Abstract
Treatment-resistant schizophrenia (TRS) will affect about one in three patients with schizophrenia. Clozapine is the only treatment approved for TRS, and patients should be treated as soon as possible to improve their chances of achieving remission. Despite its effectiveness, concern over side effects, monitoring requirements, and inexperience with prescribing often result in long delays that can expose patients to unnecessary risks and compromise their chances of achieving favorable long-term outcomes. We critically reviewed the literature on clozapine use in TRS, focusing on guidelines, systematic reviews, and algorithms to identify strategies for improving clozapine safety and tolerability. Based on this, we have provided an overview of strategies to support early initiation of clozapine in patients with TRS based on the latest evidence and our clinical experience, and have summarized the key elements in a practical, evidence-based checklist for identifying and managing patients with TRS, with the aim of increasing confidence in prescribing and monitoring clozapine therapy.
Key Points
| Early and sustained treatment with clozapine represents the best available strategy for achieving and maintaining remission in patients with treatment-resistant schizophrenia. |
| Common side effects including sialorrhea, constipation and weight gain may result in poor adherence to treatment, while the existence of rare severe adverse events and the associated monitoring burden may result in delays in starting therapy. |
| Strategies for optimizing treatment and managing side effects are summarized and a checklist is provided. |
Introduction
Schizophrenia is a serious mental illness involving positive and negative symptoms, as well as cognitive impairment [1]. It has a median incidence of 287 (uncertainty interval 246–331)/100,000 [2] and median standardized mortality ratio of 2.6 [3]. Therapies targeting postsynaptic dopamine receptors are not effective in all cases, especially regarding negative symptoms and in patients with treatment-resistant schizophrenia (TRS). TRS is defined as patients who do not respond sufficiently to sequential trials of at least two different antipsychotics administered at appropriate doses, duration, and with adequate adherence from the patient [4–7].
TRS is a major clinical challenge that can occur early in the treatment pathway or develop later in patients who respond initially to antipsychotic treatment [8–10]. Several lines of evidence indicate that it has a different neurobiology to schizophrenia that responds to dopamine D2 receptor blockers [11, 12]. It has been hypothesized that TRS could represent a neurobiologically distinct sub-type of schizophrenia [13, 14]. Some data point to a sub-type of schizophrenia associated with TRS characterized by unaltered dopamine function [15–17], and glutamate dysregulation [18–20], which would explain why these patients do not respond to dopamine D2 blockers [21]. TRS occurs in 20–50% of patients with schizophrenia [22–24], including in community settings [25]. It is associated with higher disease burden [26, 27] and poorer outcomes [28], especially involving persistent positive symptoms despite adherence with treatment [29].
Clozapine is the only drug approved for TRS by regulators in North America, Europe, and many other jurisdictions. It is a tricyclic dibenzodiazepine derivative that interacts with multiple neuroreceptors, including dopamine, serotonin and muscarinic receptors [30, 31]. Its low affinity for D2 dopamine receptors may explain its relative lack of extrapyramidal side effects [30], whilst its actions to modulate glutamate levels may contribute to its superior efficacy in TRS [32]. Clozapine is more effective than other antipsychotics for TRS [33], and it reduces rates of hospital readmission and all-cause mortality [34–36]. A systematic review and meta-analysis of long-term studies (median follow-up 5.4 years) revealed that continuous clozapine treatment was associated with a significantly lower all-cause mortality rate compared to other antipsychotics (mortality rate ratio = 0.56, 95% confidence interval (CI) = 0.36–0.85, P = 0.007) [37], which may be due to reduced suicidality [38].
Real-world evidence has confirmed its effectiveness [39–41]. Meta-analysis of cohort studies found that clozapine resulted in greater symptom improvement compared to other second-generation antipsychotics, fewer treatment discontinuations, and a lower risk of hospitalization [42]. Based on evidence from nearly 50,000 patients, the risk ratio for hospitalization in patients taking clozapine relative to patients taking other antipsychotics was 0.817 (95% CI 0.725–0.920; P = 0.001) [42].
A systematic review of evidence-based guidelines for antipsychotic treatment of patients with schizophrenia found that all 17 of the guidelines reviewed recommend initiating treatment with the antipsychotic drug clozapine when patients are diagnosed with TRS [43].
However, despite this strong recommendation, clozapine is underutilized in this setting [44–46], and many patients initiate clozapine treatment after delays associated with trials of antipsychotic polypharmacy [27, 47, 48]. This may be due to the side-effect profile of clozapine, the monitoring requirements associated with its use, and a lack of prescribing experience [49, 50]. A systematic review that investigated facilitators of guideline adherence in treating TRS identified institutional directives/audits, integrated clozapine clinics, facilitated monitoring, interaction with experts, educational programs, and distribution of educational material as effective strategies for increasing access to clozapine [51]. Delaying initiation of clozapine treatment is associated with higher economic burden [52], and poorer response rates [53–56].
It may be possible to detect or predict TRS at the first psychotic episode [57–60]; however, because of the associated risk of potentially serious adverse effects [61], clozapine is not generally indicated in the first-line treatment of schizophrenia [62, 63]. Moreover, patients receiving clozapine are recommended to have hematological monitoring, particularly in the first 6 months of treatment when the risk of agranulocytosis is highest [64]. Clinicians should also be alert for possible cardiovascular, metabolic, gastrointestinal, and neurological adverse effects, some of which may be managed by reducing the dose or titration rate while monitoring frequently [65].
The objective of this review is to provide an overview and guide for clinicians and researchers on clozapine initiation, and to propose a practical checklist for patient selection, clozapine initiation (titration) and maintenance, side-effect monitoring and management, with the aid to facilitate appropriate clozapine use in clinical care.
Literature Search
In conducting this review, we have searched Medline using the query (((algorithm OR checklist OR tool OR recommendation OR guide* OR policy)) AND ((review OR systematic OR meta-analysis OR metaanalysis))) AND ((clozapine AND (resistan* OR refractor* OR treatment-resistant schizophrenia OR TRS OR "treatment failure"))) to identify systematic reviews, meta-analyses, guidelines, and treatment algorithms related to TRS published in English before March 2022; we manually searched the reference lists of retrieved recent publications for additional relevant publications.
Patient Selection
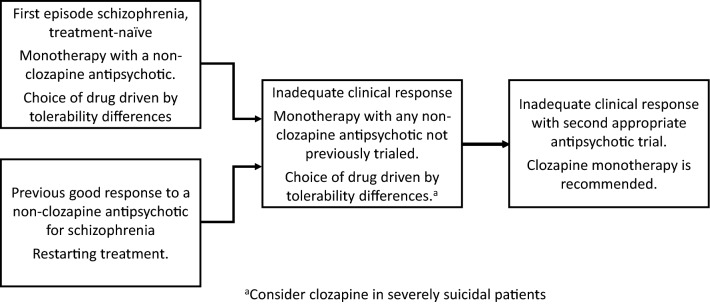
The US Food and Drug Administration (FDA) indications for clozapine include TRS and suicidal behavior in patients with schizophrenia or schizoaffective disorder [62], while the European Medicines Agency (EMA) indication includes TRS and schizophrenia patients who have severe, untreatable neurological adverse reactions to other antipsychotic agents, including second-generation antipsychotics, as well as psychotic disorders occurring during the course of Parkinson's disease, in cases where standard treatment has failed [63], whereas clozapine is indicated only for treating TRS in Australia, Japan, and Canada [66–68]. Patient selection should begin by confirming the presence of TRS (Fig. 1) [4], (Fig. 2) [7, 69–71], and the absence of contraindications for clozapine treatment, which may differ by country.
Fig. 1.
Definition and identification of treatment-resistant schizophrenia. TRS treatment-resistant schizophrenia, CRS clozapine-resistant schizophrenia [7]; BPRS Brief Psychiatric Rating Scale, CGI-S-TRS Clinical Global Impressions-Severity TRS Scale, PANSS Positive and Negative Syndrome Scale, SANS Scale for the Assessment of Negative Symptoms, SAPS Scale for the Assessment of Positive Symptoms
(adapted from reference [4], permission not required)
Fig. 2.
Algorithm for initial schizophrenia treatment and determination of treatment-resistance schizophrenia (TRS) [7, 69–71] (drawn from published information, permission not required)
Where available, treatment should take place in a context that supports the psychosocial needs of the patient and provides care for psychiatric comorbidities and any medical problems, as well as providing access to psychiatric pharmacotherapy and monitoring [69–71].
Special Clinical Populations
Pediatric Setting/Early-Onset Schizophrenia
Both the EMA and the FDA state that the safety and effectiveness of clozapine have not been established in pediatric patients [62, 63]; however, guidelines from the UK National Institute for Health and Care Excellence (NICE) [6] and the Canadian guidelines recommend offering clozapine treatment for patients, including children, with early-onset schizophrenia (EOS) meeting criteria for TRS [6, 72]. A systematic review of the efficacy and tolerability of clozapine in this setting revealed that efficacy, tolerability, and dose-blood level relationships are similar to those in adults [73]. Sedation and sialorrhea were reported by most patients treated for EOS. A network meta-analysis established clozapine as more efficacious than other antipsychotics in children and adolescents with schizophrenia [74].
Older Adults
Few geriatric patients were included in studies that informed the FDA label, therefore, caution is warranted regarding the side effects of tachycardia and orthostatic hypotension in patients with compromised cardiovascular functioning, and anticholinergic effects of constipation and urinary retention in older adults in general [62]. The EMA specifically recommends that treatment be initiated at a low dose and titrated slowly [63]. A systematic review of clozapine use in older adults confirmed the lack of controlled studies and reported modest effectiveness based on observational studies, suggesting that the decision must carefully weigh the potential benefits and risks in each individual patient considering their comorbidities and concomitant treatments [75].
Pregnancy
Clozapine treatment is not contraindicated by the EMA or FDA for pregnant women who clearly require treatment (FDA pregnancy class B) [62, 63]; however, caution and additional monitoring are warranted because of the relative lack of studies. The World Federation of Societies of Biological Psychiatry does not recommend clozapine treatment during pregnancy because its side-effect profile includes agranulocytosis, metabolic effects, and seizures [76]. A systematic review of outcomes after clozapine continuation in pregnancy based on very limited evidence concluded that a thorough benefit-risk analysis is needed, followed by close monitoring when clozapine is continued during pregnancy [77]. The decision should consider the substantial risks to the mother and fetus/infant from untreated schizophrenia [78]. Clozapine use during breastfeeding is widely contraindicated because clozapine is excreted into breast milk [63, 76, 79–81].
Clozapine Treatment
Initiating Treatment
Major factors influencing blood clozapine concentrations include those significantly elevating clozapine blood levels, such as female sex (estrogen), older age, Asian/Amerindian ancestry, obesity, inflammation (from infection, rapid clozapine titration), high levels of caffeine use, coadministration of valproic acid, low CYP1A2 expression, and coadministration of CYP1A2 inhibitors; conversely, clozapine blood levels are lowered by smoking and other CYP1A2 inducers, including phenobarbital, phenytoin, and topiramate dosage > 400 mg/day (Table 1) [62, 82–86].
Table 1.
Clozapine dose adjustment in patients taking concomitant medications [62]
| Co-medications (selected examples) | Scenarios | ||
|---|---|---|---|
| Initiating clozapine while taking a co-medication | Adding a co-medication while taking clozapine | Discontinuing a co-medication while continuing clozapine | |
| Strong CYP1A2 inhibitors (e.g., fluvoxamine, ciprofloxacin, enoxacin) | Use one-third of the clozapine dose | Increase clozapine dose based on clinical response | |
| Moderate or weak CYP1A2 inhibitors (e.g., oral contraceptives, caffeine) | Monitor for adverse reactions. Consider reducing the clozapine dose if necessary | Monitor for lack of effectiveness. Consider increasing clozapine dose if necessary | |
| CYP2D6 or CYP3A4 inhibitors (e.g., cimetidine, escitalopram, erythromycin, paroxetine, bupropion, fluoxetine, quinidine, duloxetine, terbinafine, sertraline) | |||
| Strong CYP3A4 Inducers (e.g., phenytoin, carbamazepine, St. John’s wort, rifampin) | Concomitant use is not recommended. However, if the inducer is necessary, it may be necessary to increase the clozapine dose. Monitor for decreased effectiveness | Reduce clozapine dose based on clinical response. | |
| Moderate or weak CYP1A2 or CYP3A4 inducers (e.g., tobacco smoking, omeprazole, dexamethasone, famotidine) | Monitor for decreased effectiveness. Consider increasing the clozapine dose if necessary | Monitor for adverse reactions. Consider reducing the clozapine dose if necessary | |
CYP3A4 cytochrome P450 3A4, CYP1A2 cytochrome P450 1A2
This table was reproduced from the FDA document "HIGHLIGHTS OF PRESCRIBING INFORMATION - CLOZARIL®”, copyright HLS Therapeutics (USA), Inc. Any changes made to the table are not endorsed by HLS Therapeutics (USA), Inc. CLOZARIL® is a registered trademark of Novartis Pharmaceuticals Corporation [62]
Caffeine and tobacco consumption are common and, although their effects on clozapine metabolism can be compensated through drug titration, as with the introduction or removal of concomitant medications, patients should be cautioned about changing their daily intake of these substances. Studies identifying a clinically relevant change in caffeine intake are lacking, but a relevant change may be > 1 cup of coffee or > 2 cans of caffeinated beverages per day in non-smoking patients (> 3 cups of coffee or > 6 cans of caffeinated beverages in smokers) [82].
Inflammation reduces CYP1A2 enzyme activity and increases clozapine blood levels; therefore, it is recommended to halve the clozapine dose in people with severe infection, including symptomatic COVID-19, until the patient has been 3 days without fever, after which the prior clozapine dose can be resumed [87].
The minimum trough level for clozapine in plasma associated with a good therapeutic response is generally thought to be 350 ng/mL, whilst levels above 600 ng/mL are associated with higher risk for dose-dependent side effects such as seizures [88, 89]; therefore, the lowest effective clozapine level should be targeted when therapeutic drug monitoring is used (see Sect. 4.6, Therapeutic Drug Monitoring). Some patients only respond to higher clozapine levels, and this may warrant higher dosages, combined with the use of prophylactic anticonvulsive therapy [62, 63].
When clozapine treatment is started in an in-patient setting, it is recommended to start at 12.5 mg once or twice a day on day one, followed by daily dosage incrementation by 25–50 mg up to a minimum therapeutic dosage (in adults generally 250–450 mg/day depending on sex, race, and smoking status), if tolerated. Older adults should start with 12.5 mg on the first day and daily dosage incrementation by 25 mg [63]. In the community or other settings where patients are not under continuous monitoring, slower titration should be used (see Beck et al. for a community titration schedule [90]).
Most patients with TRS will be switching from a failed antipsychotic therapy. The dosage of the failed antipsychotic should be tapered down during the initiation of treatment with clozapine. Our experience indicates that the best way to cross-taper is to keep the dose of the failed antipsychotic constant during the first week as clozapine titration is initiated, and then to gradually reduce the dose by about 25% per week, depending on the degree of symptomatic stability, the adverse effect burden during the overlap, and the ability to increase clozapine to a therapeutic dose [90]. However, the rate of reduction of the insufficiently effective antipsychotic may need to be adjusted depending on the rate of the clozapine up-titration, the side-effect profile of the failed antipsychotic and overlapping treatment, and how well the patient tolerates and responds to clozapine.
The rate of clozapine titration may influence the occurrence of some adverse events, including benign hyperthermia, tachycardia, orthostatic hypotension, and risk for myocarditis, pneumonia, agranulocytosis, and seizures [65, 82]. More gradual titration combined with careful monitoring may reduce the occurrence and severity of these events. A 2022 guideline proposes personalized titration schedules based on patient ancestry, sex, smoking status and other characteristics that influence clozapine metabolism [82]. These detailed schedules, which include weekly dosing targets and blood clozapine levels during titration are summarized in Table 2. During titration, excessive sedation, orthostatic hypotension, blood clozapine levels exceeding the limit for that week, and signs of inflammation (e.g., elevation in C-reactive protein) are reasons for pausing dosage escalation [82]. See Sect. 5 Side-Effect Monitoring and Management, and follow clozapine clinical monitoring recommendations.
Table 2.
Summary of clozapine titration schedules based on ancestry and clozapine metabolism [82]
| Patient characteristics | Expected dosage, (concentration/ dosage ratio, ng per mL/mg per d) | First Week | Second week | Third week | Fourth and subsequent weeks |
|---|---|---|---|---|---|
| Asia/Amerindian with lower clozapine metabolisma |
75–150 mg/day (4.7 to 2.3) |
First dose 6.25 mg at nightb Increase daily dosage by 6.25 mg Target 25 mg/day |
2 dose increases of 12.5 mg/day Target 50 mg/day TDM < 118 ng/mL on day 7 |
2 dose increases of 12.5 mg/day Target 75 mg/day TDM < 235 ng/mL on day 14 |
Target dosage from 75 mg/day for female non-smokers to 75 to 150 mg/day for male smokersc TDM < 353 ng/mL on day 21 |
| Asia/Amerindian with average clozapine metabolism |
175–300 mg/day (2.1 to 1.3) |
First dose 12.5 mg at night Increase daily dosage by 12.5 mg Target 50 mg/day |
2 dose increases of 12.5 mg/day Target 100 mg/day TDM < 105 ng/mL on day 7 |
2 dose increases of 25 mg/day Target 150 mg/day TDM < 210 ng/mL on day 14 |
Target dosage from 175 mg/day for female non-smokers to 300 mg/day for male smokers TDM < 315 ng/mL on day 21 |
| European/Western Asiand ancestry with lower clozapine metabolism |
100–200 mg/day (3.5 to 1.75) |
First dose 12.5 mg at night Increase daily dosage by 12.5 mg Target 50 mg/day |
2 dose increases of 50 mg/day Target 75 mg/day TDM < 175 ng/mL on day 7 |
2 dose increases of 25 mg/day Target 100 mg/day for female non-smokers; 150 mg/day for others TDM < 263 ng/mL on day 14 |
Target dosage from 100 mg/day for female non-smokers to 200 mg/day for male smokers TDM < 350 ng/mL on day 21 |
| European/Western Asiand ancestry with average clozapine metabolism |
250–400 mg/day (1.4 to 0.88) |
First dose 25 mg at night Increase daily dosage by 25 mg Target 100 mg/day |
2 dose increases of 50 mg/day Target 200 mg/day at the end of the second week. TDM < 140 ng/mL on day 7 |
2 dose increases of 25 mg/day Target 250 mg/day for female non-smokers; 300 mg/day for others TDM < 280 ng/mL on day 14 |
Target dosage from 250 mg/day for female non-smokers to 400 mg/day for male smokers TDM < 350 ng/mL on day 21 |
| US patients with non-European ancestries other than Asia/Amerindian with lower clozapine metabolism |
150–300 mg/day (2.33 to 1.17) |
First dose 12.5 mg at night Increase daily dosage by 12.5 mg Target 50 mg/day |
2 dose increases of 25 mg/day Target 100 mg/day at the end of the second week. TDM < 117 ng/mL on day 7 |
2 dose increases of 25 mg/day Target 125 mg/day for female non-smokers; 150 mg/day for others TDM < 233 ng/mL on day 14 |
Target dosage from 150 mg/day for female non-smokers to 300 mg/day for male smokers TDM < 291 ng/mL on day 21 |
| US patients with non-European ancestries other than Asia/Amerindian with average clozapine metabolism |
300–600 mg/day (1.17 to 0.58) |
First dose of 25 mg at night Increase daily dosage by 25 mg Target 100 mg/day |
2 dose increases of 50 mg/day Target 200 mg/day TDM < 117 ng/mL on day 7 |
2 dose increases of 25 mg/day Target 300 mg/day TDM < 234 ng/mL on day 14 |
Target dosage from 300 mg/day for female non-smokers to 600 mg/day for male smokers TDM < 351 ng/mL on day 21 |
TDM therapeutic drug monitoring of clozapine concentration, which may be useful for personalizing titration rates
aLower clozapine metabolism, e.g., obesity, oral contraceptives, valproate, use of caffeine (for caffeine-containing beverages, see https://www.caffeineinformer.com/the-caffeine-database)
bAdminister most of dosage at night to avoid daytime sedation and orthostatic hypotension
cIncreased clozapine metabolism by cigarette smoking
dWestern Asians are those whose ancestry is from Asian countries west of Pakistan
Drawn from information in reference [82], permission not required
Measuring Response to Therapy
Where possible, use a standardized symptom-rating scale, such as the 30-item Positive and Negative Symptom Scale (PANSS) [91], or a standardized brief psychiatric rating scale, such as the six-item PANSS-6 [92–95] (Table 3) at baseline and subsequently to determine responses to therapy.
Table 3.
Positive and Negative Syndrome Scale-6 (PANSS-6), comprises three positive and three negative items from the PANSS-30 [91, 92]
| PANSS-30 Item | Itema |
|---|---|
| P1 | Delusions |
| P2 | Conceptual disorganization |
| P3 | Hallucinations |
| N1 | Blunted affect |
| N4 | Social withdrawal |
| N6 | Lack of spontaneity and flow of conversation |
aEach item is scored 1–7 and values are combined; remission corresponds to PANSS-6 total scores < 14 (reproduced from reference [92], permission not required)
Monitoring for Side Effects During the Titration Phase
Clozapine is associated with side effects (e.g., constipation, dizziness, drowsiness, leukopenia, sialorrhea, tachycardia, and weight gain), as well as potentially life-threatening adverse events (e.g., agranulocytosis, myocarditis, cardiomyopathy, neutropenia, agranulocytosis, paralytic ileus, pneumonia, and seizures) that warrant vigilant monitoring following established protocols, especially while titrating up to the therapeutic dose and during the first months of treatment (see Sect. 5) [31]. During this critical period, therapeutic drug monitoring (TDM) provides useful feedback for dosing and confirmation of adherence (see Sect. 4.6). Careful monitoring is also critical for detection and proactive management of side effects before they impact on treatment adherence [65].
Clozapine Trial Duration
After therapeutic plasma levels are achieved, Treatment Response and Resistance in Psychosis (TRRIP) Consensus Guidelines indicate that patients should receive clozapine with careful monitoring for at least 12 weeks, and ideally longer, before evaluating response, unless it cannot be tolerated [7]. In patients with strong negative symptoms, aggression, and/or those at high risk of suicide, a minimum trial duration of at least 16 weeks, and ideally longer, is recommended before evaluating response, unless it is not tolerated [96].
In patients with clozapine-resistant schizophrenia (CRS) [7], various pharmacologic augmentation strategies have been studied and can be tried [97–99]. In a naturalistic, nationwide database study, the only antipsychotic augmentation of clozapine that was superior to clozapine monotherapy for reducing the risk of hospitalization was the addition of the partial D2 agonist aripiprazole [100]. However, the most convincing evidence for efficacious augmentation of clozapine in CRS exists for electroconvulsive treatment [101].
Discontinuing Clozapine
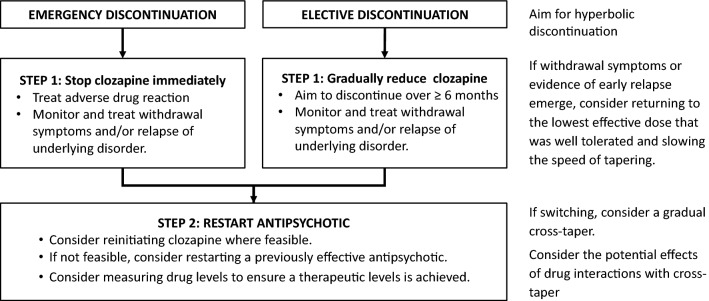
A decision to discontinue clozapine may be based on issues with efficacy, adherence, or tolerability, or on the emergence of life-threatening adverse effects (see Sect. 5). When the decision is not based on an emergency, the clozapine dosage should ideally be tapered over 6 months if possible, with cross-titration to an alternative antipsychotic chosen based on history of response, tolerability, and side-effect profile (Fig. 3) [102]. Regardless of the reason for discontinuation, patients should be monitored closely for the onset or worsening of psychosis, and absolute neutrophil count monitoring should continue for 2 weeks after discontinuation.
Fig. 3.
Provisional protocol for discontinuation of clozapine (
adapted from reference [102], permission not required)
Therapeutic Drug Monitoring
TDM is not needed if the patient is tolerating and responding to clozapine. However, it can be useful during initial titration of clozapine and during maintenance when there are signs of relapse, onset of side effects that are known to be dose dependent, or changes in factors known to influence blood levels, such as concomitant medications or cigarette use [103, 104]. Poor adherence to therapy is a frequent cause of non-response that can be detected with TDM; however, low plasma levels may also be due to pharmacokinetic effects. Typical indications for TDM are listed in Table 4 [103].
Table 4.
Typical indications for therapeutic drug monitoring (TDM) in psychiatric or neurologic patients [103]
|
Obligatory TDM for drugs with high levels of recommendation to use TDM Dosage optimization after initial prescription or after dosage change |
|
Specific indications for TDM for any drug independent of its level of recommendation to use TDM Uncertain adherence to medication Lack of clinical improvement under recommended dosage Relapse under maintenance treatment Relapse prevention because of uncertain adherence to medication Recurrence of symptoms under adequate dosage Adverse effects and clinical improvement under recommended dosage Combination treatment with a drug known for its interaction potential or suspected drug interaction Patient with abnormally high or low body weight Pregnant or breast-feeding patient Child or adolescent patient Older adult patient (> 65 years old) Patient with pharmacokinetically relevant comorbidity (hepatic or renal insufficiency, cardiovascular disease) Patient with acute or chronic inflammations or infections Patient with restrictive gastrointestinal resection or bariatric surgery Problem occurring after switching from an original preparation to a generic form (and vice versa) |
Adapted from reference [103]
The clozapine dose required to achieve and maintain blood levels in the therapeutic range varies greatly among patients. There may be high intra-individual variation in plasma levels even in stable patients [105], and some patients only respond after achieving blood clozapine levels above the reference range (350–600 ng/mL) [106].
TDM may be a useful tool for supporting dosing decisions because it provides direct feedback on blood concentrations without waiting for symptoms to respond or for side effects to develop [4]. This information can be used to optimize clozapine dosage (Table 5) [104]. Moreover, TDM may potentially increase prescriber confidence [107]. Lower doses/dose reductions may be necessary in patients with clinically significant renal or hepatic impairment (Fig. 4).
Table 5.
Therapeutic drug monitoring (TDM)-informed decision-making algorithm for clozapine-treated patientsa [104]
| Clozapine levela | Response | Tolerability | Action |
|---|---|---|---|
| Subtherapeutic (< 350 ng/mL) | Insufficient | Intolerable | Increase dose slowly to reference range and treat side effects if possible |
| Insufficient | Tolerable | Increase dose to reference range | |
| Sufficient | Intolerable | Consider decreasing dose | |
| Sufficient | Tolerable | No changes needed, continue standard side-effect monitoring | |
| Within reference range (350–600 ng/mL) | Insufficient | Intolerable | Treat side effects and increase dose slowly remaining in the reference range, if tolerated |
| Insufficient | Tolerable | Increase dose slowly remaining in the reference range, if possible | |
| Sufficient | Intolerable | If tolerability does not improve, decrease dose, monitor to remain in the reference range, if possible | |
| Sufficient | Tolerable | Continue to monitor | |
| Supratherapeutic (> 600 ng/mL) | Insufficient | Intolerable | Consider decreasing dose, monitor. Consider prophylactic anticonvulsant |
| Insufficient | Tolerable | Consider cautious dose increase or augmentation. Consider prophylactic anticonvulsant in both cases | |
| Sufficient | Intolerable | Decrease dose slowly, monitor to remain in the reference range, if possible | |
| Sufficient | Tolerable | Continue to monitor concentrations. Be vigilant for tolerability issues and consider prophylactic anticonvulsant |
aRefers to clozapine only, not clozapine plus norclozapine
Adapted by permission from reference [104]
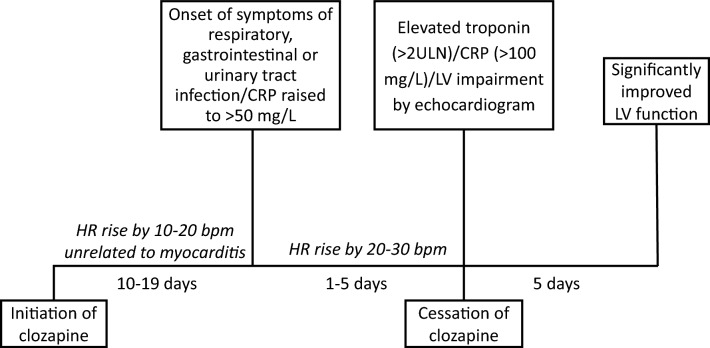
Fig. 4.
The typical evolution of clozapine-induced myocarditis. bpm beats per minute, CRP C-reactive protein, HR heart rate, LV left ventricular, ULN upper limit of normal [131] (reprinted by permission of SAGE Publications)
Side Effect Monitoring and Management
Clozapine is associated with side effects of varying severity, including FDA “black box” warnings for severe neutropenia, orthostatic hypotension, bradycardia, and syncope, seizure, myocarditis, cardiomyopathy, and mitral valve incompetence, as well as increased mortality in older adults with dementia-related psychosis (see prescribing information for complete list) [62]. Risk-benefit considerations should be weighed against the fact that clozapine is the only approved treatment for TRS and that clozapine treatment significantly lowers all-cause mortality [34–36].
Adverse effects should be monitored regularly according to local guidelines, which may vary between jurisdictions. Several countries require enrolment in clozapine patient-monitoring registries. Other side effects like nocturnal enuresis or incontinence can be troubling to the patient, and have major consequences if their burden leads to nonadherence and psychotic relapse [108].
Patient-compiled side-effect rating scales can provide a starting point for discussing the topic with patients. A systematic review of rating scales for side effects associated with antipsychotic medications suggested that the Udvalg for Kliniske Undersøgelser Side Effects Rating Scale for Patients (UKU-SERS-Pat), Liverpool University Neuroleptic Side Effect Rating Scale (LUNSERS), and the Glasgow Antipsychotic Side-effects Scale (GASS) are useful for this purpose [109]. A validated, clozapine-specific version of the GASS (GASS-C) has been developed [110]. However, several potentially life-threatening side effects are not detected by patient-compiled rating scales. An audit conducted in UK mental health services found that patient-compiled rating scales may be underutilized [111].
Agranulocytosis: Laboratory Tests, Clinical Complications
Clozapine-induced agranulocytosis (severe neutropenia) is a potentially life-threatening decrease in absolute neutrophil count to levels below 500/mm3 and increases susceptibility to infections [112]. It occurs most frequently during the first month of treatment and affects approximately 1% of treated patients [113, 114]. These reactions are unpredictable and do not appear to be dose dependent [115]. Findings from genomic studies suggest the involvement of multiple genes, including the solute carrier organic anion transporter 1B3/1B7 and members of the human leukocyte antigen complex [116]. People of African, Middle Eastern, or West Indian descent may present with benign ethnic neutropenia (BEN; absolute neutrophil counts 1000–1800/mm3) [117]. BEN is not associated with frequent or severe infections, or a higher risk of agranulocytosis with clozapine treatment [118]; however, the lower baseline neutrophil levels require a separate monitoring scheme [62].
Agranulocytosis: Detection and Clinical Complications
Blood cell counts have been successful for preventing fatalities and are required in all treated patients. The FDA requires weekly monitoring during the first 6 months of treatment; if absolute neutrophil count remains ≥ 1500/µL (≥ 1000/µL for BEN), then monitoring may be reduced to every 2 weeks for the next 6 months, and monthly thereafter (Tables 6 and 7) [62].
Table 6.
Clozapine treatment recommendations based on absolute neutrophil count monitoring for the general patient population [62]
| ANC level | Treatment recommendations | ANC monitoring |
|---|---|---|
| Normal range (≥ 1,500/μL) |
Initiate treatment If treatment interrupted: ≤ 30 days, continue monitoring as before ≥ 30 days, monitor as if new patient |
Weekly from initiation to 6 months Every 2 weeks from 6 to 12 months Monthly after 12 months |
| Mild neutropenia (1,000–1,499/μL)a | Continue treatment; consider low-dose lithium augmentation to increase white cell count |
Three times weekly until ANC ≥ 1,500/μL Once ANC ≥ 1,500/μL, return to patient’s last “normal range” ANC monitoring intervalb |
| Moderate neutropenia (500–999/μL)a |
Recommend hematology consultation Interrupt treatment for suspected clozapine induced neutropenia Resume treatment once ANC ≥ 1,000/μL; consider low-dose lithium augmentation to increase white cell count |
Daily until ANC ≥ 1,000/μL, then Three times weekly until ANC ≥ 1,500/μL Once ANC ≥ 1,500/μL, check ANC weekly for 4 weeks, then return to patient’s last “normal range” ANC monitoring intervalb |
| Severe neutropenia (< 500/μL)a |
Recommend hematology consultation Interrupt treatment for suspected clozapine-induced neutropenia Do not rechallenge unless prescriber determines benefits outweigh risks |
Daily until ANC ≥ 1,000/μL, then Three times weekly until ANC ≥ 1,500/μL If patient rechallenged, resume treatment as a new patient under “normal range” monitoring once ANC ≥ 1,500/μL |
ANC absolute neutrophil count, BEN benign ethnic neutropenia
aConfirm all initial reports of ANC less than 1,500/μL with a repeat ANC measurement within 24 h
bIf clinically appropriate
This table was adapted from the FDA document "HIGHLIGHTS OF PRESCRIBING INFORMATION - CLOZARIL®”, copyright HLS Therapeutics (USA), Inc. Any changes made to the table are not endorsed by HLS Therapeutics (USA), Inc. CLOZARIL® is a registered trademark of Novartis Pharmaceuticals Corporation [62]
Table 7.
Clozapine treatment recommendations for patients with benign ethnic neutropenia, based on absolute neutrophil count (ANC) monitoring [62]
| ANC level | Treatment recommendations | ANC monitoring |
|---|---|---|
| Normal BEN range (established ANC baseline ≥ 1,000/μL) |
Obtain at least two baseline ANC levels before initiating treatment, consider low-dose lithium augmentation to increase white cell count If treatment interrupted ≤ 30 days, continue monitoring as before; if > 30 days, monitor as if new patient |
Weekly from initiation to 6 months Every 2 weeks from 6 to 12 months Monthly after 12 months |
| Discontinuation of treatment for reasons other than neutropenia |
Reduce dose over 1–2 weeks if not urgent For abrupt discontinuation, continue existing monitoring until ANC ≥ 1000/μL or > baseline Additional monitoring is required if fever (≥ 38.5 °C) within 2 weeks after discontinuation |
|
|
BEN neutropenia 500–999/μLa |
Recommend hematology consultation Continue treatment; consider low-dose lithium augmentation to increase white cell count |
Three times weekly until ANC ≥ 1,000/μL or ≥ patient’s known baseline Once ANC ≥ 1,000/μL or at patient’s baseline, check ANC weekly for 4 weeks, then return to patient’s last “normal BEN range” ANC monitoring intervalb |
| BEN severe neutropenia < 500/μLa |
Recommend hematology consultation Interrupt treatment for suspected clozapine-induced neutropenia Do not rechallenge unless prescriber determines benefits outweigh risks; consider low-dose lithium augmentation to increase white cell count |
Daily until ANC ≥ 500/μL, then Three times weekly until ANC ≥ patient’s baseline If patient rechallenged, resume treatment as a new patient under “normal range” monitoring once ANC ≥ 1,000/μL or at patient’s baseline |
BEN benign ethnic neutropenia
aConfirm all initial reports of ANC < 1,000/µL with a repeat ANC measurement within 24 h
bIf clinically appropriate
This table was adapted from the FDA document "HIGHLIGHTS OF PRESCRIBING INFORMATION - CLOZARIL®”, copyright HLS Therapeutics (USA), Inc. Any changes made to the table are not endorsed by HLS Therapeutics (USA), Inc. CLOZARIL® is a registered trademark of Novartis Pharmaceuticals Corporation [62]
The EMA requires weekly monitoring of absolute neutrophil count for the first 18 weeks of treatment, followed by monthly for the duration of treatment [63].
Rechallenge after recovery from clozapine-induced neutropenia is envisioned in guidance from the FDA. A summary of 259 cases of clozapine rechallenge after major adverse events reported a success rate of 63.0% (128/203) for patients with neutropenia, and 17.7% (3/17) for those with agranulocytosis [119]. According to the reviewed cases, rechallenge may be more successful with slow titration and coadministration of low-dose lithium (300–600 mg/day), which stimulates the bone marrow production of leucocytes. Although lithium stimulates leucocyte production, it may also mask the onset of agranulocytosis. Preliminary data suggest that granulocyte-colony stimulating factor, widely used to reduce the incidence and severity of chemotherapy-induced neutropenia, may be useful for stimulating leukopoiesis during clozapine rechallenge [120–123].
Myocarditis and Other Cardiological Adverse Events
Clozapine is associated with the risk of rare myocarditis and cardiomyopathy. Myocarditis is an acute condition involving inflammation of the myocardium that usually occurs during the first month of treatment [124, 125], while cardiomyopathy involves structural changes to the left ventricle that occur over months to years [126]. These conditions do not appear to be dose dependent. In a meta-analysis of cardiac adverse drug reactions among 258,961 people exposed to clozapine, the myocarditis rate was 0.7% (95% CI 0.3–1.6) and the rate of cardiomyopathy was 0.6% (95% CI 0.2–2.3) [127]. The meta-analysis included mainly retrospective studies that used a variety of definitions and markers. Subsequent reports of presumptive myocarditis based on sensitive laboratory tests [128] or retrospectively identified myocarditis (cases defined as flu-like symptoms plus ≥ 1 symptom/sign of cardiac dysfunction plus ≥ 1 indicative diagnostic abnormality and no evidence of a viral cause) [129], suggest that the incidence may be higher. The risk of myocarditis is higher among patients commencing clozapine treatment while receiving valproate [130]. In a review of case reports, the success rate of clozapine rechallenge after clozapine-associated myocarditis was 64.7% (11/17) [119].
Presentation of Early Cardiological Adverse Events
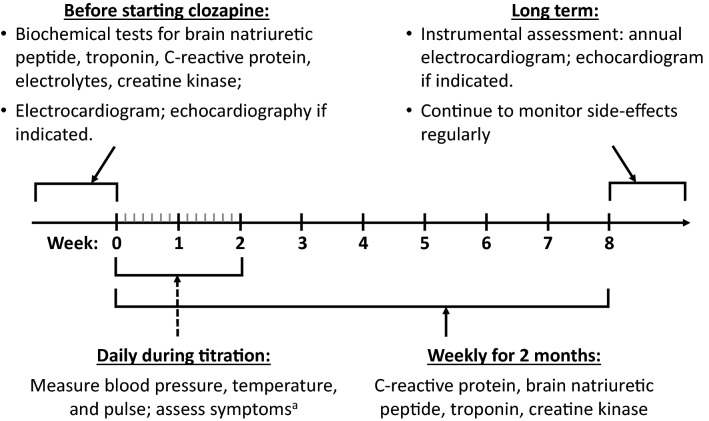
Although rare, cardiological adverse events are potentially life-threatening and their onset can be difficult to diagnose. They may occur more frequently in the first weeks after treatment initiation [124]; however, they are not strictly related to clozapine titration or dosage dependent, therefore vigilance and a proactive attitude are imperative over the entire course of clozapine treatment (Fig. 5) [131].
Fig. 5.
Monitoring for clozapine-induced cardiotoxicity [90, 131–133]. Less or more frequent monitoring may be appropriate depending on the clinical situation and local guidelines. aSymptoms may include chest pain, edema, shortness of breath, and other signs associated with heart failure. If signs or symptoms develop, or cardio markers are elevated, increase the frequency of troponin and C-reactive protein tests until normalized
Signs and symptoms of myocarditis may include fever, malaise, tachycardia/palpitations, arrhythmias, chest pain, and/or symptoms associated with heart failure like dyspnea, tachypnea, and fatigue [63]; however, the clinical presentation is variable and many of the symptoms are not specific to myocarditis [124], highlighting the need for monitoring markers of cardiotoxicity and cardiovascular risk factors (Fig. 5) [90, 131–133]. Troponin I is a sensitive and specific marker for myocardial damage. Echocardiography may be indicated when clinical signs or abnormal biomarkers are present.
Cardiomyopathy often presents with decreased exercise tolerance, peripheral edema, and myocardial congestion, and can lead to the development of malignant arrhythmias, cardiac insufficiency, and pump failure [132]. B-type natriuretic peptide (BNP) and N-terminal proBNP are sensitive and specific markers for heart failure and cardiomyopathy.
In most cases clozapine will be discontinued once myocarditis or cardiomyopathy develops, but there may be circumstances where the benefits outweigh the risks. Any decision to rechallenge should be made with the consultation of a cardiologist or heart failure specialist, with the rechallenge attempted at least 6 months after the episode of clozapine-associated myocarditis using a titration in which dosage is increased slowly (e.g., by 25 mg per week) [133].
Gastrointestinal Hypomotility
Clozapine use can cause gastrointestinal hypomotility in any part of the digestive tract and, depending on the location, this can lead to constipation with increased risk of paralytic ileus and pseudo-obstruction, gastroparesis, or dysphagia and aspiration pneumonia and death [134]. A meta-analysis of 32 studies (n = 2,013 patients) showed that the pooled prevalence of constipation associated with clozapine was 31.2% [135]. A comprehensive review of publications up to 2010 revealed a mortality rate for life-threatening gastrointestinal hypomotility of 15.0–27.5% [136], while mortality in patients who develop paralytic ileus was 43.7% [137]. The risk is highest during the first 4 months of treatment, but remains throughout treatment [138]. The risk increases with age and clozapine dose, and is higher in patients receiving concomitant treatment with anticholinergic medications, other medications with anticholinergic properties, or opioids [139].
Symptoms and Signs
Symptoms may include abdominal pain or distention, infrequent/difficult defecation, and other well-known symptoms of constipation; however, patients with schizophrenia may not be aware of abdominal symptoms, and detection should not rely on spontaneous reporting. Simply asking the patient whether they are experiencing constipation may not be reliable. Evaluation can use a brief constipation scale such as the eight-item Constipation Assessment Scale (Table 8) [140], or the ROME IV instrument [141].
Table 8.
Constipation assessment scale [140]
| Item | No problem | Some problem | Severe problem |
|---|---|---|---|
| Abdominal distention or bloating | 0 | 1 | 2 |
| Change in amount of gas passed rectally | 0 | 1 | 2 |
| Less frequent bowel movements | 0 | 1 | 2 |
| Oozing liquid stool | 0 | 1 | 2 |
| Rectal fullness or pressure | 0 | 1 | 2 |
| Rectal pain with bowel movement | 0 | 1 | 2 |
| Small volume of stool | 0 | 1 | 2 |
| Unable to pass stool | 0 | 1 | 2 |
| Total score (severity = sum of scores) | |||
From reference [140], permission not required
Comparing the results from patient-reported constipation with objective data from gastrointestinal motility testing revealed that self-reporting greatly underestimates the presence of this frequent and potentially life-threatening side effect, highlighting the importance of educating the patient and family on the need for vigilance [142, 143].
Protocols and Procedures to Prevent Gastrointestinal Hypomotility
The best strategy for preventing serious gastrointestinal outcomes is to inform and remind the patient of the potential risks, and counsel them on the importance of a healthy diet, adequate fluid intake, and physical activity. Concomitant medications should be monitored to avoid drugs known to promote constipation or reduce gastrointestinal motility (e.g., opioids, antihistamines, other anticholinergic drugs). This approach should be combined with frequent constipation screening (e.g., weekly administration of a brief scale during the first few months). Abdominal auscultation, palpation, and percussion may be useful for identifying blockage.
Proactive measures might include the co-prescription of prophylactic laxatives in patients with risk factors (e.g., advanced age, history of constipation). A targeted approach in which laxatives are administered only when needed may avoid unnecessary treatment but requires timely diagnosis of constipation to prevent serious/fatal adverse events. Together, the prevalence and severity of potential adverse outcomes, insensitivity of self-reported screening, and impracticality of screening with gastrointestinal motility test argue for the use of prophylactic laxatives. There are potential side effects with long-term laxative use, and these need to be weighed against the benefits. The FDA recommends “considering prophylactic laxatives in high-risk patients” [62].
Treatment
Management includes clozapine dose reduction, osmotic laxative, stimulant laxative, or docusate stool softeners. Patients not responding to these should be referred to gastroenterology. When a bowel obstruction is suspected, stimulants and bulk-forming laxatives should not be used, and the patient should be referred for urgent surgical review.
Clozapine-Associated Pneumonia
The risk of pneumonia is higher with clozapine than with other second-generation antipsychotics [144–146], and pneumonia associated with clozapine has a higher mortality rate than its better-recognized cardiovascular and hematological adverse effects [145, 147]. Several clozapine-associated effects may contribute to increasing the risk of aspiration and the onset of pneumonia, including sialorrhea, sedation, and dysphagia/decreased gastrointestinal motility; moreover, clozapine blood levels may increase once an infection is established, and this may be compounded in smokers who are forced to stop smoking because of pneumonia. A finding of pneumonia may require a reduction or interruption of clozapine therapy until the infection is resolved [147]. This highlights the importance of reducing the chances of side effects by using the lowest effective clozapine dosage, while remaining vigilant for signs of respiratory tract infection. Update and maintain vaccination against seasonal influenza and COVID-19. Family members and caregivers should be informed of the dangers, risk factors, and signs of aspiration pneumonia.
Sialorrhea
Sialorrhea is one the most frequent adverse events of clozapine therapy (prevalence up to 90%), and may reduce treatment tolerance and adherence [148, 149]. Clozapine-induced sialorrhea may increase the risk of aspiration pneumonia in fragile patients and should be monitored at every visit. It is addressed in brief side-effect scales (e.g., the GASS-C) and can be further monitored using, for example, the two-item Drooling Severity and Frequency Scale (DSFS) [150], or the five-point Nocturnal Hypersalivation Rating Scale (NHRS) [151].
Treatment may include clozapine dose reduction, when possible, and anticholinergics. A meta-analysis of 19 studies suggests that diphenhydramine, propantheline, chlorpheniramine, and benzamide derivatives can reduce the rates of clozapine-induced sialorrhea, although caution is warranted because some agents (e.g., propantheline) increase the risk of constipation [152]. Thus, locally applied anticholinergic preparations with limited systemic exposure should be used [153, 154], but care should be taken to prevent accidental or intentional ingestion of toxic quantities, and they should be avoided in patients who struggle to follow administration instructions. In severe cases, when other approaches have failed, injection of botulinum toxin B into the salivary glands can be considered [155].
Clozapine-Induced Convulsions
Clozapine may cause EEG changes, but any association between EEG changes and the onset of seizures is unclear and generally prophylactic anticonvulsant therapy is not indicated in the absence of a history of or risk factors for seizures [156]. Clozapine lowers the seizure threshold in a dose-dependent fashion [157], and is associated with seizures at an annual incidence of approximately 5% [158, 159]. Whilst seizures have been reported in patients receiving low doses, the risk is increased at higher doses [160], and the FDA label urges caution when prescribing clozapine in patients with a history of seizures or other predisposing risk factors for seizure (e.g., CNS pathology, medications that lower the seizure threshold, alcohol abuse) [62].
Management of clozapine-induced seizures requires measuring serum drug concentrations and reducing the dose, as well as introducing an anticonvulsant. Consider adding valproate (not in women of child-bearing potential) or lamotrigine as seizure prophylaxis at higher clozapine doses [159]. Dose reduction and/or addition of antiepileptic therapy allows most patients to continue clozapine therapy [161].
Metabolic Syndrome and Clozapine
Cardiovascular mortality contributes significantly to the reduced life expectancy of people with schizophrenia [162, 163]. Metabolic changes include hyperglycemia, dyslipidemia, and weight gain [62, 164], which predispose patients to diabetes mellitus and cardiovascular disease. Clozapine is the antipsychotic with the highest risk of weight gain and other metabolic effects [164]. Clozapine-associated changes in glucose, insulin, and weight begin within 6–10 weeks of starting treatment [164, 165].
Clozapine’s action at numerous receptors has been implicated in these effects [166, 167], and the presence of one component of the metabolic syndrome is a risk factor for the other components [168].
FDA recommendations include regular monitoring of glucose levels, body weight, and symptoms of hyperglycemia (e.g., polydipsia, polyuria, polyphagia, weakness). Hemoglobin A1C levels are more sensitive than fasting glucose levels to detect prediabetes and diabetes, and do not require fasting status [169].
Options for management include considering metformin treatment to improve hyperglycemia, elevated triglycerides, and obesity [170, 171]. Glucagon-like peptide receptor agonists can increase insulin secretion while decreasing glucagon secretion and food intake, and have a larger effect on weight loss than metformin [172]. Lifestyle changes should clearly be promoted [173].
Conclusion
Patients meeting the criterial for TRS should be treated as soon as possible to improve their chances of achieving remission with clozapine; however, concern over side effects, monitoring requirements, and inexperience with prescribing often result in long delays that can expose patients to unnecessary risks and compromise their chances of achieving favorable long-term outcomes [4]. We have provided an overview of strategies to support early initiation of clozapine based on the latest evidence and our clinical experience, and have summarized the key elements in a checklist. A practical, evidence-based checklist for identifying and managing patients with TRS, such as provided here, should increase confidence in prescribing and monitoring clozapine, reducing treatment delays, and improving outcomes.
Acknowledgements
Medical writing support for revisions was provided by Richard Vernell, on behalf of Edra S.p.A., and was funded by Viatris.
Declarations
Funding
This project was supported by an unconditional grant from Viatris to support medical writing provided by EDRA S.p.A.; the sponsor had no role in study design, collection of evidence, interpretation of data, writing the manuscript, or the decision to publish. Open access publication was funded by EDRA S.p.A. with an unconditional grant from Viatris.
Conflict of interest
Dr. Correll has been a consultant and/or advisor to or has received honoraria from: AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Axsome, Cardio Diagnostics, Compass, Damitsa, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Medscape, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Pharmabrain, Recordati, Relmada, Reviva, Rovi, Seqirus, Servier, SK Life Science, Sumitomo Dainippon, Sunovion, Supernus, Takeda, Teva, and Viatris. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Relmada, Reviva, Rovi, and Teva. He has received grant support from Janssen and Takeda. He received royalties from UpToDate and holds stock options for Cardio Diagnostics, Mindpax, and LB Pharma. Dr. Agid has been a consultant and/or advisor to or has received honoraria as follows: Advisory Board/Consultant: Janssen-Ortho (Johnson & Johnson); Otsuka; Lundbeck; Allergan/Abbvie; Speaker: Janssen-Ortho (Johnson & Johnson); Lundbeck, Otsuka, Mylan Pharmaceuticals; HLS Therapeutics; Research Contracts: Janssen-Ortho (Johnson & Johnson); Otsuka; Boehringer Ingelheim; Neurocrine Bioscience; DiaMentis. Prof. Crespo-Facorro has received honoraria as a consultant and/or advisor to Angelini, Janssen/J&J, Lundbeck, Otsuka and Viatris. Dr. de Bartolomeis has participated in advisory boards, lectures and unrestricted presentations sponsored by Trivia- Mylan. Dr. Fagiolini is /has been a consultant and/or a speaker and/or has received research grants from: Angelini, Apsen, Boehringer Ingelheim, Lundbeck, Janssen, Viatris, Otsuka, Recordati, Sanofi Aventis, Sunovion, Glaxo Smith Kline. Dr. Seppala is a part-time employee of Viatris Finland as a Consultant counselling doctors on clozapine-related problems. He has participated as advisor/speaker in meetings organized by Viatris, Recordati, and Janssen-Cilag. Dr. Howes is a part-time employee of H. Lundbeck A/S and has received investigator-initiated research funding from and/or participated in advisory/ speaker meetings organized by Angellini, Autifony, Biogen, Boehringer-Ingelheim, Eli Lilly, Heptares, Global Medical Education, Invicro, Jansenn, Lundbeck, Neurocrine, Otsuka, Sunovion, Rand, Recordati, Roche and Viatris/ Mylan.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
CC and OH initiated the project. All authors were involved in literature searching and analysis for one or more of the main topics, and initial drafting of the corresponding sections. All authors critically revised the work and approved the final version of the manuscript for publication.
Footnotes
The original online version of this article was revised. The reference citation was corrected from 103 to 104 in few occurrences.
Change history
8/17/2022
A Correction to this paper has been published: 10.1007/s40263-022-00946-w
References
- 1.Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, et al. Schizophrenia. Nat Rev Dis Primer. 2015;1:15067. doi: 10.1038/nrdp.2015.67. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2019 Mental Disorders Collaborators Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137–150. doi: 10.1016/S2215-0366(21)00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev US. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- 4.Kane JM, Agid O, Baldwin ML, Howes O, Lindenmayer J-P, Marder S, et al. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry. 2019 doi: 10.4088/JCP.18com12123. [DOI] [PubMed] [Google Scholar]
- 5.Buckley PF. Treatment-resistant schizophrenia. Focus (Am Psychiatr Publ). 2020;18:364–367. doi: 10.1176/appi.focus.20200025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Collaborating Centre for Mental Health (UK). Psychosis and Schizophrenia in Adults: Treatment and Management: Updated Edition 2014 [Internet]. London: National Institute for Health and Care Excellence (UK); 2014 [cited 2022 Jan 20]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK248060/ [PubMed]
- 7.Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJM, Birnbaum ML, et al. Treatment-resistant schizophrenia: Treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174:216–229. doi: 10.1176/appi.ajp.2016.16050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lally J, Ajnakina O, Di Forti M, Trotta A, Demjaha A, Kolliakou A, et al. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol Med. 2016;46:3231–3240. doi: 10.1017/S0033291716002014. [DOI] [PubMed] [Google Scholar]
- 9.Correll CU, Brevig T, Brain C. Exploration of treatment-resistant schizophrenia subtypes based on a survey of 204 US psychiatrists. Neuropsychiatr Dis Treat. 2019;15:3461–3473. doi: 10.2147/NDT.S234813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siskind D, Orr S, Sinha S, Yu O, Brijball B, Warren N, et al. Rates of treatment-resistant schizophrenia from first-episode cohorts: systematic review and meta-analysis. Br J Psychiatry J Ment Sci. 2022;220:115–120. doi: 10.1192/bjp.2021.61. [DOI] [PubMed] [Google Scholar]
- 11.Mouchlianitis E, McCutcheon R, Howes OD. Brain-imaging studies of treatment-resistant schizophrenia: a systematic review. Lancet Psychiatry. 2016;3:451–463. doi: 10.1016/S2215-0366(15)00540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potkin SG, Kane JM, Correll CU, Lindenmayer J-P, Agid O, Marder SR, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6:1. doi: 10.1038/s41537-019-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howes OD, Kapur S. A neurobiological hypothesis for the classification of schizophrenia: type A (hyperdopaminergic) and type B (normodopaminergic) Br J Psychiatry J Ment Sci. 2014;205:1–3. doi: 10.1192/bjp.bp.113.138578. [DOI] [PubMed] [Google Scholar]
- 14.Brugger SP, Angelescu I, Abi-Dargham A, Mizrahi R, Shahrezaei V, Howes OD. Heterogeneity of striatal dopamine function in schizophrenia: meta-analysis of variance. Biol Psychiatry. 2020;87:215–224. doi: 10.1016/j.biopsych.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 2012;169:1203–1210. doi: 10.1176/appi.ajp.2012.12010144. [DOI] [PubMed] [Google Scholar]
- 16.Jauhar S, McCutcheon R, Borgan F, Veronese M, Nour M, Pepper F, et al. The relationship between cortical glutamate and striatal dopamine in first-episode psychosis: a cross-sectional multimodal PET and magnetic resonance spectroscopy imaging study. Lancet Psychiatry. 2018;5:816–823. doi: 10.1016/S2215-0366(18)30268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veronese M, Santangelo B, Jauhar S, D’Ambrosio E, Demjaha A, Salimbeni H, et al. A potential biomarker for treatment stratification in psychosis: evaluation of an [18F] FDOPA PET imaging approach. Neuropsychopharmacology. 2021;46:1122–1132. doi: 10.1038/s41386-020-00866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demjaha A, Egerton A, Murray RM, Kapur S, Howes OD, Stone JM, et al. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry. 2014;75:e11–13. doi: 10.1016/j.biopsych.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Pillinger T, Rogdaki M, McCutcheon RA, Hathway P, Egerton A, Howes OD. Altered glutamatergic response and functional connectivity in treatment resistant schizophrenia: the effect of riluzole and therapeutic implications. Psychopharmacology. 2019;236:1985–1997. doi: 10.1007/s00213-019-5188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mouchlianitis E, Bloomfield MAP, Law V, Beck K, Selvaraj S, Rasquinha N, et al. Treatment-resistant schizophrenia patients show elevated anterior cingulate cortex glutamate compared to treatment-responsive. Schizophr Bull. 2016;42:744–752. doi: 10.1093/schbul/sbv151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jauhar S, Veronese M, Nour MM, Rogdaki M, Hathway P, Turkheimer FE, et al. Determinants of treatment response in first-episode psychosis: an 18F-DOPA PET study. Mol Psychiatry. 2019;24:1502–1512. doi: 10.1038/s41380-018-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elkis H, Buckley PF. Treatment-resistant schizophrenia. Psychiatr Clin North Am. 2016;39:239–265. doi: 10.1016/j.psc.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Wimberley T, Støvring H, Sørensen HJ, Horsdal HT, MacCabe JH, Gasse C. Predictors of treatment resistance in patients with schizophrenia: a population-based cohort study. Lancet Psychiatry. 2016;3:358–366. doi: 10.1016/S2215-0366(15)00575-1. [DOI] [PubMed] [Google Scholar]
- 24.Stokes I, Griffiths SL, Jones R, Everard L, Jones PB, Fowler D, et al. Prevalence of treatment resistance and clozapine use in early intervention services. BJPsych Open. 2020;6:e107. doi: 10.1192/bjo.2020.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck K, McCutcheon R, Stephenson L, Schilderman M, Patel N, Ramsay R, et al. Prevalence of treatment-resistant psychoses in the community: a naturalistic study. J Psychopharmacol. 2019;33:1248–1253. doi: 10.1177/0269881119855995. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy JL, Altar CA, Taylor DL, Degtiar I, Hornberger JC. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol. 2014;29:63–76. doi: 10.1097/YIC.0b013e32836508e6. [DOI] [PubMed] [Google Scholar]
- 27.Correll CU, Brevig T, Brain C. Patient characteristics, burden and pharmacotherapy of treatment-resistant schizophrenia: results from a survey of 204 US psychiatrists. BMC Psychiatry. 2019;19:362. doi: 10.1186/s12888-019-2318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iasevoli F, Giordano S, Balletta R, Latte G, Formato MV, Prinzivalli E, et al. Treatment resistant schizophrenia is associated with the worst community functioning among severely-ill highly-disabling psychiatric conditions and is the most relevant predictor of poorer achievements in functional milestones. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:34–48. doi: 10.1016/j.pnpbp.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Velligan DI, Brain C, Bouérat Duvold L, Agid O. Caregiver burdens associated with treatment-resistant schizophrenia: a quantitative caregiver survey of experiences, attitudes, and perceptions. Front Psychiatry. 2019;10:584. doi: 10.3389/fpsyt.2019.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaar SJ, Natesan S, McCutcheon R, Howes OD. Antipsychotics: mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. 2020;172:107704. doi: 10.1016/j.neuropharm.2019.107704. [DOI] [PubMed] [Google Scholar]
- 31.Wagner E, Siafis S, Fernando P, Falkai P, Honer WG, Röh A, et al. Efficacy and safety of clozapine in psychotic disorders-a systematic quantitative meta-review. Transl Psychiatry. 2021;11:487. doi: 10.1038/s41398-021-01613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McQueen G, Sendt K-V, Gillespie A, Avila A, Lally J, Vallianatou K, et al. Changes in brain glutamate on switching to clozapine in treatment-resistant schizophrenia. Schizophr Bull. 2021;47:662–671. doi: 10.1093/schbul/sbaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuno Y, McCutcheon RA, Brugger SP, Howes OD. Heterogeneity and efficacy of antipsychotic treatment for schizophrenia with or without treatment resistance: a meta-analysis. Neuropsychopharmacol. 2020;45:622–631. doi: 10.1038/s41386-019-0577-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168:603–609. doi: 10.1176/appi.ajp.2011.10081224. [DOI] [PubMed] [Google Scholar]
- 35.Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study) Lancet. 2009;374:620–627. doi: 10.1016/S0140-6736(09)60742-X. [DOI] [PubMed] [Google Scholar]
- 36.Taipale H, Tanskanen A, Mehtälä J, Vattulainen P, Correll CU, Tiihonen J. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20) World Psychiatry. 2020;19:61–68. doi: 10.1002/wps.20699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vermeulen JM, van Rooijen G, van de Kerkhof MPJ, Sutterland AL, Correll CU, de Haan L. Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1–12.5 years. Schizophr Bull. 2019;45:315–329. doi: 10.1093/schbul/sby052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A, et al. Clozapine treatment for suicidality in schizophrenia: international suicide prevention trial (InterSePT) Arch Gen Psychiatry. 2003;60:82–91. doi: 10.1001/archpsyc.60.1.82. [DOI] [PubMed] [Google Scholar]
- 39.Vanasse A, Blais L, Courteau J, Cohen AA, Roberge P, Larouche A, et al. Comparative effectiveness and safety of antipsychotic drugs in schizophrenia treatment: a real-world observational study. Acta Psychiatr Scand. 2016;134:374–384. doi: 10.1111/acps.12621. [DOI] [PubMed] [Google Scholar]
- 40.Wimberley T, MacCabe JH, Laursen TM, Sørensen HJ, Astrup A, Horsdal HT, et al. Mortality and self-harm in association with clozapine in treatment-resistant schizophrenia. Am J Psychiatry. 2017;174:990–998. doi: 10.1176/appi.ajp.2017.16091097. [DOI] [PubMed] [Google Scholar]
- 41.Cho J, Hayes RD, Jewell A, Kadra G, Shetty H, MacCabe JH, et al. Clozapine and all-cause mortality in treatment-resistant schizophrenia: a historical cohort study. Acta Psychiatr Scand. 2019;139:237–247. doi: 10.1111/acps.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masuda T, Misawa F, Takase M, Kane JM, Correll CU. Association with hospitalization and all-cause discontinuation among patients with schizophrenia on clozapine vs other oral second-generation antipsychotics: a systematic review and meta-analysis of cohort studies. JAMA Psychiat. 2019;76:1052–1062. doi: 10.1001/jamapsychiatry.2019.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimomura Y, Kikuchi Y, Suzuki T, Uchida H, Mimura M, Takeuchi H. Antipsychotic treatment strategies for acute phase and treatment resistance in schizophrenia: a systematic review of the guidelines and algorithms. Schizophr Res. 2021;236:142–155. doi: 10.1016/j.schres.2021.07.040. [DOI] [PubMed] [Google Scholar]
- 44.Bachmann CJ, Aagaard L, Bernardo M, Brandt L, Cartabia M, Clavenna A, et al. International trends in clozapine use: a study in 17 countries. Acta Psychiatr Scand. 2017;136:37–51. doi: 10.1111/acps.12742. [DOI] [PubMed] [Google Scholar]
- 45.Howes OD, Vergunst F, Gee S, McGuire P, Kapur S, Taylor D. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201:481–485. doi: 10.1192/bjp.bp.111.105833. [DOI] [PubMed] [Google Scholar]
- 46.Thien K, O’Donoghue B. Delays and barriers to the commencement of clozapine in eligible people with a psychotic disorder: a literature review. Early Interv Psychiatry. 2019;13:18–23. doi: 10.1111/eip.12683. [DOI] [PubMed] [Google Scholar]
- 47.Taylor DM, Young C, Paton C. Prior antipsychotic prescribing in patients currently receiving clozapine: a case note review. J Clin Psychiatry. 2003;64:30–34. doi: 10.4088/jcp.v64n0107. [DOI] [PubMed] [Google Scholar]
- 48.Nielsen J, Dahm M, Lublin H, Taylor D. Psychiatrists’ attitude towards and knowledge of clozapine treatment. J Psychopharmacol. 2010;24:965–971. doi: 10.1177/0269881108100320. [DOI] [PubMed] [Google Scholar]
- 49.Farooq S, Choudry A, Cohen D, Naeem F, Ayub M. Barriers to using clozapine in treatment-resistant schizophrenia: systematic review. BJPsych Bull. 2019;43:8–16. doi: 10.1192/bjb.2018.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh B, Hughes AJ, Roerig JL. Comfort level and barriers to the appropriate use of clozapine: a preliminary survey of US psychiatric residents. Acad Psychiatry. 2020;44:53–58. doi: 10.1007/s40596-019-01134-7. [DOI] [PubMed] [Google Scholar]
- 51.Verdoux H, Quiles C, Bachmann CJ, Siskind D. Prescriber and institutional barriers and facilitators of clozapine use: a systematic review. Schizophr Res. 2018;201:10–19. doi: 10.1016/j.schres.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 52.Gören JL, Rose AJ, Smith EG, Ney JP. The business case for expanded clozapine utilization. Psychiatr Serv. 2016;67:1197–1205. doi: 10.1176/appi.ps.201500507. [DOI] [PubMed] [Google Scholar]
- 53.Nielsen J, Nielsen RE, Correll CU. Predictors of clozapine response in patients with treatment-refractory schizophrenia: results from a Danish Register Study. J Clin Psychopharmacol. 2012;32:678–683. doi: 10.1097/JCP.0b013e318267b3cd. [DOI] [PubMed] [Google Scholar]
- 54.Üçok A, Çikrikçili U, Karabulut S, Salaj A, Öztürk M, Tabak Ö, et al. Delayed initiation of clozapine may be related to poor response in treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2015;30:290–295. doi: 10.1097/YIC.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 55.Yada Y, Yoshimura B, Kishi Y. Correlation between delay in initiating clozapine and symptomatic improvement. Schizophr Res. 2015;168:585–586. doi: 10.1016/j.schres.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 56.Yoshimura B, Yada Y, So R, Takaki M, Yamada N. The critical treatment window of clozapine in treatment-resistant schizophrenia: secondary analysis of an observational study. Psychiatry Res. 2017;250:65–70. doi: 10.1016/j.psychres.2017.01.064. [DOI] [PubMed] [Google Scholar]
- 57.Demjaha A, Lappin JM, Stahl D, Patel MX, MacCabe JH, Howes OD, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med. 2017;47:1981–1989. doi: 10.1017/S0033291717000435. [DOI] [PubMed] [Google Scholar]
- 58.Ortiz BB, Higuchi CH, Noto C, Joyce DW, Correll CU, Bressan RA, et al. A symptom combination predicting treatment-resistant schizophrenia—a strategy for real-world clinical practice. Schizophr Res. 2020;218:195–200. doi: 10.1016/j.schres.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Chan SKW, Chan HYV, Honer WG, Bastiampillai T, Suen YN, Yeung WS, et al. Predictors of treatment-resistant and clozapine-resistant schizophrenia: a 12-year follow-up study of first-episode schizophrenia-spectrum disorders. Schizophr Bull. 2021;47:485–494. doi: 10.1093/schbul/sbaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dempster K, Li A, Sabesan P, Norman R, Palaniyappan L. Treatment resistance: a time-based approach for early identification in first episode psychosis. J Pers Med. 2021;11:711. doi: 10.3390/jpm11080711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Berardis D, Rapini G, Olivieri L, Di Nicola D, Tomasetti C, Valchera A, et al. Safety of antipsychotics for the treatment of schizophrenia: a focus on the adverse effects of clozapine. Ther Adv Drug Saf. 2018;9:237–256. doi: 10.1177/2042098618756261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.US Food and Drug Administration. Clozaril (clozapine) Prescribing information [Internet]. 2021. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/019758s088lbl.pdf
- 63.European Agency for the Evaluation of Medicinal Products (EMEA). SmPC Leponex (clozapine) [Internet]. 2002. Available from: https://www.ema.europa.eu/en/documents/referral/summary-information-referral-opinion-following-arbitration-pursuant-article-30-council-directive/83/ec-leponex-associated-names-international-non-proprietary-name-inn-clozapine-background-inform_en.pdf
- 64.Nielsen J, Young C, Ifteni P, Kishimoto T, Xiang Y-T, Schulte PFJ, et al. Worldwide differences in regulations of clozapine use. CNS Drugs. 2016;30:149–161. doi: 10.1007/s40263-016-0311-1. [DOI] [PubMed] [Google Scholar]
- 65.Nielsen J, Correll CU, Manu P, Kane JM. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74:603–613. doi: 10.4088/JCP.12r08064. [DOI] [PubMed] [Google Scholar]
- 66.Australia - National Prescribing Service MedicineWise. Clozaril [Internet]. [cited 2022 Jan 3]. Available from: https://www.nps.org.au/medicine-finder/clozaril-tablets#full-pi
- 67.Canada - AA PHARMA. CLOZAPINE PRODUCT MONOGRAPH [Internet]. Available from: https://www.aapharma.ca/downloads/en/PIL/2020/Clozapine-PrMono-ENG-May_6_2020.pdf
- 68.Japan Pharmaceuticals and Medical Devices Agency. Clozapine [Internet]. 2021. Available from: https://www.pmda.go.jp/files/000241004.pdf
- 69.Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World federation of societies of biological psychiatry (wfsbp) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13:318–378. doi: 10.3109/15622975.2012.696143. [DOI] [PubMed] [Google Scholar]
- 70.Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, Fischer BA, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36:71–93. doi: 10.1093/schbul/sbp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dixon LB, Dickerson F, Bellack AS, Bennett M, Dickinson D, Goldberg RW, et al. The 2009 schizophrenia PORT psychosocial treatment recommendations and summary statements. Schizophr Bull. 2010;36:48–70. doi: 10.1093/schbul/sbp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abidi S, Mian I, Garcia-Ortega I, Lecomte T, Raedler T, Jackson K, et al. Canadian guidelines for the pharmacological treatment of schizophrenia spectrum and other psychotic disorders in children and youth. Can J Psychiatry. 2017;62:635–647. doi: 10.1177/0706743717720197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schneider C, Corrigall R, Hayes D, Kyriakopoulos M, Frangou S. Systematic review of the efficacy and tolerability of clozapine in the treatment of youth with early onset schizophrenia. Eur Psychiatry. 2014;29:1–10. doi: 10.1016/j.eurpsy.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Krause M, Zhu Y, Huhn M, Schneider-Thoma J, Bighelli I, Chaimani A, et al. Efficacy, acceptability, and tolerability of antipsychotics in children and adolescents with schizophrenia: A network meta-analysis. Eur Neuropsychopharmacol. 2018;28:659–674. doi: 10.1016/j.euroneuro.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 75.Renzenbrink M, Wand APF. A systematic review of clozapine’s effectiveness for primary psychotic and bipolar disorders in older adults. Int Psychogeriatr. 2021;2:1–13. doi: 10.1017/S1041610220004172. [DOI] [PubMed] [Google Scholar]
- 76.Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthøj B, Gattaz WF, et al. World federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of schizophrenia. Part 3: Update 2015 Management of special circumstances: depression, suicidality, substance use disorders and pregnancy and lactation. World J Biol Psychiatry. 2015;16:142–170. doi: 10.3109/15622975.2015.1009163. [DOI] [PubMed] [Google Scholar]
- 77.Thanigaivel R, Bretag-Norris R, Amos A, McDermott B. A systematic review of maternal and infant outcomes after clozapine continuation in pregnancy. Int J Psychiatry Clin Pract. 2021 doi: 10.1080/13651501.2021.1936070. [DOI] [PubMed] [Google Scholar]
- 78.Romaine E, McAllister-Williams RH. Guidelines on prescribing psychotropic medication during the perinatal period. Br J Hosp Med. 2019;80:27–32. doi: 10.12968/hmed.2019.80.1.27. [DOI] [PubMed] [Google Scholar]
- 79.Fortinguerra F, Clavenna A, Bonati M. Psychotropic drug use during breastfeeding: a review of the evidence. Pediatrics. 2009;124:e547–556. doi: 10.1542/peds.2009-0326. [DOI] [PubMed] [Google Scholar]
- 80.McAllister-Williams RH, Baldwin DS, Cantwell R, Easter A, Gilvarry E, Glover V, et al. British Association for Psychopharmacology consensus guidance on the use of psychotropic medication preconception, in pregnancy and postpartum 2017. J Psychopharmacol. 2017;31:519–552. doi: 10.1177/0269881117699361. [DOI] [PubMed] [Google Scholar]
- 81.Payne JL. Psychopharmacology in pregnancy and breastfeeding. Med Clin North Am. 2019;103:629–650. doi: 10.1016/j.mcna.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 82.de Leon J, Schoretsanitis G, Smith RL, Molden E, Solismaa A, Seppälä N, et al. An international adult guideline for making clozapine titration safer by using six ancestry-based personalized dosing titrations, CRP, and clozapine levels. Pharmacopsychiatry. 2021 doi: 10.1055/a-1625-6388. [DOI] [PubMed] [Google Scholar]
- 83.González-Esquivel DF, Jung-Cook H, Baptista T, de Leon J. Amerindians may need clozapine dosing similar to that of Asians. Rev Psiquiatr Salud Ment. 2021;14:177–179. doi: 10.1016/j.rpsmen.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 84.Ruan C-J, Zang Y-N, Wang C-Y, Cheng Y-H, Sun C, Spina E, et al. Clozapine metabolism in east asians and caucasians: a pilot exploration of the prevalence of poor metabolizers and a systematic review. J Clin Psychopharmacol. 2019;39:135–144. doi: 10.1097/JCP.0000000000001018. [DOI] [PubMed] [Google Scholar]
- 85.Clark SR, Warren NS, Kim G, Jankowiak D, Schubert KO, Kisely S, et al. Elevated clozapine levels associated with infection: A systematic review. Schizophr Res. 2018;192:50–56. doi: 10.1016/j.schres.2017.03.045. [DOI] [PubMed] [Google Scholar]
- 86.Albitar O, Harun SN, Zainal H, Ibrahim B, Sheikh Ghadzi SM. Population pharmacokinetics of clozapine: a systematic review. BioMed Res Int. 2020;2020:9872936. doi: 10.1155/2020/9872936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siskind D, Honer WG, Clark S, Correll CU, Hasan A, Howes O, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci JPN. 2020;45:222–223. doi: 10.1503/jpn.200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Remington G, Agid O, Foussias G, Ferguson L, McDonald K, Powell V. Clozapine and therapeutic drug monitoring: is there sufficient evidence for an upper threshold? Psychopharmacology. 2013;225:505–518. doi: 10.1007/s00213-012-2922-7. [DOI] [PubMed] [Google Scholar]
- 89.Siskind D, Sharma M, Pawar M, Pearson E, Wagner E, Warren N, et al. Clozapine levels as a predictor for therapeutic response: a systematic review and meta-analysis. Acta Psychiatr Scand. 2021;144:422–432. doi: 10.1111/acps.13361. [DOI] [PubMed] [Google Scholar]
- 90.Beck K, McCutcheon R, Bloomfield MAP, Gaughran F, Reis Marques T, MacCabe J, et al. The practical management of refractory schizophrenia–the Maudsley Treatment REview and Assessment Team service approach. Acta Psychiatr Scand. 2014;130:427–438. doi: 10.1111/acps.12327. [DOI] [PubMed] [Google Scholar]
- 91.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 92.Østergaard SD, Lemming OM, Mors O, Correll CU, Bech P. PANSS-6: a brief rating scale for the measurement of severity in schizophrenia. Acta Psychiatr Scand. 2016;133:436–444. doi: 10.1111/acps.12526. [DOI] [PubMed] [Google Scholar]
- 93.Østergaard SD, Opler MGA, Correll CU. Bridging the measurement gap between research and clinical care in schizophrenia: positive and negative syndrome scale-6 (PANSS-6) and other assessments based on the simplified negative and positive symptoms interview (SNAPSI) Innov Clin Neurosci. 2017;14:68–72. [PMC free article] [PubMed] [Google Scholar]
- 94.Østergaard SD, Foldager L, Mors O, Bech P, Correll CU. The validity and sensitivity of PANSS-6 in treatment-resistant schizophrenia. Acta Psychiatr Scand. 2018;138:420–431. doi: 10.1111/acps.12952. [DOI] [PubMed] [Google Scholar]
- 95.Kølbæk P, Dines D, Holm T, Blicher AB, Sørensen RD, O’Leary KM, et al. Clinical validation of ratings on the six-item positive and negative syndrome scale obtained via the simplified negative and positive symptoms interview. J Psychopharmacol. 2021;35:1081–1090. doi: 10.1177/0269881121996890. [DOI] [PubMed] [Google Scholar]
- 96.Wagner E, Kane JM, Correll CU, Howes O, Siskind D, Honer WG, et al. Clozapine combination and augmentation strategies in patients with schizophrenia-recommendations from an international expert survey among the treatment response and resistance in psychosis (TRRIP) working group. Schizophr Bull. 2020;46:1459–1470. doi: 10.1093/schbul/sbaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wagner E, Löhrs L, Siskind D, Honer WG, Falkai P, Hasan A. Clozapine augmentation strategies—a systematic meta-review of available evidence. Treatment options for clozapine resistance. J Psychopharmacol. 2019;33:423–435. doi: 10.1177/0269881118822171. [DOI] [PubMed] [Google Scholar]
- 98.Siskind DJ, Lee M, Ravindran A, Zhang Q, Ma E, Motamarri B, et al. Augmentation strategies for clozapine refractory schizophrenia: a systematic review and meta-analysis. Aust N Z J Psychiatry. 2018;52:751–767. doi: 10.1177/0004867418772351. [DOI] [PubMed] [Google Scholar]
- 99.Correll CU, Rubio JM, Inczedy-Farkas G, Birnbaum ML, Kane JM, Leucht S. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiat. 2017;74:675–684. doi: 10.1001/jamapsychiatry.2017.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tiihonen J, Taipale H, Mehtälä J, Vattulainen P, Correll CU, Tanskanen A. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiat. 2019;76:499–507. doi: 10.1001/jamapsychiatry.2018.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang G, Zheng W, Li X-B, Wang S-B, Cai D-B, Yang X-H, et al. ECT augmentation of clozapine for clozapine-resistant schizophrenia: a meta-analysis of randomized controlled trials. J Psychiatr Res. 2018;105:23–32. doi: 10.1016/j.jpsychires.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 102.Blackman G, Oloyede E, Horowitz M, Harland R, Taylor D, MacCabe J, et al. Reducing the risk of withdrawal symptoms and relapse following clozapine discontinuation-is it feasible to develop evidence-based guidelines? Schizophr Bull. 2022;48:176–189. doi: 10.1093/schbul/sbab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hiemke C, Bergemann N, Clement HW, Conca A, Deckert J, Domschke K, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51:9–62. doi: 10.1055/s-0043-116492. [DOI] [PubMed] [Google Scholar]
- 104.Schoretsanitis G, Kane JM, Correll CU, Marder SR, Citrome L, Newcomer JW, et al. Blood levels to optimize antipsychotic treatment in clinical practice: a joint consensus statement of the American society of clinical psychopharmacology and the therapeutic drug monitoring task force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry. 2020;81:19. doi: 10.4088/JCP.19cs13169. [DOI] [PubMed] [Google Scholar]
- 105.Turrion MC, Perez J, Bernardo M, Fernandez-Egea E. Intra-individual variation of clozapine and norclozapine plasma levels in clinical practice. Rev Psiquiatr Salud Ment. 2020;13:31–35. doi: 10.1016/j.rpsm.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 106.Stark A, Scott J. A review of the use of clozapine levels to guide treatment and determine cause of death. Aust N Z J Psychiatry. 2012;46:816–825. doi: 10.1177/0004867412438871. [DOI] [PubMed] [Google Scholar]
- 107.Kitchen D, Till A, Xavier P. Routine clozapine assay monitoring to improve the management of treatment-resistant schizophrenia. BJPsych Bull. 2021;2:1–4. doi: 10.1192/bjb.2021.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tanzer T, Warren N, McMahon L, Barras M, Kisely S, Brooks E, et al. Treatment strategies for clozapine-induced nocturnal enuresis and urinary incontinence: a systematic review. CNS Spectr. 2022 doi: 10.1017/S1092852922000050. [DOI] [PubMed] [Google Scholar]
- 109.van Strien AM, Keijsers CJPW, Derijks HJ, van Marum RJ. Rating scales to measure side effects of antipsychotic medication: a systematic review. J Psychopharmacol. 2015;29:857–866. doi: 10.1177/0269881115593893. [DOI] [PubMed] [Google Scholar]
- 110.Hynes C, Keating D, McWilliams S, Madigan K, Kinsella A, Maidment I, et al. Glasgow antipsychotic side-effects scale for clozapine—development and validation of a clozapine-specific side-effects scale. Schizophr Res. 2015;168:505–513. doi: 10.1016/j.schres.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 111.Barnes TRE, MacCabe JH, Kane JM, Delgado O, Paton C. The physical health and side-effect monitoring of patients prescribed clozapine: data from a clinical audit conducted in UK mental health services. Ther Adv Psychopharmacol. 2020;10:2045125320937908. doi: 10.1177/2045125320937908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mijovic A, MacCabe JH. Clozapine-induced agranulocytosis. Ann Hematol. 2020;99:2477–2482. doi: 10.1007/s00277-020-04215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329:162–167. doi: 10.1056/NEJM199307153290303. [DOI] [PubMed] [Google Scholar]
- 114.Myles N, Myles H, Xia S, Large M, Kisely S, Galletly C, et al. Meta-analysis examining the epidemiology of clozapine-associated neutropenia. Acta Psychiatr Scand. 2018;138:101–109. doi: 10.1111/acps.12898. [DOI] [PubMed] [Google Scholar]
- 115.Flanagan RJ, Dunk L. Haematological toxicity of drugs used in psychiatry. Hum Psychopharmacol. 2008;23(Suppl 1):27–41. doi: 10.1002/hup.917. [DOI] [PubMed] [Google Scholar]
- 116.Legge SE, Walters JT. Genetics of clozapine-associated neutropenia: recent advances, challenges and future perspective. Pharmacogenomics. 2019;20:279–290. doi: 10.2217/pgs-2018-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oloyede E, Dzahini O, Barnes N, Mijovic A, Gandhi S, Stuart-Smith S, et al. Benign ethnic neutropenia: an analysis of prevalence, timing and identification accuracy in two large inner-city NHS hospitals. BMC Psychiatry. 2021;21:502. doi: 10.1186/s12888-021-03514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Manu P, Sarvaiya N, Rogozea LM, Kane JM, Correll CU. Benign ethnic neutropenia and clozapine use: a systematic review of the evidence and treatment recommendations. J Clin Psychiatry. 2016;77:e909–916. doi: 10.4088/JCP.15r10085. [DOI] [PubMed] [Google Scholar]
- 119.Manu P, Lapitskaya Y, Shaikh A, Nielsen J. Clozapine rechallenge after major adverse effects: clinical guidelines based on 259 cases. Am J Ther. 2018;25:e218–e223. doi: 10.1097/MJT.0000000000000715. [DOI] [PubMed] [Google Scholar]
- 120.Lally J, Malik S, Krivoy A, Whiskey E, Taylor DM, Gaughran FP, et al. The use of granulocyte colony-stimulating factor in clozapine rechallenge: a systematic review. J Clin Psychopharmacol. 2017;37:600–604. doi: 10.1097/JCP.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 121.Myles N, Myles H, Clark SR, Bird R, Siskind D. Use of granulocyte-colony stimulating factor to prevent recurrent clozapine-induced neutropenia on drug rechallenge: a systematic review of the literature and clinical recommendations. Aust N Z J Psychiatry. 2017;51:980–989. doi: 10.1177/0004867417720516. [DOI] [PubMed] [Google Scholar]
- 122.Silva E, Higgins M, Hammer B, Stephenson P. Clozapine rechallenge and initiation despite neutropenia—a practical, step-by-step guide. BMC Psychiatry. 2020;20:279. doi: 10.1186/s12888-020-02592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Béchard L, Corbeil O, Plante M, Thivierge M-A, Lafrenière C-É, Roy M-A, et al. Clozapine rechallenge following neutropenia using granulocyte colony-stimulating factor: a Quebec case series. J Psychopharmacol. 2021;35:1152–1157. doi: 10.1177/02698811211029737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bellissima BL, Tingle MD, Cicović A, Alawami M, Kenedi C. A systematic review of clozapine-induced myocarditis. Int J Cardiol. 2018;259:122–129. doi: 10.1016/j.ijcard.2017.12.102. [DOI] [PubMed] [Google Scholar]
- 125.De Las CC, Sanz EJ, Ruan C-J, de Leon J. Clozapine-associated myocarditis in the World Health Organization’s pharmacovigilance database: Focus on reports from various countries. Rev Psiquiatr Salud Ment. 2021;S1888–9891(21):00070–77. doi: 10.1016/j.rpsm.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 126.Alawami M, Wasywich C, Cicovic A, Kenedi C. A systematic review of clozapine induced cardiomyopathy. Int J Cardiol. 2014;176:315–320. doi: 10.1016/j.ijcard.2014.07.103. [DOI] [PubMed] [Google Scholar]
- 127.Siskind D, Sidhu A, Cross J, Chua Y-T, Myles N, Cohen D, et al. Systematic review and meta-analysis of rates of clozapine-associated myocarditis and cardiomyopathy. Aust N Z J Psychiatry. 2020;54:467–481. doi: 10.1177/0004867419898760. [DOI] [PubMed] [Google Scholar]
- 128.Sandarsh S, Bishnoi RJ, Shashank RB, Miller BJ, Freudenreich O, McEvoy JP. Monitoring for myocarditis during treatment initiation with clozapine. Acta Psychiatr Scand. 2021;144:194–200. doi: 10.1111/acps.13328. [DOI] [PubMed] [Google Scholar]
- 129.Bellissima BL, Vara A, Helsby N, Garavan F, Tingle MD. Incidence and investigation of potential risk-factors for clozapine-associated myocarditis and cardiomyopathy in a New Zealand cohort. Psychiatry Res. 2021;299:113873. doi: 10.1016/j.psychres.2021.113873. [DOI] [PubMed] [Google Scholar]
- 130.Vickers M, Ramineni V, Malacova E, Eriksson L, McMahon K, Moudgil V, et al. Risk factors for clozapine-induced myocarditis and cardiomyopathy: a systematic review and meta-analysis. Acta Psychiatr Scand. 2022;145:442–455. doi: 10.1111/acps.13398. [DOI] [PubMed] [Google Scholar]
- 131.Ronaldson KJ, Fitzgerald PB, Taylor AJ, Topliss DJ, McNeil JJ. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45:458–465. doi: 10.3109/00048674.2011.572852. [DOI] [PubMed] [Google Scholar]
- 132.Patel RK, Moore AM, Piper S, Sweeney M, Whiskey E, Cole G, et al. Clozapine and cardiotoxicity—a guide for psychiatrists written by cardiologists. Psychiatry Res. 2019;282:112491. doi: 10.1016/j.psychres.2019.112491. [DOI] [PubMed] [Google Scholar]
- 133.Griffin JM, Woznica E, Gilotra NA, Nucifora FC. Clozapine-associated myocarditis: a protocol for monitoring upon clozapine initiation and recommendations for how to conduct a clozapine rechallenge. J Clin Psychopharmacol. 2021;41:180–185. doi: 10.1097/JCP.0000000000001358. [DOI] [PubMed] [Google Scholar]
- 134.Every-Palmer S, Inns SJ, Grant E, Ellis PM. Effects of clozapine on the gut: cross-sectional study of delayed gastric emptying and small and large intestinal dysmotility. CNS Drugs. 2019;33:81–91. doi: 10.1007/s40263-018-0587-4. [DOI] [PubMed] [Google Scholar]
- 135.Shirazi A, Stubbs B, Gomez L, Moore S, Gaughran F, Flanagan RJ, et al. Prevalence and predictors of clozapine-associated constipation: a systematic review and meta-analysis. Int J Mol Sci. 2016;17:E863. doi: 10.3390/ijms17060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cohen D, Bogers JPAM, van Dijk D, Bakker B, Schulte PFJ. Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy. J Clin Psychiatry. 2012;73:1307–1312. doi: 10.4088/JCP.11r06977. [DOI] [PubMed] [Google Scholar]
- 137.Cohen D. Clozapine and gastrointestinal hypomotility. CNS Drugs. 2017;31:1083–1091. doi: 10.1007/s40263-017-0481-5. [DOI] [PubMed] [Google Scholar]
- 138.Palmer SE, McLean RM, Ellis PM, Harrison-Woolrych M. Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry. 2008;69:759–768. doi: 10.4088/jcp.v69n0509. [DOI] [PubMed] [Google Scholar]
- 139.West S, Rowbotham D, Xiong G, Kenedi C. Clozapine induced gastrointestinal hypomotility: A potentially life threatening adverse event A review of the literature. Gen Hosp Psychiatry. 2017;46:32–37. doi: 10.1016/j.genhosppsych.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 140.McMillan SC, Williams FA. Validity and reliability of the constipation assessment scale. Cancer Nurs. 1989;12:183–188. doi: 10.1097/00002820-198906000-00012. [DOI] [PubMed] [Google Scholar]
- 141.Drossman DA, Hasler WL. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016;150:1257–1261. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 142.Every-Palmer S, Inns SJ, Ellis PM. Constipation screening in people taking clozapine: a diagnostic accuracy study. Schizophr Res. 2020;220:179–186. doi: 10.1016/j.schres.2020.03.032. [DOI] [PubMed] [Google Scholar]
- 143.Every-Palmer S, Ellis PM. Clozapine-induced gastrointestinal hypomotility: A 22-year bi-national pharmacovigilance study of serious or fatal “slow gut” reactions, and comparison with international drug safety advice. CNS Drugs. 2017;31:699–709. doi: 10.1007/s40263-017-0448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stoecker ZR, George WT, O’Brien JB, Jancik J, Colon E, Rasimas JJ. Clozapine usage increases the incidence of pneumonia compared with risperidone and the general population: a retrospective comparison of clozapine, risperidone, and the general population in a single hospital over 25 months. Int Clin Psychopharmacol. 2017;32:155–160. doi: 10.1097/YIC.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 145.de Leon J, Sanz EJ, Norén GN, De Las CC. Pneumonia may be more frequent and have more fatal outcomes with clozapine than with other second-generation antipsychotics. World Psychiatry. 2020;19:120–121. doi: 10.1002/wps.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chang C-K, Chen P-H, Pan C-H, Su S-S, Tsai S-Y, Chen C-C, et al. Antipsychotic medications and the progression of upper respiratory infection to pneumonia in patients with schizophrenia. Schizophr Res. 2020;222:327–334. doi: 10.1016/j.schres.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 147.De Leon J, Sanz EJ, De Las CC. Data from the world health organization’s pharmacovigilance database supports the prominent role of pneumonia in mortality associated with clozapine adverse drug reactions. Schizophr Bull. 2020;46:1–3. doi: 10.1093/schbul/sbz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maher S, Cunningham A, O’Callaghan N, Byrne F, Mc Donald C, McInerney S, et al. Clozapine-induced hypersalivation: an estimate of prevalence, severity and impact on quality of life. Ther Adv Psychopharmacol. 2016;6:178–184. doi: 10.1177/2045125316641019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ishikawa S, Kobayashi M, Hashimoto N, Mikami H, Tanimura A, Narumi K, et al. Association between n-desmethylclozapine and clozapine-induced sialorrhea: involvement of increased nocturnal salivary secretion via muscarinic receptors by N-desmethylclozapine. J Pharmacol Exp Ther. 2020;375:376–384. doi: 10.1124/jpet.120.000164. [DOI] [PubMed] [Google Scholar]
- 150.Thomas-Stonell N, Greenberg J. Three treatment approaches and clinical factors in the reduction of drooling. Dysphagia. 1988;3:73–78. doi: 10.1007/BF02412423. [DOI] [PubMed] [Google Scholar]
- 151.Spivak B, Adlersberg S, Rosen L, Gonen N, Mester R, Weizman A. Trihexyphenidyl treatment of clozapine-induced hypersalivation. Int Clin Psychopharmacol. 1997;12:213–215. doi: 10.1097/00004850-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 152.Chen S-Y, Ravindran G, Zhang Q, Kisely S, Siskind D. Treatment strategies for clozapine-induced sialorrhea: a systematic review and meta-analysis. CNS Drugs. 2019;33:225–238. doi: 10.1007/s40263-019-00612-8. [DOI] [PubMed] [Google Scholar]
- 153.Mubaslat O, Lambert T. The effect of sublingual atropine sulfate on clozapine-induced hypersalivation: a multicentre, randomised placebo-controlled trial. Psychopharmacology. 2020;237:2905–2915. doi: 10.1007/s00213-020-05627-4. [DOI] [PubMed] [Google Scholar]
- 154.Schoretsanitis G, Kuzin M, Kane JM, Hiemke C, Paulzen M, Haen E. Elevated clozapine concentrations in clozapine-treated patients with hypersalivation. Clin Pharmacokinet. 2021;60:329–335. doi: 10.1007/s40262-020-00944-5. [DOI] [PubMed] [Google Scholar]
- 155.Steinlechner S, Klein C, Moser A, Lencer R, Hagenah J. Botulinum toxin B as an effective and safe treatment for neuroleptic-induced sialorrhea. Psychopharmacology. 2010;207:593–597. doi: 10.1007/s00213-009-1689-y. [DOI] [PubMed] [Google Scholar]
- 156.Jackson A, Seneviratne U. EEG changes in patients on antipsychotic therapy: a systematic review. Epilepsy Behav. 2019;95:1–9. doi: 10.1016/j.yebeh.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 157.Pisani F, Oteri G, Costa C, Di Raimondo G, Di Perri R. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25:91–110. doi: 10.2165/00002018-200225020-00004. [DOI] [PubMed] [Google Scholar]
- 158.Varma S, Bishara D, Besag FMC, Taylor D. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1:47–66. doi: 10.1177/2045125311405566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29:101–111. doi: 10.1007/s40263-014-0222-y. [DOI] [PubMed] [Google Scholar]
- 160.Rohde C, Nielsen J. Managing common adverse effects of clozapine [Internet]. Oxford University Press; 2018. Available from: http://www.oxfordmedicine.com/view/10.1093/med/9780198828761.001.0001/med-9780198828761-chapter-7. 10.1093/med/9780198828761.003.0007
- 161.Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55:153–156. [PubMed] [Google Scholar]
- 162.Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry. 2017;4:295–301. doi: 10.1016/S2215-0366(17)30078-0. [DOI] [PubMed] [Google Scholar]
- 163.Westman J, Eriksson SV, Gissler M, Hällgren J, Prieto ML, Bobo WV, et al. Increased cardiovascular mortality in people with schizophrenia: a 24-year national register study. Epidemiol Psychiatr Sci. 2018;27:519–527. doi: 10.1017/S2045796017000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:64–77. doi: 10.1016/S2215-0366(19)30416-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Howes OD, Bhatnagar A, Gaughran FP, Amiel SA, Murray RM, Pilowsky LS. A prospective study of impairment in glucose control caused by clozapine without changes in insulin resistance. Am J Psychiatry. 2004;161:361–363. doi: 10.1176/appi.ajp.161.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Correll CU, Lencz T, Malhotra AK. Antipsychotic drugs and obesity. Trends Mol Med. 2011;17:97–107. doi: 10.1016/j.molmed.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yuen JWY, Kim DD, Procyshyn RM, Panenka WJ, Honer WG, Barr AM. A focused review of the metabolic side-effects of clozapine. Front Endocrinol. 2021;12:609240. doi: 10.3389/fendo.2021.609240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bernardo M, Rico-Villademoros F, García-Rizo C, Rojo R, Gómez-Huelgas R. Real-world data on the adverse metabolic effects of second-generation antipsychotics and their potential determinants in adult patients: a systematic review of population-based studies. Adv Ther. 2021;38:2491–2512. doi: 10.1007/s12325-021-01689-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Manu P, Correll CU, Wampers M, van Winkel R, Yu W, Mitchell AJ, et al. Prediabetic increase in hemoglobin A1c compared with impaired fasting glucose in patients receiving antipsychotic drugs. Eur Neuropsychopharmacol. 2013;23:205–211. doi: 10.1016/j.euroneuro.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 170.Liu Z, Zheng W, Gao S, Qin Z, Li G, Ning Y. Metformin for treatment of clozapine-induced weight gain in adult patients with schizophrenia: a meta-analysis. Shanghai Arch Psychiatry. 2015;27:331–340. doi: 10.11919/j.issn.1002-0829.215071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Siskind DJ, Leung J, Russell AW, Wysoczanski D, Kisely S. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0156208. doi: 10.1371/journal.pone.0156208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Siskind D, Hahn M, Correll CU, Fink-Jensen A, Russell AW, Bak N, et al. Glucagon-like peptide-1 receptor agonists for antipsychotic-associated cardio-metabolic risk factors: a systematic review and individual participant data meta-analysis. Diabetes Obes Metab. 2019;21:293–302. doi: 10.1111/dom.13522. [DOI] [PubMed] [Google Scholar]
- 173.Speyer H, Jakobsen AS, Westergaard C, Nørgaard HCB, Jørgensen KB, Pisinger C, et al. Lifestyle interventions for weight management in people with serious mental illness: a systematic review with meta-analysis, trial sequential analysis, and meta-regression analysis exploring the mediators and moderators of treatment effects. Psychother Psychosom. 2019;88:350–362. doi: 10.1159/000502293. [DOI] [PubMed] [Google Scholar]