Abstract
Purpose
To assess knowledge of obesity-associated cancer risk, self-awareness of BMI status, and willingness to engage in weight loss intervention in breast cancer survivors with overweight and obesity as a companion study for a novel weight loss program using a telehealth platform (NCT04855552).
Methods
Breast cancer survivors with BMI ≥ 25 kg/m2 were surveyed to assess self-perception of BMI, knowledge of obesity-related cancer risk, and willingness to participate in weight loss programs. Multivariable logistic regression was used to assess factors associated with willingness to participate.
Results
Of the 122 participants, 73 (59.8%) had BMI 25.0–29.9 kg/m2 (overweight) and 49 (40.2%) had BMI ≥ 30 (obesity). Patients with obesity were more likely to underestimate their BMI than those with overweight, 40.8% vs. 23.3% (p = 0.03). The majority (82.0%) indicated awareness that obesity increases breast cancer risk and 57.4% expressed interest in a weight loss program. Patients with knowledge of obesity-related breast cancer risk (91.4% willing vs. 69.2% not willing, p < 0.01) were more willing to participate in a weight loss program on univariable and multivariable analyses (p < 0.01).
Conclusion
Our results underscore the importance of raising patients’ awareness of obesity-related health risks and individual BMI category. Future work in the development of better education and communication tools to improve awareness will likely improve the adoption rate of healthy lifestyles in at-risk patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10549-022-06546-y.
Keywords: Breast cancer, Obesity, Cancer risk, Patient education, Weight loss
Introduction
Obesity and the metabolic consequences related to obesity have been linked to increased incidence of cancer and worse cancer outcomes [1–6]. The relationship between obesity and development of postmenopausal breast cancer is well established [7–10], with an increased relative risk (RR) of 1.13 and 1.25 for women with overweight and obesity, respectively, compared to women of normal weight [11]. Furthermore, multiple studies have shown that obesity is associated with worse overall and cancer-specific survival in breast cancer patients [9, 10]. One meta-analysis of 213,075 women with 41,477 deaths across 82 studies found that obesity increased the risk of total mortality and breast cancer-specific mortality in breast cancer survivors by 41% and 35%, respectively [10]. Breast cancer survivors with obesity also have a significantly greater risk of developing a second primary breast cancer (RR 1.40) or a contralateral breast cancer (RR 1.37) [12]. Conversely, intentional weight loss has been shown to be associated with a decreased breast cancer risk among pre- and postmenopausal women [13–17]. In the Iowa Women’s Health Study, women who intentionally lost 20 or more pounds through lifestyle modification had a 21% lower incidence of breast cancer [13]. Such evidence affirms that obesity is a modifiable risk factor for breast cancer, and more generally, weight losses as low as 5% of initial weight produce more general health benefits, such as improvements in cardiovascular disease and diabetes markers [18].
Despite abundant evidence on the negative impact of obesity on breast cancer risk and outcomes, studies examining knowledge of the association between obesity and breast cancer in the general population have found many women to be uninformed of this link [19–21]. Two studies surveying women in urban centers found that up to 54% were aware that obesity increases the risk for breast cancer [19, 20], while a study of 410 women with overweight and obesity found that 53% were aware of this association [21]. To our knowledge, no studies have evaluated awareness of this association in a population of breast cancer survivors. As for participation in weight loss trials in the breast cancer population, a recent study has shown that 546 of 667 eligible patients (81%) who were approached gave consent to participate in a weight loss trial, and of those, 62% were randomized, but it is unknown if the knowledge of this link influenced those who consented [22]. We recently completed a survey study as a companion to a pilot weight loss program (NCT04855552) to be conducted using a virtual telehealth platform for breast cancer survivors at our institution. The purpose of the survey study was to evaluate knowledge of the link between obesity and breast cancer in a population of breast cancer survivors with overweight or obesity, to gauge interest in weight loss intervention, and to understand the clinical characteristics associated with willingness to participate in an institutional weight loss intervention to address this modifiable risk factor.
Methods
Participant recruitment
This study was reviewed and approved by the Institutional Review Board (IRB). Participants were recruited from a breast surgery clinic at our breast center. Patients routinely follow up in the breast surgery clinic every 6 months for two years and then yearly after their breast cancer surgery. In this clinic, among patients who have completed their breast cancer treatment, around 28% have normal weight, 39% have overweight, and 22% have obesity. The clinical characteristics of this study cohort were similar to a larger cohort described in a prior study [23]. Prior to scheduled outpatient appointments, we prescreened the electronic medical record for female breast cancer survivors with most recent body mass index (BMI) ≥ 25 kg/m2. Patients were eligible for this study if they had an ECOG performance status of 0 (defined as those who are fully active, able to carry on all pre-disease performance without restriction) or 1 (restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work), had a history of biopsy-proven ductal carcinoma in situ or invasive breast cancer, had undergone surgery, had completed any indicated adjuvant cytotoxic chemotherapy and/or radiation therapy at least 6 months prior to enrollment, were currently cancer free, and had overweight (BMI 25.0–29.9 kg/m2) or obesity (BMI ≥ 30 kg/m2) by World Health Organization (WHO) BMI classification. Patients were excluded from the study if younger than 18 years of age, currently pregnant, or unable to provide informed consent. Eligible patients were approached following clinic appointments.
Materials
After obtaining written informed consent from each participant, surveys were administered. Five surveys (Patient Health Questionnaire, MBSRQ-AS, SF12, International Physical Activity Questionnaire, and Breast Cancer Questionnaire (BCQ) (supplementary materials)) were administered to participants who provided informed consent [24, 25]. The BCQ queries demographic and clinical characteristics, such as age, weight, height, and income, as well as assessing patients’ self-perception of their BMI classification, knowledge of the effect of obesity on cancer risk, and willingness to participate in an institutional weight loss intervention. Patients were defined as being willing to participate if they were interested and would like to be contacted in the future to participate in our institutional weight loss program once it was formally organized. Patients were asked to self-assess their BMI classification using categorical descriptors of World Health Organization (WHO) classifications without the corresponding numerical values (i.e., “underweight, average weight, overweight, slightly obese, moderately obese, or very obese,” representing BMI ranges of < 18.5, 18.5–24.9, 25.0–29.9, 30.0–34.9, 35.0–39.9, and ≥ 40 kg/m2). Knowledge of the effect of obesity on cancer risk was assessed for breast, lung, and ovarian cancers, with responses rated on five-level Likert scale from “1—decreases the risk a lot” to “5—increases the risk a lot.” Questions on the association between obesity and lung and ovarian cancers served as distractor questions as described [25]. Participants who indicated interest in an institutional weight loss intervention were queried about preferred mode of delivery for an intervention, including in-person meetings, virtual modes (telephone, web based, or cell phone application based), or weight loss surgery. The BCQ was modeled on a similar form used for a study of endometrial cancer survivors at our institution [25], and questions were selected from a bank of previously validated questions available from the Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System and the Harvard Forms of Health Survey [20, 26, 27].
Patient height was obtained by self-report, and weight was measured in office on the day of survey completion, per clinic protocol. BMI was calculated for each patient using the formula (weight [kg])/(height [m])2 and was categorized according to WHO classifications. Clinical information including tumor stage and histology, surgery, adjuvant therapy, and comorbidities was obtained through the electronic medical record.
Statistical methods
Descriptive statistics were used to characterize the demographic and clinical characteristics of our cohort, stratified by participants with overweight vs. obesity. Demographic and clinical characteristics of those willing and unwilling to participate in weight loss intervention were compared using χ2 or ANOVA, as appropriate. Multivariable logistic regression was carried out to determine which factors predict willingness to engage in weight loss intervention. Variables with a p value < 0.4 on bivariate analysis were included in our multivariable model (i.e., age, household income, marital status, lymphedema, depression score, tumor stage, nodal stage, axilla management, accuracy of BMI self-assessment, and knowledge of obesity-related breast cancer). All p values were two tailed with p < 0.05 considered statistically significant. Statistical analyses were performed using STATA version 16/SE (StataCorp LLC, College Station, TX).
Results
Demographics and clinical characteristics of survey participants
Between January 2019 and March 2020, 264 patients met eligibility criteria on prescreen, 187 patients were approached, and 124 (66.3%) consented to enroll. Of 187 eligible patients approached, a total of 124 (66.3%) consented to participate in this study. Two participants were later excluded from analysis due to incomplete questionnaires (< 25% complete), resulting in a final cohort of 122 patients (Fig. 1). Clinical characteristics of our study cohort are summarized in Table 1. The median age of participants was 62 (range 34–84), and median BMI was 29.3 kg/m2 (range 25.0–50.5 kg/m2). Seventy-three (59.8%) participants had an overweight BMI, while 49 (40.2%) had an obese BMI. The clinical characteristics of this study cohort were similar to those of our general breast cancer patients cohort described in a prior study [23].
Fig. 1.
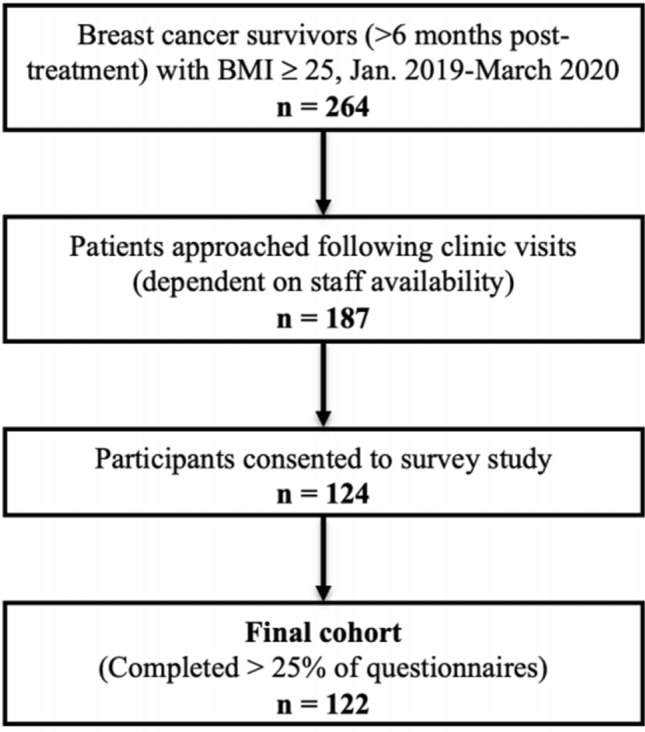
Consort diagram
Table 1.
Demographic and clinical characteristics of survey respondents by BMI category
| Total | Overweight (25.0–29.9) | Obese (≥ 30.0) | p | ||||
|---|---|---|---|---|---|---|---|
| N | 122 | 100.0% | 73 | 59.8% | 49 | 40.2% | |
| Age, median (range) | 62 (34–84) | 60 (34–83) | 63 (40–84) | 0.59 | |||
| Race | |||||||
| White | 97 | 79.5% | 59 | 80.8% | 38 | 77.6% | 0.27 |
| Black | 16 | 13.1% | 7 | 9.6% | 9 | 18.4% | |
| Asian/PI* | 6 | 4.9% | 4 | 5.5% | 2 | 4.1% | |
| Other/NA | 3 | 2.5% | 3 | 4.1% | 0 | 0.0% | |
| Household income | |||||||
| < $25,000 | 9 | 7.4% | 5 | 6.8% | 4 | 8.2% | 0.58 |
| $25,000–$50,000 | 11 | 9.0% | 5 | 6.8% | 6 | 12.2% | |
| $50,000–$75,000 | 15 | 12.3% | 10 | 13.7% | 5 | 10.2% | |
| > $75,000 | 72 | 59.0% | 43 | 58.9% | 29 | 59.2% | |
| Did not disclose | 13 | 10.7% | 10 | 13.7% | 3 | 6.1% | |
| Marital status | |||||||
| Married | 76 | 62.3% | 43 | 58.9% | 33 | 67.3% | 0.38 |
| Not married | 44 | 36.1% | 28 | 38.4% | 16 | 32.7% | |
| NA | 2 | 1.6% | 2 | 2.7% | 0 | 0.0% | |
| Parity | |||||||
| 0 | 29 | 23.8% | 20 | 27.4% | 9 | 18.4% | 0.48 |
| 1–2 | 57 | 46.7% | 32 | 43.8% | 25 | 51.0% | |
| ≥ 3 | 31 | 25.4% | 17 | 23.3% | 14 | 28.6% | |
| NA | 5 | 4.1% | 4 | 5.5% | 1 | 2.0% | |
| Employment status | |||||||
| Employed | 75 | 61.5% | 46 | 63.0% | 29 | 59.2% | 0.37 |
| Not employed | 6 | 4.9% | 5 | 6.8% | 1 | 2.0% | |
| Retired | 33 | 27.0% | 19 | 26.0% | 14 | 28.6% | |
| NA | 8 | 6.6% | 3 | 4.1% | 5 | 10.2% | |
| Comorbidities | |||||||
| Cardiovascular disease | 37 | 30.3% | 19 | 26.0% | 18 | 36.7% | 0.18 |
| Lung disease | 10 | 8.2% | 4 | 5.5% | 6 | 12.2% | 0.19 |
| Diabetes | 16 | 13.1% | 6 | 8.2% | 10 | 20.4% | 0.05 |
| Joint disease | 22 | 18.0% | 9 | 12.3% | 13 | 26.5% | 0.05 |
| Lymphedema | 14 | 11.5% | 8 | 11.0% | 6 | 12.2% | 0.84 |
| Depression score | |||||||
| Minimal or None (0–4) | 81 | 66.4% | 49 | 67.1% | 32 | 65.3% | 0.13 |
| Mild (5–9) | 16 | 13.1% | 12 | 16.4% | 4 | 8.2% | |
| Moderate (10–14) | 11 | 9.0% | 3 | 4.1% | 8 | 16.3% | |
| Moderately Severe (15–19) | 5 | 4.1% | 3 | 4.1% | 2 | 4.1% | |
| Severe (20–27) | 1 | 0.8% | 1 | 1.4% | 0 | 0.0% | |
| Tumor stage | |||||||
| T0 | 42 | 34.4% | 29 | 39.7% | 13 | 26.5% | 0.05 |
| T1 | 51 | 41.8% | 32 | 43.8% | 19 | 38.8% | |
| T2 | 23 | 18.9% | 11 | 15.1% | 12 | 24.5% | |
| T3/4 | 6 | 4.9% | 1 | 1.4% | 5 | 10.2% | |
| Nodal stage | |||||||
| N0 | 102 | 83.6% | 66 | 90.4% | 36 | 73.5% | 0.04 |
| N1 | 18 | 14.8% | 6 | 8.2% | 12 | 24.5% | |
| N2/3 | 2 | 1.6% | 1 | 1.4% | 1 | 2.0% | |
| Surgery type | |||||||
| Lumpectomy | 62 | 50.8% | 39 | 53.4% | 23 | 46.9% | 0.48 |
| Mastectomy | 60 | 49.2% | 34 | 46.6% | 26 | 53.1% | |
| Reconstruction type | |||||||
| Free flap reconstruction | 36 | 29.5% | 19 | 26.0% | 17 | 34.7% | 0.13 |
| Implant reconstruction | 14 | 11.5% | 11 | 15.1% | 3 | 6.1% | |
| No reconstruction | 10 | 8.2% | 4 | 5.5% | 6 | 12.2% | |
| Axilla management | |||||||
| SLNB only | 87 | 71.3% | 53 | 72.6% | 34 | 69.4% | 0.02 |
| AND | 16 | 13.1% | 5 | 6.8% | 11 | 22.4% | |
| None | 19 | 15.6% | 15 | 20.5% | 4 | 8.2% | |
| Duration since adjuvant therapy (mean, SD) | |||||||
| < 1 year | 41 | 33.6% | 27 | 37.0% | 14 | 28.6% | 0.34 |
| ≥ 1 year | 81 | 66.4% | 46 | 63.0% | 35 | 71.4% | |
| Accuracy of BMI Self-assessment | |||||||
| Underestimate | 37 | 30.3% | 17 | 23.3% | 20 | 40.8% | 0.03 |
| Accurate assessment | 68 | 55.7% | 48 | 65.8% | 20 | 40.8% | |
| Overestimate | 17 | 13.9% | 8 | 11.0% | 9 | 18.4% | |
| Knowledge of obesity-related breast cancer risk | |||||||
| Yes | 100 | 82.0% | 59 | 80.8% | 41 | 83.7% | 0.69 |
| No | 22 | 18.0% | 14 | 19.2% | 8 | 16.3% | |
| Willingness to participate in weight loss intervention | |||||||
| Yes | 70 | 57.4% | 40 | 54.8% | 30 | 61.2% | 0.48 |
| No | 52 | 42.6% | 33 | 45.2% | 19 | 38.8% | |
| Distance to hospital | |||||||
| < 25 miles | 72 | 59.0% | 43 | 58.9% | 29 | 59.2% | 0.975 |
| ≥ 25 miles | 50 | 41.0% | 30 | 41.1% | 20 | 40.8% | |
*PI pacific islander, T tumor stage, N nodal stage, SLNB sentinel lymph node biopsy, AND axilla node dissection, SD standard deviation
The majority of participants were white (79.5%) and had a household income greater than $75,000 (59.0%). All participants had an ECOG performance status of 0 or 1. The cohorts with overweight and obesity did not differ in demographic characteristics, comorbidities, or distance to the hospital. They also did not differ in surgical management of the breast or time since completion of adjuvant therapy. However, participants with obesity were more likely than participants with overweight to present with larger T2 or T3/4 tumors (24.5% vs. 15.1%; 10.2% vs. 1.4%, p = 0.05), have 1–3 involved lymph nodes (N1 nodal stage) (24.5% vs. 8.2%, p = 0.04), and to undergo axillary node dissection (AND) (22.4% vs 6.8%, p = 0.02).
To determine if participants were able to accurately classify their weight, calculated BMI was compared to self-assessed BMI category by survey response. Sixty-eight (55.7%) patients accurately categorized their BMI (Table 1). Among patients who incorrectly estimated their true BMI category (n = 54), more than twice as many underestimated (68.5%) as overestimated (31.5%) their BMI. Patients with overweight BMI were significantly more likely to categorize their BMI appropriately than patients with obese BMI (p = 0.03), of whom 40.8% underestimated their true BMI (Table 1).
Participants were also asked whether obesity increases the risk of breast, lung, and ovarian cancers (Supplementary Table 1). Of all patients surveyed, 82.0% indicated that obesity increases the risk of breast cancer. Responses were considered affirmative if the participant selected “increases the risk a little” or “increases the risk a lot.” By contrast, more than half of participants selected “I don’t know” to the distractor questions regarding the effect of obesity on lung cancer and ovarian cancer risk (55.7% and 51.6%, respectively). Knowledge of obesity-related breast cancer risk did not vary significantly by participant BMI group (p = 0.69; Table 1) but did differ between those willing vs. unwilling to participate in weight loss intervention (91.4% vs. 69.2%, p < 0.01; Table 2).
Table 2.
Demographic and clinical characteristics of survey respondents by willingness to participate in an institutional weight loss intervention
| Total | Not willing to participate | Willing to participate | p | ||||
|---|---|---|---|---|---|---|---|
| N | 122 | 100.0% | 52 | 42.6% | 70 | 57.4% | |
| Age, median (range) | 62 (34–84) | 63 (34–84) | 60 (38–82) | 0.38 | |||
| Race | |||||||
| White | 97 | 79.5% | 40 | 76.9% | 57 | 81.4% | 0.66 |
| Black | 16 | 13.1% | 9 | 17.3% | 7 | 10.0% | |
| Asian/PI | 6 | 4.9% | 2 | 3.8% | 4 | 5.7% | |
| Other/NA | 3 | 2.5% | 1 | 1.9% | 2 | 2.9% | |
| Household income | |||||||
| < $25,000 | 9 | 7.4% | 4 | 7.7% | 5 | 7.1% | 0.15 |
| $25,000–$50,000 | 11 | 9.0% | 8 | 15.4% | 3 | 4.3% | |
| $50,000–$75,000 | 15 | 12.3% | 7 | 13.5% | 8 | 11.4% | |
| > $75,000 | 72 | 59.0% | 25 | 48.1% | 47 | 67.1% | 0.15 |
| Did not disclose | 13 | 10.7% | 7 | 13.5% | 6 | 8.6% | |
| Marital status | |||||||
| Married | 76 | 62.3% | 30 | 57.7% | 46 | 65.7% | 0.25 |
| Not married | 44 | 36.1% | 22 | 42.3% | 22 | 31.4% | |
| NA | 2 | 1.6% | 0 | 0.0% | 2 | 2.9% | |
| Parity | |||||||
| 0 | 29 | 23.8% | 16 | 30.8% | 13 | 18.6% | 0.45 |
| 1–2 | 57 | 46.7% | 23 | 44.2% | 34 | 48.6% | |
| ≥ 3 | 31 | 25.4% | 11 | 21.2% | 20 | 28.6% | |
| NA | 5 | 4.1% | 2 | 3.8% | 3 | 4.3% | |
| Employment status | |||||||
| Employed | 75 | 61.5% | 32 | 61.5% | 43 | 61.4% | 0.94 |
| Not employed | 6 | 4.9% | 2 | 3.8% | 4 | 5.7% | |
| Retired | 33 | 27.0% | 15 | 28.8% | 18 | 25.7% | |
| NA | 8 | 6.6% | 3 | 5.8% | 5 | 7.1% | |
| Comorbidities | |||||||
| Cardiovascular Disease | 37 | 30.3% | 15 | 28.8% | 22 | 31.4% | 0.72 |
| Lung disease | 10 | 8.2% | 4 | 7.7% | 6 | 8.6% | 0.84 |
| Diabetes | 16 | 13.1% | 7 | 13.5% | 9 | 12.9% | 0.92 |
| Joint disease | 22 | 18.0% | 10 | 19.2% | 12 | 17.1% | 0.77 |
| Lymphedema | 14 | 11.5% | 8 | 15.4% | 6 | 8.6% | 0.28 |
| Depression score | |||||||
| Minimal or None (0–4) | 81 | 66.4% | 36 | 69.2% | 45 | 64.3% | 0.39 |
| Mild (5–9) | 16 | 13.1% | 9 | 17.3% | 7 | 10.0% | 0.39 |
| Moderate (10–14) | 11 | 9.0% | 3 | 5.8% | 8 | 11.4% | |
| Moderately Severe (15–19) | 5 | 4.1% | 1 | 1.9% | 4 | 5.7% | |
| Severe (20–27) | 1 | 0.8% | 0 | 0.0% | 1 | 1.4% | |
| Tumor stage | |||||||
| T0 | 42 | 34.4% | 24 | 46.2% | 18 | 25.7% | 0.09 |
| T1 | 51 | 41.8% | 16 | 30.8% | 35 | 50.0% | |
| T2 | 23 | 18.9% | 10 | 19.2% | 13 | 18.6% | |
| T3/4 | 6 | 4.9% | 2 | 3.8% | 4 | 5.7% | |
| Nodal stage | |||||||
| N0 | 102 | 83.6% | 46 | 88.5% | 56 | 80.0% | 0.31 |
| N1 | 18 | 14.8% | 6 | 11.5% | 12 | 17.1% | |
| N2/3 | 2 | 1.6% | 0 | 0.0% | 2 | 2.9% | |
| Surgery type | |||||||
| Lumpectomy | 62 | 50.8% | 28 | 53.8% | 34 | 48.6% | 0.56 |
| Mastectomy | 60 | 49.2% | 24 | 46.2% | 36 | 51.4% | |
| Reconstruction type | |||||||
| Free flap reconstruction | 36 | 29.5% | 16 | 30.8% | 20 | 28.6% | 0.26 |
| Implant reconstruction | 14 | 11.5% | 3 | 5.8% | 11 | 15.7% | |
| No reconstruction | 10 | 8.2% | 5 | 9.6% | 5 | 7.1% | |
| Axilla management | |||||||
| SLNB only | 87 | 71.3% | 34 | 65.4% | 53 | 75.7% | 0.14 |
| AND | 16 | 13.1% | 6 | 11.5% | 10 | 14.3% | |
| None | 19 | 15.6% | 12 | 23.1% | 7 | 10.0% | |
| Duration since adjuvant therapy | |||||||
| < 1 year | 41 | 33.6% | 18 | 34.6% | 23 | 32.9% | 0.84 |
| ≥ 1 year | 81 | 66.4% | 34 | 65.4% | 47 | 67.1% | |
| Accuracy of BMI Self-assessment | |||||||
| Underestimate | 37 | 30.3% | 22 | 42.3% | 15 | 21.4% | < 0.01 |
| Accurate assessment | 68 | 55.7% | 28 | 53.8% | 40 | 57.1% | |
| Overestimate | 17 | 13.9% | 2 | 3.8% | 15 | 21.4% | |
| Knowledge of obesity-related breast cancer risk | |||||||
| Yes | 100 | 82.0% | 36 | 69.2% | 64 | 91.4% | < 0.01 |
| No | 22 | 18.0% | 16 | 30.8% | 6 | 8.6% | |
| Distance to hospital | |||||||
| < 25 miles | 72 | 59.0% | 32 | 61.5% | 40 | 57.1% | 0.625 |
| ≥ 25 miles | 50 | 41.0% | 20 | 38.5% | 30 | 42.9% | |
*PI pacific islander, T tumor stage, N nodal stage, SLNB sentinel lymph node biopsy, AND axilla node dissection, SD standard deviation
Demographics and clinical characteristics associated with willingness to participate in institutional weight loss program
One of the questions in the Breast Cancer Questionnaire was to assess if participants would be interested in participating in a formal weight loss program (question 19, supplementary materials). Among the 122 participants who completed the surveys, 70 (57.4%) indicated willingness to participate. Interest did not differ significantly between those with overweight (54.8%) vs. obesity (61.2%, p = 0.48). Of those willing to participate, 68.9% preferred a virtual platform for intervention (telephone, internet, or cell phone application), rather than in-person meetings (21.3%) or weight loss surgery (9.8%).
Demographic and clinical characteristics were also compared between groups based on willingness to participate in a weight loss trial (Table 2). There were no significant differences between groups in patient age, race, income, or BMI. Employment status and distance from home to hospital defined categorically as < 25 miles or ≥ 25 miles were not different between the two groups. In addition, clinical characteristics, including tumor and nodal stage, surgery, chemotherapy, radiation, and comorbidities (including lymphedema), also did not differ in univariable analysis (Table 2). Patient self-assessment of BMI did differ significantly (p < 0.01); those who underestimated their true BMI were less willing to participate in a weight loss intervention (15 of 37 patients [40.5%] willing vs. 22 of 37 patients [59.5%] unwilling), while nearly all patients (15 of 17 patients [88.2%]) who overestimated their true BMI agreed to participate (p < 0.01). Patients endorsing knowledge of obesity-related breast cancer risk were significantly more willing to engage in weight loss intervention than those without such knowledge (p < 0.01). Knowledge of obesity-related breast cancer risk (OR 8.37, p = 0.01) remained a significant predictor of willingness to participate in weight loss intervention on multivariable logistic regression analysis (Table 3).
Table 3.
Multivariable logistic regression of demographic and clinical characteristics influencing willingness to participate in an institutional weight loss intervention
| OR | (95% CI) | p | |||
|---|---|---|---|---|---|
| Age | 1.00 | 0.94 | to | 1.05 | 0.87 |
| Household income | |||||
| < $25,000 | 1.00 | – | to | – | – |
| $25,000–$50,000 | 0.29 | 0.01 | to | 7.25 | 0.45 |
| $50,000–$75,000 | 3.73 | 0.20 | to | 70.25 | 0.38 |
| > %75,000 | 1.69 | 0.12 | to | 24.06 | 0.70 |
| Did not disclose | 0.62 | 0.03 | to | 13.64 | 0.76 |
| Marital status | |||||
| Not married | 1.00 | – | to | – | – |
| Married | 2.52 | 0.71 | to | 8.89 | 0.15 |
| Unknown | – | – | to | – | – |
| Comorbidities* | |||||
| Lymphedema | 0.26 | 0.05 | to | 1.36 | 0.11 |
| Depression score | |||||
| Minimal or none (0–4) | 1.00 | – | to | – | – |
| Mild (5–9) | 0.35 | 0.09 | to | 1.42 | 0.14 |
| Moderate (10–14) | 1.06 | 0.15 | to | 7.53 | 0.95 |
| Moderately severe/severe (15 +) | 2.22 | 0.15 | to | 32.34 | 0.56 |
| Tumor stage | |||||
| T0 | 1.00 | – | to | – | – |
| T1 | 2.37 | 0.64 | to | 8.74 | 0.19 |
| T2 | 1.42 | 0.23 | to | 8.61 | 0.71 |
| T3/4 | 2.47 | 0.05 | to | 111.75 | 0.64 |
| Nodal stage | |||||
| N0 | 1.00 | – | to | – | – |
| N1 | 2.98 | 0.30 | to | 29.93 | 0.35 |
| N2/3 | – | – | to | – | – |
| Axilla management | |||||
| None | 1.00 | – | to | – | – |
| SNB | 2.45 | 0.52 | to | 11.60 | 0.26 |
| AND | 1.15 | 0.10 | to | 13.41 | 0.91 |
| BMI self-assessment | |||||
| Accurate | 1.00 | – | to | – | – |
| Underestimate | 0.58 | 0.18 | to | 1.86 | 0.36 |
| Overestimate | 4.83 | 0.67 | to | 34.89 | 0.12 |
| Knowledge of obesity-related breast cancer risk | |||||
| No | 1.00 | – | to | – | – |
| Yes | 8.37 | 1.77 | to | 39.56 | 0.01 |
PI pacific islander, T tumor stage, N nodal stage, SLNB sentinel lymph node biopsy, AND axilla node dissection
*Reference group for each comorbidity is composed of patients without that comorbidity
Discussion
We completed this companion survey study to determine the willingness of breast cancer survivors with overweight and obesity treated at our institution to participate in a pilot weight loss program using a virtual telehealth platform (NCT04855552). The goals of this study were to assess knowledge of the association between obesity and breast cancer risk, self-perception of BMI, and willingness to engage in a weight loss intervention offered at our institution. Our patient population demonstrated a higher-than-expected knowledge of obesity-related breast cancer risk, with over 80% of participants indicating knowledge of this association. However, even in our population with high awareness, there was a significant proportion who underestimated their own BMI, which was associated with lower willingness to participate in weight loss intervention. Finally, multivariable analysis showed that knowledge of obesity-related breast cancer risk significantly increases willingness to participate in weight loss intervention, even when controlling for true and self-perceived BMI.
While well known within the medical community, there is limited public knowledge that obesity is linked to increased risk of breast cancer. A survey of 1545 women in Houston, TX found that 54% were aware that obesity increases the risk of breast cancer [20], while a study of 207 women at an urban health fair reported that only 28% of participants were aware of this risk, despite a high degree of awareness (> 70%) of the cardiometabolic risks associated with obesity [19]. Knowledge of obesity-related cancer risk is similarly limited in populations of women with overweight and obesity [21]. In contrast to these previous studies, 82% of our study participants were aware of the association between obesity and breast cancer risk.
Despite the high proportion of patients in our study cohort indicating awareness of the association between obesity and breast cancer risk, only 57.4% reported willingness to participate in weight loss intervention. We believe our findings also shed light on the need to develop better communication tools for healthcare providers to have frank discussions with cancer patients regarding their BMI category, associated cancer risks, and mitigation strategies. This discussion could also include the broader range of health improvements, such as cardiovascular and diabetic complications, which are associated with weight loss [18].
Our study has several limitations. One of the limitations included sampling and selection bias. While we are encouraged by the high degree of awareness of the association between obesity and breast cancer risk in our study cohort, this finding may not be generalizable to the broader community of breast cancer survivors. As participants were drawn primarily from a single surgeon’s practice, the high degree of knowledge regarding obesity-related breast cancer risk in our patient population may be due, at least in part, to clinician-specific patient education practices, thereby highlighting the sampling/selection bias of our study. Our survey study was aimed at gauging interest in our breast cancer patients in participating in a weight loss program. Our study was not designed to address the impact of obesity and weight loss on breast cancer outcomes, a limitation of this study. Our cohort was also disproportionately composed of white and high-income participants, which is not representative of the general US population. Strengths of this study include our moderate sample size, though plans for further enrollment were curtailed by the onset of the COVID-19 pandemic, and the original nature of this qualitative research. To our knowledge, this is the first study to evaluate knowledge of the link between obesity and breast cancer risk in a population of breast cancer survivors, as well as the first to assess predictors of willingness to engage in a weight loss intervention in this population.
The effect of intentional weight loss on recurrence risk for breast cancer survivors with obesity is uncertain and is currently under investigation in prospective studies such as the ongoing Breast Cancer Weight Loss (BWEL) trial [28]. However, obesity is known to increase both disease-specific and all-cause mortality in breast cancer survivors [10], highlighting the need for effective weight loss strategies in this population. Our study identified knowledge of the association between obesity and breast cancer risk as a predictor of willingness to engage in weight loss intervention, suggesting that effective communication and education tools may enhance participation and adherence to lifestyle modification strategies. While our findings suggest that improved education regarding BMI and cancer risk may increase engagement with weight loss, there has been limited research on how to communicate such information to this patient population. As we initiate our pilot weight loss program for our breast cancer survivors, we are mindful that future work in the development of better education and communication tools/aids to improve awareness will likely improve the adoption rate of healthy lifestyles in at-risk patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research was funded, in part, through a FOCUS Medical Student Fellowship in Women’s Health supported by the Bertha Dagan Berman Award and the Breast Cancer Research Funds from the Abramson Cancer Center.
Funding
This research was funded, in part, through a FOCUS Medical Student Fellowship in Women’s Health supported by the Bertha Dagan Berman Award and the Breast Cancer Research Funds from the Abramson Cancer Center.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was approved by the Institutional Review Board at the University of Pennsylvania and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in this study.
Consent for publication
Patients signed informed consent regarding use of their data for publication. No identifying information is included in this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Laura Burkbauer, Email: Laura.Burkbauer@pennmedicine.upenn.edu.
Kelly Allison, Email: kca@pennmedicine.upenn.edu.
Julia Tchou, Email: Julia.tchou@pennmedicine.upenn.edu.
References
- 1.Basen-Engquist K, Chang M. Obesity and cancer risk: recent review and evidence. Curr Oncol Rep. 2011;13:71–76. doi: 10.1007/s11912-010-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. New England J Med 14 [DOI] [PubMed]
- 3.Colditz GA, Peterson LL. Obesity and cancer: evidence, impact, and future directions. Clin Chem. 2018;64:154–162. doi: 10.1373/clinchem.2017.277376. [DOI] [PubMed] [Google Scholar]
- 4.Steele CB, Thomas CC, Henley SJ, et al (2017) Vital Signs : Trends in Incidence of Cancers Associated with Overweight and Obesity — United States, 2005–2014. MMWR Morb Mortal Wkly Rep 66:1052–1058. 10.15585/mmwr.mm6639e1 [DOI] [PMC free article] [PubMed]
- 5.Renehan AG, Tyson M, Egger M, et al (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. 371:10 [DOI] [PubMed]
- 6.Goodwin PJ, Chlebowski RT. Obesity and cancer: insights for clinicians. JCO. 2016;34:4197–4202. doi: 10.1200/JCO.2016.70.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: evidence, challenges, and opportunities. JCO. 2016;34:4203–4216. doi: 10.1200/JCO.2016.68.4480. [DOI] [PubMed] [Google Scholar]
- 8.Neuhouser ML, Aragaki AK, Prentice RL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol. 2015;1:611. doi: 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 10.Chan DSM, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer—systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eheman C, Henley SJ, Ballard-Barbash R, et al. Annual report to the nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity: 2011 report on the status of cancer. Cancer. 2012;118:2338–2366. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Druesne-Pecollo N, Touvier M, Barrandon E, et al. Excess body weight and second primary cancer risk after breast cancer: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat. 2012;135:647–654. doi: 10.1007/s10549-012-2187-1. [DOI] [PubMed] [Google Scholar]
- 13.Parker ED, Folsom AR. Intentional weight loss and incidence of obesity-related cancers: the Iowa Women’s Health Study. Int J Obes. 2003;27:1447–1452. doi: 10.1038/sj.ijo.0802437. [DOI] [PubMed] [Google Scholar]
- 14.Birks S, Peeters A, Backholer K, et al. A systematic review of the impact of weight loss on cancer incidence and mortality: weight loss and cancer. Obes Rev. 2012;13:868–891. doi: 10.1111/j.1467-789X.2012.01010.x. [DOI] [PubMed] [Google Scholar]
- 15.Hardefeldt PJ, Penninkilampi R, Edirimanne S, Eslick GD. Physical activity and weight loss reduce the risk of breast cancer: a meta-analysis of 139 prospective and retrospective studies. Clin Breast Cancer. 2018;18:e601–e612. doi: 10.1016/j.clbc.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Howell A, Anderson AS, Clarke RB, et al. Risk determination and prevention of breast cancer. Breast Cancer Res. 2014;16:446. doi: 10.1186/s13058-014-0446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eliassen AH, Colditz GA, Rosner B, et al Adult weight change and risk of postmenopausal breast cancer. 9 [DOI] [PubMed]
- 18.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 19.Cardozo ER, Dune TJ, Neff LM, et al. Knowledge of obesity and its impact on reproductive health outcomes among urban women. J Community Health. 2013;38:261–267. doi: 10.1007/s10900-012-9609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soliman PT, Bassett RL, Wilson EB, et al. Limited public knowledge of obesity and endometrial cancer risk: what women know. Obstet Gynecol. 2008;112:835–842. doi: 10.1097/AOG.0b013e318187d022. [DOI] [PubMed] [Google Scholar]
- 21.Winston GJ, Caesar-Phillips E, Peterson JC, et al. Knowledge of the health consequences of obesity among overweight/obese Black and Hispanic adults. Patient Educ Couns. 2014;94:123–127. doi: 10.1016/j.pec.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodwin PJ, Segal RJ, Vallis M, et al. The LISA randomized trial of a weight loss intervention in postmenopausal breast cancer. npj Breast Cancer. 2020;6:6. doi: 10.1038/s41523-020-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gershuni V, Li YR, Williams AD, et al. Breast cancer subtype distribution is different in normal weight, overweight, and obese women. Breast Cancer Res Treat. 2017;163:375–381. doi: 10.1007/s10549-017-4192-x. [DOI] [PubMed] [Google Scholar]
- 24.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haggerty AF, Sarwer DB, Schmitz KH, et al. Obesity and endometrial cancer: a lack of knowledge but opportunity for intervention. Nutr Cancer. 2017;69:990–995. doi: 10.1080/01635581.2017.1359313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.HPFS Questionnaires. Harvard school of public health health professionals follow up study
- 27.Centers for Disease Control and Prevention (CDC). Behavioral risk factor surveillance system survey questionnaire
- 28.Ligibel JA, Barry WT, Alfano C, et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): study design. npj Breast Cancer. 2017;3:37. doi: 10.1038/s41523-017-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


