Abstract
Hepatitis B surface antigen (HBsAg) clearance is considered as functional cure in patients with chronic hepatitis B (CHB). This study aimed to assess the durability of HBsAg clearance achieved by interferon-based therapies in patients with CHB who were originally positive for hepatitis B envelope antigen (HBeAg). In this prospective study, HBeAg-positive CHB patients with confirmed HBsAg loss under interferon-based therapies were enrolled within 12 weeks from end of treatment and followed up for 48 weeks. Virological markers, biochemical indicators, and liver imaging examinations were observed every 3–6 months. Sustained functional cure was analysed as primary outcome. Factor associated with sustained HBsAg loss or reversion was also investigated. The rate of HBsAg loss sustainability was 91.8% (212/231). Patients receiving consolidation treatment for 12–24 weeks or ≥ 24 weeks had higher rates of sustained HBsAg negativity than those receiving consolidation treatment for < 12 weeks (98.3% and 91.2% vs. 86.7%, P = 0.068), and the former groups had significantly higher anti-HBs levels than the later (P < 0.05). The cumulative incidence of HBsAg reversion and HBV DNA reversion was 8.2% and 3.9%, respectively. Consolidation treatment of ≥ 12 weeks [odd ratio (OR) 3.318, 95% confidence interval (CI) 1.077–10.224, P = 0.037) was a predictor of sustained functional cure, and HBeAg-positivity at cessation of treatment (OR 12.271, 95% CI 1.076–139.919, P = 0.043) was a predictor of HBsAg reversion. Interferon-alpha induced functional cure was durable and a consolidation treatment of ≥ 12–24 weeks was needed after HBsAg loss in HBeAg-positive CHB patients.
Keywords: Chronic hepatitis B (CHB), Functional cure, HBeAg positive, HBsAg loss, Interferon (IFN)
Highlights
-
•
It is a large prospective study of clinical cure in HBeAg-positive CHB patients.
-
•
It showed the functional cure induced by interferon-alpha was durable.
-
•
The extended IFN therapy is critical for HBsAg loss in HBeAg-positive CHB patients.
-
•
Therapy of IFN consolidation ≥12–24 weeks is necessary in the clinical cure of CHB.
1. Introduction
Chronic hepatitis B virus (HBV)-infected individuals are at high risk of developing liver cirrhosis, decompensation of liver function, and hepatocellular carcinoma (HCC) (EASL, 2017; Omata et al., 2017; Terrault et al., 2018). Current antiviral therapy could effectively reduce hepatic necroinflammation and liver fibrosis through suppressing viral replication or clearing HBV by immune control. Oral nucleos(t)ide analogues (NAs) are currently the mainstay of HBV treatment; however, insufficient hepatitis B surface antigen (HBsAg) loss has been a conundrum in NA therapies. Interferon (IFN) based therapy is associated with better sustained virological and serological response and a higher chance of HBsAg loss (Marcellin et al., 2016). Moreover, IFN-based therapy is more capable to reduce incidence of HCC than NAs therapy (Liang et al., 2016; APASL, 2017). The National Institute for Health and Care Excellence (NICE) Hepatitis B clinical guideline recommended pegylated IFN-α (PEG-IFN) as the first-line antiviral drug for patients with chronic hepatitis B (CHB) in view of a higher rate of hepatitis B envelope antigen (HBeAg) seroconversion (National Clinical Guideline, 2013).
HBsAg loss is a desired treatment endpoint in CHB management because its value, as the surrogate marker, is association with better long-term outcome in comparison to those with persistent HBsAg antigenemia. For patients with CHB, HBsAg loss is the most important indicator of good long-term outcome of liver disease, regardless of route spontaneous clearance or via antiviral therapy (Pan and Zhang, 2005; Moucari et al., 2009; EASL, 2017; Tong et al., 2018). Therefore, HBsAg loss is regarded as the hallmark of functional cure and an ideal treatment endpoint in CHB patients (EASL, 2017; Omata et al., 2017; Terrault et al., 2018). However, the HBsAg loss response achieved after treatment is not always long-lasting, and there have been reported cases of recurrence which may cause liver disease relapse (Li et al., 2019; Alawad et al., 2020). The pattern of HBsAg reappearance, risk factors associated, and ways of intervention, remain to be fully elucidated. The purpose of our study was to evaluate the durability of HBsAg clearance obtained by IFN-based therapies and to investigate factors associated with HBsAg loss maintenance or reversion in HBeAg-positive CHB patients.
2. Materials and methods
2.1. Study design and data source
The study population was from our prospective cohort study at Hepatology Division 2, Beijing Ditan Hospital from October 2009 to June 2018 (Pan et al., 2021). CHB patients received personalized standard IFNα 5 MU injection every other day or weekly injection of PEG-IFNα-2a 180 μg, targeting maximal HBsAg reduction until seroclearance. HBeAg-positive CHB patients who achieved HBsAg loss by IFN or PEG-IFN based therapy and stopped antiviral drugs were eligible for recruitment into this clinical observational study. The enrolment criteria were: 1) HBeAg-positive CHB before treatment initiation; 2) patients who achieved functional cure as pre-specified: HBsAg-negative (quantitative HBsAg < 0.05 IU/mL) and undetectable HBV DNA levels (< 20 IU/mL) confirmed by two consecutive tests 1–3 months apart before treatment cessation; 3) enrolment within 12 weeks post-treatment. The exclusion criteria were: 1) patients co-infected with other viruses such as human immunodeficiency virus (HIV), hepatitis D virus (HDV), hepatitis C virus (HCV), and without other confounding liver diseases such as alcoholic liver disease and autoimmune hepatitis; 2) no using of hormone or immunomodulatory medication during the 48-week follow-up period (Li et al., 2020). The study was approved by the Human Research Ethics Committee of Beijing Ditan Hospital (Approval number: JDL2017-073-02). Written informed consent was obtained from all participating patients, and the study was registered at clinicaltrials.gov (Clinical Trials. gov ID: NCT02336399).
2.2. Data collection
Baseline data was defined by the date of enrolment into follow-up treatment (IFN or PEG-IFN). Demographic data include date of birth (age), gender, liver and renal biochemistry, haematological readings, and virological levels (HBV DNA, HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc). Particularly, the medication used (IFN or PEG-IFN), their dosage and adjustment, commencement and cessation dates, were all recorded. Liver ultrasound and CT examination were carried out during treatment and at the time of enrolment. During the follow-up period, HBV DNA and serological markers (HBsAg, anti-HBs, HBeAg, anti-HBe), liver biochemistries, renal function, blood glucose, alpha fetoprotein (AFP), and blood cells count were performed every three months. Liver fibrosis test (FibroScan 502, Echosens™, Paris, France), ultrasound (Acuson Sequoia, Siemens, Erlangen, Germany), or CT examination (Computed Tomography System, LightSpeed VCT, LightSpeed Pro32, Tokyo, Japan) were performed every 3–6 months.
2.3. Clinical indicator detection
HBV serological markers were measured by ARCHITECT i2000 HBsAg and HBeAg detection reagents (Architect i2000 analyzer; Abbott Diagnostics, Abbott Park, IL, USA). HBsAg negativity was defined as HBsAg level < 0.05 IU/mL, and HBsAg seroconversion was defined as HBsAg < 0.05 IU/mL plus anti-HBs ≥ 10 mIU/mL; serum HBV DNA was quantitated by using CobasTaqMan96 real-time quantitative PCR detection reagent (Limit of detection: 20 IU/mL) (Roche, Pleasanton, CA, USA). Tests of liver biochemistries, renal function, AFP, blood glucose, and blood lipids were performed by using an automated biochemical analyzer. Full blood test was carried out by using automated blood cell analyzer (Wako Pure Chemical Industries, Ltd, Tokyo, Japan).
2.4. Study endpoint
The primary endpoint was the rate of sustained functional cure at the end of the 48-week follow-up. For observation of long term outcomes, such as development of cirrhosis, liver function failure and the occurrence of HCC, 48 weeks of observation is not enough. For diagnosing sustained viral response or inactive HBsAg carrier, the observation period was usually 48 weeks. In our study, the sustained functional cure was defined as HBsAg negative maintained to 48 weeks after cessation of treatment. The secondary endpoints were as follows: 1) the rate of HBsAg reversion (HBsAg level return to ≥ 0.05 IU/mL); 2) percentage of HBeAg reversion (HBeAg level return to ≥ 1.0 S/CO); 3) the rate of virological reversion (HBV DNA level return to ≥ 20 IU/mL); 4) hepatitis recurrence (HBV DNA ≥ 2000 IU/mL, HBsAg ≥ 0.05 IU/mL, ALT level ≥ 80 U/L); 5) incidence of progressive liver diseases (ascites due to cirrhosis, gastrointestinal bleeding, hepatic encephalopathy, hepatorenal syndrome) and hepatocellular carcinoma.
2.5. Statistical analysis
Categorical variables were expressed as percentages. Chi-square test and nonparametric tests were used for comparison between groups. The normal distribution continuous variables were represented by mean ± standard deviation (SD), and the non-normal distribution continuous variables were represented by median and inter-quartile ranges (median, Q1–Q3). The comparison between groups was performed by Student's t-test, analysis of variance, and Kruskal Wallis test. The survival rate was calculated by Kaplan-Meire methodology and compared by log-rank test. The univariate and multivariate logistic regression analysis were used to analyze the factors associated with HBsAg reversion with statistical significance. The cut-off value of the anti-HBs level, which predicts sustained clinical cure, was determined by receiver operating characteristic curve (ROC) analysis.
3. Results
3.1. Demographics and treatments
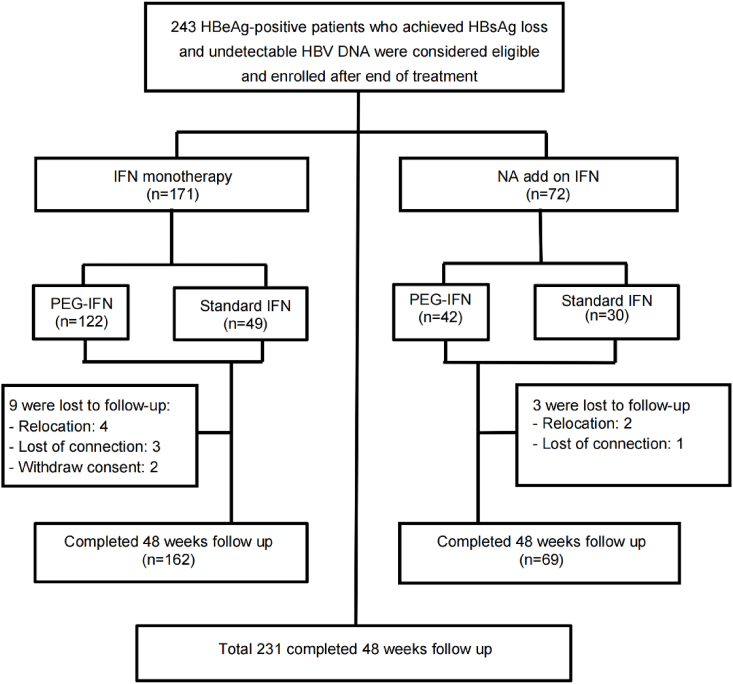
In total, 243 patients who had achieved functional cured were enrolled in this study (168 male, 75 female, mean age 37.3 ± 9.1 years). Among them, 164 patients were treated with PEG-IFN, the other 79 received standard IFN. In regards to treatment history, 171 patients were treatment naïve for any antiviral therapy and the other 72 patients were undergoing NAs therapy before add-on of PEG-IFN or standard IFN [adefovir dipivoxil (ADV): 20, entecavir (ETV): 29, lamivudine (LAM): 18, telbivudine (LdT): 56]. Patients treated with LdT were switched to ETV prior to add-on of IFN to avoid potential peripheral neuropathy. In patients received NA adding on PEG-IFN or standard IFN, NA was stopped as soon as completion of IFN therapy. The consolidation treatment time was defined as from the time of HBsAg loss confirmed to treatment cessation. The length of consolidation treatment was determined as follows: after both HBsAg and HBV DNA were negative twice at an interval of 12 weeks, the consolidation treatment was conducted for 12–24 weeks according to the levels of HBsAb. Because the HBsAb level varies from individual to individual, the length of consolidation treatment is different. All patients initiated with dosage according to label were eligible for personalized treatment adjustment. Although the recommended time of PEG-IFN treatment is 48 weeks, in this study, if the HBsAg levels kept decreasing during treatment, patients will receive extended treatment to pursuit HBsAg loss, followed by a consolidation treatment for seroconversion or high HBsAb levels. The median treatment duration of IFN based therapy was 123.0 weeks (58.0, 189.0), while the median treatment duration before HBsAg reached undetectable levels was 93.0 weeks (37.0, 149.5). In our study, 12 cases were lost to follow up. Statistical analysis was performed on the 231 patients (95.1%) who completed the full 48-week follow-up (Fig. 1).
Fig. 1.
Patient study flow diagram. HBsAg, hepatitis B surface antigen; PEG-IFN, pegylated interferon; IFN, interferon; NA, nucleos(t)ide analogue.
3.2. Baseline characteristics
For the 231 patients who completed 48 weeks of follow-up, the median consolidation treatment period, defined as the duration between HBsAg clearance and treatment cessation, was 23.5 weeks (12.0, 40.3). There were 60 (25.9%), 58 (25.1%), and 113 (48.9%) patients who received consolidation treatment for ≤ 12, 12–24, and ≥ 24 weeks, respectively. At the end of IFN treatment, when HBsAg was below the detection limit, 85.3% (197/231) patients had concomitant HBsAg-seroconversion. In terms of HBeAg status, 15 patients remained HBeAg-positive at 2.47 ± 1.08 S/CO level, 50 patients cleared HBeAg without appearance of anti-HBe, while the majority 166 (71.9%, 166/231) patients had HBeAg-seroconversion (Table 1).
Table 1.
Baseline clinical characteristics in patients with sustained functional cure and HBsAg reversion.
| Clinical characteristics at baseline | All patients (n = 231) | Sustained HBsAg response (n = 212) | HBsAg reversion (n = 19) | t/Z/χ2 | P value |
|---|---|---|---|---|---|
| Male gender, n (%) | 159 (68.83) | 146 (68.88) | 13 (68.42) | 0.002 | 0.968 |
| Age (y) | 37.32 ± 9.10 | 37.37 ± 9.11 | 36.74 ± 9.18 | 0.291 | 0.771 |
| Alanine aminotransferase (IU/L), median (IQR) | 31.80 (19.10, 45.80) | 31.40 (19.13, 45.73) | 33.80 (18.40, 48.20) | −0.133 | 0.895 |
| Aspartate aminotransferase (IU/L), median (IQR) | 27.50 (22.40, 36.20) | 27.40 (22.33, 36.15) | 28.30 (23.40, 36.20) | 0.204 | 0.839 |
| Total bilirubin (μmol/L), mean ± SD | 11.32 ± 5.19 | 11.50 ± 5.31 | 9.26 ± 2.83 | 1.815 | 0.071 |
| Direct bilirubin (μmol/L), mean ± SD | 4.16 ± 2.65 | 4.25 ± 2.74 | 3.27 ± 1.08 | 1.542 | 0.124 |
| Albumin (g/L), mean ± SD | 46.40 ± 3.35 | 46.46 ± 3.32 | 45.65 ± 3.73 | 1.010 | 0.313 |
| Glu level (mmol/L), mean ± SD | 5.63 ± 0.70 | 5.63 ± 0.69 | 5.57 ± 0.77 | 0.370 | 0.712 |
| PEG-IFN based therapy, n (%)a | 158 (68.40) | 143 (67.45) | 15 (78.95) | 1.012 | 0.314 |
| NA add on PEG-IFN or standard IFN, n (%)b | 69 (29.87) | 62 (29.25) | 7 (36.84) | 0.480 | 0.488 |
| Treatment time before HBsAg loss (wk), median (IQR) | 93.00 (38.00, 152.00) | 93.00 (37.00, 148.75) | 119.00 (81.00, 198.00) | −1.334 | 0.196 |
| Consolidation treatment time after HBsAg loss (wk), median (IQR) | 23.50 (12.00, 40.26) | 23.00 (12.00, 39.00) | 24.00 (0.00, 41.00) | 0.403 | 0.687 |
| Consolidation treatment time ≥ 12 weeks after HBsAg loss, n (%) | 171 (74.03) | 160 (75.47) | 11 (57.89) | 2.802 | 0.094 |
| Consolidation treatment time ≥ 24 weeks after HBsAg loss, n (%) | 113 (48.92) | 103 (48.58) | 10 (52.63) | 0.114 | 0.735 |
| Baseline HBeAg positive, n (%) | 15 (6.49) | 12 (5.66) | 3 (15.79) | 2.946 | 0.086 |
| Baseline HBeAb negative, n (%) | 65 (28.13) | 61 (28.77) | 4 (21.05) | 0.514 | 0.473 |
| Baseline HBeAg seroconversion, n (%) | 166 (71.86) | 151 (71.23) | 15 (78.95) | 0.514 | 0.473 |
| Baseline HBsAg seroconversion, n (%) | 197 (85.28) | 180 (84.91) | 17 (89.47) | 0.290 | 0.590 |
| Anti-HBs level (log10 mIU/mL), mean ± SD | 2.02 ± 0.86 | 2.00 ± 0.87 | 2.14 ± 0.81 | 1.076 | 0.283 |
| Negative (< 10 mIU/mL), n (%) | 34 (14.72) | 32 (15.09) | 2 (10.53) | 2.898 | 0.408 |
| Low (10–100 mIU/mL), n (%) | 59 (25.54) | 53 (25.00) | 6 (31.58) | ||
| Middle (100–1000 mIU/mL), n (%) | 114 (49.35) | 103 (48.58) | 11 (57.89) | ||
| High (> 1000 mIU/mL), n (%) | 23 (9.96) | 23 (10.85) | 0 (0.00) | ||
| Cirrhosis, n (%) | 5 (2.16) | 5 (2.36) | 0 (0.00) | / | / |
| NAFLD, n (%) | 34 (14.72) | 31 (14.62) | 3 (15.79) | 0.019 | 0.891 |
Statistical analysis was performed by chi-square test for count data and t-test or non-parametric test for measurement data in the comparison of difference in variables between the two groups.
PEG-IFN, pegylated interferon; IFN, interferon; HBsAg, hepatitis B surface antigen; HBsAb, hepatitis B surface antibody; HBeAg, hepatitis B envelope antigen; HBeAb, hepatitis B e antibody; NA, nucleos(t)ide analogue; NAFLD, non-alcoholic fatty liver disease; IQR, inter-quartile range; SD, standard deviation.
PEG-IFN based therapy vs. standard IFN based therapy.
NA add on PEG-IFN or standard IFN vs. monotherapy of PEG-IFN or standard IFN.
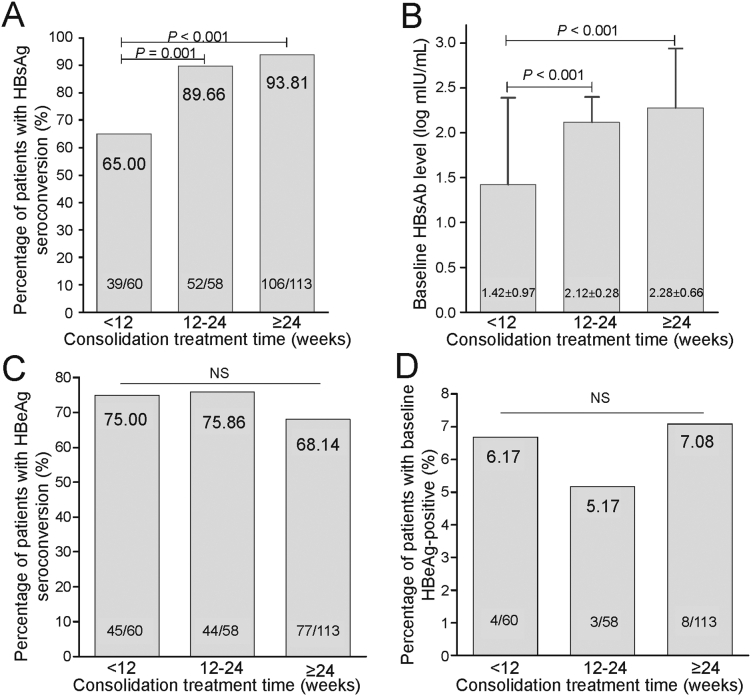
To understand whether the various consolidation treatment schemes affected the rate of HBsAg loss reversion or seroconversion, we carried out a sub-analysis in patients received different consolidation treatment time (< 12, 12–24, and ≥ 24 weeks). Compared to the group receiving consolidation treatment for < 12 weeks, the groups with 12–24 weeks and ≥ 24 weeks demonstrated significantly higher rates of HBsAg-seroconversion (89.7% and 93.8% vs. 65.0%, both P < 0.001), although there was no difference between the 12–24 weeks and ≥ 24 weeks groups (P > 0.05). In terms of HBsAg seroconversion outcome (appearance of anti-HBs), patients who received consolidation treatment for 12–24 weeks and ≥ 24 weeks also demonstrated higher anti-HBs quantification than patients who received consolidation time < 12 weeks (P < 0.001). At the withdrawal of consolidation treatment, the proportion of patients with HBeAg-positivity or with HBeAg-seroconversion was similar among the three groups (Fig. 2).
Fig. 2.
HBsAg and HBeAg responses in patients who received different consolidation treatment. HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; NS, not significant.
3.3. Sustained functional cure and changes of viral biomarkers
After the 48-week follow-up, 212 (91.8%, 212/231) patients had a sustained functional cure. In the subgroup analysis according the treatments strategies, there was no difference in the rate of sustained HBsAg negative response either between mono IFN treatment (PEG-IFN or standard IFN) and combination of treatment (NA add on PEG-IFN or standard IFN) [92.5% (150/162) vs. 89.8% (62/69), χ2 = 0.480, P = 0.488], or between PEG-IFN based therapy and standard IFN based treatment [90.5% (143/158) vs. 94.5% (69/73), χ2 = 1.066, P = 0.302]. Similarly, no difference was found between mono PEG-IFN treatment and NA add on PEG-IFN treatment [90.6% (106/117) vs. 90.2% (37/41), Fisher, P > 0.999], mono standard IFN treatment and NA add on standard IFN treatment [97.8% (44/45) vs. 89.3% (25/28), χ2 = 2.403, P = 0.121], mono PEG-IFN and standard IFN [90.6% (106/117) vs. 97.8% (44/45), Fisher, P = 0.182], or NA add on PEG-IFN and NA add on standard IFN [90.2% (37/41) vs. 89.3% (25/28), Fisher, P > 0.999].
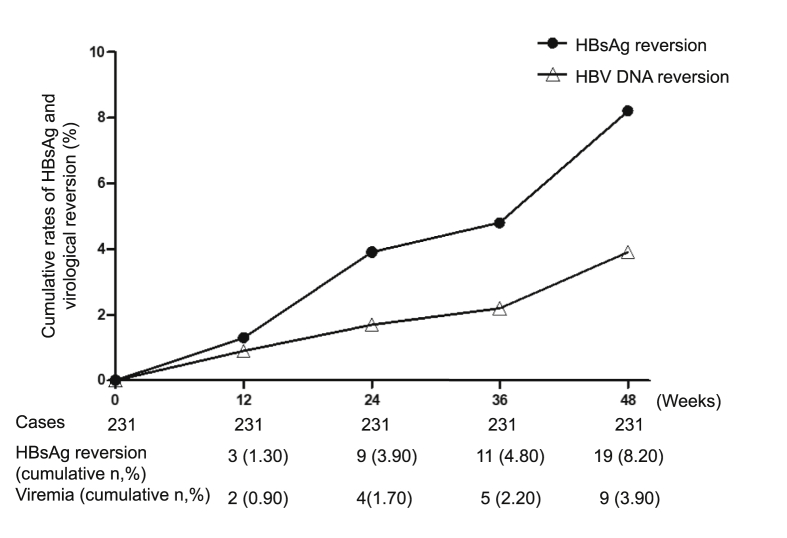
The rates of sustained functional cure among patients receiving consolidation treatment < 12 weeks, 12–24 weeks and ≥ 24 weeks were 86.7% (52/60), 98.3% (57/58) and 91.2% (103/113), respectively. Multivariate logistic regression analysis identified consolidation treatment ≥ 12 weeks was an independent factor for the sustained functional cure [odd ratio (OR) 3.318, 95% confidence interval (CI) 1.077–10.224, P = 0.037). The cumulative incidence of HBsAg reversion and virological reversion was 8.2% (19/231) and 3.9% (9/231), with average quantification of 0.15 (0.08, 0.86) IU/mL and 2.52 ± 1.42 log10 IU/mL, respectively (Fig. 3).
Fig. 3.
Kaplan-Meier analysis of the cumulative rates of HBsAg reversion and virologic reversion during follow-up period. HBsAg, hepatitis B surface antigen.
Among the 197 (85.2%, 197/231) patients with an anti-HBs presence at enrolment (anti-HBs ≥ 10 mIU/mL), 15 (7.6%) had disappearance of anti-HBs at the end of follow-up. Among these, 5 lost anti-HBs but maintained functional cure, while the other 10 had a concomitant recurrence of serum HBsAg. The rates of anti-HBs disappearance were significantly different between the groups receiving < 12 and ≥ 12 weeks consolidation treatment [20.5% (8/39) vs. 4.4% (7/158), χ2 = 11.501, P = 0.001].
On the contrary, among the 34 (14.8%, 34/231) patients without anti-HBs at enrolment, 19 (55.9%, 19/34) developed detectable anti-HBs during the follow-up period. Intriguingly, the proportion of anti-HBs development during follow-up in the group receiving consolidation treatment for < 12 weeks was numerically higher than that from the group undergoing consolidation treatment for ≥ 12 weeks [66.7% (14/21) vs. 38.5% (5/13), χ2 = 2.591, P = 0.107].
Regarding HBeAg status, there were 216 patients in total who achieved HBeAg loss while the rest 15 remained HBeAg positive as at enrolment. During the full follow-up period, 1 patient experienced HBeAg reappearance, while 2 of the 15 patients who were originally HBeAg positive achieved HBeAg clearance. In this study, two patients had confirmed hepatitis B recurrence per pre-specified definition. One was HBeAg positive, and the other was HBeAg negative before treatment initiation. No patient developed an indication of liver decompensation or HCC during the full treatment and follow-up period.
3.4. Factors associated with HBsAg and virological reversion
In order to identify any host or viral parameters which are potentially associated with HBsAg or HBV DNA reversion, we conducted a stratification analysis in patients with or without HBsAg seroreversion during the follow-up period. Numerically, the group with durable HBsAg clearance had a relatively higher proportion of patients receiving consolidation treatment ≥ 12 weeks and fewer were HBeAg positive at the end of treatment. Univariate logistic regression analysis, however, did not demonstrate that consolidation treatment duration or HBeAg positivity at treatment cessation was associated with the sustainability of HBsAg clearance (Table 2). For multi-variables logistics regression analysis of HBsAg seroreversion after follow-up, we further conducted a multivariate logistic regression analysis incorporating other host, treatment, and viral factors, including age, sex, type of IFN (PEG-IFN), IFN combined with NA (NA add on IFN), baseline HBeAg-positive, baseline HBeAb-negative, baseline HBsAg seroconversion, baseline HBsAb level, and consolidation treatment time < 12 weeks. Results showed that consolidation treatment < 12 weeks (OR 3.142, 95% CI 1.037–9.520, P = 0.043) and HBeAg-positivity (OR 12.271, 95% CI 1.076–139.919, P = 0.043) at the cessation of treatment were independent factors for HBsAg seroreversion (Table 2).
Table 2.
Logistic regression analyses for predictors of HBsAg reversion.
| Variables for HBsAg reversion | OR | 95% CI | P-values |
|---|---|---|---|
| Male gender | 1.021 | 0.372–2.803 | 0.968 |
| Age (y) | 0.992 | 0.941–1.046 | 0.770 |
| Alanine aminotransferase (IU/L) | 1.001 | 0.989–1.013 | 0.915 |
| Aspartate aminotransferase (IU/L) | 1.002 | 0.984–1.020 | 0.838 |
| Total bilirubin (μmol/L) | 0.869 | 0.752–1.005 | 0.058 |
| Direct bilirubin (μmol/L) | 0.710 | 0.499–1.010 | 0.057 |
| Albumin (g/L) | 0.936 | 0.823–1.064 | 0.313 |
| Glu level (mmol/L) | 0.874 | 0.429–1.778 | 0.710 |
| PEG-IFN based therapya | 1.809 | 0.579–5.656 | 0.308 |
| NA add on PEG-IFN or standard IFNb | 1.411 | 0.531–3.753 | 0.490 |
| Treatment time before HBsAg loss (wk) | 1.003 | 0.998–1.009 | 0.204 |
| Consolidation treatment time after HBsAg loss (wk) | 1.000 | 0.987–1.013 | 0.999 |
| Consolidation treatment time ≥ 12 weeks after HBsAg loss | 0.447 | 0.171–1.171 | 0.101 |
| Consolidation treatment time ≥ 24 weeks after HBsAg loss | 1.176 | 0.459–3.010 | 0.736 |
| Baseline HBeAg positive | 3.125 | 0.799–12.222 | 0.102 |
| Baseline HBeAb negative | 0.660 | 0.211–2.069 | 0.476 |
| Baseline HBeAg seroconversion | 1.515 | 0.483–4.748 | 0.476 |
| Baseline HBsAg seroconversion | 1.511 | 0.333–6.858 | 0.593 |
| Anti-HBs level (log10 mIU/mL) | 1.222 | 0.681–2.192 | 0.501 |
| Cirrhosis | / | / | / |
| NAFLD | 1.044 | 0.441–2.471 | 0.922 |
| Multi-variables logistics regression analysis for HBsAg seroreversion after follow-up c | |||
| Baseline HBeAg-positive for HBsAg seroreversion | 12.271 | 1.076–139.919 | 0.043 |
| Consolidation treatment time < 12 weeks after HBsAg loss for HBsAg seroreversion | 3.142 | 1.037–9.520 | 0.043 |
Statistical analysis was performed by single-factor logistics regression analysis for single-factor analysis, and variables with P < 0.1 in the single-factor analysis were included. Independent factors associated with HBsAg seroreversion were obtained by multi-factor logistics regression analysis.
PEG-IFN, pegylated interferon; IFN, interferon; HBsAg, hepatitis B surface antigen; HBsAb, hepatitis B surface antibody; HBeAg, hepatitis B envelope antigen; HBeAb, hepatitis B e antibody; NA, nucleos(t)ide analogue; NAFLD, non-alcoholic fatty liver disease.
PEG-IFN based therapy vs. standard IFN based therapy.
NA add on PEG-IFN or standard IFN vs. monotherapy of PEG-IFN or standard IFN.
Including age, sex, type of interferon (PEG-IFN), NA add on IFN, basline HBeAg-positive, baseline HBeAb-negative, baseline HBsAg seroconversion, baseline HBsAb level, and consolidation treatment time < 12 weeks for analysis.
We also investigated HBV DNA reversion during follow-up. Univariate logistic regression analysis demonstrated that HBeAg positivity at the end of treatment was a relevant risk factor (OR 8.750, 95% CI 1.946–39.334, P = 0.005). Multivariate logistic regression analysis did not identify any factor correlated to HBV DNA reversion. All nine cases of HBV DNA reversion occurred in patients with concurrent HBsAg reversion. Due to insufficient sample size, the correlation analysis was not implemented to investigate factors attributing to hepatitis recurrence.
4. Discussion
Viral clearance of HBV after acute infection or functional cure from previously established chronic infection mainly depends on the host immune responses (Li et al., 2017a; Li et al., 2018; Li M. et al., 2021; Li et al., 2021a; Li et al., 2021b). HBsAg seroclearance is a surrogate marker and may represent the enhanced or even successful immune control by host of HBV covalently closed circular DNA (cccDNA) moiety (Chu and Liaw, 2010; Li et al., 2017b; Li M. et al., 2021). Previous studies have demonstrated that HBsAg seroclearance could be augmented in certain patients under NA- or IFN-based treatment; however, an uncertain proportion of patients may experience HBsAg seroreversion (Li et al., 2017b; Yip et al., 2017). The present study aimed to assess the sustained functional cure response in HBeAg positive CHB patients who achieved HBsAg loss and undetectable HBV DNA under IFN-based therapy. We further investigated the risk factors associated with HBsAg reversion or virological relapse during a 48-week follow-up after treatment cessation.
Based on the WHO reference standard, 1 IU/mL of HBsAg is equivalent to 1–10 ng/mL, 2 × 108 subviral particles, or 5 × 107 progeny virions (Hollinger, 2008). Therefore, clinical cut-off using < 0.05 IU/mL to indicate HBsAg seroclearance may not rule out the possibility of very low HBsAg antigenemia. In our study, quantitative HBsAg was performed on any patients who were HBsAg negative. From a clinical perspective, we thus analysed the sustainability of HBsAg loss that reflects an effective immune control against viral replication. It's reported that the rate of HBsAg reversion at one year off-treatment was 7.6% and 6.8% in NA- and IFN-based therapies, respectively (Kim et al., 2014; Wu et al., 2020). To the best of our knowledge, the present study is one of the largest cohorts rendering a sufficient number of qualified patients for assessment of sustained functional cure achieved under IFN-based therapy in HBeAg positive CHB patients. The proportion of patients with sustained functional cure after one year since treatment cessation was 91.8%. Although there were four kinds of treatments in this study, they were all IFN-based therapy, and there was no significant difference in the rate of sustained HBsAg negative response among these groups. Moreover, the association of treatments regiment with sustained HBsAg negative after treatment cessation was not found by logistic regression analysis. The results indicated that various IFN-based treatment options may not affect the maintenance of HBsAg negative after treatment was stopped. All patients who maintained HBsAg negativity simultaneously displayed undetectable HBV DNA. These findings indicate that a functional cure resulting from IFN treatment was durable in most HBeAg-positive CHB patients.
The presence of anti-HBs showing B-cell functional restoration indicates significant immune control in HBV-infected patients following HBsAg clearance (van Campenhout and Janssen, 2015). Among HBV inactive carriers who spontaneously cleared HBsAg, subjects harboring anti-HBs had a lower chance of detectable HBV viremia compared to patients without anti-HBs during long-term follow-up (Chu and Liaw, 2012). In the present study, 85.3% patients achieved both HBsAg loss and production of anti-HBs after receiving personalized IFN-based treatment. Under the NA treatment scheme, such HBsAg seroconversion was reported as 67.4% in those who cleared HBsAg (Yip et al., 2017), which is lower than our results. Subsequent analysis of subsets under various consolidation treatment lengths revealed that patients who underwent ≥ 12 weeks of treatment were associated with an augmented HBsAg seroconversion rate and greater quantitative anti-HBs than those who received consolidation treatment for < 12 weeks. As expected, the group who received consolidation treatment for ≥ 12 weeks had a significantly lower rate of anti-HBs disappearance after treatment discontinuation. HBsAg-negativity has been shown to be more durable in patients with accompanied presence of anti-HBs (Yip et al., 2017). Our results revealed an association of anti-HBs with higher durability of HBsAg loss, although not statistically significant. Our data also implied for the first time, that consolidation treatment of ≥ 12–24 weeks might be optimal in sustaining anti-HBs and HBsAg loss introduced after IFN-based regimens. This finding provides a guide to clinical practice for avoiding the typical lengthy consolidation treatment of 12 month after HBsAg loss is achieved under NA therapies.
The cumulative incidence of HBsAg reversion in this study was 8.2%, and the rate of virological reversion was 3.9% during 48 weeks of follow-up. All virological reversion occurred in patients who had concurrent HBsAg reversion. Univariate logistic regression analysis did not find any factor predicting HBsAg reversion, However, multivariate logistic regression analysis showed consolidation treatment < 12 weeks and HBeAg positivity were independent factors associated with incidental HBsAg reversion. Also, univariate logistic regression analysis showed HBeAg positive at the end of treatment was a relevant risk factor for virological reversion. Results indicated that signal parameter might neglect the effects of other factors and was not enough to predict HBsAg seroreversion during follow up. In order to reduce the occurrence of HBsAg seroreversion after cessation of treatment, patients should receive ≥ 12 weeks of consolidation treatment and get HBeAg loss. HBeAg production is dependent upon cccDNA moiety activation and transcription (Zhou et al., 2006). In case with wild type virus strain and without nucleic acid mutation, the HBV DNA load, HBeAg and HBsAg level are matched, and the HBsAg loss usually occurs after HBeAg loss and HBV DNA turn negative. The reason for HBeAg positive after HBsAg loss could be considered the mutation of nucleic acid of viral genome to reduce the production of HBsAg (Hao et al., 2019; Shen et al., 2019), thus leads to HBeAg being positive at the time of HBsAg loss during antiviral therapy. Excessive HBe antigenemia may impair the generation, maturation, and functioning of dendritic cells (Hatipoglu et al., 2014; Li et al., 2021b). HBeAg may also induce activation of Treg while inhibiting the secretion of IFN-γ by nature killer cells (Jegaskanda et al., 2014). In our study, there were 6.5% of patients remained HBeAg positive despite having achieved HBsAg clearance under interferon treatment, suggesting that patients may not have reached complete immune control of HBV. Our results therefore provided additional evidence that HBsAg clearance did not necessarily accompany HBeAg loss. To further maximize the durability of HBsAg seroclearance, we suggest that patients may require a consolidation treatment of ≥ 12–24 weeks if pursuit of HBeAg loss is deemed necessary. In patients who achieved HBsAg loss under NA treatment, 21.2% experienced detectable HBV DNA (Yip et al., 2017), and in some patients, HBsAg loss was induced by resistance HBV mutant (Kim et al., 2014). In our study, all patients who achieved HBsAg loss had undetectable HBV DNA before the cessation of treatment. All virological relapse occurred in the patients who simultaneously experienced HBsAg reversion. Our results indicate that HBsAg clearance induced by IFN-based therapy is more a result of control of viral cccDNA activities. Instead, the observation of undetectable HBsAg during NA treatment may be attributed to a potential mutation in pre-S1, pre-S2, or the major hydrophilic region (MHR) of the S gene. In our study, among those patients who achieved only HBsAg loss, 55.9% developed anti-HBs during the follow-up period. Surprisingly, patients receiving consolidation treatment for < 12 weeks actually demonstrated a relatively higher chance of development of anti-HBs compared to those receiving treatment for ≥ 12 weeks.
In patients who had HBsAg reversion, except two patients occurred hepatitis B recurrence, the others remain at very low HBsAg and/or HBV DNA levels, which means they are unable to maintain the status of functional cure, but are still in the immune control period. The long term outcome need be further followed up. Patients with HBsAg seroreversion, even patients with sustained functional cure, should take liver function and imaging examination, and the viral biomarker be monitored regularly.
In patients with CHB, relapse of hepatitis after therapy discontinuation depends on the degree of clearance of cccDNA and immune control to viral replication. Some studies demonstrated that hepatitis B core-related antigen (HBcrAg) level alone or combined with HBsAg level can predict the hepatitis relapse after discontinuation of antiviral drugs. In patients treated with LAM, especially in HBeAg negative cases, HBcrAg level was a better predictor in non-reactivation of hepatitis after discontinued treatment than HBV DNA load at end of treatment. Patients with lower HBcrAg levels had a lower rate of hepatitis relapse, and the ability for predicting non hepatitis relapse was of sensitivity 0.8 and specificity 0.8 (Matsumoto et al., 2007). In another study in HBeAg negative patients who achieved HBV DNA < 3.0 log10 copiers/mL after NA treatment, it was demonstrated that HBsAg and HBcrAg levels were independent predictors for hepatitis relapse after treatment discontinuation, and the lower antigen levels, the lower rates of hepatitis relapse (Matsumoto et al., 2012). In CHB patients switching NA treatment to PEG-IFN, the baseline HBsAg and HBcrAg levels at PEG-IFN initial treatment were associated with the response and were the independent predictors for the efficacies of PEG-IFN, and the combination can enhance the predicting ability. In group of patients with response, the HBsAg and HBcrAg levels decreased continually. In contrast, the HBsAg and HBcrAg levels increased in non-responders during post-PEG-IFN therapy follow-up. Meanwhile, levels of HBsAg and HBcrAg at the end of PEG-IFN therapy can still predict response to the subsequent treatment (Matsumoto et al., 2018). In our study, all patients achieved HBsAg loss and undetectable HBV DNA load. Results showed that the duration of consolidation therapy and HBeAg negative at stop treatment were correlated with sustained HBsAg negative response. Unfortunately, HBcrAg level was not detected. Since the level of HBcrAg correlates with the cccDNA level in liver cell, and in peripheral blood the rate of virus particles to subvirus particles, which is composed of HBsAg, differs greatly among individuals and among virus strain (1:102–105 times) (Seeger et al., 2000; Schaller et al., 1991), it is reasonable to believe that the predictive ability for sustained functional cure would be improved if HBcrAg level is combined. To further improve the predictive ability of sustained HBsAg-negative response, HBcrAg and pgRNA testing should be performed at the end of treatment.
There are some limitations in our study. Since some subjects had started antiviral therapy before entering our research, a number of baseline factors were failed to collect. First, HBV genotypes were not well recorded due to technical restriction when the study was initially designed. As such, we could not neglect potential impact of genotypes on the sustained functional cure, although the major prevalent genotypes in China were B and C (Su et al., 2020). Second, this study did not collect data of HBV DNA, HBsAg, and HBeAg before initiation of treatment. Therefore, any association of virological parameters with sustained functional cure could not be analysed. Third, the follow-up period was initially designed for 48 weeks. We'll collect the data beyond 48 weeks and report the long term outcomes in future studies.
Our study explored some factors associated with the durability of HBsAg clearance achieved by IFN-based therapies. The results showed consolidation treatment of ≥ 12 weeks was a predictor of sustained functional cure, and HBeAg-positivity at cessation of treatment was a predictor of HBsAg reversion. The high proportion of HBsAg sustainability with consolidation treatment of ≥ 12 weeks may provide more evidence to guide clinical practice.
5. Conclusions
In summary, IFN-α induced functional cure was durable and a consolidation treatment of ≥ 12–24 weeks was needed after HBsAg loss in HBeAg-positive CHB patients.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement
The study was approved by the Human Research Ethics Committee of Beijing Ditan Hospital (Approval number: JDL2017-073-02). Written informed consent was obtained from all participating patients, and the study was registered at clinicaltrials.gov (Clinical Trials. gov ID: NCT02336399).
Author contributions
Minghui Li: conceptualization, data curation, formal analysis, writing-original draft preparation. Fangfang Sun: data curation, visualization, investigation. Xiaoyue Bi: data curation, investigation. Yanjie Lin: data curation. Liu Yang: visualization. Yao Lu: supervision. Lu Zhang: supervision. Gang Wan: software. Wei Yi: funding acquisition, supervision, formal analysis. Linqing Zhao: conceptualization, supervision, formal analysis. Yao Xie: lead, funding acquisition, writing-reviewing and editing.
Conflict of interest
The authors have no conflict of interests related to this publication.
Acknowledgments
The research group thanks all study patients and the staff in the study area. This study was funded in part by the Beijing Municipal Science and Technology Commission (No.Z151100004015122), Beijing Municipal Administration of Hospitals’ Clinical Medicine Development of special funding support (No. XMLX 201706 and XMLX202127), National Science and Technology Major Project of China (2017ZX10203202-003, 2017ZX10201201-001-006, and 2017ZX10201201-002-006), Beijing Science and Technology Commission (No.D161100002716002), Digestive Medical Coordinated Development Center of Beijing Municipal Administration of Hospitals (No.XXZ0302 and XXT28), and Special public health project of Capital health development(2021-1G-4061 and 2022-1-2172).
Contributor Information
Wei Yi, Email: yiwei1215@163.com.
Linqing Zhao, Email: linqingz525@163.com.
Yao Xie, Email: xieyao00120184@sina.com.
References
- Alawad A.S., Auh S., Suarez D., Ghany M.G. Durability of spontaneous and treatment-related loss of hepatitis B s antigen. Clin. Gastroenterol. Hepatol. 2020;18:700–709.e703. doi: 10.1016/j.cgh.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APASL Abstracts of the 26th annual conference of APASL, February 15-19, 2017, Shanghai, China. Hepatol. Int. 2017;11:1–1093. doi: 10.1007/s12072-016-9783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Liaw Y.F. Hepatitis B surface antigen seroclearance during chronic HBV infection. Antivir. Ther. 2010;15:133–143. doi: 10.3851/IMP1497. [DOI] [PubMed] [Google Scholar]
- Chu C.M., Liaw Y.F. Prevalence of and risk factors for hepatitis B viremia after spontaneous hepatitis B surface antigen seroclearance in hepatitis B carriers. Clin. Infect. Dis. 2012;54:88–90. doi: 10.1093/cid/cir755. [DOI] [PubMed] [Google Scholar]
- EASL EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- Hao R., Xiang K., Shi Y., Zhao D., Tian H., Xu B., Zhu Y., Dong H., Ding H., Zhuang H., Hu J., Li T. Naturally occurring mutations within HBV surface promoter II sequences affect transcription activity, HBsAg and HBV DNA levels in HBeAg-positive chronic hepatitis B patients. Viruses. 2019;11:78. doi: 10.3390/v11010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatipoglu I., Ercan D., Acilan C., Basalp A., Durali D., Baykal A.T. Hepatitis B virus e antigen (HBeAg) may have a negative effect on dendritic cell generation. Immunobiology. 2014;219:944–949. doi: 10.1016/j.imbio.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Hollinger F.B. Hepatitis B virus infection and transfusion medicine: science and the occult. Transfusion. 2008;48:1001–1026. doi: 10.1111/j.1537-2995.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- Jegaskanda S., Ahn S.H., Skinner N., Thompson A.J., Ngyuen T., Holmes J., De Rose R., Navis M., Winnall W.R., Kramski M., Bernardi G., Bayliss J., Colledge D., Sozzi V., Visvanathan K., Locarnini S.A., Kent S.J., Revill P.A. Downregulation of interleukin-18-mediated cell signaling and interferon gamma expression by the hepatitis B virus e antigen. J. Virol. 2014;88:10412–10420. doi: 10.1128/JVI.00111-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.A., Lim Y.S., An J., Lee D., Shim J.H., Kim K.M., Lee H.C., Chung Y.H., Lee Y.S., Suh D.J. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut. 2014;63:1325–1332. doi: 10.1136/gutjnl-2013-305517. [DOI] [PubMed] [Google Scholar]
- Li M., Zhang L., Lu Y., Chen Q., Lu H., Sun F., Zeng Z., Wan G., Zhao L., Xie Y. Early serum HBsAg kinetics as predictor of HBsAg loss in patients with HBeAg-negative chronic hepatitis B after treatment with pegylated interferonα-2a. Virol. Sin. 2021;36:311–320. doi: 10.1007/s12250-020-00290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.H., Chen Q.Q., Zhang L., Lu H.H., Sun F.F., Zeng Z., Lu Y., Yi W., Xie Y. Association of cytokines with hepatitis B virus and its antigen. J. Med. Virol. 2020;92:3426–3435. doi: 10.1002/jmv.26301. [DOI] [PubMed] [Google Scholar]
- Li M.H., Lu H.H., Chen Q.Q., Lin Y.J., Zeng Z., Lu Y., Zhang L., Dong J.P., Yi W., Xie Y. Changes in the cytokine profiles of patients with chronic hepatitis B during antiviral therapy. Biomed. Environ. Sci. 2021;34:443–453. doi: 10.3967/bes2021.061. [DOI] [PubMed] [Google Scholar]
- Li M.H., Lu Y., Sun F.F., Chen Q.Q., Zhang L., Lu H.H., Zeng Z., Yi W., Xie Y. Transforming growth factor β as a possible independent factor in chronic hepatitis B. Arch. Virol. 2021;166:1853–1858. doi: 10.1007/s00705-021-05062-6. [DOI] [PubMed] [Google Scholar]
- Li M.H., Yi W., Zhang L., Lu Y., Lu H.H., Shen G., Wu S.L., Hao H.X., Gao Y.J., Chang M., Liu R.Y., Hu L.P., Cao W.H., Chen Q.Q., Li J.N., Wan G., Xie Y. Predictors of sustained functional cure in hepatitis B envelope antigen-negative patients achieving hepatitis B surface antigen seroclearance with interferon-alpha-based therapy. J. Viral Hepat. 2019;26(Suppl. 1):32–41. doi: 10.1111/jvh.13151. [DOI] [PubMed] [Google Scholar]
- Li M.H., Zhang D., Zhang L., Qu X.J., Lu Y., Shen G., Wu S.L., Chang M., Liu R.Y., Hu L.P., Hao H.X., Hua W.H., Song S.J., Wan G., Liu S.A., Xie Y. Ratios of T-helper 2 cells to T-helper 1 cells and cytokine levels in patients with hepatitis B. Chin. Med. J. 2017;130:1810–1815. doi: 10.4103/0366-6999.211541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.H., Zhang L., Qu X.J., Lu Y., Shen G., Li Z.Z., Wu S.L., Liu R.Y., Chang M., Hu L.P., Hua W.H., Song S.J., Wan G., Xie Y. The predictive value of baseline HBsAg level and early response for HBsAg loss in patients with HBeAg-positive chronic hepatitis B during pegylated interferon alpha-2a treatment. Biomed. Environ. Sci. 2017;30:177–184. doi: 10.3967/bes2017.025. [DOI] [PubMed] [Google Scholar]
- Li M.H., Zhang L., Zhang D., Cao W.H., Qi T.L., Hao H.X., Wang X.Y., Ran C.P., Qu X.J., Liu S.A., Lu Y., Shen G., Wu S.L., Chang M., Liu R.Y., Hu L.P., Hua W.H., Wan G., Cheng J., Xie Y. Plasmacytoid dendritic cell function and cytokine network profiles in patients with acute or chronic hepatitis B virus infection. Chin. Med. J. 2018;131:43–49. doi: 10.4103/0366-6999.221275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K.H., Hsu C.W., Chang M.L., Chen Y.C., Lai M.W., Yeh C.T. Peginterferon is superior to nucleos(t)ide analogues for prevention of hepatocellular carcinoma in chronic hepatitis B. J. Infect. Dis. 2016;213:966–974. doi: 10.1093/infdis/jiv547. [DOI] [PubMed] [Google Scholar]
- Marcellin P., Ahn S.H., Ma X., Caruntu F.A., Tak W.Y., Elkashab M., Chuang W.L., Lim S.G., Tabak F., Mehta R., Petersen J., Foster G.R., Lou L., Martins E.B., Dinh P., Lin L., Corsa A., Charuworn P., Subramanian G.M., Reiser H., Reesink H.W., Fung S., Strasser S.I., Trinh H., Buti M., Gaeta G.B., Hui A.J., Papatheodoridis G., Flisiak R., Chan H.L. Combination of Tenofovir disoproxil fumarate and peginterferon α-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology. 2016;150:134–144. doi: 10.1053/j.gastro.2015.09.043. e110. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Tanaka E., Minami M., Okanoue T., Yatsuhashi H., Nagaoka S., Suzuki F., Kobayashi M., Chayama K., Imamura M., Yotsuyanagi H., Nakaoka S., Maki N., Kawata S., Kumada H., Iino S., Kiyosawa K. Low serum level of hepatitis B core-related antigen indicates unlikely reactivation of hepatitis after cessation of lamivudine therapy. Hepatol. Res. 2007;37:661–666. doi: 10.1111/j.1872-034X.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Tanaka E., Suzuki Y., Kobayashi M., Tanaka Y., Shinkai N., Hige S., Yatsuhashi H., Nagaoka S., Chayama K., Tsuge M., Yokosuka O., Imazeki F., Nishiguchi S., Saito M., Fujiwara K., Torii N., Hiramatsu N., Karino Y., Kumada H. Combination of hepatitis B viral antigens and DNA for prediction of relapse after discontinuation of nucleos(t)ide analogs in patients with chronic hepatitis B. Hepatol. Res. 2012;42:139–149. doi: 10.1111/j.1872-034X.2011.00910.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Nishiguchi S., Enomoto H., Kang J.H., Tanaka Y., Shinkai N., Kurosaki M., Enomoto M., Kanda T., Yokosuka O., Yatsuhashi H., Nagaoka S., Okuse C., Kagawa T., Mine T., Takaguchi K., Saito S., Hino K., Ikeda F., Sakisaka S., Morihara D., Miyase S., Tsuge M., Chayama K., Hiramatsu N., Suzuki Y., Murata K., Tanaka E. Combinational use of hepatitis B viral antigens predicts responses to nucleos(t)ide analogue/peg-interferon sequential therapy. J. Gastroenterol. 2018;53:247–257. doi: 10.1007/s00535-017-1360-z. [DOI] [PubMed] [Google Scholar]
- Moucari R., Korevaar A., Lada O., Martinot-Peignoux M., Boyer N., Mackiewicz V., Dauvergne A., Cardoso A.C., Asselah T., Nicolas-Chanoine M.H., Vidaud M., Valla D., Bedossa P., Marcellin P. High rates of HBsAg seroconversion in HBeAg-positive chronic hepatitis B patients responding to interferon: a long-term follow-up study. J. Hepatol. 2009;50:1084–1092. doi: 10.1016/j.jhep.2009.01.016. [DOI] [PubMed] [Google Scholar]
- National Clinical Guideline C. Hepatitis B (Chronic): Diagnosis and Management of Chronic Hepatitis B in Children, Young People and Adults. National Institute for Health and Care Excellence (UK) Copyright © National Clinical Guideline Centre; London: 2013. National Institute for health and Care excellence: clinical guidelines. 2013. [Google Scholar]
- Omata M., Cheng A.L., Kokudo N., Kudo M., Lee J.M., Jia J., Tateishi R., Han K.H., Chawla Y.K., Shiina S., Jafri W., Payawal D.A., Ohki T., Ogasawara S., Chen P.J., Lesmana C.R.A., Lesmana L.A., Gani R.A., Obi S., Dokmeci A.K., Sarin S.K. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol. Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C.Q., Li M.H., Yi W., Zhang L., Lu Y., Hao H.X., Wan G., Cao W.H., Wang X.Y., Ran C.P., Shen G., Wu S.L., Chang M., Gao Y.J., Xie Y. Outcome of Chinese patients with hepatitis B at 96 weeks after functional cure with IFN versus combination regimens. Liver Int. 2021;41:1498–1508. doi: 10.1111/liv.14801. [DOI] [PubMed] [Google Scholar]
- Pan C.Q., Zhang J.X. Natural history and clinical consequences of hepatitis B virus infection. Int. J. Med. Sci. 2005;2:36–40. doi: 10.7150/ijms.2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller H., Fischer M. Transcriptional control of hepadnavirus gene expression. Curr. Top. Microbiol. Immunol. 1991;168:21–39. doi: 10.1007/978-3-642-76015-0_2. [DOI] [PubMed] [Google Scholar]
- Seeger C., Mason W.S. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 2000;64:51–68. doi: 10.1128/mmbr.64.1.51-68.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Chen C., Ye C., Zhang H., Hang S., Chen M., Zhu Z., Xue Y., Liu L. Mutations in reverse transcriptase region of HBV affect Hepatitis B surface antigen titers and its correlation with HBV DNA. J. Infect. Dev. Ctries. 2019;13:1062–1067. doi: 10.3855/jidc.11447. [DOI] [PubMed] [Google Scholar]
- Su Q.D., Zhang S., Wang F., Liu H., Zhang G.M., Zheng H., Qiu F., Sun X.J., Liang X.F., Bi S.L., Shen L.P., Wang F.Z. Epidemiological distribution of hepatitis B virus genotypes in 1-29-year-olds in the mainland of China. Vaccine. 2020;38:8238–8246. doi: 10.1016/j.vaccine.2020.09.083. [DOI] [PubMed] [Google Scholar]
- Terrault N.A., Lok A.S.F., McMahon B.J., Chang K.M., Hwang J.P., Jonas M.M., Brown R.S., Jr., Bzowej N.H., Wong J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M.J., Pan C.Q., Han S.B., Lu D.S., Raman S., Hu K.Q., Lim J.K., Hann H.W., Min A.D. An expert consensus for the management of chronic hepatitis B in Asian Americans. Aliment. Pharmacol. Ther. 2018;47:1181–1200. doi: 10.1111/apt.14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Campenhout M.J., Janssen H.L. How to achieve immune control in chronic hepatitis B? Hepatol. Int. 2015;9:9–16. doi: 10.1007/s12072-014-9571-3. [DOI] [PubMed] [Google Scholar]
- Wu Y., Liu Y., Lu J., Cao Z., Jin Y., Ma L., Geng N., Ren S., Zheng Y., Shen C., Chen X. Durability of interferon-induced hepatitis B surface antigen seroclearance. Clin. Gastroenterol. Hepatol. 2020;18:514–516. doi: 10.1016/j.cgh.2019.04.020. e512. [DOI] [PubMed] [Google Scholar]
- Yip T.C., Wong G.L., Wong V.W., Tse Y.K., Lui G.C., Lam K.L., Chan H.L. Durability of hepatitis B surface antigen seroclearance in untreated and nucleos(t)ide analogue-treated patients. J. Hepatol. 2017;68:63–72. doi: 10.1016/j.jhep.2017.09.018. [DOI] [PubMed] [Google Scholar]
- Zhou T., Guo H., Guo J.T., Cuconati A., Mehta A., Block T.M. Hepatitis B virus e antigen production is dependent upon covalently closed circular (ccc) DNA in HepAD38 cell cultures and may serve as a cccDNA surrogate in antiviral screening assays. Antivir. Res. 2006;72:116–124. doi: 10.1016/j.antiviral.2006.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.