Dear Sirs,
Anti-LGI1 encephalitis is a rare homogeneous clinical syndrome, showing early faciobrachial dystonic seizures (FBDS) and other focal seizures, associated with cognitive and behavioral disturbances in the context of limbic encephalitis [1].
COVID-19 is an ongoing pandemic, for which the Italian Ministry of Health adopted a massive vaccination campaign starting January 2021 (https://salute.gov.it). The most frequently employed vaccines are based on mRNA (BNT122b2 and CX-024414) or viral vectors (ChAdOx1-S and Ad26.COV2.S). A few patients presenting with autoimmune encephalitis following COVID-19 vaccinations were reported, including a case diagnosed with anti-LGI1 encephalitis [2–4].
Herein, we report a case series of four Italian patients who developed anti-LGI1 encephalitis temporally associated with prior different COVID-19 vaccinations.
The demographic and clinical characteristics of the four patients are summarized in Table 1. The mean age was 56 years (18–66 years), and two were females. Disease onset occurred after a mean of 13 days (6–23 days) following COVID-19 vaccination (3 mRNA vaccines, 1 viral vector vaccine). Patients presented with FBDS (n = 2) (Video), other focal seizures (n = 2), behavioral disturbances (n = 3), cognitive impairment (n = 2), and hypersomnia (n = 1), and hyponatremia was detected in two cases. EEG revealed epileptiform and/or slow abnormalities in the fronto-temporal region in all patients, while MRI revealed T2/FLAIR hyper-intensity of the mesial temporal lobes in two cases (Fig. 1). CSF analysis was unremarkable in three patients and traumatic in case 2. Serum and CSF were analyzed with a standardized cell-based assay (CBA) kit (Euroimmun) for detecting antibodies against neuronal surface antigens (LGI1, CASPR2, NMDAR, AMPAR, GABABR, and DPPX). The diagnosis of anti-LGI1 encephalitis was based on anti-LGI1 positivity in serum (n = 4) and CSF (n = 2) and consistent anatomo-electro-clinical features. Patients were treated with anti-seizure medications (n = 4), steroid pulse therapy (n = 4), and intravenous immunoglobulin (n = 1), resulting in clinical recovery in all subjects. Case 1 died eight months after disease onset due to vanishing bile duct syndrome.
Table 1.
Patients’ demographic and clinical features
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age (years), sex | 73, female | 66, male | 18, female | 66, male |
| City | Rimini | Bologna | Bologna | Bologna |
| Past medical history | Unremarkable | Hypertension | Unremarkable | Polyallergic |
| Previous COVID-19 infection | No | No | No | No |
| Vaccination | Viral vector (ChAdOx1-S), 1st dose | mRNA (BNT62b2) 2nd dose | mRNA (CX-024414) 3rd dose | mRNA (BNT62b2) 2nd dose |
| Days from vaccination to disease onset | 14 days | 6 days | 23 days | 9 days |
| Clinical features | FBDS, behavioral disturbances | Cognitive impairment, behavioral disturbances | Focal seizures, short-term memory impairment | FBDS, focal seizures, behavioral disturbances, hypersomnia |
| Hyponatremia | Yes | No | No | Yes |
| EEG | Bilateral fronto-temporal sharp waves; electrographic temporal seizures | Right fronto-temporal sharp waves; electrographic temporal seizures | Right fronto-temporal sharp waves | Bilateral fronto-temporal epileptiform discharges |
| Brain MRI | Bilateral mesial temporal lobe T2-weighted hyper-intensity with swelling in the left hippocampus | Bilateral mesial temporal lobe T2-weighted hyper-intensity with swelling and contrast enhancement in the right amygdala and hippocampus | Normal | Normal |
| CSF analysis | Normal | Traumatic (protein 83 mg/dl, 11,000/cc erythrocytes, 12/cc leukocytes) | Normal | Normal |
| Anti-LGI1 positivity | Serum and CSF | Serum | Serum | Serum and CSF |
| Immunotherapy | Methylprednisolone 1000 mg for 5 days, subsequent oral steroid tapering | Methylprednisolone 1000 mg for 5 days, subsequent oral steroid tapering | Methylprednisolone 1000 mg for 5 days, subsequent oral steroid tapering | Methylprednisolone 500 mg for 5 days, IVIg 0.4/kg/day for 5 days |
| Other therapies | Valproate | Levetiracetam | Lacosamide levetiracetam | Lacosamide levetiracetam |
| Outcome (time at last follow-up) |
Seizure-free, normal mental status Died after 8 months due to vanishing bile duct syndrome |
Normal mental status (7 months) | Seizure-free, normal mental status (3 months) | Seizure-free, normal mental status (3 months) |
FBDS, faciobrachial dystonic seizures; IVIg, intravenous immunoglobulin
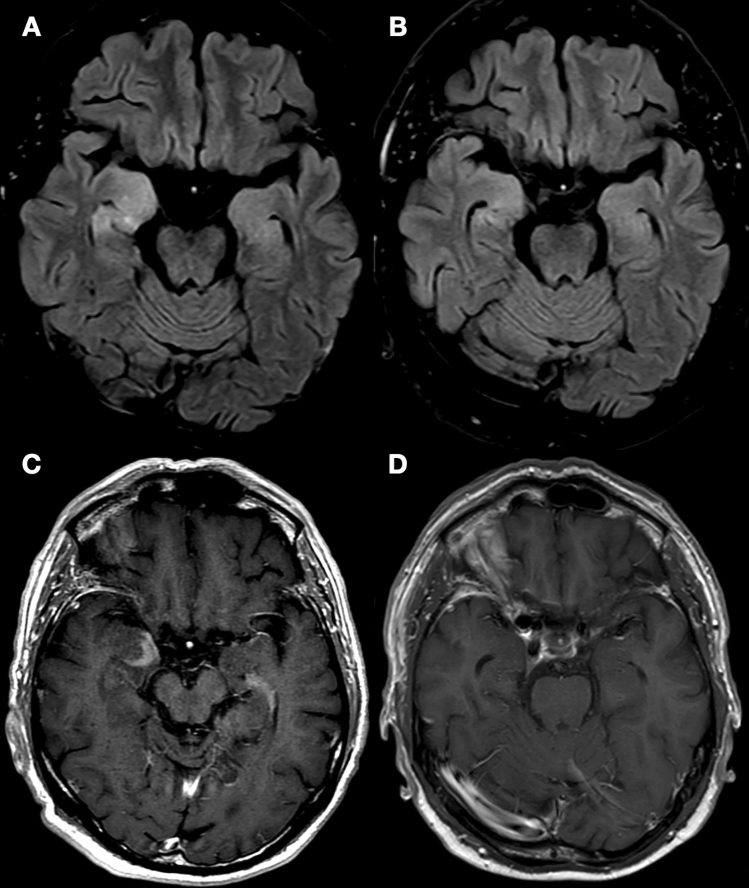
Fig. 1.
Brain MRI in Case 2. A Axial fluid-attenuated inversion recovery (FLAIR) images showed a right-predominant hyper-intensity of the mesial temporal lobes and swelling of the right amygdala. B Post-contrast T1-weighted sequences showed enhancement in the right amygdala. After 5 months from the introduction of steroid therapy, a control brain MRI shows a significant reduction of mesial temporal lobes hyper-intensity in FLAIR sequences (C) and a complete resolution of pathological contrast enhancement (D)
Three patients were living in the metropolitan area of Bologna, northern Italy, with approximately 1.000.000 inhabitants, where only one laboratory performs autoimmune anti-neuronal antibodies testing. In the last year, these were the only patients who tested positive for anti-LGI1 antibodies. During the previous year, only one patient with new-onset anti-LGI1 encephalitis was detected. Informed written consent was obtained for all patients but case 1, who is deceased; ethical committee approval was not required for this study.
We described four patients who developed anti-LGI1 encephalitis with classic clinical features following different COVID-19 vaccinations. The mean time from vaccination to disease onset was approximately 2 weeks, as in the only previously reported case [4]. Anti-LGI1 encephalitis represents an extremely rare disease, with a reported annual incidence ranging from 0.4/million (95% CI 0.3–0.5) in a French study [5] to 0.83/million (95% CI 0.45–1.40) in a nationwide Dutch study [1]. To our knowledge, it has not been previously associated with any other vaccination. Considering the population of Bologna, the crude incidence of anti-LGI1 encephalitis during the last year was 3/million (95% CI 0.6–8.8), with positive anti-LGI1 testing detected only in the three herein reported cases from Bologna. On the other hand, in the same area, there was just one patient with new-onset anti-LGI1 encephalitis during the previous year. We did not observe any further cases of autoimmune encephalitis following COVID-19 vaccinations. Even though this epidemiological data should be considered cautiously, the presence of a strict temporal relationship between disease onset and vaccination lets us hypothesize that anti-LGI1 encephalitis may represent a rare complication of COVID-19 vaccination. Four patients developed anti-LGI1 encephalitis after the vaccination with two different mRNA vaccines (three cases from this report, one previously reported [4]), while case 1 was administered a viral vector vaccine; therefore, should this association be confirmed, the trigger would not be a specific COVID-19 vaccine, but rather the immune response generated by the encoded SARS-CoV-2 spike protein or by shared adjuvants [6]. This might speculatively happen due to molecular mimicry and immune cross reaction, a process by which several other vaccines have been suspected to trigger autoimmunity, likely in genetically predisposed subjects [7]. However, as for other reported neurological and systemic autoimmune manifestations, whether the association of anti-LGI1 encephalitis with COVID-19 vaccines is coincidental or causal remains to be definitely determined [2–4, 6]. Additionally, the extraordinary benefits of mass COVID-19 vaccination in preventing disease morbidity and mortality surely outweigh the risk of developing autoimmune disorders in general and anti-LGI1 encephalitis specifically, especially considering the good clinical response of this condition to immunotherapies [1].
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 EEG findings in Case 1. (A) Interictal EEG showed right-predominant intermittent sharp and slow waves activity in the fronto-temporal regions. (B) Ictal EEG showed a focal electrographic seizure characterized by a 10 seconds rhythmic theta discharge followed by repetitive sharp waves in the right temporal regions, without clinical correlates. EEG acquisition settings: 10-20 system, longitudinal montage; recording speed 30 sec/page; sensitivity 7 μV/mm; time constant 0.1 sec; high-frequency filter 35 Hz. (PNG 2052 kb)
Supplementary file2 Case 4. The video shows three brief dystonic seizures involving the left arm, with corresponding muscle activity on surface electromyography, preceded by a slow wave predominant in the right fronto-central region on EEG, more evident in the third seizure. The three electromyography channels record, from top to bottom, the left orbicularis oris, biceps brachii, and wrist/hand extensors. (MP4 18162 kb)
Acknowledgements
We would like to thank Lara Alvisi for her assistance in preparing the Video of Case 4 and all patients for giving their consent to publication.
Author contributions
GMA and LM drafted the manuscript; All authors contributed to to either the acquisition, analysis, or interpretation of data; MG and PC supervised the study and critically revised the manuscript for intellectual content.
Funding
We declare no funding.
Declarations
Conflicts of interest
The authors declare that they have no competing interests.
Consent for publication
Informed written consent was obtained for all patients but case 1, who is deceased.
Footnotes
Gian Maria Asioli and Lorenzo Muccioli contributed equally.
References
- 1.van Sonderen A, Thijs RD, Coenders EC, et al. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology. 2016;87(14):1449–1456. doi: 10.1212/WNL.0000000000003173. [DOI] [PubMed] [Google Scholar]
- 2.Zuhorn F, Graf T, Klingebiel R, Schäbitz WR, Rogalewski A. Postvaccinal encephalitis after ChAdOx1 nCov-19. Ann Neurol. 2021;90(3):506–511. doi: 10.1002/ana.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaulen LD, Doubrovinskaia S, Mooshage C, et al. Neurological autoimmune diseases following vaccinations against SARS-CoV-2: a case series. Eur J Neurol. 2022;29(2):555–563. doi: 10.1111/ene.15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zlotnik Y, Gadoth A, Abu-Salameh I, Horev A, Novoa R, Ifergane G. Case report: anti-LGI1 encephalitis following COVID-19 vaccination. Front Immunol. 2022;12:813487. doi: 10.3389/fimmu.2021.813487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hébert J, Riche B, Vogrig A, et al. Epidemiology of paraneoplastic neurologic syndromes and autoimmune encephalitides in France. Neurol Neuroimmunol Neuroinflamm. 2020;7(6):e883. doi: 10.1212/NXI.0000000000000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Xu Z, Wang P, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165(4):386–401. doi: 10.1111/imm.13443. [DOI] [PubMed] [Google Scholar]
- 7.Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018 doi: 10.1038/cmi.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 EEG findings in Case 1. (A) Interictal EEG showed right-predominant intermittent sharp and slow waves activity in the fronto-temporal regions. (B) Ictal EEG showed a focal electrographic seizure characterized by a 10 seconds rhythmic theta discharge followed by repetitive sharp waves in the right temporal regions, without clinical correlates. EEG acquisition settings: 10-20 system, longitudinal montage; recording speed 30 sec/page; sensitivity 7 μV/mm; time constant 0.1 sec; high-frequency filter 35 Hz. (PNG 2052 kb)
Supplementary file2 Case 4. The video shows three brief dystonic seizures involving the left arm, with corresponding muscle activity on surface electromyography, preceded by a slow wave predominant in the right fronto-central region on EEG, more evident in the third seizure. The three electromyography channels record, from top to bottom, the left orbicularis oris, biceps brachii, and wrist/hand extensors. (MP4 18162 kb)