Abstract
Background
Ovarian hyperstimulation syndrome (OHSS) is a serious and potentially fatal complication of ovarian stimulation which affects 1% to 14% of all in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) cycles. A number of clinical studies with conflicting results have reported on the use of plasma expanders such as albumin, hydroxyethyl starch (HES), mannitol, polygeline and dextran as a possible intervention for the prevention of OHSS. Women with very high estradiol levels, high numbers of follicles or oocytes retrieved, and women with polycystic ovary syndrome (PCOS), are at particularly high risk of developing OHSS. Plasma expanders are not commonly used nowadays in ovarian hyperstimulation. This is mainly because clinical evidence on their effectiveness remains sparse, because of the low incidence of moderate and severe ovarian hyperstimulation syndrome (OHSS) and the simultaneous introduction of mild stimulation approaches, gonadotropin‐releasing hormone (GnRH) antagonist protocols and the freeze‐all strategy for the prevention of OHSS.
Objectives
To review the effectiveness and safety of administration of volume expanders for the prevention of moderate and severe ovarian hyperstimulation syndrome (OHSS) in high‐risk women undergoing IVF or ICSI treatment cycles.
Search methods
We searched databases including the Cochrane Gynaecology and Fertility Group Specialised Register of controlled trials, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase and trial registers to September 2015; no date restrictions were used as new comparators were included in this search. The references of relevant publications were also searched. We attempted to contact authors to provide or clarify data that were unclear from trial or abstract reports.
Selection criteria
We included randomised controlled trials (RCTs) comparing volume expanders versus placebo or no treatment for the prevention of OHSS in high‐risk women undergoing ovarian hyperstimulation as part of any assisted reproductive technique.
Data collection and analysis
Two review authors independently selected the studies, assessed risk of bias and extracted relevant data. The primary review outcome was moderate or severe OHSS. Other outcomes were live birth, pregnancy and adverse events. We combined data to calculate pooled Peto odds ratios (ORs) and 95% confidence intervals (CIs) for each intervention. Statistical heterogeneity was assessed using the I2 statistic. We assessed the overall quality of the evidence for each comparison, using GRADE methods.
Main results
We included nine RCTs (1867 women) comparing human albumin (seven RCTs) or HES (two RCTs) or mannitol (one RCT) versus placebo or no treatment for prevention of OHSS. The evidence was very low to moderate quality for all comparisons. The main limitations were imprecision, poor reporting of study methods, and failure to blind outcome assessment.
There was evidence of a beneficial effect of intravenous albumin on OHSS, though heterogeneity was substantial (Peto OR 0.67 95% CI 0.47 to 0.95, seven studies, 1452 high risk women; I² = 69%, very low quality evidence) . This suggests that if the rate of moderate or severe OHSS with no treatment is 12%, it will be about 9% (6% to12%) with the use of intravenous albumin. However, there was evidence of a detrimental effect on pregnancy rates (Peto OR 0.72 95% CI 0.55 to 0.94, I² = 42%, seven studies 1069 high risk women, moderate quality evidence). This suggests that if the chance of pregnancy is 40% without treatment, it will be about 32% (27% to 38%) with the use of albumin.
There was evidence of a beneficial effect of HES on OHSS (Peto OR 0.27 95% CI 0.12 to 0.59, I² = 0%, two studies, 272 women, very low quality evidence). This suggests that if the rate of moderate or severe OHSS with no treatment is 16%, it will be about 5% (2% to 10%) with the use of HES. There was no evidence of an effect on pregnancy rates (Peto OR 1.20 95% CI 0.49 to 2.93, one study, 168 women, very low quality evidence).
There was evidence of a beneficial effect of mannitol on OHSS (Peto OR 0.38, 95% CI 0.22 to 0.64, one study, 226 women with PCOS, low quality evidence). This means that if the risk of moderate or severe OHSS with no treatment is 52%, it will be about 29% (19% to 41%) with mannitol. There was no evidence of an effect on pregnancy rates (Peto OR 0.85 95% CI 0.47 to 1.55; one study, 226 women, low quality evidence).
Live birth rates were not reported in any of the studies. Adverse events appeared to be uncommon, but were too poorly reported to reach any firm conclusions.
Authors' conclusions
Evidence suggests that the plasma expanders assessed in this review (human albumin, HES and mannitol) reduce rates of moderate and severe OHSS in women at high risk. Adverse events appear to be uncommon, but were too poorly reported to reach any firm conclusions, and there were no data on live birth. However, there was evidence that human albumin reduces pregnancy rates. While there was no evidence that HES, or mannitol had any influence on pregnancy rates, the evidence of effectiveness was based on very few trials which need to be confirmed in additional, larger randomised controlled trials (RCTs) before they should be considered for routine use in clinical practice.
Plain language summary
Intravenous plasma expanders for preventing ovarian hyperstimulation syndrome (OHSS)
Review question
Researchers in the Cochrane Collaboration reviewed the evidence on different types of volume expanders in women at high risk of OHSS undergoing ovarian hyperstimulation as part of any assisted reproductive technique (ART). Women with very high estradiol levels, high numbers of follicles or oocytes retrieved, and women with polycystic ovary syndrome (PCOS), are at particularly high risk of developing OHSS.
Background
OHSS is a serious complication of ovarian stimulation, which affects up to 14% of ART cycles. It is characterised by enlarged ovaries following excessive hormonal stimulation resulting in a shift of fluid from the blood vessels to the extracellular space. It can cause abdominal bloating, blood clots (thrombosis) and reduced perfusion of vital organs like the kidneys and liver. Several clinical studies have reported on the use of intravenous fluids such as albumin and hydroxyethyl starch (HES), as a possible way of preventing OHSS.
Study characteristics
We found nine randomised controlled trials (RCTs) which compared the use of volume expanders (albumin, HES and mannitol) for preventing moderate or severe OHSS. Control groups received no treatment or placebo. The studies included 1867 women at high risk of OHSS. The evidence is current to September 2015.
Key results
Evidence suggests that plasma expanders (human albumin, HES and mannitol) reduce rates of moderate and severe OHSS in women at high risk.
If the rate of OHSS without treatment is 12%, it will be about 9% (6% to 12%) with the use of intravenous albumin. If the rate of OHSS without treatment is 16%, it will be about 5% (2% to 10%) with the use of HES, and if the rate without treatment is 52%, it will be about 29% (19% to 41%) with mannitol.
Adverse events appear to be uncommon, but were too poorly reported to reach firm conclusions. No studies reported live birth, but there was evidence that human albumin reduces pregnancy rates. While there was no evidence that HES, or mannitol had any influence on pregnancy rates, the evidence of effectiveness was based on very few trials, and better evidence is needed before they should be considered for routine use in clinical practice.
Quality of the evidence
The evidence was very low to moderate quality for all comparisons. The main limitations were imprecision, poor reporting of study methods, and failure to blind outcome assessment.
Summary of findings
Background
Description of the condition
Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic, serious and potentially fatal complication of ovarian stimulation which affects 1% to 14% of all in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) cycles (Garcia‐Velasco 2003). OHSS may be associated with massive ovarian enlargement, extracellular exudate accumulation combined with profound intravascular volume depletion, ascites, hydrothorax, haemoconcentration, liver dysfunction and renal failure (Aboulghar 2003; Vloeberghs 2009). It can lead to cancellation of an IVF cycle and prolonged bed rest or hospitalisation, which may have significant emotional, social, and economic impacts (Delvigne 2002; Engmann 2008). OHSS can be classified into an early form that is related to the ovarian response and exogenous human chorionic gonadotrophin (hCG) administration, and is detected three to nine days after hCG administration. A late form of OHSS, diagnosed 10 to 17 days later, is due to endogenous hCG (Mathur 2000) and is categorised as mild, moderate, severe or life‐threatening. The aetiology of OHSS is not completely clear at this moment; however the syndrome is strongly associated with serum hCG and certain vasoactive substances (Enskog 2001; Rizk 1997).
Description of the intervention
Many strategies have been tried to prevent OHSS and cycle cancellation such as coasting (withholding gonadotrophins before the ovulation trigger is given) (D'Angelo 2011), gonadotropin‐releasing hormone (GnRH) agonist as an oocyte trigger in GnRH antagonist cycles (Kol 2008; Youssef 2014), natural cycle IVF (Edwards 2007), cabergoline (Tang 2012), embryo freezing (D'Angelo 2007), and in vitro oocyte maturation (Loutradis 2006). Unfortunately, none of the strategies currently employed completely prevent OHSS after hCG administration (Egbase 2000). Recently the role of vascular endothelial growth factor (VEGF), as mediator of hCG‐dependent ovarian angiogenesis, has emerged (Cerrilo 2009). VEGF is expressed in human ovaries (Yan 1993) and levels significantly increase after hCG administration leading to increased vascular permeability (Foong 2006). It has been proposed that the administration of intravenous fluids such as human albumin, hydroxyethyl starch (HES), dextran or polygeline might result in a restoration of intravascular volume and inactivation of the vasoactive intermediates responsible for the pathogenesis of OHSS (Asch 1993; Chen 1997; Isik 1997; Kissler 2001; Shalev 1995).
How the intervention might work
Albumin has both osmotic and transport functions. It contributes about 75% of the plasma oncotic pressure and administration of 50 g human albumin solution will draw more than 800 mL of extracellular fluid into the circulation within 15 minutes (McClelland 1990). It has been suggested that the binding and transport properties of human albumin play a major role in the prevention of severe OHSS, as albumin may result in binding and inactivation of the vasoactive intermediates responsible for the pathogenesis of OHSS. The osmotic function is responsible for maintaining the intra‐vascular volume in the event of capillary leakage, thus preventing the sequelae of hypovolaemia, ascites and haemoconcentration (Shalev 1995).
Hydroxyethyl starch (HES) is a plasma expander that has gained recent attention as an alternative to albumin in reducing the incidence of severe OHSS. Because HES is a non‐biological substance, its use avoids any potential concern about viral transmission that may be present with albumin (Abramov 2001; Chen 2003a).
Dextran is a complex, branched glucan composed of chains of varying lengths. It is used as an antithrombotic, to reduce blood viscosity, and as a volume expander in anaemia (Endo 2004).
Polygeline is a type of intravenous colloid with 3.5% urea‐linked gelatin used to treat OHSS (Gamzu 2002). It is used in the prevention or treatment of shock associated with reduction in effective circulating blood volume due to haemorrhage, loss of plasma or loss of water and electrolytes from persistent vomiting and diarrhoea.
Mannitol is a naturally occurring sugar alcohol used for its osmotic diuretic purposes; it is used as plasma expander for the protection of renal failure and in cases of intracerebral oedema (Shawkat 2012).
Why it is important to do this review
OHSS is one of the most common adverse effects of assisted reproductive technology‐controlled ovarian hyperstimulation (ART‐COH) cycles. OHSS can result in hospital admission and in some cases in critical illness. Therefore the aim of this review is to evaluate the evidence from randomised controlled trials (RCTs) to determine whether volume expanders can reduce the incidence of moderate and severe OHSS in high‐risk women undergoing IVF/ICSI treatment This review provides a new evidence base for physicians and stakeholders considering the use of plasma expanders in women at high risk of developing OHSS who are undergoing IVF/ICSI treatment.
This is an update of a Cochrane review first published in 1999, and previously updated in 2002 and 2011. This is the first update that aims to report 'moderate or severe OHSS' as a primary outcome, as opposed to the original primary outcome of 'severe OHSS'.
Objectives
To investigate the effectiveness and safety of administration of volume expanders in the prevention of moderate and severe ovarian hyperstimulation syndrome (OHSS) in high‐risk women undergoing in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs).
We excluded non‐randomised and quasi‐randomised studies (e.g. studies with evidence of inadequate sequence generation such as alternate days, patient numbers) as they are associated with a high risk of bias.
Cross‐over studies were not eligible for inclusion, as the design is not valid in this context.
Types of participants
Women of reproductive age who were having controlled ovarian hyperstimulation as part of any assisted reproductive technique and were considered to be at high risk of moderate or severe OHSS (as determined by either a diagnosis of polycystic ovary syndrome (PCOS) (criterion new to the 2016 update), a specific threshold serum estradiol level, a threshold number of follicles on day of human chorionic gonadotrophin (hCG) administration or threshold number of retrieved oocytes (as defined in individual studies).
Types of interventions
All kinds of volume expanders used in the prevention of OHSS versus placebo or no treatment. Administration can be at any time in the cycle (e.g. prior to or after oocyte pick‐up).
For example:
Intravenous albumin versus placebo or no treatment
Hydroxyethyl starch (HES) versus placebo or no treatment
Mannitol versus placebo or no treatment
Polygeline versus placebo or no treatment
Dextran versus placebo or no treatment
Types of outcome measures
Primary outcomes
1. Moderate or severe OHSS (as determined by established clinical criteria, such as Humaidan 2010; Rizk 1999; Navot 1992; Golan 1989; Schenker 1978; WHO 1973)
Secondary outcomes
2. Live birth rate per woman randomised
3. Pregnancy rate per woman randomised (as confirmed by β‐hCG or pregnancy test or ultrasonic visualisation of fetal heart beat at a certain gestational age (as defined in the separate studies))
4. Adverse effects of treatment (e.g. allergic reaction)
Search methods for identification of studies
We searched for published and unpublished RCTs of diverse intravenous fluids versus placebo or no treatment using a systematic search strategy, without date or language restriction and in consultation with the Cochrane Gynaecology and Fertility (formerly Menstrual Disorders and Subfertility Group (MDSG)) Information Specialist.
Electronic searches
The following electronic databases, trial registers and websites were searched to September 2015.
Ovid Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 2)
Ovid MEDLINE (Appendix 3)
Ovid Embase: Embase (Appendix 4)
Ovid PsycINFO (Appendix 5)
MEDLINE and Embase search strategies use different filters for identifying randomised trials. The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomised trials which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011).
The Embase and CINAHL searches were combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/mehodology/filters.html#random)
Trial registers for ongoing and registered trials including www.controlled‐trials.com/,https://clinicaltrials.gov and www.who.int/trialsearch/Default.aspx
The Web of Knowledge (for conference abstracts, and to check citations of included studies)
Searching other resources
We hand searched the reference lists of all primary studies and review articles retrieved by the search, and checked the citation lists of relevant publications. We contacted known experts in the field and personal contacts regarding any unpublished materials.
Data collection and analysis
Selection of studies
Thereview authors (MY or SM) independently reviewed the titles and abstracts of studies using the a priori criteria for inclusion. The full‐text manuscripts of studies were obtained for the short‐listed papers that were considered potentially eligible for inclusion. We sought further information from the authors of study reports that did not contain sufficient information to make a decision about eligibility. Thereview authors independently critically appraised these studies and any disagreements were resolved by discussion. The studies that were determined to be suitable for inclusion were assessed for risk of bias and data were extracted. Subsequently, a detailed 'Characteristics of excluded studies' table was constructed for those studies that did not satisfy the inclusion criteria. A similar 'Characteristics of included studies' table was constructed for those studies considered suitable for inclusion.
The 2016 selection process was documented with a study flow diagram (Figure 1).
1.
Study flow diagram.
Data extraction and management
A standardised data extraction form was developed and piloted for consistency and completeness. Two review authors (MY, HG or SM) independently performed data extraction. The two sets of extracted data were compared and discrepancies were resolved by discussion. Where studies had multiple publications, the review authors collated multiple reports so that each study rather than each report was the unit of interest in the review; overlapping data were thus identified and duplicates were excluded.
We requested extra information about the methodological quality of some studies (Bellver 2003; Gokmen 2005; Isik 1996; Isikoglu 2007; Saremi 2003; Shalev 1995; Shoham 1994), however the only response we received was from the authors of Isikoglu 2007 and Saremi 2003.
Assessment of risk of bias in included studies
The review authors independently evaluated the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool (Higgins 2011) to assess: selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias. Disagreements were resolved by discussion. We described all judgements fully and presented the conclusions in 'Risk of bias' tables (Figure 2; Figure 3), which were incorporated into the interpretation of review findings by means of sensitivity analyses.
2.
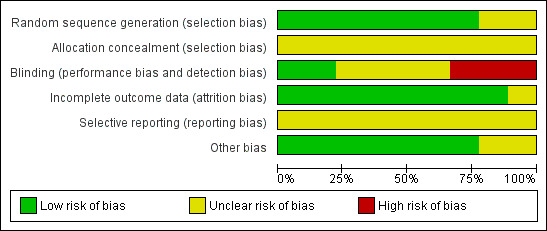
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
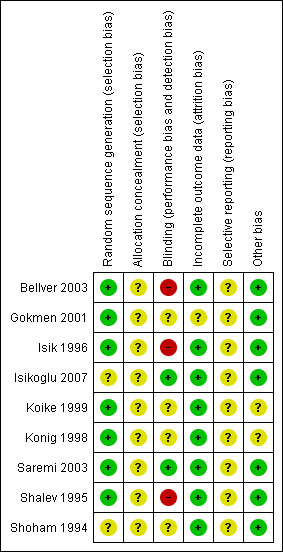
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Measures of treatment effect
All data were dichotomous. The numbers of events in the control and intervention groups of each study were used to calculate Peto odds ratios (ORs) for each comparison, with 95% confidence intervals (CIs).
Unit of analysis issues
The primary analysis was per woman randomised. Only one cycle per woman could be included.
Dealing with missing data
The data were analysed on an intention‐to‐treat basis as far as possible and attempts were made to obtain missing data from the original trials. Where these were unobtainable, only the available data were analysed.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by visual inspection of the forest plots, and used the I2 statistic (Higgins 2011) to quantify any apparent inconsistency, interpreted in the broad terms:
0% to 40% might not be important;
30% to 60% represented moderate heterogeneity;
50% to 90% represented substantial heterogeneity;
75% to 100% represented considerable heterogeneity.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, the review authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. Had there been 10 or more studies in an analysis, we planned to use a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies)
Data synthesis
If the studies were sufficiently similar, data from primary studies were combined using a fixed‐effect model to compare intravenous (IV) intervention fluids versus no treatment or placebo. We considered each type of volume expander in a separate comparison.
Subgroup analysis and investigation of heterogeneity
We considered whether the effect of the intervention on OHSS rates was different in studies that only measured severe (as opposed to moderate or severe) OHSS.
We also considered whether the effect of the intervention on pregnancy rates was different in studies that diagnosed pregnancy by ultrasonic visualisation of fetal heart beat rather than by pregnancy test.
Had we detected substantial heterogeneity, we planned to explore possible explanations in sensitivity analyses and to take any statistical heterogeneity into account when interpreting the results.
Sensitivity analysis
Sensitivity analyses were conducted for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding eligibility and in the analysis. These analyses included consideration of whether conclusions would have differed if:
the summary effect measure was risk ratio rather than Peto odds ratio;
eligibility was restricted to studies without high risk of bias;
eligibility was restricted to full‐text published studies.
Overall quality of the body of evidence: 'Summary of findings' table
Two review authors working independently prepared a 'Summary of findings' table using Guideline Development Tool software and Cochrane methods (Higgins 2011)This table evaluated the overall quality of the body of evidence for all outcomes for the three review comparisons using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). Judgements about evidence quality (high, moderate, low or very low) were justified, documented, and incorporated into reporting of results for each outcome.
Results
Description of studies
Results of the search
2016 update
Two hundred and eighty‐nine studies were retrieved by the search; a total of 124 duplicates were discarded, leaving 165 studies for screening of title and abstract. Two new studies were identified concerning the infusion of mannitol (Saremi 2003) and calcium gluconate (El‐Khayat 2015). However, calcium gluconate has a very different pathophysiological mechanism than the other IV fluids used, which all are volume expanders. We excluded El‐Khayat 2015. A discussion on this topic took place between the authors and led to a decision to maintain a focus on volume expanders only; therefore, we amended the protocol and changed the title accordingly.
The data from Gokmen 2005 (conference abstract) were excluded from this update of the review, as there was serious concern that the data overlapped with the Gokmen 2001 publication, which was published as a full paper. We tried to contact the author to clarify potential duplication of data but did not succeed.
In total, nine studies were included in this review (Figure 1).
2014 update
Two hundred and seventy‐three records were retrieved by the search and were screened. One unpublished study was deemed potentially eligible and was awaiting classification. The rest were discarded as clearly ineligible based on the title or abstract.
Searches prior to 2014
In earlier versions of the review a total of 43 articles were identified as potentially eligible and retrieved in full text. Of these, 34 articles (32 studies) were further assessed and excluded. Eight studies (nine articles) were included.
Included studies
See Characteristics of included studies
Design
The nine included studies (n = 1867 women) were all single‐centre studies. Eight studies were parallel‐group RCTs comparing a single intervention versus placebo or a no treatment group; the Gokmen 2001 study had a three‐armed design with two different intervention groups versus placebo.
Eight studies were published as full papers and one as a 'letter to the editor' (Koike 1999).
Participants
All the studies used strict inclusion criteria for participant selection (see Characteristics of included studies).
Two studies used estradiol (E2) as the basic risk factor of ovarian hyperstimulation syndrome (OHSS) (7000 pmol/L in Shoham 1994, 11,010 pmol/L in Isik 1996), both in combination with 'multifollicular response'; however no specific number of follicles was mentioned.
Two studies used the number of retrieved oocytes as the main risk factor (Bellver 2003; Koike 1999).
Four studies used both E2 level and number of follicles or oocytes as inclusion criteria; high E2 or number of follicles on day of human chorionic gonadotrophin (hCG) administration/number of retrieved oocytes in Gokmen 2001, Isikoglu 2007 and Konig 1998; high E2 level and number of follicles in Shalev 1995.
Where E2 levels were (partly) used to define the 'high‐risk' population, cut‐off levels varied (1906 pg/mL in Shoham 1994, > 2506 pg/mL in Shalev 1995, > 3000 pg/mL in Gokmen 2001, > 1500 pg/mL in Konig 1998, > 3000 pg/mL in Isik 1996, > 4000 pg/mL in Isikoglu 2007, > 9200 pg/mL in Koike 1999 ; where necessary pmol/L was converted to pg/mL).
One study included women with polycystic ovary syndrome (PCOS) as their 'high‐risk' group (Saremi 2003), without taking E2 levels or number of follicles/oocytes into account.
One study excluded women with very high E2 levels and performed cycle cancellation for this extremely high‐risk group for E2 > 7000 pmol/L (Shoham 1994).
Mean female age was around 30 years in all studies (mean age range was 27.5 to 32.6 years). The intervention and control groups were largely similar regarding the number of ampoules of human menopausal gonadotropin (hMG) used for controlled ovarian hyperstimulation, number of oocytes retrieved and number of embryos. However, in five studies (Bellver 2003; Gokmen 2001; Koike 1999; Shalev 1995; Saremi 2003), there was no mention of the number of ampoules between intervention and control group.
See the table Characteristics of included studies for details.
Interventions
Seven studies compared albumin versus placebo (Gokmen 2001; Isikoglu 2007; Koike 1999; Shoham 1994) or no treatment (Bellver 2003; Isik 1996; Shalev 1995).
Two studies compared hydroxyethyl starch (HES) versus placebo (Gokmen 2001; Konig 1998).
One study made both comparisons (Gokmen 2001).
One study compared mannitol versus no treatment (Saremi 2003).
Placebo (saline) was used in the control group of five studies (Gokmen 2001; Isikoglu 2007; Koike 1999; Konig 1998; Shoham 1994).
The dose and timing of the interventions varied between studies but all were given in a relatively early phase (i.e. day of hCG administration or oocyte retrieval) to try to prevent early onset OHSS: Shoham 1994 used 50 g albumin two hours before oocyte retrieval; Koike 1999 used 37.5 g albumin just after oocyte retrieval; Shalev 1995 used 20 g albumin just after oocyte retrieval; and Isik 1996 used 10 g albumin two hours before oocyte retrieval; Gokmen 2001 and Isikoglu 2007 used 10 g albumin immediately after oocyte retrieval; and Bellver 2003 used 40 g of albumin immediately after oocyte retrieval. In Gokmen 2001, 6% hydroxyethyl starch (200/0.5) (500 mL) was used immediately after oocyte retrieval, and in Konig 1998 it was used 48 hours after the oocyte retrieval. Saremi 2003 used mannitol 3 g/kg bodyweight daily infusions (each 100 mL infusion containing 20 g mannitol and water for injection), starting the day after hCG injection until the third day after embryo transfer (lasting five to seven days depending on day of embryo transfer).
Outcomes
All nine studies reported the incidence of severe OHSS and five studies additionally reported on the incidence of moderate OHSS (Bellver 2003; Gokmen 2001; Isik 1996; Konig 1998; Saremi 2003). Diagnosis of severity of OHSS was done according to the criteria of Schenker 1978 in three studies (Gokmen 2001; Isik 1996; Shoham 1994), while Shalev 1995 and Isikoglu 2007 used the criteria of Navot 1988, Bellver 2003 used the criteria of Golan 1989, and Koike 1999 used those of Ben‐Rafael 1995. Konig 1998 used the criteria of WHO 1973. Saremi 2003 did not explicitly state the reference but describes criteria similar to Golan 1989.
Eight studies reported pregnancy rates. Diagnosis of pregnancy was determined using serum ß‐hCG in all studies except Isikoglu 2007 and Saremi 2003, which used "presence at ultrasound of a gestational sac with positive fetal heart rate".
Most studies mentioned adverse events in their results or discussion sections, but none of the studies prespecified adverse events as an outcome, or reported comparative data on adverse effects in all study groups.
Excluded studies
We excluded 39 studies which did not meet the inclusion criteria for this review, in most cases because they did not make any comparisons of interest or were not randomised. See Characteristics of excluded studies for details.
Risk of bias in included studies
Allocation
Sequence generation
Seven studies used adequate methods of sequence generation and were rated as at low risk of bias (Bellver 2003; Gokmen 2001; Isik 1996; Koike 1999; Konig 1998; Saremi 2003; Shalev 1995). Two studies did not report details of the methods used and were rated as at unclear risk of bias (Isikoglu 2007; Shoham 1994).
Allocation concealment
None of the studies clearly described an acceptable method of allocation concealment and all were rated as at unclear risk of bias in this domain.
Blinding
We considered that lack of blinding might influence outcome assessment for the outcomes of OHSS and adverse events.
One study clearly blinded both participants and outcomes assessors, and was rated as at low risk of bias (Isikoglu 2007). One study stated that "physicians were blinded to the allocated groups and a nurse performed the protocol" (Saremi 2003), and we considered this study also to be at low risk of bias. Four studies did not mention blinding, and were rated as at unclear risk (Gokmen 2001; Koike 1999; Konig 1998; Shoham 1994). Three studies were unblinded and were rated as at high risk of bias (Bellver 2003; Isik 1996; Shalev 1995)
Incomplete outcome data
All studies appeared to include all randomised women in analysis. All were rated as at low risk of attrition bias apart from one (Gokmen 2001). An abstract from a conference held in 2005 (Gokmen 2005) appears to relate to the same study but has a larger sample size and reports two additional events. We attempted to contact the study authors to query this but did not receive a reply. We therefore rated this study as at unclear risk of attrition bias.
Selective reporting
Although most studies either stated that no events were observed, or mentioned events in one or both arms, it was unclear whether data on adverse events were collected prospectively or systematically. All studies were rated as at unclear risk of bias in this domain.
Other potential sources of bias
No other potential source of bias was identified in any of the studies, and they were rated as at low risk in this domain.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Human albumin versus placebo or no treatment for the prevention of OHSS.
| Human albumin versus placebo or no treatment for the prevention of OHSS | |||||
| Population: women at high risk of OHSS Setting: ART clinics Intervention: Human albumin Comparison: placebo / no treatment | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with placebo / no treatment | Risk with Human albumin | ||||
| Moderate or severe OHSS | 122 per 1000 | 85 per 1000 (61 to 117) | OR 0.67 (0.47 to 0.95) | 1452 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 |
| Live birth | Not reported in the included studies | ||||
| Pregnancy rate assessed with: serum β‐hCG or heartbeat on ultrasound |
396 per 1000 | 321 per 1000 (265 to 381) | OR 0.72 (0.55 to 0.94) | 1069 (7 RCTs) | ⊕⊕⊕⊝ MODERATE 1 |
| Adverse effects | No reliable data as none of the studies specified adverse effects as an outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded one level for serious risk of bias: associated with lack of blinding, inadequate reporting of allocation concealment, unclear risk of attrition bias
2 Downgraded one level for serious imprecision: number of events < 300
3 Downgraded one level for serious inconsistency: I2= 69%
Summary of findings 2. HES versus placebo or no treatment for the prevention of OHSS.
| HES versus placebo or no treatment for the prevention of OHSS | |||||
| Population: Women at high risk of OHSS Setting: ART clinics Intervention: HES Comparison: placebo / no treatment | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with placebo / no treatment | Risk with HES | ||||
| Moderate or severe OHSS | 164 per 1.000 | 50 per 1000 (23 to 104) | OR 0.27 (0.12 to 0.59) | 272 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| Live birth | Not reported in the included studies | ||||
| Pregnancy rate assessed with: serum β‐hCG or heartbeat on ultrasound |
120 per 1.000 | 141 per 1000 (63 to 286) | OR 1.20 (0.49 to 2.93) | 168 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| Adverse effects | No reliable data as none of the studies specified adverse effects as an outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded two levels for very serious risk of bias: associated with inadequate reporting of allocation concealment and blinding, and unclear risk of attrition bias
2 Downgraded one level for serious imprecision: number of events <300
Summary of findings 3. Mannitol versus placebo / no treatment for the prevention of OHSS.
| Mannitol versus placebo or no treatment for the prevention of OHSS | |||||
| Population: women at high risk of OHSS Setting: ART clinics Intervention: Mannitol Comparison: placebo / no treatment | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with placebo / no treatment | Risk with Mannitol | ||||
| Moderate or severe OHSS | 517 per 1000 | 289 per 1000 (191 to 407) | OR 0.38 (0.22 to 0.64) | 226 (1 RCT) | ⊕⊕⊝⊝1,2 LOW |
| Live birth | Not reported in the included studies | ||||
| Pregnancy rate assessed with: serum β‐hCG or heartbeat on ultrasound | 276 per 1000 | 245 per 1000 (152 to 371) | OR 0.85 (0.47 to 1.55) | 226 (1 RCT) | ⊕⊕⊝⊝1,2 LOW |
| Adverse effects | No reliable data as none of the studies specified adverse effects as an outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded one level for risk of bias: inadequate reporting of allocation concealment,
2 Downgraded one level for serious imprecision: number of events < 300
1. Human albumin versus placebo or no treatment
Seven studies compared albumin versus placebo (Gokmen 2001; Isikoglu 2007; Koike 1999; Shoham 1994) or no treatment (Bellver 2003; Isik 1996; Shalev 1995).
Primary outcome
1.1 Moderate or severe OHSS
There was evidence of a difference between the groups, though (Peto OR 0.67 95% CI 0.47 to 0.95, seven studies, 1452 women; I² = 69%, very low quality evidence). Heterogeneity was substantial, and there was no obvious explanation for this (Figure 4; Analysis 1.1). This suggests that if the rate of moderate or severe OHSS with no treatment is 12%, it will be about 9% (6% to 12%) with the use of intravenous albumin.
4.
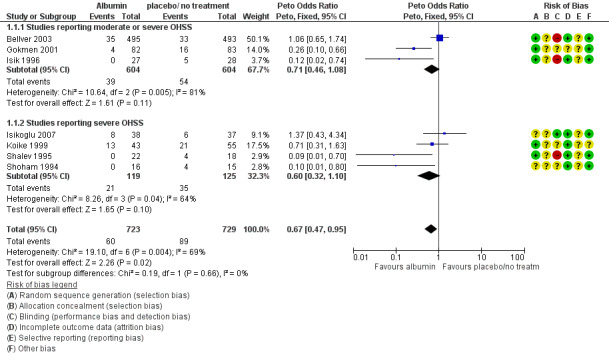
Forest plot of comparison: 1 Human Albumin vs placebo / no treatment, outcome: 1.1 Total OHSS.
1.1. Analysis.
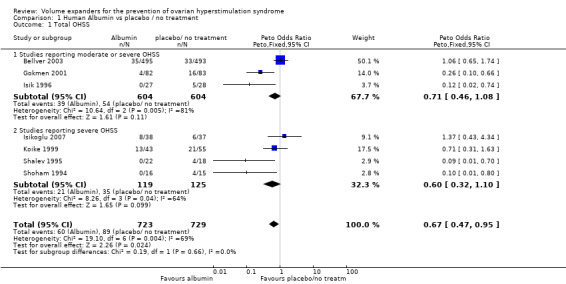
Comparison 1 Human Albumin vs placebo / no treatment, Outcome 1 Total OHSS.
Subgroup and sensitivity analyses
Subgroup analysis showed no evidence that findings differed in studies that only reported severe OHSS (test for subgroup differences: Chi² = 0.19, df = 1 (P = 0.66), I² = 0%).
Use of a risk ratio reduced heterogenity to I2=57%. Sensitivity analysis by study risk of bias was not possible as no studies were at low risk of bias in most domains.
Secondary outcomes
1.2 Live birth
None of the studies reported live birth as an outcome.
1.3 Pregnancy
Seven studies reported data for this outcome.
There was evidence of a detrimental effect on pregnancy rates for the use of albumin (Peto OR 0.72 95% CI 0.55 to 0.94, I² = 42%, seven studies, 1069 women, moderate quality evidence) (Figure 5;Analysis 1.2; Table 1).
5.
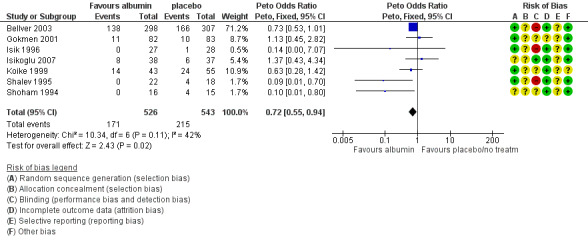
Forest plot of comparison: 1 Human Albumin vs placebo / no treatment, outcome: 1.2 Pregnancy rate.
1.2. Analysis.
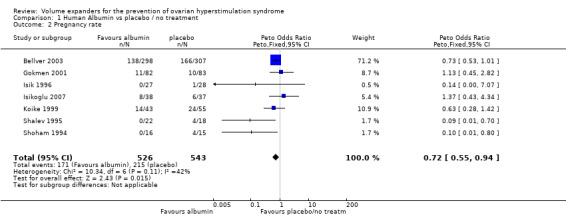
Comparison 1 Human Albumin vs placebo / no treatment, Outcome 2 Pregnancy rate.
Subgroup analysis
We examined whether effects differed according to the method used for diagnosing pregnancy (by β‐hCG: Gokmen 2001; Isik 1996; Koike 1999; Shalev 1995; Shoham 1994) or heartbeat on ultrasound (Bellver 2003 (only pregnancy data for autologous cycles considered); Isikoglu 2007) and found no evidence of a difference between the subgroups (test for subgroup differences: Chi² = 0.78, df = 1 (P = 0.38), I² = 0%
1.4 Adverse effects
None of the studies explicitly mentioned 'adverse effects' as a prespecified outcome, though most commented (in the results or discussion section) on adverse events related to the interventions. Among women receiving albumin, in one study (Gokmen 2001), a woman developed an anaphylactoid reaction with hypotension and laryngospasm. In a second study (Isik 1996), two women developed mild urticaria. Four studies (Bellver 2003, Isikoglu 2007, Shalev 1995, Shoham 1994), noted that no side effects or hypersensitivity reactions were detected in association with the intervention. Koike 1999 did not mention adverse effects.
2. Hydroxyethyl starch (HES) versus placebo or no treatment
Two studies compared hydroxyethyl starch (HES) versus placebo (Gokmen 2001; Konig 1998). Both reported overall rates of moderate or severe OHSS.
Primary outcome
2.1 Moderate or severe OHSS
There was evidence of a beneficial effect of HES on OHSS (Peto OR 0.27 95% CI 0.12 to 0.59, I² = 0%, two studies, 272 women, very low quality evidence). This suggests that if the rate of moderate or severe OHSS with no treatment is 16%, it will be about 5% (2% to 10%) with the use of HES (Analysis 2.1; Figure 6; Table 2).
2.1. Analysis.
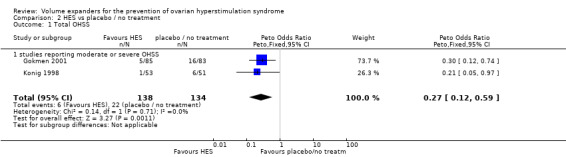
Comparison 2 HES vs placebo / no treatment, Outcome 1 Total OHSS.
6.
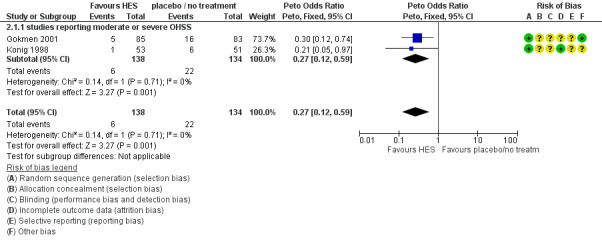
Forest plot of comparison: 2 HES vs placebo / no treatment, outcome: 2.1 Total OHSS.
Sensitivity analysis
Use of a risk ratio did not materially change the main findings. Sensitivity analysis by study risk of bias was not possible as neither studies was at low risk of bias in most domains.
Secondary outcomes
2.2 Live birth
Neither of the studies reported live birth as an outcome.
2.3 Pregnancy
There was no evidence of a difference in pregnancy rates (diagnosed by serum β‐hCG level) between women receiving IV HES and those receiving placebo (Peto OR 1.20 95% CI 0.49 to 2.93, one study, 168 women, very low quality evidence) (Analysis 2.2; Figure 7; Table 2).
2.2. Analysis.
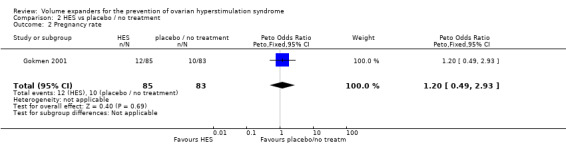
Comparison 2 HES vs placebo / no treatment, Outcome 2 Pregnancy rate.
7.
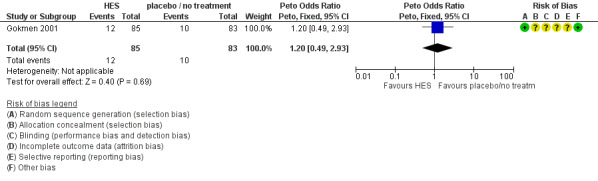
Forest plot of comparison: 2 HES vs placebo / no treatment, outcome: 2.2 Pregnancy rate.
2.4 Adverse effects
Neither of the studies explicitly mentioned 'adverse effects' as a prespecified outcome. There were two mild cases of urticaria in one study (Gokmen 2001) and one case of urticaria in the other (Konig 1998).
3. Mannitol versus placebo or no treatment
One study compared mannitol versus no treatment (Saremi 2003).This study reported overall rates of moderate or severe OHSS.
Primary outcome
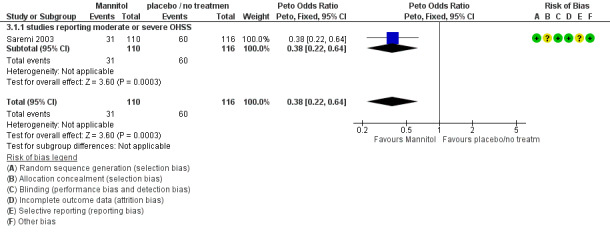
3.1 Moderate or severe OHSS
There was evidence of a beneficial effect of mannitol on OHSS (Peto OR 0.38, 95% CI 0.22 to 0.64, one study, 226 women, low quality evidence).
This suggests that if the rate of OHSS with no treatment is 52%, it will be about 29% (19% to 41%) with mannitol (Analysis 3.1; Figure 8; Table 3).
3.1. Analysis.
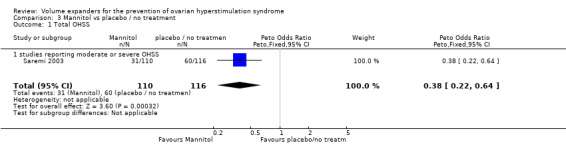
Comparison 3 Mannitol vs placebo / no treatment, Outcome 1 Total OHSS.
8.
Forest plot of comparison: 3 Mannitol vs placebo / no treatment, outcome: 3.1 Total OHSS.
Sensitivity analysis
Use of a risk ratio did not materially change the main findings.
Secondary outcomes
3.2 Live birth
Live birth was not reported as an outcome.
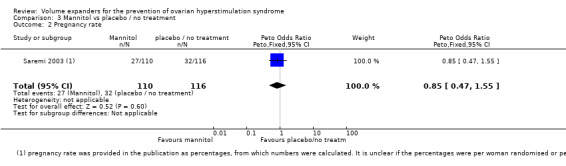
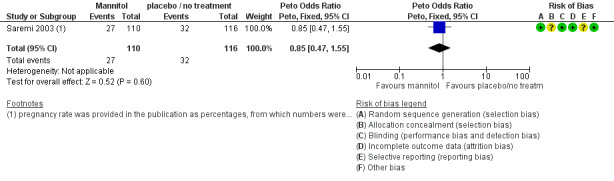
3.3 Pregnancy
There was no evidence of a difference in pregnancy rates (diagnosed by ultrasound) between the groups (Peto OR 0.85 95% CI 0.85 to 1.55; one study, 226 women, low quality evidence) (Analysis 3.2; Figure 9; Table 3).
3.2. Analysis.
Comparison 3 Mannitol vs placebo / no treatment, Outcome 2 Pregnancy rate.
9.
Forest plot of comparison: 3 Mannitol vs placebo / no treatment, outcome: 3.2 Pregnancy rate.
3.4 Adverse effects
Adverse events were not mentioned in this study. In private correspondence the authors stated that data on adverse events were collected, but they did not specify what type or report any events.
Assessment of publication bias
There were insufficient studies to construct a funnel plot to assess the risk of publication bias.
Discussion
Summary of main results
Nine randomised controlled studies compared the effectiveness of intravenous (IV) administration of the volume expanders human albumin, hydroxyethyl starch (HES), or mannitol versus placebo or no treatment for the prevention of ovarian hyperstimulation syndrome (OHSS) in women at high risk of OHSS. The overall quality of the evidence ranged from very low to moderate (Summary of findings table 1).
There was evidence that intravenous albumin administration around the time of oocyte retrieval has a beneficial effect on the incidence of moderate or severe OHSS. However, there was substantial unexplained heterogeneity between studies for this outcome (I² = 69%).
Our analysis suggested that HES might be associated with reduced rates of moderate or severe OHSS, but this was only based on two studies reporting only 28 events. Mannitol also appeared to have a beneficial effect on the incidence of OHSS, but this finding was based on a single study, so this moderate quality evidence remains to be confirmed in additional, large RCTs.
Live birth rate was not reported in any of the studies.There was moderate quality evidence that administration of human albumin lowered pregnancy rates in women at high risk for OHSS. For HES or mannitol there was no evidence of an effect on pregnancy rates.
Adverse effects were poorly reported and no firm conclusions could be drawn; although uncommon, clinicians should be aware that administration of IV fluids could cause severe morbidity.
Overall completeness and applicability of evidence
There were no randomised controlled studies (RCTs) that compared other volume expanders such as dextran or polygeline versus placebo or no treatment.
There was significant clinical heterogeneity between the included studies. The criteria for selecting women with a risk of OHSS varied across trials. Some used the number of oocytes at the day of hCG administration or the number of retrieved oocytes. Other studies used E2 level as a basic risk marker, but cut‐off levels varied greatly amongst studies; for example, inclusion E2 level for the study matched cycle cancellation (and therefore exclusion level) in another study. The method or criteria of OHSS diagnosis also varied widely between studies. With regard to studies of human albumin, the dosage of gonadotropins used for controlled ovarian hyperstimulation was not stated in most of the studies; the dose and timing of administration of albumin or starch varied between studies, between 10 g albumin immediately after oocyte retrieval to 50 g albumin one or two hour before oocyte retrieval, whilst 52.5 g albumin is required to accomplish a normal plasma albumin level of 4.2 to 4.5 g/100 mL in a well‐hydrated patient (Kissler 2001).
All of the plasma expanders were given either on the day of hCG administration or day of oocyte retrieval, therefore implicitly aiming to prevent early onset OHSS. The included studies made no distinction between early and late onset OHSS.
Quality of the evidence
All the studies were at serious risk of bias: none adequately described their method of allocation concealment, none prospectively collected comparative data on adverse events and only one clearly reported blinded outcome assessment.
The quality of the evidence was rated as very low to moderate for all comparisons. The main limitations in the evidence were risk of bias, statistical heterogeneity, imprecision, and lack of data for most comparisons. (Table 1; Table 2; Table 3).
Potential biases in the review process
Previous versions of the review included both Gokmen 2001 and a subsequent study by the same group (Gokmen 2005). We understand from personal communication with Christos Venetis (first author of Venetis 2011) that these two studies overlap and so we have removed the data for Gokmen 2005 from this updated version of the review. However, we have been unable to contact the study authors directly. No other potential biases were identified in the review process.
Agreements and disagreements with other studies or reviews
Two older systematic reviews of human albumin for the prevention of OHSS reached slightly different conclusions, but both focused on severe OHSS only. Both reviews reported no difference in the occurrence of severe OHSS between those who received intravenous albumin and those who did not (Venetis 2011: odds ratio (OR) 0.80; 95% confidence interval (CI), 0.52 to 1.22, eight RCTs, 1199 women; Jee 2010: risk ratio (RR) 0.80, 95% CI 0.57 to1.12, 9 RCTs, 1613 women). These reviews however included quasi‐randomised studies which were not eligible for the current review (for Jee 2010 this was Panay 1999; for Venetis 2011 this was Ben‐Chetrit 2001) and Venetis 2011 also included data from Gokmen 2005, that we considered possibly biased. The slightly deleterious effect of albumin on pregnancy rate reported by Jee 2010 was confirmed in our review.
We have not identified any other systematic reviews comparing HES or mannitol with placebo or no treatment for prevention of OHSS.
Authors' conclusions
Implications for practice.
Evidence suggests that the plasma expanders assessed in this review (human albumin, HES and mannitol) reduce rates of moderate and severe OHSS in women at high risk. Adverse events appear to be uncommon, but were too poorly reported to reach any firm conclusions, and there were no data on live birth. However, there was evidence that human albumin reduces pregnancy rates. While there was no evidence that HES, or mannitol had any influence on pregnancy rates, the evidence of effectiveness was based on very few trials which need to be confirmed in additional, larger randomised controlled trials (RCTs) before they should be considered for routine use in clinical practice.
.
Implications for research.
High‐quality, well‐designed and adequately powered RCTs are needed to assess the effectiveness of volume expanders in women at high risk of developing OHSS.
Future studies should take into consideration the cost‐effectiveness and psychological impact of treatment with volume expanders and should systematically report on adverse events.
What's new
| Date | Event | Description |
|---|---|---|
| 4 November 2016 | Review declared as stable | Further evidence is unlikely to change the conclusions of this review. |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 4, 1999
| Date | Event | Description |
|---|---|---|
| 29 August 2016 | New search has been performed | The revision of evidence and addition of a new comparison have led to a change in the conclusions of this review. |
| 9 September 2015 | New citation required and conclusions have changed | New search. The title has been amended to reflect differences in the inclusion criteria and change to moderate or severe OHSS as the primary outcome (previously just severe OHSS). One study added for one new comparison (mannitol versus no treatment; Saremi 2003). Exclusion of suspected duplicate data. Extra author added. 'Summary of findings' table added. |
| 2 November 2010 | New citation required and conclusions have changed | Substantive amendment |
| 24 September 2009 | Amended | The title was changed from Intra‐venous albumin for preventing severe ovarian hyperstimulation syndrome to Intra‐venous fluids for the prevention of severe ovarian hyperstimulation syndrome |
| 1 August 2009 | New search has been performed | Five RCTs were added New comparisons 1‐ Between hydroxyethyl starch versus placebo 2‐ Overall comparison of IV fluid stratified by the nature of intervention |
| 1 August 2009 | New citation required and conclusions have changed | The conclusion has changed and a new author was added with change of authors' order |
| 7 November 2008 | Amended | Converted to new review format. |
Acknowledgements
Dr Saremi is acknowledged for providing additional information on his publication. Special thanks also to the highly supportive team at the Cochrane office in Auckland, especially editor Jane Marjoribanks and the Information Specialist Marian Showell for designing and running the search strategies.
We thank Professor Johannes Evers, Professor Hesham Al‐Inany and Professor Mohamed Aboulghar for their contributions to previous versions of this review. Professor Al‐Inany took the lead in writing the protocol and the first version of the full review. Prof Aboulghar screened studies and extracted and interpreted data in the first version of the full review.
Appendices
Appendix 1. CGF database search (formerly MDSG)
MDSG search strategy iv fluids for OHSS 15.09.15
Keywords CONTAINS "ovarian hyperstimulation" or "ovarian hyperstimulation syndrome " or "OHSS" or Title CONTAINS" ovarian hyperstimulation" or "ovarian hyperstimulation syndrome " or "OHSS"
AND
Keywords CONTAINS "human albumin" or "human serum albumin" or "human sera" or "albumin" or "hydroxyethyl starch" or "hydroxyethyle starch" or "intravenous albumin" or "intravenous fluids" or "intravenous" or "Calcium" or "calcium gluconate" or "starch solution" or "mannitol" or Title CONTAINS "human albumin" or "human serum albumin" or "human sera" or "albumin" or "hydroxyethyl starch" or "hydroxyethyle starch" or "intravenous albumin" or "intravenous fluids" or "intravenous" or "Calcium" or "calcium gluconate" or "starch solution" or "mannitol"
Appendix 2. Cochrane Central Register of Controlled Trials
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <02.09.2015> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Ovarian Hyperstimulation Syndrome/ (143) 2 Ovarian Hyperstimulation Syndrome.tw. (309) 3 OHSS.tw. (227) 4 or/1‐3 (408) 5 exp Serum Albumin/ (1044) 6 Albumin$.tw. (6084) 7 human sera.tw. (24) 8 colloid$.tw. (1276) 9 exp starch/ or exp hetastarch/ (1018) 10 hydroxyethyl$.tw. (1018) 11 (starch or hetastarch).tw. (1567) 12 HES.tw. (583) 13 haemacel.tw. (1) 14 Haemaccel.tw. (52) 15 exp Calcium Gluconate/ (43) 16 Calcium.tw. (13188) 17 intravenous fluid$.tw. (533) 18 iv fluid$.tw. (204) 19 exp Infusions, Intravenous/ (8755) 20 exp Fluid Therapy/ (1127) 21 exp mannitol/ (375) 22 mannitol.tw. (782) 23 (osmitrol or osmofundin).tw. (0) 24 or/5‐23 (32416) 25 4 and 24 (41)
Appendix 3. Ovid MEDLINE®
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>02.09.2015 Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Ovarian Hyperstimulation Syndrome/ (1881) 2 Ovarian Hyperstimulation Syndrome.tw. (2167) 3 OHSS.tw. (1288) 4 or/1‐3 (2789) 5 exp Serum Albumin/ (71943) 6 Albumin$.tw. (126452) 7 human sera.tw. (6398) 8 colloid$.tw. (41622) 9 exp starch/ or exp hetastarch/ (33340) 10 hydroxyethyl$.tw. (12274) 11 (starch or hetastarch).tw. (27745) 12 HES.tw. (4360) 13 haemacel.tw. (6) 14 Haemaccel.tw. (249) 15 exp Calcium Gluconate/ (937) 16 Calcium.tw. (311984) 17 intravenous fluid$.tw. (4082) 18 iv fluid$.tw. (1017) 19 exp Infusions, Intravenous/ (49843) 20 exp Fluid Therapy/ (15810) 21 exp mannitol/ (11611) 22 mannitol.tw. (15517) 23 (osmitrol or osmofundin).tw. (1) 24 or/5‐23 (649695) 25 4 and 24 (178) 26 randomized controlled trial.pt. (411031) 27 controlled clinical trial.pt. (91630) 28 randomized.ab. (334732) 29 placebo.tw. (173207) 30 clinical trials as topic.sh. (178556) 31 randomly.ab. (241080) 32 trial.ti. (147726) 33 (crossover or cross‐over or cross over).tw. (66041) 34 or/26‐33 (1021994) 35 exp animals/ not humans.sh. (4113129) 36 34 not 35 (941700) 37 25 and 36 (36)
Appendix 4. EMBASE
Database: Embase <1980 to 2015 Week 37> 02.09.2015 Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp ovary hyperstimulation/ (6927) 2 Ovarian Hyperstimulation.tw. (5643) 3 OHSS.tw. (2090) 4 or/1‐3 (8564) 5 exp Serum Albumin/ (28111) 6 Albumin$.tw. (147231) 7 colloid$.tw. (42324) 8 exp starch/ or exp hetastarch/ (25916) 9 exp starch/ (20467) 10 exp hetastarch/ (5626) 11 hydroxyethyl.tw. (10802) 12 (starch or hetastarch).tw. (31085) 13 HES.tw. (6162) 14 haemacel.tw. (32) 15 Haemaccel.tw. (436) 16 exp gluconate calcium/ (4508) 17 calcium.tw. (353894) 18 intravenous fluid$.tw. (5388) 19 iv fluid$.tw. (2002) 20 exp infusion fluid/ (22326) 21 exp mannitol/ (25703) 22 mannitol.tw. (17118) 23 (osmitrol or osmofundin).tw. (63) 24 or/5‐23 (647541) 25 4 and 24 (270) 26 Clinical Trial/ (850170) 27 Randomized Controlled Trial/ (382698) 28 exp randomization/ (67925) 29 Single Blind Procedure/ (20923) 30 Double Blind Procedure/ (123268) 31 Crossover Procedure/ (44330) 32 Placebo/ (262629) 33 Randomi?ed controlled trial$.tw. (123047) 34 Rct.tw. (18107) 35 random allocation.tw. (1447) 36 randomly allocated.tw. (23181) 37 allocated randomly.tw. (2054) 38 (allocated adj2 random).tw. (736) 39 Single blind$.tw. (16320) 40 Double blind$.tw. (154326) 41 ((treble or triple) adj blind$).tw. (482) 42 placebo$.tw. (220002) 43 prospective study/ (305847) 44 or/26‐43 (1499732) 45 case study/ (33720) 46 case report.tw. (290494) 47 abstract report/ or letter/ (936759) 48 or/45‐47 (1254488) 49 44 not 48 (1459953) 50 49 and 25 (76)
Appendix 5. Ovid PsycINFO
Database: PsycINFO <1806 to September Week 1 2015> 02.09.2015 Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 Ovarian Hyperstimulation Syndrome.tw. (4) 2 OHSS.tw. (6) 3 or/1‐2 (8) 4 exp Serum Albumin/ (210) 5 Albumin$.tw. (1205) 6 colloid$.tw. (193) 7 (starch or hetastarch).tw. (286) 8 hydroxyethyl.tw. (47) 9 HES.tw. (436) 10 haemacel.tw. (0) 11 Haemaccel.tw. (0) 12 calcium/ or calcium.tw. (11395) 13 intravenous fluid$.tw. (103) 14 iv fluid$.tw. (35) 15 Intravenous Infusion$.tw. (531) 16 mannitol.tw. (127) 17 or/4‐16 (14271) 18 3 and 17 (0)
Appendix 6. CINAHL
CINAHL search strategy for PMA481 02.09.15
| # | Query | Results |
| S22 | S4 AND S21 | 10 |
| S21 | S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 | 45,131 |
| S20 | TX Fluid* N2 Therap* | 4,743 |
| S19 | (MM "Infusions, Intravenous") | 1,111 |
| S18 | TX iv fluid* | 291 |
| S17 | TX intravenous fluid* | 1,060 |
| S16 | TX Calcium or (MM "mannitol")or TX mannitol | 26,628 |
| S15 | (MM "Calcium") | 3,739 |
| S14 | TX Haemaccel | 22 |
| S13 | TX haemacel | 1 |
| S12 | TX hetastarch | 73 |
| S11 | TX starch | 1,393 |
| S10 | TX hydroxyethyl | 696 |
| S9 | (MM "Hydroxyethyl Starch") | 388 |
| S8 | TX colloid* | 1,185 |
| S7 | TX human sera | 89 |
| S6 | TX Albumin* | 10,636 |
| S5 | (MM "Serum Albumin") | 836 |
| S4 | S1 OR S2 OR S3 | 278 |
| S3 | TX OHSS | 79 |
| S2 | TX Ovarian Hyperstimulation Syndrome | 265 |
| S1 | (MM "Ovarian Hyperstimulation Syndrome") | 140 |
Appendix 7. Pubmed
search up to 29.07.2015
ohss and albumin (16 hits)
ohss and calcium (5 hits)
ohss and intravenous (18 hits)
ohss and starch (4 hits)
Appendix 8. ICTRP
search up to 29.07.2015
ohss and albumin (4 hits)
ohss and calcium (3 hits)
ohss and mannitol (0 hits)
ohss and intravenous (1 hits)
ohss and starch (2 hits)
ohss and fluid* (1 hits)
Appendix 9. Clinicaltrials.gov
search up to 29.07.2015
ohss and albumin (4 hits)
ohss and mannitol (0 hits)
ohss and calcium (3hits)
ohss and intravenous (2 hits)
ohss and starch (1 hits)
ohss and fluid* (13 hits)
Data and analyses
Comparison 1. Human Albumin vs placebo / no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total OHSS | 7 | 1452 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.47, 0.95] |
| 1.1 Studies reporting moderate or severe OHSS | 3 | 1208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.46, 1.08] |
| 1.2 Studies reporting severe OHSS | 4 | 244 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.60 [0.32, 1.10] |
| 2 Pregnancy rate | 7 | 1069 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.55, 0.94] |
Comparison 2. HES vs placebo / no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total OHSS | 2 | 272 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.12, 0.59] |
| 1.1 studies reporting moderate or severe OHSS | 2 | 272 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.12, 0.59] |
| 2 Pregnancy rate | 1 | 168 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.49, 2.93] |
Comparison 3. Mannitol vs placebo / no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total OHSS | 1 | 226 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.22, 0.64] |
| 1.1 studies reporting moderate or severe OHSS | 1 | 226 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.22, 0.64] |
| 2 Pregnancy rate | 1 | 226 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.47, 1.55] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bellver 2003.
| Methods | Randomised controlled trial
Parallel design
Single centre Not blind Power calculation: done |
|
| Participants | 988 women (infertile women n = 605 and donors n = 383) who were all considered to be at risk of moderate or severe OHSS (495 albumin/493 control)
> 20 retrieved oocytes was the basic risk factor of OHSS
Age (29.9 versus 29.7years)
Duration of infertility (not stated)
No. of FSH and hMG ampoules (not stated)
No. of oocytes (28.7 versus 27.8)
No. of embryos (15 versus 19) No. of transferred embryos (2.8 versus 3.0) |
|
| Interventions |
Study group: 40 g human albumin infused intravenously at a slow rate, immediately after oocyte retrieval Duration: during 30 min Control group: no treatment |
|
| Outcomes | Method of diagnosing severe OHSS: criteria for (Golan 1989) Severe OHSS: 25/495 versus 23/493 Pregnancy: 138/298 versus 166/307 Method of diagnosing pregnancy: fetal heart rate Tolerability: yes Safety: yes Cost‐benefit: stated (195 euros/patient) |
|
| Notes | Two kinds of GnRH agonist were used and only early OHSS was assessed. Placebo was not used in their control group, which eliminates the placebo effect of albumin administration. Serum E2 level, which was previously reported as an independent risk factor for severe OHSS, was not considered a high‐risk factor in this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 966/988 (98%) randomised women included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether data on adverse events were collected prospectively as it was not a prespecified outcome, though mentioned in the discussion section |
| Other bias | Low risk | No other potential bias identified |
Gokmen 2001.
| Methods | Randomised controlled trial, three‐arm trial, parallel design, single centre Power calculation: not done | |
| Participants | 250 infertile women at risk of severe OHSS (82 albumin/83 control/85 HES) E2 (the basic risk factor of OHSS) was 3000 pg/mL or the presence of > 20 follicles on the day of hCG. Age: albumin 29.6 versus HES 31.2 versus placebo 32.3 years Duration of infertility (not mentioned) No. of hMG ampoules (not mentioned) No.of oocytes (albumin 12.0 versus HES 13.2 versus placebo 11.1) No.of embryos ( albumin 3.3 versus HES 3.1 versus placebo 3.0) | |
| Interventions | Study group: 10 g albumin immediately after oocyte retrieval, or 6% HES (200/0.5) 500 mL Duration of infusion: over 30 minutes Control group: received saline | |
| Outcomes | Method of diagnosing severe OHSS: criteria of Schenker and Weinstein, 1978 Severe OHSS: albumin versus control 0/82 versus 4/83, and HES versus control 0/85 versus 4/83 Pregnancy: albumin versus control 11/82 versus 10/83, and HES versus control 12/85 versus 10/83 Method of diagnosing pregnancy: serum β‐hCG Tolerability: yes Safety: one case of anaphylactic reaction with albumin and two cases of urticaria with HES Cost‐benefit: not stated | |
| Notes | Source of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 250 women randomised and all included in analysis ‐ but later unpublished report (Gokmen 2005) apparently refers to same study and includes a larger sample and two additional events. Attempts to contact the study authors were unsuccessful. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether data on adverse events were collected prospectively as it was not a prespecified outcome |
| Other bias | Low risk | No other potential bias identified |
Isik 1996.
| Methods | Randomised controlled trial, parallel design, single centre Power calculation: done |
|
| Participants | 55 infertile women who were all considered to be at risk of severe OHSS (27 albumin/28 control) E2 (the basic risk factor of OHSS) was 11010 pmol/L Age (31.2 versus 30.8 years) Duration of infertility (7.8 versus 7.3) No. of hMG ampoules (25.9 versus 26.1) No. of oocytes (15.3 versus 16.4) No. of embryos (3.2 versus 3) | |
| Interventions | Study group: 10 g albumin 2 hours before oocyte retrieval Duration of infusion: over 1 hour Control group: no treatment | |
| Outcomes | Method of diagnosing severe OHSS: criteria of Schenker and Weinstein, 1978 Severe OHSS: 0/27 versus 1/28 Pregnancy: 5/27 versus 5/28 Method of diagnosing pregnancy: serum β‐hCG Tolerability: yes Safety: 2 urticaria Cost‐benefit: not stated | |
| Notes | Source of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random table |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether data on adverse events were collected prospectively as it was not a prespecified outcome, though mentioned in the results section |
| Other bias | Low risk | No other potential bias identified |
Isikoglu 2007.
| Methods | Randomised, placebo‐controlled trial, parallel design, single centre No sample size calculation |
|
| Participants |
Study group: 75 infertile women who were all considered to be at risk of severe OHSS (38 albumin/37control)
High blood E2 level (> 4000 pg/mL) and > 20 follicles R14 mm on the day of hCG administration have been defined as the basic risk factors for severe OHSS
Age (29.3 versus 29.1 years)
Duration of infertility (not stated)
No. of hMG ampoules ( no significant difference between both groups) No. of FSH ampoules (no significant difference between both groups) No.of oocytes (22.6 versus 20.5) No.of embryos (3.4 versus 3.2) |
|
| Interventions | Study group: 10 g (50 cc, 20% HSA diluted in 100 mL 0.9% NaCl or 100 mL 0.9% NaCl) just after oocyte retrieval Duration: 1 hour Control group: 100 mL 0.9% NaCl | |
| Outcomes | Method of diagnosing severe OHSS: according to Navot et al, 1992 Severe OHSS: 8/38 versus 6/37 Pregnancy: 21/38 versus 23/37 Method of diagnosing clinical pregnancy: heartbeat at ultrasound 3 weeks after ET Tolerability: yes Safety: yes Cost‐benefit: not calculated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods unclear: "Patients were randomised by third‐party sealed envelope entry" |
| Allocation concealment (selection bias) | Unclear risk | Methods unclear: "Patients were randomised by third‐party sealed envelope entry" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The outcome assessors were blind to the interventions. The patients were also blind to the infusions" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether data on adverse events were collected prospectively as it was not a prespecified outcome, though mentioned in the results section |
| Other bias | Low risk | No other potential bias identified |
Koike 1999.
| Methods | Randomised, placebo‐controlled trial, single centre, unpublished | |
| Participants | 98 infertile women at risk of severe OHSS ( 43 albumin plus saline versus 55 saline alone) undergoing IVF/ICSI/GIFT > 20 oocytes were retrieved from patients at high risk.The estradiol in their serum was not measured Age (32.1 versus 31.0 years) Duration of infertility (not stated) No. of hMG ampoules (not stated) No. of oocytes (27 versus 26.1) No. of embryos (19 versus 17) |
|
| Interventions | Study group: 37.5 g albumin plus 1000 mL electrolyte solution just after oocyte retrieval Duration: consecutive 3 days Control group: 1000 mL electrolyte solution for consecutive 3 days | |
| Outcomes | Method of diagnosing severe OHSS: marked haemoconcentration (haematocrit ≥ 45) and/or hypoproteinaemia (serum total protein < 6.0 g/dL) in addition to marked ascites on the upper abdomen at least 4 days after oocyte retrieval (Orvieto and Ben‐Rafael,1998) Severe OHSS: 13/43 versus 21/55 Pregnancy: 14/43 versus 24/55 Method of diagnosing pregnancy: serum β‐hCG Tolerability: not stated Safety: not stated Cost‐benefit: not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Methods not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects not reported as an outcome |
| Other bias | Unclear risk | No other potential bias identified |
Konig 1998.
| Methods | Randomised, placebo‐controlled, double blind | |
| Participants | 104 infertile women at risk of severe OHSS (53 HES/51 control) E2 the basic risk factor of OHSS) was > 1500 pg/mL and more than 10 follicles on the day of hCG injection Age (HES 32.6 versus placebo 30.7 years) Duration of infertility (not mentioned) No. of hMG ampoules (not mentioned, similar in both groups ) No. of oocytes (not mentioned, similar in both groups) No. of embryos (not mentioned) | |
| Interventions |
Study group: 1000 mL of 6% HES solution shortly after embryo transfer (48 hr after the oocyte retrieval) originally 104 pt randomised: HES 53 control 51; data presented only for 51 and 50 patients respectively. dropout 2.9% (3 women; 2 missing data, 1 allergic reaction) Duration of infusion: over 2 hours Control group: 1000 mL NaCl 0.9% solution |
|
| Outcomes | Method of diagnosing severe OHSS: criteria of WHO, 1973 Severe OHSS 0/53 versus 1/51 Pregnancy: not stated but similar between both group Method of diagnosing pregnancy: not stated (clinical pregnancy) Tolerability: yes Safety: one case of urticaria Cost‐benefit: not stated | |
| Notes | Commercially funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Methods not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double blinded; both patients and physicians blinded, but outcome assessors not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 101/104 (97%) randomised women were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether data on adverse events were collected prospectively as it was not a prespecified outcome, though mentioned in the results section |
| Other bias | Unclear risk | No other potential bias identified |
Saremi 2003.
| Methods | Randomised controlled trial; simple randomisation per random number table | |
| Participants | 226 patients with endocrine and sonographic evidence of PCOS. 110 women study group, 116 women control group E2 : no threshold for inclusion. Exclusion and cancelling when E2 > 6000 pg/mL Age ( mannitol 27.5yr vs control 28.6yr)) Duration of infertility not mentioned No. of hMG ampoules not mentioned No. of oocytes (mannitol 25.5 vs control 23.6) similar in both groups No. of embryos (not mentioned) |
|
| Interventions |
Study group (A): 3 gr/kg bodyweight mannitol (20 g mannitol/100 mL water for injection) daily starting on the day after hCG injection until third day after embryo transfer ( lasting 5‐7 days depending on day of transfer) Duration of infusion: 30‐60 minutes Control group (B): no drugs |
|
| Outcomes | Incidence of OHSS (mild, moderate and severe), pregnancy rates (defined as heartbeat at ultrasound 3 weeks after ET), blood pressure, Hb levels, white blood cell count, vital sign per 4 hrs after administration, serum osmolality and osmolar gap, intake and output volumes before and during mannitol administration & renal functionality, serum electrolytes | |
| Notes | Funding not stated; additional information from correspondence with author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation per random number table |
| Allocation concealment (selection bias) | Unclear risk | Methods not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Physicians were blinded to the allocated groups, nurse performed protocol |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women included in analysis, no drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | Data on adverse events were collected though not explicitly mentioned what kind of events. "mannitol protocol, all of the lab tests and patient's signs and symptoms were recorded; including vital sign Q4h, serum osmolality and osmolar gap, intake and output volumes before and during mannitol administration & renal functionality, serum electrolytes and etc… " (private correspondence) |
| Other bias | Low risk | No other potential bias detected |
Shalev 1995.
| Methods | Randomised, placebo‐controlled trial, parallel design, single centre No power of calculation |
|
| Participants | 40 infertile women who were all considered to be at risk of severe OHSS (22 albumin/18control) E2 (the basic risk factor of OHSS) was 9200 pmol/L Age (29.7 versus 28.3 years) Duration of infertility (not stated) No. of hMG ampoules (not stated) No. of oocytes (21 versus 19.3) No. of embryos (19 versus 17) | |
| Interventions | Study group: 20 g albumin just after oocyte retrieval Duration: not stated Control group: no treatment | |
| Outcomes | Method of diagnosing severe OHSS: according to Navot et al, 1992 Severe OHSS: 0/22 versus 4/18 Pregnancy: 6/22 versus 2/18 Method of diagnosing pregnancy: serum β‐hCG Tolerability: yes Safety: yes Cost‐benefit: not stated | |
| Notes | Source of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Methods not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether data on adverse events were collected prospectively as it was not a prespecified outcome, though mentioned in the results section |
| Other bias | Low risk | No other potential bias identified |
Shoham 1994.
| Methods | Randomised, placebo‐controlled trial, parallel design, single centre | |
| Participants | 31 infertile women who were all considered to be at risk of severe OHSS (16 albumin/15control) E2 (the basic risk factor of OHSS was 7000 pmol/L) Age (32.5 versus 29.7 years) Duration of infertility (6.9 versus 7) No. of hMG ampoules: (23.3 versus 22.7) No. of oocytes: (13.4 versus 12.6) No. of embryos: (3.4 versus 3.2) | |
| Interventions | Study group: 50 g albumin 2 hours before oocyte retrieval Duration: over 1 hour Control group: 500 mL of 9% NaCl | |
| Outcomes | Method of diagnosing severe OHSS: criteria for Schenker and Weinstein, 1978 Severe OHSS (0/16 versus 4/15) Pregnancy: (4/16 versus 2/15) Method of diagnosing pregnancy: serum β‐hCG Tolerability: yes Safety: yes Cost‐benefit: not stated | |
| Notes | Source of funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether data on adverse events were collected prospectively as it was not a prespecified outcome, though mentioned in the results section |
| Other bias | Low risk | No other potential bias identified |
ET: embryo transfer FSH: follicle‐stimulating hormone GIFT: gamete intra‐fallopian transfer GnRH: gonadotropin‐releasing hormone Hb: haemoglobin hCG: human chorionic gonadotrophin HES: hydroxyethyl starch hMG: human menopausal gonadotropin ICSI: intracytoplasmic sperm injection IVF: in vitro fertilisation NaCl: sodium chloride OHSS: ovarian hyperstimulation syndrome PCOS: polycystic ovary syndrome
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abramov 2001 | Case control study |
| Abuzeid 2010 | Prospective cohort clinical trial |
| Ahmadi 2010 | RCT, compared human albumin with cabergoline as prophylaxis of OHSS |
| Asch 1993 | Case series |
| Ben‐Chetrit 2001 | Quasi‐randomised study |
| Ben‐Rafael 1995 | Case series |
| Cambiaghi 2005 | RCT compared oral whey protein versus albumin |
| Chen 1997 | Prospective non‐randomised trial with historical control |
| Chen 2003 | Retrospective study, compared coasting versus intravenous albumin |
| Costabile 2000 | Albumin infusion versus intra‐muscular progesterone |
| Dmitrieva 2010 | RCT, compared starch solution versus calcium gluconate in prevention of OHSS |
| Egbase 1997 | Non‐controlled randomised study |
| El‐Khayat 2015 | RCT, excluded because calcium gluconate is not a volume expander |
| Endo 2004 | Quasi‐randomised study, comparing human albumin versus dextran |
| Fan 2006 | Randomised, placebo‐controlled trial comparing human albumin versus hydroxyethyl starch versus placebo. Abstract and the data were not reported clearly |
| Fluker 2000 | Prospective cohort study |
| Gamzu 2002 | Non‐randomised study, comparing hydroxyethyl starch versus Haemaccel |
| Gokmen 2005 | Data appear to overlap with Gokmen 2001. Attempts to obtain clarification from author were unsuccessful |
| Graf 1997 | Cohort study |
| Halme 1995 | Case report |
| Isik 1997 | Non‐controlled randomised study |
| Isik 2001 | Non‐randomised study, used combined approach for OHSS prevention |
| Kissler 2001 | Case report |
| Koike 2000 | Randomised controlled trial comparing human albumin versus continuous autotransfusion system of ascites (CASTA) |
| Kumbak 2005 | Retrospective study |
| Lewit 1996 | Retrospective case series |
| Lincolin 2002 | Retrospective study |
| Matorras 2013 | comparison of HES vs cabergoline and HES |
| Milacic 1996 | Method of randomisation was unclear |
| Mukherjee 1995 | Case reports |
| Naredi 2013 | Quasi‐randomised study (patients appointed to groups by odd/ even registration numbers) |
| Ndukwe 1997 | Retrospective review and data analysis |
| Ng 1995 | Cohort study |
| Panay 1999 | RCT, quasi‐randomised |
| Shaker 1996 | Compared albumin infusion versus embryo cryopreservation |
| Tan 1992 | The trial did not study the relevant intervention, instead it studied the use of glucocorticoids |
| Tehraninejad 2012 | Comparison of cabergoline with albumin |
| Torabizadeh 2013 | Comparison of cabergoline versus albumin |
| Yakovenko 2009 | Comparison of calcium gluconate versus HES |
HES: hydroxyethyl starch OHSS: ovarian hyperstimulation syndrome RCT: randomised controlled trial
Differences between protocol and review
The original plan for the review was to only study the preventive effect of human albumin administration on OHSS. During the 2010 update process it was decided that the review should be broadened to include all kinds of intravenous fluids being used in the prevention of OHSS. The only other intravenous fluid for which randomised controlled trials with a placebo or 'no treatment' control group could be found at that time, was hydroxyethyl starch (HES).
For the 2016 update, there were two new comparisons found, i.e. calcium gluconate and mannitol. However, calcium gluconate has a very different pathophysiological mechanism than the other fluids used, which all are volume expanders. A discussion on this topic took place between the authors and led to a decision to maintain a focus on volume expanders only. Therefore, we amended the protocol and changed the title accordingly.
The 2016 update also is the first version of the review to include 'moderate OHSS' as part of the primary outcome measure, as this is a clinically relevant degree of OHSS that could easily proceed to severe OHSS. Moreover, other interventional Cochrane reviews on the prevention of OHSS also use 'moderate OHSS' as an outcome.
Also in 2016 we added a subgroup analysis for the outcome clinical pregnancy, to explore whether the effect of the intervention on pregnancy rates was different in studies that diagnosed pregnancy by ultrasonic visualisation of fetal heart beat rather than by pregnancy test.
Contributions of authors
Mohamed AFM Youssef: performed searches of databases for new trials for the first review update, was involved in selecting trials for inclusion, performed independent data extraction and quality assessment of the included trials, was involved in writing the review, statistical analysis and interpretation of the data.
Selma Mourad: contributed to the 2016 update. Performed searches of databases for new trials, selecting trials for inclusion, corresponding with authors of primary studies, performed independent data extraction and quality assessment of the included trials, was responsible for interpretation of the data, adding the summary of findings tables and updating the main review text.
Sources of support
Internal sources
None specified by review author, Other.
External sources
No sources of support supplied
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bellver 2003 {published data only}
- Bellver J, Munoz EA, Ballesteros A, Soares SR, Bosch E, Simon C, et al. Intravenous albumin does not prevent moderate–severe ovarian hyperstimulation syndrome in high‐risk IVF patients: a randomized controlled study. Human Reproduction 2003;18(11):2283–8. [DOI] [PubMed] [Google Scholar]
Gokmen 2001 {published data only}
- Gokmen O, Ugur M, Ekin M, Keles G, Turan C, Oral H. Intravenous albumin versus hydroxyethyl starch for the prevention of ovarian hyperstimulation in an in‐vitro fertilization programme: a prospective randomised placebo controlled study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2001;96(2):187‐92. [DOI] [PubMed] [Google Scholar]
Isik 1996 {published data only}
- Isik AZ, Gokmen O, Zeyneloglu HB, Kara S, Keles G, Gulekli B. Intravenous albumin prevents moderate‐severe ovarian hyperstimulation in in vitro fertilisation patients: a prospective randomized and controlled study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1996;70:170‐83. [DOI] [PubMed] [Google Scholar]
Isikoglu 2007 {published data only}
- Isikoglu M, Berkkanoglu M, Senturk Z, Ozgur K. Human albumin does not prevent ovarian hyperstimulation syndrome in assisted reproductive technology program: a prospective randomized placebo‐controlled double blind study. Fertility and Sterility 2007;88(4):982‐5. [DOI] [PubMed] [Google Scholar]
Koike 1999 {published data only}
- Koike E, Araki S, Ogawa S, Minakami H, Sato I. Does i.v albumin prevent ovarian hyperstimulation syndrome?. Human Reproduction 1999;14(7):1920. [DOI] [PubMed] [Google Scholar]
Konig 1998 {published data only}
- Konig E, Bussen S, Sütterlin M, Steck T. Prophylactic intravenous hydroxyethyle starch solution prevents moderate‐severe ovarian hyperstimulation in in‐vitro fertilization patients: a prospective, randomized, double‐blind and placebo‐controlled study. Human Reproduction 1998;13(9):2421‐4. [DOI] [PubMed] [Google Scholar]
Saremi 2003 {published and unpublished data}
- Saremi A, Alam M, Motaghi M. Administration of mannitol to prevent severe ovarian hyperstimulation syndrome: A randomized controlled trial. Middle East Fertility Society Journal 2003;8(2):159‐163. [Google Scholar]
Shalev 1995 {published data only}
- Shalev E, Giladi Y, Matilsky M, Ben‐Ami M. Decreased incidence of severe ovarian hyperstimulation syndrome in high risk in‐vitro fertilisation patients receiving intravenous albumin: a prospective study. Human Reproduction 1995;10:1373‐6. [DOI] [PubMed] [Google Scholar]
Shoham 1994 {published data only}
- Shoham Z, Weissman A, Barash A, Borenstein R, Schachter M, Insler V. Intravenous albumin for the prevention of severe ovarian hyperstimulation syndrome in an invitro fertilisation program: a prospective, randomised, placebo‐controlled study. Fertility and Sterility 1994;62:137‐42. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abramov 2001 {published data only}
- Abramov Y, Fatum M, Abrahamove D, Schenker JC. Hydroxyethyl starch versus human albumin for the treatment of severe ovarian hyperstimulation syndrome: a preliminary report. Fertility and Sterility 2001;75:1228‐30. [DOI] [PubMed] [Google Scholar]
- Abramov Y, Fatum M, Fasouliotis S, Abrahamov D, Schenker JC. Hydroxyethylstarch versus human albumin for the treatment of severe ovarian hyperstimulation syndrome. Fertility and Sterility. Abstracts of the 17th Annual meeting of the ESHRE 2001;16(1):121. [DOI] [PubMed] [Google Scholar]
Abuzeid 2010 {published data only}
- Abuzeid M, Abed S, Salem W, Joseph S, Ashraf M, Rizk P.B. The effectiveness of 6% hydroxyethyle starch solution in reducing the incidence of severe hyperstimulation syndrome in polycystic ovarian disease patients: A prospective cohort clinical trial. Journal of Reproductive Medicine and Endocrinology, IFFS 2010 September 12‐16 Munich;7(4):240‐383. [Google Scholar]
Ahmadi 2010 {published data only}
- Ahmadi Sh, Rahmani E, Oskouian H. Cabergoline versus human albumin in prophylaxis ovarian hyperstimulation syndrome. Reproductive BioMedicine Online 2010;Suppl. 3:S41–S42. [Google Scholar]
Asch 1993 {published data only}
- Asch R, Ivery G, Goldsman M. The use of intravenous albumin in patients at high risk for severe ovarian hyperstimulation syndrome. Human Reproduction 1993;8:1015‐20. [DOI] [PubMed] [Google Scholar]
Ben‐Chetrit 2001 {published data only}
- Ben Chetrit A, Eldar‐Geva T, Gal M, Huerta M, Mimon T, Algur N, et al. The questionable use of albumin for the prevention of ovarian hyperstimulation syndrome in an IVF programme: a randomized placebo‐controlled trial. Human Reproduction 2001;16(9):1880‐4. [DOI] [PubMed] [Google Scholar]
Ben‐Rafael 1995 {published data only}
- Ben‐Rafael Z, Orvieto R, Dekel A, Peleg D, Gruzman C. Intravenous albumin and the prevention of severe ovarian hyperstimulation syndrome. Human Reproduction 1995;10(10):2750‐2. [DOI] [PubMed] [Google Scholar]
Cambiaghi 2005 {published data only}
- Cambiaghi AS, Castellotti DS. Oral whey protein for preventing ovarian hyperstimulation syndrome. Fertility and Sterility. Abstracts from the annual meeting of ASRM 84 Suppl 1;84(1):315. [Google Scholar]
Chen 1997 {published data only}
- Chen C, Chen S, Wu M, Ho H, Yang J, Yang Y. Intravenous albumin does not prevent the development of severe ovarian hyperstimulation syndrome. Fertility and Sterility 1997;68:287‐91. [DOI] [PubMed] [Google Scholar]
Chen 2003 {published data only}
- Chen CD, Chao KH, Yang JH, Chen SU, Ho HN, Yang YS. Comparison of coasting and intravenous albumin in the prevention of ovarian hyperstimulation syndrome. Fertility and Sterility 2003;80(1):86‐90. [DOI] [PubMed] [Google Scholar]
Costabile 2000 {published data only}
- Costabile L, Unfer V, Manna C, Gerli S, Rossetti D, Renzo GC. Use of intramuscular progesterone versus intravenous albumin for the prevention of ovarian hyperstimulation syndrome. Gynecologic and Obstetric Investigation 2000;50(3):182‐5. [DOI] [PubMed] [Google Scholar]
Dmitrieva 2010 {published data only}
- Dmitrieva NV, Yakovenko SA, Zorina IV, Voznesenskaya YV, Apryshko VP. Comparison of calcium gluconate and starch solution in the treatment of ovarian hyperstimulation syndrome. Reproductive BioMedicine Online 2010;3:S41–S42. [Google Scholar]
Egbase 1997 {published data only}
- Egbase PE, Makhseed M, Al Sharhan M, Grdzinskas JG. Timed unilateral ovarian follicular aspiration prior to administration of human chorionic gonadotrophin for the prevention of severe ovarian hyperstimulation syndrome in invitro fertilisation: a prospective randomised study. Human Reproduction 1997;12:2603‐6. [DOI] [PubMed] [Google Scholar]
El‐Khayat 2015 {published data only}
- El‐Khayat W, Elsadek M. Calcium infusion for the prevention of ovarian hyperstimulation syndrome: a double‐blind randomized controlled trial. Fertility and Sterility 2015;103(1):101‐5. [DOI] [PubMed] [Google Scholar]
Endo 2004 {published data only}
- Endo T, Kitajima Y, Hayashi T, Fujii M, Hata H, Azumaguchi A. Low‐molecular‐weight dextran infusion is more effective for the treatment of hemoconcentration due to severe ovarian hyperstimulation syndrome than human albumin infusion. Fertility and Sterility 2004;82(5):1449‐51. [DOI] [PubMed] [Google Scholar]
Fan 2006 {published data only}
- Fan YH, Qiao J, Chen XN, Wang HY, Ma CH, Liu P. Assisted reproductive Intravenous hydroxyethyl starch versus human albumin for prevention of the ovarian hyperstimulation syndrome. VIII FIGO World Congress of Gynecology and Obstetrics 2006;2:34. [Google Scholar]
- Fan YH, Qiao J, Chen XN, Wang HY, Ma CH, Liu P. Intravenous hydroxyethyl starch versus human albumin for prevention of the ovarian hyperstimulation syndrome. Fertility and Sterility 2006;86 Suppl 2:136. [Google Scholar]
Fluker 2000 {published data only}
- Fluker MR, Copeland JE, Yuzpe AA. An ounce of prevention: outpatient management of the ovarian hyperstimulation syndrome. Fertility and Sterility 2000;73(4):821‐4. [DOI] [PubMed] [Google Scholar]
Gamzu 2002 {published data only}
- Gamzu R, Almog B, Levin Y, Avni A, Lessing BJ, Baram A. Comparative efficacy of hydroxyethyl starch and Haemaccel for the treatment of severe ovarian hyperstimulation syndrome. Fertility and Sterility 2002;77(6):1302‐3. [DOI] [PubMed] [Google Scholar]
Gokmen 2005 {published data only}
- Gokmen O, Ozcan S, Erman Akar M, Ugur M. A randomized prospective placebo‐controlled study of intravenous albumin vs. hydroxyethyl starch for the prevention of ovarian hyperstimulation in an in‐vitro fertilization programme. Fertility and Sterility: Abstracts of ASRM 2005;84(Suppl 1):308. [Google Scholar]
Graf 1997 {published data only}
- Graf AM, Fischer R, Naether ONJ, Baukloh V, Tafel J, Nuckel M. Reduced incidence of ovarian hyperstimulation syndrome by prophylactic infusion of hydroxyethyl starch solution in an in‐vitro fertilization programme. Human Reproduction 1997;12(12):2599‐602. [DOI] [PubMed] [Google Scholar]
Halme 1995 {published data only}
- Halme J, Toma SK, Talbert LM. A case of severe ovarian hyperstimulation in a healthy oocyte donor. Fertility and Sterility 1995;64(4):857‐9. [DOI] [PubMed] [Google Scholar]
Isik 1997 {published data only}
- Isik AZ, Ozgun OD, Kahraman S, Polat G, Vicdan K, Biberoglu K, et al. Intravenous albumin combined with low dose human chorionic gonadotrophin and late step‐down administration of menotropins are effective in prevention of severe ovarian hyperstimulation syndrome in high‐risk patients in an in vitro fertilisation program. Middle East Fertility Society Journal 1997;2/3:238‐42. [Google Scholar]
Isik 2001 {published data only}
- Isik AZ, Vicdan K. Combined approach as an effective method in the prevention of severe ovarian hyperstimulation syndrome. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2001;97(2):208‐12. [DOI] [PubMed] [Google Scholar]
Kissler 2001 {published data only}
- Kissler S, Neidhardt B, Siebzehnrübl E, Schmitt H, Tschaikowsky K, Wildt L. The detrimental role of colloidal volume substitutes in severe ovarian hyperstimulation syndrome: a case report. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2001;99(1):131‐4. [DOI] [PubMed] [Google Scholar]
Koike 2000 {published data only}
- Koike E, Araki S, Ogawa S, Minakami H, Sayama H, Shibahara H, et al. Clinical efficacy of peritoneovenous shunting for the treatment of sever ovarian hyperstimulation syndrome. Human Reproduction 2000;15(1):113‐7. [DOI] [PubMed] [Google Scholar]
Kumbak 2005 {published data only}
- Kumbak B, Kahraman S, Karlikaya G, Karagozoglu H, Sen T, Hatirnaz S. Intravenous albumin prophylaxis in high risk patients for moderate to severe ovarian hyperstimulation syndrome. Fertility and Sterility 2005;84 Suppl(1):264. [Google Scholar]
Lewit 1996 {published data only}
- Lewit N, Kol S, Ronen N, Itskovitz‐Eldor J. Does intravenous administration of human albumin prevent severe ovarian hyperstimulation syndrome?. Fertility and Sterility 1996;66(4):654‐6. [DOI] [PubMed] [Google Scholar]
Lincolin 2002 {published data only}
- Lincoln SR, Opsahl MS, Blauer KL, Black SH, Schulman JD. Aggressive outpatient treatment of ovarian hyperstimulation syndrome with ascites using transvaginal culdocentesis and intravenous albumin minimizes hospitalization. Journal of Assisted Reproduction and Genetics 2002;19:159‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matorras 2013 {published and unpublished data}
- Matorras R, Andrés M, Mendoza R, Prieto B, Pijoan JI, Expósito A. Prevention of ovarian hyperstimulation syndrome in GnRH agonist IVF cycles in moderate risk patients: randomized study comparing hydroxyethyl starch versus cabergoline and hydroxyethyl starch. European Journal of Obstetrics Gynecology and Reproductive Biology 2013;170(2):439‐43. [DOI] [PubMed] [Google Scholar]
Milacic 1996 {published data only}
- Milacic D, Radonjic G, Matijasevic S, Kontic O. Prevention of ovarian hyperstimulation syndrome with iv albumin. Human Reproduction 1996;11(1):216. [Google Scholar]
Mukherjee 1995 {published data only}
- Mukherjee T, Copperman AB, Sandler B, Bustillo M, Grunfeld L. Severe ovarian hyperstimulation despite prophylactic albumin at the time of oocyte retrieval for in vitro fertilization and embryo transfer. Fertility and Sterility 1995;64(3):641‐3. [DOI] [PubMed] [Google Scholar]
Naredi 2013 {published and unpublished data}
- Naredi N, Karunakaran S. Calcium gluconate infusion is as effective as the vascular endothelial growth factor antagonist cabergoline for the prevention of ovarian hyperstimulation syndrome. Journal of Human Reproductive Sciences 2013;6(4):248‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ndukwe 1997 {published data only}
- Ndukwe G, Thornton S, Fishel S, Dowell K, Aloum M. Severe ovarian hyperstimulation syndrome: is it really preventable by prophylactic intravenous albumin?. Fertility and Sterility 1997;68(5):851‐4. [DOI] [PubMed] [Google Scholar]
Ng 1995 {published data only}
- Ng E, Leader A, Claman P, Domingo M, Spence JEH. Intravenous albumin does not prevent the development of severe ovarian hyperstimulation syndrome in an in‐vitro fertilization programme. Human Reproduction 1995;10(4):807‐10. [DOI] [PubMed] [Google Scholar]
Panay 1999 {published data only}
- Iammarone E, Panay N, ZosmerA, TozerA, Hussain S, Wilson C, et al. Does the prophylactic use of intravenous albumin prevent ovarian hyperstimulation syndrome? A randomised prospective study. Human Fertility 1999;2(2):179‐180. [Google Scholar]
- Panay NA, Iammorrone E, Zosmer A, Tozer A, Hussain S. Does the prophylactic use of intravenous albumin prevent ovarian hyperstimulation syndrome? A randomized prospective study. Abstracts from the 15th Annual Meeting of the ESHRE, Tours, France 1999;14:105. [Google Scholar]
Shaker 1996 {published data only}
- Shaker AG, Zosmer A, Dean N, Berik JS, Jacob HS, Tan SL. Comparison of intravenous albumin and transfer of fresh embryos with cryopreservation of all embryos for subsequent transfer in prevention of ovarian of ovarian hyperstimulation syndrome. Fertility and Sterility 1996;65:992‐6. [DOI] [PubMed] [Google Scholar]
Tan 1992 {published data only}
- Tan Sl, Balen A, Hussein E, Campbell S, Jacobs HS. The administration of glucocorticoids for the prevention of ovarian hyperstimulation syndrome in invitro fertilisation: a prospective randomised study. Fertility and Sterility 1992;58:378‐83. [DOI] [PubMed] [Google Scholar]
Tehraninejad 2012 {published and unpublished data}
- Tehraninejad E, Hafezi M, Arabipoor A, Aziminekoo E, Chehrazi M, Bahmanabadi A. Comparison of cabergoline and intravenous albumin in the prevention of ovarian hyperstimulation syndrome: a randomized clinical trial.. Journal of Assisted Reproduction & Genetics. 2012;29(3):259‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Torabizadeh 2013 {published and unpublished data}
- Torabizadeh A, Vahidroodsari F, Ghorbanpour Z. Comparison of albumin and cabergoline in the prevention of ovarian hyperstimulation syndrome: A clinical trial study.. Iranian Journal of Reproductive Medicine 2013;11(10):837‐42. [PMC free article] [PubMed] [Google Scholar]
Yakovenko 2009 {published and unpublished data}
- Yakovenko SA, Zorina IV, Dmitrieva NV, Troshina MN, Apryshko VP, Voznesenskaya JV. Intravenous administration of calcium in ovarian hyperstimulation syndrome (OHSS) prevention. European Journal of Obstetrics Gynecology and Reproductive Biology 2011;1:s20. [Google Scholar]
Additional references
Aboulghar 2003
- Aboulghar MA, Mansour RT. Ovarian hyperstimulation syndrome: classifications and critical analysis of preventive measures. Human Reproduction Update 2003;9(3):275‐89. [DOI] [PubMed] [Google Scholar]
Cerrilo 2009
- Cerrillo M, Pacheco A, Rodríguez S, Mayoral M, Ruiz M, García Velasco JA. Differential regulation of VEGF, Cadherin, and Angiopietin 2 by trigger oocyte maturation with GnRHa vs hCG in donors: try to explain the lower OHSS incidence. Abstracts of the 25th annual meeting of the ESHRE 2009;24(1):i60. [Google Scholar]
Chen 2003a
- Chen D, Burmeister L, Goldschlag D, Rosenwaks Z. Ovarian hyperstimulation syndrome: strategies for prevention. Reproductive Biomedicine Online 2003;7(1):43‐9. [DOI] [PubMed] [Google Scholar]
D'Angelo 2007
- D'Angelo A, Amso NN. Embryo freezing for preventing ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD002806.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Angelo 2011
- D'Angelo A, Brown J, Amso NN. Coasting (withholding gonadotrophins) for preventing ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD002811.pub3] [DOI] [PubMed] [Google Scholar]
Delvigne 2002
- Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Human Reproduction Update 2002;8:559‐77. [DOI] [PubMed] [Google Scholar]
Edwards 2007
- Edwards RG. IVF, IVM, natural cycle IVF, minimal stimulation IVF ‐ time for a rethink. Reproductive Biomedicine Online 2007;15:106‐19. [DOI] [PubMed] [Google Scholar]
Egbase 2000
- Egbase PE. Severe OHSS: how many cases are preventable?. Human Reproduction 2000;15:8‐10. [DOI] [PubMed] [Google Scholar]
Engmann 2008
- Engmann L, DiLugie A, Schmidt D, Nulsen J, Maier D, Benavida C. The use of gonadotropin‐releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high‐risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomised controlled study. Fertility and Sterility 2008;89(1):84‐91. [DOI] [PubMed] [Google Scholar]
Enskog 2001
- Enskog A, Nilsson L, Brännström M. Plasma levels of free vascular endothelial growth factor(165) (VEGF(165)) are not elevated during gonadotropin stimulation in in vitro fertilization (IVF) patients developing ovarian hyperstimulation syndrome (OHSS): results of a prospective cohort study with matched controls. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2001;96:196‐201. [DOI] [PubMed] [Google Scholar]
Foong 2006
- Foong LC, Bhagavath BA, Kumar J, Ng SC. Ovarian hyperstimulation syndrome is associated with reversible impairment of vascular reactivity. Fertility and Sterility 2002;78(6):1159‐63. [DOI] [PubMed] [Google Scholar]
Garcia‐Velasco 2003
- Garcia‐Velasco JA, Pellicer A. New concepts in the understanding of the ovarian hyperstimulation syndrome. Current Opinion in Obstetrics and Gynecology 2003;15(3):251‐6. [DOI] [PubMed] [Google Scholar]
Golan 1989
- Golan A, Ron‐el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstetrical & Gynecological Survey 1989;44(6):430‐40. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011; Vol. Available from www.cochrane‐handbook.org..
Humaidan 2010
- Humaidan P, Quartarolo J, Papanikolaou EG. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertility and Sterility 2010;94(2):389–400. [DOI] [PubMed] [Google Scholar]
Jee 2010
- Jee BC, Suh CS, Kim YB, Kim SH, Choi YM, Kim JG, et al. Administration of intravenous albumin around the time of oocyte retrieval reduces pregnancy rate without preventing ovarian hyperstimulation syndrome: a systematic review and meta‐analysis. Gynecologic and Obstetric Investigation 2010;70(1):47‐54. Epub 2010 Feb 19. [DOI] [PubMed] [Google Scholar]
Kol 2008
- Kol S, Solt I. GnRH agonist for triggering final oocyte maturation in patients at risk of ovarian hyperstimulation syndrome: still a controversy?. Journal of Assisted Reproduction and Genetics 2008;25:63‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loutradis 2006
- Loutradis D, Kiapekou E, Zapanti E, Antsaklis A. Oocyte maturation in assisted reproductive techniques. Annals of the New York Academy of Sciences 2006;1092:235‐46. [DOI] [PubMed] [Google Scholar]
Mathur 2000
- Mathur RS, Jenkins JM. Is ovarian hyperstimulation syndrome associated with a poor obstetric outcome?. BJOG 2000;107:943‐6. [DOI] [PubMed] [Google Scholar]
McClelland 1990
- McClelland DB. Human albumin solutions. BMJ 1990;300:35‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Navot 1988
- Navot D, Relou A, Birkenfeld A, Rabinowitz R, Brzezinski A, Margalioth EJ. Risk factors and prognostic variables in the ovarian hyperstimulation syndrome. American Journal of Obstetrics and Gynecology 1988;159:210‐5. [DOI] [PubMed] [Google Scholar]
Navot 1992
- Navot D, Bergh PA, Laufer N. Ovarian hyperstimulation syndrome in novel reproductive technologies:prevention and treatment. Fertility & Sterility 1992;58:249‐61. [DOI] [PubMed] [Google Scholar]
Rizk 1997
- Rizk B, Aboulghar M, Smitz J, Ron‐El R. The role of vascular endothelial growth factor and interleukins in the pathogenesis of severe ovarian hyperstimulation syndrome. Human Reproduction Update 1997;3:255‐6. [DOI] [PubMed] [Google Scholar]
Rizk 1999
- Rizk B, Aboulghar MA. Classification, pathophysiology and management of ovarian hyperstimulation syndrome. In: Brinsden P editor(s). In‐Vitro Fertilization and Assisted Reproduction. New York, London: The Parthenon Publishing Group, 1999:131‐55. [Google Scholar]
Schenker 1978
- Schenker JG, Weinstein D. Ovarian hyperstimulation syndrome: a current survey. Fertility and Sterility 1978;30:225‐68. [DOI] [PubMed] [Google Scholar]
Shawkat 2012
- Shawkat H, Westwood M, Mortimer A. Mannitol: a review of its clinical uses. Continuing Education in Anaesthesia, Critical Care & Pain 2012;12(2):82‐5. [Google Scholar]
Tang 2012
- Tang H, Hunter T, Hu Y, Zhai SD, Sheng X, Hart RJ. Cabergoline for preventing ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008605.pub2] [DOI] [PubMed] [Google Scholar]
Venetis 2011
- Venetis CA, Kolibianakis EM, Toulis KA, Goulis DG, Papadimas I, Tarlatzis BC. Intravenous albumin administration for the prevention of severe ovarian hyperstimulation syndrome: a systematic review and metaanalysis. Fertility and Sterilility 2011;95(1):188‐96. [DOI] [PubMed] [Google Scholar]
Vloeberghs 2009
- Vloeberghs V, Peeraer K, Pexsters A, D’Hooghe T. Ovarianhyperstimulation syndrome and complications of ART. Best Practice & Research. Clinical Obstetrics & Gynaecology 2009;23(5):691‐709. [DOI] [PubMed] [Google Scholar]
WHO 1973
- WHO. Agents stimulating gonadal function in human. World Health Organization Technical Report Series 1973:514‐20. [PubMed]
Yan 1993
- Yan Z, Weich HA, Bernart W, Breckwoldt M, Neulen J. Ascular endothelial growth factor (VEGF) messenger ribonucleic acid (mRNA) expression in luteinized human granulosa cells in vitro. Journal of Clinical Endocrinology and Metabolism 1993;77:1723‐5. [DOI] [PubMed] [Google Scholar]
Youssef 2014
- Youssef MAFM, Veen F, Al‐Inany HG, Mochtar MH, Griesinger G, Nagi Mohesen M, et al. Gonadotropin‐releasing hormone agonist versus HCG for oocyte triggering in antagonist‐assisted reproductive technology. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD008046.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Youssef 2002
- Youssef MA, Al‐Inany HG, Evers JHANSLH, Aboulghar M. Intra‐venous fluids preventing severe ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD001302] [DOI] [Google Scholar]
Youssef 2011
- Youssef MAFM, Al‐Inany HG, Evers JLH, Aboulghar M. Intra‐venous fluids for the prevention of severe ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD001302.pub2] [DOI] [PubMed] [Google Scholar]


