Abstract
In yeast, trehalose accumulation and its hydrolysis, which is catalyzed by neutral trehalase, are believed to be important for thermotolerance. We have shown that trehalose is one of the important factors for barotolerance (resistance to hydrostatic pressure); however, nothing is known about the role of neutral trehalase in barotolerance. To estimate the contribution of neutral trehalase in resisting high hydrostatic pressure, we measured the barotolerance of neutral trehalase I and/or neutral trehalase II deletion strains. Under 180 MPa of pressure for 2 h, the neutral trehalase I deletion strain showed higher barotolerance in logarithmic-phase cells and lower barotolerance in stationary-phase cells than the wild-type strain. Introduction of the neutral trehalase I gene (NTH1) into the deletion mutant restored barotolerance defects in stationary-phase cells. Furthermore, we assessed the contribution of neutral trehalase during pressure and recovery conditions by varying the expression of NTH1 or neutral trehalase activity with a galactose-inducible GAL1 promoter with either glucose or galactose. The low barotolerance observed with glucose repression of neutral trehalase from the GAL1 promoter was restored during recovery with galactose induction. Our results suggest that neutral trehalase contributes to barotolerance, especially during recovery.
In general, hydrostatic pressure affects almost all physiological activities in living cells. Bett and Cappi (3) studied the viscosity of water as a function of pressure up to 10,000 kg/cm2. They found that relative and absolute viscosities decrease with pressure increases from 0 to 2,000 kg/cm2 at ambient temperature (3). Decreased viscosity due to high pressure results in the destruction of hydrogen bonding, and this has been reported to be a consequence of increased temperature (3). Thus, the effects of high temperature and high hydrostatic pressure can be analogous for organisms.
To understand the effect of high temperature and high hydrostatic pressure, we have been studying the analogy between hydrostatic pressure and temperature by using Saccharomyces cerevisiae as a model system (11). We have shown that the molecular chaperone Hsp104, as well as the nonreducing disaccharide trehalose, plays important role in barotolerance and thermotolerance (8, 9). However, thermotolerance and barotolerance are essentially different (8). Hsp104 has an optimum temperature for its role in barotolerance, and the temperature for hydrostatic pressure treatment is below this optimum. This lower temperature decreases the importance of Hsp104 to barotolerance (9). Several lines of evidence suggest that Hsp104 (12, 18) and trehalose (4) are important factors in thermotolerance.
Recent studies have shown that neutral trehalase I encoded by NTH1, as well as its homolog, NTH2 (encoding putative neutral trehalase II), is important for thermotolerance, especially for recovery of cells after severe heat shock (14, 15). Neutral trehalase is responsible for breaking down trehalose in the cell. Apparently, it is difficult to understand the mechanism of the contribution of neutral trehalase to thermotolerance, because this enzyme breaks down trehalose, which contributes to thermotolerance. Two models have been proposed to explain the role of neutral trehalase in heat shock recovery (14, 15, 19). The latest model states that trehalose protects cellular proteins against denaturation and subsequent aggregation, but inhibits the solubilization of protein aggregates and the refolding of the partially denatured proteins during recovery from heat shock (19). The inhibition of refolding of protein by trehalose can cause a delay in recovery from heat denaturation. Thus, neutral trehalase can contribute to thermotolerance, especially during recovery.
In this report, we show that neutral trehalase contributes to barotolerance. The deletion of NTH1 decreased barotolerance, and reintroduction of NTH1 by transformation increased barotolerance. Furthermore, induction of neutral trehalase activity under recovery conditions significantly increased barotolerance. Thus, neutral trehalase contributed to barotolerance, especially during recovery conditions.
MATERIALS AND METHODS
Strains and growth conditions.
The strains and plasmids used for this study are shown in Table 1. Information about some of the parental strains may be seen in references 13, 15 and 16. For precise experiments, the control strains were constructed by introducing URA3, LEU2, or plasmids without the NTH1 gene into the corresponding parent strains (Tables 2 to 4) so that the effect of artificial factors would be minimal. The strains were constructed according to general methods (5, 17) and were grown in YPD medium (2% polypeptone, 1% yeast extract, 2% glucose) or SD medium (0.67% yeast nitrogen base without amino acids) (7) containing 2% glucose or galactose. A preculture grown for 2 days was used to inoculate experimental cultures at a dilution rate of 20 to 5,000. The cells were grown overnight at 30°C in a shaker until the stationary or logarithmic phase of growth (A660 of 1.0).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype | Characteristic | Source or reference |
|---|---|---|---|
| Strains | |||
| YS18 | MATa his3 leu2 ura3 CanR Δgal | Wild type | Nwaka et al. (15) |
| YS18-URALEU | MATa his3 CanR Δgal | Wild type | This work |
| YSN1 | MATa his3 leu2 ura3 CanR Δgal nth1::LEU2 | Nth1− | Nwaka et al. (15) |
| YSN1-URA | MATa his3 leu2 CanR Δgal nth1::LEU2 | Nth1− | This work |
| YSN01 | MATa his3 leu2 ura3 CanR Δgal nth2::URA3 | Nth2− | Nwaka et al. (15) |
| YSN01-LEU | MATa his3 ura3 CanR Δgal nth2::URA3 | Nth2− | This work |
| YSN1-01 | MATa his3 leu2 ura3 CanR Δgal nth1::LEU2 nth2::URA3 | Nth1− Nth2− | Nwaka et al. (15) |
| YSN1-pSEY8 | MATa his3 leu2 ura3 CanR Δgal nth1::LEU2 pSEY8 | Control | This work |
| YSN1-pSEY8-NTH1 | MATa his3 leu2 ura3 CanR Δgal nth1::LEU2 pSEY8-NTH1 | High Nth1 | This work |
| YSN1A-nth1 | MATa his3 leu2 ura3 trp1 ade2 suc2 nth1::LEU2 | Nth1− | Nwaka et al. (15) |
| YSN1A-pYES2 | MATa his3 leu2 ura3 trp1 ade2 suc2 nth1::LEU2 pYES2 | Control | This work |
| YSN1A-p2.253 | MATa his3 leu2 ura3 trp1 ade2 suc2 nth1::LEU2 p2.253 | Controled Nth1 | This work |
| Plasmids | |||
| pSEY8 | AprURA3 ori(2μm DNA) | Nwaka (13) | |
| pSEY8-NTH1 | AprURA3 ori(2μm DNA) NTH1 | Nwaka and Holzer (16) | |
| pYES2 | AprURA3 ori(2μm DNA) | Nwaka et al. (15) | |
| p2.253 | AprURA3 ori(2μm DNA) NTH1 (open reading frame) | Nwaka et al. (15) |
TABLE 2.
Trehalose contents and barotolerance of the neutral trehalase-deficient strainsa
| Strain | Trehalose content (μg/mg of protein)
|
Barotolerance (% at 180 MPa for 2 h)
|
||
|---|---|---|---|---|
| Log phase | Stationary phase | Log phase | Stationary phase | |
| YS18-URALEU (wild type) | 43 ± 5.0 | 257 ± 47 | 0.068 ± 0.050 | 3.4 ± 1.0 |
| YSN1-URA (nth1 mutant) | 192 ± 30 | 519 ± 80 | 0.12 ± 0.11 | 0.30 ± 0.09 |
| YSN01-LEU (nth2 mutant) | 29 ± 1.0 | 316 ± 66 | 0.046 ± 0.069 | 5.0 ± 2.0 |
| YSN1-01 (nth1 nth2 mutant) | 179 ± 24 | 337 ± 59 | 0.029 ± 0.027 | 0.18 ± 0.01 |
Strains were grown in YPD medium until the logarithmic and stationary phases, and trehalose content and barotolerance were measured as described in Materials and Methods. To make strains show the same requirement, we introduced URA3 or LEU2 genes into the corresponding strains. Values are means ± standard deviations.
TABLE 4.
Contribution of neutral trehalase activity to barotolerance during recovery conditions
| Growth conditionsa | Barotolerance (%) of strainb:
|
|
|---|---|---|
| SEY6211-pYES2 | SEY6211-p2.253 | |
| From glucose to glucose | 0.0041 ± 0.0059 | 0.031 ± 0.011 (7.5) |
| From glucose to galactose | 0.0031 ± 0.0028 | 0.083 ± 0.024 (26) |
| From galactose to glucose | 0.0079 ± 0.0026 | 0.075 ± 0.019 (9.5) |
| From galactose to galactose | 0.026 ± 0.015 | 0.26 ± 0.068 (10) |
Yeast cells were grown in glucose or galactose until the stationary phase (from) and spread on plate medium containing glucose or galactose (to) after pressure treatment.
Values are means ± standard deviations. Values in parentheses represent barotolerance of SEY6211-p2.253/barotolerance of SEY6211-pYES2.
Barotolerance, neutral trehalase activity, and trehalose content.
Hydrostatic pressure treatment of cells was performed at 180 MPa (2 h at 25°C). Yeast cells in the growth medium were poured into 3 ml of a glass syringe, the syringe was transferred to a stainless steel vessel (KT-0422; High Pressure Chemical Co., Ltd., Hiroshima, Japan), and the vessel was pressurized with a pressure-generating pump (wp3000; High Pressure Equipment Co., Ltd., Erie, Pa.). Barotolerance was expressed as a percentage of the CFU of the high-pressure-treated cells relative to the untreated control. Neutral trehalase activity in the crude extract (10) was measured in the reaction mixture containing 34 mM imidazole-HCl (pH 7.0) and 0.11 M trehalose (1) as described previously (10). One unit of neutral trehalase is the amount of enzyme that hydrolyzes 1 μmol of trehalose in 1 min. Trehalose content was measured as glucose after hydrolysis with acid trehalase (6). The data shown are mean values of three independent and reproducible experiments.
RESULTS AND DISCUSSION
Deletion of NTH1 decreased barotolerance.
As a first step in studying the contribution of neutral trehalases to barotolerance, we measured the barotolerance of the NTH1 and/or NTH2 deletion mutants by using logarithmic-phase cells and stationary-phase cells. In Table 2, the trehalose content and barotolerance of the YS18-URALEU (wild type), YSN1-URA (nth1), YSN-LEU (nth2), and YSN1-01 (nth1 nth2) strains are shown. As expected, the strains that had the deletion in the NTH1 gene showed a higher trehalose content in logarithmic-phase cells and stationary-phase cells than the strains that had the NTH1 gene (see references 14 and 15). This is a consequence of a decrease or absence of neutral trehalase activity due to deletion of the NTH1 gene. The NTH2 gene did not affect the accumulation of trehalose. This result also agrees with the result shown by Nwaka et al. (15). They found that although the NTH2 gene is a homolog of NTH1, it shows no neutral trehalase activity.
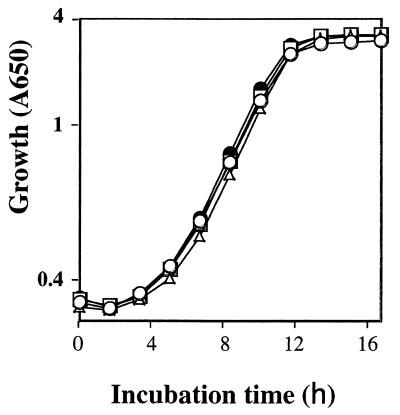
In logarithmic-phase cells, the NTH1 deletion strain showed the highest barotolerance among the strains examined. This reflects that deletion of NTH1 increases the relatively low trehalose content of logarithmic-phase cells and probably for this reason improves their barotolerance as previously reported (8). In contrast to logarithmic-phase cells, the NTH1 deletion strains showed lower barotolerance than the strains that had the NTH1 gene in the stationary phase. This result shows that neutral trehalase I contributes to barotolerance. Growth curve data show that the strains used for this study grew equally (Fig. 1). This suggests that the barotolerance differences were not due to growth differences.
FIG. 1.
Growth curve of the strains constructed. YS18-URALEU (wild type; ●), YSN1-URA (nth1::LEU2; ▵), YSN-LEU (nth2::URA3; □), and YSN1-01 (nth1::LEU2/nth2::URA3; ○) were grown in YPD medium. The preculture was diluted at a dilution rate of 100, and growth was monitored as the A650.
Reintroduction of neutral trehalase to the NTH1 deletion strain increased barotolerance.
To confirm the contribution of neutral trehalase I to barotolerance, we transformed the neutral trehalase I deletion strain (nth1) with plasmid pSEY8 containing the NTH1 gene and its promoter region (pSEY8-NTH1) (13, 16). The resulting strain, called YSN1-pSEY8-NTH1 (nth1 mutant expressing NTH1 from the plasmid), had restored neutral trehalase activity compared to the control strain YSN1-pSEY8 (nth1 mutant containing pSEY8 plasmid alone) (Table 3). Interestingly, YSN1-pSEY8-NTH1 cells, which accumulated less trehalose, showed higher barotolerance than the control strain. This result corresponds with the data in Table 2. It should be mentioned that the barotolerance values shown in Table 3 are lower than those in Table 2. This is possibly because of the strains, the differences in the medium, and the selective pressure of the plasmids used in Table 3.
TABLE 3.
Effect of introduction of the neutral trehalase I gene into the NTH1 deletion straina
| Strain | Trehalase activity (mU/mg of protein) | Trehalose content (μg/mg of protein)b | Barotolerance (%)b |
|---|---|---|---|
| YSN1-pSEY8 (nth1 mutant) | <0.1 | 185 ± 28 | 0.000078 ± 0.000011 |
| YSN1-pSEY8-NTH1 (nth1 gene on plasmid) | 23 | 108 ± 32 | 0.00078 ± 0.00013 |
Strains were grown in SD medium until the stationary phase.
Values are means ± standard deviations.
Neutral trehalase activity contributes to the recovery of cells after pressure treatment.
Although our data suggest that neutral trehalase I plays a role in barotolerance, it remains unclear how neutral trehalase performs this function. The finding that trehalose may prevent refolding of heat-denatured proteins (after heat shock) implies that trehalose hydrolysis catalyzed by neutral trehalase is essential for recovery (15, 19). However, there is no direct evidence to support this. Generally, thermotolerance or barotolerance means total tolerance during stress and recovery. Therefore, we tried to estimate the contribution of neutral trehalase during and/or after pressure treatment.
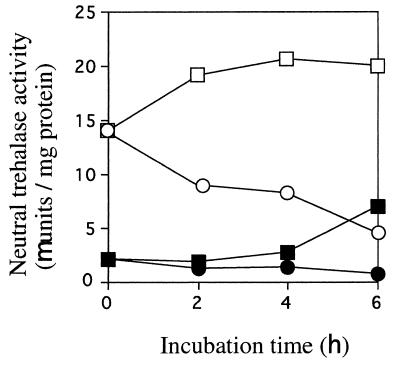
The pYES2 plasmid is a yeast-expression vector containing the GAL1/GAL10 promoter region for inducible expression by galactose. The plasmid p2.253 is derived from pYES2 containing the complete open reading frame of the gene NTH1 (15). Thus, we can control the activity of neutral trehalase in strain YSN1A-p2.253 (15) by changing the carbon. In Fig. 2, neutral trehalase activities in glucose- and galactose-grown cells are summarized. Yeast cells grown on glucose showed a lower neutral trehalase activity than cells grown on galactose. Transfer of cells from galactose to glucose decreased activity, while transfer from glucose to galactose or galactose to galactose increased activity. Although this experiment was carried out with a liquid SD medium, similar induction and repression may take place on plates.
FIG. 2.
Neutral trehalase activity in cells grown with glucose and galactose. Strain YSN1A-p2.253 grown with glucose or galactose was transferred to glucose or galactose medium. Neutral trehalase activities were measured at the indicated time, as described in the text. ●, transfer from glucose to glucose; ■, transfer from glucose to galactose; ○, transfer from galactose to glucose; □, transfer from galactose to galactose.
Data showing the effect of varying the activity of neutral trehalase on barotolerance are presented in Table 4. In this experiment, yeast cells were grown in glucose (repression) or galactose (induction) to the stationary phase and exposed to a high hydrostatic pressure of 180 MPa. Equal dilutions of the pressure-treated cells were plated and then allowed to recover on the plate with glucose (repression) or galactose (induction) as the carbon source. The YNS1A-p2.253 strain showed a higher barotolerance than the YSN1A-pYES2 control strain, even under repressed conditions. It seems that the low trehalase activity measured under repressed conditions (Fig. 2) is enough to increase barotolerance (Table 4). The YNS1A-pYES2 control cells grown on galactose showed higher basal barotolerance than the same cells grown on glucose. Galactose-grown cells are known to be more stress resistant than glucose-grown cells, because they grow more slowly (2). The YSN1A-p2.253 strain showed higher barotolerance (26-fold) than the control strain, especially when transferred from growth on glucose (repressed) to growth on galactose (induced) (Table 4). Although the standard deviation is relatively higher in this experiment, the feature was same in three independent experiments. Thus, this result implies that the induction of neutral trehalase after pressure treatment can contribute to barotolerance. This is the first direct evidence showing that neutral trehalase contributes to stress tolerance after stress treatment.
REFERENCES
- 1.App H, Holzer H. Purification and characterization of neutral trehalase from the yeast ABYS mutant. J Biol Chem. 1989;264:17583–17588. [PubMed] [Google Scholar]
- 2.Arguelles J-C. Heat-shock response in a yeast tps1 mutant deficient in trehalose synthesis. FEBS Lett. 1994;350:266–270. doi: 10.1016/0014-5793(94)00786-1. [DOI] [PubMed] [Google Scholar]
- 3.Bett K E, Cappi J B. Effect of pressure on the viscosity of water. Nature. 1965;207:620–621. [Google Scholar]
- 4.Hottiger T, Schmutz P, Wiemken A. Heat-induced accumulation and futile cycling of trehalose in Saccharomyces cerevisiae. J Bacteriol. 1987;169:5518–5522. doi: 10.1128/jb.169.12.5518-5522.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito H, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1984;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwahashi H, Obuchi K, Fujii S, Komatsu Y. The correlative evidence suggesting that trehalose stabilizes membrane structure in the yeast Saccharomyces cerevisiae. Cell Mol Biol. 1995;41:763–769. [PubMed] [Google Scholar]
- 7.Iwahashi H, Yang W, Tanguay R M. Detection and expression of the 70 kDa heat shock protein SSB1P at different temperatures in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1995;213:484–489. doi: 10.1006/bbrc.1995.2157. [DOI] [PubMed] [Google Scholar]
- 8.Iwahashi H, Obuchi K, Fujii S, Komatsu Y. Barotolerance is dependent on both trehalose and heat shock protein 104 but is essentially different from thermotolerance in Saccharomyces cerevisiae. Lett Appl Microbiol. 1997;25:43–47. doi: 10.1046/j.1472-765x.1997.t01-1-00069.x. [DOI] [PubMed] [Google Scholar]
- 9.Iwahashi H, Obuchi K, Fujii S, Komatsu Y. Effect of temperature on the role of Hsp104 and trehalose in barotolerance of Saccharomyces cerevisiae. FEBS Lett. 1997;416:1–5. doi: 10.1016/s0014-5793(97)01141-1. [DOI] [PubMed] [Google Scholar]
- 10.Iwahashi H, Nwaka S, Obuchi K, Komatsu Y. Evidence for the interplay between trehalose metabolism and Hsp104 in yeast. Appl Environ Microbiol. 1998;64:4614–4617. doi: 10.1128/aem.64.11.4614-4617.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwahashi H. Microorganisms under the pressure conditions. Nippon Nougeikagaku Kaishi. 2000;74:609–611. [Google Scholar]
- 12.Lindquist S, Kim G. Heat-shock protein 104 expression is sufficient for thermotolerance in yeast. Proc Natl Acad Sci USA. 1996;93:5301–5306. doi: 10.1073/pnas.93.11.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nwaka S. Trehalose hydrolysis in the yeast S. cerevisiae: expression and function of trehalases. Ph.D. thesis. Freiburg, Germany: University of Freiburg; 1995. [Google Scholar]
- 14.Nwaka S, Mechlen B, Destruelle M, Holzer H. Phenotypic features of trehalase mutants in Saccharomyces cerevisiae. FEBS Lett. 1995;360:286–290. doi: 10.1016/0014-5793(95)00105-i. [DOI] [PubMed] [Google Scholar]
- 15.Nwaka S, Kopp M, Holzer H. Expression and function of the trehalase genes NTH1 and YBR0106 in Saccharomyces cerevisiae. J Biol Chem. 1995;270:10193–10198. doi: 10.1074/jbc.270.17.10193. [DOI] [PubMed] [Google Scholar]
- 16.Nwaka S, Holzer H. Molecular biology of trehalose and the trehalases in the yeast S. cerevisiae. Prog Nucleic Acid Res Mol Biol. 1998;58:197–237. doi: 10.1016/s0079-6603(08)60037-9. [DOI] [PubMed] [Google Scholar]
- 17.Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez Y, Taulien J, Borkovich K A, Lindquist S. Hsp104 is required for tolerance to many forms of stress. EMBO J. 1992;11:2357–2364. doi: 10.1002/j.1460-2075.1992.tb05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer M A, Lindquist S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol Cell. 1998;1:639–648. doi: 10.1016/s1097-2765(00)80064-7. [DOI] [PubMed] [Google Scholar]