Abstract
Objective
High-sensitivity C-reactive protein (hs-CRP) is an inflammatory marker. This study aimed to identify the correlation between hs-CRP levels and diabetic kidney disease (DKD) in patients with type 2 diabetes mellitus (T2DM).
Materials/Methods
This cross-sectional and observational study included 927 patients with T2DM. We collected the data of patients based on their medical data, including sociodemographic characteristics, concomitant diseases, laboratory results, and medical therapy. Multivariate logistic regression analysis was conducted to assess the relationship between hs-CRP levels and DKD. A restricted cubic spline (RCS) was used to assess the correlation of hs-CRP levels on a continuous scale with the DKD.
Results
In total, 927 patients were recruited in our study. The median age of the recruited patients was 55 years, and there were 346 female patients and 581 male patients. The hs-CRP levels were evidently higher in patients with DKD than those without DKD. After adjusting for age, sex, diastolic blood pressure, systolic blood pressure, body mass index, neck circumference, waist circumference, hypertension, duration of diabetes, common carotid artery plaque, fasting plasma glucose, glycated hemoglobin, hemoglobin, erythrocyte, leukocyte, γ-glutamyl transferase, albumin, urea nitrogen, uric acid and triglyceride, a significant increase in the odds ratios (ORs) for DKD in the fourth hs-CRP quartile compared with the first quartile was observed (P value for trend= 0.003), and the ORs (95% confidence intervals) in the fourth quartile of hs-CRP were 1.968 (1.244–3.114) for DKD compared to the first quartile.. Moreover, the RCS curves presented a positive association between hs-CRP and DKD in total subjects, male subjects and female subjects, respectively.
Conclusions
The results of our study indicated that hs-CRP levels were significantly and positively correlated with the presence of DKD, which may provide predictive and diagnostic values in clinical practice.
Keywords: high-sensitivity C-reactive protein, diabetic kidney disease, type 2 diabetes mellitus, microvascular complications, inflammation
Introduction
Diabetes mellitus (DM) has become a major and serious threat to global human health. In the population aged 20 to 79 years, approximately 536.6 million people were diagnosed with DM worldwide in 2021, and this number will rapidly grow to 783.2 million in 2045 worldwide (1). Type 2 DM (T2DM) has 90% proportion of DM, which is featured by insulin resistance and relatively insufficient insulin secretion (2). T2DM can lead to many chronic complications, one of which is microvascular complications. In addition, approximately one in four patients with T2DM will have comorbid diabetic kidney disease (DKD) in the progression of T2DM (3). DKD is the most common cause of leading to end-stage renal disease worldwide (4, 5) and is a primary cause of mortality among patients with DM (4). Therefore, early intervention plays a vital role in preventing DKD development. To establish effective intervention strategies for DKD in clinical practice, identifying risk factors related to the presence of DKD is available (6).
The pathogenesis of DKD is multifactorial, where chronic inflammation and immune response are considered the major factors (7, 8). High-sensitivity C-reactive protein (hs-CRP) is a systemic inflammation marker that has been revealed to be correlated with DKD development (9, 10). CRP is a reactant and sensitive marker in acute-phase of inflammation, and its level increases sharply during the process of inflammation and recovers to normal level when inflammation has ceased (11, 12). Although several studies have indicated that elevated CRP levels are found in DKD (6, 13), the evidence of relationship between CRP and DKD is insufficient, and related studies are limited.
Therefore, we conducted this cross-sectional and observational study that aimed to identify the correlation between hs-CRP levels and DKD in patients with T2DM.
Materials and Methods
Study Design
This study was a cross-sectional and observational one comprising 927 patients with T2DM, and all the subjects were recruited from the National Metabolic Management Center in Shanghai General Hospital (Songjiang district). The study was approved by the Ethics Committee of Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine.
Patient Data Collection
All the data analyzed in this study were extracted from the National Metabolic Management Center. In total, 927 patients with a prior diagnosis of T2DM were included in this study. The inclusion criteria of this study were as follows: with a prior diagnosis of T2DM, the diagnostic criteria for T2DM that were in accordance with the guidelines of the 1999 World Health Organization criteria (14), age ≥18 years. The exclusion criteria were as follows: presence of chronic nephritis, presence of immune deficiency syndrome, pregnancy, presence of malignant tumor, receiving chemotherapy, presence of active infection, presence of acute diabetic complications, and missing data.
Study Variables
We collected data on patients from the National Metabolic Management Center based on their medical data, including sociodemographic characteristics, concomitant diseases, laboratory results, and medical therapy. The study variables were as follows: age; sex (male or female); educational level (under high school/high school or above); body mass index (BMI); diastolic blood pressure (DBP); systolic blood pressure (SBP); heart rate; head, neck, waist, and hip circumferences, duration of diabetes; hemoglobin, erythrocyte, leukocyte, albumin, urea nitrogen, creatinine, uric acid, triglyceride, total cholesterol, fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), alanine transaminase, aspartate aminotransferase, γ-glutamyl transferase (γ-GT), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) levels, urinary albumin-to-creatinine ratio (UACR), hs-CRP levels and estimated glomerular infiltration rate (eGFR), hypertension (no/yes), hyperlipidemia (no/yes), and common carotid artery (CCA) plaque (no/yes).
Definition of Variables
Hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg following repeated examination (15) or prior diagnosis of hypertension by a physician. Hyperlipidemia was defined as total cholesterol ≥ 240 mg/dL, triglycerides ≥ 200 mg/dL, LDL-C ≥ 160 mg/dL, and HDL-C < 40 mg/dL or prior diagnosis of hyperlipidemia by a physician. The scores of eGFR were computed by using the formula of Chronic Kidney Disease Epidemiology Collaboration (16). The UACR was calculated as the urinary albumin/creatinine ratio. DKD was defined as ACR ≥ 30 mg/g and/or eGFR < 60 mL/min/1.73 m2 (17).
Statistical Analyses
Data are presented as numbers or medians (interquartile range). Continuous variables with skewed distribution were analyzed using Mann–Whitney U test, and categorical variables were evaluated using the χ2 to identify intergroup differences. Multivariate logistic regression models were used to identify the independent effects of hs-CRP level on the presence of DKD. In the univariate analysis, variables with P value < 0.05 were enrolled in the multivariate logistic regression model. A two-sided P-value < 0.05 was regarded as that the difference has statistical significance between the two groups.
Moreover, multivariate logistic regression analysis was conducted to assess the relationship between hs-CRP levels and DKD, and the odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Model 1 was adjusted for age and sex. Model 2 was further adjusted for DBP, SBP, BMI, neck circumference, and waist circumference. Model 3 was further adjusted for hypertension, duration of diabetes, and CCA plaque. Model 4 was further adjusted for FPG, HbA1c, hemoglobin, erythrocyte, leukocyte, γ-GT, albumin, urea nitrogen, uric acid, and triglyceride.
The restricted cubic spline (RCS) with four knots at the 5th, 35th, 65th, and 95th percentiles was executed on a continuous scale with the presence of DKD. Furthermore, the associations between hs-CRP and DKD in different subgroups by grouping age, sex, BMI, hypertension, CCA plaque and HbA1c were identified by multivariate logistic regression models, and the ORs and 95% CI were calculated. In addition, the interaction between these subgroup variables and hs-CRP were calculated. All statistical analyses in our study were executed by IBM SPSS (version 25.0) and R statistical software (version 4.0.5).
Results
Characteristics of Study Subjects
A total of 927 patients were enrolled in our study. The median age of the recruited patients including 346 female patients and 581 male patients was 55 years, and there were 322 patients with DKD and 605 patients without DKD in our study. The characteristics of the patients with and without DKD were summarized in Table 1 . Age, sex, DBP, SBP, BMI, neck circumference, waist circumference, hypertension, duration of diabetes, CCA plaque, FPG, HbA1c, hemoglobin, erythrocyte, leukocyte, γ-GT, albumin, urea nitrogen, uric acid, triglyceride, hs-CRP, UACR and eGFR were significantly different between the two groups.
Table 1.
Characteristics of the study subjects.
| Variables | No DKD (n = 605; 65.26%) | DKD (n = 322; 34.74%) | P value |
|---|---|---|---|
| Age (years) | 54 (42-63) | 55 (46-65) | 0.017 |
| DBP (mmHg) | 76 (67-80) | 78 (70-84) | <0.001 |
| SBP (mmHg) | 120 (114-130) | 128(120-140) | <0.001 |
| Heart rate (beats per minute) | 78 (75-86) | 78 (76-88) | 0.072 |
| BMI (kg/m2) | 24.96 (23.00-27.35) | 25.80 (23.70-28.70) | <0.001 |
| Head circumference (cm) | 54 (52-56) | 54 (52-56) | 0.272 |
| Neck circumference (cm) | 39 (36-41) | 40 (37-42) | 0.007 |
| Waist circumference (cm) | 92 (85-97) | 93 (88-100) | 0.001 |
| Hip circumference (cm) | 96 (93-100) | 96 (93-101) | 0.106 |
| Duration of diabetes (months) | 61 (1-141) | 98 (12-171) | 0.001 |
| FPG (mmol/L) | 6.91 (5.62-8.53) | 7.67 (5.98-9.48) | <0.001 |
| HbA1c (%) | 8.50 (7.20-10.45) | 9.25 (7.50-11.00) | 0.002 |
| Hemoglobin (g/L) | 145 (132-154) | 137 (124-149) | <0.001 |
| Erythrocyte (×1012/L) | 4.75 (4.38-5.05) | 4.53 (4.14-4.92) | <0.001 |
| Leukocyte (×109/L) | 5.99 (5.09-7.07) | 6.54 (5.49-7.75) | <0.001 |
| ALT (IU/L) | 20 (14-35) | 20 (14-30) | 0.305 |
| AST (IU/L) | 18 (15-25) | 18 (15-25) | 0.365 |
| γ-GT (IU/L) | 24 (16-40) | 27 (18-41) | 0.003 |
| Albumin (g/L) | 43.2 (40.8-45.5) | 42.3 (39.3-45.0) | 0.001 |
| Urea nitrogen (mmol/L) | 5.22 (4.15-6.86) | 5.72 (4.45-7.70) | 0.007 |
| Creatinine (μmol/L) | 58.4 (47.8-67.5) | 59.4 (45.4-80.6) | 0.134 |
| Uric acid (μmol/L) | 317.0 (256.5-376.0) | 328.6 (277.0-401.3) | 0.002 |
| Triglyceride (mmol/L) | 1.58 (1.14-2.27) | 1.87 (1.40-2.77) | <0.001 |
| Total cholesterol (mmol/L) | 4.56 (3.71-5.23) | 4.69 (3.83-5.49) | 0.060 |
| HDL-C (mmol/L) | 0.91 (0.79-1.09) | 0.90 (0.78-1.05) | 0.366 |
| LDL-C (mmol/L) | 2.60 (1.91-3.17) | 2.57 (1.92-3.33) | 0.734 |
| Hs-CRP (mg/L) | 1.2 (0.5-2.6) | 1.9 (0.8-4.5) | <0.001 |
| UACR (μg/mg) | 12.99 (8.79-19.02) | 74.87 (41.88-244.78) | <0.001 |
| eGFR (mL/min per 1.73 m2) | 111.00 (101.15-122.95) | 105.68 (87.21-119.38) | <0.001 |
| Sex(male/female) | 402/203 | 179/143 | 0.001 |
| Educational level (under high school/high school or above) | 305/300 | 143/179 | 0.082 |
| Hypertension(no/yes) | 361/244 | 118/204 | <0.001 |
| Hyperlipidemia(no/yes) | 454/151 | 225/97 | 0.091 |
| CCA plaque(no/yes) | 344/261 | 147/175 | 0.001 |
Data were numbers or median (interquartile range). Continuous variables used Mann-Whitney U test and categorical variables used chi-squared test for comparing the baseline characteristics of patients with diabetic kidney disease and without diabetic kidney disease.
DBP, diastolic blood pressure; SBP, systolic blood pressure; BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; ALT, alanine transaminase; AST, aspartate aminotransferase; γ-GT, γ-glutamyl transferase; HDL-C - high-density lipoprotein cholesterol; LDL-C - low-density lipoprotein cholesterol; Hs - CRP, high-sensitivity C-reactive protein; UACR, urinary albumin to creatinine ratio; eGFR, estimated glomerular infiltration rate; CCA plaque, common carotid artery plaque; DKD, diabetic kidney disease.
Association Between hs-CRP Levels and DKD
Table 2 demonstrates that increased hs-CRP was correlated with higher odds of DKD after adjusting for several confounding factors. In our study, a significant increase in the ORs for DKD from the first to the fourth hs-CRP quartiles in the study subjects was observed after adjusting for age, sex, DBP, SBP, BMI, neck circumference, waist circumference, hypertension, duration of diabetes, and CCA plaque in the multivariate regression model (P value for trend< 0.001). When the multivariate regression model was further adjusted for FPG, HbA1c, hemoglobin, erythrocyte, leukocyte, γ-GT, albumin, urea nitrogen, uric acid and triglyceride, a similar increase in ORs for DKD from the first to the fourth hs-CRP quartiles was still observed (P value for trend = 0.003). Compared with the lowest quartile, the ORs (95% CIs) of the fourth quartile for DKD were 1.968 (95% CI, 1.244–3.114) after adjusting for age, sex, DBP, SBP, BMI, neck circumference, waist circumference, hypertension, duration of diabetes, CCA plaque, and FPG, HbA1c, hemoglobin, erythrocyte, leukocyte, γ-GT, albumin, urea nitrogen, uric acid and triglyceride.
Table 2.
Relations of high-sensitivity C-reactive protein with diabetic kidney disease in patients with type 2 diabetes mellitus.
| hs-CRP, mg/L | P value for trend | ||||
|---|---|---|---|---|---|
| Q1 (n=246) | Q2 (n=229) | Q3 (n=223) | Q4 (n=229) | ||
| Model 1 - OR (95% CI) | Ref. | 1.249 (0.832–1.875) | 1.718 (1.148–2.572) | 2.839 (1.914–4.210) | <0.001 |
| Model 2 - OR (95% CI) | Ref. | 1.153 (0.757–1.756) | 1.509 (0.988–2.305) | 2.472 (1.633–3.743) | <0.001 |
| Model 3 - OR (95% CI) | Ref. | 1.288 (0.836–1.984) | 1.568 (1.017–2.419) | 2.767 (1.805–4.243) | <0.001 |
| Model 4 - OR (95% CI) | Ref. | 1.169 (0.741–1.843) | 1.436 (0.909–2.270) | 1.968 (1.244–3.114) | 0.003 |
Model 1 was adjusted for age, sex. Model 2 was further adjusted for diastolic blood pressure, systolic blood pressure, body mass index, neck circumference and waist circumference. Model 3 was further adjusted for hypertension, duration of diabetes and common carotid artery plaque. Model 4 was further adjusted for fasting plasma glucose, glycated hemoglobin, hemoglobin, erythrocyte, leukocyte, γ-glutamyl transferase, albumin, urea nitrogen, uric acid and triglyceride. The quartile ranges of Q1, Q2, Q3, and Q4 of hs-CRP level were < 0.6, 0.6-1.4, 1.4-3.1,> 3.1 mg/L, respectively. Q1 is the reference group. Multivariate logistic regression analyses were performed to estimate the ORs and corresponding 95% CIs for diabetic kidney disease.
Q, quartile, OR, odds ratio, CI, confidence interval, hs - CRP, high-sensitivity C-reactive protein.
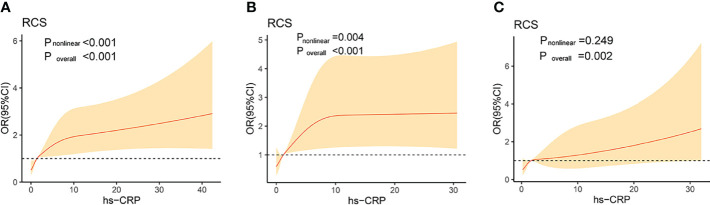
In our study, we further executed the RCS to flexibly model the correlation of hs-CRP on a continuous scale with DKD in total subjects, male subjects and female subjects, respectively. The RCS curves showed a positive association in total subjects (P for non-linearity <0.001), male subjects (P for non-linearity =0.004) and female subjects (P for non-linearity =0.249), respectively ( Figure 1 ).
Figure 1.
Association of high-sensitivity C-reactive protein levels on a continuous scale and diabetic kidney disease in total subjects (A), male subjects (B) and female subjects (C). The solid lines represent the odds ratios and the shaded areas represent the 95% confidence intervals. Model was adjusted for age, body mass index, neck circumference,waist circumference, hypertension, duration of diabetes, common carotid artery plaque, fasting plasma glucose, glycated hemoglobin. OR, odds ratio; CI, confidence interval; RCS, restricted cubic splines; hs - CRP, high-sensitivity C-reactive protein.
Subgroup Analyses
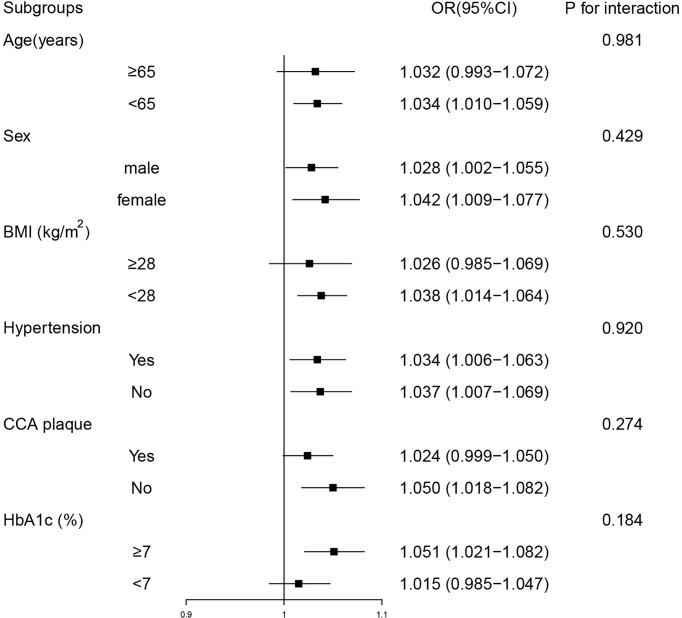
Subgroup analyses were performed to determinate the potential effect modifiers, and subjects were stratified by age (< 65 or ≥ 65 years), sex (male or female), BMI (< 28 or ≥ 28 kg/m2), hypertension (no or yes), CCA plaque (no or yes) and HbA1c (< 7 or ≥ 7%), respectively. The results of subgroup analyses showed that hs-CRP was positively correlated with DKD in most categories. No significant interaction was found between hs-CRP level and age, sex, BMI, hypertension, CCA plaque, or HbA1c, respectively ( Figure 2 ).
Figure 2.
Stratified analyses of the associations between high-sensitivity C-reactive protein levels and diabetic kidney disease. Model was adjusted for age, sex, body mass index, neck circumference,waist circumference, hypertension, duration of diabetes, common carotid artery plaque, fasting plasma glucose, glycated hemoglobin. Subgroup variable was excluded from the model. OR, odds ratio; CI, confidence interval; BMI, body mass index; CCA plaque, common carotid artery plaque; HbA1c, glycated hemoglobin.
Discussion
In this study, we identified the correlation between hs-CRP levels and DKD in patients with T2DM. We found that hs-CRP levels were evidently higher in subjects with DKD when compared with subjects with non-DKD. In addition, from the lowest quartile to highest quartile of hs-CRP levels, the ORs for DKD were significantly increase. Moreover, our results indicated a significant positive correlation between hs-CRP levels and DKD in the study subjects, which may provide scientific evidence for further basic and clinical research.
CRP levels are elevated in acute-phase inflammation. A previous study suggested that the normal population with elevated CRP levels have a higher risk of T2DM (18), and accumulated evidence suggests that circulating CRP levels have predictive value for the incidence of DM (18–20). Moreover, CRP levels were correlated with endothelial dysfunction among DM populations, and CRP can predict DM complications (19). In our study, we found a significantly positive correlation between hs-CRP levels and the presence of DKD in patients with T2DM, which is consistent with several published studies (10, 21, 22). However, the controversial view proposed by a previous study was that no correlation was found between hs-CRP levels and diabetic vascular complications (23), which may be inadequate for providing adequate evidence because of the limitations of the small sample size. A meta-analysis was executed to explore the correlation between the concentration of hs-CRP and DKD, and it was verified that elevated hs-CRP levels were correlated with the prevalence of DKD, and that hs-CRP levels can be a predictor of DKD in patients with DM (10). In addition, in the results of the RCS curves, although there was a significant positive correlation between hs-CRP and DKD after adjusting confounders, a difference was observed in female and male subjects with increasing hs-CRP levels, which may provide scientific evidence for further research.
The pathogenesis of DKD is multifactorial, and analysis studies of the genome-wide transcriptome have shown that there is a strong prevalence of inflammatory signaling pathways in DKD (24). Inflammation in the kidney activated by mononuclear phagocytic lineage cells is one of the most significant biological mechanisms underlying DKD (25–27), and several inflammatory cytokines, such as interleukin (IL)-1, IL-6, IL-18, tumor necrosis factor-α (TNF-α) and CRP, may lead to the pathogenesis of DKD (9, 28–32). As CRP is related with insulin resistance and hyperglycemia, higher CRP levels are correlated with the development of DKD by aggravating glycemic control in DM populations (33). Previous studies have indicated that the nuclear transcription factor-kappa B (NF-κB) signaling hs-CRP pathway is activated in DKD, and hs-CRP regulates many proinflammatory cytokines through the NF-κB signaling pathway (34, 35). Moreover, hs-CRP can also be induced by hyperglycemia, which accelerates inflammation in the kidneys (10). Therefore, hs-CRP level can serve as a predictor of DKD in T2DM populations.
This study has some limitations. First, the main limitation was its cross-sectional and observational nature, and longitudinal studies are necessary to test the association over time, and future prospective studies are necessary. Second, the sample size was small because all the data of this study were from a single hospital; therefore, the quality of the results may be negatively affected. Further studies with a larger population are required to examine this association. Third, our study did not explore the underlying mechanisms of hs-CRP and DKD, and whether high hs-CRP levels are a cause or result of DKD should be clarified. In addition, our study did not include several inflammatory cytokines, such as IL-6, IL-18, and TNF-α, which are correlated with DKD, which may be potential confounding factors affecting the results.
Conclusion
In our study, we found that hs-CRP levels were significantly higher in study subjects with DKD than in the subjects with non-DKD, and hs-CRP levels have been proven to be correlated with the presence of DKD in T2DM patients, which might provide predictive and diagnostic values in clinical practice.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine. Written informed consent was obtained from all participants.
Author Contributions
Study design: N-GF and Y-DP; Collection and assembly of data: MT and HC; Data analysis: MT and HC; data interpretation: N-GF and Y-DP; Manuscript writing: All authors; final approval of manuscript: All authors.
Funding
This study was supported by the National Natural Science Foundation of China (81870596, 81400785) and the Natural Science Foundation of Shanghai (21ZR1451200).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res Clin Pract (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results From the International Diabetes Federation Diabetes Atlas, 9(Th) Edition. Diabetes Res Clin Pract (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 3. Valencia WM, Florez H. How to Prevent the Microvascular Complications of Type 2 Diabetes Beyond Glucose Control. BMJ (Clin Res Ed.) (2017) 356:i6505. doi: 10.1136/bmj.i6505 [DOI] [PubMed] [Google Scholar]
- 4. Lin YC, Chang YH, Yang SY, Wu KD, Chu TS. Update of Pathophysiology and Management of Diabetic Kidney Disease. J Formosan Med Assoc = Taiwan yi zhi (2018) 117(8):662–75. doi: 10.1016/j.jfma.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 5. Chen C, Wang C, Hu C, Han Y, Zhao L, Zhu X, et al. Normoalbuminuric Diabetic Kidney Disease. Front Med (2017) 11(3):310–8. doi: 10.1007/s11684-017-0542-7 [DOI] [PubMed] [Google Scholar]
- 6. Hayashino Y, Mashitani T, Tsujii S, Ishii H. Serum High-Sensitivity C-Reactive Protein Levels are Associated With High Risk of Development, Not Progression, of Diabetic Nephropathy Among Japanese Type 2 Diabetic Patients: A Prospective Cohort Study (Diabetes Distress and Care Registry at Tenri [Ddcrt7]). Diabetes Care (2014) 37(11):2947–52. doi: 10.2337/dc14-1357 [DOI] [PubMed] [Google Scholar]
- 7. García-García PM, Getino-Melián MA, Domínguez-Pimentel V, Navarro-González JF. Inflammation in Diabetic Kidney Disease. World J Diabetes (2014) 5(4):431–43. doi: 10.4239/wjd.v5.i4.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donate-Correa J, Martín-Núñez E, Muros-de-Fuentes M, Mora-Fernández C, Navarro-González JF. Inflammatory Cytokines in Diabetic Nephropathy. J Diabetes Res (2015) 2015:948417. doi: 10.1155/2015/948417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kang ES, Kim HJ, Ahn CW, Park CW, Cha BS, Lim SK, et al. Relationship of Serum High Sensitivity C-Reactive Protein to Metabolic Syndrome and Microvascular Complications in Type 2 Diabetes. Diabetes Res Clin Pract (2005) 69(2):151–9. doi: 10.1016/j.diabres.2004.11.014 [DOI] [PubMed] [Google Scholar]
- 10. Liu Q, Jiang CY, Chen BX, Zhao W, Meng D. The Association Between High-Sensitivity C-Reactive Protein Concentration and Diabetic Nephropathy: A Meta-Analysis. Eur Rev Med Pharmacol Sci (2015) 19(23):4558–68. [PubMed] [Google Scholar]
- 11. Pepys MB, Hirschfield GM. C-Reactive Protein: A Critical Update. J Clin Invest (2003) 111(12):1805–12. doi: 10.1172/JCI200318921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verma S, Szmitko PE, Yeh ET. C-Reactive Protein: Structure Affects Function. Circulation (2004) 109(16):1914–7. doi: 10.1161/01.CIR.0000127085.32999.64 [DOI] [PubMed] [Google Scholar]
- 13. Menon V, Greene T, Wang X, Pereira AA, Marcovina SM, Beck GJ, et al. C-Reactive Protein and Albumin as Predictors of All-Cause and Cardiovascular Mortality in Chronic Kidney Disease. Kidney Int (2005) 68(2):766–72. doi: 10.1111/j.1523-1755.2005.00455.x [DOI] [PubMed] [Google Scholar]
- 14. Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, et al. The 1997 American Diabetes Association and 1999 World Health Organization Criteria for Hyperglycemia in the Diagnosis and Prediction of Diabetes. Diabetes Care (2000) 23(8):1108–12. doi: 10.2337/diacare.23.8.1108 [DOI] [PubMed] [Google Scholar]
- 15. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension (2020) 75(6):1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
- 16. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Diabetes Association. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2021. Diabetes Care (2021) 44(Suppl 1):S151–s67. doi: 10.2337/dc21-S011 [DOI] [PubMed] [Google Scholar]
- 18. Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, et al. Inflammatory Markers and Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Care (2013) 36(1):166–75. doi: 10.2337/dc12-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chuengsamarn S, Rattanamongkolgul S, Sittithumcharee G, Jirawatnotai S. Association of Serum High-Sensitivity C-Reactive Protein With Metabolic Control and Diabetic Chronic Vascular Complications in Patients With Type 2 Diabetes. Diabetes Metab synd (2017) 11(2):103–8. doi: 10.1016/j.dsx.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 20. Roy MS, Janal MN, Crosby J, Donnelly R. Plasma Markers of Inflammation and Prediction of Cardiovascular Disease and Mortality in African Americans With Type 1 Diabetes. Diabetes Res Clin Pract (2016) 114:117–25. doi: 10.1016/j.diabres.2015.12.014 [DOI] [PubMed] [Google Scholar]
- 21. Sinha SK, Shaheen M, Rajavashisth TB, Pan D, Norris KC, Nicholas SB. Association of Race/Ethnicity, Inflammation, and Albuminuria in Patients With Diabetes and Early Chronic Kidney Disease. Diabetes Care (2014) 37(4):1060–8. doi: 10.2337/dc13-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phosat C, Panprathip P, Chumpathat N, Prangthip P, Chantratita N, Soonthornworasiri N, et al. Elevated C-Reactive Protein, Interleukin 6, Tumor Necrosis Factor Alpha and Glycemic Load Associated With Type 2 Diabetes Mellitus in Rural Thais: A Cross-Sectional Study. BMC Endocr Disord (2017) 17(1):44. doi: 10.1186/s12902-017-0189-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jenkins AJ, Rothen M, Klein RL, Moller K, Eldridge L, Zheng D, et al. Cross-Sectional Associations of C-Reactive Protein With Vascular Risk Factors and Vascular Complications in the DCCT/EDIC Cohort. J Diabetes Complications (2008) 22(3):153–63. doi: 10.1016/j.jdiacomp.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 24. Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K. Transcriptome Analysis of Human Diabetic Kidney Disease. Diabetes (2011) 60(9):2354–69. doi: 10.2337/db10-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tuttle KR. Linking Metabolism and Immunology: Diabetic Nephropathy is an Inflammatory Disease. J Am Soc Nephrol (2005) 16(6):1537–8. doi: 10.1681/ASN.2005040393 [DOI] [PubMed] [Google Scholar]
- 26. Mora C, Navarro JF. Inflammation and Diabetic Nephropathy. Curr Diabetes Rep (2006) 6(6):463–8. doi: 10.1007/s11892-006-0080-1 [DOI] [PubMed] [Google Scholar]
- 27. Galkina E, Ley K. Leukocyte Recruitment and Vascular Injury in Diabetic Nephropathy. J Am Soc Nephrol (2006) 17(2):368–77. doi: 10.1681/ASN.2005080859 [DOI] [PubMed] [Google Scholar]
- 28. Shikata K, Makino H. Role of Macrophages in the Pathogenesis of Diabetic Nephropathy. Contrib Nephrol (2001), (134):46–54. doi: 10.1159/000060147 [DOI] [PubMed] [Google Scholar]
- 29. Moriwaki Y, Yamamoto T, Shibutani Y, Aoki E, Tsutsumi Z, Takahashi S, et al. Elevated Levels of Interleukin-18 and Tumor Necrosis Factor-Alpha in Serum of Patients With Type 2 Diabetes Mellitus: Relationship With Diabetic Nephropathy. Metabol Clin Exp (2003) 52(5):605–8. doi: 10.1053/meta.2003.50096 [DOI] [PubMed] [Google Scholar]
- 30. Wong CK, Ho AW, Tong PC, Yeung CY, Kong AP, Lun SW, et al. Aberrant Activation Profile of Cytokines and Mitogen-Activated Protein Kinases in Type 2 Diabetic Patients With Nephropathy. Clin Exp Immunol (2007) 149(1):123–31. doi: 10.1111/j.1365-2249.2007.03389.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shikano M, Sobajima H, Yoshikawa H, Toba T, Kushimoto H, Katsumata H, et al. Usefulness of a Highly Sensitive Urinary and Serum IL-6 Assay in Patients With Diabetic Nephropathy. Nephron (2000) 85(1):81–5. doi: 10.1159/000045634 [DOI] [PubMed] [Google Scholar]
- 32. Navarro JF, Milena FJ, Mora C, León C, García J. Renal Pro-Inflammatory Cytokine Gene Expression in Diabetic Nephropathy: Effect of Angiotensin-Converting Enzyme Inhibition and Pentoxifylline Administration. Am J Nephrol (2006) 26(6):562–70. doi: 10.1159/000098004 [DOI] [PubMed] [Google Scholar]
- 33. Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a Disease of the Innate Immune System: Association of Acute-Phase Reactants and Interleukin-6 With Metabolic Syndrome X. Diabetologia (1997) 40(11):1286–92. doi: 10.1007/s001250050822 [DOI] [PubMed] [Google Scholar]
- 34. Liang YJ, Shyu KG, Wang BW, Lai LP. C-Reactive Protein Activates the Nuclear factor-kappaB Pathway and Induces Vascular Cell Adhesion Molecule-1 Expression Through CD32 in Human Umbilical Vein Endothelial Cells and Aortic Endothelial Cells. J Mol Cell Cardiol (2006) 40(3):412–20. doi: 10.1016/j.yjmcc.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 35. Hanriot D, Bello G, Ropars A, Seguin-Devaux C, Poitevin G, Grosjean S, et al. C-Reactive Protein Induces Pro- and Anti-Inflammatory Effects, Including Activation of the Liver X Receptor Alpha, on Human Monocytes. Thromb Haemost (2008) 99(3):558–69. doi: 10.1160/TH07-06-0410 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.