Abstract
Purpose
Autoantibodies (aAbs) to type I interferons (IFNs) have been found in less than 1% of individuals under the age of 60 in the general population, with the prevalence increasing among those over 65. Neutralizing autoantibodies (naAbs) to type I IFNs have been found in at least 15% of patients with life-threatening COVID-19 pneumonia in several cohorts of primarily European descent. We aimed to evaluate the prevalence of aAbs and naAbs to IFN-α2 or IFN-ω in Japanese patients who suffered from COVID-19 as well as in the general population.
Methods
Patients who suffered from COVID-19 (n = 622, aged 0–104) and an uninfected healthy control population (n = 3,456, aged 20–91) were enrolled in this study. The severities of the COVID-19 patients were as follows: critical (n = 170), severe (n = 235), moderate (n = 112), and mild (n = 105). ELISA and ISRE reporter assays were used to detect aAbs and naAbs to IFN-α2 and IFN-ω using E. coli-produced IFNs.
Results
In an uninfected general Japanese population aged 20–91, aAbs to IFNs were detected in 0.087% of individuals. By contrast, naAbs to type I IFNs (IFN-α2 and/or IFN-ω, 100 pg/mL) were detected in 10.6% of patients with critical infections, 2.6% of patients with severe infections, and 1% of patients with mild infections. The presence of naAbs to IFNs was significantly associated with critical disease (P = 0.0012), age over 50 (P = 0.0002), and male sex (P = 0.137). A significant but not strong correlation between aAbs and naAbs to IFN-α2 existed (r = − 0.307, p value < 0.0001) reinforced the importance of measuring naAbs in COVID-19 patients, including those of Japanese ancestry.
Conclusion
In this study, we revealed that patients with pre-existing naAbs have a much higher risk of life-threatening COVID-19 pneumonia in Japanese population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10875-022-01308-3.
Keywords: COVID-19, Antibodies to type I IFNs, IFN-α2, IFN-ω, Neutralization assay, IFN-α2 concentration
Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The clinical spectrum of COVID-19 varies in severity: approximately 80% of cases are asymptomatic or presenting mild to moderate (nonhypoxemic pneumonia) disease while 20% of cases develop severe pneumonia (15%) or critical pneumonia (5%) [1]. As this virus is highly contagious and virulent, healthcare systems globally faced a crisis. Therefore, establishing a rapid examination system to identify the patients who are at high risk of life-threatening COVID-19 disease are desired.
To date, age of the patient remains the strongest epidemiological risk factor for life-threatening COVID-19, especially among patients over 65 years old [2–5]. By contrast, other variable factors, such as male sex, cardiovascular disease, chronic obstructive pulmonary disease, chronic pulmonary disease, obesity, type 2 diabetes mellitus, and smoking are modestly associated with COVID-19 aggravation [6–8]. However, there is inter-individual variability among severe cases of COVID-19 and some patients developed severe COVID-19 disease in the absence of these risk factors. Patients with inherited impairments to the innate immune system displayed rapid viral replication early in the infection followed by excessive inflammatory cytokine production that exacerbated the disease [9–14]. Indeed, genetic abnormalities in TLR3, IRF7, and TLR7 that affect type I interferon (IFN) signaling have been reported in severe COVID-19 [15, 16]. On the other hand, neutralizing autoantibodies (naAbs) to type I IFNs have also been identified as risk factors for life-threatening COVID-19. These naAbs predate the infection and represent a serious risk factor in COVID-19 aggravation. The naAbs to type I IFNs have also been associated with life-threatening adverse reactions to yellow fever vaccine (YFV) [17]. Bastard et al. reported that 10.2% of patients with life-threatening COVID-19 pneumonia had naAbs to type I IFNs, compared to 0.33% of healthy individuals and 0% of patients with asymptomatic/mild disease [18]. Further, 20% of patients with critical COVID-19 over the age of 80 years and deceased patients of all ages had naAbs to type I IFNs [19]. Importantly, the clinical impact of these naAbs is not apparent until infection with SARS-CoV-2, which makes it difficult to predict the risk of severe COVID-19 disease due to naAbs to type I IFNs.
These previous studies suggest that approximately 5% of younger patients have a risk of aggravation due to genetic abnormalities associated with type I IFNs while approximately 20% of older patients have a risk of aggravation due to naAbs to type I IFNs. While these observation have been supported by subsequent studies [20–26], further analysis are needed to clarify the pathophysiology of life-threatening COVID-19 pneumonia in individual ethnic groups that have similar genetic background and lifestyles for precise characterization of the role of antibodies to type I IFNs in COVID-19 aggravation.
Our current study aimed to determine the prevalence of neutralizing autoantibodies to type I IFNs in Japanese COVID-19 patients and their contribution to severe COVID-19 disease. In addition, we studied the prevalence of aAbs in the uninfected Japanese population to clarify the differences of the prevalence among ethnic groups.
Materials and Methods
COVID-19 Patients and Individuals in the General Population Subjected to Analysis
We conducted the study at Hiroshima University Hospital, Tokyo Medical and Dental University Medical Hospital, and Osaka City University Hospital. We enrolled 622 COVID-19 patients admitted to our institutes as well as 3456 individuals from the general population which included 1000 previously reported individuals [19] (Fig. 1A, B, Table 1). The details of the patients and the general population are described in the Supplemental materials and methods.
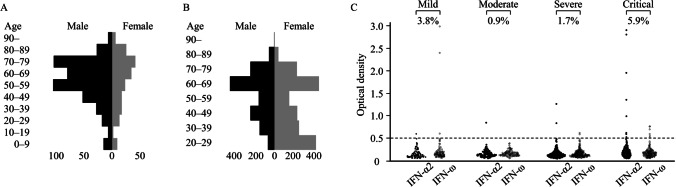
Fig. 1.
Characteristics of 622 patients with COVID-19 and 3456 individuals from the general population. A Age and sex distribution of patients with COVID-19 (n = 622). The median age of the COVID-19 patients was 61 years (IQR: 46–73 years); 70.2% were males, and 29.8% were females. B Age and sex distribution of individuals from the general population (n = 3456). The median age of subjects from the general population was 56 years (IQR: 37–67 years); 43.5% were males, and 56.5% were females. C The prevalence of aAbs to type I IFNs of patients with COVID-19 according to its severity. aAbs to IFNs were detected by ELISA in 622 patients with COVID-19 including 170 critical, 235 severe, 112 moderate, and 105 mild infections. The cutoff value of ELISA was 0.5 (O.D.)
Table 1.
Characteristics of 622 patients with COVID-19 and 3456 general population in this study
| 622 patients with COVID-19 | 3456 general population in this study | |||||
|---|---|---|---|---|---|---|
| Age (years) | Total cases [n = 622] (%) | Male [n = 439] | Female [n = 183] | Total cases [n = 3456](%) | Male [n = 1502] |
Female [n = 1954] |
| 0–9 | 22 (3.5%) | 15 | 7 | - | - | - |
| 10–19 | 8 (1.3%) | 6 | 2 | - | - | - |
| 20–29 | 31 (5.0%) | 18 | 13 | 536 (15.5%) | 72 | 464 |
| 30–39 | 45 (7.2%) | 28 | 17 | 439 (12.7%) | 164 | 275 |
| 40–49 | 69 (11.1%) | 52 | 17 | 522 (15.1%) | 267 | 255 |
| 50–59 | 127 (20.4%) | 104 | 23 | 340 (9.8%) | 174 | 166 |
| 60–69 | 112 (18.0%) | 79 | 33 | 992 (28.7%) | 495 | 497 |
| 70–79 | 144 (23.1%) | 103 | 41 | 519 (15.0%) | 267 | 252 |
| 80–89 | 51 (8.2%) | 27 | 24 | 105 (3.0%) | 60 | 45 |
| 90– | 13 (2.1%) | 7 | 6 | 3 (0.1%) | 3 | 0 |
| Severity | Total cases [n = 622](%) | Male [n = 439] | Female [n = 183] | |||
| Mild | 105 (16.9%) | 67 | 38 | |||
| Moderate | 112 (18.0%) | 68 | 44 | |||
| Severe | 235 (37.8%) | 166 | 69 | |||
| Critical | 170 (27.3%) | 138 | 32 | |||
We assessed the severity of COVID-19 based on the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia described previously [18]. “Critical” included patients who required mechanical ventilation (including intubation, high flow nasal cannula, continuous positive airway pressure and bilevel positive airway pressure, etc.), septic shock, any other organ failure and/or use of ECMO in the intensive care unit. “Severe” were defined as patients required oxygen therapy < 6 L/min because of pneumonia. The patients with mild pneumonia but no requirement for oxygen therapy were classified into “Moderate.” “Mild” were defined as patients with some mild symptoms without pneumonia.
Neutralization Assay of aAbs to Type I IFNs
We performed luciferase reporter assays as described previously [18]. The detailed method of neutralization assay is described in the Supplemental materials and methods.
Measurement of aAbs to Type I IFNs and IFN-α2 Concentration
The details of ELISA and ProQuantum™ Immunoassay are described in the Supplemental materials and methods.
Statistical Analysis
The detailed method of statistical analysis is described in the Supplemental materials and methods.
Results
The Frequency of aAbs to Type I IFNs Was High in Patients with Critical COVID-19
We first measured aAbs to type I IFNs by ELISA in 622 Japanese COVID-19 patients aged 0–104 years, including 170 critical, 235 severe, 112 moderate, and 105 mild cases. We detected aAbs to IFN-α2 or IFN-ω at the following frequencies: 5.9% critical cases, 1.7% severe cases, 0.9% moderate cases, 3.8% mild case (Fig. 1C, Table 2). In detail, 4.7% (95% CI: 2.4–9.0) of patients with critical disease had aAbs to IFN-α2, 3.5% (95% CI: 1.6–7.5) to IFN-ω, and 2.4% (95% CI: 0.9–5.9) to both IFN-α2 and IFN-ω (Table 2). Among patients who had IFN-α2 or IFN-ω aAbs, there were several patients who had isolated aAb solely to IFN-α2 or IFN-ω (Table S2). The aAbs to IFN-α2 or IFN-ω were also detected in 3.8% of patients with mild disease and 0.9% of those with moderate disease. Unlike patients with critical COVID-19, none of the patients with mild to severe disease had aAbs to both interferon subtypes (Table 2). Among patients over 50 years old, 3.6% (95% CI: 2.2–5.7) had aAbs to IFN-α2 or IFN-ω, while 1.7% (95% CI: 0.6–4.9) of patients younger than 50 years had these aAbs (Table 2). Overall, these aAbs to type I IFNs were detected more frequently in patients with critical disease and patients over 50 years old. However, isolated aAbs to IFN-α2 or IFN-ω was also detected in some of the patients with mild or moderate disease in the current study.
Table 2.
The prevalence of aAbs to type I IFNs in 622 patients with COVID-19 according to disease severity or age
| aAbs detected by ELISA | |||||
| Severity | No. of patients | IFN-α2 | IFN-ω | IFN-α2 and IFN-ω | IFN-α2 or IFN-ω |
| Mild | 105 | 1 (1.0% [0.2–5.2]) | 3 (2.9% [1.0–8.1]) | 0 (0.0%) | 4 (3.8% [1.5–9.4]) |
| Moderate | 112 | 1 (0.9% [0.2–4.9]) | 0 (0.0%) | 0 (0.0%) | 1 (0.9% [0.2–4.9]) |
| Severe | 235 | 2 (0.9% [0.2–3.1]) | 2 (0.9% [0.2–3.1]) | 0 (0.0%) | 4 (1.7% [0.7–4.3]) |
| Critical | 170 | 8 (4.7% [2.4–9.0]) | 6 (3.5% [1.6–7.5]) | 4 (2.4% [0.9–5.9]) | 10 (5.9% [3.2–10.5]) |
| Total | 627 | 12 | 11 | 4 | 19 |
| Age (years) | No. of patients | IFN-α2 | IFN-ω | IFN-α2 and IFN-ω | IFN-α2 or IFN-ω |
| 0–49 | 175 | 1 (0.6% [0.1–3.2]) | 2 (1.1% [0.3–4.1]) | 0 (0.0%) | 3 (1.7% [0.6–4.9]) |
| 50– | 447 | 11 (2.5% [1.4–4.4]) | 9 (2.0% [1.1–3.8]) | 4 (0.9% [0.3–2.3]) | 16 (3.6% [2.2–5.7]) |
| 50–59 | 127 | 5 (3.9% [1.7–8.9]) | 4 (3.2% [1.2–7.8]) | 2 (1.6% [0.4–5.6]) | 7 (5.5% [2.7–10.9]) |
| 60–69 | 112 | 1 (0.9% [0.2–4.9]) | 1 (0.9% [0.2–4.9]) | 0 (0.0%) | 2 (1.8% [0.5–6.3]) |
| 70– | 208 | 5 (2.4% [1.0–5.5]) | 4 (1.9% [0.8–4.8]) | 2 (1.0% [0.3–3.4]) | 7 (3.4% [1.6–6.8]) |
naAbs to Type I IFNs Were Frequently Detected in Patients with Critical COVID-19
aAbs which react with type I IFNs were detected by ELISA; however, their neutralizing activity could not be assessed by ELISA. We thus measured neutralizing activity against type I IFNs using the ISRE reporter assay in sera from 622 patients with COVID-19 [19]. Sera were considered to have neutralizing activity if the induction of ISRE activity, which was normalized to Renilla luciferase activity, was less than 15% of the median values of healthy controls [19]. These data are summarized in Table 3 and Table S3. Strongly neutralizing naAbs, capable of neutralizing 10 ng/mL of IFN-α2 or IFN-ω, were found in 5.9% of critical cases, 2.1% of severe cases, 0.9% of moderate cases, and 0% of mild cases (Fig. 2A, Table 3). In patients with critical disease, antibody prevalence was as follows: 5.9% (95% CI: 3.2–10.5) had naAbs to IFN-α2, 4.1% (95% CI: 2.0–8.3) to IFN-ω, and 4.1% (95% CI: 2.0–8.3) to both IFN-α2 and IFN-ω (Table 3). On the other hand, less than 1% of patients with mild to moderate disease had naAbs to type I IFNs (Table 3). Among patients over 50 years old, 3.6% (95% CI: 2.2–5.7) had naAbs to IFN-α2, 2.2% (95% CI: 1.2–4.1) to IFN-ω, 2.2% (95% CI: 1.2–4.1) to both IFN-α2 and IFN-ω, and 3.6% (95% CI: 2.2–5.7) to IFN-α2 or IFN-ω (Table 3). By contrast, none of the patients younger than 50 years old had naAbs to type I IFNs (Table 3). These results are summarized according to disease severity in Fig. 2B. All patients having neutralizing activity against IFN-ω had neutralizing activity against IFN-α2. Of note, in contrast to the prevalence of aAb (Table S2), no patients had isolated naAbs to IFN-ω (Table S3, Fig. S1).
Table 3.
The prevalence of naAbs to type I IFNs in 622 patients with COVID-19 according to disease severity and age
| naAbs detected by Neutralization assay | |||||||||
| 10 ng/mL | 100 pg/mL | ||||||||
| Severity | No. of patients | IFN-α2 | IFN-ω | IFN-α2 and IFN-ω | IFN-α2 or IFN-ω | IFN-α2 | IFN-ω | IFN-α2 and IFN-ω | IFN-α2 or IFN-ω |
| Mild | 105 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
0 (0.0%) |
1 (1.0% [0.2–5.2]) | 0 (0.0%) | 0 (0.0%) | 1 (1.0% [0.2–5.2]) |
| Moderate | 112 | 1 (0.9% [0.2–4.9]) | 1 (0.9% [0.2–4.9]) | 1 (0.9% [0.2–4.9]) |
1 (0.9%[0.2–4.9]) |
1 (0.9% [0.2–4.9]) | 1 (0.9% [0.2–4.9]) | 1 (0.9% [0.2–4.9]) | 1 (0.9% [0.2–4.9]) |
| Severe | 235 | 5 (2.1% [0.9–4.9]) | 2 (0.9% [0.2–3.0]) | 2 (0.9% [0.2–3.0]) |
5 (2.1%[0.9–4.9]) |
6 (2.6% [1.2–5.5]) | 3 (1.3% [0.4–3.7]) | 3 (1.3% [0.4–3.7]) | 6 (2.6% [1.2–5.5]) |
| Critical | 170 | 10 (5.9% [3.2–10.5]) | 7 (4.1% [2.0–8.3]) | 7 (4.1% [2.0–8.3]) | 10 (5.9% [3.2–10.5]) | 12 (7.1% [4.1–11.9]) | 17 (10.0% [6.3–15.4]) | 11 (6.5% [3.7–11.2]) | 18 (10.6% [6.8–16.1]) |
| Total | 622 | 6 | 0 | 10 | 16 | 5 | 6 | 15 | 26 |
| Age (years) | No. of patients | IFN-α2 | IFN-ω | IFN-α2 and IFN-ω | IFN-α2 or IFN-ω | IFN-α2 | IFN-ω | IFN-α2 and IFN-ω | IFN-α2 or IFN-ω |
| 0–49 | 175 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 50– | 447 | 16 (3.6% [2.2–5.7]) | 10 (2.2% [1.2–4.1]) | 10 (2.2% [1.2–4.1]) | 16 (3.6% [2.2–5.7]) | 20 (4.5% [2.9–6.8]) | 21 (4.7% [3.1–7.1]) | 15 (3.4% [2.0–5.5]) | 26 5.8% [4.0–8.4]) |
| 50–59 | 127 | 8 (6.3% [3.2–11.9]) | 6 (4.7% [2.2–9.9]) | 6 (4.7% [2.2–9.9]) | 8 (6.3% [3.2–11.9]) | 8 (6.3% [3.2–11.9]) | 10 (7.9% [4.3–13.9]) | 7 (5.5% [2.7–10.9]) | 11 (8.7% [4.9–14.8]) |
| 60–69 | 112 | 2 (1.8% [0.5–6.3]) | 1 (0.9% [0.2–4.9]) | 1 (0.9% [0.2–4.9]) | 2 (1.8% [0.5–6.3]) | 5 (4.5% [1.9–10.0]) | 5 (4.5% [1.9–10.0]) | 3 (2.7% [0.9–7.6%]) | 7 (6.3% [3.1–12.3]) |
| 70– | 208 | 6 (2.9% [1.3–6.1]) | 3 (1.4% [0.5–4.2]) | 3 (1.4% [0.5–4.2]) | 6 (2.9% [1.3–6.1]) | 7 (3.4% [1.6–6.8]) | 6 (2.9% [1.3–6.1]) | 5 (2.4% [1.0–5.5]) | 8 (3.8% [2.0–7.4]) |
Fig. 2.
naAbs to type I IFNs were detected by the neutralization assay in 622 patients with COVID-19 at a cytokine concentration of 10 ng/mL. A Dot plot of the neutralization assay stimulated by 10 ng/mL of type I IFNs. The samples showing less than 15% of luciferase activity were defined as having neutralization activity. The prevalence of naAbs was high in patients with critical COVID-19. B Neutralizing activity against type I IFNs was compared between IFN-α2 and IFN-ω stimulated by 10 ng/mL. All patients having neutralizing activity against IFN-ω had neutralizing activity against IFN-α2. C The odds ratio (OR) associated with COVID-19 aggravation among patients in critical disease compared to mild/moderate disease
Next, we analyzed serum neutralizing activity under more sensitive conditions by stimulating cells at lower concentrations (100 pg/mL) of IFN-α2 or IFN-ω. Under this condition, consistent with previous reports [19], the prevalence of naAbs was observed in 10.6% of critical cases, 2.6% of severe cases, 0.9% of moderate cases, and 1.0% of mild cases (Fig. 3A, Table 3, Table S3). In detail, 7.1% (95% CI: 4.1–11.9) of critical cases had naAbs to IFN-α2, 10.0% (95% CI: 6.3–15.4) to IFN-ω, and 6.5% (95% CI: 3.7–11.2) to both IFN-α2 and IFN-ω (Table 3). Only 1% or less of the patients with mild to moderate disease had these naAbs to IFN-α2 or IFN-ω (Table 3). Among patients over 50 years old, 4.5% (95% CI: 2.9–6.8) had naAbs to IFN-α2, 4.7% (95% CI: 3.1–7.1) of them to IFN-ω, 3.4% (95% CI: 2.0–5.5) to both IFN-α2 and IFN-ω, and 5.8% (95% CI: 4.0–8.4%) to IFN-α2 or IFN-ω. By contrast, none of the patients younger than 50 years old had naAbs to IFN-α2 or IFN-ω (Table 3).
Fig. 3.
naAbs to type I IFNs detected by the neutralization assay in 622 patients with COVID-19 at a cytokine concentration of 100 pg/mL. A Dot plot of the neutralization assay stimulated by 100 pg/mL of type I IFNs. The samples showing less than 15% of luciferase activity were defined as having neutralization activity. The prevalence of naAbs was high in patients with critical COVID-19. B Neutralizing activity against type I IFNs was compared between IFN-α2 and IFN-ω stimulated by 100 pg/mL. C The odds ratio (OR) associated with COVID-19 aggravation among patients in critical disease compared to mild/moderate disease
Using this more sensitive condition, the percentage of the patients with naAbs to IFNs was higher than in the condition with 10 ng/mL (Table 3). We detected naAbs against IFN-α2 in an additional 4 patients at the 100 pg/mL condition compared to the 10 ng/ml condition. Among these 4 patients, 3 had critical/severe disease and 1 patient had mild disease (Fig. 4A, Fig. S3). Regarding naAbs to IFN-ω, an additional 11 patients showed neutralizing activity only against 100 pg/mL. All 11 patients had critical/severe disease (Fig. 4B, Fig. S4). It is known that the concentration of type I IFNs in the blood of patients with acute and benign SARS-CoV-2 infections ranges from 1 to 100 pg/mL [13, 27]. Moreover, it has been experimentally proven that 100 pg/mL of type I IFNs can impair SARS-CoV-2 replication in epithelial cells [19]. Therefore, a neutralization assay using 100 pg/mL of type I IFNs, which reflects physiological conditions, detected naAbs more precisely than the assay using 10 ng/mL, especially naAbs to IFN-ω.
Fig. 4.
Comparison of the results of the neutralization assay and ELISA. A, B Neutralizing activity against type I IFNs was compared between type I IFN concentrations of 100 pg/mL and 10 ng/mL stimulated by IFN-α2 (A) or IFN-ω (B). C–F aAbs to type I IFNs by ELISA were compared with naAbs by the neutralization assay at concentrations of 10 ng/mL IFN-α2 (C), 10 ng/mL IFN-ω (D), 100 pg/mL IFN-α2 (E), and 100 pg/mL IFN-ω (F). The cutoff value of ELISA was 0.5 (O.D.). In neutralization assay, samples showing less than 15% of luciferase activity were defined as having neutralization activity
The prevalence of naAbs by sex was 5.5% at 100 pg/mL and 3.4% at 10 ng/mL for males and 1.1% at 100 pg/mL and 0.5% at 10 ng/mL for females (Table S4, Fig. S5). NaAbs to IFNs were significantly associated with critical disease (P = 0.0152 at 10 ng/mL, P = 0.0012 at 100 pg/mL) compared to mild disease, age over 50 (P = 0.0085, P = 0.0002), and male sex (P = 0.0488, P = 0.137) (Table 4). COVID-19 aggravation was strongly associated with naAbs among critical patients using both assay conditions (at 10 ng/mL, IFN-α2 and IFN-ω odds ratio (OR) = 9.3, IFN-α2 or IFN-ω OR = 13.5; at 100 pg/mL, IFN-α2 and IFN-ω OR = 14.9, IFN-α2 or IFN-ω OR = 12.7) (Figs. 2C and 3C). These data are consistent with previous reports that identified a high prevalence, 10.2–18% in patients with critical disease, of naAbs to type I IFNs (Table S5) [18–26].
Table 4.
Comparison of patients with and without naAbs according to disease severity, age, and sex
Comparison of the Results of the Neutralization Assay and ELISA
While the IFN neutralization assay is the gold standard in assessing the biological effect of aAbs and the ISRE reporter assay is a sensitive method, it is time-consuming. On the other hand, ELISA is more high-throughput with faster turnaround times. We thus compared the results of neutralizing activity against type I IFNs measured by the ISRE reporter assay with the results of aAbs to type I IFNs measured by ELISA. When the presence of naAbs to IFN-α2 was predicted by the results of aAbs to IFN-α2, the sensitivity was 50%, the specificity was 99.3%, the positive predictive value (PPV) was 66.7%, the negative predictive value (NPV) was 98.7% at 10 ng/mL (Fig. 4C), and these two detection methods had a weak negative correlation (a correlation coefficient − 0.307 (95% CI: − 0.376 to − 0.234, P value < 0.0001)). For the 100 pg/mL condition, the sensitivity was 40%, the specificity was 99.3% (PPV of 66.7% and NPV of 98.0%), and these two detection methods had a weak negative correlation (a correlation coefficient − 0.199 (95% CI: − 0.273 to − 0.123, P value < 0.0001)) (Fig. 4E). We thus realized that ELISA-based detection of aAbs to IFN-α2 can be an alternative method to enable testing of multiple samples, e.g., screening tests for the general population, and to evaluate antibodies to type I IFNs in sera. In contrast, for IFN-ω, ELISA failed to adequately detect the presence of naAbs to IFN-ω. Indeed, ELISA-based detection of aAbs to IFN-ω pointed out the presence of naAbs to IFN-ω (10 ng/mL condition) with a sensitivity of 10% and specificity of 98.4% (PPV of 9.1% and NPV of 98.5%) (Fig. 4D). Regarding the 100 pg/mL condition, aAbs to IFN-ω only indicated naAbs to IFN-ω with a sensitivity of 9.5% and a specificity of 98.5% (PPV of 18.2% and NPV of 96.9%) (Fig. 4F).
Sera from COVID-19 Patients with naAbs to IFN-α2 Show Low Concentrations of IFN-α2
We analyzed the concentration of IFN-α2 using 269 samples for which the exact time of specimen collection could be determined with the ProQuantum™ Human IFN alfa Immunoassay, which is a qPCR-based technique. The level of IFN-α2 in sera in patients with naAbs was significantly lower compared to those without naAbs. The serum IFN-α2 levels were below detection limit (< 4 pg/mL) in all but one 1 patient with naAbs detected by the high sensitivity condition (Fig. 5A, B). However, there is no correlation between disease severity and the concentration of IFN-α2 (P = 0.2238). We also compared the level of IFN-α2 between the samples collected from onset to day 4 and those from day 5 to day 7 after onset. We found that the concentration of IFN-α2 were significantly higher in samples from onset to day 4 compared to those from day 5 to day 7 (P = 0.0009) (data not shown). These results are consistent with a previous report [18].
Fig. 5.
IFN-α2 concentration of patients with COVID-19 and prevalence of aAbs to IFN-α2 in 3456 individuals in the general population. The IFN-α2 concentration in most of the patients with naAbs to IFN-α2 and/or IFN-ω was below the limit of quantification (< 4 pg/mL). A Patients with naAbs to 100 pg/mL of IFN-α2 and/or IFN-ω (n = 8) and patients without naAbs (n = 261) were compared. B Patients with naAbs to 10 ng/mL of IFN-α2 and/or IFN-ω (n = 5) and patients without naAbs (n = 264) were compared. C aAbs to IFN-α2 in the general population were detected using ELISA. The prevalence of aAbs were calculated according to age and sex
Prevalence of aAbs to IFN-α2 in Uninfected Individuals from the General Japanese Population
In order to understand the risk of the general Japanese population to severe COVID-19 and other viral infections, we sought to determine the prevalence of naAbs to type I IFNs in the Japanese population by detecting aAbs to IFN-α2 via ELISA. We studied 3456 Japanese individuals aged 20–91 years and unaffected by COVID-19. In this population, 3 individuals had aAbs to IFN-α2 (0.087% (95% CI: 0.0295–0.255%)) (Fig. 5C). These 3 individuals consisted of an 86-year-old female, a 78-year-old male, and a 42-year-old male. These data suggest that the prevalence of aAbs, and by inference, that of naAbs, is low in the healthy general Japanese population.
Discussion
The current study investigated aAbs and naAbs to type I IFNs in 622 patients with COVID-19 before the Delta variant became predominant. This is the second largest study on the scale of the samples, also the largest study focusing on a single ethnic group, and the first in Asia. To minimize selection bias, we collected sera from COVID-19 patients from three geographically different areas (Tokyo, Osaka, and Hiroshima) in Japan. The prevalence of naAbs to type I IFNs was high among patients with critical disease, elderly patients, and male COVID-19 patients. These observations were consistent with a previous study [18], providing strong evidence to support the risk of COVID-19 aggravation in individuals with naAbs to type I IFNs. The modest risk factors that are well known so far are male sex (OR = 1.457) [7], cardiovascular disease (adjusted risk = 2.6) [6], chronic pulmonary disease (OR = 1.089) [7], and diabetes mellitus with chronic complications (rate ratio = 1.295) [7]. Although it is impossible to compare the odds ratios directly between different cohort studies, the risk of COVID-19 aggravation among individuals with naAbs to type I IFNs was estimated to be relatively high (100 pg/mL of IFN-α2 and IFN-ω OR = 14.9, IFN-α2 or IFN-ω OR = 12.7). A recent review article also described that aAbs to IFNα, IFNβ, and/or IFNω are found in about 15–20% of patients with critical COVID-19 pneumonia over 70 years old and regarded aAbs against IFNs as a major risk factor for critical COVID-19 disease [5]. As shown in this study and a previous study [3, 5, 19], the prevalence of naAbs to type I IFNs increased with age, especially high in the population over age of 50. This might be one of the reasons why age is the most striking epidemiological risk factor. Consistent with this, naAbs to type I IFNs are found in 1% or less of patients with mild to moderate COVID-19. Therefore, although the presence of naAbs to type I IFNs is a strong risk factor for aggravation, not all patients with these naAbs developed severe or critical COVID19 disease [28].
Approximately 1% of the patients with naAbs to IFN-α2 and ω also have naAbs to IFN-β [19]. Therefore, IFN-β therapy might be effective in severe COVID-19 cases with naAbs to type I IFNs [29–32]. In addition, the removal of naAbs to type I IFNs with plasma exchange may be beneficial in the treatment of COVID-19 patients [33]. Since these treatments may be effective only in the early stage of the infection [20], establishing rapid test system to evaluate naAbs to type I IFNs are necessary for appropriate therapeutic interventions. Therefore, we evaluated the utility of a rapid ELISA instead of the ISRE reporter-based neutralization assay. ELISA data correlated well with neutralization assay for aAbs and naAbs for IFN-α2 but not for IFN-ω. Indeed, a strong association exists between the severity of COVID-19 and the presence of naAbs to IFN-α2, whereas the risk of aggravation by naAbs to IFN-ω alone was not clear [19].
We thus performed a systematic study by ELISA in 3456 individuals without COVID-19 and found that 0.087% of this population were positive for aAbs to IFN-α2. Since the examination of aAbs to IFN-α2 by ELISA predicted the presence of naAbs to IFN-α2 with sensitivities of 50% (10 ng/dL condition) and 40% (100 pg/mL condition) as shown in this study, the prevalence of naAbs to IFN-α2 was assumed to be 0.17–0.22%. This prevalence in the general population in Japan was slightly lower than that in a previous international study (0.33%) [18]. The lower prevalence of naAbs in patients with critical disease in Japan compared to that in previous international study (10.6% vs 13.6%) can be explained by this lower prevalence of naAbs in general population.
In our study, we also found that some patients with high titer aAbs did not exhibit neutralizing activity against type I IFNs as reported elsewhere [26]. This may be explained by binding of aAbs to non-neutralizing epitopes. Another explanation is that these aAbs may have neutralizing activity at concentrations lower than 100 pg/mL of stimulation. We used 10% sera in our neutralization assay, so this assay using 100 pg/mL of stimulation can detect only naAbs which neutralize 1000 pg/mL of cytokines. On the other hand, the IFN-α2 concentrations of most patients in this study were below 100 pg/mL in sera. Therefore, it is worthwhile to extend this study with neutralization conditions with lower cytokine concentrations, e.g., 10 pg/mL. Despite these limitations, this study was the first study to characterize the relationship between naAbs to type I IFNs and COVID-19 aggravation in a Japanese population and the second largest study on this theme, providing strong evidence to support the contribution of naAbs to type I IFNs to the risk of COVID-19 aggravation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all patients, their families and all physicians dedicated to COVID-19, especially in Hiroshima University Hospital, Tokyo Medical and Dental University Hospital, and Osaka City University Hospital. We thank the patients and their families for placing their trust in us. We warmly thank the members of both branches of the Laboratory of Human Genetics of Infectious Diseases. We warmly thank Y. Nemirovskaya, M. Woollett, D. Liu, S. Boucherit, C. Rivalain, M. Chrabieh, and L. Lorenzo for administrative assistance and Michael Ciancanelli for English editing. We thank Yuko Nitahara at Osaka City University Hospital for collecting sera and characteristics of patients with COVID-19.
Author Contribution
Shohei Eto, Miyuki Tsumura, and Yoko Mizoguchi performed ELISA experiment, neutralizing assay, and measured IFN-a2 concentration. Shohei Eto prepared the first draft. Shintaro Nagashima and Junko Tanaka collected samples of general population before the appearance of COVID-19 and revised the draft. Yoko Nukui, Kenichi Kashimada, Keisuke Okamoto, Akifumi Endo, Kohsuke Imai, Hirokazu Kanegane, Tomohiro Morio, Yu Nakagama, Yasutoshi Kido, Hidenori Ohnishi, Masanori Ito, and Hiroki Ohge collected samples of patients with COVID-19 and general population after the appearance of COVID-19 and revised the draft. Paul Bastard, Jean-Laurent Casanova, and Osamu Ohara analyzed and interpreted the data and revised it critically for important intellectual content. Satoshi Okada designed and supervised the study and approved the final manuscript.
Funding
This study was supported in part by JSPS KAKENHI (grant number: JP19H03620 to S.O., JP19K07940 to S.H., JP19K08908 to E.S., and JP21K19440 to T.M.), MHLW (grant number: JP19HC1001 to J.T.) and Research Program on Emerging and Re-emerging Infectious Diseases from AMED (grant number: JP20fk0108531 to S.O., JP20fk0108453 to J.T., and JP20fk0108104j0002 to T.M.). This study was supported in part by Osaka City University Strategic Research Grant (grant number OCU-SRG2021_YR09 to Y.N.). The Laboratory of Human Genetics of Infectious Diseases is supported by the Howard Hughes Medical Institute, the Rockefeller University, the St. Giles Foundation, the National Institutes of Health (NIH) (R01AI088364 and R01AI163029), the National Center for Advancing Translational Sciences (NCATS), NIH Clinical and Translational Science Award (CTSA) program (UL1 TR001866), a Fast Grant from Emergent Ventures, Mercatus Center at George Mason University, the Fisher Center for Alzheimer’s Research Foundation, the Meyer Foundation, the JPB Foundation, the French National Research Agency (ANR) under the “Investments for the Future” program (ANR-10-IAHU-01), the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), the French Foundation for Medical Research (FRM) (EQU201903007798), the ANRS-COV05, ANR GENVIR (ANR-20-CE93-003), ANR AABIFNCOV (ANR-20-CO11-0001), and ANR GenMISC (ANR-21-COVR-0039) projects, the European Union’s Horizon 2020 research and innovation programme under grant agreement No 824110 (EASI-genomics), the Square Foundation, Grandir—Fonds de solidarité pour l’enfance, the Fondation du Souffle, the SCOR Corporate Foundation for Science, The French Ministry of Higher Education, Research, and Innovation (MESRI-COVID-19), Institut National de la Santé et de la Recherche Médicale (INSERM), REACTing-INSERM and the University of Paris. PB was supported by the French Foundation for Medical Research (FRM, EA20170638020) and by the MD-PhD program of the Imagine Institute (with the support of the Fondation Bettencourt-Schueller).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Declarations
Conflict of Interest
The authors declare no competing interests.
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committees and Institutional Review Board of Hiroshima University.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Included subjects or their representatives have consented to publication of their data.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shohei Eto and Yoko Nukui contributed equally to this work.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.O'Driscoll M, Ribeiro Dos Santos G, Wang L, Cummings DAT, Azman AS, Paireau J, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590(7844):140–5. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q, Bastard P, Bolze A, Jouanguy E, Zhang SY, Cobat A, et al. Life-threatening COVID-19: Defective interferons unleash excessive inflammation. Med (N Y) 2020;1(1):14–20. doi: 10.1016/j.medj.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casanova JL, Su HC. A global effort to define the human genetics of protective immunity to SARS-CoV-2 infection. Cell. 2020;181(6):1194–1199. doi: 10.1016/j.cell.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Q, Bastard P, Cobat A, Casanova JL. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature. 2022;603(7902):587–98. doi: 10.1038/s41586-022-04447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricoca Peixoto V, Vieira A, Aguiar P, Sousa P, Carvalho C, Thomas D, et al. Determinants for hospitalisations, intensive care unit admission and death among 20,293 reported COVID-19 cases in Portugal, March to April 2020. Euro Surveill. 2021;26(33):2001059. doi: 10.2807/1560-7917.ES.2021.26.33.2001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navaratnam AV, Gray WK, Day J, Wendon J, Briggs TWR. Patient factors and temporal trends associated with COVID-19 in-hospital mortality in England: An observational study using administrative data. Lancet Respir Med. 2021;9(4):397–406. doi: 10.1016/S2213-2600(20)30579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patanavanich R, Glantz SA. Smoking is associated with COVID-19 progression: A meta-analysis. Nicotine Tob Res. 2020;22(9):1653–1656. doi: 10.1093/ntr/ntaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ku CL, Chen IT, Lai MZ. Infection-induced inflammation from specific inborn errors of immunity to COVID-19. FEBS J. 2021;288(17):5021–5041. doi: 10.1111/febs.15961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoagland DA, Moller R, Uhl SA, Oishi K, Frere J, Golynker I, et al. Leveraging the antiviral type I interferon system as a first line of defense against SARS-CoV-2 pathogenicity. Immunity. 2021;54(3):557–70 e5. doi: 10.1016/j.immuni.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galani IE, Rovina N, Lampropoulou V, Triantafyllia V, Manioudaki M, Pavlos E, et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat Immunol. 2021;22(1):32–40. doi: 10.1038/s41590-020-00840-x. [DOI] [PubMed] [Google Scholar]
- 12.Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Moller R, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–45 e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Jiang L, Li X, Lin F, Wang Y, Li B, et al. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight. 2020;5(12):e138070. doi: 10.1172/jci.insight.138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4570. [DOI] [PMC free article] [PubMed]
- 16.Asano T, Boisson B, Onodi F, Matuozzo D, Moncada-Velez M, Maglorius Renkilaraj MRL, et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci Immunol. 2021;6(62):eabl4348. [DOI] [PMC free article] [PubMed]
- 17.Bastard P, Michailidis E, Hoffmann HH, Chbihi M, Le Voyer T, Rosain J, et al. Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J Exp Med. 2021;218(4):e20202486. [DOI] [PMC free article] [PubMed]
- 18.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. [DOI] [PMC free article] [PubMed]
- 19.Bastard P, Gervais A, Le Voyer T, Rosain J, Philippot Q, Manry J, et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci Immunol. 2021;6(62):eabl4340. [DOI] [PMC free article] [PubMed]
- 20.Troya J, Bastard P, Planas-Serra L, Ryan P, Ruiz M, de Carranza M, et al. Neutralizing autoantibodies to type I IFNs in >10% of patients with severe COVID-19 pneumonia hospitalized in Madrid. Spain J Clin Immunol. 2021;41(5):914–922. doi: 10.1007/s10875-021-01036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vazquez SE, Bastard P, Kelly K, Gervais A, Norris PJ, Dumont LJ, et al. Neutralizing autoantibodies to type I interferons in COVID-19 convalescent donor plasma. J Clin Immunol. 2021;41(6):1169–1171. doi: 10.1007/s10875-021-01060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goncalves D, Mezidi M, Bastard P, Perret M, Saker K, Fabien N, et al. Antibodies against type I interferon: detection and association with severe clinical outcome in COVID-19 patients. Clin Transl Immunology. 2021;10(8):e1327. doi: 10.1002/cti2.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang EY, Mao T, Klein J, Dai Y, Huck JD, Jaycox JR, et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595(7866):283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- 24.Koning R, Bastard P, Casanova JL, Brouwer MC, van de Beek D, with the Amsterdam UMCC-BI Autoantibodies against type I interferons are associated with multi-organ failure in COVID-19 patients. Intensive Care Med. 2021;47(6):704–6. doi: 10.1007/s00134-021-06392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Wijst MGP, Vazquez SE, Hartoularos GC, Bastard P, Grant T, Bueno R, et al. Longitudinal single-cell epitope and RNA-sequencing reveals the immunological impact of type 1 interferon autoantibodies in critical COVID-19. bioRxiv. 2021;2021.03.09.434529.
- 26.Chauvineau-Grenier A, Bastard P, Servajean A, Gervais A, Rosain J, Jouanguy E, et al. Autoantibodies neutralizing type I interferons in 20% of COVID-19 deaths in a French hospital. J Clin Immunol. 2022;42(3):459–470. doi: 10.1007/s10875-021-01203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trouillet-Assant S, Viel S, Gaymard A, Pons S, Richard JC, Perret M, et al. Type I IFN immunoprofiling in COVID-19 patients. J Allergy Clin Immunol. 2020;146(1):206–8.e2. doi: 10.1016/j.jaci.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meisel C, Akbil B, Meyer T, Lankes E, Corman VM, Staudacher O, et al. Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type 1. J Clin Invest. 2021;131(14):e150867. doi: 10.1172/JCI150867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahmani H, Davoudi-Monfared E, Nourian A, Khalili H, Hajizadeh N, Jalalabadi NZ, et al. Interferon beta-1b in treatment of severe COVID-19: A randomized clinical trial. Int Immunopharmacol. 2020;88:106903. doi: 10.1016/j.intimp.2020.106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bastard P, Levy R, Henriquez S, Bodemer C, Szwebel TA, Casanova JL. Interferon-beta therapy in a patient with incontinentia pigmenti and autoantibodies against type I IFNs infected with SARS-CoV-2. J Clin Immunol. 2021;41(5):931–933. doi: 10.1007/s10875-021-01023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monk PD, Marsden RJ, Tear VJ, Brookes J, Batten TN, Mankowski M, et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(2):196–206. doi: 10.1016/S2213-2600(20)30511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hung IF-N, Lung K-C, Tso EY-K, Liu R, Chung TW-H, Chu M-Y, et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Prost N, Bastard P, Arrestier R, Fourati S, Mahevas M, Burrel S, et al. Plasma exchange to rescue patients with autoantibodies against type I interferons and life-threatening COVID-19 pneumonia. J Clin Immunol. 2021;41(3):536–544. doi: 10.1007/s10875-021-00994-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.