Abstract
Background:
In Crohn’s disease and ulcerative colitis, the anti-α4β7 integrin antibody vedolizumab has demonstrated efficacy in phase III trials and has been successfully used under real-world conditions. Occasionally, it has also been used in other forms of inflammatory bowel disease (IBD) such as microscopic colitis (MC). However, the mechanisms of vedolizumab in MC have not been studied to date. Therefore, we aimed to investigate the expression and functional role of gut-homing integrins and in particular α4β7 integrin in a cohort study in MC.
Methods:
We studied the expression of gut homing integrins on T cells from patients with MC and healthy controls by flow cytometry. To investigate the function of α4β7 integrin in MC and the potential of vedolizumab to block it, we used dynamic adhesion assays and transmigrations assays. Moreover, we describe two clinical cases of MC patients treated with vedolizumab.
Results:
A specific profile of gut homing markers can be found on T cells from patients with MC. α4β7 integrin functionally leads to firm adhesion to MAdCAM-1 and supports transmigration. Vedolizumab is able to block both processes. In two cases of MC, we observed reduced clinical symptoms and histologic improvement upon therapy with vedolizumab.
Conclusion:
Our data suggest that α4β7 mediates gut homing of T cells also in MC and that, on single cell level, vedolizumab blocks the function of α4β7 in MC. Thus, we provide mechanistic evidence supporting vedolizumab as promising therapeutic option for MC.
Keywords: α4β7 integrin, microscopic colitis, T cell trafficking, vedolizumab
Introduction
Inflammatory bowel diseases (IBD) comprise several entities of chronic immune-mediated disorders of the gastrointestinal tract. 1 In addition to Crohn’s disease (CD) and ulcerative colitis (UC), microscopic colitis (MC) has recently attracted increasing attention. 2
MC describes disease entities marked by chronic watery, non-bloody diarrhea and often unremarkable endoscopic findings,3,4 but specific histopathological alterations. 5 More precisely, it is an umbrella term for lymphocytic colitis (LC) and collagenous colitis (CC), which are characterized by increased intraepithelial lymphocytes and a thickened subepithelial collagen band, respectively.6,7 Despite these morphological differences, MC is often treated as a single entity in clinical practice8,9 due to similar clinical appearance and management of LC and CC.
The standard treatment for MC is budesonide, which has proven efficacy for the induction10,11 and maintenance of remission.12–14 However, it is not effective in all patients and the long-term use of corticosteroids is controversial.
Thus, alternative therapeutic options for MC have been explored, particularly drugs modulating the immune system such as azathioprine or anti-tumor necrosis factor alpha (TNF-α) antibodies that are also used in other forms of IBD. 15 One of these is vedolizumab, an anti-α4β7 integrin antibody, which has been approved for the treatment of CD and UC in 2014.16,17 α4β7 integrin is the functional ligand of mucosal addressing cell adhesion molecule (MAdCAM)-1, 18 which is almost exclusively found on the endothelium in the gut. 19 It is expressed on several immune cell populations and specifically induced on T and B lymphocytes during priming in the gut-associated lymphoid tissue by retinoic acid derived from retinaldehyde dehydrogenase-expressing intestinal dendritic cells. 20
The interaction of α4β7 integrin with MAdCAM-1 is a central step of the so called gut homing procedure. Gut homing is the process of immune cell extravasation from the blood stream to the intestinal tissue. 21 It is initiated by loose interactions of circulating immune cells with endothelial selecting ligand and cell adhesion molecules (CAMs) leading to slow rolling along the vessel wall. Following cell activation by tissue mediators, an active conformation of surface integrins is induced and these may then firmly bind to CAMs such as MADCAM-1 or vascular cell adhesion molecule (VCAM)-1 on the endothelium. This binding leads to arrest of the immune cells and is a prerequisite for subsequent transmigration to the tissue. 22 Since α4β7-MAdCAM-1 interactions are almost completely confined to the intestine, interruption of immune cell homing by vedolizumab is considered a gut-specific approach.
Although the exact pathogenesis of MC is unclear so far, the immune system and a dysbalanced immune response seem to play a key role. 6 Thus, blocking intestinal immune cell extravasation with vedolizumab has been proposed as a treatment for MC.23,24
However, the expression and function of α4β7 integrin and other gut-homing markers has not been determined in MC yet. Therefore, in this study, we aimed to explore the expression of gut homing integrins on T cells and to determine their dynamic adhesion to MAdCAM-1 as well as their transmigration capacities in a cohort of patients with MC. We show that MC is characterized by specific integrin expression profiles on T cells and that α4β7 integrin on T cells from patients with MC is functional for adhesion and transmigration. Our data substantiate, on a mechanistic level, vedolizumab as a promising alternative treatment option in MC.
Materials and methods
Patients and samples
We performed a cohort study with patients with MC. Peripheral blood from patients with MC (total n = 13) and from healthy control donors (total n = 13) was collected following informed written consent according to the approval of the Ethics Committee of the Friedrich-Alexander-Universität Erlangen-Nürnberg (40_16B, 426_20B). The patients were recruited in the IBD Outpatient Clinic of the Department of Medicine 1 of the University Hospital Erlangen from April 2020 to November 2021. 53.8% of the included patients were diagnosed with lymphocytic colitis and 46.2% with collagenous colitis. Clinical data are summarized in Table 1.
Table 1.
Patient and control cohort.
| Microscopic colitis | Healthy controls | ||
|---|---|---|---|
| Number of patients | 13 | 13 | |
| Lymphocytic colitis (%) | 7 (53.8) | ||
| Collagenous colitis (%) | 6 (46.2) | ||
| Female | (%) | 92.3 | 62 |
| Age | Age (mean, range) | 53.3 (25–82) | 32.1 (24–56) |
| Current therapy (%) | Infliximab | 30.8 | / |
| Budesonide | 38.5 | / | |
| None | 30.8 | / |
Characteristics of patients with microscopic colitis and healthy controls providing samples for the study.
Depending on material availability, patient samples were used for one or more of the experimental approaches described below.
STROBE guideline documentation is included as Supplementary Material.
Flow cytometry
To analyze the expression of the integrins α4β7, α4β1, αLβ2, α2β1, and αMβ2 on CD3+, CD4+, and CD8+ T cells, we performed flow cytometry of peripheral blood mononuclear cells (PBMCs). PBMCs were isolated using standard density gradient centrifugation with Lymphocyte Separation Medium (Anprotec). After PBMC isolation, the cells were stained with Fixable Viability Dye eFluor 780. Afterwards, the cells were washed with PBS and stained with antibodies against CD3 (VioGreen, REA613, Miltenyi), CD4 (FITC, VIT4, Miltenyi), CD8 (PerCP/Cy5.5, RPA-T8, Biolegend), α4 (VioBlue, MZ18-24A9, Miltenyi), β7 (PE, FIB27, Biolegend), β1 (AF647, TS2/16, Biolegend), α2 (PE-Vio770, REA188, Miltenyi), αL (PE/Cy7, HI111, Biolegend), αM (PE, ICRF44, Biolegend), and β2 (APC, TS1/18, Biolegend). Samples were fixed with the Foxp3/Transcription Factor Staining Buffer Set (eBioscience) and flow cytometry was performed on LSR Fortessa (BD) and MACSQuant (Miltenyi Biotec) instruments.
Dynamic adhesion assay
To assess dynamic T cell adhesion, CD4+ T cells were isolated with immunomagnetic beads (Miltenyi Biotec) according to the instructions of the manufacturer and stained with carboxyfluorescein succinimidyl ester (CFSE) for 15 min at 37°C. Subsequently, the cells were resuspended at 1.5 million cells/ml in RPMI 1640 medium (Thermo Fisher) with 1% penicillin/streptomycin (Biochrom) and 10% FBS (Pan Biotech). Cells were then treated with or without 10 µg/ml of the anti-α4β7 integrin antibody vedolizumab (Takeda) for 1 h at 37°C. Cells were harvested and resuspended in 1 ml adhesion buffer (pH 7.4; 150 mM NaCl, 10 mM HEPES, 1 mM CaCl2, 1 mM MgCl2, 1 mM MnCl2) at 1.5 million cells/ml.
In parallel, borosilicate glass capillaries (Vitrocom) were coated with or without Fc-Chimera of rhMAdCAM-1 (R&D Systems) at a concentration of 5 µg/ml in 150 mM NaCl with 20 mM HEPES and incubated at 37°C. After at least 1 h of incubation, the coating solution was removed from the glass capillaries and 20 µl of 10% fetal bovine serum (FBS) were inserted to block unspecific binding sites. After another incubation for at least 1 h at 37°C, the blocking solution was removed again. The capillaries were then connected to plastic tubes on both ends.
These tubes were inserted into a peristaltic pump (Baoding ShenChen Precision Pump Company) and perfused with the prepared cell solutions at a flow rate of 10 µl/min. During perfusion, video clips of 3 min were recorded using time-lapse confocal microscopy. The first and last three sequential pictures of the video clips were colored in green, red, and blue and overlaid using ImageJ (NIH) to specifically visualize stationary adhering cells in white color. By calculating the difference of adhering cells between both pictures dynamic adhesion during the 3-min-period was quantified.
Transmigration assay
To assess T cell transmigration, CD4+ T cells were as well isolated as mentioned above. In parallel, wells of a 3 µm 96-well transwell plate (Corning) were coated with or without 50 µl of rhMAdCAM-1 (R&D Systems) at a concentration of 5 µg/ml in 150 mM NaCl with 20 mM HEPES. After 60 min of incubation at 37°C, the solution was removed. In a next step, 160.000 cells CD4+ T cells were resuspended in serum-free X-Vivo 15 medium (Lonza) with 1% penicillin/streptomycin (Biochrom) and 1 mM MnCl2 at a concentration of 2 million cells/ml and were filled into the inserts of the transwell plate. In addition, vedolizumab at a concentration of 10 µg/ml was added or not. The lower wells of the transwell plate were filled with 235 µl serum-free X-Vivo 15 medium with 100 ng/ml FBS. Subsequently, the transwell plate was incubated for a transmigration period of 4 h at 37°C and, afterwards, the number of cells in the lower wells was quantified using a MACSQuant flow cytometer (Miltenyi Biotec). In all experiments, conditions were analyzed in duplicates.
Statistical analyses
GraphPad Prism (GraphPad Software, Inc.) was used for statistical analyses. Results are presented as box plots indicating median and interquartile range. Whiskers show 5th and 95th percentile. Statistical differences between two test groups were analyzed by one sample Wilcoxon test for normalized data and with Mann–Whitney U test for not normalized data. Statistically significant differences were defined as an α value of p < 0.05. In the figures, significance levels are denoted by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001).
Results
Specific integrin expression profiles on circulating T cells from patients with MC
The expression of gut homing integrins on T cells in patients with MC had been unclear so far. To address this question, we performed flow cytometry of circulating T cells from patients with MC (naïve for anti-α4β7 therapy) and healthy controls.
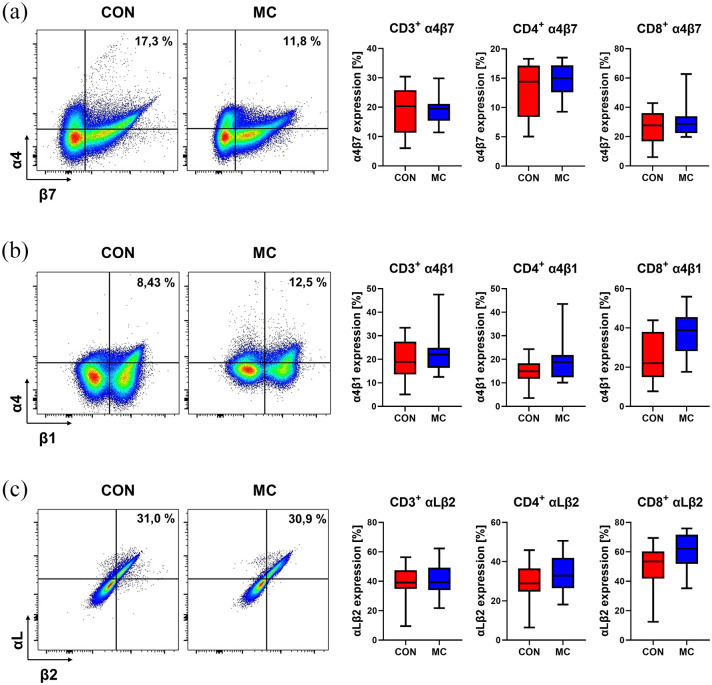
The expression of the vedolizumab target molecule α4β7 was not significantly different on CD3+ and CD4+ T cells between MC patients and healthy donors, although it was numerically increased on CD8+ T cells in MC (Figure 1(a)). Thus, T cells equipped for interacting with MAdCAM-1 to home to the gut are present in the peripheral blood of patients with MC in substantial numbers.
Figure 1.
Gut-homing integrins on T cells in MC. Left panels: Representative flow cytometry data of healthy control donors and patients with MC showing the expression of α4β7 (a), α4β1 (b), and αLβ2 (c) on CD4+ T cells from the peripheral blood. Right panels: Quantitative flow cytometry of α4β7 (a), α4β1 (b), and αLβ2 (c) expression on CD3+, CD4+, and CD8+ T cells from healthy control donors (n = 13) and MC patients (n = 13).
CON, control donors; MC, microscopic colitis.
For the integrin heterodimer α4β1, which interacts with VCAM-1 and also contributes to gut homing, 25 there was a trend toward higher expression on CD3+ and CD4+ T cells in MC. This difference was significant for CD8+ T cells (Figure 1(b)).
While we did not observe relevant differences in the expression of αLβ2 integrin interacting with endothelial intercellular cell adhesion molecule (ICAM)-1 (Figure 1(c)), the expression of αMβ2, which also binds to ICAM-1, was numerically higher on CD8+, but not on CD4+ T cells (Suppl. Fig. 1). To the contrary, integrin α2β1 serving as a collagen receptor during cell migration, was numerically reduced on all T cell populations investigated (Suppl. Fig. 1).
Collectively, these data suggested the particular integrin expression profiles can be found on T cells in the peripheral blood of patients with MC. This supported the notion that intestinal cell trafficking might be important in the context of MC.
Vedolizumab blocks the adhesion of CD4+ T cells from patients with MC to MAdCAM-1 via α4β7 integrin
Since the abundance of integrins in the peripheral blood does not necessarily correlate with their function in mediating firm adhesion to cell adhesion molecules, 26 we next aimed to determine α4β7-dependent dynamic adhesion to MAdCAM-1
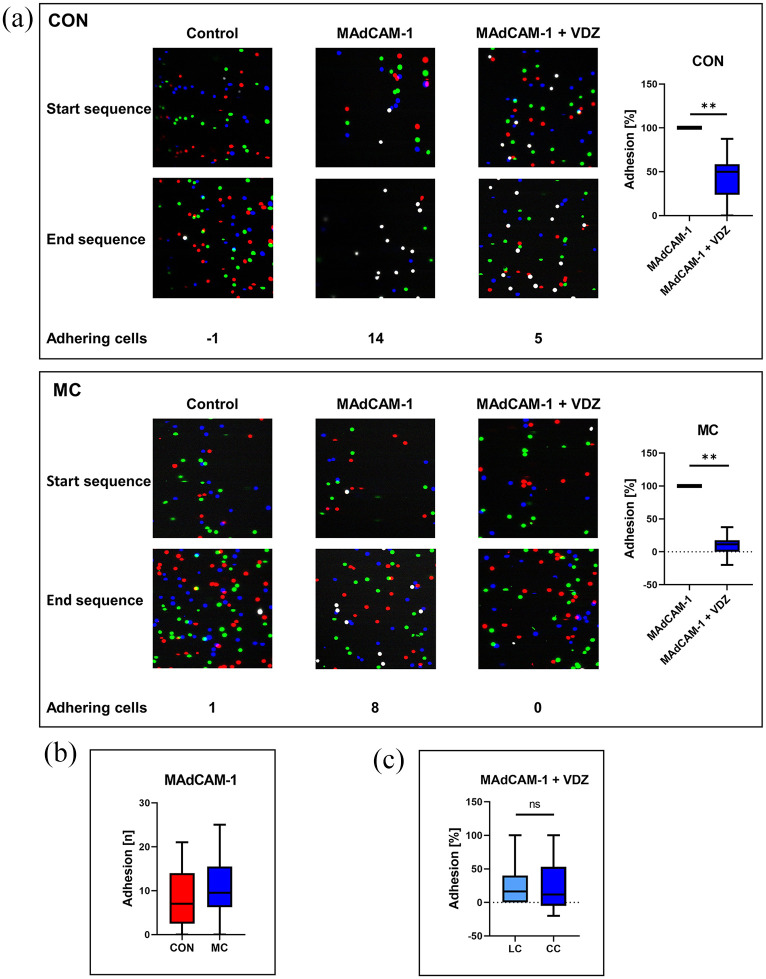
We employed a previously established dynamic adhesion assay 27 and coated MAdCAM-1 to the inside of glass capillaries, through which we perfused CD4+ T cells.
Not surprisingly, we observed specific adhesion of CD4+ T cells from the peripheral blood of healthy controls to MAdCAM-1 via α4β7 as demonstrated by substantial inhibition by the anti-α4β7 integrin antibody vedolizumab. These findings could largely be recapitulated with CD4+ T cells from the peripheral blood of patients with MC (Figure 2(a)). It is worth mentioning that the absolute level of adhesion of untreated cells (Figure 2(b)) and the extent of the reduction of adhesion induced by vedolizumab was numerically higher in MC. There was no difference between CD4+ T cells from MC patients with lymphocytic or collagenous colitis (Figure 2(c)).
Figure 2.
Impact of vedolizumab on dynamic adhesion of CD4+ T cells to MAdCAM-1 in microscopic colitis (MC): (a) left panels: representative images showing the dynamic adhesion of peripheral blood CD4+ T cells from control donors (upper panels) and patients with MC (lower panels) to uncoated capillaries (Control) and to MAdCAM-1-coated capillaries following treatment with and without vedolizumab (VDZ). Overlays of three differently colored consecutive images collected at the beginning and the end of 3 min videos are shown. Adhering cells are displayed in white color. Right panels: quantitative dynamic adhesion of CD4+ T cells from control donors (n = 13) and MC patients (n = 12) to MAdCAM-1. (b) Absolute numbers of untreated CD4+ T cells from healthy controls (n = 13) and patients with MC (n = 12) adhering to MAdCAM-1. (c) Reduction of dynamic adhesion of CD4+ T cells from patients with lymphocytic colitis (n = 6) and collagenous colitis (n = 6) to MAdCAM-1 upon treatment with VDZ.
CC, collagenous colitis; LC, lymphocytic colitis; MC, microscopic colitis; VDZ, Vedolizumab.
Vedolizumab blocks the transmigration of CD4+ T cells from patients with MC via α4β7 integrin
As firm adhesion to the endothelium is a necessary prerequisite for tissue infiltration, but requires subsequent transmigration to the tissue to be effective, we further explored the function of α4β7 for this process.
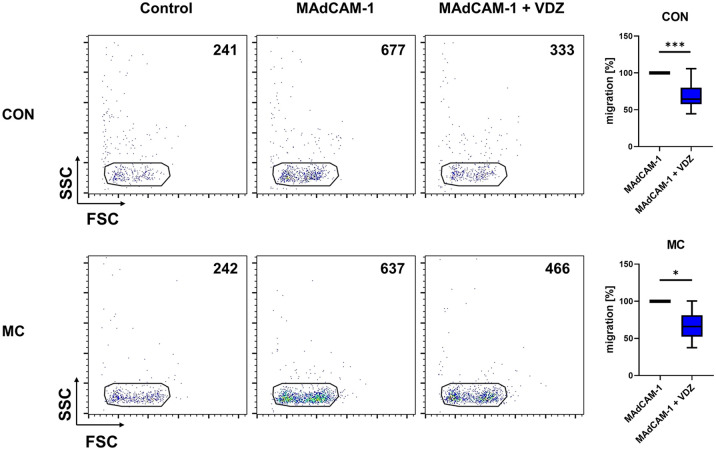
Accordingly, we used transmigration assays investigating the capacity of peripheral blood CD4+ T cells to transmigrate over small MAdCAM-1-coated pores. Similar to the dynamic adhesion assays, MAdCAM-1-coating substantially increased transmigration compared with uncoated conditions, indicating that α4β7 and MAdCAM-1 are implicated in the regulation of transmigration. Vedolizumab significantly reduced transmigration both when using cells from control donors and from patients with MC, and the level of reduction was comparable (Figure 3).
Figure 3.
Impact of vedolizumab on transmigration of CD4+ T cells via α4β7 and MAdCAM-1 in MC. Left panels: Representative flow cytometry depicting the number of CD4+ T cells from healthy control donors and patients with MC transmigrated over pores coated with and without MAdCAM-1 and after treatment with and without vedolizumab. Right panel: Quantification of the transmigration of CD4+ T cells from healthy control donors (n = 12) and patients with MC (n = 8) over MAdCAM-1-coated pores after treatment with and without vedolizumab.
MC: microscopic colitis; VDZ: vedolizumab.
Two cases of successful vedolizumab treatment for MC
These data suggested that α4β7 expressed on T cells in the peripheral blood of patients with MC is functional and that vedolizumab is a valid tool to block α4β7-mediated gut homing in these patients. However, clinical evidence on the use of vedolizumab for this indication is scarce.
Here, we report additional two cases, in which vedolizumab was effective for the treatment of MC.
One female patient suffered from lymphocytic colitis with frequent watery diarrhea. She also reported mucus discharge and abdominal pain. Prior to vedolizumab therapy, a massive increase in intraepithelial lymphocyte (IEL) counts was observed on histology and immunohistochemistry for CD3. Following the initiation of vedolizumab therapy, stool consistency and frequency improved and mucus was absent. Consistently, IEL numbers were diminishing (Figure 4(a)). She was successfully treated for more than 1 year, when she noticed a deterioration of her condition and was later switched to infliximab.
Figure 4.
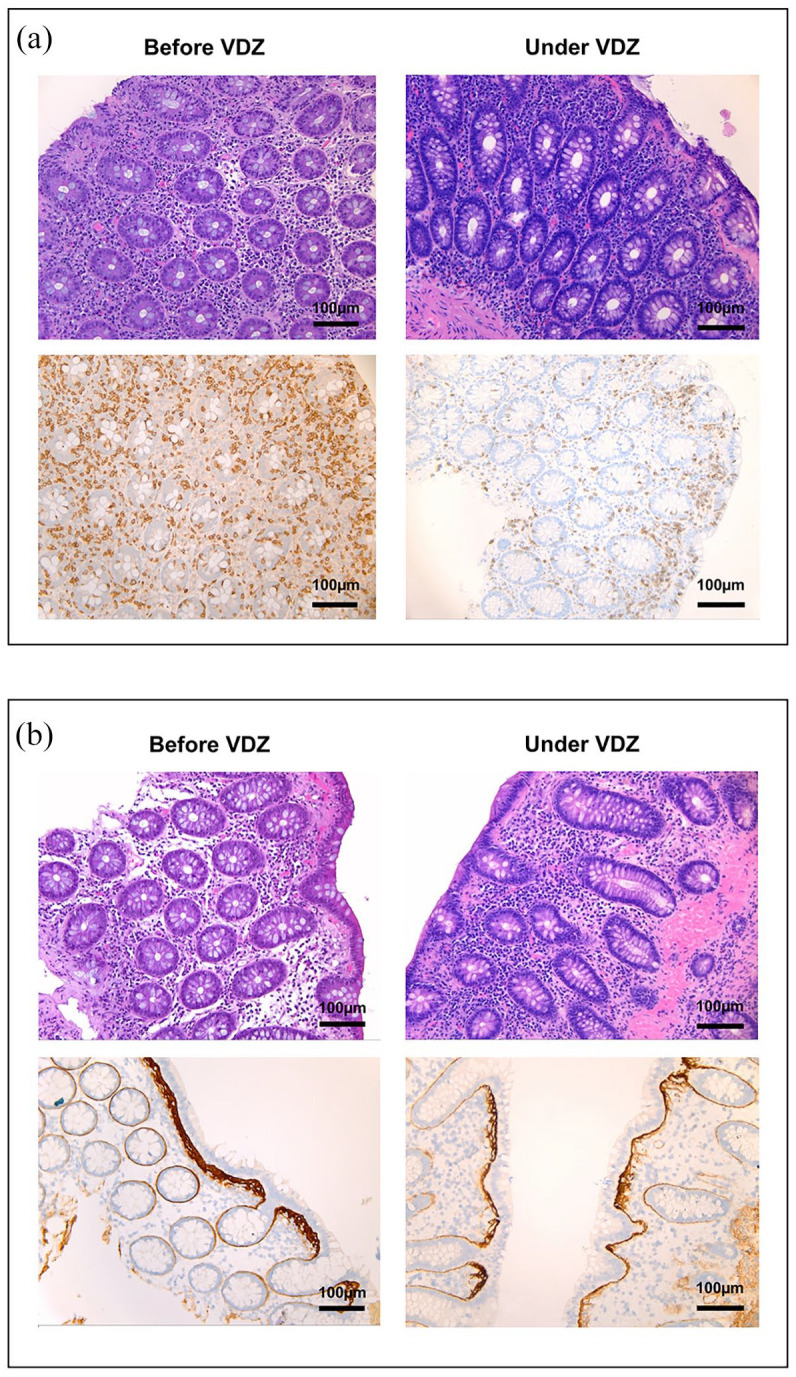
Histological and immunohistochemical findings in patients with MC treated with vedolizumab: (a) hematoxylin/eosin staining and immunohistochemistry for CD3 on sections from colon biopsies of a patient with lymphocytic colitis before and under treatment with vedolizumab as indicated. (b) Hematoxylin/eosin staining and immunohistochemistry for tenascin C on sections from colon biopsies of a patient with collagenous colitis before and under treatment with vedolizumab as indicated.
MC, microscopic colitis; VDZ, vedolizumab.
Another female patient with the diagnosis of collagenous colitis suffered from frequent watery diarrhea and abdominal pain. Prior to the initiation of vedolizumab tenascin staining showed a clearly thickened basal membrane (Figure 4(b)). Under vedolizumab therapy, she noticed substantially improved stool consistency, less abdominal pain and was able to gain 5 kg of weight. Hematoxylin/eosin and tenascin staining performed on biopsies obtained during follow-up colonoscopy demonstrated a regressive inflammatory infiltrate and an almost normal basal membrane. The patient continues to clinically benefit from therapy for more than 18 months now.
Discussion
Immune cell trafficking from the peripheral blood to tissues has been identified as a key driver of inflammation. 28 While this is a necessary prerequisite to fight intruding pathogens at the body’s surfaces, it may also predispose to chronic and harmful inflammation in the case of dysregulated immune responses such as in IBDs. 21
The gut is a special organ with regard to immune cell trafficking, since it is the only organ so far, for which an almost exclusive set of molecules regulating tissue access has been identified. The unique feature of intestinal dendritic cells to metabolize nutritional vitamin A to its derivate retinoic acid, 29 which serves as a transcription factor to induce α4β7 integrin, 20 together with the expression of its ligand MAdCAM-1 being largely confined to the gut and its associated lymphoid tissues, 19 drive a closed system directing gut-imprinted lymphocytes back to intestinal tissues.
Although other integrins and cell adhesion molecules also contribute to gut homing, 30 this α4β7-MAdCAM-1 interaction offers the opportunity to specifically interfere with trafficking processes to the intestine without affecting immunosurveillance of other organs. Consistently, in contrast to the pan-α4 antibody natalizumab, which is effective for the treatment of CD,31,32 but led to several cases of progressive multifocal leucencephalopathy due to decreased α4β1-mediated T cell homing to the central nervous system (CNS), 33 no impairment of CNS homing or associated pathology has been observed with the anti-α4β7 antibody vedolizumab so far.
Thus, it was a logical development to investigate anti-α4β7 antibodies for the therapy of immune-mediated chronic inflammation of the intestine as it occurs in IBD. Eventually, this led to the approval of vedolizumab for the treatment of CD and UC in 2014.16,17
However, CD and UC are only two out of many forms of IBD. In particular, MC has been overshadowed by these entities, also due to the absence of characteristic macroscopic disease features raising suspicion during colonoscopy. 2 Thus, no randomized clinical trials of vedolizumab in MC have been performed to date, although an important role of immune cells and a dysregulated immune response have also been postulated in MC. 6
To date, only case reports34–36 and small case series on the use of vedolizumab in MC have been published. The largest one has been described by Rivière et al., 24 who collected 11 cases of MC (five LC, six CC) treated with vedolizumab. Most of the patients had previously received immunosuppressants and/or antitumor necrosis factor (TNF) antibodies. After induction therapy, five out of eleven patients had entered clinical remission. Shipley et al. 37 report on nine patients with MC, who all showed response to vedolizumab treatment, which could be maintained in six of them. Another case series describes three cases of MC, which were successfully treated with vedolizumab. 23 Thus, the favorable effect of vedolizumab in the two patients portrayed here is in line with observations made by other authors.
While it cannot be excluded that these data are skewed by reporting bias, it can at least be concluded that blocking α4β7 integrin might be a promising strategy to treat MC. However, not only prospective trials, but also mechanistic investigations on the role of gut homing integrins in general and α4β7 in special are lacking for MC so far.
Thus, our data are the first to show that functional α4β7 is expressed on T cells in the peripheral blood from patients with MC. They demonstrate that α4β7 allows these cells to firmly adhere to MAdCAM-1 and to transmigrate through small pores following interaction with MAdCAM-1. Moreover, we show that vedolizumab is able to block these processes on T cells from patients with MC. In consequence, although the rather small sample size is a limitation of our study, these data suggest that there do not seem to exist major differences in α4β7-dependent gut homing compared with other forms of IBD and provide mechanistic evidence to support the above-mentioned preliminary clinical observations. Thus, they underscore the promising potential of vedolizumab as a therapeutic option for MC.
One might object that neither the expression of α4β7 integrin nor the extent of α4β7-dependent adhesion or transmigration were substantially different compared with healthy controls. However, such differences do also not exist in UC or CD,26,38 suggesting that it is rather the quality than the quantity of cells homing via α4β7 that is essential for disease pathogenesis and therapeutic effects.
In summary, we provide unprecedented data on the in vitro mechanisms of vedolizumab for the treatment of MC on single cell level. Further translational and clinical studies are necessary to characterize the mechanisms in vivo and to determine the efficacy in MC.
Supplemental Material
Supplemental material, sj-tif-1-tag-10.1177_17562848221098899 for Vedolizumab blocks α4β7 integrin-mediated T cell adhesion to MAdCAM-1 in microscopic colitis by Laura Besendorf, Tanja M. Müller, Carol-Immanuel Geppert, Ines Schneider, Laura Mühl, Imke Atreya, Francesco Vitali, Raja Atreya, Markus F. Neurath and Sebastian Zundler in Therapeutic Advances in Gastroenterology
Acknowledgments
The authors acknowledge support by Deutsche Forschungsgemeinschaft and Friedrich-Alexander- Universität Erlangen-Nürnberg (FAU) within the funding program Open Access Publishing. The research of TMM, IA, RA, MFN, and SZ was supported by the Interdisciplinary Center for Clinical Research (IZKF) and the ELAN program of the Friedrich-Alexander- Universität Erlangen- Nürnberg, the Else Kröner-Fresenius-Stiftung, the Fritz Bender-Stiftung, the Thyssen-Stiftung, the Dr. Robert Pfleger Stiftung, the Litwin IBD Pioneers Initiative of the Crohn’s and Colitis Foundation of America (CCFA), the Kenneth Rainin Foundation, the Ernst Jung-Stiftung for Science and Research, the German Crohn’s and Colitis Foundation (DCCV) and the German Research Foundation (DFG) through individual grants (ZU 377/4-1) and the Collaborative Research Centers TRR241 (TP C04), 643, 796, and 1181. This work was performed in fulfillment of the requirements for obtaining the degree ‘Dr. med.’ for LB. The authors thank Julia Derdau, Julia Marcks, Julia Schuster, and Dorothee Dziony for excellent technical assistance.
Footnotes
Ethics approval and consent to participate: Peripheral blood from patients with MC was collected following informed written consent according to the approval of the Ethics Committee of the Friedrich-Alexander-Universität Erlangen-Nürnberg (40_16B, 426_20B)
Author contribution(s): Laura Besendorf: Formal analysis; Investigation; Validation; Visualization; Writing – original draft; Writing – review & editing.
Tanja M. Müller: Formal analysis; Investigation; Validation; Visualization; Writing – original draft; Writing – review & editing.
Carol-Immanuel Geppert: Formal analysis; Investigation; Visualization; Writing – review & editing.
Ines Schneider: Formal analysis; Investigation; Writing – review & editing.
Laura Mühl: Formal analysis; Investigation; Writing – review & editing.
Imke Atreya: Methodology; Resources; Writing – review & editing.
Francesco Vitali: Methodology; Resources; Writing – review & editing.
Raja Atreya: Methodology; Resources; Writing – review & editing.
Markus F. Neurath: Formal analysis; Methodology; Resources; Validation; Writing – review & editing.
Sebastian Zundler: Conceptualization; Formal analysis; Funding acquisition; Investigation; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
ORCID iDs: Tanja M. Müller  https://orcid.org/0000-0003-2768-277X
https://orcid.org/0000-0003-2768-277X
Raja Atreya  https://orcid.org/0000-0002-8556-8433
https://orcid.org/0000-0002-8556-8433
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: German Research Foundation (grant no. DFG, ZU 377/4-1) and Interdisciplinary Center for Clinical Research (IZKF, scholarship to LB).
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MFN has served as an advisor for Pentax, Giuliani, MSD, Abbvie, Janssen, Takeda and Boehringer. SZ received honoraria from Takeda, Roche, Galapagos, Ferring, Lilly and Janssen. MFN and SZ received research support from Takeda, Shire (a part of Takeda) and Roche. The remaining authors declare no conflicts of interest.
Availability of data and materials: Data are available from the authors upon reasonable request.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Laura Besendorf, Department of Medicine 1, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany.
Tanja M. Müller, Department of Medicine 1, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany Deutsches Zentrum Immuntherapie (DZI), University Hospital Erlangen, Erlangen, Germany.
Carol-Immanuel Geppert, Institute of Pathology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany.
Ines Schneider, Department of Medicine 1, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany.
Laura Mühl, Department of Medicine 1, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany.
Imke Atreya, Department of Medicine 1, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany; Deutsches Zentrum Immuntherapie (DZI), University Hospital Erlangen, Erlangen, Germany.
Francesco Vitali, Department of Medicine 1, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany; Deutsches Zentrum Immuntherapie (DZI), University Hospital Erlangen, Erlangen, Germany.
Raja Atreya, Department of Medicine 1, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany; Deutsches Zentrum Immuntherapie (DZI), University Hospital Erlangen, Erlangen, Germany.
Markus F. Neurath, Department of Medicine 1, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany Deutsches Zentrum Immuntherapie (DZI), University Hospital Erlangen, Erlangen, Germany.
Sebastian Zundler, Department of Medicine 1, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Ulmenweg 18, D-91054 Erlangen, Germany; Deutsches Zentrum Immuntherapie (DZI), University Hospital Erlangen, Erlangen, Germany.
References
- 1. Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol 2010; 28: 573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miehlke S, Verhaegh B, Tontini GE, et al. Microscopic colitis: pathophysiology and clinical management. Lancet Gastroenterol Hepatol 2019; 4: 305–314. [DOI] [PubMed] [Google Scholar]
- 3. Larsson JK, Sjöberg K, Vigren L, et al. Chronic non-bloody diarrhoea: a prospective study in Malmö, Sweden, with focus on microscopic colitis. BMC Res Notes 2014; 7: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tontini GE, Pastorelli L, Spina L, et al. Microscopic colitis and colorectal neoplastic lesion rate in chronic nonbloody diarrhea: a prospective, multicenter study. Inflamm Bowel Dis 2014; 20: 882–891. [DOI] [PubMed] [Google Scholar]
- 5. Langner C, Aust D, Ensari A, et al. Histology of microscopic colitis-review with a practical approach for pathologists. Histopathology 2015; 66: 613–626. [DOI] [PubMed] [Google Scholar]
- 6. Park T, Cave D, Marshall C. Microscopic colitis: a review of etiology, treatment and refractory disease. World J Gastroenterol 2015; 21: 8804–8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis 2013; 7: 827–851. [DOI] [PubMed] [Google Scholar]
- 8. Miehlke S, Guagnozzi D, Zabana Y, et al. European guidelines on microscopic colitis: United European Gastroenterology and European Microscopic Colitis Group statements and recommendations. United European Gastroenterol J 2021; 9: 13–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nguyen GC, Smalley WE, Vege SS, et al. American Gastroenterological Association Institute guideline on the medical management of microscopic colitis. Gastroenterology 2016; 150: 242–246; quiz e17–e18. [DOI] [PubMed] [Google Scholar]
- 10. Miehlke S, Madisch A, Kupcinskas L, et al. Budesonide is more effective than mesalamine or placebo in short-term treatment of collagenous colitis. Gastroenterology 2014; 146: 1222–1230.e12. [DOI] [PubMed] [Google Scholar]
- 11. Miehlke S, Aust D, Mihaly E, et al. Efficacy and safety of budesonide, vs mesalazine or placebo, as induction therapy for lymphocytic colitis. Gastroenterology 2018; 155: 1795–1804.e3. [DOI] [PubMed] [Google Scholar]
- 12. Münch A, Bohr J, Miehlke S, et al. Low-dose budesonide for maintenance of clinical remission in collagenous colitis: a randomised, placebo-controlled, 12-month trial. Gut 2016; 65: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonderup OK, Hansen JB, Teglbjaerg PS, et al. Long-term budesonide treatment of collagenous colitis: a randomised, double-blind, placebo-controlled trial. Gut 2009; 58: 68–72. [DOI] [PubMed] [Google Scholar]
- 14. Miehlke S, Madisch A, Bethke B, et al. Oral budesonide for maintenance treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2008; 135: 1510–1516. [DOI] [PubMed] [Google Scholar]
- 15. Townsend T, Campbell F, O’Toole P, et al. Microscopic colitis: diagnosis and management. Frontline Gastroenterol 2019; 10: 388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- 17. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369: 711–721. [DOI] [PubMed] [Google Scholar]
- 18. Berlin C, Berg EL, Briskin MJ, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 1993; 74: 185–195. [DOI] [PubMed] [Google Scholar]
- 19. Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol 1997; 151: 97–110. [PMC free article] [PubMed] [Google Scholar]
- 20. Iwata M, Hirakiyama A, Eshima Y, et al. Retinoic acid imprints gut-homing specificity on T-cells. Immunity 2004; 21: 527–538. [DOI] [PubMed] [Google Scholar]
- 21. Zundler S, Becker E, Schulze LL, et al. Immune cell trafficking and retention in inflammatory bowel disease: mechanistic insights and therapeutic advances. Gut 2019; 68: 1688–1700. [DOI] [PubMed] [Google Scholar]
- 22. Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007; 7: 678–689. [DOI] [PubMed] [Google Scholar]
- 23. Jennings JJ, Charabaty A. Vedolizumab-induced remission in 3 patients with refractory microscopic colitis: a tertiary care center case series. Inflamm Bowel Dis 2019; 25: e97. [DOI] [PubMed] [Google Scholar]
- 24. Rivière P, Münch A, Michetti P, et al. Vedolizumab in refractory microscopic colitis: an international case series. J Crohns Colitis 2019; 13: 337–340. [DOI] [PubMed] [Google Scholar]
- 25. Zundler S, Fischer A, Schillinger D, et al. The α4β1 homing pathway is essential for ileal homing of Crohn’s disease effector T cells in vivo. Inflamm Bowel Dis 2017; 23: 379–391. [DOI] [PubMed] [Google Scholar]
- 26. Binder M-T, Becker E, Wiendl M, et al. Similar inhibition of dynamic adhesion of lymphocytes from IBD patients to MAdCAM-1 by vedolizumab and etrolizumab-s. Inflamm Bowel Dis 2018; 24: 1237–1250. [DOI] [PubMed] [Google Scholar]
- 27. Becker E, Schramm S, Binder M-T, et al. Dynamic adhesion assay for the functional analysis of anti-adhesion therapies in inflammatory bowel disease. J Vis Exp 2018; 139: 58210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neurath MF. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol 2019; 20: 970–979. [DOI] [PubMed] [Google Scholar]
- 29. Iwata M, Yokota A. Retinoic acid production by intestinal dendritic cells. Vitam Horm 2011; 86: 127–152. [DOI] [PubMed] [Google Scholar]
- 30. Habtezion A, Nguyen LP, Hadeiba H, et al. Leukocyte trafficking to the small intestine and colon. Gastroenterology 2016; 150: 340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med 2005; 353: 1912–1925. [DOI] [PubMed] [Google Scholar]
- 32. Targan SR, Feagan BG, Fedorak RN, et al. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE trial. Gastroenterology 2007; 132: 1672–1683. [DOI] [PubMed] [Google Scholar]
- 33. Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med 2005; 353: 362–368. [DOI] [PubMed] [Google Scholar]
- 34. Casper M, Zimmer V, Hübschen U, et al. Vedolizumab for refractory collagenous colitis: another piece of the puzzle. Dig Liver Dis 2018; 50: 1099–1100. [DOI] [PubMed] [Google Scholar]
- 35. Cushing KC, Mino-Kenudson M, Garber J, et al. Vedolizumab as a novel treatment for refractory collagenous colitis: a case report. Am J Gastroenterol 2018; 113: 632–633. [DOI] [PubMed] [Google Scholar]
- 36. Wenzel AA, Strople J, Melin-Aldana H, et al. Vedolizumab for the induction of remission in treatment-refractory microscopic colitis in a pediatric patient. J Pediatr Gastroenterol Nutr 2020; 71: e47–e48. [DOI] [PubMed] [Google Scholar]
- 37. Shipley LC, Ravi S, Russ KB, et al. Vedolizumab therapy in refractory microscopic colitis: a single center case series. Clin Gastroenterol Hepatol 2022; 20: 455–457. [DOI] [PubMed] [Google Scholar]
- 38. Fischer A, Zundler S, Atreya R, et al. Differential effects of α4β7 and GPR15 on homing of effector and regulatory T cells from patients with UC to the inflamed gut in vivo. Gut 2016; 65: 1642–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-tag-10.1177_17562848221098899 for Vedolizumab blocks α4β7 integrin-mediated T cell adhesion to MAdCAM-1 in microscopic colitis by Laura Besendorf, Tanja M. Müller, Carol-Immanuel Geppert, Ines Schneider, Laura Mühl, Imke Atreya, Francesco Vitali, Raja Atreya, Markus F. Neurath and Sebastian Zundler in Therapeutic Advances in Gastroenterology