Abstract
The immune system interacts with cancer cells in multiple intricate ways that can shield the host against hyper-proliferation but can also contribute to malignancy. Understanding the protective roles of the immune system in its interaction with cancer cells can help device new and alternate therapeutic strategies. Many immunotherapeutic methodologies, including adaptive cancer therapy, cancer peptide vaccines, monoclonal antibodies, and immune checkpoint treatment, have transformed the traditional cancer treatment landscape. However, many questions remain unaddressed. The development of personalized combination therapy and neoantigen-based cancer vaccines would be the avant-garde approach to cancer treatment. Desirable chemotherapy should be durable, safe, and target-specific. Managing both tumor (intrinsic factors) and its microenvironment (extrinsic factors) are critical for successful immunotherapy. This review describes current approaches and their advancement related to monoclonal antibody-related clinical trials, new cytokine therapy, a checkpoint inhibitor, adoptive T cell therapy, cancer vaccine, and oncolytic virus.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11033-022-07525-8.
Keywords: Chimeric Antigen Receptor (CAR), Programmed death ligand (PD-L1), Peripheral Blood Mononuclear Cells (PBMCs), Interferon gamma (IFN-ɣ), Tumor Necrosis Factor (TNF)
Introduction
Cancer remains one of the principal causes of death among humans worldwide. According to the American Cancer Society in, 2020, total 1,806,590 new cases and 606,520 deaths have been reported due to cancer in the United States. Men report a higher incidence of prostate cancer, and women have a higher incidence of breast cancer. However, the highest number of deaths have been reported due to lung and bronchiolar cancer [1]. The current therapeutic approaches include surgery, chemotherapy, and/or radiation therapy. While these approaches have proven successful in reducing tumor burden and destroying cancer cells, they come with harsh side effects and high chances of recurrence [2]. Considering these problems, other long-term strategies to treat cancer are needed. Immunotherapy is the alternative therapeutic strategy to fight cancer. Immunotherapy can be broadly defined as therapeutic measures that boost or suppress the immune responses to fight against cancer. It can either aim to directly activate the immune system to fight against the cancer cells or may augment general immune responses. Monoclonal antibody treatment, chimeric antigen receptor (CAR)-T cell therapy, and immune checkpoint inhibitors are the key immunotherapies that are being used against many cancers [3, 4]. Many clinical trials are in the pipeline to investigate cancer immunotherapies’ potential [5, 6]. Cancer immune reprogramming can be classified in three phases: (a) stimulation of adaptive and innate immune system to eradicate cancer cells (eradication phase), (b) survival of irregular malignant cells which can activate immune reprogramming (equipoise phase), (c) establishing immunosuppressive microenvironment and low-immunogenic tumors (escape phase) [7, 8].
This review summarizes the different types of immunotherapies, the ongoing and/or past successful clinical trials, the current trends and research and the challenges in this field. This review will be useful for both cancer researchers and clinicians working in this direction.
Current cancer immunotherapies
Both adaptive and innate immune systems play a crucial role in the immune response against cancers [9, 10]. The adaptive immune system comprises CD8+ cytotoxic T cells (CTLs), CD4+ helper T cells, and B cells [11]. The innate immune system can regulate the adaptive immune system by secreting various signals to activate both T and B cells [12]. Antigen-presenting cells (APCs) connect both systems and identify external antigens in the body [13]. CTLs are known to play a very important role in the immune response against cancer [14]. After being cross primed by pAPC, naïve CTLs stimulate a cascade of events that results in CTL attack on tumor cells through granzymes or perforin and/or through ligands of tumor necrosis factor (TNF) superfamily [15]. In the same direction, the anti-tumor effect can also be activated by specific antigens or co-stimulation signals to CTLs followed by secretion of TNF-α and Interferon gamma (IFN-ɣ) [7, 16]. In fact, Adoptive cell therapy (ACT) is a promising approach that involves the intervention of the patient’s immune system to fight against cancer/tumor cells. NK cells, for instance, can bind cancer cells, and several ACT approaches have been developed using this method, for example: Natural Killer Cell Therapy, other include Tumor-Infiltrating Lymphocyte Therapy (TILT), Engineered T-Cell Receptor Therapy (ETCR), Chimeric Antigen Receptor T-Cell Therapy (CARTCT). (Some of which are also shown in Supplementary Fig. 1). Following are the various kind of immunotherapies that are currently available or in the process of development.
Cytokine therapy
Cytokines are small messengers that facilitate communication between cells of the immune system to generate a coordinated response to a target antigen. Cytokines can directly activate effector cells and stromal cells at the site of the tumor and potentiate tumor cell recognition by CD8+T cells. Two cytokines have received FDA approval for treatment against cancer i.e., high doses of IL-2 are administered for metastatic melanoma and renal cell carcinoma and IFN- α2b has been used as an adjuvant in the treatment of Stage III melanoma [17]. Cytokines were the first immunotherapeutic agents that were approved by FDA in late twentieth century [18]. High doses of cytokine IFN-α have pleiotropic effects such as enhancing apoptosis, dendritic cell maturation, augmentation of CTL response against tumor cells, etc. [19]. A lot of work is being done in the neutralization of immunosuppressive cytokines such as IL-10 and TGF-β to enhance anti-tumor immune responses. Understanding the multiple roles of various cytokines in enhancing anti-tumor responses is critical for the development of immunotherapies against cancer [20]. IL-2 is another important cytokine that has been used extensively studied for its potential use in immunotherapy. IL-2 is required for the expansion of NK cells and T cells. Its utility has been limited by its severe systemic toxicity and new IL-2 based therapies are required with improved pharmacokinetics and pharmacodynamics. Such improvements include the addition of PEG moieties to improve the half-life of IL-2 in circulation. This modified cytokine is being tested in clinical trials in conjunction with various other immune checkpoint inhibitors such as atezolizumab (NCT03138889), nivolumab plus ipilimumab (NCT02983045) and nivolumab (NCT02983045, NCT03282344 and NCT03435640). Proinflammatory cytokines such as IFN-α, IL-2, IL-15, IL-21, IL-10, IL-12, and GM-CSF enhance antigen priming, promote infiltration of effector cells into tumor sites leading to cytotoxicity. Cytokines such as TNF- α, TGF-β have inhibiting effects and leads to immunosuppressive or anti-tumor activity in tumor microenvironments [21] (Fig. 1). Some examples of recently completed clinical trials using cytokines include—a TNF based immunotherapy clinical trial (NCT03348891) (www.clinicaltrials.gov) that was completed in 2021 in melanoma patients. Another such example of a recently completed clinical trial is that based on IL-10 cytokine combined with adenovirus which has been shown to have beneficial effects in pancreatic cancer [22, 23]. A high dose of IL-2 cytokine has been used in the treatment of metastatic renal carcinoma. High dose of IL-2 activates high affinity and intermediate affinity IL-2 receptor (IL-2Rβ and γc) and leads to massive pro-inflammatory side effects. Hence, this therapy is recommended for terminal stage patients. The cytokine IFN-α has been used in the treatment of hematological tumors, AIDS-related Kaposi’s sarcoma, malignant melanoma (stage 2 and 3), follicular lymphoma and renal cell cancer. However the usage of IFN-α therapy comes with many toxic and adverse cytotoxic side effects [24]. The IL-12 cytokine stimulates IFN-gamma production in cytotoxic T cells and Th1 cells. Combined usage of IL-12 with oncolytic therapy has been shown to effectively kill tumor cells with limited side effects in some clinical trials (NCT02555397, NCT00406939, NCT03281382, NCT00849459, and NCT01397708). Phase 1 clinical trials showed that recombinant human IL-15 (ALT-803 complex) activates cytotoxic NK cells and CD8+T cells and have less cytotoxic side effects than usage of unmodified IL15. The cytokine GM-CSF help with the proliferation and differentiation of myeloid cells. Clinical trials for the combined use of cell or DNA based vaccine plus GM-CSF with checkpoint inhibitors (NCT04013672, NCT03600350) or oncolytic virus plus GM-CSF with checkpoint inhibitors(NCT02977156, NCT04197882, NCT03206073, and NCT03003676) are now being tested in immunotherapy [25].
Fig. 1.
IFNs, and TGFIL-2, IL-10, IL-12, IL-15, and IL-21 have been tested in clinical trials for the immunotherapy of cancer
Monoclonal antibody (MAb) therapy
Monoclonal antibody therapy is the most successful therapeutic strategy for treating hematologic malignancies and solid tumors. The development of the hybridoma technology by Köhler and Milstein paved the path for the generation of murine antibodies targeted against specific tumor antigens [26]. However, immune responses directed against the murine region (Human anti-mouse antibodies- HAMA) limited their use in cancer treatment. The development of the humanized antibodies completely revolutionized the field of monoclonal antibody therapy. This approach was developed by Winter et. al. where the murine Fv and Fc regions were replaced by the human germ line amino acids [27]. Monoclonal antibodies work by recognizing specific tur antigen and mediate their action either by activating or inhibiting a cell surface receptor, or by activating antibody-dependent cell-mediated cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC) [28]. Some of the tumor associated antigens recognized by monoclonal antibodies can either be cluster differentiation (CD) makers, glycoproteins, glycolipids, carbohydrates, vascular targets, growth factor and stroma and extracellular matrix antigens [28]. In 1997, FDA granted approval to Rituxan®, Genentech/Biogen Idec, the first monoclonal antibody against relapsed/refractory CD20+B-cell, low-grade or follicular non-Hodgkin’s lymphoma [29, 30]. Examples of each of these categories are listed in Supplementary Table 1. Another category of immunotherapy monoclonal antibody is known as bispecific T cell engagers (BiTEs). These antibodies are constructed to target both CD3 and antigen on cancer cells and enhance T cell cytotoxicity. Blinatumomab was the first FDA approved CD3/CD19 BiTE antibody in 2017, used in the treatment of B-ALL and NHL malignancies as shown in Fig. 2 [31]. Monoclonal antibodies are now being in conjunction with other therapies and/or adjuvants. Some examples of recently concluded clinical trials are listed in Supplementary Table 2 [32–35].
Fig. 2.
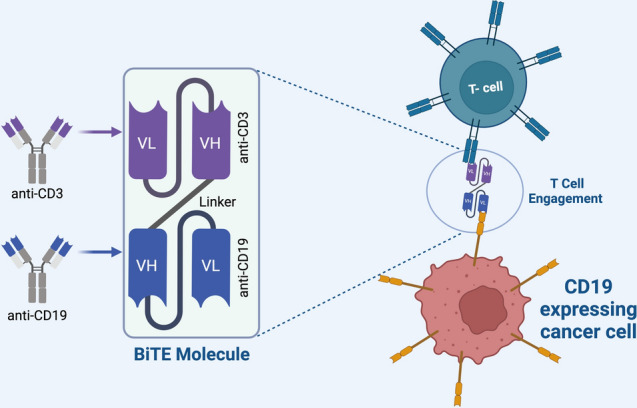
Schematic diagram showing the linkage of a tumor cell to T cell
Checkpoint inhibitors
T-cells have molecules on them that can turn off immune response thereby preventing an exaggerated response to an infection. However, cancer cells use these checkpoints to prevent being attacked by T cells [36]. Checkpoint inhibitors work by blocking the receptors utilized by cancer cells to send signals to T-cells. PD-1 (Programmed death-1) is one such checkpoint inhibitor on T cells that interacts with PD-L1, a protein on normal and cancer cells [8]. Monoclonal Antibodies directed against either PD-1 or PD-L1 can block the interaction of PD-1 and PDL-1 and augment T cell responses [37]. PD-1 inhibitors include Pembrolizumab (Keytruda), Cemiplimab (Libtayo), and Nivolumab (Opdivo) (Supplementary Table 1). PD-1 has shown promising results in treating several types of cancer, including non-small cell lung cancer, skin melanoma, kidney cancer, Hodgkin lymphoma, bladder cancer, and head and neck cancers. Examples of PD-L1 inhibitors include Atezolizumab (Tecentriq), Avelumab (Bavencio) Durvalumab (Imfinzi) (Supplementary Table 1). PD-L1 inhibitors have been beneficial in the treatment of bladder cancer, Merkel cell skin cancer (Merkel cell carcinoma) and non-small cell lung cancer. CTLA-4 is another checkpoint inhibitor found on T cells that prevent an excessive immune response. Ipilimumab (Yervoy) is a monoclonal antibody that inhibits the action of CTLA-4. A meta-analysis study shows that survival post Ipilimumab treatment in patients suffering from advanced melanoma increases by more than 20% for 3–10 years [38]. Besides targeting PD-1/PD-L1 and CTLA4, alternative T cell inhibitors such as TIGIT are the new immunotherapeutic drug targets. TIGIT binds to CD155 and CD112 present on tumor cells and/or APC cells in tumor microenvironment. Combined PD1/TIGIT is also getting attention in the new era of cancer therapy [39]. A major concern with the checkpoint inhibitors is that immune responses can run rampant and can attack innocuous cells of the body [36]. To avoid toxic side effects new alternatives are emerging concerns for cancer treatment. In this direction Lag3 marker can serve as a better alternative target as a checkpoint inhibitor. Lag3 is expressed on activated immune cells and exhausted T cells in cancer conditions. Lag3 is co-expressed with PD-1 marker, so dual blockades have great potential in cancer immunotherapy [40]. In continuation with that, other new generation checkpoint inhibitors include TIM-3, VISTA, or PD-1H, B7-H3 have been used in different clinical trials and in combination with various monoclonal antibodies [41]. VISTA negatively regulates T cells activity and belongs to the B7 family and is expressed on neutrophils, T cells and macrophage. CA-170 is a small molecule antagonist of VISTA-PDL1 axis and is currently being used in clinical trials for the treatment of solid tumors and lymphomas. To prevent cytotoxic side effects, immune checkpoint inhibitor drugs are used in delivered using nanoparticles. An example of this is the lipid coated or PLGA or micelles to deliver anti-PD1 or anti-PDL1 reagent. Supplementary Table 3 summarizes the various co-stimulatory and co-inhibitory interactions that can be utilized in developing futuristic checkpoint inhibitor therapies [42].
Adaptive T cell engineering and therapies
An emerging area of immune therapy is the use of a patients’ own cells to treat cancer. The adoptive cell transfer procedure (ACT) [43]. There are several types of ACT, but the most popular is (CAR) T-cell therapy. (CAR) T-cell therapy involves isolating autologous T cells from the patients, which are then manipulated in vitro by genetic engineering [44].
The T cell receptor extracellular domain (ScFv) can bind and recognize specific tumor antigens, hinge or spacer regions, transmembrane domains and intracellular domains which consist of the signaling domain with or without co-stimulatory CD28 domain and its recognition is MHC independent [45]. The new T cell receptor is called chimeric antigen receptor (CAR) and T-cells bearing this receptor are called (CAR) T-cells. (CAR) T-cells are grown in large numbers in the laboratory and then administered to patients as shown in Fig. 3. This therapy has been used in the treatment of advanced blood cancers. This therapy is most specific, has fewer side effects, and has the advantage of no drug resistance. In 2017, two (CAR) T-cell therapies were approved by FDA; one was Axicabtagene ciloleucel for the treatment of children with acute lymphoblastic leukemia (ALL) and has coupled with anti CD28 and other was Tisagenlecleucel for adults with advanced lymphomas coupled with 4-1BB [46]. Association with these costimulatory domains makes their response persistent due to repetitive antigenic stimulation [47]. It has shown spectacular results especially in terminal patients non-responsive to all other forms of treatment. Steven Rosenberg is one of the pioneers in the field of (CAR) T-cell therapy and he believes that even though (CAR) T-cell therapy is in its nascent stages of development, it has a lot of promise [48]. However, this therapy also comes with its own set of side effects. While we there have been some promising results in the case of hematologic malignancies, we have seen considerably less success (less therapeutic efficacy) in the case of solid tumors using CAR-T-based immunotherapy. The reasons include abnormal tumor vasculature, aberrant adhesion molecules expression, hypoxia, acidity and immunosuppressive microenvironment (stromal barrier) higher expression of immune suppressive cytokines, higher metabolism of tumor cells compared to other cells in the body, tumor cells heterogeneity all add on as obstacles in CAR T cell migration, survival and persistence [45, 49, 50]. Alternative strategy is to combine radiotherapy with CAR T cells to overcome these above mentioned obstacles in solid tumor treatment [50]. Other methods for enhancing CAR T cell therapeutic efficiency, safety, and feasibility in case of solid tumor includes CAR T cell genome editing or modification using CRISPR-Cas9, TALEN nucleases and other endonucleases to improve specificity and tackle inhibitory microenvironments. Split CAR T cell constructs facilitate additional binding with small molecules along with tumor antigens and help in activation. The use of anti-FITC Scfv universal extracellular domain, or biotinylated immune receptor enhances activation and flexibility to target specific tumor associated antigens. Physiological CAR T cells have been developed in which the extracellular domain is modified to act as a ligand/receptor domain that connected to CD3z signaling domain. Similarly tandem CAR, dual CAR, Supra CAR and CAR T cells that can release various cytokines are being developed that give better immune protection in case of solid tumor [45].
Fig. 3.
The diagram shows the procedure of chimeric antigen receptor T cell therapy (CAR). In this process, autologous T cells are removed from the patient body and the genes that encodes for the specific antigen receptors are introduced into the T cells which is called CAR. The new T cells are then cultured in the lab and then re-introduced into the patients
Cancer vaccines
Tumors express antigens that are mutated and/or are unique to the tumor or are differentially expressed or processed in the tumors compared to normal cells. These antigens uniquely expressed on cancer cells have been used to develop therapeutic cancer vaccines [51]. With decades of research on developing therapeutic cancer vaccines, the US Food and Drugs Administration (FDA) has so far approved only one cancer vaccine called Sipuleucel-T for metastatic prostate cancer. This vaccine was manufactured with autologous APCs in the patients’ peripheral blood mononuclear cells (PBMCs). PBMCs obtained from the patients were co-cultured with the peptide PA2024 prior to re-infusion [52] as shown in Fig. 4. Dendreon’s Provenge (sipuleucel-T) was the FDA approved cancer vaccine in 2010. It is a dendritic cell vaccine and is used for prostate cancer treatment. OncoVAX and GVAX are other potential emerging cancer vaccine [47]. However, there are several limitations to developing a good cancer vaccine. Some of which is a low abundance of the tumor antigen; most tumor antigens are shared. This has limited further development in this field. Successful Immunotherapeutic treatment depends on tumor neoantigens quality such as high mutational burden, clonal distribution such as high sub clonal mutation, presentation on MHC I and/or MHC II (Lower HLA heterozygosity), foreignness and higher T cell avidity [53]. The future direction to make personalized cancer vaccines is to combine DNA, RNA or target antigen encoded peptides. It is specific to neoantigens and combined with adjuvants so that it can be presented by APC and specific T cells get activated, clonally expand, and target specific tumors. Coding RNA (mRNA) bind to RNA binding protein and regulate tumor microenvironment. As has been shown by CRISPER-CAS9 screening that 57 RNA binding proteins promote the MYC driven breast cancer pathway. YTHDF2-dependent mRNA degradation causes apoptosis in tumor cells [54]. Besides this non-coding RNA such as microRNA (miRNA) circulating RNA (cir-RNA), and long noncoding RNA (lncRNA) also have been shown to modulate tumor microenvironment. Another strategy for RNA based immunotherapy is targeting tumor derived neoantigens. PTEN mRNA have been delivered in nanoparticles and it has shown to cause reactivation of the tumor suppressor PTEN leading to anti-tumor effects such as infiltration of CD8+CTLs and reversal of the immune-suppressive microenvironment by reducing T-reg and MDSC infiltration in melanoma and prostate cancers [55]. FixVac (BNT111) makes use of mRNA that targets immunogenic neoantigens packaged in nanoparticles and this has completedPhase-1 clinical trial (NCT02410733) for melanomas. Rocapuldencel-T has also shown promising effects in phase 2 clinical trial (NCT00678119) but not in phase 3 clinical trial (NCT01582672). Rocapuldencel-T makes use of monocyte derived dendritic cells transfected with tumor derived neoantigens and activated by co-transfection with CD40L mRNA. It is then combined with Sunitinib to treat stage IV renal cell carcinoma (RCC). Another approach for RNA based immunotherapy is alternative mRNA splicing which has the potential to change the neoantigen RNA pool leading to anti-tumor effects. RNA aptamer such as NOX-A12 and NOX-E36 have been tested under clinical trials and have roles in targeting chemokines in tumor microenvironment. NOX-A12 that targets stromal cell derived factor (SDF-1) has been used in combination with pembrolizumab in treatment of pancreatic and colorectal cancer. NOX-E36 targets the CCL-2 chemokine which helps in the migration of macrophage and MDSCs to the tumor sites and is used in the treatment of solid tumors [56].
Fig. 4.
Diagram showing that cancer vaccines the procedure by which induce anti-tumor immunity in patients
Oncolytic virus
Another way to lyse cancer cells is by using oncolytic viruses and this approach belongs to both biological therapy and immunotherapy. This is an advanced and innovative approach to kill cancer cells. In this technique, the virus is modified such that it is non-virulent to normal body cells but lyses cancerous cells as one of the mechanisms of action as shown in Fig. 5. An example of such a modified virus approved by the FDA in 2015 is Talimogene laherparepvec (T-VEC: a modified Herpes simplex virus that expresses GM-CSF) for advanced melanoma treatment [57]. Another example of oncolytic virus-based immunotherapy is ONCOS-102 which is based on Adenovirus. This activates dendritic cells, expresses GM-CSF, and mediates the tumor microenvironment. It is now combined with cyclophosphamide and tested in clinical trials in various cancers such as Melanoma, advanced peritoneal malignancies, and prostate cancer [47]. Oncolytic virotherapy is an advanced immunotherapy that uses replication competent viruses to target cells. Tumor cells can be targeted either directly by the virus leading to the killing of the infected cells or indirectly by activation of immune effector cells leading to cytotoxicity. Virus that are used in oncolytic therapy includes Adenovirus, Coxsackie virus, Herpes Simplex virus, Measles virus, Newcastle disease virus, Parvovirus, Poliovirus, Reovirus, VSV, Vaccinia, Retro virus, Seneca Valley virus [58]. Oncolytic virus can also be used in imaging and tumor localization in which the reporter gene in modified oncolytic virus replicates and emits fluorescence during its expression. In cancerous cells, due to virus replication the mechanism of oncolytic virotherapy induces release of tumor associated antigen along with viral mediated danger signal that leads to the activation of the adaptive immune system and recruitment of antigen presenting cells (APCs) and effector T cells and promotes cytotoxicity in tumors [59]. Recent study has shown that antigenic peptides can be used as an adjuvant along with oncolytic virus in immune therapy and this is an important step in the direction of personalized medicine [60]. Recent Clinical trials: Examples of some recently concluded clinical trials per the ClinicalTrials.gov in the year 2021 are listed in Supplementary Table 4.
Fig. 5.
Diagram enlisting the essential properties of oncolytic viruses. (1) The virus must selectively replicate in the tumor cells, (2) The virus must be able to replicate efficiently in the tumor microenvironment and (3) The oncolytic virus must function as a therapeutic agent to stimulate the immune system
Combination immunotherapy
To enhance the effectiveness and beneficial effects of immunotherapy combined usage of two or more immunotherapies are now in practice [61]. Combinational use of traditional therapy and advanced immunotherapy has shown synergistic results and are effective treatment modalities. Strategies using dual checkpoint inhibitors such as anti-PD1 and anti-CTLA4 was the first breakthrough success in treating metastatic melanoma patients [62, 63]. Combinatorial immunotherapy leads to the activation of immune system by targeting immune cells as well as by inhibiting the immunosuppressive microenvironment leading to durable anti-tumor effects [64, 65]. Different approaches have been used for combining immunotherapies such as T cell inhibition block by combining checkpoint inhibitor such as anti PD-1 with anti CTLA-4/anti-LAG3/anti-TIM3 or combining T cell co-stimulatory molecules such as anti-PD1 or anti-CTLA4 with agonistic anti 4-IBB/anti-OX-40/anti- CD27 etc. [66]. New approaches such as formation of therapeutic cancer vaccine by combining anti-PD1 or anti-CTLA4 with peptide vaccine/tumor cell vaccine/DNA vaccine pTVG-HP plasmid/Tuberculosis vaccine BCG/Dendritic cell vaccine Sipuleucel T helps in the activation of antigen presenting cells and enhances recognition of tumor cells by T cells [67, 68]. Other approaches include virotherapy where oncolytic virus T-VEC and IDO inhibitor in combination with check point inhibitors enhance tumor immunogenic potential [69]. Examples of targeted therapy include kinase inhibitors such as BRAFi+MEKi, EGFRi and drugs that inhibit DNA methylation and histone de-acetylation such as DNMTi, HDACi in conjunction with checkpoint inhibitors that block survival and proliferation of tumor cells by disrupting metabolic activity [64]. Similarly, angiogenesis inhibitors target VEGF, suppress TGF-β and IL-10 and provide synergistic effects when they work in combination with checkpoint inhibitors [61]. There are several successful examples of such therapies. Chemotherapy+Monoclonal Antibody [70–72]. Similarly, Monoclonal Antibody+Kinase inhibitor [73–75]. In addition to that, combination of two different checkpoints [76] Examples of these categories are shown in Supplementary Table 1. Oncolytic virus combined with checkpoint inhibitors are also in clinical phase trial I/II. Examples are one modified HSV in which there is a spontaneous deletion in UL56 promoter combined with Ipilimumab is in phase II clinical trial for melanoma cancer treatment. Another example of this category is the usage of Vaccinia virus in which deletion of thymidine kinase and modified to express GM-CSF combined with either Anti-CTLA4 inhibitor for solid tumor treatment or an Anti-PD1 inhibitor for CRC and they both are in phase 1 trial now [77]. Combination of oncolytic virus+CAR T cell: example in this category is the vaccinia virus expressing truncated CD19 combined with CD19 CAR T cells to have more specific targeting for solid tumor treatment [78]. Various combination of G207, 1716, and NV1020 with Cis platin chemotherapy for head and neck squamous cell carcinoma, with Mitomycin for human lung cancer has been reported [79]. These combination therapies have additive or synergistic effects in mitigating tumor cells.
Adverse effects, challenges and future direction of immunotherapy
Immunotherapy can also have a negative impact on the body if it targets healthy tissue collectively known as immune related adverse events (irAE). Patients with autoimmune disease have negative effects post immunotherapy. The symptoms include from mild skin rash, headache, fatigue, joint pain to severe, affecting organ such as gut, lungs and liver as well as endocrine system [80]. Different treatments can cause different histological iARE symptoms: for example, metastatic cancer immunotherapy that uses anti CTLA-4 leads to granulomas while anti PD-1/PDL1 generate lobular hepatitis [81]. Microbiota also influence potential adverse side-effects of immunotherapy such as high level of Bifidobacterium, Rhuminococcus, species of Bacteroidetes and in general greater immune diversity have positive anti-tumor effects. In contrast several factors such as microbiota driven bacterial polyamine transport, high level of serum IL-17 cytokine and tissue expressing inhibitor such as CTLA4 are negatively correlated with irAEs [82]. As per clinical guidelines, the management of irAE depends on the grade of immunotherapy treatment as shown in Supplementary Table 5. Grade 1 to 4 is the increasing order of irAEs symptoms from mild to severe [83]. To mitigate the adverse effects of cytotoxicity, multiple drug resistance and side effects of cancer immunotherapeutic reagents, combination therapy and nanocarrier-based delivery systems such as liposomes, nanoparticle, dendrimers and micelles can be used [84].
There are few challenges associated with immunotherapy. The primary one being why immunotherapy works well in some patients but not in others and how tumors that were once sensitive to immunotherapy acquire resistance. For cancer immunotherapy to be effective, one needs to find methods to manipulate the immune system of patients who fail to mount an immune response to their tumors. One way to effectively predict patient response to the immunotherapy drugs is to identify the biomarkers that can predict the patient outcome and to develop experimental models to test drug responsiveness. Besides these, there are other challenges towards developing and translating the immunotherapies such as genetic instability (e.g., heterogeneity, altered ploidy, and various mutations) in the genome of the cancer cells. This can addressed with the help of Next-generation sequencing (NGS) that allows the complete sequencing of each cell in the cancerous mass [85]. The use of state-of-the-art bioinformatics algorithms can help predict certain protein’s antigenicity to develop a safe and more effective personalized immunotherapy [86–88]. To this effect, peptide based therapeutic vaccines have received much attention and involve CD4+T cells and CD8+T cells to target tumor associated antigens and tumor specific antigens [89]. Clonal mutations and intra and inter tumor heterogeneity are the other challenges in personalized therapy [90]. Usage of high throughput techniques in immunotherapy intervention such as scRNA-seq to study heterogeneity in population and to identify rare tumor populations which have altered gene expression and are resistant to killing by conventional therapies is important for the success of the immunotherapy treatment. Research is ongoing and major advancements can be expected in the field of immunotherapy in the near future. Active research is now dedicated to exploring the role of Tregs, MDSCs, NK cells and TAMs in cancer. Further manipulation of the gut microbiome is expected to enhance the efficiency of immunotherapy. A clinical trial study of combined usage of cancer immunotherapeutic drugs along with SARS-CoV2 neutralizing antibodies provides insight for further research [91].
Conclusion
Cancer immunotherapy has emerged as one of the main pillars of cancer treatment. This is because it is personalized, targeted, and safe as compared to the other methods such as surgery, radiotherapy and chemotherapy. Immune checkpoint inhibitors such as PD-L1, PD-1 and CTLA-4 have proven to be successful in clinical trials against several cancer types. Other important immunotherapy strategies that have been sanctioned include VEGFR2, EGFR and combination strategies targeting PD-1, PD-L1, CTLA-4 and VEGFR2, EGFR. This has resulted in several FDA approved single or combination cancer immunotherapies. Alternative options of cancer immunotherapy such as CAR T cell therapy, oncolytic virus therapy, cancer vaccine provide new avenues in the direction of targeted killing tumor cells and provide better strategies to deal with toxic side effects of conventional therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- CAR
Chimeric Antigen Receptor
- APCs
Antigen presenting cells
- CTLs
CD8+ cytotoxic T cells
- TNF
Tumor Necrosis Factor
- IFN-ɣ
Interferon gamma
- NGS
Next-generation sequencing
- SNV
Single nucleotide variants
- CRC
Colorectal cancer
- FDA
Food and Drug Administration
- irRECIST
Immune-related response criteria
- HAMA
Human anti-mouse antibodies
- ADCC
Activating antibody-dependent cell-mediated cytotoxicity
- CDC
Complement-dependent cytotoxicity
- PD-1
Programmed death-1
- PD-L1
Programmed death ligand
- ACT
Adoptive Cell Transfer
- ALL
Acute Lymphoblastic Leukaemia
- PBMCs
Peripheral Blood Mononuclear Cells
Author contributions
VS, SB, SLG and RKJ designed, wrote and edited the manuscript.
Funding
The author(s) received no financial support for the authorship, and/or publication of this article.
Declarations
Conflict of interest
The authors declare no conflict of interests
Ethical approval
No animal has been used in this study.
Informed consent
All authors are agree to publish this manuscript in your valuable journal.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sneh Lata Gupta, Srijani Basu and Vijay Soni have contributed equally to this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Rius M, Lyko F. Epigenetic cancer therapy: rationales, targets, and drugs. Oncogene. 2012;31(39):4257–4265. doi: 10.1038/onc.2011.601. [DOI] [PubMed] [Google Scholar]
- 3.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voena C, Chiarle R. Advances in cancer immunology and cancer immunotherapy. Discov Med. 2016;21(114):125–133. [PubMed] [Google Scholar]
- 5.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribas A. Releasing the brakes on cancer immunotherapy. N Engl J Med. 2015;373(16):1490–1492. doi: 10.1056/NEJMp1510079. [DOI] [PubMed] [Google Scholar]
- 7.Mittal D, et al. New insights into cancer immunoediting and its three component phases—elimination, equilibrium, and escape. Curr Opin Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 9.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4(1):11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 10.Lakshmi Narendra B, et al. Immune system: a double-edged sword in cancer. Inflamm Res. 2013;62(9):823–834. doi: 10.1007/s00011-013-0645-9. [DOI] [PubMed] [Google Scholar]
- 11.Binder RJ. Functions of heat shock proteins in pathways of the innate and adaptive immune system. J Immunol. 2014;193(12):5765–5771. doi: 10.4049/jimmunol.1401417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. 2015;33:445–474. doi: 10.1146/annurev-immunol-032414-112043. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Zeng G. Cancer and innate immune system interactions: translational potentials for cancer immunotherapy. J Immunother. 2012;35(4):299–308. doi: 10.1097/CJI.0b013e3182518e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy–revisited. Nat Rev Drug Discov. 2011;10(8):591–600. doi: 10.1038/nrd3500. [DOI] [PubMed] [Google Scholar]
- 15.Hanlon DJ, Aldo PB, Devine L, Alvero AB, Engberg AK, Edelson R, Mor G. Enhanced stimulation of anti-ovarian cancer CD8(+)T cells by dendritic cells loaded with nanoparticle encapsulated tumor antigen. Am J Reprod Immunol. 2011;65(6):597–609. doi: 10.1111/j.1600-0897.2010.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Z, et al. CD4+T cell help selectively enhances high-avidity tumor antigen-specific CD8+T cells. J Immunol. 2015;195(7):3482–3489. doi: 10.4049/jimmunol.1401571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkwood JM, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14(1):7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 18.Eno J. Immunotherapy through the years. J Adv Pract Oncol. 2017;8(7):747–753. [PMC free article] [PubMed] [Google Scholar]
- 19.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15(7):405–414. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers. 2011;3(4):3856–3893. doi: 10.3390/cancers3043856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berraondo P, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120(1):6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsay JO, et al. Local delivery of adenoviral vectors encoding murine interleukin 10 induces colonic interleukin 10 production and is therapeutic for murine colitis. Gut. 2003;52(7):981–987. doi: 10.1136/gut.52.7.981. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Rallis KS, Corrigan AE, Dadah H, George AM, Keshwara SM, Sideris M, Szabados B. Cytokine-based cancer immunotherapy: challenges and opportunities for IL-10. Anticancer Res. 2021;41(7):3247–3252. doi: 10.21873/anticanres.15110. [DOI] [PubMed] [Google Scholar]
- 24.Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. 2018;10(12):a028472. doi: 10.1101/cshperspect.a028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chulpanova DS, Kitaeva KV, Green AR, Rizvanov AA, Solovyeva VV. Molecular aspects and future perspectives of cytokine-based anti-cancer immunotherapy. Front Cell Dev Biol. 2020;8:402. doi: 10.3389/fcell.2020.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freysd'ottir J. Production of monoclonal antibodies. Methods Mol Med. 2000;40:267–279. doi: 10.1385/1-59259-076-4:267. [DOI] [PubMed] [Google Scholar]
- 27.Riechmann L, Clark M, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature. 1988;332(6162):323–327. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- 28.Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Cancer Immunol. 2012;12:14. [PMC free article] [PubMed] [Google Scholar]
- 29.Klener P, Jr, Otáhal P, Lateckova L, Klener P. Immunotherapy approaches in cancer treatment. Curr Pharm Biotechnol. 2015;16(9):771–781. doi: 10.2174/1389201016666150619114554. [DOI] [PubMed] [Google Scholar]
- 30.Goel G, Sun W. Cancer immunotherapy in clinical practice—the past, present, and future. Chin J Cancer. 2014;33(9):445–457. doi: 10.5732/cjc.014.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian Z, Liu M, Zhang Y, et al. Bispecific T cell engagers: an emerging therapy for management of hematologic malignancies. J Hematol Oncol. 2021;14:75. doi: 10.1186/s13045-021-01084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fountzilas E, et al. Real-world safety and efficacy data of immunotherapy in patients with cancer and autoimmune disease: the experience of the Hellenic Cooperative Oncology Group. Cancer Immunol Immunother. 2022;71(2):327–337. doi: 10.1007/s00262-021-02985-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen PHD, et al. Intratumoural immune heterogeneity as a hallmark of tumour evolution and progression in hepatocellular carcinoma. Nat Commun. 2021;12(1):227. doi: 10.1038/s41467-020-20171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fritsche J, et al. Translating immunopeptidomics to immunotherapy-decision-making for patient and personalized target selection. Proteomics. 2018;18(12):e1700284. doi: 10.1002/pmic.201700284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Catenacci DVT, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b–2 trial. Lancet Oncol. 2020;21(8):1066–1076. doi: 10.1016/S1470-2045(20)30326-0. [DOI] [PubMed] [Google Scholar]
- 36.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 37.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa120069036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schadendorf D, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chauvin J, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. 2020;8:e000957. doi: 10.1136/jitc-2020-000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puhr HC, Ilhan-Mutlu A. New emerging targets in cancer immunotherapy: the role of LAG3. ESMO Open. 2019;4(2):e000482. doi: 10.1136/esmoopen-2018-000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin S, et al. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18(1):155. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yadav D, Kwak M, Chauhan PS, Puranik N, Lee P, Jin JO. Cancer immunotherapy by immune checkpoint blockade and its advanced application using bio-nanomaterials. Semin Cancer Biol. 2022 doi: 10.1016/j.semcancer.2022.02.016. [DOI] [PubMed] [Google Scholar]
- 43.Miliotou AN, Papadopoulou LC. CAR T-cell therapy: a new era in cancer immunotherapy. Curr Pharm Biotechnol. 2018;19(1):5–18. doi: 10.2174/1389201019666180418095526. [DOI] [PubMed] [Google Scholar]
- 44.June CH, et al. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 45.Tahmasebi S, Elahi R, Esmaeilzadeh A. Solid tumors challenges and new insights of CAR T cell engineering. Stem Cell Rev Rep. 2019;15(5):619–636. doi: 10.1007/s12015-019-09901-7. [DOI] [PubMed] [Google Scholar]
- 46.Knochelmann HM, Smith AS, Dwyer CJ, Wyatt MM, Mehrotra S, Paulos CM. CAR T cells in solid tumors: blueprints for building effective therapies. Front Immunol. 2018;9:1740. doi: 10.3389/fimmu.2018.01740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020;30(6):507–519. doi: 10.1038/s41422-020-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tran E, Robbins PF, Rosenberg SA. 'Final common pathway' of human cancer immunotherapy: targeting random somatic mutations. Nat Immunol. 2017;18(3):255–262. doi: 10.1038/ni.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J, Jiang H. Current challenges and strategies for chimeric antigen receptor-T-cell therapy for solid tumors. Crit Rev Immunol. 2021;41(1):1–12. doi: 10.1615/CritRevImmunol.2020036178. [DOI] [PubMed] [Google Scholar]
- 50.Hauth F, Ho AY, Ferrone S, Duda DG. Radiotherapy to enhance chimeric antigen receptor T-cell therapeutic efficacy in solid tumors: a narrative review. JAMA Oncol. 2021;7(7):1051–1059. doi: 10.1001/jamaoncol.2021.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butterfield LH. Cancer vaccines. BMJ. 2015;350:h988. doi: 10.1136/bmj.h988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 53.Saxena M, van der Burg SH, Melief C, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21(6):360–378. doi: 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- 54.Einstein JM, et al. Inhibition of YTHDF2 triggers proteotoxic cell death in MYC-driven breast cancer. Mol Cell. 2021;81(15):3048–3064.e9. doi: 10.1016/j.molcel.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin YX, et al. Reactivation of the tumor suppressor PTEN by mRNA nanoparticles enhances antitumor immunity in preclinical models. Sci Transl Med. 2021;13(599):eaba9772. doi: 10.1126/scitranslmed.aba9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pandey PR, Pandey PR, Young KH, Kumar D, Jain N, et al. RNA-mediated immunotherapy regulating tumor immune microenvironment: next wave of cancer therapeutics. Mol Cancer. 2022;21(1):58. doi: 10.1186/s12943-022-01528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oiseth SJ, Aziz MS. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J Cancer Metastasis Treat. 2017;3:250–261. doi: 10.20517/2394-4722.2017.41. [DOI] [Google Scholar]
- 58.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li L, Liu S, Han D, Tang B, Ma J. Delivery and biosafety of oncolytic virotherapy. Front Oncol. 2020;10:475. doi: 10.3389/fonc.2020.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roy DG, et al. Adjuvant oncolytic virotherapy for personalized anti-cancer vaccination. Nat Commun. 2021;12(1):2626. doi: 10.1038/s41467-021-22929-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD. Combination immunotherapy: a road map. J Immunother Cancer. 2017;5:16. doi: 10.1186/s40425-017-0218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drake CG. Combination immunotherapy approaches. Ann Oncol. 2012;23(Suppl 8):41–46. doi: 10.1093/annonc/mds262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gellrich FF, Schmitz M, Beissert S, Meier F. Anti-PD-1 and novel combinations in the treatment of melanoma-an update. J Clin Med. 2020;9(1):223. doi: 10.3390/jcm9010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vilgelm AE, Johnson DB, Richmond A. Combinatorial approach to cancer immunotherapy: strength in numbers. J Leukoc Biol. 2016;100(2):275–290. doi: 10.1189/jlb.5RI0116-013RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joshi S, Durden DL. Combinatorial approach to improve cancer immunotherapy: rational drug design strategy to simultaneously hit multiple targets to kill tumor cells and to activate the immune system. J Oncol. 2019;2019:5245034. doi: 10.1155/2019/5245034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu-Lieskovan S, Ribas A. New combination strategies using programmed cell death 1/programmed cell death ligand 1 checkpoint inhibitors as a backbone. Cancer J. 2017;23(1):10–22. doi: 10.1097/PPO.0000000000000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 2022;15(1):28. doi: 10.1186/s13045-022-01247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson LE, Brockstedt D, Leong M, Lauer P, Theisen E, Sauer JD, McNeel DG. Heterologous vaccination targeting prostatic acid phosphatase (PAP) using DNA and Listeria vaccines elicits superior anti-tumor immunity dependent on CD4+T cells elicited by DNA priming. Oncoimmunology. 2018;7(8):e1456603. doi: 10.1080/2162402X.2018.1456603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang G, et al. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses. Nat Commun. 2020;11(1):1395. doi: 10.1038/s41467-020-15229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL, Chen L, Melero I, Schalper KA, Herbst RS. Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res. 2019;25(15):4592–4602. doi: 10.1158/1078-0432.CCR-18-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmid P, et al. KEYNOTE-522: Phase III study of pembrolizumab (pembro) + chemotherapy (chemo) vs placebo + chemo as neoadjuvant therapy followed by pembro vs placebo as adjuvant therapy for triple-negative breast cancer (TNBC) J Clin Oncol. 2018;36(15_suppl):TPS602–TPS602. doi: 10.1200/JCO.2018.36.15_suppl.TPS602. [DOI] [Google Scholar]
- 72.Schmid P, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 73.Rini BI, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 74.Motzer RJ, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Makker V, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):711–718. doi: 10.1016/S1470-2045(19)30020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hellmann MD, et al. Nivolumab plus Ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 77.Shi T, et al. Combining oncolytic viruses with cancer immunotherapy: establishing a new generation of cancer treatment. Front Immunol. 2020;11:683–683. doi: 10.3389/fimmu.2020.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park AK, Fong Y, Kim SI, Yang J, Murad JP, Lu J, Jeang B, Chang WC, Chen NG, Thomas SH, Forman SJ, Priceman SJ. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci Transl Med. 2020;12(559):eaaz1863. doi: 10.1126/scitranslmed.aaz1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gopisankar MG, Surendiran A. Oncolytic virotherapy—a novel strategy for cancer therapy. Egypt J Med Hum Genet. 2018;19(3):165–169. doi: 10.1016/j.ejmhg.2017.10.006. [DOI] [Google Scholar]
- 80.Brown TJ, Mamtani R, Bange EM. Immunotherapy adverse effects. JAMA Oncol. 2021;7(12):1908–1908. doi: 10.1001/jamaoncol.2021.5009. [DOI] [PubMed] [Google Scholar]
- 81.De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O, Roche B, Antonini TM, Coilly A, Laghouati S, Robert C, Marabelle A, Guettier C, Samuel D. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68(6):1181–1190. doi: 10.1016/j.jhep.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 82.Gerson JN, Ramamurthy C, Borghaei H. Managing adverse effects of immunotherapy. Clin Adv Hematol Oncol. 2018;16(5):364–374. [PubMed] [Google Scholar]
- 83.Barber FD. Adverse events of oncologic immunotherapy and their management. Asia Pac J Oncol Nurs. 2019;6(3):212–226. doi: 10.4103/apjon.apjon_6_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raikwar S, Jain A, Saraf S, Bidla PD, Panda PK, Tiwari A, Verma A, Jain SK. Opportunities in combinational chemo-immunotherapy for breast cancer using nanotechnology: an emerging landscape. Expert Opin Drug Deliv. 2022 doi: 10.1080/17425247.2022.2044785. [DOI] [PubMed] [Google Scholar]
- 85.Charoentong P, et al. Bioinformatics for cancer immunology and immunotherapy. Cancer Immunol Immunother. 2012;61(11):1885–1903. doi: 10.1007/s00262-012-1354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miao D, Van Allen EM. Genomic determinants of cancer immunotherapy. Curr Opin Immunol. 2016;41:32–38. doi: 10.1016/j.coi.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 87.Sathyanarayanan V, Neelapu SS. Cancer immunotherapy: strategies for personalization and combinatorial approaches. Mol Oncol. 2015;9(10):2043–2053. doi: 10.1016/j.molonc.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Borghaei H, Smith MR, Campbell KS. Immunotherapy of cancer. Eur J Pharmacol. 2009;625(1–3):41–54. doi: 10.1016/j.ejphar.2009.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dhiman G, et al. Metadherin peptides containing CD4(+) and CD8(+)T cell epitopes as a therapeutic vaccine candidate against cancer. Microbiol Immunol. 2016;60(9):646–652. doi: 10.1111/1348-0421.12436. [DOI] [PubMed] [Google Scholar]
- 90.Zhou L, Wang K, Li Q, Nice EC, Zhang H, Huang C. Clinical proteomics-driven precision medicine for targeted cancer therapy: current overview and future perspectives. Expert Rev Proteomics. 2016;13(4):367–381. doi: 10.1586/14789450.2016.1159959. [DOI] [PubMed] [Google Scholar]
- 91.Gupta SL, Jaiswal RK. Neutralizing antibody: a savior in the Covid-19 disease. Mol Biol Rep. 2022;49:2465–2474. doi: 10.1007/s11033-021-07020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






