Abstract
Introduction
Gestational diabetes mellitus (GDM) is one of the most prevalent diseases during pregnancy, which is closely associated with many short-term and long-term maternal and neonatal complications and can incur heavy financial burden on both families and society. Web-based interventions have been used to manage GDM because of the advantages of high accessibility and flexibility, but their effectiveness has remained inconclusive. This systematic review and meta-analysis aims to comprehensively investigate the multidimensional effectiveness of web-based interventions for pregnant women with GDM, thereby aiding implementation decisions in clinical settings.
Methods and analysis
This systematic review protocol strictly adheres to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols guidelines. Six electronic databases (PubMed, Web of Science, Cochrane Central Register of Controlled Trials, Embase, CINAHL and PsycINFO) will be comprehensively searched from their inception to 26 January 2022 to identify randomised controlled trials and controlled clinical trials regarding the efficacy of web-based interventions for pregnant women with GDM on glycaemic control, behavioural outcomes, cognitive and attitudinal outcomes, mental health, maternal and neonatal clinical outcomes, and medical service utilisation and costs. Two reviewers will independently conduct the study selection, data extraction and quality assessment. The methodological quality of included studies will be assessed using the Effective Public Health Practice Project assessment tool. The overall meta-analyses for each of the interested outcomes will be performed if the outcome data are sufficient and provides similar effect measures, as well as subgroup analyses for glycaemic control indicators based on the different types of intervention format, interactivity and technology. We will conduct a qualitative synthesis for studies that cannot be quantitatively synthesised.
Ethics and dissemination
Ethics approval is not required for this review as no human participants will be involved. The results will be disseminated via a peer-reviewed journal or an academic conference.
PROSPERO registration number
CRD42022296625.
Keywords: telemedicine, maternal medicine, diabetes in pregnancy
Strengths and limitations of this study.
This systematic review protocol follows the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols guidelines.
Rigorous methods of review will be followed with at least two independent reviewers to conduct study selection, data extraction and quality assessment.
Subgroup analyses will be performed if possible to elaborate on the type of intervention format, interactivity and technology correlating with the increased effectiveness.
Anticipated high heterogeneity across studies may increase the difficulty in interpreting a meta-analysis.
There may be language bias as this review will only include studies published in English.
Introduction
Gestational diabetes mellitus (GDM) is one of the most common comorbidities in pregnant women, which is initially diagnosed in the second or third trimester of pregnancy and features as hyperglycaemic of variable severity without overt pregestational diabetes.1 According to the International Diabetes Federation, the worldwide prevalence of hyperglycaemic in pregnancy ranged from 8.6% to 28.0% up to 2021, which affected 21.1 million of live births (16.7%), with the majority of the cases presenting with GDM (80.3%).2 Moreover, as well-established risk factors for developing GDM, the rising obesity rates prior to pregnancy, excess weight gain during pregnancy, sedentary lifestyle and older maternal age have resulted in the increasing prevalence of GDM in recent years,3 which can potentially challenge medical resources and exert great impact on individuals and society. Actually, GDM has become an important public health issue both in developed and low-income and middle-income countries.4
It has been demonstrated that GDM is closely related to obstetrical problems at the time of delivery as well as subsequent perinatal morbidity.5 6 The potential short-term impacts for mothers include increased risk of pre-eclampsia, polyhydramnios, shoulder dystocia, stillbirth and infectious complications.7 Worse still, although GDM will resolve within a short period after delivery, it is an independent risk factor for many diseases. Specifically, the long-term maternal effects of GDM include but are not limited to GDM recurrence in the next pregnancy,8 as well as higher risks of developing type 2 diabetes (a ninefold increased risk)9 and cardiovascular diseases (2.3 times the risk).10
Likewise, a significant association between maternal anomalous hyperglycaemic and many fetal and neonatal complications has also been clearly established,7 which includes fetal intrauterine growth retardation, macrosomia, neonatal hypoglycaemia, respiratory distress syndrome and so on. Furthermore, according to the concept of transgenerational programming, offsprings who are exposed to hyperglycaemic when in the uterus have an increased risk of obesity and metabolic syndrome in their childhood and early adulthood.11
Therefore, in view of the considerable short-term and long-term complications that GDM may cause, it is of great importance to manage GDM effectively via strategies aiming to maintain the maternal glycaemia as close to normal as possible. Lifestyle interventions, typically including healthy eating and physical exercise, have been widely adopted to help optimise blood glucose levels during the prenatal period.12 Actually, these interventions are the mainstay of therapy for GDM and may suffice for most pregnant women with GDM (65%–90%).13 14 When non-pharmacological regimens fail to affect, pharmacotherapies (oral hypoglycaemic agents and insulin) will be added. However, regardless of whether drugs are involved, traditional GDM management is achieved by intensive clinic attendance for receiving disease education, reporting symptoms and glycaemic control levels, and adjusting therapeutic regimens,15–17 which will place increased demands on clinical services for providing diabetes care and aggravate the economic burden on individuals.15 18 Meanwhile, multiple barriers and disadvantages exist in the traditional mode of GDM management, such as unequal hospital resource distribution, high costs for transportation, time and energy lacking for both patients and health professionals, time-consuming waiting before seeing a doctor, and a limited intervention time window, which can reduce the efficiency of GDM management, decrease patients’ satisfaction and cause poor pregnancy outcomes.17–19 Consequently, it is essential to identify a more practical, scalable, sustainable and cost-effective mode of care to manage GDM effectively and simultaneously ease the medical service burden on the premise of not interfering with the current healthcare system or compromising the quality of care.
The rapid development and wide spread of information and communication technology worldwide has precisely provided an innovative perspective for disease management. In particular, due to the advantages of high accessibility, convenience, flexibility and efficiency,20 web-based interventions delivered by smartphones, computers, laptops and other internet-connected devices have attracted a great deal of attention and have been widely used in recent years for health and well-being promotion in patients with cardiovascular diseases,21 metabolic syndrome22 and other diseases.23–25 These technologies can help to close the loop between patients and health professionals, overcome the inequivalent distribution of medical resources, and realise the vision of pervasive healthcare,26 27 which is therefore regarded as an ideal medical and public health practice mode for disease management. Notably, pregnant women with GDM seemed to be an ideal population to target for using web-based technologies to improve health outcomes because of the high penetration rates and excellent grasp of web-based devices among the reproductive-aged population, who have limited time to attend conventional health services.16 28–30 At present, attempts have been made to improve health outcomes in pregnant women with GDM via web-based interventions; nonetheless, clinical trials on this topic have yielded mixed results. Some studies found that web-based interventions could significantly ameliorate glycaemic control,27 31 increase compliance with self-monitoring of blood glucose (SMBG),32 reduce the incidence of premature delivery33 and medical service costs,15 as well as improve satisfaction with care15 for this crowd, while others demonstrated a null effect.32 34 35 Hence, it is critical to systematically evaluate the effectiveness of web-based interventions for pregnant women with GDM.
To date, two systematic reviews and meta-analyses have been conducted to evaluate the effectiveness of web-based interventions for pregnant women with GDM.36 37 One previous review37 included pregnant women with GDM, type 1 diabetes and type 2 diabetes, while the results of the GDM subgroup (N=5 studies) showed no significant between-group effect on glycated haemoglobin (HbA1c), caesarean rate, neonatal birth weight or hypoglycaemia. On the contrary, a recent review (N=6 studies)36 focused on the effectiveness of disease-specific mobile applications and reported that fasting blood glucose (FBG), 2-hour postprandial blood glucose (2hBG) and caesarean rate in pregnant women with GDM significantly improved after intervention compared with the control group. In addition, another five systematic reviews38–42 investigated the effects of telemedicine on GDM, which included medical interventions both delivered via internet and early mobile technologies (eg, phone calls, short message service (SMS) and digital video disk (DVD)); but these reviews generated conflicting results on glycaemic control and maternal and neonatal clinical outcomes. In general, the existing systematic reviews of relevant topics reported mixed results on glycaemic control and clinical outcomes, whereas the other outcomes (such as maternal behavioural outcomes and medical service utilisation and costs) were hardly assessed. Beyond that, in the majority of these reviews,38–42 web-based technologies were conflated with early labour-intensive technologies that have become less popular in the rapidly evolving landscape of technology. More importantly, a growing number of primary studies15 32 34 43–47 with conflicting results regarding this topic have emerged after the above reviews, which may provide new evidence. Therefore, it is necessary to conduct a new systematic review that focuses on web-based technologies and includes evidence from all existing studies to comprehensively evaluate the effectiveness of web-based interventions in pregnant women with GDM, so as to provide scientific and conclusive evidence for future clinical practice.
Objectives
This systematic review and meta-analysis aims to:
Investigate the effectiveness of web-based interventions on maternal glycaemic control, behavioural outcomes, cognitive and attitudinal outcomes, mental health, maternal and neonatal clinical outcomes, as well as medical service utilisation and costs in pregnant women with GDM by integrating all available evidence from randomised controlled trials (RCTs) and controlled clinical trials (CCTs).
Innovatively gain insight into whether the type of interactivity, the type of format and the type of technology of web-based interventions can influence the intervention effects and which type of intervention regimen is the most effective, thereby finding out an optimal web-based intervention regimen.
Methods and analysis
Registration and study design
This paper presents a systematic review protocol that has been registered in PROSPERO, and any future changes will be registered as amendments. We will complete and report the study protocol following the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines48 (see online supplemental table S1 for details). The research questions are developed based on the PICOS framework (population, intervention, comparator/control, outcome and study design), which are described in detail as follows.
bmjopen-2022-061151supp001.pdf (101.4KB, pdf)
Eligibility criteria for selecting studies
Types of study
We will include RCTs and CCTs that have been published in peer-reviewed English journals, which are good standards for evidence-based clinical research.49 Single-group studies, reviews, case reports, cohort studies, letters to editors, conference abstracts and study protocols will be excluded.
Types of participant
Pregnant women with GDM but without any severe diseases (such as severe symptoms of psychological disorders or fetal abnormalities) will be included, regardless of whether she had been diagnosed with GDM in previous pregnancies. Studies that included mixed types of diabetes mellitus (including GDM, type 1 diabetes and type 2 diabetes) but reported the data specific to GDM separately will be included as well. Moreover, the present review is part of a research project aimed at developing a theoretically informed and web-assisted behaviour change intervention for pregnant adult women. Therefore, pregnant women ≥18 years old will be considered eligible for this study.
Types of intervention
The intervention should be a digital one delivered by any type of web-based modalities, which may include but are not restricted to websites and mobile applications. However, studies that only used web-based interventions for observing the maintenance of outcome changes from previously administered health interventions, incorporating web-based components with face-to-face components and lacked real web-based interventions for participants (eg, conducting interventions via video, DVD, television, radio, SMS or telephone calls) will be excluded.
Types of comparator/control
The following comparators will be regarded as eligible: a wait-list control, usual care and no interventions.
Types of outcome
Primary outcome: the glycaemic control indicators during pregnancy including HbA1c, FBG, 1-hour postprandial blood glucose (1hBG) and 2hBG.
-
The following five categories of secondary outcomes are interested:
Maternal behavioural outcomes: insulin treatment rate, oral hypoglycaemic agents treatment rate and self-care behaviours (mainly including the compliance with SMBG, healthy diet and physical activity).
Maternal cognitive and attitudinal outcomes: knowledge of disease, risk-perception of disease, self-efficacy and satisfaction with care.
Maternal mental health: depression and anxiety.
Maternal and neonatal clinical outcomes: gestational weight gain, induction of labour, vaginal delivery, normal vaginal delivery, assisted vaginal delivery, caesarean section, planned caesarean section, emergency caesarean section, gestational weeks at delivery, premature delivery, shoulder dystocia, pre-eclampsia/gestational hypertension, premature rupture of the membranes, macrosomia, admission to the neonatal intensive care unit, low birth weight, birth weight, large for gestational age, small for gestational age, neonatal hypoglycaemia, 1 min Apgar scores, 5 min Apgar scores, neonatal jaundice/hyperbilirubinaemia, respiratory morbidity, composite neonatal complications, phototherapy and neonatal death.
Medical service utilisation and costs.
Studies that included at least one of the above outcomes will be considered eligible.
Search methods for the identification of studies
PubMed, Web of Science, Embase, Cochrane Library, CINAHL and PsycINFO are anticipated to be comprehensively searched from the inception of each database to 26 January 2022. The search strategies of electronic databases are a combination of Medical Subject Heading and free-text words to represent the definitions of GDM, web-based interventions, RCTs and CCTs. The search strategies will be developed in collaboration with an academic librarian. The detailed retrieval strategies of all databases are available in online supplemental table S2. Two authors (PPG and DDC) will conduct the search process independently. Additionally, a snowball hand-search will be undertaken to retrieve additional eligible studies after database searches by reviewing the reference lists of included studies and the existing systematic reviews related to this topic.
Study selection
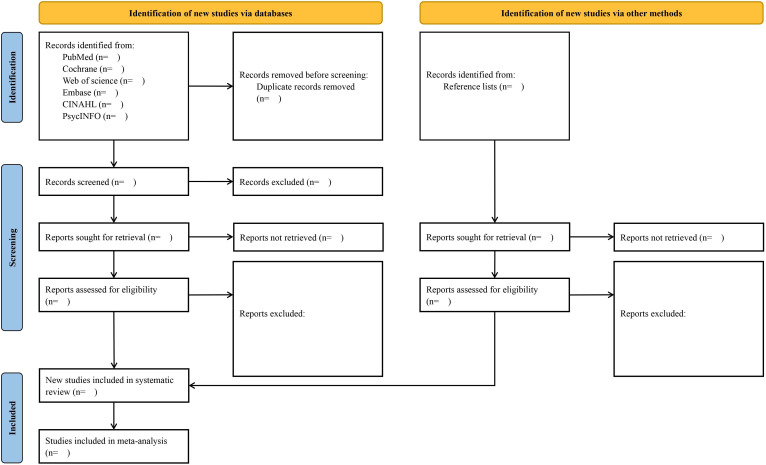
After the initial systematic searches, all retrieved records will be exported to Endnote X 8.2 reference management software. Then, the automated ‘Find Duplicates’ function of this software will be used to eliminate duplicate studies. Two authors (YJ and PX) will independently assess the remaining titles and abstracts and remove irrelevant citations in accordance with the selection criteria. Then, the full text of studies will be obtained if either of the two authors judges a publication to be potentially eligible for inclusion. Independent full text reading by the same authors will follow. Any discrepancies between the authors will be discussed first. When consistency cannot be reached, a senior reviewer (SF) will resolve the controversy. Reasons for excluding studies will be detailed on a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart (figure 1).
Figure 1.
Flow diagram of article selection process.
Data extraction and management
Data extraction will be carried out with a purpose-built, predesigned and structured template. We will first pilot the data extraction process using a subsample of included studies and make further refinements to the extraction sheet as necessary. The corresponding authors of included studies with any missing, uncertain or incomplete information will be contacted. The data from the final included studies will be independently extracted by two reviewers (ZX and XW) and checked for accuracy by a third reviewer (QZ).
From all included studies, we will collect the following information:
The general study information: the first author, year of publication, country and study design.
Participants’ details: mean age, diagnostic criteria of GDM, gestational weeks at allocation and sample size (intervention/control).
Intervention details: name of intervention, detailed regimen, duration, main technology (such as mobile application and website), interactivity (interactive/non-interactive) and format (personalised/non-personalised).
Control group regimen.
Outcomes: the primary and secondary outcomes (between-group significance: +/−).
Attrition rates.
Methodological quality assessment
The Effective Public Health Practice Project assessment tool will be applied to appraise the methodological quality of included studies,50 which showed better inter-rater agreement than the Cochrane Collaboration Risk of Bias tool.51 Studies will be evaluated on the following six aspects: selection bias, study design, confounders, blinding, data collection methods, as well as withdrawals and drop-outs. Finally, each aspect as well as the global rating will be rated as strong, moderate or weak. What needs to be pointed out specially is that the aspect of blinding will be rated as ‘strong’ for studies only evaluating objective outcomes, as objective outcomes are unlikely to be affected by actual blinding implementation.49 The methodological quality evaluation will be performed independently by two reviewers (WZ and MM), and any controversial evaluation differences will be discussed for a final decision.
Grading the quality of evidence
The Grades of Recommendation, Assessment, Development and Evaluation guidelines52 will be used to assess the level of evidence for each indicator of the primary outcome (glycaemic control). After evaluations of the risk of bias, consistency, directness of evidence, imprecision and publication bias, a body of evidence across the outcome indicators will be specified as very low, low, moderate and high quality.
Data analysis
Data synthesis
A meta-analysis will be conducted when there are sufficient studies (no less than two studies) with available data investigating the same outcome by similar effect measures. For outcomes that could not be quantitatively synthesised due to insufficient studies, unavailable data or high heterogeneity of effect measures, a narrative approach will be applied for analysis. Stata V.12.0 (Stata Corporation) will be used for all statistical calculations, and a p value <0.05 will be set as the significance level. For continuous variables, the mean differences (MDs) with 95% CIs will be selected only when the unit and the instrument of measurement are the same across trials; otherwise, the standardised MD (SMDs) with 95% CIs will be chosen.53 According to Cohen’s definition, the effect size of SMD is considered small (<0.2), moderate (0.2–0.8) or large (>0.8).54 For dichotomous variables, we will use relative risks with 95% CIs for point estimates, and the cut-off values of 1.22, 1.86 and 3.00 represent small, medium and large effects, respectively.55 The inverse variance method will be used to pool continuous outcome data and the Mantel Haenszel method for dichotomous outcome data.
Assessment of heterogeneity
The level of heterogeneity across studies will be evaluated by χ2 test and I2 test. According to the Cochrane Handbook, an I2 value of 0%–40% represents insignificant heterogeneity; 30%–60% represents moderate heterogeneity; 50%–90% represents substantial heterogeneity; >75% represents high heterogeneity.56 We will use a fixed-effect model for analysis if there is no substantial heterogeneity (p value ≥0.1 of the χ2 test and an I2 value ≤50%). Otherwise, a random-effect model will be employed, which can yield more conservative summary effect estimates and is more recommended when there is unexplained heterogeneity across studies.57
Additional analysis for the primary outcome
(1) Subgroup analyses based on the intervention format (personalised and non-personalised), interactivity (interactive and non-interactive) and technology (such as mobile applications and websites) will be performed if possible to explore an optimal web-based intervention regimen and to identify the potential sources of heterogeneity; (2) the funnel plot and Egger’s test will be employed to detect the potential publication bias if there is a sufficient number of included studies (N≥10).54
Patient and public involvement
Neither patients nor the public will be directly involved in the design, conducting, reporting or dissemination of this study because this systematic review will be based on publicly available studies.
Validity, reliability and rigour
The systematic review protocol was completed following the PRISMA-P guidelines.48 We will strictly follow the requirements of Cochrane Handbook54 and the best practise PRISMA guidelines58 when performing and reporting this systematic review.
Discussion
GDM has been demonstrated to be closely associated with considerable maternal and neonatal short-term and long-term complications.5 7 9 11 The traditional mode of GDM management is effective but requires intensive clinical input.15 18 In recent years, web-based interventions have become increasingly popular in the field of GDM management due to making treatments more accessible and affordable.16 31 33 However, the benefit of web-based interventions for pregnant women with GDM is controversial,15 27 32 and the existing systematic reviews36 37 also did not reach a consensus on this issue, which leads to confusion for clinical decision-making and restricts the application of these interventions. Hence, this paper presents a protocol for a systematic review based on all existing evidence from RCTs and CCTs to comprehensively investigate the multidimensional effectiveness of web-based interventions among pregnant women with GDM.
It is well known that maternal hyperglycaemic of variable severity is the most important clinical manifestation of GDM and the pathological basis of related complications.1 To this end, maternal glycaemic control will be used as the primary outcome in this review, reflected by four commonly measured parameters (HbA1c, FBG, 1hBG and 2hBG). Meanwhile, in order to elevate the comprehensive understanding of the effectiveness of web-based interventions, extensive secondary outcomes will also be assessed, including maternal behavioural outcomes, cognitive and attitudinal outcomes, mental health, maternal and neonatal clinical outcomes, as well as medical service utilisation and costs. The conclusions of this study will provide objective evidence on whether web-based interventions should be widely recommended for GDM management in future clinical practice.
In addition, three subgroup analyses regarding intervention format (personalised and non-personalised), interactivity (interactive and non-interactive) and technology (such as mobile applications and websites) will be performed. It is anticipated that the findings of subgroup analyses can enlighten health professionals on developing and implementing an optimal web-based intervention regimen for pregnant women with GDM and bring maximum benefits to the targeted crowd, clinicians and other relevant personnel.
However, several potential limitations to this study should be considered. First, the emerging use of web-based technologies in healthcare is relatively recent; hence, the number of studies regarding this topic may be limited. Second, given that the diagnostic criteria of GDM, gestational weeks at allocation and web-based intervention programmes are likely to be quite different, there may be high heterogeneity across studies; therefore, we plan to conduct subgroup analyses to overcome this heterogeneity. Finally, since we will only include RCTs and CCTs published in English, there may be a loss of studies written in other languages.
Ethics and dissemination
Ethical approval will not be required for this study as no identifiable patient information or privacy will be involved. The findings will be published and diffused in a peer-reviewed English journal or disseminated through an academic conference.
Supplementary Material
Acknowledgments
The authors thank Dr Cui Nianqi from the School of Medicine, Zhejiang University for the guidance on the development of this article.
Footnotes
Contributors: PPG, YJ and SWF contributed to the initial conception and design of the systematic review protocol. DDC, PX, XJW and MNM reviewed the initial framework and provided input. ZZX, WZ and QZ developed the database search strategy. PPG drafted the manuscript. PPG and ZZX critically revised the manuscript for important intellectual content. All authors approved the final version. SWF is the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.McIntyre HD, Catalano P, Zhang C, et al. Gestational diabetes mellitus. Nat Rev Dis Primers 2019;5:1–19. 10.1038/s41572-019-0098-8 10.1038/s41572-019-0098-8 [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation . IDF diabetes atlas, 2021. Available: https://diabetesatlas.org/atlas/tenthedition [Accessed 17 Feb 2022].
- 3.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007;30:S141–6. 10.2337/dc07-s206 [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep 2016;16:7. 10.1007/s11892-015-0699-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Relph S, Patel T, Delaney L, et al. Adverse pregnancy outcomes in women with diabetes-related microvascular disease and risks of disease progression in pregnancy: a systematic review and meta-analysis. PLoS Med 2021;18:e1003856. 10.1371/journal.pmed.1003856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kosinski C, Rossel J-B, Gross J, et al. Adverse metabolic outcomes in the early and late postpartum after gestational diabetes are broader than glucose control. BMJ Open Diabetes Res Care 2021;9:e002382. 10.1136/bmjdrc-2021-002382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Preda A, Pădureanu V, Moța M, et al. Analysis of maternal and neonatal complications in a group of patients with gestational diabetes mellitus. Medicina 2021;57:1170. 10.3390/medicina57111170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotzaeridi G, Blätter J, Eppel D, et al. Recurrence of gestational diabetes mellitus: to assess glucose metabolism and clinical risk factors at the beginning of a subsequent pregnancy. J Clin Med 2021;10:4794. 10.3390/jcm10204794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You H, Hu J, Liu Y, et al. Risk of type 2 diabetes mellitus after gestational diabetes mellitus: A systematic review & meta-analysis. Indian J Med Res 2021;154:62–77. 10.4103/ijmr.IJMR_852_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia 2019;62:905–14. 10.1007/s00125-019-4840-2 [DOI] [PubMed] [Google Scholar]
- 11.Eberle C, Ament C. Diabetic and metabolic programming: mechanisms altering the intrauterine milieu. ISRN Pediatr 2012;2012:975685. 10.5402/2012/975685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moholdt T, Hayman M, Shorakae S, et al. The role of lifestyle intervention in the prevention and treatment of gestational diabetes. Semin Reprod Med 2020;38:398–406. 10.1055/s-0040-1722208 [DOI] [PubMed] [Google Scholar]
- 13.Klein J, Charach R, Sheiner E. Treating diabetes during pregnancy. Expert Opin Pharmacother 2015;16:357–68. 10.1517/14656566.2015.988140 [DOI] [PubMed] [Google Scholar]
- 14.Carolan M, Gill GK, Steele C. Women’s experiences of factors that facilitate or inhibit gestational diabetes self-management. BMC Preg Child 2012;12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemelin A, Paré G, Bernard S, et al. Demonstrated cost-effectiveness of a telehomecare program for gestational diabetes mellitus management. Diabetes Technol Ther 2020;22:195–202. 10.1089/dia.2019.0259 [DOI] [PubMed] [Google Scholar]
- 16.Mackillop L, Hirst JE, Bartlett KJ, et al. Comparing the efficacy of a mobile phone-based blood glucose management system with standard clinic care in women with gestational diabetes: randomized controlled trial. JMIR Mhealth Uhealth 2018;6:e71. 10.2196/mhealth.9512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-Ferre N, Galindo M, Fernández MD, et al. The outcomes of gestational diabetes mellitus after a telecare approach are not inferior to traditional outpatient clinic visits. Int J Endocrinol 2010;2010:386941. 10.1155/2010/386941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig L, Sims R, Glasziou P, et al. Women’s experiences of a diagnosis of gestational diabetes mellitus: a systematic review. BMC Pregnancy Childbirth 2020;20:76. 10.1186/s12884-020-2745-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song S, Yuan B, Zhang L, et al. Increased inequalities in health resource and access to health care in rural China. Int J Environ Res Public Health 2018;16:49. 10.3390/ijerph16010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Im E-O, Chang SJ. Web-based interventions in nursing. Comput Inform Nurs 2013;31:94–102. 10.1097/NXN.0b013e3182771868 [DOI] [PubMed] [Google Scholar]
- 21.Al-Arkee S, Mason J, Lane DA, et al. Mobile apps to improve medication adherence in cardiovascular disease: systematic review and meta-analysis. J Med Internet Res 2021;23:e24190. 10.2196/24190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen D, Ye Z, Shao J, et al. Effect of electronic health interventions on metabolic syndrome: a systematic review and meta-analysis. BMJ Open 2020;10:e036927. 10.1136/bmjopen-2020-036927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sjöström M, Umefjord G, Stenlund H, et al. Internet-based treatment of stress urinary incontinence: 1- and 2-year results of a randomized controlled trial with a focus on pelvic floor muscle training. BJU Int 2015;116:955–64. 10.1111/bju.13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Feng H, Hu M, et al. Web-based interventions to improve mental health in home caregivers of people with dementia: meta-analysis. J Med Internet Res 2019;21:e13415. 10.2196/13415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calvache-Mateo A, López-López L, Heredia-Ciuró A, et al. Efficacy of web-based supportive interventions in quality of life in COPD patients, a systematic review and meta-analysis. Int J Environ Res Public Health 2021;18:182312692. 10.3390/ijerph182312692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mastrogiannis DS, Igwe E, Homko CJ. The role of telemedicine in the management of the pregnancy complicated by diabetes. Curr Diab Rep 2013;13:1–5. 10.1007/s11892-012-0352-x [DOI] [PubMed] [Google Scholar]
- 27.Bromuri S, Puricel S, Schumann R, et al. An expert personal health system to monitor patients affected by gestational diabetes mellitus: a feasibility study. J Ambient Intell Smart Environ 2016;8:219–37. 10.3233/AIS-160365 [DOI] [Google Scholar]
- 28.The National strategic roadmap for action. Chin Foreign Trade 2015;7. [Google Scholar]
- 29.Masao K. Measuring asia’s mobile transformation. Google asia pacific. Available: https://www.thinkwithgoogle.com/intl/en-apac/tools-resources/research-studies/measuring-asias-mobile-transformation/ [Accessed 1 Mar 2019].
- 30.Sayakhot P, Carolan-Olah M, Steele C. Use of a web-based educational intervention to improve knowledge of healthy diet and lifestyle in women with gestational diabetes mellitus compared to standard clinic-based education. BMC Preg Child 2016;16:208. 10.1186/s12884-016-0996-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo H, Zhang Y, Li P, et al. Evaluating the effects of mobile health intervention on weight management, glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus. J Endocrinol Invest 2019;42:709–14. 10.1007/s40618-018-0975-0 [DOI] [PubMed] [Google Scholar]
- 32.Yew TW, Chi C, Chan S-Y, et al. A randomized controlled trial to evaluate the effects of a smartphone application–based lifestyle coaching program on gestational weight gain, glycemic control, and maternal and neonatal outcomes in women with gestational diabetes mellitus: the SMART-GDM study. Diabetes Care 2021;44:456–63. 10.2337/dc20-1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang P, Lo W, He Z-lin, et al. Medical nutrition treatment of women with gestational diabetes mellitus by a telemedicine system based on smartphones. J Obstet Gynaecol Res 2018;44:1228–34. 10.1111/jog.13669 [DOI] [PubMed] [Google Scholar]
- 34.Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth 2021;9:e22881. 10.2196/22881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasekaba TM, Furler J, Young D, et al. Using technology to support care in gestational diabetes mellitus: quantitative outcomes of an exploratory randomised control trial of adjunct telemedicine for gestational diabetes mellitus (TeleGDM). Diabetes Res Clin Pract 2018;142:276–85. 10.1016/j.diabres.2018.05.049 [DOI] [PubMed] [Google Scholar]
- 36.Eberle C, Loehnert M, Stichling S. Effectivness of specific mobile health applications (mHealth-apps) in gestational diabtetes mellitus: a systematic review. BMC Pregnancy Childbirth 2021;21:808. 10.1186/s12884-021-04274-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau Y, Htun TP, Wong SN, et al. Efficacy of Internet-based self-monitoring interventions on maternal and neonatal outcomes in perinatal diabetic women: a systematic review and meta-analysis. J Med Internet Res 2016;18:e220. 10.2196/jmir.6153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie W, Dai P, Qin Y, et al. Effectiveness of telemedicine for pregnant women with gestational diabetes mellitus: an updated meta-analysis of 32 randomized controlled trials with trial sequential analysis. BMC Pregnancy Childbirth 2020;20:198. 10.1186/s12884-020-02892-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eberle C, Stichling S. Managing gestational diabetes mellitus during COVID-19 - maternal and neonatal outcomes using telemedical Approaches. JMIR Pediatr Parent 2021;4:e28630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li S-Y, Ouyang Y-Q, Qiao J, et al. Technology-supported lifestyle interventions to improve maternal-fetal outcomes in women with gestational diabetes mellitus: a meta-analysis. Midwifery 2020;85:102689. 10.1016/j.midw.2020.102689 [DOI] [PubMed] [Google Scholar]
- 41.Rasekaba TM, Furler J, Blackberry I, et al. Telemedicine interventions for gestational diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 2015;110:1–9. 10.1016/j.diabres.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 42.Ming W-K, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res 2016;18:e290. 10.2196/jmir.6556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Ofi EA, Mosli HH, Ghamri KA, et al. Management of postprandial hyperglycaemia and weight gain in women with gestational diabetes mellitus using a novel telemonitoring system. J Int Med Res 2019;47:754–64. 10.1177/0300060518809872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sung J-H, Lee DY, Min KP, et al. Peripartum management of gestational diabetes using a digital health care service: a pilot, randomized controlled study. Clin Ther 2019;41:2426–34. 10.1016/j.clinthera.2019.09.005 [DOI] [PubMed] [Google Scholar]
- 45.Garnweidner-Holme L, Henriksen L, Torheim LE, et al. Effect of the Pregnant+ smartphone APP on the dietary behavior of women with gestational diabetes mellitus: secondary analysis of a randomized controlled trial. JMIR Mhealth Uhealth 2020;8:e18614. 10.2196/18614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghasemi F, Vakilian K, Khalajinia Z. Comparing the effect of individual counseling with counseling on social application on self-care and quality of life of women with gestational diabetes. Prim Care Diabetes 2021;15:842–7. 10.1016/j.pcd.2021.05.009 [DOI] [PubMed] [Google Scholar]
- 47.Huang F, Zhang S, Tian Y, et al. Effect of mobile health based Peripartum management of gestational diabetes mellitus on postpartum diabetes: a randomized controlled trial. Diabetes Res Clin Pract 2021;175:108775. 10.1016/j.diabres.2021.108775 [DOI] [PubMed] [Google Scholar]
- 48.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang TS, Zhong WZ BL. Applied methodology for evidence-based medicine (2th version). Zhongnan University Press, 2014. [Google Scholar]
- 50.Thomas B, Ciliska D, Dobbins M. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews on evidence-based nursing 2004;1:176–84. [DOI] [PubMed] [Google Scholar]
- 51.Armijo-Olivo S, Stiles C, Hagen NA, et al. Assessment of study quality for systematic reviews: a comparison of the Cochrane collaboration risk of bias tool and the effective public health practice project quality assessment tool: methodological research. J Eval Clin Pract 2012;18:12–18. 10.1111/j.1365-2753.2010.01516.x [DOI] [PubMed] [Google Scholar]
- 52.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 53.Marshall S, Petocz P, Duve E, et al. The effect of replacing refined grains with whole grains on cardiovascular risk factors: a systematic review and meta-analysis of randomized controlled trials with grade clinical recommendation. J Acad Nutr Diet 2020;120:1859–83. 10.1016/j.jand.2020.06.021 [DOI] [PubMed] [Google Scholar]
- 54.Higgins JPT, Thomas J, Chandler J. Cochrane handbook for systematic reviews of interventions version 6.0. Available: https://training.cochrane.org/handbook2019
- 55.Olivier J, May WL, Bell ML. Relative effect sizes for measures of risk. Commun Stat Theory Methods 2017;46:6774–81. 10.1080/03610926.2015.1134575 [DOI] [Google Scholar]
- 56.Higgins JPT, Thomas J, Chandler J. Cochrane handbook for systematic reviews of interventions version 6.3, 2022. Available: www.training.cochrane.org/handbook2022
- 57.Chen H, Manning AK, Dupuis J. A method of moments estimator for random effect multivariate meta-analysis. Biometrics 2012;68:1278–84. 10.1111/j.1541-0420.2012.01761.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Page M, McKenzie J, Bossuyt P. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-061151supp001.pdf (101.4KB, pdf)