Abstract
Introduction:
Novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection resulting in COVID-19 disease is associated with widespread inflammation and a prothrombotic state, resulting in frequent venous thromboembolic (VTE) events. It is currently unknown whether anticoagulation is protective for VTE events. Therefore, we conducted a systematic review to identify predictors of VTE in COVID-19.
Methods:
We searched PubMed, EMBASE, Google Scholar, and Ovid databases for relevant observational studies of VTE in COVID-19 disease. The effect size for predictors of VTE was calculated using a random-effects model and presented as forest plots. Heterogeneity among studies was expressed as Q statistics and I2. Bias was assessed using the Newcastle Ottawa Scale for all identified observational studies. Publication bias was assessed with funnel plot analysis.
Results:
We identified 28 studies involving 6053 patients with suspected or confirmed COVID-19. The overall pooled prevalence of VTE events was 20.7%. Male sex was associated with a higher risk of VTE events, whereas prior history of VTE, smoking, and cancer were not. VTE events were significantly higher in severely ill patients, mechanically ventilated patients, those requiring intensive care admission, and those with a low PaO2/FiO2 ratio (P/F ratio). Chronic comorbidities, including cardiovascular disease, heart failure, renal disease, and pulmonary disease, did not increase the risk of VTE events. Patients with VTE had higher leukocyte counts and higher levels of D-dimer, C-reactive protein, and procalcitonin. The occurrence of VTE was associated with increased length of stay but did not impact mortality. Therapeutic and prophylactic doses of anticoagulation were not protective against VTE.
Conclusion:
VTE in COVID-19 is associated with male gender and severe disease but not with traditional risk factors for VTE. The occurrence of VTE does not appear to be mitigated by either prophylactic or therapeutic anticoagulation. The occurrence of VTE in this population is associated with an increased length of stay but does not appear to impact mortality.
Keywords: anticoagulation, COVID-19, mortality risk, thromboembolism, thrombosis
Introduction
Novel coronavirus infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with inflammation and a prothrombotic state, with resultant increases in fibrin, fibrin degradation products (FDP), fibrinogen, and D-dimer.1,2 These inflammatory markers are associated with more severe illness and worse clinical outcomes in coronavirus (COVID-19)-affected individuals.3,4 Clinically, an increased risk of thromboembolic disease has been observed in association with COVID-19 infection in severely ill patients.5–7 However, the relationship between inflammation, severity of COVID-19 disease, and thromboembolism remains unclear, as are the predictors of thromboembolism. Patients with COVID-19 have an increased incidence of venous thromboembolism (VTE) [deep vein thrombosis (DVT) and pulmonary embolism (PE)] despite prophylactic anticoagulation (AC).6,8 Indeed, routine ultrasound surveillance has reported a high incidence of VTE events in COVID-19 patients. 9 Current guidelines recommend using standard thromboprophylaxis for all patients admitted to the hospital with COVID-19 infection.10,11 However, as the incidence of VTE events remains high, strategies such as intermediate dose (double prophylaxis) or therapeutic parenteral anticoagulation (AC) have been recommended by national societies.8,12,13 Furthermore, there are limited data regarding routine monitoring of coagulation markers in predicting VTE events in COVID-19 infection. Whether typical risk factors, risk scores, or monitoring of coagulation factors can predict VTE events remains unanswered. Therefore, we conducted a systematic review and meta-analysis of all available observational studies to determine risk factors associated with VTE events in patients with COVID-19 infection.
Methods
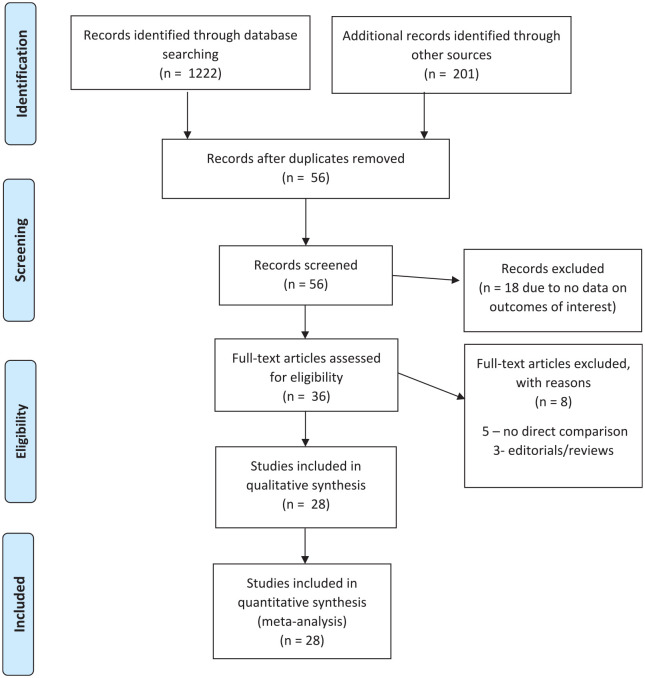
This systematic review and meta-analysis were conducted according to the Cochrane collaboration guidelines and reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Figure 1).14,15
Figure 1.
PRISMA flow diagram showing search strategy for meta-analysis comparing COVID-19 patients with and without venous thromboembolism.
Manuscript search and identification strategy
We performed a comprehensive search of PubMed, EMBASE, Google Scholar, and Ovid databases using the following search terms: ‘coronavirus’ or ‘SARS-CoV2’ or ‘COVID-19’ AND ‘venous thromboembolism’ or ‘pulmonary embolism’ or ‘deep venous thrombosis’ and their various combinations through 15 July 2020. Obtained studies were screened by title and abstract. Manuscripts describing VTE in patients with coronavirus were retrieved in their entirety and reviewed for potential inclusion. In addition, reference lists of selected studies, relevant editorials, and review articles were hand-searched to look for additional related publications.
The following inclusion criteria were used:
Any published studies of patients >18 years of age with COVID-19.
Comparison of COVID-19 patients with and without VTE.
Reported outcomes of interest.
Reported event rates with sample size or odds ratio (OR) with a 95% confidence interval (CI).
Patients with non-diagnostic or equivocal imaging, and patients on AC for other reasons prior to admission, were excluded.
‘Severe disease’ was clinically defined as patients requiring life support, mechanical ventilation or intensive care unit (ICU) admission, and those who died during hospitalization.
Data extraction
Two authors (G.A., S.C.) separately extracted data regarding baseline patient characteristics and outcomes from the selected manuscripts using a standardized data collection form. Any discrepancies in extracted data and quality assessment were resolved by discussion and consensus among all authors.
Bias analysis
Bias was assessed using the Newcastle Ottawa Scale. The funnel plot analysis estimated publication bias. Most of the studies were under the ‘excellent’ category.
Data analysis
Continuous data for baseline characteristics are presented as means with standard deviation. Categorical data are presented as frequencies with absolute numbers as well as percentages. For each study, the following information was extracted: the surname of the first author, the geographical region where the study was performed, the type of study, sample size, baseline demographic characteristics, proportion of patients receiving prophylactic or therapeutic AC, the proportion of patients with underlying VTE/PE/DVT/arterial thromboembolic events, and D-dimer results within VTE and non-VTE groups. A consensus resolved any variances. The pooled risk ratio (RR) with corresponding 95% confidence intervals (CIs) was calculated using the DerSimonian-Laird random-effects model. 15 Heterogeneity was assessed using the chi-square-based Cochrane Q test and quantified using the I2 statistic. I2 statistic values of 25%, 50%, and 75% were used to define low, moderate, and high heterogeneity, respectively, signifying the proportion of observed variance that reflects the difference in true effects. The statistical analysis was carried out using Comprehensive Meta-Analysis (Englewood, NJ, USA) with a random-effects model. A p-value of <0.05 was considered significant.
Predictors of VTE events were calculated using the random-effects model using a forest plot (Figures 2–16, Supplementary File 2). The calculated effect size was the pooled RR and the raw difference in mean between VTE and no VTE events. The primary outcome of our study was to identify the risk of VTE based on the use of prophylactic or therapeutic AC, history of previous VTE, presence or absence of cardiovascular disease (CVD), hypertension, heart failure (HF), smoking, atrial fibrillation (AF), diabetes mellitus, cancer, chronic pulmonary disease, or renal disease. We also reported the association of VTE with the severity of illness, ICU admission, and the use of mechanical ventilation. The association of baseline laboratory values, inflammatory markers, D-dimer, and demographic parameters with the risk of VTE has been demonstrated. Finally, we tried to determine whether the presence of VTE is associated with higher mortality risk. Prevalence of VTE events was expressed as a percentage with VTE events among patients with COVID-19 infection, or the percentage of computed tomography pulmonary angiography (CTPA) or compression ultrasonography (CUS) positivity.
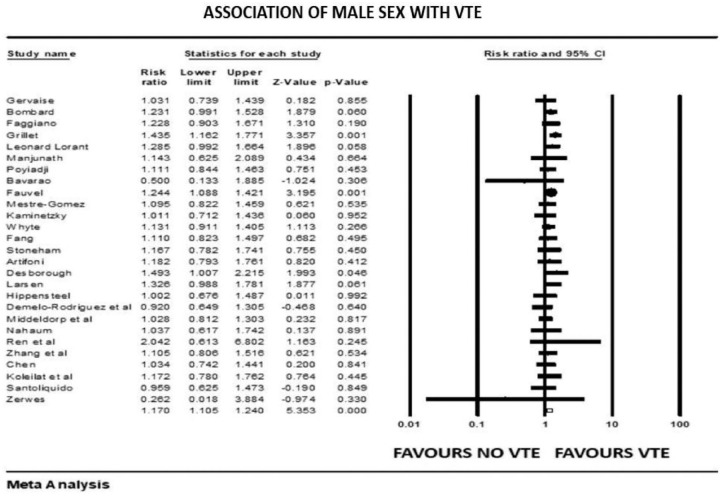
Figure 2.
Forest plot showing results of meta-analysis comparing COVID-19 patients with and without venous thromboembolism and association with male sex.
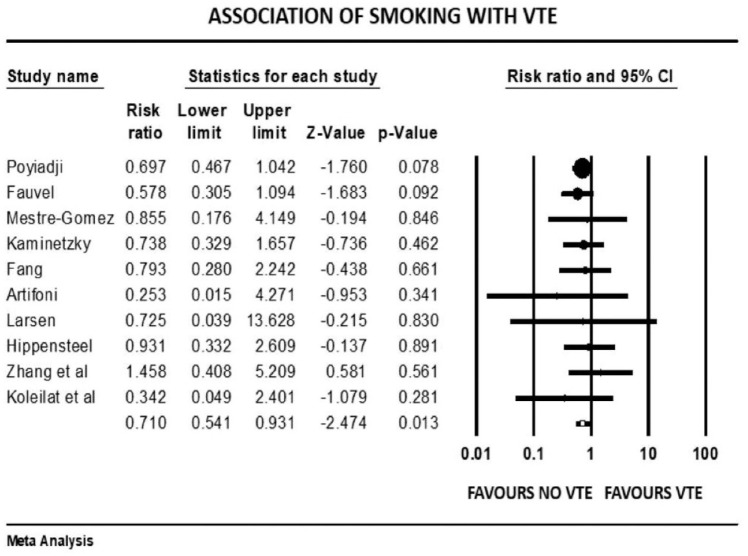
Figure 3.
Forest plot showing results of meta-analysis comparing COVID-19 patients with and without venous thromboembolism and association with smoking.
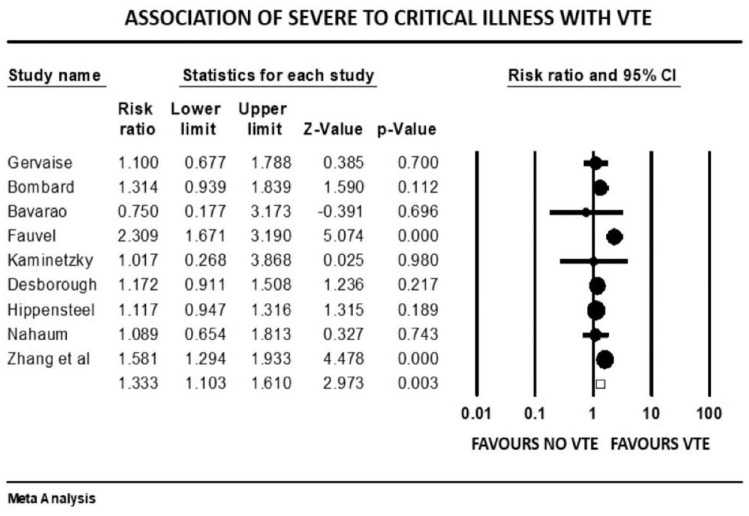
Figure 4.
Forest plot showing results of meta-analysis comparing COVID-19 patients with and without venous thromboembolism and association with severity or critical illness.
Figure 5.
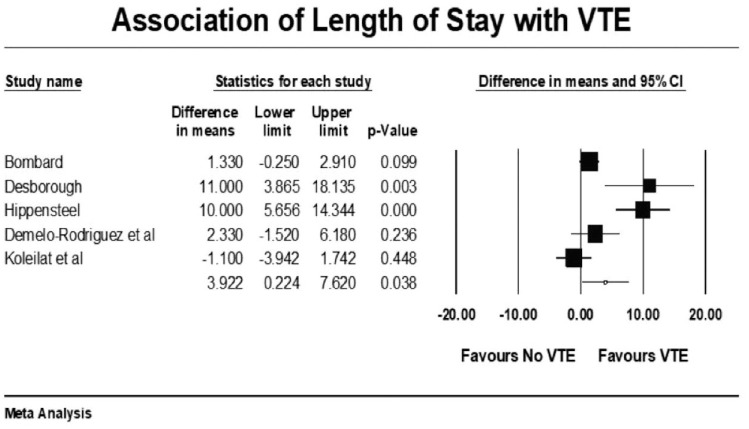
Forest plot showing results of meta-analysis comparing COVID-19 patients with and without venous thromboembolism and association with length of stay.
Figure 6.
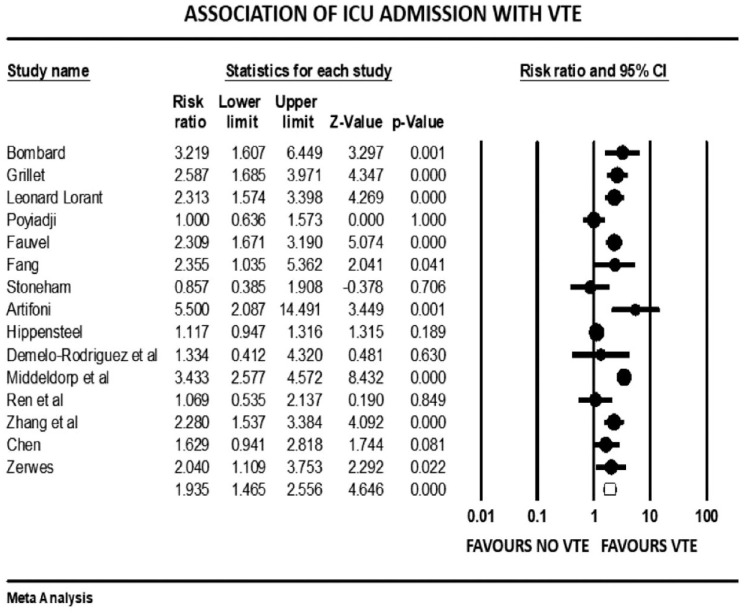
Forest plot showing results of meta-analysis comparing COVID-19 patients with and without venous thromboembolism and association with ICU admission.
Figure 7.
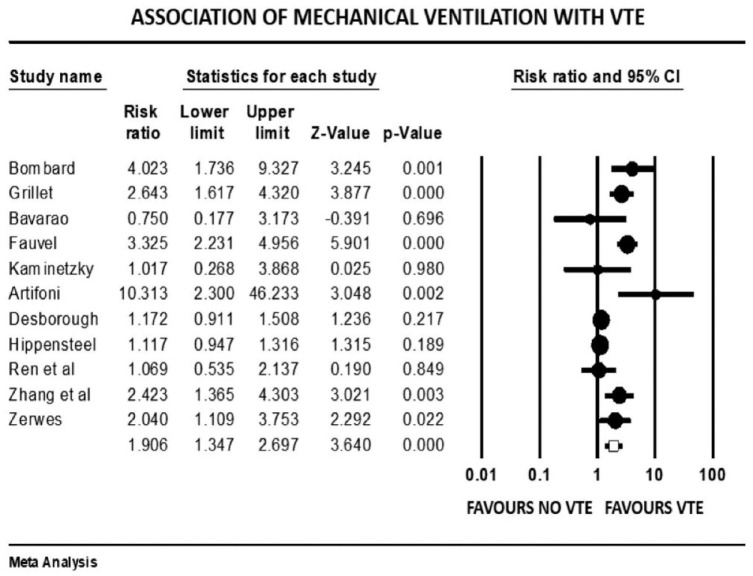
Forest plot showing results of meta-analysis comparing COVID-19 patients with and without venous thromboembolism and association with mechanical ventilation.
Figure 8.
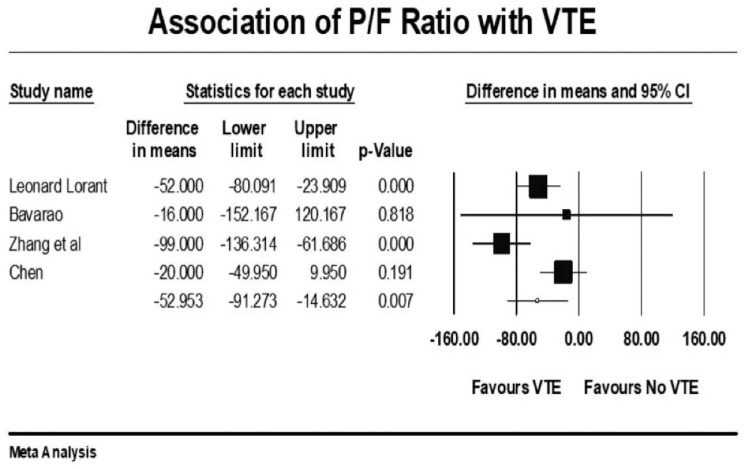
Forest plot showing results of meta-analysis comparing COVID-19 patients with and without venous thromboembolism and association with P/F ratio.
Figure 9.
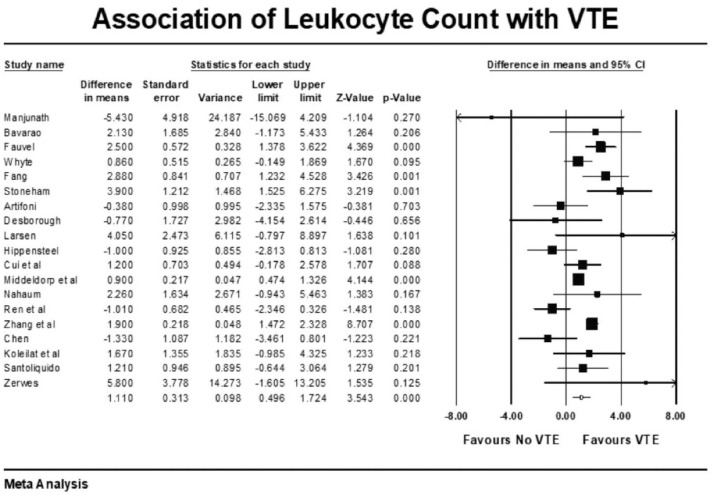
Forest plot showing results of meta-analysis comparing COVID-19 patients with and without venous thromboembolism and association with leukocyte count.
Figure 10.
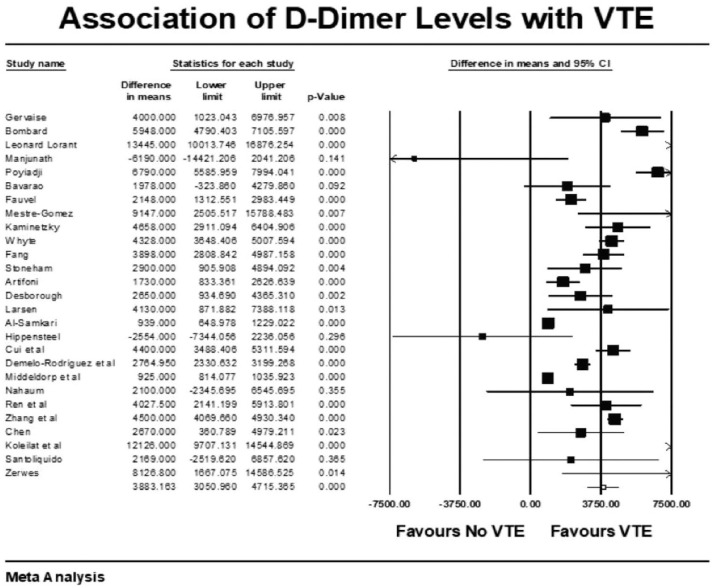
Forest plot showing results of meta-analysis comparing COVID-19 patients with and without venous thromboembolism and association with D-Dimer levels.
Figure 11.
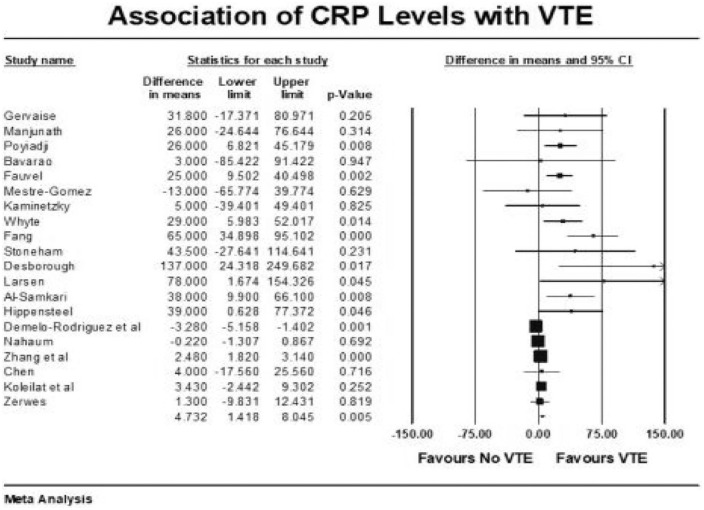
Forest plot showing results of meta-analysis comparing COVID-19 patients with and without venous thromboembolism and association with CRP levels.
Figure 12.
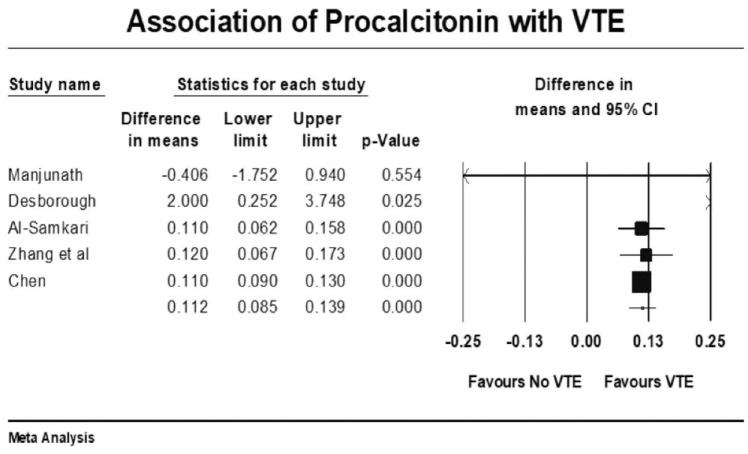
Forest plot showing results of meta-analysis comparing COVID-19 patients with and without venous thromboembolism and association with procalcitonin levels.
Figure 13.
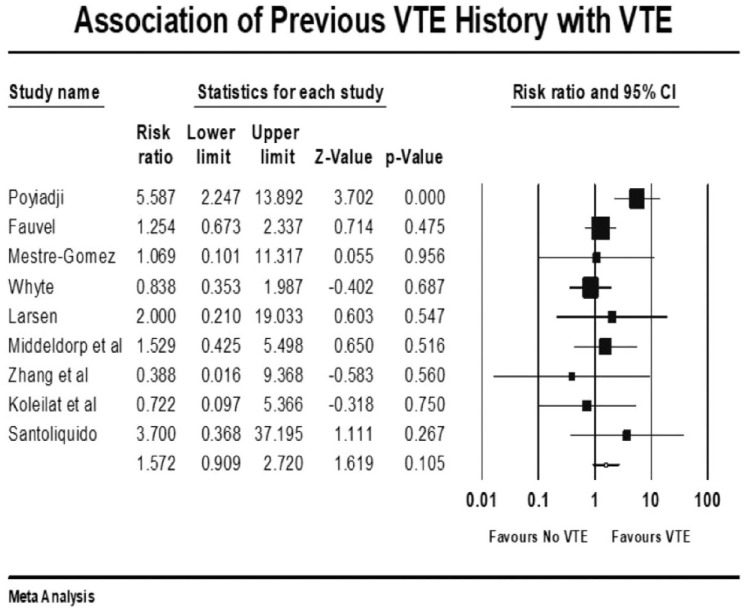
Forest plot showing results of meta-analysis comparing COVID-19 patients with and without venous thromboembolism and association with h/o VTE.
Figure 14.
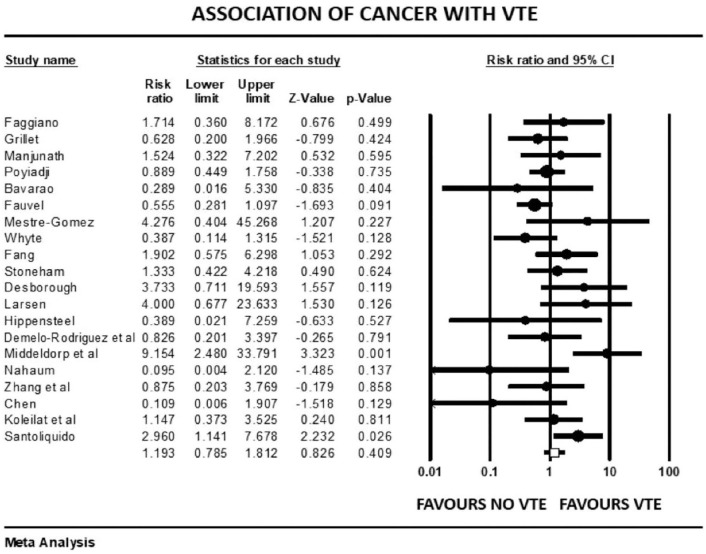
Forest plot showing results of meta-analysis comparing COVID-19 patients with and without venous thromboembolism and association with h/o cancer.
Figure 15.
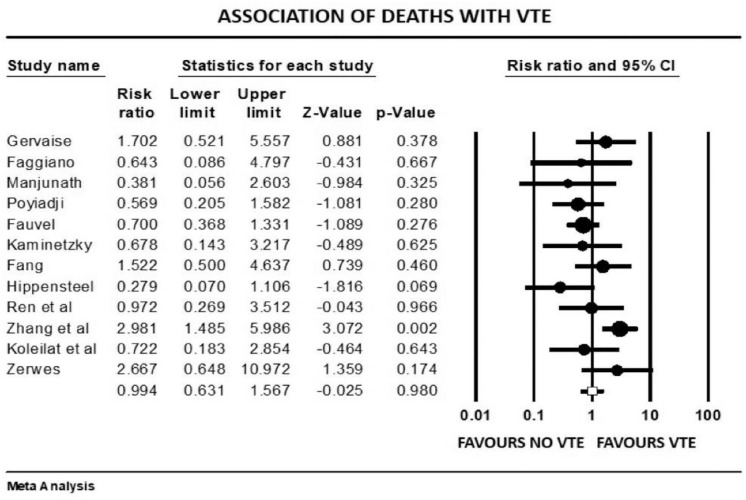
Forest plot showing results of meta-analysis comparing COVID-19 patients with and without venous thromboembolism and association with in-hospital mortality.
Figure 16.
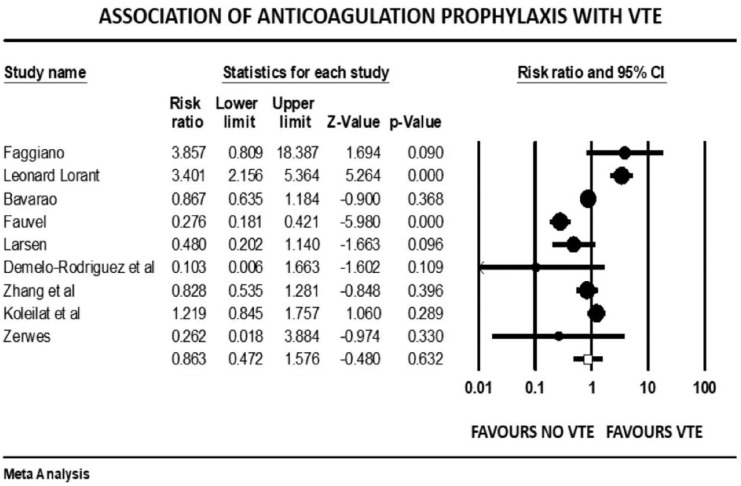
Forest plot showing results of meta-analysis comparing COVID-19 patients with and without venous thromboembolism and association with venous thromboprophylaxis.
Results
We identified 28 studies, comprising 6053 patients with confirmed or suspected COVID-19 infection. In all studies, the diagnosis of COVID-19 was made by either reverse-transcriptase polymerase chain reaction (PCR) of a nasopharyngeal swab or by high clinical suspicion combined with radiological features highly suggestive of COVID-19 infection. In all studies, CTPA or CUS was used to detect PE and DVT, respectively. The percentage of prophylactic AC use varied widely among studies as shown in Table 1.16–41 All patients had a D-dimer level or imaging during admission, and this was compared between patients with or without VTE events. Studies have overall male preponderance (65%). The baseline characteristics of the study population are presented in Table 2.16–41
Table 1.
| Study name | Use of anticoagulation |
|---|---|
| Bompard et al. | Enoxaparin 40 mg once daily in medical floor Enoxaparin 40 mg every 12 h in ICU Enoxaparin 40 mg every 12 h in obese patients irrespective of floor or ICU |
| Al-Samkari et al. | Enoxaparin 40 mg SC daily OR Unfractionated heparin 5000 U SC
every 8–12 h in medical floor Enoxaparin 40 mg SC daily OR Unfractionated heparin 5000 U SC every 8–12 h in ICU Enoxaparin 40 mg SC every 12 h OR Unfractionated heparin 5000- 7500 U SC every 8 h in obese patients |
| Chen et al. | Low-molecular-weight heparin |
| Faggiano et al. | Unfractionated or low-molecular-weight heparin |
| Fauvel et al. | Daily low-molecular-weight heparin or twice daily subcutaneous unfractionated heparin; intermediate dose (double the preventive dose); and therapeutic dose |
| Koleilat et al. | Low-molecular-weight heparin prophylaxis dose, subcutaneous heparin prophylaxis, therapeutic anticoagulation (unfractionated heparin, direct oral anticoagulant), therapeutic bivalirudin, prophylactic apixaban |
| Larsen et al. | Low-molecular-weight heparin prophylaxis |
| Léonard-Lorant et al. | Low-molecular-weight heparin therapeutic dose |
| Manjunath et al. | Prophylaxis using subcutaneous, unfractionated heparin (5000 U two times a day), low-molecular-weight heparin (40 mg daily), or home regimen of novel oral anticoagulants (two patients with atrial fibrillation) |
| Gómez et al. | Unfractionated heparin or low-molecular-weight heparin for prophylaxis dose |
| Kaminetzky et al. | Prophylactic anticoagulation: subcutaneous enoxaparin (40–60 mg
according to body mass index) or subcutaneous
heparin Therapeutic anticoagulation: subcutaneous enoxaparin (1 mg/kg) twice daily or intravenous heparin |
| Middeldorp et al. | Low-molecular-weight heparin prophylaxis dose |
| Ren et al. | 30 to 40 mg low-molecular-weight heparin (subcutaneous injection) once daily |
| Santoliquido et al. | Prophylactic dose of anticoagulant (either enoxaparin 40 mg once daily or fondaparinux 2.5 mg daily) |
| Stoneham et al. | Therapeutic anticoagulation with low-molecular-weight heparin |
| Whyte et al. | Therapy with enoxaparin, rivaroxaban, apixaban, edoxaban, and unfractionated heparin infusion |
| Zhang et al. | Therapy with low-molecular-weight heparin |
ICU, intensive care unit.
Table 2.
Baseline characteristics of patients in studies included in the meta-analysis comparing COVID-19 patients with and without venous thromboembolism.
| Study name | Study type | Study location | Inclusion criteria | Exclusion criteria | Patients included in analysis (N) | Male (%) | Median/mean age | Therapeutic/prophylactic anticoagulation | Pulmonary embolism (%) | Total VTE events/DVT | Arterial thrombotic events | Median/mean D-dimer (ng/ml) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bompard et al. | Multicenter retrospective | France | Patients with COVID-19 pneumonia being referred for CT pulmonary angiography | None | 135 | 70 | 64 | All hospitalized patients | 24% | NA | NA | 984 PE+ versus 1285 PE− |
| Al-Samkari et al. | Multicenter retrospective | USA | Patients >18 years with COVID-19 by RT-PCR of nasopharyngeal/oropharyngeal swab and D-dimer test performed on initial presentation | None | 400 | 69 | 65 | 97% | 2.50% | 4.80% | 2.80% | 1538 (initial) |
| Fiore et al. | Single-center retrospective | Italy | Confirmed COVID-19 with elevated D-dimer and at least one of the following inclusion criteria (risk factors for PE, clinical sings of PE, severe pneumonia) | None | 20 | 40 | 58 | 85% | 40.00% | NA | NA | 1692 PE+ versus 741 PE− |
| Hippensteel et al. | Single-center retrospective | USA | Critically ill patients with laboratory confirmed COVID-19 infection > 18 year. | none | 91 | 58 | 55 | NA | 5.50% | 26.10% | NA | 1071 |
| Artifoni et al. | Single-center retrospective | French | Patients hospitalized with COVID-19 in non-ICU setting receiving adequate thromboprophylaxis | Prior anticoagulation and contraindication to thromboprophylaxis | 71 | 61 | 64 | 99% | 9.80% | 22.50% | NA | 1630 VTE+ versus 670 VTE− |
| Chen et al. | Single-center retrospective | China | Critically ill COVID-19 patients requiring ICU admission | No surveillance result on DVT or pharmacologic thromboprophylaxis before ICU admission | 88 | 61 | 63 | All inpatients | NA | 46% (DVT) | NA | 6410 DVT+ versus 3100 DVT− |
| Cui et al. | Single-center retrospective | China | Patients with novel coronavirus pneumonia in ICU | None | 81 | 46 | 60 | No patients receive anticoagulation | NA | 25% (DVT) | NA | 5200 VTE+ versus 800 VTE− |
| Demelo-Rodriguez et al. | Single-center retrospective | Spain | COVID-19 patients admitted in non-intensive care unit if they are >18 years, D-dimer >1000 ng/ml, and hospitalized for at least 48 h | Patients receiving therapeutic doses of
anticoagulation History of DVT of lower extremity or signs and symptoms of DVT before inclusion |
156 | 65 | 68 | 98% prophylaxis anticoagulation | NA | 14.7% (DVT) | NA | 4527 DVT+ versus 2050 DVT− |
| Desborough | Single-center retrospective | UK | All patients with COVID-19 admitted in critical care | Discharged from critical care before developing symptoms of COVID-19 | 66 | 73 | 59 | 17% therapeutic AC, rest prophylactic AC | 8% | 15% | NA | 6910 VTE+ versus 2100 VTE− |
| Faggiano et al. | Single-center retrospective | Italy | Admitted patients with COVID-19 pneumonia | None | 25 | 100 | 70 | 43% of PE group, 11% no PE group | 28% | 8% (DVT) | NA | 4368 PE+ versus 1455 PE− |
| Fang et al. | Single-center retrospective | UK | COVID-19 patients undergoing CT pulmonary angiography | None | 100 | 68 | 62 | NA | 44% | NA | NA | 7465 PE+ versus 2450 PE− |
| Fauvel et al. | Multicenter retrospective | France | All adult patients admitted to hospital with a diagnosis of SARS-CoV2 infection | Patients who had no CT pulmonary angiogram, direct admission to ICU, and those who are still in hospital and not experienced PE on study completion | 1240 | 58 | 64 | 71% | 8.30% | 1.5% (DVT) | NA | 3519 PE+ versus 1371 PE− |
| Gervaise et al. | Single-center retrospective | France | Non-hospitalized patients referred by the ED for CT pulmonary angiogram for COVID-19 pneumonia | None | 72 | 75 | 62 | NA | 18% | NA | NA | 7290 PE+ versus 3290 PE− |
| Grillet et al. | Single-center retrospective | France | Patients >18 years with confirmed or suspected COVID-19 who had chest CT | Patients with non-contrast chest CT | 100 | 70 | 66 | NA | 23% | NA | NA | NA |
| Koleilat et al. | Single-center retrospective | USA | All adult patients COVID-19 infection who underwent duplex scanning | None | 135 | 61 | 59 | 81.2% in negative DVT group, 100% in + group | 3.7% (confirmed), 5.2% high suspicion | 13.3% (DVT) | NA | 1361 DVT+ group, 3580 DVT− group |
| Larsen et al. | Single-center retrospective | France | Returning travelers with hypoxemic COVID-19 pneumonia with confirmed COVID-19 infection | None | 35 | 77 | 66 | NA | NA | 20% | NA | 3010 PE group, 990 no PE group |
| Lorant-Leonard et al. | Single-center retrospective | France | Patients with COVID-19 who underwent CT pulmonary angiogram | None | 106 | 78 | 64 | 84% in PE group, 30% in no PE group | 30% | NA | NA | NA |
| Manjunath et al. | Single-center retrospective | USA | Critically ill COVID-19 patients receiving ICU level of care | None | 23 | 71 | 61.7 | 100% | 30% | NA | NA | 8790 PE+, 2600 PE− |
| Gomez et al. | Single-center retrospective | Spain | COVID-19 patients admitted to hospital and having CT pulmonary angiography | None | 452 | 72 | 65 | 79% PE group | 6.4% of all patients, 32% of CT scan | 0.4% (DVT) | NA | 14,480 PE+ versus 7230 PE− |
| Kaminetzky et al. | Single-center retrospective | USA | COVID-19 patients who underwent CT pulmonary angiography | Technically inadequate CT | 62 | 64 | 55 | 48% | 37% (52% in patients receiving prophylactic AC) | 38.70% | 1.60% | 6432 PE+ versus 1774 PE− |
| Middledrop et al. | Single-center retrospective | Netherland | Confirmed COVID-19 patients or suspected COVID-19 patients based on symptoms and CT finding | COVID-19 patients admitted for other medical reason | 198 | 66 | 61 | 100% | 6.60% | 20% total, 13% DVT | NA | 2600 VTE+ versus 1000 VTE− |
| Nahum et al. | Single-center retrospective | Paris | Severe COVID-19 pneumonia patients admitted to ICU | None | 34 | 78 | 62 | 100% | NA | 65% at admission, 79% after 48 h | NA | 5400 DVT+ versus 3300 DVT− |
| Poyiadji et al. | Multicenter retrospective | USA | COVID-19 patients who had CT pulmonary angiogram | CT studies limited by motion artifact and poor contrast opacification | 337 | 49 | 59 | 36% | 22% overall, 23% in patients on prophylactic AC | NA | NA | 9330 PE+ versus 2540 PE− |
| Ren et al. | Cross sectional | China | Confirmed COVID-19 patients admitted in ICU | Prior DVT or recent surgery | 48 | 54 | 70 | 98% | NA | 85% (DVT) | NA | 3480 |
| Santoliquido et al. | Single-center retrospective | Italy | Non-ICU patients admitted with COVID-19 infection | Age <18 years, ICU admission, receiving full dose AC for atrial fibrillation or prior DVT | 84 | 73 | 67 | 100% | NA | 11.9% (DVT) | NA | 6009 DVT+ versus 3840 DVT− |
| Stoneham et al. | Multicenter retrospective | UK | Confirmed or suspected COVID-19 patients based on RT-PCR/CT scan admitted to the hospital | None | 274 | 67 | 67 | NA | 5.80% | 7.70% | NA | 4100 DVT+ versus 1200 DVT− |
| Whyte et al. | Single-center retrospective | UK | Suspected or confirmed COVID-19 hospitalized patients who had CT pulmonary angiogram | None | 1477 | 52 | 63 | 100% | 5.4% overall, 37% CTPE positivity | NA | NA | 8000 PE+ versus 2060 PE− |
| Zhang et al. | Single-center retrospective | China | Hospitalized patients with COVID-19 | 143 | 52 | 63 | 37.1% lower extremity US patients | NA | 8.8% (DVT), 46.1% of US positive | NA | NA |
AC, anticoagulation; CT, computed tomography; CTPE, computed tomography pulmonary embolus; DVT, deep vein thrombosis; ED, emergency department; ICU, intensive care unit; PE, pulmonary embolism; RT-PCR, reverse transcriptase polymerase chain reaction; US, ultrasound; VTE, venous thromboembolism.16–41
The overall pooled prevalence of VTE reported in 4196 patients was 20.7%. There was no significant association between VTE events and prophylactic (mean RR: 0.86, 95% CI, 0.47 to 1.57; p = 0.63) or therapeutic AC (mean RR: 0.78, 95% CI, 0.31 to 1.95; p = 0.60), with high (I2 = 81%) and moderate (I2 = 41%) levels of heterogeneity, respectively. The risk of VTE was 17% higher in males (mean RR: 1.17, 95% CI, 1.10 to 1.24; p < 0.001, I2 = 0%). Patients with previous history of VTE trended to higher risk of developing VTE in COVID-19 infection, although this outcome did not reach statistical significance (mean RR: 1.57, 95% CI, 0.90 to 2.72; p = 0.10, I2 = 33%). Smokers had a reduced risk of VTE compared with non-smokers (mean RR: 0.71, 95% CI, 0.54 to 0.93; p = 0.013), with studies showing low level of heterogeneity (I2 = 0%, Q = 3, df = 9). VTE events were not associated with previous AF (mean RR: 0.68, p = 0.72), HF (mean RR: 1.02, p = 0.93), CVD (mean RR: 0.87, p = 0.36), hypertension (mean RR: 1.01, p = 0.90), diabetes mellitus (mean RR: 0.93, p = 0.42), pulmonary disease (mean RR: 0.90, p = 0.61), or renal disease (mean RR: 0.94, p = 0.77) (Supplemental File 3>). Patients with cancer were also not at increased risk of COVID-19-associated VTE (mean RR: 1.19, 95% CI, 0.78 to 1.81; p = 0.40; I2 = 46%).
Patients with severe illness had a 33% increased risk of developing VTE (mean RR: 1.33, 95% CI, 1.10 to 1.61; p = 0.003). Those requiring ICU admission (mean RR: 1.93, 95% CI, 1.46 to 2.55; p < 0.001) or mechanical ventilation (mean RR: 1.90, 95% CI, 1.34 to 2.69; p < 0.001) were both twice as likely to develop VTE events. There was a significant variation in true effect sizes based on a high level of heterogeneity (I2 = 62% critically ill group, I2 = 82% ICU admission group, I2 = 81% mechanical ventilation group). Patients with VTE events had a mean P/F ratio 53 points less than those who did not have VTE (raw difference in mean: −52.95, 95% CI, −91.27 to −14.63; p = 0.007; I2 = 72%).
VTE events were not associated with increased mortality among COVID-19 patients (mean RR: 0.99, p = 0.98, I2 = 44%). COVID-19 patients with VTE events had a higher mean length of stay (LOS) of approximately 4 days compared with patients without VTE (raw difference in mean: 3.9, p = 0.038). Patients with VTE events had statistically significant higher mean D-dimer levels as compared with patients who did not have VTE (raw difference in mean: 3883, 95% CI, 3050 to 4715; p < 0.001). The prediction interval was widely distributed among studies, hence a high level of heterogeneity (I2 = 96%).
Other coagulation markers such as prothrombin time-international normalized ratio (PT/INR), activated partial thromboplastin time (APTT), and fibrinogen were no different in those with and without VTE (Supplementary File). VTE patients had higher mean leukocyte count (raw difference in mean: 1.11, p < 0.001), C-reactive protein (CRP) levels (raw difference in mean: 4.73, p = 0.005), and procalcitonin (raw difference in mean: 0.11, p < 0.001) compared with the group without VTE. There was no correlation between elevated ferritin (raw difference in mean: 93, p = 0.44), lactate dehydrogenase (raw difference in mean: 12.34, p = 0.52), and interleukin-6 (raw difference in mean: 16.32, p = 0.26) with incident VTE events. VTE events were also not associated with elevated cardiac markers such as troponin and brain natriuretic peptide (BNP).
Discussion
The present study is one of the most comprehensive systematic reviews of predictors of VTE events, including biochemical, demographic, comorbidity, and severity of illness, in patients with COVID-19 infection, and its association with mortality and LOS. To assess the publication bias, we did the funnel plot that demonstrated publication bias to be low among studies included in the meta-analysis.
Our analysis revealed the following key findings: (1) Overall prevalence of VTE was 20.7%. (2) Male sex was associated with a higher risk of VTE events. (3) The presence of traditional VTE risk factors such as previous history of VTE, smoking, and cancer were not associated with an increased risk of VTE events. (4) VTE events were significantly higher in those who were severely ill, required ICU admission, had low P/F ratio, and were mechanically ventilated. (5) The presence of chronic comorbidities like CVD, HF, renal disease, and pulmonary disease did not increase the risk of a VTE event. (6) Patients with VTE had higher D-dimer levels, leukocyte count, CRP, and procalcitonin. (7) Mortality was not affected by VTE events, but LOS was increased by 4 days. (8) Therapeutic and prophylactic AC were not protective.
The mechanism of thrombotic complications in COVID-19 infection is poorly understood. Thrombotic complications have been reported in 27–69% of patients with COVID-19 who are critically ill,5,8,34,42,43 which is higher than published VTE rates for H1N1 infection. 44 Studies have shown reduced mortality in COVID-19 patients with thromboprophylaxis.4,45,46 This finding has prompted the International Society of Thrombosis and Hemostasis (ISTH) to recommend systematic prophylaxis in all hospitalized patients with COVID-19 infection. 47 Whether thromboprophylaxis significantly reduces thrombotic complications is unknown, particularly across different illness severity and clinico-biologic spectrum.
About one in four ICU patients with COVID-19 are found to have VTE events without thromboprophylaxis. 21 There was a high incidence of VTE events in ICU patients with appropriate thromboprophylaxis even if they did not require initial ICU admission.7,8,18 Studies showing the mortality benefits of thromboprophylaxis are mostly confined to medically stable patients admitted in the general medical ward.4,45 In our analysis, we did not find a significant reduction in VTE events with either prophylactic or therapeutic AC. We also reported that the risk of VTE events is higher in critically ill, mechanically ventilated ICU patients and those with a low P/F ratio.
An autopsy series from COVID-19 patients suggests pulmonary intravascular coagulopathy (PIC) as the potential pathogenic mechanism of PE. 48 SARS-CoV-2 induced downregulation of ACE2 receptors with resulting upregulation of lymphocyte function is the contributing factor for PIC. 49 PE seen in the context of COVID-19 infection is segmental or subsegmental, suggesting the possibility of in situ microthrombi from severe inflammation described as immunothrombosis. 50 Another study supported the fact of immunothrombosis. By showing that most of the DVTs seen in the cohort are line-related, it was proposed that all segmental and subsegmental PE are related to immunothrombosis. 23 Another study reported CT finding of vascular occlusion within the alveolar infiltrates, suggesting alveolar and vascular inflammation as a potential cause of in situ thrombosis. 24 The study by Desborough et al. 23 has not found any evidence that anticoagulation effectively prevents the immunothrombosis associated with COVID-19. Another case series showed that the recommended prophylaxis dose of AC lacked efficacy in preventing VTEs in COVID-19 patients with advanced disease. 24
Similarly, in our study, we have not found any correlation among VTE events with prophylactic and therapeutic anticoagulation in COVID-19 infection. The possible explanation may be that the pathogenesis of the VTE is likely associated with hypercoagulability and inflammation. Although these factors may be interrelated, additional anti-inflammatory therapy may prove superior to the AC alone. On the contrary, the AC dose may be an important issue determining the effect of the treatment.
Recently, one study has shown that therapeutic enoxaparin has improved respiratory function and oxygen requirement for patients with COVID-19. Although the study did not show any data regarding VTE events, the data support the benefit of a therapeutic dose of AC, rather than prophylactic dose. 51
We found that elevated D-dimer is associated with higher rates of VTE events. D-dimer has demonstrated prognostic value in COVID-19 pneumonia with higher levels during admission predictive of illness severity and high mortality.52,53 Interestingly, elevated D-dimer does not necessarily correlate with other inflammatory markers, as highlighted in other studies. 18 D-dimer can also be used for prognostic information with decreasing trends suggestive of the effectiveness of the AC strategy in COVID-19 patients, as shown in a study by Cui et al. 21
Moreover, it appears that the risk of VTE in COVID-19-affected patients is unrelated to traditional risk factors such as previous VTE, cancer, and smoking. CRP and procalcitonin, which are markers of inflammation, are elevated in COVID-affected patients with VTE, further suggesting that active inflammation plays a significant role in clot generation. It appears that the thrombi generation in COVID-19 infection has dual pathology with inflammation and coagulopathy playing roles simultaneously. 13 AC only addresses the coagulopathy and may therefore be only partially protective for VTE events. In ICU patients with severe disease, active inflammation is probably a principal driver for thrombogenicity, explaining the lack of benefit from AC alone in this subset of patients. The potential beneficial role of steroids in COVID-19 patients can be explained by its anti-inflammatory action. 54 Whether a combination of steroids and therapeutic AC reduces VTE events and improves outcome in high-risk COVID-19 patients remains to be seen but should be the subject of further investigation.
In comparison to other meta-analysis published on this topic, our topic has some interesting unique features: (1) if we compare the other meta-analysis, studies that we had included, comprises a large number of patients (6053) in that specific timeline used here; (2) the pooled prevalence of VTE found in our study was higher than the other studies; and (3) we also found some new findings such as smokers having reduced risk of VTE, AC whether therapeutic or prophylactic not being protective, and no association between VTE events and inpatient mortality, which makes scope for further research.55–62
Our study has shown moderate to high degree of heterogeneity, which makes it difficult to interpret the results. Interestingly, other meta-analysis has shown that thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality in contrast to our findings. 61 But the study by Henrina et al. 60 showed that VTE in patients with COVID-19 was not associated with an increased in-hospital mortality like our study. Another meta-analysis has showed lower VTE prevalence in studies with mixed dosing of anticoagulation in comparison to studies with standard prophylactic dosing of anticoagulation. 62 We emphasize on the fact that more studies followed by guidelines are necessary to know the actual effect of the anticoagulation on the VTE events in COVID-19 patients.
Limitations
Our study has certain limitations that should be considered. Most of the studies are retrospective, resulting in a bias. Studies are heterogeneous with respect to study setting (stable versus critically ill patients), inclusion criteria (all COVID positive patients versus patients who had CTPA or CUS), anticoagulation used, and a limited degree of follow-up; during the analysis, many patients were still in the hospital. Routine screening use of CTPA and CUS as opposed to using these tests based on clinical suspicion can underestimate or overestimate results.
Conclusion
There is a high rate of VTE events in hospitalized patients with COVID-19 infection. This is particularly so in patients in the ICU and those who are critically ill. Predictors of VTE events are male sex, critical illness, high D-dimer, and a presence of higher inflammatory markers. Traditional risk factors for VTE and baseline comorbidity are not predictive for VTE events. There is possibly a dual mechanism of coagulopathy and inflammation in the generation of VTE in COVID-19 infection; thus, AC alone does not appear to be protective. There is no association between VTE events and inpatient mortality; however, VTE events are associated with increased LOS, which can further increase the risk of additional VTE events. More randomized controlled trials are required to better understand the predictors of VTE events and methods of VTE prevention in SARS-CoV-2 infection, including the combination role with immunomodulators such as steroids.
Supplemental Material
Supplemental material, sj-docx-1-tak-10.1177_17539447221105013 for Predictors and mortality risk of venous thromboembolism in patients with COVID-19: systematic review and meta-analysis of observational studies by Gaurav Agarwal, Adrija Hajra, Sandipan Chakraborty, Neelkumar Patel, Suman Biswas, Mark K. Adler and Carl J. Lavie in Therapeutic Advances in Cardiovascular Disease
Supplemental material, sj-docx-2-tak-10.1177_17539447221105013 for Predictors and mortality risk of venous thromboembolism in patients with COVID-19: systematic review and meta-analysis of observational studies by Gaurav Agarwal, Adrija Hajra, Sandipan Chakraborty, Neelkumar Patel, Suman Biswas, Mark K. Adler and Carl J. Lavie in Therapeutic Advances in Cardiovascular Disease
Footnotes
Ethics approval and consent to participate: Not applicable (retrospective study).
Author contributions: Gaurav Agarwal: Conceptualization; Data curation; Formal analysis; Methodology; Writing – original draft.
Adrija Hajra: Conceptualization; Writing – original draft; Writing – review & editing.
Sandipan Chakraborty: Conceptualization; Methodology; Supervision; Writing – original draft.
Neelkumar Patel: Investigation; Methodology.
Suman Biswas: Writing – original draft.
Mark K. Adler: Methodology; Supervision; Writing – original draft.
Carl J. Lavie: Supervision.
ORCID iD: Adrija Hajra  https://orcid.org/0000-0003-4972-9073
https://orcid.org/0000-0003-4972-9073
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Gaurav Agarwal, Jersey City Medical Center, Jersey City, NJ, USA.
Adrija Hajra, Jacobi Medical Center and Albert Einstein College of Medicine, Bronx, 2562 Laconia Avenue, Bronx, NY 10469, USA.
Sandipan Chakraborty, Miami Valley Hospital, Columbus, OH, USA.
Neelkumar Patel, The University of Kansas, Kansas City, KS, USA.
Suman Biswas, Rochester Regional Health, Rochester, NY, USA.
Mark K. Adler, Interfaith Medical Center, Brooklyn, NY, USA
Carl J. Lavie, Ochsner Clinical School, The University of Queensland School of Medicine, New Orleans, LA, USA
References
- 1. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med 2020; 58: 1116–1120. [DOI] [PubMed] [Google Scholar]
- 2. Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol 2020; 75: 2352–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020; 18: 1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost 2020; 18: 1743–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020; 46: 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bandyopadhyay D, Akhtar T, Hajra A, et al. COVID-19 pandemic: cardiovascular complications and future implications. Am J Cardiovasc Drugs 2020; 20: 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020; 191: 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tavazzi G, Civardi L, Caneva L, et al. Thrombotic events in SARS-CoV-2 patients: an urgent call for ultrasound screening. Intensive Care Med 2020; 46: 1121–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The Task Force for the management of COVID-19 of the European Society of Cardiology, European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 1—epidemiology, pathophysiology, and diagnosis. Eur Heart J 2022; 43: 1033–1058, 10.1093/eurheartj/ehab696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected. Interim guidance, 13 March 2020, https://apps.who.int/iris/handle/10665/331446
- 12. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 2020; 75: 2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hajra A, Mathai SV, Ball S, et al. Management of thrombotic complications in COVID-19: an update. Drugs 2020; 80: 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019; 10: ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 16. Bompard F, Monnier H, Saab I, et al. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J 2020; 56: 2001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020; 136: 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Artifoni M, Danic G, Gautier G, et al. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis 2020; 50: 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hippensteel JA, Burnham EL, Jolley SE. Prevalence of venous thromboembolism in critically ill patients with COVID-19. Br J Haematol 2020; 190: e134–e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen S, Zhang D, Zheng T, et al. DVT incidence and risk factors in critically ill patients with COVID-19. J Thromb Thrombolysis 2021; 51: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cui S, Chen S, Li X, et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res 2020; 192: 23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Desborough MJR, Doyle AJ, Griffiths A, et al. Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom. Thromb Res 2020; 193: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Faggiano P, Bonelli A, Paris S, et al. Acute pulmonary embolism in COVID-19 disease: preliminary report on seven patients. Int J Cardiol 2020; 313: 129–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J 2020; 41: 3058–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gervaise A, Bouzad C, Peroux E, et al. Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Eur Radiol 2020; 30: 6170–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grillet F, Behr J, Calame P, et al. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology 2020; 296: E186–E188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koleilat I, Galen B, Choinski K, et al. Clinical characteristics of acute lower extremity deep venous thrombosis diagnosed by duplex in patients hospitalized for coronavirus disease 2019. J Vasc Surg Venous Lymphat Disord 2021; 9: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larsen K, Coolen-Allou N, Masse L, et al. Detection of pulmonary embolism in returning travelers with hypoxemic pneumonia due to COVID-19 in Reunion Island. Am J Trop Med Hyg 2020; 103: 844–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Léonard-Lorant I, Delabranche X, Séverac F, et al. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to D-dimer levels. Radiology 2020; 296: E189–E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manjunath M, Miranda J, Fraenkel L, et al. Acute pulmonary embolism in critically ill patients with COVID-19. medRxiv 2020, https://www.medrxiv.org/content/10.1101/2020.05.22.20110270v1
- 32. Gómez CA, Sun CK, Tsai IT, et al. Mortality and risk factors associated with pulmonary embolism in coronavirus disease 2019 patients: a systematic review and meta-analysis. Sci Rep 2021; 11: 16025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaminetzky M, Moore W, Fansiwala K, et al. Pulmonary embolism at CT pulmonary angiography in patients with COVID-19. Radiol Cardiothorac Imaging 2020; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020; 18: 1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nahum J, Morichau-Beauchant T, Daviaud F, et al. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19). JAMA Netw Open 2020; 3: e2010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poyiadji N, Cormier P, Patel PY, et al. Acute pulmonary embolism and COVID-19. Radiology 2020; 297: E335–E338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ren B, Yan F, Deng Z, et al. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID-19 in Wuhan. Circulation 2020; 142: 181–183. [DOI] [PubMed] [Google Scholar]
- 38. Santoliquido A, Porfidia A, Nesci A, et al. Incidence of deep vein thrombosis among non-ICU patients hospitalized for COVID-19 despite pharmacological thromboprophylaxis. J Thromb Haemost 2020; 18: 2358–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stoneham SM, Milne KM, Nuttall E, et al. Thrombotic risk in COVID-19: a case series and case-control study. Clin Med 2020; 20: e76–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Whyte MB, Kelly PA, Gonzalez E, et al. Pulmonary embolism in hospitalised patients with COVID-19. Thromb Res 2020; 195: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang L, Feng X, Zhang D, et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: prevalence, risk factors, and outcome. Circulation 2020; 142: 114–128. [DOI] [PubMed] [Google Scholar]
- 42. Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 2020; 191: 148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020; 191: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bunce PE, High SM, Nadjafi M, et al. Pandemic H1N1 influenza infection and vascular thrombosis. Clin Infect Dis 2011; 52: e14–e17. [DOI] [PubMed] [Google Scholar]
- 45. Yin S, Huang M, Li D, et al. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis 2021; 51: 1107–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spyropoulos AC, Ageno W, Barnathan ES. Hospital-based use of thromboprophylaxis in patients with COVID-19. Lancet 2020; 395: e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 2020; 18: 1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 2020; 20: 1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McGonagle D, O’Donnell JS, Sharif K, et al. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol 2020; 2: e437–e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cattaneo M, Bertinato EM, Birocchi S, et al. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb Haemost 2020; 120: 1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lemos ACB, do Espírito Santo DA, Salvetti MC, et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: a randomized phase II clinical trial (HESACOVID). Thromb Res 2020; 196: 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alessi J, de Oliveira GB, Schaan BD, et al. Dexamethasone in the era of COVID-19: friend or foe? An essay on the effects of dexamethasone and the potential risks of its inadvertent use in patients with diabetes. Diabetol Metab Syndr 2020; 12: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Longchamp G, Manzocchi-Besson S, Longchamp A, et al. Proximal deep vein thrombosis and pulmonary embolism in COVID-19 patients: a systematic review and meta-analysis. Thrombosis J 2021; 19: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Suh YJ, Hong H, Ohana M, et al. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology 2021; 298: E70–E80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang R, Ni L, Di X, et al. Systematic review and meta-analysis of the prevalence of venous thromboembolic events in novel coronavirus disease-2019 patients. J Vasc Surg Venous Lymphat Disord 2021; 9: 289–298.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tan BK, Mainbourg S, Friggeri A, et al. Arterial and venous thromboembolism in COVID-19: a study-level meta-analysis. Thorax 2021; 76: 970–979. [DOI] [PubMed] [Google Scholar]
- 59. Gratz J, Wiegele M, Maleczek M, et al. Risk of clinically relevant venous thromboembolism in critically ill patients with COVID-19: a systematic review and meta-analysis. Front Med 2021; 8: 647917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Henrina J, Putra IC, Cahyadi I, et al. Clinical characteristics and outcomes of venous thromboembolism in patients hospitalized for COVID-19: systematic review and meta-analysis. Thrombosis Update 2021; 2: 100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Malas MB, Naazie IN, Elsayed N, et al. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine 2020; 29: 100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kollias A, Kyriakoulis KG, Lagou S, et al. Venous thromboembolism in COVID-19: a systematic review and meta-analysis. Vasc Med 2021; 26: 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tak-10.1177_17539447221105013 for Predictors and mortality risk of venous thromboembolism in patients with COVID-19: systematic review and meta-analysis of observational studies by Gaurav Agarwal, Adrija Hajra, Sandipan Chakraborty, Neelkumar Patel, Suman Biswas, Mark K. Adler and Carl J. Lavie in Therapeutic Advances in Cardiovascular Disease
Supplemental material, sj-docx-2-tak-10.1177_17539447221105013 for Predictors and mortality risk of venous thromboembolism in patients with COVID-19: systematic review and meta-analysis of observational studies by Gaurav Agarwal, Adrija Hajra, Sandipan Chakraborty, Neelkumar Patel, Suman Biswas, Mark K. Adler and Carl J. Lavie in Therapeutic Advances in Cardiovascular Disease