Before the widespread introduction of positive-pressure ventilation in intensive care after the polio epidemics in the 1950’s, most forms of ventilatory support have been “non invasive” or “external” without direct access to the lower airways, using face mask for positive pressure, or techniques generating negative pressure around the chest from thoracic cuirass to whole body tank. In the late 19th century, ventilators simulating negative pressure around the chest have been designed, including the first workable iron lung designed by Alfred Woillez, to help save drowning victims. The first iron lung to be widely used was developed in Boston by Drinker and Shaw in 1928, and largely used to treat patients with polio. It was difficult, however, to nurse patients in the iron lungs or to clear secretions, and airways were not protected. In parallel, the short-term application of positive end-expiratory pressure (PEEP) by face mask was first described in the 1930s, especially to treat cardiogenic pulmonary edema [1].
In modern intensive care medicine, after the introduction of positive-pressure ventilation, it took some time to “rediscover” noninvasive positive pressure ventilation while some rare institutions were continuing to use face masks for hypercapnic patients [2]. At the end of the 1980’s, home ventilation was started in patients with chronic respiratory disorders, with the main objective of improving quality of life compared to tracheostomy. The recognition that major sleep disturbances could be caused by abnormal respiration also contributed to the widespread use of home ventilatory support. Manufacturers have devoted considerable technological effort to develop home ventilators for “leaky” ventilation and to provide comfortable interfaces.
In parallel with the development of home ventilators, intensivists applied noninvasive ventilation (NIV) techniques in the early 1990s to avoid endotracheal intubation in the acute care setting, especially for patients with hypercapnic ventilatory failure [3, 4]. The development of pressure support ventilation on intensive care unit (ICU) ventilators and a better understanding of the pathophysiology of ventilatory failure and respiratory muscle activity facilitated the introduction of noninvasive ventilation (NIV-PEEP providing inspiratory assistance with pressure support) [3]. Indications were rapidly extended to more heterogeneous clinical scenarios, from post-operative settings to hypoxemic respiratory failure [5]. Recognizing the complications of invasive mechanical ventilation was a strong incentive for developing NIV to prevent intubation. This was recently emphasized again by the pandemic caused by coronavirus disease 2019 (COVID-19), as the shortage of equipment and personnel has fostered the use of techniques such as high-flow nasal cannula (HFNC), NIV and continuous positive airway pressure (CPAP-providing no inspiratory assistance) to prevent intubation [6, 7]. Noninvasive support can also play a role in palliative care to relieve dyspnea in patients deemed non-eligible for invasive ventilation.
Noninvasive positive pressure ventilation
Facemask NIV improves gas exchange, reduces work of breathing and improves clinical outcome in patients with hypercapnic respiratory failure due to chronic obstructive pulmonary disease (COPD), obesity-hypoventilation, and in patients with cardiogenic pulmonary edema (either NIV or CPAP) [8]. NIV has been successfully used in post-operative hypoxemia [9], and to facilitate weaning from mechanical ventilation in ICU patients at high-risk of reintubation [10, 11]. Use of NIV during de novo hypoxemic respiratory failure has been successful in several trials, but also controversial, especially in observational studies. Patients who avoid endotracheal intubation with noninvasive support show a good clinical outcome, patients intubated after a failing trial of noninvasive support are burdened by higher mortality, possibly as consequence of delayed intubation and self-inflicted lung injury [12]. CPAP has been studied in different indications. Previous studies did not show benefits for hypoxemic respiratory failure [13] and more studies are needed in this field. By contrast CPAP has been repeatedly shown to be beneficial in post-operative respiratory failure [14].
NIV is therefore strongly recommended in hypercapnic respiratory failure due to COPD exacerbation and cardiogenic pulmonary edema and has been conditionally recommended as a prophylaxis for extubation failure in high-risk patients, and to facilitate weaning in hypercapnic patients [8]. Several factors determine the success of NIV application such as good patient selection, patient-ventilator interface, personnel expertise, and patient monitoring. The inspiratory pressure setting is essential to increase ventilation while small levels of PEEP may help to counteract auto-PEEP, combat hypoxemia and atelectasis (moderate PEEP levels) or maintain airway patency in obese patients (higher PEEP levels).
Recent guidelines made no recommendation for the use of facemask NIV in de novo hypoxemic respiratory failure for the reasons discussed above [8]. Thus, finding new strategies for noninvasive support is an important objective in this specific population. In this regard, the helmet represents an attractive interface for CPAP or NIV in hypoxemic patients; it is a cylinder-shaped hood made of transparent plastic. The helmet is generally well tolerated for prolonged treatments. One single-centre randomized trial reported a clinical benefit of helmet compared to facemask NIV in hypoxemic patients [15]. A small subsequent multicentre trial conducted in patients with COVID-19 reported a possible reduction in the rate of endotracheal intubation by helmet NIV compared to HFNC [16]. The use of this interface is still limited by the lack of conclusive data to enable clinical recommendations but offers a possible attractive alternative as suggested by a network metanalysis [17].
High-flow nasal cannula
Low-flow oxygen therapy via nasal cannula or oxygen mask is routinely used as an initial management; however, the maximum flow rate of 15 L/min is inadequate to control FiO2 or to reduce work of breathing in patients with acute respiratory failure in whom peak inspiratory flows often largely exceed 30 L/min. Insufficient heating and humidification with low-flow oxygen device lead to discomfort and poor tolerance and preclude from increasing the flow subsequently, the technology of HFNC has been developed and current systems can deliver high flow rates of heated and humidified air-oxygen mixture up to 60 L/min via a large-bore nasal cannula that are very well tolerated.
HFNC offers several physiological benefits in patients with acute respiratory failure by improving gas exchange and modulating inspiratory effort via the following mechanisms (1) flow-dependent small positive-pressure effect (up to 7 cm H2O with mouth closed [18]); (2) upper airway wash-out, reducing this part of the dead-space; (3) active heating and humidification, favoring comfort and airway mucosal integrity. A multicenter randomized trial [19] demonstrated that HFNC reduced the rate of endotracheal intubation in patients with moderate-to-severe hypoxemia compared to conventional oxygen therapy and facemask NIV and improved survival. The study has not been replicated but aggregated data in hypoxemic patients seem to confirm benefit over low-flow oxygen [7].
HFNC should be quickly titrated to high flows, rapidly increasing the flow rate up to 50–60 L/min if well tolerated; FiO2 should then be adjusted according to a target SpO2. Humidification is optimal at a temperature of 37 °C but some patients may request a lower temperature. Current guidelines recommend HFNC as the first-line treatment of de novo hypoxemic respiratory failure [20]. In critically ill patients weaned from invasive mechanical ventilation, HFNC has been shown to prevent post-extubation respiratory failure compared to conventional oxygen therapy in low-risk patients and to perform as well as prophylactic NIV in patients at high-risk of post-extubation respiratory failure [20]. NIV may be preferable in obese patients, however, alone or in combination with HFNC [21].
Take-home message
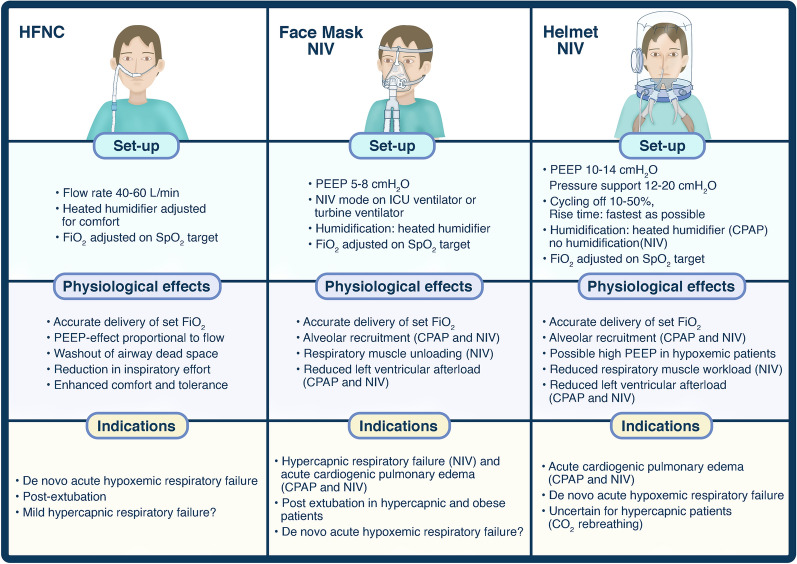
As illustrated on Fig. 1, in the acute care setting, NIV should be considered as the first-line approach to treat patients with hypercapnic respiratory failure and CPAP or NIV for respiratory failure due to acute cardiogenic pulmonary edema. Weaning or extubation can be facilitated by HFNC and NIV especially for high-risk or obese patients. The optimal noninvasive support strategy for de novo hypoxemic respiratory failure remains debated: HFNC and helmet support are promising techniques, but careful patient selection and clinical monitoring remain always warranted to best balance between the benefits and risks of these approaches [17].
Fig. 1.
Settings, physiological effects of noninvasive respiratory support and clinical applications in acute respiratory failure
Funding
LB’s research is funded by a Keenan Chair in Critical Care and Acute Respiratory Failure.
Declarations
Conflicts of interest
LB’s laboratory received research grants or equipment from Draeger, Medtronic, Stimit, Air Liquide, Fisher Paykel and Sentec. DLG has received payments for travel expenses by Getinge and Air Liquide, speaking fees by Intersurgical, GE, Fisher and Paykel and Gilead, and discloses a research grant by GE.
Footnotes
The original online version of this article was revised: Figure 1 has been corrected.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/2/2022
A Correction to this paper has been published: 10.1007/s00134-022-06828-5
Contributor Information
Nuttapol Rittayamai, Email: nuttapol.rit@mahidol.ac.th.
Domenico L. Grieco, Email: dlgrieco@outlook.it
Laurent Brochard, Email: Laurent.brochard@unityhealth.to.
References
- 1.Poulton EP. Left-sided heart failure with pulmonary edema: its treatment with the “Pulmonary plus machine”. Lancet. 1936;228:981–983. doi: 10.1016/S0140-6736(00)47948-1. [DOI] [Google Scholar]
- 2.Sadoul P, Aug MC, Gay R. Traitement par ventilation instrumentale de 100 cas d’insuffisance respiratoire aiguë sévère (PaCO2 égale ou supérieure à 70 mm Hg) chez des pulmonaires chroniques. Bull Eur Physiopathol Respir. 1965;1:489–505. [Google Scholar]
- 3.Brochard L, Isabey D, Piquet J, et al. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med. 1990;323:1523–1530. doi: 10.1056/NEJM199011293232204. [DOI] [PubMed] [Google Scholar]
- 4.Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 5.Antonelli M, Conti G, Rocco M, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med. 1998;339:429–435. doi: 10.1056/NEJM199808133390703. [DOI] [PubMed] [Google Scholar]
- 6.Perkins GD, Ji C, Connolly BA, et al. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS Randomized Clinical Trial. JAMA. 2022;327:546–558. doi: 10.1001/jama.2022.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ospina-Tascón GA, Calderón-Tapia LE, García AF, et al. Effect of high-flow oxygen therapy vs conventional oxygen therapy on invasive mechanical ventilation and clinical recovery in patients with severe COVID-19: a randomized clinical trial. JAMA. 2021;326:2161–2171. doi: 10.1001/jama.2021.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017 doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 9.Jaber S, Lescot T, Futier E, Paugam-Burtz C, Seguin P, Ferrandiere M, Lasocki S, Mimoz O, Hengy B, Sannini A, Pottecher J, Abback PS, Riu B, Belafia F, Constantin JM, Masseret E, Beaussier M, Verzilli D, De Jong A, Chanques G, Brochard L, Molinari N. Effect of noninvasive ventilation on tracheal reintubation among patients with hypoxemic respiratory failure following abdominal surgery: a randomized clinical trial. JAMA. 2016;315(13):1345–1353. doi: 10.1001/jama.2016.2706. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer M, Esquinas A, Arancibia F, et al. Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med. 2003;168:70–76. doi: 10.1164/rccm.200209-1074OC. [DOI] [PubMed] [Google Scholar]
- 11.Ferrer M, Valencia M, Nicolas JM, et al. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med. 2006;173:164–170. doi: 10.1164/rccm.200505-718OC. [DOI] [PubMed] [Google Scholar]
- 12.Bellani G, Laffey JG, Pham T, et al. Noninvasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE study. Am J Respir Crit Care Med. 2017;195:67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 13.Delclaux C, L’Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA. 2000;284:2352–2360. doi: 10.1001/jama.284.18.2352. [DOI] [PubMed] [Google Scholar]
- 14.Squadrone V, Coha M, Cerutti E, et al. Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA. 2005;293:589–595. doi: 10.1001/jama.293.5.589. [DOI] [PubMed] [Google Scholar]
- 15.Patel BK, Wolfe KS, Pohlman AS, et al. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315:2435–2441. doi: 10.1001/jama.2016.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grieco DL, Menga LS, Cesarano M, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA. 2021;325:1731–1743. doi: 10.1001/jama.2021.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreyro BL, Angriman F, Munshi L, et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. JAMA. 2020;324:57–67. doi: 10.1001/jama.2020.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieira F, Bezerra FS, Coudroy R, et al. High flow nasal cannula compared to continuous positive airway pressure: a bench and physiological study. J Appl Physiol Bethesda Md. 2022 doi: 10.1152/japplphysiol.00416.2021. [DOI] [PubMed] [Google Scholar]
- 19.Frat J-P, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 20.Rochwerg B, Einav S, Chaudhuri D, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46:2226–2237. doi: 10.1007/s00134-020-06312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thille AW, Coudroy R, Nay M-A, et al. Beneficial effects of noninvasive ventilation after extubation in obese or overweight patients: a post hoc analysis of a randomized clinical trial. Am J Respir Crit Care Med. 2022;205:440–449. doi: 10.1164/rccm.202106-1452OC. [DOI] [PubMed] [Google Scholar]