Abstract
Background:
Birthweight is an indicator of fetal growth and environmental-related alterations of birthweight have been linked with multiple disorders and conditions progressing into adulthood. Although a few studies have assessed the association between birthweight and the totality of exogenous exposures and their downstream molecular responses in maternal urine and cord blood; no prior research has considered a) the maternal serum prenatal metabolome, which is enriched for hormones, and b) non-linear and synergistic associations among exposures.
Methods:
We measured the maternal serum metabolome during pregnancy using an untargeted metabolomics approach and birthweight for gestational age (BWGA) z-score in 410 mother-child dyads enrolled in the PRogramming of Intergenerational Stress Mechanisms (PRISM) cohort. We leveraged a Bayesian factor analysis for interaction to select the most important metabolites associated with BWGA z-score and to evaluate their linear, non-linear and non-additive associations. We also assessed the primary biological functions of the identified proteins using the MetaboAnalyst, a centralized repository of curated functional information. We compared our findings with those of a traditional metabolite-wide association study (MWAS) in which metabolites are individually associated with BWGA z-score.
Results:
Among 1110 metabolites, 46 showed evidence of U-shape associations with BWGA z-score. Most of the identified metabolites (85%) were lipids primarily enriched for pathways central to energy production, immune function, and androgen and estrogen metabolism, which are essential for pregnancy and parturition processes. Metabolites within the same class, i.e. steroids and phospholipids, showed synergistic relationships with each other.
Conclusions:
Our results support that the aspects of the maternal metabolome during pregnancy contribute linearly, non-linearly and synergistically to variation in newborn birthweight.
Keywords: Exposome, Birthweight, Prenatal metabolomics, Factor analysis for interaction, Nonlinear associations, Non-additive associations
1. Introduction
The human exposome summarizes the totality of endogenous and exogenous exposures encountered throughout life and reflects the contribution of these exposures to human health (Niedzwiecki et al., 2019; Vermeulen et al., 2020). Untargeted metabolomics assays enable the concurrent detection of thousands of small molecules, hereafter metabolites, in human biofluids and have become a powerful tool for characterizing exogenous exposures and their downstream molecular responses (Niedzwiecki et al., 2020; Niedzwiecki et al., 2019). A notable advantage of the metabolomics approach is the ability to not only study associations with manifest disease, but to also interrogate underlying pathways linking circulating small molecules with cellular health. Given the relevance of environmental and molecular links with fetal growth and newborn conditions, maternal metabolomic profiles during pregnancy have attracted much interest (Rager et al., 2020).
Birthweight is considered an indicator of cumulative fetal growth and is linked to health risk later in life (Barker and Thornburg, 2013; Howe et al., 2020). Indeed, low and/or high birthweight for gestational age has been associated with subsequent childhood morbidity (Wu et al., 2011), including childhood asthma (Brooks et al., 2001), and multiple disorders progressing into adulthood, such as cognitive deficits (Oudgenoeg-Paz et al., 2017), cardio-metabolic diseases (Fagerberg et al., 2004; Johansson et al., 2008; Leeson et al., 2001), respiratory conditions (Walter et al., 2009) and osteoporosis (Harvey et al., 2014; Metrustry et al., 2018). Abnormal fetal growth results from a combination of genetic factors and, more importantly, in utero conditions arising from the endogenous and exogenous environments, such as maternal diet and toxic chemical exposures. Notably, inter-individual variability of birthweight can be explained by only a small proportion by genetic variants (Lunde et al., 2007), while maternal exposures and their biological responses have been shown to be key determinants of newborn characteristics (Howe et al., 2020). Yet, despite the putative importance of the prenatal environment and the availability of metabolomics for investigating a breadth of molecular responses to environmental and endogenous stressors, few studies have examined untargeted metabolomics signature in relation to birthweight. Moreover, the majority of analyses have been cross-sectional at birth, using cord blood or newborn dry blood metabolites, with only two studies prospectively examining maternal urine metabolites during pregnancy in relation to birthweight (Maitre et al., 2014; Maitre et al., 2016; Team et al., 2020). Although the urinary metabolome can capture lipids and hormone levels (Coburn et al., 2019; Marcos et al., 2014; Marcos et al., 2015; McLeod et al., 2017; Pozo et al., 2018 Raro et al., 2016), which both play critical roles in gestation and parturition, the metabolome measured in blood or blood-derived tissues is particularly enriched by those compounds and its use can unveil novel associations (Coburn et al., 2019). In addition, existing studies that have investigated metabolomics in relation to birthweight have considered metabolites separately and linearly through a more traditional metabolomic-wide association study (MWAS) analysis. This approach is limited as it precludes evaluating potential non-linear and synergistic associations between metabolites, thus restricting the set of final findings.
Herein we leveraged a longitudinal pregnancy cohort study to analyze associations between the metabolomics profile assessed in maternal prenatal serum and birthweight. Specifically, we adapted the recently developed Bayesian factor analysis for interactions framework (Ferrari and Dunson, 2020) for use with metabolomics data and implemented this novel method to examine non-linear and non-additive associations between maternal serum metabolites measured during pregnancy and sex-specific birthweight for gestational age (BWGA) z-score. This approach accounts for the complex correlation among all metabolites and considers all metabolites jointly, thereby minimizing multiple comparison issues. We additionally compared the results with those from the traditional MWAS analysis, in which metabolites are individually associated with BWGA z-score and findings are corrected for multiple testing.
2. Methods
2.1. Study sample
The PRogramming of Intergenerational Stress Mechanisms (PRISM) study is an urban, ethnically diverse pregnancy cohort that was designed to study a range of chemical and non-chemical stressors in relation to maternal health, pregnancy outcomes, and child development. Pregnant women were enrolled from Boston and New York City hospitals and affiliated prenatal clinics beginning in 2011. Eligibility criteria included English or Spanish-speaking, over 18 years of age at enrollment, and singleton pregnancy. Exclusion criteria included HIV + status or self-reported drinking ≥7 alcoholic drinks per week before pregnancy or any alcohol after pregnancy recognition. At the time of metabolomics profiling (March 2018), 843 women had delivered a live born infant. The analytic sample includes a random subset of 410 mother-child pairs with maternal metabolomics measured during pregnancy (week of collection: 25th, 50th, 75th percentiles: 26, 29, 33 weeks, and overall range: 11–10 weeks) and birthweight data.
2.2. Ethics
All study protocols were approved by the human studies’ committees at the Icahn School of Medicine at Mount Sinai (ISMMS) in New York City or the Brigham and Women’s Hospital (BWH) in Boston; Beth Israel Deaconess Medical Center in Boston relied on BWH for review and oversight of the study protocol. All participants provided written informed consent in their primary language of preference.
2.3. Sex-specific birthweight for gestational age z-score
We extracted data on birthweight, gestational age at birth, and sex from newborn electronic medical records. Gestational age was calculated based on: (1) date of delivery and self-reported last menstrual period and (2) ultrasound estimates from the first-trimester examination. If the discrepancy between the two sources was greater than 2 weeks, data from obstetrical estimates were used. We calculated sex-specific birthweight for gestational age (BWGA) z-scores according to the validated international infant growth charts developed by Fenton (Chou et al., 2020; Fenton and Kim, 2013).
2.4. Untargeted metabolomics phenotyping during pregnancy
Maternal blood was collected by venipuncture (mean ± standard deviation (SD): 29.6 ± 4.90 weeks) and serum aliquots were stored at −80 °C until assayed. Untargeted metabolomics analysis was conducted on 100 μl of serum at Metabolon, Inc (Durham, NC, USA) with ultrahigh performance liquid chromatography-tandem mass spectroscopy (UPLC-MS/MS). The method utilized an ultra-performance liquid chromatography (UPLC) and a Thermo Scientific Q-Exactive high resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution. One aliquot was analyzed using acidic positive ion conditions, chromatographically optimized for more hydrophilic compounds; the extract compound was gradient eluted from a C18 column using water and methanol, containing 0.05% perfluoropentanoic acid (PFPA) and 0.1% formic acid (FA). Another aliquot was analyzed with the prior approach but it was chromatographically optimized for more hydrophobic compounds and operated at an overall higher organic content. A third aliquot was analyzed using basic negative ion optimized conditions using a separate dedicated C18 column. The basic extracts were gradient eluted from the column using methanol and water, however with 6.5 mM Ammonium Bicarbonate at pH 8. The fourth aliquot was analyzed via negative ionization following elution from a HILIC column using a gradient consisting of water and acetonitrile with 10 mM Ammonium Formate, pH 10.8. The MS analysis alternated between MS and data-dependent MSn scans using dynamic exclusion. The scan range between methods covered 70–1000 m/z. Raw data was extracted, peak-identified and QC processed using Metabolon’s hardware and software.
Peaks were quantified using area-under-the-curve. Batch adjustment to correct variation resulting from instrument inter-day tuning differences was performed for each compound in run-day blocks by dividing by the median of the values for the experimental samples for each instrument run day, then multiplying these values by the original median. In one serum sample with a lower volume (65 μl instead of 80 μl), metabolite intensities were scaled accounting for the volume of serum available, under the assumption that metabolite signal intensities scale linearly with the sample volume. We normalized all metabolomic data using first the natural base for log-scaling, thus removing skewness of the data. We then used a Pareto scaling approach, which incorporates a scaling factor equal to the square root of the standard deviation of individual metabolites so that larger fold changes were scaled more than smaller fold changes (Grace and Hudson, 2016). A total of 1110 biochemicals were detected across all four assays. Potential sample outliers were examined using principal component analysis (PCA), though none were identified. Final data were presented as normalized levels to facilitate both linear and non-linear analyses and to harmonize all variables on a common scale.
2.5. Covariates
Maternal age (continuous in years), self-reported race/ethnicity (White/Black/Hispanic/other), and education level (<high school/high school or more) were determined by questionnaires administered during a structured in-person interview during pregnancy; pre-pregnancy body mass index (BMI, kg/m2) was derived from height and weight reported via questionnaire at the first prenatal visit. Gestational week of serum collection was recorded during the in-person visit. Missing information for race/ethnicity (n = 4), education level (n = 7), and week of serum collection (n = 14) were imputed using the mice R-package (van Buuren and Groothuis-Oudshoorn, 2017).
2.6. Statistical methods
2.6.1. Metabolite-wide association study (MWAS)
We performed a MWAS metabolite-by-metabolite analysis by fitting linear regression models between each metabolite and BWGA z-score. In all MWAS models, we adjusted for a core set of covariates (maternal age, ethnicity, and education levels and week of serum collection) selected a priori based on previous literature. There is a consensus that increased maternal age and minority ethnicity are linked to low birthweight (Nardozza et al., 2017; Shmueli and Cullen, 1999), while education is considered a proxy of social-economic status, which has been linked to a variety of toxic exposures and economic disparities influencing birth-weight (Shmueli and Cullen, 1999; Silvestrin et al., 2013). Week of serum collection was included as a measure of precision for the metabolomics data, after evaluating the linear association between individual metabolites and the timing of serum collection. We inspected results and identified potential p-value inflation using 1) volcano plots of the estimated coefficients and p-values, and 2) quantile–quantile plots of observed and expected p-values. We also estimated the inflation lambda factor and its 95% confidence interval (95% CI) calculated using a permutation approach. We corrected all results for multiple testing using the False Discovery Rate (FDR); we evaluated statistical significance using a 5% FDR-adjusted p-value. Finally, we assessed Pearson correlations between all metabolites. This analysis precludes assessing non-linear and non-additive relationships, and metabolites functional for BWGA z-score may be not uncovered using this approach solely. Therefore, we compared MWAS results with those from the Bayesian factor analysis for interactions, which evaluates simultaneously not only linear, but also quadratic and synergistic relationships.
2.6.2. Bayesian factor analysis for metabolite interactions
We assessed linear, non-linear and non-additive associations between metabolites and BWGA z-score using a Bayesian latent factor joint model, also known as factor analysis for interactions, which is particularly suitable when predictors are highly correlated due to their cooccurrence in the environment. A major advantage of this approach is that shrinkage and interaction can be performed simultaneously, while also quantifying uncertainty by leveraging the Bayesian framework (Ferrari and Dunson, 2020). Briefly, the model provides dimensionality reduction by identifying groups (i.e. latent factors) of continuous and normalized predictors while also characterizing the associations between those factors and a continuous outcome (Ferrari and Dunson, 2020). We considered interactions by including pairwise cross-product terms between the latent factors, which can be decomposed to the initial predictors. We ran models with 10,000 iterations of the MCMC chain and 9000 iterations of burn-in. To facilitate the interpretation of the latent factors, we resolved rotational ambiguity by applying the MatchAlign algorithm, which re-assigns the contribution of each metabolite to the factors (Ferrari and Dunson, 2020). We report the individual and multiplicative association of metabolites with BWGA z-score at a 5% significance threshold, controlling for the core set of covariates. A detailed description of all analytical results of the Bayesian factor analysis for interaction are in the Supplemental Material (A).
2.6.3. Annotation, evaluation of metabolite similarity, and enrichment analysis
Annotation.
Metabolites were identified by comparison to the Metabolon library of 3300 commercially-available purified standards. Biochemical identification was based on three criteria: retention index (RI) within a narrow RI window of the proposed identification, accurate mass match to the library +/−10 ppm, and the MS/MS forward and reverse scores between the experimental data and authentic standards. Proprietary Metabolon quality control and curation processes that have been designed to ensure accurate and consistent identification of true chemical entities and to remove those representing system artifacts, misassignments, and background noise were applied. Known metabolites were assigned to super pathways (e.g., lipids, amino acids) and sub pathways (e.g., medium-chain fatty acids, lysine metabolism), as defined by Metabolon, and were also assigned KEGG, HMDB, CAS, and PubChem identifiers when available.
Evaluation of metabolite similarity.
We employed a dissimilarity matrix to evaluate the similarity between metabolites significantly associated with BWGA z-score. This approach allowed us to identify potential pairwise dissimilarities between metabolites with and without annotation, thus enabling classification of potential super-pathways without the need for annotation.
Enrichment analysis.
A functional and integrative enrichment analysis was conducted using MetaboAnalyst software (Chong et al., 2019; Pang et al., 2020). Briefly, we compared the metabolites significantly associated with BWGA z-score to 99 metabolite sets based on normal human metabolic pathways contained in a curated library. The enrichment ratio was computed as the number of significant metabolites to the expected number of metabolites under the null hypothesis of no association (Chong et al., 2019; Pang et al., 2020). The software also provided p-values and multiple testing corrections for all results (Chong et al., 2019; Pang et al., 2020). Finally, we evaluated the over-representation of each annotated subclass using a hypergeometric test (Subramanian et al. 2005).
3. Results
3.1. Study sample
The analyzed sample was racially/ethnically diverse (Black 30%, Hispanic, 14%, White 45%, Other 11%). Mothers had an average age (SD) of 31 (5.2) years at the time of delivery and 80% had a high school education or more. The average gestational length was 38.99 (SD: 1.66) weeks. BWGA z-scores were normally distributed (Kolmogorov-Smirnov test statistic D = 0.04; p-value = 0.44) and centered (SD) in −0.13 (0.91) (Table 1). The linear analysis between individual metabolites and week of serum collection showed that only a small proportion (15%, n = 166) of metabolites were significantly linked to the timing of collection after correcting for multiple comparisons using the Bonferroni approach (Table B1); to limit the impact of the timing of collection we included week of serum collection as a precision variable in all analyses.
Table 1.
Descriptive statistics of the PRogramming of Intergenerational Stress Mechanisms (PRISM) participants (N=410)
| Characteristic | Mean (SD) or N (%) |
|---|---|
| Fenton BWGA z-score | 0.13 (0.91) |
| Maternal Age | 30.5 (5.22) |
| Fetal Sex | |
| Female | 199 (48.5%) |
| Male | 211 (51.5%) |
| Maternal Education | |
| <12th grade | 82 (20.0%) |
| High school degree or more | 328 (80.0 %) |
| Maternal Race | |
| Black/Black-Hispanic | 123 (30.0%) |
| Hispanic | 135 (32.9%) |
| White | 122 (29.8%) |
| Other | 30 (7.3%) |
3.2. MWAS: Linear individual associations
We identified 91 metabolites that were individually and linearly associated with BWGA z-scores at a 5% nominal p-value threshold (Fig. 1.A, Table B2); however, only three (C32413: 2-hydroxymyristate; C52464: 1-palmitoyl-2-arachidonoyl-GPE (16:0/20:4)*; C38125: 4-cholesten-3-one) survived multiple testing correction at the 5% FDR threshold (Fig. 1.A, Table B2). While a SD increase in levels of C52464 (Estimate (Est.): 0.20; 95 %CI: 0.10; 0.30) and C38125 (Est.: 0.20; 95 % CI: 0.11; 0.30) was associated with an increase in BWGA z-scores, a SD increase in C32413 levels was linked to a decrease of 0.18 in BWGA z-score (95 %CI: −027; 0.09) (Fig. 1.A, Table B2). The quantile-quantile plot showed no large discrepancies of the observed p-value from the distribution of expected p-values under the null hypothesis of no association between individual metabolites and BWGA z-score (Fig. 1.B), confirming that most of the metabolites have no significant linear association with BWGA z-scores. The lambda factor λ = 1.31 (95% CI = 0.48; 2.13) did not show inflation of the results at the median p-value, thus indicating no systematic bias (Fig. 1.B). Pearson correlations between metabolites identified clusters with strong positive and negative correlations between them, suggesting the presence of latent factors (Figure B1).
Fig. 1. Linear association between individual metabolites and birthweight-for-gestational age Fenton z-score.
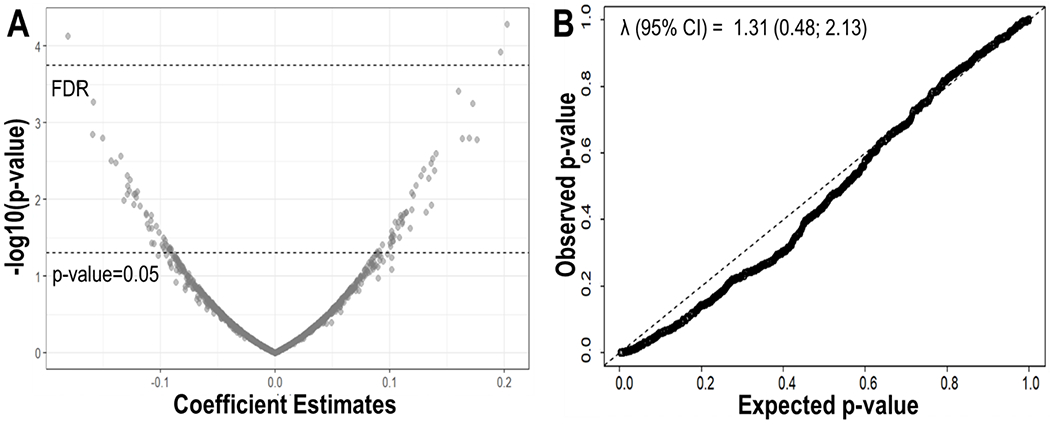
A) Volcano plot showing the estimated coefficient of the metabolite-z-score association (x-axis) and its significance (y-axis). Multiple testing correction was performed using a 5% False Discovery Rate (FDR) threshold. All analyses were adjusted for maternal age, ethnicity, education level, pre-pregnancy body mass index, and week of serum collection. B) Quantile-Quantile (QQ) plot of the expected p-value under the null hypothesis of no metabolite-z-score association (x-axis) and observed p-value (y-axis). The lambda factor identified the departure from the diagonal line at the median level of the expected p-value.
3.3. Bayesian factor analysis for interactions of metabolites
3.3.1. Linear associations and annotation
We performed factor analysis for interaction using 25 factors identified via unsupervised principal component analysis (see supplemental material A). Using these factors, we identified 46 metabolites that showed strong evidence of individual linear associations with BWGA z-scores (Fig. 2, Table B3), with 26 showing a positive direction. For a SD increase in individual metabolite levels, BWGA z-score changes ranged between −0.068 (C57463: linoleoylcholine) and 0.036 (C62695: un-annotated). Among the 46 metabolites that showed a significant association with BWGA z-score, 41 (89%) were previously annotated, of which 35 (85%) belonged to the lipid super-pathway; however those metabolites showed differences in sub-pathways (Table B3, Fig. 2). We identified 10 Androgenic Steroids, 2 Pregnenolone Steroids, 4 Diacylglycerols, 3 Fatty Acid Metabolism metabolites, 1 Monohydroxy Fatty Acid, 2 Lysophospholipids, 2 Phosphatidylcholines (PC), 9 Phosphatidylethanolamines (PE), 1 Phospholipids , and 1 Sphingolipid Synthesis metabolite (Table B3). Metabolites within the same sub-pathway showed strong correlation patterns (Figure B2).
Fig. 2.
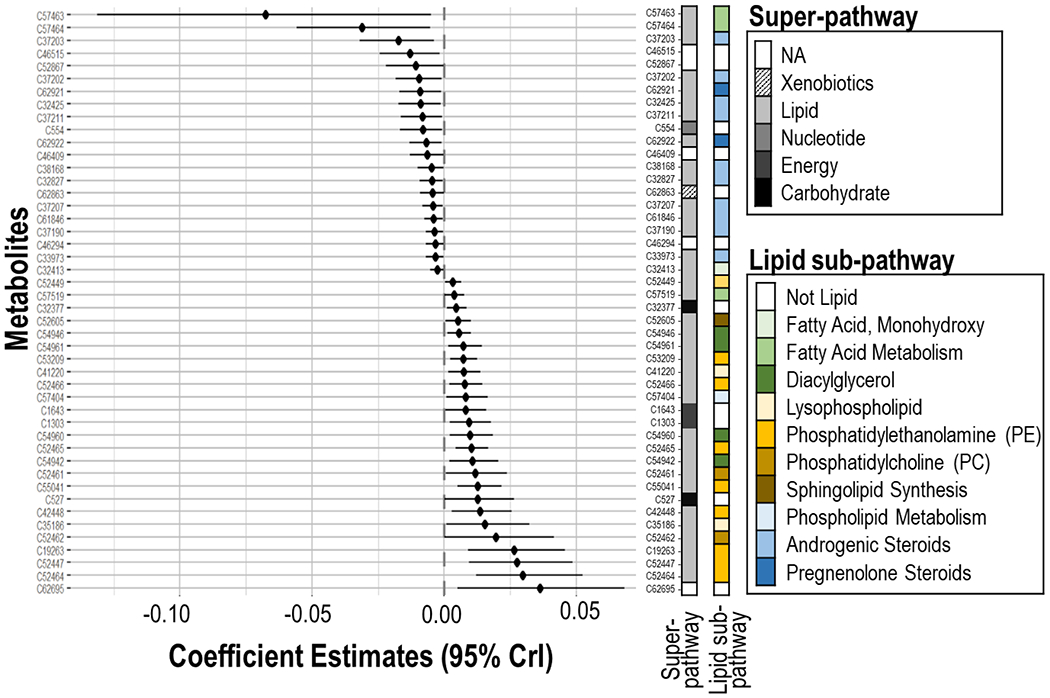
Linear association between individual maternal serum metabolites and birthweight-for-gestational age Fenton z-score. Super-pathways and sub-pathways were identified using the annotation mapping file. Significant associations between metabolites and z-scores were identified at a 5% significance threshold, while accounting for all metabolites and their correlation. All analyses were adjusted for maternal age, ethnicity, education level, pre-pregnancy body mass index, and week of serum collection.
The steroids, majority (75%) of fatty acids, and four unannotated metabolites showed a negative linear term with BWGA z-scores; while PE, PC, diacylglycerol, sphingolipids, and phospholipids had a positive relationship with BWGA. Metabolites related to energy production and carbohydrate production were also positively associated to BWGA z-score (Table B3, Fig. 2).
Two (C32413, C52464) out of three metabolites identified as significant with MWAS were included in the set of results from the Bayesian factor analysis. The direction of linear terms from the Bayesian factor analysis was consistent with most (89%) of the individual linear associations derived from MWAS analyses (Fig. 3).
Fig. 3. Comparison between the metabolite-wide association study (A) and Bayesian factor analysis for interaction (B).
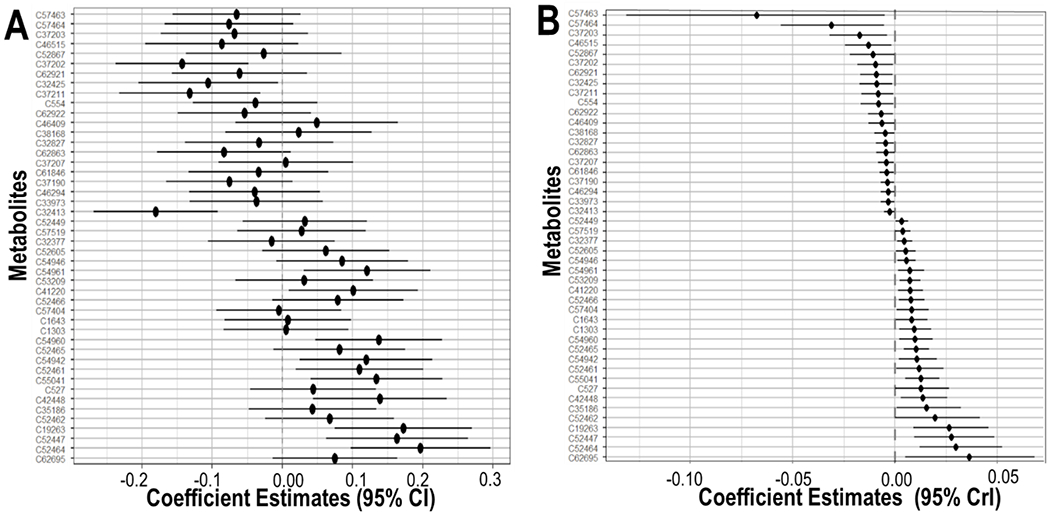
Each plot shows the association between individual prenatal maternal serum metabolites and sex-specific birthweight for gestational age z-score at a 5% significance threshold. All analyses were adjusted for maternal age, ethnicity, education level and week of serum collection.
3.3.2. Non-linear and synergistic associations
All 46 metabolites that showed strong evidence of a linear term also had a non-linear, quadratic (i.e. U-shaped) association with the BWGA z-score, which is represented by the diagonal of the heat map presented in (Fig. 4; Table B3). The quadratic associations were always larger than the absolute value of the linear terms (Table B3), thus implying that both low and high metabolite levels were associated higher BWGA z-score, while moderate levels reflected the negative or positive relationships indicated by the linear terms (Table B3, Fig. 2). Assuming a strong heredity constraint, such that the interaction term between two metabolites was included in the model only if both main associations were significant, we identified some evidence of interactions between metabolites within the same super-pathway (i.e. lipids) with similar functionalities (i.e. steroids, PE), with strongest interactions between two metabolites (C37425 and C62921) that had linear negative associations with BWGA z-score. (Fig. 4).
Fig. 4.
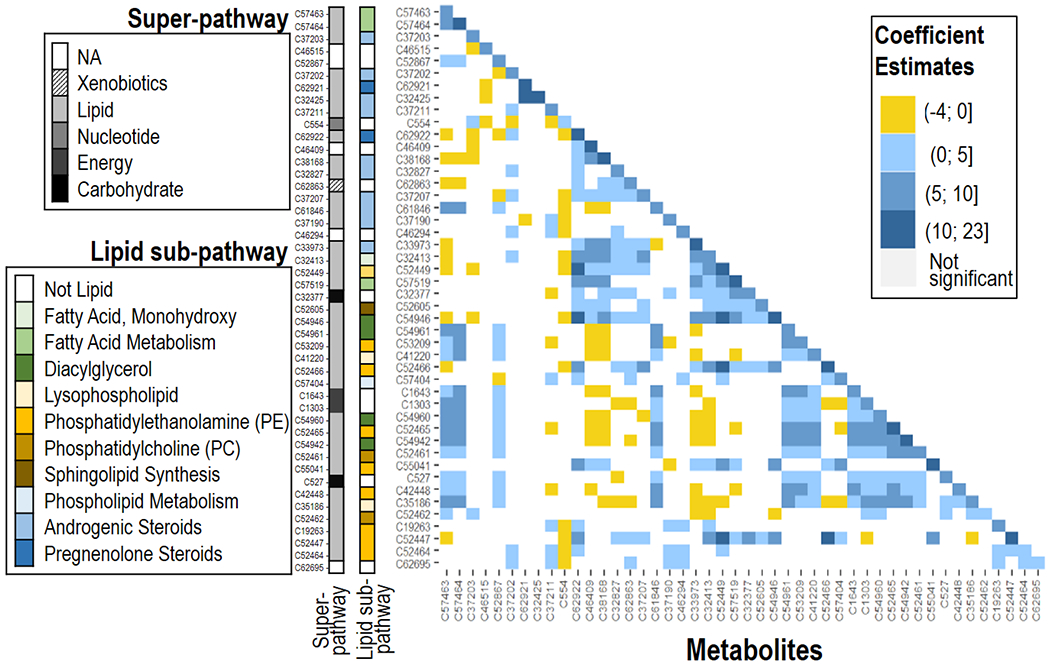
Nonlinear and synergistic relationships between individual metabolites and the birthweight Fenton z-score. Squares on left-to-right diagonal identify the coefficients of the quadratic term; squares outside the left-to-right diagonal identify the coefficients of the interaction between the corresponding metabolites. Individual and multiplicative associations between metabolites and birthweight Fenton z-score were at 5% significance threshold. All analyses were adjusted for maternal age, ethnicity, education levels, pre-pregnancy body mass index, and week of serum collection.
3.4. Metabolite similarity, and enrichment
Metabolite similarity.
We identified sets of metabolites, which had strong similarity (dissimilarity score <0.3) within the group while poor similarity (dissimilarity score >0.6) between groups. Most of the metabolites with no annotation were similar to metabolites belonging to the lipid super-pathway and more specifically, C46294, C46409, C46515 and C52867 were similar to C32827, C38168 and C37203, which were annotated as androgenic steroids (Fig. 5). While C62695 were similar to the amino acid C62863, annotated as a xenobiotic (Fig. 5).
Fig. 5.
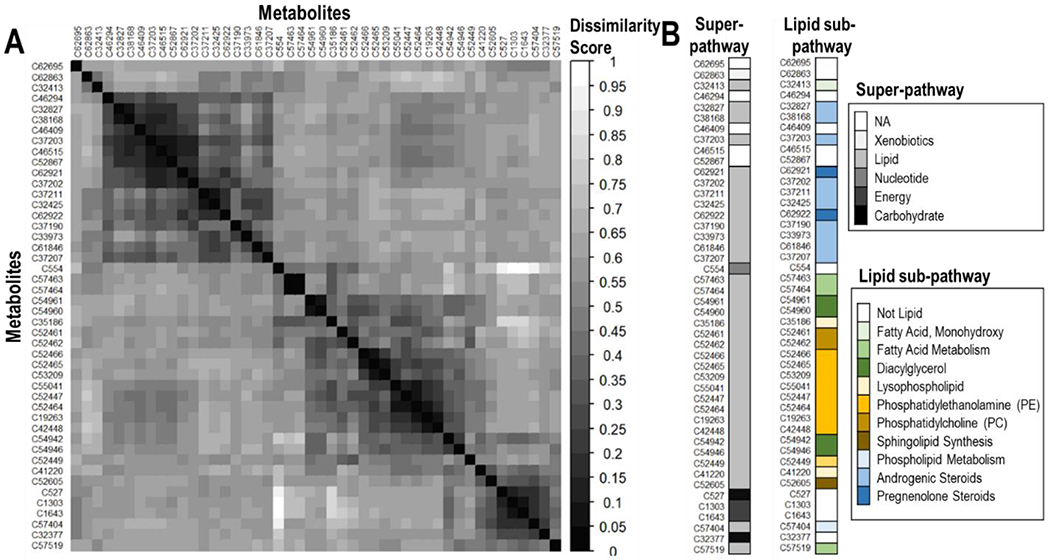
A) Dissimilarity matrix, identifying the pairwise dissimilarity between metabolites identified by the Bayesian factor analysis for interaction. The darker is the color of the dissimilarity score, the more similar are the metabolites. This matrix allows to cluster metabolites based on their similarities. B) Super-pathways and lipid sub-pathways of the identified metabolites, ordered as in A). Similar metabolites belong to the same sub-pathway.
Enrichment analysis.
With regard to BWGA z-score, we identified enrichment for the Warburg effect (p-value = 0.15), the citric acid cycle (p-value = 0.17) and androgen and estrogen metabolism (p-value 0.18) pathways. Although those results were significant at a 20% nominal p-value, they were not significant after multiple testing correction (Fig. 6; Table B4).
Fig. 6.
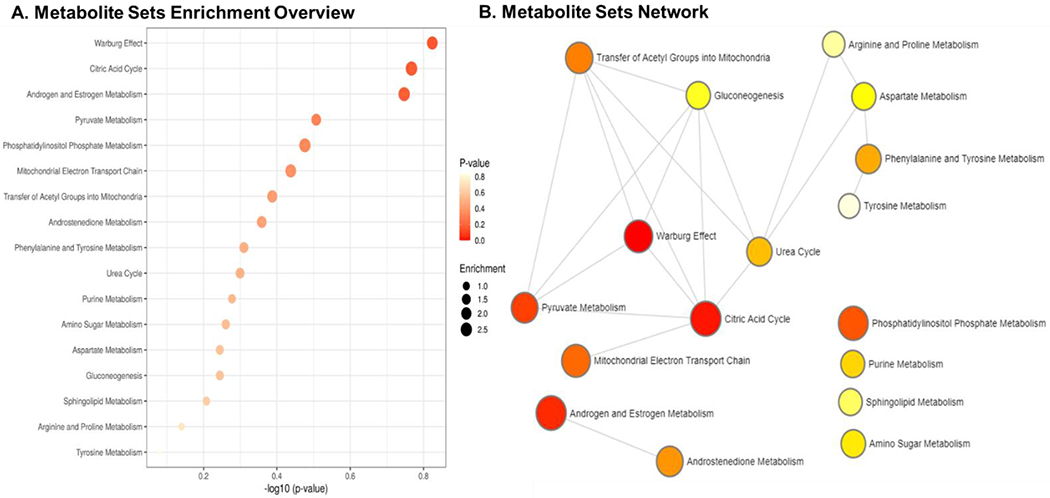
Enrichment analysis via MetabolAnalyst. A) Metabolite Sets Enrichment B) Metabolite Set Enrichment Network.
The hypergeometric tests showed an over-representation of androgens (p < 0.001), pregnenolone steroids (p = 0.07), diacylglycerol (p = 0.06) and TCA cycle subclasses (p = 0.10) (Table B5), thus supporting the central role of steroids and cellular energy support in fetal growth.
4. Discussion
This is the first prospective study to assess the relationship between maternal serum metabolites measured during pregnancy and BWGA z-score considering all metabolites jointly and allowing for non-linear and non-additive associations. We identified 46 metabolites that were significantly associated with BWGA z-score and all metabolites showed a U-shape relationship with the outcome, such that both low and high metabolite levels were associated with higher BWGA z-score. Moderate levels of steroids, a few fatty acids and four unannotated metabolites showed a negative association with BWGA z-scores; while moderate levels of phospholipids—phosphatidylethanolamines, phosphatidylcholines, phospholipids— diacylglycerols, and sphingolipids, showed an overall strong positive association with BWGA. Metabolites linked to energy production and carbohydrates were also positively associated with BWGA z-scores. Most (85%) of the identified metabolites were lipids mainly enriched in the pathways for the Warburg effect, the citric (tricarboxylic) acid (TCA) cycle and hormonal androgen and estrogen metabolism. Most of the lipophilic metabolites within the same subclassification (i.e. steroids, phospholipids) showed synergistic relationships, thus suggesting that metabolites act jointly and potentiate their effects on birthweight. A few (five) of the remaining metabolites were not mapped to any annotations. Four of the unmapped novel metabolites were similar to some metabolites mapped to the androgenic steroids, implying similar functions. Results from the traditional MWAS analysis identified only three metabolites significantly associated with the BWGA z-score, two of which were also significant in our novel Bayesian factor analysis for interaction.
Most of the significant metabolites were annotated as various lipids, supporting a role of lipids in fetal growth and their relation to other characteristics of newborn health. Indeed, during pregnancy, mothers are the sole provider of lipids to the fetus, and those lipids are selectively transported across the placenta at an increasing rate across gestation (Innis, 2007; LaBarre et al., 2020). Long-chain fatty acids have been shown to have critical roles in lipid accumulation, growth, and brain development during the third trimester (Fang et al., 2005; Robinson and Martin, 2017; Tam et al., 2016). Metabolites derived from fatty acids and/or long chain fatty acids, such as phospholipids and their derivative products (diacylglycerols), and sphingolipids showed a positive association with BWGA z-score in our findings, supporting previous research that identified higher levels of PC, PE and sphingolipids during gestation are critical for typical fetal growth (LaBarre et al., 2020). The majority of lipids enriched for long-chain fatty acid residues are confirmed to cross placenta and be transported to the fetal circulation (Innis, 2007; LaBarre et al., 2020). It has been speculated that placental endothelial lipases cleave fatty acids, thus facilitating the transport of phospholipids, which contain long-chain fatty acids, to the fetal circulation, and enhancing delivery to the fetus and the developing brain. In addition, phospholipids and their derivatives constitute a vast store of potential energy that can fuel energetically intensive processes, such as cellular replication (LaBarre et al., 2020). Sphingolipids also participate in a wide variety of metabolic, neurological, and intracellular signaling processes, including trophoblast cell turnover, thus supporting the positive link between sphingolipids and fetal growth (Breslow and Weissman, 2010). We also identified four other fatty acids that associated with BWGA z-scores. Specifically, moderate levels of acyl-choline and monohydroxy fatty acids (linoleoylcholine, stearoylcholine and 2-hydroxymyristate) were negatively linked to BWGA z-score, which is consistent with previous findings showing that linoleoylcholine and stearoylcholine are associated with birth outcomes (Petrick et al., 2017).
Our findings showed that most of the metabolites linked positively to birthweight are potentially involved in the Warburg effect and the TCA cycle, which are pathways for producing cellular energy and are associated with immune functions. Specifically, the Warburg effect, also referred to as glycolysis shift, is a metabolic mechanism that naturally occurs during blastulation and in other periods of rapid cellular proliferation (Krisher and Prather, 2012). The Warburg effect is a shift in energy production from mitochondrial oxidative phosphorylation (i.e. ATP generation) to aerobic glycolysis, despite the availability of oxygen. However, this process is inefficient compared to conventional glucose metabolism and TCA cycle pathways, which can generate up to 36 molecules of ATP per molecule of glucose in adult tissues (Metrustry et al., 2018). It is thought that this counter-intuitive process reflects the need of proliferating cells to attain critical biomass requirements, including proteins and lipids, beyond only ATP production from glucose. Indeed, macromolecular synthesis is the most pressing cellular need of proliferative cells (Krisher and Prather, 2012). The resulting metabolic intermediates are also key factors for the biosynthesis of immune proteins and downstream effectors, which are critical for orchestrating intrauterine processes, including fetal growth and development (Fu et al., 2017; Yockey and Iwasaki, 2018). Notably, it has also been hypothesized that the Warburg effect may also occur due to the malfunction of mitochondria (Kim et al., 2009). Mitochondria, which produce cellular energy in the form of ATP via oxidative phosphorylation, are essential for fueling rapid placental and fetal growth (Sferruzzi-Perri et al., 2019). It is thus plausible that Warburg-related enrichment of metabolites associated with abnormal fetal growth reflects mitochondrial dysfunction in these pregnancies. This possibility is further supported by several studies that have linked reduced mitochondria copy number and/or increased mitochondrial DNA mutational load with abnormal (small and large) birth size for gestational age (Gemma et al., 2006).
Mitochondria are also relevant to the TCA cycle, which takes place in their matrix. Levels of TCA intermediates increase as pregnancy progresses, due to an increased need for energy production through TCA cycle activity (Lindsay et al., 2015). Fumarate, one of our identified metabolites, and other TCA intermediates can act as a signal for processes involved in inflammation (Patil et al., 2019). Indeed, succinate, a clinically-relevant precursor of fumarate, accumulates in immune cells and has been shown to produce anti-inflammatory effects via inhibition of aerobic glycolysis (Kornberg, 2020). Additionally, limited research has shown that fumarate is cardio-protective (Patil et al., 2019) and that dimethyl fumarate, a cell-permeable analog, has immunomodulatory and anti-inflammatory properties (Moharregh-Khiabani et al., 2009; Mrowietz et al., 1999). However, the specific roles of fumarate and other TCA cycle intermediates during pregnancy requires further investigation.
A growing body of literature supports the idea that maternal pro- and anti-inflammatory processes during gestation are a potential pathway linking maternal environmental exposures with pregnancy progression and fetal development. Healthy pregnancies indeed show immunological shifts across the course of gestation, culminating in a proinflammatory period prior to parturition (Kuzawa et al., 2013; McDade et al., 2016). Other research supports that reduced maternal immune functions can lead to impaired placental invasion and restricted fetal growth resulting in small birth size (Denney et al., 2011; Moffett et al., 2015). Notably, maternal anti-inflammatory and immune modulatory properties improve metabolism of lipids and glucose during pregnancy, leading to embryo growth (Li et al., 2015).
Steroids, especially estrogen and progesterone, also contribute to both maternal health and fetal development throughout pregnancy (Solano and Arck, 2019). Among the identified lipid metabolites, 12 (26%) were steroids and their moderate levels were negatively linked to fetal growth, confirming previous findings showing that 16a-hydroxyD-HEAS, androsterone sulfate and 17-hydroxyprogesterone measured in newborn dry blood spots are negatively associated with birthweight (Petrick et al., 2017), while high and low levels of the identified steroids were positively associated with fetal growth supporting prior evidence on urinary metabolites measured in the late stages of pregnancy (Maitre et al., 2016; Maitre et al., 2014). Levels of pregnanediol, a steoroid congiugate, measured in maternal urine during pregnancy have also been linked to adverse birth outcomes, such as spontaneous preterm birth and restricted fetal growth (Maitre et al., 2014).
Steroids identified in our analysis were enriched for androgen and estrogen metabolism pathways. Androgen and estrogen concentrations increase in maternal circulation across the course of pregnancy (Licciardi et al., 2013). Hormones are secreted not only from the maternal adrenal gland, ovaries and myometrium but also from the placenta, which is an additional site for de novo synthesis of androstenedione and testosterone (Makieva et al., 2014). The advantage of increasing hormone levels is relevant for the maintenance of pregnancy; however an excess of hormones can be detrimental for fetal growth. For example, maternal testosterone levels cross the placenta have an effect on both placenta and the fetus. Indeed, maternal testosterone levels can affect fetal growth by increasing fetal testosterone levels and changing both maternal and placenta metabolism (Svensson et al., 2019). Also maternal estrogens and the estrogen (aromatase) complex within the placenta facilitate the increase of blood flow to the gravid uterus and placenta (Evans, 2007; Makieva et al., 2014), thus contributing to a normal fetal growth (Escobar-Morreale et al., 2012). However, impaired placental aromatase activity leads to an increase of circulation of maternal testosterone levels which can results in abnormal fetal growth (Chen et al., 2017).
There are several notable strengths of this study, including the implementation of novel statistical methodology to assess and annotate an untargeted panel of metabolites in relation to birthweight. This approach improves traditional MWAS methodology as it can be used to jointly identify linear, non-linear and interactive relationships between metabolites and their relationship with an outcome. This is an improvement as individual linear analyses captured few metabolite-birthweight relationships compared to the approach using Bayesian factor analysis for interactions framework. Further, the results from linear MWAS analyses may be affected by spurious correlations among metabolites belonging to the same class. We also leveraged an ethnically diverse population, thus making our results more generalizable. In addition, our study is unique in that we measured metabolomic profiles in maternal serum, which is enriched for several metabolites relevant to pregnancy and fetal development, such as certain hormones and lipids. Collectively, these findings support the overall hypothesis that metabolites belonging to lipid super-pathways play important roles for fetal growth and that long-chain fatty acid and steroids, which can cross the placenta and can influence infant outcomes.
We also note some limitations. First, we cannot rule out the effect of unmeasured confounders. However, we accounted for the diversity of the population with race/ethnicity, socioeconomic status, body mass index, and maternal age. While we have also considered week of serum collection as a precision variable to account for the timing of collection, a residual effect could remain and we acknowledge the variability in timing of serum collection across the course of pregnancy as a limitation of this design. In addition, our novel Bayesian approach captured only pairwise interactions between metabolites and more complex feature combinations, such as three-way interactions, cannot be ruled out by this approach. Further larger studies should explore the sex-specific and ethic-specific influence of the maternal metabolomics on the developing fetus. In addition, future targeted research investigating how these metabolites change across the pregnancy and life course, relate to external environmental exposures, and correlate to child growth is needed to more comprehensively understand the underlying biological mechanisms at play.
5. Conclusions
Our findings provide evidence that the maternal metabolome during pregnancy are associated linearly, non-linearly and synergistically to inter-individual variation of newborn fetal growth. Most of the identified metabolites were lipids enriched for pathways related to energy production, immune function, or androgen and estrogen metabolism, which are notably central to key processes involved in pregnancy and parturition.
Supplementary Material
Funding
This work was supported by the National Institutes of Health [grant numbers: R01 HL095606, R01 HL114396, and UG3 OD023337 supporting the cohort follow-up and data collection and statistical support through P30 ES023515 and UL1 TR001433. During the preparation of this manuscript, EC was supported by R01 ES032242, U2CES026555, U2CES026561, P30ES023515; FF was supported by R01 ES028804; and WC was supported by T32 HD049311 and K99ES032029].
Footnotes
CRediT authorship contribution statement
E. Colicino: Conceptualization, Methodology, Writing - original draft. F. Ferrari: Methodology, Software. W. Cowell: Writing - review & editing. M.M. Niedzwiecki: Writing - review & editing. N. Foppa Pedretti: Data curation, Software, Visualization. A. Joshi: Data curation, Software. R.O. Wright: Funding acquisition, Supervision. R.J. Wright: Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2021.106750.
References
- Barker D, Thornburg K, 2013. The obstetric origins of health for a lifetime. Clin. Obstet. Gynecol 56, 511–519. [DOI] [PubMed] [Google Scholar]
- Breslow DK, Weissman JS, 2010. Membranes in balance: mechanisms of sphingolipid homeostasis. Mol Cell. 40, 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A, et al. , 2001. Impact of low birth weight on early childhood asthma in the United States. Arch. Pediatr. Adolesc. Med 155, 401–406. [DOI] [PubMed] [Google Scholar]
- Chen L, et al. , 2017. Ziram inhibits aromatase activity in human placenta and JEG-3 cell line. Steroids 128, 114–119. [DOI] [PubMed] [Google Scholar]
- Chong J, et al. , 2019. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protocols Bioinform 68. [DOI] [PubMed] [Google Scholar]
- Chou J, et al. , 2020. PediTools electronic growth chart calculators: applications in clinical care, research, and quality improvement. J. Med. Internet Res 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn S, et al. , 2019. Comparability of serum, plasma, and urinary estrogen and estrogen metabolite measurements by sex and menopausal status. Cancer Causes & Control: CCC 30, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denney JM, et al. , 2011. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine 53, 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Morreale HF, et al. , 2012. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum. Reprod. Update 18, 146–170. [DOI] [PubMed] [Google Scholar]
- Evans TJ, 2007. Chapter 14. Reproductive Toxicity and Endocrine Disruption. In: Gupta RC, (Ed.), Veterinary Toxicology. Academic Press, pp. 206–244. [Google Scholar]
- Fagerberg B, et al. , 2004. Low birth weight in combination with catch-up growth predicts the occurrence of the metabolic syndrome in men at late middle age: the Atherosclerosis and Insulin Resistance study. J. Intern. Med 256, 254–259. [DOI] [PubMed] [Google Scholar]
- Fang P, et al. , 2005. The effect of supplementation of docosahexaenoic acid and arachidonic acid on visual acuity and neurodevelopment in larger preterm infants. Chang Gung Med. J 28. [PubMed] [Google Scholar]
- Fenton TR, Kim JH, 2013. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 13, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F, Dunson DB, 2020. Bayesian factor analysis for inference on interactions. J. Am. Stat. Assoc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu B, et al. , 2017. Natural killer cells promote fetal development through the secretion of growth-promoting factors. Immunity 47, 1100–1113. [DOI] [PubMed] [Google Scholar]
- Gemma C, et al. , 2006. Mitochondrial DNA depletion in small- and large-for-gestational-age newborns. Obesity (Silver Spring, Md.) 14, 2193–2199. [DOI] [PubMed] [Google Scholar]
- Stephen C Grace SC, Hudson DA, 2016. Processing and Visualization of Metabolomics Data Using R. doi: 10.5772/65405. [DOI] [Google Scholar]
- Harvey N, et al. , 2014. Osteoporosis: a lifecourse approach. J. Bone Miner Res 29, 1917–1925. [DOI] [PubMed] [Google Scholar]
- Howe C, et al. , 2020. Prenatal metal mixtures and birth weight for gestational age in a predominately lower-income Hispanic pregnancy Cohort in Los Angeles. Environ. Health Perspect 128, 117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis SM, 2007. Dietary (n-3) fatty acids and brain development. J. Nutr 137, 855–859. [DOI] [PubMed] [Google Scholar]
- Johansson S, et al. , 2008. The association between low birth weight and type 2 diabetes: contribution of genetic factors. Epidemiology 19, 659–665. [DOI] [PubMed] [Google Scholar]
- Kim HH, et al. , 2009. The Mitochondrial Warburg Effect: A Cancer Enigma - Open Access Library. Interdisciplinary Bio Central. [Google Scholar]
- Kornberg M, 2020. The immunologic Warburg effect: Evidence and therapeutic opportunities in autoimmunity. Wiley interdisciplinary reviews. Syst. Biol. Med 12, e1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisher R, Prather R, 2012. A role for the Warburg effect in preimplantation embryo development: metabolic modification to support rapid cell proliferation. Mol. Reprod. Dev 79, 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa C, et al. , 2013. C-reactive protein by pregnancy and lactational status among Filipino young adult women. American journal of human biology: the official journal of the Human Biology Council. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBarre JL, et al. , 2020. Maternal lipid levels across pregnancy impact the umbilical cord blood lipidome and infant birth weight. Sci. Rep 10, 14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson CP, et al. , 2001. Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation 103, 1264–1268. [DOI] [PubMed] [Google Scholar]
- Li SM, et al. , 2015. Fibroblast growth factor 21 expressions in white blood cells and sera of patients with gestational diabetes mellitus during gestation and postpartum. Endocrine 48, 519–527. [DOI] [PubMed] [Google Scholar]
- Licciardi F, et al. , 2013. Using the oocyte donation model to identify early trophoblast pregnenolone production. J. Assist. Reprod. Genet 30, 699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay KL, et al. , 2015. Longitudinal metabolomic profiling of amino acids and lipids across healthy pregnancy. PLoS ONE 10, e0145794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde A, et al. , 2007. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. Am. J. Epidemiol 165, 734–741. [DOI] [PubMed] [Google Scholar]
- Maitre L, et al. , 2014. Urinary metabolic profiles in early pregnancy are associated with preterm birth and fetal growth restriction in the Rhea mother-child cohort study. BMC Med. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre L, et al. , 2016. Maternal urinary metabolic signatures of fetal growth and associated clinical and environmental factors in the INMA study. BMC Med. 14, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makieva S, et al. , 2014. Androgens in pregnancy: roles in parturition. Hum. Reprod. Update 20, 542–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos J, Pol M, Fabregat A, Ventura R, Renau N, Hanzu FA, Casals G, Marfà S, Barceló B, Barceló A, Robles J, Segura J, Pozo O*, 2015. Urinary cysteinyl progestogens: occurrence and origin. J. Steroid. Biochem. Mol. Biol, 152, 53–61. [DOI] [PubMed] [Google Scholar]
- Marcos J, Renau N, Casals G, Segura J, Ventura R, Pozo O*, 2014. Investigation of endogenous corticosteroids profiles in human urine based on liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 812, 92–104. [DOI] [PubMed] [Google Scholar]
- McDade TW, et al. , 2016. Adiposity and chronic inflammation in young women predict inflammation during normal pregnancy in the Philippines. J. Nutr 146, 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod MD, Waller CC, Esquivel A, Balcells G, Ventura R, Segura J, Pozo O*, 2017. A constant ion loss method for the untargeted detection of bissulfate metabolites. Anal. Chem 89(3), 1602–1609. [DOI] [PubMed] [Google Scholar]
- Metrustry S, et al. , 2018. Metabolomic signatures of low birthweight: pathways to insulin resistance and oxidative stress. PLoS ONE 13, e0194316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett A, et al. , 2015. The role of the maternal immune system in the regulation of human birthweight. Philos. Trans. R Soc. Lond. B Biol. Sci 370, 20140071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moharregh-Khiabani D, et al. , 2009. Fumaric Acid and its esters: an emerging treatment for multiple sclerosis. Curr. Neuropharmacol 7, 60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrowietz U, et al. , 1999. Treatment of severe psoriasis with fumaric acid esters: scientific background and guidelines for therapeutic use. The German Fumaric Acid Ester Consensus Conference. Br. J. Dermatol 141, 424–429. [DOI] [PubMed] [Google Scholar]
- Nardozza L, et al. , 2017. Fetal growth restriction: current knowledge. Arch. Gynecol. Obstet 295, 1061–1077. [DOI] [PubMed] [Google Scholar]
- Niedzwiecki MM, et al. , 2020. High-resolution metabolomic profiling of Alzheimer’s disease in plasma. Ann. Clin. Transl. Neurol 7 (36), 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiecki MM, et al. , 2019. The exposome: molecules to populations. Annu. Rev. Pharmacol. Toxicol 59, 107–127. [DOI] [PubMed] [Google Scholar]
- Oudgenoeg-Paz O, et al. , 2017. The link between motor and cognitive development in children born preterm and/or with low birth weight: a review of current evidence. Neurosci. Biobehav. Rev 80, 382–393. [DOI] [PubMed] [Google Scholar]
- Pang Z, et al. , 2020. MetaboAnalystR 3.0: toward an optimized workflow for global metabolomics. Metabolites 10, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil N, et al. , 2019. Regulation of leukocyte function by citric acid cycle intermediates. J. Leukoc. Biol 106, 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick L, et al. , 2017. An untargeted metabolomics method for archived newborn dried blood spots in epidemiologic studies. Metabolomics 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo OJ, Marcos J, Khymenets O, Pranata A, Fitzgerald C, McLeod MD, Shackleton C, 2018. Steroid sulfation pathways targeted by disulfates determination. Application to prenatal diagnosis. J. Mol. Endocrinol 61 (2), M1–M12. [DOI] [PubMed] [Google Scholar]
- Rager JE, et al. , 2020. Review of the environmental prenatal exposome and its relationship to maternal and fetal health. Reprod. Toxicol 98, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raro M, Portoles T, Pitarch E, Sancho JV, Hernández F, Garrostas L, Marcos J, Ventura R, Segura J, Pozo O*, 2016. Potential of atmospheric pressure chemical ionization source in gas chromatography tandem mass spectrometry for the screening of urinary exogenous androgenic anabolic steroids. Anal. Chim. Acta 906, 128–138. [DOI] [PubMed] [Google Scholar]
- Robinson DT, Martin CR, 2017. Fatty acid requirements for the preterm infant. Seminars in Fetal and Neonatal Med. 22, 8–14. [DOI] [PubMed] [Google Scholar]
- Sferruzzi-Perri AN, et al. , 2019. Placental mitochondria adapt developmentally and in response to hypoxia to support fetal growth. PNAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmueli A, Cullen M, 1999. Birth weight, maternal age, and education: new observations from Connecticut and Virginia. The Yale J. Biol. Med 72, 245–258. [PMC free article] [PubMed] [Google Scholar]
- Silvestrin S, et al. , 2013. Maternal education level and low birth weight: a meta-analysis. Jornal de pediatria. 89, 339–345. [DOI] [PubMed] [Google Scholar]
- Solano ME, Arck PC, 2019. Steroids, pregnancy and fetal development. Front Immunol. 10, 3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette M, Paulovich A, Pomeroy S, Golub T, Lander E, Mesirov JP, 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci 102 (43), 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson K, et al. , 2019. Prenatal salivary sex hormone levels and birth-weight-for-gestational age. J. Perinatol 39, 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam EWY, et al. , 2016. Early postnatal docosahexaenoic acid levels and improved preterm brain development. Pediatr. Res 79, 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team CMA, et al. , 2020. Quantitative methods for metabolomic analyses evaluated in the Children’s Health Exposure Analysis Resource (CHEAR). J. Expo. Sci. Environ. Epidemiol 30, 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K, 2017. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw 45, 1–67. [Google Scholar]
- Vermeulen R, et al. , 2020. The exposome and health: where chemistry meets biology. Science 367, 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter E, et al. , 2009. Low birth weight and respiratory disease in adulthood: a population-based case-control study. Am. J. Respir. Crit. Care Med 180, 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, et al. , 2011. Racial, ethnic, and socioeconomic disparities in the prevalence of cerebral palsy. Pediatrics 127, e674–e681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey L, Iwasaki A, 2018. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity 49, 397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


