Abstract
Chronic cough is globally prevalent across all age groups. This disorder is challenging to treat because many pulmonary and extrapulmonary conditions can present with chronic cough, and cough can also be present without any identifiable underlying cause or be refractory to therapies that improve associated conditions. Most patients with chronic cough have cough hypersensitivity, which is characterized by increased neural responsivity to a range of stimuli that affect the airways and lungs, and other tissues innervated by common nerve supplies. Cough hypersensitivity presents as excessive coughing often in response to relatively innocuous stimuli, causing significant psychophysical morbidity and affecting patients’ quality of life. Understanding of the mechanisms that contribute to cough hypersensitivity and excessive coughing in different patient populations and across the lifespan is advancing and has contributed to the development of new therapies for chronic cough in adults. Owing to differences in the pathology, the organs involved and individual patient factors, treatment of chronic cough is progressing towards a personalized approach, and, in the future, novel ways to endotype patients with cough may prove valuable in management.
Subject terms: Respiratory tract diseases, Drug development
This Primer by Mazzone and colleagues summarizes the epidemiology, pathophysiology, diagnosis and treatment of chronic cough and cough hypersensitivity. This Primer also discusses how cough hypersensitivity and chronic cough affect patients’ quality of life and future research directions for the field.
Introduction
Cough is one of the most common symptoms for which people present to primary care and is a chief complaint for patients seeking medical attention in respiratory or allergy specialist clinics1,2. The definition of chronic cough in clinical guidelines is cough persisting for >8 weeks in adults and >4 weeks in children3–5; however, in many epidemiological studies, chronic cough is defined as cough that lasts >3 months6. The definition of chronic cough is guided by expert opinion, as definitive clinical criteria to distinguish acute cough from chronic cough are lacking.
In practice, chronic cough is often a long-lasting and burdensome condition, persisting for several years and sometimes decades for a substantial number of patients, despite exhaustive medical intervention7–9. Many pulmonary and some extrapulmonary diseases and disorders can present with chronic cough, making diagnosis and treatment challenging. Moreover, 40% of adults with chronic cough referred for specialist evaluation have no identified cause (known as unexplained chronic cough) or have persistent cough despite optimal treatment of conditions associated with chronic cough (known as refractory chronic cough)10.
Chronic cough of any aetiology in adults is widely believed to reflect a hypersensitivity condition, characterized by coughing that is often triggered by low levels of thermal, mechanical or chemical exposure11. The considerable burden of persistent chronic cough has led to an appreciation of cough hypersensitivity as a distinct clinical entity in adults. Distinct mechanisms that involve both peripheral and central neural pathways have a role in this hypersensitivity, and have motivated advances in cough suppressant (antitussive) drug discovery12. The aetiology and management of chronic cough differs in children from that in adults, and the relevance of cough hypersensitivity as an underpinning mechanism in children remains unclear.
This Primer discusses the global prevalence and mechanisms of cough, with a focus on the most common causes of cough hypersensitivity. This Primer provides an overview of the current state-of-the-art recommendations for cough diagnosis and management, and presents a viewpoint of recent advances in cough hypersensitivity and chronic cough that may have a future effect on treatment.
Epidemiology
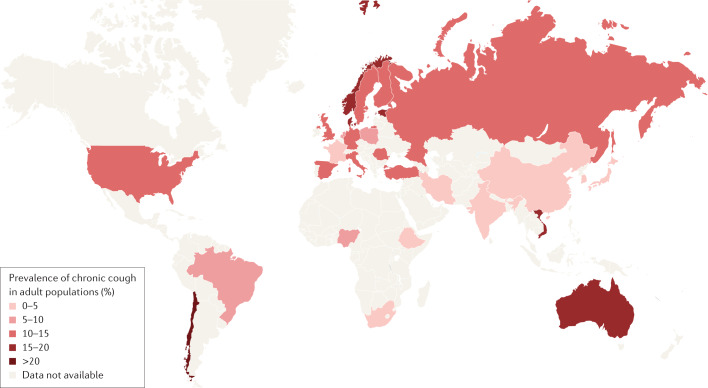
Chronic cough affects ~10% of adults in various general populations13. The prevalence of chronic cough is higher in Europe, America and Australia (10–20%) than in Asia (<5%)13 (Fig. 1). In the general population of Copenhagen, Denmark, the prevalence of chronic cough was 4% overall, with a prevalence of 3% in never-smokers, 4% in former smokers and 8% in current smokers14. Moreover, the prevalence of chronic cough in a meta-analysis was 6.2% in adults in China15.
Fig. 1. Global prevalence of chronic cough.
Map showing the results of a meta-analysis of 90 published studies assessing the regional pooled prevalence of chronic cough in adult populations. Reprinted with permission from ref.13, ERS.
The incidence of chronic cough ranges from 1.2 to 5.7 per 100 person-years in population-based studies of adults ≥45 years of age in Belgium and Canada16,17. However, no global or continental-level data are available. The longitudinal epidemiology of chronic cough and cough hypersensitivity is largely unknown, but cough may persist for longer than 5 years despite treatment in adults with chronic cough8,9,18. Certain patient traits, such as comorbid obesity, gastro-oesophageal reflux disease (GERD) and genetic background are associated with longer disease duration but warrant further investigation9,18.
The prevalence of chronic cough in children is not as clearly defined as in adults. Overall, prevalence of chronic cough in children ranges from 1.1% to 21.9%19,20; the difference in these estimates is likely due to differences in the method of data collection, definition of chronic cough used, setting studied (such as high-income versus low-income country) and age of children studied (Table 1). Few data are available on the incidence of chronic cough following an acute respiratory infection; one study21 reported that 171 of the 839 children (20.4%, 95% CI 17.7–23.1) recruited from the emergency department of a specialist children’s hospital had chronic cough (defined as cough for >4 weeks) but 63 of these children already had chronic cough at presentation. Thus, the incidence is likely 108 of 839 (12.9%). Of the children with chronic cough who were reviewed by paediatric pulmonologists, 30.8% had a new and serious chronic lung disease and 47.0% had protracted bacterial bronchitis21.
Table 1.
Prevalence of chronic cough in children
| Setting | Chronic cough definition | Age, years (n) | Prevalence, % | Ref. |
|---|---|---|---|---|
| Cross-sectional survey of Seattle middle school students using written and video respiratory-symptom questionnaires | Chronic productive cough: “daily cough productive of phlegm for at least 3 months out of the year” | 11–15 (2,397) | 7.2 (all), 3.4 (excluding those with asthma) | 220 |
| Two groups of children: one enrolled from public schools within a 10 km radius of Royal Prince Alfred Hospital, Sydney, Australia, and another enrolled from six schools in Nigeria; both questionnaire based | “In the last 12 months has your child had a cough that lasted more than 3 weeks and was not associated with a cold or flu?” | 5–7 (511) | 10.4 | 221 |
| 8–11 (654) | 9.6 | |||
| 8–11 (566) | 5.1 | |||
| Suva City schoolchildren, Fiji; questionnaire based | “Has this child coughed mucus on most mornings in the last 12 months” | Mean 9.6 (2,173) | 21.9 | 20 |
| 12 centres in northern, central and southern Italy; self-administered questionnaires completed by parents | Cough or phlegm for ≥4 days a week (in the absence of a cold) for ≥1 month per year | 6–7 (20,016); 13–14 (13,616) | 6.8 | 222 |
| Whole-population prospective study undertaken in four remote communities in north Western Australia | Parent-reported daily wet cough for ≥4 weeks with clinician researcher confirmation (with physiotherapist using non-invasive techniques to elicit a cough if necessary) | Median 3.5 (203) | 13 | 223 |
| 18 districts of six cities in Liaoning province, China; Chinese language translation of the Epidemiologic Standardization Project Questionnaire of American Thoracic Society; self-completed by parents | Cough on most days (>4 days per week) for as long as 3 months per year, either together with or separately from colds | 3–12 (11,860) | 9.5 (persistent cough) | 60 |
| Seemed congested or brought up phlegm or mucus from the chest on most days (>4 days per week) for as long as 3 months per year, either together with or separately from colds | 4.6 (persistent phlegm) | |||
| Children who participated in CAPS and BOLD-Chikhwawa studies, Chikhwawa District, rural Malawi; electronic questionnaire in Chichewa, the local language | “Does your child usually have a cough when they don’t have a cold?” and “Are there months in which they cough on most days?” | Mean 7.1 (804) | 8 | 224 |
| Five villages in Dehlon Block of Ludhiana, Punjab, India | Cough lasting for >3 weeks | 1–15 (2,275) | 1.1 | 19 |
| Follow-up of a birth cohort in which parents completed questionnaires or survey in various years | Score of ≥3 to question “How often has this child been bothered by cough?” at least 2–3 episodes in the past year | Mean 1.1 (1,064) | 6.7 | 225 |
| Mean 2.1 (945) | 4.5 | |||
| Mean 5.8 (1,024) | 12.2 | |||
| Mean 8.1 (841) | 12.1 | |||
| Mean 10.4 (956) | 12.4 |
Risk factors
Various environmental and host factors, such as respiratory infection, air pollutants, occupational irritants, allergens, eosinophils or refluxate, can sensitize and trigger cough and are potential risk factors for chronic cough22,23. Biological traits, such as age or sex hormonal status, also interact with these triggers in developing chronic cough24–26. However, the definition of chronic cough used in population-based studies is based on cough duration, does not differentiate protective cough responses from hypersensitivity and does not represent the key nature that defines cough as the disease, such as the impact, severity and hypersensitivity, and thus the epidemiological research definition should be refined for elucidating the risk factors6,27–29.
Patient factors
Age and sex underlie the burden and prevalence of chronic cough, although the mechanisms that underlie this association are unknown. In one survey, two-thirds of the patients presenting with chronic cough to specialist clinics were female, and the most common decade for presentation was 60–69 years24. The high proportion of women in specialist clinics may be due to greater effects of cough in older women, as they have more frequent complications such as stress urinary incontinence30. Another possible explanation for the increased prevalence of chronic cough in female adults is that women have a more sensitive cough reflex than men, as demonstrated by inhalation capsaicin cough reflex sensitivity tests25 or that women have greater activation of the somatosensory brain cortex in response to capsaicin inhalation than men24.
Notably, sex differences in capsaicin cough reflex sensitivity are not observed during prepubertal ages26,31, suggesting that mechanisms that predispose to hypersensitivity do not occur in children. In support of this, the prevalence of Arnold’s nerve cough reflex (evoked by mechanical stimulation of vagal fibres innervating the external auditory canal) is 11-fold higher in adults with chronic cough than in healthy adults and those with respiratory disease without cough, indicative of vagal hypersensitivity, whereas prevalence of this reflex is similar in children with chronic cough and in healthy children32.
Other populations can have unique cough epidemiology. For example, in China, most patients with chronic cough are ~40 years of age, with an equal sex proportion, despite the enhanced cough sensitivity in women24,33.
Relatively few studies have identified specific genetic risk factors for chronic cough. Identified genetic risk factors include mutations in TRPV1 (encodes transient receptor potential cation channel subfamily V member 1)34 and TAC2R (encodes neurokinin 2 receptor)35 and a RFC1 (encodes replication factor complex subunit 1) expansion associated with sensory neuropathy36,37.
Clinical factors
Cigarette smokers are three times more likely to report chronic cough than never-smokers and ex-smokers, and the cough is usually due to chronic bronchitis38. However, most patients in cough specialist clinic are non-smokers.
Infection with respiratory viruses (such as rhinovirus or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)) is a common cause of acute cough and is usually self-limiting, but post-infectious cough may persist for months in some individuals. For example, 10–20% of patients after SARS-CoV-2 infection39 and 8.5–43% of patients after H1N1 influenza40,41 have persistent cough, which may be related to cough hypersensitivity. The viruses that are likely to induce post-infectious cough are unclear. Infection with Bordetella pertussis may also be associated with a prolonged and debilitating cough (whooping cough), which can be difficult to treat42,43.
Common pulmonary causes of chronic cough in non-smokers with normal chest X-rays and spirometry are corticosteroid-responsive cough such as eosinophilic conditions, including cough variant asthma, non-asthmatic eosinophilic bronchitis and atopic cough44,45. Extrapulmonary conditions are also commonly associated with cough, including GERD and upper airway cough syndrome (previously called ‘post-nasal drip syndrome’) due to rhinitis or rhinosinusitis22. Indeed, cough variant asthma, eosinophilic bronchitis, upper airway cough syndrome and GERD account for 51–92% of cases of adult chronic cough globally45,46. However, the existence of upper airway cough syndrome as a distinct clinical entity has been debated and is proposed to reflect generalized airway inflammation resulting from asthma or airway reflux4, potentially underestimating the true incidence of cough in these conditions. Of note, the proportion of patients with chronic cough with GERD is lower in East Asia than in Europe and the USA45,46. Chronic obstructive pulmonary disease (COPD), bronchiectasis, lung cancer, interstitial lung disease and obstructive sleep apnoea are also associated with chronic cough and cough hypersensitivity but chest radiology and/or lung physiology measurements are usually abnormal47–52. Chronic cough is widely recognized as an adverse effect of angiotensin-converting enzyme (ACE) inhibitors, which are prescribed as anti-hypertensives or for heart failure53.
Rare causes of chronic cough in adults account for <15% of cases and commonly include protracted bacterial bronchitis, somatic cough syndrome (which is more common in children), diffuse panbronchiolitis and obstructive sleep apnoea syndrome54. However, only limited high-quality evidence is available regarding their prevalence and clinical implications in adults with chronic cough. Less common extrapulmonary conditions include atypical cardiac failure and cardiac arrhythmias and tracheobronchomalacia (Table 2).
Table 2.
Additional conditions associated with chronic cough in adults and children
| Condition | Possible mechanism | Clinical picture |
|---|---|---|
| Obstructive sleep apnoea | Airway inflammation associated with excessive snoring, GERD, increased cough reflex sensitivity (cough hypersensitivity) and tracheobronchomalacia, a condition in which the walls of the trachea and bronchi are weak, leading to dilation and easy collapsibility of the airways | Presence of nocturnal cough, snoring and nocturnal heartburn. Raised BMI and excessive daytime somnolence in older children or adults. Behavioural issues, tonsillar adenohypertrophy and facial abnormality in young children. Prevalence range 33–68% in patients with confirmed obstructive sleep apnoea52. CPAP therapy may be effective in alleviating cough52 |
| Ear diseases or obstructions, including excessive wax or foreign body | Activation of Arnold’s nerve cough reflex and vagal neuropathy | Cough is triggered by mechanical stimulation of the external auditory meatus. This occurs in 2% of the adult population but in 25% of people with chronic cough32. Can occasionally be identified as a cause of chronic cough when mechanical stimulation of the external auditory meatus is accompanied by features of cough hypersensitivity such as throat irritation and allotussia226 |
| ‘Cardiac cough’: premature ventricular contractions, cardiac arrhythmias and heart failure | Haemodynamic changes in the pulmonary circulation, activation of cardiopulmonary C fibres or effect of pulmonary oedema | Ventricular arrhythmia-induced cough and of cough syncope may be present in 5% of cases of ventricular arrhythmia227. Nocturnal cough can be a symptom of patients with cardiac failure and could represent the effect of airway oedema on cough receptors in large airways or the pressure of enlarged left atrium on cough receptors in airways |
| Peripheral sensory neuropathy with or without ataxia | Genetic mutations and/or nerve pathology leading to altered sensory neuron function | A rare autosomal dominant hereditary sensory neuropathy associated with chronic cough, cough hypersensitivity and gastro-oesophageal reflux228 |
| Tracheobronchomalacia or expiratory central airway collapse | Possible problems clearing airway secretions or changes in mechanical properties of the trachea during breathing | An excessive dynamic airway collapse of the posterior membrane presenting with a seal-like barking cough caused by excessive vibration of posterior tracheal wall. This condition can mimic or coexist with asthma, COPD and bronchiectasis. This condition is often associated with poor airway clearance of secretions |
| Diffuse panbronchiolitis | Airway and lung inflammation | Chronic cough may be the sole or predominant symptom. Patients can have normal respiratory function or mild airflow limitation, normal chest X-ray findings and mild dilation of the bronchiolar passages and a ‘tree-in-bud’ pattern on chest high-resolution CT. Cough can improve with long-term macrolide antibiotic therapy in some patients |
| Lung and airway tumours | Lung cancer causes of cough include the direct effect of tumour mass leading to obstruction, collapse of lung or pleural or pericardial effusion, treatment of cancer with thoracic irradiation and chemo- and/or immunotherapy229 | A change in cough pattern in a smoker can indicate lung cancer |
| ILDs including interstitial pulmonary fibrosis and systemic sclerosis-associated ILD | Airway and lung inflammation or activation of cough receptors in fibrosis by neuroinflammatory factors | Cough and dyspnoea are the main presenting features with, often, chronic cough being the main distressing symptom. Other causes of chronic cough need to be excluded such as GERD, obstructive sleep apnoea, emphysema, lung cancer and asthma. Often accompanied by features of cough hypersensitivity with an increase in capsaicin cough sensitivity |
| Somatic cough syndrome (psychogenic cough) and tic cough (habit cough) | Tic or habit cough in children (rare in adults) is possibly anxiety related, whereas somatic cough syndrome could be caused by psychological–functional disorder (transfer of psychological distress into a physical symptom) | Tic cough is a single repetitive cough, of maybe barking/honking character, usually absent in sleep. DSM-5 diagnostic criteria must be met for a diagnosis of somatic cough syndrome: “disruption of daily life; excessive thoughts about the seriousness of the symptoms, persistent anxiety about health or symptoms, or excessive time and energy devoted to symptoms or health concerns; and persistence of symptoms (typically more than six months)”109 |
| Parasitosis | Airway and lung eosinophilic inflammation and mechanical stimulation | Parasitosis such as paragonimiasis, caused by a lung fluke, Paragonimus westermani, which infects the lungs after eating an infected raw or undercooked crab or crayfish, or mammomonogamosis, caused by Mammomonogamus laryngeus, which inhabits the upper respiratory region in the human trachea, bronchi or larynx, is a rare but relevant cause in some tropical regions or in travellers. These disorders can often present as a dry persistent cough with normal chest X-rays. Blood eosinophilia is frequent230,231 |
| Hypereosinophilic syndrome | Airway eosinophilic inflammation with or without FIP1L1–PDGFRA fusion gene and aberrant tyrosine kinase activity | Presents with chronic cough as the sole or predominant symptom, hypereosinophilia in blood and sputum, and responds well to imatinib118. |
COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, 5th edition; GERD, gastro-oesophageal reflux disease; ILD, interstitial lung disease.
Environmental factors
Occupational irritants such as fumes, gases, cleaning products or dust55 may cause cough, either by triggering cough reflex or by inducing oxidative stress and eosinophilic inflammation56–58. However, the precise effect of environmental factors on chronic cough remains elusive. Air pollution is an important risk factor for chronic cough particularly in East Asia where air pollution levels are high15,59,60. At the population level, the annual level of fine particulate matter with a diameter of ≤2.5 μm (known as PM2.5) is higher in Asian countries than in European or North American countries, but the prevalence of chronic cough is lower61. Data from within-population studies suggest that the degree of air pollution is associated with the incidence of chronic cough or bronchitis62,63, highlighting potential host–environmental interactions in developing chronic cough.
Mechanisms/pathophysiology
The sensorimotor phenomenology of cough (Box 1) suggests the involvement of a complex suite of neurobiological processes involving the peripheral nervous system, brainstem and higher brain in this phenomenon. Cough can be a reflex or can be under volitional and cognitive control. It is typically initiated by irritant stimuli that activate airway mucosal sensory nerve fibres. These fibres convey this information to brainstem circuitry, which alters the normal breathing cycle to a cough motor pattern. Volitional and cognitively controlled cough is often accompanied by an irritant sensation known as the urge to cough. Volitional and cognitive control of cough and the perception of an urge to cough involve neural processing in subcortical and cortical brain sites.
Box 1 Sensorimotor phenomenology.
Cough is an observable respiratory event in which the pattern of normal eupneic breathing is temporarily altered to allow for a forceful expiration, the normal purpose of which is to clear the airways of foreign materials, chemicals or secretions.
A typical cough consists of three respiratory phases: first, a brief inspiratory phase to prime the lungs with a volume of air; second, a compression phase characterized by expiratory muscle contraction against a closed glottis, needed to ramp up intrapulmonary pressure; and finally third, the expiratory phase during which the glottis opens and high-velocity expiratory airflow occurs233.
Some types of cough may not include an inspiratory phase, especially during bouts of repetitive coughing. In events with glottic closure and expiratory effort, without preceding inspiration, the event is referred to as an expiration reflex. In the clinic, expiration reflexes cannot be distinguished from cough as they both produce similar sounds. The identification of an expiration reflex requires assessment of airflow with a pneumotachograph in the laboratory. The clinical relevance of the expiration reflex has therefore been difficult to study233.
The induction of cough motor patterning is often linked to a reflex action, initiated by sensory detection of irritant stimuli in the airways leading to a brainstem-mediated activation of cough motor pathways234, in much the same way that painful stimuli initiate spinal withdrawal reflexes. However, cough can also be a purely volitional act, initiated at will in the absence of any peripheral sensory stimuli. Similarly, voluntary control can be exerted to behaviourally change the intensity of an evoked cough effort, or to suppress coughing entirely107,235,236.
Airway irritation can also give rise to perceivable sensations (such as an itchy or scratchy throat) referred clinically as the urge to cough. These sensations are thought to provide an awareness of the presence of irritating airway stimuli, and often contribute as much to patient morbidity as does cough itself. The urge to cough may be an important determinant of behavioural cough induction or regulation81,237.
Studying cough and cough hypersensitivity
Human neurophysiological studies have contributed to our understanding of cough and cough hypersensitivity, but they are limited by the types of measurements and interventions possible. Detailed mechanistic studies into cough neurophysiology have, therefore, relied heavily on laboratory animals, especially guinea pigs and cats. Cough in these species can be reliably induced with stimuli that also evoke cough in humans64, and, as such, studies in these models have identified mechanisms that lead to sensory nerve activation, normal cough induction and neural pathways that mediate reflex coughing65. However, the use of animals to model the development and maintenance of cough hypersensitivity in humans has been debated64 as animals used to study pathological cough are largely devoid of any spontaneous coughing, requiring cough induction with an inhaled stimulus to assess the hypersensitivity. Moreover, although reflex cough hypersensitivity can be demonstrated in animal models, therapies that reverse this hypersensitivity have mostly not been clinically effective in humans12.
Peripheral neurophysiology
In guinea pigs, two peripheral sensory fibre subtypes can initiate cough when stimulated: thinly myelinated Aδ-fibre subtype and nociceptive unmyelinated C-fibre subtype65–68 (Fig. 2). The cell bodies of cough Aδ fibres and C fibres arise from distinct vagal ganglia65,66 (nodose ganglion for Aδ fibres and jugular ganglion for C fibres), and their peripheral terminations are believed to reside predominantly in the major airways (larynx, trachea and large bronchi)65, although terminations in the lung parenchyma cannot be discounted, as several parenchymal lung diseases present with chronic cough51. Molecular analyses show differing patterns of gene expression and novel mechanisms of regulation69,70. Aδ-fibre-mediated cough is triggered by aspirated particulates, accumulated secretions and mucosal acidification66, as might happen after aspiration of gastric contents. Conversely, C-fibre-mediated cough is triggered by a range of irritant environmental chemicals and mediators of inflammation or tissue damage65. Cough challenge studies and histochemical staining of airway biopsy samples suggest that these two cough pathways also exist in humans64,68,71. A combination of animal and human studies has provided a comprehensive understanding of the functioning of ion channels and receptors responsible for excitation of cough sensory fibres12 (Fig. 2).
Fig. 2. Neural pathways and mechanisms that contribute to the generation of cough.
(1) Vagal sensory neurons that are involved in cough innervate the larynx, trachea and main bronchi and, possibly, the lung parenchyma (blue and green dashed lines). Vagal Aδ fibres (whose cell bodies reside in the nodose ganglia) are activated by mechanical stimuli (such as inhaled particulate matter, mucus and aspirated gastric contents) and protons whereas vagal C fibres (whose cell bodies reside in the jugular ganglia) are activated by irritant chemicals and inflammatory mediators. (2) Vagal fibres involved in cough express several ion channels and receptors needed for transduction of diverse sensory stimuli and the formation, conduction and regulation of action potentials and (3) project to brainstem nuclei to coordinate cough motor patterning. (4) Distinct networks in the higher brain are involved in the behavioural regulation of cough, encoding of the urge to cough and for cognitive and affective processing. (5) Central mechanisms allow for volitional and cognitive modulation of cough through top-down regulation of brainstem processing (black dashed lines). AITC, allyl isothiocyanate; ASICs, acid sensing ion channel subtypes; B2, bradykinin type 2 receptor; CLC, chloride channel subtypes; H+, protons/acid; Nav, voltage-gated sodium channel subtypes; NGF, nerve growth factor; NKCC1, sodium (Na+) potassium (K+) chloride (Cl−) co-transporter; P2X, purinergic receptor subtypes; PG, prostaglandin; PGR, prostaglandin receptor; TrkA, tyrosine receptor kinase A; TRP, transient receptor potential cation channel.
Central neurophysiology
In mammals, many vagal sensory neurons terminate in the nucleus of the solitary tract, an important sensory processing nucleus in the medulla oblongata65 (Fig. 2). Studies in guinea pigs suggest that the regions involved in integrating signals from cough sensory neurons have little overlap with other vagal neuronal subtypes that innervate the airways and lungs65,72. The paratrigeminal nucleus also receives inputs from vagal cough sensory neurons73. Different subtypes of vagal sensory neuron terminate in the nucleus of the solitary tract and the paratrigeminal nucleus, and neurons from these brain regions have differing output connectivity65,72,74,75. In guinea pigs, cough mediated by jugular C fibres is reduced by targeted lesioning of the paratrigeminal nucleus, whereas Aδ-fibre-mediated cough is unaffected75. Although precision mapping of sensory terminations in the human brainstem is not feasible, functional brain imaging studies using stimuli that differentially activate nodose and jugular neural pathways support the conservation of this wiring in humans71.
Glutamate (mediated by post-synaptic NMDA (N-methyl-d-aspartate) receptors)76,77 and, possibly, neurokinin (NK) receptors67,75,78 are involved in cough in guinea pigs. Indeed, NMDA and NK1 receptor blockade in animals and humans has antitussive actions, supporting a role for these receptors in cough79,80. In rodents, brainstem neurons receiving cough sensory inputs are involved in autonomic, limbic and somatosensory processing73 (Fig. 2). In humans, functional brain imaging studies have found widely distributed brain activity accompanying inhalation of cough-evoking stimuli, in primary and secondary sensory cortical areas, and in the cingulate, insula and orbitofrontal cortices. This activity likely reflects the diverse autonomic responses, and the affective, hedonic and discriminative sensory experiences that accompany cough81.
Peripheral sensitization of cough
Experimental induction of lung inflammation, for example, with allergens or respiratory viruses, in animals and in several airway diseases or treatments in humans, is accompanied by alterations in the excitability of the peripheral terminals of vagal sensory fibres that regulate cough65 (Fig. 3). For example, impaired metabolism of bradykinin, a potent activator of vagal C fibres is linked to coughing in patients using ACE inhibitor antihypertensive therapy82. Similarly, excess release and/or impaired metabolism of ATP is associated with refractory chronic cough12.
Fig. 3. Peripheral and central processes contributing to cough hypersensitivity.
(1) Preclinical studies have described potential mechanisms that affect vagal sensory nerve fibres that are driven by the inflammatory pathology of the underlying diseases and potentially reversed by disease-specific therapy. (2) Functional synergy may also exist between sensory neurons innervating the various tissues shown. These interactions likely occur at the level of the brainstem, where convergence of vagal and/or trigeminal inputs leads to enhanced cough sensitivity. Peripheral organ pathologies have also been shown to alter synaptic efficacy in the brainstem, indicative of state of central sensitization. Patients with cough hypersensitivity have (3) increased activity in midbrain areas, and (4) a reduced ability to suppress coughing owing to a failure to recruit descending brain networks that subserve cough suppression. (5) Patients with chronic cough have a range of effects in the cognitive domain, suggestive of altered cortical processing of airway sensory information. Drugs that target vagal sensory neurons and inhibit their activity, neuromodulatory drugs that target brain processes involved in maintaining hypersensitive states, and speech and language therapy aimed at improving cough control, are all clinically useful antitussive options for patients with troublesome cough.
Inflammation can also cause plasticity of airway mucosal innervation, including changes in receptors, ion channels, neurochemistry, fibre density or the cells that contribute to fibre excitation65,83,84. For example, patients with chronic cough had a ~30-fold increase in cough responsiveness to inhaled capsaicin that was accompanied by an increased density of TRPV1-expressing fibres in bronchial biopsy samples85. In accordance with these data, studies in animal models have demonstrated a central role of upregulated TRP channel expression or activity, including TRPV1, TRPA1 and TRPV4, in development of hypersensitivity to inhaled cough challenges86,87. Of note, however, clinical trials of TRPV1, TRPA1 and TRPV4 antagonists have failed to demonstrate any benefit against natural cough in patients12,87, suggesting a disparity between animal and patient studies.
Animal studies also suggest that neural plasticity during pulmonary pathologies may relate to neuroinflammation within the vagus nerve or ganglia, characterized by increased inflammatory cell influx, upregulated inflammatory gene transcription and the release of inflammatory molecules from sensory neurons and resident or infiltrating immune cells88–90. The cause of this neuroinflammation is unclear, but likely relates to both peripheral vagal detection of tissue inflammation and adverse effects of inflammation-induced persistent firing of action potentials in sensory neurons88,91. Whether this occurs in humans is not proven, but is hypothesized as a cause of cough in some patients92.
Functional interactions between sensory fibre subtypes in the brainstem can also affect cough. In humans, mechanical stimulation of the external ear can evoke coughing (Arnold’s reflex)32, which is attributed to activation of the auricular branch of the vagus nerve, which projects to the paratrigeminal nucleus74,93. Co-activation of cough Aδ fibres and C fibres may induce cough hypersensitivity94, whereas coughing is inhibited by activation of nasal menthol-sensitive sensory fibres, lung stretch receptors and a subtype of C fibres innervating the lungs76,95,96.
Central sensitization of cough
Airway inflammation in animal models of lung disease is accompanied by altered synaptic transmission, and glial cell mobilization and activation within the nucleus of the solitary tract, resulting in elevated inflammatory mediators in the brainstem, including neurotrophic factors (NGF, BDNF and GDNF) and cytokines (IL-1β, IL-6 and TNF)97–100. These processes are expected to contribute to cough hypersensitivity via amplifying inputs from cough sensory fibres. Consistent with this, upregulated cough network activity in the midbrain has been demonstrated in patients with cough hypersensitivity101, in regions reportedly involved in the development of other sensory hypersensitivities102,103.
In humans, cough and the urge to cough are highly responsive to placebo104,105. The mechanisms of this phenomenon are comparable to those of placebo analgesia and involve recruitment of a descending neural pathway enacting opioid-dependent suppression of sensory processing in the brainstem105,106.
Cough in humans can be voluntarily induced (or enhanced) and suppressed through higher brain motor control pathways107,108. In some patients, especially children, behavioural coughing (somatic cough syndrome) may be the primary cause of chronic cough109. Volitional cough suppression involves a brain network important for general motor response inhibition107,110,111. Patients with refractory chronic cough have attenuated volitional cough suppression112,113 and impaired engagement of this cough inhibition network101. Cough and related brain activity are also modulated by acute painful stimuli applied to the skin86,114, via an extension of the conditioned pain modulation (CPM) phenomenon whereby noxious stimuli applied to one part of the body inhibit the processing of noxious stimuli applied elsewhere. CPM of cough is also reduced in patients with refractory chronic cough114. These observations suggest that the pathophysiology of refractory chronic cough involves altered efficacy of multiple central cough suppression processes. Components of these inhibitory systems that regulate cough use the inhibitory neurotransmitter GABA, and GABA receptor agonists modify evoked coughing in animals and in humans115,116. Patients with COPD do not have altered volitional cough suppression112, suggesting that distinct neural endotypes contribute to chronic cough81.
Cough in common diseases
Chronic cough in adults is commonly associated with asthma, non-asthmatic eosinophilic bronchitis, GERD, upper airway conditions (including nasal and sinus disease) and laryngeal dysfunction46. However, chronic cough does not occur in all patients with these diseases, suggesting that additional pathophysiological processes are involved. The specific aetiology of cough hypersensitivity and chronic cough likely differs both between and within patient groups, indicating that unique cough endotypes exist81. However, several general processes are thought to be important (Fig. 3).
Direct sensitization or activation of cough
In asthma, the mediators of bronchopulmonary inflammation might directly affect airway nerve fibre activity (Fig. 3). In some patients with asthma, chronic cough is the sole presenting symptom (known as cough variant asthma), whereas other patients with chronic cough have eosinophilic disease but not asthma, including non-asthmatic eosinophilic bronchitis and hypereosinophilic syndrome117,118. Whether different inflammatory processes contribute to cough across this spectrum of patients is unclear (Box 2). In patients with GERD, chronic cough could occur directly through refluxate stimulating vagal sensory fibres in the larynx and airways. However, studying this theory is difficult as the detection of laryngopharyngeal reflux is technically challenging with no objective measures for validation119,120, and detection of microaspiration through pepsin or bile acids in saliva, sputum or airway samples may not be reliable121. Of note, pepsin levels in patients with chronic cough are not different from those in healthy controls122–124. Gaseous reflux might also be important in chronic cough, but supportive evidence is lacking. In patients with upper airway cough syndrome, inflammation or mucus from the nose or sinuses may extend or drip down the pharyngeal wall to the larynx and activate cough sensory fibres. However, although many patients with chronic cough complain of a sensation of post-nasal drip, there is a paucity of research exploring the relevance of these processes125.
Box 2 Cough in asthma and related disorders.
Cough is a common symptom of asthma. Patients with less well-controlled asthma than those with well-controlled asthma usually cough more frequently238, and bronchoconstriction and allergen exposure are known to sensitize and/or provoke coughing in people with asthma239,240. However, in the laboratory, bronchoconstriction and cough pathways can be separately inhibited241,242, and the severity of cough does not always reflect asthma severity. Some patients with well-controlled asthma may experience chronic cough. Chronic cough is the primary presenting symptom in patients with cough variant asthma238, while in the paediatric literature isolated cough is rarely asthma143.
Patients with classical asthma have heightened responses to inhaled irritants such as capsaicin compared with healthy controls, although not to the same degree as those presenting with chronic cough240,243. The mechanisms leading to this remain elusive. Airway nerve density and neuronal branching are increased in bronchoscopy samples obtained from patients with classical asthma, like those seen in patients with chronic cough without asthma84,244.
The precise mechanisms that underlie why some patients develop cough variant asthma as opposed to other asthma phenotypes is unclear, and few studies have compared cough variant asthma with other phenotypes. Sputum eosinophilia might be less in cough variant asthma than in typical asthma, and patients with cough variant asthma have normal ventilation function or less severe impairment of lung function245,246. However, patients with non-asthmatic eosinophilic bronchitis, without variable airway obstruction or hyper-responsiveness characteristic of asthma, also present with chronic cough247.
The localization of mast cells within airway smooth muscle in patients with asthma but not in patients with non-asthmatic eosinophilic bronchitis has been proposed as an explanation for the lack of bronchial hyper-responsiveness in non-asthmatic eosinophilic bronchitis. However, as other histological features are similar, it is unclear why patients with non-asthmatic eosinophilic bronchitis present with chronic cough without airway obstruction and hyper-responsiveness248. The difference in airway function observed in subjects with eosinophilic bronchitis and asthma could be due to differences in mediator (such as prostaglandin E2 (ref.249)) production in the airways. Eosinophils may interact with airway sensory nerve fibres in people with asthma and promote increased airway sensory fibre density, nerve remodelling and airway hyper-reactivity244, while no relationship between eosinophils and increased nerve fibre density was noted in patients with chronic cough without asthma84.
Indirect facilitation of cough
Direct stimulation of the oesophagus or nose rarely evokes coughing. However, activating extrapulmonary sensory fibres can act synergistically with cough sensory fibres to produce cough hypersensitivity65,126–128 (Fig. 3). Oesophageal acid instillation, for example, via a naso-oesophageal catheter, sensitizes cough evoked by inhaled capsaicin in healthy volunteers and causes coughing in patients with chronic cough129,130, while application of capsaicin to the nose of healthy volunteers sensitizes cough, and nasal menthol inhibits coughing95,131. Coughing follows reflux events in patients with chronic cough more often than would be expected by chance alone132–134, of which both acid and non-acid reflux events are equally likely to precede coughing whereas reflux events extending to the proximal oesophagus are not more likely to precede coughing than distal events. These observations suggest that reflux, mucus or inflammatory mediators need not reach the airways to modulate coughing.
Diagnosis, screening and prevention
Initial evaluation of patients with chronic cough generally occurs in primary care, where treatment is commenced in those with symptoms, clinical signs and/or investigations that point to one or more potential underlying causes. Referral to secondary care specialists often occurs for more detailed investigation in those with an elusive cause of cough and in those who do not respond to treatment in primary care. Referrals are typically to pulmonary specialists, but may also be to allergists, ear, nose and throat specialists or gastroenterologists, depending on the clinical presentation. Adults with multiple comorbid conditions can be referred to multiple specialities, making the patient journey from diagnosis to assessment and treatment long. Patients with persistent cough despite detailed evaluation and further treatment trials in secondary care can be referred to specialist cough clinics. These clinics review the patients’ work-up to ensure optimal treatment of comorbid conditions that might be driving cough. In adults, attendance at a specialist cough clinic is often required to confirm a diagnosis of refractory cough or unexplained chronic cough, to use therapies that are of benefit in this patient group and often to provide opportunities for patients to participate in clinical research including trials of novel therapies. However, the provision of such services varies substantially between countries.
Clinical characteristics
Cough is an explosive effort associated with a characteristic sound (Box 1). The distinct quality of the sound can be characterized, for example, wet cough (moist, loose, productive or rattling) or dry (barking or hoarse). Wet chronic cough is thought to be associated with diagnoses characterized by mucus production such as chronic bronchitis, COPD and bronchiectasis. Although clinicians can distinguish reliably wet from dry cough, the ability to diagnose the cause of cough from sound is poor; only 34% of cases in one case series135. In some patients, two or more distinct types of cough can coexist, for example, a wet and dry cough. In the clinic, the frequency of cough sounds can be counted and the intensity of individual cough efforts measured136. However, patients seldom describe the frequency of cough and are more likely to describe clusters of cough or bouts, cough intensity and cough triggers, typically providing subjective accounts of when they occur and how much it bothers them. The pattern of chronic cough may show waxing and waning over weeks or months.
Adults with chronic cough also frequently report throat clearing. Throat clearing, like cough, is thought to be an action to clear an unpleasant sensation or irritation and may represent an aspect of laryngeal dysfunction in chronic cough. Throat clearing may also result from the feeling of mucus stuck at the back of the throat that needs to be cleared, leading to mucus being swallowed. Throat clearing may be part of the spectrum of cough events but whether its presence is associated with specific diagnoses is not known. Whether patients distinguish throat clearing from cough, and the effect of this on patients’ perception of their morbidity is unknown.
Adults with chronic cough often display characteristic signs of cough hypersensitivity. Clinically this presents as allotussia, with patients reporting cough triggered by innocuous stimuli such as talking, eating and perfumes, and/or hypertussia, a heightened cough sensitivity to known tussive stimuli such as smoke, fumes and bleach11,120. Another common symptom of cough hypersensitivity is the presence of an uncontrollable urge to cough. Sensations in the larynx (laryngeal paraesthesia) or chest such as tickle, itch or irritation can trigger coughing and can be more bothersome than the cough itself137. The presence of triggers and sensory symptoms may be the only feature to suggest the diagnosis of cough hypersensitivity. Although sensory symptoms are present in most adults with chronic cough, <5% report no triggers or urge to cough138. Many patients with chronic cough, in addition to showing signs of cough hypersensitivity, also display laryngeal hypersensitivity139. Laryngeal hypersensitivity and dysfunction often present with chronic cough that is associated with vocal cord dysfunction, muscle tension dysphonia and globus (a sensation of a ‘lump’ in the throat (Box 3)).
Box 3 Laryngeal hypersensitivity and dysfunction in chronic cough.
Laryngeal hypersensitivity refers to the excessive, abnormal, laryngeal adduction of the vocal cords during breathing or exercise, resulting in laryngeal dysfunction250. Laryngeal hypersensitivity and dysfunction represent an increased responsiveness of laryngeal protective reflexes triggered by mechanical or chemical stimuli and are considered to be part of the cough hypersensitivity syndrome.
Laryngeal hypersensitivity and dysfunction are present in many patients with chronic cough and cough hypersensitivity139,251. They are often associated with comorbid post-nasal drip, rhinosinusitis, gastro-oesophageal reflux disease and asthma252.
Symptoms are usually localized to the laryngeal area, for example, ‘scratchy’ or ‘tickly’ feeling of an urge to cough, or sometimes inspiratory stridor of airflow or feeling of suffocation or difficulty in breathing.
Laryngeal dysfunction in patients with refractory chronic cough has been associated with paradoxical vocal fold movement manifesting as vocal cord dysfunction with episodes of suffocation or difficulty in breathing or laryngospasm253. Other aspects of laryngeal dysfunction include muscle tension dysphonia that can be revealed during vocalization250.
Investigations include direct laryngoscopic examination of vocal fold motion during challenge (using external triggers such as exercise or scents), laryngeal electromyogram and voice assessment254.
Laryngeal hypersensitivity and dysfunction in patients with chronic refractory cough may respond to speech pathology intervention and behavioural management of cough, with the use of voice therapy techniques and breathing exercises255. Cough neuromodulators such as amitriptyline and gabapentin might also be beneficial.
Diagnostic work-up
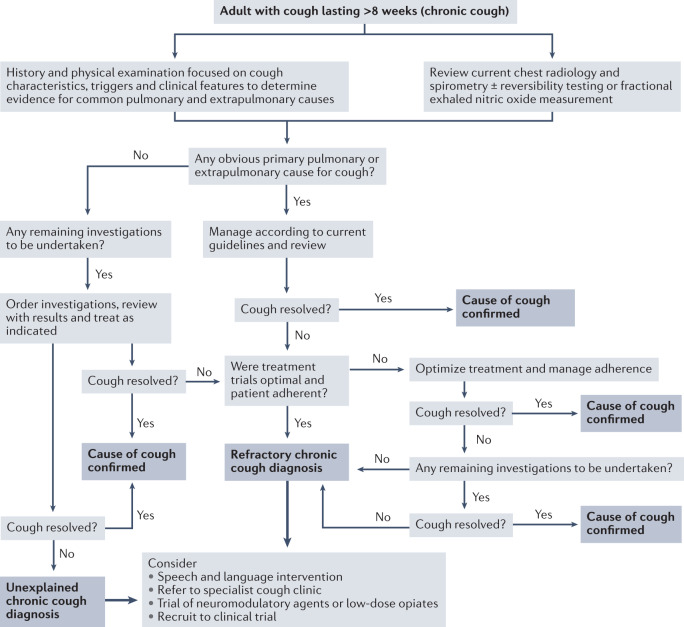
Recommendations for the evaluation of adults with chronic cough (Fig. 4) are based on the anatomical diagnostic protocol first proposed more than 40 years ago125 and founded on the principle that structures innervated by vagal sensory fibres represent potential sites for generation of chronic cough3,140. This remains a useful approach as it can assist with identifying possible treatable conditions in some patients. Any comorbid conditions involving these structures are initially presumed to be the cause of cough, recognizing that treatment of the presumed cause does not always improve the cough. Consequently, initial clinical assessment, investigations and trials of therapy tend to be focused on asthma, GERD and upper airway conditions (notably rhinitis, rhinosinusitis). A clinical history, focused on cough characteristics, associated sensations (such as urge to cough and need to clear throat), common cough triggers, concomitant symptoms, combined with physical examination, represent the first important steps in evaluation of a patient with chronic cough3,140. Concomitant symptoms associated with gastro-oesophageal reflux, and rhinosinusitis may indicate the causes of chronic cough, but a reliance solely on symptoms to guide management may be misleading. For example, the presence of upper airway symptoms may reflect only co-existent rhinitis or rhinosinusitis, and the absence of heartburn does not exclude reflux as the cause of the cough3,141. Clinical findings are frequently unremarkable in patients referred with chronic cough but finger clubbing, evidence of inflamed and/or obstructed nasal passages or the presence of wheeze or crackles on chest auscultation should inform consequent investigations and treatment. Chest radiography and spirometry are mandatory for patients undergoing assessment for chronic cough3. Measurement of markers of type 2 inflammation, such as fractional exhaled nitric oxide (FeNO) or sputum eosinophil count, may be useful in the early stage of work-ups of patients with chronic cough. Additional tests such as chest CT, polysomnography and bronchoscopy should be requested depending on the physician’s review of the case as set out in Supplementary Table 1.
Fig. 4. Evaluation and management of chronic cough in adults.
A proposed algorithm for the clinical management of patients with chronic cough, including recommendations for managing difficult-to-treat cough. The algorithm was devised using recommendations contained in existing clinical guidelines and other reference material3,4,10,46,232.
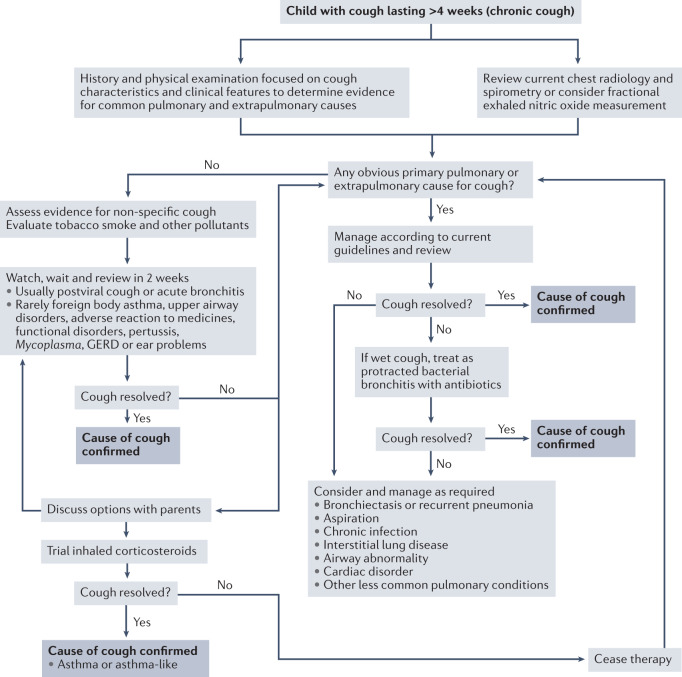
To help identify the underlying cause of chronic cough in children, paediatricians use the basic constructs of cough characterization, pointers (such as traits) and red flags (Fig. 5). These concepts are useful in current clinical practice, evidenced by calculation of likelihood ratios142 and incorporation of these facets in paediatric cough algorithms143,144. Using cough algorithms to manage paediatric chronic cough is efficacious in improving clinical outcomes, demonstrated in cohort studies145, randomized controlled trials (RCTs) based in specialist clinics145 and primary care144. Further details of the paediatric diagnostic protocols are contained in the current guidelines3,143.
Fig. 5. Evaluation and management of chronic cough in children.
A proposed algorithm for the clinical management of paediatric patients with chronic cough. The algorithm was devised using recommendations contained in existing clinical guidelines3,143. GERD, gastro-oesophageal reflux disease.
Cough assessment tools
Several validated tools are available to assess cough in clinical practice (Supplementary Box 1). Subjective assessments of cough evaluate the patient’s perspective and include cough severity, intensity, effects on health-related quality of life (HRQOL) and triggers and symptoms suggestive of cough hypersensitivity. Objective assessments of cough measure cough frequency with sound or physiological measures (electromyography (EMG), airflow or chest wall movement), intensity and the sensitivity of the cough reflex. Objective measures are important to ensure that measurements are specific to the disease and not influenced by comorbid conditions or traits such as anxiety and depression. The objective assessment of cough is largely confined to research and clinical trial use because the tools involve time-consuming analysis, are expensive, and their clinical usefulness has not been established. The relationship between objective and subjective measures of cough is moderate at best146, suggesting that subjective and objective measures assess unique aspects of cough and should be viewed as complementary and equally valuable tools for evaluation of patients.
Cough monitoring tools have been useful to evaluate the efficacy of cough medicines, owing to the smaller sample size required for studies than for subjective tools. Moreover, monitoring tools address the preference of some medicines regulatory agencies to include objective end points in clinical trials147. Cough reflex sensitivity tests have been used extensively in preclinical mechanistic studies and for drug development and dosing148. They are often not useful for assessing the efficacy of treatment as they are not predictive of a reduction in coughing in patients149. Although patients with cough have significantly lower thresholds for coughing during challenge tests compared with healthy individuals, the ranges can overlap, limiting their diagnostic potential150. Improving the sensitivity and specificity of cough challenge tests might be possible by standardizing equipment, protocols and analysis methods151.
Screening and prevention
Screening for chronic cough is not carried out in clinical practice. How screening could be done and whether it would lead to clinical benefit is unclear. Screening patients with chronic respiratory disease may be beneficial as cough is often overlooked during clinical evaluation. Moreover, early identification may improve the quality of life (QOL) of patients and possibly avoid over-treatment by specifically targeting cough. One simple screening method is a numerical rating scale that assesses cough severity and ascertains the duration of cough. Screening the general population could identify patients with respiratory disorders such as COPD, asthma, lung cancer and smoking-related chronic bronchitis at an earlier stage. The most important diagnosis is that of lung cancer, where development of a cough may be the first symptom, particularly in a smoker.
Whether non-smoking-related chronic cough is preventable is unknown. A greater understanding of the mechanism of cough, particularly cough hypersensitivity, is needed.
Management
The management of patients with chronic cough can be prolonged and complex, especially in patients with multiple comorbidities requiring treatment and when the cough may ultimately be refractory to such interventions. Therefore, management includes treatment of comorbid conditions that are potentially driving chronic cough and therapies directed at cough hypersensitivity in patients with refractory or unexplained cough.
Disease-specific therapy
For many adults with chronic cough, treatment of comorbid asthma, GERD or nasal disease (upper airway cough syndrome) improves their cough. However, few RCTs have assessed the efficacy of treatments compared with placebo in reducing cough in those with these conditions.
Asthma
Several RCTs have assessed the efficacy of asthma therapies in patients with asthma and chronic cough, but few trials have been performed in the past decade, so more recent inhaled therapies are poorly represented44. The CHEST and European Respiratory Society guidelines suggest increasing inhaled corticosteroid dose and considering trials of leukotriene inhibitors and β-agonists in those for whom the treatment response to inhaled corticosteroids is incomplete, on the basis of evidence obtained in classical asthma3,44. The use of inhaled corticosteroid is considered the first-line treatment in adults with cough variant asthma. Of note, the efficacy of inhaled therapies for asthma in patients with chronic cough can be subverted if the inhaled treatment evokes coughing. In this situation, a short course of oral prednisolone can be the simplest means of assessing the response of chronic coughing to asthma therapy. In children, chronic cough should not be attributed to asthma unless other symptoms or signs are present152, and cough in paediatric asthma should not be regarded as a marker of severity.
GERD
Treatments for GERD include proton pump inhibitors (PPIs, which reduce the acidity of refluxate), histamine (H2) receptor blockers (which have a similar, but more prolonged, effect to PPIs) and lifestyle measures such as weight loss, elevating the head of the bed and avoidance of eating before bedtime. Small RCTs of acid-suppressing therapy (PPIs and H2 receptor blockers) in patients with chronic cough have been performed but did not report positive results; a pooled analysis of these data suggests that adults with acid reflux on 24 h pH monitoring or symptoms of heartburn are most likely to experience benefit, which is now reflected in clinical guidelines4,141,153. Observational data support this approach, but response rates were low (28%) even in patients with chronic cough who reported heartburn154. Treatments for non-acid reflux are limited. Promotility agents (such as the dopamine antagonists metoclopramide and domperidone) can be prescribed only for short-term use owing to their adverse effects and lack of evidence of efficacy in reflux-related chronic cough. Macrolide antibiotics promote gastric emptying, but small studies in patients with refractory chronic cough failed to show benefit155,156. Baclofen, a GABAB agonist, blocks relaxation of the lower oesophageal sphincter, and therefore all types of reflux, but has unacceptable adverse effects for long-term use, whereas lesogaberan (a peripherally acting GABAB agonist with fewer adverse effects) did not reduce cough frequency in patients with refractory chronic cough in a RCT. Laparoscopic fundoplication, a keyhole surgical procedure whereby the upper part of the stomach is wrapped around the lower oesophagus so as to prevent the reflux of acid from the stomach into the oesophagus, in patients with GERD and chronic cough is rarely indicated owing to limited evidence for efficacy and high risk of long-term complications.
The management of children with GERD is dependent on age and disease severity. Acid-suppressive therapy should not be used solely for chronic cough in children with GERD and chronic cough157. Also, treatment of children with chronic cough is dependent on their age, feeding regimen and symptoms. PPIs and H2 receptor antagonists should not be used for longer than 4–8 weeks in paediatric patients, regardless of effectiveness, without further evaluation157. Should they prove to be successful in controlling the cough, they can be continued for longer periods, although the guidelines for managing paediatric GERD recommend a gastroscopy with biopsies and other tests to confirm anatomy and exclude other causes by histology of the biopsy samples158.
Upper airway cough syndrome
The treatment of patients with chronic cough and nasal disease is standard care for the diagnosed nasal condition. Allergic or nonallergic rhinitis is treated with nasal corticosteroids, first or second generation and intranasal antihistamines, decongestants and, if sinusitis is present, antibiotics. The potential role of nasal surgery in patients with chronic cough is unclear159,160. Clinical trials evaluating the effectiveness of targeting concurrent nasal diseases to control chronic cough are lacking; therefore, predictors of improvement in chronic cough remain uncertain161.
COPD, bronchiectasis and interstitial lung disease
Cough, typically productive, is a common first symptom of COPD and is usually attributed to cigarette smoking and also as a result of exposure to environmental pollutants162. Chronic bronchitis is a distinct phenotype of COPD and is defined as chronic cough productive of sputum for 3 months over the course of a year for two consecutive years163. In established disease, cough is reported in 70% of patients47 and many consider it to be extremely severe. Moreover, cough is a prominent feature of disease exacerbations and is associated with adverse clinical outcomes48,164,165. Measuring the severity and burden of cough using symptom-based questionnaires is now recommended in the routine clinical evaluation of patients with COPD166. Chronic cough accompanied by the expectoration of large quantities of mucopurulent sputum is a central clinical feature of bronchiectasis. Typically coughing is much worse during exacerbations and contributes to impaired health status167. Impaired airway clearance, mucus retention and bacterial colonization cause inflammation and lung damage, which contribute to cough severity but are independent of cough reflex sensitivity168. Cough is a common and disabling symptom in idiopathic pulmonary fibrosis and may be due to inflammatory consequences of the fibrosis itself or to comorbid reflux disease. No medicines are approved to treat cough in these patients, and current consensus is based on limited evidence51.
Nonspecific pharmacological therapies
Pharmacological therapies directed at eliminating cough are required in cases in which treatment of comorbid disease associated with cough is unsuccessful at relieving coughing, or in those with no obvious cause for a chronic cough. These therapies are restricted to use in adults as, for cough management purposes, the age cut-off for use in children is usually 14 years. Whether patients other than those with refractory or unexplained chronic cough would benefit from adjunct nonspecific cough therapy is unclear, and controlled trials are needed.
Opiates
Codeine and morphine are commonly used antitussives in adult-based clinical practice and have antitussive effects via central opioid receptors169. Accordingly, effective antitussive doses of opiates are likely to cause sedation. The opioid subtype by which opiates inhibit cough remains debatable, as μ-, κ- or δ-opioid receptor agonists are all antitussive170. Opioid receptors are also localized to vagal sensory neurons, and their activation can suppress sensory fibre activity; however, unlike oral codeine or intravenous morphine, inhaled morphine or codeine does not inhibit inhaled capsaicin-induced cough, arguing against a peripheral antitussive effect of opiates171. Opiates have no role in the management of children with chronic cough as they can cause death143.
Codeine has a rapid onset of action. Although widely used, the efficacy of codeine is not supported by clinical studies. Several placebo-controlled RCTs for cough have been published without objective cough measurements172,173, and other trials did not report significant benefits of codeine over placebo174,175. Moreover, codeine has several concerns regarding safety, inter-individual variability in metabolism and dependence176, although risk of dependence may be low in those without vulnerability to dependence177. Adverse effects of codeine, such as nausea, constipation, dyspepsia, dizziness or somnolence, occur in up to 50% of patients and are mostly non-critical in adults178.
Morphine is ~10 times more potent than codeine and is most often considered in patients with severe intractable cough. One trial demonstrated that low-dose slow-release morphine therapy was associated with significant improvements in subjective cough measures (Leicester Cough Questionnaire and daily cough severity scores) in patients with refractory chronic cough179. The most common adverse effects were constipation and drowsiness. The effects of morphine were mostly observed within a week, but benefit was observed in <50% of patients. Morphine is associated with several safety concerns, including respiratory depression, drowsiness and addiction, depending on the dose180.
Gabapentinoids
The GABA derivatives gabapentin and pregabalin are inhibitors of α2δ subunit-containing voltage-dependent calcium channels and possibly NMDA receptors181,182. Gabapentinoids freely pass the blood–brain barrier and are commonly used for treatment of seizures and neuropathic pain, although the sites of central action are poorly understood. In one 10-week RCT183, gabapentin significantly improved subjective cough measures and objective cough frequency in patients with chronic refractory cough; however, clinical benefits were not sustained after treatment cessation. No improvement in capsaicin cough reflex sensitivity was observed, suggesting a lack of effect on cough hypersensitivity. Of note, some patients do not experience any improvement in cough with gabapentin. Pregabalin, as an add-on to speech therapy, significantly improved subjective cough measures compared with speech therapy alone at week 14 (ref.184). However, the effects of pregabalin on objective cough frequency were not significant. Gabapentinoids cause common, and sometimes intolerable, adverse effects, including dizziness, disorientation, confusion, fatigue and blurred vision. The benefit of long-term use of gabapentinoids is difficult to predict given these adverse events.
Tricyclic antidepressants
Amitriptyline increases noradrenergic or serotonergic neurotransmission by blocking presynaptic noradrenaline or serotonin transporters and has strong binding affinities for α-adrenergic, histamine (H1) and muscarinic (M1) receptors185. In one randomized trial, treatment with low-dose amitriptyline at bedtime was significantly more effective than codeine and guaifenesin combinatorial treatment in improvement of subjective measures of cough in those with post-viral cough hypersensitivity186. Moreover, one small observational study found an improvement of >50% in 67% of patients with idiopathic (refractory or unexplained) cough 2–3 months after starting treatment; however, only one-third of patients were still on the treatment after 2–3 years, with 53% reporting an improvement of >50%187. A controlled, double-blind study of amitriptyline in chronic cough is needed. The most common adverse effects of amitriptyline are dry mouth, dizziness, headache and somnolence.
Nonspecific cough suppression and the risk of dystussia
Cough has an important protective function in the respiratory tract in healthy individuals and patients as it is needed to expel excessive airway secretions, prevent aspiration and protect against inhaled irritant stimuli, such as smoke. An ideal cough suppressant, therefore, would reduce unwanted, excessive coughing without suppression of protective cough, essentially targeting the hypersensitive state. Studies in laboratory animals and humans have demonstrated that opiates and other centrally acting neuromodulators (gabapentin and baclofen) can inhibit cough evoked by a broad range of chemical and mechanical stimuli188–191. This action is a dose-dependent generalized suppression of the nervous system (sedation), inhibition of the brainstem neurons involved in generating respiratory rhythm and/or direct suppression of cough sensory nerve activity169,192,193. Consequently, some nonspecific cough suppressants might cause dystussia, although the prevalence of this is not well documented. One post hoc analysis of patients receiving morphine and codeine for cough suppression suggests that sedation is unlikely to contribute to their cough-suppressing properties194, whereas gabapentin improved cough-specific QOL in patients with refractory cough without suppressing capsaicin cough reflex sensitivity183. Risk of dystussia and aspiration is likely related to dosing195,196, and some patient groups who are more prone to dystussia (such as the elderly and patients with spinal trauma or neurological disease) may be more susceptible to generalized cough suppression by centrally acting nonspecific cough therapies197,198. At least one new peripherally acting nonspecific cough therapy in clinical trial (the P2X3 antagonist, gefapixant) has been shown not to produce generalized cough suppression at therapeutic doses148.
Speech and language therapy
Speech and language therapy consists of education, cough control and suppression techniques, breathing exercises, vocal hygiene, hydration strategies and counselling199 (Box 4). Two randomized controlled studies have evaluated the effectiveness of speech and language therapy for adults with unexplained or refractory chronic cough. One study200 found that 88% of participants in the treatment group showed improvement in symptom frequency and severity scores for breathing, cough, voice and upper airway symptoms, compared with 14% of participants in the placebo group. Another multicentre RCT201 found an improvement in cough-specific QOL and a reduction in cough frequency in the treatment group compared with the control group. No significant difference was found between therapy and control groups regarding subjective measures of cough and cough reflex sensitivity.
The antitussive mechanisms of action of speech and language therapy remain unclear. One hypothesis is that improved understanding of the condition through education and counselling, along with training in suppression strategies, may affect the decreased inhibitory control of cough that is present in such patients65,92,101. However, empirical studies to address this have not been conducted. An improvement in paradoxical vocal fold movement and dysfunctional breathing may also contribute to control of cough202.
Box 4 Speech and language therapy management of chronic cough.
The approach to cough-specific speech and language therapy involves four steps.
Education. Patients are provided education on the biology of coughing, chronic cough and cough hypersensitivity, and the negative effects of repeated coughing and throat clearing are explained.
Vocal hygiene. Vocal and laryngeal hygiene and hydration are advised with a reduction in caffeine and alcohol intake. Nasal breathing with nasal douching may be recommended with nasal steam inhalation.
Cough control/suppression training. Following identification of patient cough triggers, patients are taught a range of suppression strategies including forced/dry swallow, sipping water, chewing gum or sucking non-medicated sweets. Breathing pattern re-education is used to promote relaxed abdominal breathing while inhaling through the nose.
Psycho-educational counselling. Behaviour modification is used to reduce over-awareness of the need to cough and facilitate an individual’s internalization of control over their cough and to help manage stress and anxiety.
Treatment of children
Successfully managing a child with chronic cough (leading to cough resolution) is dependent on identifying the aetiology of the cough and treating it (see current clinical guidelines3,143). Therefore, the suggested approach for managing children with chronic cough aims to identify the underlying cause, provide attention to contributing factors (such as tobacco smoke exposure) and understand the effect of the cough on the child and their parents or guardians3,143,145. Children aged >14 years are usually managed in accordance with adult pathways.
Quality of life
QOL is one of the key end points in clinical trials with novel antitussives, and it also aids clinical practice guideline decision-making3. Cough has significant effects on physical and mental health. Effects in adults include urinary incontinence, pain, sleep disturbance, interference with speech, anxiety and depression, avoidance of social situations and inability to work203. Less common but severe complications include syncope and head injury, hernia, suicidal ideation and rib fracture203. Stress urinary incontinence is under-recognized in adults with cough: ~65% of patients with chronic cough are female and 65% will experience cough-induced urinary incontinence204. Patients with cough-induced urinary incontinence are often too embarrassed to mention it to their physician. In some patients, the inability to control the urge to cough or throat tickle sensation can be worse than the cough itself138. In children, the key effects of chronic cough are annoyance, frustration, tiredness and effects on activities205, whereas effects on parents of children with chronic cough are worries on aetiology of cough, helplessness and sleep disturbance206. Data on the economic burden of chronic cough are lacking.
QOL can be assessed informally in the clinic by asking patients about the effects that are known to be associated with cough. HRQOL questionnaires can be used to quantify QOL in a validated and standardized manner. QOL tools have broad applicability, even in conditions with considerable heterogeneity that can limit the usefulness of objective tools. They capture aspects of disease severity that is not possible with objective tools, such as general health effects like fatigue and sleep disturbance, among others. In patients with cough, QOL tools can assess intensity and urge to cough, which cannot be assessed with cough frequency monitors207. This is particularly important for patients who cough infrequently but are greatly bothered by it.
Studies of QOL in patients with chronic cough using validated tools have found significantly worse QOL in women than in men30. Women had lower QOL owing to physical complaints, psychosocial issues and severe physical complaints. The greatest disparity between the sexes was due to stress urinary incontinence in women. Patients with a longer duration of cough, depression, younger age and interference with speech owing to cough also have worse QOL208. The presence of urge to cough is also highly correlated with impairment in QOL208. One longitudinal study of patients undergoing treatment for chronic cough found that improvement in cough was associated with a significant improvement in QOL at 3 months and continued improvement at 6 months208–210. This improvement was associated with improvements in urinary incontinence, urge to cough and anxiety symptoms208. Future studies to address gaps in knowledge should establish the frequency and characteristics of complications of cough to improve management and understand the direction of the relationship between cough, anxiety and depression. The economic impact of cough on individuals also needs to be investigated.
The Leicester Cough Questionnaire is the most widely used HRQOL tool in adults. This tool consists of 19 items that address physical, psychological and social domains211, has good repeatability and responsiveness, and the minimal important clinical difference has been established212. This questionnaire was used to demonstrate improvement in HRQOL in a randomized, placebo-controlled and double-blind phase III trial assessing antitussive activity of the P2X3 receptor antagonist, gefapixant213. The Cough QOL Questionnaire (CQLQ) is another validated tool for adult patients with chronic cough210. The CQLQ comprises 28 items, has good repeatability and responsiveness, and the minimal important clinical difference has been established214. HRQOL assessments are also valuable in the paediatric setting and are performed using acute cough215 or child-specific chronic cough205 HRQOL tools. As a child’s illness and management affects the QOL of their family, 27-item and 8-item generic parent-proxy HRQOL tools are available for use in conjunction with cough-specific HRQOL assessments206,216.
Little is known about the long-term outcomes of patients with chronic cough. In a study following patients for 7 years, only 14% of patients had resolution of cough although 26% reported a reduction in cough severity. No predictors of improvement in cough were found8. Chronic cough may be associated with long-term health risks, such as risks of mortality, morbidity or drug adverse effects, although these have not been elucidated in patients with chronic cough.
Outlook
Key gaps in knowledge
Despite advances in understanding the mechanisms of chronic cough and improvements in therapeutic development, our understanding of the relationship between cough hypersensitivity and chronic cough is incomplete. For example, although some therapeutic benefit has been shown using treatments that target mechanisms putatively involved in establishing or maintaining hypersensitivity, these treatments do not work for all patients and they do not provide complete resolution of cough in most patients who do show responsivity12. Whether this observation reflects the existence of multiple concurrent processes involved in cough hypersensitivity, yet-to-be-discovered ‘lynch pin’ processes or a lack of involvement of cough hypersensitivity in all patients is unclear. This is notable in children where evidence for cough hypersensitivity is lacking and targeting specific clinical conditions results in effective cough resolution.
We do not know whether any relationships exist with respect to the presentation of chronic cough across the lifespan. For example, an important question to answer is whether cough hypersensitivity evolves in some patients owing to the presence of a troublesome cough earlier in life. As such, whether children with chronic cough have a higher risk of cough hypersensitivity in adulthood is unknown. Indeed, genetic determinants might affect the risk of cough hypersensitivity, although this area of research is understudied. One challenge in investigating future health effects of chronic cough lies in the lack of a distinct diagnostic code for chronic cough in the International Classification of Diseases (ICD) system217. Establishment of a code that categorizes chronic cough as a disease will facilitate large-scale routinely collected health data to further understand the burden and epidemiology of chronic cough.
The answers to many of the lingering questions are dependent on improved understanding of the multiple clinical dimensions of cough. As mentioned earlier, subjective patient reports of disease severity and effects on QOL do not always track linearly with objective measures of cough frequency. Moreover, the aspects of chronic cough that have the greatest effects on patients’ lives are poorly understood. This lack of knowledge is problematic in trials of therapies that rely on only one output as the primary end point measure; for example, only short-term (≤24 h) objective cough frequency is used by drug regulators in clinical trials of new antitussive therapies, meaning that drugs that bring about clinically important improvements in patient-reported outcomes, but not cough frequency, are unlikely to advance. Yet in other clinical hypersensitivities, for example, chronic pain, subjective measures of pain severity and QOL are accepted as gold standard end point measures. Newer, less-invasive, devices for counting coughs over longer periods of time in natural settings, potentially assessing other aspects of cough including the variability of cough, and analysis of the sound, intensity, cough bout duration and the time of day218 may disentangle the relationships between objective and subjective measures, but ultimately an acceptance of the clinical importance of the subjective dimensions of cough is needed.
Endotypes and personalized medicine
Multiple mechanisms and aetiologies may underlie cough hypersensitivity. Recent suggestions of cough endotypes may help clinical management of cough. Conventionally, these endotypes relate to the underlying clinical condition (such as asthma, GERD or upper airway cough syndrome)81 but there may be other ways to endotype patients with cough; however, this needs to be explored. Clinical trials with P2X3 antagonists suggest that a subset of patients with refractory chronic cough respond well to purinergic inhibition, perhaps reflecting a currently unrecognized endotype213. Trials of other antitussives have similarly shown heterogeneity in responsivity that may reflect differing pathological processes driving cough in different patients. A more careful assessment of this heterogeneity may identify clear endotypes, which then allows more personalized approaches to cough management.
Emerging therapies
Improved understanding of cough neurophysiology has advanced the development of new antitussive therapies, several of which are progressing through clinical trials (Supplementary Table 2). Molecules targeting P2X3 and P2X2/3 ATP receptors have demonstrated efficacy in reducing cough counts, and at least four companies have molecules in advanced clinical trials. Gefapixant is the first such compound to have completed phase III trials and demonstrated efficacy in adults with refractory chronic cough213, while three other molecules have completed phase II trials (Supplementary Table 2). Trials have also been conducted with centrally acting neurokinin 1 receptor antagonists, which have demonstrated some clinical benefit, notably in patients with lung cancer presenting with chronic cough80. Drugs in earlier phases of clinical development are those targeting sodium channels involved in cough sensory neuron action potential conduction219. Whether these therapies will show efficacy in patients is unknown. Trials with various TRP channel agents have been negative (Supplementary Table 2), and enthusiasm for these drugs as therapeutic targets has waned.
One challenge for these antitussive studies is overcoming the large placebo effect that accompanies current trial designs. The development of improved animal models that better recapitulate the processes that underpin chronic cough and cough hypersensitivity, along with reimagined clinical trial designs and/or improved end point measures may be required to identify true therapeutic efficacies. Furthermore, clinical trials for new compounds have only been assessed in adults, mostly those with refractory chronic cough, and it will be important to ascertain the clinical use of these new antitussives in other patients and in children.
Supplementary information
Author contributions
Introduction (S.B.M. and K.F.C.), Epidemiology (W.J.-S., A.B.C. and K.L.), Mechanisms/pathophysiology (S.B.M., B.J.C. and J.A.S.), Diagnosis, screening and prevention (K.F.C., L.M., A.B.C. and S.S.B.), Management (K.F.C. and J.A.S.), Quality of life (S.S.B. and A.B.C.), Outlook (S.B.M.).
Peer review
Peer review information
Nature Reviews Disease Primers thanks Jonathan Abram Bernstein, Lu-Yuan Lee, Anne Vertigan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
K.F.C. has received honoraria for participating on Advisory Board meetings of GSK, AstraZeneca, Novartis, Merck, Nocion, Shionogi and Reckitt Benckiser and on the Scientific Advisory Board of the Clean Breathing Institute supported by GSK Health Care Consumer Products. He has also been remunerated for speaking engagements by AstraZeneca, Novartis and Merck. L.M. reports honoraria from Chiesi, GSK, Merck, NeRRe Therapeutics, and Shionogi Inc.; grant support from Merck; and other support from AstraZeneca, Boehringer Ingelheim, Chiesi and Reckitt Benckiser. W.-J.S. declares grants from Merck and AstraZeneca, consulting fees from Merck, AstraZeneca, Shionogi and GSK, and lecture fees from Merck, AstraZeneca, GSK and Novartis. A.B.C. reports fees to her institution as IDMC Member of an unlicensed vaccine (GSK), an unlicensed molecule for chronic cough (Merck), IDMC Member of an unlicensed monoclonal antibody (AstraZeneca) and personal fees from being an author of two Up-to-date chapters. K.L. has received honoraria from AstraZeneca, Chiesi, Circassia, Daiichi Sankyo (China), GSK, Merck, Shionogi Inc. and Novartis; grant and other support from AstraZeneca, Chiesi, Circassia, Daiichi Sankyo (China), GSK and Merck. B.J.C. has received honoraria from Merck, GSK, Nocion, Axalbion, Menlo and Attenua, and grant support from Site One and Merck. S.S.B. reports honoraria from Merck, Bellus Health, Bayer, Shionogi, NeRRe, Menlo, Boehringer Ingelheim and Reckitt Benckiser. J.A.S. is an inventor on a patent describing methods for detecting cough from sound recordings, licensed to Vitalograph Ltd. J.A.S. has received funding for consultancy and research funds from Afferent Pharmaceuticals, Merck Inc., Bayer, Bellus, GSK, Xention Ltd, Ario Pharma Ltd, Glenmark, Almirall, AstraZeneca, Axalbion, Patara, Verona Pharma, NeRRe Pharmaceuticals, Menlo Pharmaceuticals and Attenua Inc. S.B.M. declares honoraria from Merck, NeRRe Therapeutics, Reckitt Benckiser and Bellus Health and grant support from Merck and Bellus Health. None of the disclosed entities above had any involvement in the conceptualization, design, data collection, analysis, decision to publish or preparation of the manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41572-022-00370-w.
References
- 1.Irwin RS, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:1s–23s. doi: 10.1378/chest.129.1_suppl.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherry DK, Burt CW, Woodwell DA. National ambulatory medical care survey: 2001 summary. Adv. Data. 2003;337:1–44. [PubMed] [Google Scholar]
- 3.Morice AH, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur. Respir. J. 2020;55:1901136. doi: 10.1183/13993003.01136-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morice AH, et al. ERS guidelines on the assessment of cough. Eur. Respir. J. 2007;29:1256–1276. doi: 10.1183/09031936.00101006. [DOI] [PubMed] [Google Scholar]
- 5.Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:260s–283s. doi: 10.1378/chest.129.1_suppl.260S. [DOI] [PubMed] [Google Scholar]
- 6.Song WJ, et al. Defining chronic cough: a systematic review of the epidemiological literature. Allergy Asthma Immunol. Res. 2016;8:146–155. doi: 10.4168/aair.2016.8.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everett CF, Kastelik JA, Thompson RH, Morice AH. Chronic persistent cough in the community: a questionnaire survey. Cough. 2007;3:5. doi: 10.1186/1745-9974-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousaf N, Montinero W, Birring SS, Pavord ID. The long term outcome of patients with unexplained chronic cough. Respir. Med. 2013;107:408–412. doi: 10.1016/j.rmed.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Koskela HO, Lätti AM, Purokivi MK. Long-term prognosis of chronic cough: a prospective, observational cohort study. BMC Pulm. Med. 2017;17:146. doi: 10.1186/s12890-017-0496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson P, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149:27–44. doi: 10.1378/chest.15-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morice AH, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur. Respir. J. 2014;44:1132–1148. doi: 10.1183/09031936.00218613. [DOI] [PubMed] [Google Scholar]
- 12.Mazzone SB, McGarvey L. Mechanisms and rationale for targeted therapies in refractory and unexplained chronic cough. Clin. Pharmacol. Ther. 2021;109:619–636. doi: 10.1002/cpt.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song WJ, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur. Respir. J. 2015;45:1479–1481. doi: 10.1183/09031936.00218714. [DOI] [PubMed] [Google Scholar]
- 14.Çolak Y, et al. Risk factors for chronic cough among 14,669 individuals from the general population. Chest. 2017;152:563–573. doi: 10.1016/j.chest.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 15.Liang H, et al. Prevalence of chronic cough in China: a systematic review and meta-analysis. BMC Pulm. Med. 2022;22:62. doi: 10.1186/s12890-022-01847-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arinze JT, et al. Prevalence and incidence of, and risk factors for chronic cough in the adult population: the Rotterdam study. ERJ Open Res. 2020;6:00300-2019. doi: 10.1183/23120541.00300-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satia I, et al. Prevalence, incidence and characteristics of chronic cough among adults from the Canadian longitudinal study on aging. ERJ Open Res. 2021;7:00160-2021. doi: 10.1183/23120541.00160-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang SY, et al. Cough persistence in adults with chronic cough: a 4-year retrospective cohort study. Allergol. Int. 2020;69:588–593. doi: 10.1016/j.alit.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Singh D, Arora V, Sobti PC. Chronic/recurrent cough in rural children in Ludhiana, Punjab. Indian Pediatrics. 2002;39:23–29. [PubMed] [Google Scholar]
- 20.Flynn MG. Respiratory symptoms, bronchial responsiveness, and atopy in Fijian and Indian children. Am. J. Respir. Crit. Care Med. 1994;150:415–420. doi: 10.1164/ajrccm.150.2.8049824. [DOI] [PubMed] [Google Scholar]
- 21.O’Grady KF, et al. Chronic cough postacute respiratory illness in children: a cohort study. Arch. Dis. Child. 2017;102:1044–1048. doi: 10.1136/archdischild-2017-312848. [DOI] [PubMed] [Google Scholar]
- 22.Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet. 2008;371:1364–1374. doi: 10.1016/S0140-6736(08)60595-4. [DOI] [PubMed] [Google Scholar]
- 23.McGovern AE, Short KR, Kywe Moe AA, Mazzone SB. Translational review: neuroimmune mechanisms in cough and emerging therapeutic targets. J. Allergy Clin. Immunol. 2018;142:1392–1402. doi: 10.1016/j.jaci.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Morice AH, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur. Respir. J. 2014;44:1149–1155. doi: 10.1183/09031936.00217813. [DOI] [PubMed] [Google Scholar]
- 25.Dicpinigaitis PV, Rauf K. The influence of gender on cough reflex sensitivity. Chest. 1998;113:1319–1321. doi: 10.1378/chest.113.5.1319. [DOI] [PubMed] [Google Scholar]
- 26.Varechova S, Plevkova J, Hanacek J, Tatar M. Role of gender and pubertal stage on cough sensitivity in childhood and adolescence. J. Physiol. Pharmacol. 2008;59:719–726. [PubMed] [Google Scholar]
- 27.Meltzer EO, et al. Prevalence and burden of chronic cough in the United States. J. Allergy Clin. Immunol. Pract. 2021;9:4037–4044.e4032. doi: 10.1016/j.jaip.2021.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Kang MG, et al. Point prevalence and epidemiological characteristics of chronic cough in the general adult population: the Korean National Health and Nutrition Examination Survey 2010–2012. Medicine. 2017;96:e6486. doi: 10.1097/MD.0000000000006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantar A, Seminara M. Why chronic cough in children is different. Pulm. Pharmacol. Ther. 2019;56:51–55. doi: 10.1016/j.pupt.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 30.French CT, Fletcher KE, Irwin RS. Gender differences in health-related quality of life in patients complaining of chronic cough. Chest. 2004;125:482–488. doi: 10.1378/chest.125.2.482. [DOI] [PubMed] [Google Scholar]
- 31.Chang AB, et al. Do sex and atopy influence cough outcome measurements in children? Chest. 2011;140:324–330. doi: 10.1378/chest.10-2507. [DOI] [PubMed] [Google Scholar]
- 32.Dicpinigaitis PV, Enilari O, Cleven KL. Prevalence of Arnold nerve reflex in subjects with and without chronic cough: relevance to cough hypersensitivity syndrome. Pulm. Pharmacol. Ther. 2019;54:22–24. doi: 10.1016/j.pupt.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Lai K, et al. Age and sex distribution of chinese chronic cough patients and their relationship with capsaicin cough sensitivity. Allergy Asthma Immunol. Res. 2019;11:871–884. doi: 10.4168/aair.2019.11.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smit LA, et al. Transient receptor potential genes, smoking, occupational exposures and cough in adults. Respir. Res. 2012;13:26. doi: 10.1186/1465-9921-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park HK, et al. Association of genetic variations in neurokinin-2 receptor with enhanced cough sensitivity to capsaicin in chronic cough. Thorax. 2006;61:1070–1075. doi: 10.1136/thx.2005.054429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortese A, et al. Cerebellar ataxia, neuropathy, vestibular areflexia syndrome due to RFC1 repeat expansion. Brain. 2020;143:480–490. doi: 10.1093/brain/awz418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar KR, et al. RFC1 expansions can mimic hereditary sensory neuropathy with cough and Sjögren syndrome. Brain. 2020;143:e82. doi: 10.1093/brain/awaa244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zemp E, et al. Long-term ambient air pollution and respiratory symptoms in adults (SAPALDIA study). The SAPALDIA Team. Am. J. Respir. Crit. Care Med. 1999;159:1257–1266. doi: 10.1164/ajrccm.159.4.9807052. [DOI] [PubMed] [Google Scholar]
- 39.Song WJ, et al. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir. Med. 2021;9:533–544. doi: 10.1016/S2213-2600(21)00125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan NM, Vertigan AE, Ferguson J, Wark P, Gibson PG. Clinical and physiological features of postinfectious chronic cough associated with H1N1 infection. Respir. Med. 2012;106:138–144. doi: 10.1016/j.rmed.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Lin L, et al. The duration of cough in patients with H1N1 influenza. Clin. Respir. J. 2017;11:733–738. doi: 10.1111/crj.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang K, et al. Symptomatic treatment of the cough in whooping cough. Cochrane Database Syst. Rev. 2014;2014:Cd003257. doi: 10.1002/14651858.CD003257.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore A, Harnden A, Grant CC, Patel S, Irwin RS. Clinically diagnosing pertussis-associated cough in adults and children: CHEST guideline and expert panel report. Chest. 2019;155:147–154. doi: 10.1016/j.chest.2018.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Côté A, et al. Managing chronic cough due to asthma and NAEB in adults and adolescents: CHEST guideline and expert panel report. Chest. 2020;158:68–96. doi: 10.1016/j.chest.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 45.Lai K, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest. 2013;143:613–620. doi: 10.1378/chest.12-0441. [DOI] [PubMed] [Google Scholar]
- 46.Morice AH, et al. The diagnosis and management of chronic cough. Eur. Respir. J. 2004;24:481–492. doi: 10.1183/09031936.04.00027804. [DOI] [PubMed] [Google Scholar]
- 47.Rennard S, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of confronting COPD international survey. Eur. Respir. J. 2002;20:799–805. doi: 10.1183/09031936.02.03242002. [DOI] [PubMed] [Google Scholar]
- 48.Kessler R, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur. Respir. J. 2011;37:264–272. doi: 10.1183/09031936.00051110. [DOI] [PubMed] [Google Scholar]
- 49.McCallion P, De Soyza A. Cough and bronchiectasis. Pulm. Pharmacol. Ther. 2017;47:77–83. doi: 10.1016/j.pupt.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Harle AS, Blackhall FH, Smith JA, Molassiotis A. Understanding cough and its management in lung cancer. Curr. Opin. Support. Palliat. Care. 2012;6:153–162. doi: 10.1097/SPC.0b013e328352b6a5. [DOI] [PubMed] [Google Scholar]
- 51.Birring SS, et al. Treatment of interstitial lung disease associated cough: CHEST guideline and expert panel report. Chest. 2018;154:904–917. doi: 10.1016/j.chest.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 52.Chan KK, et al. Chronic cough in patients with sleep-disordered breathing. Eur. Respir. J. 2010;35:368–372. doi: 10.1183/09031936.00110409. [DOI] [PubMed] [Google Scholar]
- 53.Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:169s–173s. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- 54.Lai K, et al. The spectrum, clinical features and diagnosis of chronic cough due to rare causes. J. Thorac. Dis. 2021;13:2575–2582. doi: 10.21037/jtd-20-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moscato G, et al. EAACI position paper on assessment of cough in the workplace. Allergy. 2014;69:292–304. doi: 10.1111/all.12352. [DOI] [PubMed] [Google Scholar]
- 56.Gordon SB, et al. Glass bottle workers exposed to low-dose irritant fumes cough but do not wheeze. Am. J. Respir. Crit. Care Med. 1997;156:206–210. doi: 10.1164/ajrccm.156.1.9610042. [DOI] [PubMed] [Google Scholar]
- 57.Wiszniewska M, et al. Characterization of occupational eosinophilic bronchitis in a multicenter cohort of subjects with work-related asthma symptoms. J. Allergy Clin. Immunol. Pract. 2021;9:937–944.e934. doi: 10.1016/j.jaip.2020.08.056. [DOI] [PubMed] [Google Scholar]
- 58.Fang Z, et al. Traffic-related air pollution induces non-allergic eosinophilic airway inflammation and cough hypersensitivity in guinea-pigs. Clin. Exp. Allergy. 2019;49:366–377. doi: 10.1111/cea.13308. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Q, Qiu M, Lai K, Zhong N. Cough and environmental air pollution in China. Pulm. Pharmacol. Ther. 2015;35:132–136. doi: 10.1016/j.pupt.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Pan G, et al. Air pollution and children’s respiratory symptoms in six cities of Northern China. Respir. Med. 2010;104:1903–1911. doi: 10.1016/j.rmed.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 61.Jo EJ, Song WJ. Environmental triggers for chronic cough. Asia Pac. Allergy. 2019;9:e16. doi: 10.5415/apallergy.2019.9.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hooper LG, et al. Ambient air pollution and chronic bronchitis in a cohort of U.S. women. Environ. Health Perspect. 2018;126:027005. doi: 10.1289/EHP2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schindler C, et al. Improvements in PM10 exposure and reduced rates of respiratory symptoms in a cohort of Swiss adults (SAPALDIA) Am. J. Respir. Crit. Care Med. 2009;179:579–587. doi: 10.1164/rccm.200803-388OC. [DOI] [PubMed] [Google Scholar]
- 64.Adner M, et al. Back to the future: re-establishing guinea pig in vivo asthma models. Clin. Sci. 2020;134:1219–1242. doi: 10.1042/CS20200394. [DOI] [PubMed] [Google Scholar]
- 65.Mazzone SB, Undem BJ. Vagal afferent innervation of the airways in health and disease. Physiol. Rev. 2016;96:975–1024. doi: 10.1152/physrev.00039.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Canning BJ, et al. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J. Physiol. 2004;557:543–558. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mazzone SB, et al. Selective expression of a sodium pump isozyme by cough receptors and evidence for its essential role in regulating cough. J. Neurosci. 2009;29:13662–13671. doi: 10.1523/JNEUROSCI.4354-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.West PW, Canning BJ, Merlo-Pich E, Woodcock AA, Smith JA. Morphologic characterization of nerves in whole-mount airway biopsies. Am. J. Respir. Crit. Care Med. 2015;192:30–39. doi: 10.1164/rccm.201412-2293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J, et al. Distinct and common expression of receptors for inflammatory mediators in vagal nodose versus jugular capsaicin-sensitive/TRPV1-positive neurons detected by low input RNA sequencing. PLoS ONE. 2017;12:e0185985. doi: 10.1371/journal.pone.0185985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mazzone SB, et al. Transcriptional profiling of individual airway projecting vagal sensory neurons. Mol. Neurobiol. 2020;57:949–963. doi: 10.1007/s12035-019-01782-8. [DOI] [PubMed] [Google Scholar]
- 71.Farrell MJ, et al. Evidence for multiple bulbar and higher brain circuits processing sensory inputs from the respiratory system in humans. J. Physiol. 2020;598:5771–5787. doi: 10.1113/JP280220. [DOI] [PubMed] [Google Scholar]
- 72.Canning BJ, Mori N. An essential component to brainstem cough gating identified in anesthetized guinea pigs. FASEB J. 2010;24:3916–3926. doi: 10.1096/fj.09-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGovern AE, et al. Distinct brainstem and forebrain circuits receiving tracheal sensory neuron inputs revealed using a novel conditional anterograde transsynaptic viral tracing system. J. Neurosci. 2015;35:7041–7055. doi: 10.1523/JNEUROSCI.5128-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Driessen AK, et al. Reflex regulation of breathing by the paratrigeminal nucleus via multiple bulbar circuits. Brain Struct. Funct. 2018;223:4005–4022. doi: 10.1007/s00429-018-1732-z. [DOI] [PubMed] [Google Scholar]
- 75.Driessen AK, et al. A role for neurokinin 1 receptor expressing neurons in the paratrigeminal nucleus in bradykinin-evoked cough in guinea-pigs. J. Physiol. 2020;598:2257–2275. doi: 10.1113/JP279644. [DOI] [PubMed] [Google Scholar]
- 76.Canning BJ, Mori N. Encoding of the cough reflex in anesthetized guinea pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300:R369–R377. doi: 10.1152/ajpregu.00044.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith JA, Hilton ECY, Saulsberry L, Canning BJ. Antitussive effects of memantine in guinea pigs. Chest. 2012;141:996–1002. doi: 10.1378/chest.11-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hewitt MM, et al. Pharmacology of bradykinin-evoked coughing in guinea pigs. J. Pharmacol. Exp. Ther. 2016;357:620–628. doi: 10.1124/jpet.115.230383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dicpinigaitis PV, Canning BJ, Garner R, Paterson B. Effect of memantine on cough reflex sensitivity: translational studies in guinea pigs and humans. J. Pharmacol. Exp. Ther. 2015;352:448–454. doi: 10.1124/jpet.114.221218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith JA, et al. Aprepitant for cough in lung cancer. a randomized placebo-controlled trial and mechanistic insights. Am. J. Respir. Crit. Care Med. 2021;203:737–745. doi: 10.1164/rccm.202006-2359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mazzone SB, Chung KF, McGarvey L. The heterogeneity of chronic cough: a case for endotypes of cough hypersensitivity. Lancet Respir. Med. 2018;6:636–646. doi: 10.1016/S2213-2600(18)30150-4. [DOI] [PubMed] [Google Scholar]
- 82.Fox AJ, et al. Bradykinin-evoked sensitization of airway sensory nerves: a mechanism for ACE-inhibitor cough. Nat. Med. 1996;2:814–817. doi: 10.1038/nm0796-814. [DOI] [PubMed] [Google Scholar]
- 83.Fischer A, McGregor GP, Saria A, Philippin B, Kummer W. Induction of tachykinin gene and peptide expression in guinea pig nodose primary afferent neurons by allergic airway inflammation. J. Clin. Invest. 1996;98:2284–2291. doi: 10.1172/JCI119039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shapiro CO, et al. Airway sensory nerve density is increased in chronic cough. Am. J. Respir. Crit. Care Med. 2021;203:348–355. doi: 10.1164/rccm.201912-2347OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Groneberg DA, et al. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am. J. Respir. Crit. Care Med. 2004;170:1276–1280. doi: 10.1164/rccm.200402-174OC. [DOI] [PubMed] [Google Scholar]
- 86.Abubakar AB, Bautista TG, Dimmock MR, Mazzone SB, Farrell MJ. Behavioral and regional brain responses to inhalation of capsaicin modified by painful conditioning in humans. Chest. 2021;159:1136–1146. doi: 10.1016/j.chest.2020.08.2105. [DOI] [PubMed] [Google Scholar]
- 87.Koivisto AP, Belvisi MG, Gaudet R, Szallasi A. Advances in TRP channel drug discovery: from target validation to clinical studies. Nat. Rev. Drug Discov. 2022;21:41–59. doi: 10.1038/s41573-021-00268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verzele NAJ, et al. The impact of influenza pulmonary infection and inflammation on vagal bronchopulmonary sensory neurons. FASEB J. 2021;35:e21320. doi: 10.1096/fj.202001509R. [DOI] [PubMed] [Google Scholar]
- 89.Kaelberer MM, Caceres AI, Jordt SE. Activation of a nerve injury transcriptional signature in airway-innervating sensory neurons after lipopolysaccharide-induced lung inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2020;318:L953–L964. doi: 10.1152/ajplung.00403.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mazzone SB, et al. Modulation of vagal sensory neurons via high mobility group box-1 and receptor for advanced glycation end products: implications for respiratory viral infections. Front. Physiol. 2021;12:744812. doi: 10.3389/fphys.2021.744812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang H, et al. HMGB1 released from nociceptors mediates inflammation. Proc. Natl Acad. Sci. USA. 2021;118:e2102034118. doi: 10.1073/pnas.2102034118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chung KF, McGarvey L, Mazzone SB. Chronic cough as a neuropathic disorder. Lancet Respir. Med. 2013;1:414–422. doi: 10.1016/S2213-2600(13)70043-2. [DOI] [PubMed] [Google Scholar]
- 93.Mahadi KM, Lall VK, Deuchars SA, Deuchars J. Cardiovascular autonomic effects of transcutaneous auricular nerve stimulation via the tragus in the rat involve spinal cervical sensory afferent pathways. Brain Stim. 2019;12:1151–1158. doi: 10.1016/j.brs.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 94.Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J. Physiol. 2005;569:559–573. doi: 10.1113/jphysiol.2005.093153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Plevkova J, et al. The role of trigeminal nasal TRPM8-expressing afferent neurons in the antitussive effects of menthol. J. Appl. Physiol. 2013;115:268–274. doi: 10.1152/japplphysiol.01144.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chou YL, Mori N, Canning BJ. Opposing effects of bronchopulmonary C-fiber subtypes on cough in guinea pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;314:R489–R498. doi: 10.1152/ajpregu.00313.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spaziano G, et al. Exposure to allergen causes changes in NTS neural activities after intratracheal capsaicin application, in endocannabinoid levels and in the glia morphology of NTS. BioMed. Res. Int. 2015;2015:980983. doi: 10.1155/2015/980983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen CY, et al. Extended allergen exposure in asthmatic monkeys induces neuroplasticity in nucleus tractus solitarius. J. Allergy Clin. Immunol. 2001;108:557–562. doi: 10.1067/mai.2001.118132. [DOI] [PubMed] [Google Scholar]
- 99.Sekizawa S, et al. Extended secondhand tobacco smoke exposure induces plasticity in nucleus tractus solitarius second-order lung afferent neurons in young guinea pigs. Eur. J. Neurosci. 2008;28:771–781. doi: 10.1111/j.1460-9568.2008.06378.x. [DOI] [PubMed] [Google Scholar]
- 100.Chen Z, et al. Glial activation and inflammation in the NTS in a rat model after exposure to diesel exhaust particles. Env. Toxicol. Pharmacol. 2021;83:103584. doi: 10.1016/j.etap.2021.103584. [DOI] [PubMed] [Google Scholar]
- 101.Ando A, et al. Neural correlates of cough hypersensitivity in humans: evidence for central sensitisation and dysfunctional inhibitory control. Thorax. 2016;71:323–329. doi: 10.1136/thoraxjnl-2015-207425. [DOI] [PubMed] [Google Scholar]
- 102.Chen Z, et al. A descending pathway emanating from the periaqueductal gray mediates the development of cough-like hypersensitivity. iScience. 2022;25:103641. doi: 10.1016/j.isci.2021.103641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zambreanu L, Wise RG, Brooks JCW, Iannetti GD, Tracey I. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain. 2005;114:397–407. doi: 10.1016/j.pain.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 104.Leech J, Mazzone SB, Farrell MJ. The effect of placebo conditioning on capsaicin-evoked urge to cough. Chest. 2012;142:951–957. doi: 10.1378/chest.12-0362. [DOI] [PubMed] [Google Scholar]
- 105.Leech J, Mazzone SB, Farrell MJ. Brain activity associated with placebo suppression of the urge-to-cough in humans. Am. J. Respir. Crit. Care Med. 2013;188:1069–1075. doi: 10.1164/rccm.201306-1079OC. [DOI] [PubMed] [Google Scholar]
- 106.McGovern AE, Ajayi IE, Farrell MJ, Mazzone SB. A neuroanatomical framework for the central modulation of respiratory sensory processing and cough by the periaqueductal grey. J. Thorac. Dis. 2017;9:4098–4107. doi: 10.21037/jtd.2017.08.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mazzone SB, Cole LJ, Ando A, Egan GF, Farrell MJ. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J. Neurosci. 2011;31:2948–2958. doi: 10.1523/JNEUROSCI.4597-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Simonyan K, Saad ZS, Loucks TM, Poletto CJ, Ludlow CL. Functional neuroanatomy of human voluntary cough and sniff production. NeuroImage. 2007;37:401–409. doi: 10.1016/j.neuroimage.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vertigan AE, et al. Somatic cough syndrome (previously referred to as psychogenic cough) and tic cough (previously referred to as habit cough) in adults and children: CHEST Guideline and Expert Panel Report. Chest. 2015;148:24–31. doi: 10.1378/chest.15-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mazzone SB. Chronic cough: a disorder of response inhibition? Eur. Respir. J. 2019;53:1900254. doi: 10.1183/13993003.00254-2019. [DOI] [PubMed] [Google Scholar]
- 111.Farrell MJ, et al. Functionally connected brain regions in the network activated during capsaicin inhalation. Hum. Brain Mapp. 2014;35:5341–5355. doi: 10.1002/hbm.22554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cho PSP, et al. Cough hypersensitivity and suppression in COPD. Eur. Respir. J. 2021;57:2003569. doi: 10.1183/13993003.03569-2020. [DOI] [PubMed] [Google Scholar]
- 113.Cho PSP, Fletcher HV, Turner RD, Jolley CJ, Birring SS. Impaired cough suppression in chronic refractory cough. Eur. Respir. J. 2019;53:2003569. doi: 10.1183/13993003.02203-2018. [DOI] [PubMed] [Google Scholar]
- 114.Hilton E, et al. The effect of pain conditioning on experimentally evoked cough: evidence of impaired endogenous inhibitory control mechanisms in refractory chronic cough. Eur. Respir. J. 2020 doi: 10.1183/13993003.01387-2020. [DOI] [PubMed] [Google Scholar]
- 115.Badri H, et al. A double-blind randomised placebo-controlled trial investigating the effects of lesogaberan on the objective cough frequency and capsaicin-evoked coughs in patients with refractory chronic cough. ERJ Open Res. 2022;8:00546-2021. doi: 10.1183/23120541.00546-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dicpinigaitis PV, Rauf K. Treatment of chronic, refractory cough with baclofen. Respiration. 1998;65:86–88. doi: 10.1159/000029232. [DOI] [PubMed] [Google Scholar]
- 117.Xie J, et al. Cough in hypereosinophilic syndrome: case report and literature review. BMC Pulm. Med. 2020;20:90. doi: 10.1186/s12890-020-1134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chung KF, et al. Cough and hypereosinophilia due to FIP1L1-PDGFRA fusion gene with tyrosine kinase activity. Eur. Respir. J. 2006;27:230–232. doi: 10.1183/09031936.06.00089405. [DOI] [PubMed] [Google Scholar]
- 119.Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI) J. Voice. 2002;16:274–277. doi: 10.1016/s0892-1997(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 120.Morice AH, Faruqi S, Wright CE, Thompson R, Bland JM. Cough hypersensitivity syndrome: a distinct clinical entity. Lung. 2011;189:73–79. doi: 10.1007/s00408-010-9272-1. [DOI] [PubMed] [Google Scholar]
- 121.Trinick R, Johnston N, Dalzell AM, McNamara PS. Reflux aspiration in children with neurodisability–a significant problem, but can we measure it? J. Pediatr. Surg. 2012;47:291–298. doi: 10.1016/j.jpedsurg.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 122.Grabowski M, et al. Pepsin and bile acids in induced sputum of chronic cough patients. Respir. Med. 2011;105:1257–1261. doi: 10.1016/j.rmed.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 123.Stovold R, et al. Pepsin, a biomarker of gastric aspiration in lung allografts: a putative association with rejection. Am. J. Respir. Crit. Care Med. 2007;175:1298–1303. doi: 10.1164/rccm.200610-1485OC. [DOI] [PubMed] [Google Scholar]
- 124.Decalmer S, et al. Chronic cough: relationship between microaspiration, gastroesophageal reflux, and cough frequency. Chest. 2012;142:958–964. doi: 10.1378/chest.12-0044. [DOI] [PubMed] [Google Scholar]
- 125.Irwin RS, Corrao WM, Pratter MR. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am. Rev. Respir. Dis. 1981;123:413–417. doi: 10.1164/arrd.1981.123.4.413. [DOI] [PubMed] [Google Scholar]
- 126.Hennel M, Brozmanova M, Kollarik M. Cough reflex sensitization from esophagus and nose. Pulm. Pharmacol. Ther. 2015;35:117–121. doi: 10.1016/j.pupt.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Houghton LA, Lee AS, Badri H, DeVault KR, Smith JA. Respiratory disease and the oesophagus: reflux, reflexes and microaspiration. Nat. Rev. Gastroenterol. Hepatol. 2016;13:445–460. doi: 10.1038/nrgastro.2016.91. [DOI] [PubMed] [Google Scholar]
- 128.Plevkova J, et al. Convergence of nasal and tracheal neural pathways in modulating the cough response in guinea pigs. J. Physiol. Pharmacol. 2009;60:89–93. [PubMed] [Google Scholar]
- 129.Javorkova N, et al. Acidification of the oesophagus acutely increases the cough sensitivity in patients with gastro-oesophageal reflux and chronic cough. Neurogastroenterol. Motil. 2008;20:119–124. doi: 10.1111/j.1365-2982.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- 130.Ing AJ, Ngu MC, Breslin AB. Pathogenesis of chronic persistent cough associated with gastroesophageal reflux. Am. J. Respir. Crit. Care Med. 1994;149:160–167. doi: 10.1164/ajrccm.149.1.8111576. [DOI] [PubMed] [Google Scholar]
- 131.Plevkova J, Brozmanova M, Pecova R, Tatar M. Effects of intranasal capsaicin challenge on cough reflex in healthy human volunteers. J. Physiol. Pharmacol. 2004;55:101–106. [PubMed] [Google Scholar]
- 132.Smith JA, et al. Acoustic cough–reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology. 2010;139:754–762. doi: 10.1053/j.gastro.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 133.Sifrim D, et al. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut. 2005;54:449–454. doi: 10.1136/gut.2004.055418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Blondeau K, Dupont LJ, Mertens V, Tack J, Sifrim D. Improved diagnosis of gastro-oesophageal reflux in patients with unexplained chronic cough. Aliment. Pharmacol. Ther. 2007;25:723–732. doi: 10.1111/j.1365-2036.2007.03255.x. [DOI] [PubMed] [Google Scholar]
- 135.Smith JA, Ashurst HL, Jack S, Woodcock AA, Earis JE. The description of cough sounds by healthcare professionals. Cough. 2006;2:1. doi: 10.1186/1745-9974-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee KK, et al. Sound: a non-invasive measure of cough intensity. BMJ Open. Respir. Res. 2017;4:e000178. doi: 10.1136/bmjresp-2017-000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Won HK, et al. Cough-related laryngeal sensations and triggers in adults with chronic cough: symptom profile and impact. Allergy Asthma Immunol. Res. 2019;11:622–631. doi: 10.4168/aair.2019.11.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vertigan AE, Gibson PG. Chronic refractory cough as a sensory neuropathy: evidence from a reinterpretation of cough triggers. J. Voice. 2011;25:596–601. doi: 10.1016/j.jvoice.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 139.Sundar KM, Stark AC, Hu N, Barkmeier-Kraemer J. Is laryngeal hypersensitivity the basis of unexplained or refractory chronic cough? ERJ Open Res. 2021;7:00793-2020. doi: 10.1183/23120541.00793-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Irwin RS, French CL, Chang AB, Altman KW. Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest. 2018;153:196–209. doi: 10.1016/j.chest.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kahrilas PJ, et al. Chronic cough due to gastroesophageal reflux in adults: CHEST guideline and expert panel report. Chest. 2016;150:1341–1360. doi: 10.1016/j.chest.2016.08.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chang AB, et al. Children with chronic cough: when is watchful waiting appropriate? development of likelihood ratios for assessing children with chronic cough. Chest. 2015;147:745–753. doi: 10.1378/chest.14-2155. [DOI] [PubMed] [Google Scholar]
- 143.Chang AB, Oppenheimer JJ, Irwin RS. Managing chronic cough as a symptom in children and management algorithms: CHEST guideline and expert panel report. Chest. 2020;158:303–329. doi: 10.1016/j.chest.2020.01.042. [DOI] [PubMed] [Google Scholar]
- 144.O’Grady KF, et al. Effectiveness of a chronic cough management algorithm at the transitional stage from acute to chronic cough in children: a multicenter, nested, single-blind, randomised controlled trial. Lancet Child. Adolesc. Health. 2019;3:889–898. doi: 10.1016/S2352-4642(19)30327-X. [DOI] [PubMed] [Google Scholar]
- 145.Chang AB, et al. Use of management pathways or algorithms in children with chronic cough: systematic reviews. Chest. 2016;149:106–119. doi: 10.1378/chest.15-1403. [DOI] [PubMed] [Google Scholar]
- 146.Birring SS, et al. Cough frequency, cough sensitivity and health status in patients with chronic cough. Respir. Med. 2006;100:1105–1109. doi: 10.1016/j.rmed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 147.Lee KK, et al. A longitudinal assessment of acute cough. Am. J. Respir. Crit. Care Med. 2013;187:991–997. doi: 10.1164/rccm.201209-1686OC. [DOI] [PubMed] [Google Scholar]
- 148.Morice AH, et al. The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo-controlled study. Eur. Respir. J. 2019;54:1900439. doi: 10.1183/13993003.00439-2019. [DOI] [PubMed] [Google Scholar]
- 149.Belvisi MG, et al. XEN-D0501, a novel transient receptor potential vanilloid 1 antagonist, does not reduce cough in patients with refractory cough. Am. J. Respir. Crit. Care Med. 2017;196:1255–1263. doi: 10.1164/rccm.201704-0769OC. [DOI] [PubMed] [Google Scholar]
- 150.Prudon B, et al. Cough and glottic-stop reflex sensitivity in health and disease. Chest. 2005;127:550–557. doi: 10.1378/chest.127.2.550. [DOI] [PubMed] [Google Scholar]
- 151.Koskela HO, Nurmi HM, Birring SS. Utility of cough provocation tests in chronic cough and respiratory diseases: a comprehensive review and introduction of new reference ranges for the capsaicin test. Allergy Asthma Immunol. Res. 2021;13:833–849. doi: 10.4168/aair.2021.13.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chang AB, et al. Etiologies of chronic cough in pediatric cohorts: CHEST guideline and expert panel report. Chest. 2017;152:607–617. doi: 10.1016/j.chest.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kahrilas PJ, Howden CW, Hughes N, Molloy-Bland M. Response of chronic cough to acid-suppressive therapy in patients with gastroesophageal reflux disease. Chest. 2013;143:605–612. doi: 10.1378/chest.12-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Badri H, et al. Heartburn as a marker of the success of acid suppression therapy in chronic cough. Lung. 2021;199:597–602. doi: 10.1007/s00408-021-00496-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yousaf N, et al. Long-term low-dose erythromycin in patients with unexplained chronic cough: a double-blind placebo controlled trial. Thorax. 2010;65:1107–1110. doi: 10.1136/thx.2010.142711. [DOI] [PubMed] [Google Scholar]
- 156.Hodgson D, et al. The effects of azithromycin in treatment-resistant cough: a randomized, double-blind, placebo-controlled trial. Chest. 2016;149:1052–1060. doi: 10.1016/j.chest.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chang AB, et al. Chronic cough and gastroesophageal reflux in children: CHEST guideline and expert panel report. Chest. 2019;156:131–140. doi: 10.1016/j.chest.2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vandenplas Y, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) J. Pediatr. Gastroenterol. Nutr. 2009;49:498–547. doi: 10.1097/MPG.0b013e3181b7f563. [DOI] [PubMed] [Google Scholar]
- 159.Prasad S, Fong E, Ooi EH. Systematic review of patient-reported outcomes after revision endoscopic sinus surgery. Am. J. Rhinol. Allergy. 2017;31:248–255. doi: 10.2500/ajra.2017.31.4446. [DOI] [PubMed] [Google Scholar]
- 160.Chester AC, Sindwani R. Symptom outcomes in endoscopic sinus surgery: a systematic review of measurement methods. Laryngoscope. 2007;117:2239–2243. doi: 10.1097/MLG.0b013e318149224d. [DOI] [PubMed] [Google Scholar]
- 161.Lee JH, et al. Efficacy of non-sedating H1-receptor antihistamines in adults and adolescents with chronic cough: a systematic review. World Allergy Organ. J. 2021;14:100568. doi: 10.1016/j.waojou.2021.100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang J, et al. Risk factors for chronic cough in adults: a systematic review and meta-analysis. Respirol. 2022;27:36–47. doi: 10.1111/resp.14169. [DOI] [PubMed] [Google Scholar]
- 163.[No authors listed] Definition and classification of chronic bronchitis for clinical and epidemiological purposes. A report to the Medical Research Council by their committee on the aetiology of chronic bronchitis. Lancet. 1965;1:775–779. [PubMed] [Google Scholar]
- 164.Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am. J. Respir. Crit. Care Med. 1996;153:1530–1535. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]
- 165.Burgel PR, et al. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009;135:975–982. doi: 10.1378/chest.08-2062. [DOI] [PubMed] [Google Scholar]
- 166.Singh D, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur. Respir. J. 2019;53:1900164. doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 167.Polley L, et al. Impact of cough across different chronic respiratory diseases: comparison of two cough-specific health-related quality of life questionnaires. Chest. 2008;134:295–302. doi: 10.1378/chest.07-0141. [DOI] [PubMed] [Google Scholar]
- 168.Torrego A, et al. Capsaicin cough sensitivity in bronchiectasis. Thorax. 2006;61:706–709. doi: 10.1136/thx.2005.049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bolser DC. Mechanisms of action of central and peripheral antitussive drugs. Pulm. Pharm. 1996;9:357–364. doi: 10.1006/pulp.1996.0047. [DOI] [PubMed] [Google Scholar]
- 170.Takahama K, Shirasaki T. Central and peripheral mechanisms of narcotic antitussives: codeine-sensitive and -resistant coughs. Cough. 2007;3:8. doi: 10.1186/1745-9974-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fuller RW, Karlsson J-A, Choudry NB, Pride NB. Effect of inhaled and systemic opiates on responses to inhaled capsaicin in humans. J. Appl. Physiol. 1988;65:1125–1130. doi: 10.1152/jappl.1988.65.3.1125. [DOI] [PubMed] [Google Scholar]
- 172.Sevelius H, McCoy JF, Colmore JP. Dose response to codeine in patients with chronic cough. Clin. Pharmacol. Ther. 1971;12:449–455. doi: 10.1002/cpt1971123449. [DOI] [PubMed] [Google Scholar]
- 173.Aylward M, Maddock J, Davies DE, Protheroe DA, Leideman T. Dextromethorphan and codeine: comparison of plasma kinetics and antitussive effects. Eur. J. Respir. Dis. 1984;65:283–291. [PubMed] [Google Scholar]
- 174.Freestone C, Eccles R. Assessment of the antitussive efficacy of codeine in cough associated with common cold. J. Pharm. Pharmacol. 1997;49:1045–1049. doi: 10.1111/j.2042-7158.1997.tb06039.x. [DOI] [PubMed] [Google Scholar]
- 175.Smith J, Owen E, Earis J, Woodcock A. Effect of codeine on objective measurement of cough in chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2006;117:831–835. doi: 10.1016/j.jaci.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 176.Foley M, Deluca P, Kimergård A. Time to review the provision of addiction treatment for codeine dependence. BMJ. 2017;359:j5311. doi: 10.1136/bmj.j5311. [DOI] [PubMed] [Google Scholar]
- 177.Sproule BA, Busto UE, Somer G, Romach MK, Sellers EM. Characteristics of dependent and nondependent regular users of codeine. J. Clin. Psychopharmacol. 1999;19:367–372. doi: 10.1097/00004714-199908000-00014. [DOI] [PubMed] [Google Scholar]
- 178.Ćelić I, et al. Resolving issues about efficacy and safety of low-dose codeine in combination analgesic drugs: a systematic review. Pain. Ther. 2020;9:171–194. doi: 10.1007/s40122-020-00162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Morice AH, et al. Opiate therapy in chronic cough. Am. J. Respir. Crit. Care Med. 2007;175:312–315. doi: 10.1164/rccm.200607-892OC. [DOI] [PubMed] [Google Scholar]
- 180.Walsh TD. Prevention of opioid side effects. J. Pain. Symptom Manag. 1990;5:362–367. doi: 10.1016/0885-3924(90)90031-e. [DOI] [PubMed] [Google Scholar]
- 181.Kimos P, et al. Analgesic action of gabapentin on chronic pain in the masticatory muscles: a randomized controlled trial. Pain. 2007;127:151–160. doi: 10.1016/j.pain.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 182.Hendrich J, et al. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc. Natl Acad. Sci. USA. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380:1583–1589. doi: 10.1016/S0140-6736(12)60776-4. [DOI] [PubMed] [Google Scholar]
- 184.Vertigan AE, et al. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149:639–648. doi: 10.1378/chest.15-1271. [DOI] [PubMed] [Google Scholar]
- 185.Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;45:54–63. doi: 10.1016/j.pnpbp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 186.Jeyakumar A, Brickman TM, Haben M. Effectiveness of amitriptyline versus cough suppressants in the treatment of chronic cough resulting from postviral vagal neuropathy. Laryngoscope. 2006;116:2108–2112. doi: 10.1097/01.mlg.0000244377.60334.e3. [DOI] [PubMed] [Google Scholar]
- 187.Ryan MA, Cohen SM. Long-term follow-up of amitriptyline treatment for idiopathic cough. Laryngoscope. 2016;126:2758–2763. doi: 10.1002/lary.25978. [DOI] [PubMed] [Google Scholar]
- 188.Olsen WL, et al. Intra-arterial, but not intrathecal, baclofen and codeine attenuates cough in the cat. Front. Physiol. 2021;12:640682. doi: 10.3389/fphys.2021.640682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Simera M, Poliacek I, Jakus J. Central antitussive effect of codeine in the anesthetized rabbit. Eur. J. Med. Res. 2010;15:184–188. doi: 10.1186/2047-783X-15-S2-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fuller RW, Karlsson JA, Choudry NB, Pride NB. Effect of inhaled and systemic opiates on responses to inhaled capsaicin in humans. J. Appl. Physiol. 1988;65:1125–1130. doi: 10.1152/jappl.1988.65.3.1125. [DOI] [PubMed] [Google Scholar]
- 191.Badri H, et al. Effect of centrally and peripherally acting GABA(B) agonism on the healthy human cough reflex. Pulm. Pharmacol. Ther. 2021;71:102079. doi: 10.1016/j.pupt.2021.102079. [DOI] [PubMed] [Google Scholar]
- 192.Poliacek I, Wang C, Corrie LW, Rose MJ, Bolser DC. Microinjection of codeine into the region of the caudal ventral respiratory column suppresses cough in anesthetized cats. J. Appl. Physiol. 2010;108:858–865. doi: 10.1152/japplphysiol.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. J. Appl. Physiol. 1999;86:1017–1024. doi: 10.1152/jappl.1999.86.3.1017. [DOI] [PubMed] [Google Scholar]
- 194.Dickinson RS, Morjaria JB, Wright CE, Morice AH. Is opiate action in cough due to sedation? Ther. Adv. Chronic Dis. 2014;5:200–205. doi: 10.1177/2040622314543220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nicolakis J, Gmeiner G, Reiter C, Seltenhammer MH. Aspiration in lethal drug abuse-a consequence of opioid intoxication. Int. J. Leg. Med. 2020;134:2121–2132. doi: 10.1007/s00414-020-02412-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hårdemark Cedborg AI, et al. Effects of morphine and midazolam on pharyngeal function, airway protection, and coordination of breathing and swallowing in healthy adults. Anesthesiology. 2015;122:1253–1267. doi: 10.1097/ALN.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 197.Ebihara S, Sekiya H, Miyagi M, Ebihara T, Okazaki T. Dysphagia, dystussia, and aspiration pneumonia in elderly people. J. Thorac. Dis. 2016;8:632–639. doi: 10.21037/jtd.2016.02.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ebihara S, Ebihara T. Cough in the elderly: a novel strategy for preventing aspiration pneumonia. Pulm. Pharmacol. Ther. 2011;24:318–323. doi: 10.1016/j.pupt.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 199.Vertigan AE, Haines J, Slovarp L. An update on speech pathology management of chronic refractory cough. J. Allergy Clin. Immunol. Pract. 2019;7:1756–1761. doi: 10.1016/j.jaip.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 200.Vertigan AE, Theodoros DG, Gibson PG, Winkworth AL. Efficacy of speech pathology management for chronic cough: a randomised placebo controlled trial of treatment efficacy. Thorax. 2006;61:1065–1069. doi: 10.1136/thx.2006.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chamberlain Mitchell SA, et al. Physiotherapy, and speech and language therapy intervention for patients with refractory chronic cough: a multicentre randomised control trial. Thorax. 2017;72:129–136. doi: 10.1136/thoraxjnl-2016-208843. [DOI] [PubMed] [Google Scholar]
- 202.Ryan NM, Vertigan AE, Gibson PG. Chronic cough and laryngeal dysfunction improve with specific treatment of cough and paradoxical vocal fold movement. Cough. 2009;5:4. doi: 10.1186/1745-9974-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Brignall K, Jayaraman B, Birring SS. Quality of life and psychosocial aspects of cough. Lung. 2008;186:S55–S58. doi: 10.1007/s00408-007-9034-x. [DOI] [PubMed] [Google Scholar]
- 204.Dicpinigaitis PV. Prevalence of stress urinary incontinence in women presenting for evaluation of chronic cough. ERJ Open. Res. 2021;7:00012-2021. doi: 10.1183/23120541.00012-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Newcombe PA, et al. A child chronic cough-specific quality of life measure: development and validation. Thorax. 2016;71:695–700. doi: 10.1136/thoraxjnl-2015-207473. [DOI] [PubMed] [Google Scholar]
- 206.Newcombe PA, Sheffield JK, Chang AB. Parent cough-specific quality of life: development and validation of a short form. J. Allergy Clin. Immunol. 2013;131:1069–1074. doi: 10.1016/j.jaci.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 207.Vernon M, Kline Leidy N, Nacson A, Nelsen L. Measuring cough severity: development and pilot testing of a new seven-item cough severity patient-reported outcome measure. Ther. Adv. Respir. Dis. 2010;4:199–208. doi: 10.1177/1753465810372526. [DOI] [PubMed] [Google Scholar]
- 208.Irwin RS, Dudiki N, French CL. Life-threatening and non-life-threatening complications associated with coughing: a scoping review. Chest. 2020;158:2058–2073. doi: 10.1016/j.chest.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.French CL, Irwin RS, Curley FJ, Krikorian CJ. Impact of chronic cough on quality of life. Arch. Intern. Med. 1998;158:1657–1661. doi: 10.1001/archinte.158.15.1657. [DOI] [PubMed] [Google Scholar]
- 210.French CT, Irwin RS, Fletcher KE, Adams TM. Evaluation of a cough-specific quality-of-life questionnaire. Chest. 2002;121:1123–1131. doi: 10.1378/chest.121.4.1123. [DOI] [PubMed] [Google Scholar]
- 211.Birring SS, et al. Development of a symptom specific health status measure for patients with chronic cough: leicester cough questionnaire (LCQ) Thorax. 2003;58:339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Raj, A. A., Pavord, D. I. & Birring, S. S. in Pharmacology and Therapeutics of Cough (eds Kian, F. C. & John, W.) 311–320 (Springer, 2009).
- 213.McGarvey LP, et al. Efficacy and safety of gefapixant, a P2X 3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet. 2022;399:909–923. doi: 10.1016/S0140-6736(21)02348-5. [DOI] [PubMed] [Google Scholar]
- 214.Fletcher KE, French CT, Irwin RS, Corapi KM, Norman GR. A prospective global measure, the Punum Ladder, provides more valid assessments of quality of life than a retrospective transition measure. J. Clin. Epidemiol. 2010;63:1123–1131. doi: 10.1016/j.jclinepi.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 215.Anderson-James S, Newcombe PA, Marchant JM, Turner CT, Chang AB. Children’s acute cough-specific quality of life: revalidation and development of a short form. Lung. 2021;199:527–534. doi: 10.1007/s00408-021-00482-2. [DOI] [PubMed] [Google Scholar]
- 216.Marchant JM, et al. What is the burden of chronic cough for families? Chest. 2008;134:303–309. doi: 10.1378/chest.07-2236. [DOI] [PubMed] [Google Scholar]
- 217.McGarvey L, Gibson PG. What is chronic cough? Terminology. J. Allergy Clin. Immunol. Pract. 2019;7:1711–1714. doi: 10.1016/j.jaip.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 218.Hall JI, Lozano M, Estrada-Petrocelli L, Birring S, Turner R. The present and future of cough counting tools. J. Thorac. Dis. 2020;12:5207–5223. doi: 10.21037/jtd-2020-icc-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tochitsky I, et al. Inhibition of inflammatory pain and cough by a novel charged sodium channel blocker. Br. J. Pharmacol. 2021;178:3905–3923. doi: 10.1111/bph.15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Carter ER, Debley JS, Redding GR. Chronic productive cough in school children: prevalence and associations with asthma and environmental tobacco smoke exposure. Cough. 2006;2:11. doi: 10.1186/1745-9974-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Faniran AO, Peat JK, Woolcock AJ. Measuring persistent cough in children in epidemiological studies: development of a questionnaire and assessment of prevalence in two countries. Chest. 1999;115:434–439. doi: 10.1378/chest.115.2.434. [DOI] [PubMed] [Google Scholar]
- 222.Migliore E, et al. Respiratory symptoms in children living near busy roads and their relationship to vehicular traffic: results of an Italian multicenter study (SIDRIA 2) Env. Health. 2009;8:27. doi: 10.1186/1476-069X-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Laird P, Totterdell J, Walker R, Chang AB, Schultz A. Prevalence of chronic wet cough and protracted bacterial bronchitis in Aboriginal children. ERJ Open Res. 2019;5:00248-2019. doi: 10.1183/23120541.00248-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rylance S, et al. Lung health and exposure to air pollution in Malawian children (CAPS): a cross-sectional study. Thorax. 2019;74:1070–1077. doi: 10.1136/thoraxjnl-2018-212945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Stein RT, et al. Influence of parental smoking on respiratory symptoms during the first decade of life: the Tucson children’s respiratory study. Am. J. Epidemiol. 1999;149:1030–1037. doi: 10.1093/oxfordjournals.aje.a009748. [DOI] [PubMed] [Google Scholar]
- 226.Ryan NM, Gibson PG, Birring SS. Arnold’s nerve cough reflex: evidence for chronic cough as a sensory vagal neuropathy. J. Thorac. Dis. 2014;6:S748–S752. doi: 10.3978/j.issn.2072-1439.2014.04.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Stec SM, et al. Diagnosis and management of premature ventricular complexes-associated chronic cough. Chest. 2009;135:1535–1541. doi: 10.1378/chest.08-1814. [DOI] [PubMed] [Google Scholar]
- 228.Kok C, et al. A locus for hereditary sensory neuropathy with cough and gastroesophageal reflux on chromosome 3p22-p24. Am. J. Hum. Genet. 2003;73:632–637. doi: 10.1086/377591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Molassiotis A, Smith JA, Mazzone P, Blackhall F, Irwin RS. Symptomatic treatment of cough among adult patients with lung cancer: CHEST guideline and expert panel report. Chest. 2017;151:861–874. doi: 10.1016/j.chest.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jeon K, et al. Clinical features of recently diagnosed pulmonary paragonimiasis in Korea. Chest. 2005;128:1423–1430. doi: 10.1378/chest.128.3.1423. [DOI] [PubMed] [Google Scholar]
- 231.Agossou M, et al. Mammomonogamus laryngeus: an unusual cause of acute and chronic cough in the Caribbean area. ERJ Open Res. 2021;7:00814-2020. doi: 10.1183/23120541.00814-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.McGarvey L. The difficult-to-treat, therapy-resistant cough: why are current cough treatments not working and what can we do? Pulm. Pharmacol. Ther. 2013;26:528–531. doi: 10.1016/j.pupt.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 233.Lee KK, et al. Global physiology and pathophysiology of cough: part 1: cough phenomenology–CHEST guideline and expert panel report. Chest. 2021;159:282–293. doi: 10.1016/j.chest.2020.08.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Shannon R, et al. Production of reflex cough by brainstem respiratory networks. Pulm. Pharmacol. Ther. 2004;17:369–376. doi: 10.1016/j.pupt.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 235.Hegland KW, Bolser DC, Davenport PW. Volitional control of reflex cough. J. Appl. Physiol. 2012;113:39–46. doi: 10.1152/japplphysiol.01299.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hutchings HA, Morris S, Eccles R, Jawad MS. Voluntary suppression of cough induced by inhalation of capsaicin in healthy volunteers. Respir. Med. 1993;87:379–382. doi: 10.1016/0954-6111(93)90052-2. [DOI] [PubMed] [Google Scholar]
- 237.Davenport PW. Urge-to-cough: what can it teach us about cough? Lung. 2008;186:S107–S111. doi: 10.1007/s00408-007-9045-7. [DOI] [PubMed] [Google Scholar]
- 238.Marsden PA, et al. Objective cough frequency, airway inflammation, and disease control in asthma. Chest. 2016;149:1460–1466. doi: 10.1016/j.chest.2016.02.676. [DOI] [PubMed] [Google Scholar]
- 239.Satia I, et al. The interaction between bronchoconstriction and cough in asthma. Thorax. 2017;72:1144–1146. doi: 10.1136/thoraxjnl-2016-209625. [DOI] [PubMed] [Google Scholar]
- 240.Satia I, et al. Capsaicin-evoked cough responses in asthmatic patients: evidence for airway neuronal dysfunction. J. Allergy Clin. Immunol. 2017;139:771–779.e710. doi: 10.1016/j.jaci.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 241.Choudry NB, Fuller RW, Anderson N, Karlsson JA. Separation of cough and reflex bronchoconstriction by inhaled local anaesthetics. Eur. Respir. J. 1990;3:579–583. [PubMed] [Google Scholar]
- 242.Fujimura M, Sakamoto S, Kamio Y, Matsuda T. Effects of methacholine induced bronchoconstriction and procaterol induced bronchodilation on cough receptor sensitivity to inhaled capsaicin and tartaric acid. Thorax. 1992;47:441–445. doi: 10.1136/thx.47.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hilton EC, Baverel PG, Woodcock A, Van Der Graaf PH, Smith JA. Pharmacodynamic modeling of cough responses to capsaicin inhalation calls into question the utility of the C5 end point. J. Allergy Clin. Immunol. 2013;132:847–855.e1–5. doi: 10.1016/j.jaci.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 244.Drake MG, et al. Eosinophils increase airway sensory nerve density in mice and in human asthma. Sci. Transl. Med. 2018;10:eaar8477. doi: 10.1126/scitranslmed.aar8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gao J, Wu F, Wu S, Yang X. Inflammatory subtypes in classic asthma and cough variant asthma. J. Inflamm. Res. 2020;13:1167–1173. doi: 10.2147/JIR.S269795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Gao J, Wu HG, Wu F. Small airways dysfunction and bronchial hyper-responsiveness in cough variant asthma. Int. J. Gen. Med. 2020;13:1427–1434. doi: 10.2147/IJGM.S286144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gibson PG, Dolovich J, Denburg J, Ramsdale EH, Hargreave FE. Chronic cough: eosinophilic bronchitis without asthma. Lancet. 1989;1:1346–1348. doi: 10.1016/s0140-6736(89)92801-8. [DOI] [PubMed] [Google Scholar]
- 248.Brightling CE, et al. Mast-cell infiltration of airway smooth muscle in asthma. N. Engl. J. Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 249.Brightling CE, et al. Induced sputum inflammatory mediator concentrations in eosinophilic bronchitis and asthma. Am. J. Respir. Crit. Care Med. 2000;162:878–882. doi: 10.1164/ajrccm.162.3.9909064. [DOI] [PubMed] [Google Scholar]
- 250.Vertigan AE, Bone SL, Gibson PG. Laryngeal sensory dysfunction in laryngeal hypersensitivity syndrome. Respirology. 2013;18:948–956. doi: 10.1111/resp.12103. [DOI] [PubMed] [Google Scholar]
- 251.Vertigan AE, Kapela SM, Kearney EK, Gibson PG. Laryngeal dysfunction in cough hypersensitivity syndrome: a cross-sectional observational study. J. Allergy Clin. Immunol. Pract. 2018;6:2087–2095. doi: 10.1016/j.jaip.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 252.Bucca CB, et al. Chronic cough and irritable larynx. J. Allergy Clin. Immunol. 2011;127:412–419. doi: 10.1016/j.jaci.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 253.Ryan NM, Gibson PG. Characterization of laryngeal dysfunction in chronic persistent cough. Laryngoscope. 2009;119:640–645. doi: 10.1002/lary.20114. [DOI] [PubMed] [Google Scholar]
- 254.Hull JH, Backer V, Gibson PG, Fowler SJ. Laryngeal dysfunction: assessment and management for the clinician. Am. J. Respir. Crit. Care Med. 2016;194:1062–1072. doi: 10.1164/rccm.201606-1249CI. [DOI] [PubMed] [Google Scholar]
- 255.Chamberlain S, Birring SS, Garrod R. Nonpharmacological interventions for refractory chronic cough patients: systematic review. Lung. 2014;192:75–85. doi: 10.1007/s00408-013-9508-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.