Abstract
Multiple sclerosis (MS) is a progressive disease that often affects the cerebellum. It is characterised by demyelination, inflammation, and neurodegeneration within the central nervous system. Damage to the cerebellum in MS is associated with increased disability and decreased quality of life. Symptoms include gait and balance problems, motor speech disorder, upper limb dysfunction, and oculomotor difficulties. Monitoring symptoms is crucial for effective management of MS. A combination of clinical, neuroimaging, and task-based measures is generally used to diagnose and monitor MS. This paper reviews the present and new tools used by clinicians and researchers to assess cerebellar impairment in people with MS (pwMS). It also describes recent advances in digital and home-based monitoring for people with MS.
Keywords: Multiple sclerosis, Cerebellum, Neuroimaging, Acoustic speech analysis, Home-based monitoring
Introduction
Multiple sclerosis (MS) is a debilitating disease of the central nervous system [1] and is one of the leading causes of disability in young and middle-aged adults [2]. The disease has been described since the 1800s, with fluctuating speech impairments, muscle weakness, pain, and vision impairment being among the symptoms mentioned in the earliest accounts [3–6]. The clinical presentation of MS is highly heterogeneous, which makes individual clinical outcomes difficult to predict. Possible symptom combinations at various severity levels differ between disease types and individual people with MS (pwMS) [7]. This is further impacted by other factors such as age at onset, mental and physical health, and socioeconomic status [8–10].
Nonetheless, symptoms can be linked back to specific regions of the central nervous system. Among regions of interest, the cerebellum plays a crucial role in sensory, motor, cognitive, and behavioural processes and is often impacted during MS by inflammatory demyelinating lesions [11]. Symptoms associated with cerebellar injury include ataxia, upper limb incoordination, dysarthria, and tremor [1]. Cerebellar symptoms are common in MS, with up to one third of pwMS experiencing these [12]. When present, cerebellar dysfunction contributes significantly to an increased rate of disability, reduced mobility, and impaired quality of life [11]. Cerebellar dysfunction experienced in the first 2 years after onset is related to a 20% increase in future overall disability [13]. There are several ways of diagnosing and monitoring cerebellar symptoms in MS. Here, we review and summarise the current methods for measuring cerebellar dysfunction in pwMS with a focus on emerging technologies including advanced neuroimaging, automated speech analysis, and home-based electronic testing.
The Cerebellum
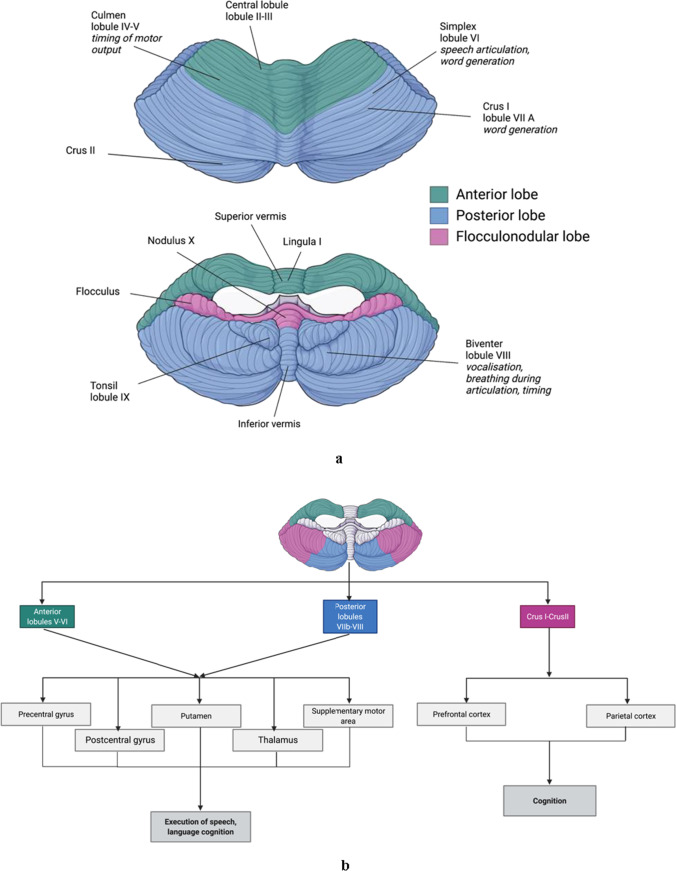
Anatomy and Role
The cerebellum is neuronally dense and accumulates several functions within the central nervous system. Despite its small size relative to the cerebrum, the cerebellum contains over 100 billion neurons compared to just 86 billion in the cerebrum [11]. The cerebellum integrates multiple circuits throughout the brain and is involved in motor, cognitive, and emotional functions. It is connected to the parietal, somatosensory, visual, auditory, prefrontal, motor, and premotor areas within the cerebral cortex through parallel connectivity loops [14]. Functional connectivity (FC) studies show distinct neural networks comprising of the cerebellum and specific regions of the cerebrum. For example, the motor cortex is associated with lobules IV-VI and VIIIB of the cerebellum, whereas areas of the prefrontal cortex connect to Crus I and Crus II [14, 15]. Purkinje cells form the output system of the cerebellum to the rest of the CNS [16]. Purkinje cell activity is associated with motor learning, coordination, and control. Furthermore, they are crucial in integrating sensory and motor signals, thus controlling sensorimotor behaviours [17]. In addition, the cerebellum plays a vital role in motor learning and memory and is highly plastic, with many forms of neural plasticity being reported [11]. Droby and colleagues [18] found an increase in FC during an acute MS relapse associated with a new white matter (WM) lesion. They believe this to be indicative of recruiting intact regions of the brain to carry out tasks [18]. A further correlation between structural damage and increased FC backs up this conclusion. Rocca et al. [19] suggest an increase in FC is an adaptive response to damage to WM bundles. During a motor task using the right hand, their study found increased activity in the left supplementary motor area, left primary sensorimotor cortex (PSMC), and right cerebellum. Rocca and colleagues also found increased FC between the right PSMC and right cerebellum, which correlated with tissue damage in the dentatothalamic and corticospinal tracts [19]. The cerebellum may therefore have a specific role in mediating FC changes following structural damage related to MS [19, 20]. See Fig. 1 for the summarised cerebellar motor connectivity.
Fig. 1.
Motor networks involving the cerebellum. Created with BioRender.com
Cerebellar Dysfunction in MS
Cerebellar impairment can start at any stage of the MS disease course [21]. Cerebellar pathology includes both grey matter (GM) and WM lesions, reduced Purkinje cell density, and neuronal loss [22]. Infratentorial lesions are associated with long-term disability [23], with the cerebellar peduncles being among the most impacted regions in terms of lesion density [24]. Recent studies have also shown that the pons and cerebellar peduncles specifically have higher lesion frequency than other areas in people with clinically isolated syndrome (CIS, the precursor to MS) [24, 25]. Autopsy research has shown an average of 38.7% of the cerebellar cortical area is affected by demyelination in pwMS, with the most severe cases reaching over 90% [26].
Cerebellar dysfunction can occur as part of an acute relapse or, perhaps more commonly, as a feature of progressive worsening in advanced MS [1]. In fact, the presence of cerebellar symptoms of MS is associated with an increased risk of developing a progressive disease course [27]. Lower cerebellar volume and higher T2 lesion load are associated with increased cognitive and motor difficulties and are correlated with higher clinical disability as measured by the Expanded Disability Status Scale (EDSS) [21]. T2 lesions in the middle and superior cerebellar peduncles are common in pwMS and are associated with disease severity and upper limb function [1, 28]. Furthermore, the cerebellar cortex is also affected by demyelination which increases in persons with progressive MS [26]. At the other end of the disease spectrum, decreases in cerebellar WM and total volume compared to controls have been described in early MS and in clinically isolated syndrome (CIS, the precursor to MS) [29].
Clinical cerebellar dysfunction (tremor, limb and gait ataxia, dysarthria, etc.) more often persist after a relapse than, for instance, sensory changes [21, 30] and can be challenging to manage, thus further adding to morbidity. Because of the organisation of the cerebellum and its different network connections, it is possible to identify location-specific deficits. For example, lesions to the midline area of the cerebellum cause dysfunction of simple motor tasks. Conversely, damage to the lateral cerebellum results in impairment of more complex motor tasks and cognitive deficits. Cognitive deficits include motor planning and language production [31–33]. Injury to the superior cerebellar peduncle, identified using diffusion tensor MRI-derived fractional anisotropy, is associated with reduced upper limb function and walking speed in pwMS [34]. Additionally, attention, verbal, and visual memory impairments correlate with reduced regional resting-state FC in cerebellar networks [35]. The dentate nucleus, a large cluster of neurons in the cerebellum, is involved in motor control, cognition, language, and sensory functions. It also connects to motor and cognitive association areas in the cerebral cortex [36]. In pwMS, researchers using light microscopy have found a significant reduction in afferent dentate synapses in areas both with and without demyelination in post-mortem cerebellar tissue [27]. They also observed atrophy and reduction of dentate neurons in pwMS, thus providing further information regarding cerebellar pathology in MS. Moreover, neuroimaging has shown altered dentate FC to frontal regions at rest in pwMS with an inverse correlation between FC and both T2 lesion volume and clinical impairment [37]. Additionally, any damage that disrupts communication between the cerebellum and higher-level cortical areas can contribute partly to cognitive impairment seen in pwMS [30, 38]. This manifests clinically as executive dysfunction and a decline in memory and language performance.
Clinical Measures of Cerebellar Dysfunction
Subjective Scoring Measures (Table 1)
Table 1.
Clinical measures of cerebellar dysfunction in pwMS
| Clinical score used | Author | Number of participants | Method/design | Findings |
|---|---|---|---|---|
| 9HPT, EDSS | Goodkin, Hertsguard [130] | 89 | Compare the 9HPT and box-and-block test to EDSS to determine sensitivity | 9HPT is sensitive to changes in functional status associated with upper limb dysfunction as measured by the EDSS |
| EDSS, KFSS | Noseworthy, Vandervoort [131] | 168 | Assess inter-rater variability in EDSS and KFSS in pwMS | Change in degree of disability associated with a 1 point change in EDSS score and a 2 point change in KFSS score |
| EDSS, KFSS, 9HPT | Cutter, Baier [132] | 5,457 from 15 datasets | Assess EDSS, KFSS, and MSFC (including the 9HPT) over time | Significant correlation between the EDSS, 9HPT, and disease duration. A strong correlation was found between the 9HPT and cerebellar FSS |
| 9HPT | Erasmus, Sarno [133] | 482 (N = 240 pwMS, 140 controls) | Repeated measures design using clinical scales and kinematic and spectral analysis to determine level of ataxic symptoms | Able to distinguish between pwMS and controls and able to distinguish those with clinical cerebellar dysfunction |
| KFSS | Kalron and Givon [134] | 289 (N = 147 with cerebellar scores) | Assess gait using pyramidal, sensory, and cerebellar scores | Pyramidal function plays the highest role in gait. No significant differences with added cerebellar dysfunction |
| ICARS, SARA | Salcı, Fil [41] | 80 | Assessed pwMS with ataxia using SARA and ICARS, correlated with EDSS and cerebellar KFSS | High inter-rater reliability, ICARS has sig correlations with EDSS and KFSS cerebellar scores, suggesting high validity |
| 9HPT | Solaro, Cattaneo [135] | 363 | Determine correlation between 9HPT scores, EDSS scores and MS type using a cross-sectional study involving multiple MS centres | Floor and ceiling effects for mild and severe cases of MS. Higher EDSS and people with primary progressive MS showed more asymmetry in hand function |
| EDSS | Le, Malpas [13] | 10,513 | Data from MSBase registry. A mixed-effects model used to determine associations between early cerebellar presentations and EDSS scores |
Cerebellar symptoms early on are associated with higher EDSS scores independent of pyramidal dysfunction They may be used as markers for disease progression |
Disease severity and neurological impairment in MS are commonly defined using a standardised clinical assessment, the EDSS. This scale was designed to describe disease progression in pwMS and uses an ordinal scale from 0 (normal neurological status) to 10 (death due to MS) [39]. The associated subscores, or so-called Kurtzke functional system (KFS) scores, were designed to address different neurological areas of dysfunction, including the cerebellar and brain stem functional systems [40]. The KFS score for these two functional systems incorporates symptoms of ataxia, nystagmus, dysarthria, swallowing difficulties, and extraocular weakness [40]. Higher cerebellar KFS scores predict a shorter time to reach an EDSS score of 6, where one requires aid to walk 100 m [12]. The other available clinical scores, although often used in MS studies, were all developed with different diseases in mind. The international cooperative ataxia rating scale (ICARS) measures ataxia-related symptoms on four subscales: posture and gait disturbances, speech disorders, kinetic functions, and oculomotor disorders [41]. The ICARS has 19 items used to assess ataxia severity and is scored out of 100 [42]. A third clinical rating system specific to cerebellar ataxia is the scale for the assessment and rating of ataxia (SARA). This measure is scored out of 40 and comprises eight different items that evaluate gait, speech, truncal postural, and limb kinetic function [43] The SARA has been validated in MS and demonstrates high test–retest reliability and internal consistency for pwMS with ataxia [41]. In addition, the score increases as cerebellar ataxia symptoms worsen, making it a valid measure of cerebellar ataxia [43].
Objective Measures
The nine-hole peg test (9HPT) assesses upper limb dexterity in pwMS [44]. It accurately distinguishes between controls and pwMS with different levels of impairment. The 9HPT is a common part of the multiple sclerosis functional composite (MSFC) alongside walking, visual, and cognition tasks [45].
We have summarised the clinical measures of cerebellar dysfunction in pwMS in Table 1.
Kinematic Analysis of Gait and Balance
Gait and balance dysfunctions are common in MS and correlate with cerebellar damage [46]. Subtle changes to gait and balance are also precursors to a more severe loss of mobility in pwMS [47]. Therefore, early detection of subtle gait changes can be used to predict mobility loss later in the disease course. There are several ways to measure gait and balance in pwMS, including wearable and non-wearable options. Non-wearable measures such as the instrumented treadmill and the butterfly diagram are more accurate and reliable but tend to require specialised equipment while also being inconvenient [47, 48]. On the other hand, wearable systems, although perhaps providing less detailed information, can be used in community settings and at home and give real-time feedback to patients. One example is the use of inertial measurement units (IMUs). IMUs are small, light integrated systems that measure the linear and angular motion of the wearer. These systems can be attached anywhere on the body but are commonly positioned on the lower back, sternum, calf, wrist, or ankle [49]. IMU harmonic ratios in people with cerebellar ataxia — a common symptom of MS — correlate with ataxia severity and gait features such as stance, swing, and double support duration [50]. This has also been found in pwMS with gait dysfunction where IMUs can quantify speed, step length, and step time. These measures correlate with EDSS scores [49]. IMUs can also be used to measure postural sway — an aspect of balance control — in pwMS [51]. Increased standing postural sway is associated with higher EDSS scores, specifically higher cerebellar KFSS subsystem scores [52]. New technology now also allows for inertial/passive data collection on smartphones and watches, making them more accessible and user-friendly for patients [47]. Inertial sensors show that postural sway deficits are associated with reduced WM integrity in the superior and inferior cerebellar peduncles in pwMS [53]. There are also simple standing and walking assessment options, including the 2- and 6-min walking tests that are frequently included in clinical trials. However, these tests can be limited by inter-test variability and lack of sensitivity to subtle changes in gait.
Limitations of Current Clinical Assessments
Clinical assessments, especially the EDSS and its subscores, remain the gold standard for monitoring MS disease status and progression. However, there are several limitations of current clinical assessments of cerebellar dysfunction. Firstly, the ICARS and SARA are not MS-specific [41], and their scores are primarily related to the level of ataxic symptoms such as posture, gait, and limb kinetic function. These items make up a possible 86 of the 100 points in the ICARS, and 30 out of 40 points of the SARA [41]. In addition, the ICARS is not always sensitive to change over time, especially with long disease durations [45, 54]. The cerebellar KFSS also focuses on gait ataxia and an increase in score requires a higher level of interference with daily functioning. While ataxia is an important symptom of cerebellar dysfunction to monitor, it is important not to underestimate the impact of other cerebellar symptoms such as tremor and dysarthria on quality of life and patient function. Furthermore, the EDSS is known to have limited inter-rater reliability [45], while the 9HPT has practice effects to consider when used alone or as part of the MSFC [45]. The 9HPT also solely assesses upper limb function and does not measure other cerebellar features such as gait [55]. Moreover, walking tests of gait have high variability depending on the precision and accuracy of measurement devices [56] and variation in task protocol [57, 58]. There is also little research on whether IMUs can indicate changes in MS disease severity over time [49]. The evidence thus far underscores that no single clinical measure provides enough information on both cerebellar function and overall MS disease-related impairments. It is, therefore, crucial to extend disease diagnosis and monitoring into paraclinical measures.
Neuroimaging Measures of Cerebellar Dysfunction
Magnetic resonance imaging (MRI) has a well-established role in research and clinical practice in MS. MRI is sensitive to different pathological substrates of MS including inflammatory demyelination and neuro-axonal loss [22]. More advanced MRI methods can derive quantitative objective measures that provide pathophysiological insights into MS pathogenesis. The cerebellum, as part of the infratentorial regions of the brain, is commonly assessed for the dissemination in space criterion of MS diagnosis [59]. MRI can be used to measure structural abnormalities and changes in cerebellar volume. Furthermore, MRI can be used to monitor connectivity between the cerebellum and cerebrum and changes in metabolism and blood flow. This allows us to monitor cerebellar function.
Lesion and Volumetric MRI
Demyelinating lesions and brain atrophy in MS are universal features of the disease across every stage of evolution, and the cerebellum is no exception. Cerebellar WM volume decreases in pwMS compared to healthy controls [30, 34, 60], whereas T1-weighted MRI can differentiate between groups of people with RRMS, SPMS, CIS, and healthy controls through analysis of mean cerebellar GM volume [60]. Similarly, cerebellar lesions are frequently detected by MRI [22]. Cerebellar leukocortical or WM lesions correlate with cerebellar volume loss and dysfunction in pwMS [61]. Additionally, overall increased T2-weighted cerebellar lesion volume and lower anterior cerebellar volume are associated with slower performance on the 9HPT [21]. There is higher volume and frequency of T2 lesions in the middle and superior cerebellar peduncles in pwMS with cerebellar and brainstem symptoms [62]. This damage is more precisely related to walking impairments in pwMS than measures of lesion volume or cerebellar atrophy [62]. Altered attention, verbal fluency, and motor performance are associated with total lesion load and mean lesion volume [63]. While this correlation is visible at 3 T, 7 T scanner findings showed significantly higher lesion load than lower level scanners [63]. A 2020 study showed that, compared to 3 T, 7 T scanners have up to 134% higher sensitivity for lesion detection. This led to better discrimination between cortical and WM lesions, and between leukocortical and WM lesions within the cerebellum in pwMS [64]. However, while lesion characterisation and volumetric MRI measures at all field strengths are useful for assessing structural changes associated with MS disease activity, they do not address any changes in FC or activity. Furthermore, they do not address microstructural changes in pwMS.
Advanced Neuroimaging Techniques
Diffusion-Weighted MRI
Diffusion MRI tracks the motion (i.e., diffusion) of water molecules in the brain [65]. Diffusion occurs with greater ease along tracts (parallel to axons) and less so when perpendicular to microstructural barriers (e.g., cell walls, extracellular sheets). Abnormalities in diffusion are often found in lesions and normal-appearing WM (NAWM) in pwMS [66, 67]. Diffusion abnormalities in the middle and superior cerebellar peduncles correlate with T2 lesion load in these regions, as well as with whole-brain T2 lesion load and cerebellar GM volume [62]. Research has found significant differences in diffusion in MS lesions when compared to contralateral and healthy tissue in the cerebrum [66]. The residual signal fraction, a measure of the volume fraction of axons, was also able to distinguish between NAWM and lesions in pwMS [66]. Additionally, normal-appearing GM (NAGM) in pwMS has microstructural damage, the extent of which correlates with the number of lesions throughout the brain and with cognitive impairment [67]. NAGM mean diffusivity and fractional anisotropy also positively correlate with EDSS scores [67]. Diffusion imaging of the cerebellum can accurately group mean differences between pwMS and controls or between pwMS with and without clinical impairment measured by the EDSS, cerebellar, and brain stem FSS [62]. Cerebellar diffusion metrics such as fractional anisotropy and radial diffusivity are correlated with EDSS scores in pwMS [62, 68]. Thus, diffusion metrics are associated with how microstructures are arranged in the CNS.
Functional MRI
Changes in functional activity and connectivity throughout the brain are some of the neurophysiological characteristics of MS [68]. Functional MRI (fMRI) uses blood oxygen level-dependent (BOLD) contrasts to track blood flow associated with neural activity [65]. BOLD contrasts show differences between pwMS and controls in connectivity, level of activation, and areas of activation in the brain [65]. fMRI can therefore demonstrate various functional abnormalities in the brains of pwMS that can be maladaptive, for example, reduced activation in the sensorimotor network. Adaptive function can also be evidenced by fMRI, such as increased activation and recruitment of additional brain areas during cognitive tasks [69]. Longitudinal research has shown that functional abnormalities vary, both during relapse and during periods of clinical stability [69]. Functional abnormalities strongly correlate both with disease severity and structural MRI findings [70].
fMRI studies involving the cerebellum can provide unique insights into its complex connections and functions. One study found a reduction in regional homogeneity of BOLD signal changes in pwMS within the left cerebellar hemisphere. In Crus I, Crus II, and dentate nucleus specifically, abnormal regional homogeneity also correlates with clinical disability [71]. Abnormal FC in the cerebellum identified through fMRI, both during active tasks and at resting-state (RS), has been linked to more severe disability and a higher number of inflammatory lesions [11]. RS fMRI has an extra advantage in MS research in that it allows us to perform functional imaging studies with pwMS who struggle completing tasks [72]. Higher cerebellar RS FC correlates with less severe disability in pwMS, which suggests an adaptive role for preserving clinical function [72]. Increased RS FC in the dentate nucleus is similarly linked to better motor performance, shorter disease duration and lower T2 lesion volume [73]. However, reduced RS FC in the dentate nucleus is associated with longer disease duration, cognitive impairment and higher T2 lesion volume in paediatric MS cases, possibly reflecting a loss of adaptive neuroplasticity [73].
Using task-based fMRI, motor dysfunction such as tremor has also been linked to cerebellar damage in pwMS [74, 75]. Additionally, fMRI has been used alongside speech analysis to identify the cerebellar function in the motor control of speech production in people with dysarthria [76]. Ackermann and Hertrichh’s 2000 study found preliminary evidence that cerebellar activation occurs at or above a speech tempo of 3 Hz during a syllable repetition task, which suggests that the cerebellum plays a role in the speed of articulatory movements after a certain base-level [76]. Like the increased sensitivity in structural imaging, ultra-high field fMRI can detect more minute changes in cerebellar functioning. While subtle impairments may not be picked up in clinical tasks, 7 T fMRI is able to detect changes in brain activity associated with upper and lower limb movement changes in minimally disabled pwMS (EDSS score < 4, pyramidal and cerebellar KFSS scores ≤ 2) [75].
Limitations of Neuroimaging Measures
In comparison to the cerebrum, the cerebellum has been less studied in MS. This is partly explained by contrast and resolution limitations of clinical MRI [77]. Additionally, optimal imaging of the cerebrum often takes precedence over and consequently limits that of the cerebellum in the clinical management of MS [78]. It is therefore not surprising that there are only a limited number of automated cerebellar segmentation algorithms available [77].
While sensitive to various pathological processes associated with MS, MRI cannot identify all underlying disease pathology [79]. For example, NAWM in T1- and T2-weighted images may still have widespread histopathological abnormalities [79]. Secondly, clinical MRI alone does not adequately explain the gradual disease progression typical in SPMS [79, 80].
Biological confounds can significantly influence both clinical MRI and fMRI. Volumetric MRI can be impacted by natural atrophy occurring with aging, level of hydration, and lifestyle factors including smoking and alcohol consumption [81]. Measurement inaccuracy in volumetric MRI therefore limits its clinical use for short-term assessment in individual pwMS. Additionally, biological artefacts such as cardiac and breathing cycles are more pronounced in cerebellar fMRI than cerebral fMRI. There are very few longitudinal fMRI studies in MS and even fewer that focus on the cerebellum [11, 82]. Furthermore, uncertainty remains about what type of neural activity is reflected in the cerebellar BOLD signal [83]. Consequently, additional research in this area is crucial for developing methods for monitoring cerebellar injury over time in pwMS.
It is also important to note that neuroimaging is less accessible than, for instance, clinical cerebellar monitoring. Imaging equipment is not always available, and when it is, it can be costly [84]. New MRI sequences can increase the scan-time of MRI, leading to patient discomfort that is further increased in those with more advanced disability [85].
Speech Measures of Cerebellar Dysfunction
Speech disorders are relatively common in pwMS, with 40–50% of pwMS experiencing difficulties with motor speech production (i.e., dysarthria) [86]. The resulting difficulties in communication often impact self-image, cause feelings of isolation, and decreased quality of life in pwMS [87]. White and GM loss, and damage to the bilateral corticobulbar tracts, cerebellum and midbrain are linked to dysarthria in MS [88, 89]. Furthermore, increased severity and frequency of dysarthric symptoms are associated with higher disability [90]. Specific speech subsystems are often affected, including deviations in articulation, prosody and respiratory support, and voice quality [91]. These deficits are linked to function of specific areas of the cerebellum and connected regions of the cerebrum (Fig. 2a and b) and are associated with other measures of disease severity in MS. For example, an increase in neurofilament light (NfL) levels following symptom onset correlate with dysarthria severity [92]. NfL is a protein associated with myelinated axons that is found in cerebrospinal fluid in amounts proportional to the level of axonal damage [93]. Speech profiles also change in line with performance on the 9HPT [94] and EDSS [91].
Fig. 2.
a Regions of the cerebellum and their motor speech functions. Created with BioRender.com. b Cerebellar connectivity networks and their role in speech production. Created with BioRender.com
Speech as a Potential Marker of Cerebellar Dysfunction in MS
Speech has shown potential as a clinical marker of disease in several other progressive neurological conditions, especially those with cerebellar involvement. These include Huntington’s disease [95], Friedreich’s ataxia (FA) [96, 97], and Parkinson’s disease (PD) [98, 99]. This research, along with recent studies regarding motor speech dysfunction in pwMS [88, 91] and people with cerebellar ataxia [100, 101], highlight the value of objective measures of dysarthria for monitoring MS disease progression associated with cerebellar dysfunction. While perceptual analysis (clinical listening and rating) of speech is the most used method for speech evaluation in clinical settings, it poses limitations such as low reproducibility, subjectivity, and confirmation bias [102, 103]. Acoustic speech analysis has been suggested to overcome the limitations of perceptual assessment [94, 103, 104]. It provides objective data linking the level of disability and speech impairment, suggesting that it is a suitable measure of MS-related neurological impairment [90]. Furthermore, acoustic speech analysis shows promise in detecting subclinical dysarthria in pwMS [91, 94, 104]. However, Noffs et al. [90] note that longitudinal research is required to determine whether acoustic speech analysis can be used as a marker for progression in pwMS.
As with any new potential biomarker, it is crucial to ensure that associations with speech variables and associations with MS function are relevant. This is determined by ecological validity, which must integrate any proposed translation of research tools into clinical use. To improve ecological validity of speech metrics, for instance, continuous or spontaneous speech should be analysed in addition to purposefully created speech tasks, such as sustained vowels or syllable repetition [105]. In doing this, the results will be more representative of natural speech and will therefore be more generalisable.
Measuring the Subsystems of Speech
Impaired motor control and weakness of muscles involved in speech can lead to dysarthria, impacting all speech subsystems: respiration, articulation, phonation, prosody, and resonance [87]. Persons with cerebellar dysarthria often have reduced articulatory accuracy and slower articulation [87, 106], which can be measured using a combination of electroglottography (monitoring the vibration of the vocal folds) and acoustic speech analysis [106]. Articulation rate specifically could be used as a marker of progression in MS [107]. Furthermore, spectral and cepstral analysis focuses on the spectral representation of speech and can pinpoint the location of formants, which can be defined as the concentration of acoustic energy around peak frequencies in speech. Cepstral analysis has been used to monitor abnormalities in resonance and voice quality due to cerebellar ataxia [100] and dysarthria in persons with PD [108] and cerebral palsy [109]. Composite acoustic measures involving prosodic features (such as intonation, stress rhythm), feature selection, and support vector machines (SVMs) and dysarthria severity diagnosis can accurately select dysarthric features and predict diagnosis [110]. Additionally, combining SVM with a Gaussian mixture model (GMM) to develop an objective measure of dysarthria severity can be used to assess prosody [111]. There are also systems specifically for dysarthric speech, which use SVMs and hidden Markov models (HMMs) to increase the level of speech recognition [112]. Automatic speech recognition (ASR) systems, which are trained on databases of healthy speech, can be used to estimate the level of intelligibility of pathological speech [113]. “Dysarthria phenotyping” may also be achievable. As different causes of dysarthria can produce different speech recognition errors, ASR can differentiate between pwMS and people with FA, for example, by identifying dysarthric speech patterns [114].
Acoustic Speech Analysis in pwMS
Objective methods of speech and voice analysis have allowed for better detection and characterisation of speech changes caused by MS [86, 94, 115]. Acoustic analysis can enhance our understanding of how neuromotor dysfunction in MS affects speech and for detecting and monitoring changes resulting from disease progression or treatment [86]. Research thus far has indicated that acoustic analysis of speech can produce valuable metrics related to neurological status in pwMS. See Table 2 for a summary of the current findings.
Table 2.
Summary of research on acoustic speech analysis and cerebellar dysfunction
| Author | Disorder | Number of participants | Method/design | Key findings |
|---|---|---|---|---|
| Hartelius, Buder [136] | MS | 20 pwMS, 20 age- and gender-matched controls | Total variance, a magnitude-based analysis, and a frequency-based analysis were used to assess long-term phonatory instability in pwMS. Phonatory instability was measured both in Hz (frequency) and dB (sound intensity/loudness) | Instability of sound intensity can be used to discriminate between pwMS and healthy controls |
| Jannetts and Lowit [108] | PD, ataxia | 43 pwPD, ten pw ataxia | Sustained phonation of /a/, passage reading, and spontaneous speech was recorded and analysed using acoustic measures and perceptual analysis | Cepstral peak prominence is an adequate predictor of breathiness and dysphonia in persons with motor speech disorders |
| Kuo and Tjaden [137] | MS, PD | 15 pwMS, 12 pwPD, 14 controls | Participants were recorded reading a 192-word passage three times in a cross-sectional design. The passage was read normally, loudly, and slowly. The latter two conditions’ orders were counterbalanced and randomised | Passage reading is associated with naturally occurring acoustic variation both in pwMS with dysarthria and in controls |
| Novotný, Rusz, Spálenka, Klempír, Horáková and Ruzicka [138] | MS, Multiple system atrophy, Cerebellar ataxia | 74 | Analyse nasality of speech in people with cerebellar disorders causing ataxic dysarthria using 1/3 octave spectra method | There can be abnormal fluctuations in nasality in pwMS with ataxic dysarthria. This was more prominent than differences in nasality between control and cerebellar disorder groups |
| Noffs, Perera, Kolbe, Shanahan, Boonstra, Evans, Butzkueven, van der Walt and Vogel [91] | MS | - | A systematic review of literature | Acoustic measurement of vowel instability can be used to discriminate between pwMS and controls. An increase in pausing, slower maximum speech rate, and subclinical voice tremor are predictive of cerebellar dysfunction in pwMS |
| Rusz, Tykalová, Salerno, Bancone, Scarpelli and Pellecchia [139] | MSA, PD | 40 with probable MSA, 20pwPD, 20 controls | Use quantitative acoustic analysis to distinguish between MSA, PD, and controls | Speech disorders reflect underlying pathophysiology of MSA. Acoustic speech analysis can distinguish between people with MSA and PD due to differing dysarthric features |
| Kashyap, Pathirana [100] | Cerebellar ataxia | 42 pwCA, 23 age-matched controls | A composite cepstral analysis comprising 12 measures was used to distinguish between people with cerebellar ataxia and control participants | Phase-based and magnitude-based cepstral analysis of speech performs were better than more traditional, time-based acoustic analysis in terms of discrimination between patients and controls |
| Noffs, Boonstra, Perera, Butzkueven, Kolbe, Maldonado, et al. [90] | MS | 119 pwMS (68 completed MRI), 22 controls | Acoustic speech analysis assessed timing, control, voice quality, naturalness, and intelligibility. Additional perceptual analysis was used for comparison. PwMS also completed the EDSS and MS Impact Scale to assess quality of life. T1-weighted MRI was used to measure brain volume and lesion load | Composite speech scores correlate with disease severity, quality of life, and total lesion load. Measures of pause percentage and frequency instability correlate with EDSS scores in pwMS, even with no perceivable dysarthria. The perceptual analysis only picked up speech impairment in pwMS with established neurological impairment (EDSS ≥ 3) |
Electronic and Home-Based Monitoring Systems
Electronic and home-based services can improve healthcare by removing accessibility constraints. Digital healthcare, in particular self-monitoring tools, can provide patients with real-time feedback, increased support, awareness of their current status, and a sense of control over their disease [116, 117]. Those are particularly appealing attributes for long-term monitoring of symptoms [118]. Many tools have been developed to monitor and assess cerebellar MS symptoms outside clinical settings, including smartphone applications and activity trackers [116]. There is also the patient determined disease steps (PDDS), which is a measure of disability in MS that can be administered online. The PDDS significantly correlates with the EDSS, pyramidal, and cerebellar functional systems scores [119], making it a useful tool for monitoring cerebellar dysfunction-based disability in pwMS.
Floodlight is a smartphone app-based system for monitoring MS disease management and progression. This tool includes a collection of tasks designed to assess mood, information processing, hand motor function, gait, and balance [120, 121]. Both the pinching test and the draw a shape test included in Floodlight correlate significantly with the 9HPT [122]. Furthermore, smartphone-based versions of the U-turn speed test and 2-min walk test also included in Floodlight have been determined as reliable and valid measures of gait and balance in pwMS [123, 124]. Recently, there has also been interest in using speech as a biomarker of neurodegenerative diseases such as MS and PD, as well as for mental health, cardiovascular diseases and COVID-19 [125]. Speech data collection is flexible and can be done in clinic, and also at home via telephone, smartphone, and web-based recording systems [125]. Tablet-based analysis of acoustic speech measures shows promise for diagnosis, monitoring, and risk prediction in pwMS [126, 127]. However, it is essential to note that digital and home-based interventions tend to have low usage and high dropout rates [128] especially outside research situations. Patients are more likely to use digital monitoring consistently if the system tracks progress, are personalised and targeted, can adapt to changing needs, provide self-management techniques, and, most importantly, have the additional support of a clinician [129]. In using digital systems, there is the additional consideration of device issues that may impact the continued monitoring of symptoms. For example, different mobile devices may have different technical issues with the same health application. Furthermore, with at-home monitoring, assistance with technical issues is less readily available than in the clinic. Given this is a more recent area of MS research and management, there is not much validation data, but home-based measures show promise.
Concluding Statements
Current tools for monitoring cerebellar dysfunction in pwMS present major limitations for both the detection of subclinical progression in cerebellar dysfunction and long-term tracking of disease progression. Cerebellar symptoms are associated with faster disease progression and earlier onset of SPMS, as well as reduced relapse recovery. For optimal monitoring of MS, we suggest working to determine the best combination of available measures for the patient. Furthermore, we must continue to explore new ways of assessing cerebellar dysfunction in pwMS. Conventional tools such as clinical tests and structural MRI are crucial for diagnosis, understanding MS and supporting pwMS with cerebellar dysfunction. However, they often do not meet our needs as researchers, or the disease management needs of the patients. While advanced neuroimaging provides additional information regarding functional and microstructural changes in the cerebellum, it is not without its limitations, and, like all imaging, it is not accessible to all pwMS due to cost, travel, and comfort. The step into digital and home-based monitoring has given rise to wearable monitors and smartphone applications to assess cerebellar symptoms such as gait and balance disruptions. In turn, this has provided pwMS with more accessible management and monitoring of their disease. Speech data is ideal for digital monitoring due to its ease of collection and ability to provide objective results in real time. The research to date suggests that speech would make a good marker of cerebellar dysfunction in pwMS. However, further research is required, particularly in terms of using acoustic speech analysis to monitor cerebellar changes over time associated with MS disease progression.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Declarations
Conflict of Interest
Anneke van der Walt served on advisory boards and receives unrestricted research grants from Novartis, Biogen, Merck, and Roche. She has received speaker’s honoraria and travel support from Novartis, Roche, and Merck. She receives grant support from the National Health and Medical Research Council of Australia and MS Research Australia. Adam Vogel is Chief Science Officer of Redenlab Inc. Gustavo Noffs works in scientific development for Redenlab Inc. Scott Kolbe receives grant income from MS Research Australia and has received honoraria from Novartis, Biogen, and Merck. Helmut Butzkueven’s institution receives compensation for serving on scientific advisory boards and speaker bureaus for Biogen, Novartis, Roche, Merck, and UCB, steering committee duties for trials conducted by Biogen, Merck, Roche, and Novartis and his institution receives research support from Merck, Roche, Novartis, ALexion, and Biogen.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilkins A. Cerebellar dysfunction in multiple sclerosis. Front Neurol. 2017;8:312. doi: 10.3389/fneur.2017.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9(5):520–532. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- 3.Brola W, Mitosek-Szewczyk K, Opara J. Symptomatology and pathogenesis of different types of pain in multiple sclerosis. Neurol Neurochir Pol. 2014;48(4):272–279. doi: 10.1016/j.pjnns.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Charcot J. Disseminated sclerosis: its symptomatology. Charcot JM Lectures on the diseases of the nervous system. London: New Syndenham Society; 1877. pp. 209–217. [Google Scholar]
- 5.Charcot JM, Sigerson G, Savill TD. Lectures on the diseases of the nervous system. La Salpêtrière: New Sydenham Society. Br Foreign Med Chir Rev. 1877;60(119):180–1.
- 6.Pearce JMS. Historical descriptions of multiple sclerosis. Eur Neurol. 2005;54(1):49–53. doi: 10.1159/000087387. [DOI] [PubMed] [Google Scholar]
- 7.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7(12):1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 8.Briggs FBS, Thompson NR, Conway DS. Prognostic factors of disability in relapsing remitting multiple sclerosis. Mult Scler Relat Disord. 2019;30:9–16. doi: 10.1016/j.msard.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 9.Cossburn M, Ingram G, Hirst C, Ben-Shlomo Y, Pickersgill TP, Robertson NP. Age at onset as a determinant of presenting phenotype and initial relapse recovery in multiple sclerosis. Mult Scler J. 2011;18(1):45–54. doi: 10.1177/1352458511417479. [DOI] [PubMed] [Google Scholar]
- 10.Marrie RA, Horwitz RI. Emerging effects of comorbidities on multiple sclerosis. Lancet Neurol. 2010;9(8):820–828. doi: 10.1016/S1474-4422(10)70135-6. [DOI] [PubMed] [Google Scholar]
- 11.Parmar K, Stadelmann C, Rocca MA, Langdon D, D'Angelo E, D’Souza M, et al. The role of the cerebellum in multiple sclerosis—150 years after Charcot. Neurosci Biobehav Rev. 2018;89:85–98. doi: 10.1016/j.neubiorev.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Weinshenker BG, Issa M, Baskerville J. Long-term and short-term outcome of multiple sclerosis: a 3-year follow-up study. Arch Neurol. 1996;53(4):353–358. doi: 10.1001/archneur.1996.00550040093018. [DOI] [PubMed] [Google Scholar]
- 13.Le M, Malpas C, Sharmin S, Horáková D, Havrdova E, Trojano M, et al. Disability outcomes of early cerebellar and brainstem symptoms in multiple sclerosis. Mult Scler J. 2020;0(0):1352458520926955. [DOI] [PubMed]
- 14.O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2009;20(4):953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19(10):2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glickstein M, Sultan F, Voogd J. Functional localization in the cerebellum. Cortex. 2011;47(1):59–80. doi: 10.1016/j.cortex.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Knogler LD, Kist AM, Portugues R. Motor context dominates output from Purkinje cell functional regions during reflexive visuomotor behaviours. Elife. 2019;8:e42138. doi: 10.7554/eLife.42138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Droby A, Yuen KSL, Muthuraman M, Reitz S-C, Fleischer V, Klein J, et al. Changes in brain functional connectivity patterns are driven by an individual lesion in MS: a resting-state fMRI study. Brain Imaging Behav. 2016;10(4):1117–1126. doi: 10.1007/s11682-015-9476-3. [DOI] [PubMed] [Google Scholar]
- 19.Rocca MA, Pagani E, Absinta M, Valsasina P, Falini A, Scotti G, et al. Altered functional and structural connectivities in patients with MS. Neurology. 2007;69(23):2136. doi: 10.1212/01.wnl.0000295504.92020.ca. [DOI] [PubMed] [Google Scholar]
- 20.Saini S, DeStefano N, Smith S, Guidi L, Amato MP, Federico A, et al. Altered cerebellar functional connectivity mediates potential adaptive plasticity in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2004;75(6):840. doi: 10.1136/jnnp.2003.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Ambrosio A, Pagani E, Riccitelli GC, Colombo B, Rodegher M, Falini A, et al. Cerebellar contribution to motor and cognitive performance in multiple sclerosis: an MRI sub-regional volumetric analysis. Mult Scler J. 2017;23(9):1194–1203. doi: 10.1177/1352458516674567. [DOI] [PubMed] [Google Scholar]
- 22.Filippi M, Brück W, Chard D, Fazekas F, Geurts JJG, Enzinger C, et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2019;18(2):198–210. doi: 10.1016/S1474-4422(18)30451-4. [DOI] [PubMed] [Google Scholar]
- 23.Minneboo A, Barkhof F, Polman CH, Uitdehaag BMJ, Knol DL, Castelijns JA. Infratentorial lesions predict long-term disability in patients with initial findings suggestive of multiple sclerosis. Arch Neurol. 2004;61(2):217–221. doi: 10.1001/archneur.61.2.217. [DOI] [PubMed] [Google Scholar]
- 24.Droby A, Fleischer V, Carnini M, Zimmermann H, Siffrin V, Gawehn J, et al. The impact of isolated lesions on white-matter fiber tracts in multiple sclerosis patients. NeuroImage Clin. 2015;8:110–6. doi: 10.1016/j.nicl.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giorgio A, Battaglini M, Rocca MA, De Leucio A, Absinta M, Van Schijndel R, et al. Location of brain lesions predicts conversion of clinically isolated syndromes to multiple sclerosis. Neurology. 2013;80(3):234–241. doi: 10.1212/WNL.0b013e31827debeb. [DOI] [PubMed] [Google Scholar]
- 26.Kutzelnigg A, Faber-Rod JC, Bauer J, Lucchinetti CF, Sorensen PS, Laursen H, et al. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 2007;17(1):38–44. doi: 10.1111/j.1750-3639.2006.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert M, Barrantes-Freer A, Lohrberg M, Antel JP, Prineas JW, Palkovits M, et al. Synaptic pathology in the cerebellar dentate nucleus in chronic multiple sclerosis. Brain Pathol. 2017;27(6):737–747. doi: 10.1111/bpa.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boonstra F, Gajamange S, Noffs G, Perera T, Strik M, Vogel A, et al. Evaluation of cerebellar function scores in relation to cerebellar axonal loss in multiple sclerosis. bioRxiv. 2020. 10.1101/2020.05.15.094938.
- 29.Ramasamy DP, Benedict RHB, Cox JL, Fritz D, Abdelrahman N, Hussein S, et al. Extent of cerebellum, subcortical and cortical atrophy in patients with MS: a case-control study. J Neurol Sci. 2009;282(1):47–54. doi: 10.1016/j.jns.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 30.Weier K, Banwell B, Cerasa A, Collins DL, Dogonowski A-M, Lassmann H, et al. The role of the cerebellum in multiple sclerosis. Cerebellum. 2015;14(3):364–374. doi: 10.1007/s12311-014-0634-8. [DOI] [PubMed] [Google Scholar]
- 31.Grodd W, Hülsmann E, Lotze M, Wildgruber D, Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp. 2001;13(2):55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konczak J, Timmann D. The effect of damage to the cerebellum on sensorimotor and cognitive function in children and adolescents. Neurosci Biobehav Rev. 2007;31(8):1101–1113. doi: 10.1016/j.neubiorev.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Wildgruber D, Ackermann H, Grodd W. Differential contributions of motor cortex, basal ganglia, and cerebellum to speech motor control: effects of syllable repetition rate evaluated by fMRI. Neuroimage. 2001;13(1):101–109. doi: 10.1006/nimg.2000.0672. [DOI] [PubMed] [Google Scholar]
- 34.Anderson VM, Wheeler-Kingshott CAM, Abdel-Aziz K, Miller DH, Toosy A, Thompson AJ, et al. A comprehensive assessment of cerebellar damage in multiple sclerosis using diffusion tractography and volumetric analysis. Mult Scler J. 2011;17(9):1079–1087. doi: 10.1177/1352458511403528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rocca MA, Valsasina P, Leavitt VM, Rodegher M, Radaelli M, Riccitelli GC, et al. Functional network connectivity abnormalities in multiple sclerosis: correlations with disability and cognitive impairment. Mult Scler J. 2017;24(4):459–471. doi: 10.1177/1352458517699875. [DOI] [PubMed] [Google Scholar]
- 36.de Leon AS, Das JM. Neuroanatomy, Dentate Nucleus. Treasure Island: StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554381/. [PubMed]
- 37.Sbardella E, Upadhyay N, Tona F, Prosperini L, De Giglio L, Petsas N, et al. Dentate nucleus connectivity in adult patients with multiple sclerosis: functional changes at rest and correlation with clinical features. Mult Scler J. 2016;23(4):546–555. doi: 10.1177/1352458516657438. [DOI] [PubMed] [Google Scholar]
- 38.Schoonheim MM, Douw L, Broeders TAA, Eijlers AJC, Meijer KA, Geurts JJG. The cerebellum and its network: disrupted static and dynamic functional connectivity patterns and cognitive impairment in multiple sclerosis. Mult Scler J. 2021;27(13):2031–2039. doi: 10.1177/1352458521999274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurtzke JF. A new scale for evaluating disability in multiple sclerosis. Neurology. 1955;5(8):580. doi: 10.1212/WNL.5.8.580. [DOI] [PubMed] [Google Scholar]
- 40.Kurtzke JF. Rating neurologic impairment in multiple sclerosis. Neurology. 1983;33(11):1444. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 41.Salcı Y, Fil A, Keklicek H, Çetin B, Armutlu K, Dolgun A, et al. Validity and reliability of the International Cooperative Ataxia Rating Scale (ICARS) and the Scale for the Assessment and Rating of Ataxia (SARA) in multiple sclerosis patients with ataxia. Mult Scler Relat Disord. 2017;18:135–140. doi: 10.1016/j.msard.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 42.Winser S, Smith CM, Hale LA, Claydon LS, Whitney SL, Klatt B, et al. Psychometric properties of a core set of measures of balance for people with cerebellar ataxia secondary to multiple sclerosis. Arch Phys Med Rehabil. 2017;98(2):270–276. doi: 10.1016/j.apmr.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 43.Subramony SH. SARA—a new clinical scale for the assessment and rating of ataxia. Nat Clin Pract Neurol. 2007;3(3):136–137. doi: 10.1038/ncpneuro0426. [DOI] [PubMed] [Google Scholar]
- 44.Feys P, Lamers I, Francis G, Benedict R, Phillips G, LaRocca N, et al. The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult Scler J. 2017;23(5):711–720. doi: 10.1177/1352458517690824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer-Moock S, Feng Y-S, Maeurer M, Dippel F-W, Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14(1):58. doi: 10.1186/1471-2377-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tramontano M, Grasso MG, Soldi S, Casula EP, Bonnì S, Mastrogiacomo S, et al. Cerebellar intermittent theta-burst stimulation combined with vestibular rehabilitation improves gait and balance in patients with multiple sclerosis: a preliminary double-blind randomized controlled trial. Cerebellum. 2020;19(6):897–901. doi: 10.1007/s12311-020-01166-y. [DOI] [PubMed] [Google Scholar]
- 47.Shanahan C, Boonstra F, Cofré Lizama LE, Strik M, Moffat B, Khan F, et al. Technologies for advanced gait and balance assessments in people with multiple sclerosis. Front Neurol. 2017;8:708. [DOI] [PMC free article] [PubMed]
- 48.Kalron A, Frid L. The, “butterfly diagram”: a gait marker for neurological and cerebellar impairment in people with multiple sclerosis. J Neurol Sci. 2015;358(1):92–100. doi: 10.1016/j.jns.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 49.Vienne-Jumeau A, Quijoux F, Vidal P-P, Ricard D. Wearable inertial sensors provide reliable biomarkers of disease severity in multiple sclerosis: a systematic review and meta-analysis. Ann Phys Rehabil Med. 2020;63(2):138–147. doi: 10.1016/j.rehab.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Castiglia SF, Trabassi D, Tatarelli A, Ranavolo A, Varrecchia T, Fiori L, et al. Identification of gait unbalance and fallers among subjects with cerebellar ataxia by a set of trunk acceleration-derived indices of gait. Cerebellum. 2022. 10.1007/s12311-021-01361-5. [DOI] [PubMed]
- 51.Sun R, Moon Y, McGinnis RS, Seagers K, Motl RW, Sheth N, et al. Assessment of postural sway in individuals with multiple sclerosis using a novel wearable inertial sensor. Digit Biomark. 2018;2(1):1–10. doi: 10.1159/000485958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McLoughlin J, Barr C, Crotty M, Lord SR, Sturnieks DL. Association of postural sway with disability status and cerebellar dysfunction in people with multiple sclerosis: a preliminary study. Int J MS Care. 2015;17(3):146–151. doi: 10.7224/1537-2073.2014-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gera G, Fling BW, Horak FB. Cerebellar white matter damage is associated with postural sway deficits in people with multiple sclerosis. Arch Phys Med Rehabil. 2020;101(2):258–264. doi: 10.1016/j.apmr.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 54.Perez-Lloret S, van de Warrenburg B, Rossi M, Rodríguez-Blázquez C, Zesiewicz T, Saute JAM, et al. Assessment of Ataxia Rating Scales and cerebellar functional tests: critique and recommendations. Mov Disord. 2021;36(2):283–297. doi: 10.1002/mds.28313. [DOI] [PubMed] [Google Scholar]
- 55.Yozbatıran N, Baskurt F, Baskurt Z, Ozakbas S, Idiman E. Motor assessment of upper extremity function and its relation with fatigue, cognitive function and quality of life in multiple sclerosis patients. J Neurol Sci. 2006;246(1):117–122. doi: 10.1016/j.jns.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 56.Creagh AP, Simillion C, Bourke AK, Scotland A, Lipsmeier F, Bernasconi C, et al. Smartphone- and smartwatch-based remote characterisation of ambulation in multiple sclerosis during the two-minute walk test. IEEE J Biomed Health Inform. 2021;25(3):838–849. doi: 10.1109/JBHI.2020.2998187. [DOI] [PubMed] [Google Scholar]
- 57.Bennett SE, Bromley LE, Fisher NM, Tomita MR, Niewczyk P. Validity and reliability of four clinical gait measures in patients with multiple sclerosis. Int J MS Care. 2017;19(5):247–252. doi: 10.7224/1537-2073.2015-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gijbels D, Dalgas U, Romberg A, de Groot V, Bethoux F, Vaney C, et al. Which walking capacity tests to use in multiple sclerosis? A multicentre study providing the basis for a core set. Mult Scler J. 2011;18(3):364–371. doi: 10.1177/1352458511420598. [DOI] [PubMed] [Google Scholar]
- 59.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 60.Anderson VM, Fisniku LK, Altmann DR, Thompson AJ, Miller DH. MRI measures show significant cerebellar gray matter volume loss in multiple sclerosis and are associated with cerebellar dysfunction. Mult Scler J. 2009;15(7):811–817. doi: 10.1177/1352458508101934. [DOI] [PubMed] [Google Scholar]
- 61.Calabrese M, Mattisi I, Rinaldi F, Favaretto A, Atzori M, Bernardi V, et al. Magnetic resonance evidence of cerebellar cortical pathology in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2010;81(4):401. doi: 10.1136/jnnp.2009.177733. [DOI] [PubMed] [Google Scholar]
- 62.Preziosa P, Rocca MA, Mesaros S, Pagani E, Drulovic J, Stosic-Opincal T, et al. Relationship between damage to the cerebellar peduncles and clinical disability in multiple sclerosis. Radiology. 2014;271(3):822–830. doi: 10.1148/radiol.13132142. [DOI] [PubMed] [Google Scholar]
- 63.Fartaria MJ, O'Brien K, Sorega A, Bonnier G, Roche A, Falkovskiy P, et al. An ultra-high field study of cerebellar pathology in early relapsing-remitting multiple sclerosis using MP2RAGE. Invest Radiol. 2017;52(5):265–73. 10.1097/RLI.0000000000000338. [DOI] [PubMed]
- 64.Louapre C, Treaba CA, Barletta V, Mainero C. Ultra-high field 7 T imaging in multiple sclerosis. Curr Opin Neurol. 2020;33(4):422–9. 10.1097/WCO.0000000000000839. [DOI] [PubMed]
- 65.Bakshi R, Thompson AJ, Rocca MA, Pelletier D, Dousset V, Barkhof F, et al. MRI in multiple sclerosis: current status and future prospects. Lancet Neurol. 2008;7(7):615–625. doi: 10.1016/S1474-4422(08)70137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Santis S, Granberg T, Ouellette R, Treaba CA, Herranz E, Fan Q, et al. Evidence of early microstructural white matter abnormalities in multiple sclerosis from multi-shell diffusion MRI. NeuroImage Clin. 2019;22:101699. doi: 10.1016/j.nicl.2019.101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han X, Wang X, Wang L, Zheng Z, Gu J, Tang D, et al. Investigation of grey matter abnormalities in multiple sclerosis patients by combined use of double inversion recovery sequences and diffusion tensor MRI at 3.0 Tesla. Clin Radiol. 2018;73(9):834.e17–.e23. doi: 10.1016/j.crad.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 68.Mormina E, Petracca M, Bommarito G, Piaggio N, Cocozza S, Inglese M. Cerebellum and neurodegenerative diseases: beyond conventional magnetic resonance imaging. World J Radiol. 2017;9(10):371–388. doi: 10.4329/wjr.v9.i10.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Filippi M, Rocca MA. Present and future of fMRI in multiple sclerosis. Expert Rev Neurother. 2013;13(sup2):27–31. doi: 10.1586/14737175.2013.865871. [DOI] [PubMed] [Google Scholar]
- 70.Rocca MA, Amato MP, De Stefano N, Enzinger C, Geurts JJ, Penner I-K, et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14(3):302–317. doi: 10.1016/S1474-4422(14)70250-9. [DOI] [PubMed] [Google Scholar]
- 71.Dogonowski A-M, Andersen KW, Madsen KH, Sørensen PS, Paulson OB, Blinkenberg M, et al. Multiple sclerosis impairs regional functional connectivity in the cerebellum. NeuroImage Clin. 2014;4:130–8. doi: 10.1016/j.nicl.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rocca MA, De Meo E, Filippi M. Resting-state fMRI in multiple sclerosis. In: Ulmer S, Jansen O, editors. fMRI: Basics and Clinical Applications. Cham: Springer International Publishing; 2020. pp. 335–353. [Google Scholar]
- 73.Cirillo S, Rocca MA, Ghezzi A, Valsasina P, Moiola L, Veggiotti P, et al. Abnormal cerebellar functional MRI connectivity in patients with paediatric multiple sclerosis. Mult Scler. 2016;22(3):292–301. doi: 10.1177/1352458515592191. [DOI] [PubMed] [Google Scholar]
- 74.Boonstra FMC, Perera T, Noffs G, Marotta C, Vogel AP, Evans AH, et al. Novel functional MRI task for studying the neural correlates of upper limb tremor. Front Neurol. 2018;9(513). 10.3389/fneur.2018.00513. [DOI] [PMC free article] [PubMed]
- 75.Strik M, Shanahan CJ, van der Walt A, Boonstra FMC, Glarin R, Galea MP, et al. Functional correlates of motor control impairments in multiple sclerosis: a 7 Tesla task functional MRI study. Hum Brain Mapp. 2021;42(8):2569–2582. doi: 10.1002/hbm.25389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ackermann H, Hertrich I. The contribution of the cerebellum to speech processing. J Neurolinguistics. 2000;13(2):95–116. doi: 10.1016/S0911-6044(00)00006-3. [DOI] [Google Scholar]
- 77.Park MTM, Pipitone J, Baer LH, Winterburn JL, Shah Y, Chavez S, et al. Derivation of high-resolution MRI atlases of the human cerebellum at 3T and segmentation using multiple automatically generated templates. Neuroimage. 2014;95:217–231. doi: 10.1016/j.neuroimage.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 78.Desmond JE, Fiez JA. Neuroimaging studies of the cerebellum: language, learning and memory. Trends Cogn Sci. 1998;2(9):355–362. doi: 10.1016/S1364-6613(98)01211-X. [DOI] [PubMed] [Google Scholar]
- 79.Fox RJ, Beall E, Bhattacharyya P, Chen JT, Sakaie K. Advanced MRI in multiple sclerosis: current status and future challenges. Neurol Clin. 2011;29(2):357–380. doi: 10.1016/j.ncl.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Filippi M, Absinta M, Rocca MA. Future MRI tools in multiple sclerosis. J Neurol Sci. 2013;331(1):14–18. doi: 10.1016/j.jns.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 81.Sinnecker T, Granziera C, Wuerfel J, Schlaeger R. Future brain and spinal cord volumetric imaging in the clinic for monitoring treatment response in MS. Curr Treat Options Neurol. 2018;20(6):17. doi: 10.1007/s11940-018-0504-7. [DOI] [PubMed] [Google Scholar]
- 82.Pantano P, Mainero C, Lenzi D, Caramia F, Iannetti GD, Piattella MC, et al. A longitudinal fMRI study on motor activity in patients with multiple sclerosis. Brain. 2005;128(9):2146–2153. doi: 10.1093/brain/awh549. [DOI] [PubMed] [Google Scholar]
- 83.Diedrichsen J, Verstynen T, Schlerf J, Wiestler T. Advances in functional imaging of the human cerebellum. Curr Opin Neurol. 2010;23(4):382–387. doi: 10.1097/WCO.0b013e32833be837. [DOI] [PubMed] [Google Scholar]
- 84.Geethanath S, Vaughan JT., Jr Accessible magnetic resonance imaging: a review. J Magn Reson Imaging. 2019;49(7):e65–e77. doi: 10.1002/jmri.26638. [DOI] [PubMed] [Google Scholar]
- 85.Story MF, Schwier E, Kailes JI. Perspectives of patients with disabilities on the accessibility of medical equipment: examination tables, imaging equipment, medical chairs, and weight scales. Disabil Health J. 2009;2(4):169–79.e1. doi: 10.1016/j.dhjo.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 86.Hartelius L, Nord L, Buder EH. Acoustic analysis of dysarthria associated with multiple sclerosis. Clin Linguist Phon. 1995;9(2):95–120. doi: 10.3109/02699209508985327. [DOI] [Google Scholar]
- 87.Enderby P. Chapter 22 - Disorders of communication: dysarthria. In: Barnes MP, Good DC, editors. Handbook of clinical neurology, vol. 110. Amsterdam: Elsevier; 2013. p. 273–81. [DOI] [PubMed]
- 88.Rusz J, Vaneckova M, Benova B, Tykalova T, Novotny M, Ruzickova H, et al. Brain volumetric correlates of dysarthria in multiple sclerosis. Brain Lang. 2019;194:58–64. doi: 10.1016/j.bandl.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 89.Li Y, Zeng C, Luo T. Paroxysmal dysarthria and ataxia in multiple sclerosis and corresponding magnetic resonance imaging findings. J Neurol. 2011;258(2):273–276. doi: 10.1007/s00415-010-5748-4. [DOI] [PubMed] [Google Scholar]
- 90.Noffs G, Boonstra FMC, Perera T, Butzkueven H, Kolbe SC, Maldonado F, et al. Speech metrics, general disability, brain imaging and quality of life in MS. Eur J Neurol. 2020;n/a(n/a). [DOI] [PubMed]
- 91.Noffs G, Perera T, Kolbe SC, Shanahan CJ, Boonstra FMC, Evans A, et al. What speech can tell us: a systematic review of dysarthria characteristics in Multiple Sclerosis. Autoimmun Rev. 2018;17(12):1202–1209. doi: 10.1016/j.autrev.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 92.Barro C, Zetterberg H. Neurological symptoms and blood neurofilament light levels. Acta Neurol Scand. 2021;144(1):13–20. doi: 10.1111/ane.13415. [DOI] [PubMed] [Google Scholar]
- 93.Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90(8):870. doi: 10.1136/jnnp-2018-320106. [DOI] [PubMed] [Google Scholar]
- 94.Rusz J, Benova B, Ruzickova H, Novotny M, Tykalova T, Hlavnicka J, et al. Characteristics of motor speech phenotypes in multiple sclerosis. Multi Scler Relat Disord. 2018;19:62–69. doi: 10.1016/j.msard.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 95.Chan JC, Stout JC, Vogel AP. Speech in prodromal and symptomatic Huntington’s disease as a model of measuring onset and progression in dominantly inherited neurodegenerative diseases. Neurosci Biobehav Rev. 2019;107:450–460. doi: 10.1016/j.neubiorev.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 96.Schirinzi T, Sancesario A, Bertini E, Castelli E, Vasco G. Speech and language disorders in Friedreich ataxia: highlights on phenomenology, assessment, and therapy. Cerebellum. 2020;19(1):126–130. doi: 10.1007/s12311-019-01084-8. [DOI] [PubMed] [Google Scholar]
- 97.Vogel AP, Wardrop MI, Folker JE, Synofzik M, Corben LA, Delatycki MB, et al. Voice in Friedreich ataxia. J Voice. 2017;31(2):243.e9–.e19. doi: 10.1016/j.jvoice.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 98.Magee M, Copland D, Vogel AP. Motor speech and non-motor language endophenotypes of Parkinson’s disease. Expert Rev Neurother. 2019;19(12):1191–1200. doi: 10.1080/14737175.2019.1649142. [DOI] [PubMed] [Google Scholar]
- 99.Rusz J, Hlavnička J, Tykalová T, Novotný M, Dušek P, Šonka K, et al. Smartphone allows capture of speech abnormalities associated with high risk of developing Parkinson’s disease. IEEE Trans Neural Syst Rehabil Eng. 2018;26(8):1495–1507. doi: 10.1109/TNSRE.2018.2851787. [DOI] [PubMed] [Google Scholar]
- 100.Kashyap B, Pathirana PN, Horne M, Power L, Szmulewicz D. Quantitative assessment of speech in cerebellar ataxia using magnitude and phase based cepstrum. Ann Biomed Eng. 2020;48(4):1322–1336. doi: 10.1007/s10439-020-02455-7. [DOI] [PubMed] [Google Scholar]
- 101.Vogel AP, Magee M, Torres-Vega R, Medrano-Montero J, Cyngler MP, Kruse M, et al. Features of speech and swallowing dysfunction in pre-ataxic spinocerebellar ataxia type 2. Neurology. 2020;95(2):e194. doi: 10.1212/WNL.0000000000009776. [DOI] [PubMed] [Google Scholar]
- 102.Kent RD. Hearing and believing. Am J Speech Lang Pathol. 1996;5(3):7–23. doi: 10.1044/1058-0360.0503.07. [DOI] [Google Scholar]
- 103.Laaridh I, Kheder W, Fredouille C, Meunier C, editors. Automatic prediction of speech evaluation metrics for dysarthric speech. Interspeech. 2017. 10.21437/Interspeech.2017-1363.
- 104.Kent RD, Weismer G, Kent JF, Vorperian HK, Duffy JR. Acoustic studies of dysarthric speech: methods, progress, and potential. J Commun Disord. 1999;32(3):141–186. doi: 10.1016/S0021-9924(99)00004-0. [DOI] [PubMed] [Google Scholar]
- 105.Maryn Y, Corthals P, Van Cauwenberge P, Roy N, De Bodt M. Toward improved ecological validity in the acoustic measurement of overall voice quality: combining continuous speech and sustained vowels. J Voice. 2010;24(5):540–555. doi: 10.1016/j.jvoice.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 106.Pützer M, Barry WJ, Moringlane JR. Effect of deep brain stimulation on different speech subsystems in patients with multiple sclerosis. J Voice. 2007;21(6):741–753. doi: 10.1016/j.jvoice.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 107.Konstantopoulos K, Charalambous M, Verhoeven J, editors. Sequential motion rates in the dysarthria of multiple sclerosis: a temporal analysis. ICPhS. 2011:1138–41.
- 108.Jannetts S, Lowit A. Cepstral analysis of hypokinetic and ataxic voices: correlations with perceptual and other acoustic measures. J Voice. 2014;28(6):673–680. doi: 10.1016/j.jvoice.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 109.Polur PD, Miller GE. Experiments with fast Fourier transform, linear predictive and cepstral coefficients in dysarthric speech recognition algorithms using hidden Markov model. IEEE Trans Neural Syst Rehabil Eng. 2005;13(4):558–561. doi: 10.1109/TNSRE.2005.856074. [DOI] [PubMed] [Google Scholar]
- 110.Vyas G, Dutta MK, Prinosil J, Harár P, editors. An automatic diagnosis and assessment of dysarthric speech using speech disorder specific prosodic features. 2016 39th International Conference on Telecommunications and Signal Processing (TSP); 2016.
- 111.Kadi K, Selouani S, Boudraa B, Boudraa M, editors. Discriminative prosodic features to assess the dysarthria severity levels. Proceedings of the World Congress on Engineering. 2013:3.
- 112.Caballero-Morales S-O, Trujillo-Romero F. Evolutionary approach for integration of multiple pronunciation patterns for enhancement of dysarthric speech recognition. Expert Syst Appl. 2014;41(3):841–852. doi: 10.1016/j.eswa.2013.08.014. [DOI] [Google Scholar]
- 113.Janbakhshi P, Kodrasi I, Bourlard H, editors. Pathological speech intelligibility assessment based on the short-time objective intelligibility measure. ICASSP 2019 - 2019 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP); 2019.
- 114.Schultz BG, Tarigoppula VSA, Noffs G, Rojas S, van der Walt A, Grayden DB, et al. Automatic speech recognition in neurodegenerative disease. Int J Speech Technol. 2021;24(3):771–9.
- 115.Konstantopoulos K, Vikelis M, Seikel JA, Mitsikostas D-D. The existence of phonatory instability in multiple sclerosis: an acoustic and electroglottographic study. Neurol Sci. 2010;31(3):259–268. doi: 10.1007/s10072-009-0170-3. [DOI] [PubMed] [Google Scholar]
- 116.Marziniak M, Brichetto G, Feys P, Meyding-Lamadé U, Vernon K, Meuth SG. The use of digital and remote communication technologies as a tool for multiple sclerosis management: narrative review. JMIR Rehabil Assist Technol. 2018;5(1):e5. doi: 10.2196/rehab.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wendrich K, van Oirschot P, Martens MB, Heerings M, Jongen PJ, Krabbenborg L. Toward digital self-monitoring of multiple sclerosis: investigating first experiences, needs, and wishes of people with MS. Int J MS Care. 2019;21(6):282–291. doi: 10.7224/1537-2073.2018-083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leddy S, Hadavi S, McCarren A, Giovannoni G, Dobson R. Validating a novel web-based method to capture disease progression outcomes in multiple sclerosis. J Neurol. 2013;260(10):2505–2510. doi: 10.1007/s00415-013-7004-1. [DOI] [PubMed] [Google Scholar]
- 119.Moccia M, Lanzillo R, Brescia Morra V, Bonavita S, Tedeschi G, Leocani L, et al. Assessing disability and relapses in multiple sclerosis on tele-neurology. Neurol Sci. 2020;41:1369–1371. doi: 10.1007/s10072-020-04470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Beek J, Freitas R, Bernasconi C, Montalban X, Butzkueven H, Kappos L, et al., editors. FLOODLIGHT Open–a global, prospective, open-access study to better understand multiple sclerosis using smartphone technology. 2019 Annual Meeting of the Consortium of Multiple Sclerosis Centers; 2019: CMSC.
- 121.van der Walt A, Butzkueven H, Shin RK, Midaglia L, Capezzuto L, Lindemann M, et al. Developing a digital solution for remote assessment in multiple sclerosis: from concept to software as a medical device. Brain Sci. 2021;11(9):1247. 10.3390/brainsci11091247. [DOI] [PMC free article] [PubMed]
- 122.Montalban X, Graves J, Midaglia L, Mulero P, Julian L, Baker M, et al. A smartphone sensor-based digital outcome assessment of multiple sclerosis. Mult Scler J. 2021:28(4):654–64. 10.1177/13524585211028561. [DOI] [PMC free article] [PubMed]
- 123.Bourke AK, Scotland A, Lipsmeier F, Gossens C, Lindemann M. Gait characteristics harvested during a smartphone-based self-administered 2-minute walk test in people with multiple sclerosis: test-retest reliability and minimum detectable change. Sensors. 2020;20(20):5906. doi: 10.3390/s20205906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cheng W-Y, Bourke AK, Lipsmeier F, Bernasconi C, Belachew S, Gossens C, et al. U-turn speed is a valid and reliable smartphone-based measure of multiple sclerosis-related gait and balance impairment. Gait Posture. 2021;84:120–126. doi: 10.1016/j.gaitpost.2020.11.025. [DOI] [PubMed] [Google Scholar]
- 125.Fagherazzi G, Fischer A, Ismael M, Despotovic V. Voice for health: the use of vocal biomarkers from research to clinical practice. Digit Biomark. 2021;5(1):78–88. doi: 10.1159/000515346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dillenseger A, Weidemann ML, Trentzsch K, Inojosa H, Haase R, Schriefer D, et al. Digital biomarkers in multiple sclerosis. Brain Sci. 2021;11(11):1519. 10.3390/brainsci11111519. [DOI] [PMC free article] [PubMed]
- 127.Noffs G, Boonstra FMC, Perera T, Kolbe SC, Stankovich J, Butzkueven H, et al. Acoustic speech analytics are predictive of cerebellar dysfunction in multiple sclerosis. Cerebellum. 2020;19(5):691–700. doi: 10.1007/s12311-020-01151-5. [DOI] [PubMed] [Google Scholar]
- 128.Gulliford M, Jessop E, Yardley L. Digital healthcare public health. Healthcare public health: improving health services through population science. Oxford: Oxford University Press; 2020. p. 187–200.
- 129.Birkhoff SD, Smeltzer SC. Perceptions of smartphone user-centered mobile health tracking apps across various chronic illness populations: an integrative review. J Nurs Scholarsh. 2017;49(4):371–378. doi: 10.1111/jnu.12298. [DOI] [PubMed] [Google Scholar]
- 130.Goodkin DE, Hertsguard D, Seminary J. Upper extremity function in multiple sclerosis: improving assessment sensitivity with box-and-block and nine-hole peg tests. Arch Phys Med Rehabil. 1988;69(10):850–854. [PubMed] [Google Scholar]
- 131.Noseworthy JH, Vandervoort MK, Wong CJ, Ebers GC. Interrater variability with the Expanded Disability Status Scale (EDSS) and Functional Systems (FS) in a multiple sclerosis clinical trial. Neurology. 1990;40(6):971. doi: 10.1212/WNL.40.6.971. [DOI] [PubMed] [Google Scholar]
- 132.Cutter GR, Baier ML, Rudick RA, Cookfair DL, Fischer JS, Petkau J, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122(5):871–882. doi: 10.1093/brain/122.5.871. [DOI] [PubMed] [Google Scholar]
- 133.Erasmus L-P, Sarno S, Albrecht H, Schwecht M, Pöllmann W, König N. Measurement of ataxic symptoms with a graphic tablet: standard values in controls and validity in Multiple Sclerosis patients. J Neurosci Methods. 2001;108(1):25–37. doi: 10.1016/S0165-0270(01)00373-9. [DOI] [PubMed] [Google Scholar]
- 134.Kalron A, Givon U. Gait characteristics according to pyramidal, sensory and cerebellar EDSS subcategories in people with multiple sclerosis. J Neurol. 2016;263(9):1796–1801. doi: 10.1007/s00415-016-8200-6. [DOI] [PubMed] [Google Scholar]
- 135.Solaro C, Cattaneo D, Brichetto G, Castelli L, Tacchino A, Gervasoni E, et al. Clinical correlates of 9-hole peg test in a large population of people with multiple sclerosis. Mult Scler Relat Disord. 2019;30:1–8. doi: 10.1016/j.msard.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 136.Hartelius L, Buder EH, Strand EA. Long-term phonatory instability in individuals with multiple sclerosis. J Speech Lang Hear Res. 1997;40(5):1056–1072. doi: 10.1044/jslhr.4005.1056. [DOI] [PubMed] [Google Scholar]
- 137.Kuo C, Tjaden K. Acoustic variation during passage reading for speakers with dysarthria and healthy controls. J Commun Disord. 2016;62:30–44. doi: 10.1016/j.jcomdis.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Novotný M, Rusz J, Spálenka K, Klempír J, Horáková D, Ruzicka E, editors. Acoustic evaluation of nasality in cerebellar syndromes. Interspeech; 2017:3132–6. 10.21437/Interspeech.2017-381.
- 139.Rusz J, Tykalová T, Salerno G, Bancone S, Scarpelli J, Pellecchia MT. Distinctive speech signature in cerebellar and parkinsonian subtypes of multiple system atrophy. J Neurol. 2019;266(6):1394–1404. doi: 10.1007/s00415-019-09271-7. [DOI] [PubMed] [Google Scholar]