Abstract
The course of sickle cell disease (SCD) is modified by polymorphisms boosting fetal hemoglobin (HbF) synthesis. However, it has remained an open question how these polymorphisms affect patients who are treated with the HbF-inducing drug hydroxyurea/ hydroxycarbamide. The German SCD registry offers the opportunity to answer this question, because >90% of patients are treated according to national guidelines recommending the use of hydroxyurea in all patients above 2 years of age. We analyzed the modifying effect of HbF-related genetic polymorphisms in 417 patients with homozygous SCD >2 years old who received hydroxyurea. HbF levels were correlated with higher total hemoglobin levels, lower rates of hemolysis, a lower frequency of painful crises and of red blood cell transfusions. The minor alleles of the polymorphisms in the γ-globin promoter (rs7482144), BCL11A (rs1427407) and HMIP (rs66650371) were strongly associated with increased HbF levels. However, these associations did not translate into lower frequencies of vaso-occlusive events which did not differ between patients either carrying or not carrying the HMIP and BCL11A polymorphisms. Patients on hydroxyurea carrying the γ-globin promoter polymorphism demonstrated substantially higher hemoglobin levels (P<10-4) but also higher frequencies of painful crises and hospitalizations (P<0.01) when compared to patients without this polymorphism. Taken together, these data indicate that the γ-globin, HMIP and BCL11A polymorphisms correlate with increased HbF in SCD patients on hydroxyurea. While HbF is negatively correlated with the frequency of painful crises and hospitalizations, this was not observed for the presence of known HbF-boosting alleles.
Introduction
Sickle cell disease (SCD) is a multiorgan disorder with a broad spectrum of clinical presentations. While some patients reach adulthood with few symptoms, others die early from complications such as acute anemia, infection or acute chest syndrome. Besides the β-globin and α-globin genotypes, the most important modifier of SCD is the persisting expression of fetal hemoglobin (HbF), a heritable quantitative trait determined mainly by the three loci BCL11A, HMIP and HBG.1 While α-thalassemia, if co-inherited with the HbS mutation, slows down HbS polymerization by reducing the cellular hemoglobin concentration,2 persisting HbF can interfere with the polymerization of HbS.3 Genetic modifiers of HbF synthesis have generally shown a beneficial effect on the phenotype of SCD.1,2,4,5 However, some studies have yielded conflicting results and most exclusively included patients who did not receive the disease-modifying drug hydroxy urea.5 Several studies analyzing the effects of α-thalassemia and HbF-modifiers in patients treated with hydroxyurea suggested that the effect of hydroxyurea on laboratory and clinical outcomes largely supersedes the effect of the genetic modifiers.6-13 Both the effects of polymorphisms identified by genomewide association studies to modify the phenotype of SCD and the pharmacological effects of hydroxy urea were originally considered to be mediated via the expression and distribution of HbF.14,15 Genetic modifiers of HbF synthesis generally interfere with the expression or the binding activity of BCL11A. While this transcription factor is required for the perinatal “hemoglobin switch”, its actions are not limited to the β-globin locus,16 leaving room for effects on red blood cells and on the phenotype of SCD that are independent from HbF expression. Similarly, hydroxyurea does not exclusively target HbF expression but in addition exerts effects such as myelosuppression and nitric oxide release that may be equally important in ameliorating SCD.17
Considering the pleiotropic effects of both genetic modifiers and pharmacotherapy, the association of genetic modifiers with a certain phenotype may vary with the treatment that is applied. Thus, the actual importance of genetic modifiers for the clinical course of SCD needs to be assessed in the context of pharmacological treatment in addition to previous studies in treatment-naïve patients. In Germany, treatment guidelines18 encourage the use of hydroxyurea in all symptomatic patients with SCD, starting at the age of 2 years. Therefore, approximately 90% of patients with homozygous SCD registered in the German SCD registry are prescribed hydroxyurea.19 We made use of laboratory and clinical data collected in this registry to identify the effect of genetic modifiers on the phenotype of homozygous SCD. To this end, we selected polymorphisms known to modify either HbF expression or the phenotype of SCD, to be independent from each other and to be functionally relevant, for instance by altering transcription factor binding.15,20-23 We focused on the polymorphisms rs1427407 and rs7606173 in BCL11A, on the 3 bp deletion delCTA rs66650371 in HMIP and on the XmnI polymorphism in the -globin promoter, rs7482144. The allele BCL11A rs1427407 T was associated with increased HbF in genomewide association studies and was shown to reduce binding of GATA1 and TAL1 to a DNase-hypersensitive site that regulates BCL11A expression.20 After conditioning for the association with rs1427407, rs7606173 G is the BCL11A allele that remains most significantly associated with high HbF expression. It is located in a DNase hypersensitive site 7 kb closer to the BCL11A transcription start site than rs1427407.20 The deletion of three base pairs, CTA, in HMIP rs66650371 is considered to cause the strong association of the HMIP locus on chr6 with HbF levels by augmenting an enhancer-like activity located between the HBS1L and the MYB genes.21 The polymorphism in the γ-globin promoter that creates an XmnI restriction site, HGB2 rs7482144 A, has long been a candidate for being the HbF-boosting sequence within the high-HbF |3-globin haplotypes (‘Senegal’ and ‘Arab-Indian’).24,25 Together, polymorphisms in BCL11A, HMIP and the y-globin promoter were estimated to explain approximately 22% of the variability in HbF expression.26 In addition, we analyzed the coinheritance of the α-thalassemia trait that has been shown in multiple studies to be associated with a reduced risk of cerebrovascular complications, but an increased risk of painful crises.5 We restricted our analyses to patients at least 2 years of age with homozygous SCD who are treated with hydroxy urea, independently of the dose used.
Methods
Patients’ recruitment and data collection
Patients were recruited through the nationwide German SCD registry (NCT03327428) which collects prospective and retrospective data on patients with SCD in Germany. The study was performed according to the Declaration of Helsinki and approved by the institutional review board of the Medical Faculty of Heidelberg University (S□416/2014). Written informed consent was obtained from patients or legal guardians.
The data collected included demographic information, diagnosis and genotype, treatment, laboratory parameters and clinical events. At the time of the data cutoff, May 13, 2020, 425 patients with homozygous SCD from 28 different institutions were enrolled in the registry. Data were analyzed by PA, JK, NA and AK-S. All co-authors had access to all registry data.
Treatment guidelines implemented in 2014 recommend parental education, the use of penicillin prophylaxis at least until the age of 5 years, and annual screening with transcranial Doppler ultrasound starting from 2 until 18 years of age. The use of hydroxyurea is encouraged in all patients with SCD who have ever experienced a painful vaso-occlusive crisis, including mild ones. The recommended starting dose of hydroxyurea is 15 mg/kg/day for adults and 20 mg/kg/day for children. In the case of insufficient efficacy, a dose escalation up to the maximum tolerated dose or to 35 mg/kg/day is recommended.
For the analysis of the frequency and distribution of genetic modifiers, patients with homozygous SCD of all ages and irrespective of treatment were included. Patients with compound heterozygous SCD (HbSC, HbS/|3-thalassemia, others) were excluded. For the analysis of laboratory parameters and complications of SCD, only patients at least 2 years of age and with ongoing treatment with hydroxyurea were considered. Patients’ data collected after stem cell transplantation were excluded. Data on complications and treatment of SCD were documented annually, together with routine laboratory parameters (hemoglobin, mean corpuscular volume [MCV], reticulocytes, lactate dehydrogenase [LDH], bilirubin and HbF) (Table 1). The laboratory parameters were only considered if the patient had not received red blood cell transfusions within 100 days before the assessment. All laboratory parameters were determined while the patient was on hydroxyurea and followed for clinical complications. If more than one laboratory data point fulfilled these criteria, we used the average of all available data.
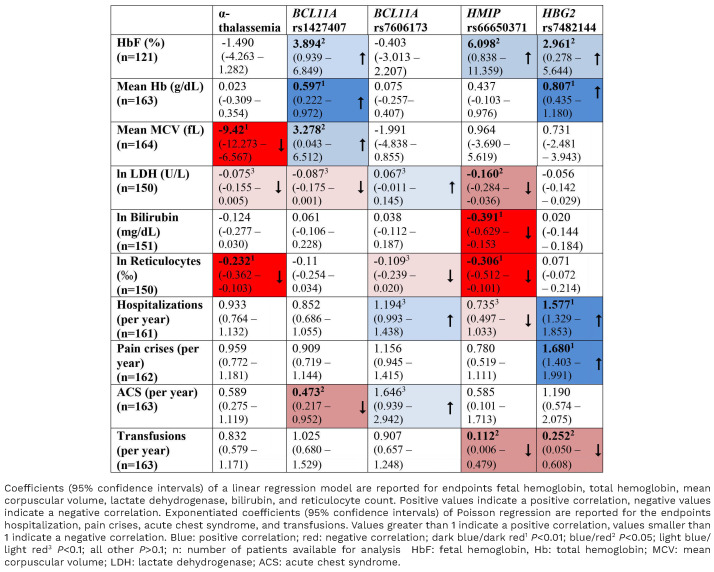
Patients registered by May 13, 2020 were included in the analysis. For the correlation of HbF with laboratory parameters and clinical course, all patients with available data were analyzed (n=193 to n=202) (Figures 1 and 2). All patients for whom the complete set of genetic traits of interest (α-thalassemia, HBG2-polymorphism rs7482144, BCL11A polymorphisms rs1427407 and rs7606173, HMIP polymorphism rs66650371) was available in combination with the respective laboratory parameter (n=121 for HbF to n=164 for MCV) (Table 2) were included in the analyses that correlated genetics with laboratory parameters and clinical course.
In order to investigate geographic and ethnic differences in the phenotypic expression of SCD, we categorized patients according to the origins of their parents from one of the three regions Mediterranean Sea, Sub-Saharan Africa and “rest of the world”. The last included mainly patients from Iraq (n=13), all other countries contributed at most two patients.
Pain crises were defined as pain requiring pharmacological treatment and hospitalization with no other obvious cause besides SCD. This definition does not include visits in the emergency department that did not result in hospital admission. Clinical events were considered from the first dose of hydroxy urea until last follow-up (median/mean observation period per patient 1.8/1.1 years; range, 0.5-5.3 years), independently of changes in the hydroxyurea dose. In 313 patients with at least 1 year of follow-up on hydroxyurea, the severity of SCD was graded as “severe” (n=83) if 1.5 or more pain crises requiring hospitalization per year or 0.5 or more episodes of acute chest syndrome (ACS) per year were documented. In addition, any stroke, sepsis, chronic pain or need of chronic red blood cell transfusions occurring on hydroxyurea defined a severe course.
Genetic analysis
The HBG2-polymorphism rs7482144 was identified by polymerase chain reaction (PCR) amplification (forward primer: ATA GCA CTT CTT ATT TGG AAA CCA A, reverse primer: TGT CTA AGT TGC CTC GAG ACT AAA G), XmnI digestion and restriction fragment length analysis.22 The BCL11A polymorphisms (rs1427407 and rs7606173) were analyzed using sequence-specific TaqMan genotyping.20 The 3 bp deletion in HMIP (rs66650371) was diagnosed by sequence-specific PCR and agarose gel electrophoresis.21 α-thalassemia deletions (-α3.7, -α4.2, -α20.5, --SEA and --MED) were detected by PCR and subsequent agarose gel electrophoresis.27
Statistical analysis
To evaluate the effect of HbF levels on laboratory parameters we used linear model analysis. To obtain approximately normally distributed variables, the values of LDH, bilirubin and reticulocytes were log-transformed. The association of HbF and clinical course was analyzed using a Poisson regression. The impact of the combination of single nucleotide polymorphisms on HbF and on the laboratory parameters was analyzed by linear multivariable regression analysis. The presence of a single nucleotide polymoprhism-variant was coded with 0 (no polymorphism - wildtype), 1 (heterozygous) and 2 (homozygous) for each studied polymorphism. A Poisson regression was used to estimate the contribution of each polymorphism on the clinical course. All statistical analyses were conducted using R version 4.0.2 (The R Foundation for Statistical Computing 2020).
Results
Patients’ characteristics
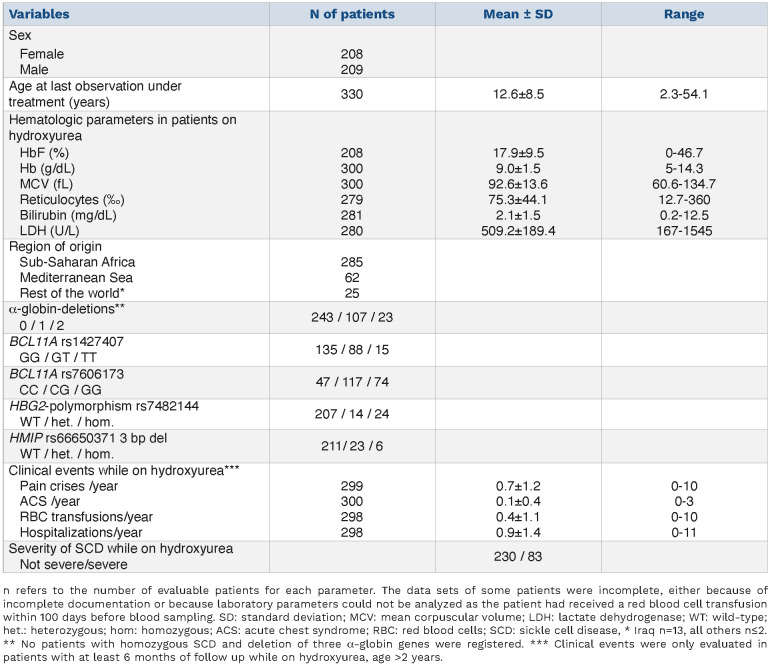
We restricted our analysis to patients at least 2 years of age on hydroxyurea, corresponding to 77.6% (n=330) of all patients with homozygous SCD in the registry. Of all patients with homozygous SCD (n=425), 19 were excluded because of young age (<2 years), 36 were excluded because they were not on hydroxyurea, 16 were excluded from the analysis of laboratory parameters because they had received red blood cell transfusions within 100 days before blood sampling, three were excluded because no data prior to allogeneic stem cell transplantation were documented and 13 did not have complete data on clinical complications. The age cut-off was set at 2 years because hydroxyurea is licensed for use in Europe starting at this age and because HbF levels were not correlated with age and sex in this group of patients (Online Supplementary Figures S1 and S2). The mean daily hydroxyurea dose was 23.2 mg/kg (standard deviation 5.9; range, 7.7-39.0).
Mean HbF levels among these patients were higher than those reported in hydroxyurea-naïve patients of West African origin28,29 and comparable to those in hydroxyureanaïve patients from India.30 Most patients had initiated hydroxyurea treatment before they were enrolled in the registry, but in 25 patients paired HbF measurements from before and after initiation of hydroxyurea treatment were available. During hydroxyurea treatment, HbF was on average 1.8-fold higher than before (mean HbF 23.7% vs. 13.2%, P<10-4 (t-test) (Online Supplementary Figure S3).
While α-thalassemia deletions and polymorphisms in BCL11A were present in at least a third of all patients, the HBG2-polymorphism rs7482144 and the polymorphism rs66650371 in HMIP affected only a minority of 10 to 15% of patients (Table 1). These less frequent traits were exceedingly rare among patients originating from sub-Saharan Africa but enriched in patients originating from the Mediterranean region (28% allele frequency for HMIP rs66650371) and from the rest of the world (47% allele frequency for the HBG2-polymorphism rs7482144) (Online Supplementary Table S1). Of note, the minor allele frequencies of these polymorphisms were paralleled by higher levels of HbF and total hemoglobin in patients not originating from sub-Saharan Africa (Online Supplementary Table S2). As expected for polymorphisms that are differentially enriched in specific ethnicities and, as is the case for the HBG2-polymorphism rs7482144, are linked to the HbS mutation, the frequency of homozygous carriers of the minor allele significantly (P<10-4) exceeded that predicted by the Hardy-Weinberg equilibrium. In contrast, α-thalassemia deletions were detected significantly more frequently among patients originating from sub-Saharan Africa in comparison to all other patients (allele frequency 25.2% vs. 7.1%, P<10-4). Compatible with a linkage dis -equilibrium, both polymorphisms in BCL11A were significantly associated with each other (P<10-4). As expected for independently inherited traits, we did not identify any significant associations between polymorphisms in BCL11A and in HMIP or HBG2. However, the polymorphisms that occurred preferentially in the non-sub-Saharan patients, HMIP and HGB2, were significantly associated with each other (P=0.008, c2 test).
Table 1.
Patients’ characteristics.
High HbF levels are associated with a milder phenotype of sickle cell disease
All hematologic parameters tested were strongly associated with HbF levels (Figure 1). While patients with an HbF of 5% had a mean total hemoglobin of 7.9 g/dL (MCV 85 fL), patients with an HbF of 25% had a mean total hemoglobin of 9.4 g/dL (MCV 94 fL). Indicators of hemo lysis showed an inverse relation (patients with HbF 5% vs. 25%: LDH 560 vs. 441 U/L, bilirubin 2.34 vs. 1.51 mg/dL, reticulocytes 87‰ vs. 53 ‰) (Figure 1).
Figure 1.
Linear regression of HbF on laboratory parameters.
(A) Linear regression of fetal hemoglobin (HbF) on total hemoglobin (n=202). (B) Linear regression of HbF on mean corpuscular volume (n=202). (C) Linear regression of HbF on ln lactate dehydrogenase (n=194). (D) Linear regression of HbF on ln bilirubin (n=195). (E) Linear regression of HbF on ln reticulocyte count (n=195). Hb: total hemoglobin; MCV; mean corpuscular volume; LDH: lactate dehydrogenase.
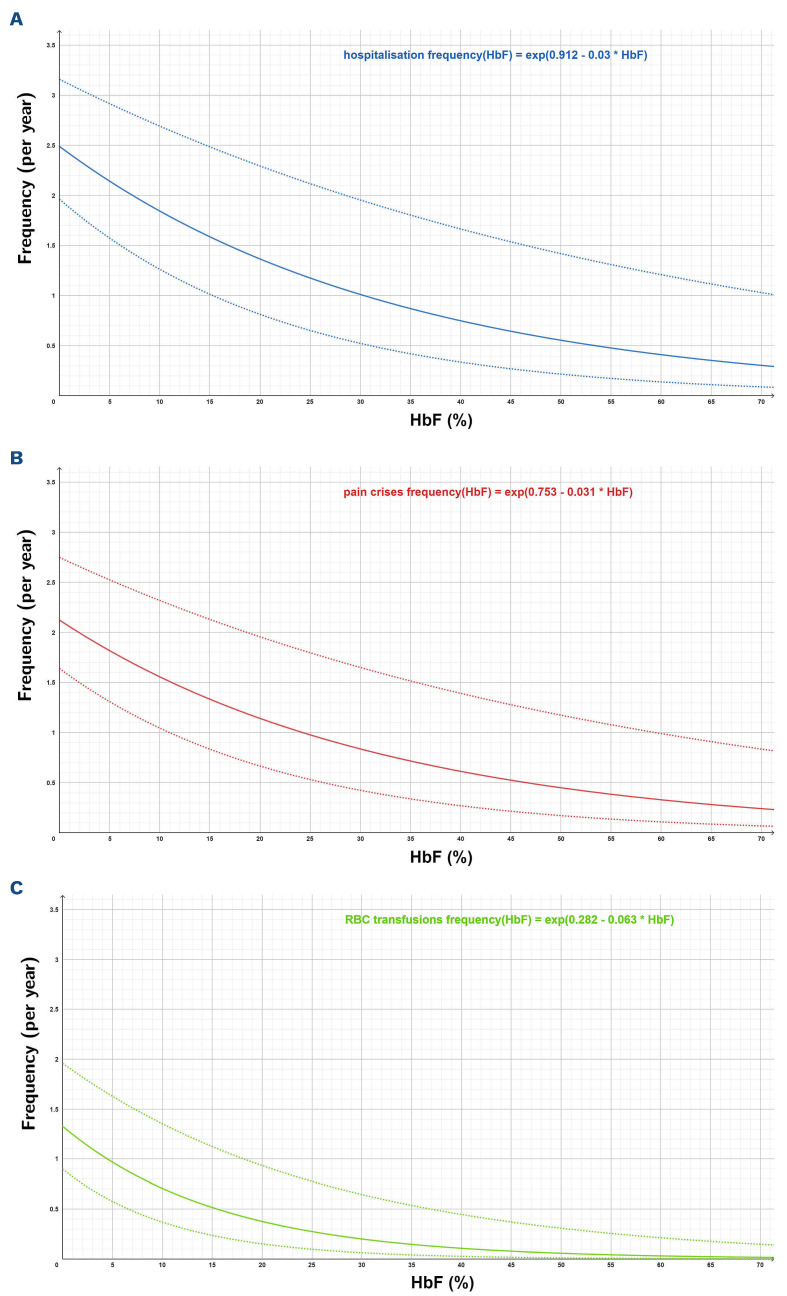
Simultaneously, the frequency of pain crises and of red blood cell transfusions decreased with increasing HbF (Figure 2). The frequency of hospitalizations paralleled that of pain crises, reflecting our definition of “pain crisis” as requiring hospitalization and the fact that most admissions in patients with SCD are due to acute pain. In contrast, ACS was not correlated with HbF levels, indicating a differential effect of HbF on these complications.
In order to analyze whether adherence to hydroxyurea - reflected by increased MCV, but also HbF and total hemoglobin - is associated with the phenotype, we compared laboratory and clinical parameters in patients with severe SCD to those in patients with non-severe SCD (Online Supplementary Table S3). While severely affected patients were slightly older than non-severely affected patients and carried more HbF-modifying alleles, they did not differ in any other laboratory parameter, most importantly MCV, indicating that potential differences in adherence to hydroxyurea were too small to be detected.
Table 2.
Multivariable analysis of the effect of genetic traits on laboratory parameters and complications of sickle cell disease.
Effects of genetic modifiers can be either mediated by HbF or independent of HbF
In order to assess whether the effects of genetic modifiers on SCD are mediated by boosting HbF expression, we next compared the correlation of genetic traits with HbF levels, with laboratory parameters and with complications of SCD (Table 2, Online Supplementary Figures S4-S11, Online Supplementary Tables S4-S7).
As expected, the co-inheritance of α-thalassemia resulted in lower MCV and reduced rates of hemolysis without significantly affecting HbF or total hemoglobin. However, the α-thalassemia trait was not linked to the frequency of complications in these patients.
The rarer alleles of the polymorphic loci BCL11A rs1427407, HMIP rs66650371 and HBG2-polymorphism rs7482144 were significantly associated with increased HbF levels. In addition, the BCL11A rs1427407 and HBG2 rs7482144 polymorphisms were associated with increased total hemoglobin concentrations. Consistent with this observation, patients carrying the HBG2-polymorphism rs7482144 received red blood cell transfusions less frequently than others. However, neither the increases in HbF nor those in total hemoglobin were reflected in a reduced frequency of complications of SCD. In contrast, pain crises and hospitalization were strikingly and highly significantly (P<0.01) more frequent in patients carrying the HBG2-polymorphism rs7482144 than in others (Table 2, Figure 3C, D). The coinheritance of two or more HbF-boosting alleles appears to be associated with an additive effect on HbF levels (Online Supplementary Figure S12). While small numbers of patients precluded definitive statistical analyses combining patients’ origins and genotypes, the association of rs7482144 with increased HbF and frequent complications appears to be independent of the region of origin (Online Supplementary Figure S13).
Figure 2.
Correlation between HbF level and complications of sickle cell disease. Poisson regression. (A-C) Numbers of patients available for analysis: 193 for hospitalization (A), 194 for pain crises (B), and red blood cell transfusion (C). Thick lines represent the predicted values, thin lines represent the 95% confidence interval.
The association of genetic traits with HbF does not correlate with the association of genetic traits and the rate of hemolysis or the frequency of complications of SCD (Table 2). While decreased LDH levels in patients with BCL11A rs1427407 and HMIP rs66650371 were concordant with increased HbF, BCL11A rs7606173 was not correlated with HbF levels but with increased LDH. At the same time, the HBG2-polymorphism rs7482144, which was clearly associated with high HbF, was not significantly associated with changes in any of the parameters that are informative of hemolysis. Without having any detectable effect on HbF levels, BCL11A rs7606173 showed a trend towards higher LDH and more frequent hospitalizations and ACS. Although there was no association between increased HbF and a reduced frequency of ACS, patients carrying BCL11A rs1427407 T showed a significant reduction in the frequency of ACS (Table 2), suggesting an HbF-independent modulation of the SCD phenotype.
We conclude that the induction of HbF expression is only one of several effects that modify the clinical phenotype of SCD in patients treated with hydroxyurea. As BCL11A, a key player that is influenced by polymorphisms in HMIP and BCL11A itself, is a pleiotropic transcription factor, its effects will not be limited to the regulation of HbF expression. In contrast, the HBG2-polymorphism rs7482144 is localized in the γ-globin promoter and shows a strong association with HbF but was not correlated with a milder course of SCD in this group of patients on hydroxyurea.
The HBG2-polymorphism rs7482144 defines a group of patients with high risk of painful crises
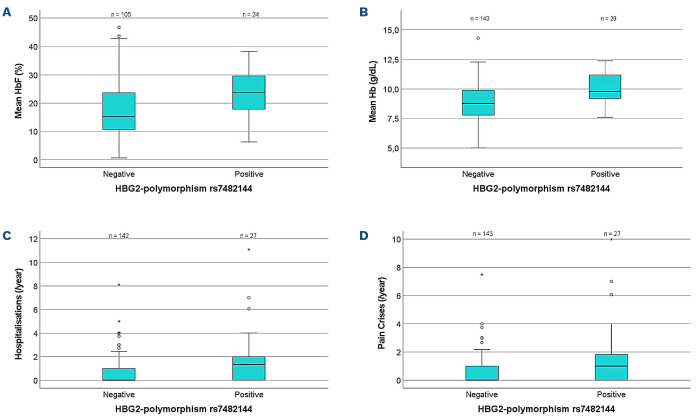
As has been observed in several studies of patients not on hydroxyurea, patients on hydroxyurea and with the HBG2-polymorphism rs7482144 (heterozygous or homozygous) had significantly higher mean HbF (23.6±8.6% vs. 17.5±10%, P=0.0061) (Figure 3A) and mean total hemoglobin (10.1±1.3 g/dL vs. 8.8±1.4 g/dL, P<10-4) (Figure 3B) when compared to all other patients. At the same time, MCV, reticulocyte counts, bilirubin and LDH levels did not differ between patients who did or did not carry at least one HBG2-polymorphism rs7482144, indicating that the rate of hemolysis is not reduced by the HBG2-polymorphism rs7482144. While among patients who did not carry the HBG2-polymorphism rs7482144 higher levels of HbF and total hemoglobin were significantly associated with fewer hospitalizations (Online Supplementary Tables S8 and S9), such a correlation was not detected among carriers of the HBG2-polymorphism rs7482144 (Online Supplementary Tables S10 and S11). In contrast, among patients carrying the HBG2-polymorphism rs7482144, there was a slight trend towards a higher total hemoglobin in those who suffered from severe disease (10.8±1.6 g/dL vs. 10.0±1.1 g/dL, P=0.21). The comparison of hydroxyurea doses prescribed to patients with or without the HBG2-polymorphism rs7482144 A confirmed that hydroxyurea doses were similar in both groups, indicating that the higher rate of complications in patients carrying rs7482144 A was not related to low hydroxyurea doses (Online Supplementary Table S7). In contrast, patients who carried either BCL11A rs1427407 T or HMIP rs66650371 delCTA were prescribed lower doses of hydroxyurea (Online Supplementary Tables S4 and S6), compatible with the notion that the hydroxyurea dose may be titrated according to HbF response.
Figure 3.
Mean values of laboratory parameters and complications comparing patients positive or negative for the γ-globin promoter polymorphism rs7482144. (A) Mean HbF ± standard deviation (SD): negative patients (n=105) versus positive patients (n=24): 17.5±10% versus 23.6±8.6%; t-test P=0.0061. (B) Mean hemoglobin ± SD: negative patients (n=143) versus positive patients (n=29): 8.9±1.5 g/dL versus 10.1±1.3 g/dL; t-test P<10-4. (C) Mean frequency of hospitalizations per year ± SD: negative patients (n=142) versus positive patients (n=27): 0.8±1.3 versus 1.9±2.6; t-test P=0.0011. (D) Mean frequency of pain crises per year ± SD: negative patients (n=143) versus positive patients (n=27): 0.6±1.1 versus 1.7±2.5; t-test P=0.0003. HbF: fetal hemoglobin; Hb: total hemoglobin.
Discussion
The negative correlation between HbF levels and the frequency of pain crises and of red blood cell transfusions confirms that the HbF level is a favorable prognostic marker in patients with SCD who are on treatment with hydroxyurea. At the same time, the polymorphisms rs1427407 in BCL11A, rs66650371 in HMIP and rs7482144 in the γ-globin promoter were strongly correlated with HbF levels in patients on hydroxyurea, indicating that γ-globin induction by hydroxyurea did not override the effects of genetic modifiers on HbF levels in this cohort of patients. This observation contrasts with results from trial cohorts that did not identify a significant effect of the genetic modifiers analyzed here on HbF levels in patients who had been treated with hydroxyurea.6-8,31 A possible explanation for this discrepancy may be the younger age of patients in these studies in comparison to our registry patients.
Despite the strong effect of HbF on the frequency of complications of SCD, the correlation of genetic modifiers with HbF did not translate into a reduced frequency of pain crises and hospitalizations in those patients who carry HbF-boosting alleles. In contrast, the presence of rs7482144 in the γ-globin promoter was associated with higher total hemoglobin but, unexpectedly, also with increased frequencies of pain crises and hospitalizations. This is in contrast to several earlier series of patients with SCD not treated with hydroxyurea who carry rs7482144 in the γ-globin promoter. These patients were characterized by increased HbF levels9,22,32,33 and, in contrast to our findings, also less frequent vaso-occlusive events.1,34-37 Studies on the association of rs7482144 with complications of SCD in patients treated with hydroxyurea are scarce.38 Consistent with our observations, the results of the BABY HUG trial showed a trend towards a higher frequency of vaso-occlusive events in patients who carry rs7482144 if treated with hydroxyurea.6 We can only speculate on the reason for the discordant effect of rs7482144 on HbF and on the frequency of pain crises. Possibly the protective effect of HbF induction is counterbalanced by the increase in total hemoglobin that results in high blood viscosity and precipitates vasoocclusion. Such an effect would result in an optimal dose level for hydroxycarbamide below the frequently used maximum tolerated dose. Patients presenting with frequent pain episodes despite high HbF and high total hemoglobin may benefit from a transient reduction of blood viscosity by cautious phlebotomy. Another possible explanation why high HbF levels and also the HbF-boosting polymorphism BCL11A rs1427407 are associated with a lower frequency of vaso-occlusive complications but rs7482144 in contrast is associated with a higher frequency of vaso-occlusive complications may involve the distribution of HbF. If HbF were distributed in a heterocellular manner in patients carrying the minor allele of rs7482144, the protection against vaso-occlusive crises would be inferior to that in patients with elevated HbF that is distributed pancellularly.39 As we do not have any data on the cellular distribution of HbF in our patients, we were not able to test this hypothesis.
The major limitation of our study is the selection of few genetic markers that, with the exception of α-thalassemia trait,2 focus on the expression of HbF,20-22 but do not consider other mechanisms that may modify the phenotype of SCD.40 As HbF-boosting polymorphisms are enriched in patients who do not originate from sub-Saharan Africa, we cannot fully discriminate between the effects of single polymorphisms, of the genetic background or even of social and behavioral factors. For future studies, a genome-wide characterization of patients may help in the identification of genetic traits causally related to the phenotype of SCD even in ethnically heterogeneous groups of patients. In addition, we observed a relatively short period of a maximum of 4 years in young patients, who were enrolled in a registry, not in a controlled clinical trial. Because the registry design does not intend source data verification, we do not expect the laboratory parameters and the data on clinical complications to be as complete as in a clinical trial. Similarly, we do not have direct data monitoring patients’ adherence to hydroxyurea treatment. However, HbF levels and MCV in these patients were consistently higher than those in hydroxyurea-naïve patients,29,41 compatible with a good adherence to treatment in the majority of patients.
The γ-globin promoter polymorphism rs7482144 was strongly associated with both pain crises and a reduced need for transfusions, indicating that the same genetic trait may have divergent effects on different phenotypic aspects of SCD. This is one reason why general conclusions on the course of SCD in an individual patient cannot be drawn from the presence of certain genetic markers. Second, while the genetic signature specifies relative risks in the subgroups, the overlap between subgroups is too large to allow for the prediction of individual risks for complications of SCD. Third, in the present study we only considered acute complications of SCD in a young group of patients but cannot yet evaluate the impact of the polymorphisms on long-term sequelae such as chronic kidney failure or pulmonary hypertension. Therefore, the decision as to whether hydroxyurea or even curative treatment options such as allogeneic stem cell transplantation or gene therapy should be offered cannot be confidently based on the genetic profile analyzed here.
We conclude that polymorphisms in BCL11A, HMIP and HGB2 increase HbF levels in patients with SCD on hydroxyurea. However, the impact of these polymorphisms on the complications of SCD treated with hydroxyurea was not observed to be correlated with their effect on HbF.
Supplementary Material
Acknowledgments
The authors thank Margit Happich and Gabriele Tolle for excellent support with genetic analyses.
Funding Statement
Funding: The German SCD registry is supported by the grants DKS 2016.12 and 2020.03 to JBK from the German Childhood Cancer Foundation.
References
- 1.Lettre G, Sankaran VG, Bezerra MA, et al. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci U S A. 2008;105(33):11869-11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgs DR, Aldridge BE, Lamb J, et al. The interaction of alpha-thalassemia and homozygous sickle-cell disease. N Engl J Med. 1982;306(24):1441-1446. [DOI] [PubMed] [Google Scholar]
- 3.Singer K, Singer L. Studies on abnormal hemoglobins : VIII. The gelling phenomenon of sickle cell hemoglobin: its biologic and diagnostic significance. Blood. 1953;8(11):1008-1023. [PubMed] [Google Scholar]
- 4.Lettre G. The search for genetic modifiers of disease severity in the beta-hemoglobinopathies. Cold Spring Harb Perspect Med. 2012;2(10):a015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meier ER, Fasano RM, Levett PR. A systematic review of the literature for severity predictors in children with sickle cell anemia. Blood Cells Mol Dis. 2017;65:86-94. [DOI] [PubMed] [Google Scholar]
- 6.Sheehan VA, Luo Z, Flanagan JM, et al. Genetic modifiers of sickle cell anemia in the BABY HUG cohort: influence on laboratory and clinical phenotypes. Am J Hematol. 2013;88(7):571-576. [DOI] [PubMed] [Google Scholar]
- 7.Aleluia MM, Santiago RP, da Guarda CC, et al. Genetic modulation of fetal hemoglobin in hydroxyurea-treated sickle cell anemia. Am J Hematol. 2017;92(5):E70-E72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheehan VA, Crosby JR, Sabo A, et al. Whole exome sequencing identifies novel genes for fetal hemoglobin response to hydroxyurea in children with sickle cell anemia. PloS One. 2014;9(10):e110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ware RE, Despotovic JM, Mortier NA, et al. Pharmacokinetics, pharmacodynamics, and pharmacogenetics of hydroxyurea treatment for children with sickle cell anemia. Blood. 2011;118(18):4985-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedrisch JR, Sheehan V, Flanagan JM, et al. The role of BCL11A and HMIP-2 polymorphisms on endogenous and hydroxyurea induced levels of fetal hemoglobin in sickle cell anemia patients from southern Brazil. Blood Cells Mol Dis. 2016;62:32-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adekile A, Menzel S, Gupta R, et al. Response to hydroxyurea among Kuwaiti patients with sickle cell disease and elevated baseline HbF levels. Am J Hematol. 2015;90(7):E138-139. [DOI] [PubMed] [Google Scholar]
- 12.Green NS, Barral S. Genetic modifiers of HbF and response to hydroxyurea in sickle cell disease. Pediatr Blood Cancer. 2011;56(2):177-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green NS, Ender KL, Pashankar F, et al. Candidate sequence variants and fetal hemoglobin in children with sickle cell disease treated with hydroxyurea. PloS One. 2013;8(2):e55709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dover GJ, Charache S, Boyer SH. Increasing fetal hemoglobin in sickle cell disease: comparisons of 5-azacytidine (subcutaneous or oral) with hydroxyurea. Trans Assoc Am Physicians. 1984;97:140-145. [PubMed] [Google Scholar]
- 15.Menzel S, Thein SL. Genetic modifiers of fetal haemoglobin in sickle cell disease. Mol Diagn Ther. 2019;23(2):235-244. [DOI] [PubMed] [Google Scholar]
- 16.Bauer DE, Orkin SH. Hemoglobin switching's surprise: the versatile transcription factor BCL11A is a master repressor of fetal hemoglobin. Curr Opin Genet Dev. 2015;33:62-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGann PT, Ware RE. Hydroxyurea for sickle cell anemia: what have we learned and what questions still remain? Curr Opin Hematol. 2011;18(3):158-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cario H, Grosse R, Jarisch A, Kulozik AE, Kunz JB, Lobitz S. AWMF-Leitlinie 025/016 Sichelzellkrankheit. 2014. [Google Scholar]
- 19.Kunz JB, Lobitz S, Grosse R, et al. Sickle cell disease in Germany: results from a national registry. Pediatr Blood Cancer. 2019;67(4):e28130. [DOI] [PubMed] [Google Scholar]
- 20.Bauer DE, Kamran SC, Lessard S, et al. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science. 2013;342(6155):253-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrell JJ, Sherva RM, Chen ZY, et al. A 3-bp deletion in the HBS1L-MYB intergenic region on chromosome 6q23 is associated with HbF expression. Blood. 2011;117(18):4935-4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilman JG, Huisman TH. DNA sequence variation associated with elevated fetal G gamma globin production. Blood. 1985;66(4):783-787. [PubMed] [Google Scholar]
- 23.Bhatnagar P, Purvis S, Barron-Casella E, et al. Genome-wide association study identifies genetic variants influencing F-cell levels in sickle-cell patients. J Hum Genet. 2011;56(4):316-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labie D, Dunda-Belkhodja O, Rouabhi F, Pagnier J, Ragusa A, Nagel RL. The -158 site 5' to the G gamma gene and G gamma expression. Blood. 1985;66(6):1463-1465. [PubMed] [Google Scholar]
- 25.Kulozik AE, Kar BC, Satapathy RK, Serjeant BE, Serjeant GR, Weatherall DJ. Fetal hemoglobin levels and beta (s) globin haplotypes in an Indian populations with sickle cell disease. Blood. 1987;69(6):1742-1746. [PubMed] [Google Scholar]
- 26.Gardner K, Fulford T, Silver N, et al. g(HbF): a genetic model of fetal hemoglobin in sickle cell disease. Blood Adv. 2018;2(3):235-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowden DK, Vickers MA, Higgs DR. A PCR-based strategy to detect the common severe determinants of α thalassaemia. Br J Haematol. 1992;81(1):104-108. [DOI] [PubMed] [Google Scholar]
- 28.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332(20):1317-1322. [DOI] [PubMed] [Google Scholar]
- 29.Borba R, Lima CS, Grotto HZ. Reticulocyte parameters and hemoglobin F production in sickle cell disease patients undergoing hydroxyurea therapy. J Clin Lab Anal. 2003;17(2):66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kar BC, Satapathy RK, Kulozik AE, et al. Sickle cell disease in Orissa State, India. Lancet. 1986;2(8517):1198-1201. [DOI] [PubMed] [Google Scholar]
- 31.Marahatta A, Flanagan JM, Howard TA, et al. Genetic variants that influence fetal hemoglobin expression from hydroxyurea treatment. Blood. 2020;136(Suppl 1):8-9.32614959 [Google Scholar]
- 32.Mtatiro SN, Makani J, Mmbando B, Thein SL, Menzel S, Cox SE. Genetic variants at HbF-modifier loci moderate anemia and leukocytosis in sickle cell disease in Tanzania. Am J Hematol. 2015;90(1):E1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicolau M, Vargas S, Silva M, et al. Genetic modulators of fetal hemoglobin expression and ischemic stroke occurrence in African descendant children with sickle cell anemia. Ann Hematol. 2019;98(12):2673-2681. [DOI] [PubMed] [Google Scholar]
- 34.Pandey S, Pandey S, Mishra RM, Saxena R. Modulating effect of the -158 gamma (C-->T) Xmn1 polymorphism in Indian sickle cell patients. Mediterr J Hematol Infect Dis. 2012;4(1):e2012001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gueye Tall F, Martin C, Ndour EHM, et al. Combined and differential effects of alpha-thalassemia and HbF-quantitative trait loci in Senegalese hydroxyurea-free children with sickle cell anemia. Pediatr Blood Cancer. 2019;66(10):e27934. [DOI] [PubMed] [Google Scholar]
- 36.Al-Allawi N, Qadir SMA, Puehringer H, Chui DHK, Farrell JJ, Oberkanins C. The association of HBG2, BCL11A, and HMIP polymorphisms with fetal hemoglobin and clinical phenotype in Iraqi Kurds with sickle cell disease. Int J Lab Hematol. 2018;41(1):87-93. [DOI] [PubMed] [Google Scholar]
- 37.Dadheech S, Jain S, Madhulatha D, et al. Association of Xmn1-158 gammaG variant with severity and HbF levels in beta-thalassemia major and sickle cell anaemia. Mol Biol Rep. 2014;41(5):3331-3337. [DOI] [PubMed] [Google Scholar]
- 38.Italia K, Jain D, Gattani S, et al. Hydroxyurea in sickle cell disease--a study of clinico-pharmacological efficacy in the Indian haplotype. Blood Cells Mol Dis. 2009;42(1):25-31. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg MH, Chui DH, Dover GJ, Sebastiani P, Alsultan A. Fetal hemoglobin in sickle cell anemia: a glass half full? Blood. 2014;123(4):481-485. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg MH, Sebastiani P. Genetic modifiers of sickle cell disease. Am J Hematol. 2012;87(8):795-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown AK, Sleeper LA, Miller ST, Pegelow CH, Gill FM, Waclawiw MA. Reference values and hematologic changes from birth to 5 years in patients with sickle cell disease. Cooperative Study of Sickle Cell Disease. Arch Pediatr Adolesc Med. 1994;148(8):796-804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.