Plant Cell DOI: https://doi.org/10.1105/tpc.111.090993
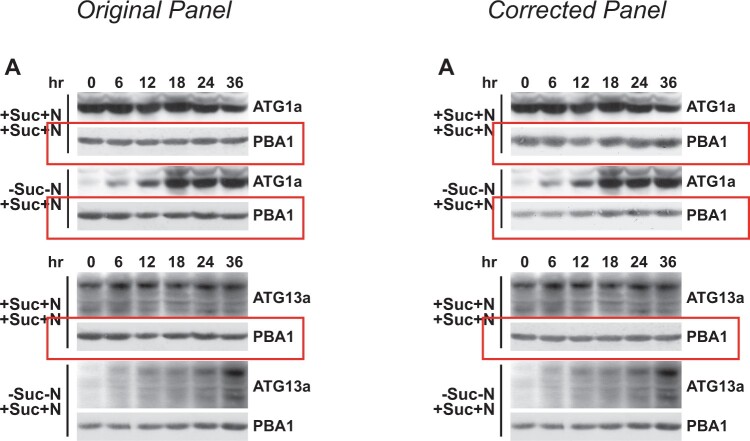
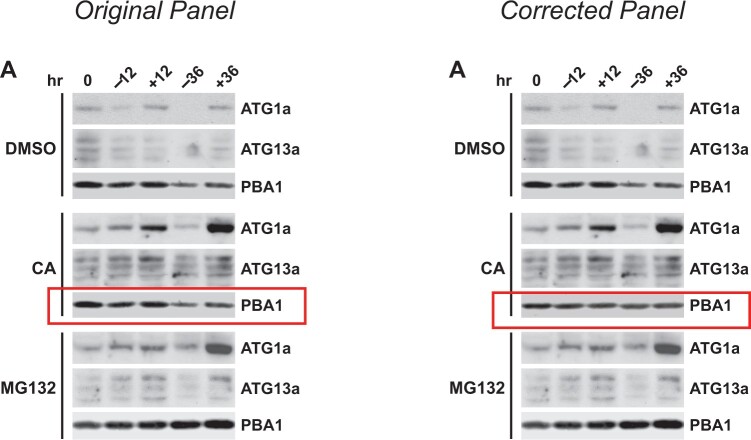
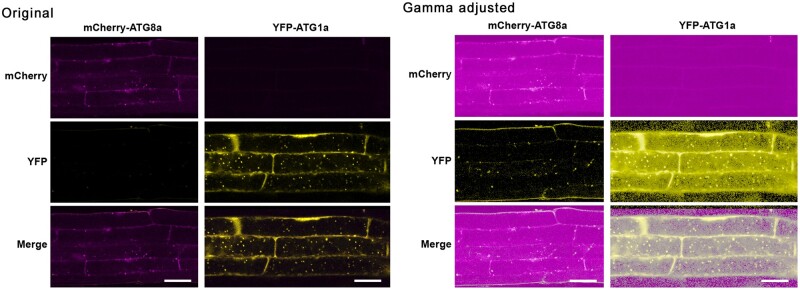
The original manuscript included inadvertant duplications and incorrect placement of control blots shown in Figures 8A and 9A. The original and corrected figures are shown below, with the duplicated and corrected panels boxed in red in the original and corrected figures, respectively. In Supplemental Figure 10, compression of the original file to reduce the file size may have rendered the faint background signal invisible in the YFP channel of roots expressing mCherry-ATG8a. A higher resolution version of the original Supplemental Figure 10 image is shown below, together with a copy that was gamma adjusted to show the underlying background signal. The results and conclusions of this work are unaffected by these corrections.
Figure 8.
ATG1a and ATG13a Proteins Rapidly Reappear after Suc/N-Limited Plants Are Refed. Five-day-old wild-type seedlings were either kept in +Suc+N medium or switched to −Suc−N for 36 h and then switched back to +Suc+N medium for the indicated times before extraction of total protein. (A) Immunoblot analysis of the refeeding time course with anti-ATG1a or ATG13a antibodies. Each lane contains an equivalent amount of tissue fresh weight. Immunoblot analyses with anti-PBA1 antibodies were used to confirm near equal protein loading.
Figure 9.
Turnover and Phosphorylation States of ATG1a and ATG13a Proteins Are Regulated by Autophagy. (A) to (C) Effect of inhibitors and mutants on the turnover of ATG1a and ATG13a during starvation. Five-day-old seedlings of the various genetic backgrounds were either kept in +Suc+N medium (+) or switched to –Suc−N medium (−) at t = 0 and then incubated for the indicated times before extraction of total protein. ATG1a and ATG13a proteins levels were detected by immunoblot analysis with anti-ATG1a and ATG13a antibodies. Immunoblot analyses with anti-PBA1 antibodies were used to confirm near equal protein loading. Each lane contains an equivalent amount of tissue fresh weight. (A) Effect of various inhibitors. CA (0.5 μM), MG132 (50 μM), or an equivalent volume of DMSO were added at t = 0.
Supplemental Figure 10.
Selective Detection of mCherry-ATG8a and YFP-ATG1a in Transgenic Arabidopsis Plants by Fluorescence Confocal Microscopy. Six-d-old wild-type plants expressing each transgene by itself were grown on +Suc-N medium and then treated for 8 hr with 0.5 µM CA. Root tip cells were imaged by fluorescence confocal microscopy for both YFP and mCherry. Merged represents the superimposition of the two images. Bars = 10 µm.
In addition, Figures 7 and 10 appear to have duplicated images, but these figures are correct as presented in the original paper as described below.
Figure 7 has a duplicated and rotated image corresponding to the 0 time point for +Suc/−N and −Suc/+N treatments probed with PBA1. For this experiment, a seedling culture grown in +Suc/+N conditions was split in two for the +Suc/–N and –Suc/+N treaments. A sample was taken a time 0 before the split and loaded at the center of a gel with time points for +Suc/–N running left to right and time points for –Suc/+N running right to left from the zero time point; therefore the zero time point is the same for both treatments, and the row corresponding to –Suc/+N was rotated to present the time points from left to right in the figure.
For Figure 10A, the same blot was probed with two antibodies (with intervening stringent washes). As these anti-ATG1a and anti-YFP antibodies were supposed to recognize the same ATG1a-YFP protein, the blots should look identical.
Note: The corrected figure and accompanying text were reviewed by members of The Plant Cell editorial board. The authors are responsible for providing a complete listing and accurate explanations for all known errors or instances of inappropriate data handling or image manipulation associated with the original publication.
Contributor Information
Anongpat Suttangkakul, Viikki Plant Science Centre, Organismal and Evolutionary Biology Research Programme, Faculty of Biological and Environmental Sciences, University of Helsinki, Helsinki, Finland.
Faqiang Li, Viikki Plant Science Centre, Organismal and Evolutionary Biology Research Programme, Faculty of Biological and Environmental Sciences, University of Helsinki, Helsinki, Finland.
Taijoon Chung, Viikki Plant Science Centre, Organismal and Evolutionary Biology Research Programme, Faculty of Biological and Environmental Sciences, University of Helsinki, Helsinki, Finland.
Richard D Vierstra, Viikki Plant Science Centre, Organismal and Evolutionary Biology Research Programme, Faculty of Biological and Environmental Sciences, University of Helsinki, Helsinki, Finland.