Abstract
Background and Aim:
Portal hypertension (PH) is a syndrome associated with cirrhosis and characterized by a progressive increase in portal pressure, with consequent compensatory vascular dilation. Gastric vascular changes associated with oxidative and nitrosative stress characterize the clinical presentation of portal hypertensive gastropathy (PHG). In addition, the inflammatory process is considered an aggravating factor for severity by contributing to gastric tissue injury. The aim of this study was to investigate the synergistic anti-inflammatory and antioxidant action of N-acetylcysteine (NAC) in the stomach of rats with PH.
Materials and Methods:
Eighteen Wistar male rats were used in this experimental protocol and were divided into three groups with six in each group: sham-operated (SO), partial portal vein ligation (PPVL), and PPVL + NAC. Treatment with NAC at a dose of 10 mg/kg (i.p.) was initiated on day 8 after surgery and continued for 7 days. We evaluated the expression of iNOS, NQO-1, HSP-90, and SOD by Western blot, as well as nuclear factor-kappa B (NF-κB) and tumor necrosis factor (TNF)-α staining by immunohistochemistry, in the rat stomach.
Results:
The PPVL group exhibited increased expression of HSP-90, iNOS, SOD, and NQO-1 when compared with controls. NAC reduced the expression of all studied proteins. Similarly, NF-κB and TNF-α staining was increased in PPVL animals versus controls and reduced in PPVL + NAC versus PPVL animals, respectively.
Conclusion:
These results suggest the effectiveness of NAC as a dual anti-inflammatory and antioxidant in animals with experimental PHG induced by partial ligation of the portal vein.
Keywords: Inflammation, N-acetylcysteine, oxidative stress, portal hypertension
Introduction
The portal system is responsible for leading blood from the intra-abdominal portion of the gastrointestinal tract, pancreas, spleen, and gallbladder to the liver sinusoids via the terminal branches of the portal vein.[1]
Pre-hepatic abnormalities (such as splenic vein thrombosis), intrahepatic abnormalities (such as cirrhosis), and post-hepatic conditions (such as Budd-Chiari syndrome) may all influence blood pressure within the portal system. The only shared characteristic of the aforementioned examples is the emergence of an anatomical barrier to blood flow. This obstacle triggers a compensatory mechanism to reduce blood pressure, promoting the development of hyperdynamic collateral circulation. This condition, characterized by a progressive increase of pressure in the portal system, is called portal hypertension (PH).[2]
The resulting bypass of blood flow from the site of obstruction directly into the systemic circulation triggers serious complications, such as ascites, hepatic encephalopathy, and gastrointestinal bleeding, with the latter accounting for the high mortality rate of 50% among patients with PH.[3]
Microcirculatory alterations in the gastric mucosa as a result of the characteristic vasodilation of HP were described by McCormack et al. in 1985.[4] Several different terms have been used to describe these changes, such as inflammatory gastritis, gastric mucosal vasculopathy, portal hypertensive mucosa, and portal hypertensive gastropathy (PHG).[5]
PHG is characterized by vasodilation secondary to obstruction of gastric blood flow, and nitric oxide (NO) is the main mediator in this vascular activation pathway.[6] NO is an endothelium-derived relaxing factor produced from L-arginine and molecular oxygen, in a process catalyzed by enzymes in the nitric oxide synthase (NOS) family. The three major NOS isoforms, neuronal NOS (nNOS and NOS1), inducible NOS (iNOS and NOS2), and endothelial NOS (eNOS and NOS3), are described in the literature, with well-established roles. NOS2 can be expressed in various cell types, and it plays an important role in inflammatory diseases and septic shock. NOS3 is expressed in endothelial cells and acts on endothelial smooth muscle relaxation.[7] All three isoforms have been associated with PH; however, the most influential isoforms in this syndrome are NOS2 and NOS3.[8] NOS3 is closely related to several factors and proteins that have positive and negative impacts on its production. Proteins that positively influence the production of this enzyme include the positive regulator molecular chaperone heat shock protein 90 (HSP90).[9]
Shear stress and vascular endothelial growth factor (VEGF) also act on the pathway that stimulates the production of NOS3, and both have been studied previously in investigations conducted by our research group.[10,11]
NOS2 produces a relatively high level of NO. This isoform is expressed in response to stimulation from lipopolysaccharides or inflammatory cytokines, and its expression is modulated by transcriptional mechanisms. Nuclear factor-kappa B (NF-κB) is the primary mediator for the induction of iNOS, which, in turn, is activated mainly by tumor necrosis factor (TNF)-α and oxidative stress.[8]
In PH, overproduction of NO via NOS2 and NOS3 determines its reaction with the superoxide anion radical (O2–º), leading to the formation of peroxynitrite (ONOO–). This, in turn, is a highly reactive species that can contribute to cellular damage, thus aggravating the overall clinical picture.[12]
The inflammation characteristic of PH affects gastric regeneration and defense, thus increasing the risk of bleeding.[13] Currently, one hypothetical treatment strategy involves the direct reduction of proinflammatory chemokines and cytokines, which contribute to the progression of PH.[14]
Thus, treatment with both antioxidant and anti-inflammatory action would represent a promising candidate for minimizing the consequences of PHG. Previous work published by our research group has demonstrated the antioxidant properties of N-acetylcysteine (NAC), a molecule used extensively in clinical practice, in an experimental model of PH.[11] The aim of this study was to evaluate the synergistic action of NAC as an antioxidant and anti-inflammatory in PHG.
Materials and Methods
Ethics
All procedures were carried out in accordance with current Brazilian legislation for the practice of scientific research using animals (Law 11.794 of October 8, 2008, 2013 Brazilian Guidelines for the Care and Use of Animals for Scientific and Educational Purposes, and 2013 CONCEA Guidelines for the Practice of Euthanasia) and followed the recommendations of the Principles of Care for Laboratory Animals formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Animal Groups and Experimental Protocols
The animals used in this study were obtained through vivarium, in accordance with institutional specifications. Throughout the experiment, animals were kept in plastic bin-type cages (47 × 34 × 18 cm) lined with wood shavings, under a 12-h light/dark cycle, at a temperature of 22 ± 4°C. They had access to water ad libitum and were fed commercially available rodent chow (Purina®–Nutripal, Porto Alegre, RS, Brazil), approximately 16 g/day.
On the first day of the experiment, the Wistar male rats were randomly divided into three groups (n=18 animals): sham-operated (SO) (n=6), partial portal vein ligation (PPVL) (n=6), and PPVL + NAC (n=6). All animals were weighed (±250 g) and anesthetized with ketamine (100 mg/kg i.p.) and xylazine (10 mg/kg i.p.). Once a proper plane of anesthesia had been achieved, a midline laparotomy was performed, and the bowel was gently retracted with a gauze pad soaked in saline. In group SO, only manipulation of the portal vein was performed, whereas in the other groups, the vein was ligated for experimental induction of PH. Briefly, the portal vein was isolated with 3-0 silk thread, partially blocked using a 20G needle placed in front of the vessel, and tied off. After ligation, the needle was removed, thus leaving the vein partially obstructed. Bowel loops were gently replaced into the abdomen, 10 mL of saline solution infused the abdominal cavity, and the muscle closed with running sutures. The experimental model used in this study was first described by Sikuler et al.[15] in 1985 and induces pre-hepatic PH.
After the surgical procedures, animals were kept in individual cages. Metamizole was administered for postoperative analgesia, with the first dose given via intramuscular injection (200 mg/kg) and subsequent doses given orally (500 mg/kg every 8 h for 72 h).
Seven days were allowed to elapse for the establishment of PHG before starting treatment. NAC (Sigma Chemical Co., St. Louis, MO, USA; CAS registry number 616-91-1) was administered intraperitoneally at a dose of 10 mg/kg, in agreement with previous studies.[11,12] The drug was dissolved in 0.6 mL of saline (0.9% NaCl), and the same volume was administered to groups that received vehicle only (SO and PPVL). Treatment was continued for 7 days, thus completing the full 15-day experimental period.
Euthanasia and Collection of Tissue Samples
On day 15, animals were again weighed and anesthetized with ketamine (100 mg/kg i.p.) and xylazine (10 mg/kg i.p.). Once adequate anesthesia had been achieved, a midline laparotomy was performed, and the stomach was resected for analysis. One portion of the stomach was frozen at -80°C, and a second fragment was fixed in 10% buffered formalin for 24 h. Then, 3-mm sections were cut from the paraffin block using a rotary microtome.
Western Blot
Western blot analysis was performed using cytosolic extract prepared from stomach homogenates. The protein concentration of each sample was measured by the Bradford method. Then, lysate proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were blocked by submerging in Tris–buffered saline and 0.05% Tween (TTBS) with 5% nonfat dry milk for 1 h at room temperature and probed overnight at 4°C. The following antibodies were used: mouse polyclonal antibody [NOS2 (sc-7271), Santa Cruz Biotechnology, Santa Cruz, CA, USA]; mouse monoclonal antibody [HSP90 (sc-101494), Santa Cruz Biotechnology, Santa Cruz, CA, USA]; goat monoclonal antibody [NQO1 (sc-16464), Santa Cruz Biotechnology, Santa Cruz, CA, USA]; goat monoclonal antibody [SOD (sc-8637), Santa Cruz Biotechnology, Santa Cruz, CA, USA], at 1:200 dilution with TTBS in 5% nonfat dry milk; and anti-β-actin (42-kDA) antibody (Sigma Aldrich, St. Louis, MO, USA) at 1:1000 dilution with TTBS in 5% nonfat dry milk. After overnight incubation, the membranes were washed with TTBS and incubated for 1 h at room temperature with secondary goat anti-mouse IgG-HRP sc-2005, donkey anti-goat antibody (sc-2020, Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:4000). Protein detection was performed via chemiluminescence using a commercial ECL kit (Amersham Pharmacia Biotech, Little Chalfont, England). The density of the specific bands was quantified with imaging densitometry software (Scion Image, Maryland, MA, USA).[16]
Immunohistochemistry
The expression of NF-κB and TNF-α in stomach tissue was analyzed using immunohistochemical techniques. Buffer at 100°C was used for antigen retrieval, and endogenous peroxidase activity was blocked by incubation in absolute methanol. Slides were then incubated with rabbit polyclonal antibody (NF-κB [sc-9072], 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and goat monoclonal antibody (TNF-α [sc-1351], 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C, followed by washing with buffer and incubation with secondary goat anti-rabbit IgG-HRP (sc-2004) for 30 min at room temperature. The slides were analyzed under a microscope equipped with a digital camera, and images were captured using Image-Plus software (Media Cybernetics, Bethesda, MD, USA). Quantification of staining for both markers was performed by digital image analysis in Adobe Photoshop® CS3 Extended 10.0, by counting the number of pixels stained. The level of expression was determined by multiplying the average density of the image by the percentage of positively stained areas (those colored brown).[17]
Statistical Analysis
Analysis of Variance was used to evaluate the quantitative data, followed by the Student–Newman–Keuls test for the analysis of multiple comparisons. The significance level was set at 5% (p<0.05). All data are presented as mean ± SE. The GraphPad InStat version 3.0 program for Windows was used.
Results
Western Blotting Analysis
Analysis of HSP90 protein expression revealed a significant increase in the PPVL group compared with controls (p<0.01). NAC effectively reduced these values in the LPVP + NAC group (p<0.01) (Fig. 1).
Figure 1.
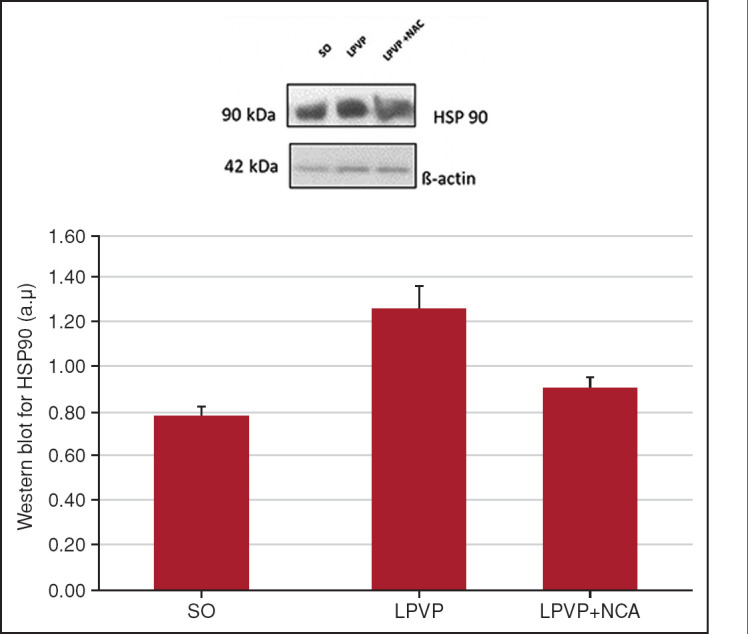
Western blot for HSP90. Effects of PPVL and NAC administration on HSP90, quantified by the Western blot technique.
*: P<0.01; **: P<0.01 (n=6); SO: Sham-operated group; PPVL: Partial portal vein ligation group; PPVL + NAC: Partial portal vein ligation group plus N-acetylcysteine treatment.
When evaluating the expression of iNOS, a decrease was observed in the LPVP + NAC group (p<0.05) and a significant increase in the LPVP group (p<0.01) (Fig. 2).
Figure 2.
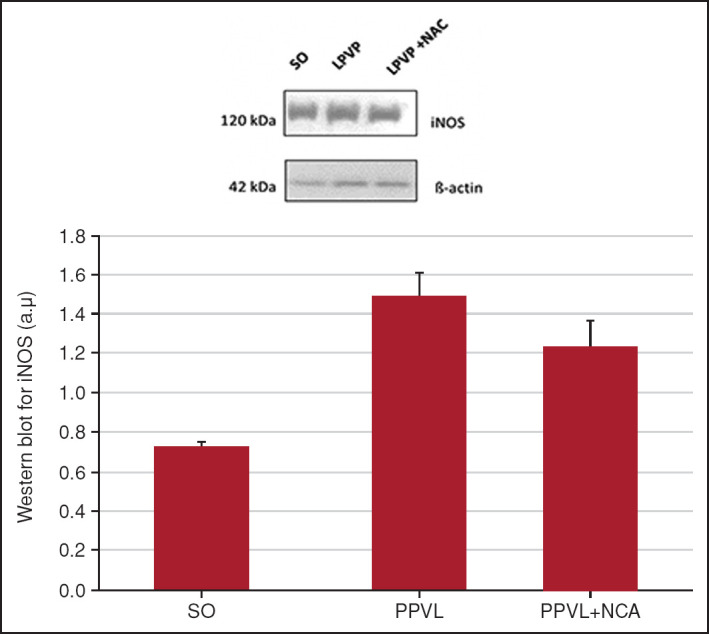
Western blot for iNOS. Effects of PPVL and NAC administration on iNOS, quantified by the Western blot technique.
*: P<0.01; **: P<0.05 (n=6).
NAC was able to substantially reduce SOD values in the treated group, up to control levels (p<0.05). In the LPVP group, SOD was overexpressed when compared with the SO group (p<0.05) (Fig. 3).
Figure 3.
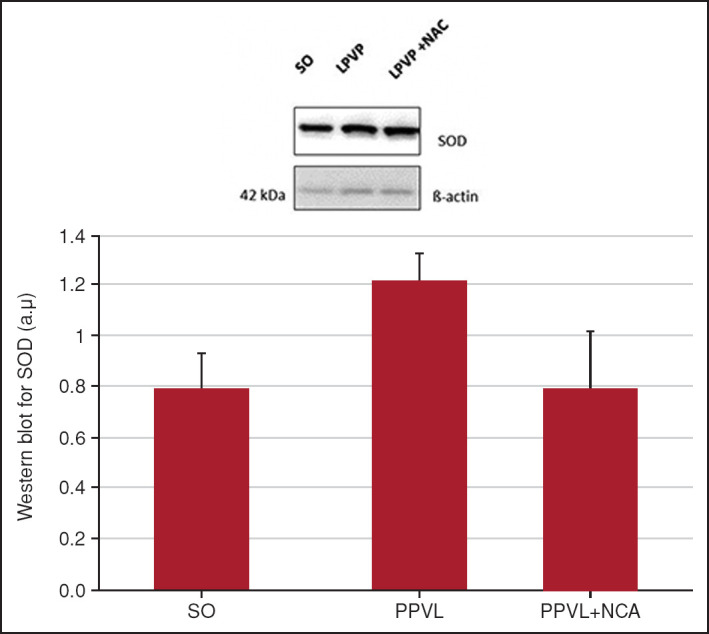
Western blot for SOD. Effects of PPVL and NAC administration on SOD, quantified by the Western blot technique.
*: P<0.05; **: P<0.05 (n=6).
In the analysis of NQO1 protein expression, NAC significantly reduced the values in the LPVP + NAC group when compared with the LPVP group (p<0.05), which had a significantly increased expression when compared with the control group (p<0.05) (Fig. 4).
Figure 4.
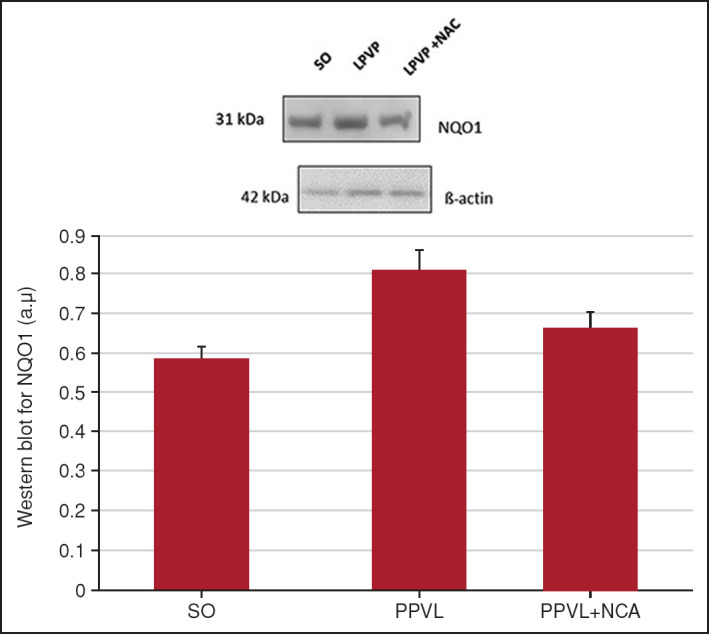
Western blot for NQO1. Effects of PPVL and NAC administration on NQO1, quantified by the Western blot technique.
*: P<0.05; **: P<0.05 (n=6).
Immunohistochemistry
Animals in the PPVL group showed a significant increase in positive staining for NF-κB as compared with controls (p<0.01). NAC was effective in reducing this immunoreactivity in the PPVL + NAC group (p<0.01) (Fig. 5).
Figure 5.
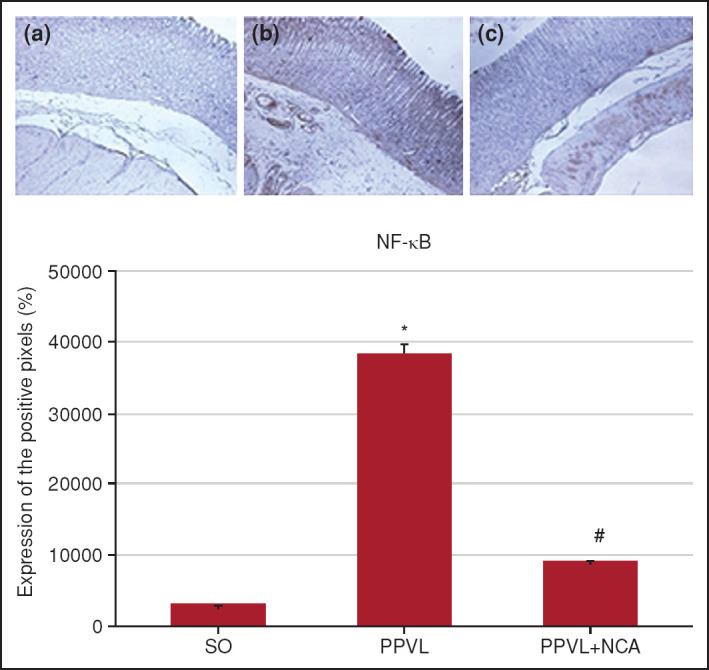
Immunohistochemical staining for NF-κB. Effects of PPVL and NAC administration on NF-κB expression. (a) SO group, (b) PPVL group, and (c) PPVL + NAC group.
*: P<0.001 (n=6).
The same pattern of expression was observed in the analysis of TNF-α. In the PPVL group, there was intense staining as compared with the SO group (p<0.01); in the PPVL + NAC group, this immunoreactivity was reduced (p<0.01) (Fig. 6).
Figure 6.
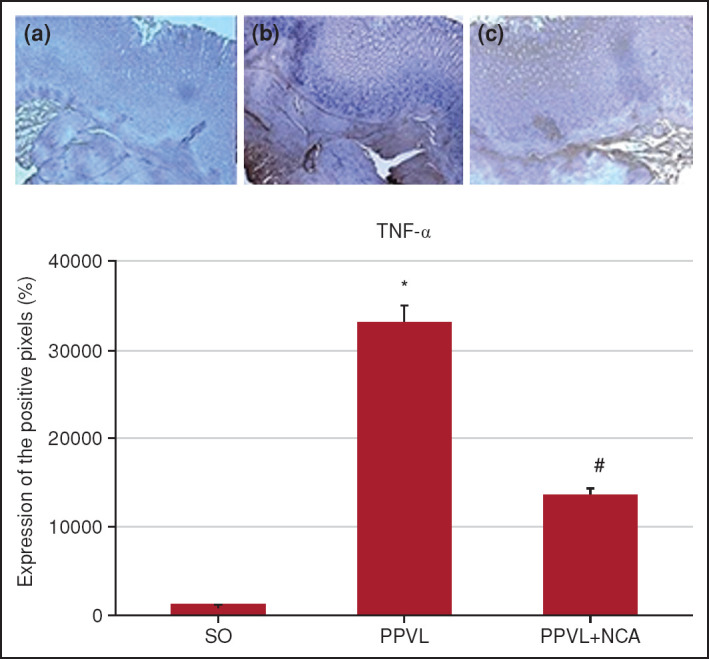
Immunohistochemical staining for TNF-α. Effects of PPVL and NAC administration on TNF-α expression. (a) SO group, (b) PPVL group, and (c) PPVL + NAC group.
*: P<0.001 (n=6).
Discussion
PH, often described as a complication of chronic liver disease, is characterized by increased blood pressure in the portal system, splanchnic vasodilation, and subsequent formation of portosystemic collaterals.[18]
The stomach is one of the main sites affected by this marked increase in vascular diameter; accordingly, gastropathy is one of the most frequent complications of PH. In practice, gastric lesions are typically located at the fundus and superior portion of the body of the stomach[19] and are characterized by a mosaic-like pattern, with or without cherry-red spots.[20] Still, the release of inflammatory mediators and consequent gastric inflammation appears to contribute to the development and worsening of gastropathy of PH.[21]
The involvement of NO in the development of hyperdynamic collateral circulation was originally proposed in 1991 by Vallance and Moncada.[22] This hypothesis has since been confirmed by several studies that demonstrated NO as the main mediator of vascular abnormalities in PH.[9,23] In previous investigations, our research group found increased expression of two NOS isoforms predominantly involved in the activation of NO synthesis (eNOS and iNOS), using an animal model of PH.[10,24] An increase in eNOS expression is already detectable in the early stages of PH induced by carbon tetrachloride (CCl4),[25] and eNOS appears to be the major enzymatic pathway for NO synthesis.[26] Agonists in the eNOS activation pathway include shear stress, VEGF, and HSP90, among others. Furthermore, inflammatory cytokines such as TNF-α appear to influence NO production by eNOS.[9]
Another modulator of NO upregulation is VEGF, which is activated by increased portal pressure. Through its angiogenic activity, VEGF ultimately contributes to increased blood flow in the splanchnic territory and to local vasodilation.[27] In the present study, animals subjected to the same experimental model had increased expression of HSP90 when compared with controls (Fig. 1), which demonstrates the influence of this chaperone in the eNOS activation pathway and confirms previous results published by Ai et al.,[28] in which HSP90 was overexpressed in the endothelium of mesenteric vessels in PPVL animals.
HSP90 in PH exerts an agonist effect on NO production and vasodilation via eNOS.[29] NAC was able to reduce the expression of HSP90 in the model tested herein. Therefore, the chosen treatment appears to fully modulate the studied pathway, reducing the levels not only of VEGF but also of HSP90, thereby contributing to a reduction in vasodilation mediated by eNOS (Fig. 1).
In PH, iNOS is also upregulated. The inducible form of NOS is activated during disease progression and may even be regulated by NF-κB. Stimulation of iNOS expression occurs along with the activation of the innate and adaptive immune systems, infiltration by polymorphonuclear leukocytes, and recruitment of lymphocytes. The latter produce large amounts of NO and cytokines that modulate the inflammatory process, such as TNF-α.[8] Our results showed higher expression of iNOS in PPVL animals (Fig. 2). This enzyme is overexpressed in the gastric mucosa of animals with PHG. Our results suggest that gastric inflammation is clearly established in this experimental model, which is consistent with the literature.[24]
NAC has modulatory activity at different stages of the inflammatory and phagocytic process, stimulating immune functions and reducing levels of proinflammatory cytokines. Furthermore, it inhibits the production of NO via iNOS.[30] In this study, animals treated with NAC exhibited reductions in iNOS expression in the stomach and, therefore, an attenuation of gastric inflammation (Fig. 2). The same phenomenon was reported in a previous study using an animal model of PH induced by dimethylnitrosamines.[31]
This finding was reaffirmed in our analysis of NF-κB and TNF-α immunoreactivity in gastric tissue: both were significantly increased in PPVL animals as compared with controls (Figs. 5, 6). NF-κB is currently considered a typical proinflammatory signaling molecule, due to its action on the expression of proinflammatory genes, chemokines, cytokines, and adhesion molecules.[32] In the cytoplasm, this factor is bound to its inhibitory protein, IκB; only after phosphorylation and degradation by specific protein kinases, such as the IκB kinase complex, does translocation of NF-κB to the nucleus become possible.[33] The stimulus for NF-κB translocation occurs via neurotransmitters such as glutamate, viral and bacterial products, reaction products (via iNOS), increased intracellular calcium levels, and oxidative stress.[34]
TNF-α, in turn, is a cytokine with an important role in the activation of NF-κB. It participates in a wide spectrum of biological activities, including inflammation, apoptosis, and cell growth and differentiation. By extracellular stimuli, TNF causes phosphorylation of IκB and subsequent release of NF-κB, which can then act on target genes in the cell nucleus.[34]
In the literature, the anti-inflammatory properties of NAC have been associated with its ability to modulate the immune response and its actions on leukotriene and prostaglandin metabolism, among other mechanisms.[35] All of these effects are related to the nature of NAC as a donor source of –SH groups, inducing the synthesis of glutathione (GSH), which increases cellular protection against oxidative stress and inflammatory processes. NAC also acts to reduce reactive oxygen species produced by leukocytes.[36]
NAC inhibits the activation of NF-κB and also blocks TNF-α by reducing its affinity for the TNF receptor.[37] It is well known that not only the inflammatory process but also oxidative stress is an aggravating factor in chronic liver disease.[30] Prolonged NO synthesis at high levels may induce oxidative stress due to the production of peroxynitrite (ONOO–), a powerful oxidant. This compound, generated by the NO and peroxynitrite reaction, can cause oxidative and nitrosative damage to proteins, lipids, and DNA.[38] The presence of a high concentration of peroxynitrite in the gastric mucosa of animals subjected to the PPVL model of PH was previously demonstrated by immunohistochemistry.[11] Yet, in the same experimental model, an increase in the thiobarbituric acid reactive substances and NO levels and a reduction in the activity of the antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPx) have been demonstrated, results that suggest increased oxidative stress.[39]
In the present study, we evaluated the possibility that NAC would act synergistically as a dual antioxidant and anti-inflammatory. To determine its antioxidant role, we used the Western blot technique to assess the expression of SOD and NADPH quinone oxidoreductase-1 (NQO1). SOD is an antioxidant enzyme responsible for catalyzing the dismutation of superoxide (O2–º) into hydrogen peroxide (H2O2). Increased levels of this enzyme are indicative of increased O2–º production and hence of oxidative stress.[30,40] In the present study, expression of this enzyme was significantly increased in PPVL animals as compared with controls, and NAC was able to reduce such overexpression (Fig. 3). The reactive oxygen species can be removed from tissue by endogenous antioxidants such as thiols, components which are found, e.g., in GSH.[41] As a precursor of this molecule, NAC acts as an antioxidant and can thus be beneficial in disorders aggravated by oxidative stress. In this study, NAC had a clear antioxidant effect, as demonstrated in the analysis of SOD expression (Fig. 3) in the PPVL + NAC group. Levels of this enzyme returned to near-control values, demonstrating a reduction in oxidative stress and, consequently, in the expression of the SOD enzyme.
Another antioxidant molecule, which is less effective than SOD but acts as a direct scavenger of the superoxide anion, is NQO1.[39] The NQO1 gene encodes enzymes responsible for cell protection, which catalyze the reduction of electrons and prevent the formation of reactive oxygen species.[42] Increased expression of NQO1 was demonstrated in PPVL animals (Fig. 4) when compared with controls. This finding is probably related to the cellular response to oxidative stress caused by inflammation,[42] and NAC showed a synergistic antioxidant and anti-inflammatory activity in reducing expression of this marker in the gastric mucosa of PPVL + NAC animals (Fig. 4).
From the findings of this study, we conclude that the experimental model of PPVL successfully induces PH with subsequent inflammation and oxidative stress in the gastric mucosa. Furthermore, we suggest that a synergistic antioxidant and anti-inflammatory effect of NAC was able to attenuate the damage caused by these processes, contributing to the integrity of gastric tissue and mitigating the injuries caused by this experimental model.
Footnotes
Ethics Committee Approval: Institutional Committee for the Care and Use of Animals, Hospital de Clínicas de Porto Alegre, Animal Experimentation Unit (date: 01.13.2014, number: 2014-0012).
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – FL, RMH, ES, JRC, CM, HF, NPM; Design – FL, RMH, ES, JRC, CM, HF, NPM; Supervision – HF, NPM; Fundings – HF, NPM; Materials – FL, RMH, ES, JRC; Data Collection and/or Processing – FL, RMH, ES, JRC; Analysis and/or Interpretation – FL, RMH, ES, JRC, CM, HF, NPM; Literature Search – FL, RMH, ES, JRC; Writing – FL, RMH, ES, JRC, CM, HF, NPM; Critical Reviews – FL, RMH, ES, JRC, CM, HF, NPM.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: Supported by Fundo de Incentivo à Pesquisa e Eventos (FIPE/HCPA).
References
- 1.Martinelli ALC. Portal hypertension. Medicina. 2004;37(3/4):253–261. [Português] [Google Scholar]
- 2.Bari K, Garcia-Tsao G. Treatment of portal hypertension. World J Gastroenterol. 2012;18(11):1166–1175. doi: 10.3748/wjg.v18.i11.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goossens N, Nakagawa S, Hoshida Y. Molecular prognostic prediction in liver cirrhosis. World J Gastroenterol. 2015;21(36):10262–10273. doi: 10.3748/wjg.v21.i36.10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormack TT, Sims J, Eyre-Brook I, Kennedy H, Goepel J, Johnson AG, et al. Gastric lesions in portal hypertension: inflammatory gastritis or congestive gastropathy? Gut. 1985;26(11):1226–1232. doi: 10.1136/gut.26.11.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mekaroonkamol P, Cohen R, Chawla S. Portal hypertensive enteropathy. World J Hepatol. 2015;7(2):127–138. doi: 10.4254/wjh.v7.i2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gracia-Sancho J, Maeso-Díaz R, Bosch J. Pathophysiology and a rational basis of therapy. Dig Dis. 2015;33(4):508–514. doi: 10.1159/000374099. [DOI] [PubMed] [Google Scholar]
- 7.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. 837a-837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu LS, George J, Wang JH. Current concepts on the role of nitric oxide in portal hypertension. World J Gastroenterol. 2013;19(11):1707–1717. doi: 10.3748/wjg.v19.i11.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martell M, Coll M, Ezkurdia N, Raurell I, Genescà J. Physiopathology of splanchnic vasodilation in portal hypertension. World J Hepatol. 2010;2(6):208–220. doi: 10.4254/wjh.v2.i6.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marques C, Licks F, Zattoni I, Borges B, de Souza LE, Marroni CA, Marroni NP. Antioxidant properties of glutamine and its role in VEGF-Akt pathways in portal hypertension gastropathy. World J Gastroenterol. 2013;19(28):4464–4474. doi: 10.3748/wjg.v19.i28.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Licks F, Hartmann RM, Marques C, Schemitt E, Colares JR, Soares Mdo C, et al. N-acetylcysteine modulates angiogenesis and vasodilation in stomach such as DNA damage in blood of portal hypertensive rats. World J Gastroenterol. 2015;21(43):12351–12360. doi: 10.3748/wjg.v21.i43.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vercelino R, Tieppo J, Dias AS, Marroni CA, Garcia E, Meurer L, et al. N-acetylcysteine effects on genotoxic and oxidative stress parameters in cirrhotic rats with hepatopulmonary syndrome. Basic Clin Pharmacol Toxicol. 2008;102(4):370–376. doi: 10.1111/j.1742-7843.2007.00181.x. [DOI] [PubMed] [Google Scholar]
- 13.Urrunaga NH, Rockey DC. Portal hypertensive gastropathy and colopathy. Clin Liver Dis. 2014;18(2):389–406. doi: 10.1016/j.cld.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garbuzenko DV. Contemporary concepts of the medical therapy of portal hypertension under liver cirrhosis. World J Gastroenterol. 2015;21(20):6117–6126. doi: 10.3748/wjg.v21.i20.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sikuler E, Kravetz D, Groszmann RJ. Evolution of portal hypertension and mechanisms involved in its maintenance in a rat model. Am J Physiol. 1985;248(6 Pt 1):G618–G25. doi: 10.1152/ajpgi.1985.248.6.G618. [DOI] [PubMed] [Google Scholar]
- 16.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaffey MJ, Mills SE, Swanson PE, Zarbo RJ, Shah AR, Wick MR. Immunoreactivity for BER-EP4 in adenocarcinomas, adenomatoid tumors, and malignant mesotheliomas. Am J Surg Pathol. 1992;16(6):593–599. doi: 10.1097/00000478-199206000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Sauerbruch T, Trebicka J. Future therapy of portal hypertension in liver cirrhosis - a guess. F1000Prime Rep. 2014;6:95. doi: 10.12703/P6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ripoll C, Garcia-Tsao G. Management of gastropathy and gastric vascular ectasia in portal hypertension. Clin Liver Dis. 2010;14(2):281–295. doi: 10.1016/j.cld.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cubillas R, Rockey DC. Portal hypertensive gastropathy: A review. Liver Int. 2010;30(8):1094–1102. doi: 10.1111/j.1478-3231.2010.02286.x. [DOI] [PubMed] [Google Scholar]
- 21.Aller MA, Arias JL, Cruz A, Arias J. Inflammation: A way to understanding the evolution of portal hypertension. Theor Biol Med Model. 2007;4:44. doi: 10.1186/1742-4682-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallance P, Moncada S. Hyperdynamic circulation in cirrhosis: a role for nitric oxide? Lancet. 1991;337(8744):776–778. doi: 10.1016/0140-6736(91)91384-7. [DOI] [PubMed] [Google Scholar]
- 23.Bolognesi M, Di Pascoli M, Verardo A, Gatta A. Splanchnic vasodilation and hyperdynamic circulatory syndrome in cirrhosis. World J Gastroenterol. 2014;20(10):2555–2563. doi: 10.3748/wjg.v20.i10.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreira AJ, Fraga C, Alonso M, Collado PS, Zetller C, Marroni C, et al. Quercetin prevents oxidative stress and NF-kappaB activation in gastric mucosa of portal hypertensive rats. Biochem Pharmacol. 2004;68(10):1939–1946. doi: 10.1016/j.bcp.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Bolognesi M, Sacerdoti D, Di Pascoli M, Angeli P, Quarta S, Sticca A, et al. Haeme oxygenase mediates hyporeactivity to phenylephrine in the mesenteric vessels of cirrhotic rats with ascites. Gut. 2005;54(11):1630–1636. doi: 10.1136/gut.2004.063735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cahill PA, Redmond EM, Hodges R, Zhang S, Sitzmann JV. Increased endothelial nitric oxide synthase activity in the hyperemic vessels of portal hypertensive rats. J Hepatol. 1996;25(3):370–378. doi: 10.1016/s0168-8278(96)80124-3. [DOI] [PubMed] [Google Scholar]
- 27.Iwakiri Y. Endothelial dysfunction in the regulation of cirrhosis and portal hypertension. Liver Int. 2012;32(2):199–213. doi: 10.1111/j.1478-3231.2011.02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ai JH, Yang Z, Qiu FZ, Zhu T. Heat shock protein 90 is responsible for hyperdynamic circulation in portal hypertensive rats. World J Gastroenterol. 2003;9(11):2544–2547. doi: 10.3748/wjg.v9.i11.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moleda L, Jurzik L, Froh M, Gäbele E, Hellerbrand C, Straub RH, et al. Role of HSP-90 for increased nNOS-mediated vasodilation in mesenteric arteries in portal hypertension. World J Gastroenterol. 2010;16(15):1837–1844. doi: 10.3748/wjg.v16.i15.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Andrade KQ, Moura FA, dos Santos JM, de Araújo OR, de Farias Santos JC, Goulart MO. Oxidative stress and inflammation in hepatic diseases: Therapeutic possibilities of n-acetylcysteine. Int J Mol Sci. 2015;16(12):30269–30308. doi: 10.3390/ijms161226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhuang RX, Wang FG, Zhou HP, Shi TT, Liu SR. [The protective effect of N-acetylcysteine magnesium against liver cirrhosis with portal hypertension in rat] Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2012;26(5):366–369. [Chinese] [PubMed] [Google Scholar]
- 32.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6) doi: 10.1101/cshperspect.a001651. a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 34.Glezer I, Marcourakis T, Avellar MCW, Gorenstein C, Scavone C. The role of the transcription factor NF-kB in the molecular mechanisms of action of psychoactive drugs. Rev Bras Psiquiatr. 2000;22(1):26–30. [Portuguese] [Google Scholar]
- 35.Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013;1830(8):4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Arranz L, Fernández C, Rodríguez A, Ribera JM, De la Fuente M. The glutathione precursor N-acetylcysteine improves immune function in postmenopausal women. Free Radic Biol Med. 2008;45(9):1252–1262. doi: 10.1016/j.freeradbiomed.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Oka S, Kamata H, Kamata K, Yagisawa H, Hirata H. N-acetylcysteine suppresses TNF-induced NF-kappaB activation through inhibition of IkappaB kinases. FEBS Lett. 2000;472:196–202. doi: 10.1016/s0014-5793(00)01464-2. [DOI] [PubMed] [Google Scholar]
- 38.Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22(37):5734–5754. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 39.Licks F, Marques C, Zetler C, Martins MI, Marroni CA, Marroni NP. Antioxidant effect of N-acetylcysteine on prehepatic portal hypertensive gastropathy in rats. Ann Hepatol. 2014;13(3):370–377. [PubMed] [Google Scholar]
- 40.Moreira AJ, Rodrigues G, Bona S, Cerski CT, Marroni CA, Mauriz JL, et al. Oxidative stress and cell damage in a model of precancerous lesions and advanced hepatocellular carcinoma in rats. Toxicol Rep. 2014;2:333–340. doi: 10.1016/j.toxrep.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marí M, Colell A, Morales A, von Montfort C, Garcia-Ruiz C, Fernández-Checa JC. Redox control of liver function in health and disease. Antioxid Redox Signal. 2010;12(11):1295–1331. doi: 10.1089/ars.2009.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93(25):14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]


