Abstract
Reactivation of UV-C-inactivated Pseudomonas aeruginosa bacteriophages D3C3, F116, G101, and UNL-1 was quantified in host cells infected during the exponential phase, during the stationary phase, and after starvation (1 day, 1 and 5 weeks) under conditions designed to detect dark repair and photoreactivation. Our experiments revealed that while the photoreactivation capacity of stationary-phase or starved cells remained about the same as that of exponential-phase cells, in some cases their capacity to support dark repair of UV-inactivated bacteriophages increased over 10-fold. This enhanced reactivation capacity was correlated with the ca. 30-fold-greater UV-C resistance of P. aeruginosa host cells that were in the stationary phase or exposed to starvation conditions prior to irradiation. The dark repair capacity of P. aeruginosa cells that were infected while they were starved for prolonged periods depended on the bacteriophage examined. For bacteriophage D3C3 this dark repair capacity declined with prolonged starvation, while for bacteriophage G101 the dark repair capacity continued to increase when cells were starved for 24 h or 1 week prior to infection. For G101, the reactivation potentials were 16-, 18-, 10-, and 3-fold at starvation intervals of 1 day, 1 week, 5 weeks, and 1.5 years, respectively. Exclusive use of exponential-phase cells to quantify bacteriophage reactivation should detect only a fraction of the true phage reactivation potential.
Numerous studies have demonstrated that significant numbers of viruses exist throughout the biosphere (3, 23, 29). For instance, bacteriophages are estimated to account for ca. 1% of the dissolved organic carbon in the open ocean (3). This relative abundance raises the question whether these bacteriophages merely persist or are functional and infective or whether, as found for the cyanophages (15), both possibilities are correct. We are interested in suitable methods for studying phage dynamics in aquatic ecosystems, i.e., suitable methods for monitoring the numbers and types of phages present over time. In this regard, there should be a dynamic equilibrium between the production of new phages following infection and the disappearance of phages, usually from one of three primary causes (21, 28): (i) consumption by grazing protozoans; (ii) attachment to labile colloids; and (iii) inactivation by UV radiation. Accurate estimates of the infective phage potential of a given sample or environment must take into consideration both the extent of the solar UV damage to the phage population and the availability of suitable bacterial hosts which could repair the damaged phage DNA.
Previously, we addressed the question of phage reproductive potential in changing ecosystems by looking at the prevalence of broad-host-range bacteriophages able to infect multiple bacterial species, including Sphaerotilus natans, Escherichia coli, and Pseudomonas aeruginosa (11). It is probable that any UV-damaged broad-host-range bacteriophages are capable of undergoing reactivation in several different host species. In this study we examined bacteriophage multiplication and reactivation by incorporating into our experimental design two important features of most aquatic ecosystems. First, we employed UV-damaged bacteriophages, and second, we infected nondividing starved or stationary-phase cells. It is well established that much of the biosphere is oligotrophic and that some, perhaps many, bacteria in nature are in the starvation survival mode (12, 20) (i.e., they divide rather infrequently). Thus, the repair capabilities of an exponentially growing, laboratory culture would likely be a poor predictor of the repair capacity of the same bacterium in nature.
One of the reasons that researchers may have avoided doing phage repair studies in stationary cells or starved cells stems from observations (1, 32) that many bacteriophages do not multiply in nongrowing bacteria. The view that all bacteriophage replication ceases in bacterial hosts that are not in the exponential phase of growth was challenged recently by Moebus (18, 19) and Schrader et al. (25). For bacteriophages specific for P. aeruginosa, it was found that bacteriophage ACQ had a mean burst size of 1,000 in exponential-phase hosts, compared to a mean burst size of 102 in starved hosts, while bacteriophage UT1 had mean burst sizes of 211 in exponential-phase hosts and 11 in starved hosts (25). The corresponding latent periods increased from 65 to 210 min for bacteriophage ACQ and from 90 to 165 min for bacteriophage UT1 (25).
The present experiments demonstrated that P. aeruginosa cells infected while they were in the stationary phase of growth or during maintenance under starvation conditions were able to reactivate UV-damaged bacteriophages by dark repair at levels that were greater than those observed for the identical hosts that were infected during the exponential phase. The levels of photoreactivation supported in stationary-phase or starved infected cells remained comparable to those in exponential-phase infected hosts. The widespread distribution of a capacity to reactivate UV-irradiated bacteriophages using host cell DNA repair enzymes suggests that repair of viral DNA damage is important to viral ecology in many environments.
MATERIALS AND METHODS
Bacteria and bacteriophages.
The bacterial strains employed in this study are listed in Table 1. Bacteriophages D3, D3C3, F116, and G101 are members of the Siphoviridae family and were obtained from R. V. Miller, Oklahoma State University. Bacteriophages F116 and G101 are temperate generalized transducing phages (16), bacteriophage D3 is a temperate specialized transducing phage (16), and D3C3 is a clear plaque mutant of phage D3. Bacteriophage UNL-1 is a lytic nontransducing phage and a member of the Myoviridae family (26). Bacteriophage lysates were produced by modifications of the soft-agar overlay method of Arber et al. (2).
TABLE 1.
P. aeruginosa strains employed in this study
| Strain | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| PAO1 | Prototroph | 27 |
| UA11079 | PAO1 carrying uvrA::ΩHg | J. Barbé |
| PAO303 | argB21 | 27 |
Media and growth conditions.
Bacteria were grown in Luria-Bertani (LB) broth obtained from Difco (Detroit, Mich.) or on LB agar (LBA) plates containing 1.3% (wt/vol) agar at 37°C. Saline (0.85% [wt/vol] NaCl) was used for cell and bacteriophage suspensions during starvation and irradiation. The soft agar used for overlays contained 1% (wt/vol) tryptone, 0.5% (wt/vol) NaCl, and 0.65% (wt/vol) agar.
Bacteriophage inactivation with UV-C radiation.
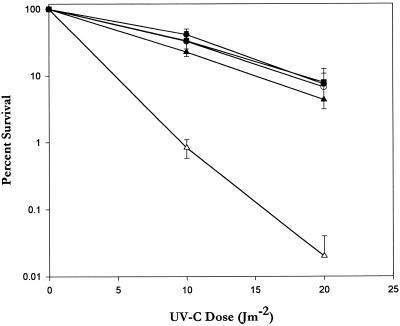
Bacteriophage inactivation with UV-C radiation was performed by the method of Shaffer et al. (26), with the modification that saline was used to dilute the bacteriophage. The bacteriophage lysates were diluted 1,000-fold in sterile saline, and 0.5-ml samples were irradiated with a General Electric 15-W germicidal lamp (G15T8) producing a flux of 0.14 J m−2 s−1. The UV lamp output was determined with an Ultra-Violet Products (San Gabriel, Calif.) UVX radiometer equipped with a UV-25 probe. Phage PFU were quantified immediately after irradiation. The UV-C doses required to obtain 0.1% survival differed markedly for the four phage types used (Fig. 1).
FIG. 1.
Inactivation kinetics for four bacteriophages capable of infecting P. aeruginosa PAO303. The phages were plaque assayed immediately after UV-C irradiation. The values are the averages of three measurements. The error bars indicate the standard errors. For phage UNL-1, some of the error bars are so small that they fall within the symbols. Symbols: ●, UNL-1; ■, D3C3; ▴, F116; ▾, G101.
Preparation of exponential-phase, stationary-phase, and starved cultures for bacteriophage infection.
P. aeruginosa PAO303 host cells were grown up overnight in a 37°C rotary shaker water bath (60 rpm) and were used as the phage hosts for the stationary-phase cell infection experiments. A 100-μl sample from the overnight culture was transferred to 10 ml of fresh LB broth and grown to the exponential phase (approximately 45 Klett units at 660 nm). Approximately 2 ml was taken from this culture and used in exponential-phase host cell infection experiments. The remainder of the culture was harvested by centrifugation, and the cell pellet was suspended in an equal volume of saline. The saline-suspended cells were returned to the rotary shaker water bath and incubated further at 37°C. After 24 h, 1 week, and 5 weeks of incubation the culture was removed and used in starved host cell infection experiments.
Bacteriophage reactivation in host cells that were infected while in the exponential phase, while in the stationary phase, or during maintenance under starvation conditions.
The irradiated bacteriophage lysates were further diluted in saline to yield an estimated 100 to 200 plaques on the assay plates. Based on the original titer of the diluted lysates prior to UV-C irradiation employed for these infections, this experimental design gave final multiplicities of infection (MOI) of approximately 0.01 for the exponential-phase and starved cells and 0.001 for the stationary-phase cells. A 100-μl sample of the appropriately diluted bacteriophage was added to 100 μl of host cells, the bacteriophage were allowed to attach for 15 min, and the infected cells were plated by the soft-agar overlay procedure onto LBA. The plates were incubated at 37°C, and PFU were counted after 16 h. The dark repair experiments were performed under amber light to prevent photoreactivation.
The comparative levels of UV-damaged bacteriophage reactivation in the various host cells were calculated by determining the reactivation factor (equation 1). This factor was the ratio of the number of PFU obtained after infection of stationary-phase or starved P. aeruginosa PAO303 host cells to the number of PFU obtained with the identical bacteriophage lysate when exponential-phase host cells were used.reactivation factor =
 |
l |
Photoreactivation of UV-damaged bacteriophages.
The capacity of P. aeruginosa host cells to support photoreactivation (4) of UV-C-irradiated bacteriophages was quantified by the technique of Shaffer et al. (26). Titrations were performed in quadruplicate, with two of the plates incubated in a 37°C incubator at a distance of 45 cm from two 15-W General Electric cool white bulbs (photo reactivating conditions) and the other two plates incubated at 37°C in the dark (nonphotoreactivated controls). For photoreactivation experiments, the reactivation factor was simply the ratio of the number of plaques obtained with plates incubated in the light to the number of plaques obtained with plates incubated in the dark, in which photoreactivation of the damaged phage was impossible.
UV-C survival of P. aeruginosa PAO303.
Survival of P. aeruginosa PAO303 was quantified by the method of Simonson et al. (27). Cultures at the appropriate phase of growth were harvested by centrifugation, and the pellets were suspended in an equal volume of saline. Stationary-phase cultures were diluted 1:100 in sterile 0.85% saline prior to irradiation. The saline-suspended samples (0.5 ml) were placed in a plastic petri dish and exposed to UV-C radiation with the lid removed. The UV-C flux was determined to be 0.14 J m−2 s−1, so exposure for 7 s yielded a total dose of 1 J m−2. Irradiated cells were diluted and plated on LBA. The plates were placed in a 37°C incubator, and the CFU were counted after 16 h. The experiments were conducted under amber light to prevent photoreactivation.
RESULTS
Our overall experimental design was to examine the plating efficiencies (PFU per milliliter) of four UV-C-irradiated bacteriophages on P. aeruginosa PAO303 hosts that had been maintained in different physiological states immediately prior to infection. UV-C radiation (190 to 290 nm) is absorbed by nucleic acids, which results in rapid inactivation of viruses (8). While the UV-C component of solar radiation is absorbed by the stratospheric ozone layer and does not reach the biosphere (9), longer-wavelength solar UV radiation (UV-A [320 to 400 nm] and UV-B [290 to 320 nm]) does penetrate the ozone layer and has significant effects on many aquatic ecosystems (9). Since both UV-B radiation and UV-C radiation result in the formation of thymine dimers in irradiated DNA (17), the use of UV-C in our experiments served as a rapid and convenient way to partially mimic a portion of the damage which in nature is caused by UV-B radiation.
Bacteriophages were irradiated with UV-C light to reduce the number of PFU to approximately 0.1% of the number present in the unirradiated lysate, and the relative UV-C inactivation rates varied substantially for the different bacteriophages (Fig. 1). The plating and repair capabilities of P. aeruginosa hosts were compared after infection of cells that had been maintained under five physiologically distinct conditions (exponential phase versus stationary phase and starved for 24 h, 1 week, or 5 weeks prior to infection). Bacteriophage titrations were performed in duplicate, with one set of plates incubated in the dark and the matching set of plates incubated in the light, where DNA damage photoreactivation was possible. The results are summarized in Tables 2 and 3.
TABLE 2.
Reactivation and plating efficiency factors for four bacteriophages on physiologically distinct P. aeruginosa PAO303 hosts incubated in the dark
| Phage | Reactivation factors for UV-C-irradiated phage with the following hostsa:
|
Plating factors for unirradiated phage with the following hostsa:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Stationary phase | Starved for 24 h | Starved for 1 week | Starved for 5 weeksb | Stationary phase | Starved for 24 h | Starved for 1 week | Starved for 5 weeksb | |
| D3C3 | 13.45 (0.12)c | 10.93 (0.18)c | 3.53 (0.81)c | 6.38 (6.27) | 1.06 (0.04) | 4.28 (0.31)c | 4.22 (0.43)c | 3.11 (0.79)c |
| F116 | 8.77 (3.84)c | 8.26 (0.95)c | 3.75 (1.19)c | 8.21 (4.13) | 0.85 (0.29) | 1.26 (0.26) | 1.18 (0.56) | 1.47 (0.47) |
| G101 | 9.79 (4.64)c | 16.48 (2.74)c | 18.73 (4.40)c | 9.84 (1.96)c | 1.99 (0.79) | 2.49 (1.26) | 2.96 (0.73)c | 1.44 (1.01) |
| UNL-1 | 2.44 (0.40)c | 2.27 (1.38) | 1.21 (0.08)c | 0.38 (0.01)c | 0.90 (0.12) | 0.66 (0.13)c | 0.29 (0.08)c | 0.18 (0.12)c |
The factors were each calculated by comparison to the plating (plaque-forming) efficiency of the same phage sample on exponential-phase cells. For example, the stationary-phase factor was the ratio of the number of PFU obtained on stationary phase cells to the number of PFU obtained on exponential phase cells. All experiments with a given phage type were performed in duplicate, with two to four separate trials conducted on different days. The standard errors are in parentheses.
All data for the cells starved for 5 weeks were obtained by using bacteriophages which had been UV-C inactivated within 36 h of the experiment.
The z values that were significantly different (P < 0.05) from the control values.
TABLE 3.
Relative photoreactivation and plating efficiency factors for four bacteriophages on physiologically distinct P. aeruginosa PAO303 hosts incubated in the light
| Phage | Photoreactivation factors for UV-C-irradiated phage with the following hostsa:
|
Plating factors for unirradiated phage with the following hostsa:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exponential phase | Stationary phase | Starved for 24 h | Starved for 1 week | Starved for 5 weeksb | Exponential phase | Stationary phase | Starved for 24 h | Starved for 1 week | Starved for 5 weeksb | |
| D3C3 | 9.07 (0.32)c | 8.97 (0.03)c | 8.35 (2.33)c | 7.72 (1.46)c | 7.95 (0.20)c | 0.87 (0.06)c | 1.13 (0.12) | 0.99 (0.07) | 0.92 (0.04)c | 0.87 (0.08) |
| F116 | 15.74 (6.64)c | 11.32 (2.06)c | 13.27 (3.83)c | 12.47 (1.43)c | 5.52 (2.83) | 0.95 (0.36) | 1.00 (0.15) | 0.97 (0.06) | 1.26 (0.39) | 0.99 (0.11) |
| G101 | 10.76 (1.44)c | 16.01 (5.45)c | 13.34 (0.97)c | 13.72 (2.37)c | 10.07 (1.75)c | 0.96 (0.15) | 1.07 (0.06) | 1.14 (0.34) | 0.86 (0.08) | 1.12 (0.17) |
| UNL-1 | 5.81 (0.64)c | 5.74 (2.26)c | 7.92 (4.17) | 4.88 (0.28)c | 6.69 (0.54)c | 0.86 (0.15) | 0.88 (0.09) | 0.71 (0.04) | 1.05 (0.17) | 0.93 (0.01)c |
The factors were each calculated by comparing the plating (plaque-forming) efficiency obtained on plates incubated in the light, where photoreactivation was possible, to the plating (plaque-forming) efficiency of the same phage sample on the same type of host cells incubated in the dark, where photoreactivation was not possible. For example, the stationary-phase factor was the ratio of the number of PFU on stationary-phase cells incubated in the light to the number of PFU obtained on stationary-phase cells incubated in the dark. All experiments with a given phage type were conducted by using duplicate plate assays, with two to four separate trials conducted on different days. The standard errors are in parentheses.
All data for the cells starved for 5 weeks were obtained by using bacteriophages which had been UV-C inactivated within 36 h of the experiment.
The z values were significantly different (P < 0.05) from the control values.
Enhanced plating efficiencies for UV-damaged bacteriophages infecting stationary-phase and starved host cells.
Bacteria entering the stationary phase become significantly more resistant to a wide range of stresses, including UV irradiation (5, 9, 10, 12). Thus, the transition of host cells to a stress-hyperresistant state should influence their potential for repair of UV-damaged bacteriophages. To test this hypothesis, the plaque-forming abilities of UV-irradiated bacteriophages were quantified after infection of host cells during the exponential phase, during maintenance in the stationary phase, or under starvation conditions. In these experiments, cultures were grown to the exponential phase, and a sample of each culture was used for phage titrations. The remainder of the culture was divided into two portions; one portion was allowed to grow until saturation, and the other was exposed to starvation conditions. The numbers of plaques obtained with the stationary-phase and starved hosts were compared to the number obtained with the original exponential-phase culture. For plates incubated in the dark, the number of plaques obtained after infection of stationary-phase hosts was always greater than the number obtained for the same phage lysate on exponential-phase host cells. All of the stationary-phase hosts exhibited significantly enhanced levels of damaged-phage reactivation compared to exponential-phase cells. For phages UNL-1, F116, G101, and D3C3, the reactivation factors were 2.4, 8.8, 9.8, and 13.5, respectively (Table 2). For host cells that were infected after incubation in saline for 24 h, the reactivation factors were 2.3, 8.3, 16.5, and 10.9 for phages UNL-1, F116, G101, and D3C3, respectively (Table 2). While bacteriophage G101 reactivation remained significantly enhanced for the entire period of the study, reactivation of phages D3C3, F116, and UNL-1 was significantly enhanced for shorter periods. While in some cases a significantly greater plating efficiency for undamaged virus was noted with stationary-phase or starved host cells (Table 2), all the increases in reactivation which we observed could not be explained by a simple, general increase in plating efficiency associated with hosts that were in the stationary phase or starved at the time of infection.
Experiments to quantify the levels of photoreactivation supported by host bacteria revealed that the repair pathway was effective in exponential-phase hosts and allowed enhanced reactivation compared to dark-incubated infected hosts of all the bacteriophages examined in this study (Table 3). Photoreactivation was clearly possible, and the relative levels remained roughly comparable to those observed in exponential-phase hosts when both stationary-phase and starved host cells were infected with phages D3C3, G101, and UNL-1. For phage F116 the relative photoreactivation rates ultimately declined with extended starvation some two- to four-fold compared to the rates observed in exponential-phase hosts (Table 3).
The enhanced phage reactivation in cells infected while they are under starvation conditions clearly involves a virus-host interaction since the reactivation factor trended downward for bacteriophage UNL-1 (Table 2) whereas it increased for up to 1 week of starvation for bacteriophage G101. This continued capacity for reactivation of G101 following prolonged starvation was shown more dramatically by using P. aeruginosa PAO303 cells which had been starved for 1.5 years. For UV-irradiated bacteriophage G101 the reactivation factor obtained with these cells was still three-fold greater than that obtained with exponential-phase cells. It seems probable that maintenance of a culture under starvation conditions for such an extended period must ultimately select cells that have a better capacity to survive in low-nutrient environments. While the selected changes that occurred in the starved cells are unknown, the extended starvation did not result in selection of cells with an intrinsically enhanced ability to support phage replication since during this period the efficiency of plating with these cells declined 33-fold. It is possible that environmental conditions in this culture selected cells which lacked suitable phage receptors or that these conditions did not allow optimal expression of required virus receptors, thus making productive infection less probable. Despite the decrease in plating efficiency, it is noteworthy that the capacity of these infected starved cells to allow plaque formation with damaged phage still exceeded that of exponential-phase cells.
Stationary-phase and starved P. aeruginosa cells exhibit enhanced UV radiation resistance.
Bacteria entering the stationary phase are known to become significantly more resistant to a wide range of stresses, including UV irradiation (5, 9, 10, 12). Thus, the transition of P. aeruginosa PAO303 host cells to the stationary phase should result in host cells that have an increased capacity for DNA damage repair, resulting in a greater level of resistance to UV radiation. Accordingly, we compared the kinetics of UV-C lethality for P. aeruginosa cells that were maintained under the same physiological conditions that we used to study phage reactivation. At a dose of 10 J m−2 the stationary-phase and starved P. aeruginosa PAO303 cells were approximately 30-fold more UV-C resistant than P. aeruginosa PAO303 cells in the exponential phase of growth (Fig. 2).
FIG. 2.
Kinetics of UV-C lethality for physiologically distinct cells of P. aeruginosa PAO303. Each experiment was conducted three times. The error bars indicate the standard errors. Symbols: ▵, exponential-phase cells; ▴, cells starved for 1 day; ●, cells starved for 1 week; ■, cells starved for 5 weeks; ○, stationary-phase cells.
Reactivation in uvrA-deficient host cells.
The reactivation of UV-C-irradiated bacteriophages was quantified in P. aeruginosa PAO1 and a genetically modified derivative (kindly provided by J. Barbé, Autonomous University of Barcelona) in which the uvrA gene was inactivated by insertion of a mercury resistance cassette (24) and was introduced via marker exchange into the P. aeruginosa chromosome. The cells containing an inactivated uvrA gene were unable to perform dark repair and were confirmed to be extremely sensitive to UV-C radiation (data not shown). Little reactivation of UV-C-irradiated bacteriophages occurred in these mutants, and the reactivation factors (in this case the ratio of the number of plaques obtained on the uvrA mutant to the number of plaques obtained on the P. aeruginosa PAO1 parental strain) were very low (Table 4). The efficiency of plating of undamaged viruses on the mutant uvrA strain was lower than that on the parental strain (Table 4), but the difference was not great enough to account for the dramatic differences in plating of damaged bacteriophages on the two hosts. Bacteriophage F116 was not examined in this study because the uvrA mutant, although not a lysogen, is insensitive to F116 infection (unpublished observations).
TABLE 4.
Relative bacteriophage reactivation and plating efficiency factors on stationary-phase P. aeruginosa PAO1 and a uvrA knockout derivative of P. aeruginosa PAO1
| Phage | Reactivation factor for UV-C-irradiated phage under the following incubation conditionsa:
|
Plating factor for unirradiated phagea | |
|---|---|---|---|
| Dark | Photoreactivating | ||
| D3C3 | 7.5 × 10−6 | 2.3 × 10−3 | 0.46 |
| F116 | NDb | ND | ND |
| G101 | 1.2 × 10−4 | 2 × 10−3 | 1.07 |
| UNL-1 | 1.3 × 10−5 | 3.1 × 10−2 | 0.97 |
The factors were each calculated by comparing the plating (plaque-forming) efficiency of a phage sample on stationary-phase uvrA knockout host cells to the plating (plaque-forming) efficiency of stationary-phase P. aeruginosa PAO1 host cells. The values are means based on two experiments.
ND, not determined.
When the same uvrA knockout strain was infected in parallel experiments with the identical irradiated bacteriophage lysates but incubated under conditions in which photoreactivation was allowed, the results were quite different. In this situation some phage DNA damage was eliminated in the mutant cells through the activity of photolyase, and the bacteriophage reactivation levels were significantly increased under such conditions (Table 4). Thus, allowing some of the DNA damage to be repaired via the alternative photoreactivation pathway led to a 17-fold increase in the number of G101 plaques, a 307-fold increase in D3C3 plating, and a greater-than-2,000-fold increase in the number of UNL-1 plaques detectable.
DISCUSSION
Reactivation of UV-irradiated bacteriophages of P. aeruginosa was quantified in cells that were in distinct physiological conditions at the moment of infection. We examined four previously characterized bacteriophages representing two different taxonomic families and both lytic and lysogenic modes of replication. The UV sensitivities of the four phages studied reflect the full range of relative UV sensitivities observed in our collection of P. aeruginosa viruses that have been isolated over a more-than-8-year period from across the United States. The bacteriophages studied clearly differ from one another with respect to UV radiation resistance, and they should not be lumped together. Our evidence supporting such bacteriophage diversity is three-fold: (i) four-fold-different doses of UV-C were required to inactivate the four bacteriophages to the same levels (Fig. 1); (ii) UNL-1 is unique as it is the only bacteriophage known which exhibits Weigle reactivation in response to UV-A radiation (26); and (iii) the dark repair capacity of P. aeruginosa cells which had been starved for prolonged periods varied greatly depending on the bacteriophage examined. For bacteriophages UNL-1 and D3C3 the repair factors decreased with time, while for bacteriophage G101 the repair factor continued to increase for a longer period (Tables 2 and 3). The observed differences in innate bacteriophage UV sensitivity cannot be ascribed to discernible features of the viruses, such as replication mode or morphology, but such differences in UV sensitivity have been found to exist between bacteriophage strains in studies conducted by others (31).
P. aeruginosa PAO1 host cells infected while they were in the stationary phase or during incubation under starvation conditions were still capable of reactivation of DNA-damaged bacteriophages via dark repair and/or photoreactivation. Indeed, infection of these cells resulted in up to a nearly 20-fold-greater reactivation capacity than did infection of exponential-phase cells (Table 2), while the photoreactivation levels observed were roughly the same as those of exponential-phase cells.
Because our experimental design employed a very low MOI (0.01 to 0.001), the observed plaques cannot have resulted from multiplicity reactivation (i.e., genetic recombination between multiple UV-C-inactivated phage which happen to have simultaneously infected the same bacterium) (14). The experiment with P. aeruginosa PAO1 and an engineered uvrA knockout derivative of this strain incapable of performing excision repair but still possessing an intact recA gene (Table 4) revealed that rescue of incoming damaged bacteriophages by homologous recombination with prophages residing in the host chromosome was limited. The difference between the two host strains is the capacity to support dark (excision) repair of DNA damage. Recombination mediated by the host recA system or phage systems, if present, would be supported in both strains, and if this were the dominant mechanism that allowed phage plaque formation, the numbers of plaques produced on the two strains would be predicted to be roughly equivalent, yielding reactivation factors near 1.00. Instead, the reactivation factors obtained in this experiment were extremely small due to the very low levels of plaques produced by damaged viruses on the uvrA host cells that were incubated in the dark (Table 4). A comparison of the plating capacities of stationary-phase uvrA hosts incubated in the dark or under conditions in which photoreactivation was possible likewise revealed that recombination did not confound our experiments. The sole difference between the two sets of infected uvrA host cells in this case was the level of DNA damage repair that was allowed by the environmental conditions. Under conditions in which some repair of DNA damage was supported (i.e., in light-exposed cells in which photoreactivation was possible), a sharp increase in phage reactivation was observed, revealing that the level of host cell DNA damage repair is the key factor influencing reactivation of damaged infecting bacteriophages. If recombination were the dominant mechanism producing the virus plaques enumerated in this experiment, it is reasonable to expect that roughly the same levels of phage would be recovered in this experiment regardless of whether the infected cells were incubated in the dark or in the presence of photoreactivating light.
While cultures maintained in the stationary phase for extended periods may be subject to takeover by more favorably adapted mutants (33), it seems unlikely that the appearance and selection of a mutant having an enhanced capacity for DNA damage repair can explain the reactivation differences which we observed between exponential-phase and stationary-phase or starved infected hosts. Such a takeover of the culture by mutants would have had to be complete within 24 h, since the peak levels of reactivation were evident at this point and the MOI employed in all the reactivation experiments were quite low. In addition, under the conditions employed in our cultures, there was no direct selection for mutants with enhanced DNA damage repair levels. The fact that the increased reactivation phenotype was consistently demonstrated in replicate experiments and the fact that reactivation levels slowly declined with time further suggest that mutational changes in the host cell populations do not account for our observations.
In order to examine experimentally the potential for mutants with enhanced UV resistance capacity to be generated and take over aged cultures, we prepared a stationary-phase culture of P. aeruginosa PAO303 by allowing the cells to grow to saturation in LB broth. This stationary-phase culture was incubated for 2 weeks at 37°C, and then cells were recovered and examined for UV sensitivity and the capacity to allow phage infection. In two replicate experiments, the level of UV sensitivity of the cells from the aged culture was found to be essentially the same as that of the parental strain (Fig. 2), with mean levels of survival of 12, 0.25, and 0.125% at UV doses of 6, 12, and 20 J m−2, respectively. This level of UV resistance was considerably less than that evident in the P. aeruginosa PAO303 cells that were simply allowed to reach the stationary phase by incubation for 24 h in growth medium or that had been starved at the time of irradiation (Fig. 2). In addition, examination of the efficiency of plating of the phages employed in this study revealed that the capacity of the cells derived from the aged culture to allow phage plaque formation was essentially equivalent to that of the parental strain (data not shown). It is important to note that while the takeover of our cultures by mutants does not explain our results, if natural selection in situ favors the replication or persistence of more UV damage repair-proficient hosts, there is an increased likelihood of a difference between laboratory estimates of reactivation potentials and the true reactivation levels in such environments.
We believe that the increased phage DNA damage reactivation which we observed when P. aeruginosa cells were infected while they were in the stationary phase or were starved was due to an enhanced constitutive host cell DNA repair capability expressed as the cells exited the exponential phase of growth. The level of bacteriophage reactivation supported in host cells that have not been deliberately exposed to SOS system-inducing agents is thought to reveal the relative level of host DNA damage repair activity (7), and the enhanced phage reactivation exhibited by P. aeruginosa cells that were infected while they were in the stationary phase or were maintained under starvation conditions (Table 2) was correlated with an increased UV-C resistance phenotype in the same cells (Fig. 2). Several species of bacteria are known to exhibit a range of stress-induced hyperresistance phenotypes associated with entry into the stationary phase of growth (10, 12, 22). Enhanced UV resistance is likely a common characteristic of stationary-phase and starved bacteria and has been reported for both Vibrio spp. (22) and E. coli (9). Even though we believe that an enhanced host cell DNA repair capacity is responsible for both the enhanced phage reactivation (Tables 2 and 3) and the UV-C resistance (Fig. 2) of stationary-phase or starved cells, we are aware that there are alternative explanations for this host phenotype. Enhanced UV-C resistance of intact bacteria could also be due to (i) shielding of the sensitive DNA chromophore so that less of the 254-nm light actually reaches the DNA (30) or (ii) protection of the DNA in a manner similar to that found in bacterial spores in which the DNA is both dehydrated and wrapped tightly with proteins throughout its length (6).
Our experimental protocol employed the same population of cells throughout; i.e., the exponential-phase population in which reactivation was initially quantified either was allowed to grow until saturation (24 h of incubation) or was subjected to starvation conditions, after which reactivation of the same UV-irradiated bacteriophage lysates was again quantified. For each reactivation experiment, control plates with undamaged, nonirradiated phage revealed that the basic plating capacity of the stationary-phase and starved cells remained comparable in most cases to that of exponential-phase host cells and that entry into the stationary phase or starvation did not in itself cause a gross increase in phage plating efficiency sufficient to account for the increased number of plaques produced on all of the hosts. It is possible that an apparent enhancement in phage reactivation after infection of starved and stationary-phase hosts could result from (i) more productive interactions between bacteriophages and bacterial cell receptors or (ii) more efficient transit of the periplasm by the phage DNA, due possibly to the existence of a more appropriate membrane potential for optimal entry of phage DNA (13) in these hosts. However, the fact that the enhanced plating efficiency for undamaged phage (Tables 2 and 3) on starved and stationary-phase hosts was insufficient to account for all of the observed differences in reactivation capacity between hosts in the exponential phase and the identical stationary-phase or starved hosts rules out these alternative explanations.
Bacteriophage reactivation experiments have typically been conducted with exponential-phase hosts. While the use of rapidly growing hosts is sometimes an experimental necessity since many bacteriophages fail to replicate in stationary-phase hosts, it is clear that virus multiplication is not universally confined to the exponential phase (18, 19, 25). The increased reactivation potential evident in bacterial hosts that were infected while they were in the stationary phase or during maintenance under starvation conditions strongly suggests that the viruses capable of replication in stationary-phase hosts encounter an environment in which DNA damage may be readily reversed, thus allowing any infecting damaged phage to fully exploit the hosts. Because plaque assays demand that infected cells be plated and thus unavoidably returned to growth conditions prior to bursting, it was not possible to ascertain the exact moment that phage reactivation occurred in relation to the host cell physiological status in our experiments. While our experiments did not rule out the possibility that the actual phage reactivation process took place during growth or, more properly, during an interval in which growth might ordinarily have been possible since the period that infected cells actually grew would have arguably been quite brief, they did reveal that the growth status of the host cells at the time of infection substantially influenced the probability of bacteriophage reactivation. An enhanced DNA damage repair capacity actually expressed either in stationary-phase hosts or at the time that infected cells are returned to conditions that support growth may positively influence the persistence of all viruses, including those that are incapable of multiplication in stationary-phase hosts. For such viruses, the infecting damaged phage genomes may be repaired and maintained quiescently until the host resumes rapid growth.
Our results support the hypothesis that stationary-phase and starved bacterial hosts express enhanced constitutive levels of DNA damage repair and thereby support enhanced reactivation of infecting UV-damaged bacteriophages. Infection of stationary-phase or starved hosts does not necessarily hinder repair, but rather may actually increase the probability of effective elimination of viral DNA damage, thus increasing phage fitness. In effect, host cell DNA damage repair systems extend the infective lifetimes of bacteriophages in environments exposed to UV radiation.
ACKNOWLEDGMENTS
This work was supported in part by cooperative agreement CR822163 with the U.S. Environmental Protection Agency Gulf Breeze Environmental Research Laboratory, by cooperative agreement 9255225 from the National Science Foundation EPSCoR program (to T.A.K.), and by grants to K.W.N. from the Nebraska Corn Board, the University of Nebraska Center for Biotechnology, and the Consortium for Plant Biotechnology Research and to J.J.S. from the Research Council at the University of Nebraska—Kearney.
K.W.N. and T.A.K. contributed equally to this work.
REFERENCES
- 1.Adams M H. Bacteriophages. New York, N.Y: Interscience Publishers; 1959. [Google Scholar]
- 2.Arber W, Enquist L, Hohn B, Murray N E, Murray K. Experimental methods for use with lambda. In: Hendrix R W, Roberts J W, Stahl F W, Weisberg R A, editors. Lambda II. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1983. pp. 433–466. [Google Scholar]
- 3.Bergh O, Borsheim K, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340:467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- 4.Dulbecco R. Reactivation of ultraviolet-inactivated bacteriophage by visible light. Nature. 1949;163:949–950. doi: 10.1038/163949b0. [DOI] [PubMed] [Google Scholar]
- 5.Eisenstark A, Calcutt M J, Becker-Hapak M, Ivanova A. Role of Escherichia coli rpoS and associated genes in defense against oxidative damage. Free Radic Biol Med. 1996;21:975–993. doi: 10.1016/s0891-5849(96)00154-2. [DOI] [PubMed] [Google Scholar]
- 6.Fairhead H, Setlow B, Setlow P. Prevention of DNA damage in spores and in vitro by small, acid-soluble proteins from Bacillus species. J Bacteriol. 1993;175:1367–1374. doi: 10.1128/jb.175.5.1367-1374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedberg E C, Walker G C, Siede W. DNA repair and mutagenesis. Washington, D.C.: ASM Press; 1995. [Google Scholar]
- 8.Harm W. Biological effects of ultraviolet radiation. New York, N.Y: Cambridge University Press; 1980. [Google Scholar]
- 9.Jagger J. Solar-UV actions on living cells. New York, N.Y: Praeger Publishers; 1985. [Google Scholar]
- 10.Jenkins D E, Schultz J E, Matin A. Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol. 1988;170:3910–3914. doi: 10.1128/jb.170.9.3910-3914.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen E C, Schrader H S, Rieland B, Thompson T L, Lee K W, Nickerson K W, Kokjohn T A. Prevalence of broad-host-range lytic bacteriophages of Sphaerotilus natans, Escherichia coli, and Pseudomonas aeruginosa. Appl Environ Microbiol. 1998;64:575–580. doi: 10.1128/aem.64.2.575-580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolter R, Siegele D A, Tormo A. The stationary phase of the bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 13.Labedan B, Goldberg E B. Requirement for membrane potential in injection of phage T4 DNA. Proc Natl Acad Sci USA. 1979;76:4669–4673. doi: 10.1073/pnas.76.9.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luria S E. Reactivation of ultraviolet-irradiated bacteriophage by multiple infection. J Cell Comp Physiol. 1952;39(Suppl. 1):119–123. [PubMed] [Google Scholar]
- 15.Martin E L, Kokjohn T A. Cyanophages. In: Granoff A, Webster R G, editors. Encyclopedia of virology. 2nd ed. London, United Kingdom: Academic Press; 1999. pp. 324–332. [Google Scholar]
- 16.Miller R V, Pemberton J M, Richards K E. F116, D3, and G101: temperate bacteriophages of Pseudomonas aeruginosa. Virology. 1974;59:566–569. doi: 10.1016/0042-6822(74)90466-8. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell D L, Nairn R S. The biology of the (6-4) photoproduct. Photochem Photobiol. 1989;49:805–819. doi: 10.1111/j.1751-1097.1989.tb05578.x. [DOI] [PubMed] [Google Scholar]
- 18.Moebus K H. Marine bacteriophage reproduction under nutrient-limited growth of host bacteria. I. Investigations with six phage-host systems. Mar Ecol Prog Ser. 1996;144:1–12. [Google Scholar]
- 19.Moebus K H. Marine bacteriophage reproduction under nutrient-limited growth of host bacteria. II. Investigations with phage-host system [H3: H3/1] Mar Ecol Prog Ser. 1996;144:13–22. [Google Scholar]
- 20.Morita R Y. Bacteria in oligotrophic environments: starvation-survival lifestyle. New York, N.Y: Chapman & Hall; 1997. [Google Scholar]
- 21.Noble R T, Fuhrman J A. Virus decay and its causes in coastal waters. Appl Environ Microbiol. 1997;63:77–83. doi: 10.1128/aem.63.1.77-83.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyström T, Olsson R M, Kjelleberg S. Survival, stress resistance, and alterations in protein expression in the marine Vibrio sp. strain S14 during starvation for different individual nutrients. Appl Environ Microbiol. 1992;58:55–65. doi: 10.1128/aem.58.1.55-65.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul J H, Jiang S C, Rose J M B. Concentration of viruses and dissolved DNA from aquatic environments by vortex flow filtration. Appl Environ Microbiol. 1991;57:2197–2204. doi: 10.1128/aem.57.8.2197-2204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivera E, Vila L, Barbé J. Expression of the Pseudomonas aeruginosa uvrA gene is constitutive. Mutat Res. 1997;377:149–155. doi: 10.1016/s0027-5107(97)00061-4. [DOI] [PubMed] [Google Scholar]
- 25.Schrader H S, Schrader J O, Walker J J, Wolf T A, Nickerson K W, Kokjohn T A. Bacteriophage infection and multiplication occur in Pseudomonas aeruginosa starved for 5 years. Can J Microbiol. 1997;43:1157–1163. doi: 10.1139/m97-164. [DOI] [PubMed] [Google Scholar]
- 26.Shaffer J J, Jacobsen L M, Schrader J O, Lee K W, Martin E L, Kokjohn T A. Characterization of Pseudomonas aeruginosa bacteriophage UNL-1, a bacterial virus with a novel UV-A-inducible DNA damage reactivation phenotype. Appl Environ Microbiol. 1999;65:2606–2613. doi: 10.1128/aem.65.6.2606-2613.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonson C S, Kokjohn T A, Miller R V. Inducible UV repair potential of Pseudomonas aeruginosa PAO. J Gen Microbiol. 1990;136:1241–1249. doi: 10.1099/00221287-136-7-1241. [DOI] [PubMed] [Google Scholar]
- 28.Suttle C A, Chen F. Mechanisms and rates of decay of marine viruses in seawater. Appl Environ Microbiol. 1992;58:3721–3729. doi: 10.1128/aem.58.11.3721-3729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torrella F, Morita R Y. Evidence by electron micrographs for a high incidence of bacteriophage particles in the waters of Yaquina Bay, Oregon: ecological and taxonomical interpretations. Appl Environ Microbiol. 1979;37:774–778. doi: 10.1128/aem.37.4.774-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waaland J R, Waaland S D, Branton D. Gas vacuoles: light shielding in blue green algae. J Cell Biol. 1970;48:212–215. doi: 10.1083/jcb.48.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wommack K E, Colwell R R. Virioplankton: viruses in aquatic systems. Microbiol Mol Biol Rev. 2000;64:69–114. doi: 10.1128/mmbr.64.1.69-114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woody M A, Cliver D O. Effects of temperature and host cell growth phase on replication of F-specific RNA coliphage Qβ. Appl Environ Microbiol. 1995;61:1520–1526. doi: 10.1128/aem.61.4.1520-1526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zambrano M M, Siegle D A, Almiron M, Tormo A, Kolter R. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science. 1993;259:1757–1760. doi: 10.1126/science.7681219. [DOI] [PubMed] [Google Scholar]