Abstract
Phosphate uptake by the phosphate-accumulating denitrifier Pseudomonas sp. JR12 was examined with different combinations of electron and carbon donors and electron acceptors. Phosphate uptake in acetate-supplemented cells took place with either oxygen or nitrate but did not take place when nitrite served as the final electron acceptor. Furthermore, nitrite reduction rates by this denitrifier were shown to be significantly reduced in the presence of phosphate. Phosphate uptake assays in the presence of the H+-ATPase inhibitor N,N′-dicyclohexylcarbodiimide (DCCD), in the presence of the uncoupler carbonyl cyanide 3-chlorophenylhydrazone (CCCP), or with osmotic shock-treated cells indicated that phosphate transport over the cytoplasmic membrane of this bacterium was mediated by primary and secondary transport systems. By examining the redox transitions of whole cells at 553 nm we found that phosphate addition caused a significant oxidation of a c-type cytochrome. Based on these findings, we propose that this c-type cytochrome serves as an intermediate in the electron transfer to both nitrite reductase and the site responsible for active phosphate transport. In previous studies with this bacterium we found that the oxidation state of this c-type cytochrome was significantly higher in acetate-supplemented, nitrite-respiring cells (incapable of phosphate uptake) than in phosphate-accumulating cells incubated with different combinations of electron donors and acceptors. Based on the latter finding and results obtained in the present study it is suggested that phosphate uptake in this bacterium is subjected to a redox control of the active phosphate transport site. By means of this mechanism an explanation is provided for the observed absence of phosphate uptake in the presence of nitrite and inhibition of nitrite reduction by phosphate in this organism. The implications of these findings regarding denitrifying, phosphate removal wastewater plants is discussed.
Biological phosphate removal from wastewater is a generally accepted, less costly alternative to chemical phosphate removal (27). Microorganisms involved in this process are capable of phosphate uptake in excess of their metabolic requirements and store phosphate internally as polyphosphate (polyP) polymers. Microorganisms thought to underlie phosphate removal in wastewater plants are collectively known as phosphate accumulating organisms (PAO). The general consensus concerning their metabolism is that they require alternating aerobic and anaerobic (or anoxic) conditions. Under such conditions phosphate is released in the anaerobic zone and stored as polyP in the aerobic or anoxic zone. Phosphorus is subsequently removed from the process stream by harvesting a fraction of the phosphorus-rich bacterial biomass (26). Isolation of bacterial isolates with all characteristics attributed to PAO have failed so far, and information on these organisms is based on studies with crude sludge samples or enrichment cultures obtained from biological phosphate removing plants. The lack of pure cultures hampers a basic understanding of this process, and for this reason the effects of environmental factors on phosphate removal by PAO, such as the presence of different electron acceptors and the type of organic carbon source, are only partially understood.
In a recent study (4), we demonstrated that the heterotrophic denitrifier Paracoccus denitrificans, as well other denitrifying isolates, exhibits the capability of polyP synthesis under either aerobic or anoxic conditions without the need for alternating aerobic and anaerobic (or anoxic) switches. Unlike PAO, these denitrifiers were unable to use polyhydroxyalkanoates as an energy source for polyP synthesis and derived energy from oxidation of external carbon sources. In preliminary studies with one of these denitrifiers, Pseudomonas sp. strain JR12, we found that phosphate uptake changed with the type of electron acceptor used. Whereas nitrate-respiring cells were capable of phosphate uptake, cells incubated with nitrite as the sole electron acceptor were not. The ability to respire on different electron acceptors provided a possibility to examine the link between the respiratory electron transfer and the phosphate uptake mechanism in this organism. In the present study, we report on a c-type cytochrome-mediated link between nitrite reduction and active phosphate transport in Pseudomonas sp. strain JR12. It is shown how, as a result of the presence of phosphate, nitrite may accumulate in cultures of Pseudomonas sp. strain JR 12. Nitrite is known to cause severe problems in biological processes, including those in wastewater treatment plants (18, 31). Particularly in phosphate-removing plants, due to the lack of phosphate-accumulating isolates, little information is available on the physiological mechanisms underlying nitrite accumulation and, hence, the importance of the present study.
MATERIALS AND METHODS
Organism and culture conditions.
Pseudomonas sp. strain JR12, previously described as Pseudomonas stutzeri (28), was isolated from a fluidized-bed reactor used for nitrate removal in intensive fish culture systems (1). The strain, deposited in the German Collection of Microorganisms and Cell Cultures (DSM 12019), reveals similarities to P. stutzeri in its fatty acid profile and metabolic properties. Partial sequencing of the 16S rRNA gene classified this bacterium in RNA group I with 99% analogy to Pseudomonas putida and 96% analogy to P. stutzeri. The bacterium was cultured in medium containing the following (per liter): Na-acetate · 3H2O, 5.67 g; NH4Cl, 1 g; MgSO4 · 7H2O, 0.6 g; KH2PO4, 0.4 g; Na2S2O3 · 5H2O, 0.1 g; CaCl2 · 2H2O, 0.07 g; Tris buffer (hydroxymethyl aminomethane), 12 g; and 2 ml of a trace mineral solution (30). The pH was adjusted to 7.0 with 6 N HCI.
Experimental protocol.
Studies were conducted with cells harvested during the late log phase of growth (after 4 to 5 days). Cells were washed twice and resuspended in the above-described medium with the following modifications. Phosphate was omitted or supplied to the medium as indicated, and either acetate or butyrate served as the electron and carbon donor while nitrate or nitrite served as electron acceptors (at concentrations indicated for each of the different experiments). Experiments were conducted in a temperature-controlled (30°C) incubation vessel (300 ml), placed on a magnetic stirrer and fitted with nitrate, pH, and oxygen-temperature electrodes. Anaerobic conditions in the vessel were obtained by continuous flushing with prepurified nitrogen gas. Overpressure within the incubation vessel prevented oxygen penetration, as verified by continuous oxygen monitoring of the medium in the absence of cells. Aerobic conditions were obtained by continuous flushing of the incubation vessel with compressed, sterile air. The experiments were initiated by adding acetate or butyrate. Periodically, samples were withdrawn for determination of ammonia, nitrite, nitrate, phosphate, and protein. The various incubations were conduced in triplicate. Since similar trends in changes of the examined parameters were observed in all replicates (analysis of covariance), results of only one run are depicted. An increase in pH (not exceeding 0.5 units) was measured in all experiments. Ammonia concentrations decreased in correspondence with the increase in bacterial biomass in the medium. Vmax values for nitrate and nitrite during each run were obtained by nonlinear regression analyses of at least 30 data points based on Michaelis-Menten kinetics using the Enzfitter software program (Elsevier-Biosoft, Amsterdam, The Netherlands).
Phosphate uptake assays with treated cells.
Phosphate uptake by cells incubated with the H+-ATPase inhibitor, N,N′-dicyclohexylcarbodiimide (DCCD), or with the uncoupler, carbonyl cyanide 3-chlorophenylhydrazone (CCCP), or by osmotic shock-treated cells were examined after EDTA treatment of the cells. Cells were three times washed with Tris buffer (pH = 7) containing 50 μg of chloramphenicol per ml. After 3 min of preincubation of cell suspension at 30°C, a 1 mM concentration of sodium EDTA (pH = 7) was added. After 10 min, MgSO4 was added to a final concentration of 10 mM. Hereafter, cells were washed once with Tris buffer (pH = 7) containing 50 μg of chloramphenicol per ml and 10 mM MgSO4, stored on ice, and assayed within 2 h. DCCD was added at a final concentration of 5 μM and CCCP was added at a final concentration of 3 μM. Osmotic shock treatment was conducted according to the method of Neu and Heppel (19). Cells (1 g, wet weight) were suspended in 80 ml of 20% sucrose–0.03 M Tris-HCl (pH 8, 24°C). Sodium EDTA (1 mM) was added, and the cell suspension was placed on a shaker (150 rpm) for 15 min. After centrifugation (13,000 × g at 4°C), the supernatant was removed and the thoroughly drained pellet was rapidly dissolved in a volume of cold water equal to that of the original volume of the suspension. The cell suspension was placed for 10 min in an ice bath situated on a rotary shaker. Hereafter, the cells were centrifuged (13,000 × g at 4°C) and resuspended in the experimental medium.
Cytochrome studies.
Cells cultured with acetate as the electron and carbon donor and nitrate as the electron acceptor were harvested in the late log phase of growth, washed with a 50 mM concentration of Tris buffer (pH 7.1), and resuspended in the same buffer at the specified concentration. Cytochrome c redox kinetics were performed in closed 3-ml cuvettes using a Hitachi (model U-3000) double-beam spectrophotometer. Changes in absorbance at 553 nm in the sample cuvette, containing acetate-supplemented cells, were recorded against a reference cuvette containing fully oxidized cells by the addition of solid ferricyanide. Antimycin A was dissolved in N,N-dimethylformamide (13 μM) and was added to a final concentration of 20 μg/ml.
Analytical procedures.
Total ammonia (NH3 and NH4+) was determined as described by Scheiner (23), nitrite was measured according to the method of Strickland and Parsons (24), nitrate was measured with a specific nitrate electrode (Radiometer, Copenhagen, Denmark) amplified with a pH meter (model PHM92; Radiometer), and phosphate (soluble orthophosphate) was measured according to the method of Golterman et al. (11). Protein was determined according to the method of Markwell et al. (17) with bovine serum albumin as the standard. Oxygen and temperature were measured with a YSI (model 57) temperature-oxygen probe (Yellow Springs Instruments).
Statistical analysis.
Triplicate runs were made for each experimental condition tested. Variations between the mean values of each of the runs were less than 10% as determined by the analysis of covariance.
RESULTS
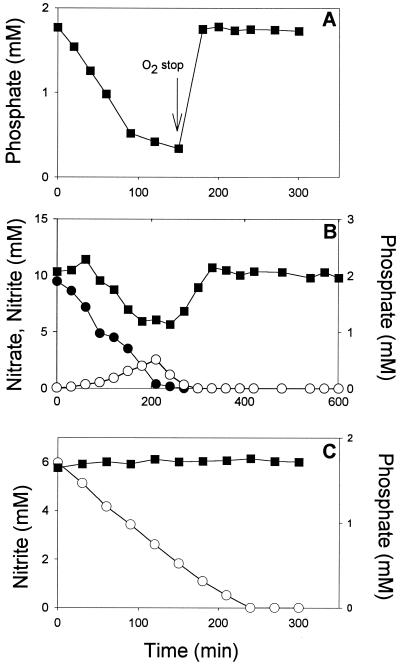
To test differences in phosphate uptake in the presence of different electron acceptors, phosphate uptake by Pseudomonas sp. strain JR 12 was examined in acetate-supplemented medium in the presence of oxygen or under anoxic conditions with either nitrate or nitrite as the electron acceptor (Fig. 1). It was found that phosphate was removed from the medium in the presence of oxygen (Fig. 1A) and nitrate (Fig. 1B) and was not removed when nitrite served as the final electron acceptor (Fig. 1C). Phosphate uptake with oxygen and nitrate took place in excess of the metabolic requirements of the cells since phosphate was released into the medium when the oxygen supply was stopped or when all nitrate was depleted (Fig. 1A and B, respectively). Phosphate had a profound effect on nitrite removal by the cells since intermediate nitrite accumulation during nitrate reduction by acetate-supplemented Pseudomonas sp. strain JR12 was considerably lower in medium devoid of phosphate than it was in medium with phosphate (not shown). Also, when nitrite was used as the sole electron acceptor, nitrite reduction was markedly depressed by the presence of phosphate (Table 1).
FIG. 1.
Changes in nitrate (●), nitrite (○), and phosphate (▪) upon incubation of Pseudomonas sp. strain JR12 (protein content, 0.3 g/liter) in phosphate-containing culture medium (see Materials and Methods) under aerobic conditions (arrow indicates time at which aeration was stopped) (A) and anoxic conditions with nitrate (B) or nitrite (C) as the final electron acceptors. Acetate (initial concentration, 15 mM) was used as the electron and carbon donor.
TABLE 1.
Effect of different phosphate concentrations on nitrite reduction rates by Pseudomonas sp. strain JR12a
| Phosphate concn (mM) | Nitrite reduction rate (mmol of nitrite/g of protein/min)b |
|---|---|
| 0 | 0.35 ± 0.02 A |
| 0.17 | 0.30 ± 0.08 A |
| 0.32 | 0.26 ± 0.02 B |
| 0.50 | 0.19 ± 0.01 C |
| 1.00 | 0.18 ± 0.03 C |
Cultures were incubated under anoxic conditions in culture medium (see Materials and Methods) at a protein concentration of 0.2 g/liter. Acetate (initial concentration, 10 mM) served as the electron and carbon donor, and nitrite (2 mM) served as the final electron acceptor. Results are presented as means ± standard deviations.
Differences found to be significant by Student's t test (P < 0.05) are followed by the same letter.
The observation that nitrate-respiring cells were capable of phosphate uptake despite the intermediate nitrite accumulation (>2 mM nitrite) in the medium (Fig. 1B) suggested that the lack of phosphate removal by nitrite-respiring cells was not caused by toxicity of nitrite exerted on the phosphate uptake mechanism. The observed lack of phosphate uptake in nitrite- but not in nitrate- or oxygen-respiring cells pointed to the involvement of the respiratory chain in phosphate uptake. To test this hypothesis, phosphate uptake assays were conducted in the presence of the H+-ATPase inhibitor DCCD. It was found that phosphate uptake by the Pseudomonas sp. strain JR 12 was an energy-dependent process, as phosphate uptake was severely inhibited when this inhibitor was added to nitrate-respiring cells (Table 2). Additional evidence for active phosphate uptake was obtained by the finding that phosphate uptake was significantly impaired in osmotic shock-treated cells (Table 2). Both treatments specifically affected phosphate uptake since cell respiration was only mildly affected as seen by the relatively small differences between nitrate removal rates of treated and control cells (Table 2). Addition of the uncoupler CCCP also resulted in reduced phosphate uptake; however, this reduction was less than that seen in DCCD or osmotic shock-treated cells (Table 2).
TABLE 2.
Effect of different treatments on phosphate uptake and nitrate reduction rates by Pseudomonas sp. strain JR12a
| Treatment | Phosphate uptake (%) | Nitrate reduction rate (mmol of nitrate/g of protein/min) |
|---|---|---|
| None (control) | 100 | 0.32 ± 0.03 |
| DCCD (0.005 mM) | 0 | 0.30 ± 0.01 |
| Osmotic shock | 15 | 0.29 ± 0.02 |
| CCCP (0.003 mM) | 64 | 0.27 ± 0.01 |
Pseudomonas sp. strain JR12 cells were pretreated as described in Materials and Methods and incubated (protein concentration, 0.15 g/liter) in culture medium (see Materials and Methods) supplemented with nitrate, acetate, and phosphate at initial concentrations of 10, 15, and 1.8 mM, respectively.
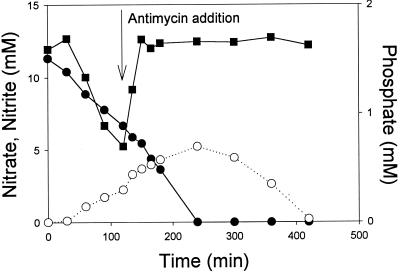
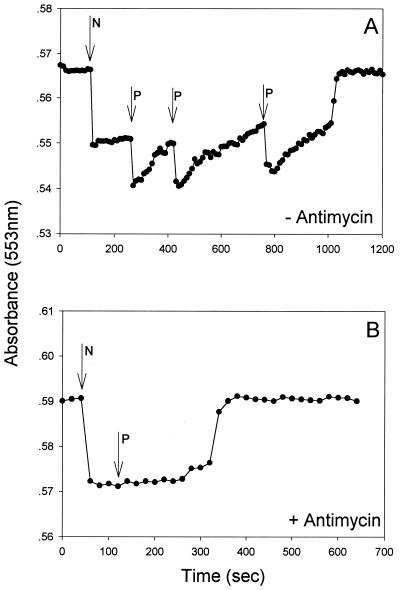
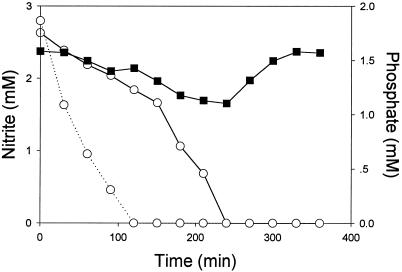
The effect of the acceptor controlled, electron transport mode and phosphate uptake were demonstrated by the following set of experiments. When antimycin A, a compound blocking the electron flow between cytochromes b and c (12), was added to acetate-supplemented cells respiring on nitrate, the cells shifted from phosphate uptake to phosphate release (Fig. 2). It was concluded, therefore, that energy transfer to cell components responsible for phosphate uptake occurred downstream of the antimycin A block. Upon examination of the oxidation state of cytochrome c in nitrate-respiring, acetate-supplemented cells, it was evident that phosphate addition caused a noticeable oxidation of this cytochrome c while no oxidation occurred when antimycin A was added to similar incubated cells (Fig. 3A and B, respectively). Additional evidence for the involvement of cytochrome c in phosphate uptake was provided with aerobically incubated cells, which shifted from phosphate uptake to phosphate release upon addition of antimycin A (not shown). We previously demonstrated (28) that butyrate-supplemented Pseudomonas sp. strain JR12 cells differ from acetate-supplemented ones in their electron transport mechanism. Whereas acetate donates electrons upstream in the electron transport chain, donation of electrons by butyrate takes place downstream in the cytochrome c region. An examination of the phosphate uptake characteristics of butyrate-supplemented cells revealed that phosphate uptake by these cells occurred not only with nitrate (not shown) but, in contrast to acetate-supplemented cells, also with nitrite as the sole electron acceptor (Fig. 4). However, as with acetate, nitrite reduction by butyrate-supplemented cells was affected by the presence of phosphate in the medium (Fig. 4).
FIG. 2.
Changes in nitrate (●), nitrite (○), and phosphate (▪) concentrations in anoxically incubated Pseudomonas sp. strain JR12 (protein content, 0.6 g/liter) in culture medium (see Materials and Methods) containing nitrate as the final electron acceptor and acetate (initial concentration, 10 mM) as the electron and carbon donor. At the indicated time antimycin A was added to the medium at a concentration of 20 μg/ml.
FIG. 3.
Absorbance at 553 nm of Pseudomonas sp. strain JR12 cultured in acetate-supplemented growth medium (see Materials and Methods), washed and resuspended in Tris buffer (pH 7.2) containing 5 mM acetate. Absorbance was monitored after the addition of nitrate (0.71 mmol of KNO3/liter) and phosphate (1.8 mmol of KH2PO4/liter) at the indicated times in the absence (A) or presence (B) of antimycin A (20 μg/ml). The absorbance was read against reference cuvettes containing ferricyanide-oxidized Pseudomonas sp. strain JR12 at the same density (protein content, 1.65 g/liter) as the sample cuvettes. Nitrate addition is indicated by N and phosphate additions are indicated by P.
FIG. 4.
Changes in nitrite (○) and phosphate (▪) concentrations in anoxically incubated Pseudomonas sp. strain JR12 (protein content, 0.4 g/liter) in culture medium (see Materials and Methods) in which nitrite served as the electron acceptor and butyrate (initial concentration, 5 mM) served as the carbon and electron donor. The dotted line indicates the decrease in nitrite concentrations during incubation of Pseudomonas sp. strain JR12 under similar conditions in medium devoid of phosphate.
DISCUSSION
Active phosphate uptake has been demonstrated in several denitrifying species (7, 16, 20, 21). Primary and secondary systems are responsible for the active transport of phosphate in these bacteria (29). In the present study, evidence for the activity of a primary, so called, phosphate-specific transport (Pst) system in Pseudomonas sp. strain JR12, was provided by the findings that (i) inorganic phosphate uptake was highly reduced by the H+-ATPase inhibitor DCCD and (ii) the uptake of phosphate was inactivated by osmotic shock, which is typical for all binding-protein-dependent systems but not for secondary transport systems (10). The presence of a secondary phosphate uptake system, driven by electrochemical ion (mostly proton) gradients over the cytoplasmic membrane (29), was demonstrated by the partial inhibition of phosphate uptake by cells incubated with the protonophore CCCP.
Very little information is available on the energy coupling between the respiratory chain and phosphate transport in bacteria. Our finding that nitrite-respiring cells, contrary to oxygen- and nitrate-respiring cells, were incapable of phosphate uptake provided a possible clue for a better understanding of the link between active phosphate uptake and the electron transport mode in this organism. Based on our results it can be concluded that phosphate uptake in excess of metabolic requirements takes place not only under conditions in which the cells are supplied with ample amounts of carbon and electron donors on one hand and electron acceptors on the other. Instead, the mode of electron transfer and in particular the involvement of specific redox carriers play an important role. This is illustrated in the present study by the finding that upon addition of antimycin A to oxygen- and nitrate-respiring cells, cells abruptly switched from phosphate uptake to phosphate release despite no noticeable changes in oxygen and nitrate respiration rates. This response can be explained by the finding that the activity of a c-type cytochrome, downstream of the antimycin A block, was an essential intermediate in the phosphate uptake by this organisms. The finding that acetate-supplemented, nitrite-respiring cells were incapable of phosphate uptake probably stems from the fact that under these specific conditions cytochrome c is impaired with respect to serving as an intermediate electron carrier in the phosphate uptake mechanism of this organism. In a previous study with Pseudomonas sp. strain JR12 (28), we found that the reduction state of cytochrome c changed with different combinations of electron donors and acceptors. More reduced cytochrome c was found in butyrate-supplemented, nitrite-respiring cells and acetate-supplemented, nitrate-respiring cells than in cells incubated with acetate and nitrite. Based on these results we suggest that at the relatively high oxidation state of cytochrome c in the latter cells, no electrons are shuttled to redox centers that operate at lower redox potentials and are involved in the active phosphate transport mechanism of this organism. A similar redox control was proposed to underlie the link between solute uptake and respiratory electron transfer in Escherichia coli (13). The observation that the presence of phosphate negatively affected the reduction rates of nitrite in butyrate-supplemented, nitrite-respiring cells and led to a relatively large accumulation of nitrite in acetate-supplemented, nitrate-respiring cells is a further indication for a cytochrome c-mediated link between the respiratory pathway and the phosphate uptake mechanism in this organism.
Several investigators (8, 15) found that nitrite impaired phosphate removal by enrichment cultures obtained from phosphate-removing wastewater plants. In these systems, nitrite accumulation often results from incomplete denitrification due to several factors—among those, the presence of oxygen (9, 14), the quantity and type of carbon source (28, 31), pH (2, 5, 25), light inhibition (3), and differential kinetics of nitrate and nitrite reductases (2, 6). In addition to denitrifiers, also nitrifiers may cause nitrite accumulation in wastewater treatment plants due to incomplete oxidation of ammonia to nitrate (22). A recent finding (18) with denitrifiers from such plants, in which the direct toxicity of nitrite was found responsible for impaired phosphate removal, could not be confirmed in the present study. We found that, despite the presence of nitrite, cells were capable of phosphate uptake when either acetate was replaced by butyrate or when, in addition to nitrite, nitrate was present in acetate-supplemented cells.
In conclusion, nitrite accumulation is a common phenomenon in wastewater plants as well as in other denitrifying environments. In this study we demonstrated that in the Pseudomonas isolate examined, the presence of phosphate inhibits nitrite reduction. Experimental evidence was provided for the role of a c-type cytochrome in both nitrite reduction and phosphate uptake in this bacterium. Based on this evidence we suggest that a cytochrome c-mediated electron shuttle to both nitrite reductase and a cytoplasmic active phosphate transport mechanism underlies the observed phosphate inhibition of nitrite reduction. To what extent these results are of wider significance in environments where both denitrification and phosphate removal occur, such as enhanced biological phosphate removal plants, remains to be examined.
REFERENCES
- 1.Aboutboul Y, Arbiv R, van Rijn J. Anaerobic treatment of fish culture effluents: volatile fatty acid mediated denitrification. Aquaculture. 1995;133:21–32. [Google Scholar]
- 2.Almeida J S, Reis M A M, Carrondo M J T. Competition between nitrate and nitrite reduction in denitrification by Pseudomonas fluorescens. Biotechnol Bioeng. 1995;46:476–484. doi: 10.1002/bit.260460512. [DOI] [PubMed] [Google Scholar]
- 3.Barak Y, Tal Y, van Rijn J. Light-mediated nitrite accumulation during denitrification by Pseudomonas sp. strain JR12. Appl Environ Microbiol. 1998;64:813–817. doi: 10.1128/aem.64.3.813-817.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barak Y, van Rijn J. Atypical polyphosphate accumulation by the denitrifying bacterium Paracoccus denitrificans. Appl Environ Microbiol. 2000;66:1209–1212. doi: 10.1128/aem.66.3.1209-1212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beccari M, Passino R, Ramadori R, Tandoi V. Kinetics of dissimilatory nitrate and nitrite reductase in suspended growth culture. J Water Pollut Control Fed. 1983;55:58–63. [Google Scholar]
- 6.Betlach M R, Tiedje J M. Kinetic explanation for accumulation of nitrite, nitric oxide and nitrous oxide during bacterial denitrification. Appl Environ Microbiol. 1981;42:1074–1084. doi: 10.1128/aem.42.6.1074-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnell J N, John P, Whatley F R. Phosphate transport in membrane vesicles of Paracoccus denitrificans. FEBS Lett. 1975;58:215–218. doi: 10.1016/0014-5793(75)80262-6. [DOI] [PubMed] [Google Scholar]
- 8.Comeau Y, Oldman W K, Hall K J. Proceedings of an IAWPRC specialized conference in Rome on biological phosphate removal in wastewaters. 1987. Dynamics of carbon reserves in biological dephosphatation of wastewater; pp. 39–55. [Google Scholar]
- 9.Coyne M S, Tiedje J M. Induction of denitrifying enzymes in oxygen limited Achromobacter cycloclastes continuous culture. FEMS Microbiol Ecol. 1990;73:263–270. [Google Scholar]
- 10.Furlong C E. Osmotic-shock-sensitive transport systems. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 1. Washington, D.C.: American Society for Microbiology; 1987. pp. 768–796. [Google Scholar]
- 11.Golterman H L, Clymo R S, Ohnstad M A M. Methods for physical and chemical analysis of fresh waters. IBP handbook no. 8, 2nd ed. Oxford, United Kingdom: Blackwell Scientific Publications; 1978. pp. 111–115. [Google Scholar]
- 12.Knobloch K, Ishaque M, Aleem M I. Oxidative phosphorylation in Micrococcus denitrificans under autotrophic conditions. Arch Microbiol. 1971;76:114–125. doi: 10.1007/BF00411785. [DOI] [PubMed] [Google Scholar]
- 13.Konings W N, Poolman B. Solute and ion transport across bacterial membranes. In: Torriani-Gorini A, Rothman F G, Silver S, Wright A, Yagil E, editors. Phosphate metabolism and cellular regulation in microorganisms. Washington, D.C.: American Society for Microbiology; 1987. pp. 197–204. [Google Scholar]
- 14.Korner H, Zumft W G. Expression of denitrification enzymes in response to the dissolved oxygen levels and respiratory substrate in continuous cultures of Pseudomonas stutzeri. Appl Environ Microbiol. 1989;137:74–78. doi: 10.1128/aem.55.7.1670-1676.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuba T, van Loosdrecht M C M, Heijnen J J. Phosphorus and nitrogen removal with minimal COD requirements by integration of denitrifying dephosphatation and nitrification in a two sludge system. Wat Res. 1996;30:1702–1710. [Google Scholar]
- 16.Lacoste A M, Cassaigne A, Neuzil E. Transport of inorganic phosphate in Pseudomonas aeruginosa. Curr Microbiol. 1981;6:115–120. [Google Scholar]
- 17.Markwell M A, Haas S M, Bieber L L, Tolbert N E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 18.Meinhold J, Arnold E, Isaacs S. Effect of nitrite on anoxic phosphate uptake in biological phosphorus removal activated sludge. Wat Res. 1999;33:1871–1883. [Google Scholar]
- 19.Neu H C, Heppel L A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965;240:3685–3692. [PubMed] [Google Scholar]
- 20.Nikata T, Sakai Y, Shibata K, Kato K, Kuroda A, Ohtaka H. Molecular analyses of the phosphate-specific transport (pst) operon of Pseudomonas aeruginosa. Mol Gen Genet. 1996;250:692–698. doi: 10.1007/BF02172980. [DOI] [PubMed] [Google Scholar]
- 21.Poole K, Hancock R E W. Phosphate-starvation-induced outer membrane proteins of members of the families Enterobacteriaceae and Pseudomonodaceae: demonstration of immunological cross-reactivity with an antiserum specific for porin protein P of Pseudomonas aeruginosa. J Bacteriol. 1986;165:987–993. doi: 10.1128/jb.165.3.987-993.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma B, Ahlert R C. Nitrification and nitrogen removal. Wat Res. 1977;11:897–925. [Google Scholar]
- 23.Scheiner D. Determinations of ammonia and Kjeldahl nitrogen by the indophenol method. Wat Res. 1976;10:31–36. [Google Scholar]
- 24.Strickland J D, Parsons T R. A practical handbook of seawater analysis. Bull Fish Res Bd Can. 1968;167:77–80. [Google Scholar]
- 25.Thomsen J K, Geest T, Cox R P. Mass spectrometric studies of the effect of pH on the accumulation of intermediates in denitrification by Paracoccus denitrificans. Appl Environ Microbiol. 1994;60:536–541. doi: 10.1128/aem.60.2.536-541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toerien D F, Gerber A, Lotter L H, Cloete T E. Enhanced biological phosphorus removal in activated sludge systems. Advances Microb Ecol. 1990;11:173–219. [Google Scholar]
- 27.van Loosdrecht M C M, Hooijmans C M, Brdjanovic D, Heijnen J J. Biological phosphate removal processes. Appl Microbiol Biotechnol. 1997;48:289–296. [Google Scholar]
- 28.van Rijn J, Tal Y, Barak Y. Influence of volatile fatty acids on nitrite accumulation by a Pseudomonas stutzeri strain isolated from a denitrifying fluidized bed reactor. Appl Environ Microbiol. 1996;62:2615–2620. doi: 10.1128/aem.62.7.2615-2620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Veen H W. Phosphate transport in prokaryotes: molecules, mediators and mechanisms. Antonie Leeuwenhoek. 1997;72:299–315. doi: 10.1023/a:1000530927928. [DOI] [PubMed] [Google Scholar]
- 30.Visniac W, Santer M. The Thiobacilli. Bacteriol Rev. 1975;21:195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilderer P A, Jones W L, Dau U. Competition in denitrification systems affecting reduction rate and accumulation of nitrite. Wat Res. 1987;21:239–245. [Google Scholar]