Abstract
Burkholderia cepacia AMMDR1 is a biocontrol agent that protects pea and sweet corn seeds from Pythium damping-off in field experiments. The goal of this work was to understand the effect of B. cepacia AMMDR1 on Pythium aphanidermatum and Aphanomyces euteiches zoospore homing events and on infection of pea seeds or roots. In vitro, B. cepacia AMMDR1 caused zoospore lysis, prevented cyst germination, and inhibited germ tube growth of both oomycetes. B. cepacia AMMDR1 also reduced the attractiveness of seed exudates to Pythium zoospores to nondetectable levels. However, when present at high levels on seeds, B. cepacia AMMDR1 had little net effect on zoospore attraction, probably because it also enhanced seed exudation. Seed-applied B. cepacia AMMDR1 dramatically reduced the incidence of infection by Pythium zoospores in situ compared with an antibiosis-deficient Tn5 mutant strain. This mutant strain also decreased Pythium infection incidence to some extent, but only when the pathogen inoculum potential was low. B. cepacia AMMDR1 did not affect attraction of Aphanomyces zoospores or Aphanomyces root rot incidence. These results suggest that B. cepacia AMMDR1 controls P. aphanidermatum largely through antibiosis, but competition for zoospore-attracting compounds can contribute to the effect. Differences in suppression of Aphanomyces and Pythium are discussed in relation to differences in the ecology of the two pathogens.
Biological control of soilborne pathogens offers a promising alternative to synthetic pesticides, in part because it is perceived as safe to the environment and to the consumers of the plants that it protects. The commercial success of biocontrol agents is expected to increase when some of its problems, such as inconsistency in performance, are improved (17). Some of the inconsistencies result from the nature of biocontrol: it is the outcome of complex interactions involving the plant, the biocontrol agent, the pathogen, and the physical and biological environments. Although in recent years exciting progress has been made in elucidating aspects of biocontrol interactions, such as the in situ production of antibiotics and siderophores (18, 28), the involvement of stationary-phase regulators in antibiotic production (29, 50, 52, 53), communication with rhizosphere bacteria through quorum sensing (42, 44), and the importance of the host component in the interactions (56), in many systems we do not yet understand all the components of disease suppression or the mechanisms involved.
Most research on the mechanisms of bacterium-mediated biocontrol has focused on antibiotics and siderophores (21, 61). Although biocontrol assays with Tn5 mutants have shown that siderophores and especially antibiotics often account for most of the biocontrol effect, the ecology of some pathogens offers opportunities for biocontrol through other mechanisms. In biocontrol of Pythium damping-off, protection of a seed or young seedling can be accomplished by inundative application of a biocontrol agent. This allows for antagonism through competition for host exudates, as different Pythium species generally depend on seed exudates for either oospore germination (58), sporangial germination (35), or zoospore attraction towards the host (14). Biocontrol can be the result of competition for stimulants of sporangial germination, such as linoleic acid (63); inducers of oospore germination (16, 60); or zoospore attractants, such as ethanol, acetaldehyde, amino acids, and sugars (40, 65). Although these are some examples of biocontrol due to a combination of competition and antibiosis, little work has focused on partitioning the effects of each process in a single system.
Antibiotic-deficient mutants often have substantially reduced biocontrol effects, but the mutant phenotypes generally do not account for all biocontrol activity. The literature on antibiosis- and/or siderophore-deficient mutants indicates that in over 90% of the more than 35 biocontrol interactions studied, a residual effect was observed (22), suggesting involvement of additional mechanisms of biocontrol. The proportion of the biocontrol effect not explainable by the mutant phenotype was more than 20% in 72% of the cases and more than 40% in 42% of the cases. Residual biocontrol activity was attributed to uncharacterized antibiotics or siderophores, lytic enzymes, and induction of systemic resistance in some but not all of the cases. Therefore, as suggested by most of the authors, the effects may have resulted from competition for space or nutrients. Moreover, since biocontrol is often tested at pathogen inoculum levels at which the pathogen-only treatment results in high levels of disease, mechanisms such as competition, which might operate only at lower pathogen inoculum levels, can be overlooked. The widespread inability to ascribe biocontrol activity to single mechanisms in so many different systems motivated us to study this phenomenon in more detail and to evaluate it at different pathogen inoculum levels.
The objectives of this study were to evaluate the effect of a biocontrol agent on zoospore homing and infection of host seeds or roots by two oomycete plant pathogens and to assess the relative importance of antibiosis and nutrient competition in biocontrol during these early events. We compared the activity of an antibiosis-positive parent strain with that of an antibiosis-deficient, seed- and root-colonizing mutant strain for (i) direct effects on the pathogens and (ii) indirect effects on zoospore attraction to seed exudates and on zoosporemediated seed or root infection. The biocontrol system described in this work involves Burkholderia cepacia AMMDR1, a bacterial biocontrol agent selected for its activity against Pythium spp. and Aphanomyces euteiches on peas (Pisum sativum L.) (6, 26, 37, 39).
MATERIALS AND METHODS
Organisms.
B. cepacia AMMD was isolated from the rhizosphere of peas grown in the Aphanomyces root rot nursery at the Arlington Agricultural Experiment Station (Arlington, Wis.) (39). This organism has been referred to as Pseudomonas cepacia AMMD (6, 26, 37, 39), B. cepacia AMMD (27, 46), and Burkholderia vietnamiensis AMMD (22), and most recently it was reclassified as a new genomovar (genomovar VII) of the B. cepacia complex (P. Vandamme, personal communication, 1999). B. cepacia AMMDR1 (= NRRL B-23396), a spontaneous rifampin-resistant (100 ppm) mutant of B. cepacia AMMD, was used throughout this study (NRRL numbers refer to voucher cultures deposited in the Agricultural Research Service Culture Collection, Peoria, Ill. [http://nrrl.ncaur.usda.gov/the_collection3.html]). B. cepacia 1324 (= NRRL B-23397) is a Tn5 mutant of B. cepacia AMMDR1 that does not exhibit antibiosis in vitro against Pythium mycelial growth (46). It contains a single Tn5 insertion, its siderophore and protease production are not affected, and it has the same doubling time in the pea spermosphere as the parent strain (46). Treatment of pea seeds with bacteria consisted of mixing seeds with bacteria scraped from 2-day-old TGE agar (5 g of tryptone, 1 g of glucose, 2.5 g of yeast extract, 15 g of agar, 1,000 ml of water) (3) cultures by using a ratio of 20 seeds per plate. Seeds were dried in a laminar flow cabinet for approximately 2 h before they were used in experiments. The numbers of bacteria per seed were determined by 30 s of sonication and 10 s of vortexing in a 10-ml water blank, followed by plating of serial dilutions on TGE agar, and were usually about 7.5 log CFU seed−1. Bacterial cultures were always started from −80°C stocks.
A. euteiches 467 (= NRRL 29264) was isolated from a diseased pea plant collected in Washara County, Wis. Zoospores were produced by a slightly modified protocol of Parke and Grau (38). Three agar plugs (no. 2 cork borer) were taken from the margins of an actively growing CMA culture and transferred to 25 ml of PG broth (38). After 2 days of static incubation at room temperature (22°C), the medium was replaced with approximately 20 ml of autoclaved rinsing solution (a 1:1 mixture of water and lake water) and the preparations were rinsed two more times at 2-h intervals. Zoospores were used 12 to 14 h after the last rinse, when the concentrations were 1 × 105 to 2 × 105 zoospores ml−1.
Pythium aphanidermatum BP-01 (= NRRL 29623) was isolated from a diseased bean plant from New Mexico. To generate zoospores, three agar plugs from the margins of an actively growing CMA culture were transferred to 25 ml of clarified V8 broth (47) and incubated statically for 24 h at 24°C. Mycelial mats were rinsed with sterile distilled water and incubated overnight, which allowed production of zoosporangia. The next morning, the mycelial mats were rinsed three times at 1-h intervals with the lake water solution described above. Zoospores were used 5 h after the last rinse, when the concentrations were approximately 5 × 104 zoospores ml−1.
Exudate production.
Exudates were generated by using commercial pea seeds (cultivar Early Perfection 77; Nunhems Seed Corp., Lewisville, Idaho). Seeds were surface sterilized for 5 min in full-strength Clorox (5.25% NaOCl) and rinsed four times in sterile water. Preliminary tests showed that seeds were completely disinfested and seed germination was not affected by this treatment. Seeds with damaged seed coats were discarded.
For bulk production of exudates, 50 surface-sterilized seeds were placed in a shallow layer of 50 ml of sterile water in a 2-liter Erlenmeyer flask. After 3 days of static incubation at 24°C, the remaining water (approximately 15 ml) was passed through a 0.45-μm-pore-size polysulfone Millipore filter and used as the seed exudate in assays. Preliminary experiments indicated that filtration resulted in sterilization of the exudates. Undiluted exudate contained up to 2.8 mg of dry matter per ml.
For some experiments, exudates were produced from seeds treated with bacteria. Sets of five surface-sterilized seeds were either not treated or treated with B. cepacia AMMDR1 or 1324 and placed in 5-ml portions of water in 125-ml Erlenmeyer flasks. Flasks containing 5 ml of water were included as controls. After 3 days of static incubation at 24°C, the remaining liquid in each flask (about 2 ml) was transferred to microcentrifuge tubes and centrifuged for 20 min at 16,000 × g, and the supernatant was passed through a 0.45-μm-pore-size polysulfone Millipore filter and used as the exudate. In some of the experiments, the bacterial population size and the electrical conductivity (EC) of the exudate were determined at the end of the 3-day incubation period as described below. EC of seed steep water is highly correlated with seed exudation (36), so this parameter was used to evaluate the effect of pretreatment of seeds with bacteria on seed exudation.
Effects of exudates from seeds pretreated with bacteria on zoospores, cysts, and germ tubes.
Aphanomyces or Pythium zoospores or zoospore cysts (obtained by 30 s of vortexing of a zoospore suspension) were combined 1:1 in flat-bottom microtiter wells with water or with exudates from nontreated seeds or seeds pretreated with B. cepacia AMMDR1 or 1324. After 4 h of incubation at 24°C, 100 μl of 4% gluteraldehyde and 10 μl of 0.05% trypan blue in lactoglycerin (containing [per liter] 412 ml of lactic acid [85%], 296 ml of glycerin, and 292 ml of distilled water) were added to the wells. The numbers of zoospores, cysts, germlings, and lysed cells on the bottoms of the wells were counted by examining 100 random cells at a magnification of ×200 with an inverted microscope (19). All experiments were conducted at least twice with a minimum of four replicates. Approximately 5.5 h after vortex-mediated encystment and following incubation at 24°C, germinated cysts were fixed with gluteraldehyde, and the germ tube lengths of randomly chosen spores were measured with an ocular micrometer. There were four replicates, and each replicate consisted of 10 spores in a separate microtiter well.
In vitro zoospore attraction assay.
The zoospore attraction assay was based on the capillary root model, in which zoospore attraction to glass capillaries that are loaded with a test compound is evaluated (48). The assay used was a modification of the agar-free version involving quantification of zoospores in open-ended capillaries (2). Zoospores were diluted with sterile water to a concentration of 5 × 104 spores ml−1. Four 150-μl aliquots of homogeneous zoospore suspensions were deposited into four flat-bottom microtiter wells; each well was a subsample. Within 2 min of distribution of the aliquots, one 2-μl glass capillary (Drummond Scientific Corp., Broomall, Pa.) that previously had been loaded with the test substance was placed at a fixed angle in each of the microtiter wells. Capillaries were placed in the zoospore suspensions at 20-s intervals and suspended for exactly 2 min. Then the outer surfaces of the capillaries were wiped clean of zoospores, and the contents of each capillary were ejected into 98 μl of 4% gluteraldehyde. For treatments in which a high number of attracted zoospores was expected, 20 μl of the mixed suspension was pipetted into a different well along with 80 μl of 4% gluteraldehyde. One additional fivefold dilution was made in treatments in which there were more than 1,500 zoospores capillary−1. After dilution, 2 μl of 0.05% trypan blue in lactoglycerin was added to each well to stain the spores and facilitate counting. Zoospores were allowed to settle to the bottoms of the wells and were counted at a magnification of ×100 with an inverted microscope. Examination of wells and capillaries with a dissecting microscope confirmed that all spores were expelled from the capillaries and ended up at the bottoms of the wells. All experiments were conducted at least twice with a minimum of three replicates.
The dose-response attractive effect of exudates on Aphanomyces and Pythium zoospores was determined in an attraction assay towards the following exudate-water dilution series: 1:0, 1:3.16, 1:10, 1:31.6, 1:100; and 1:316. The undiluted exudates each contained 1.47 mg of dry matter ml−1. The assay was performed with two replicates and four subsamples. Differences in the dose responses between the zoospores of the two species were evaluated by performing a linear regression analysis of numbers of attracted zoospores on log10-transformed exudate concentration.
Bacterial incubation in exudates or on seeds and its effect on zoospore attraction.
To determine the effect of bacterial incubation in exudates on zoospore attraction, exudate aliquots (5 ml) were placed into 125-ml Erlenmeyer flasks and inoculated with B. cepacia AMMDR1 or 1324 at a starting concentration of 4 × 106 CFU ml of exudate−1. Water aliquots served as controls. The exudates were incubated for 2 days at 24°C. After incubation, the exudates or water was transferred to microcentrifuge tubes and centrifuged for 20 min at 16,000 × g, and the supernatants were passed through 0.45-μm-pore-size Millipore filters. Each filtrate was diluted 20-fold with sterile water and used in zoospore attraction assays with Pythium and Aphanomyces zoospores. Bacterial population sizes were determined in the exudates by standard dilution plating on TGE agar. Electrical conductivity (EC) was measured by using 10-fold-diluted exudate samples and a YSI 3100 conductivity instrument (YSI Inc., Yellow Springs, Ohio). The experiments had a randomized complete block design with four replicates and time as the blocking factor. Each experiment was conducted twice. Depending on random distribution of the residuals, analysis of variance (ANOVA) was performed either with the zoospore counts or with the log10-transformed zoospore counts by using Minitab 12.1 (Minitab Inc., State College, Pa.). To test whether bacterial metabolites contained Pythium zoospore repellents, the same attraction assay was repeated with two extra treatments: exudates in which no bacteria had grown were diluted 20-fold with exudates in which bacteria (B. cepacia AMMDR1 or 1324) had grown. If a repellent was the cause of a lack of zoospore attraction towards seed exudates in which the bacteria grew, then the attraction towards the exudates subjected to the two extra treatments should have been strongly reduced compared to the attraction towards the exudates diluted with water. If the lack of zoospore attraction towards seed exudates in which the bacteria grew was due to metabolism of attractants and not due to production of a repellent, then diluting regular exudates with exudates in which the bacteria grew should not have resulted in a reduction in zoospore attraction. To test the effect of bacterial incubation on zoospore attraction over time, B. cepacia AMMDR1 was incubated in seed exudates as described above and samples were taken at zero time and after 4, 8, 12, 24, 48, and 72 h. The samples were treated as described above, and samples that were diluted 50-fold were evaluated to determine their attractiveness to Pythium zoospores. There were four replicates per time period.
To test the capacity of the biocontrol bacteria to reduce the zoospore attractiveness of exudates while new exudates were released, the Pythium zoospore attractiveness to 50-fold-diluted exudates from B. cepacia AMMDR1-treated seeds or B. cepacia 1324-treated seeds was determined and compared to the attractiveness of 50-fold-diluted exudates from nontreated seeds. The experiment was conducted three times, and the experimental design was a randomized complete block design with a minimum of three replicates.
In situ dispersal assay with Pythium zoospores.
Silica sand (Wedron Silica Company, Wedron, Ill.) was wetted to a gravimetric moisture content of approximately 5% with type I Milli-Q water and autoclaved for 1 h at 121°C. A single batch of sand that consistently allowed in situ dispersal of zoospores to untreated seeds was used for most experiments. The sand was autoclaved between experiments to prevent carryover of pathogen inoculum, which was checked by using noninoculated controls. After cooling, the sand was remixed and its moisture content was determined by drying samples at 105°C overnight. Based on the moisture content of the sand, polystyrene plastic cups (height, 8 cm; top diameter, 10.5 cm; bottom diameter, 9 cm) were filled with a quantity of sand that corresponded to 750 g of dry sand. Each cup was loosely covered with a plastic bag and incubated at 24°C for 3 days. Water was added to obtain a gravimetric moisture content of 13.6%. On the basis of a water retention curve generated for this sand, the soil water potential was −0.2 kPa and 72% of the pore space was waterfilled (effective pore diameter, 1,454 μm). The moisture content was uniform throughout the profile and among the cups. Approximately 1 h after the water content was increased, seeds were planted in circles with either a 2-, 3-, or 4-cm radius (one ring per cup) and covered with 1.2 cm of sand. There were eight seeds in the 2-cm-radius rings and 10 seeds in the 3- and 4-cm-radius rings. Within each ring, the seeds were planted at equal distances with their radicles pointing down and facing the clockwise neighboring seed. The seeds were either not treated or treated with B. cepacia AMMDR1 or 1324. Approximately 5 h after seeds were planted, 1 ml of a suspension containing 5 × 104 zoospores ml−1 was added at a depth of 1.5 cm to the center of each ring. The zoospores were added in two 0.5-ml aliquots with a Pasteur pipette. In a preliminary experiment, the in situ distribution of an added zoospore suspension was determined by twice depositing 0.5 ml of a 1% aqueous solution of trypan blue stain with the pipette and then sectioning horizontally and vertically through the deposition axis. The radius of the stained area was greatest at the location of the pipette tip. The maximum radius of the stained area was 6 mm, and the radius was relatively uniform for each depth. Negative controls consisted of cups that contained a 2-cm-radius ring of seeds and were inoculated with water. Positive controls consisted of cups that contained a 2-cm-radius ring of seeds and were inoculated at five equidistant points on a 1-cm-radius concentric ring with a fivefold-diluted zoospore suspension. After zoospore inoculation, the cups were loosely covered with plastic bags and incubated at 28°C. A preliminary experiment determined that this temperature allowed 100% infection in positive controls. Approximately 40 h after zoospore inoculation, the seeds were dug up, each seed coat and shoot were removed, and the cotyledons were separated, rinsed in 10 ml of water, blotted on sterilized paper towels, and plated with their flat sides down on 0.8% water agar (five seeds per plate). The plates were incubated at room temperature. One and 2 days after plating, every seed was checked for fungal outgrowth. For each cup, the percentage of seeds with fungal outgrowth was determined and used to calculate the average infection incidence, which was based on a minimum of four replicate cups per treatment. ANOVA was performed on arcsin of square root-transformed data. The experiment was conducted twice.
In situ dispersal assay with Aphanomyces zoospores.
The in situ dispersal assay with Aphanomyces zoospores was similar to the assay described above for Pythium zoospores, and the modifications were related to the fact that Aphanomyces is a root pathogen, not a seed pathogen (8). Silica sand was prepared as described above, but taller polystyrene cups (height, 14 cm; top diameter, 10.5 cm; bottom diameter, 9 cm) were used; each cup was filled with an amount of sand corresponding to 1,200 g of dry sand. Four days after the cups were filled, sand was brought to a gravimetric water content of 14.9% (-0.1 kPa; effective pore diameter, 2,908 μm). Seeds were planted approximately 1 h later in rings with either 2-cm (8 seeds) or 3-cm (10 seeds) radii as described above for the assay with Pythium zoospores. The seeds were either not treated or treated with B. cepacia AMMDR1 or 1324. The cups were then incubated for approximately 46 h at 24°C. At 46 h, the average root length was 2.5 cm, as determined in a separate experiment. Aphanomyces zoospores were diluted to a concentration of 5 × 104 spores ml−1 with water. One milliliter was pipetted into the center of each ring at a depth of 3.5 cm with a Pasteur pipette as described above. Negative and positive control treatments were similar to the treatments described above for the Pythium assay. The cups were loosely covered with plastic bags and incubated at 24°C for 2 days. Seedlings were carefully dug up and rinsed with tap water, and then they were incubated in moistened and rolled paper towels (four or five seedlings per paper towel). The paper towels were labeled and placed vertically in plastic test tube racks (20 towels per rack). The racks were placed in plastic tubs filled with deionized water so that the bottom 1 cm of the paper towels was submerged in the water. The seedlings were incubated at 24°C and checked every day for the next 7 days for Aphanomyces root rot symptoms. Typical symptoms were water-soaked, honey brown lesions spreading from the location where the root tip had been inoculated with zoospores. Seedlings with symptoms were removed from the rolled towels to prevent formation of zoosporangia and zoospore dispersal to neighboring seedlings. Noninoculated seedlings were placed between experimental seedlings to check for cross-contamination from infected seedlings. The design of the experiment was a completely randomized design with four replicate cups. For each evaluation day, the arcsin of square root-transformed data was subjected to ANOVA.
Bacterial populations on roots were determined 2 h after zoospore inoculation. Four random seedlings were dug up, and the size of the B. cepacia 1324 or AMMDR1 population was determined for the 1-cm root section closest to the root tip. Root sections were sonicated for 30 s and vortexed for 10 s in 10-ml water blanks, and this was followed by plating of serial dilutions on selective TBTR medium (20, 27).
Timing of in situ zoospore dispersal.
A dispersal assay was conducted with Pythium zoospores to confirm that zoospores, not cysts, were the infective units and to provide information on the time available for in situ metabolism of attractants by the bacteria. The assay was similar to that described above, except as follows. Seeds were planted only in 3-cm-radius rings. The cups were inoculated with zoospores in the center of each ring, but at zero time and 1, 2, 4, 8, 22, or 48 h later the central core of moistened sand within each ring of seeds was removed with a hollow plastic cylinder (diameter, 4.8 cm). When this cylinder of sand was removed all zoospores that had not dispersed to the seeds were removed from the system. Also, potential infection of seeds by mycelia arising from cysts germinating some distance from the seeds was prevented. After 48 h of incubation, seeds were dug up and plated as described above to determine the infection incidence. There were four replicates for each time point, and the experiment was conducted twice.
Zoospores from the same batch were placed in flat-bottom microtiter wells, and zoospore motility in vitro was determined over time. The proportion of motile zoospores was determined at each time point by fixing spores in two of the wells and counting the number of zoospores in samples of 100 propagules (zoospores, cysts, and germlings).
Effect of seed exudation level on the incidence of zoospore-mediated infection.
Low-exuding and high-exuding seeds were selected from a large population based on the EC of the steep water. Seeds were individually steeped in 5 ml of water for 4 h. Seeds with steep water EC of 40 to 70 μS cm−1 were considered low exuding, and seeds with steep water EC of 120 to 140 μS cm−1 were considered high exuding. Seeds were redried to a moisture content of 10% (dry weight basis) in a laminar flow hood before they were used in an in situ zoospore dispersal assay with Pythium zoospores. To test whether seeds exhibited the same exudation pattern after in situ reimbibition, five seeds with an exudate EC out of each 10-μS cm−1 interval between 40 and 170 μS cm−1 were selected and reimbibed for 4 h, and then the ECs were determined. The correlation coefficient between the ECs obtained after the first and second imbibitions was used as an indication of the relevance of the selection procedure. The weights of high- and low-exuding seeds were compared to test the hypothesis that the weights of high- and low-exuding seeds differed. The attraction assay used was a modified version of the standard assay performed with a ring of seeds. One high-exuding seed and one low-exuding seed were planted 8 cm away from each other on opposite sides of a central inoculation point. Pythium zoospores were inoculated at one of two inoculum levels: 2 × 104 zoospores ml−1 (high) or 2 × 103 zoospores ml−1 (low). There were 10 replicates. For each inoculum level, differences in the incidence of infection between high- and low-exuding seeds were analyzed with the McNemar test (10).
RESULTS
Effects of exudates from seeds pretreated with bacteria on zoospores, cysts, and germ tubes.
Exudates from nontreated seeds stimulated zoospore encystment, cyst germination, and germ tube growth. In exudates of seeds pretreated with B. cepacia AMMDR1, zoospores lysed within minutes, cysts did not germinate, or germ tube growth was severely restricted compared to the effects in water or in exudates from nontreated seeds (Table 1). For the most part exudates from seeds pretreated with B. cepacia 1324 had effects on zoospores and cysts similar to those of exudates from nontreated seeds.
TABLE 1.
Effects of exudates from nontreated seeds and exudates from seeds pretreated with B. cepacia AMMDR1 or 1324 on zoospore longevity, cyst germination, and germ tube growth
| Treatment | Pythium zoospore longevity (%)a | Aphanomyces zoospore longevity (%)a | Aphanomyces cyst germination (%)b | Pythium germ tube length (μm)c |
|---|---|---|---|---|
| Water | 88 ad | 76 a | 15 c | 193 b |
| Exudates from nontreated seeds | 75 b | 42 b | 37 a | 380 a |
| Exudates from seeds pretreated with B. cepacia AMMDR1 | 0 c | 0.2 c | 0 d | 34 c |
| Exudates from seeds pretreated with B. cepacia 1324 | 80 b | 42 b | 23 b | 402 a |
Longevity was calculated as follows: (average number of zoospores/total number of spores) × 100, as determined 4 h after zoospores and either water or exudates from nontreated seeds or seeds pretreated with bacteria were combined. The percentage of lysed cells did not exceed 2% in treatments without B. cepacia AMMDR1. In the treatment with B. cepacia AMMDR1, the minimum percentage of lysed cells was 94%.
Average percentage of germinated cysts 4 h after cysts and either water or exudates from nontreated or bacterium-treated seeds were combined. The effect of B. cepacia AMMDR1 on germination of Pythium cysts was more difficult to represent than the effect of this bacterium on the germination of Aphanomyces cysts. Most Pythium cysts in the preparation treated with B. cepacia AMMDR1 initiated germination (78%), but then growth quickly stalled (see data for germ tube growth). Similarly, exudates from nontreated seeds had effects on germ tube morphology but had little effect on the incidence of Pythium cyst germination (data not shown). Therefore, we chose to quantify the effects of seed exudates on germ tube length.
Average germ tube length measured 5.5 h after vortex-mediated encystment of zoospores. The effect of B. cepacia AMMDR1 on Aphanomyces germ tube length was even greater than the effect on Pythium, considering that there were few or no germinating cysts (see data for Aphanomyces cyst germination). However, quantifying the effects of the other exudate treatments on germ tube length was difficult due to greater branching of the germ tubes.
Within each column, values followed by the same letter are not significantly different according to Tukey's multiple comparisons on arcsin- (square root)-transformed values (P = 0.05).
Effect of seed exudate concentration on Aphanomyces and Pythium zoospore attraction.
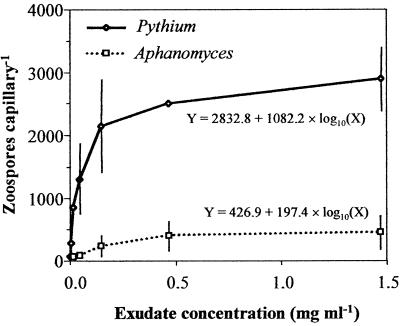
Pythium zoospores were much more strongly attracted to the exudates than Aphanomyces zoospores were (Fig. 1). Relatively small increases in zoospore attraction were observed for both oomycetes at exudate concentrations greater than 0.5 mg ml−1. Linear regression of number of zoospores versus log10-transformed exudate concentration was highly significant for both oomycetes. A comparison of the slopes and intercepts, as well as a simultaneous comparison of the slopes and intercepts of the two regression lines, revealed that each was highly significantly different (P < 0.001 for each comparison).
FIG. 1.
Effect of seed exudate concentration on zoospore attraction of two oomycetes. The data are the mean numbers of Pythium and Aphanomyces zoospores present within 2 min in glass capillaries loaded with different concentrations of pea seed exudates. The error bars indicate 2 standard errors. For the data points without error bars the standard errors were smaller than the symbols.
Bacterial incubation in exudates and its effect on zoospore attraction.
Attraction of Pythium zoospores, but not Aphanomyces zoospores, was strongly reduced towards exudates in which either of the bacterial strains had grown (Table 2). Pythium zoospores were still attracted to exudates that were diluted with exudates in which the bacteria were incubated, indicating that B. cepacia AMMDR1 and 1324 do not produce significant amounts of zoospore repellent. During the time course experiment, attraction of Pythium zoospores was reduced to insignificant levels as soon as 24 h after inoculation of seed exudates with B. cepacia AMMDR1, at which point the bacterial population size was approximately 8 log CFU ml−1 (data not shown).
TABLE 2.
Attraction of Pythium and Aphanomyces zoospores toward seed exudates in which B. cepacia AMMDR1 or 1324 was incubated
| Starting substrate | Bacterial incubation | Treatment no. | Diluting compound | No. of zoosporesa
|
|
|---|---|---|---|---|---|
| Pythium | Aphanomyces | ||||
| Water | None | 1 | Water | 19 ± 4 a | 36 ± 7 a |
| Seed exudate | None | 2 | Water | 2,456 ± 278 b | 259 ± 83 b |
| Seed exudate | B. cepacia AMMDR1 | 3 | Water | 5 ± 3 a | 204 ± 41 b |
| Seed exudate | B. cepacia 1324 | 4 | Water | 4 ± 2 a | 268 ± 99 b |
| Seed exudate | None | 5 | Treatment 3b | 1,698 ± 805 b | |
| Seed exudate | None | 6 | Treatment 4b | 1,947 ± 88 b | |
The values are the numbers of zoospores (average ± standard deviation) present in glass capillaries loaded with 20-fold-diluted test compound after 2 min of suspension in a solution containing 5 × 104 zoospores ml−1. Within each column, log10-transformed values followed by the same letter are not significantly different at the 1% level. The data are data from one of two similar experiments.
Before dilution.
The attractiveness of exudates of seeds pretreated with B. cepacia 1324 to Pythium zoospores was significantly reduced compared to that of exudates from nontreated seeds. However, in contrast to the reduced attraction to exudates in which B. cepacia AMMDR1 was incubated, exudates of seeds pretreated with B. cepacia AMMDR1 remained attractive to zoospores. Zoospore attraction, bacterial population size, and seed exudate EC were significantly greater when exudates were generated with B. cepacia AMMDR1 present than when exudates were generated with B. cepacia 1324 present (Table 3). When B. cepacia AMMDR1 was incubated in exudates that were generated in the absence of bacteria, the values for these parameters were not different as compared to the values obtained with B. cepacia 1324 treatment. A hypothesis consistent with the differences observed is that B. cepacia AMMDR1 but not B. cepacia 1324 increased seed exudation.
TABLE 3.
Attractiveness to Pythium zoospores, bacterial populations, and ECs of seed exudates in which bacteria were incubated and exudates of seeds pretreated with bacteria
| Treatment | Exudates in which bacteria were incubated
|
Exudates of seeds pretreated with bacteria
|
||||
|---|---|---|---|---|---|---|
| Attractiveness to zoosporesb | Bacterial populationc | ECd | Attractiveness to zoosporesb | Bacterial populationc | ECd | |
| Controla | 100 ah | NAg | 79.4 a | 100 a | NA | 75.3 a |
| B. cepacia 1324 | 0.7 b | 8.97 a | 64.9 a | 17 b | 10.24 a | 70.0 a |
| B. cepacia AMMDR1 | 1.0 b | 9.04 a | 66.5 a | 79 a | 10.43 b | 103.5 b |
| P valuee | <0.001 | 0.42 | 0.78 | <0.001 | 0.015 | 0.002 |
| nf | 4 | 5 | 5 | 4 | 10 | 8 |
The controls were exudates that were not inoculated with bacteria or exudates from nontreated seeds.
Normalized numbers of zoospores per capillary in attraction assays performed with 50-fold-diluted exudates. The control value (100) represented 1,030 ± 167 zoospores capillary−1 for the control treatment in which bacteria were incubated in exudates and 382 ± 157 zoospores capillary−1 for the control treatment in which exudates of seeds pretreated with bacteria were used. Differences were due to different concentrations of seed exudates produced by the two methods. Statistical tests were performed on nonnormalized data.
Bacterial population size expressed in log CFU per milliliter as determined by dilution plating on TGE agar.
Exudate EC expressed in microsiemens per centimeter.
P values of one-way ANOVAs.
Number of replicates in each experiment.
NA, not applicable.
Within each column, values followed by the same letter are not significantly different according to Fisher's multiple comparison at P = 0.05.
In situ dispersal assays with Pythium and Aphanomyces zoospores.
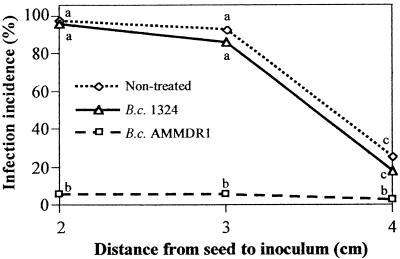
There was a sharp decrease in the incidence of Pythium disease due to treatment of seeds with B. cepacia AMMDR1; the incidence of seed infection was reduced to ≤5% at all distances from the source of the inoculum (Fig. 2). There was also a small, statistically insignificant decrease with B. cepacia 1324 treatment. However, the small reduction in the incidence of infection was statistically significant in a subsequent experiment performed with more replicates (n = 10), in which only nontreated seeds and seeds treated with B. cepacia 1324 were compared (data not shown). The incidences of infection were 100 and 95% at 3 cm (P = 0.099) and 93 and 79% at 4 cm (P = 0.049) for nontreated and B. cepacia 1324-treated seeds, respectively. When averaged over distance, the P value for the factor bacterial treatment (nontreated versus B. cepacia 1324 treated) was 0.038.
FIG. 2.
Final infection incidence levels in the first dispersal and seed infection assay with P. aphanidermatum BP-01 zoospores as a function of inoculation distance and treatment of seeds with the biocontrol agent B. cepacia AMMDR1 or its antibiosis-deficient Tn5 mutant, B. cepacia 1324. Values labeled with the same letter are not significantly different at the 5% level (n = 4).
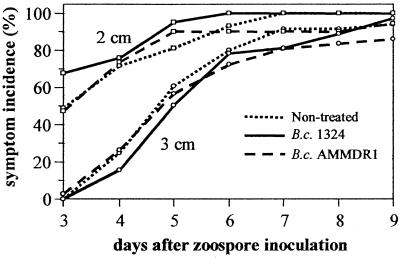
In contrast to the assay performed with Pythium zoospores, seed treatment with bacteria had practically no effect on the incidence of seedling disease in the assay performed with Aphanomyces zoospores (Fig. 3). Treatment of seeds with B. cepacia AMMDR1 did not result in a significant reduction in the incidence of symptoms at any distance. There was a minor but statistically significant decrease (5 to 10%) compared to nontreated and B. cepacia 1324-treated seeds near the end of the experiment. B. cepacia 1324 had no effect at all on the incidence of symptoms. The average population sizes of the B. cepacia AMMDR1 and 1324 populations on the 1-cm root tip sections were 4.5 and 3.9 log CFU, respectively. The P value for a Student's t test comparing these means was 0.36 (the pooled standard deviation was 0.81 [n = 4]).
FIG. 3.
Disease incidence levels over time in an in situ zoospore dispersal assay with A. euteiches 467. The factors used are planting distance from the inoculation point (2 or 3 cm) and seed treatment (nontreated or treated with B. cepacia AMMDR1 or its antibiosis-deficient Tn5 mutant, B. cepacia 1324). The means for inoculation distance (across seed treatment) were significantly different (P < 0.05) at all time points. The means for seed treatment (across inoculation distance) were not significantly different up to day 7. From that time on, symptom incidence was significantly less for the B. cepacia AMMDR1 treatment than for the other treatments.
Timing of in situ dispersal of Pythium zoospores.
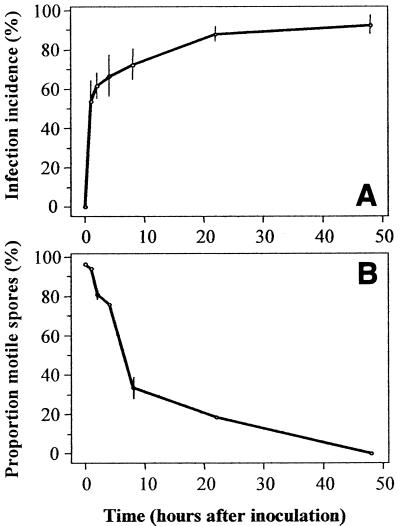
Zoospore dispersal from the inoculation site toward the periphery occurred fast; more than 50% of the seeds became infected, although the central core of substrate within the 3-cm-radius ring of seeds was removed as soon as 1 h after inoculation with zoospores, indicating that a considerable proportion of the zoospores had dispersed to the seeds within that time (Fig. 4A). Zoospore longevity in microtiter wells declined only about 20% in the first 4 h of incubation at 28°C (Fig. 4B). Even after 22 h of incubation, 18.5% of the spores were still motile.
FIG. 4.
(A) Infection incidence in an in situ zoospore dispersal assay with P. aphanidermatum BP-01. The substrate within a ring of seeds (radius, 3 cm) was removed at zero time or at 1, 2, 4, 8, 22, or 48 h after zoospore inoculation, which removed all spores which had not dispersed to the seeds by those times. (B) Zoospore longevity in an in vitro assay as reflected by the proportion of total spores (in microtiter wells) that were motile. Zoospores were incubated at the same temperature that was used in the main experiment. The error bars indicate 2 standard errors. For the data points without error bars the standard errors were smaller than the symbols.
Effect of seed exudation level on zoospore-mediated infection with Pythium.
The EC of the steep water of single seeds incubated in 5 ml of Milli-Q water for 4 h was 96 ± 50 μS cm−1 (average ± standard deviation; n = 240). A subpopulation of seeds with an EC of 40 to 70 μS cm−1 consisted of 16% of the seeds. A subpopulation with an EC of 120 to 140 μS cm−1 consisted of 10% of the seeds. The seed exudation level was not dependent on seed weight; a Student's t test on equality of the average seed weights in the two EC classes gave a P value of 0.44 (n = 80).
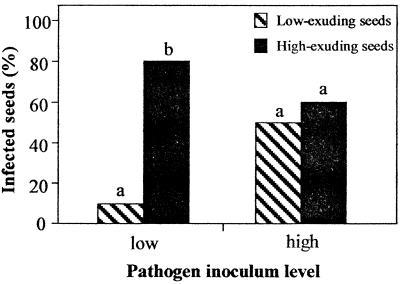
The correlation coefficient between the ECs of the steeping liquids from the first and second imbibitions was 0.81 with a P value of <0.001. Compared with high-exuding seeds, a significantly lower incidence of infection was observed with low-exuding seeds, but the difference was apparent only when the pathogen inoculum level was low (Fig. 5).
FIG. 5.
Effect of seed exudation level on Pythium infection incidence at two pathogen inoculum levels (2 × 104 and 2 × 103 zoospores ml−1) with seeds planted 4 cm away from the inoculation site. The experimental unit in this assay consisted of one low-exuding seed and one high-exuding seed per cup. Within each pathogen level, the letters indicate significant differences at P = 0.05.
DISCUSSION
This study allowed us to examine the effects of a biological control agent at specific early stages of the disease cycles of two oomycete pathogens and to partition the relative contributions of antibiosis and nutrient competition in this biological control system. The biocontrol agent B. cepacia AMMDR1 has direct effects on the zoospore homing responses of both oomycete pathogens in vitro. These effects appear to result from antibiosis, because the antibiosis-deficient mutant strain did not affect zoospore longevity or cyst germination. B. cepacia AMMDR1 and the mutant strain also had indirect effects on Pythium zoospore attraction through their effects on seed exudation, apparently by utilizing nutrients required for the zoospore homing response. However, neither bacterial strain influenced the attraction of Aphanomyces zoospores to seed exudates. In situ experiments demonstrated that B. cepacia AMMDR1 reduced the incidence of infection of seeds and roots by Pythium zoospores but not by Aphanomyces zoospores; experiments comparing the efficacies of the parent strain and the mutant strain suggested that biocontrol of infection of seeds by Pythium results largely from antibiosis.
The in vitro experiments indicated that B. cepacia AMMDR1 is able to interfere directly with several of the zoospore homing events; B. cepacia AMMDR1 but not B. cepacia 1324 caused zoospore lysis, prevented cyst germination, or inhibited germ tube growth in Aphanomyces and Pythium, indicating that there were strong direct effects due to antibiosis. The nature of the antibiotic is currently being determined by genetic and biochemical analyses, but it does not involve phenazines or 2,4-diacetylphloroglucinol (D. M. Weller, personal communication, 1998). B. cepacia AMMDR1 produces pyrrolnitrin, but considerable control of Pythium damping-off by pyrrolnitrin-deficient Tn5 mutants indicates that this antibiotic is not the major factor involved in the control of Pythium species (46).
Indirect effects of B. cepacia AMMDR1 were most clear for zoospore attraction, but only for Pythium; B. cepacia AMMDR1 and 1324 sharply reduced Pythium zoospore attraction to seed exudates when they were incubated in the exudates. Since no evidence for the presence of a zoospore repellent was found, the simplest explanation for the lack of attraction in treatments with B. cepacia AMMDR1 and 1324 is that the bacteria metabolize the zoospore attractants. In contrast to Pythium zoospore attraction, Aphanomyces zoospore attraction was only slightly reduced. The different natures of the attractants for Pythium and Aphanomyces zoospores are probably responsible for the difference in the abilities of the bacteria to reduce zoospore attractiveness. Zoospores of Pythium spp. are mainly attracted to common amino acids and sugars in seed exudates, such as glutamine, asparagine, sucrose, and mannose (14). A. euteiches is also attracted to isoflavones and flavones, such as prunetin (54). Unlike sugars and amino acids, isoflavones are difficult to metabolize (12), explaining the attractiveness to Aphanomyces zoospores remaining after microbial incubation.
Although the timing of zoospore-mediated infection with Pythium is not well known, experiments on direct germination of sporangia indicate that pea seeds can be infected as soon as 10 to 12 h after planting (30, 37). Therefore, for competition for exudates to have an effect in situ, metabolism of the exudates has to be fast (34). When B. cepacia AMMDR1 was inoculated at a concentration of 106 CFU ml of exudate−1, it took this organism 24 h to reduce the zoospore attractiveness of the exudates. This is similar to a system with Pythium ultimum sporangia and an Enterobacter cloacae strain, in which it took the bacteria 25 h to reduce the stimulatory activity of exudates to less than 10% when they were inoculated at a concentration of 5.6 × 106 CFU ml−1 (63).
When exudates were generated in the presence of the bacteria, B. cepacia 1324 still reduced the attractiveness of the exudates. In contrast to B. cepacia 1324, the parent strain not only metabolized the Pythium attractants but also stimulated exudation. This was shown by a higher exudate EC, a larger bacterial population in the exudate, and attraction of zoospores to the exudates. The exudation stimulus was apparently sufficient to partly neutralize the decrease in zoospore attraction due to metabolism. The increase in exudation may have resulted from phytotoxicity effects on the seeds and emerging seedlings, caused by the artificially high numbers of bacteria obtained in this gnotobiotic system. Depending on the host and the concentration of the antibiotic, phytotoxicity is observed with several of the antibiotics commonly produced by biocontrol agents, such as phenazine-1-carboxylic acid, 2, 4-diacetylphloroglucinol, and pyoluteorin (24, 32, 33, 55). No phytotoxic effect of B. cepacia AMMDR1 has ever been observed on pea seeds in the field, suggesting that in situ the size of the B. cepacia AMMDR1 population does not reach the size required to produce phytotoxic effects. Data on colonization of the pea rhizosphere with B. cepacia AMMDR1 support this observation (5, 27). However, phytotoxicity is occasionally observed in the field when large populations of B. cepacia AMMDR1 are applied to sweet corn seeds, especially if the seeds are also treated with captan (9).
The modified zoospore attraction assay performed with capillary tubes allowed quantitative testing of compounds at concentrations that resulted in large numbers of zoospores inside the capillaries. This was in contrast to previously described methods, in which spores were fixed and counted directly inside capillaries (2, 14, 23). The dose-response patterns for zoospore attractiveness to exudates could be fitted to the same type of curve for Aphanomyces and Pythium, but the numbers of zoospores that entered the capillaries were much larger for Pythium than for Aphanomyces. Considering that the number of zoospores that entered the capillaries plateaued at concentrations greater than 0.5 mg of exudate ml−1, it is unlikely that the difference in zoospore attraction was the result of a suboptimal concentration of attractants in the exudate. Although Aphanomyces zoospore attractants such as prunetin attract zoospores at much lower threshold levels than do certain amino acids and sugars (less than 0.1 μM versus 1 mM) (14, 54), the data obtained in this work suggest that a more sensitive receptor system does not necessarily result in accumulation of a larger number of zoospores at the source. Dose-response assays with purified chemicals are needed to confirm this.
Indirect effects due to competition for zoospore attractants could be tested with B. cepacia AMMDR1; by using at least 20-fold-diluted exudates and by restricting the assay time to 2 min, effects due to antibiosis could be avoided. However, in the other in vitro assays, antibiosis obscured potential indirect effects of B. cepacia AMMDR1. Therefore, indirect effects could be evaluated only with B. cepacia 1324. These indirect effects were not substantial and for the most part were not significant. The most important reason for this is probably that zoospore encystment and cyst germination are influenced more by Ca2+ fluxes and/or host surface compounds than by host exudates (11). Zoospores and cysts do not take up amino acids or glucose until germination (41), and the effects of these compounds on zoospores and cysts are hypothesized to be indirect by facilitating Ca2+ channeling across the membranes (11, 13). Because the role of sugars and amino acids in zoospore encystment and germination is probably secondary, bacterial competition for these compounds in exudates probably cannot result in large effects on these events. In contrast to these results, Zhou and Paulitz (65) did observe a reduction in the encystment response of P. aphanidermatum after incubation of biocontrol bacteria in exudates. However, their assay was performed in a salt solution that itself had a strong effect on encystment, which made it difficult to determine whether the effects from the bacteria were ionic or organic in nature. Germ tubes do absorb sugars and amino acids, and the effects of exudates on germ tube length were clear. Therefore, indirect effects of the bacteria on this stage were expected to be greater. However, this was not reflected in a shorter germ tube length during the first hours with the B. cepacia 1324 treatment, presumably because nutrient concentrations became suboptimal only after a few hours, when the length of the germ tubes was already considerable and became difficult to measure.
Although in vitro assays may give a good indication of the potential for antagonism in situ, the interactions observed cannot necessarily be extrapolated to natural systems; the timing and spatial dynamics of host colonization by the bacteria, the dynamics of zoospore dispersal and host infection, and effects of the host on these events can all influence the outcome of the interactions. The bioassay in sand allowed us to study such interactions in situ. By using coarse sand with a high moisture content, we provided a substrate with a high proportion of large water-filled pores, which was optimal for zoospore dispersal (15). High-moisture conditions also increase exudate diffusion and host exudation (7, 25, 59), creating a large spermosphere or rhizosphere. Under these conditions, zoospore dispersal to peas was observed over distances of at least 4 cm for Pythium and at least 3 cm for Aphanomyces. Considering that these values refer to the distance between the center of the zoospore inoculation site and the center of the seed, the actual distance over which the zoospores need to disperse is up to 1 cm smaller than the values indicated above. The test in which sand cores were removed gave indirect but convincing evidence that zoospores and not mycelia were the propagules involved in dispersal and infection. The observed level of seed infection within 1 h (>50% at a distance of 3 cm) could not have been caused by cyst germination and mycelial growth (31, 57).
In the main dispersal assay performed with zoospores, Pythium infection incidence was dramatically reduced when seeds were treated with B. cepacia AMMDR1. Only a small reduction in infection incidence was observed with B. cepacia 1324. Because B. cepacia 1324 colonizes seeds and roots (and presumably utilizes seed exudates) to the same extent as B. cepacia AMMDR1 (22, 46), biocontrol with B. cepacia AMMDR1 seems to result primarily from antibiosis. Because bacteria were applied from plate scrapings, antibiotics could have been applied together with the bacterial cells, providing immediate protection. This is an advantage over a mechanism involving nutrient competition because it does not require that the bacteria grow rapidly during the first hours after planting, when seeds are vulnerable to infection by Pythium. In addition, the strong reduction in infection incidence could in part be due to a curative effect; B. cepacia AMMDR1 can stop an ongoing infection (22).
In contrast to the results obtained for Pythium, Aphanomyces symptom incidence was barely decreased by coating seeds with B. cepacia AMMDR1. Once a pea seedling was infected with Aphanomyces, its growth through the root and expression of disease could be slowed with B. cepacia AMMDR1 but not stopped. Therefore, final symptom expression was considered representative of infection incidence. It is hypothesized that the lack of reduction in Aphanomyces infection incidence was caused by bacterial populations near the root tip, the site of Aphanomyces zoospore infection, that were not large enough for antibiosis to take place. Although roots were colonized with over 4 log CFU of B. cepacia AMMDR1 on the 1-cm root tip sections at the time of zoospore inoculation, this number is most likely too low for any appreciable amount of de novo antibiotic production to take place. Although several research groups have demonstrated with reporter genes (28) or with direct antibiotic isolation (4, 45) that in situ antibiotic production does occur and is directly related to biocontrol (62), antibiotic production often is under the control of stationary-phase regulators or is dependent on quorum sensing and therefore population size dependent (43, 49, 50, 53, 64). Populations of B. cepacia AMMDR1 near the root tip are expected to be relatively small, and cells are actively dividing, conditions under which the cells would not produce antibiotics. Once the antibiotics of B. cepacia AMMDR1 are identified, a reporter gene system can be constructed to test this directly.
Only one pea cultivar, one isolate of A. euteiches, and one isolate of P. aphanidermatum were used throughout this study. The level of biocontrol of Pythium damping-off can depend on the host genotype (26) but usually does not differ considerably except in cases of targeted breeding for improved interactions (56). The lack of biocontrol of Aphanomyces probably also does not depend on the pathogen strain, as demonstrated with a system in which up to five different Aphanomyces isolates were used (1).
B. cepacia 1324, which does not exhibit antibiosis, was not effective at reducing Pythium infection incidence except at greater inoculation distances. When zoospores were placed 4 cm from pea seeds, coating seeds with B. cepacia 1324 reduced infection slightly (10%) compared to the results obtained with nontreated seeds. This reduction may be attributed to competition for zoospore-attracting exudates leaking from the seeds; the difference in the infection incidences of low- and high-exuding seeds suggests that a reduction in nutrients diffusing from seeds could reduce infection in our system. Similar to the assay with B. cepacia 1324, this effect was significant only when pathogen inoculum pressure was reduced. There could be several reasons why B. cepacia 1324 reduced Pythium infection incidence by only 10%, even though there was complete metabolism of attractants in vitro. First, considering that infection in this system can take place as soon as 6 h after seeds are planted, the bacteria must start metabolizing the attractants in the exudates immediately. Since the timing of zoospore-mediated infection of seeds is not known, it is conceivable that the bacteria would have more time in a natural situation. Second, the nonlinear relationship between attraction and exudate concentration implies that zoospore attractants in exudates must be metabolized significantly in order to get even a small decrease in attraction. Potentially, the bacteria could be more successful in competing for nutrients from seeds that release small amounts of exudates, such as cucumber seeds, than they are in competing for nutrients from high-exuding seeds, such as corn and legume seeds (34). Third, the zoospore inoculum density might have been too great to see an effect due to competition. Under such conditions, sufficient zoospores can get close enough to the seeds to locate those with a reduced spermosphere. This was also observed in assays involving a high P. ultimum inoculum potential, in which the level of soybean damping-off was not influenced by the level of exudation by the seeds (51). Fourth, effects due to competition have no curative effect. In contrast, effects due to antibiosis can stop an ongoing infection in a portion of infected seeds (22).
Aphanomyces infection incidence was not decreased by B. cepacia 1324. This was not unexpected. First, in vitro assays revealed that Aphanomyces zoospore attractants were not metabolized by B. cepacia 1324. Second, even if the bacteria could have metabolized the attractants, the bacterial populations may have been too small (approximately 4 log CFU cm−1) to sufficiently metabolize the attractants. Successful competition by an introduced bacterium can take place only if the organism is present at concentrations higher than the concentrations in the resident population or if it can metabolize a critical compound that the resident bacteria cannot metabolize. In the system with Aphanomyces, B. cepacia 1324 was not present on root tips at concentrations higher than the concentrations of the resident bacteria, nor was it able to metabolize the critical compound(s).
In conclusion, B. cepacia AMMDR1 interfered strongly with most homing and infection events in the life cycle of Pythium but not in the homing and infection events of Aphanomyces. Most of the effects against Pythium were the result of antibiosis, but under low pathogen inoculum potential conditions, a small reduction in infection incidence was obtained as a result of competition for zoospore-attracting seed exudates. The lack of an effect against Aphanomyces was most likely due to the inability of the bacteria to colonize and exhibit antibiosis in the root tip zone, the preferred site of infection of Aphanomyces. A biocontrol strain with constitutive expression of the antibiotic(s) might be more successful in reducing infection by the pathogen.
ACKNOWLEDGMENTS
This work was supported by Hatch grant 3601 from the College of Agriculture and Life Sciences, University of Wisconsin-Madison, and by USDA-NRI-CGP grant 98-35316-80. We thank the Storkan-Hanes Foundation for additional financial support of K. H.
We thank J. Handelsman and C. Grau for helpful discussions.
REFERENCES
- 1.Alegria-Schaffer A M. Variations in host, pathogen, and environment that affect Aphanomyces root rot severity and their influence on biocontrol efficacy by Burkholderia cepacia. M. S. thesis. University of Wisconsin, Madison; 1997. [Google Scholar]
- 2.Allen R N, Newhook F J. Chemotaxis of zoospores of Phytophthora cinnamomi to ethanol in capillaries of soil pore dimensions. Trans Br Mycol Soc. 1973;61:287–302. [Google Scholar]
- 3.Atlas R M. Handbook of microbiological media. 2nd ed. Boca Raton, Fla: CRC Press; 1997. [Google Scholar]
- 4.Bonsall R F, Weller D M, Thomashow L S. Quantification of 2,4-diacetylphloroglucinol produced by fluorescent Pseudomonas spp. in vitro and in the rhizosphere of wheat. Appl Environ Microbiol. 1997;63:951–955. doi: 10.1128/aem.63.3.951-955.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowers J H, Parke J L. Colonization of pea (Pisum sativum L.) taproots by Pseudomonas fluorescens: effect of soil temperature and bacterial motility. Soil Biol Biochem. 1993;25:1693–1701. [Google Scholar]
- 6.Bowers J H, Parke J L. Epidemiology of Pythium damping-off and Aphanomyces root rot of peas after seed treatment with bacterial agents for biological control. Phytopathology. 1993;83:1466–1473. [Google Scholar]
- 7.Bratoloveanu J, Wallace H R. The influence of Pythium on the growth of barley seedlings as affected by soil water and inoculum density. Plant Soil. 1985;85:305–311. [Google Scholar]
- 8.Burke D W, Mitchell J E, Hagedorn D J. Selective conditions for infection of pea seedlings by Aphanomyces euteiches in soil. Phytopathology. 1969;590:1670–1674. [Google Scholar]
- 9.Clark A D, Parke J L. Biological control of Pythium damping-off of supersweet corn by seed-applied Burkholderia cepacia AMMDR1. Phytopathology. 1996;86:S54. [Google Scholar]
- 10.Conover W J. Practical nonparametric statistics. New York, N.Y: John Wiley & Sons; 1971. [Google Scholar]
- 11.Deacon J W, Donaldson S P. Molecular recognition in the homing responses of zoosporic fungi, with special reference to Pythium and Phytophthora. Mycol Res. 1993;97:1153–1171. [Google Scholar]
- 12.Dewick P M. Isoflavonoids. In: Harborne J B, editor. The flavonoids: advances in research since 1980. London, United Kingdom: Chapman and Hall; 1988. pp. 125–209. [Google Scholar]
- 13.Donaldson S P, Deacon J W. Role of calcium in adhesion and germination of zoospore cysts of Pythium: a model to explain infection of host plants. J Gen Microbiol. 1992;138:2051–2059. [Google Scholar]
- 14.Donaldson S P, Deacon J W. Effects of amino acids and sugars on zoospore taxis, encystment and cyst germination in Pythium aphanidermatum (Edson) Fitzp., Pythium catenulatum Matthews and Pythium dissotocum Drechs. New Phytol. 1993;123:289–295. [Google Scholar]
- 15.Duniway J M. Movement of zoospores of Phytophthora cryptogea in soils of various textures and matric potentials. Phytopathology. 1976;66:877–882. [Google Scholar]
- 16.Elad Y, Chet I. Possible role of competition for nutrients in biocontrol of Pythium damping-off by bacteria. Phytopathology. 1987;77:190–195. [Google Scholar]
- 17.Fravel D. Hurdles and bottlenecks on the road to biocontrol of plant pathogens. Australas Plant Pathol. 1999;28:53–56. [Google Scholar]
- 18.Georgakopoulos D G, Hendson M, Panopoulos N J, Schroth M N. Analysis of expression of a phenazine biosynthesis locus of Pseudomonas aureofaciens PGS12 on seeds with a mutant carrying a phenazine biosynthesis locus-ice nucleation reporter gene fusion. Appl Environ Microbiol. 1994;60:4573–4579. doi: 10.1128/aem.60.12.4573-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert G S, Handelsman J, Parke J L. Role of ammonia and calcium in lysis of zoospores of Phytophthora cactorum by Bacillus cereus strain UW85. Exp Mycol. 1990;14:1–8. [Google Scholar]
- 20.Hagedorn C, Gould W D, Bardinelli T R, Gustavson D R. A selective medium for enumeration and recovery of Pseudomonas cepacia biotypes from soil. Appl Environ Microbiol. 1987;53:2265–2268. doi: 10.1128/aem.53.9.2265-2268.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handelsman J, Stabb E V. Biocontrol of soilborne plant pathogens. Plant Cell. 1996;8:1855–1869. doi: 10.1105/tpc.8.10.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heungens K K. Pre- and post-infection interactions between the biocontrol agent Burkholderia vietnamiensis AMMDR1 and oomycete pathogens of pea. Ph.D. thesis. University of Wisconsin, Madison; 1999. [Google Scholar]
- 23.Jones S W, Donaldson S P, Deacon J W. Behavior of zoospores and zoospore cysts in relation to root infection by Pythium aphanidermatum. New Phytol. 1991;117:289–302. [Google Scholar]
- 24.Keel C, Wirthner P, Oberänsli T, Voisard C, Berger U, Haas D, Défago G. Pseudomonads as antagonists of plant pathogens in the rhizosphere: role of antibiotic 2,4-diacetylphloroglucinol in the suppression of black root rot of tobacco. Symbiosis. 1990;9:327–341. [Google Scholar]
- 25.Kerr A. The influence of soil moisture on infection of peas by Pythium ultimum. Aust J Biol Sci. 1964;17:676–685. [Google Scholar]
- 26.King E B, Parke J L. Biocontrol of Aphanomyces root rot and Pythium damping-off by Pseudomonas cepacia AMMD on four pea cultivars. Plant Dis. 1993;77:1185–1188. [Google Scholar]
- 27.King E B, Parke J L. Population density of the biocontrol agent Burkholderia cepacia AMMDR1 on four pea cultivars. Soil Biol Biochem. 1996;28:307–312. [Google Scholar]
- 28.Kraus J, Loper J E. Characterization of a genomic region required for production of the antibiotic pyoluteorin by the biological control agent Pseudomonas fluorescens Pf-5. Appl Environ Microbiol. 1995;61:849–854. doi: 10.1128/aem.61.3.849-854.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laville J, Voisard C, Keel C, Maurhofer M, Défago G, Haas D. Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root rot of tobacco. Proc Natl Acad Sci USA. 1992;89:1562–1566. doi: 10.1073/pnas.89.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lifshitz R, Windham M T, Baker R. Mechanism of biological control of preemergence damping-off of pea by seed treatment with Trichoderma spp. Phytopathology. 1986;76:720–725. [Google Scholar]
- 31.Luna L V, Hine R B. Factors influencing saprophytic growth of Pythium aphanidermatum in soil. Phytopathology. 1964;54:955–959. [Google Scholar]
- 32.Maurhofer M, Keel C, Haas D, Défago G. Influence of plant species on disease suppression by Pseudomonas fluorescens strain CHA0 with enhanced antibiotic production. Plant Pathol. 1995;44:40–50. [Google Scholar]
- 33.Maurhofer M, Keel C, Schnider U, Voisard C, Haas D, Défago G. Influence of enhanced antibiotic production in Pseudomonas fluorescens strain CHA0 on its disease suppressive capacity. Phytopathology. 1992;82:190–195. [Google Scholar]
- 34.Nelson E B. Biological control of Pythium seed rot and preemergence damping-off of cotton with Enterobacter cloacae and Erwinia herbicola applied as seed treatments. Plant Dis. 1988;72:140–142. [Google Scholar]
- 35.Nelson E B. Exudate molecules initiating fungal responses to seeds and roots. Plant Soil. 1990;129:61–73. [Google Scholar]
- 36.Pandey D K. Conductivity testing of seeds. In: Linskens H F, Jackson J F, editors. Seed analysis. New York, N.Y: Springer-Verlag; 1992. pp. 273–304. [Google Scholar]
- 37.Parke J L. Population dynamics of Pseudomonas cepacia in the pea spermosphere in relation to biocontrol of Pythium. Phytopathology. 1990;80:1307–1311. [Google Scholar]
- 38.Parke J L, Grau C R. Aphanomyces. In: Singleton L L, Mihail J D, Rush C M, editors. Methods for research on soilborne phytopathogenic fungi. St. Paul, Minn: APS Press; 1992. pp. 27–30. [Google Scholar]
- 39.Parke J L, Rand R E, Joy A E, King E B. Biological control of Pythium damping-off and Aphanomyces root rot of peas by application of Pseudomonas cepacia or Pseudomonas fluorescens to seed. Plant Dis. 1991;75:987–992. [Google Scholar]
- 40.Paulitz T C. Effect of Pseudomonas putida on the stimulation of Pythium ultimum by seed volatiles of pea and soybean. Phytopathology. 1991;81:1282–1287. [Google Scholar]
- 41.Penington C J, Iser J R, Grant B R, Gayler K R. Role of RNA and protein synthesis in stimulated germination of zoospores of the pathogenic fungus Phytophthora palmivora. Exp Mycol. 1989;13:158–168. [Google Scholar]
- 42.Pierson E A, Wood D W, Cannon J A, Blachere F M, Pierson L S. Interpopulation signaling via N-acyl-homoserine lactones among bacteria in the wheat rhizosphere. Mol Plant-Microbe Interact. 1998;11:1078–1084. [Google Scholar]
- 43.Pierson L S, III, Keppenne V D, Wood D W. Phenazine antibiotic biosynthesis in Pseudomonas aureofaciens 30–84 is regulated by PhzR in response to cell density. J Bacteriol. 1994;176:3966–3974. doi: 10.1128/jb.176.13.3966-3974.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierson L S, Wood D W, Pierson E A. Homoserine lactone-mediated gene regulation in plant-associated bacteria. Annu Rev Phytopathol. 1998;36:207–225. doi: 10.1146/annurev.phyto.36.1.207. [DOI] [PubMed] [Google Scholar]
- 45.Raaijmakers J M, Bonsall R F, Weller D M. Effect of population density of Pseudomonas fluorescens on production of 2,4-diacetylphloroglucinol in the rhizosphere of wheat. Phytopathology. 1999;89:470–475. doi: 10.1094/PHYTO.1999.89.6.470. [DOI] [PubMed] [Google Scholar]
- 46.Regner K M. The role of pyrrolnitrin in the suppression of damping-off of pea by Burkholderia cepacia AMMD. Ph.D. thesis. University of Wisconsin, Madison; 1996. [Google Scholar]
- 47.Romero S, Gallegly M E. Oogonium germination in Phytophthora infestans. Phytopathology. 1963;53:899–903. [Google Scholar]
- 48.Royle D J, Hickman C J. Analysis of factors governing in vitro accumulation of zoospores of Pythium aphanidermatum on roots. I. Behavior of zoospores. Can J Microbiol. 1964;10:151–162. [Google Scholar]
- 49.Sacherer P, Défago G, Haas D. Extracellular protease and phospholipase C are controlled by the global regulatory gene gacA in the biocontrol strain Pseudomonas fluorescens CHA0. FEMS Microbiol Lett. 1994;116:155–160. doi: 10.1111/j.1574-6968.1994.tb06694.x. [DOI] [PubMed] [Google Scholar]
- 50.Sarniguet A, Kraus J, Henkels M D, Muehlchen A M, Loper J E. The sigma factors ςs affects antibiotic production and biological control activity of Pseudomonas fluorescens Pf-5. Proc Natl Acad Sci USA. 1995;92:12255–12259. doi: 10.1073/pnas.92.26.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlub R L, Schmitthenner A F. Effects of soybean seed coat cracks on seed exudation and seedling quality in soil infested with Pythium ultimum. Phytopathology. 1978;68:1186–1191. [Google Scholar]
- 52.Schmidli-Sacherer P, Keel C, Défago G. The global regulator GacA of Pseudomonas fluorescens CHA0 is required for suppression of root diseases in dicotyledons but not in Gramineae. Plant Pathol. 1997;46:80–90. [Google Scholar]
- 53.Schnider U, Keel C, Blumer C, Troxler J, Défago G, Haas D. Amplification of the housekeeping sigma factor in Pseudomonas fluorescens CHA0 enhances antibiotic production and improves biocontrol abilities. J Bacteriol. 1995;177:5387–5392. doi: 10.1128/jb.177.18.5387-5392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sekizaki H, Yokosawa R. Studies on zoospore-attracting activity. I. Synthesis of isoflavones and their attracting activity to Aphanomyces euteiches zoospores. Chem Pharm Bull (Tokyo) 1988;36:4876–4880. doi: 10.1248/bpb.16.698. [DOI] [PubMed] [Google Scholar]
- 55.Slininger P J, Van Cauwenberge J E, Bothast R J, Weller D M, Thomashow L S, Cook R J. Effect of growth culture physiological state, metabolites, and formulation on the viability, phytotoxicity, and efficacy of the take-all biocontrol agent Pseudomonas fluorescens 2–79 stored encapsulated on wheat seeds. Appl Microbiol Biotechnol. 1996;45:391–398. [Google Scholar]
- 56.Smith K P, Handelsman J, Goodman R M. Modeling dose-response relationships in biological control: partitioning host responses to the pathogen and biocontrol agent. Phytopathology. 1997;87:720–729. doi: 10.1094/PHYTO.1997.87.7.720. [DOI] [PubMed] [Google Scholar]
- 57.Stanghellini M E, Burr T J. Effect of soil water potential on disease incidence and oospore germination of Pythium aphanidermatum. Phytopathology. 1973;63:1496–1498. [Google Scholar]
- 58.Stanghellini M E, Burr T J. Germination in vivo of Pythium aphanidermatum oospores and sporangia. Phytopathology. 1973;63:1493–1496. [Google Scholar]
- 59.Stanghellini M E, Hancock J G. Radial extent of the bean spermosphere and its relation to the behavior of Pythium ultimum. Phytopathology. 1971;61:165–168. [Google Scholar]
- 60.Tedla T, Stanghellini M E. Bacterial population dynamics and interactions with Pythium aphanidermatum in intact rhizosphere soil. Phytopathology. 1992;82:652–656. [Google Scholar]
- 61.Thomashow L S, Weller D M. Current concepts in the use of introduced bacteria for biological disease control: mechanisms and antifungal metabolites. In: Stacey G, Keen N T, editors. Plant-microbe interactions. I. New York, N.Y: Chapman and Hall; 1995. pp. 187–235. [Google Scholar]
- 62.Thomashow L S, Weller D M, Bonsall R F, Pierson L S., III Production of the antibiotic phenazine-1-carboxylic acid by fluorescent Pseudomonas species in the rhizosphere of wheat. Appl Environ Microbiol. 1990;56:908–912. doi: 10.1128/aem.56.4.908-912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Dijk K, Nelson E B. Inactivation of seed exudate stimulants of Pythium ultimum sporangium germination by biocontrol strains of Enterobacter cloacae and other seed-associated bacteria. Soil Biol Biochem. 1998;30:183–192. [Google Scholar]
- 64.Whistler C A, Corbell N A, Sarniguet A, Ream W, Loper J E. The two-component regulators GacS and GacA influence accumulation of the stationary-phase sigma factor ςs and the stress response in Pseudomonas fluorescens Pf-5. J Bacteriol. 1998;180:6635–6641. doi: 10.1128/jb.180.24.6635-6641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou T, Paulitz T C. In vitro and in vivo effects of Pseudomonas spp. on Pythium aphanidermatum: zoospore behavior in exudates and on the rhizoplane of bacteria-treated cucumber roots. Phytopathology. 1993;83:872–876. [Google Scholar]