Abstract
The majority of pulp and paper mills now biotreat their combined effluents using activated sludge. On the assumption that their wood-based effluents have negligible fixed N, and that activated-sludge microorganisms will not fix significant N, these mills routinely spend large amounts adding ammonia or urea to their aeration tanks (bioreactors) to permit normal biomass growth. N2 fixation in seven Eastern Canadian pulp and paper mill effluent treatment systems was analyzed using acetylene reduction assays, quantitative nitrogenase (nifH) gene probing, and bacterial isolations. In situ N2 fixation was undetectable in all seven bioreactors but was present in six associated primary clarifiers. One primary clarifier was studied in greater detail. Approximately 50% of all culturable cells in the clarifier contained nifH, of which >90% were Klebsiella strains. All primary-clarifier coliform bacteria growing on MacConkey agar were identified as klebsiellas, and all those probed contained nifH. In contrast, analysis of 48 random coliform isolates from other mill water system locations showed that only 24 (50%) possessed the nifH gene, and only 13 (27%) showed inducible N2-fixing activity. Thus, all the pulp and paper mill primary clarifiers tested appeared to be sites of active N2 fixation (0.87 to 4.90 mg of N liter−1 day−1) and a microbial community strongly biased toward this activity. This may also explain why coliform bacteria, especially klebsiellas, are indigenous in pulp and paper mill water systems.
Good performance of activated-sludge biotreatment systems depends on the concentrations of several key nutrients, including bioavailable “fixed” nitrogen. Unlike municipal sewage, pulp and paper mill wastewaters are typically rich in carbohydrates but poor in fixed nitrogen, due to the high C/N ratio typical of wood. Therefore, careful dosing of the raw effluent with fixed N and P prior to biotreatment is essential. This is a substantial expense. A ratio of 100:5:1 for bioavailable carbon (C) to nitrogen (N) to phosphorus (P) in the feed (raw effluent) is usually recommended. Excess fixed N is also undesirable, as it may result in fish-toxic free ammonia reaching the receiving waters and eutrophication (19). Successive nitrification and denitrification of excess N may also cause “rising sludge” in the secondary clarifier due to entrapment of N2 gas bubbles, resulting in biosolid losses to the receiving waters (16).
The biological fixation of N2 usually requires the following conditions: (i) readily available carbohydrates as an energy source (5, 12), (ii) low fixed-nitrogen concentrations (5), and (iii) absence or very low concentrations of dissolved oxygen (DO) (5, 12–14). N2 fixation has been reported from some pulp and paper mill aerated lagoons (4, 8), which may have regions supplying these key conditions. The N2 fixation in these lagoons was shown to be capable of supplying the entire N requirements of the system, corresponding to more than 600 kg of N day−1 (8). In contrast, N2 fixation is unlikely to occur in activated-sludge aeration tanks or secondary clarifiers of pulp and paper mills because (i) the DO level typical of activated-sludge operation is too high (usually 1 to 3 mg of O2 liter−1) for nitrogenase to function (8, 21), (ii) nutrient nitrogen has already been added as NH3 or urea, repressing N2 fixation (18), and (iii) levels of free sugars are usually very low because of the intense competition for carbon and energy sources by the activated-sludge biomass (16).
A mill primary clarifier is an unmixed settlement tank or basin, continuously removing suspended wood fibers and particles, and fillers or coaters such as clay, starch, and calcium carbonate from combined, pH-adjusted raw mill effluents. Primary clarifiers usually meet the three criteria given above for the growth and activity of N2-fixing microorganisms. Since their function is to mechanically settle particles and fibers, there is no aeration (low DO), and as supplemental fixed N has not been added yet in most mills, the C/N ratio is likely to be high. Previous work on the ecology of coliform bacteria in several pulp and paper mill effluent systems found permanent coliform populations, with Klebsiella strains predominant, in all primary clarifiers examined (11a). Indeed, the presence of N2-fixing members of the Enterobacteriaceae, including Klebsiella sp. strains, in pulp and paper mill water systems has long been known (2, 4, 6, 8, 9, 15, 18, 20–22).
In this study we used both acetylene reduction (AR) (an indirect measurement of nitrogenase activity) and functional gene probing (for the nifH nitrogenase gene) to detect N2-fixing bacteria and N2 fixation in seven primary-clarifier and bioreactor ecosystems. To measure the abundance and composition of N2 fixers in one particular clarifier, we enumerated the culturable bacterial community, the coliform bacteria, and we tested a subsample of the resulting colonies for the nifH gene by colony hybridization. We then identified a subset of those organisms that tested positive using biochemical characterization. This is the first report demonstrating N2-fixing activity by both cultured isolates and active in situ populations in pulp and paper mill primary clarifiers. The results also indicate the predominance of the genus Klebsiella in these nitrogen-fixing communities.
MATERIALS AND METHODS
Sampling procedures.
Seven Eastern Canadian pulp and paper mills were chosen in order to include a broad range of pulp and papermaking processes and biotreatment system designs (Table 1). Over 17 months, samples from the primary clarifiers and from the aeration tank biomass and liquor were collected. The primary-clarifier samples were collected at several depths using a 1.2-liter Kemmerer autosampler. Grab samples were transported in 500-ml polypropylene bottles on ice. AR assays and bacterial cultures were done within 4 and 24 h of sampling, respectively.
TABLE 1.
Characteristics of the mill treatment systems studied
| Mill | Process: producta | Furnishb (% recycled fibers added) | Biotreatmentc | Vol of primary clarifierd (m3) | Vol of effluent treated (m3 day−1) |
|---|---|---|---|---|---|
| A | TMP: newsprint, GWSpec | SW (15) | A/S | NAe | 68,800 |
| B-1 | GWSpec, KR papers, Bl. KR pulp | SW | SBR | 3,175 | 5,760 |
| B-2 | GWSpec, KR papers, Bl. KR pulp | SW | SBR | 11,065 | 43,200 |
| C | Bl. KR.: fine papers | HW (15) | A/S | 40,000 | 120,000 |
| D | TMP: newsprint | SW | SBR | ||
| E | TMP: newsprint, GWSpec | SW (15) | A/S | 11,000 | 61,000 |
| F | Sulfite: newsprint | SW (16) | A/S | 10,000 | 40,000 |
| G | Bl. KR: market pulp | HW | A/S | 20,000 | 71,000 |
Thermomechanical (TMP), Kraft (KR), and sulfite pulping processes were used. GWSpec, groundwood specialties; Bl. KR pulp, bleached kraft pulp.
SW, softwood; HW, hardwood.
A/S, aerobic activated sludge; SBR, sequencing batch reactor.
Approximate volume. Mill B has two primary clarifiers; B-1 treats debarking process wastewaters. The clarified B-1 effluent and the other effluent streams from the mill are treated in clarifier B-2. Mill D has no separate primary clarifier.
NA, not available.
Total counts, coliform isolates, and pure culture controls.
Total bacterial counts used triplicate serial dilutions and plating of samples onto 1/5-strength Trypticase soy agar (TSA) plates (Difco) supplemented with 1% (wt/vol) NaCl and 1% (wt/vol) glucose. Coliform counts used triplicate dilution plating on both MacConkey and eosin methylene blue agar (Difco). All plates were incubated at 37°C for 18 to 24 h. Total and fecal coliforms (TC and FC) were selected for an enumerated as described previously (11a) using the most-probable-number (MPN) methods recommended in Standard Methods for the Examination of Water and Wastewater, sections 9221B and 9221E (1). Isolations were carried out as described previously (11a) using MacConkey agar. Strains were identified using the standard API 20E biochemical test procedure (Biomérieux).
Control bacterial strains are listed in Table 2. Prior to DNA extraction, Azoarcus tolulyticus was grown aerobically in modified R2A broth (ATCC culture medium 2120). All other pure (control) cultures (Table 2) were grown aerobically in 10 ml of nutrient broth (Difco).
TABLE 2.
Pure culture DNA used as controls in this study
| Strain | Source or reference |
|---|---|
| Klebsiella pneumoniaea | |
| KPN3 (primary clarifier isolate) | This study |
| KPG1 (activated sludge isolate) | This study |
| KPG2 (primary clarifier isolate) | This study |
| KPG3 (primary clarifier isolate) | This study |
| Escherichia coli K12 (MM294A) | 11 |
| Azoarcus tolulyticus | 28 |
| Pseudomonas stutzeri JM300 | 7 |
| Pseudomonas stutzeri Zobell (ATCC 14405) | B. Ward |
| Sinorhizobium meliloti RCR 2011 (SU47) | 26 |
| Rhodobacter sphaeroides f. sp. denitrificans | 24 |
| Paracoccus denitrificans (ATCC 17749) | R. Ye |
| Corynebacterium nephridii (ATCC 11425) | R. Ye |
| Pseudomonas sp. strain G-179 | 27 |
Based on API 20E identification.
Flask cultures.
Coliform colonies isolated from MacConkey agar were transferred successively onto “high-N” glucose-thioglycolate agar plates and into 10-ml tubes of the corresponding “N-free” broth containing, per liter, 10.0 g of d-glucose, 6.3 g of K2HPO4, 1.7 g of NaH2PO4, 0.1 g of MgSO4, 0.008 g of Na2MoO4, 0.008 g of ferric citrate, 0.5 g of Na-thioglycolate, and 0.001 g of resazurin. The high-N broth also contained 0.2 g of yeast extract liter−1 and 0.5 g of Casamino Acids liter−1. In situ N2 fixation activity was measured by the AR assay as described by Knowles et al. (18). Assays were initiated by the removal of 5 ml of N2 and the addition of 5 ml of acetylene (final concentration, 10% [vol/vol]), followed by incubation at room temperature with shaking (120 rpm). To assay N2 fixation by isolates, 1 ml of a “preenrichment” culture growing on 9 ml of fresh “N-free” medium was pipetted into 50-ml flasks, and the assay was performed as for clarifier samples (above). The AR assay was used essentially as described previously (18).
nifH gene probe.
From the published sequence of the Mo-Fe nitrogenase genes of Azotobacter chroococcum (10, 17), we designed primers to amplify a portion of the nifH gene from a purified plasmid (pER4) containing nifHDK from A. chroococcum. The two primers, designated NIFN3 (5′-ATCCACCACCACTCAGAACC) and NIFC3 (5′-ATAACGCCGAACTCCATCAG) amplified a 780-bp region of nifH (sequence positions 285 to 1064; GenBank accession no. M20568). To obtain labeled probe, we amplified the probe sequence by PCR, incorporating digoxigenin (DIG)-labeled dUTP according to the manufacturer's protocol (PCR DIG probe synthesis kit; Roche Molecular Biochemicals). The amplified probe was purified after 1% agarose gel electrophoresis (with an agarose gel DNA extraction kit from Roche Molecular Biochemicals).
DNA extraction.
DNA was extracted from pure cultures using a sodium dodecyl sulfate (SDS)-based method (3). Well-mixed primary clarifier grab samples (500 ml) were blended in a Waring blender (1 min), then filtered through cheesecloth and centrifuged (10,000 × g, 10 min, 4°C). The pellet was resuspended in 1 to 3 ml of lysing solution containing 100 mM Tris-HCl (pH 8.0), 300 mM NaCl, 20 mM EDTA (pH 8.0), and 2% (wt/vol) SDS. One milliliter of resuspended cells was placed in a 2-ml screw-cap tube containing 1 g of zirconia-silica beads (diameter, 0.1 mm) (Biospec Products, Bartlesville, Okla.) and 1 ml of phenol-chloroform (1:1, vol/vol). The cells were lysed by 5 min in a beadbeater and then centrifuged (2,000 × g, 15 min), and the aqueous DNA-containing supernatant was repeatedly phenol-chloroform and chloroform extracted. Phase separation was carried out at 10,000 × g for 15 min. The purified DNA was treated with RNase, precipitated with isopropanol, washed with 70% ethanol, dried, and resuspended in 200 to 500 μl of Tris-EDTA (TE) buffer. Purified DNA was quantified on a 1% agarose gel using an AlphaImager 1200 with AlphaEase software (Alpha Innotech).
ATP extraction.
ATP was extracted from primary-clarifier and activated-sludge samples using a trichloroacetic acid-EDTA-based ATP release method and was measured using commercial luciferin-luciferase assay reagents (FL-AAM, FL-AAS, and FL-AAB; Sigma). Light production was measured using an LKB model 1250 luminometer.
Dot blot hybridization.
Purified DNA (1 μg) was alkali transferred to Hybond N+ membranes (Amersham) using a dot blot manifold. After UV cross-linking (Stratagene Stratalinker), membranes were rinsed in 0.5 M Tris-HCl (pH 7.0) to ensure removal of alkali, because the probe's DIG label is alkali labile. The hybridization and wash procedures were modified from earlier methods (25, 27). The membranes were prehybridized in roller bottles (42°C, 4 h) in 50% formamide–5× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, 1 mM EDTA [pH 7.7]–5× Denhardt's solution–0.2 mg of salmon sperm DNA ml−1. Denhardt's solution is, per liter, 0.2 g of Ficoll, 0.2 g of polyvinylpyrrolidone, and 0.2 g of bovine serum albumin. Hybridization was carried out for 14 to 16 h at 42°C in 10 ml of prehybridization solution with 10% (wt/vol) dextran sulfate and 10 ng of denatured nifH probe ml−1. The membrane was washed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% SDS (15 min at room temperature [RT]), 0.5× SSC–0.1% SDS (15 min at RT), 0.1× SSC–0.1% SDS (15 min at RT), and 0.1× SSC–1% SDS (15 min at 42°C). Detection was carried out by using nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP) as a colorimetric substrate for alkaline phosphatase according to the manufacturer's protocols (Roche Molecular Biochemicals).
Colony hybridization.
Random colonies from both TSA and MacConkey plates were chosen for hybridization analysis. These isolates and pure culture controls were transferred to TSA plates. After 24 h at 37°C, colonies were transferred to Hybond N+ membranes (Amersham) and lysed (23). Hybridization with nifH, washes, and detection were done as for the dot blots.
RESULTS AND DISCUSSION
Occurrence of N2 fixation in mill aeration tanks (bioreactors).
Existing reports of N2 fixation in pulp and paper mill treatment systems are limited to aerated lagoons (4, 8) and laboratory-scale activated-sludge systems held at abnormally low DO and fixed-N levels (11a, 20; D. J. Gapes, N. M. Frost, T. A. Clark, P. H. Dare, R. G. Hunter, and A. H. Slade, presented at the 6th IAWQ Symposium on Forest Industry Wastewaters, Tampere, Finland, 6 to 10 June, 1999). In this study, full-scale on-line activated-sludge systems and primary clarifiers were examined for N2-fixing activity. The biomass and suspended liquor from the activated-sludge aeration tanks (bioreactors) of the seven mills described in Table 1 were sampled, and AR assays were run within 4 h. While the nifH gene was detected in the samples, none showed any N2 fixation activity (data not shown).
Occurrence of N2 fixation in mill primary clarifiers.
In contrast, both the nifH gene and active N2 fixation were present in all of the six mill primary clarifiers tested (Fig. 1; Table 3). Since all dots contained the same total DNA, the nifH probe signal amplitudes (Fig. 1) indicate the relative proportion of N2-fixing genes in the total population, not the absolute population size. The amount of nifH gene probe that hybridized to these primary-clarifier DNA samples varied from very low to as much as that seen using control DNA from pure cultures of known N2-fixing organisms. This suggests that a high proportion of the total microbial consortium in the primary clarifier carries the nifH gene. We also observed a general correlation between the nifH probe signal intensity and the average in situ nitrogen-fixing activity (Table 3) calculated from all sampling sites we tested in each clarifier (Fig. 1). The maximum N2 fixation rates observed in the six primary clarifiers ranged between 0.87 and 4.90 mg of N liter−1 day−1 (Table 3), comparable to the maximum rates previously reported from an aerated lagoon system (5.5 mg of N liter−1 day−1) (8).
FIG. 1.
Dot blot hybridization of 1-μg DNA samples to the nifH gene probe. Positive controls (from left to right) are primary-clarifier isolates KPN3, KPG2, and KPG3, A. tolulyticus, Pseudomonas stutzeri JM300, Sinorhizobium meliloti, and Rhodobacter sphaeroides. Negative controls (from left to right): KPG1 (activated sludge isolate), Escherichia coli K-12, P. stutzeri Zobell, Paracoccus denitrificans, Corynebacterium nephridii, Pseudomonas sp. strain G-179, no DNA (NaOH) control. Seven primary-clarifier samples (see Table 1 for mill treatment system characteristics) were taken from mill B-1, nine from mill E, nine from mill F, eight from mill G, and two from mill C. Samples were taken at various depths at different locations across the clarifier diameter. The actual biochemical N2-fixing activity indicated, in milligrams of N fixed per liter per day, is an average of in situ activity measured at all sampling points (±, 0 to 0.5; +, 0.5 to 1.0; ++, 1.0 to 1.5; +++, 1.5 to 2.0).
TABLE 3.
Maximum observed N2 fixation rates in mill primary clarifiers examined
| Mill | Date (day-mo-yr) | Sampling depth (m) | DO level (mg liter−1) | N2 fixation rate (mg of N liter−1 day−1) |
|---|---|---|---|---|
| B-1 | 19-07-99 | 3.0 | 0.0 | 4.26 |
| 29-11-99 | 4.0 | 2.9 | 0.87 | |
| B-2 | 19-07-99 | 4.5 | 0.0 | 1.30 |
| 29-11-99 | 5.0 | 3.8 | NDa | |
| C | 6-07-99 | 4.0 | 0.0 | 4.70 |
| 22-11-99 | 4.0 | 0.0 | 3.03 | |
| 7-06-99 | 5.0 | NTb | 4.71 | |
| 4-10-99 | 5.0 | 0.0 | 1.85 | |
| 24-05-99 | 6.0 | NT | 2.19 | |
| E | 29-11-99 | 5.0 | 2.7 | ND |
| 3-08-99 | 7.5 | 0.0 | 1.00 | |
| F | 16-08-99 | 5.5 | 0.0 | 4.90 |
| G | 16-08-99 | 5.5 | 0.0 | 0.89 |
ND, no activity detected.
NT, not tested.
The primary clarifiers, which are responsible for mechanical settling, had, as expected, only a fraction of the biomass density of the corresponding activated-sludge-containing aeration tanks. At a depth of 4 m, the mill C primary clarifier had 167 ng of ATP ml−1, while the mill C aeration tank contained 3,400 to 5,200 ng of ATP ml−1.
Monitoring the mill C primary clarifier for 6 months indicated that N2 fixation probably occurs continually (Table 3). Because the primary clarifiers are unmixed and thus highly heterogeneous, the amount of N2 fixation (and biomass density) varies greatly with the sampling site selected (Table 3). To see if there was net N2 fixation in the primary clarifier, the fixed N concentrations of the mill C primary-clarifier input and output were compared (three sets of grab samples collected on three different days). The input effluent had on average a total Kjeldahl nitrogen concentration (TKN) of 3.8 ± 0.2 mg/liter, and the clarified output effluent had an average TKN of 4.8 ± 0.7 mg/liter, indicating a substantial increase in fixed N (about 120 kg, based on 120,000 m3 of raw effluent clarified day−1), despite the decrease in total C accompanying the removal of settleable solids by the primary clarifier. Because of the fluctuations in the operation of each mill and the large variations between mills, these can only be considered preliminary data for mill C, and applicable only to mill C. Since the mill measures the C-to-N ratio of the 107 tonnes of primary biosolids removed day−1 by this clarifier to be 650:1 to 800:1, it can be calculated that the settled biosolids decreased the output TKN by about 1.0 mg/liter, meaning that another 120 kg of N is produced day−1 in the primary clarifier of mill C. The structure and bioavailability of the N entering and exiting the primary clarifiers are unknown. After passage into the mill C aeration tank, most of this N appears to become part of the activated sludge, as the mill adds ammonia and ammonium pyrophosphate to a C/N/P ratio of 100:4.0 to 4.5:0.8. If all this deliberately added N is taken up, the activated (secondary) sludge should have a C/N ratio between 20:1 and 25:1. In fact, the measured ratio is typically 9:1, strongly suggesting that a large amount of the N arriving from the primary clarifier is captured in the activated sludge. Conversely, the fact that the primary-clarifier diazotrophs fix N in the presence of an influent TKN of 3.8 mg/liter suggests that the N originating in the mill is not readily available to them.
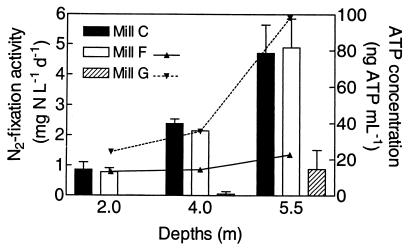
In most of the six primary clarifiers, higher N2 fixation rates were observed at greater sampling depths (Table 3 and Fig. 2). There are three likely reasons. Firstly, ATP measurements showed higher biomass concentrations in deeper samples, which contain more solids (Fig. 2). Secondly, because nitrogenase is O2 sensitive, the low oxygen tension observed in deeper samples may allow more N2 fixation per unit of biomass (Table 3). Thirdly, the settling of wood particulates in primary clarifiers may also improve carbohydrate availability at greater depths, and readily available carbohydrates are essential for N2 fixation, since a large amount of ATP is required.
FIG. 2.
Influence of depth on N2 fixation activities (bars) and ATP concentrations (lines) of three primary clarifiers. Sampling was performed at the middle of the clarifier radius except for mill G (3 m from the center). N2 fixation is reported as means of duplicate samples ± standard deviations. ATP concentrations were not measured for the mill C primary clarifier.
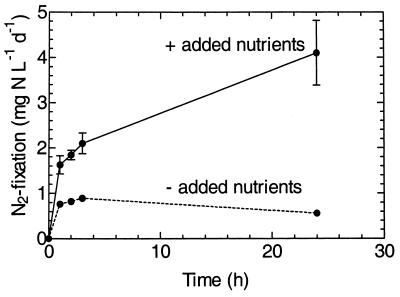
The rate of N2 fixation was shown to be nutrient limited in the mill C primary clarifier, at least in bottom sludge samples, where in situ fixation was highest (Fig. 3). Added glucose and acetate increased the N2-fixing activity more than sevenfold over 24 h. Although the Enterobacteriaceae, particularly Klebsiella pneumoniae, are metabolically versatile (20), it is likely that the glucose was the major driver of increased N2 fixation. Bruce and Clark (4) observed that the AR rate of K. pneumoniae isolates growing on Kraft mill effluent in an aerated stabilization basin (lagoon) was greatly stimulated by 5 g of glucose liter−1. Thus, the nutrient limitation that exists in the aeration tank biomass (16) also occurs in the primary clarifier, at least for N2 fixation.
FIG. 3.
Effects of added nutrients on N2 fixation in the mill C primary clarifier. The sample was obtained at a 5-m depth. Glucose and sodium acetate (final concentrations, 0.05% [wt/vol]) were added. Values are means of duplicate samples ± standard deviations. Error bars that are not visible are smaller than the symbols.
Importance of Klebsiella in the primary-clarifier N2-fixing community.
Previously, we demonstrated that coliform bacteria, predominantly Klebsiella strains, were numerous in several of the primary clarifiers examined (11a). To determine the dominant taxa in the primary-clarifier N2-fixing community, nonselective (dilute TSA) agar plate counts were done. From the same plates 188 colonies, 94 each from samples A and B (Table 4), were screened by colony hybridization. Of these isolates, 94 (50%) were nifH positive. Identification of 20 randomly selected nifH positive isolates revealed that 18 (90%) were klebsiellas. Thus, N2-fixing coliform bacteria belonging to the genus Klebsiella account for 45% of the total bacterial population culturable on a nonselective medium. The API 20E system we used for colony characterization classed all the isolated klebsiellas as K. pneumoniae. However, previous work revealed that a high proportion of the K. pneumoniae isolates found in pulp and paper mill ecosystems could be more appropriately referred to as Klebsiella terrigena or Klebsiella planticola due to their inability to produce gas from lactose at 44.5°C (11a). The high numbers and proportions of Klebsiella strains growing on the nonselective TSA plates indicate a higher proportion of coliform bacteria in primary-clarifier samples than was estimated by the TSA/MacConkey plate count ratios (Table 4). Thus, a combination of gene probing, API identification, and classical nonselective growth techniques shows that, at least in this primary-clarifier system, the MacConkey agar MPN counts seriously underestimate the total number of coliform bacteria present.
TABLE 4.
Occurrence of nifH-containing members among total culturable bacteria and coliform bacteria from a selected primary clarifier, and predominance of Klebsiella spp. among N2-fixing bacteria isolated
| Samplea | Total bacteria
|
Coliform bacteria
|
||||
|---|---|---|---|---|---|---|
| Countb (107 CFU ml−1 ± SEM) | No. hybridizing to nifH/no. tested
|
Countc (106 CFU ml−1 ± SEM) | No. hybridizing to nifH/no. tested
|
|||
| All bacteria | K. pneumoniaed | All bacteria | K. pneumoniaed | |||
| A | 3.2 ± 0.2 | 51/94 | 8/10 | 3.5 ± 0.1 | 92/94 | 10/10 |
| B | 1.2 ± 0.1 | 43/94 | 10/10 | 1.2 ± 0.1 | 94/94 | 10/10 |
| Avg | 2.2 ± 0.2 | 50% | 90% | 2.3 ± 0.2 | 98% | 100% |
Primary clarifier samples (A and B) correspond to mill C bottom sludges obtained from the center and the middle radius of the settlement basin, respectively.
On triplicate TSA plates.
On triplicate MacConkey plates.
Identified by the API 20E test.
Colony hybridization of 188 MacConkey isolates showed that 98% of the coliform population in the primary clarifier carried nifH. Of the 186 nifH-bearing isolates, 20 were randomly chosen, and all (20 of 20) were identified as Klebsiella strains (Table 4). The seven mill systems surveyed here were all shown previously to support numerous coliform bacteria (11a), but this is the first report demonstrating that primary clarifiers have large populations of bacteria actively fixing nitrogen in situ, and even larger populations with the genetic potential for N2 fixation. Unlike previous results from a lagoon treatment system (4), our findings indicate that N2-fixing Klebsiella strains are the major diazotrophs in pulp and paper mill water and treatment systems.
Does the presence of the nifH gene correlate with measured N2-fixing activity?
A total of 48 coliform isolates from several mills were tested for the nifH gene and their ability to reduce acetylene (Table 5). Of the 48 isolates, 13 (27%) showed AR activity while 24 (50%) possessed the nifH gene. No isolate testing negative for nifH could reduce acetylene, and all strains reducing acetylene tested positive for nifH. Comparable active-to-potential N2 fixation ratios were observed with K. pneumoniae isolates, with 9 of 32 (28%) and 18 of 32 (56%) displaying N2-fixing activity and the presence of the nifH gene, respectively. The percentage of N2-fixing K. pneumoniae isolates among isolates from the seven mill water systems (28%) is consistent with the findings of a previous report in which 32% of klebsiella from several ecosystems, including pulp mills, possessed N2-fixing ability (18). The low proportion of nifH-positive K. pneumoniae isolates (56%) seen in Table 5 compared with the proportion in Table 4 (100%) is presumably due to the fact that the coliform bacteria for which results are shown in Table 5 were isolated from many places in the seven pulp mill water systems, not just from primary clarifiers. Other evidence for the selection of N2 fixers by the primary-clarifier environment is the increase in the proportion of nifH-positive K. pneumoniae isolates, from 56% (18 of 32) for isolates from all sampling locations (Table 5) to 83% (15 of 18) when only K. pneumoniae isolates from primary-clarifier samples are considered. In the primary clarifier, N2-fixing klebsiellas likely possess selective growth advantages over non-N2-fixing bacteria, such as the following: (i) raw mill process effluents typically have high C/N ratios (2, 11a, 12, 18); (ii) the temperatures in primary clarifiers can rise to 40°C, also selecting for klebsiellas, since >90% of the strains from these seven paper mill water systems are thermotolerant (grow at 44.5°C) (11a); and (iii) the very low DO levels observed in primary clarifiers (Table 3) presumably select for facultative bacteria, such as coliforms (4, 21).
TABLE 5.
Distribution of coliforms, N2 fixation, and the nifH gene in several pulp and paper mill water systems
| Speciesa | No. of isolates
|
||
|---|---|---|---|
| Total tested | With the nifH geneb | With AR activity | |
| Klebsiella pneumoniae | 32 | 18 | 9 |
| Enterobacter cloacae | 8 | 2 | 2 |
| Citrobacter freundii | 4 | 2 | 2 |
| Enterobacter agglomerans | 1 | 0 | 0 |
| Escherichia coli | 3 | 2 | 0 |
| Total | 48 | 24 | 13 |
Based on API 20E results for isolates from various treatment system sources (effluent feeds, clarifiers, and aeration tanks) from seven mills.
Determined by colony hybridization.
Conclusions.
(i) The combination of in situ and isolate nifH gene probing and AR assays with classical microbial enumeration, isolation, and identification was very effective in elucidating the nature, magnitude, and microbiology of N2 fixation in mill water systems.
(ii) Klebsiella strains actively fixing N2 are major components of pulp mill primary-clarifier microbial communities. This probably explains why coliform bacteria are indigenous to pulp and paper mill water systems (2, 11a).
(iii) N2 fixation by klebsiellas and related coliform bacteria is commonplace and continuous in these primary clarifiers and may contribute significantly to the fixed N required by the activated-sludge biomass. It should also increase the value of the combined dewatered sludges as fertilizers.
(iv) Every pulp and paper mill biotreatment system is unique. The relative importance of the N2 fixed by coliforms in each primary clarifier, and whether it can be substantially increased by adjustments to the clarifier's mode of operation, remains to be shown.
ACKNOWLEDGMENTS
We thank the staff of the seven mills investigated for their cooperation. The suggestions of R. Knowles and technical support of F. Young are appreciated. We also thank B. Ward and R. Ye for supplying us with pure culture controls. Y. Chan is thanked for his generous donation of plasmid pER4.
This research was funded in part by the Pulp and Paper Research Institute of Canada (Paprican) and by support for F. Gauthier and J. D. Neufeld from the Natural Sciences and Engineering Research Council (NSERC) of Canada.
REFERENCES
- 1.American Public Health Association. Standard methods for the examination of water and wastewater. 20th ed. Washington, D.C.: American Public Health Association; 1998. [Google Scholar]
- 2.Archibald F S. The presence of coliform bacteria in Canadian pulp and paper mill water systems—a cause for concern? Water Qual Res J Can. 2000;35:1–22. [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 4.Bruce M E, Clark T A. Klebsiella and nitrogen fixation in pulp and paper mill effluents and treatment systems. Appita J. 1994;47:231–237. [Google Scholar]
- 5.Cannon M, Hill S, Kavanaugh E, Cannon F. A molecular genetic study of nif expression in Klebsiella pneumoniae at the level of transcription, translation and nitrogenase activity. Mol Gen Genet. 1985;198:198–206. doi: 10.1007/BF00382996. [DOI] [PubMed] [Google Scholar]
- 6.Caplenas N R, Kanarek M S, Dufour A P. Source and extent of Klebsiella pneumoniae in the paper industry. Appl Environ Microbiol. 1981;42:779–785. doi: 10.1128/aem.42.5.779-785.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson C A, Ingraham J L. Comparison of denitrification by Pseudomonas stutzeri, Pseudomonas aeruginosa, and Paracoccus denitrificans. Appl Environ Microbiol. 1983;45:1247–1253. doi: 10.1128/aem.45.4.1247-1253.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark T A, Dare P H, Bruce M E. Nitrogen fixation in an aerated stabilization basin treating bleached kraft mill wastewater. Water Environ Res. 1997;69:1039–1046. [Google Scholar]
- 9.Duncan D W, Razzell W E. Klebsiella biotypes among coliforms isolated from forest environments and farm produce. Appl Microbiol. 1972;24:933–938. doi: 10.1128/am.24.6.933-938.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallik E, Chan Y-K, Robson R. Detection of alternative nitrogenases in aerobic gram-negative nitrogen-fixing bacteria. J Bacteriol. 1991;173:365–371. doi: 10.1128/jb.173.1.365-371.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn T M, Kunkel B, Vos G D, Signer X E R. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J Bacteriol. 1986;167:66–72. doi: 10.1128/jb.167.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Gauthier, F., and F. S. Archibald. The ecology of “fecal indicator” bacteria commonly found in pulp and paper mill water systems. Water Res., in press. [DOI] [PubMed]
- 12.Hardy R W F, Burns R C, Holsten R D. Applications of the acetylene-ethylene assay for measurement of nitrogen fixation. Soil Biol Biochem. 1973;5:47–81. [Google Scholar]
- 13.Hill S. How is nitrogenase regulated by oxygen? FEMS Microbiol Rev. 1988;54:111–130. doi: 10.1111/j.1574-6968.1988.tb02738.x. [DOI] [PubMed] [Google Scholar]
- 14.Hill S, Turner G L, Bergensen F J. Synthesis and activity of nitrogenase in Klebsiella pneumoniae exposed to low concentrations of oxygen. J Gen Microbiol. 1984;130:1061–1067. doi: 10.1099/00221287-130-5-1061. [DOI] [PubMed] [Google Scholar]
- 15.Huntley B E, Jones A C, Cabelli V J. Klebsiella densities in waters receiving wood pulp effluents. J Water Pollut Control Fed. 1976;48:1766–1771. [PubMed] [Google Scholar]
- 16.Jenkins D, Richard M G, Daigger G T. Manual on the causes and control of activated sludge bulking and foaming. 2nd ed. Chelsea, Mich: Lewis Publishers; 1993. [Google Scholar]
- 17.Jones R L, Woodley P, Zinoni A B, Robson R L. The nifH gene encoding the Fe protein of the molybdenum nitrogenase from Azotobacter chroococcum. Gene. 1993;123:145–146. doi: 10.1016/0378-1119(93)90555-h. [DOI] [PubMed] [Google Scholar]
- 18.Knowles R, Neufeld R, Simpson S. Acetylene reduction (nitrogen fixation) by pulp and paper mill effluents and by Klebsiella isolated from effluents and environmental situations. Appl Microbiol. 1974;28:608–613. doi: 10.1128/am.28.4.608-613.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Möbius C H. Nitrogen and phosphorus limits for nutrient deficient industrial wastewaters. Water Sci Technol. 1991;24:259–267. [Google Scholar]
- 20.Neilson A H, Allard A-S. Acetylene reduction (N2-fixation) by Enterobacteriaceae isolated from industrial wastewaters and biological treatment systems. Appl Microbiol Biotechnol. 1985;23:67–74. [Google Scholar]
- 21.Neilson A H, Sparell L. Acetylene reduction (nitrogen fixation) by Enterobacteriaceae isolated from paper mill process waters. Appl Environ Microbiol. 1976;32:197–205. doi: 10.1128/aem.32.2.197-205.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niemela S I, Vaatanen P. Survival in lake water of Klebsiella pneumoniae discharged by a paper mill. Appl Environ Microbiol. 1982;44:264–269. doi: 10.1128/aem.44.2.264-269.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 24.Satoh T, Hoshino Y, Kitamura H. Rhodopseudomonas sphaeroides forma sp. denitrificans, a denitrifying strain as a subspecies of Rhodopseudomonas sphaeroides. Arch Microbiol. 1976;108:265–269. doi: 10.1007/BF00454851. [DOI] [PubMed] [Google Scholar]
- 25.Smith G B, Tiedje J M. Isolation and characterization of a nitrite reductase gene and its use as a probe for denitrifying bacteria. Appl Environ Microbiol. 1992;58:376–384. doi: 10.1128/aem.58.1.376-384.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vincent J M. Serological properties of the root-nodule bacteria. I. Strains of Rhizobium meliloti. Proc Linn Soc N S W. 1941;66:145–154. [Google Scholar]
- 27.Ye R W, Fries M R, Bezborodnikov S G, Averill B A, Tiedje J M. Characterization of the structural gene encoding a copper-containing nitrite reductase and homology of this gene to DNA of other denitrifiers. Appl Environ Microbiol. 1993;59:250–254. doi: 10.1128/aem.59.1.250-254.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J, Fries M R, Chee-Sandford J C, Tiedje J M. Phylogenetic analyses of a new group of denitrifiers capable of anaerobic growth on toluene and description of Azoarcus tolulyticus sp. nov. Int J Syst Bacteriol. 1995;45:500–506. doi: 10.1099/00207713-45-3-500. [DOI] [PubMed] [Google Scholar]