Abstract
Rhodobacter sphaeroides cells were tethered by their flagella and subjected to increasing and decreasing nutrient gradients. Using motion analysis, changes in flagellar motor rotation were measured and the responses of the cells to the chemotactic gradients were determined. The steepness and concentration ranges of increasing and decreasing gradients were varied, and the bacterial responses were measured. This allowed the limits of gradients that would invoke changes in flagellar behavior to be determined and thus predicts the nature of gradients that would evoke chemotaxis in the environment. The sensory threshold was measured at 30 nM, and the response showed saturation at 150 μM. The study determined that cells detected and responded to changing concentration rates as low as 1 nM/s for acetate and 5 nM/s for succinate. The complex sensory system of R. sphaeroides responded to both increasing and decreasing concentration gradients of attractant with different sensitivities. In addition, transition phases involving changes in the motor speed and the smoothness of motor rotation were found.
The bacterial environment is subject to constant change. Environmental factors such as nutrient concentrations, light levels, oxygen concentration, pH, and the presence of toxins all vary over time. These changes are sensed by motile bacteria, which respond by moving along concentration gradients towards optimum conditions for growth, a process known as taxis. The means of locomotion used by motile bacteria does vary, but most species use flagellum-driven motility. The bacterial flagellum is a semirigid helical filament driven at its base by a rotary motor (reviewed recently in reference 2). Unstimulated bacteria (a condition unlikely to exist outside the laboratory) swim randomly around their environment, periodically changing direction. Bacteria sense changes in their environment with time (temporal sensing), as they are too small to sense spatial difference. This means that they must compare the current environmental status with previous measurements. When a gradient of attractant is sensed, their direction-changing frequency alters to bias swimming up the gradient. The mechanism of direction changing varies between species and is controlled by the bacterial chemotaxis signal transduction (Che) pathway.
Chemotaxis has been extensively studied in Escherichia coli. In this organism counterclockwise rotation causes smooth swimming, and switching to clockwise rotation causes a tumble and a change in the subsequent direction of smooth swimming. The Che pathway controlling this switching in E. coli is well understood (1, 14). Membrane-spanning methyl-accepting chemotaxis proteins (MCPs) sense a decrease in the concentration of attractant in the periplasm (15). The MCPs transmit a signal via CheW, which interacts with the cytoplasmic domain of the MCP, to activate CheA, a histidine protein kinase. CheA autophosphorylates, and the phosphate group is then transferred to the response regulator, CheY, which interacts with the flagellar motor. The concentration of CheY-P determines whether the motors rotate counterclockwise or clockwise. The CheY-P signal is terminated by CheZ increasing the rate of autodephosphorylation. CheA also controls the activity of a methyl esterase, CheB, which is involved, along with a constitutive methyltransferase, CheR, in resetting the signaling state of the receptors.
Although there does appear to be a common central Che-type pathway for chemotaxis in most bacterial species, the pathway in E. coli appears to be one of the least complex. Experimental studies and genomic sequences show that many organisms, including a large number of environmentally important species, e.g., Pseudomonas aeruginosa, Rhodopseudomonas palustris, and Sinorhizobium meliloti (www.tigr.org), have multiple homologues of the Che proteins and multiple chemotaxis operons. This includes Rhodobacter sphaeroides, the organism used in this study (1).
R. sphaeroides is a purple nonsulfur bacterium with a single, unidirectional flagellum that rotates clockwise to propel the cell forwards. The motor stops periodically, during which time direction changing occurs. It has multiple che homologues of the E. coli che genes. To date two CheA, three CheW, two CheR, one CheB, and five CheY homologues have been identified, with up to 12 MCP-like sensors (1, 9, 23, 24, 27, 28). The che genes and some of the sensors are arranged on three chemotaxis loci, two of which are arranged as large operons, with copies of most genes encoding a single chemosensory pathway. Deletion of Che operon 1 results in very minor changes in response to chemoeffectors (27); however, deletion of Che operon 2 results in an inverted phenotype, where cells show a negative response to chemoattractant addition. This does not allow swarming on gradient plates (9, 24). CheZ is absent in many nonenteric species, including R. sphaeroides. There is evidence that some of the CheY homologues can act as phosphate sinks, terminating the signal to the flagellar motor and thus replacing the function of CheZ (25).
Probably related to having a complex sensory pathway, the behavioral responses of R. sphaeroides are also somewhat different from those of E. coli. R. sphaeroides responds chemotactically to metabolites, and the strongest attractants are weak organic acids, which are important substrates for this organism. Tactic responses to sugars, amino acids, oxygen, and light are also found. Taxis to the organic acids, sugars, and amino acids requires at least transport into the cell and probably partial metabolism (11, 13, 20). The sensors in R. sphaeroides also differ. Most of them have the conventional E. coli structure, but others are totally cytoplasmic. Indeed, immunogold labeling electron microscopy and tagging with green fluorescent protein have shown that some MCPs are localized to the poles of the cell, while others localize to the core of the cell (10, 26). These internal sensors may be involved in chemotactic signaling towards metabolites derived from transported chemoeffectors. R. sphaeroides cells have a run bias, i.e., the probability that the flagellar motor is rotating, of approximately 0.85 (6, 16, 17, 18). The major response is an increase in smooth rotation on the addition of an attractant and a stop (i.e., a direction change) on removal of the attractant or a decrease in light level or oxygen concentration. This biases the cell up a gradient (18). However, when wild-type R. sphaeroides is grown aerobically it also shows an inverted response (a stop in response to an increase in stimuli) to the increase in concentration of some chemoeffectors (e.g., succinate and malate) but not others (e.g., propionate) (17), suggesting a complex interplay of pathways linked to the current metabolic status of the cells.
Temporal sensing has been investigated in R. sphaeroides using tethered cells and stepwise changes in the concentration of chemoeffector, oxygen level, and light level (17, 18, 22). Large, saturating, step stimuli (>500 μM) gave rapid responses and recoveries taking several minutes; however, free-swimming R. sphaeroides can rapidly respond to gradients generated from 250 nM chemoeffector in agarose blocks. Investigation of the photoresponse in R. sphaeroides determined that subsaturating step and impulse responses to light stimuli were of the same duration, i.e., response and recovery within 4 s, similar to those of E. coli to subsaturating changes in chemoeffector concentration (6, 7).
Temporal step changes in chemoeffector concentration are a useful means of investigating the sensory system, but cells in the environment must also be able to sense and respond to relatively stable sensory gradients. Previous studies on E. coli have used free-swimming cells in gradients, but the interpretation of their responses is very complex, as the environment was not uniform and could not be fully defined. Block et al. (8) tethered cells and subjected them to exponential ramps in chemical concentration. These data showed that the change in bias of the motor was dependent upon the rate of change of chemoreceptor occupancy.
In this study the nature of the chemotactic response of R. sphaeroides to gradients of chemoeffectors is described, with the aim of elucidating and predicting the gradients to which cells will respond in natural environments. This will allow the data from complex sensory pathways to be interpreted in a more physiological context. It is also the first study to investigate the performance of a complex sensory system in real gradients and thus to quantify the concentration range, the kinetics, the thresholds of response, and the nature of the response itself.
MATERIALS AND METHODS
Growth conditions.
R. sphaeroides WS8 (wild type) was grown aerobically with shaking to mid-log phase (approximately 2 × 108 cells/ml) in succinate medium in the dark at 30°C as previously described (17). The cells were harvested by centrifugation and resuspended at a 10-fold concentration into 10 mM Na HEPES (pH 7.2) buffer containing 50 μg of chloramphenicol per ml.
Tethering and gradient production.
For tethering, 5 μl of cells was placed on a glass coverslip precoated with R. sphaeroides antiflagellar antibody, incubated for 10 min, and loaded into a flow chamber (5, 18). Cells were starved for 30 min by flowing past 10 mM Na HEPES (pH 7.2) buffer containing 50 μg of chloramphenicol per ml. This buffer was used as a background throughout the experiments. The attractants were added as sodium salts, as R. sphaeroides shows no behavioral response to sodium ions. The cell preparation, tethering, and experiments were conducted at room temperature.
Gradients were made with a gradient maker, using the principle of gradient formation used for centrifugation. Liquid was pumped from one of two identical, connected cylinders and replaced by fluid in the other cylinder so that both cylinders had the same volume and column height. When different concentrations of effector were placed in the columns, a gradient formed as the liquid was pumped out through the flow chamber. The system was calibrated by placing methylene blue into cylinder 1 (at 0.25 mg/ml in Na HEPES) and Na HEPES in cylinder 2. The flow chamber was placed inside a spectrophotometer, the window was aligned with the light source and detector, and measurements of optical density at 600 nm were made over time. The optical density at 600 nm was calibrated against known concentrations of methylene blue. The gradients produced were smooth and linear.
Motion analysis.
The tethered cells were viewed using a phase-contrast microscope (Optiphot microscope, Nikon Ltd., Tokyo, Japan) with an attached video camera (HYPER HAD; Sony Corp., Tokyo, Japan). Measurement of cell rotation was done using AROT7 software on a Bactracker (HTS Ltd., Sheffield, United Kingdom). The software analyzes up to 10 cells per field. Cells that were rotating without touching others were measured. Data points were taken at the video frame rate (50 Hz for interlaced images), and the raw data were downloaded as ASCII files for analysis. The raw data and data smoothed over 0.2-s intervals were analyzed. The probability of each individual cell being stopped was determined, and then the mean stop probability for the population was calculated for populations of at least 10 cells. Cells rotating at speeds of below 1.5 Hz were regarded as stopped. All experiments were repeated for at least three cell populations.
RESULTS
Gradients up to saturation.
Gradient responses to two chemoattractants, acetate and succinate, were measured, as these elicit different responses in chemoheterotrophically grown cells when given as stepwise changes in concentration (17). The stepwise addition of acetate gives an increase in smooth rotation with an apparent speed change, and removal causes a stop followed by adaptation, i.e., a return to prestimulus behavior. The addition of succinate gives an inverted response, i.e., a stop followed by adaptation.
Gradients were constructed to give a change in concentration of about 1 μM/s, as this is considered typical of the concentration range that cells may encounter in the natural environment. Smooth linear shallow gradients were used to mimic natural conditions. The attractant gradients were designed to run from 0 to 1 mM and vice versa, exposing the cells to a range of concentrations from nonstimulating through subsaturating to saturating. Concentrations of 1 mM have been found to saturate responses in tethered cells when increases were stepwise.
(i) Increasing succinate concentration gradients.
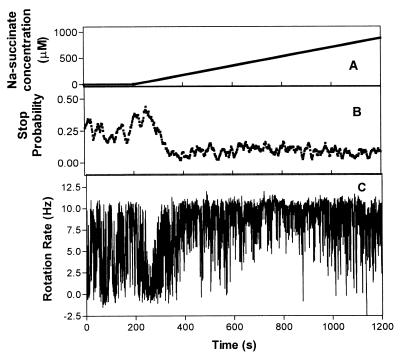
Increasing succinate gradients were passed through the flow chamber in the 0 to 1 mM concentration range. The behavior of cells in a gradient of succinate increasing at a rate of 1 μM/s from 0 to 1 mM had three phases: a predominantly stopped phase, a period of increased stop bias with slow rotation, and a phase of smooth rapid rotation (Fig. 1). The stopped phase in the example shown in Fig. 1 occurred at a threshold concentration of 33.4 μM, 38 s after the gradient flow had started, and lasted for about 30 s (over a 60 μM concentration change) with a stop bias of 0.42. However, unlike a response to a stepwise increase in concentration where the whole population was completely stopped, the population continued to have brief unsynchronized periods of slow rotation. During this phase, a typical cell was stopped for 85% of the time with 1- to 2-s runs at speeds up to 5 Hz. This suggests that the response was subsaturating, with the concentration of motor-interacting CheY-P below that required for the total stop, which would occur with a saturated response.
FIG. 1.
Response of tethered cells of R. sphaeroides WS8 to a gradient of succinate increasing at a rate of 0.9 μM/s. The graphs show the concentration of succinate (A), the mean stop probability of a population of 10 cells (B), and the rotation rate of a typical cell (C) versus time.
After the predominantly stopped period, the cells went through 124 s of decreasing stop bias, from 0.42 to 0.05, and increasing rotation rate (Fig. 1), with the speed changing from 0 (stopped) to 9.5 Hz, until 169 s after the gradient started, at 148 μM, the cell population began a phase of smooth rapid rotation with a stop bias of less than 0.1 and a mean rotation rate of 8.52 ± 2.4. This concentration, about 150 μM, is presumably the saturation point for an increasing concentration gradient.
The increasing succinate gradient was continued to a final concentration of 1 mM, and the cells continued to rotate smoothly, showing no adaptation. This phenomenon has been previously seen in free-swimming cells of R. sphaeroides and has been termed chemokinesis due to the observed increase in swimming speed of free-swimming cells (16, 21).
(ii) Decreasing succinate concentration gradient.
Succinate (1 mM) was flowed through the chamber for 20 min before the start of the experiment. The concentration of succinate was decreased at a rate of 0.9 μM/s from 1 to 0 mM. Initially the mean stop bias of the cell population was 0.38, but the reduction in succinate concentration decreased the stop bias of the population of cells to 0.15 over 150 s (Fig. 2). Analysis of individual cell data showed very little change in rotation rate at succinate concentrations of below 870 μM, with a mean population rotation rate of 4.6 ± 0.8 Hz. After this the cells rotated slightly faster, at 6.4 ± 0.7 Hz. The long, smooth runs which occurred when the gradient of succinate was increasing (Fig. 1) were absent (Fig. 2). Tethered cells of aerobic R. sphaeroides also show little response to a step decrease in succinate (17), indicating that the system is less sensitive to decreases of this attractant than to increases.
FIG. 2.
Response of tethered cells of R. sphaeroides WS8 to a gradient of succinate decreasing at a rate of 0.9 μM/s. The graphs show the concentration of succinate (A), the mean stop probability of a population of 10 cells (B), and the rotation rate of a typical cell (C) versus time.
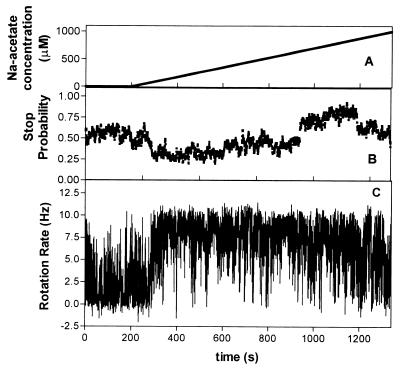
(iii) Increasing acetate concentration gradients.
The response to an increasing gradient of Na acetate is shown in Fig. 3. The increasing concentration elicited a change in rotational behavior from unstimulated behavior in two phases: a period of change with increasing rotation rate and decreasing stop bias and a second phase of smooth, fast rotation. In the gradient the acetate concentration was increasing at a rate of 0.8 μM/s from 0 to 1 mM. The phase of sensing and alteration of rotational behavior began 60 s after the gradient was initiated, at a concentration of about 46 μM, and the cells continued to increase their rate of rotation for 149 s before the response appeared to be at a maximum (115 μM). The stop probability changed from 0.6 to 0.25. Reduced stopping and smooth, fast rotation were maintained until about 1,115 s, when the stop bias increased to 0.7. This indicated that the response had saturated at 335 μM and the cells had adapted to the saturating concentration.
FIG. 3.
Response of tethered cells of R. sphaeroides WS8 to a gradient of acetate increasing at a rate of 0.9 μM/s. The graphs show the concentration of acetate (A), the mean stop probability of a population of 10 cells (B), and the rotation rate of a typical cell (C) versus time.
(iv) Decreasing acetate gradients.
The above-described experiment was conducted with the gradient of acetate decreasing from 1 to 0 mM Na-acetate. There appeared to be only one phase of behavioral response, as the rotating cell population stopped after 34 s. At this threshold the concentration of acetate was calculated to be about 958 μM and therefore had decreased by 42 μM from the start of the experiment (Fig. 4). The population remained stopped as the concentration gradient continued to decrease.
FIG. 4.
Response of tethered cells of R. sphaeroides WS8 to a gradient of acetate decreasing at a rate of 0.9 μM/s. The graphs show the concentration of acetate (A), the mean stop probability of a population of 10 cells (B), and the rotation rate of a typical cell (C) versus time.
Determining the threshold of the sensory response.
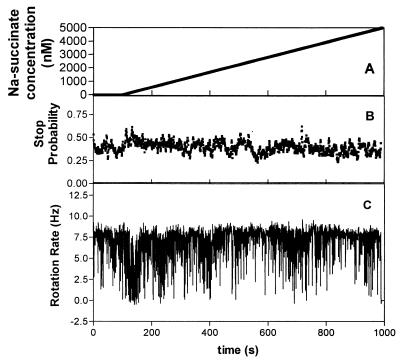
To determine the sensory thresholds of R. sphaeroides, linear increasing concentration gradients were flowed over tethered cells at reducing rates to reduce the steepness of the gradient until the cells no longer gave a measurable response. A series of gradients were designed around the range shown to cause responses in the above-described experiments. Linear gradients of increasing rates of concentration of Na-succinate from 1 to 6 nM/s were flowed past tethered cells. Rates of increase from 1 to 4 nM/s did not elicit a detectable response at low concentrations; however, a rate of increase of 5 nM/s did elicit a clear response. The response was an increase in the stop probability from 0.35 to 0.6 at 133 s (Fig. 5). The presence of this rapid response was confirmed by visual observation of the cells, and during this period the cell population was predominantly stopped, with occasional brief runs.
FIG. 5.
Response of tethered cells of R. sphaeroides WS8 to a gradient of succinate with increasing at a rate of 5 nM/s. The graphs show the concentration of succinate (A), the mean stop probability of a population of 10 cells (B), and the rotation rate of a typical cell (C) versus time.
At subsaturating levels, the stop response of the cells was not synchronized within the population. Although all cells in the population showed a period of increased stop bias, the actual stops were not synchronized, giving only small increases in the population stop bias (0.2), unlike the response to saturating levels, where there was a total stop and thus a large stop bias. The threshold of the response was between 30 and 50 nM, depending upon the individual cells. The individual stops lasted 2 to 5 s, measured from the time of the cell slowing down to the time of returning to its original rotation rate. The intervals of rotation between the stops were also short, suggesting that the refractory period of the cell population ranged from three video frames (0.06 s and a 5 to 10 nM change in concentration) to several seconds. The response times and kinetics of the system match the sensory times found for E. coli, which integrates stimuli over a few seconds, responding and recovering in 4 s (6).
The change in stop probability continued for about 50 s (up to 250 nM), when the cells reverted to prestimulus behavior. No evidence of increased smooth rotation in these shallow, low concentration gradients was found, suggesting that the signal for this response has a threshold above 1 μM.
Decreasing gradients of low concentration.
R. sphaeroides shows its strongest response to attractants which cause an increase in stop bias as their concentration is decreased (16, 17). To determine the thresholds of the sensory response to a decreasing concentration gradient, very shallow gradients of Na-acetate or Na-succinate were flowed past tethered cell populations. The rate of change of concentration was decreased until there was no response, thus determining the minimum concentration change to which the cells could respond. The cell population was not responsive to decreasing low-concentration succinate gradients at flow rates of up to 0.1 μM/s; however, R. sphaeroides was very sensitive to decreasing acetate gradients.
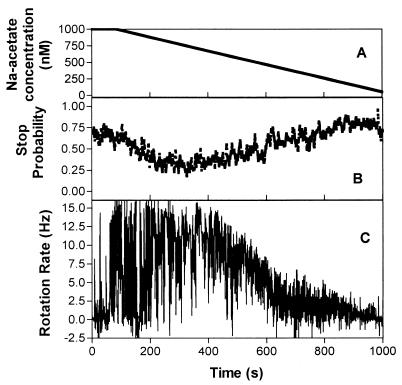
When an acetate gradient was flowed past at a decreasing rate of 1 nM/s, the cell population responded. The response had three phases: a phase where cells had smoother rotation with fewer stops at the maximum rotation rate; a second phase where the speed decreased but the stopping frequency did not increase, and a final phase where the cells stopped completely.
The gradient was started at an initial concentration of 1 μM Na-acetate (pH 7.2) in buffer and decreased to 0 μM. Cells incubated in 1 μM Na-acetate for 30 min had a stop probability of 0.7. Individual cells responded rapidly to the decreasing gradient with an increase in smooth rotation. The first response was 34 s after the gradient was initiated, at 967 nM (a decrease of 33 nM) (Fig. 6). Next, there was the phase of decreasing speed and increasing stop probability, down to 506 nM when the cells began to stop completely, presumably at a saturating concentration of CheY-P. The rotation rate of the cells also decreased (10 to 0 Hz) as the concentration decreased (this was seen visually and confirmed by analysis), with the stop probability increasing down the gradient from 0.2 to 1.0. The records of individual cells showed that their stopping frequency remained low during the phase of slowing rotation, and for most cells the proportion of time spent stopped did not increase with decreasing concentration (Fig. 6). Some cells gradually reduced speed into a stop, whereas others stopped abruptly from rotating at several hertz. Once the cells had stopped, they did not recover as the reduction in concentration continued. Cells incubated in buffer alone rotated and stopped randomly; the stopped response was therefore not due to starvation.
FIG. 6.
Response of tethered cells of R. sphaeroides WS8 to a decreasing subsaturating gradient of acetate with the acetate concentration decreasing at a rate of 1 nM/s. The graphs show the concentration of acetate (A), the mean stop probability of a population of 10 cells (B), and the rotation rate of a typical cell (C) versus time.
DISCUSSION
R. sphaeroides cells tethered by their flagella responded to shallow linear gradients of chemoeffectors and were sensitive to concentration gradients in the nanomolar to millimolar range, i.e., from subsaturating to saturating concentrations. The minimum concentration gradient to which a response was detected was a 1-nM/s decreasing gradient of acetate, while for an increasing gradient the minimum was a 5-nM/s change in succinate concentration. Combining the data from this study with those from previous studies using stepwise changes in attractant, tethered cells show responses to changes in attractant concentration ranging from 1 nM to 10 mM, giving a range of 7 orders of magnitude (16, 17, 18; C. Wood, D. S. H. Shah, H. L. Packer, and J. P. Armitage, unpublished observation). A similar broad range of sensitivity has also been found in E. coli, where cells respond chemotactically to changes over about 5 orders of magnitude (12). During this study, the sensory system appeared to be as responsive to high concentrations as to low, which suggests that it is the concentration difference that is important rather than the absolute concentration. The minimum sensory threshold of the behavioral response was found in the subsaturating range for increasing and decreasing gradients, at approximately 30 nM, although individual cells responded at between 30 and 50 nM, presumably reflecting their individual metabolic states.
In the experiments described here, the cells were under constant stimulation, probably reflecting more natural conditions. By titrating the response we have determined the minimum change that the R. sphaeroides sensory system can sense. It appears that at nanomolar concentrations it was not the absolute concentration that was important but the rate of change of effector concentration. Succinate gradients increasing at rates below 5 nM/s did not elicit a response, even when the concentration was above the sensory threshold of 30 nM. At these very low rates of change, presumably the difference in the number of molecules bound to the chemosensors was not detected over the sensory period, as the receptor binding kinetics were below the “memory” of the sensing system. Estimating the volume of R. sphaeroides to be 0.5 μm3, at 5 nM/s the cell would be exposed to a change of approximately 1.5 molecules per s, while at the threshold of 30 nM there would be a change of 9 molecules/s, an approximately 14% difference. At 4 nM/s the cell would be exposed to a change of only 1.2 molecules/s, which appears to be below the rate of change required to elicit a response (12.3% at 30 nM). The system was more sensitive to decreasing acetate concentrations and was apparently able to sense a rate of change of as little as 1 nM/s (0.3 molecule/s).
Phases of response.
R. sphaeroides showed consistent patterns, or phases, of behavior when subjected to changing gradients. The two main responses seen were the stopping of flagellar rotation and smooth, fast rotation of the motor. However, there were also phases of transition between these two states and unstimulated behavior where different patterns were observed. The size and pattern of the response depended upon the attractant concentration and the nature of the attractant. For example, a gradient of increasing succinate concentration caused an increase in stopping at low concentrations and a high concentration response (smooth, fast rotation and a decrease in stopping) as the concentration increased. The response to shallow low increasing concentrations was absent with acetate, while the chemosensory system was more responsive to a decreasing gradient of acetate than to an increasing gradient. This was the reverse of the case for a gradient of succinate. A similar phenomenon has been found for wild-type tethered cells in response to step changes, where, depending upon the chemoattractant and concentration, inverted or normal responses can occur. Surprisingly, both responses appear to lead to chemotaxis in free-swimming cell assays. Studies with che deletion mutants of R. sphaeroides have begun to elucidate the pathway and the proteins involved in normal and inverted responses. These currently point to Che operon 1 being involved in inverted responses and Che operon 2, which seems dominant, being involved in normal responses (24). This study shows that R. sphaeroides is sensitive to both increases and decreases in attractant concentration in natural gradients.
Apparent speed change.
It has consistently been reported that free-swimming (using two- and three-dimensional tracking) and tethered cells of R. sphaeroides show speed changes (chemokinesis) in response to the addition of the weak organic acids (4, 16, 18, 19). In this study sustained changes in rotation rate were found in response to increasing and decreasing concentration gradients. The chemokinetic phenomenon was absent in shallow gradients, suggesting that this response has a critical concentration threshold.
In this study, previously undetected phases of transition in response to both increasing and decreasing gradients of chemoeffector were found. There was a transition phase as the concentration increased, where the stop bias decreased and the mean speed of the individual runs increased; however, the most interesting apparent speed change was in response to the subsaturating decreasing gradients of acetate. Once the gradient was made progressively shallower, to a point where the change over time from population run to stop was increased and could be detected, a gradual decrease in the rate of rotation of the cells was observed, with rotation being quite smooth during this transition.
Recent mutational studies of the che homologues in R. sphaeroides, particularly of the five CheY products, suggest that the motor is, in default, a smoothly rotating motor with some inherent stopping (24). Two of the CheY proteins, when phosphorylated, may interact with the motor to produce stops, while the other CheY proteins might act as signal terminators or phosphate sinks (24). The rotation rates and free-swimming speeds of the CheY− mutants seem to be the same as those of the wild type showing chemokinesis.
This suggests that the speed increase may be a result of the reduction in the number of CheY-P molecules interacting with the motor. The default motor would be smooth and at maximum rotation. High concentrations of CheY-P would then result in a stop, and lower concentrations would result in a range of speeds between the two speed extremes, i.e., stop and maximum speed. There is also the possibility that the motor undergoes very brief stops and pauses which cannot be detected using video motion analysis, and their rate of occurrence may give speed changes; however, as the motor in default is smooth, these would be under the control of the Che system. During the phase of smooth rotation, the stop bias is less than the unstimulated stop bias of 0.25, which suggests that the levels of CheY-P interacting with the motor are below those found during unstimulated behavior and therefore that autophosphorylation of CheA is being repressed and the CheY proteins acting as phosphate sinks are reducing the level of motor-interacting CheY-P, causing smooth, fast rotation.
Signal input.
For simplicity, the chemotaxis system in R. sphaeroides has been considered as a system with input from periplasmic binding sites on MCPs transmitted to the Che pathway; however, both succinate and acetate require at least transport into the cell for chemotaxis to occur (3), and while CheA2 and CheB are known to be involved in chemotaxis to these compounds, the sensory receptors have not been identified. R. sphaeroides does have internal chemoreceptors, but whether one of these acts directly as a receptor for succinate, acetate, or a metabolic product is not known, and if indeed it does, the concentration kinetics for transmitting the signal and the methylation-demethylation cycle may be different from those of a classical MCP.
The response of R. sphaeroides to linear gradients has indicated that the system has some similarities to the E. coli system, such as response times and sensing range, but also has many differences. R. sphaeroides is able to sense, respond, and adapt to stimuli within the time scales known to be required for bacterial chemotaxis. The system is complex, however, allowing responses to both decreasing and increasing attractant gradients but with different levels of sensitivity. How these signals are balanced to produce accumulation of cells in a gradient is not presently understood and is under investigation. Studies of this nature on bacterial species found in natural environments will help to elucidate the type of gradients to which they will respond and the role of chemotactic behavior in the environment.
ACKNOWLEDGMENTS
We thank the NERC and the BBSRC for their support. HLP is an NERC Advanced Research Fellow.
REFERENCES
- 1.Armitage J P. Bacterial tactic responses. Adv Microb Physiol. 1999;41:229–289. doi: 10.1016/s0065-2911(08)60168-x. [DOI] [PubMed] [Google Scholar]
- 2.Armitage J P, Berry R. The bacterial flagella motor Adv. Microb Physiol. 1999;41:291–337. doi: 10.1016/s0065-2911(08)60169-1. [DOI] [PubMed] [Google Scholar]
- 3.Armitage J P, Kelly D J, Sockett R E. Bacteria p 1005–1028. R. E. Blankenship, M. T. Madigan and C. E. Bauer (ed.) 1995. Flagellate motility, behavioural responses and active transport in purple non-sulphur bacteria; pp. 1005–1028. Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands. [Google Scholar]
- 4.Armitage J P, Pitta T P, Vigeant M A-S, Packer H L, Ford R M. Transformations in flagellar structure of Rhodobacter sphaeroides and possible relationship to changes in swimming speed. J Bacteriol. 1999;181:4825–4833. doi: 10.1128/jb.181.16.4825-4833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg H C, Block S M. A minature flow cell designed for rapid exchange of media under high-power microscope objectives. J Gen Microbiol. 1984;130:2915–2920. doi: 10.1099/00221287-130-11-2915. [DOI] [PubMed] [Google Scholar]
- 6.Berry R, Armitage J P. Response kinetics of tethered Rhodobacter sphaeroides to changes in light intensity. Biophys J. 2000;78:1207–1215. doi: 10.1016/S0006-3495(00)76678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Block S M, Segall J E, Berg H C. Impulse responses in bacterial chemotaxis. Cell. 1982;31:215–226. doi: 10.1016/0092-8674(82)90421-4. [DOI] [PubMed] [Google Scholar]
- 8.Block S M, Segall J E, Berg H C. Adaptation kinetics in bacterial chemotaxis. J Bacteriol. 1983;154:312–323. doi: 10.1128/jb.154.1.312-323.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamblin P A, Maguire B A, Grishanin R N, Armitage J P. Evidence for two chemosensory pathways in Rhodobacter sphaeroides Mol. Microbiol. 1997;26:1083–1096. doi: 10.1046/j.1365-2958.1997.6502022.x. [DOI] [PubMed] [Google Scholar]
- 10.Harrison D M, Skidmore J, Armitage J P, Maddock J R. Localisation and environmental regulation of MCP-like proteins in Rhodobacter sphaeroides. Mol Microbiol. 1999;31:885–892. doi: 10.1046/j.1365-2958.1999.01226.x. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs M H J, Driessen A J M, Konings W N. Characterisation of a binding protein-dependent glutamate transport system of Rhodobacter sphaeroides. J Bacteriol. 1995;177:1812–1816. doi: 10.1128/jb.177.7.1812-1816.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jasuja R, Yu-Lin, Trentham D R, Khan S. Response tuning in bacterial chemotaxis. Proc Natl Acad Sci USA. 1999;96:11346–11351. doi: 10.1073/pnas.96.20.11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeziore S Y, Hamblin P A, Bootle W C, Poole P S, Armitage J P. Metabolism is required for chemotaxis to sugars in Rhodobacter sphaeroides. Microbiology. 1998;144:229–239. doi: 10.1099/00221287-144-1-229. [DOI] [PubMed] [Google Scholar]
- 14.Levit M N, Lui Y, Stock J B. Stimulus response coupling in bacterial chemotaxis: receptor dimers in signalling arrays. Mol Microbiol. 1998;30:459–466. doi: 10.1046/j.1365-2958.1998.01066.x. [DOI] [PubMed] [Google Scholar]
- 15.Mowbray S L, Sandgren M O J. Chemotaxis receptors: a progress report on structure and function. J Struct Biol. 1998;124:257–275. doi: 10.1006/jsbi.1998.4043. [DOI] [PubMed] [Google Scholar]
- 16.Packer H L, Armitage J P. The chemokinetic and chemotactic behaviour of Rhodobacter sphaeroides. J Bacteriol. 1994;176:206–212. doi: 10.1128/jb.176.1.206-212.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packer H L, Armitage J P. Inverted behavioural responses in wild type Rhodobacter sphaeroides to temporal stimuli. FEMS Microbiol Lett. 2000;189:299–304. doi: 10.1111/j.1574-6968.2000.tb09247.x. [DOI] [PubMed] [Google Scholar]
- 18.Packer H L, Gauden D E, Armitage J P. The behavioural response of anaerobic Rhodobacter sphaeroides to temporal stimuli. Microbiology. 1996;142:593–599. doi: 10.1099/13500872-142-3-593. [DOI] [PubMed] [Google Scholar]
- 19.Packer H L, Lawther H, Armitage J P. The Rhodobacter sphaeroides motor is a variable speed rotor. FEBS Lett. 1997;409:37–40. doi: 10.1016/s0014-5793(97)00473-0. [DOI] [PubMed] [Google Scholar]
- 20.Poole P S, Smith M J, Armitage J P. Chemotactic signalling in Rhodobacter sphaeroides requires metabolism of attractants. J Bacteriol. 1993;175:291–294. doi: 10.1128/jb.175.1.291-294.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poole P S, Williams R L, Armitage J P. Chemotactic responses of Rhodobacter sphaeroides in the absence of apparent adaption. Arch Microbiol. 1990;153:386–372. [Google Scholar]
- 22.Romagnoli S, Armitage J P. Roles of chemosensory pathways in transient changes in swimming speed of Rhodobacter sphaeroides induced by changes in photosynthetic electron transport. J Bacteriol. 1999;181:34–39. doi: 10.1128/jb.181.1.34-39.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah D S H, Porter S L, Harris D C, Wadhams G H, Hamblin P A, Armitage J P. Identification of a fourth cheY gene in Rhodobacter sphaeroides and inter-species interaction within the bacterial chemotaxis operons and cheY genes. Mol Microbiol. 2000;35:101–112. doi: 10.1046/j.1365-2958.2000.01680.x. [DOI] [PubMed] [Google Scholar]
- 24.Shah D S H, Porter S L, Martin A C, Hamblin P A, Armitage J P. Fine tuning bacterial chemotaxis: analysis of Rhodobacter sphaeroides behaviour under aerobic and anaerobic conditions by mutations of the major chemotaxis operons and cheY genes. EMBO J. 2000;19:4601–4613. doi: 10.1093/emboj/19.17.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sourjik V, Schmitt R. Phospho transfer between CheA, CheY1 and CheY2 in the chemotaxis signal transduction chain of Rhizobium meliloti. Biochemistry. 1998;37:2327–2335. doi: 10.1021/bi972330a. [DOI] [PubMed] [Google Scholar]
- 26.Wadhams G H, Martin A C, Armitage J P. Identification and localisation of a metyl-accepting chemotaxis protein in Rhodobacter sphaeroides. Mol Microbiol. 2000;36:1222–1233. doi: 10.1046/j.1365-2958.2000.01936.x. [DOI] [PubMed] [Google Scholar]
- 27.Ward M J, Bell A W, Hamblin P A, Packer H L, Armitage J P. Identification of a chemotaxis operon with 2 cheY genes in Rhodobacter sphaeroides. Mol Microbiol. 1995;17:357–366. doi: 10.1111/j.1365-2958.1995.mmi_17020357.x. [DOI] [PubMed] [Google Scholar]
- 28.Ward M J, Harrison D M, Ebner M J, Armitage J P. Identification of a methyl-accepting chemotaxis protein in Rhodobacter sphaeroides. Mol Microbiol. 1995;18:115–121. doi: 10.1111/j.1365-2958.1995.mmi_18010115.x. [DOI] [PubMed] [Google Scholar]