Abstract
Ocular cells like, retinal pigment epithelium (RPE) is a highly specialized pigmented monolayer of post-mitotic cells, which is located in the posterior segment of the eye between neuro sensory retina and vascular choroid. It functions as a selective barrier and nourishes retinal visual cells. As a result of high-level oxygen consumption of retinal cells, RPE cells are vulnerable to chronic oxidative stress and an increased level of reactive oxygen species (ROS) generated from mitochondria. These oxidative stress and ROS generation in retinal cells lead to RPE degeneration. Various sources including mtDNA damage could be an important factor of oxidative stress in RPE. Gene therapy and mitochondrial transfer studies are emerging fields in ocular disease research. For retinal degenerative diseases stem cell-based transplantation methods are developed from basic research to preclinical and clinical trials. Translational research contributions of gene and cell therapy would be a new strategy to prevent, treat and cure various ocular diseases. This review focuses on the effect of oxidative stress in ocular cell degeneration and recent translational researches on retinal degenerative diseases to cure blindness.
Keywords: Mitochondrial transfer, Oxidative stress, Retinal pigment epithelium (RPE), RPE Degeneration, Stem cell treatment
Introduction
The ocular cell system is comprised of the eye and its visual system. Atypical cell proliferation and regulation within the ocular cell system contributes to the developmental disorders. Retinal pigment epithelium (RPE) cells are a cuboidal monolayer of non-neuronal epithelial cells of the neurosensory part of the retina located between the photoreceptors and choroidal capillaries. It provides many important functions for the conversion of light energy to electrical signals by the photoreceptors for the visual process. It forms tight junction between cells and it enables passage of selective molecules between choroidal blood and neural retina.1 Degeneration of retinal epithelial cells cause severe vision loss and studies suggest that oxidative stress in the retinal cells is the major factor for RPE degeneration. Oxidative stress is an imbalance between generation of reactive oxygen species (ROS) level and detoxification of the reactive intermediates leads to cellular damage. There are several types of ROS such as superoxide anion, hydroxyl radical, singlet oxygen, and hydrogen peroxide (H2O2) that can be generated from different cellular compartments; and mitochondria is the major source of ROS generation in the cells.2 Due to the high oxygen consumption of retinal cells and phagocytic nature of RPE cells oxidative stress is more prompt in RPE cells.3 Due to the lack of histones and not well organized mitochondrial DNA (mtDNA) repair system mtDNA is more susceptible to oxidative damage by ROS than nuclear DNA (nDNA).4,5 mtDNA damage is considered as a biomarker for oxidative stress. Oxidative stress from various sources damages RPE cells and choroid capillaries. RPE degeneration happens through various cellular events and ultimately these processes induce apoptosis and reduction in RPE layer which lead to several ocular diseases and vision loss.6 Mitochondrial transfer studies using termed tunnelling nanotubes (TNTs) is an emerging research area in which it can promise the rescue of mitochondrial dysfunctional damaged cell by the mitochondrial transfer from normal cells. For retinal degenerative diseases, lot of stem cell-based cellular therapeutics have been proposed by researchers. Mitochondrial transfer studies, stem cell and gene therapy based researches all together may offer novel and successful ways of treating retinal degenerative diseases and severe blindness.
Retinal Pigmented Epithelial cells
Retinal Pigmented Epithelial cells are monolayer of polarized epithelial cells7 situated between photoreceptors and the choroid vasculature, derived from outer layer of the optic cup. The polarized organization of the RPE mediates important support functions to the neural retina and maintains access to the choroidal blood supply. They form a blood-retinal barrier and are essential for the function and viability of the photoreceptor cells, however, the number varies in different animals.8,9 Nutrient transport as well as the directional secretion of growth factors across the RPE helps to maintain the function of photoreceptors and choroids. RPE cells complement several pigments, such as melanin, lipofuscin, and flavins, which absorb excess light and protect the neuroretina from phototoxicity.10 RPE cells are phagocytic in nature but are not replaced, unlike other phagocytes and thus, any inefficiency in the degradation pathway can remain over the life of the organism. By keeping this in knowledge, Sparrow and Boulton proposed that such inefficiencies might lead to pathogenesis and age-related visual impairment.11 Recently Roni et al suggested that the degeneration of the RPE cells might lead to the death of macular photoreceptors.12 Recently, advanced RPE transplantations became a potential cell based therapy for ocular diseases.13
Oxidative stress molecules and its influence in the retina
Oxidative stress is essentially an imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects through neutralization by antioxidants. Oxidative stress is caused by the production of ROS, including superoxide anion (O2−∙), hydroxyl radical (•OH), H2O2, and singlet oxygen (O2). ROS and reactive nitrogen species (RNS) are highly reactive molecules acting in modifying proteins, nucleic acids, carbohydrates and lipids, often resulting in dysfunction of the biomolecule. At a physiological level, ROS and RNS can function as signaling molecules in crucial regulatory pathways including cell proliferation, gene expression and apoptosis.14 However, ROS/RNS levels above physiological, or an imbalance in the oxidant/antioxidant ratio, can have significant pathophysiological consequences.15 In particular, O2−∙ and •OH with an unpaired electron are also known as free radicals. H2O2 exhibits a low reactivity, but it can penetrate cell membranes, including the inner and outer membranes of mitochondria. Therefore, H2O2 can react with cellular iron and generate hydroxyl radicals, the most reactive form of oxygen, through the Fenton reaction: H2O2 + Fe2+ → ∙OH + –OH + Fe3+.
Oxidative stress plays a vital role in developing retinal diseases. The retina is one of the highest oxygen consuming tissues in the human body.16 The highest oxygen level falls dramatically across the outermost retina, creating a large gradient of oxygen towards the retina and inner segments of the photoreceptors which contain high levels of polyunsaturated fatty acids. Aging, gene abnormalities, and excess exposure to exogenous oxidative stressors (e.g., a light exposure) increase oxidative stress in the eye. The retina is located in an environment that is primed for the generation of ROS which results in oxidative damage. However, oxidative damage is normally minimized by the presence of a range of antioxidant and efficient repair systems. But as age increases oxidative damage increases, antioxidant capacity decreases and the efficiency of reparative systems become impaired whichresults in retinal dysfunction and cell loss leading to visual impairment.
ROS and mitochondria
ROS can be derived from several enzymatic and oxidation reactions. is the proximal mitochondrial ROS. In the mitochondrial respiratory chain, the electrons are transported chiefly from complexes I and III and up to 2% of electron leakage happens before reaching to complex IV. This process results in the formation of by incomplete reaction with oxygen in a one electron reduction.17 Under hypoxic conditions, this process is not performed to completion, resulting in an increased production of . Super oxides are membrane impermeable and super oxide dismutase (SOD) convert it into non radical, membrane permeable H2O2. Mitochondrial generated H2O2 is further reduced to produce hydroxyl radical •OH. This highly toxic hydroxyl radical reacts with several metabolites and induces oxidative stress in cells.18
ROS and cigarette smoking
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) is the source of ROS, derived primarily from O2−∙, through enzymatic reactions. Nitric oxide (NO) is produced by the sequential oxidation/reduction of l-arginine to l-citrulline by nitric oxide synthase (NOS), which exists in the form of inducible NOS (iNOS), neuronal NOS (nNOS), and endothelial NOS (eNOS). NO can react with and form peroxynitrite (ONOO−) which has a highly potent oxidizing and nitrosating ability. This reaction prompts eNOS uncoupling, resulting in an increase in the formation of .
Cigarette smoke is known as one of the exogenous sources of ROS. Cigarette smoke is a complex mixture of more than 4000 chemical substances. The agents present in cigarette smoke are generally subdivided into particulate and gaseous phases. The major components of the particulate phase are tar and nicotine. Nicotine and cadmium are multiple ROS producers. Nicotine is an attractive candidate molecule to explain an association of smoking with several diseases. It has been shown to be mitogenic for vascular endothelial cells and smooth muscle pericytes, to reduce apoptosis of vascular endothelial cells, and to induce the formation of capillary tubes. Nicotine promotes nitric oxide (NO) production and cadmium that accumulates preferentially in the RPE and choroid and increases ROS production. NO is produced by the sequential oxidation/reduction of l-arginine to l-citrulline by nitric oxide synthase (NOS), which exists in the form of inducible NOS (iNOS), neuronal NOS (nNOS), and endothelial NOS (eNOS). NO can react with O2−∙ and form peroxynitrite (ONOO−) which has a highly potent oxidizing and nitrosating ability. This reaction prompts eNOS uncoupling, resulting in an increase in the formation of . The gaseous phase is composed primarily of carbon monoxide, carbon dioxide and NO. Cigarette smoke tar contains numerous pro-oxidant compounds within the quinone family. Within these, hydroquinone, a benzene derivative, is an abundant oxidant in nature, found in processed foods, plastic containers, and atmospheric pollutants. High levels are detected in the plasma and urine of smokers. In addition, cigarette smoke extract (CSE) has been shown to induce alterations to mitochondrial integrity, increase in lipid peroxidation, and significant human RPE cell death. Excess light exposure is also included as a source of ROS. The energy contained in a photon of light changes electron orbitals and can break bonds directly.
Fatty acids in retina
Besides the effect of oxidative stress and ROS, long chain (LC) poly unsaturated fatty acids (PUFAs) and docosahexaenoic acid (DHA), a member of w-3 LC-PUFAs, has a role in CNS diseases. They are primarily concentrated in the nervous system, particularly in retinal photo receptors and synaptic membranes. The presence of six double bonds between carbon atoms in its polyene chain (C=C), easily undergoes non-enzymatic oxidation and fragmentation during lipid peroxidation and form numerous toxic products. In other words, PUFAs, especially long-, or very long-chain fatty acids, are targets of oxidative stress-mediated lipid peroxidation. Lipid peroxidation thus contributes to a chain reaction implicated in the generation of a variety of very reactive highly cytotoxic molecules. Retina, is rich in LC-PUFAs, particularly with DHA, which is very sensitive to oxidative damage and, unlikely to brain, retina is subjected to high level exposal to light, which can produce photochemical damage. Lipofuscin formation is another stress related mechanism occurring particularly in retina. The retinal lipofuscin is formed and accumulated by RPE cells as a consequence of both visual process taking place in photoreceptor-RPE functional complex and metabolic insufficiency of RPE lysosomal compartment.
Oxidative stress and mitochondrial dysfunction in RPE and in other ocular cells
RPE cells are composed of various pigments, such as melanin, lipofuscin, and flavins, which absorb excess light and protect the neuro-retina from phototoxicity.10 Furthermore, RPE cells secrete growth factors and additional immunosuppressive factors to protect ocular tissues from damage.19 Thus any inefficiency in one or more of the above RPE processes can cost for ocular health and vision. In an in vivo mice model, RPE has been associated with mitochondrial oxidative stress that led to metabolic dysfunction in RPE and photoreceptor cells.20 In ARPE-19 cells, sodium iodate induced oxidative stress was reported to be inhibited by ROS mediated autophagy and mitochondria that minimized RPE cell death.21 Recent studies strongly implicate oxidative stress as an unvarying threat to the structural and functional integrity of RPE.21 The experimental studies on the effects of oxidative stress in RPE have been summarized in Table 1.
Table 1.
Overall studies depicting in vitro and in vivo findings of oxidative stress on RPE.
| S.No | Country/Region | Study | Objective | Method used | Effects | References |
|---|---|---|---|---|---|---|
| 1 | California, USA | in vitro | To validate the RPE cell culture in AMD Pathology |
|
Cytology of human RPE cells are assessed and found as perfect model to study early stages of AMD. | 150 |
| 2 | Boston, USA | in vitro | To characterize the super oxide mechanisms and toxicity prevention. |
|
in vitro release superoxide and possibly H2O2 of RPE may be subject to regulation. Future studies on in vivo recommended. | 151 |
| 3 | Minnesota, USA | in vitro | To understand the role of zinc in the pathogenesis and prevention of AMD. |
|
ZPP1 is a superior probe for the detection of zinc in sub-retinal epithelial deposits in human and murine tissue. | 152 |
| 4 | Washington, USA | in vitro | To determine causative pathways which contributes to AMD |
|
The SIRT1/PGC-1α pathways contribute to AMD. | 153 |
| 5 | Florida, USA | in vitro | To study POS phagocytosis by RPE from AMD and the effect of hUTC on RPE phagocytosis, and the mechanisms involved. |
|
RPE phagocytic dysfunction in AMD and the ability of hUTC to treat the dysfunction were analysed. | 154 |
| 6 | California, USA | in vitro | To investigate the molecular mechanism of wound stimulus in RPE cells. |
|
In RPE cells, insistent mesenchymal state with wound stimulus is driven by lasting activation of the TGFβ pathway | 155 |
| 7 | Singapore | in vitro | To develop hPSC-derived RPE production and purification system that yields high-quality RPE monolayers. |
|
Pure functional RPE monolayers from hPSC using simplified 2D cultures along with RPE PLUS protocol were developed. | 156 |
| 8 | Japan | in vitro | To develop a microfluidic co-culture model of the ocular fundus tissue in a challenge to elucidate AMD pathology. |
|
Developed a microfluidic that study the development of diseases compounds that stimulate or inhibit the angiogenesis process. | 157 |
| 9 | New York, USA | in vitro | To study the impact of iron and cigarette smoke, on POS processing and its consequence for autofluorescent material accumulation in human RPE cells. |
|
Both environmental factors together inn under study can impair POS processing and leads to increased autofluorescent material accumulation in hiPSC-RPE. | 158 |
| 10 | USA | in vitro | To investigate the potential use of fucoidan for the treatment of exudative AMD. |
|
Fucoidan is safe for RPE cells and making it an interesting molecule for further studies in AMD. | 159 |
| 11 | London, UK | in vitro | To systematically develop and validate a reliable method to isolate RPE cells from adult rats. |
|
Developed an efficient method for the rapid and easy isolation of high quantities of adult rat RPE cells | 160 |
| 12 | Maryland, USA | in vitro | To study the role of Cryba1 gene in the EMT of RPE cells. |
|
Targeting Cryba 1 mutations is a potential therapeutic method for AMD. | 161 |
| 13 | Washington, USA | in vitro | To provide an evidence for altered autophagic function in the pathophysiology of AMD in an in vitro cellular model |
|
The autophagy was selectively dysregulated in AMD | 162 |
| 14 | California, USA | in vitro | To understand the molecular mechanism behind the AMD by transcriptome analysis. |
|
Discovered novel global biomarkers, phenotype-specific gene sets, and functional networks associated with AMD. | 163 |
| 15 | New Jersey, USA | in vitro | To develop a model to evaluate RPE transplantation onto human Bruch's Membrane |
|
The adherence property of RPE to normal and diseased human BrM were studied. | 164 |
| 16 | California, USA | in vitro | To investigate the expression of HN in hRPE cells and its effect on oxidative stress–induced cell death, mitochondrial bioenergetics, and senescence |
|
Suggested HN as a potential therapeutic method of AMD. | 165 |
| 17 | California, USA | in vitro | To develop a potential therapeutic for both dry and wet AMD by redesign a complement-inhibiting peptide. |
|
A novel peptide analog of compstatin is developed that become a therapeutic for the treatment of AMD. | 166 |
| 18 | Jerusalem, Israel | in vitro and in vivo | To analyse the immunosuppressive property of hESC-RPE |
|
Immune properties of hESC-RPE cells is relevant and valuable for clinical transplantation of hESC-RPE cells in retinal degenerations caused by RPE dysfunction | 167 |
| 19 | Switzerland | in vitro and in vivo | To investigate whether BMCs can be induced to express RPE cell markers in vitro and can home to the site of RPE damage after mobilization and express markers of RPE lineage in vivo. |
|
BMCs once mobilized have the ability to respond to signals from damaged RPE, migrate to the altered sub-retinal space, and form a monolayer of cells that express markers of RPE lineage. | 168 |
| 20 | Kentucky, USA | in vitro and in vivo | To gain the potentiality of RPE cells to be regenerative medicine by reprogramming of differentiated somatic cells into iPSCs |
|
By activate Hippo signaling pathway we can prepare regenerative medicines which is important in iRPESC reprogramming. | 169 |
| 21 | Finland | in vivo | To investigate the role of NRF-2 and PGC-1α in the regulation of RPE cell structure and function by using global dKO mice. |
|
The study suggests that the NRF-2/PGC-1α dKO mouse is a valuable model for investigating the role of proteasomal and autophagy clearance in the RPE and in the development of dry AMD. | 170 |
| 22 | Chinese Mainland | in vitro | To test the potentiality of paeoniflorin to prevent H2O2-induced oxidative stress in ARPE-19 cells and to elucidate the molecular pathways involved in this protection. |
|
Paeoniflorin could protect human RPE cells against H2O2-induced oxidative stress. | 171 |
| 23 | California, USA | in vitro and in vivo | To understand the molecular mechanism behind the damages caused for RPE cells. |
|
The study suggests a possible role for viral dsRNA transcripts in the development of GA and raise awareness of potential toxicity induced by siRNA therapeutics in the eye. | 172 |
| 24 | Madison, Wisconsin | in vitro and in vivo | To investigate the autonomous impact of PEDF and TSP1 on RPE cell function. |
|
Demonstrated that PEDF and TSP1 play key roles in RPE cell function and subsequently in pathogenesis of AMD. | 33 |
| 25 | Los Angeles, USA | in vivo | To describe the potential of a peptide derived from αB crystallin protein using a NaIO3 induced mouse model of GA |
|
The study shows that crySI hold promise as protective agents to prevent RPE atrophy and progressive retinal degeneration in AMD. | 173 |
| 26 | Durham, UK | in vivo | To generate a therapeutic strategy against AMD, that targets through systemic administration of anti-Aβ antibodies. |
|
The results support the feasibility of immunotherapeutic strategies targeting Aβ as treatments for both early and advanced stages of AMD, especially for those patients in whom Aβ deposition is a feature of their disease. | 174 |
| 27 | Chicago, USA | in vitro | To better understand the cellular and molecular bases for the association between smoking and AMD |
|
The cigarette smoking may be main causative agent to genetic mutations which contributes to the pathogenesis of AMD in the elderly. | 175 |
| 28 | Newcastle, UK | in vitro and in vivo | To understand the pathology of the disease and the role of environmental, dietary, and lifestyle factors. |
|
The low- and high-risk AMD-RPE cells respond very differently to UV exposure and moreover this provides evidence for UV mediated functional and cellular improvement of AMD-associated cellular changes in high-risk AMD-RPE cells. | 176 |
| 29 | Germany | in vitro and in vivo | To demonstrate the three-dimensional epithelial cyst culture of human pluripotent stem cells leads to the induction of polarized neuroepithelia |
|
The work highlights the cell biological environment of pluripotent stem cells while culturing can drastically improve differentiation and the subsequent efficacy of therapeutic outcomes. | 177 |
| 30 | New York, USA | in vitro | To compare the ability of intraocular lenses IOLs as to protect RPE cells from light damage mediated by the lipofuscin fluorophore A2E |
|
A yellow-tinted IOL that simulates the adult natural lens and protects lipofuscin-containing RPE from blue light damage may reduce the risk for or progression of AMD | 178 |
| 31 | New York, USA | in vitro | To prepare a culture model for AMD studies |
|
Culture prepared by RPE derived from patients with AMD act as a perfect model for the future studies. | 128 |
| 32 | Florida, USA | in vitro and in vivo | To study the cellular mechanisms linking oxidative stress and inflammation in AMD, |
|
The injured RPE cells may trigger progression toward CNV in smoker patients with dry AMD. | 179 |
| 33 | Maryland, USA | in vivo | To investigate the role of chemokine receptor CXCR5 in the pathogenesis of AMD. |
|
CXCR5 itself may be involved in the protection of RPE and retinal cells during aging and its loss may lead to AMD-like pathological changes in aged mice. | 180 |
| 34 | Germany | in vitro | To investigate the glycomic changes associated with EMT of RPE cells in vitro. |
|
Provide the first evidence that EMT of RPE cells in vitro confers glycomic changes and that these changes are associated with an increased responsiveness to Gal-3. | 181 |
| 35 | Durham, UK | in vivo | To test the hypothesis that the CFH H402 polymorphism contributes to the development of AMD |
|
Demonstrated a functional consequence of the Y402H polymorphism in vivo, which promotes AMD-like pathology development and affects lipoprotein levels in aged mice. | 182 |
| 36 | New York, USA | in vitro | To determine the specific role of RPE-autonomous dysfunction in drusen biogenesis and ECM alterations in maculopathies affecting the RPE–ECM complex. |
|
Distinct complement pathway genes were up-regulated in SFD, DHRD, and ADRD hiPSC-RPE cultures, potentially highlighting similar molecular change as earlier reportings in distinct maculopathies affecting the RPE–ECM complex. | 183 |
| 37 | California | in vitro | To develop an RPE cell culture model that mimics drusen formation and triggers complement activation associated with AMD |
|
Developed an RPE cell culture model that mimics various aspects of AMD pathology observed in humans. | 184 |
| 38 | Durham, UK | in vivo | To investigate the role of Complement factor H CFH in the development of AMD pathology |
|
|
185 |
| 39 | USA | in vivo | To cause mitochondrial damage in RPE cells and test for AMD characteristics |
|
Sod2 knockout decreased RPE function with an increase in oxidative stress. | 20 |
| 40 | Taiwan Region, China | in vitro | In ARPE-19 cell line, NaIO3 can cause ROS production and its effect in cell death. |
|
NaIO3 induced cytosolic ROS production and oxidative stress that resulted with activating signalling pathways that respond cell death mechanisms. | 21 |
RPE: retinal pigment epithelium; PMA: phorbol 12-myristate 13-acetate; ZPP1: Zinpyr-1; AMD: age related macular degeneration; iPSCs: induced pluripotent stem cells; PCR: polymerase chain reaction; SIRT1: sirtuin 1; PGC1α: Peroxisome proliferator-activated receptor gamma co-activator 1-alpha; POS: photoreceptor outer segments; hUTC: human umbilical tissue cells; RNA: ribonucleic acid; TGFβ: transforming growth factor beta; hPSC: human pluripotent stem cell; ELISA: enzyme linked immunosorbent assay; 2D: two dimensional; RPE PLUS: RPE purification by lipoprotein uptake-based sorting; MTT: 4,5-dimethylthiazol-2-yl; VEGF: vascular endothelial growth factor; EMT: epithelial-to-mesenchymal transition; OCM3: uveal melanoma cell line; SNPs: single nucleotide polymorphisms; ROS: reactive oxygen species; HN: humanin; hRPE: human retinal pigment epithelial; FITC: fluorescein isothiocyanate; TEM: transmission electron microscopy; PBMCs: peripheral blood mononuclear cells; hESCs: human embryonic stem cells; RCS: royal college of surgeons; BMCs: bone marrow–derived cells; GFP+: green fluorescent protein; iRPE: iPSC-derived retinal pigment epithelium; OKR: optokinetic response; qPCR: quantitative PCR; shRNA: short hairpin RNA; ChIP: chromatin immunoprecipitation; NRF2: Nuclear factor erythroid 2-related factor 2; ER: endoplasmic reticulum; dKO: double knock-out; TER: trans epithelial resistance; ARPE-19: aris-ing retinal pigment epithelium-19; ERG: electroretinography; TLR3: toll-like receptor-3; dsRNA: double stranded ribonucleic acid; GA: geographic atrophy; siRNA: short interfering RNA; PEDF: pigment epithelium derived factor; TSP1: thrombospondin 1; NO: nitric oxide; FACS: fluorescence acting cell sorting; NaIO3: sodium iodate; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labelling; ONL: outer nuclear layer; hAPP: human amyloid precursor protein; LX-PCR: long extension polymerase chain reaction; UV: ultra violet; IOLs: intraocular lenses; ECM: extracellular matrix; CNV: choroidal neovascularization; RT-PCR: real time – polymerase chain reaction; CXCR5: C-X-C chemokine receptor type 5; EMT: epithelial to mesenchymal transition; siRNA: small interfering RNA; CRISPR: clustered regularly interspaced short palindromic repeats; CFH: complement factor H; DHRD: Doyne Honeycomb Retinal Dystrophy; SFD: Sorsby's fundus dystrophy; ADRD: autosomal dominant radial drusen; BrM: Bruch's membrane; mtDNA: mitochondrial DNA; UPS: ubiquitin proteasome system; H2O2: hydrogen peroxide; SD-OCT: spectral-domain optical coherence tomography; ATP: adenosine triphosphate.
The sources of oxidative stress are miscellaneous. Primarily, the high O2 saturation levels and flow rate in choriocapillaris contribute to high O2 tension in the RPE cells. Long-term sunlight and blue light (475 nm) exposure causes free radical stress and photooxidation respectively to RPE biomolecules including pigments.22,23 Another source of intracellular oxidative stress is the age-dependent accumulation of lipofuscin, which is considered as an aging pigment in RPE.24 Young RPE cells can competently dispose lipofuscin by targeting it to lysosomal degradation. However, the lysosomal pathways and capacity to eliminate lipofuscin gets diminished as we age. As a result, this waste material builds up and produces free radicals in RPE cells.25
These various sources of free radical stress can contribute to deleterious effects inside the cells. The intracellular free radicals trigger large-scale damage to RPE biomolecules such as proteins, lipids, and DNA and finally promoting destruction of the RPE mitochondrial network. The study by Haijiang et al, (2011) showed that RPE cells from the macula have greater mtDNA damage and diminished repair capacity with respect to the cells from peripheral region. Thus, RPE in AMD is associated with increased mtDNA damage and decreased mtDNA repair. Their study found out that the mtDNA damage and reduced repair capacity, in macular RPE cells, are associated with aging and AMD pathogenesis.26 Thus, mtDNA damage is the second factor of oxidative stress in RPE. Studies in diseased postmortem eyes deliver that the accumulation of mtDNA mutations in RPE can be related to free radical stress.27, 28, 29
Recent investigations pointed out that oxidative stress in RPE induce an antioxidant response within mitochondria. The analysis by Cano et al (2014) showed that mitochondria can protect RPE from oxidative stress.30 Oxidative stress is caused by many factors which damages RPE cells and choriocapillaris. The pathology begins from the modification of various compounds in photoreceptors. As a result they get shed in the form of photoreceptor outer segments (POS). These POS are phagocytosed by RPE cells thereby results in damage and leads to dysfunction in cell metabolism. Deposition of toxic photoproducts further increases the amount of ROS thus damages mitochondria, lysosome, etc. which in turn elevates the insoluble or undigested materials in the form of lipofuscin. This process, referred to as lipofuscinogenesis, induces apoptosis and reduction in RPE cells in RPE layer. When the number of RPE is reduced, POS gets stored in between RPE layer and Bruch's membrane as drusen. This process is called drusogenesis, which stimulates inflammation and causes neovascularization and aggravateswith age leading to progression of the diseases.6,31,32 In a mice model, RPE cells with high glucose conditions increase the level of oxidative stress.33 In RPE cell lines, increase in oxidative stress leads to RPE cell death with dysregulates endogenous antioxidants.34,35 In human fetal RPE and sodium iodate (NaIO3) mouse models, toll-like receptor-2 caused oxidative stress and RPE damage.36 Recently, a review on the molecular mechanisms to determine the role of oxidative stress in ocular cells has been reported.37 Table 2 shows the studies on oxidative stress effects in RPE cells.
Table 2.
Studies on the effects of oxidative stress in RPE cells.
| S.No | Model | Method Used | Effect Of Oxidative Stress | Reference |
|---|---|---|---|---|
| 1 | Mice |
|
RPE cells were more migratory under high glucose conditions. High glucose condition increased the level of oxidative stress and has minimal effect on RPE cells proliferation and apoptosis. |
186 |
| 2 | RPE cell lines |
|
Identified GAA as a potential inhibitor of oxidative stress-induced RPE cell death by regulating FoxO3/SESN2 pathway | 34 |
| 4 | Human monocytic cell lines, hfRPE and NaIO3 mouse models |
|
TLR2 induces oxidative stress and causes retinal degeneration. | 36 |
| 5. | Mice |
|
|
20 |
| 6. | ARPE-19 cell line |
|
|
21 |
| 7 | ARPE-19 cell line |
|
|
30 |
| 8 | Human RPE cells from AMD patients |
|
|
26 |
| 9. | Human RPE cells from AMD patients |
|
|
28 |
| 10. | Human RPE cells from AMD patients |
|
|
29 |
RPE: retinal pigment epithelium; ROS: reactive-oxygen species; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium; ATP: adenosine triphosphate; SESN2: sestrin 2; FoxO3: Forkhead box O3 transcription factor; GAA: acetic acid; hfRPE: human fetal RPE; NaIO3: sodium iodate; TUNEL: terminal deoxynucleotidyltransferasedUTP nick end labeling; qPCR: quantitative polymerase chain reaction; ELISA: enzyme linked immunosorbent assay; SD-OCT: spectral-domain optical coherence tomography; RT-PCR: real-time polymerase chain reaction; mtDNA: mitochondrial DNA; TLR2: toll-like receptor 2; FITC: fluorescein isothiocyante; H2O2: hydrogen peroxide; LX-PCR: long extension polymerase chain reaction.
Corneal epithelial cells are the outer most layer of cornea and it consist of several layers. It protects from outer environment and it is transparent to reflect light properly. ROS generation and oxidative stress in corneal cells have been involved in the pathogenesis of various eye diseases including photokerato, conjunctivitis, photokeratitis, pingueculae and pterygia, cataract, glaucoma, and macular degeneration.38 Mitochondrial dysfunction and oxidative stress are the major reasons for inherited and acquired corneal diseases. Imbalance between the production of ROS and antioxidant capacity of the corneal cells is the main reason for oxidative stress in the cornea. Accumulation of ROS or RNS in the cornea affects various functions of corneal cells like signal transduction, cellular proliferation and promote cell death.39 Major transcription factors such as Nrf2 and p53 are highly involved in the stress responses of cornea. Nrf2 regulates antioxidant defense mechanism and p53 regulates cell cycle arrest, apoptosis, senescence and DNA repair.40
Due to the post mitotic nature of corneal endothelial cells it is more predominant with accumulation of damaged mtDNA. In the dry eye disease, mitochondrial damage has been involved in corneal epithelial cell death and the disease state induces apoptosis of these cells through cytochrome C mediated by apoptotic pathway.41 Kearns-Sayre Syndrome (KSS) is rare neuromuscular disorder resulting from the deletion of 5 kb mtDNA. mtDNA mutation dysregulate corneal endothelial function and corneal transparency which leads to visual impairment. Ocular diseases such as keratoconus, Fuchs Endothelial Corneal Dystrophy (FECD) and KSS are associated with mitochondrial dysfunction and oxidative stress in the corneal cell.42
Retinal Ganglion Cells (RGC) are the retinal neurons, the innermost layer of the retina which convey information directly to the brain. As a result of light exposure, retina is more vulnerable to oxidative stress. During aging, retinal mitochondrial function reduces and it causes notable increase in ROS generation.43 Oxidative stress in RGC has been the major reason for many ocular neurodegenerative diseases. Oxidative stress and mitochondrial dysfunction leads to the loss of RGC and optic nerve dysfunction which cause neurodegenerative diseases such as glucoma, Leber's Hereditary Optic Neuropathy (LHON) and progressive external ophthalmoplegia (PEO). Oxidative stress is the major reason for pathogenesis of glaucoma and it is evident from both the animal and human studies that assessed ROS production, antioxidant levels and oxidative damage markers to macromolecules which are observed under glucomatous condition. Elevated intraocular pressure (IOP) in glaucoma condition induces oxidative stress in retina and optic nerve. Many valid evidences show oxidative stress and mitochondrial dysfunctions in various ocular cells and have been the contributing factors to several diseases.
Oxidative stress and Nrf2 signaling
The nuclear factor E2-related factor 2 (Nrf2) is the master regulator of antioxidant transcription factor and Nrf2 deficiency has been associated with various disease processes caused by increased susceptibility to oxidative stress. Nrf2 pathway is a primary system employed by the RPE to neutralize oxidative stress and maintain cellular homeostasis. Nrf2 induces the expression of genes encoding ROS neutralizing enzymes, detoxifying enzymes, molecular chaperones, proteasome subunits, and enzymes essential for intermediary metabolism.44 Nrf2 knockout studies in mice reveals that Nrf2 deficiency may contribute to the pathogenic conditions. In the absence of stressed Nrf2, knockout mice are healthy, but under oxidative challenge conditions they exhibit various phenotypes.45,46 Nrf2 deficient mice manifest multiple age-dependent pathologies like progressive RPE and Bruch's membrane degeneration, drusen deposits, lipofuscin accumulation, and decreased electroretinography responses. Thus Nrf2 deficient mice were characterized as a model of retinopathy.46 The studies indicate that aging decreases the efficacy of the cytoprotective Nrf2 machinery, and in doing so, increases the susceptibility of the RPE to oxidative damage.
In another study on comparing Nrf2 signaling in the RPE of young (2 months) and old (15 months) mice under unstressed and stressed (sodium iodate) conditions. The aging RPE expressed higher levels of the Nrf2 target genes NAD(P)H:quinone oxidoreductase (NQO1), glutamate cysteine ligase subunit M (GCLM), and heme oxygenase-1 (HO1) compared with the RPE of younger mice under unstressed conditions, suggesting an age related increase in basal oxidative stress. Moreover, the RPE of older mice demonstrated impaired induction of the protective Nrf2 pathway following oxidative stress induced with sodium iodate. The RPE of old mice exposed to sodium iodate also exhibited higher levels of superoxide anion and malondialdehyde than young mice, suggesting inadequate protection against oxidative damage.47 The data indicates that the aging RPE is vulnerable to oxidative damage due to impaired Nrf2 signaling, and that Nrf2 signaling is a promising target for novel pharmacologic or genetic therapeutic strategies.
The transcriptional factor Nrf2 serves as the master regulator of a highly conserved protective molecular response to oxidative stress in all cell types, driving an expression of a coordinated suite of several antioxidant genes. Under basal conditions, Nrf2 physically interacts with the negative regulator Keap1, which targets the Nrf2 protein for ubiquitination and proteasomal degradation within the cytoplasm, thus, limiting its activity. However, under conditions of oxidative stress, Keap1 undergoes a conformational modification and releases Nrf2 for translocation to the nucleus where it binds to antioxidant response elements (AREs), thus activating transcription of its target genes, including GCLM, HO1, and NQO1.48 Nrf2 deficiency in in vivo increases susceptibility to oxidative stress in all tissues, including RPE. For example, cigarette smoke exposure induces more severe RPE damage in Nrf2−/− mice when compared with wild type controls.45
Nrf2 activation of antioxidant defense system has neuroprotective effects on RGCs in neurodegenerative diseases. There are several factors to induce Nrf2 for the RGC protection like Sox2OT, serum protease factor (SPF), l-carnithine, monomethyl fumarate (MMF), SNJ-1945, CDDO-Im (2-Cyano-3,12-dioxooleana-1,9-dien-28-imidazolide), Sulforaphane (SF), Lycium barbarum polysaccharides (LBP), Limb remote ischemic conditioning (LRIC), MicroRNA-141, Hydrogen sulfide gas (H2S) donor drugs, R-α-lipoic acid (R-LA), nipradilol, flavonoids and sulbutiamine. Induction of Nrf2/Keap1/ARE signaling pathway is considered as one of the main cell defense mechanisms against oxidative stresses for neuroprotection in RGCs.49
Effects of oxidative stress in RPE
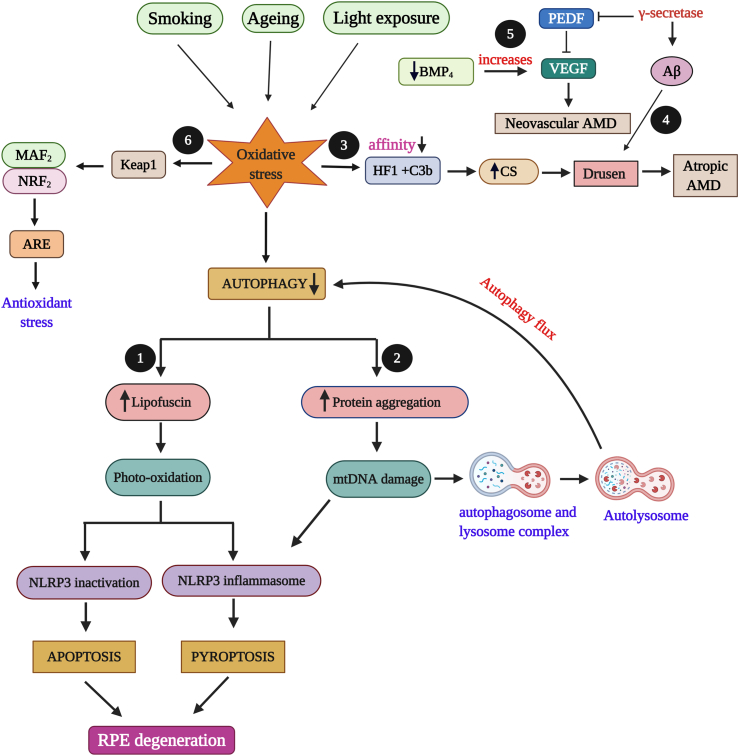
RPE undergoes oxidative stress due to external factors such as smoking, ageing or light exposure. Various mechanisms have been linked with RPE degeneration due to oxidative stress but the major contribution is by autophagy, programmed cell death. Decreased autophagy causes an increase in lipofuscin accumulation which induces photo-oxidation that results in apoptosis and pyroptosis depending upon NLRP3 (Nucleotide-binding oligomerization domain, Leucine rich Repeat and Pyrin domain containing 3) activation and inactivation.50,51 This in turn degenerates RPE cells and leads to AMD. Reduced autophagy also results in protein damage and aggregation in RPE which induces mitochondrial damage and autophagy flux through fusion of lysosome and autophagosome into autolysosome.52 Oxidative stress also lessens the affinity between compliment factor H gene (HF1) with C3b, an activator of complement system. The decreased affinity results in increased activation of alternative complement system leading to Drusen formation in RPE that ends up in atropic AMD.53 Also during oxidative stress condition, the enzyme γ-secretase function in the production of amyloid beta (Aβ) and that damages pigment epithelium derived factor (PEDF), a neurotrophic factor produced in RPE. PEDF irregulates vascular endothelial growth factor (VEGF) activity and results with the progression of neovascular AMD.53 The involvement of bone morphogenic protein 4 (BMP4), a protein functions in apoptosis and senescence has probable effects in causing neovascular AMD by increasing the expression of VEGF.53 In Nrf2 signalling, oxidative stress induces conformational change in Keap1 that leads to Nrf2 transition to the nucleus, were it interacts with MAF proteins and binds to antioxidant response elements (ARE) which results in antioxidant stress.54 These effects of oxidative stress have been depicted in Fig. 1.
Figure 1.
Oxidative stress (OS) effects in RPE degeneration. External factors (ageing, smoking, light) induce increased oxidative stress (OS) that leads to decreased autophagy. The following are the possible and reported effects of OS in RPE. 1) Decreased autophagy causes an increase in lipofusin that result in apoptosis and pyroptosis which leads to age related macular degeneration (AMD). 2) Increase in protein degradation and aggregation leads to mitochondrial damage and autophagy flux. 3) OS lessens the affinity between compliment factor H gene (HF1) and C3b which increases the activation on complement system (CS) that results in Drusen formation with atropic AMD. 4) γ-secretase involves in amyloid-β (Aβ) formation which results in Drusen formation. 5) γ-secretase damages and inhibits pigment epithelium derived factor (PEDF) which in turn irregulates vascular endothelial growth factor (VEGF) activity that leads to neovascular AMD. Decrease in bone morphogenic protein 4 (BMP4) increases VEGF and results in neovascular AMD. 6) OS alters the structure of Keap1 that leads to Nrf2 transition to the nucleus, were it interacts with MAF2 proteins and binds to antioxidant response elements (ARE) which results in antioxidant stress.
Mitochondrial studies in retinal degeneration
In 2004, a new type of cell–cell communication between animal cells, based on the formation of thin intercellular membrane channels, was reported.55 These highly sensitive nanotubular structures were termed as tunnelling nanotubes (TNT), which connects individual cells and facilitates selective long-range cell–cell communication.56 TNTs may offer a very specific and effective way of intercellular communication. The proposed functions of TNTs are a long–distance exchange of cellular compounds, ranging from small endosomes up to large organelles, cytoplasmic molecules, calcium signals, vesicles and thereby coordination of signalling between TNT connected cells.57 A general and important communication route between cells are TNTs that allow cells to functionally interact with the target cells over long distances.58 Numerous studies have been reported on TNT-like nanotubes connecting cell types thatinclude human B cells, natural killer cells and macrophages, rat astrocytes, human dendritic cells and THP-1 monocytes, neonatal rat cardiomyocytes and human progenitor cells, and Jurkat T-cells.55,57,59, 60, 61, 62, 63, 64, 65, 66
A study in 2012 investigated TNTs in the human RPE cell line ARPE-19 that investigated the formation of TNTs and they monitored ARPE-19 cells for more than 24 h by time-lapse DIC microscopy. Mitochondria are detected by membrane nanotubes. The observations of bulges along TNTs suggested the presence of organelles in TNTs. They, therefore, addressed the possibility of mitochondrial transfer within nanotubes of ARPE-19 cells. By using the specific mitochondrial dye JC-1, they observed fluorescently labeled mitochondria inside TNTs of living cells. This suggests that ARPE-19 cells have the capacity of organelle transfer through TNTs.59 Several mechanisms have been proposed to explain how organelles cross the membrane interface between TNT and connected cell, including transient membrane fusion model, multi-vesicular body fusion model and phagocytosis model.67 Spees et al2016 have shown that aerobic respiration can be rescued by the transfer of mitochondria or mtDNA from undamaged cells, in cells with dysfunctional mitochondria.68 A recent study in 2020 reported mesenchymal stem cell (MSCs) based mitochondrial transfer to ocular cells such as corneal endothelial cells (CES), photoreceptor cell line and RPE cell line through F-actin based TNTs. Transferred mitochondria from MSCs lead to an improved metabolic function in recipient ocular cells. This study helps to specify mitochondrial transfer treatment for ocular tissue regeneration.69
Several studies demonstrated occurrence of mitochondrial transfer from MSCs to various damaged cells in in vivo and in vitro. Mitochondrial transfer from bone marrow – mesenchymal stem cells (BM-MSCs) to lung epithelial cells in an airway injury model, helped to increase the ATP level in airway epithelial cells.70 In diabetic nephropathy study, mitochondria were transferred to damaged Renal Proximal Tubular epithelial cells (PTECs) in vivo and in vitro and suppressed apoptosis of PTECs.71 In an ischemia/reperfusion model and an anthracycline-induced cardiomyopathy model, BM-MSCs transferred Mt to cardiomyocytes and it showed repressed apoptosis or degeneration of cells.72,73 In vitro mitochondrial transfer from Mesenchymal Multipotent Stromal Cells (MMSCs) to neurons have reported in stroke based study and the transferred mitochondria provides better survival and function of nerve cells. This shows the neuroprotective effect of mitochondria transfer.74 and the beneficial effect of mitochondrial transfer from iPSC-MSC to epithelial cells on allergic airway inflammation.75 In vivo induced pluripotent stem cells (iPSC) -MSC mediated mitochondrial transfer could restore RGC function against mitochondrial damage that induce excessive inflammation and retinal degeneration. Healthy mitochondria preserved RGC survival and restored retinal function.76 A recent study in 2020 reported Mesenchymal Stem Cell (MSC)-based mitochondrial transfer to ocular cells such as CES, photoreceptor cell line and RPE through F-actin-based TNTs. Transferred mitochondria from MSCs lead to an improved metabolic function in recipient ocular cells. This study helped to specify mitochondrial transfer treatment for ocular tissue regeneration.69 All these studies concluded that mitochondrial transfer from MSCs increases cellular activity and energy production in recipient cells in vivo and in vitro and helps to regain the function of cells.
Cell death signals can be transmitted through the aqueous pores of gap junctions to adversely affect their neighbours,78, 79, 80, 81 this is called ‘‘bystander effect’‘.82 This suggests that RPE cells sustain very active intercellular communication under physiological conditions. Electrophysiological studies have shown that all retinal cells communicate with their neighbours through gap junctions.83, 84, 85, 86 Cx43-mediated gap-junctional intercellular communication participates in the regulation of retinal organogenesis and regeneration.87, 88, 89
Stem cell mediated ocular cell transplantation studies
In AMD conditions where patients loose both photoreceptors and RPE, both tissues should be replaced together. Transplantation of any one tissue shows limited effect in patients. But unfortunately, this technique gives more-or-less negative results due to the damage of scaffold of Muller glial cells that are responsible for organization and nourishment of the neuronal cells.90 Macular translocation surgery and RPE transplantation surgery also provides positive effects in ocular disease condition.91, 92, 93 These two in turn shows that healthy RPE can support photoreceptor survival and visual function in patients. Since allo transplantation shares genetic risks and graft rejection issues, these surgeries have many limitations practically. But a surprising good outcome can be achieved from autologous transplantation of RPE-choroid sheets.94, 95, 96 Recent studies prove that, harvesting RPE from fetal or adult donor eye tissue can overcome this situation.97
Initially, human retinal tissue and pure population of specific retinal cell types were used to diagnose and treat retinal diseases. But the availability of the tissue from donor eyes or cell lines was difficult and is not useful for genetic diagnosis of patients and cellular therapy in clinical aspect. An alternative source of patient specific retinal cell is the patient derived adult stem cells, which differentiated into retinal lineage. These cells are either unipotent or multipotent in nature. Unlike them, pluripotent stem cells (PSCs) have the capacity of infinite self-renewal and proliferate into any somatic cell type including retinal cells. One source of PSCs is the embryonic stem cells (ESCs). ESCs can be derived from the discarded embryos, but are not patient specific. Recently, human ESC-derived RPE are used in many clinical trials. In such cases the recipients are immune-suppressed in order to avoid the risk of graft rejection. Most recently, PSCs are generated by dedifferentiating patient-specific adult somatic cells into a pluripotent state by nuclear reprogramming. The ideology for differentiating iPSC into retinal progeny had been laid down by previous research on mouse and human ESCs. Still the use of iPSCs as a source for retinal cell transplantation is one of the most complex and challenging issue in all related fields.98
For retinal degenerative diseases, lots of stem cell-based cellular therapeutics have been proposed by researchers. They were mainly focused on; replacing RPE to maintain the supportive function of the RPE layer, replacement with retinal progenitor cells (RPCs) to regenerate lost retinal elements and combining RPE and RPC replacement in advanced stages where both RPE and retinal elements are lost.97 Stem cell based-treatment for retinal degeneration disease has changed from basic research to preclinical and clinical trials.99 The preclinical safety in animal models are measured by electroretinography (ERG), where it determines the overall electrical response of the retina, followed by clinical trials.100, 101, 102 These clinical studies are based on autologous or allogeneic transplantation of several kinds of stem cells. The common stem cells include human embryonic stem cell derived Retinal pigment epithelum (hESC-RPE), Induced pluripotent stem cell derived retinal pigment epithelium (iPSC-RPE), hUTC, human umbilical tissue-derived cells (hUTSC), bone marrow–derived stem cells (BMSC), Human central nervous system Stem cell (HuCNS-SC), and adipose-derived Stromal Cells.
hESCs are pluripotent stem cells that were first isolated and cultured in 1998 by Thomson et al. ESCs are derived from blastocysts and share many characteristics with epiblast cells. They can proliferate indefinitely into any of the cell types of all three germ layers.103 iPSCs share the self-renewal and pluripotency characteristics of hESCs and thus are another source for differentiated cells. It is useful in fields like regenerative medicine, disease modeling, drug testing, and exploring developmental biology. Theoretically iPSCs can be directly generated from any adult somatic tissue thus each individual could have their own iPSCs. So patients can have a unique cell source for replacing a degenerated or lost organ with no risk of immune rejection.104, 105, 106
Stem cell based treatments offers a wide range of options to replace defective retina.107 Along with many positive effects created by these cells, they have some limitations also. RPCs can differentiate and overcome the defects in retina with very less number of limitations. This is due to the lowest chances of immunological rejection.108 The similar curative effect can be obtained from BMSCs as well.109 But the methodology is time consuming and not disease specific.110,111 Q-CTS-hESC-2 cells can be incorporated in the host through a surgical procedure after its culture and differentiation.112 These cells can proliferate into any desired cell type,103 but it is limited to a narrow range of application.112 In the case of iPSCs, since its production is time consuming and expensive,113 it is reliable, because of low immune rejection rate and wide range of application.97,104,105 Recently, some studies use HuCNS-SC, because of its controlled proliferation.114 Accoring to Trevor et al (2019) and Stern and Temple. (2015) huCNS-SCs show limited area of proliferation in certain clinical trials.114,115 Adipose- Derived Stromal/Stem Cells are isolated from the adipose tissue through various procedures,116 to overcome RPE apoptosis due to oxidative stress.117 These cells are having limitations that, after transplantation they sometimes cannot directly reach to the specific site of injury.117 Stem cell based treatment on RPE cells has been represented in Fig. 2.
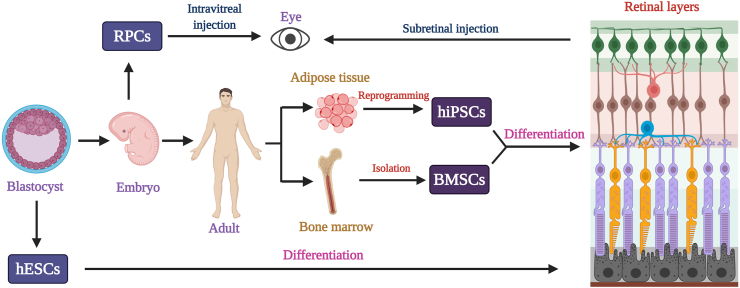
Figure 2.
Stem cell based therapeutic action in retinal cells. Different types of stem cells can be differentiated into any of the retinal cells, mainly, photoreceptor cells, bipolar cells, and ganglion cells. For that, stem cells can be isolated at any stage of life cycle. Human embryonic stem cells (hESCs) is isolated during the blastocyst stage and differentiated under various conditions in proper medium. On the other hand, retinal progenitor cells (RPCs) are extracted during the embryonic stage. It is possible to isolate stem cells from an adult body. For example, bone marrow stem cells (BMSCs) can be obtained from bone marrow and can be differentiated into any retinal cell type. Similarly, human induced pluripotent stem cells (hiPSc) can be made by reprogramming any adult somatic cells including adipose tissue. This then allowed to differentiate into any type of cells as per the requirement. RPC is injected into the patient's eye through intravitreal injection. But all other cell types mentioned above can be injected in the subretinal space of the damaged area in the eye.
In 2004, the protocol development for hESC derived RPE like cells created a great anticipation for the source of RPE cells for the treatment of retinal degenerative diseases.118 hESC derived RPE cell transplantation to retinal degenerative animal models has been manifested a great improvement in vision through reduced photoreceptor degeneration.119 Studies have been reported spontaneous differentiation of human RPE cells from hESC118,120 and more rapid differentiation through the combination of retinal inducing factors (IGF1, Noggin, Dkk1, and bFGF) and other factors (nicotinamide, Activin A, SU5402, and vasoactive intestinal peptide [VIP]), adding at appropriate, different times.121 To ensure the quality of stem cell derived RPE differentiation, various standardized and well accepted criteria have been followed such as verification of RPE morphology, identification of RPE specific genes and confirmation of RPE function.122, 123, 124
Currently, RPE transplantation is widely used as a treatment for retinal degeneration. Da Cruz et al (2018) reported transplantation of fully differentiated hESC-derived RPE monolayer patch into severe exudative AMD patients and reported successful delivery and survival of the RPE patch with improved visual acuity.125 Another study reported clinical trial of transplantation of RPE sheet cells derived from iPSCs into a patient with wet AMD.126 Study has been reported an injectable hiPSC-RPE cells after 3D spheroid culture rescued the structure and function of photoreceptors by sub-retinal transplantation.3D spheroid culture helps to maintain hiPSC-RPE properties and function. hiPSC-RPE cells rescued and improved the reduced outer nuclear layer ONL, photoreceptor loss, impaired light-avoidance behavior, and ERG visual function.127
To study the interaction between RPE and Bruch's membrane in AMD, iPSC – derived RPE cells from patient fibroblast and control sample was used with an altered extracellular matrix (ECM) that models aged Bruch's membrane. Distinct differences were found in transcriptome and physiological function in AMD and control iPSC- derived RPE. Examination of critical difference among the model should disclose more information about the disease mechanism, thus, paving way for novel therapeutic strategies.128 Photoreceptors and RPCs have been derived from various stem cells, including embryonic or fetal retinal progenitors, neurospheres, neural stem cells, and iPSCs.129,130 Researchers have transplanted various types of RPCs, originating from mouse photoreceptor precursors, rat retinal progenitor sheets, or hESC-derived photoreceptors, into animal models with degenerative retinal disease. They had shown that RPCs could migrate, differentiate and ultimately result in anatomic and functional rescue of the degenerating retina.132,99
Degeneration of RGCs causes various ocular diseases and it might be the reason for irreversible vision loss. Various studies have reported MSC-based treatment for RGC degeneration in which mitochondrial transfer from iPSC-MSC to rescue the degenerated RGC.76 Novel MSCs mediated mitochondrial transfer effectively to protect RGC from degradation and it enhances the viable therapeutic potential for retinal degenerative diseases in future.131, 132, 133, 134 Differentiation of RGC cells from hESCs and hiPSCs ensure the source for cell transplantation. Many stem cells to RGC differentiation protocols are already published and recently in a glaucoma based study it has been reported that RGC cell differentiation from hESCs using chemically defined medium and transplanted to the mouse eyes in vivo. These studies give more possibilities to cell replacement therapies in RGC related optic neuropathies.135,136 Another study has deployed spermatogonial stem cells (SSCs) as an alternative source for ESCs to generate RGCs. SSCs were first dedifferentiated to ES-like cells (SSC-ECSs) and then differentiated towards retinal lineages. Generated RGCs were eventually transplanted into the retina by intravitreal injection to glaucoma mouse model.137 The importance of each stem cell type with its advantages and disadvantages has been represented in Table 3.
Table 3.
Stem cell based treatment effects in RPE cells.
| S.No | Cell Type | Obtained from | Positive effects in RPE cells | Limitations | Study references |
|---|---|---|---|---|---|
| 1 | RPCs | Embryonic or fetal retinal progenitors, neural stem cells, and iPSCs | Migrate and differentiate to overcome anatomic and functional degeneration of the retina. | Very less limitations, due to minimized chances for immunological rejection in patients. | 97,108,130 |
| 2 | BMSCs | Human bone marrow | Safe, increased functional recovery and overcome immunologic incompatibilities. | Process is time consuming. Not disease specific. |
109, 110, 111 |
| 3 | hESCs | hESCs | Can proliferate into any of the cell types. | Limited to Wet age-related macular degeneration (wet-AMD). | 103,112 |
| 4 | iPSCs | Can be generated from any adult somatic cells | Pluripotency, low risk of immune rejection and self-renewal capacity. | Spontaneous differentiation of the iPSC are not amenable. iPSCs production is expensive and time consuming. | 97,104,105,113 |
| 5 | HuCNS-SC | Derived from fetal tissues. | Less anatomical and functional abnormalities. Controlled RPE proliferation |
RPE proliferation limited to some microscopic distance. | 114,115,187 |
| 6 | Adipose- Derived Stromal/Stem Cells. | Derived from Mesenchymal stem cells, this is obtained from adipose tissue. | Protecting RPE from damage due to oxidative stress. Prevent RPE apoptosis. |
Once transplanted in-vivo, these cells can't home to the site of injury. | 116,117,188 |
RPCs: Retinal progenitor cells; BMSCs:Bone marrow stem cells; iPSCs: induced pluripotent stem cells; hESCs: human embryonic stem cells; AMD: age related macular degeneration; HuCNS-SC: Human Central Nervous System Stem Cells; RPE: retinal pigment epithelium.
Limbal Stem Cell Deficiency (LSCD) by corneal limbal cell damage leads to depletion of corneal transparency and vision loss. Normal corneal transplantation methods are not appropriate for LSCD condition because the allografts lack limbal stem cells. In 2004, Homma et al reported transplantation of ES cell derived corneal epithelial cells and it successfully reconstructed the damaged cornea.138 A recent study have been reported corneal epithelial regeneration using human adipose mesenchymal stem cells (ADSC) through mesenchymal epithelial transition.139 These results strengthen the therapeutic application of stem cell based treatment for corneal epithelial disorders.
The anatomy of eye makes stem cell therapy a more promising treatment for retinal dysfunction due to its confined small space, so that easy local delivery of cells to the retina can be achieved with a limited number of stem cells. In addition, in vivo retinal imaging is easy due to optically clear media of eye which helps to study the effects of the cell therapy noninvasively and at cellular in detail. Finally, in immunological aspect eye allows allogeneic cell transplantation to be more feasible when compared to other organs.140 MSCs are used in cell therapy for retinal regeneration and can develop features of RPE cells141 In animal studies of retinal degeneration MSCs were reported to differentiate into photoreceptors, RPE, and express neuronal markers following administration.142,143 Various studies in stem cell treatment in eye defects could prove that there are nearly no serious side effects in the patients after the treatment. The cell type can be selected by considering its limitation and specifically for a disease. This study is suggesting more preclinical and clinical trials to improve this area of treatment.
Bone marrow–derived stem cells
Bone marrow is an alternative source of stem cells. Human bone marrow–derived stem cells (BMSC) are clinically suitable because of autologous transplantation, which overcomes immunologic incompatibilities and are easy to obtain. Early clinical investigations indicate feasibility, safety, and enhanced functional recovery after the infusion of autologous BMSC.109 Recently, it has been reported that under appropriate conditions BMCs can differentiate into not only hematopoietic cells but also epithelial cells, endothelial cells, neural cells and astrocytes both in vitro and in vivo. Furthermore, they have the capacity to differentiate into myelin-forming cells and to repair demyelinated spinal cord axons. Therefore, BMCs contain more primitive stem cells than hippocampus-derived neural stem cells. The injection of BMCs into the eye can probably rescue injured retinal tissue.106,141 More recent studies suggests that, trans-differentiation of BMCs into various cells were due to cell fusion. The multi-potency observed in BMSCs results from the heterogeneous make-up of the isolated cells.140
Bone marrow contains the highest concentration of adult stem cells. There are two groups of BMSCs; MSCs and hematopoietic stem cells. MSCs are easily harvested and expanded in tissue culture. These cells are mainly isolated from bone marrow aspirate. On the other hand hematopoietic stem cells are found in higher concentration in bone marrow. They can differentiate into all the blood cell lineages and are used in bone marrow transplantation.144 Hematopoietic stem cells are capable of self-renewal and reconstitute the bone marrow compartment of the recipient hence they are extensively used in clinical practice to treat hematologic disorders. Since these cells are also present in other tissues, they play a role beyond renewing blood cells.140 Some of the bone marrow cells have the ability to home into the damaged retina and become incorporated after intravitreal delivery. So, administration of the cells to the target tissue is easy. Unlike vitrectomy surgery for administration of cells that requires hospital admission and significant recovery time from surgery, intravitreal injection of cells into sub-retinal space can be performed in the clinic, with minimal recovery time.140,145
Among various progenitor cells within bone marrow, hMSCs are precursors of adipocytes, chondrocytes, and osteoblasts within bone marrow stroma. They are also capable of differentiating into non mesodermal tissues, including neurons and astrocytes. hMSCs are used in treating retinal degenerative diseases through various steps like, stem cell isolation and culture, obtaining cell lines, testing in animal subjects, sub-retinal injection of hMSCs, hMSC labeling, in vivo migration assay and in vitro migration assay.146 Recently the study by Alessia et al, 2012 shows that, the depletion of RGCs in eye during glaucoma can be protected by using BM-MSCs in a rat model of glaucoma.147 Even though adult stem cells are a major source of cells for tissue regeneration, age-related alterations in their numbers and function may occur. An age-related change in human BM-MSCs is a consequence for cell therapies.148 There are various kinds of BMSCs, whose potency to cure retinal dysfunction are explored through different phases of clinical trials.111 Based on the previous studies and available reports, hESC-RPE and iPSC-RPE replacement therapy are the more promising.97
Conclusion
Oxidative stress has a vital role in developing various retinal diseases and degeneration of RPE and other ocular cells. Excessive ROS production leads to severe functional and morphological changes in ocular cells. Studies on the role of retinal degeneration prove to be an essential biomarker in novel therapeutics for various diseases.149 Several therapeutic strategies are applying in ocular diseases research. Cell based therapies for the replacement of defective RPE and other ocular cells are rapidly growing research area. Stem cell based RPE and photoreceptors have reversed the vision loss in preclinical model of human regenerative disease. Stem cell-based therapies are potential alternative approach in the treatment of retinal degenerative diseases. Studies in these new emerging areas can offer most effective treatment and better understanding of degenerative diseased conditions. The overall concept of the paper has been represented in Fig. 3.
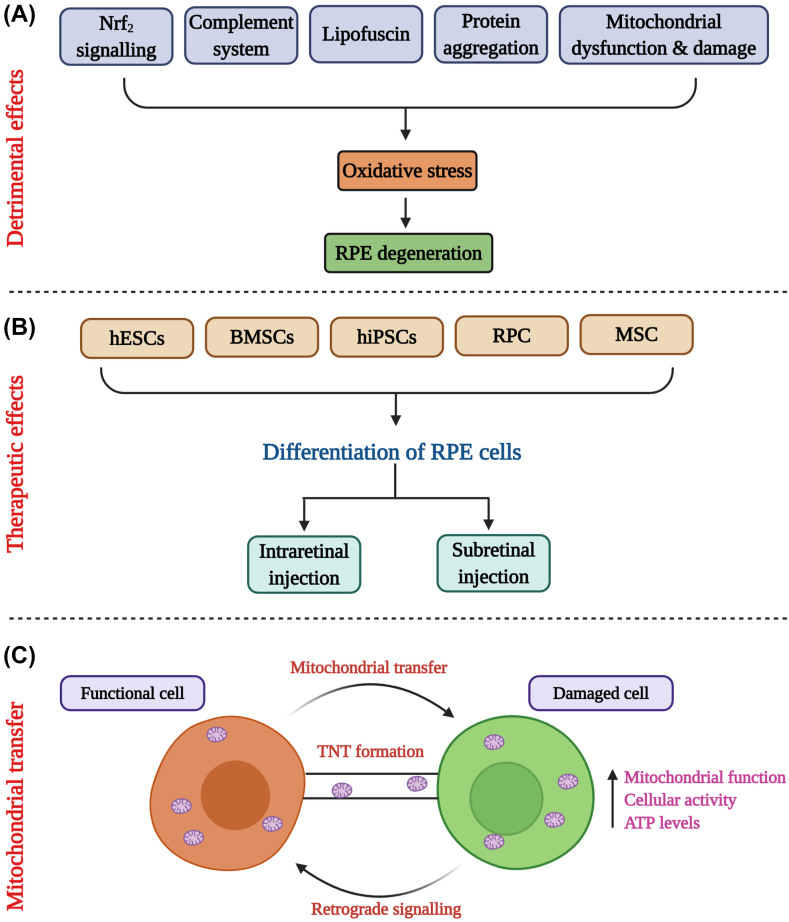
Figure 3.
Summary figure depicting significant studies carried out in the article. (A) The detrimental effects cause oxidative stress in RPE cells that leads to degeneration. Major mechanistic features involved in oxidative stress are Nrf2 signalling, complement system, lipofuscin formation, protein aggregation, mitochondrial damage and dysfunction. (B) Various types of stem cells such as human embryonic stem cells (hESCs), bone marrow stem cells (BMSCs), human induced pluripotent stem cells (hiPSCs), retinal progenitor cells (RPCs), mesenchymal stem cells (MSCs) were used to differentiate into RPE cells using transcriptional factors. The differentiated cells were injected in the eye either as intraretinal or as subretinal injections. (C) Mitochondrial cells are transferred from functional cell to damaged cells through tunnelling nanotubes (TNTs) that functions as cell-to-cell communication. The beneficial effects of mitochondrial transfer were increased mitochondrial function, cellular activity and adenosine triphosphate (ATP) levels.
Future directions
Based on the studies discussed on the effects of oxidative stress in ocular cell degeneration, it is known that oxidative stress plays a pivotal role in degeneration of ocular cells including, RGC, RPE and so on. The increased exposure to oxidative stressors due to ageing causes an excess production of ROS that leads to retinal diseases. Though there is no treatment, number of nutritional supplements are recommended to prevent the vision loss and impairment. Recently, advanced stem cell research has been concentrated as a potential target in treating retinal dysfunctions. Studies have shown promising results on transplanting regenerated retinal cells from various stem cells into the eye. Hence, in future, stem cell research studies may be focused on addressing the genetic susceptibility that might be useful to investigate the pathophysiology of ocular cell degeneration and more studies are warranted in the area of stem cell based treatments.
Author contributions
Mohana Devi Subramaniam: Resources, Concept and Supervision; Aswathy P Nair, Mahalaxmi Iyer: Data analysis, writing –original draft; Dhivya Venkatesan: Data analysis, writing –original draft; Nimmisha Eruppakotte, Soumya Kizhakkillach, Manojkumar Chandran: literature review and drafting; Ayan Roy, Abilash VG, Sinnakaruppan Mathavan: Revision of the manuscript; Balachandar Vellingiri: Conceptualization, Supervision.
Conflict of interests
Authors declare no conflict of interest.
Funding
The authors would like to thank the Science and Engineering Research Board (ECR/2018/000718), Indian Council of Medical Research (File No. 2018-2786/CMB/Adhoc-BMS) and Council for Scientific and Industrial Research ((Ref No.: 27(0353)/19/EMR-II), Government of India, New Delhi for providing necessary funding to complete this review article successfully.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Mohana Devi Subramaniam, Email: geneticmohana@gmail.com.
Balachandar Vellingiri, Email: geneticbala@buc.edu.in.
References
- 1.Lakkaraju A., Umapathy A., Tan L.X., et al. The cell biology of the retinal pigment epithelium. Prog Retin Eye Res. 2020;100846 doi: 10.1016/j.preteyeres.2020.100846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ames B.N., Shigenaga M.K., Hagen T.M. Oxidants, antioxidants, and the degenerative diseases ofmaging. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snodderly D.M. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995;62:1448S–1461S. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- 4.Sawyer D.E., Roman S.D., Aitken R.J. Relative susceptibilities of mitochondrial and nuclear DNA to damage induced by hydrogen peroxide in two mouse germ cell lines. Redox Rep. 2001;6:182–184. doi: 10.1179/135100001101536157. [DOI] [PubMed] [Google Scholar]
- 5.Jin G.F., Hurst J.S., Godley B.F. Rod outer segments mediate mitochondrial DNA damage and apoptosis in human retinal pigment epithelium. Curr Eye Res. 2001;23:11–19. doi: 10.1076/ceyr.23.1.11.5423. [DOI] [PubMed] [Google Scholar]
- 6.Malek G., Lad E.M. Emerging roles for nuclear receptors in the pathogenesis of age-related macular degeneration. Cell Mol Life Sci. 2014;71:4617–4636. doi: 10.1007/s00018-014-1709-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang M., Esteve Rudd J., Lopes V.S., et al. Microtubule motors transport phagosomes in the RPE, and lack of KLC1 leads to AMD-like pathogenesis. J Cell Biol. 2015;210:595–611. doi: 10.1083/jcb.201410112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparrow J.R., Hicks D., Hamel C.P. The retinal pigment epithelium in health and disease. Curr Mol Med. 2010;10:802–823. doi: 10.2174/156652410793937813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volland S., EsteveRudd J., Hoo J., et al. A comparison of some organizational characteristics of the mouse central retina and the human macula. PloS one. 2015;10 doi: 10.1371/journal.pone.0125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulton M., Rozanowska M., Rozanowski B. Retinal photo damage. J Photochem Photobiol B. 2001;64:144–161. doi: 10.1016/s1011-1344(01)00227-5. [DOI] [PubMed] [Google Scholar]
- 11.Sparrow J.R., Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Hazim R.A., Karumbayaram S., Jiang M., et al. Differentiation of RPE cells from integration-free iPS cells and their cell biological characterization. Stem Cell Res Ther. 2017;8:1–17. doi: 10.1186/s13287-017-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jha B.S., Bharti K. Regenerating retinal pigment epithelial cells to cure blindness: a road towards personalized artificial tissue. Curr Stem Cell Rep. 2015;1:79–91. doi: 10.1007/s40778-015-0014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leonarduzzi G., Gamba P., Gargiulo S., et al. Inflammation-related gene expression by lipid oxidation-derived products in the progression of atherosclerosis. Free Radic Bio Med. 2012;52(1):19–34. doi: 10.1016/j.freeradbiomed.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 15.Gutterridge J.M.C., Halliwell B. Free radicals and antioxidants in the year 2000: a historical look to the future. Ann NY Acad Sci. 2006;899:136–147. doi: 10.1111/j.1749-6632.2000.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 16.Yu D.Y., Cringle S.J. Retinal degeneration and local oxygen metabolism. Exp Eye Res. 2005;80(6):745–751. doi: 10.1016/j.exer.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Brand M.D., Esteves T.C. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2(2):85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Jan Ježek, Cooper Katrina F., Strich Randy. Reactive oxygen species and mitochondrial dynamics:the yin and yang of mitochondrial dysfunction and cancer progression. Antioxidants. 2018;7:13. doi: 10.3390/antiox7010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishida K., Panjwani N., Cao Z., et al. Participation of pigment epithelium in ocular immune privilege. Epithelia cultured from iris, ciliary body, and retina suppress T-cell activation by partially non-overlapping mechanisms. Ocul Immunol. 2003;11:91–105. doi: 10.1076/ocii.11.2.91.15914. [DOI] [PubMed] [Google Scholar]
- 20.Brown E.E., DeWeerd A.J., Ildefonso C.J., et al. Mitochondrial oxidative stress in the retinal pigment epithelium (RPE) led to metabolic dysfunction in both the RPE and retinal photoreceptors. Redox Biol. 2019;24:101201. doi: 10.1016/j.redox.2019.101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan C.M., Huang D.Y., Sekar P., et al. Reactive oxygen species-dependent mitochondrial dynamics and autophagy confer protective effects in retinal pigment epithelial cells against sodium iodate- induced cell death. J Biomed Sci. 2019;26(1):40. doi: 10.1186/s12929-019-0531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plafker S.M., O'Mealey G.B., Szweda L.I. Mechanisms for countering oxidative stress and damage in retinal pigment epithelium. Int Rev Cell Mol Biol. 2012;298:135–177. doi: 10.1016/B978-0-12-394309-5.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishii M., Rohrer B. Bystander effects elicited by single-cell photo-oxidative blue-light stimulation in retinal pigment epithelium cell networks. Cell Death Discov. 2017;3:16071. doi: 10.1038/cddiscovery.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sparrow J.R., Gregory-Roberts E., Yamamoto K., et al. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012;31:121–135. doi: 10.1016/j.preteyeres.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaarniranta K., Hyttinen J., Ryhanen T., et al. Mechanisms of protein aggregation in the retinal pigment epithelial cells. Front Biosci (Elite Ed) 2010;2:1374–1384. doi: 10.2741/e198. [DOI] [PubMed] [Google Scholar]
- 26.Haijiang L., Xu H., Liang F.Q., et al. Mitochondrial DNA damage and repair in RPE associated with aging and age-related macular degeneration. Invest Ophth Vis Sci. 2011;52(6):3521–3529. doi: 10.1167/iovs.10-6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai J., Nelson K.C., Wu M., et al. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 28.Feher J., Kovacs I., Artico M., et al. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol Aging. 2006;27:983–993. doi: 10.1016/j.neurobiolaging.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Karunadharma P.P., Nordgaard C.L., Olsen T.W., et al. Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Invest Ophth Vis Sci. 2010;51:5470–5479. doi: 10.1167/iovs.10-5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cano M., Wang L., Wan J., et al. Oxidative stress induces mitochondrial dysfunction and a protective unfolded protein response in RPE cells. Free Radic Biol Med. 2014;69:1–14. doi: 10.1016/j.freeradbiomed.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowak J.Z. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacolo Reports. 2006;58:353–363. [PubMed] [Google Scholar]
- 32.Tokarz P., Kaarniranta K., Blasiak J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD) Biogerontology. 2013;14:461–482. doi: 10.1007/s10522-013-9463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farnoodian M., Kinter J.B., Aghdam S.Y., et al. Expression of pigment epithelium-derived factor and thrombospondin-1 regulate proliferation and migration of retinal pigment epithelial cells. Physiol Rep. 2015;3 doi: 10.14814/phy2.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanus J., Zhang H., Chen D.H., et al. Gossypol acetic acid prevents oxidative stress-induced retinal pigment epithelial necrosis by regulating the FoxO3/sestrin2 pathway. Mol Cell Biol. 2015;35:1952–1963. doi: 10.1128/MCB.00178-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahendra C.K., Tan L.T.H., Pusparajah P., et al. Detrimental effects of UVB on retinal pigment epithelial cells and its role in age-related macular degeneration. Oxid Med Cell Longev. 2020:1904178. doi: 10.1155/2020/1904178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulfaul K., Ozaki E., Fernando N., et al. Toll-like receptor 2 facilitates oxidative damage-induced retinal degeneration. Cell Rep. 2020;30:2209–2224. doi: 10.1016/j.celrep.2020.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abokyi S., To C.H., Lam T.T., et al. Central role of oxidative stress in age-related macular degeneration: evidence from a review of the molecular mechanisms and animal models. Oxid Med Cell Longev. 2020;2020:7901270. doi: 10.1155/2020/7901270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y., Mehta G., Vasiliou V. Antioxidant defenses in the ocular surface. Ocul Surf. 2009;7:176–185. doi: 10.1016/s1542-0124(12)70185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wojcik K.A., Kaminska A., Blasiak J., et al. Oxidative stress in the pathogenesis of keratoconus and Fuchs endothelial corneal dystrophy. Int J Mol Sci. 2013;14:19294–19308. doi: 10.3390/ijms140919294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu C., Vojnovic D., Kochevar I.E., et al. UV-A irradiation activates nrf2-regulated antioxidant defense and induces p53/caspase3-dependent apoptosis in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2016;57:2319–2327. doi: 10.1167/iovs.16-19097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu Luo. Stress-induced corneal epithelial apoptosis mediated by K+ channel activation. Prog Retin Eye Res. 2006;25:515–538. doi: 10.1016/j.preteyeres.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vallabh N.A., Romano V., Willoughby C.E. Mitochondrial dysfunction and oxidative stress in corneal disease. Mitochondrion. 2017;36:103–113. doi: 10.1016/j.mito.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Eells J.T. Mitochondrial dysfunction in the aging retina. Biology. 2019;8:31. doi: 10.3390/biology8020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Cano M., Thimmalappula R., Fujihara M., et al. Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and Age-related Macular Degeneration. Vision Res. 2010;50:652–664. doi: 10.1016/j.visres.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Z., Chen Y., Wang J., et al. Age-related retinopathy in NRF2-deficient mice. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sachdeva M.M., Cano M., Handa J.T. Nrf2 signaling is Impaired in the Aging RPE given an oxidative insult. Exp Eye Res. 2014;119:111–114. doi: 10.1016/j.exer.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug metabolism reviews. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 49.Liu X.F., Zhou D.D., Xie T., et al. The Nrf2 signaling in retinal ganglion cells under oxidative stress in ocular neurodegenerative diseases. Int J Biol Sci. 2018;14:1090–1098. doi: 10.7150/ijbs.25996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaarniranta K., Tokarz P., Koskela A., et al. Autophagy regulates death of retinal pigment epithelium cells in age-related macular degeneration. Cell Biol Toxicol. 2017;33:113–128. doi: 10.1007/s10565-016-9371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaarniranta K., Sinha D., Blasiak J., et al. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013;9:973–984. doi: 10.4161/auto.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blasiak J., Petrovski G., Veréb Z., et al. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. BioMed Res Int. 2014:768026. doi: 10.1155/2014/768026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benson C., Weingeist T. Medical educational Web> medical atlases (photos of cases)> ophthalmology atlas (photos of cases)> age-related macular degeneration case with photos. Age. 2012;5:24. [Google Scholar]
- 54.Datta S., Cano M., Ebrahimi K., et al. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res. 2017;60:201–218. doi: 10.1016/j.preteyeres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rustom A., Saffrich R., Markovic I., et al. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 56.Hurtig J., Chiu D.T., Onfelt B. Intercellular nanotubes: insights from imaging studies and beyond. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:260–276. doi: 10.1002/wnan.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerdes H.H., Bukoreshtliev N.V., Barroso J.F. Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Lett. 2007;581:2194–2201. doi: 10.1016/j.febslet.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 58.Gerdes H.H., Carvalho R.N. Intercellular transfer mediated by tunneling nanotubes. Curr Opin Cell Biol. 2008;20:470–475. doi: 10.1016/j.ceb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Wittig D., Wang X., Walter C., et al. Multi-level communication of human retinal pigment epithelial cells via tunneling nanotubes. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0033195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gurke S., Barroso J.F., Gerdes H.H. The art of cellular communication: tunneling nanotubes bridge the divide. Histochem Cell Biol. 2008;129:539–550. doi: 10.1007/s00418-008-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sowinski S., Jolly C., Berninghausen O., et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 62.Onfelt B., Nedvetzki S., Yanagi K., et al. Cutting edge: membrane nanotubes connect immune cells. J Immunol. 2004;173:1511–1513. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- 63.Watkins S.C., Salter R.D. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 64.Freund D., Bauer N., Boxberger S., et al. Polarization of human hematopoietic progenitors during contact with multipotent mesenchymal stromal cells: effects on proliferation and clonogenicity. Stem Cells Dev. 2006;15:815–829. doi: 10.1089/scd.2006.15.815. [DOI] [PubMed] [Google Scholar]
- 65.Koyanagi M., Brandes R.P., Haendeler J., et al. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96:1039–1041. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- 66.Zhu D., Tan K.S., Zhang X., et al. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J Cell Sci. 2005;118:3695–3703. doi: 10.1242/jcs.02507. [DOI] [PubMed] [Google Scholar]
- 67.Wang X., Gerdes H.H. Long-distance electrical coupling via tunneling nanotubes. Biochim Biophys Acta. 2011;1818(8):2082–2086. doi: 10.1016/j.bbamem.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Spees J.L., Olson S.D., Whitney M.J., et al. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang D., Chen F.X., Zhou H., et al. Bioenergetic crosstalk between mesenchymal stem cells and various ocular cells through the intercellular trafficking of mitochondria. Theranostics. 2020;10:7260–7272. doi: 10.7150/thno.46332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Islam M.N., Das S.R., Emin M.T., et al. Mitochondrial transfer from bone marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Konari N., Nagaishi K., Kikuchi S., et al. Mitochondria transfer from mesenchymal stem cells structurally and functionally repairs renal proximal tubular epithelial cells in diabetic nephropathy in vivo. Scientific Reports. 2019;9:5184. doi: 10.1038/s41598-019-40163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han H., Hu J., Yan Q., et al. Bone marrow-derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Mol Med Rep. 2016;13:1517–1524. doi: 10.3892/mmr.2015.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y., Yu Z., Jiang D., et al. iPSC-MSCs with high intrinsic MIRO1 and sensitivity to TNF-α yield efficacious mitochondrial transfer to rescue anthracycline-induced cardiomyopathy. Stem Cell Rep. 2016;7:749–763. doi: 10.1016/j.stemcr.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Babenko V.A., Silachev D.N., Popkov V.A., et al. Miro1 enhances mitochondria transfer from multipotent mesenchymal stem cells (MMSC) to neural cells and improves the efficacy of cell recovery. Molecules. 2018;23:687. doi: 10.3390/molecules23030687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yao Y., Fan X.L., Jiang D., et al. Connexin 43-mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem Cell Rep. 2018;11:1120–1135. doi: 10.1016/j.stemcr.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang D., Xiong G., Feng H., et al. Donation of mitochondria by iPSC-derived mesenchymal stem cells protects retinal ganglion cells against mitochondrial complex I defect-induced degeneration. Theranostics. 2019;9:2395–2410. doi: 10.7150/thno.29422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frank D.K., Szymkowiak B., Josifovska Chopra O., et al. Single-cell microinjection of cytochrome c can result in gap junction-mediated apoptotic cell death of bystander cells in head and neck cancer. Head Neck. 2005;27:794–800. doi: 10.1002/hed.20235. [DOI] [PubMed] [Google Scholar]
- 79.Krutovskikh V.A., Piccoli C., Yamasaki H. Gap junction intercellular communication propagates cell death in cancerous cells. Oncogene. 2002;21:1989–1999. doi: 10.1038/sj.onc.1205187. [DOI] [PubMed] [Google Scholar]
- 80.Krysko D.V., Leybaert L., Vandenabeele P., et al. Gap junctions and the propagation of cell survival and cell death signals. Apoptosis. 2005;10:459–469. doi: 10.1007/s10495-005-1875-2. [DOI] [PubMed] [Google Scholar]
- 81.Mesnil M., Piccoli C., Tiraby G., et al. Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc Natl Acad Sci USA. 1996;93:1831–1835. doi: 10.1073/pnas.93.5.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Freeman S.M., Abboud C.N., Whartenby K.A., et al. The ‘‘bystander effect’’: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53:5274–5283. [PubMed] [Google Scholar]
- 83.Cook J.E., Becker D.L. Gap junctions in the vertebrate retina. Microsc Res Tech. 1995;31:408–419. doi: 10.1002/jemt.1070310510. [DOI] [PubMed] [Google Scholar]
- 84.Hornstein E.P., Verweij J., Li P.H., et al. Gap-junctional coupling and absolute sensitivity of photoreceptors in macaque retina. J Neurosci. 2005;25:11201–11209. doi: 10.1523/JNEUROSCI.3416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vaney D.I. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. Neurosci Lett. 1991;125:187–190. doi: 10.1016/0304-3940(91)90024-n. [DOI] [PubMed] [Google Scholar]
- 86.Vaney D.I. Retinal neurons: cell types and coupled networks. Prog Brain Res. 2001;136:239–254. doi: 10.1016/s0079-6123(02)36020-5. [DOI] [PubMed] [Google Scholar]
- 87.Becker D.L., Bonness V., Catsicas M., et al. Changing patterns of ganglion cell coupling and connexin expression during chick retinal development. J Neurobiol. 2002;52:280–293. doi: 10.1002/neu.10088. [DOI] [PubMed] [Google Scholar]
- 88.Pearson R.A., Catsicas M., Becker D.L., et al. Ca(2+) signalling and gap junction coupling within and between pigment epithelium and neural retina in the developing chick. Eur J Neurosci. 2004;19:2435–2445. doi: 10.1111/j.0953-816X.2004.03338.x. [DOI] [PubMed] [Google Scholar]
- 89.Chiba C., Hoshino A., Nakamura K., et al. Visual cycle protein RPE65 persists in new retinal cells during retinal regeneration of adult newt. J Comp Neurol. 2006;495:391–407. doi: 10.1002/cne.20880. [DOI] [PubMed] [Google Scholar]
- 90.Seiler M.J., Aramant R.B. Cell replacement and visual restoration by retinal sheet Transplants. Prog Retin Eye Res. 2012;31(6):661–687. doi: 10.1016/j.preteyeres.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fujikado T., Ohji M., Hayashi A., et al. Anatomic and functional recovery of the fovea after foveal translocation surgery without large retinotomy and simultaneous excision of a neovascular membrane. Am J Ophthalmol. 1998;126(6):839–842. doi: 10.1016/s0002-9394(98)00201-3. [DOI] [PubMed] [Google Scholar]
- 92.Pieramici D.J., De Juan E., Jr., Fujii G.Y., et al. Limited inferior macular translocation for the treatment of subfovealchoroidal neovascularization secondary to age-related macular degeneration. Am J Ophthalmol. 2000;130(4):419–428. doi: 10.1016/s0002-9394(00)00533-x. [DOI] [PubMed] [Google Scholar]
- 93.Van Meurs J.C., Van Den Biesen P.R. Autologous retinal pigment epithelium and choroid translocation in patients with exudative age-related macular degeneration: short-term follow-up. Am J Ophthalmol. 2003;136(4):688–695. doi: 10.1016/s0002-9394(03)00384-2. [DOI] [PubMed] [Google Scholar]
- 94.Heussen F.M., Fawzy N.F., Joeres S., et al. Autologous translocation of the choroid and RPE in age-related macular degeneration: 1-year follow-up in 30 patients and recommendations for patient selection. Eye (Lond) 2008;22(6):799–807. doi: 10.1038/sj.eye.6702823. [DOI] [PubMed] [Google Scholar]
- 95.van Zeeburg E.J., Maaijwee K.J., Missotten T.O., et al. A free retinal pigment epithelium-choroid graft in patients with exudative agerelated macular degeneration: results up to 7 years. Am J Ophthalmol. 2012;153(1):120–127. doi: 10.1016/j.ajo.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 96.Han L., Ma Z., Wang C., et al. Autologous transplantation of simple retinal pigment epithelium sheet for massive submacular hemorrhage associated with pigment epithelium detachment. Invest Ophthalmol Vis Sci. 2013;54(7):4956–4963. doi: 10.1167/iovs.13-11957. [DOI] [PubMed] [Google Scholar]
- 97.Nazari H., Zhang L., Zhu D., et al. Stem cell based therapies for age-related macular degeneration: the promises and the challenges. Prog Retin Eye Res. 2015:1–38. doi: 10.1016/j.preteyeres.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 98.Chen F.K., McLenachan S., Edel M., et al. iPS cells for modelling and treatment of retinal diseases. J Clin Med. 2014:1511–1541. doi: 10.3390/jcm3041511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bharti K., Rao M., Hull S.C., et al. Developing cellular therapies for retinal degenerative diseases. Invest Ophthalmol Vis Sci. 2014;55(2):1191–1202. doi: 10.1167/iovs.13-13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.da Cruz L., Chen F.K., Ahmado A., et al. RPE transplantation and its role in retinal disease. Prog Retin Eye Res. 2007;26(6):598–635. doi: 10.1016/j.preteyeres.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 101.Krohne T.U., Westenskow P.D., Kurihara T., et al. Generation of retinal pigment epithelial cells from small molecules and OCT4 reprogrammed human induced pluripotent stem cells. Stem Cells Transl Med. 2012;1(2):96–109. doi: 10.5966/sctm.2011-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thompson S., Blodi F.R., Lee S., et al. Photoreceptor cells with profound structural deficits can support useful vision in mice. Invest Ophthalmol Vis Sci. 2014;55(3):1859–1866. doi: 10.1167/iovs.13-13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 104.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 105.Fusaki N., Ban H., Nishiyama A., et al. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tomita M., Adachi Y., Yamada H., et al. Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem Cells. 2002;20:279–283. doi: 10.1634/stemcells.20-4-279. [DOI] [PubMed] [Google Scholar]
- 107.Jonesa M.K., Lua B., Girman S., et al. Cell-based therapeutic strategies for replacement and preservation in retinal degenerative diseases. Prog Retin Eye Res. 2017;58:1–27. doi: 10.1016/j.preteyeres.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu Y., Chen S.J., Li S.Y., et al. Long-term safety of human retinal progenitor cell transplantation in retinitis pigmentosa patients. Stem Cell Res Ther. 2017;8:209. doi: 10.1186/s13287-017-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dhein S., Garbade J., Rouabah D., et al. Effects of autologous bone marrow stem cell transplantation on beta-adrenoceptor density and electrical activation pattern in a rabbit model of non-ischemic heart failure. J Cardiothorac Surg. 2006;1:17. doi: 10.1186/1749-8090-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harris J.R., Brown G.A.J., Jorgensen M., et al. Bone marrow–derived cells home to and regenerate retinal pigment epithelium after injury. Invest Ophthalmol Vis Sci. 2006;47:2108–2113. doi: 10.1167/iovs.05-0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Park S.S., Moisseiev E., Bauer G., et al. Advances in bone marrow stem cell therapy for retinal dysfunction. Prog Retin Eye Res. 2017;56:148–165. doi: 10.1016/j.preteyeres.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu Y., Xu H.W., Wang L., et al. Human embryonic stem cell-derived retinal pigment epithelium transplants as a potential treatment for wet age-related macular degeneration. Cell Discovery. 2018;4:50. doi: 10.1038/s41421-018-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bracha P., Moore N.A., Ciulla T.A. Induced pluripotent stem cell-based therapy for age-related macular degeneration. Expert Opinion on Biological Therapy. 2017;17:1113–1126. doi: 10.1080/14712598.2017.1346079. [DOI] [PubMed] [Google Scholar]
- 114.McGill T.J., Osborne L., Lu B., et al. Subretinal transplantation of human central nervous system stem cells stimulates controlled proliferation of endogenous retinal pigment epithelium. Trans Vis Sci Tech. 2019;8(3):43. doi: 10.1167/tvst.8.3.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stern J., Temple S. Retinal pigment epithelial cell proliferation. Exp Biol Med. 2015;240:1079–1086. doi: 10.1177/1535370215587530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barzelay A., Algor S.W., Niztan A., et al. Adipose-derived mesenchymal stem cells migrate and rescue RPE in the setting of oxidative stress. Stem Cells Int. 2018;9682856:11. doi: 10.1155/2018/9682856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singh A.K., Srivastava G.K., Garcia-Gutierrez M.T., et al. Adipose derived mesenchymal stem cells partially rescue mitomycin C treated ARPE19 cells from death in co-culture condition. Histol Histopathol. 2013;28:1577–1583. doi: 10.14670/HH-28.1577. [DOI] [PubMed] [Google Scholar]
- 118.Klimanskaya I., Hipp J., Rezai K.A., et al. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6:217–245. doi: 10.1089/clo.2004.6.217. [DOI] [PubMed] [Google Scholar]
- 119.Lund R.D., Wang S., Klimanskaya I., et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8:189–199. doi: 10.1089/clo.2006.8.189. [DOI] [PubMed] [Google Scholar]
- 120.Hirano M., Yamamoto A., Yoshimura N.T., et al. Generation of structures formed by lens and retinal cells differentiating from embryonic stem cells. Dev Dyn. 2003;228:664–671. doi: 10.1002/dvdy.10425. [DOI] [PubMed] [Google Scholar]
- 121.Buchholz D.E., Pennington B.O., Croze R.H., et al. Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Transl Med. 2013;2:384–393. doi: 10.5966/sctm.2012-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maminishkis A., Chen S., Jalickee S., et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carr A.J., Vugler A., Lawrence J., et al. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol Vis. 2009;15:283–295. [PMC free article] [PubMed] [Google Scholar]
- 124.Carr A.J., Vugler A., Hikita S.T., et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4 doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.da Cruz L., Fynes K., Georgiadis O., et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018;36(4):1. doi: 10.1038/nbt.4114. [DOI] [PubMed] [Google Scholar]
- 126.Mandai M., Watanabe A., Kurimoto Y., et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376:1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 127.Zhu D., Xie M., Gademann F., et al. Protective effects of human iPS-derived retinal pigmented epithelial cells on retinal degenerative disease. Stem Cell Res Ther. 2020;11:98. doi: 10.1186/s13287-020-01608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gong J., Cai H., NYSCF Global Stem Cell Array Team, et al. Stem cell-derived retinal pigment epithelium from patients with age-related macular degeneration exhibit reduced metabolism and matrix interactions. Stem Cells Transl Med. 2020;9:364–376. doi: 10.1002/sctm.19-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang K., Ding S. Stem cells and eye development. N Engl J Med. 2011;365:370–372. doi: 10.1056/NEJMcibr1105280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luo J., Baranov P., Patel S., et al. Human retinal progenitor cell transplantation preserves vision. J Biol Chem. 2014;289:6362–6371. doi: 10.1074/jbc.M113.513713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Almasieh M., Wilson A.M., Morquette B., et al. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 132.Liu H., Wang M., Xia C.H., et al. Severe retinal degeneration caused by a novel rhodopsin mutation. Invest Opthalmol Vis Sci. 2010;51:1059–1065. doi: 10.1167/iovs.09-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ting D.S., Cheung G.C., Wong T.Y. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Opthalmol. 2016;44:260–277. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- 134.Yu-Wai-Man P., Griffiths P.G., Chinnery P.F. Mitochondrial optic neuropathies - disease mechanisms and therapeutic strategies. Prog Retin Eye Res. 2011;30:81–114. doi: 10.1016/j.preteyeres.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gill K.P., Hewitt A.W., Davidson K.C., et al. Methods of retinal ganglion cell differentiation from pluripotent stem cells. Tran Vis Sci Tech. 2014;3:7. doi: 10.1167/tvst.3.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang X., Tenerellib K., Wu S., et al. Cell transplantation of retinal ganglion cells derived from hESCs. Res Neurol Neurosci. 2020;38:1–10. doi: 10.3233/RNN-190941. [DOI] [PubMed] [Google Scholar]
- 137.Suen H.C., Qian Y., Liao J., et al. Transplantation of retinal ganglion cells derived from male germline stem cell as a potential treatment to glaucoma. Stem Cells Dev. 2019;28:1365–1375. doi: 10.1089/scd.2019.0060. [DOI] [PubMed] [Google Scholar]
- 138.Homma R., Yoshikawa H., Takeno M., et al. Induction of epithelial progenitors in vitro from mouse embryonic stem cells and application for reconstruction of damaged cornea in mice. Invest Opthalmol Vis Sci. 2004;45:4320–4326. doi: 10.1167/iovs.04-0044. [DOI] [PubMed] [Google Scholar]
- 139.Bandeira F., Goh T.W., Setiawan M., et al. Cellular therapy of corneal epithelial defect by adipose mesenchymal stem cell-derived epithelial progenitors. Stem Cell Res Ther. 2020;11:14. doi: 10.1186/s13287-019-1533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Park S.S. Cell therapy applications: retinal vascular diseases—diabetic retinopathy and retinal vein occlusion. Invest Ophthalmol Vis Sci. 2016;57:ORSFj1–ORSFj10. doi: 10.1167/iovs.15-17594. [DOI] [PubMed] [Google Scholar]
- 141.Duan P., Xu H., Zeng Y., et al. Human bone marrow stromal cells can differentiate to a retinal pigment epithelial phenotype when co-cultured with pigretinal pigment epithelium using a transwell system. Cell Physiol Biochem. 2013;31:601–613. doi: 10.1159/000350080. [DOI] [PubMed] [Google Scholar]
- 142.Gong J., Sagiv O., Cai H., et al. Del Priore. Effects of extracellular matrix and neighboring cells on induction of human embryonic stem cells into retinal or retinal pigment epithelial progenitors. Exp Eye Res. 2008;86(6):957–965. doi: 10.1016/j.exer.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li R., Maminishkis A., Banzon T., et al. IFN-gamma regulates retinal pigment epithelial fluid transport. Am J Physiol Cell Physiol. 2009;297:1452–1465. doi: 10.1152/ajpcell.00255.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goodell M.A., Brose K., Paradis G., et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moisseiev E., Loewenstein A., Yiu G. The suprachoroidal space: from potential space to a space with potential. Clin Ophthalmol. 2016;10:173–178. doi: 10.2147/OPTH.S89784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nakamizo A., Marini F., Amano T., et al. Human bone marrow–derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:8. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 147.Tassoni A., Gutteridge A., Barber A.C., et al. Molecular mechanisms mediating retinal reactive gliosis following bone marrow mesenchymal stem cell transplantation. Stem Cells. 2015;33:3006–3016. doi: 10.1002/stem.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stolzing A., Jones E., McGonagle D., et al. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 149.Devi S.M., Mahalaxmi I., Kaavya J., et al. Does epigenetics have a role in age related macular degeneration and diabetic retinopathy? Genes Dis. 2020;8(3):279–286. doi: 10.1016/j.gendis.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tso M., Albert D., Zimmerman L.E. Organ culture of human retinal pigment epithelium and choroid: a model for the study of cytologic behavior of RPE in vitro. Invest Opthalmol. 1973;12:554–566. [PubMed] [Google Scholar]
- 151.Dorey K., Khouri G.G., Syniuta L.A., et al. Superoxide production by procine retinal pigment epithelium in vitro. Invest Ophthalmol Vis Sci. 1989;30:1047–1054. [PubMed] [Google Scholar]
- 152.van Kuijk F.J.G.M., McPherson S.W., Roehrich H., Dipl-Biol Enhanced detection of sub-retinal pigment epithelial cell layer deposits in human and murine tissue: imaging zinc as a biomarker for age-related macular degeneration (an american ophthalmological society thesis) Trans Am Ophthalmol Soc. 2017;115:T3. [PMC free article] [PubMed] [Google Scholar]
- 153.Golestaneh N., Chu Y., Cheng S.K., et al. Repressed SIRT1/PGC-1α pathway and mitochondrial disintegration in iPSC-derived RPE disease model of age-related macular degeneration. J Transl Med. 2016;14:344. doi: 10.1186/s12967-016-1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Inana G., Murat C., An W., et al. RPE phagocytic function declines in age-related macular degeneration and is rescued by human umbilical tissue derived cells. J Transl Med. 2018;16:63. doi: 10.1186/s12967-018-1434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Radeke M.J., Radeke C.M., Shih Y.H., et al. Restoration of mesenchymal retinal pigmented epithelial cells by TGFβ pathway inhibitors: implications for age-related macular degeneration. Genome Med. 2015;7:58. doi: 10.1186/s13073-015-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Michelet F., Balasankar A., Teo N., et al. Rapid generation of purified human RPE from pluripotent stem cells using 2D cultures and lipoprotein uptake-based sorting. Stem Cell Res Ther. 2020;11:47. doi: 10.1186/s13287-020-1568-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen L.J., Ito S., Kai H., et al. Microfluidic co-cultures of retinal pigment epithelial cells and vascular endothelial cells to investigate choroidal angiogenesis. Scientific Reports. 2017;7:3538. doi: 10.1038/s41598-017-03788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dalvi S., Galloway C.A., Winschel L., et al. Environmental stress impairs photoreceptor outer segment (POS) phagocytosis and degradation and induces autofluorescent material accumulation in hiPSC-RPE cells. Cell Death Discov. 2019;5:96. doi: 10.1038/s41420-019-0171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dithmer M., Fuchs S., Shi Y., et al. Fucoidan reduces secretion and expression of vascular endothelial growth factor in the retinal pigment epithelium and reduces angiogenesis in vitro. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Heller J.P., Kwok J.C.F., Vecino E., et al. A method for the isolation and culture of adult rat retinal pigment epithelial (RPE) cells to study retinal diseases. Front Cell Neurosci. 2015;9:449. doi: 10.3389/fncel.2015.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ghosh S., Shang P., Terasaki H., et al. A role for bA3/A1-crystallin in type 2 EMT of RPE cells occurring in dry age-related macular degeneration. Invest Ophthalmol Vis Sci. 2018;59:AMD104–AMD113. doi: 10.1167/iovs.18-24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Golestaneh N., Chu Y., Xiao Y.Y., et al. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 2017;8:e2537. doi: 10.1038/cddis.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Newman, et al. Systems-level analysis of age-related macular degeneration reveals global biomarkers and phenotypespecific functional networks. Genome Med. 2012;4:16. doi: 10.1186/gm315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Castellarin A.A., Sugino I., Vargas J.A., et al. In vitro transplantation of fetal human retinal pigment epithelium cells onto human cadaver Bruch's membrane. Exp Eye Res. 1998;66:49–67. doi: 10.1006/exer.1997.0404. [DOI] [PubMed] [Google Scholar]
- 165.Sreekumar P.G., Ishikawa K., Spee C., et al. The mitochondrial derived peptide humanin protects RPE cells from oxidative stress, senescence, and mitochondrial dysfunction. Invest Ophthalmol Vis Sci. 2016;57:1238–1253. doi: 10.1167/iovs.15-17053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mohan R.R., Cabrera A.P., Harrison R.E.S., et al. Peptide redesign for inhibition of the complement system: targeting age-related macular degeneration. Mol Vis. 2016;22:1280–1290. [PMC free article] [PubMed] [Google Scholar]
- 167.Idelson M., Alper R., Obolensky A., et al. Immunological properties of human embryonic stem cell-derived retinal pigment epithelial cells. Stem Cell Reports. 2018;11:681–695. doi: 10.1016/j.stemcr.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li Y., Atmaca-Sonmez P., Schanie C.L., et al. Endogenous bone marrow–derived cells express retinal pigment epithelium cell markers and migrate to focal areas of RPE damage. Invest Ophthalmol Vis Sci. 2007;48:9. doi: 10.1167/iovs.06-1015. [DOI] [PubMed] [Google Scholar]
- 169.Chen F., Liua X., Chena Y., et al. Sphere-induced reprogramming of RPE cells into dual-potential RPE stem-like cells. EBio Medicine. 2020;52:102618. doi: 10.1016/j.ebiom.2019.102618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Felszeghy S., Viiri J., Paterno J.J., et al. Loss of NRF-2 and PGC-1α genes leads to retinal pigment epithelium damage resembling dry age-related macular degeneration. Redox Biol. 2019;20:1–12. doi: 10.1016/j.redox.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wankun X., Wenzhen Y., Min Z., et al. Protective effect of paeoniflorin against oxidative stress in human retinal pigment epithelium in vitro. Mol Vis. 2011;17:3512–3522. [PMC free article] [PubMed] [Google Scholar]
- 172.Yang Z., Stratton C., Francis P.J., et al. Toll-like receptor-3 and geographic atrophy in age-related macular degeneration. N Engl J Med. 2008;359:1456–1463. doi: 10.1056/NEJMoa0802437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sreekumar P.G., Li Z., Wang W., et al. Intra-vitreal αB crystallin fused to elastin-like polypeptide provides neuroprotection in a mouse model of age-related macular degeneration. J Control Release. 2018;283:94–104. doi: 10.1016/j.jconrel.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ding J.D., Johnson L.V., Herrmann R., et al. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. PNAS. 2011;108:E279–E287. doi: 10.1073/pnas.1100901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang A.L., Lukas T.J., Yuan M., et al. Changes in retinal pigment epithelium related to cigarette smoke: possible relevance to smoking as a risk factor for age-related macular degeneration. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hallam D., Collin j., Bojic S., et al. An induced pluripotent stem cell patient specific model of complement factor H (Y402H) polymorphism displays characteristic features of age-related macular degeneration and indicates a beneficial role for UV light exposure. Stem Cells. 2017;35:2305–2320. doi: 10.1002/stem.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhu Y., Carido M., Meinhardt A., et al. Three-dimensional neuroepithelial culture from human embryonic stem cells and its use for quantitative conversion to retinal pigment epithelium. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0054552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sparrow J.R., Miller A.S., Zhou J. Blue light-absorbing intraocular lens and retinal pigment epithelium protection in vitro. J Cataract Refract Surg. 2004;30:873–878. doi: 10.1016/j.jcrs.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 179.Pons M., Marin-Castaño M.E. Cigarette smoke-related hydroquinone dysregulates MCP-1, VEGF and PEDF expression in retinal pigment epithelium in vitro and in vivo. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0016722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang H., Liu Y., Wang L., Li W. Age-related macular degeneration phenotypes are associated with increased tumor necrosis-alpha and subretinal immune cells in aged Cxcr5 knockout mice. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Priglinger C.S., Obermann J., Szober C.M., et al. Epithelial-to-Mesenchymal transition of RPE cells in vitro confers increased β1,6-N-glycosylation and increased susceptibility to galectin-3 binding. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Landowski M., Kelly U., Klingeborn M., et al. Human complement factor H Y402H polymorphism causes an age-related macular degeneration phenotype and lipoprotein dysregulation in mice. PNAS. 2019;116(9):3703–3711. doi: 10.1073/pnas.1814014116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Galloway C.A., Dalvi S., Hung S.S.C., et al. Drusen in patient-derived hiPSC-RPE models of macular dystrophies. PNAS. 2017 doi: 10.1073/pnas.1710430114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Johnson L.V., Forest D.L., Banna C.D., et al. Cell culture model that mimics drusen formation and triggers complement activation associated with age-related macular degeneration. PNAS. 2011;108:18277–18282. doi: 10.1073/pnas.1109703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Toomey C.B., Kelly U., Saban D.R., Rickman C.B. Regulation of age-related macular degeneration-like pathology by complement factor H. PNAS. 2015:E3040–E3049. doi: 10.1073/pnas.1424391112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Farnoodian M., Halbach C., Slinger C., et al. High glucose promotes the migration of retinal pigment epithelial cells through increased oxidative stress and PEDF expression. Am J Physiol Cell Physiol. 2016;311:C418–C436. doi: 10.1152/ajpcell.00001.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Temple S., Studer L. Lessons learned from pioneering neural stem cell studies. Stem Cell Reports J. 2017;8:191–193. doi: 10.1016/j.stemcr.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sia Z., Wanga X., Sun C., et al. Adipose-derived stem cells: sources, potency, and implications for regenerative therapies. 2019;114:1087652. doi: 10.1016/j.biopha.2019.108765. [DOI] [PubMed] [Google Scholar]