Abstract
Wood extractives, commonly referred to as pitch, cause major problems in the manufacturing of pulp and paper. Treatment of nonsterile southern yellow pine chips for 14 days with Pseudomonas fluorescens, Pseudomonas sp., Xanthomonas campestris, and Serratia marcescens reduced wood extractives by as much as 40%. Control treatments receiving only water lost 11% of extractives due to the growth of naturally occurring microorganisms. Control treatments were visually discolored after the 14-day incubation, whereas bacterium-treated wood chips were free of dark staining. Investigations using P. fluorescens NRRL B21432 showed that all individual resin and fatty acid components of the pine wood extractives were substantially reduced. Micromorphological observations showed that bacteria were able to colonize resin canals, ray parenchyma cells, and tracheids. Tracheid pit membranes within bordered pit chambers were degraded after treatment with P. fluorescens NRRL B21432. P. fluorescens and the other bacteria tested appear to have the potential for biological processing to substantially reduce wood extractives in pine wood chips prior to the paper making process so that problems associated with pitch in pulp mills can be controlled.
Extractives in wood are often referred to as pitch, and these substances can consist of resin and fatty acids and other materials that are soluble in neutral, nonpolar organic solvents (17). Wood extractives can be a problem in pulp and paper production, especially mechanical pulping processes, where pitch deposits on paper-making machines result in reduced paper quality (1). Effluents discharged from pulp and paper mills with high concentrations of resin acids, a component of pitch, also may pose serious environmental concerns because of toxicity to fish and other organisms (15).
There are several methods currently used by the pulp and paper industries to reduce the amount of extractives during pulping. They include the application of additives (such as alum, talc, dispersants, and lipase enzymes) to pulp, seasoning of logs or chips, and the use of pitch-degrading fungi that may be applied as a biological treatment to wood before pulping (3, 7, 14). Sapwood-staining fungi, which are responsible for reducing extractives in logs and wood chips when they are seasoned, are considered to be detrimental since they discolor wood, reduce pulp brightness, and lower paper quality. Colorless or albino strains of sapwood-staining fungi have been used with success to treat wood before pulping to reduce pitch and the problems associated with it during the paper-making process (5, 7, 13).
In addition to fungi, some bacteria isolated from paper mill effluents have been shown to degrade resin acids (16, 26). The use of bacteria to lower the extractive content of wood before pulping could reduce pitch-related problems during paper making and lower the resin acid concentrations in effluents. Success using bacteria as a pretreatment before pulping to reduce wood extractives would provide new organisms for use in biological processing by the pulp and paper industries.
Limited information is available on the bacteria that colonize wood chips in pulp mills and their mode of action when they grow in wood. This study was done to determine if bacteria isolated from wood can be used as a treatment on wood chips to reduce wood extractives before pulping and to elucidate how bacteria colonize wood cells during the depitching process.
MATERIALS AND METHODS
Bacterial isolation.
Bacterial isolates were obtained for the initial screening from pine chips (50% Pinus taeda L., 50% Pinus virginiana Mill.) collected from a paper mill in Ashland, Va., and from Abies sp. chips collected from a paper mill in Washington State. Bacterial isolates were obtained by plating wood segments on Difco-Bacto nutrient medium. Colonies were streaked onto nutrient medium and pure cultures were obtained. Bacterial isolates were grown on Sierra medium (15 g of agar, 10 g of peptone, 5 g of NaCl, 0.1 g of CaCl2 · H2O, 10 ml of Tween 80, and 990 ml of distilled deionized water) to screen for lipase activity (2). Five bacterial isolates were selected that exhibited lipase activity. The bacterial isolates were identified by the American Type Culture Collection (Rockville, Md.) and deposited with the Northern Regional Research Laboratory (NRRL) (Peoria, Ill.) as Pseudomonas fluorescens NRRL B21432, Xanthomonas campestris NRRL B21430, and Serratia marcescens NRRL B21429. Two additional isolates not deposited with the NRRL were identified as Pseudomonas sp. strains UM-18 and UM-74. Both of these bacteria are rod shaped, oxidase positive, gram negative, and motile and did not grow at 42°C. Colonies of UM-18 and UM-74 grown on Bacto-Pseudomonas medium F were fluorescent and colonies grown on Bacto-Pseudomonas medium P using UV light were nonfluorescent.
Treatment of chips.
Fresh pine chips (50% Pinus taeda L., 50% Pinus virginiana Mill.) obtained from a paper mill in Ashland, Va., were frozen at −18°C until use. Bacteria used for treatment were grown in Difco-Bacto nutrient broth and incubated at 24°C on an orbital shaker at 30 rpm for 58 h. After 58 h bacteria and nutrient broth were added to sterile centrifuge tubes (Nalgene high-speed polypropylene tubes; 30 by 103 mm, 50-ml capacity) and centrifuged at 6,000 rpm for 5 min. Nutrient broth was then decanted and sterile deionized water was added to bring the total amount of water and cells to 100 ml. Each of four polyethylene bags received 500 g (wet weight) of nonsterile chips with 100 ml of sterile deionized water containing P. fluorescens NRRL B21432 (1.5 × 1010 cells/ml), X. campestris NRRL B21430 (1.6 × 1010 cells/ml), S. marcescens NRRL B21429 (3.3 × 1010 cells/ml), Pseudomonas sp. strain UM-18 (6.6 × 109 cells/ml), or Pseudomonas sp. strain UM-74 (1.4 × 1010 cells/ml). Cell densities were determined by viable cell counts. Also, 100 ml of sterile deionized water with no bacteria was added to each of four bags containing 500 g (wet weight) of nonsterile chips to serve as controls with natural growth of background microorganisms. Immediately after treatment, a sample of chips was removed and moisture content was determined.
P. fluorescens NRRL B21432 was further studied to determine the specific resin and fatty acids removed from the wood. A single loblolly pine tree (Pinus taeda L.) was cut from the Solan Dixon Education Center in Andalusia, Ala., chipped, and frozen at −18°C until use. Wood chips were added to polyethylene bags as previously described. Two replicates of 500 g (wet weight) of nonsterile chips were treated with 100 ml of sterile, deionized water containing P. fluorescens NRRL B21432 (3.0 × 109 cells/ml). One hundred milliliters of sterile deionized water with no bacteria was added to each of two polyethylene bags containing 500 g (wet weight) of nonsterile chips to serve as controls.
Wood extractive assay.
After 14 days of incubation, wood chips were air dried and ground in a Wiley mill to pass through a 40-mesh sieve. Wood extractives were determined from 2-g samples of treated and nontreated wood from each polyethylene bag by extraction with dichloromethane according to standard procedure T 204 om-88 of the Technical Association for the Pulp and Paper Industry, Atlanta, Ga. The amounts of resin and fatty acids present after treatment were determined using standard methods 242D01 and 081D07 (Econotech Services Ltd., Delta, British Columbia, Canada). Dichloromethane extracts from P. fluorescens-treated wood were saponified in ethanolic KOH, adjusted to pH 3, and extracted with diethyl ether. The extracts were concentrated and methylated. To determine the “free” and “total” resin and fatty acids the extract was divided and a portion was analyzed without saponification to give the free resin and fatty acids. To quantify resin and fatty acids, aqueous samples were first acidified to convert resin acid salts to free acids, which were then extracted with diethyl ether. The extracts were concentrated and the free acids were methylated, redissolved in methyl tert-butyl ether, and analyzed by gas chromatography using a capillary column and a flame ionization detector. Nonadecanoic acid was added as a surrogate standard prior to extraction, and margaric acid was added as an internal standard prior to injection. When determining the individual resin acids using gas chromatography, palustric acid coeluted with levopimaric acid and the two were reported in this study as combined levopimaric-palustric acid.
Microscopy.
Treated and nontreated wood chips were collected 7 and 14 days after treatment and fixed in 2% glutaraldehyde (50% biological grade) solution and 0.2 M sodium cacodylate buffer (pH 7.0) for 2 h, rinsed with a 0.1 M sodium calcodylate buffer (pH 7.0) three times for 15 min each, and placed in a solution of 1% osmium tetroxide in 0.2 M sodium cacodylate buffer (pH 7.0) for 90 min. The samples were processed and examined as previously described (27).
RESULTS AND DISCUSSION
Significant differences were observed in extractives from wood chips treated with bacteria compared to control wood used for the experiment (Table 1). P. fluorescens NRRL B21432 and Pseudomonas sp. strain UM-74 significantly reduced the amount of extractives by 40 and 34%, respectively, compared to the fresh chips (Table 1). Other bacteria in this study, including Pseudomonas sp. strain UM-18 and X. campestris NRRL B21430, reduced extractives by 27%, and S. marcescens NRRL B21429 reduced extractives by 22% compared to the extractives in fresh wood chips. Control treatments receiving water and aged for 14 days had an 11% reduction in extractives, compared to the fresh wood chips. This reduction can be attributed to oxidation processes and the growth of naturally occurring microorganisms present on the nonsterile wood during the 14-day incubation period (7).
TABLE 1.
Percentages of wood extractives from bacterium-treated and nontreated southern yellow pine chips incubated for 14 days
| Treatment | % Extractivesa | Comparison group(s)b | % Reduction |
|---|---|---|---|
| Freshc | 4.4 ± 0.78 | A | |
| Control with water | 3.9 ± 1.10 | A,B | 11.4 |
| S. marcescens NRRL B21430 | 3.4 ± 0.33 | B | 22.7 |
| X. campestris NRRL B21429 | 3.2 ± 0.16 | B | 27.3 |
| Pseudomonas sp. strain UM-18 | 3.2 ± 0.25 | B,C | 27.2 |
| Pseudomonas sp. strain UM-74 | 2.9 ± 0.31 | C | 34.1 |
| P. fluorescens NRRL B21432 | 2.6 ± 0.49 | C | 40.9 |
Mean ± standard deviation from four replicates.
Means within groups designated by the same letter were not significantly different at the 0.05% level using Duncan's new multiple-range test.
Represents the amount of extractives at the beginning of the experiment.
Bacteria such as P. fluorescens, X. campestris, and S. marcescens are ubiquitous in nature and have the ability to break down triglycerides, but this is the first report of these bacteria reducing pitch in nonsterile pine chips. To better understand which individual resin and fatty acid fraction of pitch were being degraded, an additional study was done with P. fluorescens NRRL B21432. This isolate was selected because it showed the greatest ability to reduce the overall pitch content (Table 1). In this experiment, total extractives were reduced by 28% and total resin acids were reduced by 25% after nonsterile loblolly pine chips were treated with P. fluorescens NRRL B21432 and compared to the fresh, control wood chips (Table 2). Treating chips with P. fluorescens NRRL B21432 substantially reduced all individual resin acids, compared to the initial amount found in the fresh chips and in chips treated with water (Table 2). Wood chips treated with water and incubated for 14 days showed a slight reduction in individual resin acids. Wood treated with P. fluorescens had greater losses of all individual resin acids than the aged and water control wood chips. The water-treated chips were also severely discolored and isolations from these chips showed that blue-staining Ophiostoma sp. was present. Isolations for bacteria indicated that the bacteria present were not Pseudomonas species (data not shown). Wood chips treated with P. fluorescens NRRL B21432 were visually free of discoloration, but substantial reductions in resin acids were determined. These results demonstrate the capacity of the bacterial treatments to inhibit detrimental blue-stain fungi and degrade greater concentrations of resin acids than the natural microflora that grows on nonsterile wood.
TABLE 2.
Total resin and fatty acids in ether extracts of loblolly pine chips inoculated with P. fluorescens NRRL B21432 and incubated for 14 daysa
| Extractive component | Acid content (mg/g of oven-dried wood)b
|
||
|---|---|---|---|
| Freshc | Control with water | P. fluorescens NRRL B21432 | |
| Resin acids | |||
| Pimaric | 1.71 ± 0.98 | 1.61 ± 0.35 | 1.42 ± 0.01 |
| Sandaracopimaric | 0.15 ± 0.11 | 0.86 ± 0.86 | 0.11 ± 0.00 |
| Isopimaric | 0.38 ± 0.18 | 0.36 ± 0.05 | 0.31 ± 0.03 |
| Levopimaric-palustric | 1.26 ± 0.37 | 1.20 ± 0.04 | 1.11 ± 0.19 |
| Dehydroabietic | 4.47 ± 2.62 | 3.93 ± 0.40 | 3.10 ± 0.17 |
| Abietic | 2.56 ± 0.39 | 2.67 ± 0.22 | 1.76 ± 0.31 |
| Total | 10.56 ± 4.66 | 9.97 ± 0.98 | 7.84 ± 0.72 |
| Fatty acids | |||
| Palmitic | 1.05 ± 0.20 | 0.75 ± 0.17 | 0.63 ± 0.16 |
| Stearic | 0.39 ± 0.00 | 0.32 ± 0.54 | 0.29 ± 0.02 |
| Oleic/linolenic | 1.50 ± 0.54 | 1.34 ± 0.56 | 1.01 ± 0.39 |
| Linoleic | 5.51 ± 2.10 | 5.61 ± 2.28 | 3.44 ± 2.17 |
| Behenic | 0.32 ± 0.20 | 0.27 ± 0.05 | 0.22 ± 0.05 |
| Total | 8.86 ± 2.95 | 8.39 ± 3.05 | 5.60 ± 2.70 |
Total extractives for fresh chips, control with only water added, and wood chips inoculated with P. fluorescens NRRL B21432 were 2.49% ± 0.64%, 2.07% ± 0.05%, and 1.79% ± 0.09%, respectively.
Mean ± standard deviation from two replicates.
Represents the amount of total resin and fatty acids at the beginning of the experiment.
Triglycerides and fatty acids are another component of pitch found in high concentrations within the problematic deposits in pulp mills (8). In our study, the total fatty acid content of extractives was reduced by 36% after nonsterile wood chips were treated with P. fluorescens NRRL B21432, compared to fresh wood chips (Table 2). This reduction in total fatty acids is similar to the results obtained when wood chips are treated with an albino strain of Ophiostoma, and the reduction appears to be sufficient to be of benefit during mechanical paper-making processes (8, 13). Treating wood chips with P. fluorescens NRRL B21432 substantially reduced all individual fatty acids, compared to the initial amount found in fresh wood chips or wood chips treated with water (Table 2). Water-treated chips also showed a slight reduction in most individual fatty acids compared to the initial amount found in the fresh chips. This reduction in both resin and fatty acids may be attributed to the growth of blue-stain fungi on the nonsterile wood during the 14 days of incubation. Blue-stain fungi, if allowed to grow in wood, have been shown to decrease the resin and fatty acid content of extractives in wood (7, 8, 9, 10). Although nonsterile chips treated with water had reduced levels of fatty acids in our study, greater reductions were observed in all fatty acid components without any visual discoloration to the wood chips after treatment with P. fluorescens NRRL B21432.
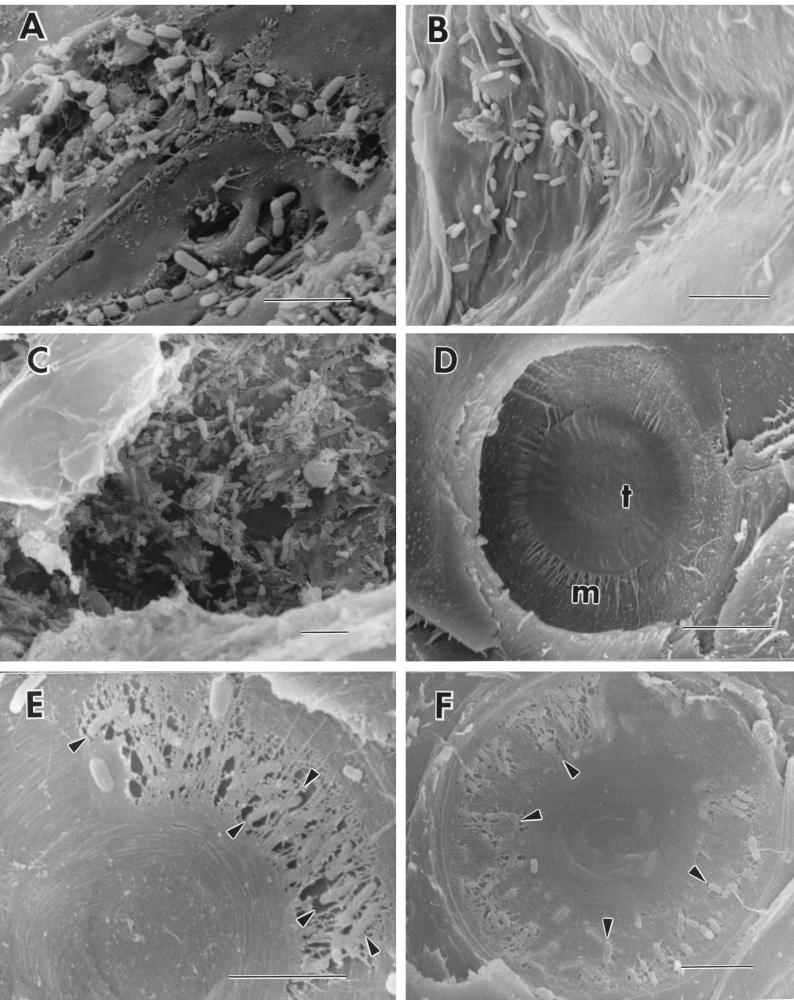
Micromorphological observations of wood treated with bacteria revealed bacteria present inside resin canals, tracheids, and ray parenchyma cells (Fig. 1). Bacteria were most often observed in areas where resin had accumulated on the wood surfaces and within resin canals (Fig. 1A and B). Bacteria also were concentrated on bordered pit membranes within tracheids. Since bacteria were observed in the innermost areas of wood chips, they apparently are able to migrate through the wood cells via the simple and bordered pits. Bordered pit membranes in pine tracheids are made of a nonlignified pectin and a cellulose-rich region in the center of the pit called a torus, which is supported by a surrounding margo that contains microfibrils of cellulose (11, 21). Minute pores less than 0.2 μm in diameter occur in the margo, and these pores restrict microbial movement from cell to cell (11, 18, 21). When wood is cut and air enters the cells, the pit membranes become aspirated and the membrane closes the pit aperture. Permeability of water or other materials through the pit chamber is impeded. For bacteria to be able to migrate into the cells within wood they must first degrade pit membranes or enter through previously degraded or damaged cells. It is known that Pseudomonas species produce a wide array of enzymes that could degrade some of the components in pit membranes (11, 20). In this study, bacteria were observed on degraded margo areas of pit membranes at both 7 and 14 days after treatment with P. fluorescens NRRL B21432. It appears that P. fluorescens NRRL B21432 was able to degrade parts of the margo and pass through the pit chamber, but no evidence of torus degradation was observed (Fig. 1E and F). Observations of pit membrane degradation in conifers have been reported previously for water-logged woods after long-term storage (4, 12, 22). In these investigations the torus often remained intact even after extensive degradation of the margo had taken place (12, 22). A unique aspect of our results is documentation that pit membrane degradation occurs in inoculated wood chips within a 14-day period. A bacterial suspension applied to freshly cut wood chips appears to be sufficient for the bacteria to become established in the wood, migrate to bordered pit chambers, and degrade pit membranes. No evidence of erosion, tunneling, or cavitation attack on the cell walls, as has been described for wood-degrading bacteria (6, 19, 23), was observed in the treated wood chips.
FIG. 1.
Pine wood inoculated with P. fluorescens NRRL B21432 and control wood. Bar = 5.0 μm. (A) Bacteria in pitch deposits that accumulated on the surface of the wood 7 days after treatment with P. fluorescens NRRL B21432. (B) Bacteria in a resin duct 7 days after inoculation with P. fluorescens NRRL B21432. (C) Bacteria in a ray parenchyma cell 7 days after treatment with P. fluorescens NRRL B21432. (D) Intact pit membrane showing the margo (m) and torus (t) from an untreated, control loblolly wood pine chip. (E) Bacteria degrading the margo region of the pit membrane (arrowheads) inside a pine wood chip 7 days after treatment with P. fluorescens NRRL B21432. (F) Bacteria degrading the margo region of a pit membrane (arrowheads) 14 days after treatment with P. fluorescens NRRL B21432.
This paper is the first report of the use of wood-colonizing bacteria to degrade extractives, including a wide range of resin and fatty acids, in wood. The bacteria are of special interest because when applied to nonsterile wood they not only remove pitch but also inhibit colonization by naturally occurring fungi that cause detrimental discoloration and degradation of the wood. The preference of bacteria for wood with a moderate to high moisture content and their fast growth and ease of application make them ideally suited for biological processing of a wide variety of woods and in different environments. The effective reduction of resin acids in wood chips treated with bacteria such as P. fluorescens NRRL B21432 could also have an effect on reducing toxic levels of these compounds in pulping effluents. The resin and fatty acid reduction caused by pretreating chips prior to pulping also will reduce the chlorinated resin and fatty acids generated during bleaching of pulp. These chlorinated derivatives are known to be more difficult to degrade in wastewaters (16, 24, 25). The degradation of pit membranes and extractive-filled cells by bacteria is an important aspect that could also have significant benefits in chemical pulping processes. The removal of pit membranes and resin could facilitate the penetration of pulping chemicals into wood, resulting in less chemical use, shorter cooking times, and more efficient and uniform delignification during the pulping process.
REFERENCES
- 1.Allen L H. Proceedings of the TAPPI Minimum Effluent Mills Symposium, 23–24 October 1997, San Francisco, Calif. Atlanta, Ga: TAPPI Press; 1997. Pitch control in pulp mills; pp. 211–224. [Google Scholar]
- 2.Atlas M R. Handbook of microbiological media. Boca Raton, Fla: CRC Press; 1997. [Google Scholar]
- 3.Behrendt C J, Blanchette R A, Farrell R L. Biological control of blue stain fungi in wood. Phytopathology. 1995;85:92–97. [Google Scholar]
- 4.Blanchette R A. Biodeterioration of archaeological wood. CAB Biodeterioration Abstr. 1995;9:113–127. [Google Scholar]
- 5.Blanchette R A, Farrell R L, Behrendt C J, White-McDougall W, Held B W. Application of biological control agents in the forest products industry. In: Kreber B, editor. Strategies for improving protection of logs and lumber. Bulletin no. 204. Rotorua, New Zealand: Forest Research Institute; 1997. pp. 81–85. [Google Scholar]
- 6.Blanchette R A, Nilsson T, Daniel G, Abad A. Biological degradation of wood. In: Rowell R M, Barbour R J, editors. Archaeological wood: properties, chemistry, and preservation. American Chemical Society Advances in Chemistry Series, no. 225. Washington, D.C.: American Chemical Society; 1990. pp. 141–174. [Google Scholar]
- 7.Blanchette R A, Farrell R L, Burnes T A, Wendler P A, Zimmerman W, Brush T S, Snyder R A. Biological control of pitch in pulp and paper production by Ophiostoma piliferum. TAPPI J. 1992;75:102–106. [Google Scholar]
- 8.Breuil C, Iverson S, Gao Y. Fungal treatment of wood chips to remove extractives. In: Young R A, Akhtar M, editors. Environmentally friendly technologies for the pulp and paper industry. New York, N.Y: John Wiley & Sons; 1998. pp. 541–565. [Google Scholar]
- 9.Brush T S, Farrell R L, Ho C. Biodegradation of wood extractives from southern yellow pine by Ophiostoma piliferum. TAPPI J. 1994;77:155–159. [Google Scholar]
- 10.Chen T, Wang Z, Gao Y, Breuil C, Hatton J V. Wood extractives and pitch problems: analysis and partial removal by biological treatment. APPITA J. 1994;47:463–466. [Google Scholar]
- 11.Daniel G, Singh A, Nilsson T. Ultrastructural and immunocytochemical studies on the window and bordered pit membranes of Pinus sylvestris L. In: Donalson L A, Singh A P, Butterfield B G, Whitehouse L J, editors. Recent advances in wood anatomy. Rotorua, New Zealand: New Zealand Forest Research Institute; 1996. pp. 373–383. [Google Scholar]
- 12.Eriksson K E, Blanchette R A, Ander P. Microbial and enzymatic degradation of wood and wood components. Heidelberg, Germany: Springer-Verlag; 1990. [Google Scholar]
- 13.Farrell R L, Blanchette R A, Brush T S, Hadar Y, Iverson S, Krisa K, Wendler P A, Zimmermann W. Cartapip: a biolpulping product for control of pitch and resin problems in pulp mills. J Biotechnol. 1993;30:112–115. [Google Scholar]
- 14.Fisher K, Messner K. Reducing troublesome pitch in pulp mills by lipolytic enzymes. TAPPI J. 1992;75:130–134. [Google Scholar]
- 15.Leach J M, Thakore A N. Toxic constituents in mechanical pulping effluents. TAPPI J. 1976;59:129–132. [Google Scholar]
- 16.Liss S N, Bicho P A, Saddler J N. Microbiology and biodegradation of resin acids in pulp mill effluents: a minireview. Can J Microbiol. 1997;75:599–611. doi: 10.1139/m97-086. [DOI] [PubMed] [Google Scholar]
- 17.Mutton D B. Wood resins. In: Hillis W E, editor. Wood extractives and their significance to the pulp and paper industry. New York, N.Y: Academic Press; 1962. pp. 331–363. [Google Scholar]
- 18.Nicholas D D, Thomas R J. The influence of enzymes on the structure and permeability of loblolly pine. Am Wood-Preserv Assoc Proc. 1968;64:70–76. [Google Scholar]
- 19.Nilsson T, Daniel G. Bacterial attack of wood cell walls. In: Houghton D R, Smith R N, Eggins R N H O H, editors. Biodeterioration 7. New York, N.Y: Elsevier Science Publishing, Inc.; 1988. pp. 739–742. [Google Scholar]
- 20.Palleroni N J. Family I Pseudomonadaceae. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams & Wilkins Co.; 1984. pp. 141–213. [Google Scholar]
- 21.Panshin A J, Zeeuw C D. Textbook of wood technology: structure, identification, properties, and uses of the commercial woods of the United States and Canada. 4th ed. New York, N.Y: McGraw-Hill Book Co.; 1980. [Google Scholar]
- 22.Schmidt O, Liese W. Occurrence and significance of bacteria in wood. Holzforschung. 1994;48:271–277. [Google Scholar]
- 23.Singh A P, Butcher J A. Bacterial degradation of wood cells: a review of degradation patterns. J Inst Wood Sci. 1991;12:143–157. [Google Scholar]
- 24.Suntio L R, Shiu W Y, Mackay D. A review of the nature and properties of chemicals present in pulp mill effluents. Chemosphere. 1988;17:1249–1290. [Google Scholar]
- 25.Wang Z, Chen T, Gao Y, Breuil C, Hiratsuka Y. Biological degradation of resin acids in wood chips by wood-inhabiting fungi. Appl Environ Microbiol. 1995;61:222–225. doi: 10.1128/aem.61.1.222-225.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Z, Martin V J J, Mohn W W. Occurrence of two resin acid-degrading bacteria and a gene encoding resin acid biodegradation in pulp and paper mill effluent biotreatment system assayed by PCR. Microb Ecol. 1999;38:114–125. doi: 10.1007/s002489900163. [DOI] [PubMed] [Google Scholar]
- 27.Zimmerman W C, Blanchette R A, Burnes T A, Farrell R L. Melanin and perithecial development in Ophiostoma piliferum. Mycologia. 1995;87:857–863. [Google Scholar]