Key Points
Question
Is the use of prothrombin complex concentrate (PCC) a safe and effective alternative to using plasma in patients undergoing cardiac surgery?
Findings
In this randomized clinical trial of 100 adult cardiac surgical patients, there was no difference in postoperative bleeding between patients receiving PCC or plasma. Fewer patients in the PCC group required intraoperative red blood cell transfusion after treatment (7 [14%] vs 15 [31%]), and adverse outcomes were similar between groups.
Meaning
These results suggest that, in patients with post–cardiopulmonary bypass coagulopathy and bleeding, PCC appears to be a safe and effective alternative to plasma transfusion.
Abstract
Importance
Post–cardiopulmonary bypass (CPB) coagulopathy and bleeding are among the most common reasons for blood product transfusion in surgical practices. Current retrospective data suggest lower transfusion rates and blood loss in patients receiving prothrombin complex concentrate (PCC) compared with plasma after cardiac surgery.
Objective
To analyze perioperative bleeding and transfusion outcomes in patients undergoing cardiac surgery who develop microvascular bleeding and receive treatment with either PCC or plasma.
Design, Setting, and Participants
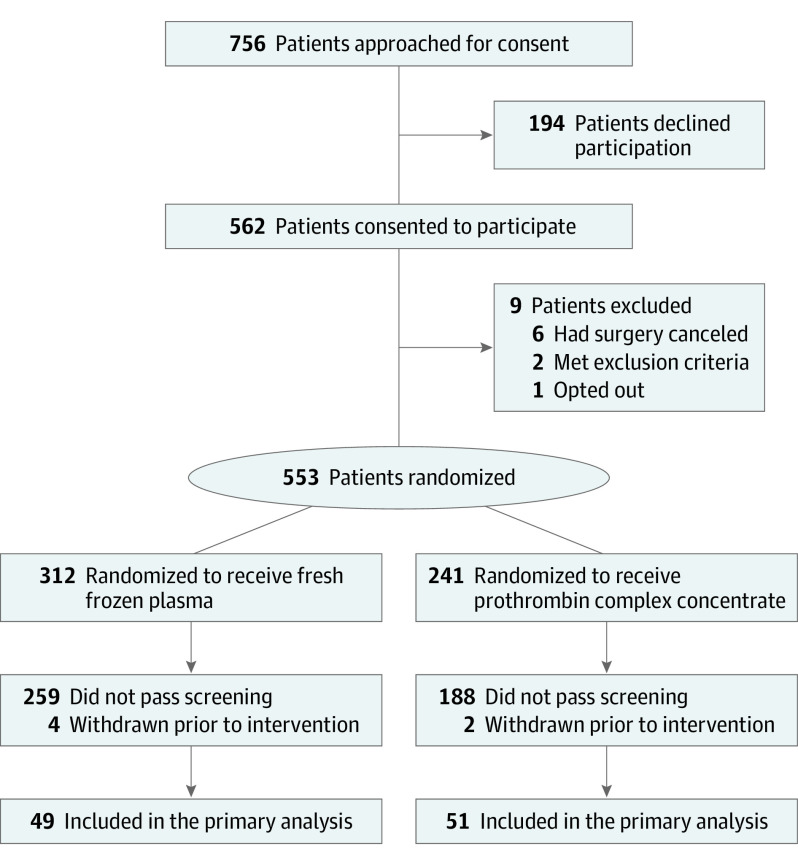
A single-institution, prospective, randomized clinical trial performed at a high-volume cardiac surgical center. Patients were aged 18 years or older and undergoing cardiac surgery with CPB. Patients undergoing complex cardiac surgical procedures (eg, aortic replacement surgery, multiple procedures, or repeated sternotomy) were preferentially targeted for enrollment. During the study period, 756 patients were approached for enrollment, and 553 patients were randomized. Of the 553 randomized patients, 100 patients met criteria for study intervention.
Interventions
Patients with excessive microvascular bleeding, a prothombin time (PT) greater than 16.6 seconds, and an international normalized ratio (INR) greater than 1.6 were randomized to receive treatment with either PCC or plasma. The PCC dose was 15 IU/kg or closest standardized dose; the plasma dose was a suggested volume of 10 to 15 mL/kg rounded to the nearest unit.
Main Outcomes and Measures
The primary outcome was postoperative bleeding (chest tube output) from the initial postsurgical intensive care unit admission through midnight on postoperative day 1. Secondary outcomes were PT/INR, rates of intraoperative red blood cell (RBC) transfusion after treatment, avoidance of allogeneic transfusion from the intraoperative period to the end of postoperative day 1, postoperative bleeding, and adverse events.
Results
One hundred patients (mean [SD] age, 66.8 [13.7] years; 61 [61.0%] male; and 1 [1.0%] Black, 1 [1.0%] Hispanic, and 98 [98.0%] White) received the study intervention (49 plasma and 51 PCC). There was no significant difference in chest tube output between the plasma and PCC groups (median [IQR], 1022 [799-1575] mL vs 937 [708-1443] mL). After treatment, patients in the PCC arm had a greater improvement in PT (effect estimate, −1.37 seconds [95% CI, −1.91 to −0.84]; P < .001) and INR (effect estimate, −0.12 [95% CI, −0.16 to −0.07]; P < .001). Fewer patients in the PCC group required intraoperative RBC transfusion after treatment (7 of 51 patients [13.7%] vs 15 of 49 patients [30.6%]; P = .04); total intraoperative transfusion rates were not significantly different between groups. Seven (13.7%) of 51 patients receiving PCCs avoided allogeneic transfusion from the intraoperative period to the end of postoperative day 1 vs none of those receiving plasma. There were no significant differences in postoperative bleeding, transfusions, or adverse events.
Conclusions and Relevance
The results of this study suggest a similar overall safety and efficacy profile for PCCs compared with plasma in this clinical context, with fewer posttreatment intraoperative RBC transfusions, improved PT/INR correction, and higher likelihood of allogeneic transfusion avoidance in patients receiving PCCs.
Trial Registration
ClinicalTrials.gov Identifier: NCT02557672
This single-institution randomized clinical trial of 100 adult cardiac surgical patients assesses the safety and efficacy of prothrombin complex concentrate vs plasma for post–cardiopulmonary bypass coagulopathy and bleeding.
Introduction
Post–cardiopulmonary bypass (CPB) coagulopathy and bleeding are among the most common reasons for blood product transfusion in surgical practices today.1 Bleeding after CPB is multifactorial and related largely to blood exposure to bypass circuit components. Coagulation aberrations include thrombocytopenia, platelet dysfunction, coagulation factor consumption and dilution, hyperfibrinolysis, and hypofibrinogenemia.2 Historically, approximately 15% of patients with factor-mediated coagulopathy and bleeding after CPB receive plasma transfusions.1,3 Coagulation factor concentrations within plasma are variable, and large transfused volumes are often necessary to correct factor deficiencies. In addition, plasma transfusion carries the risk of transfusion-related acute lung injury, transfusion-associated circulatory overload, and infectious and allergic complications, among others.4
In recent years, the off-label use of prothrombin complex concentrates (PCCs) for the treatment of coagulation factor–mediated perioperative bleeding in cardiac surgical patients has steadily increased. Distinct advantages to PCC administration include higher factor concentration with significantly less infusate volume, ambient storage (ie, at room temperature) with rapid reconstitution, not having to worry about ABO blood group compatibility, and lower risk of transfusion-related complications.5 To our knowledge, data evaluating the use of PCCs in this population are limited to retrospective studies and 2 prospective pilot studies, which have demonstrated faster return of the international normalized ratio (INR) to the target range, reduced blood loss and transfusion requirements, more effective hemostasis, and an adverse event rate similar to that of plasma transfusion.3,6,7,8,9,10,11,12,13,14,15
The purpose of this study was to analyze perioperative outcomes in patients undergoing CPB surgery, with particular focus on bleeding, transfusion, and thrombotic complications. We hypothesized that PCC use would reduce bleeding and transfusion requirements in cardiac surgical patients without increasing thrombotic complications.
Methods
Study Design and Enrollment
This prospective randomized clinical trial was approved by the Mayo Clinic College of Medicine and Science (Rochester, Minnesota) Institutional Review Board. Enrollment began on August 22, 2016, and ended May 4, 2021. The trial protocol is available in Supplement 1. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Adult patients aged 18 years and older undergoing cardiac surgery with use of CPB were eligible to be screened for inclusion in the study. Patients undergoing complex cardiac surgical procedures (eg, aortic replacement surgery, multiple procedures, or repeated sternotomy) were preferentially targeted for enrollment. Patients provided written informed consent and were enrolled at the time of a preoperative visit. Enrolled patients were randomized to receive either PCC or plasma and were included for analysis if they met the intraoperative inclusion criteria (Figure 1), which included evidence of excessive microvascular bleeding in the surgical field as determined by the surgical team and a prothrombin time (PT) greater than 16.6 seconds/INR greater than 1.6. Intraoperative exclusion criteria included a fibrinogen level less than 144 mg/dL on initial postsurgery laboratory testing (to convert milligrams per deciliter to grams per liter, multiply by 0.01), life-threatening bleeding necessitating transfusion of hemostatic products (PCC or plasma) before the study intervention time point, circumstances in which the safety of the patient could be jeopardized by continued adherence to the study protocol, and extracorporeal membrane oxygenation requirement intraoperatively or postoperatively. A full list of study inclusion and exclusion criteria is found in eAppendix 1 in Supplement 2.
Figure 1. Patient Flow Diagram.
The randomization assignments were disclosed to study staff. The study was terminated once 100 patients received treatment without exclusion. At scheduled intervals, a data and safety monitoring board not affiliated with the study reviewed aggregated data summaries with explanations of the seriousness of adverse events and their relatedness to the study.
Interventions
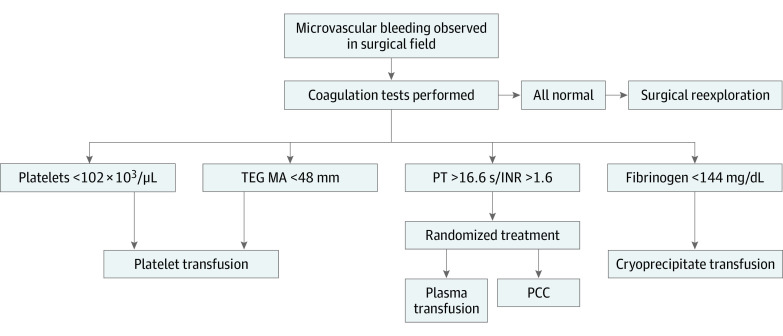
Anticoagulation included use of 400 IU/kg of heparin, with the goal of an activated clotting time (ACT) greater than 400 seconds prior to initiation of CPB. After completion of CPB, patients received protamine at a dose of 0.01 mg/unit of heparin, given with a target ACT of within 10% of the baseline value. Ten minutes after protamine administration, the ACT, platelet count, PT/INR, activated partial thromboplastin time (aPTT), and fibrinogen level were collected. Evaluation and determination of excessive microvascular bleeding in the surgical field occurred 10 minutes after return of ACT to within 10% of baseline by the surgical and anesthesiology teams. Patients with excessive microvascular bleeding and a PT/INR greater than 16.6 seconds/1.6 received treatment with either PCC or plasma. The PT/INR of 16.6 seconds/1.6 represents the cutoff for plasma transfusion within the institutional transfusion protocol used prior to and throughout the study period.1,16 The dose of prothrombin complex concentrate, human (CSL Behring), was 15 IU/kg or closest standardized dose. The plasma dosage was a suggested volume of 10 to 15 mL/kg rounded to the nearest unit. Transfusion of coagulation products other than PCC or plasma (eg, platelets and cryoprecipitate) followed the institutional algorithm (Figure 2).1
Figure 2. Institutional Intraoperative Transfusion Algorithm.
INR indicates international normalized ratio; PCC, prothrombin complex concentrate; PT, prothrombin time; and TEG MA, maximum amplitude on thromboelastography. Reprinted with permission of Wolters Kluwer Health Inc.1
SI conversion factors: To convert platelet count to ×109/L, multiply by 1; to convert fibrinogen to grams per liter, multiply by 0.01.
Patients who received treatment (PCC or plasma) and continued to demonstrate microvascular bleeding 10 minutes after product administration underwent repeated laboratory evaluation. Therapy ensued following the institutional transfusion algorithm (redosing of PCC not recommended). Resolution of microvascular bleeding was the end point for the need of transfusion rather than normalization of values on coagulation testing.
Red blood cell (RBC) transfusion triggers were not standardized in cardiac surgical patients during the study period; however, institutionally endorsed guidelines for RBC transfusion were available for all surgeons. In cardiac surgery, it is common practice to aim for a hemoglobin concentration of 8.0 g/dL or higher at surgical completion and in the early postoperative period (eg, the first 48 hours). At the completion of the operation, all patients were transferred to the intensive care unit (ICU), where an institutional transfusion algorithm was in place throughout the study period.
Data Collection and Outcomes
Baseline, intraoperative, and postoperative data were prospectively collected and recorded. All patients were followed up for 30 days after the procedure, and data related to transfusions, outcomes, and adverse events were recorded. The primary outcome was chest tube output from the initial postsurgical ICU admission through midnight on postoperative day 1. For reference, the surgical day was considered postoperative day 0. Secondary outcomes included intraoperative and postoperative transfusions, estimated blood loss, laboratory value changes, reoperation for bleeding within 48 hours of initial surgery, hospital and ICU length of stay (LOS), and 30-day mortality. A postdischarge follow-up assessment was made between 30 and 37 days.
Adverse events occurring within 30 days of operation included mortality, thromboembolic events, acute kidney injury,17 new kidney replacement therapy, acute respiratory distress syndrome,18 and transfusion-related complications.19 Adverse event definitions are provided in eAppendix 2 in Supplement 2.
Statistical Analysis
In all cases, patients were analyzed according to their randomized treatment (ie, intention to treat). Patient and procedural characteristics are summarized using median (IQR) for continuous variables and frequency counts and percentages for categorical variables, with groups compared using the Wilcoxon rank sum test for continuous variables and Fisher exact test for categorical variables. For chest tube output, a log transformation was used, and data were analyzed using a general linear model. Transfusion requirements were expressed as the number of units transfused and analyzed using proportional odds logistic regression. Laboratory values (hemoglobin level, platelet count, fibrinogen level, PT, INR, and aPTT) obtained after CBP prior to the administration of the study drug and after administration of the study drug are summarized using mean (SD), with values obtained after administration of the study drug analyzed using analysis of covariance, with the pretreatment value included as the covariate. Findings from these analyses are summarized using point estimates and 95% CIs for the effect of PCC compared with plasma. Adverse events are summarized using frequency counts and percentages and compared between groups using the Fisher exact test. Two-tailed P < .05 was considered statistically significant.
The sample size for this study was determined based on the results of a study by Arnékian et al6 that retrospectively presented a reduction in blood loss in patients receiving PCC over those receiving plasma, with a reported difference between groups of approximately 0.65 SDs. For the present study, it was determined that a sample-size of 50 patients per group would provide statistical power (2-tailed α = .05) greater than 85% to detect a difference of this magnitude.
Results
During the study period, 756 patients met inclusion criteria and were approached for enrollment; 562 provided consent, and 9 patients were withdrawn from the study after consent but prior to randomization (surgery was canceled, exclusion criteria were met after consent but prior to randomization, or patient opted out). Subsequently, 553 patients were randomized to receive either fresh frozen plasma (n = 312) or PCC (n = 241) (Figure 1). Of the 553 randomized patients, 447 patients did not meet criteria to receive the intervention, and 6 patients were withdrawn (because of surgical cancellation after randomization or life-threatening intraoperative complications necessitating study noncompliance). A total of 100 patients (mean [SD] age, 66.8 [13.7] years; 61 [61.0%] male; and 1 [1.0%] Black, 1 [1.0%] Hispanic, and 98 [98.0%] White) (Table 1) received the study intervention (49 plasma and 51 PCC, Figure 1). One patient was randomized to PCC but received plasma as a result of intraoperative surgeon error. Using the intention-to-treat principle, this patient is included in the PCC group for all analyses. One patient in the plasma group received the study drug, but because of a protocol violation (delayed reporting of a critically low fibrinogen level, which was an exclusion criterion), this patient is included in the summary of patient and procedural characteristics and the safety analyses only and excluded from the efficacy analyses per data and safety monitoring board recommendation.
Table 1. Patient Clinical and Procedural Characteristicsa.
| Characteristic | Participants, No. (%) | P value | |
|---|---|---|---|
| Plasma (n = 49) | PCC (n = 51) | ||
| Age at surgery, median (IQR), y | 69 (59-75) | 70 (62-78) | .44 |
| Female | 14 (28.6) | 25 (49.0) | .04 |
| Male | 35 (71.4) | 26 (51.0) | |
| BMI, median (IQR) | 28.9 (25.9-32.8) | 28.7 (24.4-32.6) | .44 |
| Medical history | |||
| Cerebrovascular accident | 1 (2.0) | 1 (2.0) | >.99 |
| Vascular thromboembolic disease | 0 | 0 | NA |
| Atrial fibrillation | 0 | 6 (11.8) | .03 |
| eGFR <60 mL/min/1.73 m2 | 2 (4.1) | 0 | .24 |
| Prior sternotomy | 27 (55.1) | 28 (54.9) | >.99 |
| Procedure performed | |||
| Aortic replacement | 6 (12.2) | 8 (15.7) | .50 |
| Multivalve repair or replacement | 15 (30.6) | 10 (19.6) | |
| Valve repair or replacement plus CABG | 8 (16.3) | 13 (25.5) | |
| Single valve | 6 (12.2) | 8 (15.7) | |
| Aortic replacement plus valve repair or replacement | 11 (22.4) | 10 (19.6) | |
| Aortic replacement plus CABG | 0 | 1 (2.0) | |
| Congenital defect repair | 1 (2.0) | 0 | |
| Pericardiectomy | 2 (4.1) | 0 | |
| CABG | 0 | 1 (2.0) | |
| Deep hypothermic circulatory arrest | 11 (22.4) | 16 (31.4) | .37 |
| Intraoperative antifibrinolytic | 49 (100) | 51 (100) | NA |
| CPB time, median (IQR), min | 155 (120-203) | 161 (116-215) | .94 |
| Any cross-clamp use | 44 (89.8) | 47 (92.2) | .74 |
| Cross-clamp time, median (IQR), minb | 117 (85-165) | 114 (84-158) | .77 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; eGFR, estmated glomerular filtration rate; NA, not applicable; PCC, prothrombin complex concentrate.
Continuous variables are summarized using median (IQR) and compared between groups using the rank sum test. Categorical variables are summarized using No. (%) and compared between groups using the Fisher exact test.
Cross-clamp time is summarized for patients who required cross-clamp use (n = 44 and n = 47 for the plasma and PCC groups, respectively).
Demographic, clinical, and procedural characteristics are presented in Table 1. Patient groups were similar aside from there being more male patients in the plasma group (35 of 49 patients [71.4%] vs 26 of 51 patients [51.0%]; P = .04) and more patients with a history of atrial fibrillation in the PCC group (6 of 51 patients [12%] vs no patients; P = .02).
Intraoperative laboratory data are presented in eTable 1 in Supplement 2. Patients in the plasma group had a higher baseline hemoglobin level compared with the PCC group (mean [SD], 12.47 [1.55] g/dL vs 11.65 g/dL [1.58]; P = .01; to convert to grams per liter, multiply by 10). In assessment of the pre- to posttreatment impact of the interventions on laboratory values, patients in the PCC arm had a higher posttreatment hemoglobin level (mean [SD], 9.81 [1.68] vs 10.51 [1.62]; estimated treatment effect, 0.81 g/dL [95% CI, 0.20-1.43]; P = .01), which may be explained by higher cell saver volume in the PCC group (median [IQR], 633 (484-1018) mL vs 702 [472-917] mL; eTable 2 in Supplement 2) and greater improvements in PT (effect estimate, −1.37 seconds [95% CI, −1.91 to −0.84]; P < .001) and INR (effect estimate, −0.12 [95% CI, −0.16 to −0.07]; <.001) compared with the plasma group. Intraoperative transfusion data are presented in eTable 2 in Supplement 2. Fewer patients in the PCC group required intraoperative RBC transfusion after study treatment (7 of 51 patients [13.7%] vs 15 of 49 patients in the plasma group [30.6%]; P = .04); however, total intraoperative transfusions were not significantly different. Among the 48 patients analyzed in the plasma group (1 was excluded from efficacy analyses), the median (IQR) volume of intervention plasma received was 507 (507-620) mL. Among the 51 patients in the PCC group, 1 received plasma (551 mL) instead of PCC (because of a protocol error). Among the 50 patients who received PCC, the median (IQR) dose of PCC received was 1187 (1077-1365) units. At the discretion of the anesthesiologist, 2 patients (3.9%) in the plasma group received PCC transfusion in the operating room after receiving the plasma intervention for ongoing significant microvascular bleeding.
Transfusion data from study treatment through midnight on postoperative day 1 and the primary outcome of chest tube output from the initial ICU admission to midnight on postoperative day 1 are presented in Table 2. There was no significant difference in chest tube output between the plasma and PCC groups (median [IQR], 1022 [799-1575] mL vs 937 [708-1443] mL). Although there were fewer RBC transfusions in the PCC group (15 of 51 patients [29.4%] vs 22 of 48 patients [45.8%]), this difference was not statistically significant.
Table 2. Posttreatment Chest Tube Output and Transfusionsa.
| Characteristic | Plasma, No. (%) (n = 48b) | PCC, No. (%) (n = 51) | Effect estimatec | |
|---|---|---|---|---|
| Estimate (95% CI) | P value | |||
| Chest tube output, median (IQR), mL | 1022 (799-1575) | 937 (708-1443) | 0.98 (0.81-1.19) | .84 |
| RBCs, units | ||||
| 0 | 26 (52.0) | 36 (70.6) | 0.49 (0.22-1.11) | .09 |
| 1 | 11 (22.9) | 8 (15.7) | ||
| 2 | 6 (12.5) | 5 (9.8) | ||
| ≥3 | 5 (10.4) | 2 (3.9) | ||
| Platelets, units | ||||
| 0 | 30 (62.5) | 36 (70.6) | 0.76 (0.33-1.73) | .51 |
| 1 | 13 (27.1) | 9 (17.6) | ||
| 2 | 3 (6.3) | 3 (5.9) | ||
| ≥3 | 2 (4.2) | 3 (5.9) | ||
| Cryoprecipitate, units | ||||
| 0 | 40 (83.3) | 40 (78.4) | 1.39 (0.51-3.79) | .53 |
| 1 | 2 (4.2) | 2 (3.9) | ||
| 2 | 5 (10.4) | 8 (15.7) | ||
| ≥3 | 1 (2.1) | 1 (2.0) | ||
| Plasma, units | ||||
| 0 | 43 (89.6) | 48 (94.1) | 0.52 (0.12-2.30) | .39 |
| 1 | 0 | 1 (2.0) | ||
| 2 | 4 (8.3) | 2 (3.9) | ||
| ≥3 | 1 (2.1) | 0 | ||
Abbreviations: PCC, prothrombin complex concentrate; RBCs, red blood cells.
Chest tube output was measured from initial postsurgery intensive care unit admission through midnight of the next day. Transfusions were recorded from completion of study drug administration through midnight of the next day.
One patient in the plasma group was excluded from the efficacy analysis because of a protocol violation.
For chest tube output, a log transformation was used, and the analysis was performed using linear regression. For blood product transfusions, the analysis was performed using proportional odds logistic regression. In all cases, the effect estimate is provided for PCC relative to plasma. For chest tube output, the effect estimate corresponds to the ratio of the geometric mean; for transfusions, the effect estimate corresponds to the odds ratio.
Twenty-seven patients (52.9%) in the PCC group avoided allogeneic transfusion during this period compared with 20 (41.7%) in the plasma group. Seven patients (13.7%) in the PCC group avoided any allogeneic transfusion from the intraoperative period to postoperative day 1, and 5 patients (9.8%) avoided any allogeneic transfusions when extended through postoperative day 5 (the end point for transfusion tracking) vs none in the plasma group. The 30-day adverse event data are presented in Table 3. There were no significant differences between groups for any outcome. The median (IQR) ICU LOS was 42 (23-96) hours in the plasma group and 64 (21-116) hours in the PCC group (rank sum test P = .75). The median (IQR) hospital LOS was 8 (6-10) days in the plasma group and 7 (6-11) days in the PCC group (rank sum test P = .87).
Table 3. Adverse Eventsa.
| Event | No. (%) | P value | |
|---|---|---|---|
| Plasma (n = 49) | PCC (n = 51) | ||
| Transfusion reactionb | 1 (2.0) | 0 | .49 |
| DVT | 0 | 1 (2.0) | >.99 |
| Mesenteric ischemia | 3 (6.1) | 1 (2.0) | .36 |
| Stroke/TIA | 1 (2.0) | 2 (3.9) | >.99 |
| STEMI | 1 (2.0) | 0 | .49 |
| Thromboembolic eventc | 2 (4.1) | 1 (2.0) | .61 |
| Acute kidney injury | 18 (36.7) | 21 (41.2) | .69 |
| Kidney failure with new KRT | 4 (8.2) | 5 (9.8) | >.99 |
| ARDS | 4 (8.2) | 4 (7.8) | >.99 |
| Reoperation for bleeding within 48 h | 5 (10.2) | 3 (5.9) | .48 |
| Death within 30 d | 2 (4.1) | 1 (2.0) | .61 |
Abbreviations: ARDS, acute respiratory distress syndrome; DVT, deep vein thrombosis; KRT, kidney replacement therapy; PCC, prothrombin complex concentrate; STEMI, ST-segment elevation myocardial infarction; TIA, transient ischemic attack.
Adverse events are summarized using No. (%) and compared between groups using the Fisher exact test.
Transfusion reaction denotes transfusion-related acute lung injury, hemolytic transfusion reaction, or anaphylactic or allergic transfusion reaction.
Thromboembolic event was any DVT, mesenteric ischemia, stroke or TIA, or STEMI verified as thromboembolic in nature.
Discussion
To our knowledge, this is the first prospective randomized clinical trial comparing PCC use with plasma use therapies in patients undergoing cardiac surgery. The use of PCCs in patients undergoing cardiac surgery has increased in recent years. However, prospective randomized studies evaluating their use compared with historic therapy with plasma for the treatment of factor-mediated coagulopathy induced by CPB are lacking. The main findings of this study were that perioperative bleeding and transfusion requirements were similar between those receiving plasma and PCC, except for significantly lower posttreatment intraoperative RBC transfusions for patients receiving PCCs. Patients receiving PCCs also had a greater improvement in PT/INR values compared with those receiving plasma. Perioperative allogeneic transfusion avoidance was higher in patients receiving PCCs. There were no differences in adverse outcomes between groups.
In recent years, the use of PCCs has dramatically increased and is now the treatment of choice for management of bleeding in patients taking warfarin.20,21,22 Current formulations of inactivated PCCs available in the United States include factor IX complex, human (Baxter), which contains factors 2, 9, and 10, and prothrombin complex concentrate, human (CSL Behring), which contains factors 2, 7, 9, and 10. The rationale for hemostatic therapy (such as non-RBC transfusions or PCC use) in cardiac surgery is to reduce bleeding, RBC transfusions, and associated complications. Prior retrospective studies6,9,11,23,24 and a recent meta-analysis14 have suggested that the use of PCCs reduces postoperative RBC transfusions compared with plasma among cardiac surgical patients. A recent pilot study demonstrated promising results with regard to a reduction in postoperative RBC transfusions in those receiving PCCs vs plasma.3 Our study found that patients randomized to the PCC group had fewer intraoperative RBC transfusions after treatment than those who received plasma. This was despite lower pretreatment hemoglobin values in the PCC group, which is likely explained by the dilutional impact on red cell mass seen with the higher-volume plasma transfusion. Overall, the total intraoperative RBC transfusion rates were not significantly different between groups, and the increase from pre- to posttreatment hemoglobin values seen in the PCC group can likely be explained by the higher intraoperative cell saver volume in the PCC group (702 mL vs 633 mL). There was no significant difference between groups in RBC transfusions through postoperative day 1.
The use of PCCs has been shown to lead to superior and/or quicker correction of INR derangements compared with plasma in warfarin-related coagulopathy.25 These data are not well represented in the setting of CPB-associated coagulopathy. The results of this study suggest superior correction in PT/INR values for patients receiving PCC vs plasma (eTable 1 in Supplement 2). This is not surprising, given the higher factor concentrations in PCCs known to be associated with the PT/INR26; this is important, as a greater INR correction found by Smith et al27 in patients undergoing cardiac surgery was associated with fewer intraoperative and postoperative RBC transfusions and more ICU-free days.
Widespread use of PCCs in cardiac surgery has been tempered by concern over possible thromboembolic complications. Current formulations of inactive PCCs include a variety of anticoagulants, such as heparin, proteins C and S, and antithrombin 3. Such additives may balance and/or reduce thrombotic risk when compared with PCC formulations void of these additives.28 The results of our study found no differences in major morbidity or mortality between treatment groups; more importantly, there was no difference in thromboembolic complications (Table 3). These results mirror recent retrospective studies within this patient population.11,14 In 2 recent randomized pilot studies,3,13 the use of PCC was not found to increase the risk of thromboembolic adverse events. There has been some suggestion from retrospective data that PCCs may increase risk of acute kidney injury or the need for kidney replacement therapy in cardiac surgical patients.14,24 This study did not find an increased risk of acute kidney injury or need for new kidney replacement therapy. While additional prospective studies are needed to confirm these findings, PCC dosing as described in this study does not appear to increase risk of thromboembolic complications or adverse events. Of note, the dosing of PCC in this study (15 IU/kg or closest standardized dose) is on the low side compared with doses reported in the cardiac surgical literature (typically 15-25 IU/kg)3,11,13,23,24,26 and certainly lower than doses commonly used for warfarin reversal (≥25 IU/kg).26
An important outcome of this study relates to the potential reduction or avoidance of allogeneic transfusions, including allogeneic plasma avoidance by way of PCC substitution and reduced hemodilution necessitating RBC transfusion seen with large-volume plasma transfusion. This is demonstrated by the 13.7% of patients receiving PCC who avoided any allogeneic transfusion intraoperatively through postoperative day 1 and 9.8% who avoided any allogeneic transfusion through postoperative day 5 compared with no patients in the plasma group. This is particularly relevant at a time when shortages in allogeneic blood productus are a reality and blood banks are commonly undersupplied.
Limitations
This study has limitations, including the duration for which the study spans. Several uncontrollable variables (eg, COVID-19 pandemic–related research restrictions and surgical volume fluctuations, study-staff absences, and competing studies) led to a long study duration. However, the randomized nature of the study kept the surgical year balanced, and the perioperative protocols with regard to patient blood management and transfusion did not change during the study period.
Conclusions
The use of PCCs in patients undergoing cardiac surgery has become increasingly common. The off-label use of PCCs in the setting of coagulation factor–mediated post-CPB coagulopathy, however, has been cautiously accepted, owing to a somewhat unknown safety profile and a lack of prospective randomized clinical trials. To our knowledge, this study represents the first true (nonpilot) randomized clinical trial comparing PCCs with plasma for patients with post-CPB–mediated coagulopathy. The results of this study suggest an overall similar efficacy and safety profile, with a lower rate of posttreatment intraoperative RBC transfusion, improved PT/INR correction, and higher incidence of allogeneic transfusion avoidance in patients receiving PCCs compared with plasma in this clinical context.
Trial Protocol
eAppendix 1. Study Inclusion and Exclusion Criteria
eAppendix 2. Adverse Event Definitions
eTable 1. Intraoperative Laboratory Values
eTable 2. Intraoperative Transfusions and Estimated Blood Loss
Data Sharing Statement
References
- 1.Nuttall GA, Oliver WC, Santrach PJ, et al. Efficacy of a simple intraoperative transfusion algorithm for nonerythrocyte component utilization after cardiopulmonary bypass. Anesthesiology. 2001;94(5):773-781. doi: 10.1097/00000542-200105000-00014 [DOI] [PubMed] [Google Scholar]
- 2.Thiele RH, Raphael J. A 2014 update on coagulation management for cardiopulmonary bypass. Semin Cardiothorac Vasc Anesth. 2014;18(2):177-189. doi: 10.1177/1089253214534782 [DOI] [PubMed] [Google Scholar]
- 3.Karkouti K, Bartoszko J, Grewal D, et al. Comparison of 4-factor prothrombin complex concentrate with frozen plasma for management of hemorrhage during and after cardiac surgery: a randomized pilot trial. JAMA Netw Open. 2021;4(4):e213936. doi: 10.1001/jamanetworkopen.2021.3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012;52(suppl 1):65S-79S. doi: 10.1111/j.1537-2995.2012.03663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg AD, Kor DJ. State of the art management of transfusion-related acute lung injury (TRALI). Curr Pharm Des. 2012;18(22):3273-3284. doi: 10.2174/1381612811209023273 [DOI] [PubMed] [Google Scholar]
- 6.Arnékian V, Camous J, Fattal S, Rézaiguia-Delclaux S, Nottin R, Stéphan F. Use of prothrombin complex concentrate for excessive bleeding after cardiac surgery. Interact Cardiovasc Thorac Surg. 2012;15(3):382-389. doi: 10.1093/icvts/ivs224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demeyere R, Gillardin S, Arnout J, Strengers PF. Comparison of fresh frozen plasma and prothrombin complex concentrate for the reversal of oral anticoagulants in patients undergoing cardiopulmonary bypass surgery: a randomized study. Vox Sang. 2010;99(3):251-260. doi: 10.1111/j.1423-0410.2010.01339.x [DOI] [PubMed] [Google Scholar]
- 8.Görlinger K, Dirkmann D, Hanke AA, et al. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single-center cohort study. Anesthesiology. 2011;115(6):1179-1191. doi: 10.1097/ALN.0b013e31823497dd [DOI] [PubMed] [Google Scholar]
- 9.Cappabianca G, Mariscalco G, Biancari F, et al. Safety and efficacy of prothrombin complex concentrate as first-line treatment in bleeding after cardiac surgery. Crit Care. 2016;20:5. doi: 10.1186/s13054-015-1172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdary P, Tang A, Watson D, et al. Retrospective review of a prothrombin complex concentrate (Beriplex P/N) for the management of perioperative bleeding unrelated to oral anticoagulation. Clin Appl Thromb Hemost. 2018;24(7):1159-1169. doi: 10.1177/1076029617753537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzgerald J, Lenihan M, Callum J, et al. Use of prothrombin complex concentrate for management of coagulopathy after cardiac surgery: a propensity score matched comparison to plasma. Br J Anaesth. 2018;120(5):928-934. doi: 10.1016/j.bja.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 12.Zweng I, Galvin S, Robbins R, et al. Initial experience of the use of 3-factor prothrombin complex concentrate and thromboembolic complications after cardiac surgery. Heart Lung Circ. 2019;28(11):1706-1713. doi: 10.1016/j.hlc.2018.08.016 [DOI] [PubMed] [Google Scholar]
- 13.Green L, Roberts N, Cooper J, et al. Prothrombin complex concentrate vs. fresh frozen plasma in adult patients undergoing heart surgery: a pilot randomised controlled trial (PROPHESY trial). Anaesthesia. 2021;76(7):892-901. doi: 10.1111/anae.15327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roman M, Biancari F, Ahmed AB, et al. Prothrombin complex concentrate in cardiac surgery: a systematic review and meta-analysis. Ann Thorac Surg. 2019;107(4):1275-1283. doi: 10.1016/j.athoracsur.2018.10.013 [DOI] [PubMed] [Google Scholar]
- 15.Boswell MR, Stulak JM, Tchantchaleishvili V, et al. Intraoperative prothrombin complex concentrate administration and outcomes in patients undergoing left ventricular assist device implantation. Artif Organs. 2021;45(8):E223-E303. doi: 10.1111/aor.13918 [DOI] [PubMed] [Google Scholar]
- 16.Nuttall GA, Oliver WC, Ereth MH, Santrach PJ. Coagulation tests predict bleeding after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1997;11(7):815-823. doi: 10.1016/S1053-0770(97)90112-9 [DOI] [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV, et al. ; Acute Kidney Injury Network . Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 19.Toy P, Popovsky MA, Abraham E, et al. ; National Heart, Lung and Blood Institute Working Group on TRALI . Transfusion-related acute lung injury: definition and review. Crit Care Med. 2005;33(4):721-726. doi: 10.1097/01.CCM.0000159849.94750.51 [DOI] [PubMed] [Google Scholar]
- 20.Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e152S-e184S. doi: 10.1378/chest.11-2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol. 2012;87(suppl 1):S141-S145. doi: 10.1002/ajh.23202 [DOI] [PubMed] [Google Scholar]
- 22.Levy JH, Faraoni D, Spring JL, Douketis JD, Samama CM. Managing new oral anticoagulants in the perioperative and intensive care unit setting. Anesthesiology. 2013;118(6):1466-1474. doi: 10.1097/ALN.0b013e318289bcba [DOI] [PubMed] [Google Scholar]
- 23.Ortmann E, Besser MW, Sharples LD, et al. An exploratory cohort study comparing prothrombin complex concentrate and fresh frozen plasma for the treatment of coagulopathy after complex cardiac surgery. Anesth Analg. 2015;121(1):26-33. doi: 10.1213/ANE.0000000000000689 [DOI] [PubMed] [Google Scholar]
- 24.Biancari F, Ruggieri VG, Perrotti A, et al. Comparative analysis of prothrombin complex concentrate and fresh frozen plasma in coronary surgery. Heart Lung Circ. 2019;28(12):1881-1887. doi: 10.1016/j.hlc.2018.10.025 [DOI] [PubMed] [Google Scholar]
- 25.Chai-Adisaksopha C, Hillis C, Siegal DM, et al. Prothrombin complex concentrates versus fresh frozen plasma for warfarin reversal: a systematic review and meta-analysis. Thromb Haemost. 2016;116(5):879-890. doi: 10.1160/TH16-04-0266 [DOI] [PubMed] [Google Scholar]
- 26.Ghadimi K, Levy JH, Welsby IJ. Prothrombin complex concentrates for bleeding in the perioperative setting. Anesth Analg. 2016;122(5):1287-1300. doi: 10.1213/ANE.0000000000001188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith MM, Kor DJ, Frank RD, Weister TJ, Dearani JA, Warner MA. Intraoperative plasma transfusion volumes and outcomes in cardiac surgery. J Cardiothorac Vasc Anesth. 2020;34(6):1446-1456. doi: 10.1053/j.jvca.2019.12.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreira J. DeLosSantos M. The clinical use of prothrombin complex concentrate. J Emerg Med. 2013;44(6):1201-1210. doi: 10.1016/j.jemermed.2012.12.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Study Inclusion and Exclusion Criteria
eAppendix 2. Adverse Event Definitions
eTable 1. Intraoperative Laboratory Values
eTable 2. Intraoperative Transfusions and Estimated Blood Loss
Data Sharing Statement