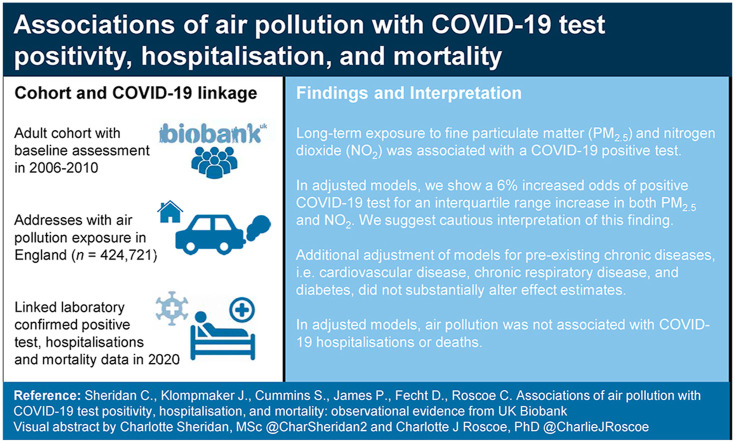
Abstract
Individual-level studies with adjustment for important COVID-19 risk factors suggest positive associations of long-term air pollution exposure (particulate matter and nitrogen dioxide) with COVID-19 infection, hospitalisations and mortality. The evidence, however, remains limited and mechanisms unclear. We aimed to investigate these associations within UK Biobank, and to examine the role of underlying chronic disease as a potential mechanism. UK Biobank COVID-19 positive laboratory test results were ascertained via Public Health England and general practitioner record linkage, COVID-19 hospitalisations via Hospital Episode Statistics, and COVID-19 mortality via Office for National Statistics mortality records from March–December 2020. We used annual average outdoor air pollution modelled at 2010 residential addresses of UK Biobank participants who resided in England (n = 424,721). We obtained important COVID-19 risk factors from baseline UK Biobank questionnaire responses (2006–2010) and general practitioner record linkage. We used logistic regression models to assess associations of air pollution with COVID-19 outcomes, adjusted for relevant confounders, and conducted sensitivity analyses. We found positive associations of fine particulate matter (PM2.5) and nitrogen dioxide (NO2) with COVID-19 positive test result after adjustment for confounders and COVID-19 risk factors, with odds ratios of 1.05 (95% confidence intervals (CI) = 1.02, 1.08), and 1.05 (95% CI = 1.01, 1.08), respectively. PM 2.5 and NO 2 were positively associated with COVID-19 hospitalisations and deaths in minimally adjusted models, but not in fully adjusted models. No associations for PM10 were found. In analyses with additional adjustment for pre-existing chronic disease, effect estimates were not substantially attenuated, indicating that underlying chronic disease may not fully explain associations. We found some evidence that long-term exposure to PM2.5 and NO2 was associated with a COVID-19 positive test result in UK Biobank, though not with COVID-19 hospitalisations or deaths.
Keywords: Air pollution, Particulate matter, PM2.5, Nitrogen dioxide, NO2, COVID-19, Coronavirus, SARS-CoV-2, Cohort study
Graphical abstract
1. Introduction
Coronavirus Disease 2019 (COVID-19) – caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) – killed 5.9 million people worldwide from December 2019, when it was first documented in humans, until December 2021. The World Health Organisation (WHO) declared COVID-19 a global pandemic on March 11, 2020, and researchers have since concluded that transmission is airborne (WHO, 2021). Individual risk factors, such as age, sex, ethnicity, education, smoking, pre-existing diseases, and residing in a care home, as well as contextual risk factors, such as area-level deprivation and outdoor air pollution levels, showed associations with COVID-19 infection and disease severity (Chadeau-Hyam et al., 2020; Davies et al., 2021; Kogevinas et al., 2021; Zhou et al., 2020). Existing epidemiological studies of the relationship between COVID-19 and long-term air pollution have been limited by ecological study design, inadequate adjustment for individual-level COVID-19 risk factors or important confounding factors, course exposure assessments, or short study periods, and have not explored potential underlying mechanisms.
Three potential pathways underlying the positive associations of long-term air pollution with COVID-19 have been suggested. Firstly, particulate matter (≤10 μm in diameter (PM10) and ≤2.5 μm in diameter (PM2.5)) may aid transportation of viral particles and therefore increase SARS-CoV-2 transmission (Domingo et al., 2020; Senatore et al., 2021). Secondly, long-term exposure to air pollution may increase an individual's susceptibility to COVID-19 infection through suppression of mucociliary clearance, phagocytosis of viral particles by alveolar macrophages, and overexpression and/or modification of the angiotensin-converting enzyme 2 (ACE-2) receptor on epithelial cells of the respiratory tract, which is the sole receptor for the attachment of SARS-CoV-2 via its spike protein (Paital and Agrawal, 2020; Woodby et al., 2021). Thirdly, air pollution exposure is associated with development of chronic diseases, including cardiovascular disease (CVD), chronic respiratory disease, and diabetes, which may increase susceptibility and the severity of COVID-19 infection (Bourdrel et al., 2021; Kogevinas et al., 2021).
Early in the pandemic, researchers reported associations of air pollutants with COVID-19 mortality using ecological study designs (Konstantinoudis et al., 2021; Lipsitt et al., 2021; Ogen, 2020; Wu et al., 2020a, Wu et al., 2020b). Ecological studies are valid for hypothesis generating purposes but cannot be used to make inferences about individual risks (Villeneuve and Goldberg, 2020). Since then, studies evaluated associations of air pollution with COVID-19 using individual-level outcome and covariate data and showed that increased severity and fatality within COVID-19 cases was associated with higher levels of long-term PM2.5 exposure, however, some studies did not adjust models for area-level deprivation (Chadeau-Hyam et al., 2020; Elliott et al., 2021; Marquès et al., 2022; Travaglio et al., 2021), which is associated with air pollution exposure (Mutz et al., 2021) and COVID-19 outcomes (Zhang et al., 2021) and may have confounded associations. In addition, some individual-level studies used spatially inaccurate or area-level estimates of long-term air pollution exposure (Bowe et al., 2021; López-Feldman et al., 2021; Marquès et al., 2022; Mendy et al., 2021; Travaglio et al., 2021), which may have resulted in exposure misclassification. Furthermore, some individual-level studies included only hospitalised patients in retrospective study designs (Bozack et al., 2022; Marquès et al., 2022; Mendy et al., 2021), which limits causal interpretation and generalisability.
A prospective, individual-level Catalan-based cohort study found that long-term residential air pollution concentrations were weakly positively associated with COVID-19 infection and strongly positively associated with severity of COVID-19, as measured by hospitalisations and self-reported symptoms (Kogevinas et al., 2021). Authors of this study suggested that effect modification by underlying chronic disease status should be assessed in future studies (Kogevinas et al., 2021), a hypothesis supported by research in the US which found a 62% increased odds of hospitalizations per 1 μg/m3 increment in long-term average PM2.5 (OR: 1.62, 95% CI: 1.00, 2.64) in patients with pre-existing respiratory diseases compared to those without (Mendy et al., 2021). Additionally, two exploratory analyses of potential risk factors for COVID-19, which included demographic, social, lifestyle, biological and medical factors, and air pollution, suggested a weak positive association of PM2.5 with COVID-19 cases or mortality (Chadeau-Hyam et al., 2020; Elliott et al., 2021). These UK Biobank studies were not focused on air pollution and, therefore, did not adjust for area-level deprivation, and were conducted earlier in the pandemic, so data was limited to COVID cases through 18 May 2020 before widespread testing was available (Chadeau-Hyam et al., 2020) and to COVID-19 mortality through 21 September 2020 (Elliott et al., 2021).
Here, we aimed to evaluate associations of long-term outdoor residential air pollution and multiple COVID-19 outcomes (laboratory-confirmed positive test cases, hospitalisations, and deaths) over the full pre-COVID vaccination time period (from March to December 2020). Additionally, we adjusted for relevant confounding factors using individual-level data from the UK Biobank cohort, which included linked general practitioner (GP) record information and area-level deprivation, and we used high spatial resolution air pollution exposure data. We also explored if pre-existing chronic diseases explained the association of air pollution with COVID-19 outcomes.
2. Methods
2.1. Study participants
UK Biobank is a prospective cohort study of 502,528 voluntary participants aged 40–69 years at baseline assessment between 2006 and 2010. At baseline, participants agreed to health and mortality record linkage and provided detailed socio-demographic and lifestyle data. We drew the study sample from UK Biobank participants in England who were alive as of 16 March 2020 (n = 430,437). We selected this date as it was the first date that Public Health England (PHE) began reporting COVID-19 laboratory test results. We excluded participants from Scotland and Wales because COVID-19 data from primary care GP records was not available through UK Biobank for these participants. We also excluded participants without information on address-level air pollution (PM2.5, PM10, nitrogen dioxide (NO2); n = 5228) or area-level deprivation (n = 525) from analyses; other missing covariate data were retained using missing indicators to minimise participant exclusions. UK Biobank has ethical approval from the North West Multi-Centre Research Ethics Committee (Reference 16/NW/0274).
2.2. Study period
The study start date was defined as 16 March 2020, the date PHE began administering, processing, and reporting COVID-19 tests on a national scale. We used 31 December 2020, as the study end date to correspond with the COVID-19 vaccine rollout. Regulators approved the first vaccine (Pfizer-BioNTech) on 2 December 2020, and the first vaccination was administered to a UK citizen on 8 December 2020. Given wait times for COVID-19 vaccination appointments and the time required to develop immunity after vaccination, however, we believe it is reasonable to assume that the vaccine rollout had a negligible impact on COVID-19 cases, hospitalisations, and deaths in UK Biobank participants up to our study end date.
2.3. Air pollution exposure assessment
The European Study of Cohorts for Air Pollution Effects (ESCAPE) project developed Land Use Regression models for annual average air pollution, PM2.5, PM10, and NO2 (Beelen et al., 2013; Eeftens et al., 2012). Modelled air pollution estimates were assigned to each UK Biobank participant's geocoded address at baseline (2010). PM2.5, PM10, and NO2 were analysed because there is well established evidence to support a possible link between these air pollutants and respiratory illnesses (Kim et al., 2018). In addition, these pollutants have distinct spatial differences in their dispersion and potentially different health effects. Specifically, NO2 exhibits high spatial contrast within small-areas, with steep drop off in concentrations away from roads and pollution sources (Sheridan et al., 2019). Meanwhile, PM2.5 concentrations are more dependent on long-range as opposed to local or mid-range emission sources. PM10 is also dependent on long-range emissions, though exhibits higher drop off in concentrations around roads than PM2.5 (Eeftens et al., 2012). We used air pollution data from 2010 to capture long-term, multi-year air pollution exposure. In England, air pollution levels have been shown to remain relatively stable since 2010 (Department for Environment, Food and Rural Affairs, 2021).
2.4. Public Health England COVID-19 cases
UK Biobank researchers linked participant records to PHE Second Generation Surveillance System (SGSS) records, which are automatically updated through continual nationwide reporting of all COVID-19 tests by laboratories. Test reporting improved over time as COVID-19 test availability increased and laboratory reporting was standardized. Initially, some laboratories only reported positive COVID-19 tests and there are no records of when individual laboratories began reporting both positive and negative results (Armstrong et al., 2020). Additionally, pillar 2 tests, i.e., those conducted in commercial laboratories for the wider public, were reported by PHE from 27 May 2020. Therefore, all COVID-19 test results prior to 27 May 2020, were pillar 1 tests, i.e. those conducted by PHE laboratories or National Health Service (NHS) hospitals for patients with clinical need or healthcare workers. Despite data limitations, previous research studies that examined UK Biobank participants and COVID-19 relied exclusively on PHE data for ascertaining COVID-19 positive test result case counts, as this data remains some of the most complete individual-level COVID-19 surveillance data available to date.
2.5. General practitioner reported COVID-19 cases
We also included COVID-19 cases from SNOMED and TPP GP records to improve the completeness of COVID-19 records, using SNOMED and TPP codes whose descriptions indicate laboratory-confirmed COVID-19 infections (n = 7605). Few COVID-19 cases appeared only in GP records and were not duplicated in PHE, hospital episode statistics (HES), or death record datasets (n = 449). SNOMED and TPP COVID-19 case codelists are included in Appendix A.
2.6. Hospitalisations
During the study period, HES data were reported using ICD-10 codes U07.1 and U07.2 to indicate confirmed and suspected COVID-19 cases, respectively. Hospitalised patients can be assigned multiple codes which are defined at each hospital admission, including each hospital transfer. We used confirmed COVID-19 ICD-10 code (U07.1), defined as COVID-19 confirmed with a laboratory test as the primary (first position) diagnosis to capture COIVD-19 hospitalisations (n = 1598).
Office for National Statistics death records are linked to UK Biobank participants and use the same ICD-10 codes for COVID-19 as HES data, described above. In total, 568 study participants died with their primary cause of death being a confirmed COVID-19 infections (U07.1) during the study period.
Age, sex, ethnicity, total household income after tax, smoking status, and body mass index (BMI) were derived by UK Biobank from participant responses to the baseline questionnaire and physical measurements. Age was operationalised in months and sex as a binary male or female. We condensed detailed ethnic definitions into a binary variable due to the low number of non-white UK Biobank participants (n = 22,486). Total household income was available from UK Biobank in 5 brackets: less than £18,000, £18,000 – £30,999, £31,000 – £51,999, £52,000 - £100,000, and greater than £100,000. We categorized smoking status as never, previous, current, or missing; and body mass index as healthy or underweight, overweight, obese, or missing. Individual-level variables for care home residency and number of COVID-19 tests taken over the study period were extracted from the PHE SGSS dataset and GP records. Care home residency status is not available in UK Biobank, however, researchers have derived care home residency from GP records using SNOMED and TTP codes, listed in Appendix B (Schultze et al., 2021). We considered care home residency status an important covariate for COVID-19 research and used this approach to create a binary variable to capture presence/absence of any care home related code in GP records (n = 2047).
Access to COVID-19 tests has been discussed as an important issue for COVID-19 analyses in UK Biobank (Carter et al., 2021; Chadeau-Hyam et al., 2020), to account for this, we summed the total number of laboratory-confirmed COVID-19 test results reported, both positive and negative results, for each participant during the study period (mean = 0.33; SD = 1.18; range = 0–50) to use as a proxy for access to tests and/or regularity of COVID-19 testing. Codelists for all COVID-19 tests in GP records including positive, negative, or inconclusive are included in Appendix A and all entries in the PHE SGSS dataset were counted as an independent test. To avoid overlapping testing records, we removed duplicate COVID-19 tests between GP records and PHE SGSS data which were administered on the same date.
Area-level deprivation and urbanicity for the census area around participants' residential addresses, which they provided at study baseline (2006–2010), were assessed by other researchers and are available via UK Biobank. Area-level deprivation was assigned using the Townsend deprivation index which encompasses four domains: unemployment, car ownership, home ownership, and household overcrowding. Townsend deprivation scores were assigned to participants at baseline (2006–2010) using the preceding Townsend release (2001) at the Output Area census level (∼125 households). Urbanicity was assigned by other researchers based on the Office of National Statistics (ONS) density classifications (2001) for the administrative area of each participant's postcode. Output Areas with a population of over 10,000 people were classed as Urban with progressively rural areas designated as Towns, Villages, then Hamlets. Although both deprivation and urbanicity variables predate the study period, the relative ranking of these area-level indices remains stable over time (Kontopantelis et al., 2018).
Regarding pre-existing chronic diseases, hospital records for cardiovascular disease (ICD-10 codes I), chronic respiratory disease (ICD-10 codes J300 – J998), and type 2 diabetes (ICD-10 codes E10 – E14 minus E10.2, E11.2, E12.2, E13.2, E14.2) are available through UK Biobank. A list of ICD-10 codes and conditions is included in Appendix C. Each of these disease groups is an important risk factor for COVID-19 hospitalisation or death (Elliott et al., 2021, Zhang et al., 2021) and represents a possible mechanistic pathway for the effect of long-term air pollution on COVID-19 outcome (Bourdrel et al., 2021). We assigned each participant a binary pre-existing disease covariate delineating those with and without hospital records (any position) for any of these disease groups prior to the study start date (March 16, 2020).
2.7. Statistical analysis
We used logistic regression models to assess the association of air pollutants with COVID-19 laboratory-confirmed positive test result (COVID-19 confirmed cases), hospitalisations, and deaths. Single-exposure models were sequentially adjusted for 1) age and sex; 2) age, sex, and other individual covariates - ethnicity, total household income after tax, smoking status, BMI, care home residency, and frequency of COVID-19 testing; 3) age, sex, other individual covariates, Townsend area-level deprivation and area-level urbanicity (main model). To test whether chronic diseases could explain the associations of air pollution and COVID-19 outcomes, we ran the main model with additional adjustment for prior disease status (hospital record of cardiovascular disease, chronic respiratory disease, or type 2 diabetes).
We conducted multiple sensitivity analyses to assess the robustness of results. Sensitivity analyses included restricting the study sample to those who received at least one COVID-19 test to exclude those who did not have access to/seek a test. In the beginning of the follow-up period, tests availability was limited and only available to individuals with more severe infections and healthcare workers. We excluded care home residents because of their increased risk of severe COVID-19 outcomes (Burton et al., 2021). Further, we included positive COVID-19 antibody tests to detect additional asymptomatic infections within the study sample. Additionally, we examined effect modification by stratifying participants with and without a hospital record of cardiovascular disease, chronic respiratory disease, or type 2 diabetes to assess whether pre-existing conditions were a potential mechanistic pathway.
We conducted a sensitivity analysis to adjust for a binary retirement variable given the advanced average age of UK Biobank. Participants who were retired would not have encountered the same occupational exposures as participants who were working, volunteering, or studying. We included those who reported remaining at home due to illness or disability, or to care for family members in the “retired” category because of their similarly reduced risk of COVID-19 occupational exposure. The retirement variable was derived from baseline questionnaires so additional participants likely retired between their baseline assessment and the onset of the pandemic, but would not have been captured.
We mutually adjusted each of the air pollution exposures – particulate matter (PM2.5 and PM10) adjusted for NO2 and NO2 adjusted for PM2.5. Lastly, we separately analysed all cases, hospitalisations, and deaths from wave 1 (March 16, 2020–May 31, 2020) of the pandemic (Davies et al., 2021), because public health measures, COVID-19 test access, and therapeutics for treating infections improved considerably after the initial wave as scientific knowledge of COVID-19 evolved (Armstrong et al., 2020; Kadri et al., 2021; Pouwels et al., 2021).
3. Results
Our study population included in total 424,721 participants who lived in England at baseline, were alive and a participant of UK Biobank at the start of this study (Table 1 ). The average age of the participants was 68 years old (SD = 8.11). There were more female (54.9%) than male (45.1%) participants, and the study population was predominantly white (93.6%), and living in urban areas (85%). Most participants were never smokers (55.1%), and the majority were with overweight or obesity (42.3% and 24.0%, respectively).
Table 1.
UK Biobank Participant COVID-19 outcomes and characteristics at baseline assessments (2006–2010).
| Outcomes and exposures | England-based participants (n = 424,721) |
|---|---|
| COVID-19 outcomes (n) | |
| COVID-19 confirmed cases | 10,790 |
| COVID-19 hospitalisations | 1598 |
| COVID-19 deaths | 568 |
| Average air pollution in 100 m circular distance buffer (mean, SD) | |
| PM2.5 (μg/m3) | 9.99 (1.05) |
| PM10 (μg/m3) | 16.2 (1.89) |
| NO2 (μg/m3) | 26.7 (7.66) |
| Covariates | |
| Age, years (mean, SD) | 68.0 (8.11) |
| Sex | |
| Female | 233,290 (54.9%) |
| Male | 191,431 (45.1%) |
| Ethnicity | |
| White | 397,601 (93.6%) |
| Non-White | 25,501 (6.0%) |
| Missing | 1619 (0.4%) |
| Income | |
| Less than 18,000 | 79,977 (18.8%) |
| 18,000 to 30,999 | 91,228 (21.5%) |
| 31,000 to 51,999 | 94,102 (22.2%) |
| 52,000 to 100,000 | 73,727 (17.4%) |
| Greater than 100,000 | 19,830 (4.7%) |
| Missing | 65,857 (15.5%) |
| Smoking status | |
| Never | 233,977 (55.1%) |
| Previous | 145,971 (34.4%) |
| Current | 42,279 (10.0%) |
| Missing | 2494 (0.6%) |
| Body Mass Index | |
| Healthy or Underweight | 140,783 (33.1%) |
| Overweight | 179,656 (42.3%) |
| Obese | 101,797 (24.0%) |
| Missing | 2485 (0.6%) |
| Care home residency | |
| Resident | 2047 (0.5%) |
| Non-resident | 422,674 (99.5%) |
| Number of COVID-19 tests over study period (mean, SD) | 0.33 (1.18) |
| Retirement status | |
| Retired | 163,175 (38.4%) |
| Not retired | 261,546 (61.6%) |
| Townsend Deprivation Tertile (2001 census) | |
| Low | 86,992 (20.5%) |
| Medium | 117,780 (27.7%) |
| High | 219,949 (53.5%) |
| Urbanicity | |
| Urban (>10k population) | 361,176 (85.0%) |
| Town | 29,514 (6.9%) |
| Village | 21,117 (5.0%) |
| Hamlet | 9202 (2.2%) |
| Missing | 3712 (0.9%) |
| Prior Hospital Record (any position) by Disease Group | |
| Cardiovascular disease | 157,793 (37.2%) |
| Chronic respiratory diseases | 66,796 (15.7%) |
| Type 2 Diabetes | 31,824 (7.5%) |
| All disease groups | 187,456 (44.1%) |
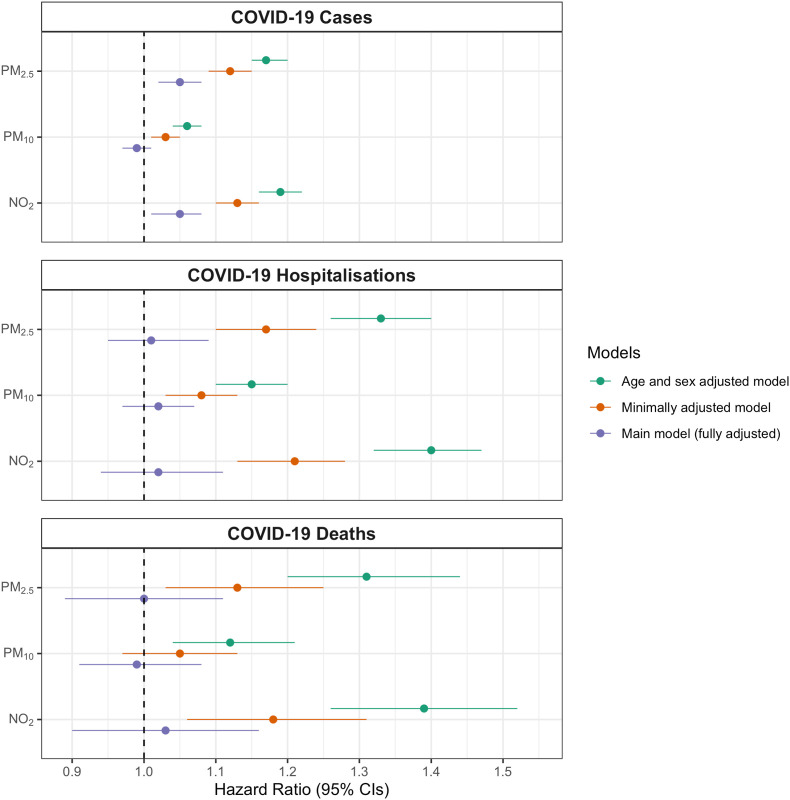
We observed strong positive associations of air pollution and COVID-19 outcomes in models adjusted only for age and sex (Fig. 1 ). These associations were attenuated when models were adjusted for other individual-level covariates, which included important COVID-19 risk factors such as ethnicity, smoking status, and care home residency. Additional adjustment in fully adjusted models for area-level covariates – deprivation and urbanicity – showed that associations between PM2.5 and NO2 with a laboratory-confirmed COVID-19 case remained positive, though associations with hospitalisations and deaths were attenuated towards the null. We observed no associations of PM10 with any of the COVID-19 outcome variables (positive test result, hospitalisation, or death) in fully adjusted models.
Fig. 1.
Odds ratios and 95% confidence intervals for an interquartile range increase in PM2.5, PM10, NO2 air pollution at UK Biobank residential addresses, with confirmed COVID-19 cases, hospitalisations, and deaths for UK Biobank participants (n = 424,721). Models were sequentially adjusted for 1) age and sex (green); 2) age, sex, and other individual-level covariates – ethnicity, average household income level, smoking status, body mass index, care home residency, and number of COVID-19 tests taken (minimally adjusted; orange); and 3) age, sex, other individual-level covariates listed in (2), Townsend deprivation (2001), and Office for National Statistics urbanicity categories (fully adjusted Main model; purple). Numeric results can be found in Supplementary material, Table E1. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Compared to the main model, further adjustment for chronic disease, as indicated by a previous hospital record of CVD, chronic respiratory disease, or type 2 diabetes, did not substantially affect the fully-adjusted results (Table 2 ). Similarly, restricting the analysis to those with a chronic disease (n = 187,456) did not substantially alter the associations of PM2.5 or NO2, with COVID-19 cases. We found associations of both PM2.5 and NO2 with COVID-19 cases were stronger for the sensitivity analysis that restricted the study sample to individuals who had received at least one laboratory-confirmed COVID-19 test. Analyses of only those who tested positive for COVID-19 to determine the effect on case severity (hospitalisations) and case fatality (deaths) showed no associations.
Table 2.
Odds ratios and 95% confidence intervals for an interquartile range increase in PM2.5, PM10, NO2 air pollution at UK Biobank residential addresses, with confirmed COVID-19 cases, hospitalisations, and deaths for UK Biobank participants. Main model is adjusted for age, sex, ethnicity, average household income level, smoking status, body mass index, care home residency, number of COVID-19 tests, Townsend deprivation (2001), and Office for National Statistics urbanicity categories. The Main model was additionally adjusted for chronic disease diagnosis prior to March 2020. Fully adjusted subset analyses for individuals hospitalised with a chronic disease diagnosis prior to March 2020, who received at least one laboratory confirmed COVID-19 test, and who tested positive with a laboratory confirmed COVID-19 test are shown.
| Outcome | Pollutant | IQR (μg/m3) | Main model |
Main model, additionally adjusted for chronic disease |
Main model for individuals previously hospitalised with a chronic disease |
Main model for individuals who received at least 1 lab-confirmed COVID-19 test |
Main model for individuals with a lab-confirmed positive COVID-19 result |
|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||
| COVID-19 Case | PM2.5 | 1.27 | 1.05 (1.02, 1.08) | 1.05 (1.02, 1.08) | 1.06 (1.02, 1.10) | 1.06 (1.03, 1.09) | . |
| PM10 | 1.75 | 0.99 (0.97, 1.01) | 0.99 (0.97, 1.01) | 0.98 (0.96, 1.01) | 1.01 (0.98, 1.03) | . | |
| NO2 | 9.93 | 1.05 (1.01, 1.08) | 1.05 (1.02, 1.08) | 1.05 (1.01, 1.10) | 1.10 (1.06, 1.14) | . | |
| COVID-19 Hospitalisation | PM2.5 | 1.27 | 1.01 (0.95, 1.09) | 1.01 (0.94, 1.09) | 1.02 (0.94, 1.10) | 1.03 (0.96, 1.11) | 0.98 (0.90, 1.05) |
| PM10 | 1.75 | 1.02 (0.97, 1.07) | 1.02 (0.97, 1.07) | 1.00 (0.95, 1.06) | 1.03 (0.98, 1.08) | 1.02 (0.97, 1.08) | |
| NO2 | 9.93 | 1.02 (0.94, 1.11) | 1.02 (0.94, 1.11) | 1.04 (0.95, 1.13) | 1.05 (0.97, 1.14) | 1.00 (0.92, 1.09) | |
| COVID-19 Death | PM2.5 | 1.27 | 1.00 (0.89, 1.11) | 0.99 (0.88, 1.11) | 1.05 (0.94, 1.19) | 1.01 (0.89, 1.13) | 0.96 (0.85, 1.08) |
| PM10 | 1.75 | 0.99 (0.91, 1.08) | 0.99 (0.91, 1.08) | 1.01 (0.93, 1.10) | 1.00 (0.91, 1.09) | 1.00 (0.91, 1.09) | |
| NO2 | 9.93 | 1.03 (0.90, 1.16) | 1.03 (0.90, 1.16) | 1.08 (0.94, 1.23) | 1.06 (0.93, 1.21) | 1.02 (0.89, 1.16) |
Sensitivity analyses showed our full study sample results were robust compared to restricted study samples of tested (Table D1) and positive laboratory confirmed COVID-19 participants (Table D2). Additionally, excluding participants living in care homes did not substantially impact our findings (Table D3); additional inclusion of participants who tested positive via antibody COVID-19 tests remained consistent with our main model findings (Table D4); additionally adjusting for hospital record of CVD, chronic respiratory disease, type 2 diabetes, or any of the above hospitalisations yielded similar results to the main model for an analysis of COVID-19 cases, hospitalizations, and deaths (Table D5) as well as COVID-19 hospitalisations and deaths amongst COVID-19 cases i.e. case fatality and mortality (Table D6); limiting the analysis to only wave 1 COVID-19 outcomes (through May 31, 2020) showed a slight increase in effect estimates and larger confidence intervals (Table D7); mutual adjustment for air pollution variables (nitrogen dioxide for particulate matter and vice versa) suggested that the association with COVID-19 cases was driven by PM2.5 (Table D8); adjusting for baseline retirement status/staying at home for other reasons did not substantially alter our findings (Table D9).
4. Discussion
Our study aimed to investigate associations between long-term air pollution and COVID-19 cases, hospitalisations, and deaths, after careful adjustment for relevant confounding factors. In minimally adjusted models, we found associations between higher levels of residential PM2.5 and NO2 in the decade prior to the pandemic and a COVID-19 positive test result (COVID-19 cases). These associations attenuated but remained after adjustment for demographic and lifestyle covariates. The positive associations of PM2.5 and NO2 with COVID-19 hospitalisations and deaths were attenuated toward the null after adjustment for relevant confounders. In subset analyses of individuals with at least one laboratory-confirmed COVID-19 test or individuals with prior hospitalisation for a chronic disease, associations of both PM2.5 and NO2 with COVID-19 hospitalisations and deaths were stronger compared to the main model. We found no association of PM10 with COVID-19 positive test result, COVID-19 hospitalisations, or COVID-19 deaths in our main analyses.
In models additionally adjusted for underlying chronic diseases we did not see a substantial decrease in the magnitude of effect estimates. Several previous studies showed associations of air pollution with CVD, chronic respiratory disease, and/or type-2 diabetes (Cai et al., 2018; Doiron et al., 2019; Yang et al., 2020), and an additional attenuation of the associations could have indicated potential mediation of air pollution and COVID-19 associations by underlying disease status. Underlying disease is strongly associated with many of the covariates included in the fully adjusted model, so the effect of underlying diseases may have been captured in the fully adjusted models through the combination of included covariates.
Previous studies have shown positive associations of long-term PM2.5 concentrations and COVID-19 case-severity (self-reported symptoms, hospitalisation or death) (Davies et al., 2021; Elliott et al., 2021; Kogevinas et al., 2021). Our study, which used primary position COVID-19 hospitalisation and death records, and adjusted for individual and area-level confounders, showed limited associations for PM2.5 and NO2. The low number of COVID-19 hospitalisations (n = 1598) and deaths (n = 568) resulted in wide confidence intervals so, while the results were not significant from a strict statistical interpretation, they may still be in concordance with previous studies. Associations of NO2 with COVID-19 cases were stronger for individuals who received at least one COVID-19 test compared to the full population. This finding may be due to the particularly harmful effects of NO2 exposure in participants who required testing, though should be interpreted with caution given that this subset of participants may contain more healthcare workers and other participants at high risk of infection. Additionally, NO2 associations with hospitalisations and deaths in the subset population with prior chronic disease hospitalisation showed larger effect estimates (insignificant) than in the full study population, which may be indicative of a stronger effect of air pollution in this population, but could also be an artifact of collider bias (Griffith et al., 2020) and should be interpreted with caution.
A UK Biobank study that used individual-level COVID-19 data from wave 1 of the COVID-19 outbreak (through April 26, 2020) found strong associations of air pollution with COVID-19 cases and deaths (Travaglio et al., 2021), however, this early UK Biobank study did not adjust for individual-level risk factors that have since been shown to greatly impact COVID-19 risk, including sex, ethnicity, and smoking status. Furthermore, the spatial resolution of the exposure assessment was relatively coarse (estimated within 2 km of residential address), which resulted in reporting of air pollution-COVID-19 effect estimates similar to earlier ecological studies. In our comparable wave 1 sensitivity analysis (through May 31, 2020), we found only borderline significant positive associations of PM2.5 and no association of NO2 with COVID-19 positive test result, echoing Chadeau-Hyam and colleagues' findings (Chadeau-Hyam et al., 2020). We found no association of PM2.5 or NO2 with COVID-19 hospitalisations or deaths during wave 1, though the low number of both outcomes resulted in wide confidence intervals. Other exploratory UK Biobank analyses included air pollution amongst many other lifestyle and demographic variables to explore potential risk factors for COVID-19 outcomes; the exploratory models used were adjusted for individual-level covariates and urbanicity, but were not adjusted for area-level deprivation (Chadeau-Hyam et al., 2020; Elliott et al., 2021). We believe that area-level deprivation, which is associated with air pollution at residential address (Mutz et al., 2021) and COVID-19 (Zhang et al., 2021) in UK Biobank, could have confounded previous UK Biobank air pollution and COVID-19 studies based on differences in effect estimates from our minimally adjusted and fully adjusted main model. In mutually adjusted analyses (NO2 and PM2.5), the association with COVID-19 cases was driven by PM2.5, though all pollutants were highly correlated, and this finding should be interpreted with caution.
In addition to the completeness of the confounding variables and the high resolution of our exposure data, our study builds upon previous analyses by incorporating detailed COVID-19 testing data, including information from general practitioners and antibody test results, as well as care home residency status. A strength of this analysis is the adjustment for COVID-19 testing frequency during the follow-up period. Testing frequency may be related to access to testing, employment, and COVID-related behaviours, and likelihood of being tested has been shown to be non-random in the UK Biobank population (Chadeau-Hyam et al., 2020). In our main analysis, we included all UK Biobank participants in England regardless of whether they had been tested using a laboratory-confirmed test for COVID-19, as excluding untested individuals may result in collider bias (Griffith et al., 2020). In sensitivity analyses, we conducted analyses using only tested participants (Table D1) and only tested participants with a positive COVID-19 test result (Table D2), which may be subject to collider bias (Griffith et al., 2020), however, results were not substantially different from the main analysis without exclusions. Furthermore, we included positive COVID-19 antibody tests as confirmed COVID-19 cases in a sensitivity analysis to capture additional, asymptomatic infections though the results were not substantially different to the main analyses. We acknowledge the potential bias in outcome misclassification due to untested individuals who did have COVID-19 not being counted as cases. This bias was likely mitigated among COVID-19 hospitalisations and deaths because testing was more universally available in hospital settings.
Unique to our UK Biobank study was adjustment for care home residency as a covariate. Living in a care home substantially increases the risk of COVID-19 infection (Jeffery-Smith et al., 2021) and, during the 1st wave of the pandemic, patients were discharged from hospitals directly to care homes without being tested for COVID-19 (Iacobucci, 2020). UK Biobank is a cohort of older adults so likely has more participants live in care homes than the general public, and their risk of COVID-19 would be elevated, irrespective of their prior long-term air pollution exposure. Additionally, accounting for the distinct risks of an older cohort, we did not adjust our main analysis for occupation due to the advanced age of study participants. At baseline (2006–2010), 33% of participants were already retired and another 6% reported looking after their home/family or remaining at home due to illness or disability. In a sensitivity analysis (Table D9), we adjusted for a baseline binary retirement status variable (including those who reported remaining at home for other reasons), despite the limitation that many employed participants at baseline may have since retired. The results for this sensitivity analysis remained consistent with the main analysis.
A limitation of this study is the temporal misalignment of annual average air pollution exposure (2010) and covariate data (2001–2010) with COVID-19 outcome data (2020). Individual covariate data was collected at UK Biobank baseline (2006–2010) and, furthermore, area-level deprivation was linked from 2001 census data, although relative ranking of area-level indices has been shown to be stable over decades in England (Kontopantelis et al., 2018). Annual average air pollution exposure at baseline assessment in 2010 may not represent air pollution exposure in 2020 and participants may have changed address, which cannot be verified in UK Biobank due to lack of longitudinal follow-up of residential address. However, in England, air pollution emissions remained relatively stable from 2010 to 2019 (Department for Environment, Food and Rural Affairs, 2021). Additionally, absolute air pollution concentrations may change over time at UK Biobank addresses, but the spatial variability that is captured by land-use regression air pollution modelling has been shown to be stable over comparable time periods to our study (long-term concentrations over 10 years) (Eeftens et al., 2011; Gulliver et al., 2016). Air pollution levels were lower in 2020 compared to prior years due to pandemic-related behaviour changes, but our analysis was designed to consider the long-term, multi-year impact of air pollution exposure (preceding COVID-19) rather than short-term exposures. Other cohorts with regular address and covariate follow-up should be considered in future analyses. Additionally, analyses to examine the effects of short-term air pollution on exacerbation of COVID-19 symptoms are required.
Finally, though our analysis did not show statistically significant increased COVID-19 severity in terms of hospitalisations and deaths, numbers of hospitalisations and deaths were small and, furthermore, severity may be measured in ways that are less severe than hospitalisation. Indeed, severity has been measured through self-report in other COVID-19 studies and showed association with air pollution (Kogevinas et al., 2021). We suggest caution in interpretation of the association of PM2.5 and NO2 with COVID-19 cases, as some residual confounding by population density may remain. NO2 varies on a small spatial scale around roads and pollution sources and is strongly, positively correlated with population density (Lamsal et al., 2013), in turn, population density is strongly, positively correlated with COVID-19 cases (Pouwels et al., 2021; Wong and Li, 2020). In UK Biobank, area-level population density is coarsely categorized as urbanicity, hence our suggestion to interpret associations with COVID-19 positive test cases with caution due to potential residual confounding. However, overall, this analysis was adjusted for important confounders and COVID-19 risk factors, had accurate spatial resolution air pollution exposure assessment, and used high quality COVID-19 surveillance data linked to a large prospective cohort of older adults, with many sensitivity analyses to support interpretation.
Given that all people are exposed to air pollution (World Health Organization, 2021) and that COVID-19 continues to impact many regions, even small harmful effects of air pollution on COVID-19 infection, severity, and fatality across the global population would result in large total health and economic impacts, we suggest that air pollution reduction benefits be considered not only in the context of the current pandemic, but also future epidemics (e.g., the climate crisis).
5. Conclusion
After adjustment for individual and area-level covariates, including adjustments for COVID-19 tests, care home residency, and area level deprivation, our results showed weak associations of PM2.5 and NO2 with COVID-19 infections, though no associations with hospitalisations or deaths. Our results suggest pre-existing conditions do not completely explain associations of air pollution with COVID-19 infection, however, additional studies with formal mediation analysis would strengthen this interpretation, providing that assumptions for formal mediation can be met.
Author statement
C.S. conceived the project and wrote the manuscript with support from C.R. C.S. and C.R. carried out statistical analyses. J.K. advised on statistical analysis and interpretation. D.F, J.K., P.J., S.C. and C.R. commented on the manuscript. C.R., D.F., S.C. supervised the project.
Ethics
Our study complies with the Declaration of Helsinki; UK Biobank has ethics approval from the North West Multi-Centre Research Ethics Committee (Reference 16/NW/0274).
Funding
This work was supported by the MRC Centre for Environment and Health, which is currently funded by the Medical Research Council (MR/S019669/1, 2019–2024). Infrastructure support for the Department of Epidemiology and Biostatistics was provided by the NIHR Imperial Biomedical Research Centre. The publication of this work was supported by a Johnson & Johnson London School of Hygiene & Tropical Medicine MSc in Public Health student scholarship.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
This paper has been recommended for acceptance by Da Chen.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envpol.2022.119686.
Appendix A. Supplementary data
The following is the supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- Armstrong J., Rudkin J.K., Allen N., Crook D.W., Wilson D.J., Wyllie D.H., O'Connell A.M. Dynamic linkage of COVID-19 test results between public health England's Second generation surveillance System and UK biobank. Microb. Genom. 2020 doi: 10.1099/mgen.0.000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelen R., Hoek G., Vienneau D., Eeftens M., Dimakopoulou K., Pedeli X., Tsai M.-Y., Künzli N., Schikowski T., Marcon A., Eriksen K.T., Raaschou-Nielsen O., Stephanou E., Patelarou E., Lanki T., Yli-Tuomi T., Declercq C., Falq G., Stempfelet M., Birk M., Cyrys J., von Klot S., Nádor G., Varró M.J., Dėdelė A., Gražulevičienė R., Mölter A., Lindley S., Madsen C., Cesaroni G., Ranzi A., Badaloni C., Hoffmann B., Nonnemacher M., Krämer U., Kuhlbusch T., Cirach M., de Nazelle A., Nieuwenhuijsen M., Bellander T., Korek M., Olsson D., Strömgren M., Dons E., Jerrett M., Fischer P., Wang M., Brunekreef B., de Hoogh K. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe – the ESCAPE project. Atmos. Environ. 2013;72:10–23. doi: 10.1016/j.atmosenv.2013.02.037. [DOI] [Google Scholar]
- Bourdrel T., Annesi-Maesano I., Alahmad B., Maesano C.N., Bind M.-A. The impact of outdoor air pollution on COVID-19: a review of evidence from in vitro, animal, and human studies. Eur. Respir. Rev. 2021;30 doi: 10.1183/16000617.0242-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe B., Xie Y., Gibson A.K., Cai M., van Donkelaar A., Martin R.V., Burnett R., Al-Aly Z. Ambient fine particulate matter air pollution and the risk of hospitalization among COVID-19 positive individuals: cohort study. Environ. Int. 2021;154 doi: 10.1016/j.envint.2021.106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozack A., Pierre S., DeFelice N., Colicino E., Jack D., Chillrud S.N., Rundle A., Astua A., Quinn J.W., McGuinn L., Yang Q., Johnson K., Masci J., Lukban L., Maru D., Lee A.G. Long-term air pollution exposure and COVID-19 mortality: a patient-level analysis from New York city. Am. J. Respir. Crit. Care Med. 2022;205:651–662. doi: 10.1164/rccm.202104-0845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton J.K., Reid M., Gribben C., Caldwell D., Clark D.N., Hanlon P., Quinn T.J., Fischbacher C., Knight P., Guthrie B., McAllister D.A. Impact of COVID-19 on care-home mortality and life expectancy in Scotland. Age Ageing. 2021;50:1029–1037. doi: 10.1093/ageing/afab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Hodgson S., Blangiardo M., Gulliver J., Morley D., Fecht D., Vienneau D., de Hoogh K., Key T., Hveem K., Elliott P., Hansell A.L. Road traffic noise, air pollution and incident cardiovascular disease: a joint analysis of the HUNT, EPIC-Oxford and UK Biobank cohorts. Environ. Int. 2018;114:191–201. doi: 10.1016/j.envint.2018.02.048. [DOI] [PubMed] [Google Scholar]
- Carter A.R., Griffith G.J., Gkatzionis A., Hughes R., Smith G.D., Lawlor D., Tilling K. P50 Time-varying selection bias in analyses of COVID-19 in UK Biobank. J. Epidemiol. Community Health. 2021;75:A64. doi: 10.1136/jech-2021-SSMabstracts.138. [DOI] [Google Scholar]
- Chadeau-Hyam M., Bodinier B., Elliott J., Whitaker M.D., Tzoulaki I., Vermeulen R., Kelly-Irving M., Delpierre C., Elliott P. Risk factors for positive and negative COVID-19 tests: a cautious and in-depth analysis of UK biobank data. Int. J. Epidemiol. 2020;49:1454–1467. doi: 10.1093/ije/dyaa134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B., Parkes B.L., Bennett J., Fecht D., Blangiardo M., Ezzati M., Elliott P. Community factors and excess mortality in first wave of the COVID-19 pandemic in England. Nat. Commun. 2021;12:3755. doi: 10.1038/s41467-021-23935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department for Environment, Food and Rural Affairs . 2021. Emissions of Air Pollutants in the UK.https://webarchive.nationalarchives.gov.uk/ukgwa/20210216021731/https://www.gov.uk/government/statistics/emissions-of-air-pollutants/emissions-of-air-pollutants-in-the-uk-1970-to-2018-particulate-matter-pm10-and-pm25#sections-in-this-release 1970 to 2019 [WWW Document]. URL. 22. 22 3. [Google Scholar]
- Doiron D., de Hoogh K., Probst-Hensch N., Fortier I., Cai Y., De Matteis S., Hansell A.L. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur. Respir. J. 2019 doi: 10.1183/13993003.02140-2018. [DOI] [PubMed] [Google Scholar]
- Domingo J.L., Marquès M., Rovira J. Influence of airborne transmission of SARS-CoV-2 on COVID-19 pandemic. A review. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeftens M., Beelen R., Fischer P., Brunekreef B., Meliefste K., Hoek G. Stability of measured and modelled spatial contrasts in NO2 over time. Occup. Environ. Med. 2011;68:765. doi: 10.1136/oem.2010.061135. [DOI] [PubMed] [Google Scholar]
- Eeftens M., Tsai M.-Y., Ampe C., Anwander B., Beelen R., Bellander T., Cesaroni G., Cirach M., Cyrys J., de Hoogh K., De Nazelle A., de Vocht F., Declercq C., Dėdelė A., Eriksen K., Galassi C., Gražulevičienė R., Grivas G., Heinrich J., Hoffmann B., Iakovides M., Ineichen A., Katsouyanni K., Korek M., Krämer U., Kuhlbusch T., Lanki T., Madsen C., Meliefste K., Mölter A., Mosler G., Nieuwenhuijsen M., Oldenwening M., Pennanen A., Probst-Hensch N., Quass U., Raaschou-Nielsen O., Ranzi A., Stephanou E., Sugiri D., Udvardy O., Vaskövi É., Weinmayr G., Brunekreef B., Hoek G. Spatial variation of PM2.5, PM10, PM2.5 absorbance and PMcoarse concentrations between and within 20 European study areas and the relationship with NO2 – results of the ESCAPE project. Atmos. Environ. 2012;62:303–317. doi: 10.1016/j.atmosenv.2012.08.038. [DOI] [Google Scholar]
- Elliott J., Bodinier B., Whitaker M., Delpierre C., Vermeulen R., Tzoulaki I., Elliott P., Chadeau-Hyam M. COVID-19 mortality in the UK Biobank cohort: revisiting and evaluating risk factors. Eur. J. Epidemiol. 2021;36:299–309. doi: 10.1007/s10654-021-00722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith G.J., Morris T.T., Tudball M.J., Herbert A., Mancano G., Pike L., Sharp G.C., Sterne J., Palmer T.M., Davey Smith G., Tilling K., Zuccolo L., Davies N.M., Hemani G. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat. Commun. 2020;11:5749. doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliver J., de Hoogh K., Hoek G., Vienneau D., Fecht D., Hansell A. Back-extrapolated and year-specific NO2 land use regression models for Great Britain - do they yield different exposure assessment? Environ. Int. 2016;92(93):202–209. doi: 10.1016/j.envint.2016.03.037. [DOI] [PubMed] [Google Scholar]
- Iacobucci G. BMJ; 2020. Covid-19: Lack of Testing Led to Patients Being Discharged to Care Homes with Virus, Say Auditors. [DOI] [PubMed] [Google Scholar]
- Jeffery-Smith A., Dun-Campbell K., Janarthanan R., Fok J., Crawley-Boevey E., Vusirikala A., Fernandez Ruiz De Olano E., Sanchez Perez M., Tang S., Rowland T.A., Wynne-Evans E., Bell A., Patel B., Amin-Chowdhury Z., Aiano F., Paranthaman K., Ma T., Saavedra-Campos M., Ellis J., Lackenby A., Whitaker H., Myers R., Höschler K., Brown K., Ramsay M.E., Shetty N., Chow J.Y., Ladhani S., Zambon M. Infection and transmission of SARS-CoV-2 in London care homes reporting no cases or outbreaks of COVID-19: prospective observational cohort study, England 2020. Lancet Reg. Health - Eur. 2021;3 doi: 10.1016/j.lanepe.2021.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadri S.S., Sun J., Lawandi A., Strich J.R., Busch L.M., Keller M., Babiker A., Yek C., Malik S., Krack J., Dekker J.P., Spaulding A.B., Ricotta E., Powers J.H., Rhee C., Klompas M., Athale J., Boehmer T.K., Gundlapalli A.V., Bentley W., Datta S.D., Danner R.L., Demirkale C.Y., Warner S. Association between caseload surge and COVID-19 survival in 558 U.S. Hospitals, March to august 2020. Ann. Intern. Med. 2021;174:1240–1251. doi: 10.7326/M21-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogevinas M., Castaño-Vinyals G., Karachaliou M., Espinosa A., de Cid R., Garcia-Aymerich J., Carreras A., Cortés B., Pleguezuelos V., Jiménez A. Ambient air pollution in relation to SARS-CoV-2 infection, antibody response, and COVID-19 disease: a cohort study in Catalonia, Spain (COVICAT study) Environ. Health Perspect. 2021;129 doi: 10.1289/EHP9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinoudis G., Padellini T., Bennett J., Davies B., Ezzati M., Blangiardo M. Long-term exposure to air-pollution and COVID-19 mortality in England: a hierarchical spatial analysis. Environ. Int. 2021;146 doi: 10.1016/j.envint.2020.106316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontopantelis E., Mamas M.A., van Marwijk H., Ryan A.M., Buchan I.E., Ashcroft D.M., Doran T. Geographical epidemiology of health and overall deprivation in England, its changes and persistence from 2004 to 2015: a longitudinal spatial population study. J. Epidemiol. Community Health. 2018 doi: 10.1136/jech-2017-209999. [DOI] [PubMed] [Google Scholar]
- Lamsal L.N., Martin R.V., Parrish D.D., Krotkov N.A. Scaling relationship for NO2 pollution and urban population size: a satellite perspective. Environ. Sci. Technol. 2013;47:7855–7861. doi: 10.1021/es400744g. [DOI] [PubMed] [Google Scholar]
- Lipsitt J., Chan-Golston A.M., Liu J., Su J., Zhu Y., Jerrett M. Spatial analysis of COVID-19 and traffic-related air pollution in Los Angeles. Environ. Int. 2021;153 doi: 10.1016/j.envint.2021.106531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Feldman A., Heres D., Marquez-Padilla F. Air pollution exposure and COVID-19: a look at mortality in Mexico City using individual-level data. Sci. Total Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.143929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquès M., Correig E., Ibarretxe D., Anoro E., Antonio Arroyo J., Jericó C., Borrallo R.M., Miret M., Näf S., Pardo A., Perea V., Pérez-Bernalte R., Ramírez-Montesinos R., Royuela M., Soler C., Urquizu-Padilla M., Zamora A., Pedro-Botet J., Masana L., Domingo J.L. Long-term exposure to PM10 above WHO guidelines exacerbates COVID-19 severity and mortality. Environ. Int. 2022;158 doi: 10.1016/j.envint.2021.106930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendy A., Wu X., Keller J.L., Fassler C.S., Apewokin S., Mersha T.B., Xie C., Pinney S.M. Long-term exposure to fine particulate matter and hospitalization in COVID-19 patients. Respir. Med. 2021;178 doi: 10.1016/j.rmed.2021.106313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutz J., Roscoe C.J., Lewis C.M. Exploring health in the UK Biobank: associations with sociodemographic characteristics, psychosocial factors, lifestyle and environmental exposures. BMC Med. 2021;19:240. doi: 10.1186/s12916-021-02097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci. Total Environ. 2020;726 doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paital B., Agrawal P.K. Air pollution by NO2 and PM2.5 explains COVID-19 infection severity by overexpression of angiotensin-converting enzyme 2 in respiratory cells: a review. Environ. Chem. Lett. 2020:1–18. doi: 10.1007/s10311-020-01091-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels K.B., House T., Pritchard E., Robotham J.V., Birrell P.J., Gelman A., Vihta K.-D., Bowers N., Boreham I., Thomas H., Lewis J., Bell I., Bell J.I., Newton J.N., Farrar J., Diamond I., Benton P., Walker Ann Sarah, Pouwels K.B., Walker A., Sarah, Crook D., Matthews P.C., Peto T., Pritchard E., Stoesser N., Vihta K.-D., Howarth A., Doherty G., Kavanagh J., Chau K.K., Hatch S.B., Ebner D., Martins Ferreira L., Christott T., Marsden B.D., Dejnirattisai W., Mongkolsapaya J., Hoosdally S., Cornall R., Stuart D.I., Screaton G., Eyre D., Bell J., Cox S., Paddon K., James T., House T., Newton J.N., Robotham J.V., Birrell P., Jordan H., Sheppard T., Athey G., Moody D., Curry L., Brereton P., Hay J., Vansteenhouse H., Bell I., Diamond I., Lambert A., Benton P., Rourke E., Hawkes S., Henry S., Scruton J., Stokes P., Thomas T., Allen J., Black R., Bovill H., Braunholtz D., Brown D., Collyer S., Crees M., Daglish C., Davies B., Donnarumma H., Douglas-Mann J., Felton A., Finselbach H., Fordham E., Ipser A., Jenkins J., Jones J., Kent K., Kerai G., Lloyd L., Masding V., Osborn E., Patel A., Pereira E., Pett T., Randall M., Reeve D., Shah P., Snook R., Studley R., Sutherland E., Swinn E., Thomas H., Tudor A., Weston J., Leib S., Tierney J., Farkas G., Cobb R., Van Galen F., Compton L., Irving J., Clarke J., Mullis R., Ireland L., Airimitoaie D., Nash C., Cox D., Fisher S., Moore Z., McLean J., Kerby M. Community prevalence of SARS-CoV-2 in England from April to november, 2020: results from the ONS coronavirus infection survey. Lancet Public Health. 2021;6:e30–e38. doi: 10.1016/S2468-2667(20)30282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze A., Bates C., Cockburn J., MacKenna B., Nightingale E., Curtis H., Hulme W., Morton C., Croker R., Bacon S., McDonald H., Rentsch C., Bhaskaran K., Mathur R., Tomlinson L., Williamson E., Forbes H., Tazare J., Grint D., Walker A., Inglesby P., DeVito N., Mehrkar A., Hickman G., Davy S., Ward T., Fisher L., Evans D., Wing K., Wong A., McManus R., Parry J., Hester F., Harper S., Evans S., Douglas I., Smeeth L., Eggo R., Goldacre B. Identifying care home residents in electronic health records - an OpenSAFELY short data report [version 1; peer review: 2 approved] Wellcome Open Res. 2021;6 doi: 10.12688/wellcomeopenres.16737.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatore V., Zarra T., Buonerba A., Choo K.-H., Hasan S.W., Korshin G., Li C.-W., Ksibi M., Belgiorno V., Naddeo V. Indoor versus outdoor transmission of SARS-COV-2: environmental factors in virus spread and underestimated sources of risk. Euro-Mediter. J. Environ. Integr. 2021;6:30. doi: 10.1007/s41207-021-00243-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan C.E., Roscoe C.J., Gulliver J., de Preux L., Fecht D. Inequalities in exposure to nitrogen dioxide in parks and playgrounds in greater London. Int. J. Environ. Res. Publ. Health. 2019;16 doi: 10.3390/ijerph16173194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglio M., Yu Y., Popovic R., Selley L., Leal N.S., Martins L.M. Links between air pollution and COVID-19 in England. Environ. Pollut. 2021;268 doi: 10.1016/j.envpol.2020.115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve Paul J., Goldberg Mark S. Methodological considerations for epidemiological studies of air pollution and the SARS and COVID-19 coronavirus outbreaks. Environ. Health Perspect. 2020;128 doi: 10.1289/EHP7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2021. Roadmap to Improve and Ensure Good Indoor Ventilation in the Context of COVID-19 (No. 9789240021280) [Google Scholar]
- Wong D.W.S., Li Y. Spreading of COVID-19: density matters. PLoS One. 2020;15 doi: 10.1371/journal.pone.0242398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodby B., Arnold M.M., Valacchi G. SARS-CoV-2 infection, COVID-19 pathogenesis, and exposure to air pollution: what is the connection? Ann. N. Y. Acad. Sci. 2021;1486:15–38. doi: 10.1111/nyas.14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva, Switzerland: 2021. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide (No. 9789240034228) [PubMed] [Google Scholar]
- Wu Xiao, Nethery R., Sabath B., Braun D., Dominici F. 2020. Exposure to Air Pollution and COVID-19 Mortality in the United States: A Nationwide Cross-Sectional Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Nethery R.C., Sabath M.B., Braun D., Dominici F. Air pollution and COVID-19 mortality in the United States: strengths and limitations of an ecological regression analysis. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B.-Y., Fan S., Thiering E., Seissler J., Nowak D., Dong G.-H., Heinrich J. Ambient air pollution and diabetes: a systematic review and meta-analysis. Environ. Res. 2020;180 doi: 10.1016/j.envres.2019.108817. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yang H., Li S., Li W.-D., Wang J., Wang Y. Association analysis framework of genetic and exposure risks for COVID-19 in middle-aged and elderly adults. Mech. Ageing Dev. 2021;194 doi: 10.1016/j.mad.2021.111433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.