Abstract
BACKGROUND
The usefulness of a mandatory joint aspiration before re-implantation in patients with a cement spacer already in place is unclear.
AIM
To evaluate the role of culturing synovial fluid obtained by joint aspiration before re-implantation in patients who underwent a two-stage septic revision.
METHODS
A retrospective observational study was conducted, including patients that underwent a two-stage septic revision (hip or knee) from 2010 to 2017. After the first stage revision and according to intraoperative culture results, all patients were treated with an antibiotic protocol for 6-8 wk. Following 2 wk without antibiotics, a culture of synovial fluid was obtained. The results of these cultures were recorded and compared with cultures obtained during re-implantation surgery.
RESULTS
Forty-one patients (20 hip and 21 knee spacers) were included in the final analysis. In 39 cases, the culture of synovial fluid was negative, while in the remaining 2 cases (knee spacers) no analysis was possible due to dry tap. In 5 of the patients, two or more intraoperative cultures taken during the re-implantation surgery were positive.
CONCLUSION
We found no evidence to support mandatory joint aspiration before re-implantation in patients with a cement spacer in place.
Keywords: Joint aspiration, Synovial fluid, Two-stage surgery, Revision surgery, Periprosthetic joint infection
Core Tip: Many parameters and diagnostic methods have been analyzed to determine the optimal time to perform the second stage of a two-staged revision surgery. Synovial fluid culture after joint aspiration seems to be a reasonable test to evaluate the presence of microorganisms in the joint. However, the effectiveness of this diagnostic test is unclear. Despite the lack of validation, synovial aspiration is a common practice before prosthesis reimplantation. With our results, we found no evidence to support mandatory joint aspiration before re-implantation in patients with a cement spacer in place.
INTRODUCTION
Periprosthetic joint infection (PJI) remains a challenging complication for all orthopedic surgeons. Despite the increase of a one-stage revision strategy, two-stage revision surgery remains the gold standard procedure for chronic PJI. Two-stage procedures using antibiotic-loaded cement spacers have reported eradication rates of over 73%[1-4]. To determine the optimal time to perform the second stage of the revision surgery, many parameters and diagnostic methods had been analyzed. Synovial fluid culture after a joint aspiration seems to be a reasonable test to evaluate the presence of microorganisms in the joint[5-7]. However, the effectiveness of this diagnostic test is unclear. Despite the lack of validation, synovial aspiration is a common practice before prosthesis reimplantation.
The purpose of our study was to evaluate the role of joint aspiration and synovial fluid culturing before re-implantation in patients with a cement spacer in place.
MATERIALS AND METHODS
A retrospective observational study was conducted. We analyzed all patients that underwent a two-stage revision surgery at our institution between 2010 and 2017 (inclusive).
The following variables were recorded for all patients: demographic parameters, results of first stage cultures, cultures of the synovial fluid between stages, results of second stage cultures, and the need for new procedures after the second stage.
All patients to whom arthrocentesis before the second stage of the surgery was not performed or the intraoperative cultures for the two stages of the surgery were not correctly analyzed were excluded from this study.
Treatment protocol
Our arthroplasty two-stage exchange protocol consisted of a first surgery where the prosthesis was explanted as well as all the cement and forage implants. A radical debridement was performed, and 5-7 samples were taken and analyzed by the microbiology laboratory. A cement spacer loaded with antibiotics (vancomycin and gentamicin), usually preformed (Vancogenx®-Space, Tecres), was then placed. After surgery, an empirical intravenous antibiotic treatment (teicoplanin, rifampin, and amikacin) was started and continued until definitive results for the microbiological cultures were obtained. Once the causative microorganisms were isolated, antibiotic therapy was tailored to its sensitivity. This antibiotic treatment was then continued for 6 to 8 wk. After which, antibiotics were stopped for 2 wk (antibiotic holidays), and an arthrocentesis was performed. Blood tests were performed to quantify acute phase reactants, such as C-reactive protein. If the patient remained afebrile, without local clinical signs of infection, and with normalized serum C-reactive protein levels, we assumed that the infection was controlled and proceeded to the second stage. During the second-stage surgery, the cement spacer was removed and submitted to the microbiology laboratory for sonication. Another thorough debridement and sampling were performed before implantation of the definitive prosthesis. After the second stage surgery, patients received antibiotic therapy based on the sensitivity of the infecting organisms for 6 mo for total knee arthroplasty or 3 mo for total hip arthroplasty.
Joint aspiration protocol
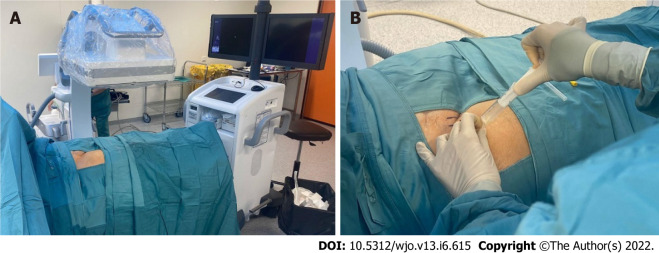
The knee is a superficial joint where after adequate skin disinfection and with proper sterility measures we performed an arthrocentesis at the outpatient clinic. Synovial fluid obtained was sent for microbiological study. On the other hand, hip arthrocentesis was performed at the operating room with the assistance of sedation by the anesthesiologist and fluoroscopic aid to localize the correct space for joint puncture (Figure 1). Sterility measures and microbiological studies were the same as for the knee joint.
Figure 1.
Hip arthrocentesis procedure. A: Hip arthrocentesis setup; B: Hip arthrocentesis.
Microbiological protocol
Following the sampling protocol at our hospital, we took between 5 and 7 intraoperative samples. Each one was taken using a clean scalpel and clamp to avoid cross-contamination. Tissue samples were introduced in sterile plastic containers and sent to the microbiological laboratory without culture media. Once received in the laboratory, the tissue samples were homogenized in thioglycolate broth before plating in the following culture media (bioMérieux Marcy-l’Étoile, France): (1) 5% blood sheep agar: 7 d at 37 ºC in 5% CO2 atmosphere; (2) Chocolate agar: 7 d at 37 ºC in 5% CO2 atmosphere; (3) McConkey agar: 2 d in a normal atmosphere; (4) Sabouraud agar: 5 d at 37 ºC in a normal atmosphere; (5) Anaerobic agar: 7 d in an anaerobic atmosphere; and (6) Thioglycolate broth: systematic spread after 5 d of incubation in a normal atmosphere, in 5% sheep blood agar, chocolate agar, and anaerobic agar with the incubation times previously described.
When the consistency of the samples did not allow homogenization, they were covered with thioglycolate broth and plated on agar plates (not in thioglycolate broth) after overnight incubation at 35 ºC. Gram stains were performed from synovial fluid samples and then inoculated into a BacT/ALERT bottle (bioMérieux Marcy-l’Étoile, France) incubated for 7 d.
Results interpretation
The results of intraoperative cultures during first-stage surgery and synovial fluid were recorded and compared with cultures obtained during re-implantation surgery. According to culture results during the second stage, patients were classified as persistent infection when second stage cultures were positive for the same microorganism that was isolated during the first stage even if only one single culture was positive. Reinfection was considered when two or more of the second stage cultures were positive for the same microorganism but differ from the ones isolated during the first stage. The presence of only one positive culture from intraoperative samples for a low virulent microorganism not isolated in the first stage was considered as a contaminant.
RESULTS
A total of 50 patients diagnosed with PJI treated with a two-stage arthroplasty revision surgery were analyzed; nine patients were excluded because joint aspiration was not performed or the sample of synovial fluid was not correctly processed. The remaining 41 patients (20 hip and 21 knee joints) were included in the final analysis.
The mean age of patients was 70.4 years (range: 40-85 years). Twenty-four of them were females (61%), and sixteen were males (31%). In 39 patients, the synovial fluid culture was negative. In the remaining 2 cases, both knee spacers, no analysis was possible due to dry tap. Five patients had two or more positive intraoperative cultures during re-implantation surgery (Table 1). Only 1 patient, number 3, had a persistent infection. In this patient, the synovial fluid culture before the second-stage surgery failed to identify the infection. The other 4 cases had a reinfection, and in all of them the synovial fluid was negative. Three of these five patients (60%) required further surgeries after the second stage, and it was due to an infection in two cases (40%) (patients 1 and 2).
Table 1.
Patients with more than two intraoperative cultures positive during second-stage surgery
|
Patient
|
Microorg. 1st stage
|
Spacer joint aspiration
|
Microorg. 2nd stage
|
Reinfection/Persistence
|
Reoperation after 2nd stage
|
Microorg. reoperation
|
| 1 | Negative | Negative | S. epidermidis; S. capitis | Reinfection | Yes (Multiple) | Klebsiella spp |
| 2 | Negative | Negative | S. epidermidis; S. cohnii | Reinfection | Yes (Debridement) | Negative |
| 3 | S. epidermidis; S. lugdunensis | Negative | S. epidermidis | Persistence | No | - |
| 4 | Negative | Negative | S. epidermidis; S. haemolyticus | Reinfection | Yes (Periprosthetic fracture) | - |
| 5 | Negative | Negative | S. epidermidis; C. acnes | Reinfection | No | - |
S. epidermidis: Staphylococcus epidermidis; S. lugdunensis: Staphylococcus lugdunensis; S. haemolyticus: Staphylococcus haemolyticus; S. capitis: Staphylococcus capitis; S. cohni: Staphylococcus cohnii; C. acnes: Cutibacterium acnes.
Thirty-six patients (87.8%) had negative cultures or one positive culture from a minimum of five intraoperative samples (considered contamination) during the second stage of the revision surgery. Of these patients, 17 (47.22%) needed new interventions after the second stage, and 12 of them (70.59%) were due to septic causes.
DISCUSSION
PJI is a challenging complication following orthopedic surgery. Two-stage revision surgery was first described by Insall et al[8], and it is considered the gold standard treatment for chronic PJI. The precise time to perform the second stage of the revision surgery remains uncertain. A combination of serum markers and synovial aspiration results is considered the best test for determining the presence of PJI persistence[5-7,9].
Although the majority of studies confirm low sensitivity for joint aspiration fluid culture before reimplantation surgery[10-14] (some as low as our data of 0%), other studies like Preininger et al[15] and Newman et al[16] reported higher rates (21% and 30%, respectively) with a maximum sensitivity of 83% in the study by Meermans et al[17]. All studies agree on its high specificity, above 90%[10-17].
Mont et al[18] and Aalirezaie et al[19] considered joint aspiration and synovial fluid culturing a useful tool. However, we found similarities in our results with other authors and agreed to not perform mandatory synovial fluid aspiration before the second stage[11-13,15].
An antibiotic-free interval before joint aspiration (antibiotic holiday) and the time until the culture result is available (a minimum of 2 wk) extends the duration between the first and second stage of the two-stage revision surgery. However, active antibiotic treatment can result in false negatives. In all the cases of our series, the cultures of the first stage, second stage, and synovial fluid obtained from joint aspiration were performed in patients without active antibiotic treatment. Despite this condition, we did not have any positive cultures. To reduce the time between stages, some authors such as Mühlhofer et al[10] and Boelch et al[11] recommend performing reimplantation surgery without antibiotic holiday.
There are some explanations for not having obtained any positive result in the synovial fluid culture in our patients. In the first place, the small sample size and the low sensitivity of the joint aspiration could explain our results. Second, the low bacterial load in the synovial fluid at the time of the joint aspiration. Third, the presence of local antibiotics due to elution of the antibiotic present in the cement spacer[20].
It is important to emphasize the differences between the knee and hip joint aspiration procedures. Knee joint aspiration is a much easier procedure as it is a more accessible joint and does not require guidance by fluoroscopy or ultrasound techniques. In some centers, when no fluid is obtained after joint aspiration, sterile saline is injected into the joint and then aspirated to obtain fluid to analyze. Injection of saline fluid into a joint that did not yield any synovial fluid (dry tap) was not recommended during the 2018 International Consensus Meeting on musculoskeletal infection.
The main limitations of our study are its retrospective nature and the limited number of cases. There are few articles published in the literature concerning the value of synovial aspiration before re-implantation surgery with a cement spacer in place. These papers present heterogeneous data and an inconsistent antibiotic-free interval, making them difficult to compare.
CONCLUSION
Although synovial fluid culture may provide useful information regarding the infection status of the joint, we found no evidence to support mandatory joint aspiration before re-implantation in patients with a cement spacer in place.
ARTICLE HIGHLIGHTS
Research background
There are few studies in the literature based on the usefulness of joint aspiration with a cement spacer in place. The importance of this type of study lies in finding useful methods for determining the appropriate timing of the second stage of revision surgery.
Research motivation
The main problem in this type of research is its heterogeneity, as the duration of antibiotic treatment, the presence of antibiotic holiday, the use or not of a physiological saline solution when a dry aspiration is obtained, etc vary according to each institution’s protocol.
Research objectives
The objective of this study was to evaluate the role of culturing synovial fluid obtained by joint aspiration before re-implantation in patients who underwent a two-stage septic revision.
Research methods
This is a retrospective study, and the research method was to observe the results obtained in the joint aspiration performed before re-implantation in the knee/hip septic replacements in our center between 2010 and 2017.
Research results
The results obtained in the study showed low sensitivity of joint aspiration for detecting infection persistence when performed prior to the second stage in a two-stage replacement.
Research conclusions
The results obtained in our study lead us to not recommend the use of joint aspiration prior to the second stage of revision surgery due to its low sensitivity.
Research perspectives
Future research should focus on obtaining reliable markers to indicate the optimal time to perform the second stage of a two-stage septic revision.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Mútua Terrassa Institutional Review Board.
Informed consent statement: No written consent was needed for this article. No identifying information is included in this article.
Conflict-of-interest statement: No conflict of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 27, 2021
First decision: January 25, 2022
Article in press: May 13, 2022
Specialty type: Orthopedics
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jamali R, Iran; Lass R, Austria S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL
Contributor Information
Sandra Huguet, Department of Traumatology and Orthopaedics, Hospital Universitari Mútua Terrassa, Terrassa 08221, Spain; Department of Traumatology and Orthopaedics, Consorci Sanitari de l’Alt Penedès - Garraf, Vilafranca del Penedès 08720, Spain. sahuguet@csap.cat.
Martí Bernaus, Department of Traumatology and Orthopaedics, Osteoarticular Infections Unit, Hospital Universitari Mútua Terrassa, Terrassa 08221, Spain.
Lucía Gómez, Osteoarticular Infections Unit, Hospital Universitari Mútua Terrassa, Terrassa 08221, Spain.
Eva Cuchí, Osteoarticular Infections Unit, Hospital Universitari Mútua Terrassa, Terrassa 08221, Spain; Department of Microbiology, CATLAB, Viladecavalls 08232, Spain.
Alex Soriano, Department of Infectious Diseases, Osteoarticular Infections Unit, Hospital Clínic, Barcelona 08036, Spain.
Lluís Font-Vizcarra, Department of Traumatology and Orthopaedics, Osteoarticular Infections Unit, Hospital Universitari Mútua Terrassa, Terrassa 08221, Spain.
Data sharing statement
No additional data are available.
References
- 1.Anagnostakos K, Fürst O, Kelm J. Antibiotic-impregnated PMMA hip spacers: Current status. Acta Orthop. 2006;77:628–637. doi: 10.1080/17453670610012719. [DOI] [PubMed] [Google Scholar]
- 2.Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am. 2007;89:871–882. doi: 10.2106/JBJS.E.01070. [DOI] [PubMed] [Google Scholar]
- 3.Kurd MF, Ghanem E, Steinbrecher J, Parvizi J. Two-stage exchange knee arthroplasty: does resistance of the infecting organism influence the outcome? Clin Orthop Relat Res. 2010;468:2060–2066. doi: 10.1007/s11999-010-1296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biring GS, Kostamo T, Garbuz DS, Masri BA, Duncan CP. Two-stage revision arthroplasty of the hip for infection using an interim articulated Prostalac hip spacer: a 10- to 15-year follow-up study. J Bone Joint Surg Br. 2009;91:1431–1437. doi: 10.1302/0301-620X.91B11.22026. [DOI] [PubMed] [Google Scholar]
- 5.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004;117:556–562. doi: 10.1016/j.amjmed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Parvizi J, Della Valle CJ. AAOS Clinical Practice Guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18:771–772. doi: 10.5435/00124635-201012000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008;90:1869–1875. doi: 10.2106/JBJS.G.01255. [DOI] [PubMed] [Google Scholar]
- 8.Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65:1087–1098. [PubMed] [Google Scholar]
- 9.Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J. Staged revision for knee arthroplasty infection: what is the role of serologic tests before reimplantation? Clin Orthop Relat Res. 2009;467:1699–1705. doi: 10.1007/s11999-009-0742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mühlhofer HML, Knebel C, Pohlig F, Feihl S, Harrasser N, Schauwecker J, von Eisenhart-Rothe R. Synovial aspiration and serological testing in two-stage revision arthroplasty for prosthetic joint infection: evaluation before reconstruction with a mean follow-up of twenty seven months. Int Orthop. 2018;42:265–271. doi: 10.1007/s00264-017-3700-2. [DOI] [PubMed] [Google Scholar]
- 11.Boelch SP, Weissenberger M, Spohn F, Rudert M, Luedemann M. Insufficient sensitivity of joint aspiration during the two-stage exchange of the hip with spacers. J Orthop Surg Res. 2018;13:7. doi: 10.1186/s13018-017-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boelch SP, Roth M, Arnholdt J, Rudert M, Luedemann M. Synovial Fluid Aspiration Should Not Be Routinely Performed during the Two-Stage Exchange of the Knee. Biomed Res Int. 2018;2018:6720712. doi: 10.1155/2018/6720712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoell S, Moeller A, Gosheger G, Hardes J, Dieckmann R, Schulz D. Two-stage revision arthroplasty for periprosthetic joint infections: What is the value of cultures and white cell count in synovial fluid and CRP in serum before second stage reimplantation? Arch Orthop Trauma Surg. 2016;136:447–452. doi: 10.1007/s00402-015-2404-6. [DOI] [PubMed] [Google Scholar]
- 14.Lonner JH, Siliski JM, Della Valle C, DiCesare P, Lotke PA. Role of knee aspiration after resection of the infected total knee arthroplasty. Am J Orthop (Belle Mead NJ) 2001;30:305–309. [PubMed] [Google Scholar]
- 15.Preininger B, Janz V, von Roth P, Trampuz A, Perka CF, Pfitzner T. Inadequacy of Joint Aspiration for Detection of Persistent Periprosthetic Infection During Two-Stage Septic Revision Knee Surgery. Orthopedics. 2017;40:231–234. doi: 10.3928/01477447-20170411-04. [DOI] [PubMed] [Google Scholar]
- 16.Newman JM, George J, Klika AK, Hatem SF, Barsoum WK, Trevor North W, Higuera CA. What is the Diagnostic Accuracy of Aspirations Performed on Hips With Antibiotic Cement Spacers? Clin Orthop Relat Res. 2017;475:204–211. doi: 10.1007/s11999-016-5093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meermans G, Haddad FS. Is there a role for tissue biopsy in the diagnosis of periprosthetic infection? Clin Orthop Relat Res. 2010;468:1410–1417. doi: 10.1007/s11999-010-1245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mont MA, Waldman BJ, Hungerford DS. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection. A comparison-group study. J Bone Joint Surg Am. 2000;82:1552–1557. doi: 10.2106/00004623-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Aalirezaie A, Bauer TW, Fayaz H, Griffin W, Higuera CA, Krenn V, Molano M, Moojen DJ, Restrepo C, Shahi A, Shubnyakov I, Sporer S, Tanavalee A, Teloken M, Velázquez Moreno JD. Hip and Knee Section, Diagnosis, Reimplantation: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34:S369–S379. doi: 10.1016/j.arth.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Anagnostakos K, Meyer C. Antibiotic Elution from Hip and Knee Acrylic Bone Cement Spacers: A Systematic Review. Biomed Res Int. 2017;2017:4657874. doi: 10.1155/2017/4657874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.