Abstract
Background
Antimicrobial stewardship (AMS) programmes in hospitals support optimal antimicrobial use by utilizing strategies such as restriction policies and education. Several systematic reviews on digital interventions supporting AMS have been conducted but they have focused on specific interventions and outcomes.
Objectives
To provide a systematic overview and synthesis of evidence on the effectiveness of digital interventions to improve antimicrobial prescribing and monitoring in hospitals.
Methods
Multiple databases were searched from 2010 onwards. Review papers were eligible if they included studies that examined the effectiveness of AMS digital interventions in an inpatient hospital setting. Papers were excluded if they were not systematic reviews, were limited to a paediatric setting, or were not in English.
Results
Eight systematic reviews were included for data extraction. A large number of digital interventions were evaluated, with a strong focus on clinical decision support. Due to the heterogeneity of the interventions and outcome measures, a meta-analysis could not be performed. The majority of reviews reported that digital interventions reduced antimicrobial use and improved antimicrobial appropriateness. The impact of digital interventions on clinical outcomes was inconsistent.
Conclusions
Digital interventions reduce antimicrobial use and improve antimicrobial appropriateness in hospitals, but no firm conclusions can be drawn about the degree to which different types of digital interventions achieve these outcomes. Evaluation of sociotechnical aspects of digital intervention implementation is limited, despite the critical role that user acceptance, uptake and feasibility play in ensuring improvements in AMS are achieved with digital health.
Introduction
Antimicrobial stewardship (AMS) is a term used to describe programmes aimed at optimizing antimicrobial use, ultimately improving patient outcomes, and reducing adverse consequences including antimicrobial resistance, in a cost-effective way.1 AMS programmes are typically hospital-wide and multifaceted, including strategies such as prescribing guidelines, formularies and approval systems, post-prescription audit and feedback, and point-of-care interventions.1 A plethora of studies have been published evaluating the impact of different AMS programmes and interventions on antimicrobial prescribing and patient outcomes.2,3 A range of outcome measures including antimicrobial use, cost, resistance patterns, secondary infections, patient outcomes (e.g. mortality and length of stay) and readmission rates have been used by studies to assess the impact of AMS in hospitals.4 AMS programmes have been shown to reduce inappropriate antimicrobial use, hospital length of stay, costs, rates of resistance and hospital-acquired infections.3,5–7
The use of digital interventions within AMS programmes is recommended by international guidelines.7–9 AMS processes that were traditionally paper-based are gradually becoming computerized with the increasing implementation of information technology in hospitals. Many AMS programme barriers previously cited in the literature can potentially be addressed with the use of digital interventions. For example, difficulty identifying patients receiving suboptimal therapy and difficulty communicating recommendations to prescribers were barriers to undertaking audit and feedback.10 These can be overcome with computerized provider order entry (CPOE) systems or electronic medical records (eMRs), which allow an AMS team to easily identify patients on an antimicrobial and enter notes into the system to document and communicate recommendations to prescribers. Integrating restriction policies and implementing guidelines can also be done more efficiently with the use of CPOE10–12 and clinical decision support (CDS). For example, an alert signalling de-escalation to narrower-spectrum agents can assist prescribers in making appropriate antimicrobial choices at the point of prescribing.13 Despite these benefits, research has also identified a range of challenges and risks associated with the use of AMS digital interventions. For example, implementing too many CDS alerts can lead to alert fatigue,14 many stand-alone systems do not interface with existing clinical information systems, and digital interventions are not always user-friendly.15 These issues can hamper uptake of systems and so reduce effectiveness of digital interventions in practice.15
Hospital-based AMS interventions1–3,16,17 and digital interventions supporting AMS18–20 have been the focus of previous reviews. These reviews have focused on specific types of interventions, like CDS, or specific outcome measures. It would therefore be challenging for those selecting or purchasing AMS digital interventions, or those embarking on an evaluation of digital interventions, to synthesize this available information and evidence. We set out to perform a review of reviews. The objective of the current study was to provide a systematic overview and synthesis of the evidence on the effectiveness of digital interventions to improve antimicrobial prescribing and monitoring in hospitals. Specifically, we aimed to answer three questions: (1) What digital interventions are used in hospitals to improve antimicrobial prescribing and monitoring? (2) What outcome measures are used to evaluate the effectiveness of digital interventions designed to improve antimicrobial prescribing and monitoring? and (3) How effective are digital interventions in improving antimicrobial prescribing and monitoring in hospitals?
Methods
Eligibility criteria
Systematic review papers were eligible if they included studies examining the effectiveness of digital interventions related to antimicrobial prescribing and monitoring of antimicrobial use in an inpatient hospital setting. Papers were excluded if they did not include a clearly defined search strategy (i.e. were not systematic) or they were not in English. Reviews limited to paediatric settings were also excluded due to the unique challenges associated with prescribing and monitoring antimicrobials in this context.21
Information sources and search strategy
The following databases were searched: MEDLINE, Embase, Scopus, CINAHL and the Cochrane Database of Systematic Reviews. The search was limited to reviews from 2010 onwards as recent systematic reviews would have included papers from older systematic reviews and contained the most up-to-date information. Keywords and subject headings relating to ‘information technology’ and ‘antimicrobials’ were defined for each database. The database search terms are provided in Table S1, available as Supplementary data at JAC Online. The search was conducted on 12 June 2020 and updated on 14 October 2021.
Study selection
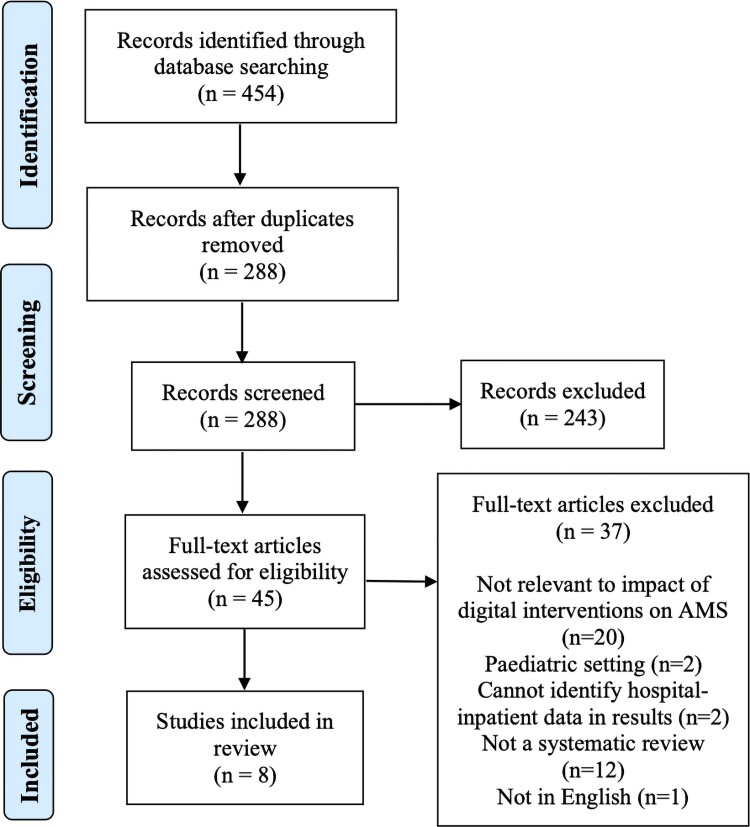
The study selection process is depicted in Figure 1. Papers were imported into Endnote and duplicates were removed. Two researchers independently screened the remaining titles and abstracts based on the eligibility criteria. Researchers met to discuss discrepancies until consensus was reached. Any titles or abstracts that the researchers were unsure of were included for full-text review. Full text of the remaining 45 potentially relevant papers were retrieved and reviewed independently by two researchers. Discrepancies were discussed and consensus was reached on included systematic reviews.
Figure 1.
PRISMA flow diagram. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Data extraction
Conducting a meta-analysis was not possible due to heterogeneity of interventions and outcomes. We therefore undertook a meta-summary, which is viewed to be an appropriate method when integrating variable quantitative and qualitative evidence.22
Data were extracted into an Excel spreadsheet by one researcher and reviewed independently by a second researcher. A third researcher with a pharmacy background also reviewed the data for clarity and accuracy of categorization. General information about the reviews was extracted, such as setting, review objective, sources and number of included papers. For reviews that included data summary tables for their included papers, digital interventions and outcome measures were extracted from these tables. When tables were not available, digital interventions and outcome measures were extracted from the results section in each review. Data on the effectiveness of the digital interventions were also extracted from the results section of the reviews.
Quality assessment
The Assessment of Multiple Systematic Reviews 2 (AMSTAR 2)23 was used to assess the quality of the systematic reviews. AMSTAR 2 is a 16-item checklist that allows for the critical appraisal of systematic reviews specifically related to healthcare interventions. It is a validated tool and was chosen as it can be used for systematic reviews and/or meta-analyses that include both randomized controlled trials and non-randomized studies of interventions.23–25 Two researchers independently applied the AMSTAR 2 checklist to the reviews and met to discuss discrepancies until a consensus was reached on the quality of the reviews. As AMSTAR 2 does not have a numerical scoring system, the overall quality of papers was determined by the number of critical and non-critical flaws (see Table S2). Overall confidence in the results of reviews was rated as high, moderate, low or critically low, as suggested by Shea et al.23
Results
Review selection
The database search returned 454 papers. Titles and abstracts of records were screened and excluded based on exclusion criteria, from which we retrieved 45 papers for full-text review. After screening, eight papers met the inclusion criteria and data were extracted for analysis (see Figure 1).
Review characteristics
The characteristics of the included reviews are presented in Table 1. Review papers reported results from between 34 and 143 studies. The majority of reviews (n = 5) were specific to antibiotics and all were published in the last 5 years.
Table 1.
Characteristics of included studies and results of quality assessment
| Author (year) | Setting/population | Objective | Sources | Number of included papers | Antimicrobial type | Quality of included papersa | Funding source reported? |
|---|---|---|---|---|---|---|---|
| Baysari et al. (2016)26 | Hospital, inpatient | To review evidence of the effectiveness of information technology interventions to improve antimicrobial prescribing in hospitals. | MEDLINE, Embase, PubMed, reference lists | 45 | Antimicrobials | Majority were low quality | Yes—Public |
| Carracedo Martinez et al. (2019)18 | Primary care and hospital | To examine whether the use of a CDSS is associated with improved antibiotic prescribing, and the secondary objective was to determine whether CDSSs are associated with lower morbidity and mortality. | MEDLINE, Embase | 34 | Antibiotics | Majority were low quality | Yes—Public |
| Cresswell et al. (2017)27 | Hospital | To identify and describe existing and emerging approaches to promoting the appropriate use of antibiotics through hospital ePrescribing systems. | MEDLINE, Embase, CDSR, Clinicaltrials.gov, ISRCTN Registry, NHS EED, PROSPERO, Google Scholar | 143 | Antibiotics | NR | Yes—Public |
| Curtis et al. (2017)19 | Hospital, inpatient | To evaluate the evidence for CDS in improving quantitative and qualitative measures of antibiotic prescribing in inpatient hospital settings. | MEDLINE, Embase, PubMed, Web of Science, CINAHL, Cochrane library, HMIC, PsycInfo | 81 | Antibiotics | Majority were low quality | Yes—Private |
| Helou et al. (2020)28 | Hospital, inpatient | To systematically review AMS apps and their impact on prescribing by physicians treating in-hospital patients. | MEDLINE, Embase, Cochrane Central, Web of Science, Google Scholar | 13 | Antimicrobials | Majority were low to medium quality | Yes—No funding |
| Laka et al. (2020)20 | Primary care and hospitals | To assess the effectiveness of CDSSs at reducing unnecessary and suboptimal antibiotic prescribing within different healthcare settings. | MEDLINE, Embase, PubMed, CENTRAL, Scopus, CINAHL, PsycInfo, Web of Science, reference lists | 57 | Antibiotics | Majority were low quality | Yes—Public |
| Rawson et al. (2017)29 | Primary care and hospitals | To understand the current scope of CDSSs for antimicrobial management and analyse existing methods used to evaluate and report such systems. | MEDLINE, Embase, HMIC, Global Health | 58 | Antimicrobials | Majority were medium to low quality | Yes—Public |
| Rittman et al. (2019)30 | Hospital | To review the current status of an interactive, patient-centred CDSS on antibiotic use. | PubMed | 45 | Antibiotics | Majority were low to medium quality | Not reported |
CDSR, Cochrane database of systematic reviews; CENTRAL, Cochrane central register of controlled trials; CINAHL, Cumulative Index to Nursing and Allied Health Literature; HMIC, Healthcare Management Information Consortium; ISRCTN, International Standard Randomised Controlled Trial Number; NHS EED, National Health Service Economic Evaluation Database; PROSPERO, international prospective register of systematic reviews.
Quality of included papers as determined by authors of the review.
Results of the AMSTAR 2 quality assessment are presented in Table S2. Three reviews were rated as moderate, two low and three critically low quality. All reviews reported potential sources of conflict; most performed the search study selection in duplicate (n = 7) and provided a discussion of the heterogeneity observed in the review results (n = 6). All reviews failed to provide a list of excluded studies and justified exclusions. The majority of reviews (n = 7) also failed to explain their selection of the study designs for inclusion. Of the four reviews that included a meta-analysis, all used appropriate statistical methods and assessed the potential impact of risk of bias in individual studies on the results of the meta-analysis.
What digital interventions are used in hospitals to improve antimicrobial prescribing and monitoring?
The digital interventions investigated in the papers contained in the reviews are collated in Table 2 and mapped to the medication management pathway cycle.31 The majority of digital interventions supported the decision to prescribe and the review of medicine orders, but many of the tools supported multiple processes. For example, CDS tools were used to support the decision to prescribe, the recording of medicine orders, reviewing medicine orders and monitoring responses. None of the included reviews compared digital interventions to other interventions.
Table 2.
Digital interventions evaluated in papers, mapped to the medicine management pathway cycle31
| Digital interventions | Medicine management pathway cycle stepsa | ||||||
|---|---|---|---|---|---|---|---|
| Decision to prescribe | Record medicine order | Review medicine order | Issue medicine | Distribution and storage of medicine | Administration | Monitor response | |
| Alerts in eMR | ✓ | ✓ | ✓ | ✓ | |||
| Applications providing local resistance maps and preliminary microbiological reports with therapeutic recommendation | ✓ | ✓ | |||||
| Automated dispensing system | ✓ | ✓ | |||||
| Automated microscopy testing | ✓ | ||||||
| Calculator (smartphone application) | ✓ | ||||||
| CDS (within CPOE/eMR, stand-alone, web-based) | ✓ | ✓ | ✓ | ✓ | |||
| Checklist in eMR | ✓ | ✓ | |||||
| Clinical dashboard | ✓ | ||||||
| Closed-loop order-processing system | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Computerized antimicrobial approval system | ✓ | ✓ | |||||
| Computerized system that links pharmacy, chemistry, microbiology and patient management data | ✓ | ✓ | |||||
| Data warehouse and monitoring system | ✓ | ✓ | |||||
| Dosing calculator in CPOE | ✓ | ||||||
| Electronic microbiology reporting | ✓ | ✓ | |||||
| Electronic overview linked to electronic health record | ✓ | ||||||
| Electronic screening tool to predict likelihood of developing C. difficile infection | ✓ | ||||||
| Guidelines (in eMR, web-based, on smartphone application) | ✓ | ✓ | |||||
| Mobile technology for pharmacists to verify medication orders | ✓ | ||||||
| Predictive models for treatment recommendations | ✓ | ✓ | |||||
| Stand-alone dose-prediction software | ✓ | ||||||
| Surveillance system | ✓ | ||||||
| Susceptibility results (smartphone application) | ✓ | ✓ | |||||
Medication management pathway cycle steps are not included in the table if they were not supported by digital interventions, as reported in papers.
What outcome measures are used to evaluate the effectiveness of digital interventions designed to improve antimicrobial prescribing and monitoring?
We identified a large number of outcome measures in the papers included in the eight reviews (Table 3). Most outcome measures could be categorized into antimicrobial use, antimicrobial appropriateness, efficiency, clinical, microbiological and economic measures; however, there was substantial variability in how these outcomes were defined and measured.
Table 3.
Outcome measures reported by studies in the eight systematic reviews
| Outcome measures | |
|---|---|
| Antimicrobial use | DDD per 100 or 1000 occupied bed-days; ICU bed-days; patient bed-days |
| Number of antimicrobials: per patient; per hospitalization; per department | |
| Number of antimicrobials: orders; requests; courses prescribed; drugs dispensed | |
| Number of: patients prescribed antimicrobial; patients receiving excessive dosages; regimen changes; interventions; sepsis interventions | |
| Number of doses per antimicrobial course | |
| Prescription rate | |
| Consumption of antimicrobials: oral; broad spectrum; restricted | |
| Antimicrobial-free days; days of excessive use; days on antimicrobial; days patient treated for infections | |
| Failure of antibiotic re-dosing | |
| Duration of exposure; duration of therapy | |
| Patients on IV >72 h; IV-to-oral switch; discharges on oral antimicrobials | |
| Change in third/fourth-generation cephalosporin use | |
| Appropriateness | Antimicrobial susceptibility mismatches; organism predication; adherence to preliminary microbiological reports; adherence to local resistance map recommendations |
| Adherence to: guidelines; recommendations; dosage targets | |
| Appropriate: dose; dosing intervals; empirical therapy; prescribing; initial levels ordered; antimicrobial coverage | |
| Monitoring-based appropriateness: initial trough concentrations; plasma concentrations within therapeutic range; appropriate TDM | |
| Proportion of: days with adherence to guidelines; appropriate courses; correct prescriptions; medication errors | |
| Antimicrobial escalation and de-escalation | |
| Antimicrobial prescribed to those with an allergy | |
| Discontinued: in appropriate time frame; within 48 h of surgery (prophylaxis) | |
| Errors; prescribing and omission error rate per order | |
| Number of orders with appropriate dosing intervals | |
| Pharmacy interventions; rejections from ID team | |
| Prophylaxis improvement | |
| Risk-appropriate antimicrobial | |
| Sensitivity, specificity, positive predictive value, negative predictive value | |
| Timely discontinuation of prophylaxis; % of surgeries where prophylaxis discontinued after surgery | |
| Usage in low-risk β-lactam allergy | |
| Efficiency | Delays in antimicrobial therapy |
| Time from culture to appropriate antimicrobial | |
| Time to: administration; dosing; prescription; prophylaxis | |
| Time savings; time spent by antimicrobial managing team; time spent managing antimicrobial utilization; time to make decision | |
| Clinical | Survival; 180 day survival rate |
| Mortality; 30 day all-cause mortality; hospital mortality; ICU mortality | |
| Length of stay; length of stay in ICU | |
| Hospital-acquired infections; hospital infection rate; patients at risk of C. difficile infection; rates of surgical site infections; detection of sepsis; ROC-predicated bloodstream infections | |
| Rates of ADEs; incidence of toxicity; rates of nephrotoxicity | |
| Fevers; severity of illness; patient complexity; response rate; catheter days | |
| Hospital readmission; 30 day readmission; transfers to ICU; ED visits within 72 h | |
| Patient disposition from ED | |
| Microbiological | Incidence of nosocomial infections with C. difficile, MRSA and MDR organisms |
| Antimicrobial resistance; drug-resistant pathogen emergence; resistance patterns; resistance rate | |
| Monthly urinalysis ordered; urine cultures ordered after urinalysis; quantity of radiological and microbiological diagnostics | |
| Economic | Case mix index |
| Cost of: antimicrobials; hospitalization; surveillance of ADEs; pharmacy | |
| Resource intensity weight of each hospitalization |
ADE, adverse drug event; ED, emergency department; ID, infectious disease; ROC, receiver operating characteristic; TDM, therapeutic drug monitoring.
In evaluating antimicrobial use, outcomes predominantly related to the quantity of antimicrobials prescribed or administered (e.g. number of antimicrobials ordered per patient32) or time (e.g. duration of therapy33,34). Outcomes related to appropriateness varied, with some classifying an antimicrobial as appropriate if the order was compliant with guidelines28,35–37 and others classifying an antimicrobial as appropriate if the microbe cultured from the patient was susceptible to the antimicrobial.38,39 There were also differences in what components of antimicrobial orders were assessed for appropriateness (e.g. appropriate dose,40 proportion of appropriate courses41).
Economic outcomes were assessed in all eight reviews but few studies in each review focused on these. The most common economic outcome evaluated was cost of antimicrobials.28,42,43 Similarly, some studies included in reviews reported outcome measures related to use and acceptance of digital interventions, such as proportion of accepted CDS system (CDSS) recommendations,44 or use of mobile apps,28 but these were reported to a lesser degree than the outcomes in Table 3.
How effective are digital interventions in improving antimicrobial prescribing and monitoring in hospitals?
The impact of digital interventions on antimicrobial use, appropriateness and clinical outcomes, as reported in the eight review papers, is presented in Table 4. Four of the included reviews18–20,26 conducted a meta-analysis and the findings from these analyses are presented in Table 4. The findings from the remaining papers were extracted from their results and conclusion sections. Seven of the eight reviews that assessed the impact of digital interventions on antimicrobial use found that implementation of an intervention was associated with a decrease in use. The majority of papers also found an increase in antimicrobial appropriateness following implementation of digital interventions. Impact on clinical outcomes was less clear. Two meta-analyses19,20 revealed that digital interventions resulted in a significant reduction in mortality and one also found a reduction in length of stay.20
Table 4.
Impact of digital interventions on antimicrobial use, appropriateness and clinical outcomes
| Author | Antimicrobial use | Appropriateness | Clinical outcomes |
|---|---|---|---|
| Baysari et al.26 | Decreased | Increased.a Appropriate use of antimicrobials: pooled RR 1.49; 95% CI 1.23–1.81; P < 0.0001. | No significant effect on mortalitya or length of stay.a Mortality: pooled RR 0.91; 95% CI 0.82–1.00; P = 0.06. Length of stay: pooled mean difference −0.84; 95% CI −2.43 to 0.76; P = 0.30. |
| Carracedo Martinez et al.18 | Decreaseda | Increased.a Accuracy of empirical treatment: random OR 1.93; 95% CI 1.21–3.08; P < 0.0711. Compliance with guidelines: random OR 4.22; 95% CI 1.89–9.42; P < 0.0001. | No significant effect on mortalitya or length of stay.a Mortality: random OR 0.98; 95% CI 0.84–1.14; P = 0.2321. Length of stay: SMD −0.24; 95% CI −0.49 to 0.02; P < 0.0001. |
| Cresswell et al.27 | Decreased | Increased | Inconsistent |
| Curtis et al.19 | Decreased | Increased.a Adequacy of antibiotic coverage: OR 2.11; 95% CI 1.67–2.66; P = 0.00001. | Reduction in mortality.a Mortality: OR 0.85, 95% CI 0.75–0.96; P = 0.01. |
| Helou et al.28 | Decreased | Inconsistent | NR |
| Laka et al.20 | Decreased | Increased.a Appropriateness of antimicrobial therapy: pooled OR 2.28; 95% CI 1.82–2.86; P = 0.000. | Reduction in mortality.a Mortality: pooled OR 0.82; 95% CI 0.73–0.91; P = 0.045. |
| Rawson et al.29 | NR | Inconsistent | Inconsistent |
| Rittman et al.30 | Decreased | Increased | Inconsistent |
NR, not reported; RR, risk ratio; SMD, standardized mean difference.
Results are based on meta-analysis.
Helou et al.,28 Baysari et al.26 and Laka et al.20 were the only reviews to examine one specific type of digital intervention or present results based on subgroups of digital interventions. Helou et al.28 only presented results specific to smartphone applications, but did not conduct a meta-analysis due to large variations in study designs and outcomes. Baysari et al.26 categorized digital interventions into stand-alone CDSSs, CDSSs embedded in CPOE, approval systems and surveillance systems. Baysari et al.26 did not conduct a subgroup analysis as part of their meta-analysis but in their description of findings they reported that antimicrobial use decreased following implementation of all these systems except surveillance systems, and appropriateness increased with stand-alone CDSSs and CDSSs embedded in CPOE. CDSSs embedded in CPOE and surveillance systems resulted in a decreased length of stay and surveillance systems also resulted in a decrease in adverse drug events. Laka et al.20 conducted a subgroup analysis of CDS types and found that all CDS types were associated with an increase in antimicrobial appropriateness.
All reviews examined the economic impact of the digital interventions. Four reviews20,27–29 found that digital interventions were associated with a reduction in cost of antimicrobials, with the remaining reviews reporting mixed or insignificant results. No reviews examined cost-effectiveness of digital interventions.
Discussion
In this review of systematic reviews, we have summarized and synthesized the evidence for digital interventions supporting AMS. Eight reviews that examined the effectiveness of digital interventions related to antimicrobial prescribing and monitoring in hospitals were identified, most of moderate quality. Information on the digital interventions and outcome measures was collated, highlighting the inconsistency across studies in what AMS component was evaluated, and in the approaches used for evaluation. Despite this variability, reviews were consistent in the conclusions drawn. Digital interventions appear to reduce antimicrobial use and improve antimicrobial appropriateness, with the majority of studies showing interventions had inconsistent findings or no significant impact on clinical outcomes.
There was a large degree of variability in the digital interventions examined, although there was a strong focus on CDS interventions. However, we identified no review or study that compared CDS interventions with one another. This result shows that there is limited information available to guide hospitals on intervention selection. Further research comparing specific digital interventions for AMS, including different CDS types, is needed to identify optimal choices for hospitals.
Outcome measures reported in papers also differed across studies but were predominantly related to antimicrobial use and appropriateness, consistent with previous reviews of AMS interventions in general.2,3 However, there was substantial variability in how these concepts were defined and measured. For example, antimicrobial consumption can be measured and expressed in various ways including duration of therapy, number of prescriptions, or number of antimicrobials dispensed, all of which were measures found in this review. This variation precluded us from conducting a meta-analysis and also makes comparison of antimicrobial consumption levels between sites or studies difficult. The WHO’s Collaborating Centre for Drug Statistics Methodology promotes the use of defined daily dose (DDD) as a drug consumption measure.45 IDSA and the Society for Healthcare Epidemiology of America advocate DDD per 1000 patient-days as an outcome measure for AMS programmes.46 However, there are some flaws to using this metric and a change in DDD is not solely suitable for drawing conclusions on an AMS intervention’s success.47 For example, DDD biases against combination therapy even if that therapy is more appropriate, and it underestimates exposure when doses are reduced for renal dysfunction.47,48 Days of therapy (DOT) or exposure days are often used and thought to be more clinically relevant to prescribers; however, they share the same limitation in biasing against combination therapy.48 Using a combination of metrics may be a more appropriate approach to measure success of an AMS intervention, as it can provide a more comprehensive evaluation.49 For example, a novel method proposed by Evans et al.50 uses multiple metrics combining clinical outcomes, safety outcomes and duration of therapy to provide an overall probability of a better outcome occurring for patients with the intervention.
In this review, a core question we set out to answer was how effective digital interventions are in improving antimicrobial prescribing and monitoring in hospitals. Unfortunately, the variability in interventions and outcome measures used in review papers prevented us from answering this. Our results indicated that digital interventions, on the whole, decrease antimicrobial use and increase antimicrobial appropriateness. Reviews that undertook meta-analyses showed that appropriateness increased by 1.9- to 4.2-fold, and one review showed that antimicrobial use declined by 70%. However, these ranges should be interpreted with caution, as variable measures were used.
The impact of digital tools on clinical outcomes is less clear. Clinical outcome measures such as mortality and length of stay are difficult to attribute directly to AMS interventions due to confounding variables such as infection control, resistance patterns, quality improvement initiatives and severity of illness.51 A modified Delphi study with an expert panel to select relevant outcome measures for AMS interventions in acute-care hospitals recommended that programmes focus on antimicrobial use and process measures.52 The reluctance to include clinical outcomes was reportedly due to the difficulty in detecting changes in clinical outcomes and attributing them to the AMS intervention.52 The Delphi study also highlighted that there are no clear standardized definitions for AMS-specific clinical outcomes such as infection-related mortality or readmission related to infectious diagnoses, a finding which also emerged from our review. It has been recommended that clinical measures be used to demonstrate that interventions do not cause harm rather than to evaluate effectiveness.52 When interventions result in a reduction in antimicrobial use, it is important to assess clinical outcomes such as mortality to ensure the intervention does not increase health risks.20,48 From the reviews in our synthesis that conducted a meta-analysis, two found a reduction in mortality19,20 and two found no significant effect on mortality or length of stay.18,26 Although a limited number of reviews, these findings are reassuring as they suggest that digital interventions did not cause harm.
It is interesting to note that we found limited assessment and reporting of sociotechnical aspects associated with implementation of AMS digital interventions. A small number of reviews briefly examined use and acceptance of digital interventions, but these were in the minority. When evaluating digital interventions, clinical outcomes are viewed to be just one aspect of effectiveness, with additional aspects recommended to be examined, such as organizational, human and social, ethical and legal, technological, clinical, cost and economical.53 Our review identified a clear evidence gap in understanding and evaluating these other components of AMS digital intervention effectiveness.
This review was limited by the lack of high-quality reviews that met our inclusion criteria, and by the quality of studies included in the eight reviews. Our results and conclusions are significantly limited by the quality of information contained in systematic reviews. Similarly, a Cochrane review of interventions to improve antibiotic prescribing found substantial design heterogeneity and all non-randomized trials and controlled before–after trials had a high risk of bias.3 This may be due to the complexity of evaluating AMS interventions as they are often implemented in ‘real-world’ settings and are consequently prone to bias, confounding and random time effects.54 More high-quality studies are needed to strengthen the evidence base on the impact of digital interventions in AMS programmes on prescribing and monitoring of antimicrobials, and in evaluating sociotechnical aspects of digital intervention implementation.
To conclude, the results of this review indicate that digital interventions reduce antimicrobial use and improve antimicrobial appropriateness in hospitals, but no firm conclusions can be drawn about the degree to which different types of digital interventions achieve these outcomes. The impact of digital interventions on clinical outcomes is less clear and high-quality evaluation, including the use of recommended outcome measures,52 is needed to assess the impact on patients and organizations. Evaluation of sociotechnical aspects of digital intervention implementation is limited, despite the critical role that user acceptance, uptake and feasibility play in ensuring improvements in AMS are achieved with digital health.
Supplementary Material
Contributor Information
Bethany A Van Dort, The University of Sydney, Biomedical Informatics and Digital Health, School of Medical Sciences, Charles Perkins Centre, Faculty of Medicine and Health, Sydney, New South Wales, Australia.
Jonathan Penm, The University of Sydney, School of Pharmacy, Faculty of Medicine and Health, Sydney, New South Wales, Australia.
Angus Ritchie, Health Informatics Unit, Sydney Local Health District, Camperdown, Australia; The University of Sydney, Faculty of Medicine and Health, Concord Clinical School, Sydney, New South Wales, Australia.
Melissa T Baysari, The University of Sydney, Biomedical Informatics and Digital Health, School of Medical Sciences, Charles Perkins Centre, Faculty of Medicine and Health, Sydney, New South Wales, Australia.
Funding
This work was supported by the Australian Government Research Training Program (RTP) Scholarship to B.A.V.D.
Transparency declarations
None to declare.
Author contributions
All authors were involved in the study conception. B.A.V.D. and M.T.B. reviewed search results and completed data extraction. B.A.V.D. drafted the manuscript and M.T.B., J.P. and A.R. critically revised it. All authors have given final approval of the published version and agree to be accountable for all aspects of the work.
Supplementary data
Tables S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. Australian Commission on Safety and Quality in Health Care . Antimicrobial Stewardship in Australian Health Care. 2018. https://www.safetyandquality.gov.au/our-work/antimicrobial-stewardship/antimicrobial-stewardship-australian-health-care-ams-book.
- 2. Wagner B, Filice GA, Drekonja Det al. . Antimicrobial stewardship programs in inpatient hospital settings: a systematic review. Infect Control Hosp Epidemiol 2014; 35: 1209–28. [DOI] [PubMed] [Google Scholar]
- 3. Davey P, Brown E, Scott CLet al. . Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2017; CD003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akpan MR, Ahmad R, Shebl NAet al. . A review of quality measures for assessing the impact of antimicrobial stewardship programs in hospitals. Antibiotics 2016; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karanika S, Paudel S, Grigoras Cet al. . Systematic review and meta-analysis of clinical and economic outcomes from the implementation of hospital-based antimicrobial stewardship programs. Antimicrob Agents Chemother 2016; 60: 4840–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baur D, Gladstone BP, Burkert Fet al. . Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17: 990–1001. [DOI] [PubMed] [Google Scholar]
- 7. Barlam TF, Cosgrove SE, Abbo LMet al. . Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62: e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Australian Commission on Safety and Quality in Health Care . AURA 2019: third Australian report on antimicrobial use and resistance in human health. 2019. https://www.safetyandquality.gov.au/our-work/antimicrobial-resistance/antimicrobial-use-and-resistance-australia-surveillance-system/aura-2019.
- 9. WHO . Antimicrobial stewardship programmes in health-care facilities in low-and middle-income countries: a WHO practical toolkit. https://www.who.int/publications/i/item/9789241515481. [DOI] [PMC free article] [PubMed]
- 10. Patel SJ, Saiman L. Principles and strategies of antimicrobial stewardship in the neonatal intensive care unit. Semin Perinatol 2012; 36: 431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kapadia SN, Abramson EL, Carter EJet al. . The expanding role of antimicrobial stewardship programs in hospitals in the United States: lessons learned from a multisite qualitative study. Jt Comm J Qual Patient Saf 2018; 44: 68–74. [DOI] [PubMed] [Google Scholar]
- 12. Tamma PD, Avdic E, Keenan JFet al. . What is the more effective antibiotic stewardship intervention: preprescription authorization or postprescription review with feedback? Clin Infect Dis 2017; 64: 537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghamrawi RJ, Kantorovich A, Bauer SRet al. . Evaluation of antimicrobial stewardship-related alerts using a clinical decision support system. Hosp Pharm 2017; 52: 679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Sijs H, van Gelder T, Vulto Aet al. . Understanding handling of drug safety alerts: a simulation study. Int J Med Inf 2010; 79: 361–9. [DOI] [PubMed] [Google Scholar]
- 15. Cowman K, Chen V, Guo Yet al. . Using technology to enhance antimicrobial stewardship impact in the acute care setting. Curr Treat Options Infect Dis 2020; 12: 145–57. [Google Scholar]
- 16. Bosso JA, Drew RH. Application of antimicrobial stewardship to optimise management of community acquired pneumonia. Int J Clin Pract 2011; 65: 775–83. [DOI] [PubMed] [Google Scholar]
- 17. Honda H, Ohmagari N, Tokuda Yet al. . Antimicrobial stewardship in inpatient settings in the Asia Pacific region: a systematic review and meta-analysis. Clin Infect Dis 2017; 64: S119–26. [DOI] [PubMed] [Google Scholar]
- 18. Carracedo-Martinez E, Gonzalez-Gonzalez C, Teixeira-Rodrigues Aet al. . Computerized clinical decision support systems and antibiotic prescribing: a systematic review and meta-analysis. Clin Ther 2019; 41: 552–81. [DOI] [PubMed] [Google Scholar]
- 19. Curtis CE, Al Bahar F, Marriott JF. The effectiveness of computerised decision support on antibiotic use in hospitals: a systematic review. PLoS One 2017; 12: e0183062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laka M, Milazzo A, Merlin T. Can evidence-based decision support tools transform antibiotic management? A systematic review and meta-analyses. J Antimicrob Chemother 2020; 75: 1099–111. [DOI] [PubMed] [Google Scholar]
- 21. Bowes J, Yasseen AS III, Barrowman Net al. . Antimicrobial stewardship in pediatrics: focusing on the challenges clinicians face. BMC Pediatr 2014; 14: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ammenwerth E. Evidence-based health informatics: how do we know what we know? Methods Inf Med 2015; 54: 298–307. [DOI] [PubMed] [Google Scholar]
- 23. Shea BJ, Reeves BC, Wells Get al. . AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017; 358: j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lorenz RC, Matthias K, Pieper Det al. . A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol 2019; 114: 133–40. [DOI] [PubMed] [Google Scholar]
- 25. Storman M, Storman D, Jasinska KWet al. . The quality of systematic reviews/meta-analyses published in the field of bariatrics: a cross-sectional systematic survey using AMSTAR 2 and ROBIS. Obesity Reviews 2020; 21: e12994. [DOI] [PubMed] [Google Scholar]
- 26. Baysari MT, Lehnbom EC, Li Let al. . The effectiveness of information technology to improve antimicrobial prescribing in hospitals: a systematic review and meta-analysis. Int J Med Inform 2016; 92: 15–34. [DOI] [PubMed] [Google Scholar]
- 27. Cresswell K, Mozaffar H, Shah Set al. . Approaches to promoting the appropriate use of antibiotics through hospital electronic prescribing systems: a scoping review. Int J Pharm Pract 2017; 25: 5–17. [DOI] [PubMed] [Google Scholar]
- 28. Helou RI, Foudraine DE, Catho Get al. . Use of stewardship smartphone applications by physicians and prescribing of antimicrobials in hospitals: a systematic review. PLoS One 2020; 15: e0239751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rawson TM, Moore LSP, Hernandez Bet al. . A systematic review of clinical decision support systems for antimicrobial management: are we failing to investigate these interventions appropriately? Clin Microbiol Infect 2017; 23: 524–32. [DOI] [PubMed] [Google Scholar]
- 30. Rittmann B, Stevens MP. Clinical decision support systems and their role in antibiotic stewardship: a systematic review. Curr Infect Dis Rep 2019; 21: 29. [DOI] [PubMed] [Google Scholar]
- 31. Stowasser DA, Allinson YM, O’Leary M. Understanding the medicines management pathway. J Pharm Pract Res 2004; 34: 293–6. [Google Scholar]
- 32. Tafelski S, Nachtigall I, Deja Met al. . Computer-assisted decision support for changing practice in severe sepsis and septic shock. J Int Med Res 2010; 38: 1605–16. [DOI] [PubMed] [Google Scholar]
- 33. Devabhakthuni S, Gonzales JP, Tata ALet al. . Evaluation of vancomycin dosing and monitoring in adult medicine patients. Hosp Pharm 2012; 47: 451–9. [Google Scholar]
- 34. Shojania KG, Yokoe D, Platt Ret al. . Reducing vancomycin use utilizing a computer guideline: results of a randomized controlled trial. J Am Med Inform Assoc 1998; 5: 554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Demonchy E, Dufour J-C, Gaudart Jet al. . Impact of a computerized decision support system on compliance with guidelines on antibiotics prescribed for urinary tract infections in emergency departments: a multicentre prospective before-and-after controlled interventional study. J Antimicrob Chemother 2014; 69: 2857–63. [DOI] [PubMed] [Google Scholar]
- 36. Fernández Urrusuno R, Flores Dorado M, Vilches Arenas Aet al. . Improving the appropriateness of antimicrobial use in primary care after implementation of a local antimicrobial guide in both levels of care. Eur J Clin Pharmacol 2014; 70: 1011–20. [DOI] [PubMed] [Google Scholar]
- 37. Rodrigues JF, Casado A, Palos Cet al. . A computer-assisted prescription system to improve antibacterial surgical prophylaxis. Infect Control Hosp Epidemiol 2012; 33: 435–7. [DOI] [PubMed] [Google Scholar]
- 38. Carman MJ, Phipps J, Raley Jet al. . Use of a clinical decision support tool to improve guideline adherence for the treatment of methicillin-resistant Staphylococcus aureus: skin and soft tissue infections. Adv Emerg Nurs J 2011; 33: 252–66. [DOI] [PubMed] [Google Scholar]
- 39. Karsies TJ, Sargel CL, Marquardt DJet al. . An empiric antibiotic protocol using risk stratification improves antibiotic selection and timing in critically ill children. Ann Am Thorac Soc 2014; 11: 1569–75. [DOI] [PubMed] [Google Scholar]
- 40. Cox ZL, Nelsen CL, Waitman LRet al. . Effects of clinical decision support on initial dosing and monitoring of tobramycin and amikacin. Am J Health Syst Pharm 2011; 68: 624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Filice GA, Drekonja DM, Thurn JRet al. . Use of a computer decision support system and antimicrobial therapy appropriateness. Infect Control Hosp Epidemiol 2013; 34: 558–65. [DOI] [PubMed] [Google Scholar]
- 42. Evans RS, Pestotnik SL, Burke JPet al. . Reducing the duration of prophylactic antibiotic use through computer monitoring of surgical patients. Ann Pharmacother 1990; 24: 351–54. [DOI] [PubMed] [Google Scholar]
- 43. McGregor JC, Weekes E, Forrest GNet al. . Impact of a computerized clinical decision support system on reducing inappropriate antimicrobial use: a randomized controlled trial. J Am Med Inform Assoc 2006; 13: 378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schulz L, Osterby K, Fox B. The use of best practice alerts with the development of an antimicrobial stewardship navigator to promote antibiotic de-escalation in the electronic medical record. Infect Control Hosp Epidemiol 2013; 34: 1259–65. [DOI] [PubMed] [Google Scholar]
- 45. WHO . WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC classification and DDD assignment. 2021. https://www.whocc.no/atc_ddd_index_and_guidelines/guidelines/.
- 46. Dellit TH, Owens RC, McGowan JEet al. . Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44: 159–77. [DOI] [PubMed] [Google Scholar]
- 47. Dik J-WH, Hendrix R, Poelman Ret al. . Measuring the impact of antimicrobial stewardship programs. Expert Rev Anti Infect Ther 2016; 14: 569–75. [DOI] [PubMed] [Google Scholar]
- 48. Morris AM. Antimicrobial stewardship programs: appropriate measures and metrics to study their impact. Curr Treat Options Infect Dis 2014; 6: 101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morris AM, Brener S, Dresser Let al. . Use of a structured panel process to define quality metrics for antimicrobial stewardship programs. Infect Control Hosp Epidemiol 2012; 33: 500–6. [DOI] [PubMed] [Google Scholar]
- 50. Evans SR, Rubin D, Follmann Det al. . Desirability of Outcome Ranking (DOOR) and Response Adjusted for Duration of Antibiotic Risk (RADAR). Clin Infect Dis 2015; 61: 800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Emberger J, Tassone D, Stevens MPet al. . The current state of antimicrobial stewardship: challenges, successes, and future directions. Curr Infect Dis Rep 2018; 20: 31. [DOI] [PubMed] [Google Scholar]
- 52. Moehring RW, Anderson DJ, Cochran RLet al. . Expert consensus on metrics to assess the impact of patient-level antimicrobial stewardship interventions in acute-care settings. Clin Infect Dis 2017; 64: 377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Enam A, Torres-Bonilla J, Eriksson H. Evidence-based evaluation of eHealth interventions: systematic literature review. J Med Internet Res 2018; 20: e10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Kraker MEA, Abbas M, Huttner Bet al. . Good epidemiological practice: a narrative review of appropriate scientific methods to evaluate the impact of antimicrobial stewardship interventions. Clin Microbiol Infect 2017; 23: 819–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.