Abstract
Objectives:
The aim of this study was to determine and compare the occurrence of adverse pregnancy outcomes in a cohort of pregnant women with interpregnancy interval of < and ⩾6 months (short and normal interpregnancy interval, respectively) following a spontaneous miscarriage in their last pregnancies.
Methods:
This was a cohort study that involved pregnant women with a spontaneous pregnancy loss in their last pregnancies. They were recruited at a gestational age of 13–15 weeks and followed up to determine the obstetric and foetal outcomes of their pregnancies at four tertiary hospitals in Nigeria from July 2018 to September 2019. Data collected were analysed using SPSS version 26.0. A Chi-square and multivariate logistic regression analysis were done, and a p-value of less than 0.05 was assumed to be statistically significant.
Results:
A total of 705 participants were studied, out of which 448 (63.5%) and 257 (36.5%) of the participants had short and normal interpregnancy interval after a spontaneous miscarriage. Over 80% of the participants had first-trimester pregnancy losses and were managed with manual vacuum aspiration in 73.3% of the cases. The majority, 87.5% for the normal interpregnancy interval cohort and 86.4% for the short interpregnancy interval cohort, had live births, while 8.5% and 10.1% of the women in the normal and short interpregnancy interval cohorts, respectively, had repeat miscarriages. There was no statistical difference in the occurrence of live births and repeat miscarriages between both cohorts (p > 0.05). There was no increased risk of occurrence of adverse foetomaternal outcomes in both groups (p > 0.05). Multivariate logistic regression analysis showed that there was no statistical difference in the occurrence adverse foetomaternal outcomes between the studied cohorts (p > 0.05).
Conclusion:
There was no significant difference in the occurrence of adverse maternal and foetal outcomes in the cohorts of mothers with short and normal interpregnancy interval following miscarriages in their last previous pregnancies.
Keywords: Interpregnancy interval, miscarriage, obstetric outcome, early pregnancy loss, low-income setting, prospective cohort study
Introduction
Grieving couples who have just experienced a miscarriage, especially for a planned pregnancy, are often eager to know the ideal time to undertake the next conception, without increased risk of a recurrent pregnancy loss and other obstetric complications. Early pregnancy loss complicates approximately 12%–15% of pregnancies, and studies have shown that previous miscarriages and interpregnancy interval (IPI) could affect the obstetric and perinatal outcomes of subsequent pregnancy.1 –4 These findings informed the World Health Organization’s (WHO) recommendation, which encourages women who had experienced a previous miscarriage to wait for a minimum of 6 months before the next conception to achieve an optimal outcome and reduce obstetric complications, such as low birth weight babies, preterm delivery, premature rupture of foetal membrane, preeclampsia, stillbirth and operative delivery.1,2,5 –10 However, this duration may be a cause of concern or anxiety for grieving couples who have just experienced a pregnancy loss and want to know how soon they can attempt the next conception, especially those with poor obstetric history or who are elderly nulliparae.
Contrary to the findings of the research on which WHO based its recommendations regarding pregnancy spacing of ⩾6 months after a miscarriage, some researchers have reported that the risk of adverse obstetric outcome was less in women who conceived less than 6 months after a pregnancy loss.3,4,11,12 There are also contemporary reports, indicating that the longer the waiting period, the higher the risk of a non-live birth in the next pregnancy.4,13
IPI is simply defined as the time interval between a live birth and the estimated time of conception of the subsequent pregnancy. 14 Also, defined as the spacing between a live birth and the beginning of the following pregnancy. 15 This interval is variously defined in studies as short or long. Some authors have defined short IPI as time interval that is shorter than 6 months, while long IPI was defined as time interval over 60 months between a live birth and the estimated time of conception of the subsequent pregnancy.14,15
It is noteworthy to observe that most of these studies were conducted in high-income countries with low fertility rates, good access to quality preconception, prenatal, intrapartum and postnatal care services, significant access and acceptance of family planning and where couples do not place an extremely high premium on childbirth and large family size.16,17 Contrarily, the reverse is the case in most low- and middle-income countries (LMICs) like Nigeria, where a huge premium is placed on early childbearing and any delay in childbearing after marriage is unacceptable and considered as a reproductive failure by peers, family members and the society.16,18 In such a setting, it is difficult for couples who have just experienced a miscarriage, to accept or adhere to long IPI before their next conception. Due to these pressing reasons, many couples and families in LMICs will want to know how soon they can safely attempt and achieve the next pregnancy.
Considering the difference in study populations, each with different cultures giving rise to the traditional recommendation to delay conception for ⩾6 months following a miscarriage, this study was underpinned. And also, due to the paucity of Nigerian studies examining the association between IPI after a prior miscarriage and adverse obstetric outcomes (maternal and foetal), the current study was designed to assess the impact of IPI <6 months versus IPI ⩾6 months on the obstetric outcome (live births, repeat miscarriages, placenta praevia, hypertensive disorders of pregnancy, preterm contractions and labour, premature rupture of membranes (PROM), intrauterine foetal deaths (IUFD) and intrauterine growth restriction (IUGR), mode/route of delivery, blood loss for vaginal and caesarean section deliveries, birth weight, need for resuscitation, admission into the newborn intensive care unit (NICU) and early neonatal deaths) in the next pregnancy following a miscarriage. This will help doctors, midwives, non-governmental organizations and governmental organization in establishing polices that will serve as bases for establishing patterns, and maternal and perinatal outcome recommendations regarding optimum interval of conception after miscarriages among parturients in the study area. In addition, it will provide non-existent local evidence that could guide health care providers in the study area during post-abortion care counsellors to assist couples to make informed decisions regarding their fertility needs and in planning future conception. Our evidence will also add to the global existing knowledge on the optimal IPI following a miscarriage in Nigeria. This study adhered to the STROBE guidelines for observational studies in the reporting of this study. 19
Methods
Study design and setting
This was a cohort study conducted between 1 July 2018 and 30 September 2019 in four tertiary health facilities: Alex-Ekwueme Federal University Teaching Hospital in Abakaliki, Ebonyi State; University of Nigeria Teaching Hospital (UNTH) in Enugu, Enugu State; Enugu State University Teaching Hospital (ESUTH) in Enugu, Enugu State; and Stella Obasanjo Hospital (SOH) in Benin City, Edo State, which are all located in the southern region of the country. The south-south and southeast geopolitical zones are among the six geopolitical zones of Nigeria. There are 11 states in the two zones; Abia, Akwa Ibom, Anambra, Bayelsa, Delta, Ebonyi, Edo, Enugu, Imo and Rivers states. The two regions were created from the old eastern and western regions of Nigeria on 27 May 1967, by the regime of General Yakubu Gowon. Edo and Delta were created from the western region, while the rest were created from the eastern region of Nigeria. The region is one of the most densely populated places in Nigeria. The region has three major vegetations; coastal south has mangrove swamps and tidal waterways, further north of the swamps is more of tropical rainforest and northernmost is the Guinea Savannah region. Most of the petroleum exploration in Nigeria occur in this region.
The total fertility rate (TFR) in Nigeria was 5.417 births per woman. 20 The obstetrics and gynaecology units of the above-named study sites were managed by consultant obstetricians and resident doctors assisted by midwives and nurses. All high-risk pregnancies and deliveries are supervised and conducted by senior resident doctors and/or consultant obstetricians, and attended by a neonatologist. The standard practice in the hospital is to admit all neonates with complications into the neonatal intensive care unit (NICU). Also, diagnostic protocols and treatment guidelines are similar in all four hospitals.
Participants
Participants were recruited at their antenatal booking visits at their earliest time of presentation up to a gestational age of 13 weeks.
Inclusion and exclusion criteria
All pregnant women with ongoing confirmed pregnancies (using ultrasound), previous spontaneous miscarriages antedating index pregnancy, confirmed singleton pregnancies and consenting parturients were eligible for recruitment for this study. The participants had ultrasound to confirm their pregnancies and if there is a disparity of more than 5 days, the ultrasound report of gestational age is accepted ahead of the calculated gestational age from the participant’s last menstrual period (LMP). Women with induced abortion, no previous miscarriage(s), parturient with medical conditions antedating pregnancy like hypertensive disorders, pre-gestational diabetes, multiple pregnancy, previous caesarean sections, placenta praevia, abruption placenta, women scheduled for elective caesarean section, women who booked after 13 weeks’ gestational age and women younger than 18 years of age were excluded.
Study sampling and selection of participants
Three states were randomly chosen among the 11 states in the south-south and southeast geopolitical zones. In the above-selected states, four centres (Alex-Ekwueme Federal University Teaching Hospital in Abakaliki, Ebonyi State; University of Nigeria Teaching Hospital (UNTH) in Enugu, Enugu State; Enugu State University Teaching Hospital (ESUTH) in Enugu, Enugu State and Stella Obasanjo Hospital (SOH) in Benin City, Edo State) with a high rate of maternal services were chosen purposively for this study. Pregnant women who met the inclusion criteria were consecutively recruited after informed written consent and were followed up during the antenatal period, intrapartum, postpartum until discharged home and for 6 weeks postpartum (during postnatal visits).
Sample size calculation
The sample size used for this study was calculated using the formula N = 1/(1–f) × (2 × (Zα + Zβ) 2 × p × (1–p)/(p0–p1) 2 ), where p = p0 + p1/2, p0 is the proportion of the participants in the unexposed group who are expected to exhibit the outcome of interest, p1 is the proportion of the participants in the exposed group who are expected to exhibit the outcome of interest, f is the proportion of the study subjects who are expected to leave the study for reasons other than the outcome under investigation. 21 The sample size calculation was based on a similar study by Bentolila et al. 22 in Beer-Sheva, Israel, where p0 was 0.297, p1 was 0.186, assumed f was 0.5 and Zα = 1.96 for 95% confidence at a power of 90% (Zβ = 1.282). The calculated sample size of 626 was enough for this cohort study, but a total of 705 participants were recruited for this study.
Outcome measures
The primary outcome measures were the rate of miscarriage, live birth, stillbirth, APGAR scores at birth, admission into the NICU and early neonatal death. Secondary outcomes were rates of caesarean section delivery, preterm delivery, low birth weight infants, PROM, IUGRs, preeclampsia, placenta praevia and placental abruption. Women were recruited blindly, that is, both the senior midwife recruiters and the doctors collecting the data did not know which category each mother will fall into. The cut-off length of the miscarriage to LMP interval used for statistical analysis was only known to the lead investigators.
Data collection
Data were collected by 16 trained research assistants, mostly junior medical doctors; four in each of the study facilities, using a pretested, specially designed pro forma. Data on socio-demographic characteristics were obtained from the participants and confirmed using the records on their medical files. These data were collected at contact, during follow-up in the antenatal clinics, during delivery/termination of pregnancy and at 6 weeks post-delivery. The phone contacts of these mothers were obtained and this helped in tracking the progress of their pregnancy outside that done in the antenatal clinics. Information obtained was the history of miscarriages antedating the current pregnancy, the number of previous miscarriages, gestational age at which miscarriage occurred, how it was managed, history of post-abortion complications, anaemia, antepartum haemorrhage, IPI, prenatal problems in the index pregnancy, duration of current pregnancy/gestational age at delivery and outcome of current pregnancy; mode of delivery, intrapartum problems, estimated blood loss, birth weight and APGAR scores of the baby, 23 need for newborn resuscitation and admissions into the NICU, status of the baby (live birth, stillbirth, early neonatal deaths) and duration of hospital stay.
Definitions
Miscarriage was defined as the termination of a pregnancy before the age of viability (24 weeks gestation in the current study, based on the level of neonatal care facilities and savage rate in the study centres). IPI was defined as the time between the last miscarriage and the first day of the LMP of the index pregnancy. Short IPI in this study is defined here as time interval that is shorter than 6 months.
Statistical analysis
Data were entered in an Excel spreadsheet (Microsoft, Redmond, WA, USA), checked for double entry, cleaned and transferred to SPSS version 26.0 (IBM, Armonk, NY, USA) for statistical analyses. Descriptive statistics were used to assess baseline characteristics. Where appropriate, continuous variables were converted to clinically applicable categories. Frequencies were calculated for categorical variables and the Chi-square test (or Fisher’s exact test) was used to test for associations. When both the Chi-square test and Fisher’s exact tests were estimated, only the p-value of the Fisher’s exact test was reported. Multivariable logistic regression analyses were performed to determine adjusted odds ratios for the primary obstetric outcomes. A p-value of less than 0.05 was used to define statistical significance at a 95% confidence interval (CI), and all tests were two-tailed.
Ethical considerations
The study was primarily approved by the Human Research and Ethics Committee (HREC) of Alex-Ekwueme Federal University Teaching Hospital, Abakaliki (FETHA/REC/VOL1/2019/540). Ethical approval was given by the Ethics Review committees of all the participating institutions. Signed written consents were obtained from all the participants for them to be interviewed and for their medical records to be used for this study. The data of parturient were made fully anonymous before they were entered into the database and analysed.
Results
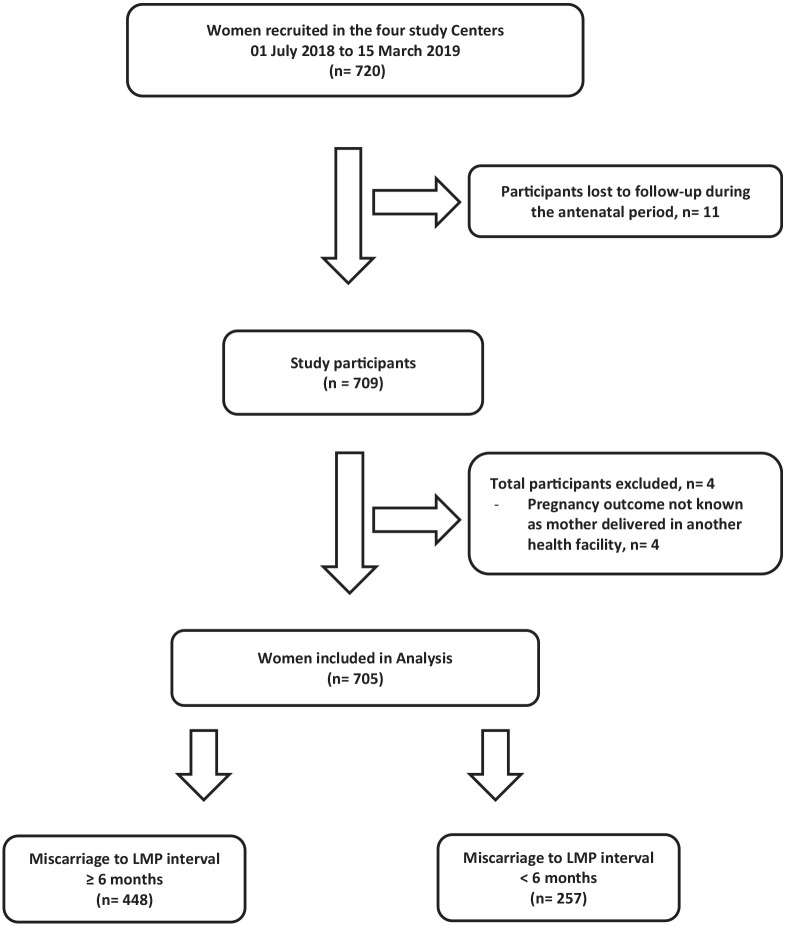
At the commencement of the study, 720 participants who met the inclusion criteria were recruited. A total of 11 participants were lost to follow-up during the antenatal period, while 4 participants delivered in other health facilities with the outcomes of their pregnancy unknown and were subsequently excluded from the study. Only 705 participants with completed data were included in this study analysis. A total of 448 (63.5%) participants belonged to those with IPI of ⩾6 months, while 257 (36.5%) participants belonged to the IPI of <6 months group (short IPI). Details are shown in Figure 1. The mean age of the respondents was 28.23 ± 5.21 years. More than four-fifths of the respondents were aged 20–34 years, and most of the participants were married and had a secondary level of education and above. Over four-fifths of the women had first-trimester pregnancy losses. Other socio-demographic characteristics, number of previous miscarriages, gestational age at which they occurred and how the miscarriages were managed with associated complications are shown in Table 1.
Figure 1.
Selection and follow-up of study cohorts.
LMP: last menstrual period.
Table 1.
Baseline socio-demographic characteristics of study participants.
| Socio-demographic characteristics | Sub-category | Miscarriage to LMP interval ⩾6 months (n = 448) |
Miscarriage to LMP interval <6 months (n = 257) |
p |
|---|---|---|---|---|
| Mothers’ age (years) | <20 | 2 (0.4%) | 3 (1.2%) | 0.309 |
| 20–34 | 352 (78.6%) | 209 (81.3%) | ||
| ⩾35 | 94 (21.0%) | 45 (17.5%) | ||
| Marital status | Single | 1 (0.2%) | 2 (0.8%) | 0.558 |
| Married | 447 (99.8%) | 255 (99.2%) | ||
| Educational qualification | Primary education and below | 37 (8.3%) | 18 (7.0%) | 0.565 |
| Secondary education and above | 411 (91.7%) | 239 (93.0%) | ||
| Occupation | Not employed | 98 (21.9%) | 65 (25.3%) | 0.309 |
| Employed | 350 (78.1%) | 192 (74.7%) | ||
| Religion | Christianity | 439 (98.0%) | 246 (95.7%) | 0.099 |
| Islam | 9 (2.0%) | 11 (4.3%) | ||
| Parity | 0 | 151 (33.7%) | 81 (31.5%) | 0.136 |
| 1–4 | 274 (61.2%) | 170 (66.1%) | ||
| ⩾5 | 23 (5.1%) | 6 (2.3%) | ||
| No. of miscarriages in the past | 1 miscarriage in the past | 311 (69.4%) | 188 (73.2%) | 0.303 |
| ⩾2 miscarriages in the past | 137 (30.6%) | 69 (26.8%) | ||
| Gestational age of last miscarriage (weeks) | 1–13 | 367 (81.9%) | 215 (83.7%) | 0.607 |
| ⩾14 | 81 (18.1%) | 42 (16.3%) | ||
| How was the last miscarriage managed? | Expectant management | 54 (12.1%) | 25 (9.7%) | 0.014 |
| Medical management with uterotonics | 56 (12.5%) | 53 (20.6%) | ||
| Manual vacuum aspiration (MVA) | 338 (75.4%) | 179 (69.7%) | ||
| Post-abortal complication in previous miscarriages a | Haemorrhage | 4 (0.9%) | 1 (0.4%) | 0.658 |
| Retained products of conception | 3 (0.7%) | 1 (0.4%) | 1.000 | |
| Cervical incompetence b | 2 (0.4%) | 1 (0.4%) | 1.000 | |
| Delayed fertility c | 27 (6.0%) | 6 (2.3%) | 0.026 | |
| Pyrexia | 8 (1.8%) | 4 (1.6%) | 1.000 | |
| Uterine synechiae | 11 (2.5%) | 3 (1.2%) | 0.277 |
LMP: last menstrual period.
More than one complication can apply in a respondent.
Cervical incompetence was diagnosed in those with two more previous miscarriages before the index pregnancy.
Delayed fertility is defined as being unable to conceive for 1 year after the last miscarriage despite desiring to conceive.
There was no increased risk of placenta praevia (p = 0.538), hypertensive disorders of pregnancy (p = 0.111), preterm contractions and labour (p = 0.133), PROM (p = 0.500), IUFD (p = 0.130) and IUGR (p = 0.557) and other obstetric morbidities during pregnancy. Other details were shown in Table 2.
Table 2.
Association between interpregnancy interval and complications of pregnancy beyond 20 weeks gestation in the subsequent pregnancy.
| Complications during index pregnancy beyond 20 weeks’ gestation (n = 641) a | Miscarriage to LMP interval ⩾6 months (n = 410) |
Miscarriage to LMP interval <6 months (n = 231) |
p |
|---|---|---|---|
| APH due to placenta praevia b | 2 (0.5%) | 0 (0.0%) | 0.538 |
| Threatened miscarriage | 8 (2.0%) | 7 (3.0%) | 0.420 |
| Required cervical cerclage for cervical incompetence | 2 (0.5%) | 1 (0.4%) | 1.000 |
| Hypertensive disorders of pregnancy | 17 (4.1%) | 4 (1.7%) | 0.111 |
| Preterm contractions and labour | 19 (4.6%) | 5 (2.2%) | 0.133 |
| Premature rupture of membranes (PROM) | 16 (3.9%) | 6 (2.6%) | 0.500 |
| Intrauterine foetal death (IUFD) | 0 (0.0%) | 2 (0.9%) | 0.130 |
| Intrauterine growth retardation | 1 (0.2%) | 2 (0.9%) | 0.557 |
LMP: last menstrual period; APH: antepartum haemorrhage.
More than one complication can apply.
Hypertensive disorders of pregnancy = non-proteinuric (gestational) hypertension, preeclampsia, eclampsia.
Majority (87.5% for the ⩾6 months cohort and 86.4% for the <6 months cohorts) had live birth, p = 0.734. About 8.5% in the ⩾6 months cohort and 10.1% in the <6 months cohort had repeat miscarriage in the index pregnancy. Table 3 shows the association between IPI and obstetric outcomes in the subsequent pregnancy. It indicates that there was also no significant statistical difference in the mode/route of delivery (p = 0.568). The mean blood loss for vaginal delivery (p = 0.174) and caesarean section (p = 0.225), birth weight (p = 0.481), need for resuscitation (p = 0.555), admission into the newborn intensive care unit, NICU (p = 0.445) and early neonatal deaths (p = 0.199) were not statistically different. The mean duration of hospital admission, APGAR scores at the first and fifth minute, indications for NICU admission and causes of early neonatal deaths are shown in Table 3.
Table 3.
Association between interpregnancy interval and obstetric outcomes in the subsequent pregnancy.
| Obstetric outcomes in the subsequent pregnancy | Sub-category | Miscarriage to LMP interval ⩾6 months (n = 448) |
Miscarriage to LMP interval <6 months (n = 257) |
p |
|---|---|---|---|---|
| Birth outcome of the subsequent pregnancy | Live birth | 392 (87.5%) | 222 (86.4%) | 0.734 |
| Stillbirth | 18 (4.0%) | 9 (3.5%) | ||
| Miscarriage | 38 (8.5%) | 26 (10.1%) | ||
| Gestational age at birth (N = 641) a | Preterm (GA <37 weeks) | 333 (81.2%) | 187 (81.0%) | 1.000 |
| Term (GA ⩾37 weeks) | 77 (18.8%) | 44 (19.0%) | ||
| Mode of delivery of index pregnancy (N = 641) a | Vaginal delivery | 306 (74.6%) | 177 (76.6%) | 0.575 |
| Caesarean section | 104 (25.4%) | 54 (23.4%) | ||
| Blood loss during delivery (N = 641) a | M ± SD (mL) for vaginal delivery | 221.04 (±99.6) | 192.50 (±111.8) | 0.174 |
| M ± SD (mL) for caesarean section | 402.14 (±140.8) | 368.9 (±115.3) | 0.225 | |
| Duration of admission for mother (N = 641) a | M ± SD (in days) | 3.67 (±2.33) | 3.42 (±2.30) | 0.429 |
| Birth weight, g (N = 641) a | <2500 | 53 (12.9%) | 25 (10.8%) | 0.481 |
| 2500–3999 | 319 (77.8%) | 189 (81.8%) | ||
| ⩾4000 | 38 (9.3%) | 17 (7.4%) | ||
| Did baby need assistance to breath? (N = 614) b | Yes | 55 (13.4%) | 35 (15.2%) | 0.543 |
| No | 355 (86.6%) | 196 (84.8%) | ||
| APGAR scores at 1 min (N = 614) b | Low/abnormal (0–6) | 57 (13.9) | 39 (16.9) | 0.310 |
| Moderate/normal (>6) | 353 (86.1%) | 192 (83.1%) | ||
| APGAR scores at 5 min (N = 614) b | Low/abnormal (0–6) | 25 (6.1%) | 22 (9.5%) | 0.110 |
| Moderate/normal (>6) | 385 (93.9%) | 209 (90.5%) | ||
| Admission into NICU (N = 614) b | Admitted | 67 (16.3%) | 44 (19.0%) | 0.385 |
| Not admitted | 343 (83.7%) | 187 (81.0%) | ||
| Indication for admission into NICU (N = 614) b | Birth asphyxia | 23 (5.6%) | 19 (8.3%) | 0.189 |
| Complication of prematurity | 19 (4.6%) | 11 (4.8%) | 0.941 | |
| Respiratory distress syndrome | 17 (4.1%) | 9 (3.9%) | 0.877 | |
| Congenital anomalies | 0 (0.0%) | 1 (0.4%) | 0.182 | |
| Foetal macrosomia | 5 (1.2%) | 0 (0.0%) | 0.165* | |
| Hypoglycaemia | 1 (0.2%) | 2 (0.9%) | 0.296* | |
| Phototherapy for neonatal jaundice | 2 (0.5%) | 2 (0.9%) | 0.622* | |
| Neonatal death (N = 614) b | Yes | 18 (4.6%) | 16 (7.2%) | 0.169 |
| No | 392 (95.6%) | 215 (93.1%) | ||
| Cause of death (N = 614) b | Birth asphyxia | 9 (2.2%) | 8 (3.5%) | 0.337 |
| Severe prematurity | 4 (1.0%) | 5 (2.2%) | 0.295* | |
| Respiratory distress syndrome | 5 (1.2%) | 2 (0.9%) | 1.000* | |
| Congenital anomalies | 0 (0.0%) | 1 (0.4%) | 0.360* |
LMP: last menstrual period; GA: gestational age; SD: standard deviation; NICU: neonatal intensive care unit.
N = 641, as miscarriage cases were deselected.
N = 614, as women who had miscarriage and stillbirths were deselected.
Fisher’s exact test.
Multivariate logistic regression analysis (Table 4) demonstrated that there was no significant difference in the primary outcomes; repeat miscarriage (p = 0.667), stillbirth (p = 0.972), low APGAR scores at the fifth minute (p = 0.233) and neonatal death (p = 0.182) even after adjusting for maternal age, parity of the mothers, number of prior miscarriages, gestational age of the immediate last miscarriage and how the last miscarriage was managed.
Table 4.
Association between interpregnancy interval and primary obstetric outcomes in the subsequent pregnancy.
| Obstetric outcomes in the subsequent pregnancy | Miscarriage to LMP interval ⩾ 6 months AOR a (95% CI) |
Miscarriage to LMP interval < 6 months AOR a (95% CI) |
p |
|---|---|---|---|
| Repeat miscarriage | 1.000 (Reference) | 1.222 (0.490–3.050) | 0.667 |
| Stillbirth | 1.000 (Reference) | 1.027 (0.236–4.472) | 0.972 |
| Low birth weight | 1.000 (Reference) | 0.544 (0.192–1.540) | 0.251 |
| Need assistance to breath | 1.000 (Reference) | 1.151 (0.503–2.635) | 0.739 |
| Low APGAR score at 5 min | 1.000 (Reference) | 3.338 (0.860–12.959) | 0.082 |
| Admission into NICU | 1.000 (Reference) | 1.439 (0.719–2.882) | 0.304 |
| Neonatal death | 1.000 (Reference) | 2.091 (0.675–6.483) | 0.201 |
LMP: last menstrual period; AOR: adjusted odds ratio; NICU: neonatal intensive care unit.
Adjusted for maternal age, parity of the mother, number of prior miscarriages, gestational age of last miscarriage and how the last miscarriage was managed.
Discussion
This study was designed to assess the impact of IPI <6 months (short IPI) versus IPI ⩾6 months on the obstetric outcome (live births, repeat miscarriages, placenta praevia, hypertensive disorders of pregnancy, preterm contractions and labour, PROM, IUFD and IUGR, mode/route of delivery, blood loss for vaginal and caesarean section deliveries, birth weight, need for resuscitation, admission into the newborn intensive care unit (NICU), and early neonatal deaths) in the next pregnancy following a miscarriage. The result showed that the two study cohorts had similar socio-demographic and obstetric characteristics, thus limiting potential bias which could arise from significant differences in characteristics of the participants. It was also observed that over 80% of the participants had first-trimester pregnancy losses and these participants were managed with manual vacuum aspiration in 73.3% of the cases. The majority, 87.5% for the normal IPI cohort and 86.4% for the short IPI cohort had live births, while 8.5% and 10.1% of the women in the normal and short IPI cohorts, respectively, had repeat miscarriages. There was no observed statistical difference in the occurrence of live births and repeat miscarriages between both cohorts (p > 0.05). There was no increased risk of occurrence of adverse foetomaternal outcomes in both groups (p > 0.05). Multivariate logistic regression analysis showed that there was no statistical difference in the occurrence adverse foetomaternal outcomes between the studied cohorts.
This study corroborated the fact that majority (80%) of miscarriages occur during the first trimester as reported in several studies globally.24 –26 In contrast, a report on the occurrence of early pregnancy loss was reported only in 43% of the participants in a retrospective study of spontaneous first-trimester miscarriage rates per woman among parous women with one or more pregnancies of 24 weeks or more by Cohain et al. 27 in Jerusalem, Israel. They blamed the low reporting of first trimester miscarriages may have occurred due to underreporting, perhaps due to denial, forgetfulness, and/or miscarriage mistaken for delayed menstruation. Early pregnancy losses before pregnancies were clinically detectable and was identified to have occurred in 22% of the women studied by Wilcox et al. 24 in a US cohort study.
Majority (73.3%) of the participants had manual vacuum aspiration in this study and this finding is much similar to the treatment method that was used among 3000 Finnish women that participated in a survey. 28 The continued prevalent use of the surgical approach for the evacuation of the uterus may not be unconnected to the possible occurrence of incomplete miscarriage and unplanned surgical evacuation which complicates the medical method in some patients. 29 Also, the poor awareness and utilization of standard treatment guidelines, 30 prevalence of supplier-induced demand, 31 poor counselling, restrictive abortion laws 32 and asymmetry of information 33 between the patients and their doctors especially in the study area may have played a significant role in making surgical method a preferred method by the health practitioners. This preponderance of treatment using surgical method was, however, different from findings in other studies34,35 where medical management was applied and found to have comparable outcomes with the surgical methods, especially since the introduction of prostaglandins as an efficient method of medical evacuation. The convenience of medical method, reduction of occurrence of uterine synechiae, absence of use of anaesthesia and the resultant comparable outcomes with surgical methods made it an easier and convenient method of uterine evacuation recently.36,37 The use of medical management also reduces drastically the need for specialized surgical skills and therefore encourages task shifting and task sharing, making it easy to use even in secondary 34 and primary health care facilities. 38
Overall, there were no significant differences in adverse obstetric and perinatal risk to the mother and baby, between the two groups studied. This is corroborated by the study in Scotland, United Kingdom, where women who conceived within 6 months of an initial miscarriage had the best reproductive outcomes and lowest complication rates in the next pregnancy. 3 The current study showed that getting pregnant within 6 months of a miscarriage did not predispose parturient to a significantly higher risk of another miscarriage in the next pregnancy (8.5% in the ⩾6 months IPI cohort versus 10.1% in the <6 months IPI cohort), this finding was higher than 7.3% repeat miscarriage for IPI <3 months reported by Sundermann et al. 39 in Tennessee, United States. However, a systematic review and meta-analysis reported that the risk of a recurrent pregnancy loss was less in women who had post-abortion IPI <6 months. 40 This was contrary to the finding in Bangladesh, where short inter-outcome intervals after a spontaneous miscarriage were associated with a high risk of a similar outcome in the next conception. 41 However, the live birth outcome for both arms was similar (87.5% for ⩾6 months IPI cohort versus 86.4% for the <6 months IPI cohort). This outcome is primarily very important to women in the study area as most of them are interested in knowing from the doctor the safest time of getting pregnant after a pregnancy loss. This outcome was in tandem with 76.5% live birth reported by Wong et al. 4 However, Love et al. 3 in Scotland, reported a live birth outcome of 85.2% in women with IPI <6 months. These reports of higher live birth outcomes are consistent with the outcome of a systematic review and meta-analysis by Kangatharan et al., 40 where the chance of having a live birth was 40% higher in women with IPI <6 months. Nigerians and Africans in general attach a lot of premium to childbirth in their families. Most of the women out of pressures from their relatives to get a child for them in the new family will most likely want to get pregnant within the window of short IPI to narrow the waiting time for a child to be born in their families.
This study also showed that there was no difference in the risk of hypertensive disorders of pregnancy, low birth weight, preterm labour, PROM, IUFD/stillbirth and IUGR, between the two groups. This finding was much the same as that by Kangatharan et al., 40 except for preterm birth which they reported to be lower in women with IPI <6 months. However, in Scotland, Love et al. 3 reported that the risk of these low birth weight and preterm delivery was less in the parturient with IPI <6 months. These results were contrary to that by Conde-Agudelo et al., 2 where these complications were more in those with IPI <6 months, except for preeclampsia and low birth weight that were not significantly different in both groups. Preterm birth was also found to have increased odds ratio in women with IPI <1 or >3 years after a live birth. 42 In Denmark, Hegelund et al. 43 found that preterm birth and small for gestational age babies were lowest in women with IPI between 18 and 23 months compared to those with short IPI; these findings were, however, comparable to the report by Zhang et al. 44 in China.
There was no difference in the mode of delivery by either caesarean section or vaginal birth between both arms. Also, blood loss for vaginal delivery and caesarean section was comparable with no higher risk of primary postpartum haemorrhage (PPH) in either group. In contrast, Love et al. 3 and El Behery et al., 13 independently reported that the risk of having a caesarean section, after an IPI of <6 months, after a miscarriage was less. Furthermore, this study showed that there was no higher neonatal resuscitation for birth asphyxia, newborn intensive care unit admissions and early neonatal deaths. Correspondingly, Morgan-Ortiz et al. 45 reported that APGAR scores were similar between the similar cohorts, with no differences in the perinatal morbidity, akin Goldstein et al. 46 reported that neonatal outcomes for the two groups were similar, although neonates in the longer IPI conception arm were more likely to have low birth APGAR score <7 at 5 minutes, or admission to the neonatal intensive care unit. However, in a similar study by DaVanzo et al., 47 which assessed late neonatal morbidity and mortality, it was found that post-miscarriage IPI of ⩽3 months was associated with significantly higher late neonatal mortality and IPI of 12–18 months was associated with a significantly lower unadjusted risk of post-neonatal mortality.
Finally, most women and at large their families in Nigeria and most low-income countries, place a high premium on children. They are also eager to know the safest possible time for them to become pregnancy after a pregnancy loss. The implication of the results of this research is that with further research works on this research topic in a similar setting as in this study, our policies in the management of women after a miscarriage should be changed to reflect the findings of this research. By implication of this research, and with proper patient selection (depending on her pre-pregnancy morbid conditions), our women can also be counselled and encouraged to get pregnant within the short IPI timing and expect good foetomaternal outcomes.
Strengths /limitations of the study: Most of the aspects of the cohort study were conducted by the generation of primary data. The reliability of primary data collected from multiple centres and proper follow-up of the participants therefore gives this study an edge. This local study is one of the very few in Nigeria and other LMICs and will help in the development of policies that will guide health practitioners in counselling mothers who had a miscarriage and are eager to get pregnant as soon as feasible. This is even more important these days where women continue childbearing into their advanced ages with the attendant adverse events. 48 However, the sample size was relatively smaller and recall of events associated with the previous miscarriage was subjective and maybe a source of bias.
Conclusion
IPI <6 months following a miscarriage was not associated with increased maternal and perinatal complications. Therefore, women who suffered uncomplicated pregnancy losses and who desires future conception should not be deterred from doing so before 6 months.
We recommend that counselling women on the optimum IPI after a spontaneous miscarriage should be individualized with proper risk assessment instead.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_20503121221105589 for Interpregnancy interval after a miscarriage and obstetric outcomes in the subsequent pregnancy in a low-income setting, Nigeria: A cohort study by Lucky Osaheni Lawani, Joseph Tochukwu Enebe, Paul Eze, Francis Nwabueze Igboke, Chukwuemeka Ikeji Ukaegbe, Monica Omosivie Ugwu, Ujunwa Justina Agu, Enebe Nympha Onyinye and Chukwuemeka Anthony Iyoke in SAGE Open Medicine
Acknowledgments
The authors thank Ms Ebere Ozota and Mr George Okafor for helping collate the datasets. They also thank research assistants and the nursing teams in the antenatal care clinics and maternity wards of the four hospitals for their inestimable support during the recruitments and interview of participants and while accessing their medical records.
Footnotes
Author contributions: L.O.L., P.E., J.T.E. and C.A.I. designed the study, oversaw its conduct, data collection, data analysis, interpretation of results, drafted the original article and reviewed the final draft. F.N.I. and N.O.E. contributed to the design of the study, data collection and review of the article. C.I.U. and U.J.A. participated in the design of the study, analysis and interpretation of data and the review of the final manuscript. All the authors read and approved the final draft of the article.
Availability of data and materials: The datasets used and/or analysed during this study are available from the corresponding author on reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval and consent to participate: The study was approved primarily by the Human Research and Ethics Committee (HREC) of Alex-Ekwueme Federal University Teaching Hospital, Abakaliki (FETHA/REC/VOL1/2019/540). Ethical approval was granted by the Ethics Review Committee of all four participating health facilities; Alex-Ekwueme Federal University Teaching Hospital in Abakaliki, Ebonyi State; University of Nigeria Teaching Hospital (UNTH) in Enugu, Enugu State; Enugu State University Teaching Hospital (ESUTH) in Enugu, Enugu State; and Stella Obasanjo Hospital (SOH) in Benin City, Edo State. All four hospital research committees determined that the study did not constitute a human subject trial and therefore approved the protocol for the research.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Written informed consents were obtained from all the parturients at the point of recruitment for them to be interviewed and for their medical records to be used for this study. Mothers younger than 18 years of age were excluded from the study.
ORCID iDs: Joseph Tochukwu Enebe  https://orcid.org/0000-0002-3517-1104
https://orcid.org/0000-0002-3517-1104
Supplemental material: Supplemental material for this article is available online.
References
- 1. World Health Organization (WHO). Report of a WHO technical consultation on birth spacing: Geneva, Switzerland 13-15 June 2005, https://www.who.int/publications/i/item/WHO-RHR-07.1 [Google Scholar]
- 2. Conde-Agudelo A, Belizán JM, Breman R, et al. Effect of the interpregnancy interval after an abortion on maternal and perinatal health in Latin America. Int J Gynaecol Obstet 2006; 89: S34–S40. [DOI] [PubMed] [Google Scholar]
- 3. Love ER, Bhattacharya S, Smith NC, et al. Effect of interpregnancy interval on outcomes of pregnancy after miscarriage: retrospective analysis of hospital episode statistics in Scotland. BMJ 2010; 341: c3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong LF, Schliep KC, Silver RM, et al. The effect of a very short interpregnancy interval and pregnancy outcomes following a previous pregnancy loss. Am J Obstet Gynecol 2015; 212(3): 375.e1–375.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Atrash HK, Hogue CJ. The effect of pregnancy termination on future reproduction. Baillieres Clin Obstet Gynaecol 1990; 4(2): 391–405. [DOI] [PubMed] [Google Scholar]
- 6. Bhattacharya S, Bhattacharya S. Effect of miscarriage on future pregnancies. Womens Health 2009; 5: 5–8. [DOI] [PubMed] [Google Scholar]
- 7. Bhattacharya S, Townend J, Shetty A, et al. Does miscarriage in an initial pregnancy lead to adverse obstetric and perinatal outcomes in the next continuing pregnancy? BJOG 2008; 115(13): 1623–1629. [DOI] [PubMed] [Google Scholar]
- 8. Moreau C, Kaminski M, Ancel PY, et al. Previous induced abortions and the risk of very preterm delivery: results of the EPIPAGE study. BJOG 2005; 112(4): 430–437. [DOI] [PubMed] [Google Scholar]
- 9. Swingle HM, Colaizy TT, Zimmerman MB, et al. Abortion and the risk of subsequent preterm birth: a systematic review with meta-analyses. J Reprod Med 2009; 54(2): 95–108. [PubMed] [Google Scholar]
- 10. Martius JA, Steck T, Oehler MK, et al. Risk factors associated with preterm (<37+0 weeks) and early preterm birth (<32+0 weeks): univariate and multivariate analysis of 106 345 singleton births from the 1994 statewide perinatal survey of Bavaria. Eur J Obstet Gynecol Reprod Biol 1998; 80: 183–189. [DOI] [PubMed] [Google Scholar]
- 11. Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Effects of birth spacing on maternal health: a systematic review. Am J Obstet Gynecol 2007; 196: 297–308. [DOI] [PubMed] [Google Scholar]
- 12. Makhlouf MA, Clifton RG, Roberts JM, et al. Adverse pregnancy outcomes among women with prior spontaneous or induced abortions. Am J Perinatol 2014; 31: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El Behery MM, Siam S, Seksaka MA, et al. Reproductive performance in the next pregnancy for nulliparous women with history of first trimester spontaneous abortion. Arch Gynecol Obstet 2013; 288(4): 939–944. [DOI] [PubMed] [Google Scholar]
- 14. Imterat M, Wainstock T, Sheiner E, et al. Inter–pregnancy interval and later pediatric cardiovascular health of the offspring – a population-based cohort study. J Dev Orig Health Dis 2021; 12(5): 819–823. [DOI] [PubMed] [Google Scholar]
- 15. Shachar BZ, Lyell DJ. Interpregnancy interval and obstetrical complications. Obstet Gynecol Surv 2012; 67(9): 584–596. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization (WHO). Postpartum family planning: essential for ensuring health of women and their babies. Geneva: World Health Organization, 2018. [Google Scholar]
- 17. World Health Organization (WHO). Family planning/contraception methods: fact sheets, https://www.who.int/news-room/fact-sheets/detail/family-planning-contraception (2020, accessed 29 September 2021).
- 18. Bongaarts J. Can family planning programs reduce high desired family size in Sub-Saharan Africa? Int Perspect Sex Reprod Health 2011; 37(4): 209–216. [DOI] [PubMed] [Google Scholar]
- 19. Vandenbroucke JP, Von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Epidemiology 2007; 18: 805–835. [DOI] [PubMed] [Google Scholar]
- 20. World Population Review. Total fertility rate 2022, https://worldpopulationreview.com/country-rankings/total-fertility-rate (2021, accessed 29 September 2021).
- 21. Taofeek I. Research methodology and dissertation writing for health and allied health professionals. 1st ed. Abuja, Nigeria: Cress Global Link Limited, 2009. [Google Scholar]
- 22. Bentolila Y, Ratzon R, Shoham-Vardi I, et al. Effect of interpregnancy interval on outcomes of pregnancy after recurrent pregnancy loss. J Matern Fetal Neonatal Med 2013; 26(14): 1459–1464. [DOI] [PubMed] [Google Scholar]
- 23. Simon LV, Hashmi MF, Bragg BN. APGAR score. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing, 2022, p. 11. [Google Scholar]
- 24. Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med 1988; 319: 189–194. [DOI] [PubMed] [Google Scholar]
- 25. Loss EP. ACOG practice bulletin no. 200 summary: early pregnancy loss. Obstet Gynecol 2018; 132(5): 1311–1313. [DOI] [PubMed] [Google Scholar]
- 26. Wang X, Chen C, Wang L, et al. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril 2003; 79(3): 577–584. [DOI] [PubMed] [Google Scholar]
- 27. Cohain JS, Buxbaum RE, Mankuta D. Spontaneous first trimester miscarriage rates per woman among parous women with 1 or more pregnancies of 24 weeks or more. BMC Pregnancy Childbirth 2017; 17: 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hemminki E. Treatment of miscarriage: current practice and rationale. Obstet Gynecol 1998; 91(2): 247–253. [DOI] [PubMed] [Google Scholar]
- 29. Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (miscarriage treatment (MIST) trial). BMJ 2006; 332: 1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ayinbuomwan AS, Isah AO. Standard treatment guidelines: perception and utilization in a tertiary health care facility in South-South, Nigeria. Ann Afr Med 2019; 18(1): 47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Onwujekwe O, Dike N, Uzochukwu B, et al. Informal payments for healthcare: differences in expenditures from consumers and providers perspectives for treatment of malaria in Nigeria. Health Policy 2010; 96(1): 72–79. [DOI] [PubMed] [Google Scholar]
- 32. Akande OW, Adenuga AT, Ejidike IC, et al. Unsafe abortion practices and the law in Nigeria: time for change. Sex Reprod Health Matters 2020; 28: 1758445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adeyele JS, Michael O, Augustine I. Asymmetric information and health-risk behavior in the National Health Insurance Scheme in Jos metropolis, Nigeria. Ekon Horizonti 2019; 21: 145–159. [Google Scholar]
- 34. Fawole AO, Diop A, Adeyanju AO, et al. Misoprostol as first-line treatment for incomplete abortion at a secondary-level health facility in Nigeria. Int J Gynaecol Obstet 2012; 119(2): 170–173. [DOI] [PubMed] [Google Scholar]
- 35. Blum J, Winikoff B, Gemzell-Danielsson K, et al. Treatment of incomplete abortion and miscarriage with misoprostol. Int J Gynaecol Obstet 2007; 99(Suppl. 2): S186–S189. [DOI] [PubMed] [Google Scholar]
- 36. Weeks A, Alia G, Blum J, et al. A randomized trial of misoprostol compared with manual vacuum aspiration for incomplete abortion. Obstet Gynecol 2005; 106(3): 540–547. [DOI] [PubMed] [Google Scholar]
- 37. Ani VC, Enebe JT, Dim CC, et al. Sublingual misoprostol versus manual vacuum aspiration for treatment of incomplete abortion in Nigeria: a randomized control study. Pan Afr Med J 2022; 41: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization (WHO). Medical management of abortion. Geneva: World Health Organization, 2018. [Google Scholar]
- 39. Sundermann AC, Hartmann KE, Jones SH, et al. Interpregnancy interval after pregnancy loss and risk of repeat miscarriage. Obstet Gynecol 2017; 130(6): 1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kangatharan C, Labram S, Bhattacharya S. Interpregnancy interval following miscarriage and adverse pregnancy outcomes: systematic review and meta-analysis. Hum Reprod Update 2017; 23: 221–231. [DOI] [PubMed] [Google Scholar]
- 41. Nonyane BAS, Norton M, Begum N, et al. Pregnancy intervals after stillbirth, neonatal death and spontaneous abortion and the risk of an adverse outcome in the next pregnancy in rural Bangladesh. BMC Pregnancy Childbirth 2019; 19: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shachar BZ, Mayo JA, Lyell DJ, et al. Interpregnancy interval after live birth or pregnancy termination and estimated risk of preterm birth: a retrospective cohort study. BJOG 2016; 123: 2009–2017. [DOI] [PubMed] [Google Scholar]
- 43. Hegelund ER, Urhoj SK, Andersen AN, et al. Interpregnancy interval and risk of adverse pregnancy outcomes: a register-based study of 328,577 pregnancies in Denmark 1994-2010. Matern Child Health J 2018; 22(7): 1008–1015. [DOI] [PubMed] [Google Scholar]
- 44. Zhang L, Shen S, He J, et al. Effect of interpregnancy interval on adverse perinatal outcomes in southern China: a retrospective cohort study, 2000–2015. Paediatr Perinat Epidemiol 2018; 32(2): 131–140. [DOI] [PubMed] [Google Scholar]
- 45. Morgan-Ortiz F, Muñoz-Acosta J, Valdez-Quevedo R, et al. Effect of post-abortion interpregnancy interval obstetric and perinatal outcomes. Ginecol Obstet Mex 2010; 78: 46–52. [PubMed] [Google Scholar]
- 46. Goldstein RRP, Croughan MS, Robertson PA. Neonatal outcomes in immediate versus delayed conceptions after spontaneous abortion: a retrospective case series. Am J Obstet Gynecol 2002; 186: 1230–1236. [DOI] [PubMed] [Google Scholar]
- 47. DaVanzo J, Hale L, Rahman M. How long after a miscarriage should women wait before becoming pregnant again? Multivariate analysis of cohort data from Matlab, Bangladesh. BMJ Open 2012; 2(4): e001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ikeanyi EM, Onyiriuka AN. Advanced maternal age at the first pregnancy and obstetric performance: a retrospective study. Pac J Med Sci 2014; 13: 21–31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_20503121221105589 for Interpregnancy interval after a miscarriage and obstetric outcomes in the subsequent pregnancy in a low-income setting, Nigeria: A cohort study by Lucky Osaheni Lawani, Joseph Tochukwu Enebe, Paul Eze, Francis Nwabueze Igboke, Chukwuemeka Ikeji Ukaegbe, Monica Omosivie Ugwu, Ujunwa Justina Agu, Enebe Nympha Onyinye and Chukwuemeka Anthony Iyoke in SAGE Open Medicine