Abstract
During adaptive immune responses, germinal centers (GC) appear as transient microstructures, in which antigen-specific B and T cells interact with each other. Because only the antigen-activated B and T cells, such as Plasmablasts or follicular T helper (Tfh) cells, are present in GC, the in depth-analysis of GC is of great interest. To identify the cells that reside within GC, the majority of studies use the expression of specific surface molecules for analysis by flow cytometry. To do so, the tissue has to be disrupted for the preparation of single-cell suspensions. Thereby, the local information regarding neighborhoods of B cells and T cells and their potential interaction is lost. To study GC in vivo within their original microenvironment, we established a protocol for the isolation of GC by laser microdissection. To enable the identification of GC for subsequent transcriptomic analysis, the degradation of mRNA was diminished by using frozen tissues and by establishing a rapid staining protocol. This procedure enables histological and transcriptomic analysis of individual GC even within one lymphoid organ.
Keywords: Secondary lymphoid organs , Germinal centers , Follicular T helper cells , Laser microdissection , Cryo-preserved lymphoid structures , In vivo analysis
Background
One hallmark of secondary lymphoid organs is their strict compartmentalization into B and T cell zones. In an inactive stage, B cells and T cells are separated from each other; while B cells populate in B cell follicles, T cells accumulate in T cell zones ( Gasteiger et al. , 2016 ). This spatial segregation breaks down upon antigen encounter. Especially those B and T cells that are specific for the given antigen initiate the formation of GC within the B cell follicles ( Qi, 2016 ). GCs are the predominant site of antibody gene somatic hypermutation and are therefore crucially important for human health by enabling the production of high-affinity antibodies against pathogens. They are the result of a well-controlled sequence of events during T dependent humoral immune responses, in which initially rare antigen-engaged B cells encounter rare cognate antigen-specific T cells to eventually generate antibody-producing long-lived plasma cells and memory B cells. GC are histological visible, transient microstructures that contain a dark zone, a light zone, and a surrounding mantle zone ( Allen et al. , 2007 ). Numerous GC emerge upon antigen exposure within secondary lymphoid organs. They are formed mainly from proliferating B cells, which undergo somatic hypermutations and class-switches in the dark zone. B cells bearing a newly formed B cell receptor migrate from the dark zone to the light zone, where they compete with other B cell clones for antigen and T cell help ( Cyster and Allen, 2019 ). T cell help is provided by Tfh cells, which are licensed to enter GC by expression of the chemokine receptor CXCR5 ( Vinuesa et al. , 2016 ).
Here, we describe a protocol for the isolation of GC-B cells and GC-Tfh cells within individual GC by laser microdissection ( Niebuhr et al. , 2021 ). By keeping the microarchitecture intact and by using a short nuclear staining protocol that preserves the RNA at a high quality, this protocol has the advantage that it enables the analysis of gene expression in vivo and the usage of conserved tissues (especially cryopreserved) from former experiments. Of note, gene expression and immune receptor repertoires from each individual GC within one lymph node can be analyzed and compared. Until today, it is not known how diverse the T-and B cell responses within individual GC of one lymph node are and whether potential high or low diversities between GC would impact the affinity of the antibodies produced. Of note, this protocol is not restricted to GC and can be used to isolate GC-adjacent and/or non-adjacent T and B cell zones in addition ( Kalies et al. , 2008 ).
Materials and Reagents
Reaction tubes, 1.5 mL, safe seal, (Sarstedt, catalog number: 72.706.400)
Coverslips thickness 1.24 × 60 mm Gerhard Menzel GmbH (Carl Roth, catalog number: H878.2)
Superfrost Plus Microscope Glass Slides (Thermo Scientific, catalog number: J1800AMNZ), store at room temperature (RT)
Membranslide 1.0 PEN (Carl Zeiss AG, catalog number: 415190-9041-000), store at RT
Microtube 500 (D) (Carl Zeiss AG, catalog number: 415190-9221-000)
Mineral oil (Trinity Biotech, catalog number: 400-5), store at RT
0.2 µm syringe filter (Macherey-Nagel, catalog number: 729022), store at RT
Acetone 99.8% (Carl Roth, catalog number: 9372.5), store at RT in a ventilated place
Aquatex (Merck, catalog number: 1.08562.0050), store at 15–25°C
Biotin hamster anti-mouse TCRβ chain (clone H57-597) (BD Biosciences, catalog number: 553169), store undiluted at 4°C
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A2153), store at 2–8°C
Chloroform 99% (Carl Roth, catalog number: Y015.1), store at RT in a ventilated place
di-Sodiumhydrogenphosphate dodecahydrate (Na 2 HPO 4 ·12H 2 O) (Merck, catalog number: 1.06579.1000), store at RT
Ethanol 99.8% (Carl Roth, catalog number: 9065.4), store at RT
ExtrAvidin Alkaline Phosphatase (Sigma-Aldrich, catalog number: E2636), store in the dark at 2–8°C
ExtrAvidin Peroxidase (Sigma, catalog number: E2886), store in the dark at 2–8°C
Fast Blue BB salt (Sigma-Aldrich, catalog number: F3378), store at -20°C
Fast Red TR salt (Sigma-Aldrich, catalog number: 368881), store at RT
Liquid DAB+ Substrate Chromogen System (Agilent Technologies, catalog number: K3468), store in the dark at 2–8°C
Methanol 99.9% (Carl Roth, catalog number: 4627.5), store at RT in a ventilated place
Normal mouse serum (Invitrogen, catalog number: 10410), store at -20°C
Paraformaldehyde (Applichem, catalog number: A3813,1000), store at 4°C
Purified Rat anti-mouse Ki-67 (clone 16A8) (BioLegend, catalog number: 652402), store at 2–8°C
Purified Rat Anti-Mouse CD45R/B220 (clone RA3-6B2) (BD Biosciences, catalog number: 553084), store in the dark at 2–8°C
Rabbit Anti-Rat IgG (H+L), Human ads-BIOT (Southern Biotech, catalog number: 6185-08), store at 2–8°C
Biotinylated Hamster anti-mouse TCRβ antibody (clone H57-597) (BD Biosciences, catalog number: 553169), store undiluted at 4°C
Sodium azide (NaN 3 ) (Sigma-Aldrich, catalog number: S2002S8032), store at RT in a dark and well-ventilated place
Sodium Chloride (NaCl) (Carl Roth, catalog number: 3957.1), store at RT
Sodium dihydrogen phosphate monohydrate (NaH 2 PO 4 ·H 2 O) (Merck, catalog number: 1.06346.1000), store at RT
Naphthol AS-MX phosphate (Sigma-Aldrich, catalog number: N4875), store at -20°C
Dimethylformamide (SERVA, catalog number: 20270), store at room temperature
(-)-Tetramisole hydrochloride (Levamisole) (Sigma-Aldrich, catalog number: L9756), store at 2–8°C
Tissue freezing medium (Leica, catalog number: 14020108926), store at RT
Toluidine blue (Fluka, catalog number: 89640), store at RT
Tris base (Sigma-Aldrich, catalog number: T1503), store at RT
Tween 20 (Merck, catalog number: 8.22184.0500), store at RT
Dako Pen (DAKO, catalog number: 5200230-2)
Antibody solution (see Recipes)
Tris Buffer (0.1 M) (see Recipes)
Alkaline Phosphatase - Anti-Alkaline Phosphatase (APAAP) substrate (see Recipes)
Fast Blue solution (see Recipes)
Fast red solution (see Recipes)
PBS (phosphate-buffered saline) (see Recipes)
PFA 4% (paraformaldehyde) (see Recipes)
TBS-Tween (Tris-buffered Saline-Tween) (see Recipes)
Toluidine blue 1% solution (see Recipes)
Equipment
Laser microdissection system (Carl Zeiss AG, model: PALM Microbeam Laser microdissection system).
Transmitted light microscope (Leitz, model: Laborlux 11)
pH211 Microprocessor pH Meter (HANNA Instruments)
Refrigerator and freezer
Stand-alone UV light lamp holder for ultraviolet germicidal lamp G30W T8 (Sylvania, catalog number: 0000518)
Vortex (Scientific Industries, model: Vortex-2 Genie)
LEICA ® manual microtome (LEICA, model: CM3050S)
Eppendorf ® microcentrifuge (Eppendorf, model: 5417R)
Heating and Magnetic Stirrer (Roth, model: MH 15)
Software
Palm Robo software, Version 4.8.0.1 (Carl Zeiss AG)
Procedure
-
Preparation of lymphoid organs for isolation of GC by laser microdissection
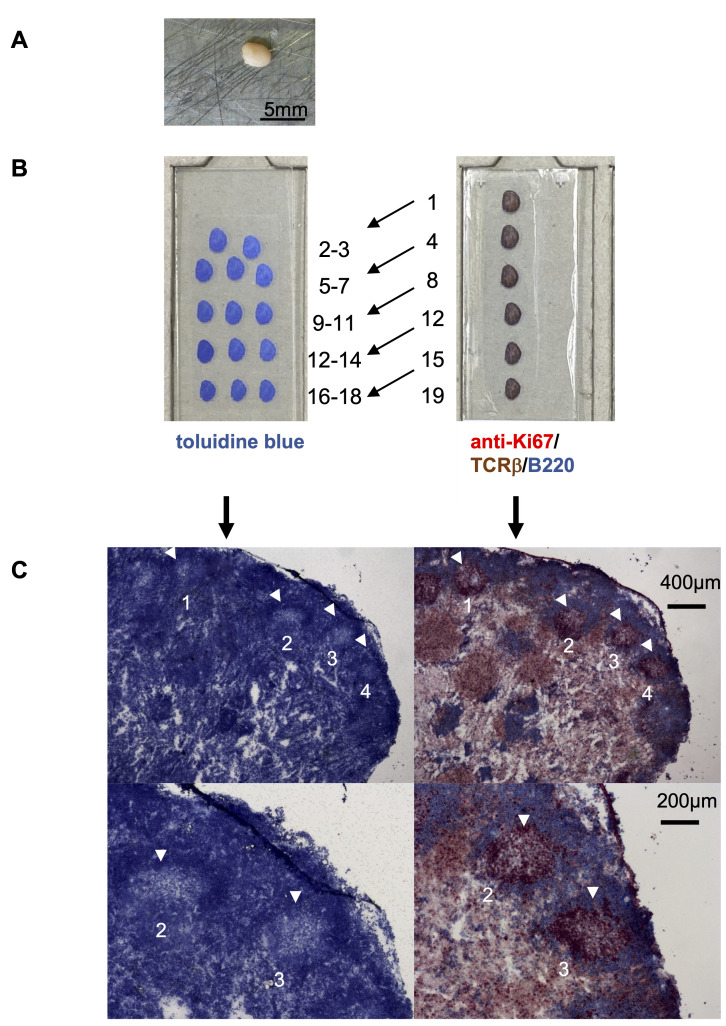
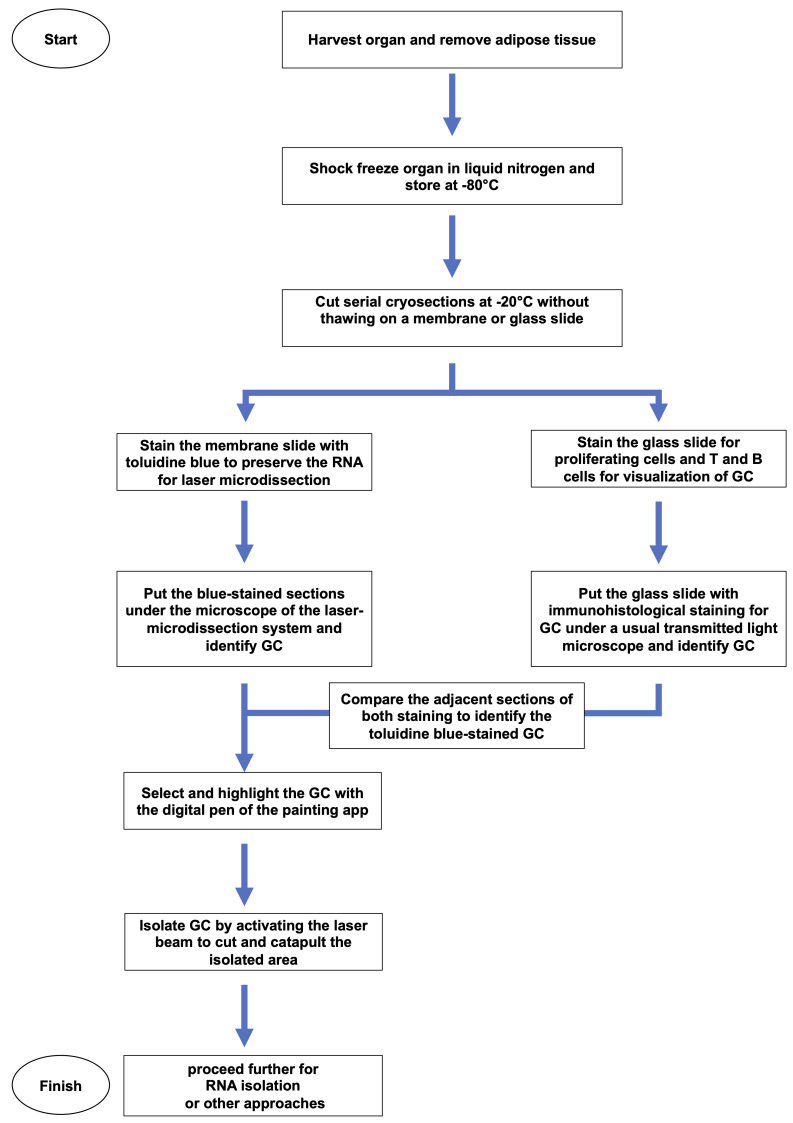
To enable transcriptional analysis, it is required to isolate GC while preserving mRNA at a high quality. Therefore, activated lymph nodes were fixed by snap-freezing in liquid nitrogen immediately after harvest and removal of adipose tissues ( Figure 1A ). Subsequently, tissues are stored in a -80°C refrigerator. Cryosections are prepared at -20°C using a cryomicrotom without allowing the tissue to thaw. To minimize the loss of RNA, two stainings are required to accurately identify GC: a short staining protocol with toluidine blue that preserves the mRNA and an immunohistochemical staining that allows the identification of GC and Tfh cells. To compare both stainings, serial cryosections were prepared on two slides: (i) a membrane slide for toluidine blue-staining and for laser microdissection; and (ii) a glass slide for immunohistochemical staining to visualize GC, T cells, and B cells ( Figure 1B –1C and Figure 2 ). Of note, to preserve the mRNA, the incubation of the tissue sections in aqueous solutions should be as short as possible, such as the 2-2-2 min washing-toluidine blue-washing incubation described here (steps e–g). Because RNases lose most of their activity in dehydrated dried tissues (step h), the slides can be stored before further processing (step i).
For troubleshooting, to optimize the staining protocol and to test for RNA integrity, it is recommended to cut one whole section from the membrane slide before and after staining and compare RNA concentrations (either by OD measurements or by PCRs).
-
Staining of cryosections on membrane slides with toluidine blue for laser microdissection
Optional: Expose membrane slides to UV light for 30 min at a distance of 400 mm for disinfection and to improve stickiness for tissue sections.
Embed collected frozen lymph nodes in tissue freezing medium and cut until a plain cut-level is present.
Cut 12 µm-sections of lymph nodes using a cryomicrotom and mount several individual sections on UV-treated membrane slides and on glass slides ( Figure 1 ).
Fix tissues in 75% ethanol for 10 min.
Rinse membrane slides in laboratory-grade H 2 O for 2 min.
Stain tissue by applying 0.1% toluidine blue for 2 min.
Rinse two times in laboratory-grade H 2 O for 2 min.
Dehydrate tissues by incubating in 96% ethanol for 15 s.
Use slides immediately or store them at -80°C until further processing.
-
Staining of cryosections for GC, T, and B cells on glass slides
Take the serial sections that have been mounted on glass slides.
Dry slides at RT for at least 1 h.
To fix tissues, incubate glass slides in chloroform for 10 min and acetone for 10 min. Afterwards, rinse tissues in TBS-Tween for 15 min and cover the lymph node section with 4% PFA and incubate them at 4°C for 45 min.
Rinse glass slides in TBS-Tween for 10 min and draw a circle around each tissue section with a Dako Pen to confine the liquids during staining.
Incubate sections with the first primary antibody anti-mouse Ki-67 at ambient temperature overnight (1:100 dilution in antibody solution).
Wash unbound antibodies away by incubation with TBS-Tween for 10 min and add the first secondary biotinylated anti-rat IgG antibody (1:500 dilution in PBS with 5% normal mouse serum) for 30 min.
Rinse with TBS-Tween for 30 min and cover tissue sections with ExtrAvidin Alkaline Phosphatase (1:100 in TBS-Tween) for 30 min.
Rinse with TBS-Tween for 10 min and apply Fast Red staining solution for 25 min.
Rinse with TBS-Tween for 10 min for removal of leftover staining solution.
Incubate sections with the second primary biotinylated anti-mouse TCRβ antibody (1:50 in TBS-Tween) for 1 h and rinse glass slides in TBS-Tween for 10 min to remove the unbound antibody.
Add ExtrAvidin Peroxidase (1:100 in TBS-Tween) for 30 min, wash slides in TBS-Tween for 30 min, and rinse glass slides in TBS-Tween for 10 min.
Incubate sections with Liquid DAB+ Substrate for 5 min to visualize T cells and wash with TBS-Tween for 10 min.
Add the third primary anti-mouse B220 antibody (1:100 in antibody solution) for 1 h and rinse with TBS-Tween for 10 min.
Incubate sections with the second secondary biotinylated anti-rat IgG antibody (1:500 dilution in PBS with 5% normal mouse serum) for 30 min and wash in TBS-Tween for 10 min.
Apply ExtrAvidin Alkaline Phosphatase for 30 min and rinse slides with TBS-Tween for 15 min.
Add Fast Blue staining solution 10 min to stain B cells. Subsequently, rinse sections again in TBS-Tween for 15 min and mount them with Aquatex and coverslips.
-
-
Isolation of GC by laser microdissection
-
Comparison of toluidine blue- and immunohistochemical-stained slides
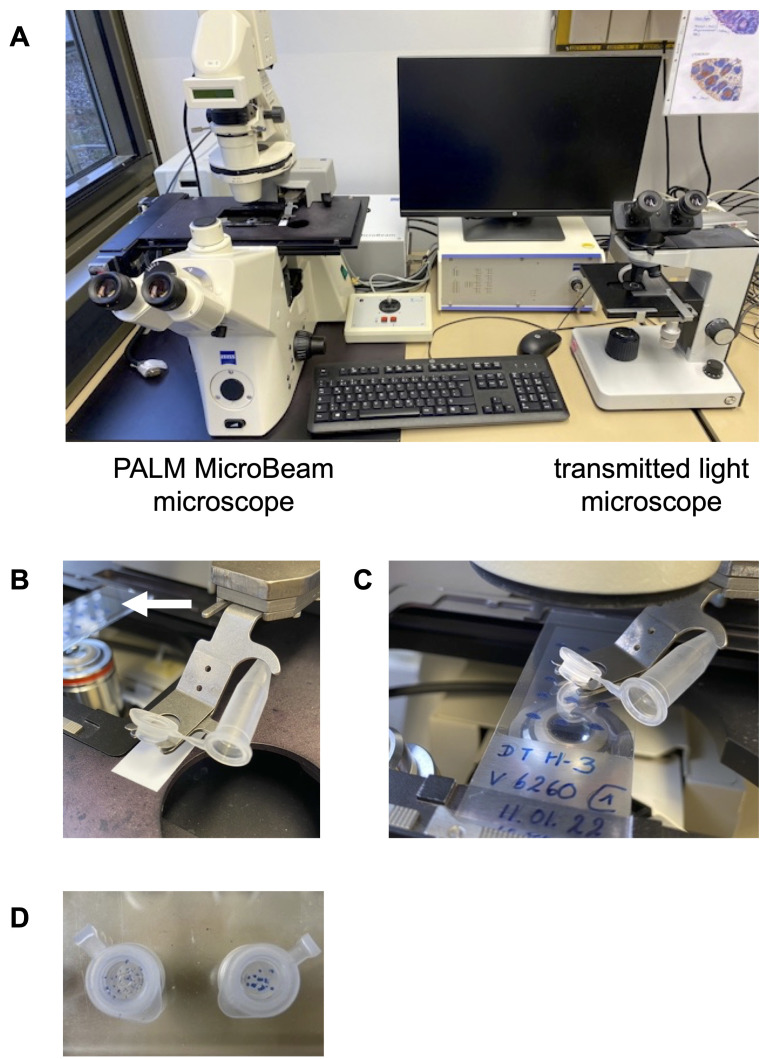
Place a usual light transmitted microscope close to the PALM MicroBeam microscope ( Figure 3A ).
Take the toluidine blue-stained slides and dry them at RT for 10 min.
Switch on the PALM MicroBeam microscope and put the slide with the toluidine blue-stained sections onto the slide holder of the object table.
Open the Palm Robo software.
Take the immunohistochemical-stained slide and put it on the object table of a usual light microscope that should stand close to the PALM MicroBeam microscope.
Carefully compare the two slides for the identification of GC. Note that samples that were closer in cryosections must be compared together ( e.g ., identify the GC by comparing cryosections 1 to 2 and 3).
-
Isolation of GC by laser microdissection
Before activating the laser beam, prepare the cap of the microtube and moisten it carefully with a very thin layer of mineral oil.
Put the cap and the microtube into the provided holder ( Figure 3B ).
Following the instruction of the manufacturer’s protocol, adjust the laser beam to the digital pen of the painting app by using the Palm Robo software. In addition, use a spot on the slide that is not covered with tissue to select the thickness of the laser beam. This can be done by fine-tuning the laser energy and the speed of the microbeam mover.
Cautiously surround the identified GC by using the digital pen of the painting app. It is required to carefully close the circled area with the digital pencil ( Video 1 ).
Place the cap holder directly over the slide to collect these predefined areas ( Figure 3C ).
Activate the laser beam to cut and catapult the isolated area by using the command “RoboLPC”. The UV-laser cuts the marked line and catapults the GC into the cap of the microtube ( Video 1 and Figure 3D ).
After collection of GC tissue (here: 2 × 10 6 –10 × 10 6 µm 2 per sample), remove the cap and microtube. Pool all microdissected and catapulted tissue pieces by closing the microtube with the cap and spinning down with a centrifuge. Collected tissues can be used for subsequent analysis.
-
Figure 1. Preparation of lymph nodes for isolation of GC by laser microdissection.
A. Activated murine popliteal lymph node. The surrounding adipose tissue was removed carefully before snap freezing. B. Serial cryosections of lymph nodes distributed on two slides, which were either stained with toluidine blue (left slide) or with anti-Ki67 (red) for proliferating cells, with anti-TCRβ for T cells (brown) and with anti-B220 for B cells (blue) (right side). The numbers indicate the order of serial sections. C. Example of two adjacent sections shown at higher magnifications. Toluidine blue-stained lymph node sections are shown on the left. Four GC are marked by white arrowheads (upper panel). Two GC are shown at a higher magnification on the lower panel. The immunohistochemical staining of the adjacent section on the right side confirms the identified GC (see Figure 2).
Figure 2. Workflow showing how the tissue is treated and processed in detail.
Figure 3. Equipment to isolate GC by laser microdissection.
A. The workplace shows the PALM MicroBeam microscope for laser capturing the tissues (left side) and the transmitted light microscope for immunohistochemical-stained sections to ensure the correct identification of GC (right side). B. A microtube is positioned into the holder. The white arrow indicates the membrane slide that contains the toluidine blue-stained sections in the slide holder. C. The cap- and microtube-holder must be placed directly over the slide to collect the catapulted tissues. D. The microdissected pieces of tissues can be clearly seen as toluidine blue-stained spots at the bottom of the microtube caps.
Video 1. Laser capturing of lymphoid tissue compartments.
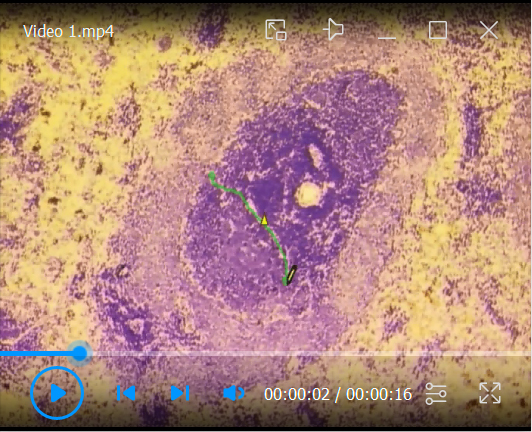
A toluidine blue-stained section of a rat spleen is used as an example for the microdissection of a B cell zone from lymphoid tissues.
1. The T- and B-cell zone can be clearly distinguished. 2. The area of interest, in this case, is a primary B cell follicle, which is circled using the drawing tool from the Palm Robo software. 3. The command “RoboLPC” from the Palm Robo software activates the laser beam to cut the marked tissue and the underlying membrane. An additional UV laser pulse catapults the isolated area into the cap of the microtube.
Recipes
-
Antibody solution
PBS + 1% BSA + 0.1% NaN 3 .
The solution can be stored at 4°C for six months.
-
Tris Buffer (0.1 M)
Dilute 12.1 g of Tris base in laboratory grade H 2 O to a final volume of 1 L. Adjust the pH to 8.2 with HCl.
The Tris buffer is stable at 4°C for six months.
-
Alkaline Phosphatase - Anti-Alkaline Phosphatase (APAAP) substrate
Dilute 20 mg of Naphthol AS-MX phosphate in 2 mL of N,N-Dimethylformamide, 100 µL of Levamisole (0.24 g/mL), and 98 mL of Tris-buffer 0.1 M.
The APAAP substrate is stable maximum for 30 days at 4°C.
-
Fast Blue staining solution
Dilute 0.002 g of Fast Blue BB salt in 4 mL of APAAP-substrate. Subject the solution to gentle shaking for 10 min. Prior to staining samples with the Fast Blue staining solution, filter with a 0.2 µm syringe filter. After treating the sample with the filtered solution, subject it to gentle shaking for 10 min. The solution should be used fresh.
Caution! The waste is hazardous, as the Fast Blue BB salt used in this solution is classified as a hazard when swallowed.
-
Fast Red staining solution
Dilute 0.01 g of Fast Red Salt in 3 mL of APAAP-substrate. After the dilution, subject the solution to gentle shaking for 5 min and then keep standing still for an additional 5 min. When added to the samples, subject the Fast Red staining solution to gentle shaking for 25 min.
Caution! Prepare the solution on a well-ventilated bench. The solution should be used fresh.
The waste is hazardous due to its last longing effect on aquatic life. Fast Red TR salt is hazardous as it causes severe skin burns and eye damage. The salt is also hazardous when swallowed.
-
PBS (phosphate-buffered saline) 5×
Dilute 90 g of NaCl, 2.704 g of NaH 2 PO 4 monohydrate, and 28.794 g of Na 2 HPO 4 ·12 H 2 O in laboratory-graded H 2 O with the final volume of 2 L. Adjust the pH to 7.4.
PBS 5× can be stored at 4°C for approximately six months.
PBS 1× should be used fresh after diluting 200 mL of PBS 5× in 800 mL of laboratory-graded H 2 O.
-
PFA 4% (paraformaldehyde) solution
Prepare the PFA 4% solution by dissolving 8 g of paraformaldehyde in 200 mL of PBS 1× solution by stirring (at 500 rpm) and heating (at 60°C) using a heating and magnetic stirrer.
Caution! Prepare the solution in a ventilated hood and use it fresh.
-
TBS-Tween (Tris-buffered Saline-Tween)
Prepare the TBS-Tween using a 10× TBS stock and a Tween-20 (5%) stock. Prepare the TBS 10× by diluting 242.28 g of Tris (hydroxymethyl aminomethane) and 344.40 g of NaCl in laboratory grade H 2 O with a final volume of 4 L; adjust the pH to 7.6 and keep at 4°C for up to six months.
Prepare the Tween-20 (5%) by adding 190 mL of laboratory-grade H 2 O to 10 mL of Tween-20. Prepare 1 L of TBS-Tween 1× by adding 890 mL of laboratory-grade H 2 O to 100 mL of TBS 10× and 10 mL of Tween-20 (5%). The TBS-tween solution can be stored for up to six months.
-
Toluidine blue 1% solution
Dilute 0.5 g of toluidine blue in 1 mL of methanol, 15 mL of ethanol, and 50 mL of DEPC-treated water.
The solution can be kept in the dark at RT for one year.
Acknowledgments
This work was supported by grants from the German Research Foundation (DFG) within the framework of the Schleswig-Holstein Excellence Cluster I and I (EXC 306, Inflammation at Interfaces, project XTP4), the graduate school GRK 1727/2, GRK2633/1, and the TR-SFB654 project C4 at the University of Luebeck to KK and JW. Part of the figures is adapted and modified from the studies of Kalies et al. (2008) and Niebuhr et al. (2020 and 2021).
Competing interests
The authors indicate no potential conflicts of interest.
Ethics
All experiments were approved by the Animal Care and Use Committee of the state Schleswig-Holstein (Ministerium fuer Energiewende, Landwirtschaft, Umwelt, Natur und Digitalisierung), proposals: V 252-72241.122-1 (24/-3/02), V 312-72241.122-1 (32-3/06), V242-35471/2016 (68-6/16) and 23/A11/05. All animal experiments were conducted by certified personnel.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
Q&A
Post your question about this protocol in Q&A and get help from the authors of the protocol and some of its users.
References
- 1. Allen C. D. , Okada T. and Cyster J. G. ( 2007 . ). Germinal-center organization and cellular dynamics . Immunity 27 ( 2 ): 190 - 202 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cyster J. G. and Allen C. D. C. ( 2019 . ). B Cell Responses: Cell Interaction Dynamics and Decisions . Cell 177 ( 3 ): 524 - 540 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gasteiger G. , Ataide M. and Kastenmüller W. ( 2016 . ). Lymph node- an organ for T-cell activation and pathogen defense . Immunol Rev 271 ( 1 ): 200 - 220 . [DOI] [PubMed] [Google Scholar]
- 4. Kalies K. , Konig P. , Zhang Y. M. , Deierling M. , Barthelmann J. , Stamm C. and Westermann J. ( 2008 . ). Nonoverlapping expression of IL10, IL12p40, and IFNgamma mRNA in the marginal zone and T cell zone of the spleen after antigenic stimulation . J Immunol 180 ( 8 ): 5457 - 5465 . [DOI] [PubMed] [Google Scholar]
- 5. Niebuhr M. , Belde J. , Fahnrich A. , Serge A. , Irla M. , Ellebrecht C. T. , Hammers C. M. , Bieber K. , Westermann J. and Kalies K. ( 2021 . ). Receptor repertoires of murine follicular T helper cells reveal a high clonal overlap in separate lymph nodes in autoimmunity . Elife 10 : e70053 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qi H. ( 2016 . ). T follicular helper cells in space-time . Nat Rev Immunol 16 ( 10 ): 612 - 625 . [DOI] [PubMed] [Google Scholar]
- 7. Vinuesa C. G. , Linterman M. A. , Yu D. and MacLennan I. C. ( 2016 . ). Follicular Helper T Cells . Annu Rev Immunol 34 : 335 - 368 . [DOI] [PubMed] [Google Scholar]