Abstract
BACKGROUND
In the molecular era, the Laurén system is still a cost-effective and widely implemented classification for gastric cancer (GC) and it has been recently associated with clinical, histological and molecular features of these tumors. Despite recent advances in the understanding of the molecular biology of GC, there is a need to develop new prognostic tools for patient stratification in clinical practice. Thus, the identification of easily available prognostic factors in patients with intestinal and diffuse-type tumors can significantly improve risk assessment and patient stratification in GC.
AIM
To identify clinicopathological differences, risk factors, and to develop cost-effective prognostic scores for patients with intestinal and diffuse-type GC.
METHODS
Retrospective study of all patients undergoing surgery for GC at a tertiary referral center from 2001 to 2019. 286 cases met inclusion criteria (intestinal: 190, diffuse: 96). Clinical data and gross findings were collected. All specimens were reviewed by two independent pathologists and a detailed protocol for histologic evaluation was followed. Five tissue microarrays (TMAs) were constructed and sections of the TMA block were immunostained for HERCEPTEST, MSH2, MSH6, MLH1 and PMS2. Statistical analyses were performed and prognostic scores were developed based on hazard ratios.
RESULTS
Intestinal and diffuse-type GC showed different epidemiological, clinicopathological and prognostic features. Diffuse tumors were significantly associated with younger age, less symptomatology, flat morphology, deeper invasion, perineural infiltration, advanced stage at diagnosis, administration of adjuvant therapy and poorer prognosis. Intestinal lesions were fungoid or polypoid, showed necrosis, desmoplasia, microsatellite instability and HERCEPTEST positivity and were diagnosed at earlier stages. Tumor depth, desmoplasia, macroscopic type and lymph node involvement were independently related to the Laurén subtype. Furthermore, intestinal and diffuse GC were associated with different risk factors for progression and death. Vascular invasion, perineural infiltration and growth pattern were important prognostic factors in intestinal-type GC. On the contrary, tumor size and necrosis were significant prognosticators in diffuse-type GC. Our recurrence and cancer-specific death scores for patients with intestinal and diffuse-type GC showed an excellent patient stratification into three (diffuse GC) or four (intestinal) prognostic groups.
CONCLUSION
Our findings support that Laurén subtypes represent different clinicopathological and biological entities. The development of specific prognostic scores is a useful and cost-effective strategy to improve risk assessment in GC.
Keywords: Gastric cancer, Clinicopathological, Score, Prognosis, Laurén, Molecular
Core Tip: In the molecular era, the Laurén system is a cost-effective and widely implemented classification. The identification of easily available prognostic factors in intestinal and diffuse-type tumors may significantly improve patient stratification in gastric cancer (GC). In this study, the authors found that intestinal and diffuse-type GC show different epidemiological, clinical and prognostic features. Laurén subtypes were also associated with different risk factors for tumor progression and death. Finally, separate clinicopathological scores for patients with intestinal and diffuse-type GC showed an excellent prognostic stratification. The development of specific prognostic scores is a useful, cost-effective strategy to improve risk assessment in GC.
INTRODUCTION
Gastric cancer (GC) is an aggressive tumor which is usually diagnosed at advanced stages in western countries[1,2]. It can be classified according to its location, macroscopical or microscopical features[3,4]. Although several histological-based classifications have been proposed, only the Laurén and the most recent WHO classification are currently widely used[5]. The Laurén system has been extensively adopted by clinicians and pathologists since its publication in 1965 and it can be easily evaluated in conventional paraffin-embedded hematoxylin-eosin-stained slides[6]. This classification divides GC into intestinal, diffuse and mixed types, depending on the tumor architecture, growth pattern and cell morphology. Intestinal-type GC is composed of glandular structures accompanied by papillary or solid components. On the other hand, diffuse-type GC is composed of loosely attached cells growing as small clusters or scattered cells with an infiltrative pattern. This classification has been variably associated with clinicopathological features[7,8]. Intestinal tumors occur more frequently in older men and they are related to Helicobacter pylori (H. pylori) infection and environmental factors. Furthermore, most studies have identified Laurén subtype as an independent prognosticator in GC[9-11].
Recent technological advances have allowed us to improve the understanding of the molecular biology of GC[12]. In 2014, The Cancer Genome Atlas (TCGA) Research Network defined four molecular subtypes of GC: tumors positive for Epstein-Barr virus, microsatellite unstable tumors (MSI), genomically stable tumors (GS) and tumors with chromosomal instability (CIN)[13]. Laurén classification has also been correlated with these molecular groups[14,15].
The only curative treatment for GC is surgery and localized tumors are treated by total or subtotal gastrectomy. However, patient prognosis is poor with estimated 5-year survival rates lower than 30%[16]. There is an urgent need to identify potential therapeutic targets and prognostic factors in GC in order to improve patient subclassification and response to therapy. It has been suggested that diffuse GC may benefit from broader surgical margins, extended lymphadenectomy and hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal metastasis[17,18]. Interestingly, diffuse GC may be more resistant to standard chemotherapeutic regimens than intestinal GC, and several investigators have recommended the use of the Laurén classification in clinical trials[19].
In summary, in the era of molecular medicine, the Laurén system is a cost-effective and widely implemented classification which has been associated with clinical, pathological, prognostic and molecular features. Thus, Laurén subtypes can be considered distinct entities that differ in their histology, biology, and clinical behavior, and the identification of easily available prognostic factors in patients with intestinal and diffuse-type tumors may significantly improve risk assessment and patient stratification in GC.
In this study, our objectives were to: (1) Assess the clinicopathological differences between Laurén subtypes; (2) Identify and compare the clinicopathological risk factors for recurrence and cancer-specific death of patients with intestinal and diffuse-type GC; and (3) Develop specific cost-effective prognostic scores for overall survival (OS) and disease-free survival (DFS) for patients with intestinal and diffuse GC. As far as we know, no other study has developed specific clinicopathological prognostic scores for patients with intestinal and diffuse-type GC.
MATERIALS AND METHODS
Study design
This is a retrospective study including all patients undergoing surgery for GC at a tertiary referral hospital in Madrid (Spain) from 2001 to 2019. All tumors were treated by total or subtotal gastrectomy with D1 or D2 lymphadenectomy. Clinical records were reviewed and demographic, clinical and follow-up information was retrieved including age at diagnosis, sex, symptoms (local and systemic), smoking and drinking habits, treatment, tumor recurrence and cause of death. Gross findings (tumor size, tumor location, macroscopic type) were collected from the database of the Department of Surgical Pathology (PatWin). The study was reviewed and approved by the institutional review board of the hospital.
Histopathological study
All tumors were formalin-fixed and embedded in paraffin. Slides were reviewed by two independent pathologists following a detailed protocol for histologic evaluation. Discordant cases were conjointly reviewed. Main microscopic features were assessed including tumor type (Laurén classification), histologic grade, presence of signet-ring cells (independently of the percentage of signet-ring cells), tumor budding, perineural infiltration, lymphovascular invasion, growth pattern (expansive or infiltrative), desmoplasia, necrosis, surgical margins, tumor depth (T stage), number of lymph node (LN) dissected, number of metastatic LN and lymph node ratio (LNR). The LNR was defined as the ratio between the number of metastatic LN and the total number of LN retrieved from the resection specimen. LNR was treated as a quantitative variable. All cases were staged according to the 8th edition of the American Joint Committee on Cancer tumor, node, metastasis (TNM) classification of GC[20].
Immunohistochemical study
Five tissue microarrays (TMAs) including a subgroup of cases from the GC cohort were assembled for immunohistochemical (IHC) analysis. TMAs were constructed using the MTA-1 tissue arrayer (Beecher Instruments, Sun Prairie, United States) and contained 2 cores per case (diameter: 1 mm). Representative areas were pre-selected by a pathologist and corresponded to the center and leading edge of each tumor. Cores were punched out from the donor block and transferred into a TMA. 2 μm sections were obtained from the TMA block for IHC study. Slides were deparaffinized by incubation at 60ºC for 10 min and incubated with Dako PT-Link for 20 min at 95ºC in a high pH buffered solution. Holders were incubated with peroxidase blocking reagent (Dako, Denmark) to block endogenous peroxidase. Sections were incubated for 20 min with the primary antibodies followed by incubation with the appropriate anti-Ig horseradish peroxidase-conjugated polymer (EnVision, Dako, Denmark) to detect antigen- antibody reaction. Then, biopsies were visualized with 3,3’-diaminobenzidine as a chromogen for 5 min and counterstained with hematoxylin. Sections of the TMA block were immunostained for HERCEPTEST, MSH2, MSH6, MLH1 and PMS2 (all antibodies prediluted; Dako, Denmark). Positive and negative controls were included. IHC markers were evaluated by two experienced pathologists. Staining intensity, location and percentage of cells stained were assessed for all antibodies. For the aims of this study, HERCEPTEST was evaluated according to the CAP recommendations. MSI tumors showed complete loss of MLH1, MSH2, MSH6 and/or PMS2 IHC expression.
Inclusion criteria
We reviewed all GC resected in our institution between 2001 and 2019. After data collection, patients receiving neoadjuvant therapy, metastatic tumors at diagnosis, patients with R1 or R2 resections and tumors of the mixed type were excluded from the study.
Statistical analysis
All data was stored in an anonymized Excel file and analyzed using IBM SPSS statistics (V20). Qualitative variables were described as frequencies. Quantitative variables were expressed as mean ± SD or median with range, as appropriate. For the analysis of the association between variables, we have performed χ2 (chi)-squared test for qualitative data and Student’s t-test for dichotomic quantitative variables. Statistical significance was settled at a P value < 0.05.
Multivariate analyses were performed by Cox regression (backward stepwise method), and regression models were adjusted for potential confounders. Two models (OS and DFS) were calculated for each Laurén subtype.
Prognostic scores for tumor progression and death were developed based on the hazard ratios of significant independent prognostic factors, as seen in other studies[21]. Two prognostic scores (one for recurrence and one for cancer-specific death) were constructed for each Laurén subtype.
OS and DFS curves according to the prognostic scores were estimated by Kaplan Meier analysis and significance was tested with the log-rank test. Receiver operating characteristic curves for cancer-specific death and progression were plotted. The area under the curve (AUC) was calculated for each prognostic score.
RESULTS
A total of 377 GC were resected in our institution between 2001 and 2019. Final analyses included 286 patients with pure intestinal-type GC (n = 190) and diffuse-type GC (n = 96). Clinicopathological features of our cases are presented in Supplementary Table 1. Mean age at diagnosis was 72 years and most patients were symptomatic (90.2%). Mean tumor size was 43 mm and most lesions were located in the gastric antrum (56.1%) and body (34.5%). According to their macroscopic appearance, tumors were mainly fungoid (36%) or ulcerative (31.8%). Microscopically, 66.4% of GC were intestinal (n = 190) and 33.6% were diffuse (n = 96). 35.1%, 35.1% and 50.5% of cases showed vascular invasion, perineural infiltration and desmoplasia, respectively. IHC was performed in 172 GC (intestinal n = 107, diffuse n = 65): 28.5% were microsatellite unstable and most cases were HERCEPTEST 0 (91.9%).Patients were diagnosed at stages I (27.2%), II (31.8%) and III (40.5%). 18% of patients received adjuvant therapy. Mean follow-up was 46.5 mo (0-208 mo). During follow-up, 36.6% of tumors recurred and 26.8% of patients died due to tumor.
Clinicopathological differences between Laurén subtypes
Clinicopathological features of our cases depending on the Laurén subtype are summarized in Supplementary Table 1. Univariate analysis (Table 1) showed a significant association between Laurén subtypes and patient age, tumor depth, macroscopic type, local symptoms, necrosis, perineural infiltration, intratumoral inflammatory infiltration, desmoplasia, MSI, HERCEPTEST, T, N, LNR, adjuvant therapy, tumor recurrence and patient death. Diffuse tumors were diagnosed at advanced stages in younger patients with less local symptoms. They infiltrated deeper into the gastric wall, had flat morphology and higher rates of perineural infiltration. MSI was infrequent and HERCEPTEST was negative (0) in all cases. Adjuvant treatment was administered more frequently to patients with diffuse GC. Intestinal lesions were more frequently fungoid or polypoid, showed necrosis and desmoplasia, and were diagnosed at earlier stages. In respect of patient prognosis, diffuse GC was significantly associated with higher rates of tumor recurrence and cancer-specific death. Multivariate analysis is presented in Table 2. DFS and OS curves according to the Laurén classification are shown as Supplementary Figures 1 and 2, respectively.
Table 1.
Univariate analyses: Differences between intestinal and diffuse subtypes (Chi-squared and Student’s t tests)
|
Feature
|
P
value
|
OR, diffuse (95%CI)1
|
| Age | < 0.001 | |
| Depth | 0.004 | |
| Macroscopic type | 0.001 | |
| Polypoid | 0.86 (0.45-1.65) | |
| Flat | 3.16 (1.49-6.71) | |
| Ulcerative | 1.54 (0.9-2.65) | |
| Fungoid | 0.38 (0.21-0.69) | |
| Local symptoms | 0.004 | 0.4 (0.2-0.75) |
| Necrosis | 0.006 | 0.39 (0.2-0.77) |
| Perineural infiltration | < 0.001 | 2.98 (1.78-5) |
| Intratumoral inflammation | 0.054 | |
| No | 0.63 (0.28-1.38) | |
| Mild | 2.08 (1.07-4.03) | |
| High | 0.68 (0.38-1.2) | |
| Desmoplasia | < 0.001 | 0.29 (0.16-0.54) |
| Microsatellite instability | 0.023 | 0.16 (0.02-0.28) |
| HERCEPTEST 2+/3+ | 0.018 | 0.07 (0.02-0.24) |
| T stage | 0.02 | |
| T1 | 0.41 (0.19-0.88) | |
| T2 | 0.74 (0.39-1.4) | |
| T3 | 1.25 (0.76-2.05) | |
| T4 | 2.08 (1.08-3.99) | |
| N stage | 0.02 | |
| N0 | 0.5 (0.3-0.85) | |
| N1 | 0.8 (0.4-1.6) | |
| N2 | 1.93 (1.04-3.55) | |
| N3 | 1.62 (0.9-2.93) | |
| Metastatic lymph nodes | 0.005 | |
| Lymph node ratio | < 0.001 | |
| Adjuvant therapy | 0.022 | 2.14 (1.11-4.15) |
| Recurrence | < 0.001 | 2.63 (1.55-4.47) |
| Death | < 0.001 | 2.82 (1.56-5.09) |
Odds ratios have been calculated for diffuse vs intestinal subtype.
Table 2.
Multivariate analysis: Variables independently related to Laurén subtypes
| Factor | P value | HR |
95%CI for HR1
|
|
|
|
|
|
Lower
|
Upper
|
| Lymph node ratio | 0.012 | 9.463 | 1.655 | 54.091 |
| Depth | 0.037 | 1.108 | 1.006 | 1.220 |
| Desmoplasia | 0.014 | 0.323 | 0.131 | 0.798 |
| Macroscopy | ||||
| Polypoid | 0.011 | |||
| Flat | 0.006 | 10.002 | 1.928 | 51.895 |
| Ulcerative | 0.027 | 4.536 | 1.189 | 17.307 |
| Fungoid | 0.686 | 1.318 | 0.345 | 5.030 |
Hazard ratios have been calculated using the intestinal type as a reference.
Prognostic factors in intestinal and diffuse-type GC
Intestinal GC: Univariate analysis is summarized in Supplementary Table 2. Tumor recurrence was significantly associated with perineural infiltration, vascular invasion, T, N, TNM stage and LNR. The relationship between tumor budding or MSI and recurrence tended to be significant (P = 0.057 and 0.084, respectively). Death due to GC was significantly related to infiltrative growth pattern, vascular invasion, T, N, TNM stage and LNR. Presence of necrosis and MSI approached significance (P = 0.088 and 0.096, respectively). Multivariate analysis is presented in Table 3: LNR, vascular invasion and T stage were independent risk factors for tumor recurrence, whereas LNR and growth pattern were independently associated with tumor death.
Table 3.
Independent risk factors for tumor recurrence and cancer-specific death in intestinal and diffuse-type gastric cancer
| Dependent variable | Factor | P value | HR |
95%CI for HR
|
|
|
|
|
|
|
Lower
|
Upper
|
| Intestinal type GC | |||||
| Tumor death (OS) | LNR | 0.004 | 32.424 | 3.057 | 343.96 |
| Growth | 0.052 | ||||
| Expansive | |||||
| Infiltrative | 4.678 | 0.987 | 22.177 | ||
| Recurrence (DFS) | LNR | 0.03 | 3.758 | 1.138 | 12.411 |
| Vascular invasion | 0.005 | 2.829 | 1.379 | 5.806 | |
| T stage | 0.056 | ||||
| T1-2 | 1 | ||||
| T3-4 | 2.193 | 0.98 | 4.909 | ||
| Diffuse type GC | |||||
| Tumor death (OS) | LNR | 0.026 | 5.729 | 1.234 | 26.599 |
| Necrosis | 0.008 | 4.234 | 1.460 | 12.278 | |
| Recurrence (DFS) | Size | 0.001 | 1.018 | 1.005 | 1.030 |
| LNR | < 0.001 | 11.420 | 3.895 | 33.477 | |
DFS: disease-free survival; GC: Gastric cancer; LNR: Lymph node ratio; OS: overall survival.
Diffuse GC: Univariate analysis is summarized in Supplementary Table 2. GC recurrence was significantly related to tumor size, T, N, TNM stage and LNR; and death due to GC was significantly associated with tumor necrosis, presence of systemic symptoms, N, TNM stage and LNR. The association between cancer-specific death and vascular invasion, T stage and tumor size tended to be significant (P = 0.059, 0.058 and 0.08, respectively). Multivariate analysis (Table 3) identified tumor size and LNR as independent predictors of tumor recurrence. Necrosis and LNR were independent risk factors for death due to GC.
Prognostic scores for patients with intestinal and diffuse-type GC
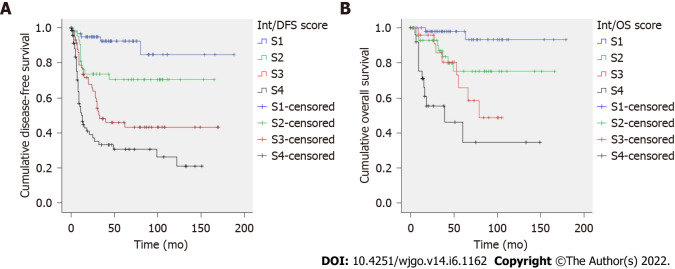
Intestinal GC: Two prognostic scores were constructed based on hazard ratios (Table 4). The recurrence score included T stage, LNR and vascular invasion; total score ranged from 0 to 9. Kaplan-Meier curves showed an excellent patient stratification into four prognostic groups (S1-S4, P < 0.001, Figure 1A). Mean DFS times were 161, 129, 83 and 61 mo for S1-S4 cases, respectively. The risk score for predicting cancer-specific death included LNR and growth pattern; total score ranged from 0 to 37. Cases were divided into four categories (S1-S4). This score showed a good prognostic performance by Kaplan-Meier analysis (P < 0.001, Figure 1B). Mean OS was 170, 132, 77 and 67 mo for S1-S4 patients. AUC values of the prognostic scores for recurrence and cancer-specific death were 0.745 and 0.763, respectively (Supplementary Table 3).
Table 4.
Prognostic scores for patients with intestinal-type gastric cancer
|
Dependent variable
|
Death
|
|
Recurrence
|
|
| Features | Growth | T stage | ||
| Expansive | 0 | T1-2 | 0 | |
| Infiltrative | 5 | T3-4 | 2 | |
| LNR | × 32 | LNR | × 4 | |
| Vascular invasion | 3 | |||
| Total score | 0-37 | 0-9 | ||
| Stages | ||||
| S1 | 0 | 0 | ||
| S2 | > 0-5 | > 0-2 | ||
| S3 | > 5-14 | > 2-< 5 | ||
| S4 | > 14 | 5-9 |
LNR: Lymph node ratio.
Figure 1.
Intestinal-type gastric cancer. A: Recurrence score; B: Cancer-specific death score. Kaplan-Meier curves of each prognostic group (S1-S4). P value by log-rank test was P < 0.001.
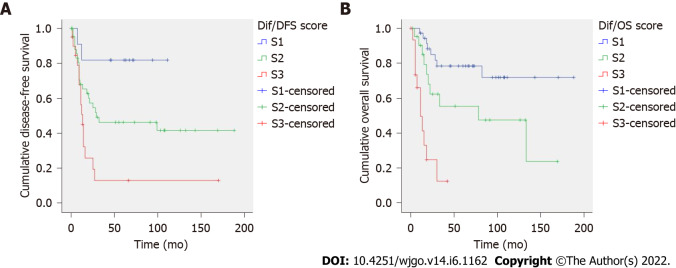
Diffuse GC: Prognostic scores for diffuse-type GC are presented in Table 5. The score for predicting tumor recurrence included tumor size and LNR; total score ranged from 0 to 120. The score for cancer-specific death included tumor necrosis and LNR; total score ranged from 0 to 10. Both prognostic scores showed an excellent risk stratification of patients into three groups (S1-S3, P < 0.005, Figure 2). Mean DFS was 93, 90 and 33 mo (S1-S3 patients) and mean OS was 145, 86 and 16 mo (S1-S3 patients). AUC values of the prognostic scores for recurrence and cancer-specific death were 0.674 and 0.710, respectively (Supplementary Table 3).
Table 5.
Prognostic scores for patients with diffuse-type gastric cancer
|
Dependent variable
|
Death
|
|
Recurrence
|
|
|
| Features | Necrosis | 4 | Size in mm | × 1 | |
| LNR | × 6 | LNR | × 11 | ||
| Total | 0-10 | 0-120 | |||
| Stages | |||||
| S1 | 0-< 1.5 | 0-20 | |||
| S2 | 1.5-4 | > 20-60 | |||
| S3 | > 4 | > 60 | |||
LNR: Lymph node ratio.
Figure 2.
Diffuse-type gastric cancer. A: Recurrence score, P value by log-rank test was P = 0.003; B: Cancer-specific death score, P value by log-rank test was P < 0.001. Kaplan-Meier curves of each prognostic group (S1-S3).
DISCUSSION
The global incidence of GC has been decreasing in recent years and this fact may be due to the detection and eradication of H. pylori and improvements in food preservation[22-24]. However, the relative incidence of diffuse GC is consequently increasing[25]. As previously mentioned, the Laurén system was developed in 1965 as a “histo-clinical classification”. After this first description of intestinal and diffuse-type GC, several studies have variably associated Laurén subtypes with clinicopathological features of GC, including patient age, sex or macroscopic morphology[7,8,26]. In our series, Laurén subtypes showed significant differences in age at diagnosis, tumor depth, macroscopic type, local symptoms, necrosis, perineural infiltration, intratumoral inflammatory infiltration, desmoplasia, T, N, LNR and administration of adjuvant therapy.
In respect of GC prognosis, the relationship between Laurén subtypes and patient outcomes is still controversial. In this study, we observed that diffuse GC showed higher rates of recurrence and cancer-specific death than intestinal tumors. Furthermore, in a previous study, we identified Laurén subtype as an independent prognostic factor for both DFS and OS in a subgroup of patients with GC from our institution[27]. Most authors have found that diffuse tumors are significantly and independently related to poor prognosis[10,28,29], but other studies have not confirmed these findings[30,31]. A recent meta-analysis including 73 publications and more than 61000 patients further confirmed the prognostic value of the Laurén classification[32].
The huge impact of technological advances on GC diagnosis and pathogenesis has led to the development of new molecular-based classifications[33]. However, molecular studies are expensive and these classifications have not been implemented in practice. The most important systems have been published by TCGA in 2014 and the Asian Cancer Research Group (ACRG) in 2015[13,34]. TCGA defined four subtypes: tumors positive for Epstein-Barr virus, MSI tumors, GS tumors and tumors with CIN[13]. ACRG divided GC into p53 active, p53 inactive, mesenchymal and MSI GC[34]. Most GS and mesenchymal tumors are diffuse and most cases of MSI and CIN GC are intestinal-type tumors[35]. Intestinal tumors are associated with MSI and show higher mutation rates and more copy-number alterations than diffuse-type GC. On the other hand, diffuse GC is related to CDH1 mutation, and approximately 9% of these tumors present MSI[36]. Recent studies have also shown that HER2 positivity is more frequently seen in intestinal-type GC[37,38]. Our results support these findings: we observed that intestinal-type tumors are associated with higher rates of MSI (34.6% vs 18.5%) and HERCEPTEST positivity. 6.7%, 3.8% and 3.8% of intestinal cases were 1+, 2+ and 3+, respectively, whereas all diffuse tumors were HERCEPTEST negative (0).
Laurén classification may also play a role in patient management and response to therapy[39]. Early GC can be treated by endoscopic resection and standard criteria include well or moderately differentiated GC confined to the mucosa, size ≤ 20 mm and absence of lymphatic or venous invasion[40,41]. Current expanded criteria for endoscopic submucosal dissection include the resection of high-grade GC ≤ 2 cm in size[42]. GC in stages IB-III is treated by gastrectomy with lymphadenectomy. Diffuse tumors may benefit from more aggressive surgical options and prevention or treatment of peritoneal metastases by HIPEC[43,44]. Regarding chemotherapeutic and radiotherapeutic regimens, most treatment protocols are based on the TNM classification. However, several authors have observed that treatment response may vary depending on the Laurén subtype and have suggested that diffuse tumors may be more resistant to standard chemotherapeutic agents than intestinal-type GC[39]. In a recent literature review, we summarized the main findings of clinical trials and comparative studies analyzing treatment regimens depending on the Laurén subtypes[11]. According to this review, studies on adjuvant therapy showed that intestinal-type GC is more chemo-sensitive than diffuse GC and treatment response may also vary depending on geographical features. The benefit of neoadjuvant therapy seems to be limited to patients with intestinal GC.
Clinicopathological prognostic features of GC patients have been previously analyzed in the literature[45]. Some authors have studied specific prognostic factors in younger patients or proximal tumors[46,47]. In this study, our second objective was to identify clinicopathological risk factors for tumor recurrence and cancer-specific death depending on the Laurén subtype. As might be expected, the TNM system was associated with tumor recurrence and death in both GC subtypes. But several differences were observed: vascular invasion, perineural infiltration and infiltrative growth pattern were important prognostic features in intestinal-type GC. On the contrary, tumor size and necrosis were significant prognosticators in diffuse-type GC. As for molecular features, we found that the relationship between MSI and prognosis tended to be significant only in intestinal-type tumors.
Finally, we constructed prognostic scores for predicting tumor recurrence and cancer-specific survival in patients with intestinal and diffuse-type GC. Our scores included only clinicopathological variables and can be easily calculated in clinical practice. All scores showed an excellent patient stratification into three (diffuse GC) or four (intestinal GC) prognostic groups by Kaplan-Meier analyses. Previous studies have developed predictive scores for GC patients and most of them included nutritional and laboratory findings[41,48]. Other authors have developed molecular signatures or scores including immunohistochemical parameters[49]. Recently, Bao et al[50] developed a three-gene signature for prognostic prediction in diffuse-type GC. Clinicopathological prognostic scores, although easy to apply, have been less frequently published[51]. As far as we know, no other study has developed separate clinicopathological risk scores for patients with intestinal and diffuse-type GC.
Strengths and limitations of our study
The results of this study should be interpreted in the context of its strengths and limitations. Strengths: This study includes patients with pure intestinal or diffuse GC treated by curative gastrectomy. Cases with neoadjuvant therapy, mixed tumors and R1-2 resections were excluded. All patients were diagnosed and treated in a western tertiary hospital. All tumors were reviewed and pathological features were independently assessed by two pathologists following a detailed protocol. Limitations: Retrospective study. GC is not frequent in western countries so this study includes less patients than Asian studies. IHC markers were performed in TMA sections and they may not represent the full heterogeneity of the tumor. In an attempt to overcome this limitation, cores were selected from the center and the leading edge of each case. Furthermore, no significant differences were observed between the two cores of each tumor.
CONCLUSION
In our series, intestinal and diffuse-type GC showed different epidemiological, clinical and prognostic features and they were associated with different risk factors for progression and death. Our specific prognostic scores for predicting tumor recurrence and cancer-specific survival in patients with intestinal and diffuse-type GC showed an excellent patient stratification into three (diffuse GC) or four (intestinal GC) prognostic groups.
Laurén classification is a cost-effective and widely implemented tool in GC and it has regained importance in the last few years due to its correlation with the molecular groups of GC. Our findings support the notion that Laurén subtypes may represent different clinicopathological and biological entities and the development of specific prognostic scores could be a useful and cost-effective strategy to improve risk assessment and patient stratification in GC. Our scores include clinicopathological variables easily available in practice and patients can be stratified according to their risk without complementary tests. However, our results should be externally validated and refined in other western and eastern cohorts of patients. Thus, more studies with a larger number of patients and other ethnic groups are needed in order to confirm the prognostic validity of the proposed prognostic scores and the current role of the Laurén classification in GC.
ARTICLE HIGHLIGHTS
Research background
In the molecular era, the Laurén system is still a cost-effective and widely implemented classification for gastric cancer (GC) and it has been recently associated with clinical, histological and molecular features of these tumors. Laurén subtypes have also shown differences in response to systemic therapy.
Research motivation
Despite recent advances in the understanding of the molecular biology of GC, there is a need to develop new prognostic tools for patient stratification in clinical practice. The implementation of specific scores for patients with intestinal and diffuse-type GC may significantly improve risk assessment and management of GC.
Research objectives
Our aims were to: (1) evaluate the clinicopathological differences between Laurén subtypes; (2) identify specific risk factors for these subtypes; and (3) develop prognostic scores for patients with intestinal and diffuse-type GC.
Research methods
This is a retrospective study of all patients undergoing surgery for GC at a tertiary referral center from 2001 to 2019. Clinical data and gross findings were collected. Histological and immunohistochemical features were assessed by two independent pathologists and prognostic scores were developed based on hazard ratios.
Research results
In our series of western patients with GC, intestinal and diffuse-type tumors showed distinctive epidemiological, clinical and prognostic features. In addition, Laurén subtypes were associated with different risk factors for tumor progression and cancer-specific death. Our prognostic scores for predicting overall survival and disease-free survival in patients with intestinal and diffuse-type GC included clinicopathological variables that can be easily calculated in clinical practice and showed an excellent patient stratification into three (diffuse GC) or four (intestinal GC) prognostic groups.
Research conclusions
The stratification of GC patients depending on Laurén subtypes and the implementation of specific clinicopathological prognostic scores in intestinal and diffuse-type tumors can be useful for patient stratification, risk assessment and treatment selection.
Research perspectives
Our prognostic scores should be externally validated in patients from both western and eastern countries due to the geographical variation of GC. In addition, this study opens a door to the development and implementation of cost-effective and specific clinicopathological prognostic scores in patients with GC in different contexts.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of the Hospital Clínico San Carlos.
Conflict-of-interest statement: We have no financial relationships to disclose.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Spanish Society of Pathology, No. 3147; Spanish Society for Documentation and Scientific Information, No. 3982.
Peer-review started: December 30, 2021
First decision: March 13, 2022
Article in press: May 27, 2022
Specialty type: Pathology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Demma JA, Israel; Lee HJ, South Korea; Rahimi-Movaghar E, Iran A-Editor: Lin FY, China S-Editor: Zhang H L-Editor: Filipodia CL P-Editor: Zhang H
Contributor Information
Cristina Díaz del Arco, Department of Surgical Pathology, Hospital Clínico San Carlos, Madrid 28040, Spain; Department of Legal Medicine, Psychiatry and Pathology, Complutense University of Madrid, Madrid 28040, Spain. crisdelarco@gmail.com.
Lourdes Estrada Muñoz, Department of Surgical Pathology, Hospital Rey Juan Carlos, Madrid 28933, Spain; Department of Basic Medical Sciences, Rey Juan Carlos University, Madrid 28933, Spain.
Luis Ortega Medina, Department of Surgical Pathology, Hospital Clínico San Carlos, Madrid 28040, Spain; Department of Legal Medicine, Psychiatry and Pathology, Complutense University of Madrid, Madrid 28040, Spain.
Elena Molina Roldán, Department of Surgical Pathology, Hospital Clínico San Carlos, Madrid 28040, Spain.
M Ángeles Cerón Nieto, Department of Surgical Pathology, Hospital Clínico San Carlos, Madrid 28040, Spain.
Soledad García Gómez de las Heras, Department of Basic Medical Sciences, Rey Juan Carlos University, Madrid 28933, Spain.
M Jesús Fernández Aceñero, Department of Surgical Pathology, Hospital Clínico San Carlos, Madrid 28040, Spain; Department of Legal Medicine, Psychiatry and Pathology, Complutense University of Madrid, Madrid 28040, Spain.
Data sharing statement
Data will be available on request.
References
- 1.Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26–38. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 4.Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251–261. doi: 10.3978/j.issn.2078-6891.2012.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S. Pathohistological classification systems in gastric cancer: diagnostic relevance and prognostic value. World J Gastroenterol. 2014;20:5679–5684. doi: 10.3748/wjg.v20.i19.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 7.Bringeland EA, Wasmuth HH, Mjønes P, Myklebust TÅ, Grønbech JE. A population-based study on incidence rates, Lauren distribution, stage distribution, treatment, and long-term outcomes for gastric adenocarcinoma in Central Norway 2001-2011. Acta Oncol. 2017;56:39–45. doi: 10.1080/0284186X.2016.1227086. [DOI] [PubMed] [Google Scholar]
- 8.Cheng HF, Huang KH, Chen MH, Fang WL, Lin CH, Chao Y, Lo SS, Li AFY, Wu CW, Shyr YM. The clinicopathological characteristics and genetic alterations of gastric cancer patients according to the Lauren classification. Int Surg. 2020;12:18137–18150. doi: 10.18632/aging.103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Zhu X, Wang Y, Wang R, Wang L, Zhu ML, Zheng L. Prognostic value and association of Lauren classification with VEGF and VEGFR-2 expression in gastric cancer. Oncol Lett. 2019;18:4891–4899. doi: 10.3892/ol.2019.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YC, Fang WL, Wang RF, Liu CA, Yang MH, Lo SS, Wu CW, Li AF, Shyr YM, Huang KH. Clinicopathological Variation of Lauren Classification in Gastric Cancer. Pathol Oncol Res. 2016;22:197–202. doi: 10.1007/s12253-015-9996-6. [DOI] [PubMed] [Google Scholar]
- 11.Díaz Del Arco C, Ortega Medina L, Estrada Muñoz L, García Gómez de Las Heras S, Fernández Aceñero MJ. Is there still a place for conventional histopathology in the age of molecular medicine? Histol Histopathol. 2021;36:587–613. doi: 10.14670/HH-18-309. [DOI] [PubMed] [Google Scholar]
- 12.Chia NY, Tan P. Molecular classification of gastric cancer. Ann Oncol. 2016;27:763–769. doi: 10.1093/annonc/mdw040. [DOI] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J, Shen H, Kapesa L, Zeng S. Lauren classification and individualized chemotherapy in gastric cancer. Oncol Lett. 2016;11:2959–2964. doi: 10.3892/ol.2016.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serra O, Galán M, Ginesta MM, Calvo M, Sala N, Salazar R. Comparison and applicability of molecular classifications for gastric cancer. Cancer Treat Rev. 2019;77:29–34. doi: 10.1016/j.ctrv.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Charalampakis N, Economopoulou P, Kotsantis I, Tolia M, Schizas D, Liakakos T, Elimova E, Ajani JA, Psyrri A. Medical management of gastric cancer: a 2017 update. Cancer Med. 2018;7:123–133. doi: 10.1002/cam4.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stiekema J, Cats A, Kuijpers A, van Coevorden F, Boot H, Jansen EP, Verheij M, Balague Ponz O, Hauptmann M, van Sandick JW. Surgical treatment results of intestinal and diffuse type gastric cancer. Implications for a differentiated therapeutic approach? Eur J Surg Oncol. 2013;39:686–693. doi: 10.1016/j.ejso.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Rawicz-Pruszyński K, Mielko J, Pudło K, Lisiecki R, Skoczylas T, Murawa D, Polkowski WP. Yield of staging laparoscopy in gastric cancer is influenced by Laurén histologic subtype. J Surg Oncol. 2019;120:1148–1153. doi: 10.1002/jso.25711. [DOI] [PubMed] [Google Scholar]
- 19.Wang K, Li E, Busuttil RA, Kong JC, Pattison S, Sung JJY, Yu J, El-Omar EM, Simpson JA, Boussioutas A. A cohort study and meta-analysis of the evidence for consideration of Lauren subtype when prescribing adjuvant or palliative chemotherapy for gastric cancer. Ther Adv Med Oncol. 2020;12:1758835920930359. doi: 10.1177/1758835920930359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amin M, Edge S, Greene F, Byrd D, Brookland R, Washington M, Gershenwald J, Compaton C, Hess K. AJCC Cancer Staging Manual. 8th ed. American Joint Committee on Cancer, 2017. [Google Scholar]
- 21.Hall RE, Porter J, Quan H, Reeves MJ. Developing an adapted Charlson comorbidity index for ischemic stroke outcome studies. BMC Health Serv Res. 2019;19:930. doi: 10.1186/s12913-019-4720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150:1113–1124.e5. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 23.Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J. 2014;55:621–628. doi: 10.11622/smedj.2014174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petryszyn P, Chapelle N, Matysiak-Budnik T. Gastric Cancer: Where Are We Heading? Dig Dis. 2020;38:280–285. doi: 10.1159/000506509. [DOI] [PubMed] [Google Scholar]
- 25.Assumpção PP, Barra WF, Ishak G, Coelho LGV, Coimbra FJF, Freitas HC, Dias-Neto E, Camargo MC, Szklo M. The diffuse-type gastric cancer epidemiology enigma. BMC Gastroenterol. 2020;20:223. doi: 10.1186/s12876-020-01354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JC, Lee YC, Kim JH, Kim YJ, Lee SK, Hyung WJ, Noh SH, Kim CB. Clinicopathological aspects and prognostic value with respect to age: an analysis of 3,362 consecutive gastric cancer patients. J Surg Oncol. 2009;99:395–401. doi: 10.1002/jso.21281. [DOI] [PubMed] [Google Scholar]
- 27.Díaz Del Arco C, Estrada Muñoz L, Molina Roldán E, Ortega Medina L, García Gómez de Las Heras S, Chávez Á, Fernández Aceñero M. Proposal for a clinicopathological prognostic score for resected gastric cancer patients. Saudi J Gastroenterol. 2021;27:44–53. doi: 10.4103/sjg.SJG_208_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu MZ, Cai MY, Zhang DS, Wang ZQ, Wang DS, Li YH, Xu RH. Clinicopathological characteristics and prognostic analysis of Lauren classification in gastric adenocarcinoma in China. J Transl Med. 2013;11:58. doi: 10.1186/1479-5876-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Xing XM, Ma LN, Liu L, Hao J, Feng LX, Yu Z. Metastatic lymph node ratio and Lauren classification are independent prognostic markers for survival rates of patients with gastric cancer. Oncol Lett. 2018;15:8853–8862. doi: 10.3892/ol.2018.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pyo JH, Ahn S, Lee H, Min BH, Lee JH, Shim SG, Choi MG, Sohn TS, Bae JM, Kim KM, Yeon S, Jung SH, Kim JJ, Kim S. Clinicopathological Features and Prognosis of Mixed-Type T1a Gastric Cancer Based on Lauren's Classification. Ann Surg Oncol. 2016;23:784–791. doi: 10.1245/s10434-016-5549-9. [DOI] [PubMed] [Google Scholar]
- 31.Lee JY, Gong EJ, Chung EJ, Park HW, Bae SE, Kim EH, Kim J, Do YS, Kim TH, Chang HS, Song HJ, Choe J, Jung HY. The Characteristics and Prognosis of Diffuse-Type Early Gastric Cancer Diagnosed during Health Check-Ups. Gut Liver. 2017;11:807–812. doi: 10.5009/gnl17033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrelli F, Berenato R, Turati L, Mennitto A, Steccanella F, Caporale M, Dallera P, de Braud F, Pezzica E, Di Bartolomeo M, Sgroi G, Mazzaferro V, Pietrantonio F, Barni S. Prognostic value of diffuse versus intestinal histotype in patients with gastric cancer: a systematic review and meta-analysis. J Gastrointest Oncol. 2017;8:148–163. doi: 10.21037/jgo.2017.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gullo I, Carneiro F, Oliveira C, Almeida GM. Heterogeneity in Gastric Cancer: From Pure Morphology to Molecular Classifications. Pathobiology. 2018;85:50–63. doi: 10.1159/000473881. [DOI] [PubMed] [Google Scholar]
- 34.Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Kim KM. Biomarkers for gastric cancer: molecular classification revisited. Precis Futur Med. 2017;1:59–68. [Google Scholar]
- 36.Hirotsu Y, Mochizuki H, Amemiya K, Ohyama H, Yoshimura D, Amano H, Miura Y, Ashizawa H, Nakagomi K, Takaoka S, Hosoda K, Suzuki Y, Oyama T, Hada M, Kojima Y, Omata M. Deficiency of mismatch repair genes is less frequently observed in signet ring cell compared with non-signet ring cell gastric cancer. Med Oncol. 2019;36:23. doi: 10.1007/s12032-019-1246-4. [DOI] [PubMed] [Google Scholar]
- 37.Baretton G, Kreipe HH, Schirmacher P, Gaiser T, Hofheinz R, Berghäuser KH, Koch W, Künzel C, Morris S, Rüschoff J Nicht-interventionelle Untersuchung (NIU) HER2 Study Group. HER2 testing in gastric cancer diagnosis: insights on variables influencing HER2-positivity from a large, multicenter, observational study in Germany. Virchows Arch. 2019;474:551–560. doi: 10.1007/s00428-019-02541-9. [DOI] [PubMed] [Google Scholar]
- 38.Wang HB, Liao XF, Zhang J. Clinicopathological factors associated with HER2-positive gastric cancer: A meta-analysis. Medicine (Baltimore) 2017;96:e8437. doi: 10.1097/MD.0000000000008437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiménez Fonseca P, Carmona-Bayonas A, Hernández R, Custodio A, Cano JM, Lacalle A, Echavarria I, Macias I, Mangas M, Visa L, Buxo E, Álvarez Manceñido F, Viudez A, Pericay C, Azkarate A, Ramchandani A, López C, Martinez de Castro E, Fernández Montes A, Longo F, Sánchez Bayona R, Limón ML, Diaz-Serrano A, Martin Carnicero A, Arias D, Cerdà P, Rivera F, Vieitez JM, Sánchez Cánovas M, Garrido M, Gallego J. Lauren subtypes of advanced gastric cancer influence survival and response to chemotherapy: real-world data from the AGAMENON National Cancer Registry. Br J Cancer. 2017;117:775–782. doi: 10.1038/bjc.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829–854. doi: 10.1055/s-0034-1392882. [DOI] [PubMed] [Google Scholar]
- 41.Gao Y, Huang D. The value of the systematic inflammation-based Glasgow Prognostic Score in patients with gastric cancer: a literature review. J Cancer Res Ther. 2014;10:799–804. doi: 10.4103/0973-1482.146054. [DOI] [PubMed] [Google Scholar]
- 42.Min YW, Min BH, Lee JH, Kim JJ. Endoscopic treatment for early gastric cancer. World J Gastroenterol. 2014;20:4566–4573. doi: 10.3748/wjg.v20.i16.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohli P, Penumadu P, Srinivas BH, M S, Dubashi B, Kate V, Kumar H, R K, Balasubramanian A. Clinicopathological profile and its association with peritoneal disease among gastric cancer patients. Surg Oncol. 2021;38:101595. doi: 10.1016/j.suronc.2021.101595. [DOI] [PubMed] [Google Scholar]
- 44.Lee JH, Chang KK, Yoon C, Tang LH, Strong VE, Yoon SS. Lauren Histologic Type Is the Most Important Factor Associated With Pattern of Recurrence Following Resection of Gastric Adenocarcinoma. Ann Surg. 2018;267:105–113. doi: 10.1097/SLA.0000000000002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potrc S, Gadiijev E, Hajdinjak T, Kavalar R. Clinicopathological and immunohistochemical markers after radical gastrectomy for gastric cancer. Hepatogastroenterology. 2007;54:308–314. [PubMed] [Google Scholar]
- 46.Santoro R, Carboni F, Lepiane P, Ettorre GM, Santoro E. Clinicopathological features and prognosis of gastric cancer in young European adults. Br J Surg. 2007;94:737–742. doi: 10.1002/bjs.5600. [DOI] [PubMed] [Google Scholar]
- 47.Erturk MS, Ciçek Y, Ersan Y, Saribeyoğlu K, Doğusoy G, Erginoz E. Analysis of clinicopathological prognostic parameters in adenocarcinoma of the gastric cardia. Acta Chir Belg. 2003;103:611–615. doi: 10.1080/00015458.2003.11679503. [DOI] [PubMed] [Google Scholar]
- 48.Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:1537–1549. doi: 10.1007/s00432-014-1714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bria E, De Manzoni G, Beghelli S, Tomezzoli A, Barbi S, Di Gregorio C, Scardoni M, Amato E, Frizziero M, Sperduti I, Corbo V, Brunelli M, Bersani S, Tortora G, Scarpa A. A clinical-biological risk stratification model for resected gastric cancer: prognostic impact of Her2, Fhit, and APC expression status. Ann Oncol. 2013;24:693–701. doi: 10.1093/annonc/mds506. [DOI] [PubMed] [Google Scholar]
- 50.Bao B, Zheng C, Yang B, Jin Y, Hou K, Li Z, Zheng X, Yu S, Zhang X, Fan Y, Qu X, Liu Y, Che X. Identification of Subtype-Specific Three-Gene Signature for Prognostic Prediction in Diffuse Type Gastric Cancer. Front Oncol. 2019;9:1243. doi: 10.3389/fonc.2019.01243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Díaz Del Arco C, Estrada Muñoz L, Ortega Medina L, Chávez Á, Ruiz Adelantado I, García Gómez de Las Heras S, Fernández Aceñero MJ. Update, validation and comparison of three different clinicopathological scores for patients with resected gastric cancer: A western experience. Ann Diagn Pathol. 2020;49:151635. doi: 10.1016/j.anndiagpath.2020.151635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available on request.