Abstract
Diffuse large B-cell lymphoma (DLBCL) represents the most common subtype of aggressive lymphoma. Depending on individual risk factors, roughly 60–65% of patients can be cured by chemoimmunotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). However, patients with primary refractory disease or relapse (R/R) after an initial response are still characterized by poor outcome. Until now, transplant-eligible R/R DLBCL patients are treated with intensive salvage regimens followed by high-dose chemotherapy and autologous stem cell transplantation (ASCT) which, however, only cures a limited number of patients. It is most likely that in patients with early relapse after chemoimmunotherapy, chimeric antigen receptor (CAR) T-cells will replace high-dose chemotherapy and ASCT. So far, transplant-ineligible patients have mostly been treated in palliative intent. Recently, a plethora of novel agents comprising new monoclonal antibodies, antibody drug conjugates (ADC), bispecific antibodies, and CAR T-cells have emerged and have significantly improved outcome of patients with R/R DLBCL. In this review, we summarize our current knowledge on the usage of novel drugs and approaches for the treatment of patients with R/R DLBCL.
Keywords: ADC, ASCT, bispecific antibodies, CAR T-cells, DLBCL, monoclonal antibodies, targeted therapies
Introduction
Diffuse large B-cell lymphoma (DLBCL) represents the most common lymphoma entity accounting for roughly 40% of all cases. 1 In the revised World Health Organization (WHO) classification, several subtypes previously summarized under the term ‘DLBCL’ now represent own entities such as primary mediastinal B-cell lymphoma, high-grade B-cell lymphoma with MYC and BCL2 and BCL6 rearrangement, T-cell histiocyte-rich B-cell lymphoma, or Epstein-Barr virus (EBV)-positive DLBCL to mention some of them. 1 In this review, we focus on the entity of ‘DLBCL not otherwise specified (NOS)’. However, it must be taken into account that various clinical DLBCL studies encompassed additional aggressive B-cell lymphoma subtypes which may have influenced the results of these trials.
Comprehensive molecular analyses showed that DLBCL represents a heterogeneous diagnostic category. Gene expression profiling defined two distinct subtypes derived from activated B-cell-like (ABC) or germinal center B-cell-like (GCB) DLBCL while roughly 15% of all DLBCL cases remained unclassifiable.2,3 Additional molecular subtypes within and beyond ABC and GCB DLBCL have been identified based on recurrent somatic mutations, copy number alterations, and structural variants.4–6
Despite these advances in the understanding of DLBCL lymphomagenesis, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) still represents the internationally accepted standard therapy of untreated DLBCL patients. Until recently, large phase III studies adding different agents to R-CHOP failed to significantly improve patients’ outcome.7–11 However, very recently, encouraging results of the POLARIX trial (NCT03274492) have been reported. 12 The combination of rituximab, cyclophosphamide, doxorubicin, and prednisone (R-CHP) and the antibody drug conjugate (ADC) polatuzumab-vedotin significantly improved progression-free survival (PFS) of untreated DLBCL patients with an international prognostic index (IPI) of 2–5 compared with R-CHOP. Also based on the evaluation and potential approval by the medical agencies, R-CHP and polatuzumab-vedotin might become the new standard of care of untreated DLBCL patients with an IPI of 2–5.
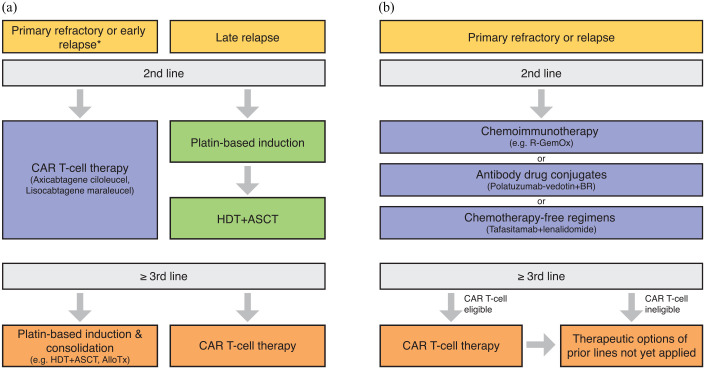
Overall, while roughly two-thirds of DLBCL patients can be cured by first-line therapy, one-third of the patients will be primary refractory or will relapse (R/R DLBCL) after an initial response. 13 These patients are characterized by poor outcome with the majority of patients succumbing to their disease. With a plethora of novel agents available, treatment algorithms become more and more complex and the sequencing of these approaches remains poorly studied. To this end, this review provides an overview of the current therapeutic algorithms for patients with R/R DLBCL (Figure 1).
Figure 1.
Therapeutic algorithm for patients with R/R DLBCL. (a) For transplant-eligible patients, depending on the time point of relapse, either an anti-CD19 CAR T-cell therapy (using axicabtagene ciloleucel or lisocabtagene maraleucel) or platin-based induction followed by high-dose therapy (HDT) and autologous stem cell transplantation (ASCT) represent the standard approach (*within 12 months after completion of first-line therapy). (b) For transplant-ineligible patients, chemoimmunotherapy, antibody drug conjugates, as well as chemotherapy-free regimens represent potential therapeutic options in second line. Third-line therapy using anti-CD19–directed CAR T-cells represents a potentially curative option for eligible patients.
Transplant-eligible patients
Primary refractory or patients relapsing after an initial response are characterized by adverse outcome. 14 Up to now, the clinical management of R/R patients largely depends on their eligibility for high-dose therapy (HDT) and autologous stem cell transplantation (ASCT). As of today, R/R patients considered transplant-eligible will normally receive platinum-based salvage chemoimmunotherapy with rituximab, gemcitabine, dexamethasone, and cisplatin (R-GDP); rituximab, ifosfamid, carboplatin, and etoposide (R-ICE); or rituximab, dexamethasone, high-dose cytarabine, and cisplatin (R-DHAP). All three regimens have been investigated prospectively achieving very similar results for the investigated outcome parameters.14,15 In the multicenter phase III CORAL trial, 396 DLBCL patients in first relapse or with primary refractory disease were assigned to either R-ICE or R-DHAP followed by HDT/ASCT. 14 Response rates for both regimens were 63.5% versus 62.8%, respectively. Overall, 3-year PFS and the overall survival (OS) were 37% and 49%, respectively, with no significant differences between the treatment arms.
In the NCIC-CTG LY.12 trial, 619 patients with R/R aggressive lymphoma were assigned to R-GDP or R-DHAP. Response rates following R-GDP were 45.2% and 44% after R-DHAP, respectively, while R-GDP showed a better toxicity profile with less febrile neutropenia and nausea. 15
If a partial remission (PR) or complete remission (CR) is achieved, consolidation with HDT/ASCT is recommended. The most often used HDT regimens are rituximab, carmustine, etoposide, cytarabine, and melphalan (R-BEAM) or rituximab, cyclophosphamide, etoposide, and carmustine (R-CVB). In the CORAL study, patients who underwent HDT/ASCT reached a 3-year PFS of 53%. 14 Importantly, survival significantly depended on the time between end of first-line therapy and relapse; patients with early relapse within the first 12 months after diagnosis had adverse outcome with 3-year OS of 39% compared with 64% for patients with a later relapse underscoring that especially patients with longer remissions and chemo-sensitive disease benefit from HDT/ASCT. 14 Patients not responding or relapsing after salvage therapy should be re-evaluated if cure remains the therapeutic goal. These patients frequently represent prime candidates for novel approaches, in particular for chimeric antigen receptor (CAR) T-cell therapies.
Very recently, the final results of three prospective randomized trials directly comparing the current standard of HDT/ASCT to CAR T-cell therapy in second-line therapy of transplant-eligible patients refractory to or relapsing early after R-CHOP-like therapy have been presented.16–18 In two out of three of these trials, CAR T-cell therapy was superior to HDT/ASCT so that in near future CAR T-cells might become the new standard of second-line therapy for patients with large B-cell lymphoma refractory to or relapsing early after R-CHOP-like therapy. Detailed results of these trials are presented in the section ‘Third-line therapy – Chimeric antigen receptor T cells’.
Transplant-ineligible patients
Chemoimmunotherapy
In the past, the vast majority of transplant-ineligible patients were treated in palliative intent with chemoimmunotherapeutic regimens such as rituximab, gemcitabine, and oxaliplatin (R-Gem-Ox) or bendamustine and rituximab (BR). However, these therapies most often failed to induce durable remissions. In a multicenter phase II trial, 59 R/R DLBCL patients received BR as salvage therapy. The overall response rate (ORR) was 62.7% with a CR rate of 37.3% and the median PFS was 6.7 months. 19 In an initial phase II trial including 46 transplant-ineligible R/R DLBCL patients, R-Gem-Ox showed very promising results with an ORR of 83% and a 2-year OS of 66%. 20 However, almost half of the included patients had not received prior rituximab treatment explaining the rather favorable outcome of these patients. 20 Accordingly, in another phase II trial including 49 transplant-ineligible R/R DLBCL patients who were pretreated with rituximab in 63% of cases, the 5-year OS rate following R-Gem-Ox salvage therapy was reported to be only 14%. 21
New monoclonal antibodies
The introduction of rituximab resulted in a significant improvement in survival of DLBCL patients.10,22–24 Besides, it also triggered the development of other monoclonal antibodies.
While the use of the anti-CD20 antibody obinutuzumab did not improve therapy of DLBCL patients, 25 the anti-CD19 antibody tafasitamab has been recently approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in combination with the immunomodulatory drug lenalidomide for transplant-ineligible patients with R/R DLBCL (Figure 2). 26 This approval is based on the recently published L-MIND trial (Table 1). 26 In this study, the combination of tafasitamab and lenalidomide was administered for up to 12 cycles every 28 days. Patients without progression after cycle 12 continued with a tafasitamab monotherapy until progression. Median age of the included study patients was 72 years and these patients had received a median of two prior lines of therapy with roughly half of patients being refractory to their last treatment. However, importantly, patients with primary refractory disease and patients with a lymphoma harboring a MYC and BCL2 and/ or BCL6 rearrangement were excluded from this study, indicating a bias toward good risk patients. Nevertheless, 48 of 80 patients (60%) showed an objective response with 43% achieving a CR and 18% a PR. 26 In a recently published follow-up study, the median duration of response was 43.9 months and median OS was 33.5 months. 27 Therapy was generally well tolerated with the most common nonhematological side effects being rash, diarrhea, asthenia, cough, peripheral edema, and fever. For the majority of transplant-ineligible patients, the combination of tafasitamab and lenalidomide represents a promising new treatment option. To what extent the anti-CD19 therapy might impair the efficacy of following CD19-targeting CAR T-cells needs further studies.
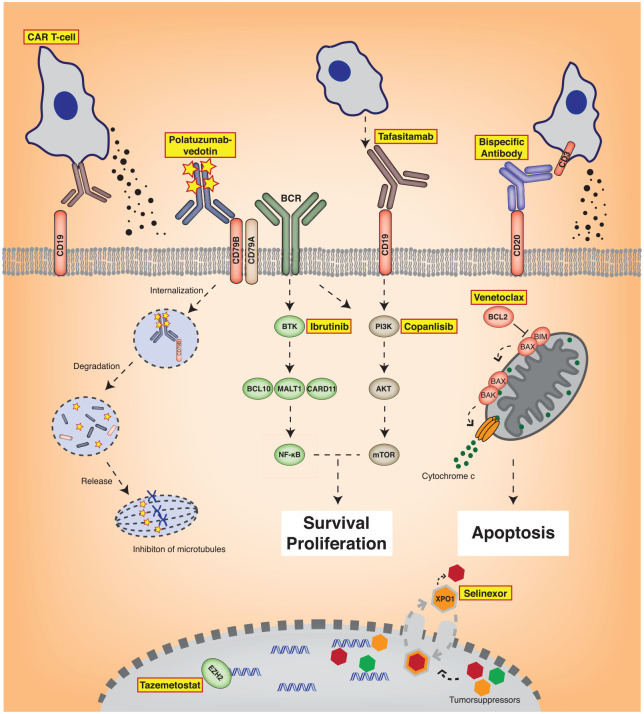
Figure 2.
Novel agents for the treatment of patients with R/R DLBCL. Depicted are targets of novel therapeutic approaches. The surface antigen CD19 can be recognized by CD19-targeting CAR T-cells or by novel antibodies such as tafasitamab. Polatuzumab-vedotin binds to CD79B associated with the B-cell receptor (BCR). Bispecific antibodies can directly link B- and T cells mediating T-cell activation. Targeted small-molecule inhibitors such as ibrutinib or copanlisib inhibit BCR or PI3K signaling, respectively. Venetoclax inhibits the anti-apoptotic protein BCL2 inducing apoptosis. Tazemetostat targets the oncogenic methyltransferase EZH2. Selinexor interrupts the export of diverse tumorsuppressor proteins out of the nucleus by inhibition of XPO1 leading to nuclear accumulation and activation of these tumor suppressors.
Table 1.
Overview of novel therapeutic options in the management of R/R DLBCL patients.
| Class and agent | Target | Study | Trial phase | Number of patients | ORR (%) | CRR (%) | Median PFS (months) | Median DOR (months) | Most frequent adverse events a |
|---|---|---|---|---|---|---|---|---|---|
| Monoclonal antibodies | |||||||||
| Tafasitamab + lenalidomide | CD19 | Salles et al. 26 | II | 80 (incl. transformation) | 60 | 43 | 12.1 | 21.7 | TEAEs (grade ⩾3): neutropenia (48%), thrombocytopenia (17%), febrile neutropenia (12%), rash (9%), anemia (7%) |
| Antibody drug conjugates | |||||||||
| Polatuzumab-vedotin + BR | CD79B | Sehn et al. 28 | II | 40 | 45 | 40 | 9.5 | 12.6 | AEs (grade 3–4): neutropenia (46%), thrombocytopenia (41%), anemia (28%), febrile neutropenia (10%) |
| Loncastuximab-tesirine | CD19 | Caimi et al. 29 | II | 145 (incl. PMBL, HGBCL) | 48.3 | 24.1 | – | 10.3 | TEAEs (grade ⩾3): neutropenia (26%), thrombocytopenia (18%), anemia (10%) |
| CAR T-cell therapy | |||||||||
| Axicabtagene ciloleucel | CD19 | Neelapu et al. 30 | II | 101 (incl. PMBL, transformed FL) | 82 | 54 | – | 8.1 | AEs (grade ⩾3): neutropenia (78%), anemia (43%), thrombocytopenia (38%), febrile neutropenia (31%), neurologic events (28%), CRS (13%), hyponatremia (10%) |
| Tisagenlecleucel | CD19 | Schuster et al. 31 | II | 93 (incl. transformed FL, HGBCL) | 52 | 40 | – | Not reached | AEs (grade 3–4, <8 weeks after infusion): cytopenia (not resolved by day 28) (32%), CRS (22%), infections (20%), febrile neutropenia (15%), neurologic events (12%) |
| Lisocabtagene maraleucel | CD19 | Abramson et al. 32 | II | 256 (incl. transformed FL, HGBCL) | 73 | 53 | 6.8 | Not reached | TEAEs (grade ⩾3): neutropenia (60%), anemia (37%), thrombocytopenia (27%), hypophosphatemia (6%); neurologic events (10%), CRS (grade 3–4) (2%) |
| Bispecific T-cell engagers | |||||||||
| Blinatumomab | CD19/CD3 | Viardot et al. 33 | II | 21 (incl. transformation) | 43 | 19 | 3.7 | 13.5 | AEs (grade ⩾3): neurologic events (22%), leukopenia (17%), thrombocytopenia (17%), pneumonia (13%), hyperglycemia (9%) |
| Mosunetuzumab | CD20/CD3 | Schuster et al. 34 | I/Ib | 124 (R/R aggressive lymphoma) | 37.1 | 19.4 | – | – | AEs (grade 3): neurologic events (3%), CRS (1%) |
| Glofitamab | CD20/CD3 | Hutchings et al. 35 | I | 73 | 41.1 | 28.8 | 2.9 b | 5.5 b | AEs (grade ⩾3): neutropenia (25%), thrombocytopenia (8%), anemia (8%), CRS (4%) |
| Epcoritamab | CD20/CD3 | Hutchings et al. 36 | I/II | 22 | 68 | 45 | 9.1 | – | TEAEs (grade ⩾3): anemia (13%), pyrexia (6%), hypotension (6%), fatigue (6%) |
| Immune checkpoint inhibitors | |||||||||
| Nivolumab | PD-1 | Ansell et al. 37 | II | 121 (incl. transformation) | 10/3 c | 3/0 c | 1.9/1.4 c | 11.4/8 c | Drug-related AEs (grade 3–4): neutropenia (4%), thrombocytopenia (3%), lipase increased (3%), fatigue (2%) |
| Magrolimab | CD47 | Advani et al. 38 | Ib | 15 (incl. transformation, HGBCL) | 40 | 33 | – | Not reached | SAEs (all grades): infections (18%), anemia (5%), dyspnea (5%), pyrexia (5%), lactic acidosis (5%), retroperitoneal mass (5%), pulmonary embolism (5%), infusion-related reaction (5%) |
| Other targeted approaches | |||||||||
| Ibrutinib | BTK | Wilson et al. 39 | I/II | 80 | 25 | 10 | 1.6 | – | TEAEs (grade ⩾3): fatigue (8%), hyponatremia (7%), thrombocytopenia (6%), anemia (5%) |
| Lenalidomide | Immunomodulatory | Czuczman et al. 40 | II/III | 51 | 27.5 | 9.8 | 3.4 | 18.5 | TEAEs (grade ⩾3): neutropenia (43%), anemia (19%), infections (19%), respiratory/thoracic/mediastinal disorders (19%), thrombocytopenia (17%), gastrointestinal disorders (17%), febrile neutropenia (7%) |
| Copanlisib | PI3K | Lenz et al. 41 | II | 67 (incl. transformed FL, HGBCL) | 19.4 | 7.5 | 1.8 | 4.3 | TEAEs (grade ⩾3): hypertension (33%), hyperglycemia (31%), neutropenia (13%), hypokalemia (6%) |
| Venetoclax | BCL2 | Davids et al. 42 | I | 34 (incl. PMBCL) | 18 | 12 | 1.0 | – | Emergent AEs (grade 3–4): anemia (15%), neutropenia (11%), fatigue (7%) |
| Tazemetostat | EZH2 | Ribrag et al. 43 | II | 157 | 17 | 3/9 d | 4/2 d | 11/7 d | TEAEs (all grades): thrombocytopenia (20%), nausea (17%), anemia (15%), neutropenia (15%), vomiting (15%), cough (14%), diarrhea (12%), fatigue (12%), pyrexia (12%), abdominal pain (11%), asthenia (10%) |
| Selinexor | XPO1 | Kalakonda et al. 44 | II | 127 (incl. transformation, HGBCL) | 28 | 12 | 2.6 | 9.3 | TEAEs (grade 3–4): thrombocytopenia (46%), neutropenia (25%), anemia (22%), fatigue (11%), hyponatremia (8%) |
AE, adverse event; BR, bendamustine and rituximab; BTK, Bruton’s tyrosine kinase; CAR, chimeric antigen receptor; CRR, complete response rate; CRS, cytokine release syndrome; DLBCL, diffuse large B-cell lymphoma; DOR, duration of response; FL, follicular lymphoma; HGBCL, high-grade B-cell lymphoma with rearrangement of MYC and BCL2 and/ or BCL6; incl., including; MCL, mantle cell lymphoma; ORR, overall response rate; PD, programmed cell death proteins; PFS, progression-free survival; PI3K, phosphatidylinositol 3-kinase; PMBL, primary mediastinal B-cell lymphoma; R/R, primary refractory/relapsed; SAE, severe adverse event; TEAE, treatment-emergent adverse event; XPO1, exportin 1.
Percentages refer to the whole study population treated with the respective drug of interest.
Referring to patients with aggressive lymphoma including DLBCL, FL grade 3B, MCL, PMBCL, transformation.
Transplant-failed/transplant-ineligible patients.
Mutated/wild-type EZH2.
ADC
ADC represent a novel class of anticancer agents. The first ADC integrated into routine treatment of lymphoma patients was brentuximab-vedotin that targets the CD30 antigen which is commonly expressed on Hodgkin lymphoma or anaplastic large T-cell lymphoma cells. 45
Polatuzumab-vedotin is an ADC binding to CD79B, a B-cell receptor (BCR)-associated protein expressed by more than 95% of DLBCLs. 46 The molecule is linked to the cytotoxic anti-microtubule agent monomethyl auristatin E (MMAE). Upon binding to CD79B, polatuzumab-vedotin is internalized and MMAE is released causing cell death (Figure 2). 46 In a phase II study, 39 patients with R/R DLBCL were treated with polatuzumab-vedotin combined with rituximab until either disease progression or until unacceptable toxicities occurred. 47 The ORR was 54% with 21% of patients achieving a CR while the median duration of response was 13.4 months. Most common adverse events were hematological toxicities and diarrhea. 47 Based on these favorable results, 80 transplant-ineligible DLBCL patients with R/R disease were randomized in a phase II trial to either the combination of polatuzumab-vedotin, rituximab, and bendamustine or to rituximab and bendamustine alone (Table 1). 28 Patients included in the trial had received a median of two prior lines of therapy and 80% of patients were refractory to the last treatment. The addition of polatuzumab-vedotin to rituximab and bendamustine significantly improved both PFS and OS. The median OS was improved from 4.7 months in patients treated with rituximab and bendamustine alone to 12.4 months in the polatuzumab-vedotin, rituximab, and bendamustine arm. The median duration of response was improved by the addition of polatuzumab-vedotin to 12.6 months compared with 7.7 months. The combination of polatuzumab-vedotin, rituximab, and bendamustine was associated with a higher rate of cytopenias. However, this did not lead to more infections or increased need of transfusions. The polyneuropathy caused by polatuzumab-vedotin was in general manageable and reversible. 28 Based on these results, the combination of polatuzumab-vedotin, rituximab, and bendamustine has recently been approved by both EMA and FDA.
Another ADC that has shown promising activity in the treatment of patients with R/R DLBCL is loncastuximab-tesirine (ADCT-402). Loncastuximab-tesirine comprises anti-CD19 antibody and pyrrolobenzodiazepine (PBD) dimers. 48 PBD is a cytotoxic sequence-selective DNA cross-linking agent. In a recently completed phase II study including 145 DLBCL patients with R/R disease, nearly half of patients showed a response (ORR = 48.3%) with 24.1% of patients achieving a CR (Table 1). 29 Most common side effects were cytopenia and increase of liver enzymes. Loncastuximab-tesirine was subsequently approved by the FDA for the treatment of patients previously treated with at least two prior systemic lines with R/R large B-cell lymphoma including DLBCL NOS, DLBCL arising from indolent lymphoma, and high-grade B-cell lymphoma.
Third-line therapy–CAR T-cells
CAR T-cells represent a new therapeutic concept for the treatment of patients with different B-cell lymphomas. CAR T-cells are curative for R/R DLBCL patients having either relapsed after ASCT or for transplant-ineligible patients. Thus, CAR T-cells represent the current standard of care for patients having received two previous lines of therapy.
Autologous T cells are genetically modified to express a CAR. 49 Each CAR consists of an antibody-derived single-chain variable fragment targeting a specific tumor antigen, a spacer and linker region, as well as a cytoplasmatic domain inducing T-cell response and regulating the persistence of CAR T-cells. 49 The currently licensed and clinically available CAR T-cells all target the CD19 antigen, but differ by their cytoplasmatic co-stimulatory domains (Figure 2). Unfortunately, early CAR T-cell experience showed that harvesting sufficient numbers of viable patients’ T cells may be compromised by previous therapies. Furthermore, organizational and process-related problems can prolong the time from leukapheresis to product delivery to an extent unacceptable for R/R DLBCL patients with rapidly progressing disease in need of immediate therapy. Therefore, allogeneic CAR T-cells which can be used ‘off the shelf’ are also under investigation. 50 Before infusion of CAR T-cells, a T cell–depleting chemotherapy mostly consisting of fludarabine and cyclophosphamide is administered.51,52 When infused to patients, the CAR T-cells can induce multiple side effects, the most significant being the so-called cytokine release syndrome (CRS) as a form of systemic inflammatory response syndrome (SIRS) and neurologic symptoms ranging from mild cognitive impairment to unconsciousness and coma.30–32 These symptoms in most instances are fully reversible and can be controlled by the early use of glucocorticoids and the interleukin 6 (IL-6) receptor antagonist tocilizumab.30–32
Three different autologous CAR T-cells have been investigated in larger clinical studies. The efficacy and safety of axicabtagene ciloleucel, which was approved in 2017 by the FDA and 2018 by the EMA, were tested in the multicenter single-arm phase II trial ZUMA-1 (Table 1). 30 In the ZUMA-1 trial, axicabtagene ciloleucel was administered to 101 patients with R/R DLBCL, primary mediastinal B-cell lymphoma, or transformed follicular lymphoma. The median age of patients was 58 years, 69% had received more than three prior lines of therapy, and roughly 80% of patients had refractory disease. The median time from leukapheresis to delivery was 17 days and a bridging therapy was not allowed. Overall, 82% of patients showed an objective response with 54% reaching a CR. 30 In a recently presented long-term follow-up, the 3-year OS still was 47% and the median OS was 25.8 months. 53 With respect to safety, 13% of patients developed a CRS of grade 3 or higher, whereas 28% of patients showed neurologic symptoms of grade 3 or higher. 30 The median time from infusion to onset of CRS and neurologic symptoms was 2 and 5 days, respectively. Nearly all CRS and neurologic events resolved. 30
Tisagenlecleucel represents another CAR T-cell product approved by FDA and EMA. 31 As axicabtagene ciloleucel, tisagenlecleucel received approval for adult patients with R/R DLBCL after two previous lines of systemic therapy by EMA. In the multicenter, international phase II JULIET trial, 93 patients with R/R DLBCL, transformed follicular lymphoma, or high-grade B-cell lymphoma with MYC, BCL2, and BCL6 rearrangement ineligible for or progressive after HDT/ASCT were treated with tisagenlecleucel (Table 1). The median age of patients was 56 years, 52% had received at least three prior lines of therapy, and 55% showed refractory disease. The median time from trial enrollment to infusion of CAR T-cells was 54 days. Best ORR was 52%, 40% of patients achieved a CR. CRS and neurologic events (both ⩾grade 3) occurred in 22% and 12% of patients, respectively. In total, 97% of all CRS events had resolved at the time of data cutoff. 31 In a recently published long-term follow-up, the median duration of response was not reached with a median follow-up of 40.3 months and the proportion of patients maintaining their response at 36 months was estimated at 60.4%. 54
Various real-life data sets confirm the promising data from the ZUMA-1 and the JULIET trials.55–57 In a recent retrospective analysis, 275 patients were treated with axicabtagene ciloleucel across 17 US centers. 55 Median time from apheresis to start of conditioning chemotherapy was 21 days. In contrast to patients treated in the ZUMA-1 trial, 53% of patients received a bridging therapy. The ORR was 82% while 64% of patients achieved a CR. With a median observation time of 12.9 months, the median PFS was 8.3 months. Rates of CRS and neurologic events were comparable with the frequency and severity observed in the ZUMA-1 trial. Patients with elevated lactate dehydrogenase (LDH) were characterized by significantly inferior PFS and OS suggesting that tumor burden and dynamics of progressive disease before CAR T-cells is an important prognostic factor. 55 Recently, data from the French national registry DESCAR-T have been presented. 57 In total, 463 patients who received commercial CAR T-cells at 14 French centers were included in this analysis. Median age was 63 years and patients received a median number of three prior lines of therapy. Overall, 65% of patients received axicabtagene ciloleucel and 35% were treated with tisagenlecleucel. Best ORR was 70.2% with 38% of patients achieving a CR and 27% showing a PR at day 30 after CAR T-cell infusion. Of patients who achieved CR at day 30, 61% maintained their CR at day 90. 57
Finally, lisocabtagene maraleucel represents the third CAR T-cell product that has been approved by the FDA in February 2021 for patients with R/R large B-cell lymphoma after two lines of therapy. In the single-arm, multicenter TRANSCEND trial, 256 patients with R/R DLBCL (including transformation from any indolent lymphoma) or high-grade B-cell lymphoma with MYC, BCL2, and BCL6 rearrangement were infused with lisocabtagene maraleucel and included in the efficacy analysis (Table 1). 32 The median age of patients was 63 years. In total, 73% of patients showed an objective response and 53% achieved a CR. Median PFS was 6.8 months. With a median follow-up of 12 months, median duration of response was not reached. Severe CRS or neurologic events (⩾grade 3) occurred in 2% and 10% of patients, respectively. Median time from leukapheresis to product delivery was 24 days. 32 In a recent presentation, preliminary data of the phase II OUTREACH trial showed comparable results in terms of efficacy and safety when lisocabtagene maraleucel was administered in an in- or outpatient setting. 58
Recently, the available CAR T-cell therapies have prospectively been compared with the current standard care of HDT/ASCT in second-line therapy. The ZUMA-7 trial was an international phase III trial randomly assigning 180 patients with refractory large B-cell lymphoma or early relapse within 12 months after front-line chemoimmunotherapy to axicabtagene ciloleucel and 179 patients to receiving standard care therapy. 16 Standard care was defined as two or three cycles of salvage therapy followed by HDT/ASCT for responding patients. As in the ZUMA-1 trial, only glucocorticoids have been allowed for bridging to CAR T-cells. Overall, 83% of patients treated with axicabtagene ciloleucel and 50% of patients receiving HDT/ASCT showed a response with CR rates of 65% and 32%, respectively. 16 The primary end point of event-free survival (EFS) significantly differed between treatment groups with a 2-year EFS of 41% in the CAR T-cell arm versus 16% in the HDT/ASCT group (hazard ratio for event or death 0.4, p < 0.001). Two-year OS was 61% versus 52%, respectively. The reported rates of toxicity corresponded to previous reports: severe CRS (⩾grade 3) occurred in 6% and neurologic toxicity (⩾grade 3) in 21% of cases. 16
The TRANSFORM trial was a global phase III trial randomizing 184 patients with primary refractory large B-cell lymphoma or early relapse within 12 months after front-line therapy to either standard care comprising salvage therapy and HDT/ASCT or CAR T-cell therapy with lisocabtagene maraleucel. 18 Baseline characteristics were well comparable between treatment groups. Patients receiving lisocabtagene maraleucel showed an ORR of 86% compared with 48% for patients receiving HDT/ASCT. In line with the data of the ZUMA-7 trial, the median EFS was significantly improved for patients of the CAR T-cell group with 10.1 months compared with 2.3 months in the standard care group (hazard ratio 0.349, p < 0.0001). 18 Median OS was not reached versus 16.4 months, respectively.
Finally, the BELINDA trial investigated the role of tisagenlecleucel in second-line therapy. 17 In this international phase III trial, 322 patients with R/R aggressive B-cell lymphoma were randomized between salvage therapy followed by HDT/ASCT and tisagenlecleucel including optional prior bridging therapy. As in the ZUMA-7 and TRANSFORM trials, eligible patients were primary refractory or showed early relapse within 12 months after front-line therapy. In total, 32.5% of patients in the standard care group responded to salvage therapy and received subsequently HDT/ASCT. In total, 95.7% of patients randomized to the CAR T-cell group received tisagenlecleucel. In contrast to the JULIET trial, patients were not excluded from CAR T-cell infusion in case of progressive disease following bridging therapy. Overall, 46.3% of patients treated with tisagenlecleucel showed a response compared with 42.5% of patients belonging to the HDT/ASCT group. The primary end point EFS reached a median of 3 months in both treatment groups (hazard ratio for event or death in the tisagenlecleucel group 1.07, p = 0.61). 17 Severe CRS and neurotoxicity (⩾grade 3) occurred in 5.2% and 1.9% after tisagenlecleucel infusion, respectively.
The encouraging results of the ZUMA-7 and TRANSFORM trials show that CAR T-cell therapy might evolve as the new standard for the treatment of patients with R/R DLBCL relapsing early after chemoimmunotherapy. However, the results of the BELINDA trial also underscore that selection of suitable patients is of major importance for the success of CAR T-cell therapies. 59 When further analyzing and comparing these trials, it has to be considered that different populations of patients have been included in these studies. Based on the yet available data, it is premature to conclude that one CAR T-cell product is superior to the other, although this also cannot be excluded at this stage. In the ZUMA-7 trial, only glucocorticoids were allowed as bridging therapy before CAR T-cell infusion and patients with impending organ-compromising disease were excluded leading – at least to a certain degree – to a selection bias toward patients with less aggressive disease. 16 In the ZUMA-7 trial, time from leukapheresis to final product release took only 13 days. 16 In contrast, multiple rounds of bridging therapy were allowed and patients with impending organ-compromising disease were not excluded from the BELINDA trial. 17 Despite bridging therapy, 26% of patients showed progressive disease before CAR T-cell therapy. Median time from leukapheresis to CAR T-cell infusion was significantly longer with 52 days. 17 Moreover, baseline characteristics of patients show that significantly more patients with DLBCL of the ABC subtype, that are characterized by adverse prognosis, have been included in the BELINDA compared with the ZUMA-7 trial (32% versus 9%).16,17
Allogeneic stem cell transplantation
Allogeneic stem cell transplantation has been used in the treatment of patients with R/R DLBCL especially after failure of HDT and ASCT.60,61 However, with a plethora of novel alternative treatment options emerging, the role of allogeneic stem cell transplantation remains to be defined.
The curative potential of allogeneic stem cell transplantation is in part caused by the immune effect exerted by donor T cells termed graft versus lymphoma (GvL) effect. However, in DLBCL, the GvL effect seems less prominent compared with other lymphoma entities reflected by relatively high relapse rates. 62 In addition, graft versus host disease (GvHD) of various severities can occur in any allografted patient despite successful recipient/donor matching resulting in significant morbidity and mortality. 63 Registry data of R/R DLBCL patients who underwent allogeneic stem cell transplantation reported 3-year OS rates ranging from 30 to 50%.61,64,65 The only prospective trial evaluating the role of allogeneic stem cell transplantation for R/R DLBCL patients was the DSHNHL 2004-R3 trial in which 84 lymphoma and 42 DLBCL patients were included. 66 In total, 55% of enrolled patients showed refractory disease. For the whole study population, the 3-year PFS and OS (post transplant) were 39% and 42%, respectively. The non-relapse mortality (NRM) rate at 1 year was 35%. Lower NRM and better OS were reported for patients with a 10/10 human leukocyte antigen (HLA)-matched donor and patients receiving anti-thymocyte globulin (ATG) for GvHD prophylaxis. Retrospective comparison evaluating the efficacy and safety of allogeneic stem cell transplantation and CAR T-cells suggests similar efficacy but substantially less toxicity after CAR T-cell therapies. 67
In summary, the exact role of allogeneic stem cell transplantation in the treatment algorithm of DLBCL patients needs to be defined and it may remain a valid option for fit patients relapsing after CAR T-cell therapy.
Novel therapeutic approaches
Bispecific antibodies
Bispecific T-cell engagers (BITEs) are antibodies comprising two antigen-binding sites, one directed against a tumor antigen and one directed against a T-cell antigen bringing T cells and tumor cells into direct proximity (Figure 2). 68 In early clinical trials, BITEs have shown very promising anti-lymphoma activity also in heavily pretreated patients.34–36
Blinatumomab was the first BITE approved for the treatment of patients with R/R and minimal residual disease (MRD)-positive acute lymphoblastic leukemia.69,70 Blinatumomab consists of two single-chain variable fragments directed against CD19 and CD3 but lacks its own Fc domain leading to a shorter halftime and therefore necessitating prolonged periods of continuous infusion. In a phase I/II trial, blinatumomab led to encouraging anti-lymphoma activity in intensively pretreated R/R DLBCL patients with ORR ranging from 43 to 55% with some long-term remissions (Table 1).33,71,72 However, the disadvantageous pharmacokinetics and the occurrence of serious neurological side effects in some patients led to the development of alternate BITEs.
Mosunetuzumab is a full-length humanized IgG bispecific antibody targeting CD20 and CD3. 73 In a multicenter phase I/Ib trial, 124 patients with R/R aggressive lymphomas including DLBCL and transformed follicular lymphoma were treated (Table 1). 34 Patients were heavily pretreated with a median of three prior lines of therapy including ASCT and CAR T-cell therapies. The response rate in the overall cohort was 37.1% while 22.2% of patients who were previously treated with CAR T-cells responded. In the overall cohort, roughly 20% of patients achieved a CR. Overall tolerability was good and only 1.1% of patients developed a severe CRS of grade 3 or higher.
Glofitamab is a novel bispecific, bivalent antibody containing two domains targeting CD20 and one domain directed against CD3. 74 In a phase I trial, 171 R/R lymphoma patients including 73 DLBCL patients were treated with glofitamab (Table 1). 35 Overall, the median age of patients included in the study was 64 years. Patients had received a median of three prior lines of therapy with 90.6% of patients being refractory to any prior treatment. In patients with R/R DLBCL, an ORR of 41.1% with a CR in 28.8% of cases was observed. Among patients with aggressive lymphoma, median PFS was short with 2.9 months. However, of patients in CR, 72.8% sustained a CR after 12 months. Again, the tolerability was good as a severe CRS (⩾grade 3) occurred in only 3.5% of patients.
Finally, epcoritamab that can be applied subcutaneously is a bispecific antibody targeting CD20 and CD3. 75 In an early clinical dose escalation study, epcoritamab showed high activity suggesting further evaluation in a phase I/III trial (Table 1). 36 In 22 evaluable R/R DLBCL patients, the ORR was 68% with 45% of patients achieving a CR. No dose-limiting effects were observed. Most frequent adverse events were pyrexia and local reactions at the injection site. Severe CRS did not occur.
In summary, bispecific antibodies have shown impressive efficacy in heavily pretreated patients with R/R DLBCL. However, these findings need to be confirmed in larger studies and longer follow-up is required to be able to fully appreciate if the observed responses represent indeed long-term remissions.
Immune checkpoint inhibitors
Drugs targeting immune checkpoints modulate immune responses and re-establish anti-lymphoma activity of the immune system. A well-explored axis is the interaction of programmed cell death proteins 1/2 (PD-1/2) and their respective ligands (PD-L1/2).76,77 By expression of PD-L1/2, cells are able to bind to PD-1/2 receptors expressed on B and T cells and to exert inhibitory effects. 78 Despite promising results observed in other lymphoma entities such as Hodgkin lymphoma, targeting PD-1 has not been successful in the treatment of DLBCL patients. 79 In a phase II trial, R/R DLBCL patients who were transplant-ineligible or who failed HDT/ASCT were treated with the anti-PD-1 monoclonal antibody nivolumab (Table 1). 37 Overall, in only 10% of patients following HDT and ASCT and in 3% of the transplant-ineligible patients, respectively, an objective response was observable. Median duration of response was 11 and 8 months, respectively. Potentially, the combination with conventional chemoimmunotherapy might improve results of checkpoint inhibitors in DLBCL. To this end, a large international phase III trial currently investigates the combination of rituximab, gemcitabine, oxaliplatin, and nivolumab in patients with R/R aggressive B- and T-cell lymphomas (NCT03366272).
Encouraging results were achieved in an early clinical trial for patients with R/R lymphoma including 15 DLBCL patients who were treated with the macrophage immune checkpoint inhibitor magrolimab (Hu5 F9-G4) in combination with rituximab (Table 1). 38 Magrolimab blocks CD47 signaling that induces an antiphagocytic signal. CD47 is highly expressed by a variety of tumor cells including lymphoma cells. Notably, 40% of R/R DLBCL patients showed an objective response with 33% achieving a CR. At a median follow-up of 6 months, more than 90% of responses were ongoing.
Targeted small-molecule inhibitors
A profound understanding of the molecular pathogenesis of DLBCL is required to incorporate specific pathway inhibitors into the treatment of affected patients. DLBCL is a heterogeneous diagnostic category comprising several distinct molecular subtypes. Based on gene expression profiling, at least two distinct subtypes termed activated B-cell-like (ABC) and germinal center B-cell-like (GCB) DLBCL have been identified.2,3,80 Follow-up work detected subtypes within and beyond ABC and GCB DLBCL based on recurrent mutations, copy number alterations, and structural variants.4–6 However, these subtypes are not just characterized by differences in genetic abnormalities and their gene expression profile but also by their dependencies to oncogenic pathways which can be targeted by specific inhibitors.
Over the last years, multiple agents have been developed to target specific genetic aberrations. Chronic active BCR signaling represents a hallmark of ABC DLBCL biology leading to constitutive activation of the oncogenic nuclear factor-kappa B (NF-κB) pathway (Figure 2). 81 The oral small-molecule ibrutinib is an irreversible inhibitor of the Bruton’s tyrosine kinase (BTK) which is centrally involved in BCR signaling. 82 In a phase I/II trial, 80 patients with R/R DLBCL were treated with ibrutinib monotherapy (Table 1). 39 Among patients with ABC DLBCL, 37% had an objective response compared with only 5% of subjects with GCB DLBCL. In particular, patients with concomitant mutations of CD79B and MYD88 showed high response rates following BTK inhibition.39,83
The phase III trial PHOENIX investigated whether the standard of R-CHOP chemoimmunotherapy in front line could be further improved by the addition of ibrutinib for non-GCB DLBCL patients. 7 Although this study did not meet its primary end point, a subgroup analysis revealed that younger patients (age < 60 years) showed a survival benefit following R-CHOP in combination with ibrutinib. 7 Moreover, in a recent analysis, patients with DLBCL of the MCD or N1 subtypes showed 3-year EFS of 100% following R-CHOP and ibrutinib. 84 In contrast, patients with DLBCLs belonging to the MCD or N1 subgroups who had received R-CHOP alone showed a significantly worse 3-year EFS of 42.9% and 50%, respectively. 84
The immunomodulatory drug lenalidomide exerts multiple different effects on the malignant B cells as well as on bystander cells.85–88 A preclinical study investigating the molecular mechanisms of lenalidomide efficacy in DLBCL cell lines suggested predominant activity of lenalidomide in ABC DLBCL compared with other molecular DLBCL subtypes. 89 In a clinical phase II/III trial including 102 R/R DLBCL patients treated with lenalidomide or investigator’s choice, the ORR was 27.5% following lenalidomide monotherapy. 40 In the lenalidomide arm, median PFS reached 15.1 weeks for patients with non-GCB DLBCL compared with 10.1 weeks for GCB DLBCL patients. Different lenalidomide combinations yielded encouraging results (see also ‘New monoclonal antibodies’). In a small clinical study, lenalidomide was used in combination with rituximab (four cycles for induction followed by lenalidomide maintenance for 8 months) for the treatment of elderly transplant-ineligible patients. 90 Complete response rate (CRR) at the end of maintenance was 35% with some patients responding long time to this combination. 90 Based on these encouraging data, the addition of lenalidomide to standard R-CHOP therapy was investigated in untreated DLBCL patients. A randomized phase II trial showed a PFS benefit for R-CHOP plus lenalidomide (R2-CHOP), 91 while the ROBUST phase III trial failed to show a significant difference between R2-CHOP and R-CHOP plus placebo for patients with ABC DLBCL. 8 The exact reasons for these discrepant results remain unclear, but might be related to patient selection (including all molecular DLBCL subtypes versus ABC DLBCL only) and lenalidomide dosing.
Another important downstream signaling cascade of the BCR and downstream target of CD19 signaling is the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway (Figure 2). Especially, DLBCLs of the ABC subtype are addicted to signaling via the alpha- and delta-isoforms of PI3K. 92 In a single-arm phase II study, 67 patients with R/R DLBCL were treated with single-agent copanlisib, a pan PI3K inhibitor with potent activity against the PI3K-α and PI3K-δ isoforms (Table 1). 41 The ORR was 19.4%, 31.6% in ABC, and 13.3% in GCB DLBCL patients. Median PFS and duration of response were 1.8 and 4.3 months, respectively. Studies investigating the efficacy of copanlisib in combination with R-CHOP are currently running (NCT04263584).
BCL2 is an important regulator of apoptosis (Figure 2). In roughly 35% of all GCB DLBCLs, BCL2 translocations are detectable, while roughly 38% of primary ABC DLBCL samples show BCL2 gains or amplifications.93,94 In a phase I trial enrolling 34 DLBCL patients, monotherapy with the orally bioavailable BCL2 inhibitor venetoclax led to an ORR of only 18% with a short median PFS of 1 month (Table 1). 42 Clinically relevant tumor lysis syndrome as particularly seen in the treatment of patients with chronic lymphocytic leukemia (CLL) did not occur. In the phase II CAVALLI trial, R-CHOP combined with venetoclax in first-line therapy showed a trend for improved PFS and OS compared with patients with similar characteristics treated on the GOYA study with R-CHOP alone. 95
Mutations of enhancer of zeste homolog 2 (EZH2) are a frequent genetic event in GCB DLBCLs. EZH2 represents a histone methyltransferase and recurrent activating mutations in the encoding gene were reported to enhance proliferation and to block further differentiation of germinal center B-cells. 96 In a multicenter phase II trial, 157 R/R DLBCL patients were treated with a monotherapy of the oral EZH2 inhibitor tazemetostat (Table 1). 43 The ORR was 17% of all patients regardless of the mutational status of EZH2. However, the median duration of response was significantly longer in patients with DLBCL harboring EZH2 mutation (11 months versus 7 months).
Another target for the treatment of DLBCL patients that has recently been described is the protein exportin 1 (XPO1) being involved in the transport of various proteins from the nucleus to the cytoplasm (Figure 2). The oral selective XPO1 inhibitor selinexor induces the accumulation of different tumor suppressor proteins in the nucleus re-establishing and enhancing their tumor-suppressive effects. 97 In a multicenter phase II trial, 127 patients with R/R DLBCL received a monotherapy with selinexor (Table 1). ORR was 28% with 12% of patients achieving a CR. 44 Median duration of response was 9.3 months. Based on these data, in June 2020, the FDA approved selinexor for the treatment of adult R/R DLBCL patients after two prior lines of systemic treatment.
In summary, different specific targeted agents have shown efficacy to a different degree. However, these responses were generally short term. Therefore, several trials aimed to reach higher efficiency by combining different agents and by exploiting synergistic effects. In a phase Ib trial, 45 patients were treated with the combination of rituximab, ibrutinib, and lenalidomide. The ORR was 44% and the CR was 28%. 98 In patients with non-GCB DLBCL, the ORR was 65% and the median duration of response was 15.9 months. The overall toxicity was manageable. In another phase Ib/II trial, patients with R/R B-cell lymphoma were treated with 2–6 cycles of venetoclax, ibrutinib, prednisone, obinutuzumab, and lenalidomide every 21 days. 99 In 27 patients with aggressive lymphoma, the ORR was 56% and the CR 37%. Overall, with a median follow-up of 13 months, 69% of responses were ongoing. Median PFS was 9 months and median OS was not reached. 99
As outlined above, distinct biological parameters such as gene expression profiles or specific genetic alterations emerge as biomarkers to predict the success of targeted therapeutic approaches. So far, most parameters do not belong to the routine diagnostic workup of patients with lymphoma. To select the most appropriate targeted agent for the treatment of patients with DLBCL, fast and cost-effective diagnostic assays applicable in clinical routine will be needed.
Conclusion and outlook
The therapeutic armamentarium for patients with R/R DLBCL has significantly improved in the last couple of years. Various novel antibodies, ADCs, specific small-molecule inhibitors, as well as CAR T-cells have been approved for the treatment of affected patients. However, the ideal sequence and combination of these and other new approaches remain to be elucidated in future clinical trials. In addition, our understanding why some patients respond to specific therapies while others are resistant is still very limited. In the same lines, it is still unclear which patients will benefit from which therapeutic approach. To this end, a significantly better understanding of the biology of DLBCL and how these novel agents and approaches influence DLBCL biology is critically warranted. Clarifying these crucial questions might pave the way for novel combinations and optimal sequencing of our therapies to cure more patients with R/R DLBCL.
Footnotes
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Fabian Frontzek: Conceptualization; Data curation; Investigation; Methodology; Project administration; Visualization; Writing – original draft.
Imke Karsten: Conceptualization; Investigation; Writing – original draft.
Norbert Schmitz: Conceptualization; Project administration; Writing – review & editing.
Georg Lenz: Conceptualization; Project administration; Supervision; Writing – review & editing.
ORCID iD: Fabian Frontzek  https://orcid.org/0000-0003-1705-3638
https://orcid.org/0000-0003-1705-3638
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Fabian Frontzek and Imke Karsten have no conflicts of interest to declare. Norbert Schmitz received financial support by BeiGene, research support by Janssen, travel grants and honoraria by Takeda, honoraria by Riemser/Esteve, and owns stock by BMS. Georg Lenz received research grants from AGIOS, AQUINOX, AstraZeneca, Bayer, Celgene, Gilead, Janssen, Morphosys, Novartis, Roche, and Verastem. GL received honoraria from Abbvie, Amgen, AstraZeneca, Bayer, BMS, Celgene, Constellation, Genmab, Gilead, Incyte, Janssen, Karyopharm, Morphosys, NanoString, Novartis, and Roche.
Availability of data and materials: Not applicable.
Contributor Information
Fabian Frontzek, Department of Medicine A for Hematology, Oncology, and Pneumology, University Hospital Münster, Münster, Germany.
Imke Karsten, Department of Medicine A for Hematology, Oncology, and Pneumology, University Hospital Münster, Münster, Germany.
Norbert Schmitz, Department of Medicine A for Hematology, Oncology, and Pneumology, University Hospital Münster, Münster, Germany.
Georg Lenz, Department of Medicine A for Hematology, Oncology, and Pneumology, University Hospital Münster, 48149 Münster, Germany.
References
- 1. Swerdlow SC, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Geneva: World Health Organization, 2017. [Google Scholar]
- 2. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000; 403: 503–511. [DOI] [PubMed] [Google Scholar]
- 3. Wright G, Tan B, Rosenwald A, et al. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci USA 2003; 100: 9991–9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 2018; 24: 679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med 2018; 378: 1396–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wright GW, Huang DW, Phelan JD, et al. A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell 2020; 37: 551–568.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol 2019; 37: 1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nowakowski GS, Chiappella A, Gascoyne RD, et al. ROBUST: a phase III study of lenalidomide plus R-CHOP versus placebo plus R-CHOP in previously untreated patients with ABC-type diffuse large B-cell lymphoma. J Clin Oncol 2021; 39: 1317–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies A, Cummin TE, Barrans S, et al. Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large B-cell lymphoma (REMoDL-B): an open-label, randomised, phase 3 trial. Lancet Oncol 2019; 20: 649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002; 346: 235–242. [DOI] [PubMed] [Google Scholar]
- 11. Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006; 7: 379–391. [DOI] [PubMed] [Google Scholar]
- 12. Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med 2022; 386: 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017; 130: 1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010; 28: 4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol 2014; 32: 3490–3496. [DOI] [PubMed] [Google Scholar]
- 16. Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med 2022; 386: 640–654. [DOI] [PubMed] [Google Scholar]
- 17. Bishop MR, Dickinson M, Purtill D, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med 2022; 386: 629–639. [DOI] [PubMed] [Google Scholar]
- 18. Kamdar MK, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel (liso-cel), a CD19-directed chimeric antigen receptor (CAR) T-cell therapy, versus standard of care (SOC) with salvage chemotherapy (CT) followed by autologous stem cell transplantation (ASCT) As second-line (2L) treatment in patients (Pts) with relapsed or refractory (R/R) large B-cell lymphoma (LBCL): results from the randomized phase 3 transform study. Blood 2021; 138: 91.33881503 [Google Scholar]
- 19. Ohmachi K, Niitsu N, Uchida T, et al. Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2013; 31: 2103–2109. [DOI] [PubMed] [Google Scholar]
- 20. El Gnaoui T, Dupuis J, Belhadj K, et al. Rituximab, gemcitabine and oxaliplatin: an effective salvage regimen for patients with relapsed or refractory B-cell lymphoma not candidates for high-dose therapy. Ann Oncol 2007; 18: 1363–1368. [DOI] [PubMed] [Google Scholar]
- 21. Mounier N, El Gnaoui T, Tilly H, et al. Rituximab plus gemcitabine and oxaliplatin in patients with refractory/relapsed diffuse large B-cell lymphoma who are not candidates for high-dose therapy. A phase II lymphoma study association trial. Haematologica 2013; 98: 1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pfreundschuh M, Kuhnt E, Trumper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011; 12: 1013–1022. [DOI] [PubMed] [Google Scholar]
- 23. Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 2008; 9: 105–116. [DOI] [PubMed] [Google Scholar]
- 24. Schmitz N, Nickelsen M, Ziepert M, et al. Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002-1). Lancet Oncol 2012; 13: 1250–1259. [DOI] [PubMed] [Google Scholar]
- 25. Vitolo U, Trneny M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol 2017; 35: 3529–3537. [DOI] [PubMed] [Google Scholar]
- 26. Salles G, Duell J, Gonzalez Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol 2020; 21: 978–988. [DOI] [PubMed] [Google Scholar]
- 27. Duell J, Maddocks KJ, Gonzalez-Barca E, et al. Long-term outcomes from the phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Haematologica 2021; 106: 2417–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2020; 38: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 2021; 22: 790–800. [DOI] [PubMed] [Google Scholar]
- 30. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017; 377: 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019; 380: 45–56. [DOI] [PubMed] [Google Scholar]
- 32. Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 2020; 396: 839–852. [DOI] [PubMed] [Google Scholar]
- 33. Viardot A, Goebeler ME, Hess G, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 2016; 127: 1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schuster SJ, Bartlett NL, Assouline S, et al. Mosunetuzumab induces complete remissions in poor prognosis non-hodgkin lymphoma patients, including those who are resistant to or relapsing after chimeric antigen receptor T-cell (CAR-T) therapies, and is active in treatment through multiple lines. Blood 2019; 134: 6.31273004 [Google Scholar]
- 35. Hutchings M, Morschhauser F, Iacoboni G, et al. Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J Clin Oncol 2021; 39: 1959–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hutchings M, Mous R, Clausen MR, et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet 2021; 398: 1157–1169. [DOI] [PubMed] [Google Scholar]
- 37. Ansell SM, Minnema MC, Johnson P, et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: a single-arm, phase II study. J Clin Oncol 2019; 37: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Advani R, Flinn I, Popplewell L, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N Engl J Med 2018; 379: 1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 2015; 21: 922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Czuczman MS, Trneny M, Davies A, et al. A phase 2/3 multicenter, randomized, open-label study to compare the efficacy and safety of lenalidomide versus investigator’s choice in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res 2017; 23: 4127–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lenz G, Hawkes E, Verhoef G, et al. Single-agent activity of phosphatidylinositol 3-kinase inhibition with copanlisib in patients with molecularly defined relapsed or refractory diffuse large B-cell lymphoma. Leukemia 2020; 34: 2184–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol 2017; 35: 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ribrag V, Morschhauser F, McKay P, et al. Interim results from an ongoing phase 2 multicenter study of tazemetostat, an EZH2 inhibitor, in patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL). Blood 2018;132(Suppl. 1): 4196. [Google Scholar]
- 44. Kalakonda N, Maerevoet M, Cavallo F, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol 2020; 7: e511–e522. [DOI] [PubMed] [Google Scholar]
- 45. Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol 2012; 30: 631–637. [DOI] [PubMed] [Google Scholar]
- 46. Dornan D, Bennett F, Chen Y, et al. Therapeutic potential of an anti-CD79b antibody-drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkin lymphoma. Blood 2009; 114: 2721–2729. [DOI] [PubMed] [Google Scholar]
- 47. Morschhauser F, Flinn IW, Advani R, et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol 2019; 6: e254–e265. [DOI] [PubMed] [Google Scholar]
- 48. Zammarchi F, Corbett S, Adams L, et al. ADCT-402, a PBD dimer-containing antibody drug conjugate targeting CD19-expressing malignancies. Blood 2018; 131: 1094–1105. [DOI] [PubMed] [Google Scholar]
- 49. Priceman SJ, Forman SJ, Brown CE. Smart CARs engineered for cancer immunotherapy. Curr Opin Oncol 2015; 27: 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Benjamin R, Graham C, Yallop D, et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet 2020; 396: 1885–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Klebanoff CA, Khong HT, Antony PA, et al. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol 2005; 26: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kochenderfer JN, Somerville RPT, Lu T, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol 2017; 35: 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jacobson C, Locke FL, Ghobadi A, et al. Long-term survival and gradual recovery of B cells in patients with refractory large B cell lymphoma treated with axicabtagene ciloleucel (Axi-Cel). Blood 2020; 136: 40–42. [Google Scholar]
- 54. Schuster SJ, Tam CS, Borchmann P, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2021; 22: 1403–1415. [DOI] [PubMed] [Google Scholar]
- 55. Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol 2020; 38: 3119–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Riedell PA, Walling C, Nastoupil LJ, et al. A multicenter retrospective analysis of outcomes and toxicities with commercial axicabtagene ciloleucel and tisagenlecleucel for relapsed/refractory aggressive B-cell lymphomas. Biol Blood Marrow Transplant 2020; 26: S41–S42. [Google Scholar]
- 57. Le Gouill S, Bachy E, Blasi R, et al. First results of DLBCL patients treated with CAR T-cells and enrolled in DESCAR-T registry, a French real-life database for CAR T-cells in hematologic malagnancies. Hematol Oncol 2021; 39: 084(S2). [Google Scholar]
- 58. Godwin JE, Freytes CO, Maris M, et al. Outcomes of treatment with the chimeric antigen receptor (CAR) T-cell therapy lisocabtagene maraleucel (liso-cel) in the nonuniversity setting: initial results from the Outreach Study. Blood 2020; 136: 1196. [Google Scholar]
- 59. Roschewski M, Longo DL, Wilson WH. CAR T-cell therapy for large B–cell lymphoma – who, when, and how? N Engl J Med 2022; 386: 692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fenske TS, Ahn KW, Graff TM, et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br J Haematol 2016; 174: 235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Kampen RJ, Canals C, Schouten HC, et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin’s lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J Clin Oncol 2011; 29: 1342–1348. [DOI] [PubMed] [Google Scholar]
- 62. Urbano-Ispizua A, Pavletic SZ, Flowers ME, et al. The impact of graft-versus-host disease on the relapse rate in patients with lymphoma depends on the histological subtype and the intensity of the conditioning regimen. Biol Blood Marrow Transplant 2015; 21: 1746–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dreger P, Sureda A, Ahn KW, et al. PTCy-based haploidentical vs matched related or unrelated donor reduced-intensity conditioning transplant for DLBCL. Blood Adv 2019; 3: 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rigacci L, Puccini B, Dodero A, et al. Allogeneic hematopoietic stem cell transplantation in patients with diffuse large B cell lymphoma relapsed after autologous stem cell transplantation: a GITMO study. Ann Hematol 2012; 91: 931–939. [DOI] [PubMed] [Google Scholar]
- 65. Robinson SP, Boumendil A, Finel H, et al. Autologous stem cell transplantation for relapsed/refractory diffuse large B-cell lymphoma: efficacy in the rituximab era and comparison to first allogeneic transplants. A report from the EBMT Lymphoma Working Party. Bone Marrow Transplant 2016; 51: 365–371. [DOI] [PubMed] [Google Scholar]
- 66. Glass B, Hasenkamp J, Wulf G, et al. Rituximab after lymphoma-directed conditioning and allogeneic stem-cell transplantation for relapsed and refractory aggressive non-Hodgkin lymphoma (DSHNHL R3): an open-label, randomised, phase 2 trial. Lancet Oncol 2014; 15: 757–766. [DOI] [PubMed] [Google Scholar]
- 67. Dreger P, Dietrich S, Schubert ML, et al. CAR T-cells or allogeneic transplantation as standard of care for advanced large B-cell lymphoma: an intent-to-treat comparison. Blood Adv 2020; 4: 6157–6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schuster SJ. Bispecific antibodies for the treatment of lymphomas: promises and challenges. Hematol Oncol 2021; 39(Suppl. 1): 113–116. [DOI] [PubMed] [Google Scholar]
- 69. Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 2017; 376: 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gokbuget N, Zugmaier G, Klinger M, et al. Long-term relapse-free survival in a phase 2 study of blinatumomab for the treatment of patients with minimal residual disease in B-lineage acute lymphoblastic leukemia. Haematologica 2017; 102: e132–e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Goebeler ME, Knop S, Viardot A, et al. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J Clin Oncol 2016; 34: 1104–1111. [DOI] [PubMed] [Google Scholar]
- 72. Dufner V, Sayehli CM, Chatterjee M, et al. Long-term outcome of patients with relapsed/refractory B-cell non-Hodgkin lymphoma treated with blinatumomab. Blood Adv 2019; 3: 2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sun LL, Ellerman D, Mathieu M, et al. Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med 2015; 7: 287ra70. [DOI] [PubMed] [Google Scholar]
- 74. Minson A, Dickinson M. Glofitamab CD20-TCB bispecific antibody. Leuk Lymphoma 2021; 62: 3098–3108. [DOI] [PubMed] [Google Scholar]
- 75. Engelberts PJ, Hiemstra IH, de Jong B, et al. DuoBody-CD3xCD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. EBioMedicine 2020; 52: 102625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 2012; 1: 1223–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Errico A. Immunotherapy: PD-1-PD-L1 axis: efficient checkpoint blockade against cancer. Nat Rev Clin Oncol 2015; 12: 63. [DOI] [PubMed] [Google Scholar]
- 78. Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26: 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015; 372: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002; 346: 1937–1947. [DOI] [PubMed] [Google Scholar]
- 81. Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010; 463: 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rawlings DJ, Scharenberg AM, Park H, et al. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science 1996; 271: 822–825. [DOI] [PubMed] [Google Scholar]
- 83. Phelan JD, Young RM, Webster DE, et al. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature 2018; 560: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wilson WH, Wright GW, Huang DW, et al. Effect of ibrutinib with R-CHOP chemotherapy in genetic subtypes of DLBCL. Cancer Cell 2021; 39: 1643–1653.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. McDaniel JM, Pinilla-Ibarz J, Epling-Burnette PK. Molecular action of lenalidomide in lymphocytes and hematologic malignancies. Adv Hematol 2012; 2012: 513702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. LeBlanc R, Hideshima T, Catley LP, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood 2004; 103: 1787–1790. [DOI] [PubMed] [Google Scholar]
- 87. Gorgun G, Calabrese E, Soydan E, et al. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood 2010; 116: 3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhu D, Corral LG, Fleming YW, et al. Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer Immunol Immunother 2008; 57: 1849–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yang Y, Shaffer AL, 3rd, Emre NC, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell 2012; 21: 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zinzani PL, Pellegrini C, Argnani L, et al. Prolonged disease-free survival in elderly relapsed diffuse large B-cell lymphoma patients treated with lenalidomide plus rituximab. Haematologica 2016; 101: e385–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol 2015; 33: 251–257. [DOI] [PubMed] [Google Scholar]
- 92. Erdmann T, Klener P, Lynch JT, et al. Sensitivity to PI3K and AKT inhibitors is mediated by divergent molecular mechanisms in subtypes of DLBCL. Blood 2017; 130: 310–322. [DOI] [PubMed] [Google Scholar]
- 93. Iqbal J, Sanger WG, Horsman DE, et al. BCL2 translocation defines a unique tumor subset within the germinal center B-cell-like diffuse large B-cell lymphoma. Am J Pathol 2004; 165: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lenz G, Wright GW, Emre NC, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci USA 2008; 105: 13520–13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood 2021; 137: 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Beguelin W, Popovic R, Teater M, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 2013; 23: 677–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tan DS, Bedard PL, Kuruvilla J, et al. Promising SINEs for embargoing nuclear-cytoplasmic export as an anticancer strategy. Cancer Discov 2014; 4: 527–537. [DOI] [PubMed] [Google Scholar]
- 98. Goy A, Ramchandren R, Ghosh N, et al. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non-germinal center B-cell-like DLBCL. Blood 2019; 134: 1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Melani C, Lakhotia R, Pittaluga S, et al. Phase 1b/2 study of vipor (venetoclax, ibrutinib, prednisone, obinutuzumab, and lenalidomide) in relapsed/refractory B-cell lymphoma: safety, efficacy and molecular analysis. Blood 2020: Abstract 598, https://ash.confex.com/ash/2020/webprogram/Paper141447.html