 %) d’entre eux prenaient soit un IPP, soit un antagoniste H2, soit les deux; chez 23 patients (31 %) la prescription était appropriée. Après évaluation par l’équipe médicale et discussion avec les patients, 29 patients ont accepté de cesser l’IPP. Quatorze patients ont recommencé les IPP, la plupart pour un reflux gastro-œsophagien. Trois patients ont souffert d’une hémorragie gastro-intestinale, dont une s’est avérée fatale. Le taux de calcium sérique (p=0,17) et la dose de chélateurs du phosphate (p=0,075) n’ont pas changé, mais le taux de phosphate sérique a augmenté (1,55 [0,29] à 1,85 [0,34] mmol/L; p=0,0005), tout comme le taux de magnésium sérique (1,01 [0,16] à 1,06 [0,14] mmol/L; p=0,01).
Limites:
Le faible échantillon de patients et la nature observationnelle de l’étude ne permettent pas d’établir un lien de causalité dans cette population présentant un risque élevé d’hémorragie gastro-intestinale.
Conclusion:
Nos résultats suggèrent que la déprescription des IPP recommandée dans la population générale pourrait être associée à un préjudice chez les patients atteints d’IRT. Des études plus approfondies sont nécessaires.
Enregistrement de l’essai:
Non enregistré.
Introduction
Proton pump inhibitors (PPIs) and H2 receptor antagonists (H2 blockers) are commonly used in the general population for treating patients with gastroesophageal reflux disease (GERD), peptic and duodenal ulcers, upper gastrointestinal (GI) bleeding, and as prophylactic medications for those patients who are at high risk for bleeding.1,2 The most common indications for PPIs, such as GERD, only require short-term treatment for about 4 to 8 weeks. There is a high prevalence of use of these medications in the community with concerns about potential overuse. In some studies, there is a lack of documented ongoing indication for 40% to 55% of primary care patients and 40% to 65% of hospitalized patients. 3 PPIs account for more than $11 billion in expenditures annually in the United States; pantoprazole was the fifth most commonly dispensed medication with more than 11 million prescriptions in Canada in 2012.4,5 Although these medications are viewed as safe, they have been associated with numerous side effects including diarrhea (Clostridium difficile), impaired B12, magnesium and calcium absorption, infection, fractures, and pneumonia in predominately observational studies. 4 ,6-9 There is also a concern about hypergastrinemia with associated trophic effects on enterochromaffin-like cells with potential progression to dysplasia and malignancy. 2 Long-term prescription may also contribute to polypharmacy and subsequently nonadherence, medication errors, drug interactions, and hospitalizations. 4
Polypharmacy has been well described as an issue in the dialysis population. In one study, patients with end-stage kidney disease (ESKD) were taking an average of 10 to 12 prescribed and over-the-counter medications per day, average 19 pills. 10 In Canada, the major medication group is phosphate binders; commonly calcium carbonate (®Tums). Perhaps surprisingly in that context, patients with ESKD treated with hemodialysis (HD) are also commonly prescribed PPIs or H2 blockers. 11 The increase in pH from 2.0 to > 6.0 by PPIs may have a negative impact on the ability of calcium carbonate to lower serum phosphate levels as calcium carbonate binds phosphate better at lower pH levels. 12
De-prescribing is one way to address polypharmacy and its consequences for patients treated with dialysis. The purpose of this study was to apply the Choosing Wisely guidelines and others for de-prescribing PPIs (H2 blockers) for patients with ESKD treated with HD and secondarily to assess the impact on serum magnesium and mineral metabolism (serum calcium, serum phosphate, and phosphate binder dose) for patients who were able to discontinue the PPI.4,9
Methods
This quality assurance study was approved by the Ottawa Health Science Research Ethics Board (20200617-01H) and conducted in adherence with the Declaration of Helsinki. The electronic medical records (EMRs) of all adult patients with ESRD treated with HD at the Ottawa Hospital Riverside Campus, Ottawa, Ontario, Canada, were reviewed from June to October 2021. Basic demographic and comorbidity data were collected on all patients. Medication lists were reviewed for antiplatelet agents, anticoagulants, non-steroidal anti-inflammatory drugs (NSAIDS), glucocorticoids, and phosphate binder type and dose. The medication lists were also screened for use of PPI’s and H2 antagonists. Charts were extensively searched for any gastroenterology notes, procedures, and pathology for any absolute indications for PPIs as per the Choosing Wisely guidelines and others.4,9 (1) Erosive esophagitis (Grade C and D); (2) barrett’s esophagus; (3) NSAID use plus at least one of age > 65 years, prior ulcer, concurrent anti-coagulation, anti-platelet or corticosteroid; and (4) antiplatelet therapy plus at least one of a history of ulcer, concurrent anticoagulation, NSAID or 2 of the following: age greater than 60 years, corticosteroid, and GERD symptoms. Given the high risk of GI bleeding in the ESKD population, we also considered dual antiplatelet therapy (DAPT) in combination with either advanced age (>65 years) or corticosteroid as an indication for a PPI. Corticosteroid monotherapy was not considered an absolute indication for a PPI. The deprescribing team was comprised of a staff nephrologist, a gastroenterology fellow, a nephrology fellow, a physician assistant, and 2 medical students. Any uncertainties about indications for PPI/H2 blocker use were reviewed by the team’s gastroenterology fellow. The list of patients on a PPI/H2 blocker that did not have an absolute indication was then sent to the patient’s primary nephrology team for review. Patient lists were updated to reflect consensus opinion about the safety of stopping the PPI. Reasons for any discrepancies from the chart review and the primary care team were documented.
All patients, in whom stopping the PPI was felt to be safe, were approached during their usual HD treatment. The purpose of the project was explained verbally and patients were provided with a handout that explained the indications and possible risks of PPIs. For patients who were in agreement, the PPI was stopped over a 2-week period. Patients were encouraged to contact a nephrology care provider if they developed any symptoms that they felt were related to discontinuing the medication. The patients were then followed for an additional 8 weeks to determine the percentage of patients who restarted the PPI/H2 antagonist. Serum Mg, Ca, and P04 were measured at baseline and repeated in 8 to 12 weeks for those patients who did not restart their PPI.
Participants were divided into 3 groups: (1) taking a PPI without an indication, (2) taking a PPI with an indication, and (3) not taking a PPI. We report number and percentage for categorical variables. Mean with standard deviation or median with interquartile range (IQR) was reported for continuous variables. Analysis of variance (ANOVA) test and Kruskal-Wallis were used to compare means and medians among the 3 groups respectively. Chi-square test or Fisher’s Exact test was used to compare proportions where appropriate. Wilcoxon signed rank test was used for the paired change scores (from pre to post). For all statistical tests, 2-tailed test was used to determine significance at the 5% level. All statistical analyses were performed using SAS, Version 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Results
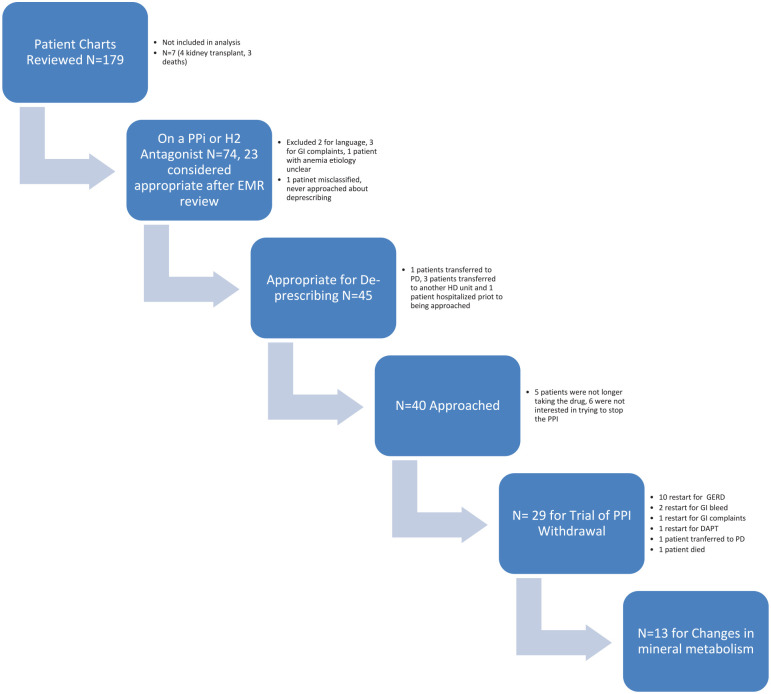
One hundred seventy-nine patient’s EMRs were available for review; 4 patients received a transplant and 3 patients died prior to review by the de-prescribing team and are not included in the analysis (Figure 1). Seventy-four patients had a PPI or H2 antagonist or both on their EMR medication list (43%); 23 (31%) were assessed as appropriate. Of note, only 4 patients were taking an H2 antagonist of which 3 were also taking a PPI. One 84-year-old frail patient had a remote history of a GI bleed secondary to a endoscopy proven ulcer; it was decided that continuing her PPI was appropriate. One patient was found to have been misclassified on data cleaning (on a PPI and anticoagulation alone). The most common absolute indications for PPI were related to antiplatelet therapy (Table 1).
Figure 1.
Flow diagram for PPI deprescribing.
Note. PPI = proton pump inhibitor; EMR = electronic medical record; GI = gastrointestinal; HD = hemodialysis; GERD = gastroesophageal reflux disease; DAPT = dual antiplatelet therapy; PD = peritoneal dialysis.
Table 1.
Absolute Indications for a PPI.
| Indication for PPI | N = 23 |
|---|---|
| Erosive esophagitis | 5 |
| Barrett’s esophagus | 0 |
| NSAID + one other (age >65 years, prior ulcer, concurrent anti-coagulation, anti-platelet, or prednisone | 1 |
| GI bleed secondary to an ulcer | 1 |
| Antiplatelet with one other (history of ulcer, concomitant anticoagulation or NSAID) or 2 other (>60 years, prednisone, GERD) | 7 |
| Dual antiplatelet therapy with one other (age >65 years, anticoagulation, prednisone or NSAIDs) | 8 |
| Misclassified (anticoagulation alone) | 1 |
Note. PPI = proton pump inhibitor; NSAID = non-steroidal anti-inflammatory drug; GI = gastrointestinal; GERD = gastroesophageal reflux disease.
After review by each patient’s primary nephrology team, 45 of 51 patients were felt to be appropriate for potential de-prescribing (Figure 1). The concerns highlighted by the primary nephrology team included language barriers (2), numerous GI complaints (3), and 1 patient with worsening anemia, etiology unclear. One patient transferred to peritoneal dialysis, 3 to another HD unit, and 1 patient was hospitalized prior to being approached.
Of the 40 patients approached, 5 stated that they were no longer taking the medication. Six patients were not interested in trying to discontinue the medication as they were concerned about possible reoccurrence of symptoms. The patients on PPIs without an indication, on PPIs with an absolute indication, and not taking PPIs differed with respect to history of coronary artery disease, dyslipidemia, use of aspirin (ASA), use of antiplatelet therapy, anticoagulation, and prednisone (Table 2).
Table 2.
Baseline Characteristics by Indication for PPI (After Full Review).
| On PPI, no indication (N = 47) |
On PPI, indication (N = 22) |
No PPI (N = 103) |
P-value | |
|---|---|---|---|---|
| Age—years (mean, SD) | 66 (16) | 69 (11) | 66 (14) | .65 |
| Sex (F/M) | 22/25 | 10/12 | 30/73 | .07 |
| Dialysis vintage (median, IQR) | 762 (322-1623) | 788 (579-1320) | 949 (516-2006) | .31 |
| Diabetes mellitus (N, %) | 27 (57) | 16 (73) | 51 (50) | .13 |
| Hypertension (N, %) | 34 (72) | 19 (86) | 87 (84) | .17 |
| Dyslipidemia (N, %) | 32 (68) | 21 (95) | 63 (61) | .008 |
| Coronary artery disease (N, %) | 11 (23) | 16 (72) | 31 (30) | .0001 |
| PVD (N, %) | 3 (6) | 6 (27) | 16 (16) | .07 |
| Aspirin (N, %) | 19 (40) | 18 (82) | 37 (36) | .0004 |
| NSAID (N, %) | 1 (2) | 1 (5) | 0 (0) | .09 |
| Antiplatelet (N, %) | 3 (6) | 10 (45) | 6 (6) | <.0001 |
| Anticoagulant (N, %) | 7 (15) | 1 (5) | 1 (1) | .002 |
| Prednisone | 7 (15) | 4 (18) | 5 (5) | .03 |
Note. PPI = proton pump inhibitor; N = number; F = female; M = male; IQR = interquartile range; PVD = peripheral vascular disease; NSAID = non-steroidal anti-inflammatory.
Twenty-nine patients agreed to a trial of PPI withdrawal (one of these patients was on a PPI and an H2 antagonist). All patients were continued on anticoagulation for HD (tinzaparin 2500 to 4500 units). During follow-up, 14 patients restarted their PPI (10 patients had a reoccurrence of GERD [1 patient restarted the PPI 3 times per week and 1 switched to an H2 antagonist]; 1 patient had nausea, vomiting, and GI complaints; 2 patients had melena and a drop in hemoglobin 2-4 weeks after discontinuing the PPI [1 with endoscopy proven Grade C esophagitis]; and 1 elderly patient was started on DAPT post cardiac procedure). One patient died from a massive GI bleed 2 days after his PPI was switched to every second day dosing. One patient transferred to peritoneal dialysis (PD). Five of the 10 patients with a reoccurrence of GERD were taking calcium carbonate as a phosphate binder, the other 5 were not taking binder; 1 of the 2 patients who had a GI bleed potentially attributable to stopping the PPI was taking calcium carbonate as a phosphate binder.
For those patients who were able to remain off the PPI, there was no difference in baseline and post PPI deprescribing serum calcium (P = .17) or calcium carbonate phosphate binder dose (P = .75). However, serum phosphate increased from 1.55 (0.29) to 1.85 (0.34) mmol/L; P = .0005 and magnesium increased from 1.01 (0.16) to 1.06 (0.14) mmol/L; P = .01 (Table 3).
Table 3.
Changes in Mineral Metabolism After Deprescribing PPI’s.
| Baseline value | Post PPI deprescribing | P-value | |
|---|---|---|---|
| Calcium mmol/L (mean; SD) | 2.34 (0.12) | 2.31 (0.18) | .17 |
| Phosphate mmol/L (mean; SD) | 1.55 (0.29) | 1.85 (0.34) | .0005 |
| Calcium carbonate dose (mg, Elemental) (median; IQR) | 900 (0-1200) | 900 (0-1400) | .75 |
| Magnesium mmol/L(mean; SD) | 1.01 (0.16) | 1.06 (0.14) | .01 |
Note. PPI = proton pump inhibitor; IQR = interquartile range.
Discussion
In this study, we found that 43% of patients with ESKD treated with HD were taking PPIs as per their EMR medication record of which only 30% were assessed as appropriate based on predefined criteria. During patient interviews, 5 patients had stopped taking the PPI decreasing the percentage of patients with ESKD on these medications to 69/172 (40%). In follow-up, 14 of 29 patients had to restart their PPI for GI symptoms including 2 patients who had a GI bleed. One patient died secondary to a GI bleed but almost immediately after decreasing the dose of his PPI making cause and effect unlikely.
In the general population, PPIs are one of the most widely prescribed medications. Due to the high prevalence of their use and overuse, they have been selected as a target medication class for deprescribing. 13 In one study of 331 patients in residential care, 34% did not have a documented indication for a PPI. 1 A similar percentage of patients on an inpatient internal medicine and family practice ward did not have a documented indication for a PPI. 14 In a long-term facility, 63% of residents were felt to be candidates for PPI deprescribing; PPI use for GERD greater than 8 weeks was the most common (53%) and no PPI indication identified (20%). 15 In a 2003 study of patients with ESKD, 41% of patients were taking an acid suppressing drug; a higher prevalence was published in a more recent study.11,16 In our study, 40% of patients were being treated with a PPI the majority of whom did not appear to have an absolute indication and were candidates for a de-prescribing trial.
Of those patients who agreed to a trial of stopping the PPI, 34% had to re-start the medication for GERD type symptoms despite stopping the PPI over 2 weeks. A further 2 patients (7%) had a GI bleed 2 to 4 weeks after stopping the PPI (4 to 6 weeks from the start of PPI weaning). In primary care, the success rate of de-prescribing PPIs (stopping or reducing the dose) is up to 95%. 17 However in a Cochrane review of 6 studies, de-prescribing was associated with worse symptoms but a reduced pill burden. Notably, there was very little data regarding the long-term benefits and harms of PPI reduction or discontinuation. 18 In an ESKD population similar to ours, 24 of 86 patients were included in a PPI deprescribing study, 13 of whom had a history of controlled GERD. 19 In spite of very careful selection, only 50% remained off the PPI at 6 months mostly due to a reoccurrence of GERD symptoms. Our results are very similar to theirs. It is unclear if patients with ESKD are at greater risk of PPI de-prescribing failure. Patients with ESKD have lower gastric pH and higher gastrin levels than people without kidney failure in some but not all studies.20-22 Hypergastrinemia has been associated with an inability to wean PPIs in the general population. 2
Although much has been written about the potential harms of PPIs, little has been written about the potential harms associated with discontinuing this class of medications. The original reason for starting the medication is often very difficult to ascertain and patients often do not remember the indication. 16 De-prescribing has been associated with mortality benefits in observational studies but not in randomized controlled trials. 23 One in 7 patients treated for ESKD will experience a major hemorrhage within 3 years of dialysis initiation; a risk about 20 fold greater than people with normal kidney function. 24 In one study, 42% of the bleeds were upper GI; it is unclear if any of these events could have been prevented by a PPI.25,26
Contrary to our hypothesis, there was an increase in serum phosphate in the small number of patients who did not restart their PPI. Not all of the patients who remained off the PPI were taking a calcium carbonate phosphate binder (9 of 13) further limiting our ability to detect an effect if it actually exits. In a small retrospective study, the concomitant administration of PPIs or H2 antagonists with calcium carbonate was associated with an attenuated hypophosphatemic effect. 27 Both studies have limitations such that further research is required to definitively answer this question. PPIs have been associated with hypomagnesemia, an effect medicated by inhibition of active magnesium absorption via transcellular magnesium channels. 28 Hypomagnesemia has been associated with vascular calcification in ESKD. 29 Although serum magnesium increased in our study, it is unclear if the change is clinically relevant.
Our study has a couple of important limitations including the relatively small number of patients who agreed to stop their PPI. The observational nature of the study does not establish causation in this population that are at such high risk to experience a GI bleed, but the endoscopy proven esophagitis in the 1 patient suggests harm. The strengths of our study include the exhaustive EMR search for absolute PPI indications, involvement of the patient’s nephrology team, and close follow-up for symptoms and complications post PPI withdrawal.
In summary, PPIs are widely used and have been associated with several rare side effects in the general population prompting recommendations for deprescribing this class of medications. In our study, we did an exhaustive search of the patient’s EMR and incorporated recommendations by the primary nephrology team prior to approaching patients about discontinuing this class of medications. About ½ of the patients who agreed to a trial of PPI withdrawal restarted the medication for a reoccurrence of GERD symptoms similar to another study of ESKD patients. Importantly in this very high risk population, 2 patients had a GI bleed within 2 to 4 weeks of stopping the PPI. Serum phosphate also increased. Our results suggest that PPI de-prescribing in patients with ESKD is time consuming and may be associated with harm. Indications for PPIs and de-prescribing safety need to be established specifically for patients with ESKD and is in need of additional study.
Acknowledgments
Dr D Zimmerman receives salary support from the Department of Medicine at the Ottawa Hospital and statistical support from the Methods Center of the Ottawa Hospital Research Institute.
Footnotes
Ethics Approval and Consent to Participate: This quality assurance study was approved by the Ottawa Health Science Research Ethics Board (20200617-01H) and conducted in adherence with the Declaration of Helsinki. Participant consent was not required for this quality assurance project.
Consent for Publication: All authors provide consent for publication.
Availability of Data and Materials: All authors were involved in the study and manuscript preparation.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. D Zimmerman has received financial support from Otsuka as a speaker and advisory board member. She has also received financial support from Bayer as an advisory board member. The other authors do not have any disclosures to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors but was supported by funds from the Jones Family Foundation.
ORCID iD: Deborah Zimmerman  https://orcid.org/0000-0003-0000-8806
https://orcid.org/0000-0003-0000-8806
References
- 1. Chan A, Liang L, Tung ACH, Kinkade A, Tejani AM. Is there a reason for the proton pump inhibitor? an assessment of prescribing for residential care patients in British Columbia. Can J Hosp Pharm. 2018;71(5):295-301. [PMC free article] [PubMed] [Google Scholar]
- 2. Helgadottir H, Bjornsson ES. Problems associated with deprescribing of proton pump inhibitors. Int J Mol Sci. 2019;20(21):5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolus NE, Farrell MB, Zimmerman J. Abdominal Imaging 2017: Quality, Safety, and Dose Optimization. Reston, VA: Society of Nuclear Medicine & Molecular Imaging; 2017. [Google Scholar]
- 4. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician. 2017;63(5):354-364. [PMC free article] [PubMed] [Google Scholar]
- 5. Nehra AK, Alexander JA, Loftus CG, Nehra V. Proton pump inhibitors: review of emerging concerns. Mayo Clin Proc. 2018;93(2):240-246. [DOI] [PubMed] [Google Scholar]
- 6. Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med. 2009;122(10):896-903. [DOI] [PubMed] [Google Scholar]
- 7. Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34(11-12):1269-1281. [DOI] [PubMed] [Google Scholar]
- 8. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors. JAMA Intern Med. 2016;176(2):172-174. [DOI] [PubMed] [Google Scholar]
- 9. Walsh K, Kwan D, Marr P, Papoushek C, Lyon WK, Cino M. Bye-bye, PPI. A toolkit for deprescribing proton pump inibitors in EMR-enabled primary care settings (Version 1.3). https://choosingwiselycanada.org/wp-content/uploads/2017/07/CWC_PPI_Toolkit_v1.2_2017-07-12.pdf. Published 2019. Accessed June 9, 2022.
- 10. St Peter WL. Management of polypharmacy in dialysis patients. Semin Dial. 2015;28(4):427-432. [DOI] [PubMed] [Google Scholar]
- 11. Strid H, Simren M, Bjornsson ES. Overuse of acid suppressant drugs in patients with chronic renal failure. Nephrol Dial Transplant. 2003;18(3):570-575. [DOI] [PubMed] [Google Scholar]
- 12. Sheikh MS, Maguire JA, Emmett M, et al. Reduction of dietary phosphorus absorption by phosphorus binders. A theoretical, in vitro, and in vivo study. J Clin Invest. 1989;83(1):66-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zed PJ. Deprescribing proton pump inhibitors. Can J Hosp Pharm. 2018;71(5):291-292. [PMC free article] [PubMed] [Google Scholar]
- 14. Wan A, Halpape K, Talkhi SC, et al. Evaluation of prescribing appropriateness and initiatives to improve prescribing of proton pump inhibitors at Vancouver General Hospital. Can J Hosp Pharm. 2018;71(5):308-315. [PMC free article] [PubMed] [Google Scholar]
- 15. Doell A, Walus A, To J, Bell A. Quantifying candidacy for deprescribing of proton pump inhibitors among long-term care residents. Can J Hosp Pharm. 2018;71(5):302-307. [PMC free article] [PubMed] [Google Scholar]
- 16. McIntyre C, McQuillan R, Bell C, Battistella M. Targeted deprescribing in an outpatient hemodialysis unit: a quality improvement study to decrease polypharmacy. Am J Kidney Dis. 2017;70(5):611-618. [DOI] [PubMed] [Google Scholar]
- 17. Odenthal DR, Philbrick AM, Harris IM. Successful deprescribing of unnecessary proton pump inhibitors in a primary care clinic. J Am Pharm Assoc. 2020;60(1):100-104. [DOI] [PubMed] [Google Scholar]
- 18. Boghossian TA, Rashid FJ, Thompson W, et al. Deprescribing versus continuation of chronic proton pump inhibitor use in adults. Cochrane Database Syst Rev. 2017;3:CD011969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bittner V, Weiner DH, Yusuf S, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. JAMA. 1993;270(14):1702-1707. [PubMed] [Google Scholar]
- 20. Cekin AH, Boyacioglu S, Gursoy M, et al. Gastroesophageal reflux disease in chronic renal failure patients with upper GI symptoms: multivariate analysis of pathogenetic factors. Am J Gastroenterol. 2002;97(6):1352-1356. [DOI] [PubMed] [Google Scholar]
- 21. Wesdorp RI, Falcao HA, Banks PB, Martino J, Fischer JE. Gastrin and gastric acid secretion in renal failure. Am J Surg. 1981;141(3):334-338. [DOI] [PubMed] [Google Scholar]
- 22. Usta M, Ersoy A, Ayar Y, et al. Comparison of endoscopic and pathological findings of the upper gastrointestinal tract in transplant candidate patients undergoing hemodialysis or peritoneal dialysis treatment: a review of literature. BMC Nephrol. 2020;21(1):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sood MM, Bota SE, McArthur E, et al. The three-year incidence of major hemorrhage among older adults initiating chronic dialysis. Can J Kidney Health Dis. 2014;1:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moayyedi P, Eikelboom JW, Bosch J, et al. Pantoprazole to prevent gastroduodenal events in patients receiving rivaroxaban and/or aspirin in a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2019;157(2):403-412.e405. [DOI] [PubMed] [Google Scholar]
- 26. Ali Khan M, Howden CW. The role of proton pump inhibitors in the management of upper gastrointestinal disorders. Gastroenterol Hepatol (N Y). 2018;14(3):169-175. [PMC free article] [PubMed] [Google Scholar]
- 27. Tatsuzawa M, Ogawa R, Ohkubo A, et al. Influence of proton pump inhibitors and histamine H2 receptor antagonists on serum phosphorus level control by calcium carbonate in patients undergoing hemodialysis: a retrospective medical chart review. J Pharm Health Care Sci. 2016;2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toh JW, Ong E, Wilson R. Hypomagnesaemia associated with long-term use of proton pump inhibitors. Gastroenterol Rep. 2015;3(3):243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Molnar AO, Biyani M, Hammond I, et al. Lower serum magnesium is associated with vascular calcification in peritoneal dialysis patients: a cross sectional study. BMC Nephrol. 2017;18(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]