Abstract
Objective
Although complications and clinical symptoms of COVID-19 have been elucidated, the prevalence of long-term sequelae of COVID-19 is less clear in previously hospitalized COVID-19 patients. This review and meta-analysis present the occurrence of different symptoms up to 1 year of follow-up for previously hospitalized patients.
Methods
We performed a systematic review from PubMed and Web of Science using keywords such as “COVID-19”, “SARS-CoV-2”, “sequelae”, “long-term effect” and included studies with at least 3-month of follow-up. Meta-analyses using random-effects models were performed to estimate the pooled prevalence for different sequelae. Subgroup analyses were conducted by different follow-up time, regions, age and ICU admission.
Results
72 articles were included in the meta-analyses after screening 11,620 articles, identifying a total of 167 sequelae related to COVID-19 from 88,769 patients. Commonly reported sequelae included fatigue (27.5%, 95% CI 22.4–33.3%, range 1.5–84.9%), somnipathy (20.1%, 95% CI 14.7–26.9%, range 1.2–64.8%), anxiety (18.0%, 95% CI 13.8–23.1%, range 0.6–47.8%), dyspnea (15.5%, 95% CI 11.3–20.9%, range 0.8–58.4%), PTSD (14.6%, 95% CI 11.3–18.7%, range 1.2–32.0%), hypomnesia (13.4%, 95% CI 8.4–20.7%, range 0.6–53.8%), arthralgia (12.9%, 95% CI 8.4–19.2%, range 0.0–47.8%), depression (12.7%, 95% CI 9.3–17.2%, range 0.6–37.5%), alopecia (11.2%, 95% CI 6.9–17.6%, range 0.0–47.0%) over 3–13.2 months of follow-up. The prevalence of most symptoms reduced after > 9 months of follow-up, but fatigue and somnipathy persisted in 26.2% and 15.1%, respectively, of the patients over a year. COVID-19 patients from Asia reported a lower prevalence than those from other regions.
Conclusions
This review identified a wide spectrum of COVID-19 sequelae in previously hospitalized COVID-19 patients, with some symptoms persisting up to 1 year. Management and rehabilitation strategies targeting these symptoms may improve quality of life of recovered patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s15010-022-01862-3.
Keywords: COVID-19, Sequelae, Consequences, Post COVID-19, Long-term, Meta-analysis
Introduction
Since its emergence in December 2019, SARS-CoV-2 has rapidly spread around the world, leading to > 480 million confirmed cases and > 6 million deaths as of March 2022 [1]. As more people have recovered from COVID-19, the long-term sequelae of the disease and its impact to the healthcare system have become an important consideration.
Evidence has shown that hospitalized COVID-19 patients would develop various complications, including pneumonia (75%), acute liver injury (19%), heart injury (7% to 17%), acute respiratory distress syndrome (15%), acute kidney injury (9%), acute heart failure, disturbance of consciousness, etc. [2] A noticeable portion of hospitalized patients would also show symptoms such as fever (70–90%), shortness of breath (53–80%), dry cough (60–86%), myalgia (15–44%), fatigue (38%), nausea/vomiting or diarrhea (15–39%), weakness (25%), headache, loss of taste, loss of smell, etc. [3] Although these complications and symptoms have been elucidated in hospitalized patients, their persistence after recovery has not been clearly described to assess how quality of life is affected among the recovered patients. Up to now, 12 meta-analyses studied the long-term sequelae with follow-up time > 3 months [4–15]. Only two of them stratified the prevalence of headache, myalgia, arthralgia, and chest pain among hospitalized patients by < 6 and > 6 months follow-up [9, 10]. However, none of them reported prevalences of a wide range of symptoms among previously hospitalized patients at different months of follow-up which showed the time trend [11]. Hospitalized patients are more likely to suffer from more severe post-COVID symptoms; hence there is a need to clarify their severity and persistence.
Here we carried out a systematic review and meta-analysis of sequelae of hospital discharged COVID-19 patients to identify all aftermaths of COVID-19, which can help to prevent and manage the long-term sequelae of the discharged COVID-19 patients. In our study we focused on previously hospitalized patients who had more severe symptoms and more sequelae, and also focused on articles with at least a 3-month follow-up time, to study sequelae which likely have a longer impact on their quality of life. Our study will also provide pooled estimates at 12 months follow-up. Such information would inform health management strategies of COVID-19 patients by identifying sequelae that may persistently affect the quality of life.
Methods
Search strategy and eligibility criteria
This systematic review and meta-analysis followed the PRISMA guidelines [16]. The protocol was registered in PROSPERO (register no: CRD42022314319). Studies about long-term sequelae of COVID-19 were identified through PubMed and Web of Science for publications up to 1 March 2022 Search queries included terms such as “COVID-19”, “SARS-CoV-2”, “2019-nCoV”, “sequelae”, “long-term effect”, “long-term consequence”, “long-term manifestations”, “persistent effect”, “persistent symptom”, “persistent manifestations”, “post COVID-19 effect”, “post COVID-19 symptom”, “post COVID-19 manifestations” and “post COVID-19 syndrome” (Appendix 1). Reference lists of systematic reviews were also reviewed. We included studies published in English.
The eligibility criteria were as follows:(1) study designs included cohort studies, case–control studies, and cross-sectional studies with a clear follow-up duration.; (2) patients should be hospital discharged adult patients aged 18 years or above representative of the general population without any age restriction, with a pathologically confirmed COVID-19 infection; (3) patients should be confirmed before 2022 to exclude patients infected with the omicron variant which may have a different symptom profile (4). Outcomes were defined as all clinical symptoms after at least 90 days after discharge, admission, diagnosis or symptom onset, consistent with case definition of post COVID-19 condition by the World Health Organization [17]. Sequelae of COVID-19 included respiratory, cardiovascular, psychosocial, neurological, dermatological, digestive, endocrinological symptoms and other symptoms. Studies reporting serological, immunological results or CT scores only were excluded. (5) Sample size > 100.
Data extraction and quality assessment
Data Extraction and Quality Assessment were conducted by two independent reviewers (TY & XL). Any disagreement was solved by a senior author (EHYL). Extracted data included the first author, year of publication, sample size, disease severity, average follow-up period, the average age of participants, and clinical symptoms defined before.
Study quality was evaluated by using the Newcastle–Ottawa Scale (NOS) [18]. Studies with 7–9 stars were perceived as high quality and studies with < 4 stars were perceived as poor quality. We assessed quality in selection strategies (representativeness of the exposed cohort, ascertainment of exposure, etc.), comparability, and outcome (outcome assessment, follow-up rate, etc.).
Statistical analysis
For those sequelae reported in two or more studies, the pooled prevalence and 95% CI were estimated in a meta-analysis using a random-effects model (the variance component was estimated using the DerSimonian–Laird estimator) and inverse variance methods following logit transformation [19]. For those sequelae reported only in a single study, the prevalence was estimated by directly dividing the number of cases who shown symptoms by the total sample size, and the 95% CI was estimated by using the Clopper-Pearson interval. Boxplots, median and interquartile ranges (IQR) were present for sequelae reported by ten or more studies.
The chi-square-based Cochran’s Q test and Higgins (I2) statistics were used to assess the heterogeneity of studies included in the meta-analysis. The significance level of Cochran’s Q test was 0.05. Studies were regarded as having low, moderate and high heterogeneity with I2 = < 30%, 30–60% and > 60%, respectively. Subgroup analysis was conducted for those meta-analyses which included 15 or more studies, by different follow-up duration, regions, ICU admission and age. Meta-regression was used to test whether the difference of estimates between subgroups is significant. The pooled follow-up duration was weighted by sample size of each study. All statistical analyses were conducted in R version 4.0.3 (R Development Core Team).
Results
Study characteristics
A total of 11,620 studies were identified from PubMed and Web of Science, and one study was identified from other systematic reviews (Fig. 1). After removing 1301 duplicates, 10,320 articles were screened against title and abstract, and 10,062 articles not meeting the eligibility criteria were excluded. Finally, a total of 72 articles were included in the meta-analysis by screening the full texts of 256 articles: 19 articles did not have clinical symptoms as outcomes; 35 articles had a less than 3-month follow-up time; 40 articles included less than 100 participants; 70 articles did not only include hospitalized patients; 4 articles did not report follow-up time; 3 articles did not report prevalence in sequelae; 13 duplicate publications were further identified.
Fig. 1.
Flow-chart for the literature selection process
All included articles were published between 2020 and 2022. Of the 72 studies included in our analysis, 15 studies were from China, 11 each from Italy and Spain, 5 from England, 4 from France, 3 each from America and Mexico, 2 each from India, Germany, Switzerland and Belgium, 1 each from Iran, Australia, Egypt, Brazil, Peru, Turkey, Pakistan, Singapore, Austria, Kingdom of Saudi Arabia, Denmark and Netherlands (Table 1). All studies were observational studies: 62 non-controlled cohort studies, 5 controlled cohort studies, 4 cross-sectional studies and 1 case–control study. Data from the control groups (non COVID-19 cases) were not included in our analysis. One article conducted two independent telephone and outpatient follow-ups [35]. The two surveys reported different sequelae, and hence were regarded as two independent studies and included in the meta-analysis, while data on cough was included from the telephone follow-up only. Four articles which included one same cohort were included in the meta-analysis as four independent studies as they reported different symptoms [21–24]. The average follow-up time ranged from 90 days to 13.2 months and the size of follow-up cohorts ranged from 101 to 47,780. Only patients hospitalized for COVID-19 were included (Fig. 2).
Table 1.
General characteristics of studies included in the meta-analysis
| Study, (year) | City/country | Sample size | Disease severity | Mean follow-up perioda | Mean age (years)/male (%) | Clinical symptoms | NOS score |
|---|---|---|---|---|---|---|---|
| Xiong et al. [20] (2020) | Wuhan, China | 538 | General, severe, critical | Median (IQR): 97.0 (95.0–102.0) days |
Median (IQR): 52.0 (41.0–62.0) Male 46% |
Physical decline/fatigue, sweating, myalgia, arthralgia, chills, limb oedema, dizziness, post activity polypnea, nonmotor polypnea, chest distress, chest pain, cough, sputum, sore throat, resting heart rate increase, discontinuous flushing, newly diagnosed hypertension, somnipathy, depression, anxiety, dysphoria, feelings of inferiority, alopecia | 7 |
| Fernández-de-las-Peñas et al. [21–24], (2021, 2022) | Madrid, Spain | 1969 |
Hospitalized ICU 7% |
11.2 ± 0.5 months |
61 ± 16 Male 54% |
Cough, chest pain, dyspnea, and fatigue | 6 |
| 8.4 ± 1.5 months | Loss memory, skin rashes, brain fog, attention disorders, palpitations, gastrointestinal disorders, ocular/vision disorders, anosmia, ageusia, sore throat, diarrhea, voice problems, musculoskeletal pain | ||||||
|
Mean (range): 13.2 (11–15) months |
Anxiety, depression, poor sleep quality | ||||||
| Bellan et al. [25], (2021) | Novara, Italy | 200 | Hospitalized ICU 12% | 12 months |
Median (IQR): 62 (51–71) Male 61% |
Fever, cough, dyspnea, ageusia, anosmia, diarrhea, arthralgia/myalgia, chest pain, sore throat, alopecia, fatigue | 5 |
| Romero-Duarte et al. [26], (2021) | Spain | 797 |
Hospitalized ICU 11% |
6 months |
63 ± 14.4 Male 54% |
Fever, fatigue, muscle weakness, musculoskeletal pain, general malaise, oedema, pressure ulcers, dyspnea, rib pain, thoracic pain, persistent cough, persistent pharyngeal symptoms, icu-related polyneuropathy, headache, paresthesia, movement disturbances, disorientation or confusion, persistent anosmia or dysgeusia, depressive symptoms, anxiety symptoms, sleep disturbances, thrombotic manifestations, pruritus, alopecia, exanthema, eczema, renal insufficiency de novo, glycaemia uncontrol, vertigo symptoms, otoacoustic symptoms, diarrhea, constipation, vomiting, abdominal pain, anorexia, hypotension or syncope, arrythmia or palpitations, urinary tract infection, pneumonia, mycosis | 6 |
| Simani et al. [27], (2021) | Iran | 120 | Hospitalized | 6 months after infection |
54.62 ± 16.94 Male 67% |
Fatigue, post-traumatic stress disorder (PTSD) | 5 |
| Mei et al. [28], (2021) | Wuhan, China | 3677 | Mild, Severe, Critical | Median (IQR): 144 (135–157) days |
Median (IQR): 59 (47–68) Male 46% |
Shortness of breath, cough/sputum, pharyngitis/foreign body feeling, dyspnea, pulmonary fibrosis, lung damage, bronchitis, COPD, hemoptysis, chest pain/tightness, palpitation, cardiac disease, tachycardia, angina pectoris, heart attack, insomnia, joint pain/back pain/lumbago, fatigue, headache/dizziness/poor memory, change of taste and smell, myalgia, impaired vision, leg numbness/finger stiffness, neuralgia, paralysis, tinnitus, confusion, coma, cerebral infarction, hair loss, bitter/dryness in mouth, high blood sugar, diabetes, gastrointestinal complaints/poor appetite, diarrhea, constipation, emesis, hidrosis, erythra, allergy, hepatic insufficiency, edema, antiadoncus, hypertension, kidney insufficiency, reduction of physical strength, dryness/excessive secretion in eye | 4 |
|
Qu et al. [29] (2021) |
China | 540 | Mild, moderate, severe | 3 months |
Median (IQR): 47.5 (37.0–57.0) Male 50% |
Fatigue, cough, sputum, dyspnea, diarrhea, shortness of breath, joint pain, dysbasia, palpitations | 4 |
| Shang et al. [30], (2021) | Wuhan, China | 796 |
Severe, critical ICU 38% |
6 months |
Median (IQR): 62.0 (51.0–69.0) Male 51% |
Cough, throat itching, shortness of breath, chest pains, dizziness/headache, muscle joint pain, backache, fatigue, sleep disorder, hypomnesia, hair loss, sweat, new hypertension | 4 |
| Suárez-Robles et al. [31], (2021) | Spain | 134 |
Hospitalized ICU 2% |
90 days |
58.53 ± 18 Male 46% |
Fatigue, dyspnea, loss of weight, loss of appetite, cough, anosmia, headaches, arthritis, palpitations, dysgeusia, general malaise, dysphonia, sensitivity disorders, sputum, walking disturbances | 4 |
| Huang et al. [32], (2021) | Wuhan, China | 1733 |
Hospitalized ICU 4% |
Median (IQR): 153.0 (146.0–160.0) days |
Median (IQR): 57.0 (47.0–65.0) Male 52% |
Fatigue or muscle weakness, sleep difficulties, hair loss, smell disorder, palpitations, joint pain, decreased appetite, taste disorder, dizziness, diarrhea or vomiting, chest pain, sore throat or difficult to swallow, skin rash, myalgia, headache, low grade fever, anxiety or depression, problems with walking around, problems with washing or dishing, problems with usual activity, pain or discomfort | 5 |
| Tarsitani et al. [33], (2021) | Rome, Italy | 115 | Hospitalized | 3 months |
Median (IQR): 57 (48–66) Male 54% |
PTSD | 5 |
| Froidure et al. [34], (2021) | Belgium | 126 |
Severe, critical ICU 22% |
Median (IQR): 95 (86–107) days |
Median (IQR): 60 (53–68) Male 59% |
Fatigue, dyspnea, chronic dry cough, chest oppression | 5 |
| Morin et al. [35], (2021) | France | 478 (telephone assessment) |
Hospitalized ICU 30% |
Median (IQR): 113 (94–128) days |
60.9 ± 16.1 Male 58% |
Dyspnea, cough, chest discomfort/pain, fatigue, anorexia, weight loss, anosmia, headaches, paresthesia, memory difficulties, mental slowness, concentration problems | 5 |
| 177 (in-person outpatient) |
Hospitalized ICU 55% |
Median (IQR): 125 (107–144) days |
56.9 ± 13.2 Male 62% |
Persistent cough, cognitive complaint, cognitive impairment, anxiety, depression, insomnia, PTSD | |||
| Ayoubkhani et al. [36], (2021) | England | 47,780 |
Hospitalized ICU 10% |
140 ± 50 days |
64.5 ± 19.2 Male 55% |
Diabetes (new onset) | 7 |
| Sykes et al. [37], (2021) | England | 134 |
Hospitalized ICU 20% |
Median (IQR): 113 (46–167) |
Median (IQR): 58 (25–89) Male 66% |
Breathlessness, myalgia, anxiety, extreme fatigue, low mood, memory impairment, sleep disturbance, cough, attention deficit, pleuritic chest pain, sore throat, fever, anosmia, cognitive impairment, taste deficiency, rash | 5 |
| Arnold et al. [38], (2020) | England | 110 | Mild, moderate, severe | Median (IQR): 90 (80–97) |
Median (IQR): 60 (46–73) Male 62% |
Fever, cough, breathlessness, anosmia, fatigue, myalgia, headache, chest pain, arthralgia, diarrhea, abdominal pain, nausea, insomnia | 6 |
| Garrigues et al. [39], (2020) | France | 120 |
Hospitalized ICU 20% |
110.9 ± 11.1 |
63.2 ± 15.7 Male 63% |
Cough, chest pain, fatigue, dyspnea, ageusia, anosmia, hair loss, attention disorder, memory loss, sleep disorder | 5 |
| Caruso et al. [40], (2021) | Rome, Italy | 118 | Moderate, severe | 6 months |
65 ± 12 Male 47% |
Dyspnea, cough, fever, fatigue, olfactory dysfunction, gustatory dysfunction, hair loss, decline of visual acuity | 5 |
| Castro et al. [41], (2021) | Boston, America | 5571 |
Hospitalized ICU 13% |
120 days |
Median (IQR): 63 (50–76) Male 53% |
Fatigue, anxiety, sleep disruption, headache, impaired cognition, anosmia, memory, language disturbance, hallucinations | 7 |
| Darcis et al. [42], (2021) | Belgium | 101 |
Moderate, severe ICU 26% |
3 months |
60.5 ± 13.9 Male 63% |
Exertional dyspnea, fatigue, dry cough, chest pain, headaches, loss of appetite, myalgia, dyspnea at rest, anosmia, ageusia, rhinorrhea, paresthesia/dysesthesias, memory impairment, diarrhea, productive cough, pharyngeal pain, confusion, nauseas, vomiting, fever | 4 |
| González-Hermosillo et al. [43], (2021) | Mexico | 130 | Moderate, severe | 6 months |
51 ± 14 Male 65% |
Fatigue, resting dyspnea, dyspnea on effort, concentration impairment, short-term memory loss, inability to focus vision, light sensitivity, anosmia, ageusia, tingling, disturbance of sleep, unrefreshing sleep, postural dizziness, lightheadedness when prolonged standing, chest pain, tachycardia, change pattern of sweating, intolerance to temperature, stomach bloated after meals, abdominal pain, diarrhea, constipation, nausea, urinary frequency, difficulty emptying bladder, difficulty with sexual function, headache, muscle pain, joint pain, anxiety, depression | 5 |
| Gramaglia et al. [44], (2021) | Novara, Italy | 237 |
Severe ICU 12% |
4 months |
Median (IQR): 61 (50–71) Male 60% |
Anxiety, depressive symptoms, changes in appetite and sleep patterns | 5 |
| Bozzetti et al. [45], (2021) | Verona, Italy | 107 | Hospitalized | 6 months |
Median (range): 63 (32–90) Male 65% |
Hyposmia, hypogeusia, vertigo, fatigue, headache, myalgia, impaired memory | 5 |
| Maestre-Muñiz et al. [46], (2021) | Spain | 445 | Severe, critical | 12 months |
71.5 ± 14.3 Male 45% |
Breathlessness, tiredness, loss of taste, loss of smell, hair loss, memory lapses, sleep difficulties, muscular weakness, headache, myalgia, low-grade fever, mood changes, gastrointestinal symptoms, chest pain, skin rash, palpitations, concentration difficulties, sore throat | 5 |
| Vincent et al. [47], (2021) | Switzerland | 108 |
Hospitalized ICU 17% |
90 days |
58.4 ± 15.8 Male 59.0% |
Anxiety, depression, PTSD | 5 |
| Sun et al. [48], (2021) | Wuhan, China | 932 | Non-severe, severe | 3 months |
Median (IQR): 58 (48–67) Male 40% |
Cough, fatigue, dysgeusia, dysosmia, anorexia, dyspnea | 5 |
|
Vlake et al. [49] (2021) |
Netherlands | 116 |
Hospitalized ICU 14% |
3 months |
Median (95% range): 61 (35–85) Male 64% |
PTSD, anxiety, depression | 7 |
|
Huang et al. [50] (2021) |
Wuhan, China | 1276 |
Hospitalized ICU 4% |
Median (IQR): 185.0 (175.0–198.0) days Median (IQR): 349.0 (337.0–361.0) days |
Median (IQR): 59.0 (49.0–67.0) Male 53% |
Fatigue or muscle weakness, sleep difficulties, hair loss, smell disorder, palpitations, joint pain, decreased appetite, taste disorder, dizziness, diarrhea or vomiting, chest pain, sore throat or difficult to swallow, skin rash, myalgia, headache, anxiety or depression, problems with walking around, problems with washing or dishing, problems with usual activity, pain or discomfort | 7 |
| Li et al. [51], (2021) | Shenzhen, China | 147 | Hospitalized | 90 days |
Not reported Male 49% |
Dyspnea, exercise limitation, cough, fatigue, chest tightness, hyposmia | 5 |
| Sibila et al. [52], (2021) | Spain | 172 |
Moderate, severe ICU 43% |
3 months |
56.1 ± 19.8 Male 57% |
Dyspnea, cough, joint pain, diarrhea, sputum production, headache, chest pain | 5 |
| Liu et al. [53], (2021) | Wuhan, China | 594 | Moderate, severe, critical 11% | 12 months |
Median (IQR): 63 (53–68) Male 46% |
Cough, fatigue, myalgia, dyspnea, chest tightness, chest pain, heart palpitations, sputum production, diarrhea, nausea, loss of appetite, abdominal pain, headache, dizziness, night sweats, insomnia, numbness in limbs, joint pain, memory loss, decreases taste, vision loss, hearing loss, smell loss, hair loss, edema, backache, skin pruritus, mouth and pharynx discomfort, thrombosis | 5 |
| Lombardo et al. [54], (2021) | Milan, Italy | 189 |
Hospitalized ICU 4% |
12 months |
Median (IQR): 57 (47–68) Male 52% |
Fatigue and weakness, muscle and joint pain, sleep disorders, respiratory disorders, neurological and cognitive impairments, sensory alterations, movement impairments, gastrointestinal symptoms | 5 |
| Maestrini et al. [55], (2021) | Rome, Italy | 118 |
Hospitalized ICU 29% |
347 ± 10 days |
Median (IQR): 70.5 (56.2–80.0) Male 57% |
Fatigue, dyspnea, impaired memory, palpitations, arthomyalgia, cutaneous manifestations, chest pain, ageusia, gastrointestinal symptoms, ocular manifestations | 4 |
| Sigfrid et al. [56], (2021) | England | 327 |
Hospitalized ICU 40% |
Median (IQR): 222 (189–269) |
Median (IQR): 59.7 (51.7–67.7) Male 59% |
Fatigue, shortness of breath, problems sleeping, headache, limb weakness, joint pain or swelling, persistent muscle pain, dizziness/light headedness, problems with balance, swollen ankle, palpitations, constipation, problems seeing, diarrhea, stomach pain, chest pains, persistent cough, erectile dysfunction, pain on breathing, loss of smell, persistent fevers, loss of taste, nausea/vomiting, loss of appetite, problems swallowing, skin rash, weight loss, problems passing urine, hemiplegia/paraesthesiae, toe lesions | 4 |
| Ahmed et al. [57], (2021) | Egypt | 182 | Non-severe, severe, critical | 6 months |
46.5 ± 17.4 Male 46% |
Somatization, obsessive–compulsive, interpersonal sensibility, depression, anxiety, anger-hostility, phobic-anxiety, paranoid ideation, psychosis | 4 |
| Anjana et al. [58], (2021) | India | 154 | Mild, moderate, severe | 3 months |
31.5 ± 18.4 Male 37% |
Fatigue, headache, myalgia, exertional dyspnea, joint pain, orthopnea, dry cough | 5 |
| Bai et al. [59], (2022) | Italy | 377 | Hospitalized | Median (IQR): 102 (86—126) days |
Median (IQR): 57 (49–68) Male 64% |
Anosmia, dysgeusia, fever, joint pain or myalgia, rest dyspnea, exertional dyspnea, fatigue, brain fog, PTSD, depression, anxiety | 4 |
| Chen et al. [60], (2021) | China | 715 |
Mild, moderate, severe, critical ICU 6% |
Median (IQR): 225.0 (222.0–228.0) days |
Median (IQR): 69 (67–73) Male 51% |
Fatigue, cough, sputum, exertional or resting dyspnea, chest tightness, palpitation, orthopnea, lower limb edema | 4 |
| Damiano et al. [61], (2021) | Brazil | 425 |
Hospitalized ICU 50% |
207 ± 20.4 days |
55.7 ± 14.2 Male 52% |
Anxiety, depression, PTSD, specific phobia with COVID, obsessive compulsive disorder, delusions, hallucinations | 4 |
| Eloy et al. [62], (2021) | French | 324 |
Hospitalized ICU 19% |
185 days 95% CI [182–191] |
Median (IQR): 61 (52–69) Male 63% |
Fatigue, myalgia, headache, cough, nasal obstruction, sore throat, feverishness, joint pain, dyspnea, anosmia, ageusia, depression, anxiety | 4 |
| Evans et al. [63], (2021) | UK | 1077 | Mild, moderate, severe, critical | Median (IQR): 5.9 (4.9–6.5) days |
57.9 ± 13.0 Male 64% |
Anxiety, depression, PTSD, cognitive impairment, aching in your muscles, physical slowing down, slowing down in your thinking, joint pain or swelling, limb weakness, difficulty with concentration, short term memory loss, headache, tingling feeling/pins and needles, confusion/fuzzy head, dizziness or lightheaded, chest tightness, problems with balance, altered personality/ behavior, chest pain, palpitations, leg/ankle swelling, skin rash, diarrhea, pain on breathing, weight loss, tremor/shakiness, constipation, erectile dysfunction, loss of sense of smell, can’t fully move or control movement, abdominal pain, loss of control of passing urine, loss of appetite, loss of taste, can’t move and/or feel one side of your body or face | 4 |
|
Gamberini et al. [64] (2021) |
Italy | 178 |
Mild, moderate, severe ICU 100% |
12 months |
Median (IQR): 64 (55–70) Male 73% |
Cough, arthromialgia, palpitations, dyspnea | 4 |
| Garcia-Abellan et al. [65], (2021) | Spain | 146 | Hospitalized | 6 months |
Median (IQR): 64 (54–76) Male 60% |
Fatigue, myalgia, dyspnea, cough, nasal congestion | 4 |
| Ghosn et al. [66], (2021) | French | 1137 |
Hospitalized ICU 29% |
Median (IQR): 194 (188—205) days |
Median (IQR): 61 (51–71) Male 63% |
Fatigue, dyspnea, joint pain, myalgia, headache, rhinorrhea, cough, sore throat, ageusia, anosmia | 4 |
| Hodgson et al. [67], (2021) | Australia | 160 | ICU 100% | 6 months |
Median (IQR): 62 (55–71) Male 61% |
Shortness of breath, loss of strength, fatigue, persistent cough, loss of taste, loss of smell, headache, persistent chest pain, palpitations, myalgia/arthralgia, loss of sensation, hair loss, weight loss, anxiety, depression, ptsd, cognitive dysfunction | 4 |
| Horwitz et al. [68], (2021) | USA | 126 |
Hospitalized ICU45% |
6 months |
Median (IQR): 62 (52–68) Male 60% |
Memory changes, brain fog, difficulty sleeping, dizziness, headache, ringing in ears, altered/loss of smell, altered/loss of taste, fatigue, weakness, muscle/body ache, joint pain, tremors, rash, palpitations, nausea, diarrhea | 5 |
| Huang et al. [69], (2021) | China | 574 | Hospitalized | 194.0 ± 15.3 days |
57.7 ± 11.4 Male 39% |
Sweating, myalgia or joint pain, chills, shortness of breath, chest distress, chest pain, cough, sputum. decreased qppetite, abdominal distention, diarrhea, forgetfulness, hypopsia, hearing losing, sleep difficulty, PTSD | 4 |
| Huarcaya-Victoria et al. [70], (2021) | Peru | 318 | Mild, moderate, severe, critical | 102.1 days (95% CI, 98.3–106.0) |
Median (IQR): 53.1 (51.8–54.4) Male 61% |
Depression, anxiety, somatic symptom, PTSD | 4 |
| Karaarslan et al. [71], (2021) | Turkey | 285 | Hospitalized | 6 months |
52.3 ± 12.1 Male 60% |
Fatigue, myalgia, joint pain, back pain, neck pain, fever, cough, lack of appetite, dyspnea, diarrhea, sore throat, headache, dizziness, absence of taste, absence of smell, sweat, hair loss | 5 |
| Boglione et al. [72], (2021) | Italy | 449 |
Hospitalized ICU 14% |
Median (IQR): 178.5 (165.5 – 211.5) days |
Median (IQR): 65.0 (56.0–75.5) Male 78% |
Fatigue, myalgias/arthralgias, fever, headache, dyspnea, cough, chest pain, brain fog, dizziness, memory impairment, anosmia, ageusia, peripheral neuropathy, tachyarrhytmias, pericarditis/myocarditis, sleeping disorders, PTSD, anxiety, depression, psychosis, behavior disorder, weight loss, hair loss, diabetes, hypertension, psoriasis, venous thromboembolism, thyroid dysfunction | 5 |
| Chand et al. [73], (2021) | USA | 103 |
Hospitalized ICU 100% |
Median (IQR): 216.5 (200–234.5) days |
Median (IQR): 54.0 (46.0–61.0) Male 52% |
Cough, shortness of breath, anosmia, PTSD, depression | 5 |
| Kumar et al. [74], (2021) | Pakistan | 817 | Hospitalized | 90 days |
41 ± 9 Male 63% |
Insomnia, altered sense of smell, headache, altered sense of taste, altered vision, dizziness, stroke | 6 |
| Mei et al. [75], (2022) | Tianjin, China | 144 | Hospitalized | 3 months |
Not reported Male 50% |
PTSD | 5 |
| Nesan et al. [76], (2021) | India | 1354 |
Mild, moderate, severe ICU 2% |
3 months |
Not reported Male 73% |
Tiredness/fatigue, stress and anxiety, change in mood, myalgia, loss of appetite, sleep disturbances, muscular weakness, constipation, loss of taste, nausea and vomiting, loss of smell, headache, abdominal discomfort, breathing difficulty, confusion, numbness, sore throat, chest pain, palpitations, burning and pricking pain sensation on body, reduced urine output | 5 |
| Ong et al. [77], (2021) | Singapore | 183 |
Mild, moderate, severe ICU 18% |
180 days |
Median (IQR): 44 (33–56) Male 75% |
Cough, dyspnea, sputum production, chest pain, myalgia, fatigue, joint pain, memory loss, limb numbness, limb weakness, diarrhea, headache, fever, sweats, abdominal pain, anosmia/ageusia, blocked nose, rhinorrhea, sore throat | 5 |
| Rass et al. [78], (2021) | Austria | 103 |
Moderate, severe ICU 30% |
3 months |
Median (IQR): 56 (48–68) Male 70% |
Hyposmia, anosmia, hypogeusia, new cephalea, vertigo/dizziness/lightheadedness, neck stiffness, myalgia, decreased consciousness, dysarthria, aphasia, positive frontal release signs, blurring vision, oculomotor nerve palsy, facial palsy, dysphagia, bradykinesia, dystonia, chorea, myoclonus/jerks, asterixis, dysmetria, tremors, abnormal muscle tone, muscle atrophy, decreased/disturbed sensibility, paresis, babinski sign, gait abnormality, PTSD, depression, anxiety, persistent fatigue, sleep disturbances, forgetfulness, trouble concentrating, difficulty thinking | 4 |
| Righi et al. [79], (2022) | Italy | 235 | Mild, moderate, severe | 9 months |
Median (IQR): 61.9 (54–71) Male 69% |
Cough, Breathlessness, Fatigue, Anosmia, Dysgeusia, Myalgia, Diarrhoea | 5 |
| Rivera-Izquierdo et al. [80], (2022) | Spain | 453 |
Hospitalized ICU 11% |
12 months |
61.2 ± 14.3 Male 57% |
Fatigue, Muscle Weakness, Muscle or Joint Pain, Dyspnoea, Chest Pain, Pharyngeal Symptoms, Headache, Sensitivity Disorders, Movement Disorders, Confusion, Memory Loss, Depressive Symptoms, Anxiety Symptoms, Sleep Disturbances, Thrombotic Events, Diarrhoea, Constipation, Abdominal Pain | 6 |
| Sibila et al. [81], (2022) | Spain | 215 |
Hospitalized ICU 44% |
6 months |
61.4 ± 11.8 Male 61% |
Dyspnea, fatigue, cough, joint pain, diarrhea, sputum production, headache, chest pain | 5 |
| Staudt et al. [82], (2022) | Germany | 101 |
Hospitalized ICU 20% |
10 months |
Median (IQR): 60.0 (50.8–66.0) Male 58% |
Shortness of breath, voice alteration, impaired taste, excessive sweating, fatigue, sleeping disorder, cognitive impairment, disturbed balance, hair loss | 4 |
| Tessitore et al. [83], (2021) | Switzerland | 165 |
Hospitalized ICU 16% |
12 months |
Median (IQR): 58 (50–69) Male 62% |
Fever, dyspnea, cough, myalgia, tiredness/fatigue, headache, expectorations, altered smell or taste sensation, runny nose, depression | 5 |
| Tleyjeh et al. [84], (2022) | Kingdom of Saudi Arabia | 222 | Hospitalized | Median (IQR): 122 (109—158) days | Not reported | Dyspnea, fatigue | 4 |
| Torres-Ruiz et al. [85], (2021) | Mexico | 103 | Hospitalized | 107.8 days |
Median (IQR): 50 (41–58) Male 46% |
Confusion, fatigue, cough, dyspnea, headache, wheezing, fever, joint pain, increased joint size, inability to dress, inability to walk, inability to open jars, chest pain, orthopnea, peripheral oedema, myalgia, dermatosis, paresthesia, decreased visual acuity, decreased concentration, memory decline, alopecia, back pain, anosmia, dysgeusia | 5 |
| Vejen et al. [86], (2022) | Denmark | 128 | Hospitalized | Median (IQR): 140 (119–157) days |
Median (IQR): 64.5 (51.0–75.0) Male 58% |
Fatigue, dyspnea, cough, memory loss, chest or muscle pain, phlegm, attention loss, loss of smell/taste | 4 |
| Wang et al. [87], (2021) | China | 199 |
Asymptomatic, mild, moderate, severe, critical ICU 3% |
6 months |
42.7 Male 47% |
PTSD | 4 |
| Wong-Chew et al. [88], (2022) | Mexico | 4670 |
Hospitalized ICU 5% |
90 days |
Median (IQR): 48 (37–58) Male 50% |
Alopecia, fatigue, insomnia, headache, desire to cry, sadness, back pain, anguish, anhedonia, anger, joint pain, paraesthesia, pharyngodynia, lack of concentration, lower back pain, rhinorrhea, hypo- or polyphagia, muscle pain, loss of memory, sweating, lethargy, cough, chest pain, difficulty breathing, dizziness, anxiety, globus, tearing, bradyphrenia, slow walking, general discomfort, anosmia, nausea, diarrhea, rash, weight loss, increase respiration, dysgeusia, chills, tremors, abdominal pain, disorientation, spots on the skin, fever, cyanosis | 4 |
| Zhang et al. [89], (2021) | China | 2433 |
Non-severe, severe ICU 2% |
Median (IQR): 364.0 (357.0–371.0) days |
Median (IQR): 60.0 (49.0–68.0) Male 50% |
Fatigue, sweating, chest tightness, anxiety, myalgia, palpitation, cough, shortness of breath, dizziness, expectoration, dyspnea, headache, edema of lower limbs, taste change, impaired sense of smell, sore throat, anorexia, diarrhea, hemoptysis, nausea, chill, vomiting, fever | 5 |
| Zhou et al. [90], (2021) | China | 120 | Non-severe, severe | Median (IQR): 314.5 (296–338) days |
51.6 ± 10.8 Male 41% |
Shortness of breath, fatigue, sleep difficulties, joint pain, loss of smell, constipation, diarrhea, anxiety, depression | 4 |
| Zuschlag et al. [91], (2022) | Germany | 162 |
Asymptomatic, mild, moderate, severe, critical ICU 7% |
12 months |
65.1 Male 54% |
Fatigue, cognitive dysfunction, shortness of breath, pain in muscles and joints, headache, cough, altered smell/taste, posttraumatic stress symptoms, sleep problems, anxiety, depression, disturbance of sensitivity in one leg, loss of appetite and weight, nausea, pain in hands and feet, pruritus, thoracic burning, vertigo, weakness of forefoot | 4 |
aFollow up after hospital discharge (mean ± SD) if not specified
Fig. 2.
The boxplots for the prevalence of post-COVID sequelae among previously hospitalized patients with ten or more studies
The quality of studies was assessed by NOS and no articles were rated as poor quality (Appendix 2). Only five studies (6.9%) were rated high quality. The majority of studies were non-controlled cohort studies and did not meet the standard of comparability.
Meta-analysis of prevalence of sequelae related to COVID-19
A total of 167 sequelae related to COVID-19 were identified in the systematic review, including respiratory/lung function, cardiovascular, psychosocial, neurological, dermatological, digestive system, endocrinological system and other sequelae. 92 sequelae were reported by two or more studies and their pooled prevalence was presented (Table 2). These sequelae were reported during 90 days to 13.2 months of follow-up.
Table 2.
167 sequelae in patients who recovered from COVID-19
| Symptom | Number of studies | Prevalence (%)a | 95 CI (%) | Range (%) | I2 (%) | PH |
|---|---|---|---|---|---|---|
| Respiratory/lung function sequelae | ||||||
| Post–activity polypnea | 3 | 29.8 | [20.5–41.3] | (21.4–42.3) | 91.7 | < 0.001 |
| Shortness of breath | 14 | 25.6 | [15.1–39.8] | (3.7–59.7) | 98.9 | < 0.001 |
| Dyspnea | 35 | 15.5 | [11.3–20.9] | (0.8–58.4) | 98.4 | < 0.001 |
| Pain on breathing | 2 | 13.9 | [11.9–16.2] | (13.1–14.3) | 0.0 | 0.628 |
| Chest distress | 8 | 10.8 | [6.8.–16.8] | (3.2–29.6) | 97.2 | < 0.001 |
| Cough | 40 | 9.8 | [7.8–12.4] | (0.0–35.2) | 95.60 | < 0.001 |
| Sputum production | 3 | 9.3 | [3.2–24.1] | (3.3–22.7) | 91.4 | < 0.001 |
| Nonmotor polypnea | 3 | 7.9 | [3.8–15.7] | (4.6–16.2) | 90.0 | < 0.001 |
| Chest pain | 29 | 6.9 | [5.3–9.0] | (0.0–27.7) | 94.9 | < 0.001 |
| Globus | 1 | 5.9 | [4.5–7.6] | – | – | – |
| Rib pain | 1 | 4.5 | [3.3–6.2] | – | – | – |
| Sputum | 9 | 4.2 | [2.8–6.2] | (1.0–10.0) | 87.8 | < 0.001 |
| Sore throat | 15 | 3.4 | [2.2–5.3] | (0.0–13.3) | 94.6 | < 0.001 |
| Swallowing problem | 2 | 2.9 | [0.2–33.5] | (0.0–8.6) | 76.7 | 0.038 |
| Persistent pharyngeal symptoms | 4 | 2.7 | [0.9–8.3] | (1.1–8.4) | 97.3 | < 0.001 |
| Wheezing | 2 | 2.7 | [1.9–3.9] | (1.9–2.8) | 0.0 | 0.613 |
| Throat itching | 1 | 1.4 | [0.8–2.5] | – | – | – |
| Pneumonia | 1 | 0.8 | [0.3–1.7] | – | – | – |
| Thoracic burning | 1 | 0.6 | [0.0–3.4] | – | – | – |
| Orthopnea | 3 | 0.6 | [0.3–1.3] | (0.0–0.6) | 0.0 | 0.984 |
| Pulmonary fibrosis | 1 | 0.6 | [0.4–0.9] | – | – | – |
| Lung damage | 1 | 0.3 | [0.2–0.6] | – | – | – |
| Hemoptysis | 2 | 0.1 | [0.0–0.4] | (0.1–0.2) | 60.4 | 0.112 |
| Bronchitis | 1 | 0.1 | [0.0–0.3] | – | – | – |
| COPD | 1 | 0.1 | [0.0–0.3] | – | – | – |
| Cardiovascular sequelae | ||||||
| Resting heart rate increase | 1 | 11.2 | [8.8–14.1] | – | – | – |
| Tachycardia | 3 | 7.5 | [0.8–44.5] | (0.4–35.4) | 99.2 | < 0.001 |
| Palpitations | 18 | 6.6 | [4.4–9.8] | (0.5–23.2) | 97.5 | < 0.001 |
| Hypotension or syncope | 2 | 5.5 | [1.5–18.6] | (2.9–10.7) | 92.4 | < 0.001 |
| Discontinuous flushing | 1 | 4.8 | [3.3–7.0] | – | – | – |
| Newly diagnosed hypertension | 4 | 1.1 | [0.1–11.4] | (0.2–14.0) | 98.2 | < 0.001 |
| Cardiac disease | 1 | 0.4 | [0.2–0.6] | – | – | – |
| Angina pectoris | 1 | 0.1 | [0.0–0.3] | – | – | – |
| Heart attack | 1 | 0.03 | [0.0–0.2] | – | – | – |
| Psychosocial sequelae | ||||||
| Somatization | 2 | 37.5 | [30.2–45.3] | (34.0–41.8) | 66.8 | 0.083 |
| Phobic–anxiety | 1 | 24.2 | [18.2–31.1] | – | – | – |
| Somnipathy | 30 | 20.1 | [14.7–26.9] | (1.2–64.8) | 98.9 | < 0.001 |
| Anxiety | 24 | 18.0 | [13.8–23.1] | (0.6–47.8) | 97.8 | < 0.001 |
| Sadness | 1 | 16.0 | [13.7–18.5] | – | – | – |
| PTSD | 18 | 14.6 | [11.3–18.7] | (1.2–32.0) | 91.8 | < 0.001 |
| Anger–hostility | 2 | 13.4 | [10.5–17.0] | (12.3–15.9) | 44.3 | 0.180 |
| Depression | 22 | 12.7 | [9.3–17.2] | (0.6–37.5) | 96.0 | < 0.001 |
| Paranoid ideation | 1 | 10.4 | [6.4–15.8] | – | – | – |
| Psychosis | 2 | 6.4 | [0.7–39.7] | (2.1–17.6) | 97.2 | < 0.001 |
| Behavior disorder | 2 | 5.9 | [0.4–52.4] | (1.4–20.8) | 97.9 | < 0.001 |
| Obsessive–compulsive | 2 | 5.7 | [0.4–49.7] | (1.4–19.8) | 97.5 | < 0.001 |
| Specific phobia—with covid | 1 | 2.8 | [1.5–4.9] | – | – | – |
| Dysphoria | 1 | 1.7 | [0.9–3.2] | – | – | – |
| Interpersonal sensibility | 1 | 0.6 | [0.0–3.0] | – | – | – |
| Feelings of inferiority | 1 | 0.6 | [0.2–1.7] | – | – | – |
| Neurological sequelae | ||||||
| Bradykinesia | 1 | 49.9 | [46.0–53.7] | – | – | – |
| Cognitive complaint | 1 | 49.7 | [42.0–57.4] | – | – | – |
| Inability to focus vision | 1 | 33.1 | [25.1–41.9] | – | – | – |
| Problems with balance | 3 | 28.3 | [20.8–37.3] | (16.8–35.6) | 85.9 | < 0.001 |
| Fatigue | 53 | 27.5 | [22.4–33.3] | (1.5–84.9) | 98.9 | < 0.001 |
| Abnormal reflex status | 1 | 26.2 | [18.0–35.8] | – | – | – |
| Sensory alterations | 1 | 25.9 | [19.8–32.8] | – | – | – |
| Brain fog | 4 | 25.0 | [10.3–49.1] | (9.6–43.9) | 99.0 | < 0.001 |
| Arthritis | 1 | 24.6 | [18.1–32.6] | – | – | – |
| Unrefreshing sleep | 2 | 21.9 | [2.6–74.9] | (7.8–48.5) | 99.2 | < 0.001 |
| Cognitive impairment | 8 | 21.2 | [11.0–36.9] | (5.8–38.6) | 98.5 | < 0.001 |
| Neck pain | 1 | 21.1 | [16.5–26.3] | – | – | – |
| Voice alteration | 1 | 20.8 | [13.4–30.0] | – | – | – |
| Light sensitivity | 1 | 20.0 | [13.5–27.9] | – | – | – |
| General malaise | 6 | 19.9 | [8.8–39.1] | (0.0–46.3) | 98.2 | < 0.001 |
| Peripheral neuropathy | 1 | 17.9 | [14.4–21.9] | – | – | – |
| Lightheadedness when prolonged standing | 1 | 16.9 | [10.9–24.5] | – | – | – |
| Concentration problem | 11 | 15.6 | [9.1–25.4] | (2.9–40.2) | 97.8 | < 0.001 |
| Discomfort | 3 | 15.5 | [7.6–29.2] | (3.7–29.2) | 98.8 | < 0.001 |
| Low mood | 3 | 14.5 | [3.8–42.4] | (5.8–39.6) | 98.4 | < 0.001 |
| Mental slowness | 3 | 14.1 | [2.7–48.8] | (5.0–42.4) | 99.3 | < 0.001 |
| Hypomnesia | 20 | 13.4 | [8.4–20.7] | (0.6–53.8) | 98.5 | < 0.001 |
| Arthralgia | 21 | 12.9 | [8.4–19.2] | (0.0–47.8) | 98.4 | < 0.001 |
| Anguish | 1 | 12.7 | [10.6–15.0] | – | – | – |
| Anhedonia | 1 | 12.6 | [10.5–14.9] | – | – | – |
| Tingling | 3 | 12.5 | [2.5.1–44.6] | (0.4–46.9) | 98.6 | < 0.001 |
| Delusions | 1 | 12.5 | [9.5–16.0] | – | – | – |
| Myalgia | 27 | 10.9 | [6.8–16.9] | (0.2–57.2) | 98.7 | < 0.001 |
| Tremors | 4 | 8.3 | [3.5–18.7] | (2.5–13.9) | 95.1 | < 0.001 |
| Muscle atrophy | 1 | 7.8 | [3.4–14.7] | – | – | – |
| Headache | 33 | 7.5 | [5.3–10.6] | (0.0–39.4) | 97.8 | < 0.001 |
| Backache | 5 | 6.9 | [2.4–18.1] | (2.0–32.3) | 97.8 | < 0.001 |
| Dizziness | 17 | 6.9 | [3.9–12.1] | (0.6–35.8) | 98.4 | < 0.001 |
| Paresthesia | 7 | 6.9 | [4.6–10.2] | (3.4–12.1) | 85.7 | < 0.001 |
| Reduction of physical strength | 2 | 6.5 | [0.5–50.8] | (1.7–21.7) | 99.1 | < 0.001 |
| Abnormal muscle tone | 1 | 5.8 | [2.2–12.3] | – | – | – |
| Gait abnormality | 1 | 5.8 | [2.2–12.3] | – | – | – |
| Ageusia | 31 | 5.8 | [3.7–8.8] | (0.0–49.9) | 97.3 | < 0.001 |
| Tearing | 1 | 5.8 | [4.4–7.5] | – | – | – |
| Anosmia | 36 | 5.7 | [3.8–8.3] | (0.0–53.8) | 97.6 | < 0.001 |
| Movement disturbances | 11 | 5.7 | [4.0–8.0] | (1.1–17.5) | 91.1 | < 0.001 |
| Sensitivity disorders | 4 | 4.4 | [1.3–14.0] | (0.6–16.5) | 91.3 | < 0.001 |
| Muscle weakness | 4 | 4.3 | [2.6–6.8] | (3.1–9.1) | 83.7 | < 0.001 |
| ICU-related polyneuropathy | 1 | 3.1 | [2.1–4.6] | – | – | – |
| Impaired vision | 9 | 2.9 | [1.3–6.3] | (0.0–18.0) | 96.0 | < 0.001 |
| Facial palsy | 2 | 2.5 | [0.2–21.9] | (0.0–5.9) | 69.0 | 0.073 |
| Dysphonia | 3 | 2.1 | [0.3–14.1] | (0.6–8.2) | 96.5 | < 0.001 |
| Hallucinations | 2 | 1.9 | [0.1–29.6] | (0.4–8.5) | 99.2 | < 0.001 |
| Dysmetria | 1 | 1.9 | [0.2–6.8] | – | – | – |
| Babinski sign | 1 | 1.9 | [0.2–6.8] | – | – | – |
| Disorientation or confusion | 7 | 1.8 | [0.4–8.5] | (0.0–30.2) | 98.6 | < 0.001 |
| Problems with usual activity | 2 | 1.5 | [1.1–2.0] | (1.4–1.6) | 0.0 | 0.766 |
| Problems with washing or dishing | 2 | 1.1 | [0.5–2.4] | (0.7–1.6) | 80.3 | 0.024 |
| Myoclonus | 2 | 1.0 | [0.5–2.1] | (0.0–1.1) | 0.0 | 0.584 |
| Tinnitus | 2 | 0.9 | [0.0–66.5] | (0.1–11.9) | 98.1 | < 0.001 |
| Paralysis | 2 | 0.7 | [0.0–44.2] | (0.1–6.8) | 97.3 | < 0.001 |
| Hearing loss | 2 | 0.7 | [0.1–4.9] | (0.2–1.6) | 73.5 | 0.052 |
| Leg numbness/finger stiffness | 4 | 0.4 | [0.2–1.1] | (0.1–0.8) | 70.1 | 0.018 |
| Coma | 2 | 0.3 | [0.0–18.1] | (0.0–2.3) | 94.6 | < 0.001 |
| Cerebral infarction | 2 | 0.1 | [0.0–1.4] | (0.0–0.4) | 80.3 | 0.024 |
| Neuralgia | 1 | 0.1 | [0.0–0.2] | – | – | – |
| Aphasia | 1 | 0.0 | [0.0–3.5] | – | – | – |
| Asterixis | 1 | 0.0 | [0.0–3.5] | – | – | – |
| Chorea | 1 | 0.0 | [0.0–3.5] | – | – | – |
| Decreased consciousness | 1 | 0.0 | [0.0–3.5] | – | – | – |
| Dystonia | 1 | 0.0 | [0.0–3.5] | – | – | – |
| Neck stiffness | 1 | 0.0 | [0.0–3.5] | – | – | – |
| Oculomotor nerve palsy | 1 | 0.0 | [0.0–3.5] | – | – | |
| Dermatological sequelae | ||||||
| Changing pattern of sweating | 1 | 28.5 | [20.9–37.0] | – | – | – |
| Sweating | 10 | 6.4 | [3.5–11.7] | (0.0–28.5) | 98.1 | < 0.001 |
| Exanthema | 11 | 4.6 | [2.7–7.7] | (0.2–16.0) | 97.0 | < 0.001 |
| Psoriasis | 1 | 4.1 | [2.5–6.5] | – | – | – |
| Pressure ulcers | 1 | 1.8 | [1.0–2.9] | – | – | – |
| Eczema | 1 | 1.5 | [0.8–2.6] | – | – | – |
| Pruritus | 3 | 1.2 | [0.4–3.6] | (0.6–2.5) | 70.0 | 0.036 |
| Cyanosis | 1 | 0.4 | [0.1–1.1] | – | – | – |
| Allergy | 1 | 0.1 | [0.0–0.2] | – | – | – |
| Sequelae related to digestive system | ||||||
| Stomach bloated after meals | 2 | 15.3 | [2.1–59.8] | (5.8–34.6) | 98.6 | < 0.001 |
| Hypolyphagia | 1 | 9.5 | [7.7–11.6] | – | – | – |
| Poor appetite | 14 | 5.7 | [3.5–9.0] | (0.0–26.9) | 96.6 | < 0.001 |
| Constipation | 8 | 4.6 | [1.9–10.7] | (0.1–27.7) | 97.8 | < 0.001 |
| Abdominal pain | 10 | 3.5 | [1.6–7.3] | (0.0–17.7) | 96.5 | < 0.001 |
| Diarrhea | 21 | 2.9 | [1.8–4.9] | (0.0–17.7) | 96.1 | < 0.001 |
| Nausea | 8 | 1.6 | [0.5–5.0] | (0.0–16.9) | 93.9 | < 0.001 |
| Anorexia | 2 | 0.9 | [0.6–1.3] | (0.8–1.0) | 0.0 | 0.632 |
| Vomiting | 4 | 0.3 | [0.0–2.2] | (0.0–2.0) | 91.3 | < 0.001 |
| Hepatic insufficiency | 1 | 0.2 | [0.1–0.4] | – | – | – |
| Sequelae related endocrinological system | ||||||
| Alopecia | 17 | 11.2 | [6.9–17.6] | (0.0–47.0) | 98.5 | < 0.001 |
| Diabetes (new onset) | 3 | 1.1 | [0.2–7.1] | (0.1–9.0) | 99.1 | < 0.001 |
| Glycaemia uncontrol | 1 | 0.9 | [0.4–1.8] | – | – | – |
| Bitter/dryness in mouth | 1 | 0.3 | [0.2–0.6] | – | – | – |
| High blood sugar | 1 | 0.2 | [0.1–0.4] | – | – | – |
| Dryness/excessive secretion in eye | 1 | 0.1 | [0.0–0.2] | – | – | – |
| Other sequelae | ||||||
| Intolerance to temperature | 1 | 30.8 | [23.0–39.5] | – | – | – |
| Urinary frequency | 1 | 23.1 | [16.1–31.3] | – | – | – |
| Musculoskeletal pain | 13 | 17.3 | [11.1–25.8] | (4.2–50.8) | 98.3 | < 0.001 |
| Erectile dysfunction | 3 | 17.2 | [12.2–23.6] | (13.8–23.4) | 76.0 | 0.015 |
| Difficulty emptying bladder | 1 | 10.8 | [6.0–17.4] | – | – | – |
| Loss of weight | 7 | 10.6 | [5.4–19.6] | (2.9–37.3) | 96.8 | < 0.001 |
| Problems passing urine | 2 | 8.9 | [6.0–13.1] | (7.0–10.6) | 68.5 | 0.075 |
| Thyroid dysfunction | 1 | 8.1 | [5.7–11.0] | – | – | – |
| Rhinorrhea | 5 | 8.0 | [5.6–11.1] | (0.0–10.9) | 71.3 | 0.008 |
| Loss of control of opening bowels | 1 | 5.5 | [3.9––7.5] | – | – | – |
| Blocked nose | 3 | 5.1 | [1.1–19.8] | (0.8–16.0) | 88.7 | < 0.001 |
| Toe lesions | 1 | 4.0 | [2.1–6.7] | – | – | – |
| Urinary tract infection | 1 | 3.9 | [2.8–5.5] | – | – | – |
| Lumpy lesions on toes | 1 | 2.9 | [1.7–4.5] | – | – | – |
| Chills | 4 | 2.7 | [0.6–11.6] | (0.1–20.0) | 98.4 | < 0.001 |
| Oedema | 9 | 2.3 | [0.7–7.4] | (0.2–28.3) | 98.8 | < 0.001 |
| Thrombotic manifestations | 4 | 1.8 | [0.9–3.6] | (0.2–3.1) | 73.2 | 0.011 |
| Mycosis | 1 | 1.4 | [0.8–2.5] | – | – | – |
| Otoacoustic symptoms | 1 | 1.3 | [0.7–2.3] | – | – | – |
| Fever | 18 | 1.1 | [0.6–2.2] | (0.0–11.0) | 89.4 | < 0.001 |
| Increased joint size | 1 | 1.0 | [0.0–5.3] | – | – | – |
| Renal insufficiency de novo | 2 | 0.4 | [0.1–2.0] | (0.2–0.9) | 89.1 | 0.002 |
| Reduced urine output | 1 | 0.3 | [0.1–0.8] | – | – | – |
| Antiadoncus | 1 | 0.03 | [0.0–0.2] | – | – | – |
aUsing estimates at the longest follow-up time for those studies have multiple follow-up time
Respiratory/lung function sequelae
The most common sequelae in the respiratory system were shortness of breath (14 studies) with a prevalence of 25.6% (95% CI 15.1–39.8%, range 3.7–59.7%) and post-activity polypnea (3 study) with the prevalence of 29.8% (95% CI 20.5–41.3%, range 21.4–42.3%), followed by dyspnea (35 studies), pain on breathing (2 studies), chest distress (8 studies), cough (40 studies), with prevalence of 15.5% (95% CI 11.3–20.9%, range 0.8–58.4%), 13.9% (95% CI 11.9–16.2%, range 13.1–14.3), 10.8% (95% CI 6.8–16.8%, range 3.2–29.6%) and 9.8% (95% CI 7.8–12.4%, range 0.0–35.2%), respectively.
Cardiovascular sequelae
The most common sequela was resting heart rate increase (1 study) with prevalence of 11.2% (95% CI 8.8–14.1%). 18 studies reported palpitations with pooled estimate of 6.6% (95% CI 4.4–9.8%, range 0.5–23.2%).
Psychosocial sequelae
Somatization (2 studies) was the most common psychosocial sequela with a prevalence of 37.5% (95% CI 30.2–45.3%, range 34.0–41.8%). More than ten studies measured somnipathy, anxiety, PTSD and depression with a pooled prevalence of 20.1% (14.6–26.9%, range 1.2–64.8%), 18.0% (95% CI 13.8–23.1%, range 0.6–47.8%), 14.6% (95% CI 11.3–18.7%, range 1.2–32.0%) and 12.7% (95% CI 9.3–17.2%, range 0.6–37.5%), respectively.
Neurological sequelae
More than ten studies reported that recovered patients had fatigue, concentration problem, hypomnesia arthralgia, myalgia, headache, dizziness, ageusia, anosmia and movement disturbances, among which fatigue had the highest prevalence of 27.5% (95% CI 22.4–33.3%, range 1.5–84.9%). Meanwhile, one study each reported that the prevalence of bradykinesia and cognitive complaint were up to 49.9% and 49.7%.
Dermatological sequelae
10 and 11 articles reported sweating (6.4% 95% CI 3.5–11.7%, range 0.0–28.5%) and exanthema (4.6% 95% CI 2.7–7.7%, range 0.2–16.0%), respectively. However, a study reported that the prevalence of changing pattern of sweating was 28.5% (95% CI 20.9–37.0%).
Sequelae related to the digestive system
Poor appetite, abdominal pain and diarrhea were reported by more than ten studies, respectively, with a pooled prevalence of 5.7% (95% CI 3.5–9.0%, range 0.0–26.9%), 3.5% (95% CI 1.6–7.3%, range 0.0–17.7%) and 2.9% (95% CI 1.8–4.9%, range 0.0–17.7%), respectively. In addition, two studies reported that 15.3% (95% CI 2.1–59.8%, range 5.8–34.6%) of patients had stomach bloated after meals.
Sequelae-related endocrinological system
The prevalence of dermatological sequelae was generally low. The most common sequela related to the endocrinological system was alopecia, with a pooled prevalence of 11.2% (95% CI 6.9–17.6%, range 0.0–47.0%) from 17 articles.
Other sequelae
Among the remaining sequelae, intolerance to temperature, urinary frequency, musculoskeletal pain and erectile dysfunction were common, with prevalences of 30.8% (95%CI 23.0–39.5%), 23.1% (95% CI 16.1–31.3%), 17.3% (95% CI 11.1–25.8%, range 4.2–50.8%) and 17.2% (95% CI 12.2–23.6%, range 13.8–23.4%), respectively.
Heterogeneity analysis and sensitivity analysis
Most of the sequelae prevalence had high heterogeneity across studies (I2 > 60%, PH < 0.05). Six meta-analyses showed low heterogeneity (pain on breathing: I2 = 0.00%, PH = 0.628, wheezing: I2 = 0.00%, PH = 0.613, orthopnea: I2 = 0.00%, PH = 0.984, problems with usual activity: I2 = 0.00%, PH = 0.766, myoclonus: I2 = 0.00%, PH = 0.584, anorexia: I2 = 0.00%, PH = 0.632), one showed moderate heterogeneity (anger-hostility: I2 = 44.3%, PH = 0.180).
Subgroup analysis
Temporal trend of symptoms
We present prevalence of symptoms at different follow-up time (3–4, 5–8 and > 9 months). Overall, the prevalence of all symptoms showed higher prevalences at 5–8 months’ follow-up (Appendix 3 and Fig. 3). The prevalence of most symptoms decreased at > 9 months, except fatigue, dyspnea, depression, arthralgia, anosmia, ageusia and dizziness. The prevalence of PTSD and chest pain decreased from 3–4 to > 9 months with significant difference, and alopecia decreased significantly from 3–4 to 5–8 months. Heterogeneity in most symptoms was still high after stratification by follow-up duration (I2 > 60.0%, PH < 0.05). Only fever at > 9 months’ follow-up had a low heterogeneity (I2 = 22.0%, PH = 0.279).
Fig. 3.
Pooled prevalence of sequelae at 3–4, 5–8 and ≥ 9 months (9–13 months) follow-up among previously hospitalized patients
Symptoms by geographic regions
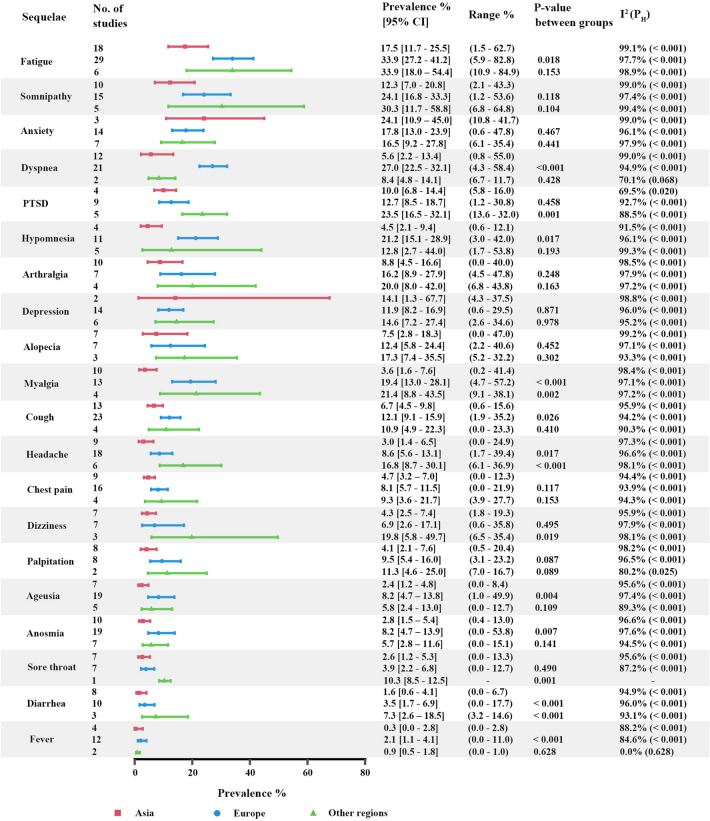
We further analyzed the prevalence of symptoms by geographic regions. Overall, the pooled prevalence of symptoms in Asia was lower than that in Europe and other regions (Appendix 4 and Fig. 4). Additionally, the heterogeneity in symptoms was still high (I2 > 60.0%, PH < 0.05), except fever in regions out of Asia and Europe (I2 = 0.0%, PH = 0.628). Europe reported higher prevalences than Asia in fatigue, dyspnea, anxiety, anosmia, ageusia and headache with significant difference.
Fig. 4.
Pooled prevalence of sequelae among previously hospitalized patients in Asia, Europe and other regions
Symptoms by age
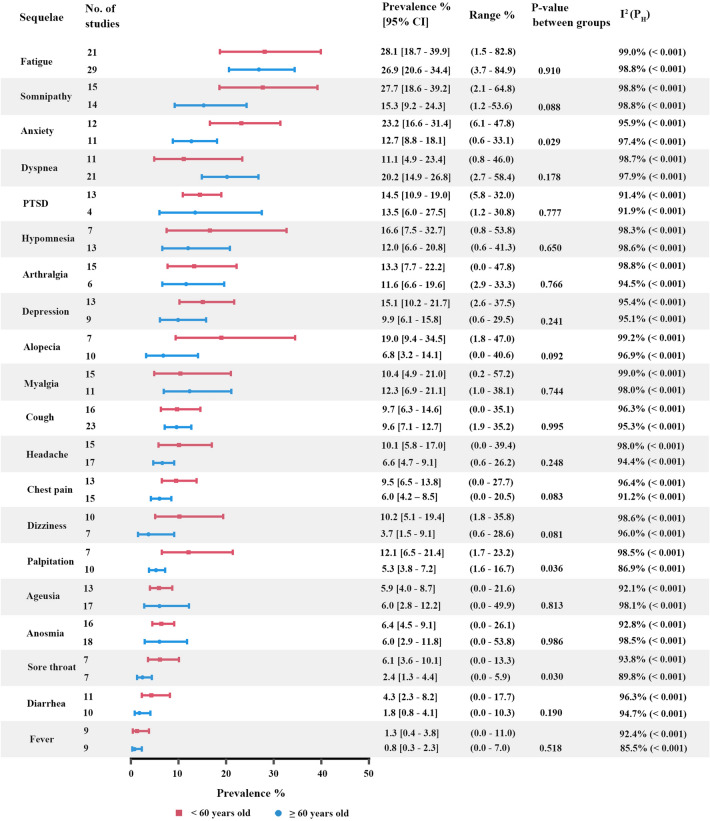
We estimated the prevalence of symptoms by age (< 60 years old and ≥ 60 years old). Overall, the pooled prevalence of symptoms in < 60 years old was higher than that in ≥ 60 years old (Appendix 5 and Fig. 5), except dyspnea, PTSD, anosmia, myalgia, cough, ageusia and headache. The heterogeneity in symptoms was still high (I2 > 60.0%, PH < 0.05). Patients < 60 years old reported higher prevalences than those ≥ 60 years old in palpitation and sore throat with significant difference.
Fig. 5.
Pooled prevalence of sequelae among previously hospitalized patients in < 60 years old and ≥ 60 years old
Symptoms by ICU admission
We showed the prevalence of symptoms by ICU admission (< 20% and ≥ 20%). Overall, the pooled prevalence of symptoms in < 20% ICU admission was lower than that in 20% ICU admission (Appendix 6 and Appendix 7), except depression and alopecia. The heterogeneity in symptoms was still high (I2 > 60.0%, PH < 0.05). Studies with < 20% ICU admission reported lower prevalences than studied ≥ 20% ICU admission in dizziness and fever.
Discussion
This systematic review and meta-analysis identified 167 sequelae related to COVID-19 from 88,769 patients at least 90 days follow-up after hospital discharge. These included respiratory/lung function, cardiovascular, psychosocial, neurological, dermatological, digestive system and endocrinological system sequelae. We identified symptoms such as fatigue, somnipathy, dyspnea, anxiety, depression, PTSD and arthralgia which persisted for 1 year in the discharged COVID-19 patients. The prevalence of some neurological symptoms (fatigue, arthralgia, anosmia, ageusia and dizziness) did not show decreasing trends up to 1 year of follow-up, while most of the other symptoms resolved over time.
Respiratory/lung function sequelae
Shortness of breath (25.6%) and post-activity polypnea (29.8%) were the 2 most common sequelae in the respiratory system. Furthermore, some patients who recovered from COVID-19 would develop symptoms, including dyspnea (15.5%), pain on breathing (13.9%), chest distress (10.8%), cough (9.8%), etc. These symptoms can be explained by the influence of COVID-19 on the lungs. The current evidence indicates that the organ most affected by COVID-19 is the lung, which may cause a variety of pathophysiological events, including capillary damage and bleeding, hyaline membrane formation, pulmonary consolidation, diffuse alveolar epithelium destruction, and alveolar septal fibrous proliferation [92, 93]. COVID-19 can extensively damage the alveolar epithelial cell and the endothelial cell with secondary fibroproliferation, which may cause lung fibrosis or pulmonary hypertension by chronic vascular and alveolar remodeling [94, 95].
Clinically, the pathological changes can significantly affect activities of daily living, by reduction of walking distance in the 6-min walking test [32]. A systematic review and meta-analysis have found that 39%, 15% and 7% of COVID-19 patients had altered diffusion capacity, restrictive pattern, and obstructive pattern, respectively, in lungs [96]. Radiological and pathological report showed pulmonary fibrosis, parenchymal bands, irregular interfaces, reticular opacities and traction bronchiectasis with or without honeycomb lung [97–100]. Additionally, varying degrees of alveolar structure destruction and pulmonary interstitial fibrosis were observed in the autopsy of patients with COVID-19 [101].
The underlying pathophysiology of post-COVID sequelae is multifaceted and subject to debate. It can be classified into lung-dependent direct tissue injury, and lung-independent pathological inflammation such as viral persistence, immune dysregulation, and autoimmunity [102, 103]. Radiologically, post-COVID decline in pulmonary function is closely associated with pulmonary fibrosis and ground glass opacification [35, 104–106]. An investigation of autopsy samples demonstrated viral persistence, resulting in multiple organ injury and various clinical presentations [107]. The role of inflammatory biomarkers in its pathogenesis is discordant since some studies have observed a persistently elevated biomarkers [103, 108, 109], while some researchers also show there is no direct correlations between them [110].
The evidence showed that the lungs have the potential to heal after severe injury, which is consistent with the downtrend of the prevalence in cough, chest pain and sore throat [111]. However, we also observed that dyspnea persisted in 13.1% of the discharged COVID-19 patients for over a year, which suggested that lung injury may affect patients for a longer duration. Evidence from SARS and MERS reported that some patients still have lung damage even 15 years later [112]. Therefore, a specialized rehabilitation program is important. One study has shown that a 6-week rehabilitation program can significantly improves pulmonary functions, quality of life and anxiety in COVID-19 survivors [113]. Early rehabilitation is beneficial to functional recovery and functional independence of patients [114, 115]. Therefore, several professional bodies advocated the need of early detection and rehabilitations. The British Thoracic Society recommended regular follow-up of high-risk patients 4 months after infection; and the Swiss COVID group and Swiss Society for Pulmonology recommended detailed pulmonary assessments and rehabilitations, if resources are available [116, 117].
Cardiovascular sequelae
Resting heart rate increase were the most common sequelae in the cardiovascular system with the prevalence of 11.2%, which may be related to heart damage caused by COVID-19. A study of recovered COVID-19 patients reported that almost 78% of patients had heart involvement and 60% of them had persistent inflammation of the heart muscle unrelated to a pre-existing condition [118]. Cardiovascular sequelae may recover as we found that the prevalence of palpitation decreased slightly over time. It is important to note that even with a significant recovery of heart function, there may still be a risk of coronary artery disease, atrial fibrillation, or ventricular arrhythmias due to myocardial injury [119].
Patients with cardiovascular sequelae require close monitoring because the post-discharge mortality rate is high, with near half of the patients dying 3 months after discharge [120]. These patients should be referred for clinical reviews and reassessment 1–2 months after discharge [121, 122]. Postural Orthostatic Tachycardia Syndrome (POTS) has been reported in COVID-19 survivors [123]. They may be presented as easy fatigue, postural tachycardia, dizziness and exercise intolerance [124]. The proposed mechanism has been an alteration of autonomic nervous system regulated by angiotensin converting enzyme 2 (ACE2) protein found on neurons [125].
Psychosocial sequelae
The sequelae related to mental health were of common occurrence in COVID-19, such as somatization (37.5%), phobic-anxiety (24.2%), somnipathy (20.1%), anxiety (18.0%), PTSD (14.6%) and depression (12.7%). Meanwhile, the experience with SARS and MERS also showed that the prevalence of depression, anxiety and post-traumatic stress disorder remained high even after 39 months, which indicates that the mental health of patients is of great concern after they recovered from COVID-19 [126]. We observed that the prevalence of somnipathy, anxiety and depression was still high after a year (15.1%, 12.9% and 12.6%, respectively). Reason of psychosocial sequelae is proposed to be inflammatory marker-mediated neuroinflammatory damages since cytokines IL-4 and IL-6 are persistently elevated in patients reported with neurological or psychological symptoms [127]. Other inflammatory markers reported include amyloid-beta, neurofilament light, neurogranin, total tau and p-T181-Tau [128]. Nearly half of survivors without prior psychiatric conditions live with depression after 3 months of recovery from severe COVID-19 associated respiratory failure [129].
Neurological sequelae
A great number of neurological sequelae have been found in this systematic review and meta-analysis. Among these sequelae, bradykinesia (49.9%), cognitive complaint (49.7%), inability to focus vision (33.1%), problems with balance (28.3%), fatigue (27.5%), abnormal reflex status (26.2%), sensory alterations (25.9%), brain fog (25.0%), arthritis (24.6%), unrefreshing sleep (21.9%), cognitive impairment (21.2%), neck pain (21.1%), voice alteration (20.8%) and light sensitivity (20.0%) had a relatively high prevalence. They may be related to viral infection, physiological impairment (e.g., hypoxia), cerebrovascular disease, immunoreaction, side effects of medication, etc. [126] Reason of neurological dysfunction can be explained macroscopically and microscopically. Macroscopically, hypoxic nerve damage leads to mitochondrial swellings in various brain sites, such as cerebral white matters, brainstem, parahippal gyrus, thalamus and sites with high metabolic requirements [130–133]. Neurons do not readily regenerate; thus, these clinical presentations may be long-lasting, leading to Long COVID-19 syndrome [134]. Microscopically, persistently elevated cytokines were observed in patients with neurological presentations. Multifocal neurological damages in patients with Long COVID-19 Syndrome may be a result from indirect T-cell and microglia damage in the brain, similar to stroke and neuroinflammatory diseases [135]. Additionally, lung sequelae are also believed to be a major cause of fatigue [136].
However, the downtrend was not observed in neurological sequelae, and the prevalence was still high in fatigue (26.2%) and arthralgia (11.5%) after a year.
Dermatological sequelae
Ten and 11 articles reported that COVID-19 recovered patients would have sweating and exanthema with a low prevalence of 6.4% and 4.6%, respectively. González-Hermosillo et al. reported that 28.5% of patients would change pattern of sweating at 6-month follow-up after discharge [43]. These dermatological sequelae may be caused by the drug used to treat COVID-19, maybe because of the long-term effect of COVID-19, or maybe because of some random effects (wrong measurement, the patient happened to have this sequela, etc.). Further studies are needed to determine whether COVID-19 can cause dermatological sequelae.
Sequelae related to the digestive system
14 studies reported poor appetite, with a pooled prevalence of 5.7%. This may be related to the anosmia and the ageusia caused by COVID-19, and elevated levels of inflammatory cytokines are also a common cause of anorexia [137]. Stomach bloated after meals and hyplyphagia occurred frequently (15.3% and 9.5%). Additionally, a small number of patients also had abdominal pain, diarrhea, vomiting, and other sequelae, which are also developed during the acute phase of COVID-19. This may be because the SARS-CoV-2 replicates in the gastrointestinal tract and then infects and destroys absorbent intestinal cells. Or the gastrointestinal system is directly damaged by the COVID-19 induced inflammation [138]. Meanwhile, we observed that the prevalence of diarrhea decreased with the increase of follow-up time.
Although the observed prevalence of hepatic insufficiency was low (0.2%), liver injury has been observed in various studies of COVID-19 survivors [139, 140]. Majority of the survivors showed gradual normalization of liver function enzymes within 2 months after discharge [141]. However, patients with chronic liver conditions (such as liver cirrhosis) are associated with a fourfold increase of mortality risk after COVID-19 infections, compared with those without [142]. Thus, COVID-19 survivors with chronic liver conditions should be prioritized for rehabilitations follow-up.
Sequelae-related endocrinological system
Alopecia was the most common sequela related to the endocrinological system with a pooled prevalence of 11.2%. However, it is apparently lower than another systematic review which reported a pooled prevalence of 25% [143]. It may be because the follow-up time of this systematic review is shorter than mine, the symptom of alopecia may have been alleviated. The alopecia developed after COVID-19 can be referred to as telogen effluvium, defined as a diffuse hair loss after significant systemic stressors or infection, which is due to premature follicular transitions from the active growth phase (anagen) to the resting phase (telogen) [144]. Generally, it will last about 3 months. Therefore, we observed that the prevalence of alopecia decreased by 20.9% from 3 – 4 months to 5 –8 months.
Another Long-COVID related endocrinopathy is diabetes mellitus (DM). Proposed pathogenic mechanism is SARS-CoV-2 binding to ACE2 receptors of pancreatic beta-islet cells, triggering autoimmune response and type I DM [145, 146]. Thus, hyperglycemia without DM and new-onset DM are associated with poor prognosis COVID-19 after excluding risk factors such as obesity and corticosteroid administration [147].
Other sequelae
Weight loss (10.6%) and malnutrition are common in patients who recover from COVID-19. Another study that evaluated the COIVD-19 patients after clinical remission, also found that more than 50% of them were at risk of malnutrition and approximately 30% of them lost more than 5% of weight compared with baseline [148]. This may be because acute systemic inflammation can severely affect several metabolic and hypothalamic pathways, leading to decreased anorexia and food intake as well as increased resting energy expenditure and muscle catabolism [149]. Additionally, erectile dysfunction (18.6%) was found as a sequela of COVID-19, which may be because of psychological distress, endothelial dysfunction, subclinical hypogonadism, or impaired pulmonary hemodynamics [149]. Moreover, the urinary symptoms (urinary frequency, difficulty emptying bladder, problems passing urine) may be because of viral cystitis caused by SARS-COV-2, and further research is needed [150]. The musculoskeletal pain is also a common post-COVID symptom. The muscular nociception is neurologically and immunologically mediated in Long COVID-19 Syndrome. Its pathogenesis is related to muscle injury secondary to hyperinflammatory state [71, 151, 152], leading to to ACE-2 mediated injury with elevated inflammatory & nociceptive immune markers, e.g. IL2, IL7, IL10, IL-6, TNFα. These are amendable by pharmacological and non-pharmacological interventions by pain-modulation pathways to facilitate future rehabilitations. High prevalence of intolerance to temperature (30.8%) reported by González-Hermosillo et al. cannot be explained yet, and needs more further researches.
Our subgroup analysis showed that the prevalence of most COVID-19 sequelae decreased after > 9 months of follow-up, except that fatigue, somnipathy, dyspnea, depression, arthralgia, anosmia and ageusia remained stable for a longer time. However, the prevalence of most sequelae increased at 5 – 8 months. A similar pattern in a meta-analysis by Alkodaymi et al., prevalences of symptoms at 6 – 9 months were higher than others and did not decrease noticeably afterwards for some symptoms such as fatigue, dyspnea and sleep disorder [11]. Other explanation could be studies reporting sequelae at 5–8 months had a higher proportion of ICU admission, and were mostly from regions out of Asia, while studies of 3–4 months and ≥ 9 months were all from Asia. Furthermore, Asia showed lower prevalence than regions out of Asia, except for anxiety and depression. As a majority of Asian studies (15/22) were from China, the lower prevalence may be explained by admission of milder patients in China, whereas most mild patients were treated at home in other regions. A study from Wuhan, China reported that the condition of 93% of patients in the general ward was not serious, compared to 77% in East London, UK [153, 154]. It may also be explained by younger age and lower pre-existing medical comorbidities among Chinese patients [155]. Additionally, some symptoms in patients aged < 60 years had significantly higher prevalence than that in patients aged ≥ 60 years, such as anxiety, palpitation and sore throat (Appendix 5 and Fig. 5), while other studies found that COVID-19 patients of different age groups may exhibit different sets of clinical symptoms [156]. Finally, the pooled prevalence of symptoms tends to be higher among studies with more severe patients (i.e. higher % of ICU admission). Other factors such as treatment, rehabilitation, race and blood type may also affect symptom persistence [157, 158].
The heterogeneity of most meta-analyses was high and from our subgroup analyses it could not be explained by follow-up time, regions, age or ICU admission. Possible source of heterogeneity may include differences in the determination of symptoms (questionnaire or self-report or diagnosis by doctors), the proportion of sex, drug use, etc. We did not perform meta-regression analysis to further identify the source of heterogeneity due to the limited number of studies for each symptom.
One strength of this study is that this systematic review and meta-analysis studied all potential long-term sequelae (> 3 months) in hospital discharged COVID-19 patients. The review presented a wide spectrum of health burdens among hospital recovered COVID-19 patients and informed management strategies of COVID-19 discharged patients. Currently, different long COVID-19 clinical assessment tools have been proposed, such as Newcastle post-COVID syndrome Follow Up Screening Questionnaire and COVID-19 Yorkshire Rehabilitation Scale [159, 160]. This study provided clues for standardization of rehabilitation baseline assessment based on symptom prevalence and severity. The focus on hospitalized patients would be of clinical significance and provided most relevant information for hospital-based rehabilitation strategies and services. The meta-analysis by Maglietta et al. described symptoms > 3 months for hospitalized COVID-19 patients, but did not further analyze the prevalence over time [14]. The meta-analysis by Alkodaymi et al. presented symptom prevalence over time, but did not stratify by hospitalized and non-hospitalized COVID-19 patients [11].
There are also some limitations. First, there was large heterogeneity in the prevalence estimates and hence the pooled prevalence should be interpreted with caution. There could be differences in the assessment or reporting of symptoms which contributed to the heterogeneity between studies. Second, some sequelae had a small sample size so that the pooled estimate may not be generalized. Third, clinical symptoms may have various diagnostic standards and symptoms such as cough and chest pain were mainly self-reported [20, 25, 29–31, 36, 38, 40–43, 46, 47, 51–58, 60, 62, 74, 80, 81, 86, 88, 89, 91], as healthcare resources were already stretched coping with the evolving pandemic. Finally, the excess burden of each sequela compared to the general population was not quantified which were available from a limited number of controlled cohort studies only.
This systematic review and meta-analysis indicated that post-COVID symptoms are common in hospital discharged patients. A noticeable proportion of discharged COVID-19 patients still reported fatigue and somnipathy after 1 year of follow-up. Therefore, it is imperative to develop and implement effective preventive and rehabilitative measures for hospitalized COVID-19 patients and COVID-19 survivors after discharge, such as inhibiting viral replication, blocking the inflammatory response and early preventive treatment. Furthermore, post-discharge monitoring is essential to monitor the progress of these sequelae and take treatment measures timely. A recent study suggested community rehabilitative approach in COVID-19 survivors stratified by different clinical severity [161]. In view of the enormous populations, patients with mild severity should be monitored with digital interventions, while moderate and severe patients should have regular follow-up and rehabilitation sessions. Additionally, the mental health of COVID-19 recovered patients is deeply influenced because of social isolation, the psychological impact of new and potentially fatal diseases, fear of infecting others and stigma [126]. Therefore, continuous psychological evaluation after hospital discharge is necessary. Individuals who have depression, anxiety, or PTSD may need psychological intervention from an occupational therapist, social worker, or rehabilitation psychologist [162]. Moreover, because recovered COVID-19 patients often experience weight loss and malnutrition, diagnosis, prevention and treatment of malnutrition should be considered in the previously hospitalized COVID-19 patient management strategy to improve short- and long-term outcomes, as suggested by the European Society of Enteral and Parenteral Nutrition (ESPEN) [163]. Meanwhile, there are still limited therapeutic options for COVID-19 infections and long COVID-19, preventive measures such as vaccinations, masks, personal hygiene and social distancing should be stressed [164, 165].
Conclusion
Post-COVID symptoms affected some of the hospital discharged patients persistently for around 1 year, such as fatigue (26.2%), somnipathy (15.1%), anxiety (12.9%), dyspnea (13.1%), arthralgia (11.5%), depression (12.6%), alopecia (10.5%), etc. A multidisciplinary approach is required to develop preventive measures, rehabilitation techniques and clinical management strategies to reduce the impact of COVID-19 sequelae on patients’ lives.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by AIR@InnoHK administered by Innovation and Technology Commission.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval is not required for this systematic review and meta-analysis as only secondary analysis of data already available in scientific databases will be conducted.
Footnotes
Tianqi Yang and Michael Zhipeng Yan shared co-first authors.
References
- 1.World Health Organization. WHO coronavirus (COVID-19) Dashboard [internet]. [2022-04-01]. https://covid19.who.int/. [PubMed]
- 2.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iqbal FM, Lam K, Sounderajah V, Clarke JM, Ashrafian H, Darzi A. Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. EClinicalMedicine. 2021;1:100899. doi: 10.1016/j.eclinm.2021.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez-Ramirez DC, Normand K, Zhaoyun Y, Torres-Castro R. Long-term impact of COVID-19: a systematic review of the literature and meta-analysis. Biomedicines. 2021;9:900. doi: 10.3390/biomedicines9080900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michelen M, Manoharan L, Elkheir N, Cheng V, Dagens A, Hastie C, O'Hara M, Suett J, Dahmash D, Bugaeva P, Rigby I. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021;6:e005427. doi: 10.1136/bmjgh-2021-005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceban F, Ling S, Lui LM, Lee Y, Gill H, Teopiz KM, Rodrigues NB, Subramaniapillai M, Di Vincenzo JD, Cao B, Lin K. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immunity. 2021 doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Premraj L, Kannapadi NV, Briggs J, Seal SM, Battaglini D, Fanning J, Suen J, Robba C, Fraser J, Cho SM. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. 2022;15:120162. doi: 10.1016/j.jns.2022.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández-de-las-Peñas C, Navarro-Santana M, Gómez-Mayordomo V, Cuadrado ML, García-Azorín D, Arendt-Nielsen L, Plaza-Manzano G. Headache as an acute and post-COVID-19 symptom in COVID-19 survivors: a meta-analysis of the current literature. Eur J Neurol. 2021;28:3820–3825. doi: 10.1111/ene.15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-de-Las-Peñas C, Navarro-Santana M, Plaza-Manzano G, Palacios-Ceña D, Arendt-Nielsen L. Time course prevalence of post-COVID pain symptoms of musculoskeletal origin in patients who had survived to SARS-CoV-2 infection: a systematic review and meta-analysis. Pain. 2021 doi: 10.1097/j.pain.0000000000002496. [DOI] [PubMed] [Google Scholar]
- 11.Alkodaymi MS, Omrani OA, Fawzy NA, Abou Shaar B, Almamlouk R, Riaz M, Obeidat M, Obeidat Y, Gerberi D, Taha RM, Kashour Z. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect. 2022 doi: 10.1016/j.cmi.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behnood SA, Shafran R, Bennett SD, Zhang AX, O'Mahoney LL, Stephenson TJ, Ladhani SN, DeStavola BL, Viner RM, Swann OV. Persistent symptoms following SARS-CoV-2 infection among children and young people: a meta-analysis of controlled and uncontrolled studies. J Infect. 2021 doi: 10.1016/j.jinf.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Q, Zheng B, Daines L, Sheikh A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. 2022;11:269. doi: 10.3390/pathogens11020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maglietta G, Diodati F, Puntoni M, Lazzarelli S, Marcomini B, Patrizi L, Caminiti C. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390/jcm11061541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao S, Benzouak T, Gunpat S, Burns RJ, Tahir TA, Jolles S, Kisely S. Fatigue symptoms associated with COVID-19 in convalescent or recovered COVID-19 patients; a systematic review and meta-analysis. Ann Behav Med. 2022;56:219–234. doi: 10.1093/abm/kaab081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1.
- 18.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www3.med.unipmn.it/dispense_ebm/2009-2010/Corso%20Perfezionamento%20EBM_Faggiano/NOS_oxford.pdf.
- 19.Wang N. How to conduct a meta-analysis of proportions in R: a comprehensive tutorial. John Jay College of Criminal Justice, New York, 0-62. 2018. 10.13140/RG.2.2.27199.00161
- 20.Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, Dong W. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández-de-Las-Peñas C, Guijarro C, Plaza-Canteli S, Hernández-Barrera V, Torres-Macho J. Prevalence of post-COVID-19 cough one year after SARS-CoV-2 infection: a multicenter study. Lung. 2021;199:249–253. doi: 10.1007/s00408-021-00450-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández-de-Las-Peñas C, Pellicer-Valero OJ, Navarro-Pardo E, Palacios-Ceña D, Florencio LL, Guijarro C, Martín-Guerrero JD. Symptoms experienced at the acute phase of SARS-CoV-2 infection as risk factor of long-term post-COVID symptoms: the LONG-COVID-EXP-CM multicenter study. Int J Infect Dis. 2022 doi: 10.1016/j.ijid.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-de-La-Speñas C, de-la-Llave-Rincón AI, Ortega-Santiago R, Ambite-Quesada S, Gómez-Mayordomo V, Cuadrado ML, Arias-Navalón JA, Hernández-Barrera V, Martín-Guerrero JD, Pellicer-Valero OJ, Arendt-Nielsen L. Prevalence and risk factors of musculoskeletal pain symptoms as long-term post-COVID sequelae in hospitalized COVID-19 survivors: a multicenter study. Pain. 2021;1:5–89. doi: 10.1097/j.pain.0000000000002564. [DOI] [PubMed] [Google Scholar]
- 24.Fernández-de-Las-Peñas C, Martín-Guerrero JD, Cancela-Cilleruelo I, Moro-López-Menchero P, Rodríguez-Jiménez J, Pellicer-Valero OJ. Trajectory curves of post-COVID anxiety/depressive symptoms and sleep quality in previously hospitalized COVID-19 survivors: the LONG-COVID-EXP-CM multicenter study. Psychol Med. 2022;10:1–2. doi: 10.1017/S003329172200006X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellan M, Baricich A, Patrucco F, Zeppegno P, Gramaglia C, Balbo PE, Carriero A, Amico CS, Avanzi GC, Barini M, Battaglia M. Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19. Sci Rep. 2021;11:1. doi: 10.1038/s41598-021-01215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero-Duarte Á, Rivera-Izquierdo M, de Alba IG, Pérez-Contreras M, Fernández-Martínez NF, Ruiz-Montero R, Serrano-Ortiz Á, González-Serna RO, Salcedo-Leal I, Jiménez-Mejías E, Cárdenas-Cruz A. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: the ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021;19:1–3. doi: 10.1186/s12916-021-02003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simani L, Ramezani M, Darazam IA, Sagharichi M, Aalipour MA, Ghorbani F, Pakdaman H. Prevalence and correlates of chronic fatigue syndrome and post-traumatic stress disorder after the outbreak of the COVID-19. J Neurovirol. 2021;27:154–159. doi: 10.1007/s13365-021-00949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mei Q, Wang F, Yang Y, Hu G, Guo S, Zhang Q, Bryant A, Zhang L, Kurts C, Wei L, Yuan X. Health issues and immunological assessment related to Wuhan's COVID-19 survivors: a multicenter follow-up study. Front Med. 2021;7:487. doi: 10.3389/fmed.2021.617689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu G, Zhen Q, Wang W, Fan S, Wu Q, Zhang C, Li B, Liu G, Yu Y, Li Y, Yong L. Health-related quality of life of COVID-19 patients after discharge: a multicenter follow-up study. J Clin Nurs. 2021;30:1742–1750. doi: 10.1111/jocn.15733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shang YF, Liu T, Yu JN, Xu XR, Zahid KR, Wei YC, Wang XH, Zhou FL. Half-year follow-up of patients recovering from severe COVID-19: analysis of symptoms and their risk factors. J Intern Med. 2021 doi: 10.1111/joim.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suárez-Robles M, del Rosario Iguaran-Bermúdez M, García-Klepizg JL, Lorenzo-Villalba N, Méndez-Bailón M. Ninety days post-hospitalization evaluation of residual COVID-19 symptoms through a phone call check list. Pan Afr Med J. 2020;37:1–89. doi: 10.11604/pamj.2020.37.289.27110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarsitani L, Vassalini P, Koukopoulos A, Borrazzo C, Alessi F, Di Nicolantonio C, Serra R, Alessandri F, Ceccarelli G, Mastroianni CM, d’Ettorre G. Post-traumatic stress disorder among COVID-19 survivors at 3-month follow-up after hospital discharge. J Gen Intern Med. 2021;36:1702–1707. doi: 10.1007/s11606-021-06731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Froidure A, Mahsouli A, Liistro G, De Greef J, Belkhir L, Gérard L, Bertrand A, Koenig S, Pothen L, Yildiz H, Mwenge B. Integrative respiratory follow-up of severe COVID-19 reveals common functional and lung imaging sequelae. Respir Med. 2021;1:106383. doi: 10.1016/j.rmed.2021.106383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morin L, Savale L, Pham T, Colle R, Figueiredo S, Harrois A, Gasnier M, Lecoq AL, Meyrignac O, Noel N, Baudry E. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayoubkhani D, Khunti K, Nafilyan V, Maddox T, Humberstone B, Diamond I, Banerjee A. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021 doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crooks MG. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113–119. doi: 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnold DT, Hamilton FW, Milne A, Morley AJ, Viner J, Attwood M, Noel A, Gunning S, Hatrick J, Hamilton S, Elvers KT. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021;76:399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garrigues E, Janvier P, Kherabi Y, Le Bot A, Hamon A, Gouze H, Doucet L, Berkani S, Oliosi E, Mallart E, Corre F. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caruso D, Guido G, Zerunian M, Polidori T, Lucertini E, Pucciarelli F, Polici M, Rucci C, Bracci B, Nicolai M, Cremona A. Postacute sequelae of COVID-19 pneumonia: 6-month chest CT follow-up. Radiology. 2021;27:210834. doi: 10.1148/radiol.2021210834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castro V, Rosand J, Giacino J, McCoy T, Perlis RH. Case-control study of neuropsychiatric symptoms following COVID-19 hospitalization in 2 academic health systems. medRxiv. 2021 doi: 10.1101/2021.07.09.21252353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darcis G, Bouquegneau A, Maes N, Thys M, Henket M, Labye F, Rousseau AF, Canivet P, Desir C, Calmes D, Schils R. Long-term clinical follow-up of patients suffering from moderate-to-severe COVID-19 infection: a monocentric prospective observational cohort study. Int J Infect Dis. 2021;1:209–216. doi: 10.1016/j.ijid.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.González-Hermosillo JA, Martínez-López JP, Carrillo-Lampón SA, Ruiz-Ojeda D, Herrera-Ramírez S, Amezcua-Guerra LM, Martínez-Alvarado MD. Post-acute COVID-19 symptoms, a potential link with myalgic encephalomyelitis/chronic fatigue syndrome: a 6-month survey in a Mexican cohort. Brain Sci. 2021;11:760. doi: 10.3390/brainsci11060760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gramaglia C, Gambaro E, Bellan M, Balbo PE, Baricich A, Sainaghi PP, Pirisi M, Baldon G, Battistini S, Binda V, Feggi A. Mid-term psychiatric outcomes of patients recovered from COVID-19 from an Italian cohort of hospitalized patients. Front Psychiatry. 2021 doi: 10.3389/fpsyt.2021.667385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bozzetti S, Ferrari S, Zanzoni S, Alberti D, Braggio M, Carta S, Piraino F, Gabbiani D, Girelli D, Nocini R, Monaco S. Neurological symptoms and axonal damage in COVID-19 survivors: are there sequelae? Immunol Res. 2021;7:1–5. doi: 10.1007/s12026-021-09220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maestre-Muñiz MM, Arias Á, Mata-Vázquez E, Martín-Toledano M, López-Larramona G, Ruiz-Chicote AM, Nieto-Sandoval B, Lucendo AJ. Long-term outcomes of patients with coronavirus disease 2019 at one year after hospital discharge. J Clin Med. 2021;10:2945. doi: 10.3390/jcm10132945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vincent A, Beck K, Becker C, Zumbrunn S, Ramin-Wright M, Urben T, Quinto A, Schaefert R, Meinlschmidt G, Gaab J, Reinhardt T. Psychological burden in patients with COVID-19 and their relatives 90 days after hospitalization: a prospective observational cohort study. J Psychosom Res. 2021;1:110526. doi: 10.1016/j.jpsychores.2021.110526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun LL, Wang J, Wang YS, Hu PF, Zhao ZQ, Chen W, Ning BF, Yin C, Hao YS, Wang Q, Wang C. Symptomatic features and prognosis of 932 hospitalized patients with coronavirus disease 2019 in Wuhan. J Dig Dis. 2021;22:271–281. doi: 10.1111/1751-2980.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vlake JH, Wesselius S, van Genderen ME, van Bommel J, Boxma-de Klerk B, Wils EJ. Psychological distress and health-related quality of life in patients after hospitalization during the COVID-19 pandemic: a single-center, observational study. PLoS ONE. 2021;16:e0255774. doi: 10.1371/journal.pone.0255774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, Hu P, Guo L, Liu M, Xu J, Zhang X. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. The Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Shen C, Wang L, Majumder S, Zhang D, Deen MJ, Li Y, Qing L, Zhang Y, Chen C, Zou R. Pulmonary fibrosis and its related factors in discharged patients with new corona virus pneumonia: a cohort study. Respir Res. 2021;22:1. doi: 10.1186/s12931-021-01798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sibila O, Albacar N, Perea L, Faner R, Torralba Y, Hernandez-Gonzalez F, Moisés J, Sanchez-Ruano N, Sequeira-Aymar E, Badia JR, Agusti A. Lung function sequelae in COVID-19 patients 3 months after hospital discharge. Arch Bronconeumol. 2021 doi: 10.1016/j.arbres.2021.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu T, Wu D, Yan W, Wang X, Zhang X, Ma K, Chen H, Zeng Z, Qin Y, Wang H, Xing M. Twelve-month systemic consequences of COVID-19 in patients discharged from hospital: a prospective cohort study in Wuhan, China. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lombardo MD, Foppiani A, Peretti GM, Mangiavini L, Battezzati A, Bertoli S, Martinelli Boneschi F, Zuccotti GV. Long term COVID-19 complications in inpatients and outpatients: a one-year follow up cohort study. SSRN 3814761. 2021. https://ssrn.com/abstract=3814761. [DOI] [PMC free article] [PubMed]
- 55.Maestrini V, Birtolo LI, Francone M, Galardo G, Galea N, Severino P, Alessandri F, Colaiacomo MC, Cundari G, Chimenti C, Lavalle C. Cardiac involvement in consecutive unselected hospitalized COVID-19 population: in-hospital evaluation and one-year follow-up. Int J Cardiol. 2021;15:235–242. doi: 10.1016/j.ijcard.2021.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sigfrid L, Drake TM, Pauley E, Jesudason EC, Olliaro P, Lim WS, Gillesen A, Berry C, Lowe DJ, McPeake J, Lone N. Long Covid in adults discharged from UK hospitals after Covid-19: a prospective, multicentre cohort study using the ISARIC WHO clinical characterisation protocol. medRxiv. 2021 doi: 10.1101/2021.03.18.21253888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmed GK, Khedr EM, Hamad DA, Meshref TS, Hashem MM, Aly MM. Long term impact of COVID-19 infection on sleep and mental health: a cross-sectional study. Psychiatry Res. 2021;1:114243. doi: 10.1016/j.psychres.2021.114243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anjana NK, Annie TT, Siba S, Meenu MS, Chintha S, Anish TS. Manifestations and risk factors of post COVID syndrome among COVID-19 patients presented with minimal symptoms—a study from Kerala, India. J Fam Med Prim Care. 2021;10:4023–4029. doi: 10.4103/jfmpc.jfmpc_851_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bai F, Tomasoni D, Falcinella C, Barbanotti D, Castoldi R, Mulè G, Augello M, Mondatore D, Allegrini M, Cona A, Tesoro D. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 2022;28:611-e9. doi: 10.1016/j.cmi.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen X, Li Y, Shao TR, Yang LL, Li SJ, Wang XJ, Li A, Wu YY, Liu XF, Liu CM, Liu YH. Some characteristics of clinical sequelae of COVID-19 survivors from Wuhan, China: a multi-center longitudinal study. Influenza Other Respir Viruses. 2022;16:395–401. doi: 10.1111/irv.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Damiano RF, Caruso MJ, Cincoto AV, de Almeida Rocca CC, de Pádua SA, Bacchi P, Guedes BF, Brunoni AR, Pan PM, Nitrini R, Beach S. Post-COVID-19 psychiatric and cognitive morbidity: preliminary findings from a Brazilian cohort study. Gen Hosp Psychiatry. 2022 doi: 10.1016/j.genhosppsych.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Philippine EL, Tardivon C, Martin-Blondel G, Isnard M, Le Turnier P, Le Marechal M, Cabié A, Launay O, Tattevin P, Senneville E, Ansart S. Severity of self-reported symptoms and psychological burden 6-months after hospital admission for COVID-19: a prospective cohort study. Int J Infect Dis. 2021;1:247–253. doi: 10.1016/j.ijid.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evans RA, McAuley H, Harrison EM, Shikotra A, Singapuri A, Sereno M, Elneima O, Docherty AB, Lone NI, Leavy OC, Daines L. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9:1275–1287. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gamberini L, Mazzoli CA, Prediletto I, Sintonen H, Scaramuzzo G, Allegri D, Colombo D, Tonetti T, Zani G, Capozzi C, Dalpiaz G. Health-related quality of life profiles, trajectories, persistent symptoms and pulmonary function one year after ICU discharge in invasively ventilated COVID-19 patients, a prospective follow-up study. Respir Med. 2021;189:106665. doi: 10.1016/j.rmed.2021.106665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.García-Abellán J, Padilla S, Fernández-González M, García JA, Agulló V, Andreo M, Ruiz S, Galiana A, Gutiérrez F, Masiá M. Antibody response to SARS-CoV-2 is associated with long-term clinical outcome in patients with COVID-19: a longitudinal study. J Clin Immunol. 2021;41:1490–1501. doi: 10.1007/s10875-021-01083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghosn J, Piroth L, Epaulard O, Le Turnier P, Mentré F, Bachelet D, Laouénan C. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect. 2021;27:1041-e1. doi: 10.1016/j.cmi.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hodgson CL, Higgins AM, Bailey MJ, Mather AM, Beach L, Bellomo R, Bissett B, Boden IJ, Bradley S, Burrell A, Cooper DJ. The impact of COVID-19 critical illness on new disability, functional outcomes and return to work at 6 months: a prospective cohort study. Crit Care. 2021;25:1–2. doi: 10.1186/s13054-021-03794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horwitz LI, Garry K, Prete AM, Sharma S, Mendoza F, Kahan T, Karpel H, Duan E, Hochman KA, Weerahandi H. Six-month outcomes in patients hospitalized with severe COVID-19. J Gen Intern Med. 2021;36:3772–3777. doi: 10.1007/s11606-021-07032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang L, Xu X, Zhang L, Zheng D, Liu Y, Feng B, Hu J, Lin Q, Xi X, Wang Q, Lin M. Post-traumatic stress disorder symptoms and quality of life of COVID-19 survivors at 6-month follow-up: a cross-sectional observational study. Front Psychiatry. 2021 doi: 10.3389/fpsyt.2021.782478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huarcaya-Victoria J, Barreto J, Aire L, Podestá A, Caqui M, Guija-Igreda R, Castillo C, Alarcon-Ruiz CA. Mental health in COVID-2019 survivors from a general hospital in Peru: sociodemographic, clinical, and inflammatory variable associations. Int J Ment Heal Addict. 2021;28:1–22. doi: 10.1007/s11469-021-00659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karaarslan F, Güneri FD, Kardeş S. Long COVID: rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin Rheumatol. 2022;41:289–296. doi: 10.1007/s10067-021-05942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boglione L, Meli G, Poletti F, Rostagno R, Moglia R, Cantone M, Esposito M, Scianguetta C, Domenicale B, Di Pasquale F, Borrè S. Risk factors and incidence of long-COVID syndrome in hospitalized patients: does remdesivir have a protective effect? QJM Int J Med. 2021;114:865–871. doi: 10.1093/qjmed/hcab297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chand S, Kapoor S, Naqvi A, Thakkar J, Fazzari MJ, Orsi D, Dieiev V, Lewandowski DC, Dicpinigaitis PV. Long-term follow up of renal and other acute organ failure in survivors of critical illness due to covid-19. J Intensive Care Med. 2021 doi: 10.1177/08850666211062582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar J, Makheja K, Rahul FN, Kumar S, Kumar M, Chand M, Kammawal Y, Khalid D, Jahangir M, Bachani P. Long-term neurological impact of COVID-19. Cureus. 2021 doi: 10.7759/cureus.18131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mei Z, Wu X, Zhang X, Zheng X, Li W, Fan R, Yu H, Zhang S, Gu Y, Wang X, Xia Y. The occurrence and risk factors associated with post-traumatic stress disorder among discharged COVID-19 patients in Tianjin, China. Brain Behav. 2022;5:1–2492. doi: 10.1002/brb3.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nesan GS, Keerthana D, Yamini R, Jain T, Kumar D, Eashwer A, Maiya GR. 3-Month symptom-based ambidirectional follow-up study among recovered COVID-19 patients from a tertiary care hospital using telehealth in Chennai, India. Inquiry J Health Care Org Provis Financ. 2021 doi: 10.1177/00469580211060165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ong SW, Fong SW, Young BE, Chan YH, Lee B, Amrun SN, Chee RS, Yeo NK, Tambyah P, Pada S, Tan SY. Persistent symptoms and association with inflammatory cytokine signatures in recovered coronavirus disease 2019 patients. In: Open forum infectious diseases, vol 8, no 6, p. ofab156. Oxford: Oxford University Press; 2021 [DOI] [PMC free article] [PubMed]
- 78.Rass V, Beer R, Schiefecker AJ, Kofler M, Lindner A, Mahlknecht P, Heim B, Limmert V, Sahanic S, Pizzini A, Sonnweber T. Neurological outcome and quality of life 3 months after COVID-19: a prospective observational cohort study. Eur J Neurol. 2021;28:3348–3359. doi: 10.1111/ene.14803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Righi E, Mirandola M, Mazzaferri F, Dossi G, Razzaboni E, Zaffagnini A, Ivaldi F, Visentin A, Lambertenghi L, Arena C, Micheletto C. Determinants of persistence of symptoms and impact on physical and mental wellbeing in long COVID: a prospective cohort study. J Infect. 2022 doi: 10.1016/j.jinf.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rivera-Izquierdo M, Láinez-Ramos-Bossini AJ, de Alba IG, Ortiz-González-Serna R, Serrano-Ortiz Á, Fernández-Martínez NF, Ruiz-Montero R, Cervilla JA. Long COVID 12 months after discharge: persistent symptoms in patients hospitalised due to COVID-19 and patients hospitalised due to other causes—a multicentre cohort study. BMC Med. 2022;20:1. doi: 10.1186/s12916-022-02292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sibila O, Perea L, Albacar N, Moisés J, Cruz T, Mendoza N, Solarat B, Lledó G, Espinosa G, Barberà JA, Badia JR. Elevated plasma levels of epithelial and endothelial cell markers in COVID-19 survivors with reduced lung diffusing capacity six months after hospital discharge. Respir Res. 2022;23:1. doi: 10.1186/s12931-022-01955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Staudt A, Jörres RA, Hinterberger T, Lehnen N, Loew T, Budweiser S. Associations of Post-Acute COVID syndrome with physiological and clinical measures 10 months after hospitalization in patients of the first wave. Eur J Intern Med. 2022;1:50–60. doi: 10.1016/j.ejim.2021.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tessitore E, Handgraaf S, Poncet A, Achard M, Höfer S, Carballo S, Marti C, Follonier C, Girardin F, Mach F, Carballo D. Symptoms and quality of life at 1-year follow up of patients discharged after an acute COVID-19 episode. Swiss Med Wkly. 2021 doi: 10.4414/smw.2021.w30093. [DOI] [PubMed] [Google Scholar]
- 84.Tleyjeh IM, Saddik B, Ramakrishnan RK, AlSwaidan N, AlAnazi A, Alhazmi D, Aloufi A, AlSumait F, Berbari EF, Halwani R. Long term predictors of breathlessness, exercise intolerance, chronic fatigue and well-being in hospitalized patients with COVID-19: a cohort study with 4 months median follow-up. J Infect Public Health. 2022;15:21–28. doi: 10.1016/j.jiph.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Torres-Ruiz J, Lomelín-Gascón J, Lira-Luna J, Pérez-Fragoso A, Tapia-Conyer R, Nuñez-Aguirre M, Alcalá-Carmona B, Absalón-Aguilar A, Maravillas-Montero JL, Mejía-Domínguez NR, Núñez-Álvarez C. FANSY POSTCOV: a composite clinical immunological predictive index for post-COVID-19 syndrome unveils distinctive features in a cohort study of mild to critical patients. Clin Transl Med. 2021;11:e623. doi: 10.1002/ctm2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vejen M, Hansen EF, Al-Jarah BN, Jensen C, Thaning P, Jeschke KN, Ulrik CS. Hospital admission for COVID-19 pneumonitis–long-term impairment in quality of life and lung function. Eur Clin Respir J. 2022;9:2024735. doi: 10.1080/20018525.2021.2024735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang B, Yang X, Fu L, Hu Y, Luo D, Xiao X, Ju N, Zheng W, Xu H, Fang Y, Chan PS. Post-traumatic stress disorder symptoms in COVID-19 survivors 6 months after hospital discharge: an application of the conservation of resource theory. Front Psychiatry. 2021 doi: 10.3389/fpsyt.2021.773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wong-Chew RM, Rodríguez Cabrera EX, Rodríguez Valdez CA, Lomelin-Gascon J, Morales-Juárez L, de la Cerda ML, Villa-Romero AR, Arce Fernández S, Serratos Fernandez M, Bello HH, Castañeda LM. Symptom cluster analysis of long COVID-19 in patients discharged from the temporary COVID-19 hospital in Mexico City. Ther Adv Infect Dis. 2022;9:20499361211069264. doi: 10.1177/20499361211069264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang X, Wang F, Shen Y, Zhang X, Cen Y, Wang B, Zhao S, Zhou Y, Hu B, Wang M, Liu Y. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open. 2021;4:e2127403. doi: 10.1001/jamanetworkopen.2021.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou F, Tao M, Shang L, Liu Y, Pan G, Jin Y, Wang L, Hu S, Li J, Zhang M, Fu Y. Assessment of sequelae of COVID-19 nearly 1 year after diagnosis. Front Med. 2021 doi: 10.3389/fmed.2021.717194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zuschlag D, Grandt D, Custodis F, Braun C, Häuser W. Spontaneously reported persistent symptoms related to coronavirus disease 2019 one year after hospital discharge. Der Schmerz. 2022;25:1–9. doi: 10.1007/s00482-022-00626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, Lei C, Chen R, Zhong N, Li S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020 doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Venkataraman T, Frieman MB. The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus-induced pulmonary fibrosis. Antiviral Res. 2017;1:142–150. doi: 10.1016/j.antiviral.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frija-Masson J, Debray MP, Gilbert M, Lescure FX, Travert F, Borie R, Khalil A, Crestani B, d'Ortho MP, Bancal C. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J. 2020 doi: 10.1183/13993003.01754-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, Solis-Navarro L, Burgos F, Puppo H, Vilaró J. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2020 doi: 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McGroder CF, Zhang D, Choudhury MA, Salvatore MM, D'Souza BM, Hoffman EA, Wei Y, Baldwin MR, Garcia CK. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax. 2021 doi: 10.1136/thoraxjnl-2021-217031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y, Dong C, Hu Y, Li C, Ren Q, Zhang X, Shi H, Zhou M. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020;296:E55–64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barisione E, Grillo F, Ball L, Bianchi R, Grosso M, Morbini P, Pelosi P, Patroniti NA, De Lucia A, Orengo G, Gratarola A. Fibrotic progression and radiologic correlation in matched lung samples from COVID-19 post-mortems. Virchows Arch. 2021;478:471–485. doi: 10.1007/s00428-020-02934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang W, Wu Q, Chen Z, Xiong Z, Wang K, Tian J, Zhang S. The potential indicators for pulmonary fibrosis in survivors of severe COVID-19. J Infect. 2021;82:e5–7. doi: 10.1016/j.jinf.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua bing li xue za zhi Chin J Pathol. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 102.Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis. 2021;53:737–754. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Phetsouphanh C, Darley DR, Wilson DB, Howe A, Munier C, Patel SK, Juno JA, Burrell LM, Kent SJ, Dore GJ, Kelleher AD. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022;13:1–7. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- 104.Zhao YM, Shang YM, Song WB, Li QQ, Xie H, Xu QF, Jia JL, Li LM, Mao HL, Zhou XM, Luo H. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;1:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.González J, Benítez ID, Carmona P, Santisteve S, Monge A, Moncusí-Moix A, Gort-Paniello C, Pinilla L, Carratalá A, Zuil M, Ferrer R. Pulmonary function and radiologic features in survivors of critical COVID-19: a 3-month prospective cohort. Chest. 2021;160:187–198. doi: 10.1016/j.chest.2021.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frija-Masson J, Debray MP, Boussouar S, Khalil A, Bancal C, Motiejunaite J, Galarza-Jimenez MA, Benzaquen H, Penaud D, Laveneziana P, Malrin R. Residual ground glass opacities three months after covid-19 pneumonia correlate to alteration of respiratory function: the post Covid M3 study. Respir Med. 2021;1:106435. doi: 10.1016/j.rmed.2021.106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maccio U, Zinkernagel AS, Schuepbach R, Probst-Mueller E, Frontzek K, Brugger SD, Hofmaenner DA, Moch H, Varga Z. Long-term persisting SARS-CoV-2 RNA and pathological findings: lessons learnt from a series of 35 COVID-19 autopsies. Front Med. 2022 doi: 10.3389/fmed.2022.778489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haffke M, Freitag H, Rudolf G, Seifert M, Doehner W, Scherbakov N, Hanitsch L, Wittke K, Bauer S, Konietschke F, Paul F. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS) J Transl Med. 2022;20:1–1. doi: 10.1186/s12967-022-03346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vianello A, Guarnieri G, Braccioni F, Lococo S, Molena B, Cecchetto A, Giraudo C, De Marchi LB, Caminati M, Senna G. The pathogenesis, epidemiology and biomarkers of susceptibility of pulmonary fibrosis in COVID-19 survivors. Clin Chem Lab Med (CCLM) 2022;60:307–316. doi: 10.1515/cclm-2021-1021. [DOI] [PubMed] [Google Scholar]
- 110.Fernández-de-Las-Peñas C, Ryan-Murua P, Rodríguez-Jiménez J, Palacios-Ceña M, Arendt-Nielsen L, Torres-Macho J. Serological biomarkers at hospital admission are not related to long-term post-COVID fatigue and dyspnea in COVID-19 survivors. Respiration. 2022;5:1–8. doi: 10.1159/000524042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheung OY, Chan JW, Ng CK, Koo CK. The spectrum of pathological changes in severe acute respiratory syndrome (SARS) Histopathology. 2004;45:119–124. doi: 10.1111/j.1365-2559.2004.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Helding L, Carroll TL, Nix J, Johns MM, LeBorgne WD, Meyer D. COVID-19 after effects: concerns for singers. J Voice. 2020 doi: 10.1016/j.jvoice.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu K, Zhang W, Yang Y, Zhang J, Li Y, Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: a randomized controlled study. Complement Ther Clin Pract. 2020;1:101166. doi: 10.1016/j.ctcp.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ali RM, Ghonimy MB. Post-COVID-19 pneumonia lung fibrosis: a worrisome sequelae in surviving patients. Egypt J Radiol Nucl Med. 2021;52:1–8. doi: 10.1186/s43055-021-00484-3. [DOI] [Google Scholar]
- 115.Stam H, Stucki G, Bickenbach J. Covid-19 and post intensive care syndrome: a call for action. J Rehabil Med. 2020 doi: 10.2340/16501977-2677. [DOI] [PubMed] [Google Scholar]
- 116.Funke-Chambour M, Bridevaux PO, Clarenbach CF, Soccal PM, Nicod LP, von Garnier C. Swiss recommendations for the follow-up and treatment of pulmonary long COVID. Respiration. 2021;100:826–841. doi: 10.1159/000517255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.British Thoracic Society. Guidance on respiratory follow up of patients with a clinical radiological diagnosis of COVID-19 pneumonia. British Thoracic Society. 2020. https://www.brit-thoracic.org.uk.
- 118.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mitrani RD, Dabas N, Goldberger JJ. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020;17:1984–1990. doi: 10.1016/j.hrthm.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gąsior M, Jaroszewicz J, Wita K, Cieśla D, Hudzik B. High post-discharge mortality in hospitalized COVID-19 patients with cardiovascular comorbidities. Pol Arch Intern Med. 2021 doi: 10.20452/pamw.16026. [DOI] [PubMed] [Google Scholar]
- 121.Lavery AM, Preston LE, Ko JY, Chevinsky JR, DeSisto CL, Pennington AF, Kompaniyets L, Datta SD, Click ES, Golden T, Goodman AB. Characteristics of hospitalized COVID-19 patients discharged and experiencing same-hospital readmission—United States, March–August 2020. Morbid Mortal Wkly Rep. 2020;69:1695. doi: 10.15585/mmwr.mm6945e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174:576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O'Sullivan JS, Lyne A, Vaughan CJ. COVID-19-induced postural orthostatic tachycardia syndrome treated with ivabradine. BMJ Case Rep CP. 2021;14:e243585. doi: 10.1136/bcr-2021-243585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res. 2021;69:205–211. doi: 10.1007/s12026-021-09191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goldstein DS. The possible association between COVID-19 and postural tachycardia syndrome. Heart Rhythm. 2021 doi: 10.1016/j.hrthm.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, Zandi MS, Lewis G, David AS. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sun B, Tang N, Peluso MJ, Iyer NS, Torres L, Donatelli JL, Munter SE, Nixon CC, Rutishauser RL, Rodriguez-Barraquer I, Greenhouse B. Characterization and biomarker analyses of post-COVID-19 complications and neurological manifestations. Cells. 2021;10:386. doi: 10.3390/cells10020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Olanipekun T, Abe T, Effoe V, Westney G, Snyder R. Incidence and severity of depression among recovered African Americans with COVID-19-associated respiratory failure. J Racial Ethn Health Disparities. 2021;6:1–6. doi: 10.1007/s40615-021-01034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cai X, Hu X, Ekumi IO, Wang J, An Y, Li Z, Yuan B. Psychological distress and its correlates among COVID-19 survivors during early convalescence across age groups. Am J Geriatr Psychiatry. 2020;28:1030–1039. doi: 10.1016/j.jagp.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miners S, Kehoe PG, Love S. Cognitive impact of COVID-19: looking beyond the short term. Alzheimers Res Ther. 2020;12:1–6. doi: 10.1186/s13195-020-00744-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coolen T, Lolli V, Sadeghi N, Rovai A, Trotta N, Taccone FS, Creteur J, Henrard S, Goffard JC, Dewitte O, Naeije G. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020;95:e2016–e2027. doi: 10.1212/WNL.0000000000010116. [DOI] [PubMed] [Google Scholar]
- 132.Stefano GB. Historical insight into infections and disorders associated with neurological and psychiatric sequelae similar to long COVID. Med Sci Monit Int Med J Exp Clin Res. 2021;27:e931447-1. doi: 10.12659/MSM.931447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sollini M, Morbelli S, Ciccarelli M, Cecconi M, Aghemo A, Morelli P, Chiola S, Gelardi F, Chiti A. Long COVID hallmarks on [18F] FDG-PET/CT: a case-control study. Eur J Nucl Med Mol Imaging. 2021;7:1–1. doi: 10.1007/s00259-021-05294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Radnis C, Qiu S, Jhaveri M, Da Silva I, Szewka A, Koffman L. Radiographic and clinical neurologic manifestations of COVID-19 related hypoxemia. J Neurol Sci. 2020;15:117119. doi: 10.1016/j.jns.2020.117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, Dodd SJ, Koretsky AP, Watts JA, Cheung V, Masliah E. Microvascular injury in the brains of patients with Covid-19. N Engl J Med. 2021;384:481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Metlay JP, Fine MJ, Schulz R, Marrie TJ, Coley CM, Kapoor WN, Singer DE. Measuring symptomatic and functional recovery in patients with community-acquired pneumonia. J Gen Intern Med. 1997;12:423–430. doi: 10.1046/j.1525-1497.1997.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morley JE, Kalantar-Zadeh K, Anker SD. COVID-19: a major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle. 2020;11:863–865. doi: 10.1002/jcsm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Patel KP, Patel PA, Vunnam RR, Hewlett AT, Jain R, Jing R, Vunnam SR. Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19. J Clin Virol. 2020;1:104386. doi: 10.1016/j.jcv.2020.104386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Iacobucci G. Long covid: damage to multiple organs presents in young, low risk patients. BMJ Br Med J. 2020 doi: 10.1136/bmj.m4470. [DOI] [Google Scholar]
- 140.Zhong P, Xu J, Yang D, Shen Y, Wang L, Feng Y, Du C, Song Y, Wu C, Hu X, Sun Y. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct Target Ther. 2020;5:1–8. doi: 10.1038/s41392-020-00373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.An YW, Song S, Li WX, Chen YX, Hu XP, Zhao J, Li ZW, Jiang GY, Wang C, Wang JC, Yuan B. Liver function recovery of COVID-19 patients after discharge, a follow-up study. Int J Med Sci. 2021;18:176. doi: 10.7150/ijms.50691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, Villapol S. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021 doi: 10.2139/ssrn.3769978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mieczkowska K, Deutsch A, Borok J, Guzman AK, Fruchter R, Patel P, Wind O, McLellan BN, Mann RE, Halverstam CP. Telogen effluvium: a sequela of COVID-19. Int J Dermatol. 2021 doi: 10.1111/ijd.15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chee YJ, Ng SJ, Yeoh E. Diabetic ketoacidosis precipitated by covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:108166. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suwanwongse K, Shabarek N. Newly diagnosed diabetes mellitus, DKA, and COVID-19: causality or coincidence? A report of three cases. J Med Virol. 2021;93:1150–1153. doi: 10.1002/jmv.26339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Papachristou S, Stamatiou I, Stoian AP, Papanas N. New-onset diabetes in COVID-19: time to frame its fearful symmetry. Diabetes Ther. 2021;12:461–464. doi: 10.1007/s13300-020-00988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Di Filippo L, De Lorenzo R, D'Amico M, Sofia V, Roveri L, Mele R, Saibene A, Rovere-Querini P, Conte C. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: a post-hoc analysis of a prospective cohort study. Clin Nutr. 2021;40:2420–2426. doi: 10.1016/j.clnu.2020.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sansone A, Mollaioli D, Ciocca G, Limoncin E, Colonnello E, Vena W, Jannini EA. Addressing male sexual and reproductive health in the wake of COVID-19 outbreak. J Endocrinol Investig. 2021;44:223–231. doi: 10.1007/s40618-020-01350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mumm JN, Osterman A, Ruzicka M, Stihl C, Vilsmaier T, Munker D, Khatamzas E, Giessen-Jung C, Stief C, Staehler M, Rodler S. Urinary frequency as a possibly overlooked symptom in COVID-19 patients: does SARS-CoV-2 cause viral cystitis? Eur Urol. 2020;78:624–628. doi: 10.1016/j.eururo.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Silva CC, Bichara CN, Carneiro FR, Palacios VR, Van den Berg AV, Quaresma JA, Falcão LF. Muscle dysfunction in the long coronavirus disease 2019 syndrome: pathogenesis and clinical approach. Rev Med Virol. 2019 doi: 10.1002/rmv.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang L, Yang N, Yang J, Zhao S, Su C. A review: the manifestations, mechanisms, and treatments of musculoskeletal pain in patients with COVID-19. Front Pain Res. 2022 doi: 10.3389/fpain.2022.826160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu J, Zhang S, Wu Z, Shang Y, Dong X, Li G, Zhang L, Chen Y, Ye X, Du H, Liu Y. Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Ann Intensive Care. 2020;10:1–21. doi: 10.1186/s13613-020-00706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cheng D, Calderwood C, Skyllberg E, Ainley A. Clinical characteristics and outcomes of adult patients admitted with COVID-19 in East London: a retrospective cohort analysis. BMJ Open Respir Res. 2021;8:e000813. doi: 10.1136/bmjresp-2020-000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fernández-de-las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Florencio LL, Cuadrado ML, Plaza-Manzano G, Navarro-Santana M. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med. 2021 doi: 10.1016/j.ejim.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Canas LS, Sudre CH, Pujol JC, Polidori L, Murray B, Molteni E, Graham MS, Klaser K, Antonelli M, Berry S, Davies R. Early detection of COVID-19 in the UK using self-reported symptoms: a large-scale, prospective, epidemiological surveillance study. Lancet Digit Health. 2021;3:e587–e598. doi: 10.1016/S2589-7500(21)00131-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mackey K, Ayers CK, Kondo KK. Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med. 2021;174:362–373. doi: 10.7326/M20-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu BB, Gu DZ, Yu JN, Yang J, Shen WQ. Association between ABO blood groups and COVID-19 infection, severity and demise: a systematic review and meta-analysis. Infect Genet Evol. 2020 doi: 10.1016/j.meegid.2020.104485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sivan M, Halpin S, Gee J. Assessing long-term rehabilitation needs in COVID-19 survivors using a telephone screening tool (C19-YRS tool) Adv Clin Neurosci Rehabil. 2020;19:14–17. doi: 10.47795/NELE5960. [DOI] [Google Scholar]
- 160.National Health Service United Kingdom. Newcastle post-COVID syndrome follow up screening questionnaire. National Health Service United Kingdom. 2021. https://www.rdash.nhs.uk/wp-content/uploads/2021/03/Newcastle-Post-Covid-Screening-Questionnaire.pdf.
- 161.Yan Z, Yang M, Lai CL. Long covid-19 syndrome: a comprehensive review of its effect on various organ systems and recommendation on rehabilitation plans. Biomedicines. 2021;9:966. doi: 10.3390/biomedicines9080966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sheehy LM. Considerations for postacute rehabilitation for survivors of COVID-19. JMIR Public Health Surveill. 2020;6:e19462. doi: 10.2196/19462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznarić Ž, Nitzan D, Pirlich M, Singer P. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Liječnički vjesnik. 2020;142:75–84. doi: 10.26800/LV-142-3-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yan ZP, Yang M, Lai CL. COVID-19 vaccines: a review of the safety and efficacy of current clinical trials. Pharmaceuticals. 2021;14:406. doi: 10.3390/ph14050406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yan Z, Yang M, Lai CL. COVID-19 vaccinations: a comprehensive review of their safety and efficacy in special populations. Vaccines. 2021;9:1097. doi: 10.3390/vaccines9101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.