Summary
Upon embryo implantation, the uterine mucosa - the endometrium - transforms into a robust decidual matrix that accommodates the fetal placenta throughout pregnancy. This transition is driven by the differentiation of endometrial fibroblasts into specialised decidual cells. A synchronised influx of circulating natural killer (NK) cells and bone marrow-derived mesenchymal stem/progenitor cells (BM-MSC) is pivotal for decidual homeostasis and expansion in early pregnancy. We hypothesise that pathological signals interfering with the recruitment or activity of extrauterine cells at the maternal-fetal interface link miscarriage to subsequent adverse pregnancy outcomes, including further pregnancy losses and preterm labour. NK cells and BM-MSC are key homeostatic regulators in multiple tissues, pointing towards a shared aetiology between recurrent miscarriage and age-related disorders, including cardiometabolic disease. We propose the term ‘miscarriage syndrome’ to capture the health risks associated with miscarriage and discuss how this paradigm can inform clinical practice and accelerate the development of preventative strategies.
Keywords: Pregnancy, Miscarriage, Ageing, Preterm birth, Cardiovascular disease, Syndrome
An estimated 60% of human embryos are lost before or soon after implantation. This high attrition rate is attributed to intrinsic chromosomal instability in preimplantation embryos and the ability of the endometrium to engage in embryo biosensing and selection.1,2 Following implantation, most pregnancy failures occur so early that they escape detection whereas the remainder present as clinical miscarriages. The population prevalence of women with one, two, or three or more self-reported miscarriages is 10·8%, 1·9%, and 0·7%, respectively.3 Over 90% of losses occur in the first trimester of pregnancy.4 Maternal age is a major determinant of miscarriage rates with the risk rising sharply after the age of 34 years, reflecting the exponential increase in chromosome error rates in oocytes and fetal tissues.1 The risk of miscarriage further increases stepwise by 7–9% with each additional loss independently of maternal age.4,5 For example, at the age of 34 years, the likelihood of a live birth after one miscarriage is 82%, 68% after three losses, and 50% after 5 miscarriages.4
Although most women with recurrent miscarriage will go on to have a live birth, these pregnancies are at risk of preterm birth, fetal growth restriction, placental abruption and stillbirth.3 Each additional pregnancy loss compounds the risk of obstetric disorders in a future ongoing pregnancy.3,6 Recurrent miscarriage is also associated with cardiometabolic disease in later life, including diabetes, hyperlipidaemia, hypertension and a five-fold higher risk of myocardial infarction.7, 8, 9 These diseases manifest at earlier ages in women who have experienced recurrent miscarriage compared to the background population.9 Women with a history of recurrent miscarriage exhibit significantly higher 10-year cardiovascular risk scores when compared to control subjects of the same age.10 Obstetric disorders, including preterm birth, also increase the risk of age-related cardiometabolic disease.11 Further, ischaemic heart disease is more prevalent in parents of women who experience recurrent miscarriage, suggesting a genetic component.12
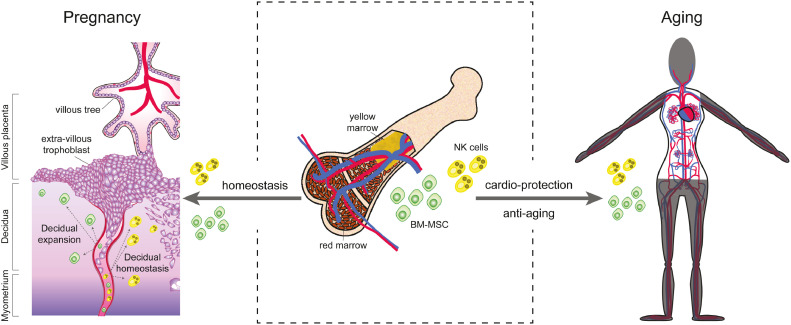
The 2021 ‘Lancet Series on Miscarriage’ summarised the physical and psychological health burdens associated with early pregnancy loss and called for research into the mechanisms that link recurrent miscarriage to obstetric disorders and age-related disorders.3,13,14 Here, we hypothesise that the critical dependency of the endometrium on the recruitment of extrauterine cells involved in tissue homeostasis and repair in early gestation explains the health implications of miscarriage beyond the index pregnancy (Figure 1). We propose the term ‘miscarriage syndrome’ to emphasise the unmet need for individualised risk assessment and tailored surveillance following miscarriage.
Figure 1.
Bone marrow-derived mesenchymal stem/progenitor cells (BM-MSC) and natural (NK) killer cells (middle panel) are critical for decidual expansion and homeostasis at the maternal-fetal interface (left panel) and confer protection against age-related cardiovascular disease (right panel). For detailed discussion, see text.
Miscarriage syndrome: a common pathological pathway
During the reproductive years, the endometrium undergoes iterative cycles of tissue breakdown and repair. In each ovulatory cycle, oestrogen-dependent proliferation of resident epithelial and stromal cells is followed by progesterone-dependent differentiation, culminating in a four-day window during which embryo implantation can take place.15 The implantation window opens with acute remodelling of glands and stroma, a process that ultimately results in menstrual breakdown or, in case of pregnancy, formation of a robust matrix, termed decidua, which accommodates the placenta. Secretion of chemokines and transient tissue oedema promote influx of innate immune cells, foremost natural killer (NK) cells, and bone marrow-derived mesenchymal stem/progenitor cells (BM-MSC).16, 17, 18 In parallel, stromal cells transform into stress-resistant, progesterone-dependent decidual cells upon closure of the window.15 Some fibroblasts damaged by replication stress during the growth phase fail to differentiate and acquire an acute senescent phenotype.17,19 Senescence refers to an evolutionarily conserved cell state characterised by permanent growth arrest, resistance to apoptosis and a complex senescence-associated secretory phenotype (SASP).20 Senescent endometrial fibroblasts secrete an abundance of extracellular matrix proteins and proteinases, chemokines, and inflammatory mediators, which create a permissive implantation environment.21 Although initially few in number, prolonged SASP production induces secondary senescence in neighbouring decidual cells, tissue inflammation, influx of neutrophils and menstrual breakdown.16,19 Upon successful embryo implantation, progesterone-dependent decidual cells are maintained and counteract the endometrial propensity for self-destruction by secreting interleukin-15 and other factors that instruct uterine NK cells to eliminate senescent fibroblasts.1,16,19In addition, differentiation of BM-MSC into distinct prolactin-producing decidual cells compensates for cellular attrition and confers plasticity on the nascent maternal-fetal interface.17,18
Thus, transformation of the cycling endometrium into the decidua of pregnancy depends critically on uterine chemokine signals, transvascular migration of circulating NK cells and BM-MSC, and their proliferative expansion and phenotypic differentiation into effective homeostatic regulators of the maternal-fetal interface.16, 17, 18,22, 23, 24 Interference at any stage of this pathway can potentially compromise placenta formation and cause miscarriage. For example, recurrent miscarriage is associated with loss of BM-MSC during the implantation window with the level of depletion correlating to the number of previous losses.25 The abundance of clonogenic stromal cells in midluteal endometrium also correlates negatively with increasing body mass index (BMI), which is in keeping with the observation that obesity is associated with higher recurrence rates of miscarriage.3,26,27 Maternal obesity also drives functional alterations in uterine NK cells. Notably, other immune cells also play important roles in maintaining the integrity of the maternal-fetal interface in early gestation, including dentritic cells, activated regulatory T cells, and anti-inflammatory (M2) macrophages.16, 17, 18,22, 23, 24,28, 29, 30
The literature is replete with reports associating recurrent miscarriage with abnormalities in circulating NK and other immune cells.31 There is, however, no consensus on the precise nature of immune cell dysfunctions nor is it clear how they impact on the maternal-fetal interface. Further, a large Norwegian registry linkage study reported that most pre-existing chronic disorders, including autoimmune disorders, do not impact significantly on the risk of miscarriage. One notable exception is cardiovascular disease, especially atherosclerosis.32 Following implantation, plugging of the decidual spiral arterioles by invading trophoblast precludes perfusion of the developing placenta during the first 10–12 weeks of pregnancy.33, 34, 35, 36 Hence, vascular disease may impact primarily on transvascular migration of extrauterine cells in early pregnancy. BM-MSC also give rise to uterine endothelial progenitors,18 suggesting an integral link between pre-existing vascular disease and aberrant decidual transformation of the endometrium. As pregnancy progresses into the second trimester, the uteroplacental arteries convert into large fibrinoid vessels devoid of endothelium and other vascular structures, thus curtailing influx of extrauterine cells into the centre of the placental bed. Therefore, the level of plasticity of the maternal-fetal interface may not only be determined in early gestation, but homeostatic imbalances below the threshold for miscarriage could become amplified beyond the second trimester, leading to preterm labour and other obstetric disorders.
Transient emergence of senescent cells causing acute inflammation and tissue remodelling is not confined to the endometrium but implicated in a myriad of physiological processes, ranging from development to wound healing. By contrast, accumulation of senescent cells and SASP-dependent chronic tissue inflammation, designated ‘inflammaging’, are hallmarks of ageing and age-related disorders, such as cardiovascular disease and type 2 diabetes.20,37, 38, 39, 40 Multiple factors contribute to inflammaging, including genetic susceptibility, chronic infection, obesity, and defective immune clearance of senescent cells.20 Intriguingly, abnormalities in circulating NK cells reportedly persist long after the pregnancy loss,31 raising the possibility that aberrant immunological ‘memory’ contributes to the susceptibility of miscarriage patients to age-related disorders. Further, pregnancy failure may unmask pre-existing deficiencies in BM-MSC. Loss of BM-MSC ‘fitness’ in terms of anti-inflammatory and immunomodulatory properties is also a hallmark of ageing and implicated in cardiovascular disease.41
Miscarriage syndrome: clinical and research implications
Current definitions of recurrent miscarriage are based on an arbitrary number of pregnancy losses, usually two or three, and do not capture the escalating risk of adverse health outcomes with each additional pregnancy loss.3 We propose that clinical management of early pregnancy loss should include individualised assessment of the risk of ‘miscarriage syndrome’ to inform women and guide healthcare professionals in determining the level of surveillance in future pregnancies and beyond. Data from nationwide, registry-based cohort studies already enable enumeration of the general recurrence risk of miscarriage based on maternal age and the number and sequence of prior losses and live births.4 We suggest that inclusion of risk factors of cardiometabolic disease (e.g., ethnicity, BMI, lifestyle factors and laboratory tests) will refine individualised predictions of health risks. In a recent scientific statement, the American Heart Association (AHA) emphasised the importance of active interventions to lower the risk of future cardiovascular disease following a pregnancy affected by preterm birth, preeclampsia or other obstetrical disorders.42 Apart from recommending (life-long) heart-healthy diet and adequate physical activity, the AHA also called for studies evaluating pharmacoprevention of primary cardiovascular disease following adverse pregnancy outcome. Adopting the term ‘miscarriage syndrome’ will not only render these recommendations integral to the management of miscarriage by healthcare professionals but - in all probability – also enhance patient compliance.
A syndromic approach to miscarriage, and especially recurrent miscarriage, highlights the need for large prospective cohort studies such as Tommy's Net in the UK, which captures clinical outcome data in real-time.43 Our ‘shared aetiology’ hypothesis calls for an integrated research approach to understand the contribution of systemic versus uterine perturbations in relaying obstetric and age-related risks associated with miscarriage. It also points towards novel treatment strategies centred on targeting endometrial homeostasis. For example, in mice, transfer of wild-type BM-MSC by bone marrow transplantation restores endometrial function and prevents pregnancy loss in animals lacking Homeobox a11, a key decidual transcription factor.18 Echoing these findings, a recent pilot trial reported that dipeptidyl-peptidase IV inhibitors enhance pre-pregnancy endometrial recruitment of BM-MSC in recurrent miscarriage patients by amplifying uterine chemokine signalling.24 Further, senolytics, an emerging class of drugs that selectivily kill senescent cells, are studied intensively for their potential to prevent age-related cardiometabolic disease and increase heatlhy lifespan.20,37,39,44, 45, 46 A recent study using a complex uterine organoid system highlighted the potential of senolytics as novel endometrial therapeutics.21 Importantly, it seems likely that therapeutic interventions that safeguard endometrial homeostasis before and after embryo implantation will not only reduce miscarriage rates but also mitigate against the risk of preterm birth and other obstetric disorders.
In summary, the plasticity and integrity of the maternal-fetal interface in pregnancy critically depends on massive recruitment of extrauterine BM-MSC and immune cells (Figure 1). We propose that this dependency makes early pregnancy a ‘stress-test’ of a physiological system also critical for tissue homeostasis upon ageing. Miscarriage may not only ‘unmask’ an underlying defect but the likelihood of a defect increases with each additional loss, thus accounting for the escalating risk of obstetrical disorders and cadiometabolic disease in later life with higher-order miscarriages. While pregnancy failure can be an isolated event, for example caused by fetal aneuploidy, it is imperative to distinguish this from ‘miscarriage syndrome’, which requires enhanced surveillance and intervention to safeguard the health of babies and their mothers in future pregnancies and beyond.
Contributors
All authors contributed equally to conceptualisation, writing-original draft, and writing-review & editing. All authors have read and authorised the final version of the manuscript.
Declaration of interests
The authors have no interests to declare.
References
- 1.Brosens JJ, Bennett PR, Abrahams VM, et al. Maternal selection of human embryos in early gestation: insights from recurrent miscarriage. Semin Cell Dev Biol [Internet] 2022 doi: 10.1016/j.semcdb.2022.01.007. https://www.sciencedirect.com/science/article/pii/S1084952122000155?via%3Dihub [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brosens JJ, Salker MS, Teklenburg G, et al. Uterine selection of human embryos at implantation. Sci Rep [Internet] 2014;4(1):3894. doi: 10.1038/srep03894. https://pubmed.ncbi.nlm.nih.gov/24503642/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet [Internet] 2021;397(10285):1658–1667. doi: 10.1016/S0140-6736(21)00682-6. https://pubmed.ncbi.nlm.nih.gov/33915094/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 4.Kolte AM, Westergaard D, Lidegaard Ø, Brunak S, Nielsen HS. Chance of live birth: a nationwide, registry-based cohort study. Hum Reprod [Internet] 2021;36(4):1065–1073. doi: 10.1093/humrep/deaa326. https://pubmed.ncbi.nlm.nih.gov/33394013/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 5.Magnus MC, Wilcox AJ, Morken N-H, Weinberg CR, Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ [Internet] 2019;364:l869. doi: 10.1136/bmj.l869. https://pubmed.ncbi.nlm.nih.gov/30894356/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu CQ, Nichols K, Carwana M, Cormier N, Maratta C. Preterm birth after recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril [Internet] 2022;117(4):811–819. doi: 10.1016/j.fertnstert.2022.01.004. https://pubmed.ncbi.nlm.nih.gov/35131102/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 7.Horn J, Tanz LJ, Stuart JJ, et al. Early or late pregnancy loss and development of clinical cardiovascular disease risk factors: a prospective cohort study. BJOG [Internet] 2019;126(1):33–42. doi: 10.1111/1471-0528.15452. https://pubmed.ncbi.nlm.nih.gov/30144277/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kharazmi E, Dossus L, Rohrmann S, Kaaks R. Pregnancy loss and risk of cardiovascular disease: a prospective population-based cohort study (EPIC-Heidelberg) Heart [Internet] 2011;97(1):49–54. doi: 10.1136/hrt.2010.202226. https://pubmed.ncbi.nlm.nih.gov/21123827/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 9.Ranthe MF, Andersen EAW, Wohlfahrt J, Bundgaard H, Melbye M, Boyd HA. Pregnancy loss and later risk of atherosclerotic disease. Circulation [Internet] 2013;127(17):1775–1782. doi: 10.1161/CIRCULATIONAHA.112.000285. https://pubmed.ncbi.nlm.nih.gov/23536362/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 10.Wagner MM, Beshay MM, Rooijakkers S, et al. Increased cardiovascular disease risk in women with a history of recurrent miscarriage. Acta Obstet Gynecol Scand [Internet] 2018;97(10):1192–1199. doi: 10.1111/aogs.13392. https://pubmed.ncbi.nlm.nih.gov/29806956/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robbins CL, Hutchings Y, Dietz PM, Kuklina EV, Callaghan WM. History of preterm birth and subsequent cardiovascular disease: a systematic review. Am J Obstet Gynecol [Internet] 2014;210(4):285–297. doi: 10.1016/j.ajog.2013.09.020. https://pubmed.ncbi.nlm.nih.gov/24055578/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith GCS, Wood AM, Pell JP, Hattie J. Recurrent miscarriage is associated with a family history of ischaemic heart disease: a retrospective cohort study: Recurrent miscarriage and family history of IHD. BJOG [Internet] 2011;118(5):557–563. doi: 10.1111/j.1471-0528.2010.02890.x. https://pubmed.ncbi.nlm.nih.gov/21244619/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 13.Coomarasamy A, Gallos ID, Papadopoulou A, et al. Sporadic miscarriage: evidence to provide effective care. Lancet [Internet] 2021;397(10285):1668–1674. doi: 10.1016/S0140-6736(21)00683-8. https://pubmed.ncbi.nlm.nih.gov/33915095/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 14.Coomarasamy A, Dhillon-Smith RK, Papadopoulou A, et al. Recurrent miscarriage: evidence to accelerate action. Lancet [Internet] 2021;397(10285):1675–1682. doi: 10.1016/S0140-6736(21)00681-4. https://pubmed.ncbi.nlm.nih.gov/33915096/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 15.Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev [Internet] 2014;35(6):851–905. doi: 10.1210/er.2014-1045. https://pubmed.ncbi.nlm.nih.gov/25141152/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 16.Brighton PJ, Maruyama Y, Fishwick K, et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. Elife [Internet] 2017;6 doi: 10.7554/eLife.31274. https://pubmed.ncbi.nlm.nih.gov/29227245/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diniz-da-Costa M, Kong C-S, Fishwick KJ, et al. Characterization of highly proliferative decidual precursor cells during the window of implantation in human endometrium. Stem Cells [Internet] 2021;39(8):1067–1080. doi: 10.1002/stem.3367. https://pubmed.ncbi.nlm.nih.gov/33764639/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 18.Tal R, Shaikh S, Pallavi P, et al. Adult bone marrow progenitors become decidual cells and contribute to embryo implantation and pregnancy. PLoS Biol [Internet] 2019;17(9) doi: 10.1371/journal.pbio.3000421. https://pubmed.ncbi.nlm.nih.gov/31513564/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas ES, Vrljicak P, Muter J, et al. Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Commun Biol [Internet] 2020;3(1):37. doi: 10.1038/s42003-020-0763-1. https://pubmed.ncbi.nlm.nih.gov/31965050/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature [Internet] 2019;571(7764):183–192. doi: 10.1038/s41586-019-1365-2. https://pubmed.ncbi.nlm.nih.gov/31292558/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rawlings TM, Makwana K, Taylor DM, et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. Elife [Internet] 2021;10 doi: 10.7554/eLife.69603. https://pubmed.ncbi.nlm.nih.gov/34487490/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang Y-Y, Lyu F, Abuwala N, et al. Chemokine C-X-C receptor 4 (CXCR4) mediates recruitment of bone marrow-derived nonhematopoietic and immune cells to the pregnant uterus. Biol Reprod [Internet] 2022 doi: 10.1093/biolre/ioac029. https://pubmed.ncbi.nlm.nih.gov/35134114/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strunz B, Bister J, Jönsson H, et al. Continuous human uterine NK cell differentiation in response to endometrial regeneration and pregnancy. Sci Immunol [Internet] 2021;6(56):eabb7800. doi: 10.1126/sciimmunol.abb7800. https://pubmed.ncbi.nlm.nih.gov/33617461/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 24.Tewary S, Lucas ES, Fujihara R, et al. Impact of sitagliptin on endometrial mesenchymal stem-like progenitor cells: a randomised, double-blind placebo-controlled feasibility trial. EBioMedicine [Internet] 2020;51(102597) doi: 10.1016/j.ebiom.2019.102597. https://pubmed.ncbi.nlm.nih.gov/31928963/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas ES, Dyer NP, Murakami K, et al. Loss of endometrial plasticity in recurrent pregnancy loss: MSC deficiency in recurrent miscarriage. Stem Cells [Internet] 2016;34(2):346–356. doi: 10.1002/stem.2222. https://pubmed.ncbi.nlm.nih.gov/26418742/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 26.Murakami K, Bhandari H, Lucas ES, et al. Deficiency in clonogenic endometrial mesenchymal stem cells in obese women with reproductive failure–a pilot study. PLoS One [Internet] 2013;8(12):e82582. doi: 10.1371/journal.pone.0082582. https://pubmed.ncbi.nlm.nih.gov/24340046/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavalcante MB, Sarno M, Peixoto AB. Obesity and recurrent miscarriage: a systematic review and meta-analysis: obesity and recurrent miscarriage. J Obstet Gynaecol Res [Internet] 2019;45(1):30–38. doi: 10.1111/jog.13799. https://pubmed.ncbi.nlm.nih.gov/30156037/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 28.Schumacher A, Sharkey DJ, Robertson SA, Zenclussen AC. Immune cells at the fetomaternal interface: how the microenvironment modulates immune cells to foster fetal development. J Immunol [Internet] 2018;201(2):325–334. doi: 10.4049/jimmunol.1800058. https://pubmed.ncbi.nlm.nih.gov/29987001/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 29.Plaks V, Birnberg T, Berkutzki T, et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest [Internet] 2008;118(12):3954–3965. doi: 10.1172/JCI36682. https://pubmed.ncbi.nlm.nih.gov/19033665/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solano ME. Decidual immune cells: guardians of human pregnancies. Best Pract Res Clin Obstet Gynaecol [Internet] 2019;60:3–16. doi: 10.1016/j.bpobgyn.2019.05.009. https://pubmed.ncbi.nlm.nih.gov/31285174/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 31.Toth B, Zhu L, Karakizlis H, et al. NK cell subsets in idiopathic recurrent miscarriage and renal transplant patients. J Reprod Immunol [Internet] 2020;138(103098) doi: 10.1016/j.jri.2020.103098. https://pubmed.ncbi.nlm.nih.gov/32045760/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 32.Magnus MC, Morken N-H, Wensaas K-A, Wilcox AJ, Håberg SE. Risk of miscarriage in women with chronic diseases in Norway: a registry linkage study. PLoS Med [Internet] 2021;18(5) doi: 10.1371/journal.pmed.1003603. https://pubmed.ncbi.nlm.nih.gov/33970911/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James JL, Saghian R, Perwick R, Clark AR. Trophoblast plugs: impact on utero-placental haemodynamics and spiral artery remodelling. Hum Reprod [Internet] 2018;33(8):1430–1441. doi: 10.1093/humrep/dey225. https://pubmed.ncbi.nlm.nih.gov/29955830/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 34.Allerkamp HH, Clark AR, Lee TC, Morgan TK, Burton GJ, James JL. Something old, something new: digital quantification of uterine vascular remodelling and trophoblast plugging in historical collections provides new insight into adaptation of the utero-placental circulation. Hum Reprod [Internet] 2021;36(3):571–586. doi: 10.1093/humrep/deaa303. https://pubmed.ncbi.nlm.nih.gov/33600565/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 35.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol [Internet] 2011;204(3):193–201. doi: 10.1016/j.ajog.2010.08.009. https://pubmed.ncbi.nlm.nih.gov/21094932/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies. Am J Obstet Gynecol [Internet] 2002;187(5):1416–1423. doi: 10.1067/mob.2002.127305. https://pubmed.ncbi.nlm.nih.gov/12439541/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 37.Sweeney M, Cook SA, Gil J. Therapeutic opportunities for senolysis in cardiovascular disease. FEBS J [Internet] 2022 doi: 10.1111/febs.16351. https://pubmed.ncbi.nlm.nih.gov/35015342/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Childs BG, Zhang C, Shuja F, et al. Senescent cells suppress innate smooth muscle cell repair functions in atherosclerosis. Nat Aging [Internet] 2021;1(8):698–714. doi: 10.1038/s43587-021-00089-5. https://pubmed.ncbi.nlm.nih.gov/34746803/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Postmus AC, Sturmlechner I, Jonker JW, van Deursen JM, van de Sluis B, Kruit JK. Senescent cells in the development of cardiometabolic disease. Curr Opin Lipidol [Internet] 2019;30(3):177–185. doi: 10.1097/MOL.0000000000000602. https://pubmed.ncbi.nlm.nih.gov/30913069/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med [Internet] 2015;21(12):1424–1435. doi: 10.1038/nm.4000. https://pubmed.ncbi.nlm.nih.gov/26646499/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li F, Guo X, Chen S-Y. Function and therapeutic potential of mesenchymal stem cells in atherosclerosis. Front Cardiovasc Med [Internet] 2017;4:32. doi: 10.3389/fcvm.2017.00032. https://pubmed.ncbi.nlm.nih.gov/28589127/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American heart association: a scientific statement from the American heart association. Circulation [Internet] 2021;143(18):e902–e916. doi: 10.1161/CIR.0000000000000961. https://pubmed.ncbi.nlm.nih.gov/33779213/ [cited 2022 May 27]; Available from: [DOI] [PubMed] [Google Scholar]
- 43.Shields R, Khan O, Lim Choi Keung S, et al. Quantitative assessment of pregnancy outcome following recurrent miscarriage clinic care: a prospective cohort study. BMJ Open [Internet] 2022;12(2) doi: 10.1136/bmjopen-2021-052661. https://pubmed.ncbi.nlm.nih.gov/35110317/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Childs BG, Li H, van Deursen JM. Senescent cells: a therapeutic target for cardiovascular disease. J Clin Invest [Internet] 2018;128(4):1217–1228. doi: 10.1172/JCI95146. https://pubmed.ncbi.nlm.nih.gov/29608141/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Childs BG, Gluscevic M, Baker DJ, et al. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov [Internet] 2017;16(10):718–735. doi: 10.1038/nrd.2017.116. https://pubmed.ncbi.nlm.nih.gov/28729727/ [cited 2022 May 27]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mkrtchyan GV, Abdelmohsen K, Andreux P, et al. ARDD 2020: from aging mechanisms to interventions. Aging (Albany NY) 2020;12(24):24484–24503. doi: 10.18632/aging.202454. https://pubmed.ncbi.nlm.nih.gov/33378272/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]