Abstract
Neurodegenerative disorders of the central nervous system (CNS) and brain traumatic insults are characterized by complex overlapping pathophysiological alterations encompassing neuroinflammation, alterations of synaptic functions, oxidative stress, and progressive neurodegeneration that eventually lead to irreversible motor and cognitive dysfunctions. A single pharmacological approach is unlikely to provide a complementary set of molecular therapeutic actions suitable to resolve these complex pathologies. Recent preclinical data are providing evidence-based scientific rationales to support biotherapies based on administering neurotrophic factors and extracellular vesicles present in the lysates of human platelets collected from healthy donors to the brain. Here, we present the most recent findings on the composition of the platelet proteome that can activate complementary signaling pathways in vivo to trigger neuroprotection, synapse protection, anti-inflammation, antioxidation, and neurorestoration. We also report experimental data where the administration of human platelet lysates (HPL) was safe and resulted in beneficial neuroprotective effects in established rodent models of neurodegenerative diseases such as Parkinson’s disease, Alzheimer's disease, traumatic brain injury, and stroke. Platelet-based biotherapies, prepared from collected platelet concentrates (PC), are emerging as a novel pragmatic and accessible translational therapeutic strategy for treating neurological diseases. Based on this assumption, we further elaborated on various clinical, manufacturing, and regulatory issues that need to be addressed to ensure the ethical supply, quality, and safety of HPL preparations for treating neurodegenerative and traumatic pathologies of the CNS. HPL made from PC may become a unique approach for scientifically based treatments of neurological disorders readily accessible in low-, middle-, and high-income countries.
Graphical abstract
Keywords: Platelet neurotrophins, Neuroprotection, Neurorestoration, Growth factors, Extracellular vesicles, Brain
Background
Neurodegenerative and traumatic diseases affecting the central nervous system (CNS) are associated with overlapping physiopathological molecular events such as neuroinflammation and oxidative stress, ultimately leading to progressive loss of vulnerable synapses and neuronal populations resulting in various clinical features encompassing movement disorders, cognitive functions, and behavioral impairments [1–4]. The most common neurodegenerative diseases are Parkinson’s disease and Alzheimer’s disease, as well as amyotrophic lateral sclerosis, frontotemporal lobal degeneration, and Huntington’s disease. The latter are proteinopathies [5] associated with various selective or overlapping neuropathological/biochemical protein abnormalities involving, among others, amyloid peptides, tau, α-synuclein, TAR DNA-binding protein (TDP)-43, and huntingtin. Stroke, repeated concussions, and traumatic brain injuries (TBIs) are another group of neurological pathologies affecting the CNS with complex clinical features that involve cascades of pathological events that trigger accelerated neurodegeneration and eventually develop into persistent cognitive and motor impairments.
Despite widespread extensive fundamental and clinical research efforts to better understand cellular and molecular mechanisms underlying these diseases [6], there is still a dramatic lack of effective therapeutic molecules or management approaches to reverse, reduce, or even stabilize neuronal degeneration. In the context of neurodegeneration, while some considerable efforts have been and are being made to develop new treatment approaches, for instance immunotherapy and gene silencing, most current therapies are at best focused on symptoms, and treating diseases of the CNS remains a therapeutic challenge. Further, it is becoming evident that a single pharmacological approach is unlikely to provide a complementary set of therapeutic actions suitable to resolve these pathologies. New mechanistic biological hypotheses to unveil disease occurrences and progression are generating explorative therapeutic strategies that aim to retard or stop the progression of neurodegenerative processes. These therapeutic options include gene therapy, small (s)RNA, DNA, micro (mi)RNA, antagomirs (antisense miRNAs), peptide therapy, monoclonal antibodies, autologous or allogeneic mesenchymal stromal cells (MSCs), and the MSC secretome containing neurotrophic factors and extracellular vesicles [7–11]. These new therapies, if embraced by the biotechnology industry and medical community, will however probably require time-consuming and demanding efforts for their development, preclinical and clinical validation, licensing, and market expansion, and due to their costs will be reserved for a minority of patients globally.
The situation described above justifies attempts to develop, whenever possible, pragmatic alternative therapeutic solutions that are safe, effective, and affordable for most patients in low- and middle-income countries (LMIC) and industrialized economies. In this regard, interest has turned to the recently identified neurorestorative and neuroregenerative capability of the complementary combination of physiological neurotrophic factors present in human platelets for treating CNS disorders. On one hand, the scientific rationale for this application lies in the well-established general contributions of platelets to wound healing [12] and most specifically to their promotion of neuronal plasticity and CNS repair and development [13, 14]. Indeed, platelets contain a plethora of neurotrophic and other bioactive factors, as well as neurotransmitters able to promote the survival, repair, and regeneration of neurons, and maintain healthy brain function. On the other hand, the clinical rationale revolves around the fact that clinical-grade platelets are already routinely produced by blood establishments worldwide for therapeutic applications in transfusion medicine, implying an existing infrastructure for the collection and supply of source materials and the repositioning of the clinical use of human platelets for applications in regenerative medicine [15].
In the present review, we aimed to summarize multifaceted molecular roles played by platelets in coagulation, tissue repair, and regeneration to explain the benefits of using human platelet lysates (HPL) in neuroregenerative medicine and cell therapy. As platelet lysates are rich in extracellular vesicles (EVs), we also elaborate on their roles and possible uses in neuroregenerative medicine. We particularly focus on recent evidence-based preclinical data in cellular and animal models of stroke, Parkinson’s disease, Alzheimer’s disease, TBIs, and amyotrophic lateral sclerosis that provide a scientific rationale to support the further development of neuroprotective and neurorestorative biotherapies based on the administration of the platelet secretome to the brain. We also discuss the clinical, manufacturing, and regulatory pathways for an ethical supply and optimal quality and safety specifications of HPLs and EV preparations for use as pragmatic treatment options for CNS diseases.
Platelets: structure and multifaceted regulatory functions
Platelets are derived from megakaryocytes that reside in the bone marrow and are continuously released into the blood circulation to maintain a count of 150–400 × 106/mL under normal physiological conditions. Platelets exhibit unique structural specificities that include (a) the lack of a nucleus, (b) a relatively small size (2–4 um) in a quiescent state, (c) the presence of multiple membrane surface markers, especially the GPIb-IX-V complex, GPVI, and GPIIb/IIIa (also known as integrin alphaIIb-beta3) that are highly reactive to activated coagulation factors, vascular endothelium proteins, and immune cells, (d) their contents of multiple granules and mitochondria, and (e) the existence of an open canalicular system that permits two-way exchanges of molecules, including proteins, with the environment [16, 17]. In addition, platelets exposed to biochemical or physical stresses are prone to release various populations of EVs [18, 19].
The most well-known physiological function of platelets is associated with hemostasis. Platelets serve as vital “sentinels” that surveil the integrity of the blood vasculature. These reactions, known as the primary and secondary hemostasis, involve platelets and several coagulation factors that jointly prevent vascular loss by forming a hemostatic platelet–fibrin plug [20–23]. Once the blood vasculature is injured, the exposure of the tissue factor (TF) glycoprotein from the vascular subendothelium to the vascular surface induces a succession of potent biochemical reactions [24]. Exposed TF binds to factor (F) VII, leading to its activation (FVIIa), generation of FIXa and of the prothrombinase complex (FXa-FVa), which converts pro-thrombin (FII) into thrombin. The small amount of thrombin generated binds to and activates platelets through their thrombin receptors (protease-activated receptors). Activated platelets expose phosphatidylserine, a negatively charged phospholipid that binds the tenase complex (FIXa and FVIIIa) and the prothrombinase complex [25]. With more FIXa and FXIa generated, these biochemical reactions jointly amplify the generation of thrombin (thrombin burst), which leads to massive conversion of fibrinogen into a fibrin plug that entraps platelets. The formation of the fibrin–platelet plug, a natural biomaterial, contributes to stopping tissue bleeding and constitutes the first stage of gradual physiological events leading to tissue healing and regenerative remodeling [15]. The components released through the degranulation of activated platelets are instrumental in orchestrating tissue repair processes in the damaged tissue microenvironment. Platelet factors released into the microenvironment trigger chemotaxis, local stem cell proliferation and differentiation into dedicated functional cells, and ultimately complete healing and tissue repair. This is the reason why, in addition to being essential for hemostasis, platelets are regarded as very instrumental “healing cells” of the body [12, 26].
Numerous studies helped unveil the nature of platelet growth-promoting biomolecules that are instrumental in tissue healing. Platelets contain 50–80 alpha-granules, with sizes ranging 200–500 nm that are an essential reservoir of functional factors [17, 27, 28]. Alpha-granules contain coagulation factors, adhesion molecules, immunological molecules, chemokines, cytokines, and growth factors. Platelet growth factors include platelet-derived growth factor-AA (PDGF-AA), -AB (PDGF-AAB), and -BB (PDGF-BB), transforming growth factor (TGF)-β, brain-derived neurotrophic factor (BDNF), vascular endothelium growth factor (VEGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and hepatocyte growth factor (HGF). Other potent intercellular signaling molecules present in alpha-granules encompass chemotactic cytokines such as CCL5 (RANTES) and C-X-C motif chemokine ligand 4 (CXCL4 or platelet factor 4 (PF4)). In addition, platelets harbor three to eight dense granules, approximately 150 nm in size, which are a reservoir of neurotransmitters, including serotonin, dopamine, histamine, epinephrine, gamma-aminobutyric acid, and glutamate, as well as polyphosphate and ATP [28]. A striking factor is the presence of numerous molecules with neurotrophic activities in the intracellular platelet compartment.
Several publications have commented on the intriguing structural similarities existing between platelets and neurons [29–33]. Both cells contain compartments that store a comparable set of functional biomolecules, suggesting their cross-communication at the interface of the blood circulation and the brain, possibly with the involvement of EVs capable of crossing the blood–brain barrier (BBB) [32, 34]. Platelet alpha-granules, which contain neurotrophic growth factors and cytokines, resemble neuronal large dense-core vesicles, whereas platelet dense granules contain serotonin, dopamine, epinephrine neurotransmitters and ATP and share common biochemical features with the small dense-core synaptic vesicles of neurons. In addition, both cell types are reactive to an increase in internal calcium ions that triggers exocytosis [35].
Platelet lysates in regenerative medicine and cell therapy: medical and logistic rationales for translation
Rationale for translational applications
There are strong scientific and pragmatic industrial rationales for the clinical use of platelet derivatives, in particular platelet proteome preparations made by a lysis process of platelets and removal of platelet membrane debris, in regenerative medicine and cell therapies. One of the most established proofs of concept of the non-toxicity and cell growth-promoting value of HPLs relies on its increasing use as a xeno-free substitute for fetal bovine serum as a supplement for growth media for propagating human cells [36, 37]. There is ample evidence that primary MSCs isolated from various tissues can be successfully expanded in vitro in media supplemented with 5–15% clinical-grade HPLs under conditions where the cell doubling time is short (therefore cell expansion is faster), the immunophenotype is not altered, and expanded cells maintain their capacity to differentiate into osteocyte, chondrocyte, and adipocyte lineages following criteria established by the International Society of Cell and Gene Therapy [36–38]. In addition, no sign of teratogenicity has been observed. Recent studies also suggested the benefits of HPLs for expanding differentiated human cells and immune cells [39], and enhancing the neurotrophic potency of adipose tissue-derived MSCs through promotion of axonal outgrowth, suggesting applications in nerve tissue engineering [40] and human cell therapies [41]. Therefore, multiple studies have unambiguously demonstrated the non-toxicity and functional activities of HPLs preparations across a large range of cell types [39].
Preparation of platelet concentrates (PCs)
PCs, the source of HPL, are available at global level, including in several LMICs. PCs are routinely produced by blood establishments either by centrifugation of whole blood, as a by-product of the preparation of erythrocyte concentrates, or by dedicated apheresis procedures to obtain in both cases a ± five-fold concentrated suspension of platelets in plasma or a mixture of plasma and a platelet-additive solution (PAS) [42, 43]. Whole blood-derived PCs are isolated from 450 mL of whole blood collection that is anticoagulated by a citrate solution containing glucose and adenine cell nutrients to preserve erythrocytes. To isolate platelets, the blood is kept at 22 ± 2 °C for a few hours before the first centrifugation. A soft-spin centrifugation procedure (approximately 1000 × g for 10 min at 22 °C), predominantly used in the USA, results in a plasma supernatant rich in platelets or platelet-rich plasma (PRP). PCs are then prepared from PRP by a second centrifugation at higher g force (approximately, 3000 × g for 5 min at 22 °C) to pelletize the platelets, followed by resuspension in a small plasma volume (50–70 ml) [44, 45]. A “hard-spin” centrifugation procedure of whole blood (approximately 3000 × g for 5 min at 22 °C), mostly used in Europe, leads to the preparation with a platelet-rich buffy coat present in-between the erythrocytes (bottom part) and cell-depleted plasma (top part) [44, 45]. The buffy coat compartment recovered in a satellite bag with 20–30 ml of plasma (and/or PAS) is further centrifuged, and the supernatant is transferred to a bag for storage. Platelets from four to six donors are typically pooled (to reach a volume of about 200 mL) to prepare a PC unit for transfusion to adults. PCs can also be prepared with automated cell separators (apheresis) that perform extracorporeal isolation procedures of platelets from anticoagulated whole blood by intermittent or continuous centrifugation [45, 46]. The procedure allows one to obtain 200–300 mL of concentrated platelets suspended in plasma or PAS from a single donor.
PCs are an established licensed cellular blood-derived product for transfusion, which is listed as an “essential medicine” by the World Health Organization (WHO) (https://www.who.int/medicines/publications/essentialmedicines/en/). This WHO listing implies a recommendation to all countries to ensure an appropriate and safe supply of PCs for domestic healthcare systems. The collection of PCs from screened healthy blood donors should be carried out by licensed blood-collection establishments applying the concept of good manufacturing practices (GMPs) and working under the supervision of national regulatory authorities. The medical devices, and anticoagulant and additive solutions used in PC production should also be licensed. The two main methods to prepare PCs (either by the centrifugation of whole blood donations, or by dedicated apheresis technology) deliver PCs of largely equivalent quality. This alternative provides flexibility in guaranteeing a sufficient supply for all therapeutic applications of PCs. Once PCs can no longer be used for transfusion, typically 5–7 days after collection, these so called “outdated” preparations suit the manufacture of lysates for regenerative medicine and cell therapy applications [36, 37, 45]. To summarize, platelet preparations can be made available globally from locally collected PCs of ensured quality, guaranteeing a minimum domestic supply level. Further, PCs complying with the strict regulations for transfusion can be used to prepare platelet trophic factors for use outside traditional applications in transfusion medicine. The advantages and limitations of using PC as source material for HPL manufacture are summarized in Table 1.
Table 1.
Advantages and limitations of human platelet concentrates as source materials for producing allogeneic platelet lysates for regenerative medicine and cell therapies
| Features | Comments | |
|---|---|---|
| Supply | Domestic collection at the global level | Whole blood collection rate is steadily increasing especially in LMICs |
| Produced from whole blood or by apheresis | About 20% of the blood collected is used to prepare PCs: possibility to expand the supply | |
| “Outdated” units can be used as source material | Platelet concentrates can be frozen until processing | |
| Quality | Listed as an “essential medicine” by the WHO | Stimulation to countries to ensure a safe supply |
| Licensed medicinal products | Qualification of the source material | |
| Blood establishments inspected by NRAs | Guarantee of compliance with good manufacturing practices | |
| Pathogen safety | Blood donor screening | Exclusion of donors with risk factors of TTI |
| Serological and NAT of viral markers | HIV, HBV, and HCV markers are mandatory | |
| Pathogen reduction by photochemical treatment to alter nucleic acids |
Made possible as platelets are anucleated Inactivation of most viruses, bacteria and protozoa |
NRA national regulatory authorities, TTI transfusion-transmitted infection, WHO World Health Organization, LMICs low- and middle-income countries, NAT nucleic acid testing, HBV hepatitis B virus, HCV hepatitis C virus
Selection of PCs to prepare HPLs for neurological applications
Numerous studies indicate that allogeneic PCs prepared from whole blood donations or by apheresis can be suitable for preparing HPLs for cell propagation [37, 47, 48]. Preclinical evaluations in cell and animal models of neurological disorders, although preliminary, suggest that both types of donation procedures could be equally suitable to prepare HPLs for brain administration [49–51]. Further evaluations are warranted to delineate any influence of the specifications of the PC donations on the neuroprotective activity of the HPL.
The quality specifications of PCs for transfusion include a minimal platelet count of 2 × 1011 per unit transfused to ensure an enrichment close to fivefold compared to blood. The content of white blood should be less than 109 in a PC unit of 200–300 mL and is less than 106 when leucofiltration is performed. PC collected by apheresis typically has fewer white blood cells than those prepared from whole blood [44, 45]. As noted above, PC can be suspended in 100% plasma or a mixture of 30–40% plasma and 60–70% PAS. In addition to lowering the protein content, PAS, a solution that contains sodium/potassium chloride, citrate, phosphate, and mannitol [52], often used in combination with pathogen reduction treatments, affects the composition of HPLs made from the whole PC donations, but its impact on neuroprotection is unknown. By contrast, PAS and pathogen reduction do not impact the neuroprotective activity of an HPL made from platelets isolated from such PC donations [53]. While outdated PC processed 7 or 8 days after collection is suitable for preparing one neuroprotective HPL [51, 53], the impact of a more extended period is still unknown and would require evaluation.
Preparation of platelet lysates
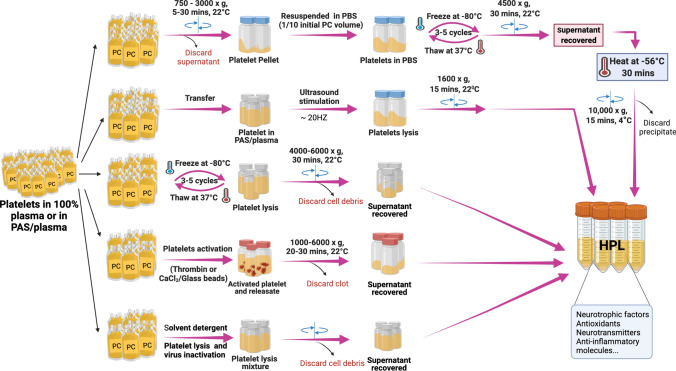
Several methods have been developed for preparing HPL from PCs allowing one to obtain a cell-free extract rich in platelet proteins [36, 45, 54], as summarized in Fig. 1.
Fig. 1.
Human platelet lysate preparation methods. They are either generated from fresh or expired platelet concentrates collected by apheresis or obtained from whole blood donations. Fresh or expired platelet concentrates can be lysed by several freeze/thaw steps, by platelet activation induced by addition of thrombin or calcium chloride (CaCl2), by sonication or by solvent/detergent (S/D) treatment. In all these methods, the cells debris are depleted by centrifugation and discarded after cells lysis and the supernatant recovered and aliquoted. HPL human platelet lysate, PBS phosphate buffer saline, PC platelet concentrates
Three freeze–thaw cycles of PC at − 80/ + 37 °C fragment platelet membranes to release platelets’ intracellular cargo without apparent alteration of the biological activity of the resulting lysate [38, 55, 56]. Sonication, combined or not with a freeze–thaw process, is another approach to fragment platelets and release their contents [57]. Activation of platelets by the addition of a calcium salt solution counterbalances the anticoagulant effect of the citrate solution and degranulates the platelets. Such “serum conversion”, due to the rapid generation of endogenous thrombin and activation of a blood coagulation cascade, converts fibrinogen into fibrin, concomitant activation and degranulation of platelets, and release of the contents [58–60]. The resulting protein lysates or releasates are further processed by centrifugation to remove cell membranes and/or fibrin clots before aseptic filtration to ensure bacterial sterility. The protein contents of the final preparation are influenced by the specificities of the starting PC material, such as a formulation in plasma or a plasma/PAS mixture [36] and by the possible pre-isolation of platelets prior to lysis [49]. Pooling of PCs from approximately 50 donors or more proved to be a major contributor to product standardization and batch consistency in applications for in vitro cell expansion [37]. Most platelet protein preparations are fully capable of supporting clinical-grade expansion of MSCs from various origins [39, 61–63] and some were evaluated using in vitro and in vivo models of neurological diseases [49, 50, 53, 64–69].
Recognizing that administration of HPL to the brain, through intracranial or intranasal administration, is a very novel therapeutic paradigm, efforts are needed to optimize its safety. HPLs dedicated to brain administration have been developed and the product characterized [49]. This HPL (termed “HPPL” for human platelet pellet lysate) is obtained by pelletizing and concentrating platelets from PCs by centrifugation, followed by three freeze—thaw cycles for lysis, and by heat treatment at 56 °C for 30 min. Interestingly, heat treatment was found to improve the neuroprotective activity of HPLs in in vitro Parkinson’s disease models [49], while also contributing to depletion of fibrinogen, a neurotoxic protein, to a decrease in the in vitro procoagulant/prothrombogenic activity [49, 68], and to an improved tolerability in primary cortical neuron culture [53]. HPL batches for brain administration will need to be controlled and meet the standard specifications of any therapeutic biologicals, such as bacterial sterility, low endotoxin content, consistent physicochemical characteristics (pH, osmolality), protein and growth factor content, low level of proteolytic and thrombogenic activity. However, there is still no standard defining the specifications of HPLs, and those may depend upon the mode of delivery to the brain.
Platelet-related bioactive molecules and EVs
For a long time regarded as having mostly a hemostatic role, platelets’ non-hemostatic functions are now being unveiled through proteome analyses. As stated above, platelets are characterized by their high contents of native growth factors, chemokines, cytokines, enzymes, antioxidants, and chemicals in their α-granules and dense granules making them a natural reservoir of bioactive molecules. Protein materials derived from these platelets have been intensively characterized by several techniques. To detect concentrations of HPL molecules, techniques such as ELISA, protein/cytokine-array analysis, multiplex technology, and biochemical analysis were initially used. They revealed a plethora of factors with variable concentrations depending on the blood donors, the number of PCs pooled, the HPL production method, etc. [70]. To have a wider and unbiased view of the cytokines, chemokines, and soluble adhesion molecules contained in HPLs, proteomics approaches were employed [71–73]. This intensive characterization of HPL contents (Table 2) has led to detection of specific groups of active molecules, as detailed below, involved mainly in cellular processes (cell–cell signaling, signal transduction, cell recognition, the cell cycle, cell component movement, cell proliferation processes, etc.), responses to stimuli, cellular component organization, biogenesis, etc. [74].
Table 2.
Bioactive molecules in human platelet lysate (HPL) with diverse roles in brain repair and neurogenesis
| HPL bioactive factors | Reported biological effects | Experimental model | References | |
|---|---|---|---|---|
| Growth factors, cytokines | BDNF | Supports NSC proliferation, migration, and differentiation | Neuronal precursors | [75, 76] |
| Favors synaptic plasticity and facilitates synapse maturation | Neuronal precursors | [77, 78] | ||
| Supports hippocampal neurogenesis | In rodents | [79, 80] | ||
| EGF | Induces NSC proliferation and migration | NSC culture | [81] | |
| Supports cortical tissue regeneration and motor function recovery | In vivo stroke model | [82–84] | ||
| FGF | Promotes proliferation and differentiation of NSCs | Cell culture and in vivo | [85] | |
| Stimulates neurogenesis | Focal ischemia model in rats | [82, 86] | ||
| Regulates Schwann cell proliferation, axonal growth, and remyelination | Nerve injury in mice | [87] | ||
| GSN | Inhibits apoptosis and is neuroprotective in murine stroke | Hippocampal neuron culture, in vivo stroke model | [88, 89] | |
| HGF | Protects dopaminergic neurons, motor neurons, and sympathetic neurons | Neuron culture | [90, 91] | |
| IGF | Promotes NSC growth and differentiation, stimulates adult hippocampal neurogenesis, has neuroprotective activity | Neuronal cell culture, in vivo administration | [31, 92–95] | |
| LGALS1 | Prevents microglial activation and promotes neuroprotection | Culture of microglia and astrocytes; in mice | [96, 97] | |
| Promotes astrocyte maturation but inhibits proliferation | In vitro cell culture | [98] | ||
| MANF | Protects rat embryonic nigral dopaminergic neurons | Rat model of PD | [99] | |
| Is neuroprotective and neurorestorative | Rat model of PD | [100] | ||
| Supports the development and sprouting of dopaminergic axonal terminals | In vitro cell culture | [99] | ||
| Decreases stress and activates the PI3K/Akt/mTOR pathway | In vitro cell culture | [101] | ||
| Activates the PI3K/Akt/GSK3β pathway and Nrf2 nuclear translocation | In vitro | [102] | ||
| Inhibits apoptosis | In vitro cell culture | [103] | ||
| Inhibits autophagic via activation of the AMPK/mTOR pathway and ameliorates ROS by maintaining mitochondrial function | In vitro cell culture | [104] | ||
| NENF | Promotes neurotrophic activity and neuronal cell proliferation and stimulates differentiation | Mouse neural precursor cells | [105] | |
| Is a novel player in the maintenance of the anxiety circuitry | Neudesin-null mice | [106] | ||
| PDGF | Regulates NSC proliferation, migration, differentiation, and survival processes, and reduces apoptosis | Cultured NSCs | [107, 108] | |
| Protects cells against MPP+-induced cell death | SH-SY5Y cell culture | [109] | ||
| Has restorative effects | Rodent model of PD | [110, 111] | ||
| PF4 | Promotes neuronal differentiation in DBA/2 mice | Mouse primary cells and in vivo infusion | [112] | |
| TGF-β | Triggers differentiation of precursor cells | In vitro and in vivo mouse mesencephalic progenitors | [113] | |
| VEGF | Promotes proliferation and migration of endothelial cells, and the formation of new blood vessels in vivo, and enhances vascular permeability | In vitro endothelial cell culture | [114] | |
| Slows progression of amyotrophic lateral sclerosis in mice by stimulating motoneuron functions | Rat model of amyotrophic lateral sclerosis | [115, 116] | ||
| Protects cultured motoneurons against death in conditions of hypoxia, oxidative stress, and serum deprivation | Neuronal cell culture | [117, 118] | ||
| Exerts protective effects on primary hippocampal neurons against glutamate toxicity | In vitro neuronal culture | [119] | ||
| Chemicals | Vit B12 | Prevents cognitive decline | Clinical trials | [120, 121] |
| Serotonin | Modulates neural activity | Ex vivo using rat brains | [122] | |
| Chemokines | RANTES | Can be neuroprotective | Primary cortical neuron culture, AD and stroke models | [123, 124] |
| Contributes to neuronal synaptic activity and memory formation | Primary neuron culture and WT and CCL5-/- mice | [125, 126] | ||
| Reduces neuronal degeneration and memory dysfunction after mTBI | Primary neuron culture, mTBI mouse model | [127] | ||
| MIF | Mediates neuroprotective effects in Parkinson’s disease | Mouse model of PD, SH-SY5Y in vitro model of PD | [128] | |
| Antioxidants | CAT | Protects against dopaminergic neuronal cell death | Mesencephalic neuronal–glial culture, rat stroke model | [129, 130] |
| CP | Inhibits lipid peroxidation and ROS | CP−/− mouse model | [131] | |
| Exerts protective activity against iron-induced oxidative damage in Alzheimer’s disease and TBIs | CP −/−, AD, focal cortical contusion injury mouse models | [131–133] | ||
| GPX | Protects mammalian cells against oxidative damage | Human cell line cultures | [134] | |
| Is protective in Huntington’s disease models (inhibits the activity of ROS-producing enzymes) | In vitro cell culture and Drosophila Huntington’s disease model | [135] | ||
| SOD | Inhibits lipid peroxidation, is neuroprotective | In vitro primary cultured cortical neurons and rat stroke model | [130, 136, 137] | |
| Trx (TXN) |
Protects against oxidative stress-associated diseases Modulates microtubule polymerization kinetics in vivo |
PC12 cell culture | [138, 139] | |
| Is involved in cell–cell communication, transcriptional regulation, cell signaling, and DNA synthesis | Focal brain ischemia in rats | [140] | ||
| Exerts a cytoprotective effect in the nervous system | RASMC and raw cell culture | [141] | ||
| GCLM | Is associated with glutathione synthesis | In vivo | [142, 143] | |
| Interleukins | TIMP-1 | Regulates neuroinflammation and neuropathic pain | In vivo in mice and rats | [144–147] |
| Modulates astrocyte function and myelination | In vivo | [148, 149] | ||
| IL-4 | Has anti-inflammatory properties | Human monocytes, murine bone marrow-derived macrophage culture | [150] | |
| Platelet-EVs | GFs | Stimulates angiogenesis and neurogenesis | Rat ischemia model | [151] |
| miR-126-3p | Exerts anti-inflammatory effects | Primary human macrophages | [152] | |
AMPK Adenosine monophosphate-activated protein kinase, AD Alzheimer disease, BDNF brain-derived neurotrophic factor, CAT catalase, CCL5 CC chemokine ligand 5, CP ceruloplasmin, EGF epidermal growth factor, EV extracellular vesicle, FGF fibroblast growth factor, GAL-1 or LGALS1 galectin 1, gsn gelsolin, GCLM glutamate-cysteine ligase regulatory subunit, GPX glutathione peroxidase, GSK3β glycogen synthase kinase 3β, GF growth factor, HGF hepatocyte growth factor, IGF‐1 insulin‐like growth factor‐1, MANF mesencephalic astrocyte-derived neurotrophic factor, MIF macrophage migration inhibitory factor, mTOR mammalian target of rapamycin, mTBI mild traumatic brain injury, MPP myelin protein peripheral, NSC neural stem cell, NENF neuron-derived neurotrophic factor or neudesin, Nrf2 nuclear factor erythroid 2-related factor, PI3K phosphatidylinositol 3-kinase, PD Parkinson’s disease, PDGF platelet-derived growth factor, ROS reactive oxygen species, SOD superoxide dismutase, TIMP-1 tissue inhibitors of metalloproteinases 1, TXN thioredoxin, TGF-β transforming growth factor β, WT wild type
Platelet lysate bioactive molecules
HPLs are particularly rich in growth factors and neurotrophins, such as BDNF, TGF-β, PDGF-AA and -BB, insulin-like growth factor (IGF)-1, EGF, VEGF, bFGF, macrophage-stimulating protein (MSP), macrophage-colony stimulating factor (M-CSF), angiopoietin-1, angiogenin, and IGF-binding protein 3 (IGF-BP3) detectable by ELISA or multiplex analyses [153]. The complex composition was revealed by proteomics approaches such as two-dimensional electrophoresis and liquid chromatography/mass spectroscopy. These findings suggest that the current lysis or activation procedures to prepare HPLs, as described above, efficiently extract the platelet granule contents. It is however noteworthy that the PC sources (donor age and sex) and their mode of preparation (apheresis, whole blood, with the addition or not of PAS, and leuco-filtration or not) may influence HPL protein and growth factor (GF) profiles and their functional capacities [36]. These criteria must be considered during HPL production since in several studies, the beneficial effects of HPL therapies are likely mediated, at least in part, by GFs (Fig. 2) [36, 62].
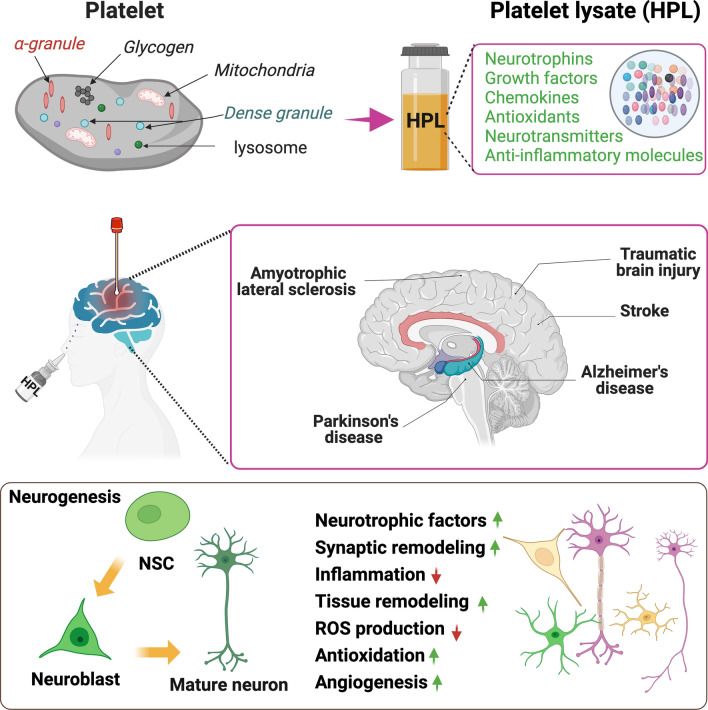
Fig. 2.
Summary of the neuroprotective and neurorestorative activities of human platelet lysate reported in animal models of neurodegenerative diseases and CNS injuries. HPL are produced from platelets. In the animal models of Alzheimer and Parkinson’s diseases, amyotrophic lateral sclerosis, stroke, and traumatic brain injury human platelet lysates are delivered either by intranasal, intracerebroventricular administration, or by topical deposition at the desired region. The main effect of the HPL is their ability to stimulate neurogenesis and their neuroprotective activity. HPL human platelet lysate, NSC neural stem cell, ROS reactive oxygen species
In addition to GFs, a wide range of chemokines, including platelet factor 4 (PF4 or CXCL4), CC-chemokine ligand 3 (CCL3 or macrophage inflammatory protein (MIP-1α)), CCL4 (MIP-1β), CCL5 (RANTES), CXCL1, MCP1/CCL2, CXCL2, and chemokine receptor-binding protein (macrophage migration inhibitory factor, MIF) has been identified in HPLs [153, 154]. Physiologically, secretion of these chemokines generally occurs during platelet activation, which explains their presence in HPL preparations obtained by platelet lysis or thrombin-generation. They play roles at the onset of inflammatory reactions by attracting leukocytes to infiltrate injured tissues. HPL contents in chemokines are commonly quantified by multiplex-based measurements. They are generally associated with chemotaxis, wound repair, and angiogenesis [153, 155], but are also linked to neuroinflammation, which is now considered not only to be a detrimental factor but also a supportive phenomenon in CNS repair as revealed in many studies [156–158]. It was also shown that platelet granules contain both proinflammatory and anti-inflammatory molecules associated with immunity. Thus, as expected, studies have highlighted the presence of interleukins in HPL preparations. Interleukin (IL)-1α, IL-1 receptor antagonist (IL-1Ra), IL-4, cell surface receptors (cluster of differentiation 14 (CD14) and CD40L) as well as proteases, such as tissue inhibitor of metalloproteinase (TIMP)-1, are among the molecules detected. These interleukins are commonly associated with inflammation modulation, but may also act as trophic factors, stimulating the migration and differentiation of cells, as well as playing an anti-inflammatory role [144, 148, 159].
Further, HPLs are enriched in various antioxidants such as glutathione peroxidase (GPx), glutathione S-transferase, catalase (CAT), ceruloplasmin (CP), superoxide dismutase (SOD), and thioredoxin (TXN) [51]. Glutathione and TXN are defined as thiol antioxidants. Glutathione can be a cofactor for many detoxifying enzymes, carries amino acids across plasma membranes, directly scavenges OH radicals and dioxygen, and produces vitamins, leading to its protective impacts against oxidative stress [160]. TXN has oxidoreductase activity, and its plasmatic level increases in diseases associated with oxidative/nitrosative stress and inflammation [161]. SOD and CAT are considered enzymatic antioxidants which strongly protect against oxidative stress. Other biochemical analyses have been used to further characterize the chemical contents of HPLs, allowing the identification of albumin, total cholesterol, triglycerides, vitamin B12, calcium, iron, and sodium [153]. Further, and quite importantly, HPLs contain a serotonin neurotransmitter, which is involved in diverse functions including vascular tone, hemostasis, immune response, bone remodeling, and energy metabolism. It is not synthesized by platelets but can accumulate in the blood [162] and be stored in dense granules.
Platelet-derived EVs
Most cells, including platelets, can release EVs [163, 164]. Although published information is still scarce, it is increasingly evident that HPLs contain, in addition to proteins, peptides and other biomolecules, a plethora of EVs. “EV” is an umbrella term defining a heterogeneous population of cell-derived nanosized vesicles delimited by a lipid bilayer [165]. EVs are typically classified into exosomes when they originate from the endosomal compartment of cells, microvesicles, or microparticles when their formation results from a budding of the cellular plasma membrane [166].
The interest in EVs lies in the fact that they are now widely thought to play important physiological and pathological roles in cell-to-cell communications as a cargo of biomolecules. EV's functions are orchestrated by (a) the “targeting” receptors or integrins present on their membrane and (b) the content of potent biomolecules, both of which represent a biological signature from the parent cells. EV cargoes include trophic factors/proteins, lipids, messenger (m)RNA, and miRNA that can be delivered to distant cellular recipients. EVs, especially those derived from MSCs, are even seen as potential stand-alone therapeutic products or drug-delivery vehicles in various fields of regenerative medicine and disease treatment [167–171]. In addition, EVs, either native or loaded with drugs, are generating much interest for applications in treating disorders of the CNS, such as Parkinson’s disease [172], gliomas [172], Batten disease [173], Alzheimer’s disease [174], hypoxia-ischemic injury [175], and lipopolysaccharide (LPS)-induced microglial proliferation [176]. The scientific rationale supporting the use of EVs in the treatment of CNS disorders includes (a) a lower risk of immunogenicity and tumorigenicity compared to MSCs [177, 178], (b) their safety and biocompatibility compared to synthetic nanocarriers [179], and (c) a demonstrated capacity to encapsulate natural and synthetic therapeutic molecules, long circulating half-lives to overcome tissue barriers [180], and an ability to cross the BBB [177, 181]. For instance, blood-derived exosomes loaded with dopamine were able to cross the BBB, reach the striatum and the substantia nigra, exert a more potent functional effect, and be less toxic than free dopamine in an animal model of Parkinson’s disease [182]. Similarly, the intravenous administration of macrophage-derived EVs led to BBB penetration and delivery of BDNF in an animal model, including under a state of brain inflammation, suggesting therapeutic potential for treating CNS diseases [183]. Zebrafish studies demonstrated the ability of brain endothelial cell-derived exosomes to deliver anticancer drugs across the BBB, supporting their potential use in treating brain cancer [184]. EVs are also attracting a lot of interest due to the possibility that they can be delivered by intranasal (IN) administration [177, 185, 186]. For instance, the IN administration of monocyte- and macrophage-derived EVs that are loaded with the CAT antioxidant led to neuroprotective effects in an in vivo Parkinson’s disease model [187]. All these findings justify recent attempts to better understand the possible presence as well as roles, either beneficial or detrimental, played by platelet EVs in HPLs [163].
Several elements support the presence of a substantial number of EVs in at least some HPLs. PCs for transfusion, which are used to produce HPLs, were found to contain numerous EVs [188–191]. Those originate from the collected blood itself or can be additionally released by platelets over the 5–7-day storage period in a liquid state after blood collection [191]. Indeed, platelets and megakaryocytes contribute close to 50% of the EV pool present in the blood circulation [192], representing an apparent concentration of up to 109/µL of plasma [193, 194]. However, accurate quantitation of EVs in PCs (pEVs) and HPLs remains highly dependent on the quantitative technique used and the capacity to discriminate from chylomicrons, low-density lipoprotein, and high-density lipoprotein. Using cryo-electron microscopy, pEV concentrations were conservatively determined to be close to 11,500/µL of plasma [193]. Platelet activation by various agonists and shear stress generated during collection, handling, and storage can lead to platelet degranulation and concomitant EV release [195–197].
PCs used for transfusion or as raw material to prepare HPLs are thus likely to contain a high proportion of EVs derived from megakaryocytes and platelets [19, 168]. In addition, the freeze–thaw process and calcium chloride-induced platelet lysis or degranulation to prepare the HPLs are likely to lead to further pEV generation. The population of EVs in HPL preparations depends upon the mode of collection of the PCs and of the production of the HPL. pEVs express various clusters of differentiation on their surface such as CD31, CD41, CD42, CD61, CD62, and CD63. Phosphatidylserine (PS), a procoagulant active phospholipid, may also be expressed on the surface of pEVs [198]. The presence of PS-expressing pEVs in HPLs may vary, suggesting an impact of the mode of production [68]. Some pEVs have binding sites for coagulation factors, such as activated factor V and factor VIII, and thrombin [199], and a surface that is 50–100-fold more procoagulant than that of activated platelets [199], which may represent a potential safety concern for brain administration. The possibility of pEVs exerting proinflammatory actions exists as they may contain cytokines such as IL-1, IL-6, and tumor necrosis factor (TNF)-α [200]. However, other studies suggested their capacity to inhibit cytokine release by macrophages [201] and plasmacytoid dendritic cells [202], and stimulation of monocyte aggregation by monocytes and neutrophil extracellular trap formation [203, 204], differ among pEV populations. Possible roles played by pEVs in the neuroprotective and neurorestorative functions and anti-ageing capacity of platelet lysates may be associated with their contents of neurotrophic GFs such as PDGF and BDNF, anti-inflammatory and antioxidative biomolecules, neurotransmitters, non-coding RNAs (mRNAs and miRNAs) [205, 206], and mitochondria, at least for some subsets [18, 19, 164, 188]. pEV lipid components promote the capacity of human umbilical vein endothelial cells (HUVECs) to proliferate, migrate, and form tubes and promote in vivo revascularization following chronic ischemia [207]. Proangiogenic effects of pEVs were also associated with their contents of VEGF, bFGF, and PDGF [208]. This suggests that the pro- and anti-inflammatory effects and other biological functions of pEVs are intimately linked to their nature, supporting future in-depth studies of the type of EVs present in HPLs as efficacy and safety factors.
Expected neuroprotective outcomes of platelet lysate bioactive molecules
The various bioactive molecules contained in HPLs and pEVs therefore have strong potential for neuroregenerative applications. Trophic factors present in HPLs were individually described several years ago as crucial signaling molecules that have specific functions in essential biological processes related to the CNS [209, 210]. For instance, using an in vitro model of neuronal cell cultures, Kim et al. [211], Pietz et al. [212]. and Nikkhah et al.[213] demonstrated the ability of BDNF and PDGF to protect neuronal cells against apoptotic cell death. Other factors, including EGF [214], VEGF [215] mesencephalic astrocyte-derived neurotrophic factor (MANF) [216], and IGF-1, were also demonstrated to improve neuronal survival or provide neuroprotection, in addition, to stem cell proliferation and differentiation into neurons. In vitro and in vivo experiments particularly elucidated the key functions of GFs and neurotrophins on neural stem cell development into neurospheres and neural differentiation. Neurotrophic factors, such as VEGF, EGF, FGF-2, PDGF, BDNF, PF-4, TGF-ß, IGF-1, connective tissue growth factor, and bone morphogenetic protein (BMP)-2, -4, and -6, are all involved in neurogenesis and neuroepithelial cell proliferation, differentiation, migration, and survival [14, 217]. Activation of the TrkB- and p75NTR signaling pathways [218, 219], known as cell surface receptors of several of these neurotrophins, is also involved in these processes, in both physiological and pathological conditions [82, 220–222]. In a developmental setting, these factors also control the formation of synapses [223]. Other investigations showed that platelet EVs enhanced neural stem cell growth and differentiation [224]. In keeping with these observations, platelet trophic factors might have a significant impact on neurogenesis, occurring mainly in adults, in the subgranular zone of the hippocampal dentate gyrus and subventricular zone (SVZ) of lateral ventricles [225–229]. In physiological conditions, there are experimental in vivo arguments that the platelet proteome might impact brain neurogenesis. Indeed, large numbers of blood proteins were recently found to physiologically cross the BBB [230]. In agreement, the blood of young mice promoted the formation of new neurons in the adult brain [231–233]. Other experiments supported the concept that in vivo activation of platelets, prone to release growth-promoting factors, improves neurogenesis in physiological conditions, presumably in part through PF4 (CXCL4), an abundant factor in platelet proteomes [234]. Another example is the chemokine CCL5, which is also abundant in platelets and recently demonstrated to promote glucose aerobic metabolism and positively impact hippocampal synaptic plasticity and cognition [125]. A more extensive view of the effects of natural molecules found in platelet lysates that are prone to exert CNS effects is displayed in Table 2.
Platelet lysates for brain administration: preclinical evidence
The above-mentioned generic potential of individual components of HPL regarding neuronal fates shows that platelet lysates may combine several properties and potentially exerts synergistic beneficial impacts in many neurological conditions. By its ability to modulate several important processes such as inflammation, neuronal death, oxidative stress, and angiogenesis, presumably occurring at different stages in many disorders, HPLs likely represent a reliable source of human trophic factors with high clinical potential. In keeping with this idea, the potential of HPL has been revealed though various experimental and recent preclinical studies, in several different pathophysiological contexts (Table 3).
Table 3.
Examples of evidence of human platelet trophic factors contributions to central nervous system (CNS) repair
| Pathology | Platelet preparations | Model | Biological outcomes | References |
|---|---|---|---|---|
| Stroke | Human platelets isolated from platelet-rich plasma, resuspended in PBS, and subjected to three freeze–thaw cycles |
• In vivo: focal ischaemia in focal Male spontaneously hypertensive rats produced by permanent distal middle cerebral artery occlusion (PMCAO) • Injection to the lateral ventricle |
• Increases eNSC proliferation and angiogenesis in the SVZ and in the peri-lesion cortex • Improved behavioral deficits |
[69] |
| Human platelet microparticles and exosomes prepared from thrombin-activated human platelets and isolated by ultracentrifugation (100′000 × g) |
• In vivo PMCAO model in adult spontaneously hypertensive rats • Topical application by biodegradable polymer |
• Triggers neurogenesis and angiogenesis at the infarct boundary zone • Improves behavioral deficits |
[151] | |
| Alzheimer’s disease | Human plasma rich in growth factors obtained by calcium chloride treatment in glass tubes and centrifuged |
• In vitro: Primary cortical and hippocampal neurons • In vivo: intranasal delivery to double-transgenic APP/PS1 mouse model |
• Enhances proliferation of and survival of primary neuronal cultures • Enhances hippocampal neurogenesis and reduces Aβ-induced neurodegeneration |
[65, 66] |
| Parkinson’s disease | Human plasma rich in growth factors obtained by calcium chloride treatment in glass tubes and centrifuged [65] |
• In vitro: human dopaminergic neuroblastoma cell line (SH-SY5Y) treated with MPP + • In vivo: MPTP mice model. Intranasal administration |
• Protects dopaminergic neurons from MPP + toxicity • Prevents striatal dopaminergic neurons and dopamine depletion from MPTP toxicity; diminishes the inflammatory responses and improves motor performance, associated with reduction in NF-κB activation, and inflammatory markers expression in the substantia nigra |
[67] |
| Human platelets isolated from clinical-grade platelet concentrates and heat-treated (56 °C, 30 min) (HPPL) |
• In vitro: dopaminergic LUHMES cells exposed to MPP + ; BV2 microglial cells with/without LPS stimulation • In vivo: MPTP mice model. Intranasal administration |
• Protects dopaminergic LUHMES neurons against MPP + neurotoxin; protects BV2 cells against inflammation • Diffuses in the striatum and cortex; protects the substantia nigra and striatum against MPTP intoxication; no neuroinflammation |
[49] | |
| HPPL preparation [49] | • In vitro: LUHMES cells exposed to various specific pro-oxidants and regulated cell death inducers: MPP + , menadione, elastin, staurosporine, and rapamycin | • Protects LUHMES cells against erastin, menadione and MPP + in part through an activation of the Akt and MEK pathways | [64] | |
| HPPL preparation [49] made from outdated pathogen-reduced (Intercept) clinical-grade platelet concentrates | • In vitro: dopaminergic Lund human mesencephalic (LUHMES) cells; primary cortical/hippocampal neurons |
• Non-toxic to LUHMES cells nor primary neurons • Enhances the expression of tyrosine hydroxylase and neuron-specific enolase in LUHMES cells, and protects against ferroptosis induced by erastin • No detrimental impact on synaptic protein expression in primary neurons • No inflammation of BV2 microglial cells |
[53] | |
| Brain injuries | Human platelet microparticles and exosomes preparation [151] | • In vitro assay of neural stem cell (NSC) proliferation, survival and differentiation | • Increases NSC proliferation survival, and differentiation, partially through ERK and Akt signalling | [224] |
| Pooled HPL prepared from outdated platelet concentrates by two freeze–thaw cycles and centrifugation at 4000 × g | • In vitro: Effect on proliferating subependymal zone (SEZ), derived NSPCs | • Increases the numbers of in vitro proliferating adult rat SEZ-derived NPCs and reduces apoptosis without affecting proliferative or lineage-differentiation capacity | [217] | |
| HPPL preparation [49] made from outdated pathogen-reduced (Intercept) clinical-grade platelet concentrates | • In vitro: non-differentiated SH-SY5Y neuroblastoma cells; EA-hy926 human endothelial cell; BV2 microglial cells stimulated or not with LPS |
• Non-toxic to SH-SY5Y, BV-2 and EA-hy926 cells • Stimulates wound healing and neuronal differentiation of SH-SY5Y into neurons • Does not trigger TNF or COX-2 inflammatory markers by BV-2 microglia, and decreases inflammation after LPS stimulation |
[50] | |
| HPPL preparation [49] |
• In vitro: scratch assay performed using differentiated SH-SY5Y neuroblastoma cell cultures • In vivo: two mouse models of TBI (controlled cortical impact and in-house cortical brain scratch) injury. One topical administration in the lesioned area followed by daily intranasal administration for 6 days |
• Stimulates wound healing of differentiated SH-SY5Y neuroblastoma • Improves mouse motor function • Mitigates cortical neuroinflammation, and oxidative stress in the injured area • Reduces loss of cortical synaptic proteins • Reverses several pathways promoted by the TBI models related to transport, postsynaptic density, mitochondria or lipid metabolism |
[51] | |
| EVs isolated from four HPL using size-exclusion chromatography | • In vitro: wound healing assay of SH-SY5Y neuroblastoma cells; mice primary neuronal cells |
• Non-toxic to SH-SY5Y neuronal cells • Differentially promotes cell growth and migration in a wound healing model of SH-SY5Y cells • Stimulates network formation in primary neuronal cultures |
[235] | |
| Amyotrophic lateral sclerosis | HPPL preparation 49 | • In vitro: NSC34 motoneurons exposed to various specific pro-oxidants and regulated cell death inducers: MPP + , menadione, elastin, staurosporine, and rapamycin | • Protects NSC34 motoneurons against STS and menadione toxicity in part through activation of the Akt and MEK pathways | [64] |
| HPL prepared from pooled human platelet concentrates, sero-converted and heat-treated (HHPL) and its sub-fractions |
• In vitro: motoneuron cultures isolated from E12.5 spinal cord of Hb9:: GFP or C57BL/6 embryos and exposed to erastin, STS or menadione) or glutamate • In vivo: FVB Tg(Sod1*G86R)M1Jwg/J mice; intracerebroventricular administration of HHPL; intranasal administration of < 3 kDa fraction |
• HPPL and sub-fractions exerts Akt-dependent neuroprotection, strong anti-apoptotic and anti-ferroptotic actions on neuronal cells • The < 3 kDa fraction has GPX4 dependent anti-ferroptotic properties • Intracerebral delivery of HHPL or intranasal administration of < 3 kDa fraction increases the lifespan of SOD1G86R mice |
[236] |
APP/PS1 Amyloid precursor protein/presenilin-1, eNSC embryonic neural stem cells, MPP + 1-methyl-4-phenylpyridinium, MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, LPS lipopolysaccharides, LUHMES Lund human mesencephalic, NF-κB nuclear transcription factor-κB, PMCAO permanent distal middle cerebral artery occlusion
Stroke
The first studies to investigate the neuroprotective and neurorestorative activity of HPLs have been conducted in stroke models. In an experimental model of focal ischemia induced by permanent distal middle cerebral artery occlusion (PMCAO), Hayon et al. tested the effect of direct HPL administration into the lateral ventricle, delivered just after ischemic injury [69]. They followed the functional outcomes of animals for 90 days and found a beneficial effect of HPL on the neurological severity score after only 1 week which was maintained over time. Histologically, such a benefit was associated by a reduced infarct volume along with enhanced angiogenesis and neurogenesis, presumably through Akt and extracellular signal-regulated kinase (ERK)-dependent mechanisms. Another study was performed by Zhang et al. using rats with focal ischemic stroke followed by local or systemic delivery (intrafemorally vein injection) of HPL [237]. As found by Hayon et al., a local HPL infusion reduced neurological deficits and infarct volumes compared to control treatment. Interestingly, systemic delivery also provided a benefit, albeit smaller [237]. Finally, pEVs have also been investigated in rat PMCAO and were found to promote angiogenesis and neurogenesis leading to improved behavioral outcomes [151], suggesting that HPL's effects are, at least in part, mediated by pEVs.
Alzheimer’s disease
Anitua et al. evaluated a specific type of HPL in Alzheimer’s disease animal model. In their study, the group prepared an HPL called “PRGF” [plasma rich in growth factor), Endoret)] from healthy young male donors’ whole blood [65]. The HPL was administrated by intranasal delivery (IN) three times per week for 4 weeks to male double-transgenic APP/PS1 mice. Behavioral tests investigating anxiety and memory were performed. As expected, APP/PS1 mice exhibited memory impairments, which were reversed in the HPL-treated group. This beneficial impact of HPL was also observed in terms of anxiety. Behavioral improvement was correlated with reduced β-amyloid (Aβ) deposition and brain levels of Aβ40 and Aβ42 but also tau hyperphosphorylation, though glycogen synthase kinase 3β (GSK3β) inhibition, all logically converging towards the enhanced expressions of synaptic markers (synaptophysin, synapsin, and postsynaptic density protein (PSD)-95). Interestingly, that study particularly suggested that HPL prevented astrocytic reactivity by Aβ and even Aβ degradation by those glial cells, using cultured astrocytes [65]. Later work by the same group [66] confirmed the in vitro ability of this HPL preparation to reduce Aβ-induced neurotoxicity of primary neurons but also provided interesting data showing the ability of HPL to increase proliferation and differentiation of neural stem cells in the subgranular zone (SGZ). These encouraging preliminary results in Alzheimer’s disease models need to be refined using curative paradigms, opening to Tau models and humanized knock-in and human induced pluripotent stem cell (hiPSC) models.
Parkinson’s disease
HPLs have been investigated as a novel biotherapy for Parkinson’s disease using both in vitro and in vivo models and various mode of administration, including IN and intracerebroventricular (ICV). In 2017, Chou et al. [49] and Gouel et al. [64] reported the in vitro neuroprotective activity of a concentrated and heat-treated HPL with low protein contents and depleted of plasma proteins, using Lund human mesencephalic (LUHMES) cells subjected to either neurotoxin 1-methyl-4-phenylpyridinium (MPP +) or erastin. The latter has been used as a model of ferroptosis [238, 239], an iron-dependent cell death involving lipid peroxidation [240] and reported to be one of the cell death pathways linked to Parkinson’s disease pathological development [240]. In those studies, the authors revealed that at up to 20% (v/v), this HPL was not toxic and that pretreatment with 5% HPL significantly alleviated ROS production and cell death promoted by both inducers through Akt and mitogen-activated protein kinase (MEK)-dependent signaling pathways [64]. Similar results were reported using LUHMES cells as well as primary neurons treated by an HPL prepared from licensed human PCs subjected to pathogen-reduction treatment using psoralen/UVA irradiation (Intercept) [53]. These data notably highlighted that this photochemical treatment of the PC raw materials used to prepare the HPL did not alter HPL neuroprotective activity. It is noteworthy that the precise mechanisms underlying the anti-ferroptosis action of HPL remain to be elucidated. It may likely be related to the presence of antioxidants such as SOD or glutathione. Importantly, beneficial effects of HPL were also demonstrated in an in vivo model of Parkinson’s disease generated following 1-methyl-4-phenyl-1,2,3,5-tetrahydropyridine (MPTP) intoxication [49]. The data particularly demonstrated that IN delivery of HPL, initiated 2 days before an MPTP injection led to a significant reduction of dopaminergic neuron degeneration as attested by enhanced tyrosine hydroxylase staining in both the substantia nigra and striatum [49]. These effects were suggested to notably arise from reduced anti-inflammatory activity of HPL since both cytochrome oxidase subunit 2 (COX-2) and Iba-1 levels were found to be reduced. That study however lacked behavioral investigations. Interestingly, Anitua et al. examining the effects of preventive IN administration of PRGF-Endoret (also used in the APP/PS1 model, see above), in the MPTP mouse model [67], reported similar improvement, including anti-inflammatory potential at the motor level. Strikingly, this work also demonstrated that HPL delivered after lesion onset by MPTP was also able to provide partial protection of dopaminergic neurons and alleviate motor alterations [67]. It is notable that in both Chou et al. and Anitua et al.'s studies, it was demonstrated that HPL neuroactive molecules readily reach the brain following IN administration. Overall, these data support HPL as a safe product able to provide neuroprotection and stimulate tissue regeneration in Parkinson’s disease models. From a clinical perspective, the potential of HPLs delivered after lesion occurrence needs to be further demonstrated as well as in additional chronic models, including non-human primates.
Traumatic brain injury (TBI)
TBI is another pathology in need of a pluripotent and multifaceted therapeutic strategy to ensure an immediate anti-inflammatory effect, neuroprotection, and neuro-restoration through enhanced angiogenesis and neurogenesis, remodeling of the neuro-vasculature, and reconstruction of neuronal cell networks [241]. Interestingly, Kazanis et al. reported in an experimental model of focal TBI that an accumulation of activated platelets surrounding the injury site correlated with a significant reduction in neuronal death, suggesting the presumable involvement of the platelet proteome in the repair process [217]. Using in vitro neural stem/progenitor cell (NSPC) culture, those authors confirmed the ability of HPL to enhance cell survival and protection against apoptosis. In agreement with such a protective ability of HPL, we recently evaluated the protective ability of HPL using an in vitro and two in vivo models of TBI. In vitro, we used a SH-SY5Y neuroblastoma model stimulated or not with HPL and found that the latter stimulation readily supported the maturation and neurite outgrowth of cells under a scratch lesion procedure [50]. Importantly, we also reported the benefits afforded by platelet lysate in a model of mild TBI resulting from a concussion and in an “in-house” developed model of penetrating TBI (cortical brain scratch or “CBS”). Animals were treated with one topical application of HPL to the wounded area followed by daily IN administration over 6 days. At the behavioral level, motor and memory functions were found to have improved by HPL in both models. At the molecular level, explaining behavioral motor improvements, HPL alleviated cortical neuroinflammation, oxidative stress, and synaptic loss. Interestingly, a non-hypothesis driven proteomic evaluation highlighted the involvement of some biological pathways such as the Wnt signaling pathway, nuclear factor-erythroid 2-related factor 2 (Nrf2), and fatty acid biosynthesis in the synergistic neuroprotective activity of the platelet preparations. These findings therefore correlate with the above-mentioned physiological roles of platelets and indicate the clinical relevance of platelet lysates in TBIs. Those studies also suggest that, in case of severe injury with brain access, HPL could be administered topically followed by continuous therapy by IN administration. Recent data indicate that in the mild TBI concussion model, IN administration of this HPL over three days is sufficient to counter-balance cortical inflammation [242]. The frequency and duration of IN administration to reach optimal recovery remain undefined, and further experimental exploration is needed.
Amyotrophic lateral sclerosis
The potential to use HPL trophic factors as a biotherapy against amyotrophic lateral sclerosis progression has been proposed [243], following encouraging results in an in vitro cell culture model by Gouel et al. using NSC-34, a mouse motor neuron-like cell line [64]. In that work, cells were intoxicated with staurosporine or menadione 1 h after HPL stimulation. In neurotoxic conditions, cell viability was found to be significantly higher with HPL treatment, an effect associated with the Akt pathway, known to be involved in cell growth, survival, proliferation, apoptosis, metabolism, and angiogenesis [64]. Another heat-treated HPL and its various fractions separated based on their molecular mass were confirmed to exert Akt-dependent anti-apoptotic and anti-ferroptotic actions in neuronal cell cultures [236]. The fraction with an apparent molecular mass below 3 kDa exerted a GPX4-dependent anti-ferroptotic action suggesting that it has a protective role that may complement those associated to the higher molecular mass neurotrophins present in the unfractionated HPL. Most importantly, the intracerebroventricular delivery of the HPL, and the IN administration of the fraction containing < 3 kDa molecules increased the lifespan of SOD1G86R mice used as model of ALS [236]. These encouraging data might be of crucial importance considering the rapid clinical deterioration of affected patients and the lack of effective treatments for ALS.
Expected quality and safety requirements for clinical translation: regulatory perspectives
Looking at current preclinical data, considerations should be given to the feasibility of bringing HPLs to the stage of clinical trials in neurological disorders. In that regard, developing safe and standardized HPLs for brain administration will necessitate a unique set of quality and safety requirements that can be somewhat more demanding than those needed for clinical-grade cell expansion and “low key” clinical applications in regenerative medicine.
Manufacturing process
The manufacturing process of clinical-grade platelet preparations is facilitated by the fact that PC units, used as starting materials, are regarded as medicines in many countries worldwide. PCs used to prepare HPLs should be collected from healthy blood donors screened by blood establishments following international requirements. In a context of the production of platelet preparations for brain administration, this is important as several neurodegenerative pathologies, such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and Huntington’s disease, may be linked to platelet dysfunctions [32].
To achieve an optimal standardization and consistency in the composition of HPLs, however, pooling of donations from at least 50 donors appears to be required based on experience generated in the production of HPL preparations for human cell propagation [36, 37]. Pooling, however, increases the statistical risk of the presence of infectious agents, most particularly viruses. Even though viral safety profiles of PC donations are currently very high, all infectious risks cannot be completely excluded by the measures in place in blood establishments for selecting and screening blood donors, or viral testing of donations. A low risk of window-phase donations for human immunodeficiency virus (HIV), hepatitis B virus (HBV), and HCV exists. Besides, some blood-borne viruses, like HAV, parvovirus B19, Dengue virus, Zika virus, West Nile virus, as well as unknown or emerging viruses, potentially including severe acute respiratory syndrome coronavirus-2 [SARS-CoV-2, also known as coronavirus disease 2019 (COVID-19)], are not routinely tested for in blood donors in most jurisdictions. Among those, several viruses, especially flaviviruses, are known to affect the brain and induce encephalitis [244–246]. In addition, the use of HPL-based therapeutic products in neuroprotection must also consider any theoretical risks of prion diseases and other transmissible spongiform encephalopathies [247]. In recent years, Alzheimer’s disease, Parkinson’s disease and many brain proteinopathies have been also considered as prion-like disorders [248]. Experimental models have explored protein seeding and spreading in prion-like disorders. Moreover, there are few evidences that seeds of prion-like proteins inoculate in the periphery may lead to brain diseases in different animal models [249–251]. Finally, there are several studies indicating that blood-derived materials and tissues from patients with different proteinopathies may contain prion-like proteins [252–254]. Altogether, such findings have highlighted new potential biological risks for blood-derived materials such as HPL that should be taken into consideration for translational developments.
The vast experience gained in the last three decades in ensuring the safety of pooled blood products can serve to guarantee that HPLs for brain administration can meet the most stringent safety criteria. Indeed, in addition to collecting platelets from healthy donors with no known risk factors and viral testing, PCs can be individually subjected to pathogen-reduction treatments by photo(chemical) inactivation, such as sporalen/ultraviolet A (UVA) and riboflavin/UVC [255–257], or short-wave UV light [258]. Such treatments are already licensed for PCs used for transfusion or under clinical evaluation. Lack of toxicity and maintenance of neuroprotective activity in neuronal cell models of one HPL made from psoralen/UVA-treated PCs were demonstrated [50, 53], as were the lack of toxicity or other negative effects on MSCs [259]. Additional measures and viral-reduction treatments of HPLs for brain administration can be considered based on experiences gained from the plasma fractionation and cell therapy industries. Such measures include testing the starting HPL pool to confirm the absence of nucleic acids of relevant viruses such as HIV, HBV, HCV, HAV, parvovirus B19 [260, 261], or emerging viruses as needed, and possible implementation of dedicated virus-inactivation or removal steps, such as solvent/detergent, gamma-irradiation, e-beam, or nanofiltration, as recently described for pooled HPLs used for ex vivo human cell propagation [39]. The above-mentioned findings related to prion transmission also highlight the importance of ensuring the health status of the blood donors, one cornerstone of transfusion safety. This also stresses the possible relevance of implementing prion removal procedures during HPL manufacture. Nanofiltration on filters of 35 nm, and preferably 20 or 15 nm, which efficiently removes viruses, has also been found in various experimental studies to remove prion infectivity from blood products [262–265]. Another pillar of the virus safety of HPLs is, as for any blood products, the need for a solid traceability system, ensured through auditing and inspections, so that immediate protective actions can be taken both ways when unexpected events are identified at the donor or patient sides [37].
Quality and safety criteria
The specific quality and safety criteria of HPLs for brain administration remain to be determined following further preclinical evaluations in animal models and eventual safety studies in humans. As for any parenteral products for human clinical use, bacterial sterility, and the absence of endotoxins (which can generate neuroinflammation) below a strict level for each batch need to be ensured. Each batch should meet specified limits for osmolality, pH, total protein content, and the presence of insoluble materials, as well as any potentially toxic chemicals used during the process of production to control their removal below acceptable levels.
Compared to applications to tissues like joints, muscles, and skin, administration to the brain, regardless of whether it is ICV, topical, or IN, is expected to require an HPL with a specific set of quality requirements, at least at the stage of process validation, with regard to the total protein content and removal of potentially toxic blood–brain proteins. As an example, one particular HPL was developed using a process that removed the plasma compartment to specifically decrease the bulk of unnecessary proteins and avoid a risk of protein overload in the cerebrospinal fluid [49]; this also avoids the presence of plasma-borne fibrinogen and coagulation factors, thereby limiting possible risks of neurotoxic fibrin deposition following ICV administration [266]. In addition, this preparation was subjected to heat treatment at 56 °C for 30 min that was shown to lower the risk of thrombin generation, and proteolytic and thrombogenic activities without altering its neuroprotective capacity [49, 68]. Dedicated inactivation or removal treatment effective against neurotoxic viruses, such as HCV, dengue, and Zika viruses, are particularly relevant.
Platelet lysates for brain administration: pending questions
Several questions will need to be addressed and resolved to translate a platelet lysate biotherapy into clinical practices.
One issue refers to the mode of administration to allow controlled delivery of the treatment into the brain. The above-mentioned preclinical studies in rodents indicate that platelet lysates can be administered directly to the brain by intracranial [69], intranasal [49, 51, 66, 67], topical [51] and/or intracerebroventricular [236] routes. These delivery modes intend to bypass, at least partially, the BBB to provide better targeting and control of the doses reaching the CNS. Direct brain delivery also avoids potential side effects associated with systemic administration. Intracranial or intracerebroventricular administrations are the best options currently available to ensure precise control of the dose of product administered to the brain. However, this mode of delivery is invasive and requests the implantation of a device allowing continuous or intermittent delivery [267]. Such procedure would be possibly attractive to patients with the most advanced cases or short pejorative outcomes, e.g., patients with amyotrophic lateral sclerosis or advanced stages of Alzheimer’s or Parkinson’s diseases, pending associated surgery remains possible. In addition of intracerebral delivery, an alternative non-invasive intranasal route of administration, assuming effective delivery procedures can be developed for human use [268], would be highly attractive for early-stage treatment of patients with moderate symptoms associated with neurodegenerative disorders or mild trauma. To our knowledge, the intravenous delivery of HPL has not been reported in animal models, potentially for three reasons: (a) high potential of immunological reactions of immunocompetent animals receiving a mixture of human proteins, thereby limiting the extent of scientific information from preclinical work; (b) risks of systemic uncontrolled adverse reactions; and (c) limited permeability of the BBB to intravenously administered active substances.
Defining the optimal dose and frequency of administration of HPL, depending upon pathologies and their severity, will require further preclinical studies and may warrant studies using non-human primates for optimal predictive assessment of what could occur in humans. With that regard, the ability to correlate the impact of administered doses with objective parameters based on brain imaging as a diagnostic biomarker, or blood biochemistry as biological biomarker will be valuable for treatment modalities.
There is little information on the possible risks linked to the long-term administration of platelet lysates into the brain by either direct or intra-nasal infusions. Even though no side effects are reported in animal models, even over close to 3 months of delivery [236], the complexity of the platelet lysate does not exclude the eventual occurrence of side effects. For instance, the presence of pro-coagulation and pro-inflammation molecules could eventually counterbalance the beneficial neuronal effects, leading to a detrimental impact on the health status of patients and worsening their neurological disorders. The potential presence of proteinopathy seeds might also warrant being evaluated. These potential risks re-emphasize the importance of developing HPLs with a specific set of quality requirements along the whole chain of production for optimal biochemical safety for brain administration.
The potential for host immune reactions against donors’ antigens when using non-autologous platelet lysates may not be excluded. However, based on the long-term clinical experience acquired with the use of allogeneic plasma and plasma protein products for transfusion, this risk appears reasonably unlikely provided the human platelet lysates protein solution is depleted of the antigen-bearing blood cell membranes by filtrations.
Conclusion and perspectives
There are objective scientific, medical, and industrial rationales supporting the evaluation of dedicated blood-derived HPL for treating neurological diseases affecting the CNS [32–34, 243]. Collected human PCs represent a valuable raw material for producing HPLs. Out of 117 million blood donations collected each year in the world [269, 270], only about 20% are currently used to isolate PCs for transfusions, indicating that excess source material is virtually available to produce HPLs for brain administration.
As HPLs dedicated to brain administration are a new therapeutic concept aiming at repositioning the traditional use of platelets for transfusion, a regulatory framework will need to be developed which considers mechanisms of action, dosing, modes of administration, and severity of the diseases. Communication with regulatory authorities will be needed particularly regarding criteria influencing the safety and efficacy considering the inherent complex biochemical compositions of HPL preparations, as is already the case of numerous therapeutic blood products. As for other blood-derived medicines, control and monitoring of the manufacturing process are key quality factors that should be scrutinized by regulatory authorities [167].
HPLs are attracting interest as a novel therapeutic approach in neuroprotective and neurorestorative medicine. Due to their origin from platelets, and not from recombinant engineering technologies, and due to the complexity of their proteomes, HPLs can trigger multiple molecular processes instrumental to neuroprotection and restoration. It will therefore be crucial to further unveil the biological functions triggered by HPL treatment and delineate impacts associated with source platelet materials and any process variations. Quality and safety requirements can be delineated for process and product validations by refined analytical tools (including proteomics) and cellular models relevant to the treatment of specific brain disorders. In conclusion, there are rational reasons to encourage further studies on the capacity of platelet extracts to exert beneficial effects in treating diseases of the CNS. This is further supported by the fact that PCs represent a source material available locally at the global level including in most LMIC, ensuring a domestic supply to treat global neurological disorders.
Acknowledgements
We express our appreciation to our colleagues conducting preclinical research on the use of HPL against neurological diseases in particular Professor David Devos and his team (Flore Gouel, Anne-Sophie Rolland, Kelly Timmerman) at the Lille Neuroscience and Cognition Center. The graphical abstract has been created with BioRender.com
Abbreviations
- BBB
Blood Brain barrier
- b-FGF
Basic fibroblast growth factor
- BMP
Bone morphogenetic protein
- CAT
Catalase
- CBS
Cortical brain scratch
- CCL3
CC-chemokine ligand 3
- CCI
Controlled cortical impact
- CD
Cluster of differentiation
- CP
Ceruloplasmin
- CNS
Central nervous system
- CXCL4
C-X-C motif chemokine ligand 4
- EGF
Epidermal growth factor
- ERK
Extracellular signal-regulated kinase
- EV
Extracellular vesicle
- FTD
Frontotemporal depression
- GF
Growth factor
- GMP
Good manufacturing practices
- GPx
Glutathione peroxidase
- GSK3β
Glycogen synthase kinase 3β
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HGF
Hepatocyte growth factor
- HPPL
Human platelet pellet lysate
- HPL
Human platelet lysate
- HUVEC
Human umbilical vein endothelial cells
- ICV
Intracerebroventricular
- IGF
Insulin-like growth factor
- IGF-BP3
IGF-binding protein 3
- IL
Interleukin
- IN
Intranasal
- LMIC
Low- and middle-income countries
- LPS
Lipopolysaccharide
- LUHMES
Lund human mesencephalic
- MANF
Mesencephalic astrocyte-derived neurotrophic factor
- M-CSF
Macrophage colony-stimulating factor
- MEK
Mitogen-activated protein kinase
- MIF
Macrophage migration inhibitory factor
- MIP
Macrophage inflammatory protein
- MPP +
1-Methyl-4-phenylpyridinium
- MPTP
1-Methyl-4-phrnyl-1,2,3,5-tetrahydropyridine
- MSC
Mesenchymal stromal cell
- MSP
Macrophage-stimulating protein
- NSPC
Neural stem/progenitor cell
- Nrf2
Nuclear factor-erythroid 2-related factor 2
- PC
Platelet concentrate
- pEVs
Platelet-extracellular vesicles
- PF4
Platelet factor 4
- PDGF
Platelet-derived growth factor
- PMCAO
Permanent distal middle cerebral artery occlusion
- PS
Phosphatidylserine
- ROS
Reactive oxygen species
- SGD
Subgranular zone
- SVZ
Subventricular zone
- TIMP
Tissue inhibitor of metalloproteinase
- SOD
Superoxide dismutase
- TF
Tissue factor
- TBI
Traumatic brain injury
- TDP-43
TAR DNA-binding protein-43
- TNF
Tumor necrosis factor
- TXN
Thioredoxin
Author contributions
All authors contributed to the writing of this manuscript and approved the final version.
Funding
We thank the Ministry of Science and Technology (MOST) of Taiwan (107-2314-B-038-and 110-2314-B-038-079), the National Health Research Institute of Taiwan (NHRI-EX110-10920EI), Taipei Medical University (TMU) Higher Education Sprout Project MoE (DP2-107-21121-01N 09) and PhD fellowship; Université de Lille, France (CABRI/MOBLILEX grant); Bilateral Orchid research project (MOST and French Association of Taiwan-Campus France; 108-2911-I-038-503); Taipei Medical University, International Collaborative Research Project Funding 108–3805-005-111; International Laboratory funding scheme supported by the University of Lille and TMU (NeuroTMULille); Inserm, CNRS, Université de Lille and CHU Lille (Programmes d’Investissements d’Avenir LabEx (excellence laboratory), DISTALZ (Development of Innovative Strategies for a Transdisciplinary approach to Alzheimer’s disease).
Availability of data and materials
Not applicable.
Declarations
Conflict of interest
TB is named as one of the inventors of patents applications by his academic institution, and is a founder of Invenis Biotherapies a start-up from University of Lille and Taipei Medical University.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
David Blum, Email: david.blum@inserm.fr.
Thierry Burnouf, Email: thburnouf@gmail.com, Email: thierry@tmu.edu.tw.
References
- 1.Muthuraju S, et al. The role of neuroinflammation in cellular damage in neurodegenerative diseases. Biomed Res Int. 2020;2020:9231452. doi: 10.1155/2020/9231452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh A, et al. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules. 2019;24(8):1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi H, Chang HY, Sang TK. Neuronal cell death mechanisms in major neurodegenerative diseases. Int J Mol Sci. 2018;19(10):3082. doi: 10.3390/ijms19103082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christidi F, et al. Social cognition dysfunctions in neurodegenerative diseases: neuroanatomical correlates and clinical implications. Behav Neurol. 2018;2018:1849794. doi: 10.1155/2018/1849794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dugger BN, Dickson DW. Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2017;9(7):a028035. doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 7.Mouhieddine TH, et al. Stem cells in neuroinjury and neurodegenerative disorders: challenges and future neurotherapeutic prospects. Neural Regen Res. 2014;9(9):901–906. doi: 10.4103/1673-5374.133129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martier R, Konstantinova P. Gene therapy for neurodegenerative diseases: slowing down the ticking clock. Front Neurosci. 2020 doi: 10.3389/fnins.2020.580179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parambi DGT, et al. Gene therapy approach with an emphasis on growth factors: theoretical and clinical outcomes in neurodegenerative diseases. Mol Neurobiol. 2022;59(1):191–233. doi: 10.1007/s12035-021-02555-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Hu Y, Ju D. Gene therapy for neurodegenerative disorders: advances, insights and prospects. Acta Pharm Sin B. 2020;10(8):1347–1359. doi: 10.1016/j.apsb.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed SL, Escayg A. Extracellular vesicles in the treatment of neurological disorders. Neurobiol Dis. 2021;157:105445. doi: 10.1016/j.nbd.2021.105445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nurden AT. The biology of the platelet with special reference to inflammation, wound healing and immunity. Front Biosci (Landmark Ed) 2018;23:726–751. doi: 10.2741/4613. [DOI] [PubMed] [Google Scholar]
- 13.Dukhinova M, et al. Platelets mediate protective neuroinflammation and promote neuronal plasticity at the site of neuronal injury. Brain Behav Immun. 2018;74:7–27. doi: 10.1016/j.bbi.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Rivera FJ, et al. Beyond clotting: a role of platelets in CNS repair? Front Cell Neurosci. 2016;9:511. doi: 10.3389/fncel.2015.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burnouf T, et al. Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. 2013;27(2):77–89. doi: 10.1016/j.blre.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 16.White JG, Michelson A. Platelet structure. Platelets. 2007;2:45–71. [Google Scholar]
- 17.Gremmel T, Frelinger III AL, Michelson AD (2016) Platelet physiology. In: Seminars in thrombosis and hemostasis. Thieme Medical Publishers [DOI] [PubMed]
- 18.Burnouf T, et al. Platelet microparticles: detection and assessment of their paradoxical functional roles in disease and regenerative medicine. Blood Rev. 2014;28(4):155–166. doi: 10.1016/j.blre.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Boilard E, Duchez AC, Brisson A. The diversity of platelet microparticles. Curr Opin Hematol. 2015;22(5):437–444. doi: 10.1097/MOH.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 20.Rapaport SI, Rao LVM. The tissue factor pathway: how it has become a “prima ballerina”. Thromb Haemost. 1995;74(07):007–017. [PubMed] [Google Scholar]
- 21.Norris LA. Blood coagulation. Best Pract Res Clin Obstet Gynaecol. 2003;17(3):369–383. doi: 10.1016/s1521-6934(03)00014-2. [DOI] [PubMed] [Google Scholar]
- 22.Schenone M, Furie BC, Furie B. The blood coagulation cascade. Curr Opin Hematol. 2004;11(4):272–277. doi: 10.1097/01.moh.0000130308.37353.d4. [DOI] [PubMed] [Google Scholar]
- 23.Davie EW. Biochemical and molecular aspects of the coagulation cascade. Thromb Haemost. 1995;74(07):001–006. [PubMed] [Google Scholar]
- 24.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27(8):1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 25.Dahlback B. Blood coagulation. Lancet. 2000;355(9215):1627–1632. doi: 10.1016/S0140-6736(00)02225-X. [DOI] [PubMed] [Google Scholar]
- 26.Nurden AT, et al. Platelets and wound healing. Front Biosci. 2008;13:3532–3548. doi: 10.2741/2947. [DOI] [PubMed] [Google Scholar]
- 27.Harrison P, Cramer EM. Platelet α-granules. Blood Rev. 1993;7(1):52–62. doi: 10.1016/0268-960x(93)90024-x. [DOI] [PubMed] [Google Scholar]
- 28.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23(4):177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canobbio I. Blood platelets: circulating mirrors of neurons? Res Pract Thromb Haemost. 2019;3(4):564–565. doi: 10.1002/rth2.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goubau C, et al. Regulated granule trafficking in platelets and neurons: a common molecular machinery. Eur J Paediatr Neurol. 2013;17(2):117–125. doi: 10.1016/j.ejpn.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Knusel B, et al. Selective and nonselective stimulation of central cholinergic and dopaminergic development in vitro by nerve growth factor, basic fibroblast growth factor, epidermal growth factor, insulin and the insulin-like growth factors I and II. J Neurosci. 1990;10(2):558. doi: 10.1523/JNEUROSCI.10-02-00558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leiter O, Walker TL. Platelets in neurodegenerative conditions-friend or foe? Front Immunol. 2020;11:747. doi: 10.3389/fimmu.2020.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burnouf T, Walker TL. The multifaceted role of platelets in mediating brain function. Blood. 2022 doi: 10.1182/blood.2022015970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leiter O, Walker TL. Platelets: the missing link between the blood and brain? Prog Neurobiol. 2019;183:101695. doi: 10.1016/j.pneurobio.2019.101695. [DOI] [PubMed] [Google Scholar]
- 35.Reed GL, Fitzgerald ML, Polgar J. Molecular mechanisms of platelet exocytosis: insights into the "secrete" life of thrombocytes. Blood. 2000;96(10):3334–3342. [PubMed] [Google Scholar]
- 36.Burnouf T, et al. Human platelet lysate: replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016;76:371–387. doi: 10.1016/j.biomaterials.2015.10.065. [DOI] [PubMed] [Google Scholar]
- 37.Schallmoser K, et al. Production and quality requirements of human platelet lysate: a position statement from the working party on cellular therapies of the international society of blood transfusion. Trends Biotechnol. 2020;38(1):13–23. doi: 10.1016/j.tibtech.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Schallmoser K, et al. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47(8):1436–1446. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 39.Barro L, et al. Human platelet lysates for human cell propagation. Platelets. 2021;32(2):152–162. doi: 10.1080/09537104.2020.1849602. [DOI] [PubMed] [Google Scholar]
- 40.Lischer M, et al. Human platelet lysate stimulated adipose stem cells exhibit strong neurotrophic potency for nerve tissue engineering applications. Regen Med. 2020;15(3):1399–1408. doi: 10.2217/rme-2020-0031. [DOI] [PubMed] [Google Scholar]
- 41.Palombella S, et al. Human platelet lysate as a potential clinical-translatable supplement to support the neurotrophic properties of human adipose-derived stem cells. Stem Cell Res Ther. 2020;11(1):1–14. doi: 10.1186/s13287-020-01949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh RP, et al. Quality assessment of platelet concentrates prepared by platelet rich plasma-platelet concentrate, buffy coat poor-platelet concentrate (BC-PC) and apheresis-PC methods. Asian J Transfus Sci. 2009;3(2):86. doi: 10.4103/0973-6247.53882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tynngård N. Preparation, storage and quality control of platelet concentrates. Transfus Apheres Sci. 2009;41(2):97–104. doi: 10.1016/j.transci.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Council of Europe - 20th Edition of the Guide to the preparation, use and quality assurance of blood components (2022) https://www.edqm.eu/en/blood-guide. C.o. Europe, Editor. Strasbourg, France
- 45.Shih DT, Burnouf T. Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion. N Biotechnol. 2015;32(1):199–211. doi: 10.1016/j.nbt.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burgstaler EA. Blood component collection by apheresis. J Clin Apheresis. 2006;21(2):142–151. doi: 10.1002/jca.20043. [DOI] [PubMed] [Google Scholar]
- 47.Strunk D, et al. International Forum on GMP-grade human platelet lysate for cell propagation: summary. Vox Sang. 2018;113(1):80–87. doi: 10.1111/vox.12593. [DOI] [PubMed] [Google Scholar]
- 48.Bieback K, et al. Gaps in the knowledge of human platelet lysate as a cell culture supplement for cell therapy: a joint publication from the AABB and the International Society for Cell & Gene Therapy. Cytotherapy. 2019;21(9):911–924. doi: 10.1016/j.jcyt.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Chou ML, et al. Tailor-made purified human platelet lysate concentrated in neurotrophins for treatment of Parkinson's disease. Biomaterials. 2017;142:77–89. doi: 10.1016/j.biomaterials.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 50.Nebie O, et al. Heat-treated human platelet pellet lysate modulates microglia activation, favors wound healing and promotes neuronal differentiation in vitro. Platelets. 2021;32(2):226–237. doi: 10.1080/09537104.2020.1732324. [DOI] [PubMed] [Google Scholar]
- 51.Nebie O, et al. Human platelet lysate biotherapy for traumatic brain injury: preclinical assessment. Brain. 2021;144(10):3142–3158. doi: 10.1093/brain/awab205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azuma H, et al. Platelet additive solution - electrolytes. Transfus Apher Sci. 2011;44(3):277–281. doi: 10.1016/j.transci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Nebie O, et al. The neuroprotective activity of heat-treated human platelet lysate biomaterials manufactured from outdated pathogen-reduced (amotosalen/UVA) platelet concentrates. J Biomed Sci. 2019;26(1):89. doi: 10.1186/s12929-019-0579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bieback K, et al. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27(9):2331–2341. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- 55.Doucet C, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205(2):228–236. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- 56.Strandberg G, et al. Standardizing the freeze-thaw preparation of growth factors from platelet lysate. Transfusion. 2017;57(4):1058–1065. doi: 10.1111/trf.13998. [DOI] [PubMed] [Google Scholar]
- 57.Bernardi M, et al. Production of human platelet lysate by use of ultrasound for ex vivo expansion of human bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2013;15(8):920–929. doi: 10.1016/j.jcyt.2013.01.219. [DOI] [PubMed] [Google Scholar]
- 58.Mojica-Henshaw MP, et al. Serum-converted platelet lysate can substitute for fetal bovine serum in human mesenchymal stromal cell cultures. Cytotherapy. 2013;15(12):1458–1468. doi: 10.1016/j.jcyt.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 59.Van Pham P, et al. Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem Cell Res Ther. 2013;4(4):91. doi: 10.1186/scrt277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kocaoemer A, et al. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 2007;25(5):1270–1278. doi: 10.1634/stemcells.2006-0627. [DOI] [PubMed] [Google Scholar]
- 61.Shih DT, Burnouf T. Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion. New Biotechnol. 2015;32(1):199–211. doi: 10.1016/j.nbt.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schallmoser K, et al. Platelet-derived growth factors for GMP-compliant propagation of mesenchymal stromal cells. Biomed Mater Eng. 2009;19(4–5):271–276. doi: 10.3233/BME-2009-0591. [DOI] [PubMed] [Google Scholar]
- 63.Reinisch A, et al. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood. 2015;125(2):249–260. doi: 10.1182/blood-2014-04-572255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gouel F, et al. The protective effect of human platelet lysate in models of neurodegenerative disease: involvement of the Akt and MEK pathways. J Tissue Eng Regen Med. 2017;11(11):3236–3240. doi: 10.1002/term.2222. [DOI] [PubMed] [Google Scholar]
- 65.Anitua E, et al. Plasma rich in growth factors (PRGF-Endoret) reduces neuropathologic hallmarks and improves cognitive functions in an Alzheimer's disease mouse model. Neurobiol Aging. 2014;35(7):1582–1595. doi: 10.1016/j.neurobiolaging.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 66.Anitua E, et al. Intranasal delivery of plasma and platelet growth factors using PRGF-endoret system enhances neurogenesis in a mouse model of Alzheimer’s disease. PLoS ONE. 2013;8(9):e73118. doi: 10.1371/journal.pone.0073118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anitua E, et al. Intranasal PRGF-Endoret enhances neuronal survival and attenuates NF-κB-dependent inflammation process in a mouse model of Parkinson's disease. J Control Release. 2015;203:170–180. doi: 10.1016/j.jconrel.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 68.Delila L, et al. Extensive characterization of the composition and functional activities of five preparations of human platelet lysates for dedicated clinical uses. Platelets. 2021;32(2):259–272. doi: 10.1080/09537104.2020.1849603. [DOI] [PubMed] [Google Scholar]
- 69.Hayon Y, et al. Platelet lysates stimulate angiogenesis, neurogenesis and neuroprotection after stroke. Thromb Haemost. 2013;110(2):323–330. doi: 10.1160/TH12-11-0875. [DOI] [PubMed] [Google Scholar]
- 70.Horn P, et al. Impact of individual platelet lysates on isolation and growth of human mesenchymal stromal cells. Cytotherapy. 2010;12(7):888–898. doi: 10.3109/14653249.2010.501788. [DOI] [PubMed] [Google Scholar]
- 71.García A, et al. Extensive analysis of the human platelet proteome by two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2004;4(3):656–668. doi: 10.1002/pmic.200300665. [DOI] [PubMed] [Google Scholar]
- 72.Crespo-Diaz R, et al. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant. 2011;20(6):797–812. doi: 10.3727/096368910X543376. [DOI] [PubMed] [Google Scholar]
- 73.Senzel L, Gnatenko DV, Bahou WF. The platelet proteome. Curr Opin Hematol. 2009;16(5):329–333. doi: 10.1097/MOH.0b013e32832e9dc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boyanova D, et al. PlateletWeb: a systems biologic analysis of signaling networks in human platelets. Blood. 2012;119(3):e22–e34. doi: 10.1182/blood-2011-10-387308. [DOI] [PubMed] [Google Scholar]
- 75.Ahmed S, Reynolds BA, Weiss S. BDNF enhances the differentiation but not the survival of CNS stem cell-derived neuronal precursors. J Neurosci. 1995;15(8):5765–5778. doi: 10.1523/JNEUROSCI.15-08-05765.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen BY, et al. Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/β-catenin signaling pathway. J Neurosci Res. 2013;91(1):30–41. doi: 10.1002/jnr.23138. [DOI] [PubMed] [Google Scholar]
- 77.Zagrebelsky M, Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology. 2014;76:628–638. doi: 10.1016/j.neuropharm.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 78.Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4(11):1093–1101. doi: 10.1038/nn735. [DOI] [PubMed] [Google Scholar]
- 79.Scharfman H, et al. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192(2):348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 80.Benraiss A, et al. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21(17):6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gonzalez-Perez O, Quiñones-Hinojosa A. Dose-dependent effect of EGF on migration and differentiation of adult subventricular zone astrocytes. Glia. 2010;58(8):975–983. doi: 10.1002/glia.20979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cooke MJ, et al. Controlled epi-cortical delivery of epidermal growth factor for the stimulation of endogenous neural stem cell proliferation in stroke-injured brain. Biomaterials. 2011;32(24):5688–5697. doi: 10.1016/j.biomaterials.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 83.Kolb B, et al. Growth factor-stimulated generation of new cortical tissue and functional recovery after stroke damage to the motor cortex of rats. J Cereb Blood Flow Metab. 2007;27(5):983–997. doi: 10.1038/sj.jcbfm.9600402. [DOI] [PubMed] [Google Scholar]
- 84.Teramoto T, et al. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J Clin Investig. 2003;111(8):1125–1132. doi: 10.1172/JCI17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dayer AG, et al. Expression of FGF-2 in neural progenitor cells enhances their potential for cellular brain repair in the rodent cortex. Brain. 2007;130(11):2962–2976. doi: 10.1093/brain/awm200. [DOI] [PubMed] [Google Scholar]
- 86.Kalluri HSG, Dempsey RJ. Growth factors, stem cells, and stroke. Neurosurgical Focus FOC. 2008;24(3–4):E14. doi: 10.3171/FOC/2008/24/3-4/E13. [DOI] [PubMed] [Google Scholar]
- 87.Jungnickel J, et al. Faster nerve regeneration after sciatic nerve injury in mice over-expressing basic fibroblast growth factor. J Neurobiol. 2006;66(9):940–948. doi: 10.1002/neu.20265. [DOI] [PubMed] [Google Scholar]
- 88.Endres M, et al. Neuroprotective effects of gelsolin during murine stroke. J Clin Investig. 1999;103(3):347–354. doi: 10.1172/JCI4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohtsu M, et al. Inhibition of apoptosis by the actin-regulatory protein gelsolin. EMBO J. 1997;16(15):4650–4656. doi: 10.1093/emboj/16.15.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wong V, et al. Hepatocyte growth factor promotes motor neuron survival and synergizes with ciliary neurotrophic factor. J Biol Chem. 1997;272(8):5187–5191. doi: 10.1074/jbc.272.8.5187. [DOI] [PubMed] [Google Scholar]
- 91.Maina F, et al. Multiple roles for hepatocyte growth factor in sympathetic neuron development. Neuron. 1998;20(5):835–846. doi: 10.1016/s0896-6273(00)80466-3. [DOI] [PubMed] [Google Scholar]
- 92.Aberg MA, et al. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20(8):2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Breese CR, et al. Expression of insulin-like growth factor-1 (IGF-1) and IGF-binding protein 2 (IGF-BP2) in the hippocampus following cytotoxic lesion of the dentate gyrus. J Compara Neurol. 1996;369(3):388–404. doi: 10.1002/(SICI)1096-9861(19960603)369:3<388::AID-CNE5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 94.Sortino MA, Canonico PL. Neuroprotective effect of insulin-like growth factor I in immortalized hypothalamic cells. Endocrinology. 1996;137(4):1418–1422. doi: 10.1210/endo.137.4.8625919. [DOI] [PubMed] [Google Scholar]
- 95.Hollis ER, II, et al. IGF-I gene delivery promotes corticospinal neuronal survival but not regeneration after adult CNS injury. Exp Neurol. 2009;215(1):53–59. doi: 10.1016/j.expneurol.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nonaka M, Fukuda M. Galectin-1 for neuroprotection? Immunity. 2012;37(2):187–189. doi: 10.1016/j.immuni.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 97.Starossom SC, et al. Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity. 2012;37(2):249–263. doi: 10.1016/j.immuni.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sasaki T, et al. Galectin-1 induces astrocyte differentiation, which leads to production of brain-derived neurotrophic factor. Glycobiology. 2004;14(4):357–363. doi: 10.1093/glycob/cwh043. [DOI] [PubMed] [Google Scholar]
- 99.Petrova PS, et al. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci. 2003;20(2):173–187. doi: 10.1385/jmn:20:2:173. [DOI] [PubMed] [Google Scholar]
- 100.Voutilainen MH, et al. Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson's disease. J Neurosci. 2009;29(30):9651–9659. doi: 10.1523/JNEUROSCI.0833-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hao F, et al. Long-term protective effects of AAV9-mesencephalic astrocyte-derived neurotrophic factor gene transfer in parkinsonian rats. Exp Neurol. 2017;291:120–133. doi: 10.1016/j.expneurol.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 102.Zhang J, et al. Nrf2-mediated neuroprotection by MANF against 6-OHDA-induced cell damage via PI3K/AKT/GSK3β pathway. Exp Gerontol. 2017;100:77–86. doi: 10.1016/j.exger.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 103.Huang J, et al. Mesencephalic astrocyte-derived neurotrophic factor reduces cell apoptosis via upregulating GRP78 in SH-SY5Y cells. Cell Biol Int. 2016;40(7):803–811. doi: 10.1002/cbin.10621. [DOI] [PubMed] [Google Scholar]
- 104.Zhang J, et al. Mesencephalic astrocyte-derived neurotrophic factor alleviated 6-OHDA-induced cell damage via ROS-AMPK/mTOR mediated autophagic inhibition. Exp Gerontol. 2017;89:45–56. doi: 10.1016/j.exger.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 105.Kimura I, et al. Neudesin, a secreted factor, promotes neural cell proliferation and neuronal differentiation in mouse neural precursor cells. J Neurosci Res. 2006;83(8):1415–1424. doi: 10.1002/jnr.20849. [DOI] [PubMed] [Google Scholar]
- 106.Novais A, et al. Neudesin is involved in anxiety behavior: structural and neurochemical correlates. Front Behav Neurosci. 2013;7:119–119. doi: 10.3389/fnbeh.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Erlandsson A, Enarsson M, Forsberg-Nilsson K. Immature neurons from CNS stem cells proliferate in response to platelet-derived growth factor. J Neurosci. 2001;21(10):3483–3491. doi: 10.1523/JNEUROSCI.21-10-03483.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gruber HE, Norton HJ, Hanley EN., Jr Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine. 2000;25(17):2153–2157. doi: 10.1097/00007632-200009010-00002. [DOI] [PubMed] [Google Scholar]
- 109.Chen H, et al. Molecular mechanism of platelet-derived growth factor (PDGF)-BB-mediated protection against MPP+ toxicity in SH-SY5Y cells. J Mol Neurosci. 2021;71(6):1131–1143. doi: 10.1007/s12031-020-01735-0. [DOI] [PubMed] [Google Scholar]
- 110.Zachrisson O, et al. Restorative effects of platelet derived growth factor-BB in rodent models of Parkinson's disease. J Parkinsons Dis. 2011;1:49–63. doi: 10.3233/JPD-2011-0003. [DOI] [PubMed] [Google Scholar]
- 111.Paul G, et al. Safety and tolerability of intracerebroventricular PDGF-BB in Parkinson’s disease patients. J Clin Investig. 2015;125(3):1339–1346. doi: 10.1172/JCI79635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leiter O, et al. Exercise-induced activated platelets increase adult hippocampal precursor proliferation and promote neuronal differentiation. Stem Cell Reports. 2019;12:667–679. doi: 10.1016/j.stemcr.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roussa E, et al. Transforming growth factor β is required for differentiation of mouse mesencephalic progenitors into dopaminergic neurons in vitro and in vivo: ectopic induction in dorsal mesencephalon. Stem Cells. 2006;24(9):2120–2129. doi: 10.1634/stemcells.2005-0514. [DOI] [PubMed] [Google Scholar]
- 114.Guo D, et al. VEGF stimulated the angiogenesis by promoting the mitochondrial functions. Oncotarget. 2017;8(44):77020–77027. doi: 10.18632/oncotarget.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Storkebaum E, et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8(1):85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 116.Lambrechts D, et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 2003;34(4):383–394. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- 117.Oosthuyse B, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28(2):131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 118.Svensson B, et al. Vascular endothelial growth factor protects cultured rat hippocampal neurons against hypoxic injury via an antiexcitotoxic, caspase-independent mechanism. J Cereb Blood Flow Metab. 2002;22(10):1170–1175. doi: 10.1097/01.wcb.0000037988.07114.98. [DOI] [PubMed] [Google Scholar]
- 119.Matsuzaki H, et al. Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: signal transduction cascades. FASEB J. 2001;15(7):1218–1220. [PubMed] [Google Scholar]
- 120.de Jager CA, et al. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int J Geriatr Psychiatry. 2012;27(6):592–600. doi: 10.1002/gps.2758. [DOI] [PubMed] [Google Scholar]
- 121.Walker JG, et al. Oral folic acid and vitamin B-12 supplementation to prevent cognitive decline in community-dwelling older adults with depressive symptoms—the Beyond Ageing Project: a randomized controlled trial. Am J Clin Nutr. 2011;95(1):194–203. doi: 10.3945/ajcn.110.007799. [DOI] [PubMed] [Google Scholar]
- 122.Tanaka E, North RA. Actions of 5-hydroxytryptamine on neurons of the rat cingulate cortex. J Neurophysiol. 1993;69(5):1749–1757. doi: 10.1152/jn.1993.69.5.1749. [DOI] [PubMed] [Google Scholar]
- 123.Tripathy D, Thirumangalakudi L, Grammas P. RANTES upregulation in the Alzheimer's disease brain: a possible neuroprotective role. Neurobiol Aging. 2010;31(1):8–16. doi: 10.1016/j.neurobiolaging.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tokami H, et al. RANTES has a potential to play a neuroprotective role in an autocrine/paracrine manner after ischemic stroke. Brain Res. 2013;1517:122–132. doi: 10.1016/j.brainres.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 125.Ajoy R, et al. CCL5 promotion of bioenergy metabolism is crucial for hippocampal synapse complex and memory formation. Mol Psychiatry. 2021 doi: 10.1038/s41380-021-01103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Musante V, et al. RANTES modulates the release of glutamate in human neocortex. J Neurosci. 2008;28(47):12231–12240. doi: 10.1523/JNEUROSCI.3212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ho M-H, et al. CCL5 via GPX1 activation protects hippocampal memory function after mild traumatic brain injury. Redox Biol. 2021;46:102067. doi: 10.1016/j.redox.2021.102067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li S, et al. Macrophage migration inhibitory factor mediates neuroprotective effects by regulating inflammation, apoptosis and autophagy in Parkinson's disease. Neuroscience. 2019;416:50–62. doi: 10.1016/j.neuroscience.2019.05.052. [DOI] [PubMed] [Google Scholar]
- 129.Baker K, et al. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther. 1998;284(1):215–221. [PubMed] [Google Scholar]
- 130.Wang T, et al. Protective effect of the SOD/catalase mimetic MnTMPyP on inflammation-mediated dopaminergic neurodegeneration in mesencephalic neuronal-glial cultures. J Neuroimmunol. 2004;147(1):68–72. doi: 10.1016/j.jneuroim.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 131.Hineno A, et al. Ceruloplasmin protects against rotenone-induced oxidative stress and neurotoxicity. Neurochem Res. 2011;36(11):2127–2135. doi: 10.1007/s11064-011-0537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao YS, et al. Ceruloplasmin, a potential therapeutic agent for Alzheimer's disease. Antioxid Redox Signal. 2018;28(14):1323–1337. doi: 10.1089/ars.2016.6883. [DOI] [PubMed] [Google Scholar]
- 133.Ayton S, et al. Ceruloplasmin and β-amyloid precursor protein confer neuroprotection in traumatic brain injury and lower neuronal iron. Free Radic Biol Med. 2014;69:331–337. doi: 10.1016/j.freeradbiomed.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 134.Bhowmick D, et al. Highly efficient glutathione peroxidase and peroxiredoxin mimetics protect mammalian cells against oxidative damage. Angew Chem Int Ed. 2015;54(29):8449–8453. doi: 10.1002/anie.201502430. [DOI] [PubMed] [Google Scholar]
- 135.Mason RP, et al. Glutathione peroxidase activity is neuroprotective in models of Huntington's disease. Nat Genet. 2013;45(10):1249–1254. doi: 10.1038/ng.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liao R, Wood TR, Nance E. Superoxide dismutase reduces monosodium glutamate-induced injury in an organotypic whole hemisphere brain slice model of excitotoxicity. J Biol Eng. 2020;14:3. doi: 10.1186/s13036-020-0226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huang H-F, et al. Neuroprotection by manganese superoxide dismutase (MnSOD) mimics: antioxidant effect and oxidative stress regulation in acute experimental stroke. CNS Neurosci Ther. 2012;18(10):811–818. doi: 10.1111/j.1755-5949.2012.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bai J, et al. Critical roles of thioredoxin in nerve growth factor-mediated signal transduction and neurite outgrowth in PC12 cells. J Neurosci. 2003;23(2):503–509. doi: 10.1523/JNEUROSCI.23-02-00503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Landino L, Skreslet T, Alston J. Modulation of microtubule assembly kinetics by the thioredoxin reductase system. J Biol Chem. 2004;279(33):35–101. doi: 10.1074/jbc.M405471200. [DOI] [PubMed] [Google Scholar]
- 140.Takagi Y, et al. Expression and distribution of redox regulatory protein, thioredoxin during transient focal brain ischemia in the rat. Neurosci Lett. 1998;251(1):25–28. doi: 10.1016/s0304-3940(98)00492-3. [DOI] [PubMed] [Google Scholar]
- 141.Wiesel P, et al. Thioredoxin facilitates the induction of heme oxygenase-1 in response to inflammatory mediators*. J Biol Chem. 2000;275(32):24840–24846. doi: 10.1074/jbc.M000835200. [DOI] [PubMed] [Google Scholar]
- 142.Yang Y, et al. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm (−/−) knockout mouse: novel model system for a severely compromised oxidative stress response. J Biol Chem. 2002;277(51):49446–49452. doi: 10.1074/jbc.M209372200. [DOI] [PubMed] [Google Scholar]
- 143.Föller M, et al. Functional significance of glutamate–cysteine ligase modifier for erythrocyte survival in vitro and in vivo. Cell Death Differ. 2013;20(10):1350–1358. doi: 10.1038/cdd.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Knight BE, et al. TIMP-1 attenuates the development of inflammatory pain through MMP-dependent and receptor-mediated cell signaling mechanisms. Front Mol Neurosci. 2019 doi: 10.3389/fnmol.2019.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dhar A, et al. Novel role of TGF-β in differential astrocyte-TIMP-1 regulation: Implications for HIV-1-dementia and neuroinflammation. J Neurosci Res. 2006;83(7):1271–1280. doi: 10.1002/jnr.20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang B, et al. Different expression of tissue inhibitor of metalloproteinase family members in rat dorsal root ganglia and their changes after peripheral nerve injury. Neuroscience. 2011;193:421–428. doi: 10.1016/j.neuroscience.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 147.Ji R-R, et al. Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol Sci. 2009;30(7):336–340. doi: 10.1016/j.tips.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Claycomb KI, et al. Astrocyte regulation of CNS inflammation and remyelination. Brain Sci. 2013;3(3):1109–1127. doi: 10.3390/brainsci3031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moore CS, et al. Astrocytic tissue inhibitor of metalloproteinase-1 (TIMP-1) promotes oligodendrocyte differentiation and enhances CNS myelination. J Neurosci. 2011;31(16):6247–6254. doi: 10.1523/JNEUROSCI.5474-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Woodward EA, et al. The anti-inflammatory effects of interleukin-4 are not mediated by suppressor of cytokine signalling-1 (SOCS1) Immunology. 2010;131(1):118–127. doi: 10.1111/j.1365-2567.2010.03281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hayon Y, et al. Platelet microparticles induce angiogenesis and neurogenesis after cerebral ischemia. Curr Neurovasc Res. 2012;9(3):185–192. doi: 10.2174/156720212801619018. [DOI] [PubMed] [Google Scholar]
- 152.Laffont B, et al. Platelet microparticles reprogram macrophage gene expression and function. Thromb Haemost. 2016;115(2):311–323. doi: 10.1160/TH15-05-0389. [DOI] [PubMed] [Google Scholar]
- 153.Viau S, et al. A highly standardized and characterized human platelet lysate for efficient and reproducible expansion of human bone marrow mesenchymal stromal cells. Cytotherapy. 2019;21(7):738–754. doi: 10.1016/j.jcyt.2019.04.053. [DOI] [PubMed] [Google Scholar]
- 154.Shanbhag S, et al. Influence of platelet storage time on human platelet lysates and platelet lysate-expanded mesenchymal stromal cells for bone tissue engineering. Stem Cell Res Ther. 2020;11(1):351. doi: 10.1186/s13287-020-01863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Westerweel PE, et al. RANTES is required for ischaemia-induced angiogenesis, which may hamper RANTES-targeted anti-atherosclerotic therapy. Thromb Haemost. 2008;99(4):794–795. doi: 10.1160/TH07-10-0628. [DOI] [PubMed] [Google Scholar]
- 156.Patel JR, et al. CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc Natl Acad Sci USA. 2010;107(24):11062–11067. doi: 10.1073/pnas.1006301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jaerve A, Müller HW. Chemokines in CNS injury and repair. Cell Tissue Res. 2012;349(1):229–248. doi: 10.1007/s00441-012-1427-3. [DOI] [PubMed] [Google Scholar]
- 158.Miron VE, Franklin RJ. Macrophages and CNS remyelination. J Neurochem. 2014;130(2):165–171. doi: 10.1111/jnc.12705. [DOI] [PubMed] [Google Scholar]
- 159.Moore CS, Crocker SJ. An alternate perspective on the roles of TIMPs and MMPs in pathology. Am J Pathol. 2012;180(1):12–16. doi: 10.1016/j.ajpath.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 160.Birk J, et al. Endoplasmic reticulum: reduced and oxidized glutathione revisited. J Cell Sci. 2013;126(Pt 7):1604–1617. doi: 10.1242/jcs.117218. [DOI] [PubMed] [Google Scholar]
- 161.Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016;15(1):71–71. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pavanetto M, et al. Regulation of serotonin transport in human platelets by tyrosine kinase Syk. Cell Physiol Biochem. 2011;27(2):139–148. doi: 10.1159/000325216. [DOI] [PubMed] [Google Scholar]
- 163.Johnson J, et al. Prospective therapeutic applications of platelet extracellular vesicles. Trends Biotechnol. 2021;39(6):598–612. doi: 10.1016/j.tibtech.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 164.Gasecka A, Nieuwland R, Siljander PR-M. Platelets. Elsevier; 2019. Platelet-derived extracellular vesicles; pp. 401–416. [Google Scholar]
- 165.Thery C, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lotvall J, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Agrahari V, et al. Extracellular microvesicles as new industrial therapeutic frontiers. Trends Biotechnol. 2019;37(7):707–729. doi: 10.1016/j.tibtech.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 168.Kao C-Y, Papoutsakis ET. Extracellular vesicles: exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr Opin Biotechnol. 2019;60:89–98. doi: 10.1016/j.copbio.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 169.Mathieu M, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 170.Baek G, et al. Mesenchymal stem cell-derived extracellular vesicles as therapeutics and as a drug delivery platform. Stem Cells Transl Med. 2019;8(9):880–886. doi: 10.1002/sctm.18-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hall J, et al. Delivery of therapeutic proteins via extracellular vesicles: review and potential treatments for Parkinson's disease, Glioma, and Schwannoma. Cell Mol Neurobiol. 2016;36(3):417–427. doi: 10.1007/s10571-015-0309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Haney MJ, et al. TPP1 delivery to lysosomes with extracellular vesicles and their enhanced brain distribution in the animal model of batten disease. Adv Healthc Mater. 2019;8(11):e1801271. doi: 10.1002/adhm.201801271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Losurdo M, et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer's disease. Stem Cells Transl Med. 2020;9(9):1068–1084. doi: 10.1002/sctm.19-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xin D, et al. Mesenchymal stromal cell-derived extracellular vesicles modulate microglia/macrophage polarization and protect the brain against hypoxia-ischemic injury in neonatal mice by targeting delivery of miR-21a-5p. Acta Biomater. 2020;113:597–613. doi: 10.1016/j.actbio.2020.06.037. [DOI] [PubMed] [Google Scholar]
- 176.Liao K, et al. Intranasal delivery of lincRNA-Cox2 siRNA loaded extracellular vesicles decreases lipopolysaccharide-induced microglial proliferation in mice. J Neuroimmune Pharmacol. 2020;15(3):390–399. doi: 10.1007/s11481-019-09864-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rufino-Ramos D, et al. Extracellular vesicles: novel promising delivery systems for therapy of brain diseases. J Control Release. 2017;262:247–258. doi: 10.1016/j.jconrel.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 178.Jiang XC, Gao JQ. Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm. 2017;521(1–2):167–175. doi: 10.1016/j.ijpharm.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 179.Agrahari V, et al. Nanoformulation properties, characterization, and behavior in complex biological matrices: challenges and opportunities for brain-targeted drug delivery applications and enhanced translational potential. Adv Drug Deliv Rev. 2019;148:146–180. doi: 10.1016/j.addr.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 180.Johnsen KB, et al. A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta. 2014;1846(1):75–87. doi: 10.1016/j.bbcan.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 181.Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Qu M, et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson's disease. J Control Release. 2018;287:156–166. doi: 10.1016/j.jconrel.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 183.Yuan D, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. doi: 10.1016/j.biomaterials.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang T, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32(6):2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rene CA, Parks RJ. Delivery of therapeutic agents to the central nervous system and the promise of extracellular vesicles. Pharmaceutics. 2021;13(4):492. doi: 10.3390/pharmaceutics13040492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stanimirovic DB, Sandhu JK, Costain WJ. Emerging technologies for delivery of biotherapeutics and gene therapy across the blood-brain barrier. BioDrugs. 2018;32(6):547–559. doi: 10.1007/s40259-018-0309-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Haney MJ, et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Marcoux G, et al. Platelet-derived extracellular vesicles convey mitochondrial DAMPs in platelet concentrates and their levels are associated with adverse reactions. Transfusion. 2019;59(7):2403–2414. doi: 10.1111/trf.15300. [DOI] [PubMed] [Google Scholar]
- 189.Black A, et al. Analysis of platelet-derived extracellular vesicles in plateletpheresis concentrates: a multicenter study. Transfusion. 2017;57(6):1459–1469. doi: 10.1111/trf.14109. [DOI] [PubMed] [Google Scholar]
- 190.Hermida-Nogueira L, et al. Proteomic analysis of extracellular vesicles derived from platelet concentrates treated with Mirasol® identifies biomarkers of platelet storage lesion. J Proteomics. 2020;210:103529. doi: 10.1016/j.jprot.2019.103529. [DOI] [PubMed] [Google Scholar]
- 191.Marcoux G, et al. Microparticle and mitochondrial release during extended storage of different types of platelet concentrates. Platelets. 2017;28(3):272–280. doi: 10.1080/09537104.2016.1218455. [DOI] [PubMed] [Google Scholar]
- 192.Berckmans RJ, et al. Extracellular vesicles and coagulation in blood from healthy humans revisited. J Extracell Vesicles. 2019;8(1):1688936. doi: 10.1080/20013078.2019.1688936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Arraud N, et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12(5):614–627. doi: 10.1111/jth.12554. [DOI] [PubMed] [Google Scholar]
- 194.Puhm F, Boilard E, Machlus KR. Platelet extracellular vesicles: beyond the blood. Arterioscler Thromb Vasc Biol. 2021;41(1):87–96. doi: 10.1161/ATVBAHA.120.314644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sung PS, Huang TF, Hsieh SL. Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2. Nat Commun. 2019;10(1):2402. doi: 10.1038/s41467-019-10360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Aatonen MT, et al. Isolation and characterization of platelet-derived extracellular vesicles. J Extracell Vesicles. 2014;3:24692. doi: 10.3402/jev.v3.24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wu YW, et al. Clinical-grade cryopreserved doxorubicin-loaded platelets: role of cancer cells and platelet extracellular vesicles activation loop. J Biomed Sci. 2020;27(1):45. doi: 10.1186/s12929-020-00633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kerris EWJ, et al. Platelets and platelet extracellular vesicles in hemostasis and sepsis. J Investig Med. 2020;68(4):813–820. doi: 10.1136/jim-2019-001195. [DOI] [PubMed] [Google Scholar]
- 199.Sinauridze EI, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97(3):425–434. [PubMed] [Google Scholar]
- 200.Słomka A, et al. Large extracellular vesicles: have we found the holy grail of inflammation? Front Immunol. 2018;9:2723. doi: 10.3389/fimmu.2018.02723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sadallah S, et al. Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. J Immunol. 2011;186(11):6543–6552. doi: 10.4049/jimmunol.1002788. [DOI] [PubMed] [Google Scholar]
- 202.Ceroi A, et al. The anti-inflammatory effects of platelet-derived microparticles in human plasmacytoid dendritic cells involve liver X receptor activation. Haematologica. 2016;101(3):e72–e76. doi: 10.3324/haematol.2015.135459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Obeid S, et al. NanoBioAnalytical characterization of extracellular vesicles in 75-nm nanofiltered human plasma for transfusion: a tool to improve transfusion safety. Nanomedicine. 2019;20:101977. doi: 10.1016/j.nano.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 204.Lin HC, et al. Platelet-derived microparticles trigger THP-1 monocytic cell aggregation and release of pro-coagulant tissue factor-expressing microparticles in vitro. Transfus Apher Sci. 2015;53(2):246–252. doi: 10.1016/j.transci.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 205.Boilard E. Extracellular vesicles and their content in bioactive lipid mediators: more than a sack of microRNA. J Lipid Res. 2018;59(11):2037–2046. doi: 10.1194/jlr.R084640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Melki I, et al. Platelet microvesicles in health and disease. Platelets. 2017;28(3):214–221. doi: 10.1080/09537104.2016.1265924. [DOI] [PubMed] [Google Scholar]
- 207.Kim HK, et al. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004;124(3):376–384. doi: 10.1046/j.1365-2141.2003.04773.x. [DOI] [PubMed] [Google Scholar]
- 208.Brill A, et al. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67(1):30–38. doi: 10.1016/j.cardiores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 209.Nabiuni M, Shokohi R, Moghaddam P. CSF protein contents and their roles in brain development. Zahedan J Res Med Sci. 2015;17(9):e1042. [Google Scholar]
- 210.Larpthaveesarp A, Ferriero DM, Gonzalez FF. Growth factors for the treatment of ischemic brain injury (growth factor treatment) Brain Sci. 2015;5(2):165–177. doi: 10.3390/brainsci5020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kim DH, et al. Prevention of apoptotic but not necrotic cell death following neuronal injury by neurotrophins signaling through the tyrosine kinase receptor. J Neurosurg. 2004;100(1):79–87. doi: 10.3171/jns.2004.100.1.0079. [DOI] [PubMed] [Google Scholar]
- 212.Pietz K, et al. Protective effect of platelet-derived growth factor against 6-hydroxydopamine-induced lesion of rat dopaminergic neurons in culture. Neurosci Lett. 1996;204(1–2):101–104. doi: 10.1016/0304-3940(96)12326-0. [DOI] [PubMed] [Google Scholar]
- 213.Nikkhah G, et al. Platelet-derived growth factor promotes survival of rat and human mesencephalic dopaminergic neurons in culture. Exp Brain Res. 1993;92(3):516–523. doi: 10.1007/BF00229041. [DOI] [PubMed] [Google Scholar]
- 214.Perez-Saad H, et al. Neuroprotective effect of epidermal growth factor in experimental acrylamide neuropathy: an electrophysiological approach. J Peripher Nerv Syst. 2017;22(2):106–111. doi: 10.1111/jns.12214. [DOI] [PubMed] [Google Scholar]
- 215.Vezzani A. VEGF as a target for neuroprotection. Epilepsy currents. 2008;8(5):135–137. doi: 10.1111/j.1535-7511.2008.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nam J, Koppinen TK, Voutilainen MH. MANF is neuroprotective in early stages of EAE, and elevated in spinal white matter by treatment with dexamethasone. Front Cell Neurosci. 2021 doi: 10.3389/fncel.2021.640084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kazanis I, et al. Lesion-induced accumulation of platelets promotes survival of adult neural stem / progenitor cells. Exp Neurol. 2015;269:75–89. doi: 10.1016/j.expneurol.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 218.Shetty AK, Turner DA. In vitro survival and differentiation of neurons derived from epidermal growth factor-responsive postnatal hippocampal stem cells: inducing effects of brain-derived neurotrophic factor. J Neurobiol. 1998;35(4):395–425. doi: 10.1002/(sici)1097-4695(19980615)35:4<395::aid-neu7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 219.Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67(3):203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 220.Alagappan D, et al. Brain injury expands the numbers of neural stem cells and progenitors in the SVZ by enhancing their responsiveness to EGF. ASN Neuro. 2009;1(2):AN20090002. doi: 10.1042/AN20090002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang X, et al. Roles of intracellular fibroblast growth factors in neural development and functions. Sci China Life Sci. 2012;55(12):1038–1044. doi: 10.1007/s11427-012-4412-x. [DOI] [PubMed] [Google Scholar]
- 222.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118(19):4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 223.Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403(6767):312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- 224.Hayon Y, et al. Platelet microparticles promote neural stem cell proliferation, survival and differentiation. J Mol Neurosci. 2012;47(3):659–665. doi: 10.1007/s12031-012-9711-y. [DOI] [PubMed] [Google Scholar]
- 225.Boldrini M, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22(4):589–599. e5. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 227.Moreno-Jiménez EP, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25(4):554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- 228.Spalding KL, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tobin MK, et al. Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell. 2019;24(6):974–982 e3. doi: 10.1016/j.stem.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yang AC, et al. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature. 2020;583(7816):425–430. doi: 10.1038/s41586-020-2453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Castellano JM, et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature. 2017;544(7651):488–492. doi: 10.1038/nature22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Villeda SA, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20(6):659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Villeda SA, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Leiter O, et al. Exercise-induced activated platelets increase adult hippocampal precursor proliferation and promote neuronal differentiation. Stem Cell Reports. 2019;12(4):667–679. doi: 10.1016/j.stemcr.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nyam-Erdene A, et al. Characterization and chromatographic isolation of platelet extracellular vesicles from human platelet lysates for applications in neuroregenerative medicine. ACS Biomater Sci Eng. 2021;7(12):5823–5835. doi: 10.1021/acsbiomaterials.1c01226. [DOI] [PubMed] [Google Scholar]
- 236.Gouel F, et al. Whole and fractionated human platelet lysate biomaterials-based biotherapy induces strong neuroprotection in experimental models of amyotrophic lateral sclerosis. Biomaterials. 2022 doi: 10.1016/j.biomaterials.2021.121311. [DOI] [PubMed] [Google Scholar]
- 237.Zhang Y, et al. Administration of human platelet-rich plasma reduces infarction volume and improves motor function in adult rats with focal ischemic stroke. Brain Res. 2015;1594:267–273. doi: 10.1016/j.brainres.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 238.Dixon SJ, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Doll S, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wu Y, et al. The potential role of ferroptosis in neonatal brain injury. Front Neurosci. 2019;13:115. doi: 10.3389/fnins.2019.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Xiong Y, et al. Investigational agents for treatment of traumatic brain injury. Expert Opin Investig Drugs. 2015;24(6):743–760. doi: 10.1517/13543784.2015.1021919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Delila L et al. (accepted) Neuroprotective activity of a virus-safe nanofiltered human platelet lysate depleted of extracellular vesicles in Parkinson’s disease and traumatic brain injury models. Bioeng Transl Med [DOI] [PMC free article] [PubMed]
- 243.Gouel F, et al. Past and future of neurotrophic growth factors therapies in ALS: from single neurotrophic growth factor to stem cells and human platelet lysates. Front Neurol. 2019;10:835. doi: 10.3389/fneur.2019.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Fletcher N, McKeating J. Hepatitis C virus and the brain. J Viral Hepatitis. 2012;19(5):301–306. doi: 10.1111/j.1365-2893.2012.01591.x. [DOI] [PubMed] [Google Scholar]
- 245.Weissenborn K, et al. Hepatitis C virus infection and the brain. Metab Brain Dis. 2009;24(1):197–210. doi: 10.1007/s11011-008-9130-5. [DOI] [PubMed] [Google Scholar]
- 246.Carod-Artal FJ, et al. Neurological complications of dengue virus infection. Lancet Neurol. 2013;12(9):906–919. doi: 10.1016/S1474-4422(13)70150-9. [DOI] [PubMed] [Google Scholar]
- 247.Watson N, et al. The importance of ongoing international surveillance for Creutzfeldt-Jakob disease. Nat Rev Neurol. 2021;17(6):362–379. doi: 10.1038/s41582-021-00488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Walker LC, Jucker M. Neurodegenerative diseases: expanding the prion concept. Annu Rev Neurosci. 2015;38:87–103. doi: 10.1146/annurev-neuro-071714-033828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Mammadova N, Cassmann E, Greenlee JJ. Successful transmission of the chronic wasting disease (CWD) agent to white-tailed deer by intravenous blood transfusion. Res Vet Sci. 2020;133:304–306. doi: 10.1016/j.rvsc.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 250.Morales R, et al. Infusion of blood from mice displaying cerebral amyloidosis accelerates amyloid pathology in animal models of Alzheimer's disease. Acta Neuropathol Commun. 2020;8(1):213. doi: 10.1186/s40478-020-01087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Clavaguera F, et al. Peripheral administration of tau aggregates triggers intracerebral tauopathy in transgenic mice. Acta Neuropathol. 2014;127(2):299–301. doi: 10.1007/s00401-013-1231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Duyckaerts C, et al. Neuropathology of iatrogenic Creutzfeldt-Jakob disease and immunoassay of French cadaver-sourced growth hormone batches suggest possible transmission of tauopathy and long incubation periods for the transmission of Abeta pathology. Acta Neuropathol. 2018;135(2):201–212. doi: 10.1007/s00401-017-1791-x. [DOI] [PubMed] [Google Scholar]
- 253.Sawyer EB, et al. Preclinical detection of infectivity and disease-specific PrP in blood throughout the incubation period of prion disease. Sci Rep. 2015;5:17742. doi: 10.1038/srep17742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lauwers E, et al. Potential human transmission of amyloid beta pathology: surveillance and risks. Lancet Neurol. 2020;19(10):872–878. doi: 10.1016/S1474-4422(20)30238-6. [DOI] [PubMed] [Google Scholar]
- 255.Jonsdottir-Buch S, et al. Expired pathogen inactivated platelet concentrates support differentiation and immunomodulation of mesenchymal stromal cells in culture. J Tissue Eng Regen Med. 2014;8:374. doi: 10.3727/096368914X683043. [DOI] [PubMed] [Google Scholar]
- 256.Fazzina R, et al. Culture of human cell lines by a pathogen-inactivated human platelet lysate. Cytotechnology. 2016;68(4):1185–1195. doi: 10.1007/s10616-015-9878-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jonsdottir-Buch SM, et al. Expired and pathogen-inactivated platelet concentrates support differentiation and immunomodulation of mesenchymal stromal cells in culture. Cell Transplant. 2015;24(8):1545–1554. doi: 10.3727/096368914X683043. [DOI] [PubMed] [Google Scholar]
- 258.Viau S, et al. Pathogen reduction through additive-free short-wave UV light irradiation retains the optimal efficacy of human platelet lysate for the expansion of human bone marrow mesenchymal stem cells. PLoS ONE. 2017;12(8):e0181406. doi: 10.1371/journal.pone.0181406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Barro L, et al. A double-virally-inactivated (Intercept-solvent/detergent) human platelet lysate for in vitro expansion of human mesenchymal stromal cells. Transfusion. 2019;59(6):2061–2073. doi: 10.1111/trf.15251. [DOI] [PubMed] [Google Scholar]
- 260.Burnouf T, Radosevich M. Reducing the risk of infection from plasma products: specific preventative strategies. Blood Rev. 2000;14(2):94–110. doi: 10.1054/blre.2000.0129. [DOI] [PubMed] [Google Scholar]
- 261.Burnouf T. Modern plasma fractionation. Transfus Med Rev. 2007;21(2):101–117. doi: 10.1016/j.tmrv.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Burnouf T, Padilla A. Current strategies to prevent transmission of prions by human plasma derivatives. Transfus Clin Biol. 2006;13(5):320–328. doi: 10.1016/j.tracli.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 263.Radomski KU, et al. Pathogen safety of a new intravenous immune globulin 10% liquid. BioDrugs. 2017;31(2):125–134. doi: 10.1007/s40259-017-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Goussen C, et al. Biological safety of a highly purified 10% liquid intravenous immunoglobulin preparation from human plasma. BioDrugs. 2017;31(3):251–261. doi: 10.1007/s40259-017-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Flan B, Arrabal S. Manufacture of plasma-derived products in France and measures to prevent the risk of vCJD transmission: precautionary measures and efficacy of manufacturing processes in prion removal. Transfus Clin Biol. 2007;14(1):51–62. doi: 10.1016/j.tracli.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 266.Petersen MA, Ryu JK, Akassoglou K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci. 2018;19(5):283–301. doi: 10.1038/nrn.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Moreau C, et al. Intraventricular dopamine infusion alleviates motor symptoms in a primate model of Parkinson's disease. Neurobiol Dis. 2020;139:104846. doi: 10.1016/j.nbd.2020.104846. [DOI] [PubMed] [Google Scholar]
- 268.Crowe TP, Hsu WH. Evaluation of recent intranasal drug delivery systems to the central nervous system. Pharmaceutics. 2022;14(3):629. doi: 10.3390/pharmaceutics14030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Burnouf T. Blood products: unmet needs for essential medicines. Lancet Haematol. 2019;6(12):e598–e599. doi: 10.1016/S2352-3026(19)30217-0. [DOI] [PubMed] [Google Scholar]
- 270.Roberts N, et al. The global need and availability of blood products: a modelling study. Lancet Haematol. 2019;6(12):e606–e615. doi: 10.1016/S2352-3026(19)30200-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.