Abstract
Background
Hospitalized COVID-19 patients are at high risk of venous thromboembolism (VTE). Standard doses of anticoagulant prophylaxis may not be sufficiently effective for the prevention of VTE. The objective of this systematic-review and meta-analysis was to compare the efficacy and safety of high-dose versus low-dose thromboprophylaxis in hospitalized patients with COVID-19.
Material and methods
MEDLINE and EMBASE were searched up to October 2021 for randomized clinical trials (RCTs) comparing high-dose with low-dose thromboprophylaxis in hospitalized adult patients with COVID-19. The primary efficacy outcome was the occurrence of VTE and the primary safety outcome was major bleeding.
Results
A total of 5470 patients from 9 RCTs were included. Four trials included critically ill patients, four non-critically ill patients, and one included both. VTE occurred in 2.9% of patients on high-dose and in 5.7% of patients on low-dose thromboprophylaxis (relative risk [RR] 0.53; 95% confidence intervals [CIs], 0.41–0.69; I2 = 0%; number needed to treat for an additional beneficial outcome, 22). Major bleeding occurred in 2.5% and 1.4% of patients, respectively (RR 1.78; 95% CI, 1.20–2.66; I2 = 0%; number needed to treat for an additional harmful outcome, 100). All-cause mortality did not differ between groups (RR 0.97; 95% CI, 0.75–1.26; I2 = 47%). The risk of VTE was significantly reduced by high-dose thromboprophylaxis in non-critically ill (RR 0.54; 95% CI, 0.35–0.86; I2 = 0%), but not in critically ill patients (RR 0.69; 95% CI, 0.39–1.21; I2 = 36%).
Discussion
In hospitalized patients with COVID-19, high-dose thromboprophylaxis is more effective than low-dose for the prevention of VTE but increases the risk of major bleeding.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11739-022-03004-x.
Keywords: Anticoagulants, COVID-19, Heparin, SARS-CoV2, Venous thromboembolism
Introduction
The combination of systemic activation of coagulation, extensive pulmonary endotheliopathy and thrombo-inflammatory process caused by SARS-CoV2 results in an increased risk of thrombotic complications, mainly occurring in the venous system [1, 2]. In hospitalized COVID-19 patients, the incidence of venous thromboembolism (VTE) appears to be considerably higher compared to the incidence reported in medical patients with infection, sepsis or septic shock [3]. This risk increases with disease severity, with an estimated incidence of VTE of 17% in non-critically ill COVID-19 patients and 24% in those admitted to intensive care units (ICUs) [3].
Several observational studies assessed different antithrombotic treatments, mostly heparin, for the prevention of VTE in COVID-19 patients [4]. Available data suggested that, despite pharmacologic thromboprophylaxis, there is a substantial residual risk of VTE and a non-negligible risk of bleeding events [3, 5]. In addition, it remains unclear whether prophylactic or higher doses of anticoagulants may reduce in-hospital mortality in COVID-19 patients [6, 7]. Interpretation of findings from retrospective observational cohorts is complicated by the potential for residual confounding as the decision about the use, intensity and duration of anticoagulation in these studies was left to the treating physicians [3]. These observations left clinicians uncertain about the need for higher doses of thromboprophylaxis and resulted in heterogeneous indications from different guidance documents, in particular for critically ill patients.
Recent randomized controlled trials (RCTs) comparing the efficacy and safety of high-dose (i.e., intermediate or therapeutic dose) with low-dose thromboprophylaxis in patients hospitalised with COVID-19 reported conflicting findings [8–13]. Although the primary objectives of these studies were to evaluate whether high-dose anticoagulation may prevent COVID-19 progression to organ failure and death, most trials reported data on VTE and bleeding events.
The objective of this systematic review and meta-analysis was to evaluate the pooled efficacy and safety of high-dose versus low-dose thromboprophylaxis for VTE in hospitalized patients with COVID-19.
Methods
This systematic review and meta-analysis was performed following the Preferred Reporting Items for Systematic reviews and meta-analysis (PRISMA) guidelines [14].
Study search and selection
MEDLINE and EMBASE were searched from January 2020 up to October 2021 for RCTs comparing different regimens of venous thromboprophylaxis in hospitalized adult patients with COVID-19. The complete search strategy is shown in Supplementary Table 1.
Two authors (EV and AP) independently screened the title and abstract of all records identified by the search. RCTs including hospitalized patients with objectively diagnosed SARS-CoV2 infection and comparing high-dose anticoagulation (experimental group) with low-dose anticoagulation (control group) were eligible. Studies with a non-randomised design or published in languages other than English were excluded. Any disagreement was resolved through discussion between the review authors or involving a third review author (MDN).
Data extraction and risk of bias assessment
Two authors (EV and AP) independently extracted the following data: study characteristics (e.g., number of included patients, health-care setting), patient characteristics (e.g., age, sex, presence of comorbidities, disease severity), anticoagulant regimens in both experimental and control groups (e.g., type, dose, and duration of anticoagulation), number of patients who experienced the outcome of interest, and follow-up duration. Any disagreement was resolved through discussion between the review authors or involving a third review author (MDN).
The primary efficacy outcome was the occurrence of any symptomatic or incidental VTE. Secondary efficacy outcomes were all-cause mortality, acute myocardial infarction, acute ischemic stroke, acute peripheral arterial ischemic events, symptomatic or incidental deep vein thrombosis (DVT), and symptomatic or incidental pulmonary embolism (PE). The primary safety outcome was major bleeding as defined by the authors, while the secondary safety outcomes were clinically relevant non-major bleeding and heparin-induced thrombocytopenia.
The risk of bias was independently evaluated by two authors (EV and AP) with the revised Cochrane risk-of-bias tool for randomized trials [15]. Any disagreement was resolved through discussion between the review authors or involving a third review author (MDN).
Statistical analysis
Categorical variables were described as counts and percentages and continuous variables presented as median (interquartile range) or mean (standard deviation), as appropriate. Pooled risk ratios (RRs) with corresponding 95% confidence intervals (CIs) and prediction intervals (PIs) were calculated using a random-effects model. PIs show the extent of between-study variation and predict the possible effect in a future study that is comparable to those included in the meta-analysis. Heterogeneity among the included studies was evaluated by visual inspection of forest plots and the DerSimonian-Laird estimator, and it was loosely defined as moderate for I2 values of 30–60%, substantial for I2 values of 50–90%, and considerable for I2 values of 75–100% [16]. The pooled absolute risk reductions, number of patients needed to treat for an additional beneficial outcome (NNTB) or for an additional harmful outcome (NNTH) with their CIs were calculated respectively for primary efficacy and safety outcomes in case of statistically significant findings.
To help the interpretation of the data, we also expressed the results in absolute terms, i.e., the number of events that would occur in 1000 patients receiving high-dose thromboprophylaxis, using the observed risk in the low-dose group and the pooled relative risk with its 95% CIs.
Sensitivity analyses were planned to compare the efficacy and safety of intermediate or therapeutic dose versus low-dose thromboprophylaxis, and of high-dose versus low-dose thromboprophylaxis in critically ill and non-critically ill COVID-19 patients. Critically ill patients included those admitted to ICU, patients requiring invasive mechanical ventilation, and patients with severe COVID-19 as defined by the study authors. All other patients were considered non-critically ill. We included a study in one of the subgroups above if more than 75% of patients in that study fulfilled these criteria. Furthermore, post hoc sensitivity analyses were performed including only RCTs in which patients received the intended high-dose or low-dose thromboprophylaxis and to evaluate the efficacy of high-dose versus low-dose thromboprophylaxis in symptomatic VTE.
The presence of publication bias was assessed by funnel plots for the primary outcomes.
Statistical analyses were performed using R studio version 1.3.959, “meta” and “forest” packages [17].
Results
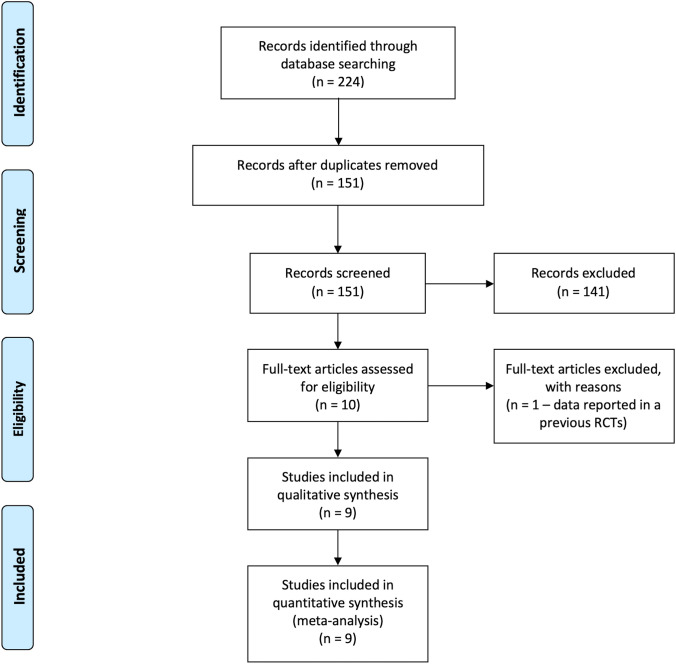
A total of 224 records were identified by the search (Fig. 1). After removing 73 duplicates, 141 records were excluded by title and abstract screening. Of the remaining 10 studies evaluated as full-text, 9 were included in the final analysis for a total of 5470 patients [8–13, 18–20]. The inter-reviewer agreement was excellent with a kappa statistic of 0.94.
Fig. 1.
PRISMA flow diagram
Characteristics of included studies
The main characteristics of the included RCTs are listed in Table 1. The size of the included studies ranged from 20 to 2226 patients and follow-up duration varied between 21 and 30 days. Four RCTs included critically ill patients [8, 11–13], four non-critically ill patients [9, 10, 18, 19], and one trial included a mixed population [20].
Table 1.
Characteristics of included studies
| Goligher EC (8) | Lawler PR (9) | Lemos ACB (11) | Lopes RD (10) | Sadeghipour P (13) | Perepu US (12) | |
|---|---|---|---|---|---|---|
| Patients | Critically ill | Non-critically ill | Critically ill | Non-critically ill | Critically ill | Critically ill |
| Experimental group, n | 536 | 1181 | 10 | 310 | 276 | 87 |
| Regimen | Therapeutic | Therapeutic | Therapeutic | Therapeutic | Intermediate | Intermediate |
| Dose | According to local protocols | According to local protocols | Enoxaparin: 1 mg/kg BIDa |
Rivaroxaban: 20 mg OD (stable patients)a Enoxaparin: 1 mg/kg BID (unstable patients)a |
Enoxaparin: 1 mg/kg ODa | Enoxaparin: 1 mg/kg ODa |
| Duration of anticoagulation | 14 days or until recovery | 14 days or until recovery | 4 up to 14 days | 30 days | 30 days | Not specified |
| Anticoagulant regimens after randomization, % | ||||||
| Therapeutic | 77.6 | 79.6 | 100 | 100 | – | – |
| Intermediate | 8.3 | 5.6 | – | – | 70.4 | 88.1 |
| Prophylactic | 3.4 | 5.8 | – | – | – | – |
| Other (not specified) | 8.3 | 8.7 | – | – | 19.6 | 11.9 |
| Control group, n | 567 | 1050 | 10 | 304 | 286 | 86 |
| Dose | According to local protocols | According to local protocols |
Enoxaparin: 40 mg ODa UFH: 5000 IU TIDa |
Enoxaparin: 40 mg ODa UFH: 5000 IU BID or TIDa |
Enoxaparin: 40 mg ODa | Enoxaparin: 40 mg ODa |
| Anticoagulant regimens after randomization, % | ||||||
| Therapeutic | 6.1 | 0.9 | – | – | – | – |
| Intermediate | 51.7 | 26.5 | – | – | – | – |
| Prophylactic | 40.4 | 71.7 | 100 | 99.7 | 66.9 | 78.8 |
| Other (not specified) | 1.8 | 0.8 | – | 0.3 | 23.1 | 21.2 |
| Duration of anticoagulation | 14 days or until recovery | 14 days or until recovery | Not specified | 30 days | 30 days | Not specified |
| VTE definition | Symptomatic DVT or PE | Symptomatic DVT or PE | Not specified | Not specified | Symptomatic DVT and PE | Not specified |
| Bleeding definition | ISTH | ISTH | TIMI bleeding criteria | ISTH | Bleeding Academy Research Consortium type 3 or 5 definition | ISTH |
| Follow-up | 21 days | 21 days | 28 days | 30 days | 30 days | 30 days |
| Sholzberg M (18) | Spyropoulos AC (20) | Marcos M (19) | |
|---|---|---|---|
| Patients | Non-critically ill | Mixed population | Non-critically ill |
| Experimental group, n | 228 | 129 | 33 |
| Regimen | Therapeutic | Therapeutic | Therapeutic |
| Dose |
Enoxaparin: 1 mg/kg BID or 1.5 mg/kg ODa Dalteparin: 200 IU/kg OD or 100 IU/Kg BIDa Tinzaparin: 175 IU/kg ODa UFH: IV bolus then continuous infusiona |
Enoxaparin: 1 mg/kg BIDa | Bemiparin: 115 IU/kg ODa |
| Duration of anticoagulation | Until the first of hospital discharge, day 28, study withdrawal, or death | During hospitalization | 10 days |
| Anticoagulant regimens after randomization, % | |||
| Therapeutic | 97.4 | 100 | 100 |
| Intermediate | – | – | – |
| Prophylactic | – | – | – |
| Other (not specified) | 2.6 | – | – |
| Control group, n | 237 | 124 | 32 |
| Dose |
Enoxaparin: 40 mg/kg ODa Dalteparin: 5000 IU ODa Tinzaparin: 4500 IU ODa Fondaparinux: 2.5 mg ODa UFH: 5000 IU BID or TIDa |
Enoxaparin: 30 or 40 mg OD or BIDa Dalteparin: 2500 IU or 5000 IU ODa UFH: up to 22500 IUa |
Bemiparin: 3500 IU ODa |
| Duration of anticoagulation | Until the first of hospital discharge, day 28, study withdrawal, or death | During hospitalization | 10 days |
| Anticoagulant regimens after randomization, % | |||
| Therapeutic | – | – | – |
| Intermediate | – | 38.7 | – |
| Prophylactic | 97.9 | 61.3 | 100 |
| Other (not specified) | 2.1 | – | – |
| VTE definition | Symptomatic or asymptomatic DVT or PE | Symptomatic or asymptomatic DVT or PE | Symptomatic DVT or PE |
| Bleeding definition | ISTH | ISTH | ISTH |
| Follow-up | 28 days | 30 ± 2 days | 10 and 30 days |
BID, twice daily; DVT, deep vein thrombosis; ISTH, international society on thrombosis and haemostasis; OD, once daily; PE, pulmonary embolism; TIMI, thrombolysis in myocardial infarction
aDose adjustment according to body weight and/or renal function
Experimental treatment consisted in intermediate or therapeutic dose anticoagulation in two and seven RCTs, respectively. All RCTs used standard in-hospital venous thromboprophylaxis as control treatment. Usual-care thromboprophylaxis was not protocol-mandated in most cases, and it was administered at a dose and duration decided by the treating clinician according to local practice, which could include either standard low-dose thromboprophylaxis or enhanced intermediate-dose thromboprophylaxis. The proportion of patients in the experimental group who did not receive the intended high-dose thromboprophylaxis varied from 0 to 22.4% (Table 1). In the control group, a variable proportion of patients ranging between 0 and 57.8% received intermediate to therapeutic doses of anticoagulation (Table 1). With regard to the type of pharmacological thromboprophylaxis, most studies used enoxaparin or unfractionated heparin in both experimental and control groups. In one RCT, the experimental group received rivaroxaban (20 mg or 15 mg once daily) if patients were clinically stable and therapeutic heparin followed by rivaroxaban in case of unstable patients [10]. Dose and duration of anticoagulation were highly variable across the studies (Table 1).
The incidence of VTE was reported by eight studies [8–13, 18, 20], while the occurrence of arterial thrombosis (i.e., acute myocardial infarction, acute ischemic stroke, and acute peripheral arterial ischemic event) was reported in seven studies [8–10, 12, 13, 18, 20]. One study used a combined outcome including both venous and arterial events [19]. All studies reported major bleeding and all-cause mortality during follow-up. The definition of the outcomes of interest was highly variable among the studies (Table 1). Only three RCTs had VTE as primary efficacy outcome [13, 19, 20], and five had major bleeding as primary safety outcome [10, 11, 18–20].
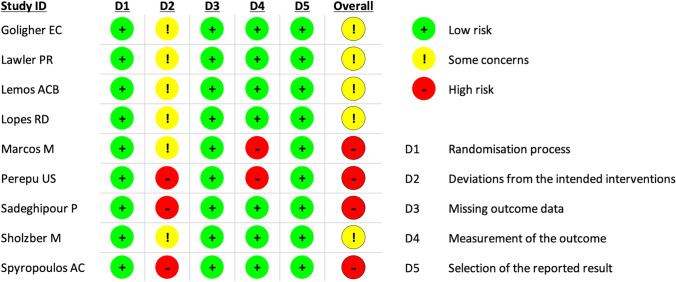
Four RCTs had an overall high risk of bias and five RCTs presented some concerns regarding the overall risk of bias (Fig. 2).
Fig. 2.
Risk of bias summary for included studies
Primary efficacy and safety outcomes
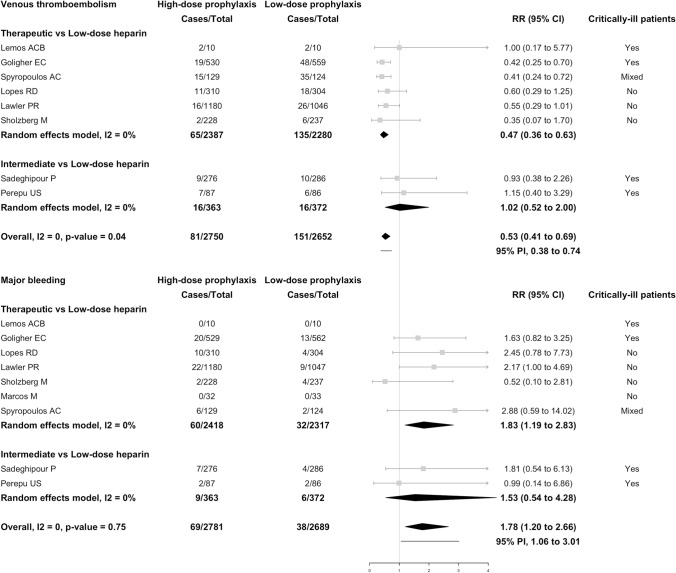
Overall, 81 of 2750 (2.9%) patients receiving high-dose thromboprophylaxis developed VTE compared with 151 of 2652 (5.7%) patients receiving low-dose thromboprophylaxis (RR 0.53; 95% CI, 0.41–0.69; I2 = 0%; Fig. 3) for a NNTB of 22 (95% CI, 17–33). Based on these findings, high-dose thromboprophylaxis would result in 27 fewer patients experiencing VTE for every 1000 treated patients (95% CI, 17 fewer to 38 fewer) compared with low-dose thromboprophylaxis. Of all VTEs, 143 were PEs (38 in the high-dose and 94 in the low-dose prophylaxis; in 11 patients the prophylaxis dose was not specified) and 84 were DVTs (37 in the high-dose and 46 in the low-dose prophylaxis; in 1 patient, the dose was not specified). Results were consistent when only symptomatic venous thromboembolic events were included in the analysis (Supplementary Fig. 1).
Fig. 3.
Venous thromboembolism and major bleeding events in hospitalized patients with COVID-19 receiving high-dose versus low-dose prophylaxis. The vertical line indicates the summary estimate. Gray squares indicate individual study estimates, whereas the gray horizontal lines indicate 95% confidence intervals of the individual studies. The diamond indicates the summary estimate with 95% confidence intervals. The horizontal black line refers to the prediction intervals which are displayed numerically under the 95% confidence intervals. Prediction interval shows the extent of between-study variation and predict the possible effect in a future study that is comparable to those included in the meta-analysis. CI, confidence interval; PI, prediction interval; RR, risk ratio
The risk of VTE was significantly reduced in patients receiving therapeutic-dose (RR 0.47; 95% CI, 0.36–0.63; I2 = 0%), but not in those receiving intermediate-dose thromboprophylaxis (RR 1.02; 95% CI, 0.52–2.00; I2 = 0%) (Fig. 3). Supplementary Fig. 2 shows the comparison between high-dose and low-dose thromboprophylaxis in non-critically ill (RR 0.54; 95% CI, 0.35–0.86; I2 = 0%) and in critically ill patients (RR 0.69; 95% CI, 0.39–1.21; I2 = 36%).
Major bleeding occurred in 69 of 2781 (2.5%) patients receiving high-dose thromboprophylaxis compared with 38 of 2689 (1.4%) patients receiving low-dose thromboprophylaxis (RR 1.78; 95% CI, 1.20–2.66; I2 = 0%; Fig. 3) for a NNTH of 100 (95% CI, 47–401). On the basis of these findings, 11 more patients for every 1000 treated patient would experience major bleeding with high-dose as compared to low-dose thromboprophylaxis (95% CI, 3 more to 17 more). Major bleeding was fatal in 6 out of 47 cases (12.8%) and in 1 out of 23 cases (4.3%) in the high-dose and low-dose groups, respectively (data reported in 7 RCTs) [9–11, 13, 18–20].
The risk of major bleeding was significantly higher in patients receiving therapeutic-dose (RR 1.83; 95% CI, 1.19–2.83; I2 = 0%), but not in patients on intermediate-dose thromboprophylaxis (RR 1.53; 95% CI, 0.54–4.28; I2 = 0%) (Fig. 3).
Supplementary Fig. 3 shows the risk of major bleeding with high-dose versus low-dose thromboprophylaxis in non-critically ill (RR 1.79; 95% CI, 0.87–3.67; I2 = 22%) and critically ill patients (RR 1.60; 95% CI, 0.90–2.84; I2 = 0%).
Funnel plots for the primary outcomes are reported in Supplementary Figs. 4 and 5.
Secondary efficacy and safety outcomes
All-cause mortality occurred in 494 of 2786 (17.7%) patients receiving high-dose thromboprophylaxis compared with 501 of 2690 (18.6%) patients receiving low-dose thromboprophylaxis (RR 0.97; 95% CI, 0.75–1.26; I2 = 47%; Supplementary Fig. 6).
The risk of PE was lower in patients receiving high-dose thromboprophylaxis than low-dose thromboprophylaxis (RR 0.41; 95% CI, 0.28–0.59; I2 = 0%; Supplementary Fig. 7). The risks of DVT (RR 0.83; 95% CI, 0.51–1.36; I2 = 0%; Supplementary Fig. 8), acute myocardial infarction (RR 0.76; 95% CI, 0.50–1.15; I2 = 0%; Supplementary Fig. 9), acute ischemic stroke (RR 0.94; 95% CI, 0.56–1.57; I2 = 0%; Supplementary Fig. 10), acute peripheral arterial ischemic events (RR 1.63; 95% CI, 0.27–9.76; I2 = 7%; Supplementary Fig. 11), and clinically relevant non-major bleeding (RR 3.05; 95% CI, 0.01–1003.84; I2 = 28%; Supplementary Fig. 12) appeared not to be significantly affected by the administration of high-dose compared to low-dose venous thromboprophylaxis. Data on heparin-induced thrombocytopenia were reported in three RCTs only (0% in both experimental and control group) [9, 18, 20].
Discussion
The results of this study showed that less than 5% of hospitalized patients with COVID-19 receiving anticoagulation experienced VTE. The use of high-dose venous thromboprophylaxis in these patients was associated with a lower risk of VTE, but a higher risk of major bleeding compared to low-dose venous thromboprophylaxis. Non-critically ill patients seemed to have greater VTE risk reduction and similar bleeding risk with high-dose venous thromboprophylaxis compared to critically ill patients. The use of high-dose thromboprophylaxis appeared not to affect all-cause mortality, acute myocardial infarction, acute ischemic stroke, or acute peripheral arterial ischemic events.
Over the last months, several studies showed that hospitalized patients with COVID-19 have a high risk of VTE despite receiving standard pharmacological thromboprophylaxis [3]. These observations raised the question of whether higher doses of anticoagulation could lower the thrombotic risk without compromising safety [3]. Observational studies reported promising results in terms of survival rate and respiratory failure reduction among patients receiving high-dose anticoagulation, however, there was a concerning signal for an increased risk of bleeding complications [6, 7]. Based on these preliminary reports, international guidelines suggested, with a very low certainty, the administration of standard venous thromboprophylaxis in all COVID-19 hospitalized patients with consideration for higher doses in patients at higher risk of VTE [21–23]. The results of this meta-analysis support a greater efficacy from high-doses of thromboprophylaxis and suggest a better benefit-to-risk profile for therapeutic rather than intermediate doses. Importantly, a relevant proportion of patients in the control group received intermediate-dose thromboprophylaxis possibly attenuating the benefits of therapeutic doses and hiding potential benefits of intermediate doses in the experimental groups. For example, in the study by Goligher and colleagues, as many as 51.7% of patients in the usual-care thromboprophylaxis group received intermediate-dose thromboprophylaxis and additional 6.1% of patients received therapeutic-dose anticoagulation, which may have diluted the differences between intervention and control groups [8]. Similarly, in the study of Lawler and colleagues, 26.5% of patients in the usual-care thromboprophylaxis group received intermediate-dose thromboprophylaxis [9]. Therefore, the results of our subgroup analysis need to be considered hypothesis generating only, also in light of the low number of patients and events (Fig. 3).
The benefits and risks of high-dose thromboprophylaxis may vary according to COVID-19 severity and a higher benefit of intermediate-dose or therapeutic-dose anticoagulation is expected in critically ill patients admitted to ICUs who are at higher risk of VTE compared to non-critically ill patients [3]. However, the results of our pooled analysis showed a greater benefit of high-dose thromboprophylaxis in non-critically ill patients than in critically ill patients. One explanation for this unexpected finding is that in patients with severe COVID-19 the thrombo-inflammatory process and activation of blood coagulation are too advanced to be effectively controlled by anticoagulation even if therapeutic-dose thromboprophylaxis is used [24]. Conversely, in non-critically ill patients this process may be still modifiable and the net clinical benefit appeared to be in favour of high-dose thromboprophylaxis with a number of VTE events prevented over twofold higher compared to the number of major bleeding complications [24].
There are some limitations which warrant discussion. First, the dose and duration of anticoagulant regimens varied widely across the studies. The sensitivity analysis including only RCTs in which patients received the intended high-dose or low-dose thromboprophylaxis yielded similar results compared to the primary analysis (Supplementary Figs. 13, 14). Furthermore, stratified analysis based on the use of intermediate- or therapeutic-dose thromboprophylaxis suggested a differential efficacy and larger effects with therapeutic-doses, in the absence of between-study heterogeneity. Second, the open-label design of all included trials represents a potential limitation and ascertainment bias cannot be excluded for VTE and major bleeding. Third, outcome definitions varied across trials, the occurrence of VTE during follow-up represented the primary efficacy outcome in only three RCTs, and all studies were underpowered to show differences in VTE or bleeding events. The relatively low incidence of VTE may be explained by the evaluation of symptomatic cases in the vast majority of the included RCTs and by the absence of protocol-mandated screening for VTE in the studies. Additional factors may include differences in study populations with inclusion of less severe cases or the effects of concomitant anti-inflammatory therapies on COVID-19-associated thrombo-inflammation [25]. The relatively low number of events may have prevented to show potentially relevant differences between intervention and control groups for the secondary outcomes. In addition, the low number of included studies limit the interpretation of all subgroup analyses which need to be interpreted with caution and regarded only as hypothesis generating. Fourth, the results of subgroup analysis on critically ill versus non-critically ill patients may be affected by the heterogeneous diagnostic criteria used for disease severity classification and by the inclusion of a mixed population in one RCT [20]. However, sensitivity analysis which considered only RCTs including critical patients yielded similar results for both VTE and major bleeding (Supplementary Figs. 15, 16).
In hospitalized patients with COVID-19, the administration of high-dose thromboprophylaxis significantly reduces the risk of VTE compared to low-dose thromboprophylaxis but increases the risk of major bleeding. There was no apparent effect of high-dose thromboprophylaxis on mortality or arterial events. Non-critically ill patients seemed to obtain a larger benefit from high-dose thromboprophylaxis with a similar risk of bleeding compared to critically ill patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
There was no funding source for this study.
Authors’ contribution
Study conception and design: E. Valeriani, A. Porfidia; data acquisition: E. Valeriani, A. Porfidia; Statistical analysis: E. Valeriani; interpretation of the data: all authors; drafting of the manuscript: E. Valeriani, A. Porfidia, W. Ageno, M. Di Nisio; critical revision of the manuscript for important intellectual content: all authors; final approval of the manuscript: all authors.
Declarations
Conflict of interest
E. Valeriani and S. Spoto have nothing to disclose. A. Porfidia reports personal fees from Bayer, Boehringer Inghelheim, Daiichi Sankyo, BMS-Pfizer, Novartis and Aspen, outside the submitted work. W. Ageno reports grants and personal fees from Bayer, and personal fees from BMS/Pfizer, Daiichi Sankyo, Sanofi, Aspen, Janssen, and Portola, outside the submitted work. R. Pola reports personal fees from Bayer, Boehringer Inghelheim, Daiichi Sankyo, BMS-Pfizer, Novartis and Aspen, outside the submitted work. M. Di Nisio reports personal fees from Bayer, Daiichi Sankyo, BMS-Pfizer, Leo Pharma, Sanofi, and Aspen, outside the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Emanuele Valeriani and Angelo Porfidia share first co-authorship.
References
- 1.Levi M, Thachil J, Iba T, et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Sullivan JM, Gonagle DM, Ward SE, et al (2020) Endothelial cells orchestrate COVID-19 coagulopathy. Lancet Haematol 7: e566–74 [DOI] [PMC free article] [PubMed]
- 3.Porfidia A, Valeriani E, Pola R, et al. Venous thromboembolism in patients with COVID-19: systematic review and meta-analysis. Thromb Res. 2020;196:67–74. doi: 10.1016/j.thromres.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020;18:1020–1022. doi: 10.1111/jth.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santoliquido A, Porfidia A, Nesci A, et al. Incidence of deep vein thrombosis among non-ICU patients hospitalized for COVID-19 despite pharmacological thromboprophylaxis. J Thromb Haemost. 2020;18:2358–2363. doi: 10.1111/jth.14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ionescu F, Jaiyesimi I, Petrescu I, et al. Association of anticoagulation dose and survival in hospitalized COVID-19 patients: a retrospective propensity score-weighted analysis. Eur J Haematol. 2021;106:165–174. doi: 10.1111/ejh.13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopes RD, de Barros E, Silva PGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemos ACB, do Espírito Santo DA, Salvetti MC, et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: a randomized phase IIclinical trial (HESACOVID) Thromb Res. 2020;196:359–366. doi: 10.1016/j.thromres.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perepu US, Chambers I, Wahab A, et al. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: a multi-center, open-label, randomized controlled trial. J Thromb Haemost. 2021;19:2225–2234. doi: 10.1111/jth.15450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadeghipour P, Talasaz AH, Rashidi F, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the inspiration randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins J, Altman D, Sterne J (2017) Assessing risk of bias in included studies. In: Cochrane Handbook for Systematic Reviews of Interventions version 520
- 16.Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook. Deeks J, Higgins J, Altma nD. [insert producer], producer. Edition ed.
- 17.R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/2019)
- 18.Sholzberg M, Tang GH, Rahhal H, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. doi: 10.1136/bmj.n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcos M, Carmona-Torre F, Vidal Laso R, et al (2021) Therapeutic vs. prophylactic bemiparin in hospitalized patients with non-severe COVID-19 (BEMICOP): an open-label, multicenter, randomized trial. Thromb Haemost 122(2):295–299 [DOI] [PubMed]
- 20.Spyropoulos AC, Goldin M, Giannis D, et al (2021) Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med 181(12):1612–1620 [DOI] [PMC free article] [PubMed]
- 21.Marietta M, Ageno W, Artoni A, et al. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET) Blood Transfus. 2020;18:167–169. doi: 10.2450/2020.0083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5:872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ten Cate H. Surviving Covid-19 with heparin? N Engl J Med. 2020;202(385):845–846. doi: 10.1056/NEJMe2111151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Nisio M, Potere N, Candeloro M, et al. Interleukin-6 receptor blockade with subcutaneous tocilizumab improves coagulation activity in patients with COVID-19. Eur J Intern Med. 2021;83:34–38. doi: 10.1016/j.ejim.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.