Abstract
The cardiotoxicity induced by arsenic trioxide (ATO) limits its clinical application in acute promyelocytic leukemia treatment. Sacubitril/valsartan (LCZ696) is an effective drug for the treatment of heart failure. In this study, we aimed to investigate the protective effect and mechanisms of LCZ696 against the ATO-induced cardiotoxicity in mice and H9c2 cells. We found that LCZ696 could alleviate the decrease of ejection fraction and fractional shortening induced by ATO, thereby improving mouse cardiac contractile function. LCZ696 could also reduce the myocardial enzyme, resist oxidative stress, mitigate myocardial fibrosis, and ameliorate myocardial structure, thereby alleviating myocardial damage caused by ATO. In addition, LCZ696 could significantly increase the cell viability and reduce the accumulation of reactive oxygen species in ATO-treated H9c2 cells. Besides, in vivo and in vitro studies have been found that LCZ696 could restore the expression of Bcl-2 and reduce Bax and Caspase-3 levels, inhibiting ATO-induced apoptosis. Meanwhile, LCZ696 decreased the levels of IL-1, IL-6, and TNF-α, alleviating the inflammatory injury caused by ATO. Furthermore, LCZ696 prevented NF-κB upregulation induced by ATO. Our findings revealed that LCZ696 has a considerable effect on preventing cardiotoxicity induced by ATO, which attributes to its capability to suppress oxidative stress, inflammation, and apoptosis.
Keywords: LCZ696, arsenic trioxide, cardiotoxicity, oxidative stress, inflammation, apoptosis
1. Introduction
Arsenic trioxide (ATO) has been proven to be an effective drug in acute promyelocytic leukemia (APL) treatment.1 However, it was found that some APL patients were hampered by adverse reactions of multiple organ systems in the process of clinical application of ATO.2 Cardiotoxicity was one of the most dangerous adverse that could cause treatment interruption and even sudden death. About 30% of APL patients treated with ATO might experience cardiac toxicity.3 Cardiac adverse greatly limited the clinical application of ATO. Studies showed that inflammation, oxidative stress, and apoptosis were related to ATO-induced cardiotoxicity.4,5 Such effects could interfere with the autonomic regulation of the heart and increase the risk of cardiovascular diseases.6 ATO produces reactive oxygen species (ROS) by interfering with mitochondrial function, and then causes oxidative damage to mitochondria and inflammatory reaction. Excessive production of inflammatory factors such as interleukin (IL) and tumor necrosis factor-α (TNF-α) tended to aggravate heart injury.7 After that, mitochondria released pro-apoptotic proteins into the cytoplasm, leading to cardiomyocyte apoptosis and damage to heart function.8 Therefore, an anti-oxidation and anti-inflammation substance may be effective for eliminating ATO-induced cardiotoxicity.
Sacubitril/valsartan (LCZ696) was consisted of the neutral lysozyme inhibitor sacubitril and angiotensin receptor blocker valsartan.9 LCZ696 has significant clinical efficacy as an anti-heart failure drug.10 In addition, LCZ696 could reduce myocardial oxidative stress and apoptosis in heart failure mouse model through regulating the Sirt3/MnSOD pathway.11 At the same time, it could also restrict nuclear factor kappa-B (NF-κB) signaling and NLRP3 inflammasome to prevent cardiac fibrosis and dysfunction.12 Besides, LCZ696 has a protective effect on both drug-induced cardiotoxicity and heart failure animal models caused by surgery. In a mouse model of dilated cardiomyopathy induced by doxorubicin, LCZ696 could partially maintain mitochondrial function through Drp1-mediated pathways, thereby improving myocardial function and reducing apoptosis.13 Thus, LCZ696 may have anti-oxidation and anti-inflammation activity to ameliorate cardiac injury. However, whether LCZ696 could prevent and treat cardiotoxicity caused by ATO remains unknown.
Therefore, this study aims to demonstrate the protective effect of LCZ696 on ATO-induced myocardial injury and to explore the mechanism through in vitro and in vivo experiments.
2. Materials and methods
2.1. Materials and animal treatment
LCZ696 was obtained from Novartis (Beijing, China). ATO was provided by Harbin YI-DA Pharmaceutical Ltd (Harbin, China). Adult male Kunming mice (8-weeks old, weighing 22 ± 2 g) were purchased from the Experimental Animal Center of Harbin Medical University (Harbin, China). The animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). The experiment was approved by the Animal Ethics Committee of The First Affiliated Hospital of Harbin Medical University. The mice were randomly divided into 4 groups (n = 10 for each group) as follows:
(a) Normal control (NC) group: Mice in this group were administered 10 mL/kg/day saline by oral gavage 1 h before 10 mL/kg/day saline by intraperitoneal injection.
(b) LCZ696 group: Mice in this group were administered 68 mg/kg/day LCZ696 by oral gavage11–13 1 h before 10 mL/kg/day saline by intraperitoneal injection.
(c) ATO group: Mice in this group were administered 10 mL/kg/day saline by oral gavage 1 h before 2 mg/kg/day ATO by intraperitoneal injection.
(d) LCZ696 + ATO group: Mice in this group were administered 68 mg/kg/day LCZ696 by oral gavage 1 h before 2 mg/kg/day ATO by intraperitoneal injection.
Cardiac function of mice was analyzed by echocardiography after 4 weeks of administration.
2.2. Echocardiographic measurements
Mice were analyzed cardiac function by echocardiography after 4 weeks of administration. 2% isoflurane was used to anesthetize mice. M-mode ultrasound images were detected and analyzed by micro-ultrasound system (General Electric Company, USA). Detection indicators include ejection fraction (EF), fractional shortening (FS), left ventricular internal diameter in diastole (LVIDd), and left ventricular internal diameter in systole (LVIDs).
2.3. Biochemical analysis
Creatine kinase (CK), lactate dehydrogenase (LDH), and aspartate aminotransferase (AST) activities in serum were tested by an automatic biochemistry analyzer (Audit Diagnostics Inc., Ireland).
2.4. Analysis of oxidative stress in hearts
The superoxide dismutase (SOD) activity, glutathione (GSH), and malondialdehyde (MDA) content in the hearts were determined with corresponding kits (Jiancheng Bioengineering Institute, Nanjing, China), respectively.14
2.5. Heart histopathological measurements
Heart tissue was harvested after the detection of echocardiography. The heart was fixed with 4% paraformaldehyde, then, embedded in paraffin blocks. Heart samples was sliced. Paraffin slices were stained with hematoxylin and eosin (H&E) and Masson trichrome. The slices were then examined by a light microscope (Zeiss, Jena, Germany). The images were captured at 200 times magnification and measured with the Image J software (Image J v1.51, USA).
2.6. Cell culture
H9c2 cells were purchased from Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM supplemented with 10% FBS. Cells were grown at 37 °C in a humidified atmosphere with 5% CO2. The cells were treated as follows: (a) NC group: Cells in this group were treated with culture medium; (b) LCZ696 group: Cells in this group were treated with 20 μM LCZ696 for 24 h; (c) ATO group: Cells in this group were treated with 5 μM ATO for 24 h; and (d) ATO + LCZ696 group. Cells in this group were treated with 20 μM LCZ696 and 5 μM ATO for 24 h.
2.7. Cell-viability assay
The Cell Counting Kit-8 (CCK-8, Beyotime Biotechnology) was used to evaluate cell viability. According to the manufacturer’s instructions, cells were seeded in 96-well plates at an average of 5,000 cells per well and cultured for 24 h. Cells were incubated with 10 μL CCK-8 solution for 1 h after drug treatment. Enzyme-labeled instrument (BioTek, Vermont, USA) was used to measure absorbance at 450 nm.
2.8. ROS detection by fluorescence microscopy
Dihydroethidium (DHE) fluorescent probe (Beyotime Biotechnology) was used to detect intracellular ROS. H9c2 cells were seeded in 24-well plates and cultured for 24 h. Cells were washed twice in PBS after drug treatment. Adherent cells were harvested and incubated with 10 μM DHE at 37 °C for 30 min. Images were captured at 200 times magnification by a light microscope (Zeiss, Jena, Germany) and measured with the Image J software (Image J v1.51, USA).
2.9. Western blot analysis
Mice heart tissue samples and H9c2 cells were lysed in RIPA lysis buffer with protease inhibitor. BCA Protein Assay Kit was used to determine protein concentration. Protein was loaded onto 12% sodium dodecyl sulfate polyacrylamide gels and then transferred to nitrocellulose membranes. The membranes were blocked with 5% skim milk for 1 h and then incubated with primary antibodies including: Caspase-3 (1:1000; Affinity Biosciences); Bcl-2 (1:1000; Affinity Biosciences); Bax (1:1000; Affinity Biosciences); β-actin (1:1000; Affinity Biosciences). The membranes were incubated with secondary antibodies (Invitrogen) for 1 h. Densitometric analysis was performed using Gel imaging system (Bio-Rad, Hercules, California, USA). Results were reported as fold changes normalized to NC group values.
2.10. Quantitative real-time PCR
Total RNA was harvested from mice heart tissue samples and H9c2 cells by TRIzol reagent (Invitrogen, CA, USA). The cDNAs were produced from the total RNA with Reverse Transcription Kit (Toyobo, Japan). Gene expressions were normalized to β-actin level. Real-time PCR was done on the ABI Prism 7500 Sequence Detection System (Applied Biosystems, USA) using SYBR Green Master kit (Roche, Germany). Relative mRNA expression was determined using the Ct method. The following primers sequence:
mouse/rat β-actin: “forward 5’-CCCTAAGGCCAACCGTGAAAAG-3’ and reverse 5’-ACCCTCATAGATGGGCACAGT-3’;”
mouse IL-1: “forward 5’-GAAATGCCACCTTTTGACAGTG-3’ and reverse 5’-TGGATGCTCTCATCAGGACAG-3’;”
mouse/rat IL-6: “forward 5’-TCTATACCACTTCACAAGTCGGA-3’ and reverse 5’-GAATTGCCATTGCACAACTCTTT-3’;”
mouse TNF-α: “forward 5’-CAGGCGGTGCCTATGTCTC-3’ and reverse 5’-CGATCACCCCGAAGTTCAGTAG-3’;”
mouse/rat NF-κB: “forward 5’- AGAGGGGATTTCGATTCCGC-3’ and reverse 5’- CCTGTGGGTAGGATTTCTTGTTC-3’;”
rat IL-1: “forward 5’-TCCCTGAACTCAACTGTGAAATA-3’ and reverse 5’-GGCTTGGAAGCAATCCTTAATC-3’;”
rat TNF-α: “forward 5’-ACCTTATCTACTCCCAGGTTCT-3’ and reverse 5’-GGCTGACTTTCTCCTGGTATG-3’.”
2.11. Statistical analysis
Data were presented as mean ± standard error of the mean (SEM) from at least 3 independent experiments. Comparisons between different groups were performed using one-way ANOVA followed by Tukey’s test with GraphPad Prism (GraphPad Prism software, USA), and graphical presentation of data was also obtained from GraphPad Prism. A P-value less than 0.05 was considered statistically significant.
3. Results
3.1. LCZ696 improved left ventricular dysfunction induced by ATO
To determine the effect of LCZ696 on left ventricular dysfunction, mice were administered with ATO and LCZ696. The cardiac function of mice was analyzed by echocardiography after 4 weeks. As shown in Figure 1A, echocardiogram images of each group were taken to examine the contractile function. After ATO treatment, the EF and FS of the mice were exacerbated compared with the NC group (P < 0.05). At the same time, LCZ696 substantially alleviated the decrease of EF and FS (P < 0.05), suggesting that LCZ696 could improve the cardiac contractile dysfunction induced by ATO (Fig. 1B).
Fig. 1.
LCZ696 ameliorated left ventricular functions after ATO treatment. (A) Representative echocardiogram images of each group; (B) echocardiography indices obtained from the image, expressed as the mean ± SEM, n = 10 (*P < 0.05 vs. NC group; #P < 0.05 vs. ATO group).
3.2. LCZ696 decreases the level of cardiac injury markers in the serum
As shown in Figure 2A, the activities of CK, LDH, and AST in the ATO group were increased significantly compared with the NC group (P < 0.05). However, the activity of CK, LDH, and AST in the ATO + LCZ696 group was significantly lower than that in the ATO group (P < 0.05).
Fig. 2.
Effect of LCZ696 on the heart function indicators and oxidative homeostasis. (A) CK, LDH, and AST activities in plasma were determined. (B) SOD activity, MDA, and GSH content in cardiac tissue were determined. Values were presented as the mean ± SEM, n = 5 (*P < 0.05, **P < 0.01 vs. the NC group; #P < 0.05 vs. the ATO group).
3.3. LCZ696 mitigates lipid peroxidation in the cardiac tissue
As shown in Figure 2B, cardiac MDA concentration was markedly increased in the cardiac tissue of ATO group compared to NC group (P < 0.05), whereas, a significant decrease in GSH and SOD were observed in the ATO group (P < 0.05). Compared with mice treated with ATO, LCZ696 significantly decreased the concentration of MDA and increased the concentration of GSH and SOD (P < 0.05).
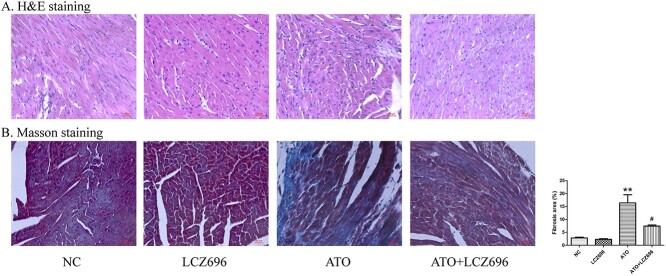
3.4. LCZ696 relieved ATO-induced cardiac histopathology changes
H&E staining was used for histological analysis. As shown in Figure 3A, the hearts after ATO treatment showed cell structural abnormalities and inflammatory cells infiltration. These pathological changes were significantly improved by LCZ696 treatment. Masson trichrome staining was used to assess cardiac fibrosis. Figure 3B showed that the fibrotic regions of mice hearts were statistically increased in the ATO group (P < 0.01), which were alleviated by LCZ696 treatment (P < 0.05).
Fig. 3.
LCZ696 alleviated ATO-induced myocardial injury and fibrosis in mice hearts. (A) Representative H&E staining micrographs of heart tissues. (B) Masson staining of 4 groups. Collagen area (%) of heart tissues were presented as fibrosis, n = 5. The values were presented as the mean ± SEM (The scale bar represents 50 μm. **P < 0.01, vs. the NC group; #P < 0.05 vs. the ATO group).
3.5. LCZ696 protects against ATO-induced apoptosis and inflammation in vivo
To elaborate whether the cardioprotective effect of LCZ696 is mediated by apoptosis and inflammation, the expression of anti-apoptosis protein Bcl-2 and pro-apoptosis protein Bax and Caspase-3 was detected through Western-blot analysis, as well as the mRNA levels of IL-1, IL-6, TNF-α, and NF-κB were detected through quantitative real-time PCR. As shown in Figure 4, ATO could reduce Bcl-2 expression and increase Bax and Caspase-3 expression in mice heart tissue samples. However, the expression of Bcl-2 was up-regulated, Bax and Caspase-3 levels were down-regulated after LCZ696 treatment (P < 0.05) compared with the ATO group. The mRNA levels of inflammatory factors IL-1, IL-6, and TNF-α were increased in the ATO group compared to the NC group (P < 0.01). In contrast, these inflammatory factors in the ATO + LCZ696 group were significantly lower than those in ATO group (P < 0.05). Importantly, the NF-κB signaling pathway had the effect on regulating inflammation and apoptosis. The results showed that LCZ696 could restore the increase of NF-κB levels induced by ATO in vivo (P < 0.01).
Fig. 4.
Effects of LCZ696 on apoptosis and inflammation in mice heart tissue. (A) Caspase-3, Bcl-2, and Bax levels in mice heart tissue were detected by Western blot, n = 3. (B) IL-1, IL-6, TNF-α, and NF-κB mRNA levels in mice heart tissue were determined by quantitative real-time PCR, n = 3.The relative expression was analyzed by normalization to β-actin. Results are reported as fold changes normalized to the NC group values. Data were presented as the mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001 vs. the NC group; #P < 0.05, ##P < 0.01 vs. the ATO group).
3.6. LCZ696 alleviated the decrease in cell viability caused by ATO
First, the effects of ATO on the viability of H9c2 cells was tested (Fig. 5A). Cell viability decreased with the increase of ATO concentration. The cell viability treated by 5 μM ATO decreased significantly (P < 0.001). However, the cell overly deteriorated when treated by 10 μM ATO. Therefore, 5 μM ATO was selected for subsequent experiments. Then, the effect of different concentration LCZ696 on restoring ATO-induced decrease in cell viability was verified (Fig. 5B). We found LCZ696 at 20 μM could significantly alleviate the decline in cell viability induced by ATO (P < 0.05). However, cell viability was not increase significantly at higher concentrations of LCZ696. Therefore, 20 μM LCZ696 were selected for subsequent in vitro experiments. Besides, we confirmed that 20 μM LCZ696 could restore the decline in cell viability induced by 5 μM ATO without causing injury to the H9c2 cells (Fig. 5C).
Fig. 5.
LCZ696 restored the decline in cell viability induced by ATO. (A) ATO (0–10 μM) decreased H9c2 cell viability. (B) LCZ696 (0–40 μM) alleviated the reduced viability of H9c2 cells induced by 5 μM ATO. (C) The effect of 20 μM LCZ696 and 5 μM ATO on the viability of H9c2 cells. Relative cell viability was determined by the CCK-8 assay. Cells were treated with LCZ696 and ATO for 24 h. Data were expressed as mean ± SEM, n = 5 (*P < 0.05, ***P < 0.001 vs. the NC group; #P < 0.05 vs. the ATO group).
3.7. LCZ696 reduced the accumulation of intracellular ROS induced by ATO
Fluorescence staining was used to assess the accumulation of ROS in H9c2 cells. The intensity of the fluorescence image indicated the content of ROS. As shown in Figure 6A, the level of intracellular ROS in the ATO group was dramatically increased compared with the NC group (P < 0.001). However, this increase was suppressed by LCZ696 (P < 0.001). In addition, LCZ696 had no effect on intracellular ROS without ATO treatment.
Fig. 6.
Effects of LCZ696 on oxidative stress, apoptosis and inflammation in H9c2 cells. (A) Fluorescence images and the ratio graph of ROS in different treatment groups. The scale bar represents 50 μm, n = 5. (B) Caspase-3, Bcl-2, and Bax levels in H9c2 cells were detected by Western blot, n = 3. (C) IL-1, IL-6, TNF-α, and NF-κB mRNA levels in H9c2 cells were determined by quantitative real-time PCR, n = 3. The relative expression was analyzed by normalization to β-actin. Results are reported as fold changes normalized to the NC group values. Data were presented as the mean ± SEM (**P < 0.01, ***P < 0.001 vs. the NC group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. the ATO group).
3.8. In vitro cardioprotective role of LCZ696 against ATO-induced apoptosis and inflammation
To further elaborate the cardioprotective effect of LCZ696, the role of LCZ696 on apoptosis and inflammation was tested in H9c2 cells. As shown in Figure 6B, LCZ696 could restore the decrease of Bcl-2 expression and the increase of Caspase-3 and Bax levels induced by ATO (P < 0.05). Besides, Figure 6C showed the mRNA levels of IL-1, IL-6, and TNF-α were reduced by LCZ696 (P < 0.01) compared with the ATO group. Furthermore, LCZ696 could restore the increase of NF-κB levels caused by ATO (P < 0.01). These findings illustrated that LCZ696 could protect against ATO-induced cardiomyocyte injury by inhibiting apoptosis and reducing the levels of inflammatory factors, which may be related to the NF-κB signaling pathway.
4. Discussion
Since the last century, ATO has been widely studied and applied because of its efficacy in the treatment of APL.15 However, many APL patients suffered serious risks during treatment due to the cardiotoxicity of ATO.5 LCZ696 is a novel medicine for treating heart failure and protecting heart function. Our research demonstrated that LCZ696 treatment could significantly prevent cardiotoxicity caused by ATO in mouse and H9c2 cell models. Its mechanism may attribute to suppressing oxidative stress, inflammatory factors, and apoptosis.
EF and FS were typically used as indicators to measure the systolic function of the heart.16 Previous studies found that intravenous injection of 2 mg/kg/day ATO for 2 consecutive weeks could induce a significant decrease in mice cardiac EF and FS.17 Proteomics analysis indicated that the proteins related to cardiac systolic and diastolic dysfunction in ATO-treated mice changed significantly.18 We have proved that LCZ696 could markedly alleviate the reduction of EF and FS caused by ATO in mouse models. However, our results showed LVIDd and LVIDs had no statistical significance. The change of ventricular internal diameter is a slow process, which requires long-term injury stimulation. It indicated that the processing time of our mouse model could be extended for more pronounced injury. The activities of LDH, CK, and AST are commonly used for clinical diagnosis of myocardial injury.7 In the present study, treatment with LCZ696 evidently decreased serum LDH, CK, and AST activities compared with ATO-treated group. These findings demonstrate LCZ696 has a protective effect on cardiac injury induced by ATO.
It is well established that myocardial cell necrosis and fibrosis were important manifestations of cardiac injury.19 H&E staining is one of the critical indicators for judging myocardial injury.20 In our study, H&E staining indicated abnormal changes in cell morphology and cardiomyocyte necrosis in the myocardial tissue of the ATO group. LCZ696 treatment could significantly improve this situation. As one of the cancer drugs with cardiotoxicity, ATO-induced cardiotoxicity was believed to be partly related to increased fibrosis.21 ATO up-regulates TGF-β and MMP in cardiac fibroblasts, and increases cardiac fibrosis in guinea pigs and humans.21,22 LCZ696 could inhibits the formation of TLR2-MyD88 complex and thus reduce doxorubicin-induced cardiac fibrosis.23 In addition, LCZ696 could reduce rat cardiac fibrosis caused by transverse aortic constriction,24 and reduce fibrosis biomarkers in patients with heart failure.25 Masson staining of heart tissue demonstrated that ATO could aggravate the degree of fibrosis of mice heart tissue compared with the NC group. This situation could be alleviated after the treatment of LCZ696.
Oxidative stress, inflammation, and apoptosis were crucial causes of ATO-induced heart damage.26 We confirmed that LCZ696 could restore the decline in cell viability induced by ATO without causing injury to the H9c2 cells. Mitochondria was subjected to oxidative damage and inflammatory damage by the ATO and then release pro-apoptotic proteins, which resulting in apoptosis.6 Cell mitochondrial apoptosis signaling pathway has been elaborated. Bcl-2 family could stabilize the mitochondrial transmembrane potential and regulate the apoptosis induction pathway.27 Apoptosis stimulator regulated the protein of Bcl-2 family, destroyed the mitochondrial membrane structure, and lead to the release of mitochondrial proteins.28 Eventually, Caspase-3 was activated, which could cleave substrates and cause cell death. It has been clarified that in the NB4 cell line, treatment with 1 μM ATO effectively down-regulated levels of the Bcl-2 gene, leading to specific degradation of the substrate.29 Studies found that Caspase-3 were up-regulated in early brain injury and gradually decreased after 24 h.30 Overexpression of Caspase-3 could be a symbol of early apoptosis. Our study confirmed that ATO could induce both the decrease of Bcl-2 expression and the increase of Bax and Caspase-3 expression in mouse and cells. Importantly, LCZ696 could play a relieving effect to the change of these proteins. That is, LCZ696 could protect ATO-induced apoptosis by regulating the mitochondrial apoptosis pathway.
As a classic apoptosis stimulator, ROS is a class of oxygen-containing substance that includes superoxide and hydroxyl radicals.31 The mechanism of ATO-induced apoptosis includes the enhancement of the ROS production system and the weakening of the ROS elimination system to induce intracellular ROS accumulation.32,33 Excessive ROS could lead to oxidative stress and lipid peroxidation, which induced production of MDA. As the major cellular antioxidant, the activity of SOD and GSH are recognized as key indicators to determine the degree of oxidative stress.7 Oxidative stress could initiate the inflammatory reaction, lipid peroxidation, cause DNA strand breaks and mutations, and ultimately lead to cell apoptosis.27,31 Our results showed that ATO treatment decreased SOD and GSH activities as well as increased MDA concentration in the heart. Immunofluorescence indicated that ATO could greatly increase the ROS in H9c2 cells. It is worth noting that these effects were attenuated by LCZ696 treatment.
It was reported that LCZ696 could regulate TLR4/NF-κB signaling pathways and inhibit inflammatory factors by down-regulating the level of ROS, thereby inhibiting inflammation and apoptosis.34,35 The NF-κB signaling pathway has been confirmed to play an important role in regulating inflammation and apoptosis.35 As a potent inflammatory mediator, NF-κB can be activated by oxidative stress. Under the stimulation of pro-inflammatory factors, NF-κB is activated and transferred to the nucleus, then accumulates and accelerates the production of pro-inflammatory factors.36 Our results showed that ATO-induced excessive oxidative stress caused the activation of NF-κB, which increased inflammatory factor. Importantly, increased IL-1, IL-6, TNF-α, and NF-κB levels induced by ATO were relieved by LCZ696. Thus, our results demonstrated that oxidative stress, inflammation, and apoptosis were involved in the protective effect of LCZ696 on cardiac damage induced by ATO.
5. Conclusion
In conclusion, our findings confirmed that LCZ696 has an obvious effect to prevent cardiotoxicity induced by ATO for the first time. The effects include improving myocardial contractility, reducing the expression of myocardial enzymes, restoring normal myocardial structure, and relieving cardiomyocyte damage. These protective effects may attribute to suppressing oxidative stress, inflammation, and apoptosis. Our data supported that LCZ696 has clinical potential to reduce cardiotoxicity caused by ATO.
Authors’ contribution
Zhiqiang Wu carried out experimental work, analyzed the data, and wrote the manuscript. Hongzhu Chen, Liwang Lin, and Jing Lu participated in the experimental investigation. Qilei Zhao and Zengxiang Dong contributed to the final preparation of this paper and submission. Xin Hai revised the manuscript, designed, and supervised this research.
Funding
This work was financially supported by grants from the National Natural Science Foundation of China (No. 81700151), Natural Science Foundation of Heilongjiang Province for Excellent Youths (No. YQ2019H016), Postdoctoral Foundation of Heilongjiang Province (No. LBH-Q20031), Heilongjiang Key R&D Program (No. GZ20210070) and Excellent Youth Foundation of First Affiliated Hospital of Harbin Medical University (No. HYD2020JQ0018).
Conflicts of interest
The authors declare they have no financial interests.
Ethics approval
The animal experimental procedures and protocols were conducted according to the Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978), and were approved by the Animal Ethics Committee of The First Affiliated Hospital of Harbin Medical University.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Contributor Information
Zhiqiang Wu, Department of Pharmacy, First Affiliated Hospital, Harbin Medical University, Harbin, China.
Hongzhu Chen, Department of Pharmacy, First Affiliated Hospital, Harbin Medical University, Harbin, China.
Liwang Lin, Department of Pharmacy, First Affiliated Hospital, Harbin Medical University, Harbin, China.
Jing Lu, Department of Pharmacy, First Affiliated Hospital, Harbin Medical University, Harbin, China.
Qilei Zhao, Department of Pharmacy, First Affiliated Hospital, Harbin Medical University, Harbin, China.
Zengxiang Dong, Department of Pharmacy, First Affiliated Hospital, Harbin Medical University, Harbin, China.
Xin Hai, Department of Pharmacy, First Affiliated Hospital, Harbin Medical University, Harbin, China.
References
- 1. Jamieson C, Martinelli G, Papayannidis C, Cortes JE. Hedgehog pathway inhibitors: a new therapeutic class for the treatment of acute myeloid leukemia. Blood Cancer Discov. 2020:1(2):134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haloupek N. The landscape of blood cancer research today-and where the field is headed. Blood Cancer Discov. 2020:1(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unnikrishnan D, Dutcher JP, Garl S, Varshneya N, Lucariello R, Wiernik PH. Cardiac monitoring of patients receiving arsenic trioxide therapy. Br J Haematol. 2004:124(5):610–617. [DOI] [PubMed] [Google Scholar]
- 4. Maimaitiyiming Y, Wang QQ, Yang C, Ogra Y, Lou Y, Smith CA, Hussain L, Shao YM, Lin J, Liu J et al. Hyperthermia selectively destabilizes oncogenic fusion proteins. Blood Cancer Discov. 2020:2(4):388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alamolhodaei NS, Shirani K, Karimi G. Arsenic cardiotoxicity: an overview. Environ Toxicol Pharmacol. 2015:40(3):1005–1014. [DOI] [PubMed] [Google Scholar]
- 6. Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014:24(10):R453–R462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baiyun R, Li S, Liu B, Lu J, Lv Y, Xu J, Wu J, Li J, Lv Z, Zhang Z. Luteolin-mediated PI3K/AKT/Nrf2 signaling pathway ameliorates inorganic mercury-induced cardiac injury. Ecotoxicol Environ Saf. 2018:161:655–661. [DOI] [PubMed] [Google Scholar]
- 8. Kumar S, Yedjou CG, Tchounwou PB. Arsenic trioxide induces oxidative stress, DNA damage, and mitochondrial pathway of apoptosis in human leukemia (HL-60) cells. J Exp Clin Cancer Res. 2014:33(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mcmurray JJV. Neprilysin inhibition to treat heart failure: a tale of science, serendipity, and second chances. Eur J Heart Fail. 2015:17(3):242–247. [DOI] [PubMed] [Google Scholar]
- 10. Owens AT, Brozena SC, Jessup M. New management strategies in heart failure. Circ Res. 2016:118(3):480–495. [DOI] [PubMed] [Google Scholar]
- 11. Peng S, Lu XF, Qi YD, Li J, Xu J, Yuan TY, Wu XY, Ding Y, Li WH, Zhou GQ et al. LCZ696 ameliorates oxidative stress and pressure overload-induced pathological cardiac remodeling by regulating the Sirt3/MnSOD pathway. Oxidative Med Cell Longev. 2020:2020:9815039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li X, Zhu Q, Wang Q, Zhang Q, Zheng Y, Wang L, Jin Q. Protection of sacubitril/valsartan against pathological cardiac remodeling by inhibiting the NLRP3 inflammasome after relief of pressure overload in mice. Cardiovasc Drugs Ther. 2020:34(5):629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xia Y, Chen Z, Chen A, Fu M, Dong Z, Hu K, Yang X, Zou Y, Sun A, Qian J et al. LCZ696 improves cardiac function via alleviating Drp1-mediated mitochondrial dysfunction in mice with doxorubicin-induced dilated cardiomyopathy. J Mol Cell Cardiol. 2017:108:138–148. [DOI] [PubMed] [Google Scholar]
- 14. Yang D, Yang Q, Fu N, Li S, Han B, Liu Y, Tang Y, Guo X, Lv Z, Zhang Z. Hexavalent chromium induced heart dysfunction via Sesn2-mediated impairment of mitochondrial function and energy supply. Chemosphere. 2021:264(Pt 2):128547. [DOI] [PubMed] [Google Scholar]
- 15. Wu HC, Rérolle D, de Thé H. PML/RARA destabilization by hyperthermia: a new model for oncogenic fusion protein degradation. Blood Cancer Discov. 2021:2(4):300–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wray DW, Amann M, Richardson RS. Peripheral vascular function, oxygen delivery and utilization: the impact of oxidative stress in aging and heart failure with reduced ejection fraction. Heart Fail Rev. 2017:22(2):149–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang R, Zhang J, Wang S, Wang M, Ye T, Du Y, Xie X, Ye J, Sun G, Sun X. The cardiotoxicity induced by arsenic trioxide is alleviated by salvianolic acid A via maintaining calcium homeostasis and inhibiting endoplasmic reticulum stress. Molecules. 2019:24(3):543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang Q, Xi G, Alamdar A, Zhang J, Shen H. Comparative proteomic analysis reveals heart toxicity induced by chronic arsenic exposure in rats. Environ Pollut. 2017:229:210–218. [DOI] [PubMed] [Google Scholar]
- 19. Burlew BS, Weber K. T, Cardiac fibrosis as a cause of diastolic dysfunction. Herz. 2002:27(2):92–98. [DOI] [PubMed] [Google Scholar]
- 20. Zheng B, Yang Y, Li J, Li J, Zuo S, Chu X, Xu S, Ma D, Chu L. Magnesium isoglycyrrhizinate alleviates arsenic trioxide-induced cardiotoxicity: contribution of Nrf2 and TLR4/NF-κB signaling pathway. Drug Design Dev Ther. 2021:15:543–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li C, Qu X, Xu W, Qu N, Mei L, Liu Y, Wang X, Yu X, Liu Z, Nie D et al. Arsenic trioxide induces cardiac fibroblast apoptosis in vitro and in vivo by up-regulating TGF-β1 expression. Toxicol Lett. 2013:219(3):223–230. [DOI] [PubMed] [Google Scholar]
- 22. Chu W, Li C, Qu X, Zhao D, Wang X, Yu X, Cai F, Liang H, Zhang Y, Zhao X et al. Arsenic-induced interstitial myocardial fibrosis reveals a new insight into drug-induced long QT syndrome. Cardiovasc Res. 2012:96(1):90–98. [DOI] [PubMed] [Google Scholar]
- 23. Ye S, Su L, Shan P, Ye B, Wu S, Liang G, Huang W. LCZ696 attenuated doxorubicin-induced chronic cardiomyopathy through the TLR2-MyD88 complex formation. Front Cell and Dev Biol. 2021:9(4):654051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burke RM, Lighthouse JK, Mickelsen DM, Small EM. Sacubitril/Valsartan decreases cardiac fibrosis in left ventricle pressure overload by restoring PKG signaling in cardiac fibroblasts. Circ Heart Fail. 2019:12(4):e005565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zile MR, O'Meara E, Claggett B, Prescott MF, Solomon SD, Swedberg K, Packer M, McMurray J, Shi V, Lefkowitz M et al. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol. 2019:73(7):795–806. [DOI] [PubMed] [Google Scholar]
- 26. Ma D, Zhang J, Zhang Y, Zhang X, Han X, Song T, Zhang Y, Chu L. Inhibition of myocardial hypertrophy by magnesium isoglycyrrhizinate through the TLR4/NF-κB signaling pathway in mice. Int Immunopharmacol. 2018:55:237–244. [DOI] [PubMed] [Google Scholar]
- 27. Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007:9(1):49–89. [DOI] [PubMed] [Google Scholar]
- 28. Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999:68:383–424. [DOI] [PubMed] [Google Scholar]
- 29. Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999:94(6):2102–2111. [PubMed] [Google Scholar]
- 30. Yu ZQ, Jia Y, Chen G. Possible involvement of cathepsin B/D and caspase-3 in deferoxamine-related neuroprotection of early brain injury after subarachnoid haemorrhage in rats. Neuropathol Appl Neurobiol. 2014:40(3):270–283. [DOI] [PubMed] [Google Scholar]
- 31. Nazari A, Mirian M, Aghaei M, Aliomrani M. 4-Hydroxyhalcone effects on cisplatin-induced genotoxicity model. Toxicol Res. 2021:10(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yi J, Gao F, Shi G, Li H, Wang Z, Shi X, Tang X. The inherent cellular level of reactive oxygen species: one of the mechanisms determining apoptotic susceptibility of leukemic cells to arsenic trioxide. Apoptosis. 2002:7(3):209–215. [DOI] [PubMed] [Google Scholar]
- 33. Chou W, Chen H, Yu S, Cheng L, Yang P, Dang C. V, Arsenic suppresses gene expression in promyelocytic leukemia cells partly through Sp1 oxidation. Blood. 2005:106(1):304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gao A, Wang Y, Gao X, Tian W. LCZ696 ameliorates lipopolysaccharide-induced endothelial injury. Aging. 2021:13(7):9582–9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bai W, Huo T, Chen X, Song X, Meng C, Dang Y, Rong C, Dou L, Qi X. Sacubitril/valsartan inhibits ox-LDL-induced MALAT1 expression, inflammation and apoptosis by suppressing the TLR4/NF-κB signaling pathway in HUVECs. Mol Med Rep. 2021:23(6):402. [DOI] [PubMed] [Google Scholar]
- 36. Li J, Jiang H, Wu P, Li S, Han B, Yang Q, Wang X, Han B, Deng N, Qu B et al. Toxicological effects of deltamethrin on quail cerebrum: weakened antioxidant defense and enhanced apoptosis. Environ Pollut. 2021:286:117319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.