Abstract
Increasing evidence supports the notion that neuroinflammation plays a critical role in the etiology of major depressive disorder (MDD), at least in a subset of patients. By virtue of their capacity to transform into reactive states in response to inflammatory insults, microglia, the brain’s resident immune cells, play a pivotal role in the induction of neuroinflammation. Experimental studies have demonstrated the ability of microglia to recognize pathogens or damaged cells, leading to the activation of a cytotoxic response that exacerbates damage to brain cells. However, microglia display a wide range of responses to injury and may also promote resolution stages of inflammation and tissue regeneration. MDD has been associated with chronic priming of microglia. Recent studies suggest that altered microglial morphology and function, caused either by intense inflammatory activation or by senescence, may contribute to depression and associated impairments in neuroplasticity. In this context, modifying microglia phenotype by tuning inflammatory pathways might have important translational relevance to harness neuroinflammation in MDD. Interestingly, it was recently shown that different microglial phenotypes are associated with distinct metabolic pathways and analysis of the underlying molecular mechanisms points to an instrumental role for energy metabolism in shaping microglial functions. Here, we review various canonical pro-inflammatory, anti-inflammatory and metabolic pathways in microglia that may provide new therapeutic opportunities to control neuroinflammation in brain disorders, with a strong focus on MDD.
Keywords: microglia, neuroinflammation, metabolic pathway, major depressive disorder, anti-inflammatory pathway, pro-inflammatory pathway, microglial pathways as therapeutic targets
Introduction
An association between inflammation and major depressive disorder (MDD) has long been hypothesized based on investigations using various approaches. Studies have reported elevated levels of both peripheral (1) and central (2–7) pro-inflammatory cytokines in depressed patients, supporting the hypothesis of an immune-mediated etiology of MDD (8–10). Indeed, subsets of MDD patients have increased concentrations of circulating cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6 (11) and increased expression of innate immune-related genes in the blood. Also, bidirectional relationships between depression and inflammatory or autoimmune disorders exist. Namely, there is a high incidence of co-morbid inflammatory disease and rheumatoid arthritis (RA) in MDD patients (12).
One of the most important cell types involved in regulating neuroinflammation are microglia, which can modulate immunological responses and play a fundamental role in maintaining homeostatic brain functions. The implication of microglia in normal brain physiology includes, but is not limited to, synaptic pruning, phagocytosis, oligodendrocyte maturation and neurogenesis (10, 13). Functionally, microglia are one of the most diverse cell types in the central nervous system (CNS), as they dynamically adapt, at both the cellular and molecular levels, to their ever-changing environment (10, 14). The heterogeneous nature of microglia has been highlighted by high-throughput approaches like single cell RNA-sequencing. Indeed, factors such as brain region, sex, age and type of pathology can significantly affect microglial phenotype, including gene expression signatures and secretory profiles (15, 16). Under physiological and pathological conditions, microglia display spatial heterogeneity in density, morphology, turnover rate, pruning, metabolism and molecular signature (17–19).
Microglial activation occurs through inflammatory insult or slight alterations in brain homeostasis. This activation is dependent on the context and the type of stressor or pathology. Microglia determine the pathological outcome of stressors through secretion of cytokines, chemokines and growth factors and psychopathologies have repeatedly been associated with long-lasting priming and sensitization of cerebral microglia (10, 20, 21). Microglia also modulate communication between the nervous and the immune system in response to different physiological, psychological and immunological stressors. They are in fact considered to be responsible for the decreased neuroplasticity observed in depression (22) and recent findings have associated microglial abnormalities with neuropsychiatric disorders such as MDD, which have been termed microgliopathies by some (23–25).
Manipulating the microglial phenotype is an intriguing strategy for developing new therapeutics for MDD. Particular attention is currently given to exploiting alternative microglial polarization as a potential therapeutic option in a wide range of neurological and neuropsychiatric disorders (15). To achieve this, canonical pathways that govern tuning of the microglial phenotype have been investigated including transforming growth factor β (TGF-β), IL-4 receptor and peroxisome proliferator-activated receptors-gamma (PPAR-γ) (26, 27). We and others previously published reviews on the roles played by microglia in psychiatric disorders (10, 13, 21, 28). However, a review focused on different canonical microglial pro-inflammatory, anti-inflammatory and metabolic pathways and their translational value in drug discovery for MDD is lacking. We aim to fill the gap.
Role of Neuroinflammation in Psychopathology
The role(s) played by the innate immune response in MDD has been the object of several experimental and clinical studies in which microglia have increasingly become the focus of investigation. Microglia have been studied in different psychiatric disorders using various approaches, including postmortem investigations in humans as well as experimental studies in animal models (21, 29, 30). The term neuroinflammation, denoting inflammatory processes in the CNS, is a rather general notion that could include both peripheral and central components of inflammation. Interestingly, a number of studies have suggested the involvement of peripheral inflammation in the pathogenesis of depression. Menard et al. associated a reduced expression of the endothelial cell tight junction protein claudin-5 with abnormal blood vessel morphology in the nucleus accumbens of stress-susceptible but not resilient mice (31). In a more recent study, the same group showed that epigenetic regulation of claudin-5 is associated with stress resilience. Indeed, they identified nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) signaling pathway and histone deacetylase 1 as mediators of stress susceptibility. Pharmacological inhibition of histone deacetylase 1 rescued claudin-5 expression in the nucleus accumbens and promoted resilience (32). It is to be noted, however, that blood–brain barrier (BBB) disruption is not a common phenomenon in animal models of depression (31) and that the mechanism underlying this region-specific BBB abnormality remains to be clarified.
Recently, neuroinflammation has been used to explain functional microglial abnormalities observed in psychopathologies. A developed mouse model of obsessive-compulsive disorder serves as an interesting example of the link between microglial abnormalities and mental illness. Mutant HOXB8 mice display unexpected behavior manifested by compulsive grooming and hair removal (33). These actions directly mirror trichotillomania seen in humans with obsessive-compulsive spectrum disorder (33). Chen et al. reported that, in the brain, the HOXB8 cell lineage exclusively labels bone marrow-derived microglia. This finding strongly fosters the theory that the excessive grooming behavior observed in HOXB8 mutant mice is a consequence of defective microglia, thus relating hematopoietic function to mouse behavior (33). Another interesting example is the effect of CX3CR1 deficiency on behavior relevant to post-traumatic stress disorder. Neuronal CX3CL1 and its microglial target CX3CR1 play an essential role in synaptic plasticity and a correlation between CX3CR1 deficiency and increased fear behavior as well as an anxiolytic-like phenotype have been reported by Schubert et al. (34). However, Milior et al. observed a contradicting finding in which microglial CX3CR1 knock-out mice were resilient to chronic unpredictable stress (CUS) suggesting microglia-neuron communication may be at the interplay of resilience or susceptibility to a depression-like phenotype (35). Colony-stimulating factor 1 receptor signaling was also shown to control cerebellar microglia and to be essential for motor function and social interaction (36).
Importance of Microglia in Major Depressive Disorder
Despite a strong correlation between microglial activation and depression in pre-clinical and clinical studies, it remains unclear whether microglial abnormities play a causal role in depression (37). Clinical findings indicate a strong correlation between disease etiology and inflammation (37). Subsets of MDD patients consistently display increased levels of pro-inflammatory cytokines such as TNF-α and IL-6 (11, 30). A previous postmortem investigation by our group has shown that the percentage of primed microglia is increased in the dorsal anterior cingulate cortex (ACC) of depressed suicides compared to matched controls (38). This observation is consistent with independent reports of microglial activation in the ACC of MDD patients (39).
There is a growing body of literature showing increased microglial activation in inflammatory and non-inflammatory rodent models of depression. The most studied inflammatory model is that of lipopolysaccharide (LPS) injection. Systematic LPS administration not only triggers peripheral immune responses but also activates microglia in the brain (37, 40). Following LPS challenge, pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 are upregulated in different brain areas (41, 42). These inflammatory changes are accompanied by decreased sucrose preference and increased immobility in the forced swim test (43). Moreover, animals that receive acute or chronic non-inflammatory stress also show microglial activation along with morphological changes and increased levels of pro-inflammatory cytokine release in different brain regions including the hippocampus, thalamus and prefrontal cortex. For example, mice subjected to chronic social defeat (CSD) exhibit increased numbers of CD68-expressing microglia that have increased phagocytic capacity. Several other groups have reported microglial dysregulation following CSD stress (44–49). Lehmann et al. have reported that depressive-like behavior throughout and following CSD involves microglia-derived reactive oxygen species (ROS). Using colony stimulating factor receptor antagonist PLX5622 to deplete microglia before and during the 14-day CSD procedure, mice were protected from the effects of stress as measured by light/dark and social interaction paradigms (10, 50). More evidence indicating the involvement of microglial activation in depression comes from minocycline studies. Minocycline is an anti-inflammatory tetracycline that inhibits microglial activation and subsequent neuroinflammation (37, 51). Minocycline treatment does not have anti-depressive behavioral effects in naïve mice (52), however, it elicits significant anti-depressive effects in the rat model of chronic unpredictable mild stress (CUMS) (53). Intriguingly, combinatorial therapy of minocycline with antidepressants provides better clinical outcomes in some MDD patients, implying the contribution of neuroinflammation and microglial activation in a subset of patients afflicted with this psychopathology (54).
In the following sections, we aim to highlight the potential roles played by microglia in the pathogenesis of MDD, with a focus on the roles played by the canonical pro-inflammatory, anti-inflammatory and metabolic pathways.
Pro-Inflammatory Pathways in Microglia
Tumor Necrosis Factor-α Mediated Pathway
TNF-α is a trimeric cytokine that is expressed either in a 27 kDa transmembrane form or a 17 kDa soluble form processed by TNF-α converting enzyme (55–57). TNF-α exerts its pleiotropic effects by binding to two primary receptors: TNF-α Receptor 1 (TNFR1) and TNF-α Receptor 2 (TNFR2). Soluble TNF-α typically binds TNFR1 after clustering at the cell membrane (58, 59). Transmembrane TNF-α preferentially binds TNFR2 as a ligand and can serve as a receptor for cell-to-cell contact (56, 59). Regardless of form, after a TNF-α ligand binds, TNFR1 and TNFR2 from homodimers to induce downstream signaling for cellular processes such as defense from foreign pathogens, enhancing inflammation and promoting cell survival or apoptosis (57, 60, 61).
TNF-α is produced by different cell types in the CNS, including neurons, astrocytes, microglia and endothelial cells (62, 63). However, monocytic immune cells like microglia are the dominant secretors and targets of TNF-α (57). TNF-α is now well understood as a critical pro-inflammatory cytokine that has an instrumental roles in the CNS, including innate immunity, sleep regulation, neuronal activity and necrotic and apoptotic cell death (58, 64). Under physiological conditions TNF expression is induced by basal activity in microglia, neurons and astrocytes. This cytokine is essential for regulating neuronal function including synaptic activity. Neurons constitutively express TNF-α receptors, which are important for mediating neuroprotection against neurotoxic stimuli (65).
Microglia-derived TNF-α is critical in innate immune responses within the CNS (66). Previous studies have documented the ability of TNF-α to influence microglial function in response to neuroinflammatory insults. For instance, to achieve a swifter recognition of foreign pathogens, TNF-α binds to its microglial receptors and upregulates the expression of toll-like receptor (TLR) 2, a pattern recognition receptor specialized for bacteria, enhancing microglia’s overall immune response (67). Aside from priming microglia for enhanced pathogen detection, this cytokine also enhances natural killer cells’ and macrophages’ ability to kill cells and phagocytose, respectively (58, 67).
TNFR1 and TNFR2 notably have varying functions within the brain (Table 1), some of which are region-specific. It was previously observed that the reparative function of TNF-α on neurons in the striatum is reliant upon TNFR1 (68). At the same time, similar capabilities of TNF-α in the hippocampus depend upon TNFR2 but the receptors’ expressions were equal in both regions (68). A more recent study found similar effects when investigating TNFR1 and TNFR2 single nucleotide polymorphisms (69). These results indicated that TNF-α regulation of striatal morphology was predominated by TNFR1 signaling while regulation of hippocampal morphology was shown to rely primarily on TNFR2 (69). It is thought that this region-specificity may be due to the two receptors’ differential impacts on cell survival since TNFR1’s downstream processes promote apoptosis and TNFR2’s pathways are more anti-apoptotic (68).
TABLE 1.
Overview of studies on TNFR1 and TNFR2’s differential processes within the brain.
| Study | Study performed | TNFR type | Cell type | Observed effects |
| (68) | TNFR1 and TNFR2 levels measured in encephalitis mouse model | TNFR1 | Striatal Neurons | TNFR1 carries out reparative function of TNF-α on striatum neurons |
| TNFR2 | Hippocampal neurons | TNFR2 carries out reparative function of TNF-α in hippocampus | ||
| (69) | Single nucleotide polymorphisms and associated brain morphology changes | TNFR1 | Striatal neurons | TNFR1 responsible for TNF-α regulation of striatal morphology through apoptosis |
| TNFR2 | Hippocampal neurons | TNFR2 responsible for TNF-α regulation of hippocampal morphology through anti-apoptotic processes | ||
| (87) | Anti-TNF-α therapy and its effects on cell survival | TNFR1 | Cholinergic neurons | When TNFR1 receptor blocked by anti-TNF-α, reduced apoptosis |
| TNFR2 | Cholinergic neurons | When TNFR2 blocked by anti-TNF-α, reduced cell protective processes | ||
| (88) | TNFR2 KO mice | TNFR2 | Microglia | KO mice displayed early onset experimental autoimmune encephalitis |
| (89) | Activating TNFR2 in cultured mouse microglia | TNFR2 | Microglia | TNFR2 regulates production of pro-regenerative and neuroprotective factors such as granulocyte colony-stimulating factor and IL-10 |
TNF-α, tumor necrosis factor-alpha; TNFR1, TNF-α receptor 1; TNFR2, TNF-α receptor 2; KO, knockout; IL-10, interleukin-10.
The distinct effects of these receptors on cell survival and inflammation can be understood through their different recruitment of signaling complexes after a ligand binds (Figure 1). Active TNFR1 homodimers allow four different complexes to form to engage various cellular processes (70). Complexes I, IIa, and IIb similarly activate NF-κB and mitogen-activated protein kinases (MAPK) to promote cell survival, cell proliferation, immune defense and inflammation (70). Complexes IIa and IIb are additionally responsible for activating the caspase apoptotic pathway (70). The last complex, complex IIc, also plays a role in inflammation but is most notable for its role in necroptosis (70). Unlike TNFR1 pathways, the current understanding of TNFR2 is not as comprehensive (71). However, previous studies with TNFR2 knockout mice have shown its importance in anti-inflammatory and cell-protective processes (71). Furthermore, it is theorized that active TNFR2 homodimers recruit adapter protein TNF receptor-associated factor 2 to activate NF-κB pathways (71). Despite TNFRs sharing similar structures and ligands there is a clear heterogeneity in their downstream effects.
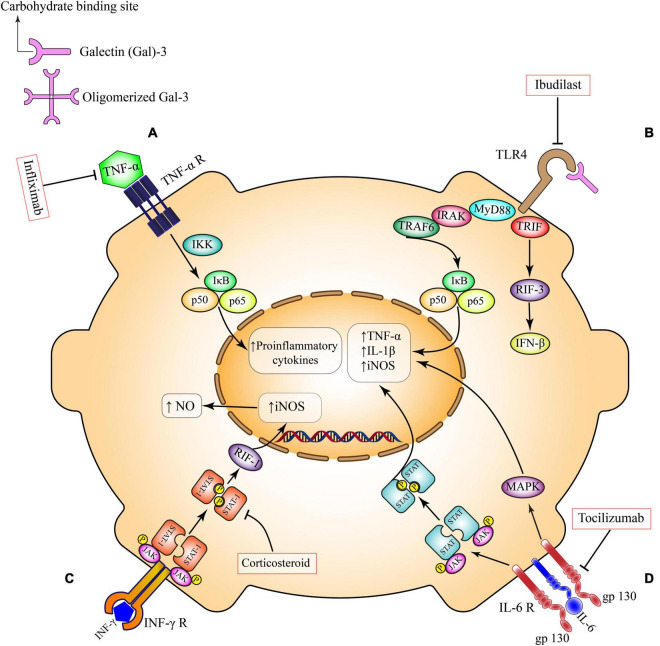
FIGURE 1.
Pro-inflammatory pathways in microglia. (A) TNF-α receptor activation, induces the canonical pro-inflammatory transcriptional factors such as NFκB and subsequent production of inflammatory mediators. This pathway can be inhibited by Infliximab. (B) TLR4 ligands and secreted Gal-3 directly bind to TLR4 on the microglial surface and exacerbates inflammatory responses through induction of different cytokines and chemokines. This pathway can be inhibited by Ibudilast. (C) Activation of INF-γ receptor on microglia triggers the overexpression of inducible nitric oxide synthase (iNOS) and overproduction of nitric oxide via Janus kinase (JAK)/signal transducer and activator of transcription (STAT)/RIF-1 pathway. Corticosteroids can suppress this pathway at the level of STAT factors. (D) IL-6 trans-signaling occurs in brain cell types that have membrane bound gp130, including microglia. IL-6 bound to soluble IL-6R activates signaling through membrane bound gp130. This trans-signaling is thought to be pro-inflammatory via the induction of JAK/STAT and MAPK signaling pathways. Tocilizumab inhibits this pro-inflammatory pathway. TNF-α, tumor necrosis factor-α; IKK, the IκB kinase; NO, nitric oxide; iNOS, inducible nitric oxide synthase; IL-1β, interleukin 1 beta; IL-6, interleukin 6; JAK, Janus kinase; STAT, signal transducer and activator of transcription; RIF-1, replication timing regulatory factor 1; MAPK, a mitogen-activated protein kinase; TLR4, toll-like receptor 4; MYD88, myeloid differentiation primary response 88; TRIF, TIR-domain-containing adapter-inducing interferon-β; IRAK, interleukin 1 receptor associated kinase; TRAF, TNF receptor associated factor; IFN-β, interferon-β; IFN-γ, interferon gamma.
TNF-α has long been implicated in several peripheral and central inflammatory conditions (58, 72, 73). Meanwhile, research on the role of TNF-α in psychiatric disorders is evolving. In this context, TNF-α’s pro-inflammatory functions may exacerbate or contribute to depressive symptoms (74). Both TNFR1 and TNFR2 pathways can modulate inflammatory pathways through downstream NFkB signaling (60). Additionally, TNF-α can induce glutamate-mediated excitotoxicity. This cytokine facilitates crosstalk between microglia and astrocytes to promote the release of astrocytic glutamate, the formation of excitatory synapses and the release of more TNF-α from microglia (75). Therefore, TNF-α mediated pathways can lead to extraneous inflammation and cell death contributing to the worsening of MDD.
In accordance, several studies have seen higher levels of serum TNF-α associated with depressive symptoms and in MDD patients compared to matched controls (76–78). A few studies measuring cytokine profiles in cerebrospinal fluid (CSF) have not found differences in TNF-α levels between depressed patients and healthy controls (79). However, there are only few such studies of CSF cytokine levels and the results seem rather inconsistent as compared to studies of peripheral (plasma) cytokines (79). In addition, plasma cytokine profiles do not necessarily represent cytokine levels within the CNS (80).
In a pioneer study, Ohgidani et al. showed that acute stress induces TNF-α secretion from hippocampal microglia resulting in mouse working memory deficits. These authors observed that morphological changes in hippocampal microglia did not occur (81). Furthermore, etanercept, a TNF-α inhibitor, rescued the working memory impairment accompanied by a reduction in hippocampal TNF-α (81). Indeed, maladaptive microglial activation may be linked to MDD and modulating microglial activation seems a promising therapeutic target for depression.
A previous study with monoclonal antibody against TNF-α revealed that this intervention quelled symptoms of anhedonia but did not affect depression scores significantly compared to placebo groups (82). Current data seem to support the effectiveness of anti-inflammatory agents as antidepressants only in patients with increased peripheral inflammation (83). This subgroup may include MDD patients with increased inflammatory markers or those with medical conditions characterized by increased levels of peripheral inflammation (84). Specifically, anti-TNF-α therapy was found not to be effective in all treatment-resistant depression patients, but it did improve depressive symptoms in those with higher baselines of inflammatory markers (85). One major reason for such failure is the double-edged role of microglial TNF-α in fundamental physiological processes such as the neuroinflammatory response to tissue damage, neuronal circuit formation, synaptic plasticity and myelin degeneration and repair (86). Indeed, any positive impact from blocking TNFR1 receptors would be nullified by blocking TNFR2 activation, adding another complex layer upon possible therapies for MDD (87). It has been shown that the TNF-α mediated activation of microglial TNFR2 is instrumental for the protective functions of these cells (86). For instance, microglia-specific TNFR2 knockout mice display early onset of experimental autoimmune encephalitis (88). In this context, it has been shown that TNFR2 regulates the production of pro-regenerative and neuroprotective factors including granulocyte colony-stimulating factor and IL-10 in microglia (89). It is noteworthy that protective aspects of TNF-α signaling in microglia have been greatly overlooked with respect to the pro-inflammatory ones. Thus, more investigations are needed to decipher the mechanisms regulating the balance between TNFR1 and TNFR2 pathways in microglia in order to limit the detrimental immune responses without blocking the protective ones (86).
IL-6 Mediated Pathway
Over the years, the cytokine IL-6 has been linked to stress-related disorders such as depression and anxiety (90). This cytokine is a small multifunctional protein (91) that can be produced by several cell types including endothelial cells, epithelial cells, astrocytes, microglia and neurons (92, 93). IL-6 belongs to a family of proteins that utilize glycoprotein 130 (gp130) as a signal transducer (Figure 1). Depending on the presence of IL-6 receptor (IL-6R) or membrane bound gp130 which are expressed differently in different cell types, IL-6 has pro- or anti-inflammatory properties (93) resulting in either inflammatory or anti-inflammatory cascades (90).
According to a few major meta-analyses, IL-6 is one of the most consistently elevated cytokines in the blood of patients with MDD (11, 94, 95). Remarkably, IL-6 blood levels might have a predictive value as a biomarker. Moreover, peripheral levels of IL-6 correlate with symptom severity of antidepressant non-responders (96). In addition, increased levels of IL-6 have been reported in the CSF of patients with MDD as well as in suicide attempters (3, 97). Unfortunately, research addressing the role of microglial derived IL-6 are lacking in human postmortem studies. Only one pioneer study indicates a non-inflammatory phenotype of microglia in MDD following single-cell mass cytometry of microglia (98). The authors performed single-cell analysis of microglia from four different postmortem brain regions including frontal lobe, temporal lobe, thalamus and subventricular zone of medicated individuals with MDD and they found no evidence for the induction of canonical pro-inflammatory (IL-1β, IL-6, and TNF-α) and anti-inflammatory cytokines such as IL-10 (98).
Various pre-clinical studies have investigated the role of microglial IL-6 in the context of stress. For instance, increased IL-6 mRNA is found in microglia isolated directly from the brains of mice that have undergone repeated social defeat (RSD) stress (99) and treatment with the antidepressant imipramine inhibits social avoidance behavior and diminished microglia IL-6 in mice exposed to stress (90, 99). In another study, Aniszewska et al. found that stress induced a significant increase in the number of IL-6-immunoreactive microglia in the hippocampus, cortex and brain stem (100).
Blocking IL-6R-mediated pathways (e.g., tocilizumab) or neutralizing IL-6 function (e.g., sirukumab) might have clinical value in a subset of MDD patients, especially in treatment-resistant cases or in patients with peripheral inflammatory diseases (101). Interestingly, the efficacy of interleukin-6 neutralizing antibodies on symptoms of MDD patients with RA has been reported (102). However, BBB penetration and adverse effects may limit their use in MDD patients without the history of peripheral inflammatory diseases such as RA (103). In addition, there are two types of IL-6 signaling: a classical anti-inflammatory signaling and a trans-signaling proinflammatory signaling. It means that general targeting of IL-6 pathways in MDD either with IL-6R inhibitors or IL-6 blocker is not an optimal choice (101, 103). A more selective intervention seems more promising for future drug discovery targeting IL-6 signaling (101).
Toll-Like Receptor 4 and Nuclear Factor-Kappa B Mediated Pathways
TLR4 is one of nine members in the TLR family of pathogen-specific pattern recognition receptors dedicated to responding to unique structural components of foreign microbial agents to trigger immune responses (104, 105). As such, this receptor is highly involved in regulating brain innate immune responses in pathophysiological conditions. TLR4 is notably the most researched receptor within the TLR family. It is well known to be primarily responsible in the reaction against Gram-negative bacteria by binding to LPS, its pathogen-associated molecular pattern (106). However, TLR4 has also been shown to recognize damage-associated molecular patterns (DAMPs) and xenobiotics (107). Additionally, TLR4 can be activated by other non-bacterial TLR4 agonists naturally present within the body such as saturated fatty acids (108). TLR4s are generally expressed in myeloid lineage cells like macrophages and other non-immune cells like endothelial cells (106). Within the CNS, TLR4 is expressed primarily by microglia but can also be found in astrocytes, oligodendrocytes and neurons (107).
NF-κB refers to a group of transcription factors that serve as significant regulators of pro-inflammatory genes and has been of particular interest as a target for pharmacological treatments in inflammatory diseases (109). They exist as two subfamilies of inducible dimers, made up of either DNA binding proteins from the ‘Nκ-kB’ or ‘Rel’ family, which can then form homodimers or heterodimers (110). Alternatively, the term NF-κB can also describe p50-RelA heterodimer, the predominant NF-κB dimer present in many cells (111). Regardless, all NF-κB dimers are constitutively inhibited by IkB proteins that are degraded to activate and translocate the transcription factor into the nucleus (109). NF-κB activity is rapidly induced in microglia following inflammatory insults (112).
During activation with LPS, the pathogen-associated molecular pattern forms a multimolecular complex with TLR4 and its accessory molecules which readies the receptor for dimerization (113). The formed dimer then induces downstream effects (Figure 1) either through the myeloid differentiation primary response 88 (MyD88)-dependent pathway or the MyD88-independent pathway (106, 114). The MyD88-dependent pathway leads to activation of transcription factors that induce the expression of pro-inflammatory cytokine genes (114). The MyD88-independent pathway, also known as the TIR-domain-containing adapter-inducing interferon-β dependent pathway, also activates transcription factors but mediates the induction of type 1 interferon-inducible genes (114). In both cases NF-κB is activated (115).
TLR4 and NF-κB are of particular interest in pathophysiological conditions due to their significant roles in innate immunity. Accordingly, many of the following studies have implicated their dysregulation within various neurodegenerative diseases and psychiatric disorders (116–118). Elevated TLR4 expression and associated overactive microglia were observed within a transgenic mouse model of Alzheimer’s Disease (AD) leading to cognitive impairment (119, 120). Furthermore, previous investigations have also characterized an increase in NF-κB activation within the CNS of animal models and patients with neurodegenerative disorders (117). Interestingly, a study of postmortem brains from patients with schizophrenia revealed that TLR4 levels are increased within the cerebellum but decreased within the prefrontal cortex (PFC) suggesting that general dysregulation, rather than upregulation, could lead to harmful effects (118). In the same study, NF-κB levels were notably altered inversely to TLR4 levels, with increased levels within the PFC and decreases in the cerebellum (118). Another investigation demonstrated increases in NF-κB levels, especially in microglia, as a neuroinflammatory mechanism in autism spectrum conditions (116). The studies above support the idea that TLR4, NF-κB and their combined pathway likely play significant roles in neuroinflammatory response in MDD. More specifically, the dysregulation of TLR4 and NF-κB inflammatory processes has been suggested to be involved in MDD. TLR4 single gene polymorphisms were associated with suicide and anxiety scores in MDD patients, while methylation levels of TLR4-associated CpGs were related to the severity of depressive symptoms (121, 122). In general, increased TLR4 expression and decreased expression of its inhibitor TNFAIP3 were associated with depressive symptoms (107). Similarly, increased NF-κB activity also appears to play a role in depression. In a rat early-life stress model, the depression-susceptible animals generally displayed more activated NF-κB. Inversely, the subgroup showing resilient phenotype had more inactivated NF-κB (123). Another notable study supported this idea by demonstrating improved depressive-like behaviors in mice when increased NF-κB expression was inhibited (124). Other studies have specifically investigated TLR4-NF-κB pathways in depression. A supporting study indicated that suppressing the TLR4-NFκB signaling pathway inhibits depression-like behavior in mice (125). These studies sparked further interest in investigating these pathways to develop new antidepressant candidates for MDD patients (Table 2).
TABLE 2.
Overview of studies performed to investigate TLR4 and NF-κB’s roles in neuropsychiatric disorders.
| Study | Method | Condition of Brain/Behavior | Finding |
| (118) | Measure TLR4 and NF-κB levels in human postmortem brains | Schizophrenia | PFC: (1) ↓ TLR4, (2) ↑ NF-κB Cerebellum: (1) ↑ TLR4, (2) ↓ NF-κB |
| (116) | Measure NF-κB levels in postmortem human brains | Autism spectrum conditions | ↑ NF-κB levels especially in microglia |
| (121) | Associative study on TLR4 single gene polymorphisms and MDD | Individuals with higher suicide and anxiety scores | ↑ TLR4 single gene polymorphisms |
| (122) | Associative Study of epigenetic effects of TLR4 on severity of symptoms | Higher severity of depressive symptoms | ↑ methylation levels of TLR4-associated CpGs |
| (123) | Measure levels of active NF-κB in an early-life stress rat model | Depression susceptible phenotype | ↑ activated NF-κB |
| Depression resilient phenotype | ↑ inactivated NF-κB | ||
| (124) | NF-κB inhibited in mice model to see behavioral changes | Improved depressive-like behaviors | ↓ overactivated NF-κB |
| (125) | Suppressing TLR4-NF-κB pathway in mice | Inhibits depression-like behavior | ↓ signaling of TLR4-NF-κB pathway |
↑, increased; ↓, decreased; TLR4, toll-like receptor 4; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; PFC, prefrontal cortex.
The current literature strongly supports the existence and importance of TLR4, NF-κB and their conjoined roles in MDD. Although TLR4 activation seems to play a role in MDD, the underlying mechanisms are unclear. Much of the previous work implicating TLR4 in MDD have primarily focused on the role of TLR4 following activation by LPS in bacterial infections (114). However, as noted previously, TLR4 can be triggered by many different ligands; thus, current perspectives are limiting and require further investigation from novel angles (106–108). More specifically, it may be useful to consider the immune signaling that coincides with other events aside from infection, such as during the critical period of development or epigenetic modifications (126, 127). These are important time points that may impart predisposing factors on individuals if TLR4 signaling is altered (126, 127). Furthermore, these deleterious inflammatory states associated with altered TLR4 pathways may also be attributed to other mechanisms such as neuroendocrine signaling and dysregulated gut microbiota (127). Therefore, to improve the efficacy of future drugs targeting TLR4 in MDD, research into more diverse mechanistic facets should be conducted. A recent study used this approach to propose polyphenols as a potential group of drug candidates for future consideration (128).
Interferon-Gamma Mediated Pathway
Interferon-gamma (IFN-γ) is a pleiotropic soluble cytokine that is produced by different immune cell types including lymphocytes, B cells and antigen-presenting cells. In the CNS, different cells such as neurons, microglia and astrocytes produce this cytokine and express its receptors. The activation of the IFN-γ receptor induces several canonical downstream pathways (Figure 1) such as the janus kinase (JAK) 1 and 2, signal transducer and activator of transcription (STAT) 1 and the extracellular-signal-regulated-kinase (ERK) 1/2. Many genes, as well as micro RNAs and long non-coding RNAs, are activated following IFN-γ receptor stimulation (129). Neuroinflammation mediated by IFN-γ has been reported in neurological disorders. However, the effects of IFN-γ on behavior in the context of stress are mostly unknown (129). Intriguingly, IFN-γ knockout mice show decreased anxiety- and depressive-like behaviors (129). These effects are accompanied by elevation of serotonergic and noradrenergic activity in the central amygdaloid nucleus, together with increased baseline plasma corticosterone, decreased neurogenesis in the hippocampus and decreased levels of nerve growth factors in the PFC, indicating that IFN-γ modulates anxiety and depressive states and is involved in CNS plasticity (130–132). In an intriguing report, Zhang et al. showed that intracerebroventricular injection of IFN-γ in mice causes impairment of adult hippocampal neurogenesis, behavioral despair, anhedonia and cognitive loss. Furthermore, IFN-γ induces microglial activation that is associated with morphological changes and upregulation of phagocytic marker CD68 and pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) (133). Inhibition of the JAK/STAT1 pathway, downstream of IFN-γ receptor, suppresses microglial-mediated neuroinflammation, diminishes depressive-like behaviors and improves memory (133).
An array of studies has demonstrated the possible effects of antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs), on inflammatory responses and microglial function (10, 134). Besides their known therapeutic mechanism involving the modulation of the serotonergic system, SSRIs can regulate the activation state and secretory profile of microglia (10). In this context, Horikawa et al. reported that paroxetine and sertraline prevent microglial activation by inhibiting IFN-γ-induced elevation of intracellular calcium (135). Interestingly, Alboni et al. found that quality of the environment effects the mechanism of action of Fluoxetine. Enriched environments coincident with Fluoxetine administration induced pro-inflammatory microglial profiles while a stressful environment resulted in anti-inflammatory secretory profiles (134).
It is established that microglia adopt reactive states in response to an inflammatory insult. However, at both transcriptional and functional levels, microglia appear to be more complex and dynamic than anticipated. This might explain why engagement of microglia can be either neuroprotective or neurotoxic, leading to attenuation or exacerbation of disease progression (10, 15, 136) depending on the context. According to the traditional classification of macrophages/microglia, during microglial activation following an inflammatory insult, cell morphology is altered either to M1, the typically activated phenotype, or to M2, an alternative activated phenotype; and this phenotypic switch depends on the type of insult. M1 microglia are considered proinflammatory and produce mediators such as TNF-α and IL-1β. It has been shown that INF is a canonical cytokine that can polarize microglia toward M1. It is noteworthy that the classification of the M1 and M2 phenotypes have been challenged (10, 136, 137). The reason is that such classification has been defined mainly based on in vitro studies of peripheral macrophages and that M1 and M2 states fail to emerge in brain resident microglia. It is now accepted that activated microglia co-express canonical gene products associated with both M1 and M2 states. Indeed, following brain injury, microglia do not simply switch to a polarized “M1-only” or “M2-only” phenotype but rather display a mixed phenotype due to the complex signaling cascades surrounding them (27, 136, 137). However, in the context of this review, we will continue to use the broad categories of activated pro-inflammatory and anti-inflammatory unless the study mentioned investigated more dynamic phenotypes.
The choroid plexus (ChP), a highly vascularized tissue that produces CSF and lacks a BBB, is an interface between peripheral and central immune responses (138). Our group previously investigated the cellular and molecular inflammatory profile of the ChP of the lateral ventricle in depressed suicides and healthy controls (138). We measured the content of several pro- and anti-inflammatory transcripts as well as the density of Iba1+ macrophages associated with the ChP epithelial cell layer. The levels of pro-inflammatory markers, ICAM1 (a protein implicated in immune cell trafficking) and Iba1, were measured to be significantly downregulated in depressed suicides as compared to controls (138). Intriguingly IFN-γ signaling has been shown as a selective key regulator of immune cell trafficking across the ChP epithelium under physiological conditions of CNS immune surveillance and following neuroinflammatory insult (139). This unique mechanism could be harnessed to adjust the interplay between the peripheral immune system and microglia in affective disorders.
Anti-Inflammatory Pathways in Microglia
Alpha-Seven Nicotinic Receptor Mediated Pathway
The alpha-7 nicotinic acetylcholine receptor (α7 nAChR) is a ligand-gated ion channel expressed by macrophage/microglia (140, 141) and has proved to be a promising target in pharmacotherapy of psychiatric disorders. α7 nAChR agonists or partial agonists are known to improve cognitive dysfunction by regulating microglial activation through inhibition of canonical pro-inflammatory transcriptional factors such as NFkB and induction of anti-inflammatory signaling pathways such as nuclear factor-erythroid factor 2-related factor 2 (Nrf2) (142–144). In fact, there is ample evidence that the cholinergic system plays a fundamental role in regulating central inflammation and glial activation via homomeric α7 nAChRs (145–147). The α7 nAChRs consist of five α subunits and are expressed by neuronal and glial cells (148, 149). These ligand-gated ion channels allow for calcium influx and subsequent ultra-rapid desensitization (150, 151). α7 nAChRs are widely expressed in the brain, including in regions such as the PFC, hippocampus and other limbic areas (150). Microglial α7 nAChRs play important roles in regulating inflammatory processes in the CNS (148, 150). Stimulation of α7 nAChR leads to a reduction in glial activation and decreases in proinflammatory cytokine levels in different brain regions (152–154).
The microglial α7 nAChRs have dual ionotropic/metabotropic properties and their intracellular signaling pathways that modulate inflammation do not only depend on transient ion influx (145, 155, 156). Indeed, neuronal α7 nAChRs mainly have an ionotropic function (145). The downstream metabotropic signaling pathways of microglial α7 nAChRs are different from neuronal α7 nAChRs (157). Activation of microglial α7 nAChRs induces phospholipase C and enhanced calcium release from intercellular stores which are sensitive to inositol trisphosphate (153). This process results in the inhibition of NF-κB transcriptional activity (Figure 2) (158). As a result of this inhibition the levels of pro-inflammatory cytokines are decreased (154).
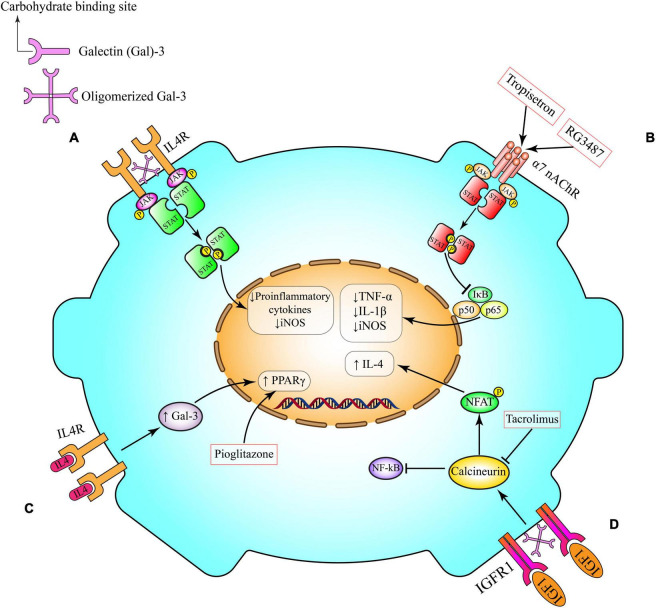
FIGURE 2.
Anti-inflammatory pathways in microglia. (A) Gal-3 induces alternative microglia activation through interaction with IL-4 receptor (IL4R). Following Gal-3 lattice formation the carbohydrate-binding site of Gal-3 molecules interacts with glycosylated IL4R and prevents their endocytosis and also over activation of IL4R and its anti-inflammatory signaling. (B) Activation of α7 nAChR on microglia triggers anti-inflammatory cascades, including Janus kinase (JAK)/signal transducer and activator of transcription (STAT) and PI3K/Akt, which potentiate the activity of transcriptional factor Nrf2 and its downstream pathways (HO-1 and CAT), and inhibits the canonical proinflammatory protein NFκB, which governs the production of proinflammatory cytokines (e.g., TNF-α) and enzymes (e.g., iNOS and COX-2) involved in neuroinflammation. This pathway can be induced by tropisetron and RG3487. (C) IL-4 can interact with its tyrosine kinase IL4R on microglia cell surface. This interaction might activate one of the canonical transcriptional factors that are involved in microglia polarization such as PPAR-γ. Pioglitazone activates PPAR-γ. (D) By crosslinking insulin-like growth factor 1 receptor (IGFR-1), secreted Gal-3 will prevent early endocytosis and over-activate the Janus kinase (JAK)/signal transducer and activator of transcription STAT pathway and the transcription of genes needed for production of anti-inflammatory cytokines such as IL-4. IL-4, interleukin 4; iNOS, inducible nitric oxide synthase; TNF-α, tumor necrosis factor-α; JAK, Janus kinase; STAT, signal transducer and activator of transcription; NFAT, nuclear factor of activated T-cells; NF-κB, nuclear factor kappa B; α7 nAChR, α7 nicotinic acetylcholine receptors; Gal-3, galectin-3; IGFR, insulin-like growth factor 1 (IGF-1) receptor; PPAR-γ, peroxisome proliferator- activated receptor-gamma.
It has been shown that chronic restraint stress (CRS) alters central cholinergic signaling in brain regions that have been implicated in MDD (159). Namely, CRS induces hippocampal choline acetyltransferase protein expression and decreases nuclear STAT3 signaling. CRS also augments signaling activity, IL-1β and TNF-α expression and microglial activation. Intriguingly, cholinergic stimulation with a selective α7 nAChR agonist significantly diminishes CRS-induced depressive-like behavior, neuroinflammation and neuronal damage. Moreover, activation of α7 nAChRs restores central cholinergic signaling function, inhibits TLR4-mediated inflammatory signaling and microglial activity and increases the number of regulatory T-cells in the hippocampus following stress (159).
α7 nAChR activation induces the transcriptional activity of Nrf2 (143). Previous studies especially by the Lopez group indicate that α7 nAChR mediated activation of Nrf2 elicits anti-inflammatory mechanisms in microglia (160–165). This anti-inflammatory axis might play an instrumental role in antidepressant aspects of α7 nAChR modulators. In this context, the efficacy of selective or promiscuous ligands that can activate α7 nAChR-Nrf2 pathway have been shown in depressive disorders (144, 164). One interesting example for the promiscuous ligand is Tropisetron. This ligand is a 5-HT3 receptor antagonist and α7 nAChR partial agonist. This serotonergic ligand has shown a great efficacy in a wide range of psychiatric disorders including MDD and schizophrenia in both experimental models and clinical trials (144, 166). The other example is RG3487 (C15H19ClN4O), the novel 5-HT3 antagonist with α7 nAChR partial agonist properties. This ligand significantly improves attentional performance in experimental models and has shown promising results in clinical trials for cognitive impairment associated with schizophrenia (142, 167).
IL-4 Receptor Mediated Pathway
IL-4 is a multifunctional cytokine secreted by Th2 cells, mast cells, eosinophils and basophils (168, 169). IL-4 is a crucial molecule for microglia and macrophage polarization and it plays pivotal roles in brain function following neuroinflammatory insult (169). The effects of IL-4 are mediated through the IL-4 receptor α-chain. Following binding to its ligand, IL-4 receptor α-chain dimerizes either with the common γ-chain to produce the type-1 signaling complex located mainly on hematopoietic cells, or with the IL-13 receptor α 1 to produce the type-2 complex, which is expressed also on non-hematopoietic cells. The type-1 signaling complex (Figure 2) is pivotal for alternatively activated macrophages (168). Upon activation, the type-1 complex signals through JAK1 and JAK3, which phosphorylate and create docking sites for the transcription factor STAT6. This transcriptional factor then dimerizes and translocates to the cell nucleus to regulate the expression of several genes (168).
IL-4 might be protective against depression due to its ability to harness inflammation and to inhibit serotonin transporter activity. Wachholz et al. demonstrated that a decreased IL-4 responsiveness of microglia is specifically related to the development of depressive-like behavior. IL-4 deficient mice show notable augmentation of depressive-like behavior in the forced swim and tail suspension test (170). In experimental models of stress, the decline in IL-4 levels in the locus coeruleus may be involved in anxiety-like behavior and an inverse relationship between IL-4 secretion and hypothalamic-pituitary-adrenal (HPA)/sympathetic-adrenal-medullary-axes activation has been reported (171). These findings suggest that modulation of the IL-4 receptor signaling pathway is required to adapt to homeostatic mechanisms in response to stressful events (171). In addition, it has been shown that microglial IL-4 receptor pathway modulates cognitive function following neuroinflammation (172).
It is well established that adult neurogenesis in the dentate gyrus of the hippocampus is regulated by specific microglia population and potentially implicated in MDD (10, 173). Very recently, Zhang et al. showed in rodents that IL-4 driven microglia modulate stress resilience through BDNF-dependent neurogenesis (173). Their findings indicated that IL-4 driven microglia are characterized by a high expression of Arg1 which is critical in maintaining hippocampal neurogenesis and stress resistance. Decreasing Arg1+ microglia in the hippocampus by knocking down the microglial IL-4 receptor inhibited hippocampal neurogenesis and enhanced stress vulnerability. Indeed, Increasing Arg1+ microglia in the hippocampus by enhancing IL4 signaling restored hippocampal neurogenesis and the resilience to stress-induced depression (173).
Following an inflammatory insult, endogenous IL-4 can interact with its tyrosine kinase IL-4 receptor on the microglia cell surface (Figure 2). This interaction induces the production of Galectin-3 (Gal-3), prostaglandin (PG) J2 and activates STAT6. Production or activation of these molecules ultimately leads to activation of canonical transcriptional factors for microglia polarization such as PPAR-γ. The transcriptional activity of PPAR-γ can induce microglia alternative activation by decreasing the production of ROS, pro-inflammatory cytokines and suppressing the activity of NF-κB (15, 27, 174). PPAR-γ is an important and canonical transcriptional factor in the induction of anti-inflammatory signaling pathways (discussed in detail in the next section).
Peroxisome Proliferator-Activated Receptor Gamma Mediated Pathway
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors of the nuclear hormone receptor superfamily. PPARs exist as three isoforms (α, γ, and δ/β). PPARs have a ligand binding domain and a DNA binding domain. When their endogenous or exogenous ligands bind to PPARs, they create a heterodimeric complex which recruits other co-activators including PPAR coactivator-1, PPAR-interacting protein, PPAR-binding protein, steroid receptor co-activator-1 and CREB binding protein. This complex binds to the promoter regions of specific genes that contain a regulatory element known as the peroxisome proliferator response element which either activates or transrepresses the target genes (175). In the mammalian body, PPARs control glucose metabolism, cell proliferation and differentiation (176). The fact that PPAR-γ is involved in the modulation of macrophage differentiation and activation in peripheral tissues led to studying of the role of PPAR-γ in CNS resident microglia. Several investigations indicate that PPAR-γ endogenous ligand and synthetic agonists might influence brain inflammation by inhibiting different functions related to microglial activation, such as production of inflammatory cytokines, chemokines, nitric oxide and prostaglandins (PGs) (177).
PPARγ ligands elicit anti-inflammatory and neuroprotective actions in various experimental models of neurodegenerative diseases (178). Indeed, PPARγ activation inhibits the activity of transcription factors including NF-κB, AP1, and STAT. Some studies indicate that IL-4 receptor signaling increases the endogenous level of PPAR-γ ligands such as PGJ2 and subsequently amplifies transcriptional activity of PPAR-γ which might polarize microglia phenotype toward the anti-inflammatory one (179).
The PPARγ-mediated pathway has been the subject of several pre-clinical and human studies of MDD. A low PPARγ level in the hippocampus and PFC has been associated with depressive-like behavior in mice (180). Selective agonists of PPAR-γ already have FDA approval for the treatment of type 2 diabetes and their potential antidepressant effects, through modulation of metabolism and inflammation, have been investigated in different models (181). Interestingly, the efficacy of PPAR-γ ligands has been shown in metabolic disorder to be associated with depressive-like behavior in rodents. Namely, obesity in rats results in downregulation of PPARγ in the PFC (182), meanwhile chronic treatment with pioglitazone reversed depressive-like behaviors associated with obesity in CUMS mouse model (183). There is an increased risk for obese patients with chronic low-grade inflammation to develop depression (184, 185). Also, obesity induces microglial activation and neuroinflammation that play crucial roles in the pathogenesis of depression (186).
In an interesting report Qin et al. demonstrated that CUMS can induce severe depressive-like behaviors, neuroinflammation and reduced expression of PPARγ in leptin-deficient (ob/ob) mice as compared to wild type mice. Administration of a selective PPARγ agonist, pioglitazone rectified the behavioral abnormalities and alleviated microglial pro-inflammatory cytokine levels and NF-κB activation in PFC and hippocampus (187). Other studies also investigated anti-inflammatory and antidepressant effects of PPAR-γ agonists. Li et al. aimed to explore the effects of pioglitazone on depressive-like behaviors of mice treated with LPS and elucidated the underlying mechanisms. Their findings indicated that PPAR-γ activation induces PI3K/AKT/JNK/p38 signaling pathway and counteract LPS mediated apoptosis in mice PFC (188).
Studies have provided evidence that the antidepressant-like effect of pioglitazone in the forced swim test is mediated partly through N-methyl-D-aspartate (NMDA) receptor signaling and nitric oxide pathway (189, 190). Cognitive impairment is a feature of both AD and psychiatric disorders. The PPARγ agonist, rosiglitazone improves hippocampus-dependent cognitive deficits. Its cognitive enhancement partly occurs through the induction of ERK cascade, a critical mediator of memory consolidation. Jahrling et al. showed that PPARγ agonism facilitated recruitment of PPARγ to pERK during memory consolidation (191). Other investigations have pointed to the involvement of NMDA receptor and nitric oxide pathway in the memory improving effects of PPARγ ligands (192, 193). These findings highlight the fact that PPARγ ligands might have therapeutic implication in MDD specifically in the patients that have memory impairments. Namely, Sepanjnia et al. showed that pioglitazone, a selective PPAR-γ agonist, is an effective and safe short-term add-on therapy to Citalopram in non-diabetic patients with MDD and was associated with a high rate of early improvement and remission (194). Taken together, these findings showcase the potential for developing new interventions that target the brain’s innate immune responses in different psychiatric disorders. However, much is still unknown about role of microglia in psychiatric disorders and why neuroinflammation is not a common phenomenon in all MDD patients (21).
Galectin-3 Mediated Pathway
Galectins are a family of soluble β-galactoside-binding proteins found in all multicellular organisms. They act as both DAMPs in innate immunity and/or as pattern-recognition receptors that bind to pathogen-associated molecular patterns. Gal-3 has recently been implicated in studies of neuroinflammatory diseases (195). This lectin is involved in cell-cell adhesion, modulation of the brain’s innate immune response and microglial activation patterns in both physiological and pathophysiological settings. Gal-3 also mediates cell proliferation and migration (196, 197). Several studies using different approaches and methods have demonstrated both protective and deleterious effects of Gal-3 in neuroinflammatory diseases making Gal-3 an attractive target in drug discovery. Among different galectin family members, Gal-3 is unique in that in addition to the carbohydrate recognition domain, it possesses a proline and glycine-rich N-terminal domain through which it forms oligomers (195, 198). Gal-3 is expressed in epithelial cells, endothelial cells, neurons and immune cells where it is synthesized as a cytosolic protein. It can be released or secreted into the extracellular space where several bind to cell surface glycoproteins (199). Originally identified as a marker of activated macrophages, there is increasing evidence suggesting its role as a modulator of microglial phenotypes in neuroinflammation (200).
Gal-3 plays important extracellular physiological roles. It uses IL-4 dependent mechanisms to mediate microglial arborization (195). In vitro studies highlight the importance of the carbohydrate-binding site of extracellular Gal-3 in microglia motility and ramification. Microglia pruning of axons and synaptic terminals might involve Gal-3 (18). Intracellular Gal-3 also holds distinct roles in physiological and pathological conditions. Following inflammatory insult, endogenous IL-4 interacts with microglial IL-4 receptors, thereby increasing the production of Gal-3 and subsequently inducing the canonical transcriptional factor PPAR-γ leading to anti-inflammatory signaling (Figure 2), as described in Section “Peroxisome Proliferator-Activated Receptor Gamma Mediated Pathway” (15, 201). In neuroinflammatory events Gal-3 elicits time-dependent protective actions. For instance, a Gal-3 feedback loop is critical for IL-4-mediated alternative polarization of peripheral macrophages (200, 202). It was also shown that following neuroinflammatory insult induction of Gal-3 in proliferating resident microglia is neuroprotective (27). Additionally, Gal-3 positive proliferating microglia are the major contributing cells of neurotrophic molecules such as insulin-like growth factor 1 (IGF-1) (200) which can also enhance the effects of trophic factors such as IGF-1 through inhibition of IGF-1 endocytosis (Figure 2) (195, 200). In 2019, Rahimian et al. studied time- and context-dependent effects of Gal-3 as a neuroprotective mediator following neuroinflammation (27). We showed that Gal-3 induces an anti-inflammatory microglial phenotype through IL-4 receptor pathway. It is likely that the polarization following neuroinflammatory insults is influenced by Gal-3 binding to glycans attached to IL-4 receptors (27).
In addition to its protective actions, Gal-3 also plays a pro-inflammatory role as reported in different animal and human studies especially in neurodegenerative disorders (195). Literature suggests that microglia-derived Gal-3 is detrimental in certain neuroinflammatory conditions. Indeed, comprehensive single-cell RNA analyses of CNS immune cells in neurodegenerative conditions including AD have led to the discovery of disease-associated microglia (DAM). This subpopulation of microglia displays a distinct transcriptional and functional signature (17). Boza-Serrano et al. showed that expression of Gal-3 in DAM in a mouse model of familial AD (5xFAD). They demonstrated that in 5xFAD mice Gal-3 is expressed solely in microglia associated with amyloid-β plaques and its deletion both decreases amyloid-β burden and improves memory function. Moreover, Gal-3 was found to be a TREM2 endogenous ligand binding through its carbohydrate-binding domain (203). Gal-3 direct interaction with TLR4 receptor may be an additional mechanism by which it regulates the severity of inflammation. Burguillos et al. showed that interaction of Gal-3 with TLR4 receptors in acute phase of neuroinflammation exacerbates neural cell death and prolongs inflammation, while its ablation elicits anti-inflammatory and neuroprotective effects (204).
Emerging experimental and clinical evidence indicates that Gal-3 may also play a role in MDD (205). Recently, Stajic et al. investigated the role of Gal-3 in modulation of anxiety levels in mice (206). The finding of this study revealed contradictory effects of Gal-3 on anxiety levels in the physiological condition and following acute inflammatory challenge with LPS. Gal-3 deficiency showed clear anxiogenic effect in basal conditions that is accompanied with lower expression of brain-derived neurotrophic factor (BDNF) and GABAA receptors. Gal-3 deficiency was also associated with anxiolytic response following acute administration of LPS (206). Intriguingly, the relationship between the novel inflammatory aspect of Gal-3 and depression symptom severity has been studied. In a large sample size, King et al. demonstrated higher Gal-3 levels were associated with higher levels of depressive symptoms. Their findings suggest that Gal-3 may be a new and useful inflammatory biomarker associated with depression (207). Another interesting clinical investigation showed that depression in type 1 diabetes is associated with high levels of circulating Gal-3 (208).
Metabolic Pathways in Microglia
Cannabinoid Signaling in Microglia
The endogenous cannabinoid (endocannabinoid) system has been implicated in synaptic communication and influences anxiety and cognition, metabolism, growth and development and response to internal and external immune insults via an array of actions mediated by their receptors (209, 210). In the CNS, endocannabinoids such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG) regulate several physiological functions via two main G-protein-coupled cannabinoid receptors 1 and 2 (CB1 and CB2) (211). Endocannabinoids can also interact with several extracellular and intracellular targets such as G-protein-coupled receptor 55 (GPR55), PPARs and transient receptor potential vanilloid 1 (212). CB1 receptors are expressed in the cortex, hippocampus, cerebellum, basal ganglia and brainstem, usually at presynaptic terminals or on axons (210). They have also been reportedly found on glial cells (213, 214). CB2 receptors are much less expressed in the CNS compared to CB1 receptors, however, they have been found in the brainstem, cerebellum and hippocampus among other areas (212) and are primarily found on immune cells, astrocytes and less commonly in neurons (215). Endocannabinoids are generally synthesized post-synaptically after Ca2+ influx or activation of Gq/11-linked G-protein-coupled receptors; they act in a retrograde fashion influencing presynaptic cell firing (210, 215).
AEA is synthesized by phospholipase D catalyzed hydrolysis of N-acylphosphatidylethanolamine (216), meanwhile, 2-AG synthesis from membrane phospholipids is catalyzed by phospholipase C and diacylglycerol lipase (217). AEA degradation occurs predominantly by the enzyme fatty acid amide hydrolase (FAAH) or by cyclooxygenase (COX)-2 oxidation creating PGs (210) while 2-AG degradation occurs mostly through monoacylglycerol lipase (MAGL) but it can sometimes be oxidized by COX-2 or hydrolyzed by FAAH (210, 218). Although some processes occur due to crosstalk between cell types (219), microglia contain the complete machinery required for a functional endocannabinoid system. Rodent microglia are known to express both CB1 and CB2 (220, 221). The presence of CB1 in human microglia is controversial (211, 222), however, a few studies describe CB1 microglial expression in active multiple sclerosis plaques of postmortem human brain samples (223, 224). Microglia also produce the enzymes responsible for hydrolysis and inactivation of AEA and 2-AG.
The endocannabinoid system has recently been implicated as a regulator of microglial migration and activity which points to cannabinoids being a useful target for modifying microglia in pathological conditions. Reusch et al. showed that the CB2 receptor is necessary for TLR-mediated microglia activation through p38 MAPK signaling (225). Other studies have revealed that the CB2 receptor is instrumental to induce the anti-inflammatory phenotype in microglia (226, 227). Tao et al. (227) found that JWH133, a selective CB2 receptor agonist promotes the anti-inflammatory phenotype in microglia through CB2 receptor stimulated cAMP/PKA pathway (227). We and others have showed that CB2 activation can trigger the activity of canonical anti-inflammatory transcriptional factors such as PPAR-γ (Figure 3) (228–230). As discussed in previous sections, the transcriptional activity of PPAR-γ is pivotal for microglia alternative activation by diminishing the production of pro-inflammatory cytokines and inhibiting the activity of NF-κB. Following neuroinflammation, CB2 receptors are upregulated (212) which has been shown to trigger microglia migration to the site of injury/lesion (231, 232). Experimental studies elucidate that neuroinflammation produces adenosine triphosphate (ATP) (discussed in the Purinergic Signaling section), which causes 2–AG production, commencing microglia migration through activation of the CB2 receptors at the microglial leading edge (233).
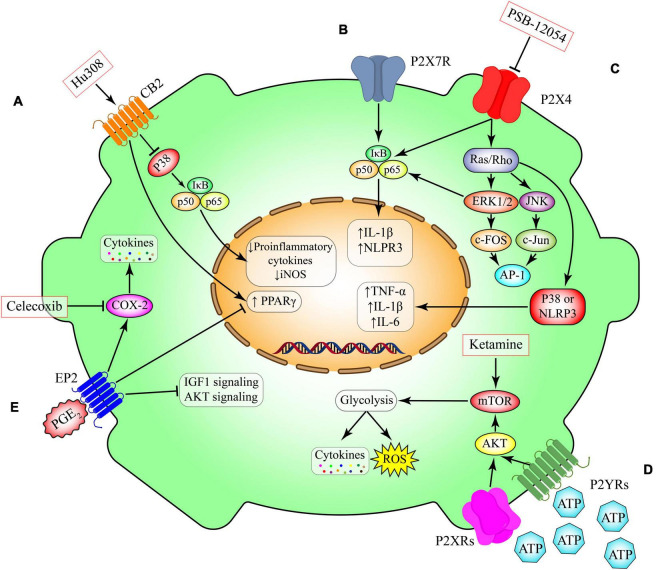
FIGURE 3.
Metabolic pathways in microglia. (A) Activation of CB2 receptors that are expressed in non-neural cells including microglia promotes anti-inflammatory cascades through inhibition of NFκB or induction of anti-inflammatory transcriptional factor such as peroxisome proliferator-activated receptor gamma (PPAR-γ). This pathway can be induced by Hu308. (B) P2X7 receptor activation, induces the canonical pro-inflammatory transcriptional factors such as NFκB and subsequent production of inflammatory mediators such as IL-1 beta and NLPR3. (C) Activation of ligand gated ion channel P2X4 triggers the switching on two canonical pathways including NFκB and Ras/ERK/JNK. These proteins induce the production of several cytokines such as TNF-α, IL-beta and IL-6. This pathway can be inhibited by PSB-12054. (D) Induction of the G-protein coupled receptors P2YRs have essential roles in modulating the expression of metabolic pathways such as mTOR and their downstream glucose metabolism. The induction of glycolysis through mTOR has been implicated in production of several cytokines and chemokines. Ketamine triggers the mTOR pathways leading to induction of glycolysis. (E) Prostaglandin E2 (PGE 2) is a lipid mediator derived from the fatty acid arachidonic acid. Its interaction with the microglial G-protein coupled receptor EP2 induces the activity of cyclooxygenase-2 (COX-2) and inhibits several intracellular pathways including PPAR-γ, AKT and IGF1. Celecoxib is s selective COX-2 inhibitor. CB2, cannabinoid type 2 (CB2) receptor; P2X4, P2X purinoceptor 4; P2X7, P2X purinoceptor 7; P2YR, purinergic receptor P2Y; EP2, prostaglandin E2 receptor 2; PGE2, prostaglandin E2; IGF1, insulin-like growth factor 1; AKT, RAC(Rho family)-alpha serine/threonine-protein kinase; COX-2, cyclooxygenase-2; TNF-α, tumor necrosis factor-α; iNOS, inducible nitric oxide synthase; IL-1β, interleukin 1 beta; IL-6, interleukin 6; PPAR-γ, peroxisome proliferator- activated receptor-gamma; mTOR, mammalian target of rapamycin; AP-1, activator protein 1; JNK, c-Jun N-terminal kinases; NLRP3, NLR family pyrin domain containing 3; ERK, extracellular-signal-regulated-kinase; IκB, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor; ROS, reactive oxygen species.
The human endocannabinoid system has been implicated in MDD (234, 235). A 2019 meta-analysis revealed a very strong association of CB2rs2501432 polymorphism with depressive disorder, but not CB1rs1049353 polymorphism (236). Moreover, peripheral serum levels of AEA and 2-AG are significantly reduced in women diagnosed with MDD (237). Few studies have investigated the endocannabinoid system in postmortem human brain of psychiatric cases. Hungund et al. (238) found that CB1 receptor protein is increased in the dorsolateral PFC of depressed suicides. Moreover, using [35S]GTPgammaS binding assays which assesses coupling of G-proteins to G-protein-coupled receptors in postmortem brain, the authors found that CB1 cannabinoid signaling was increased in the same region when compared to healthy controls (238) implicating cannabinoid signaling in both depression and suicide.
The role of the endocannabinoid system has also been studied in different stress paradigms (Table 3) (239, 240). RSD of mice has been used to model depression and anxiety. This paradigm not only has stress related behavioral outcomes and impaired fear extinction but also an increase in inflammation both peripherally and in the brain. Lisboa et al. showed that stimulating CB1/2 by injecting WIN55,212-2, a non-selective agonist, daily before the RSD paradigm, reduced IL-1β mRNA in the brain but specifically in CD68+ activated microglia. Moreover, activation of the CB1/2 receptors before RSD repaired fear extinction and stress-related behavioral deficits (240). Although interesting, this study begs the question of whether these protective effects were mediated through the CB1 or CB2 receptor. In 2011, Zoppi et al. showed that daily pre-stress administration of arachidonyl-2′-chloroethylamide (ACEA), a selective CB1 receptor agonist, prevented upregulation of pro-inflammatory markers in the PFC of wild type mice; but not in CB1–/– knockouts (239). In another study by García-Gutiérrez et al. overexpression of CB2 receptor in mice had a protective effect, providing resilience to chronic mild stress and decreased depressive-like behaviors measured by the forced swim test and novelty-suppressed feeding test. Interestingly, chronic (4-week) administration of the CB2 receptor antagonist AM630 had anti-depressant like effects in wild type mice but not those that overexpressed CB2 receptor (241). More recently, a CDS stress paradigm was used in both CB1–/– knockouts and wild type littermates. Beins et al. found that CB1 knockouts mice were much more susceptible to CSD stress and mild CSD showing significant stress behaviors. Moreover, these stressed CB1–/– mice had dysregulated HPA axes with insufficient glucocorticoid signaling and hyper-activated microglia (242). Interestingly, at baseline, CB1–/– mice have increased expression of Fkbp5, a negative regulator of glucocorticoid signaling and a gene already implicated in depression (243). Overall, it seems that the endocannabinoid system serves a protective role in counteracting neuroinflammation by induction of anti-inflammatory profiles in microglia.
TABLE 3.
Summary of cannabinoid receptors involvement in stress response.
| Study | Stress paradigm | Animal model | Treatment | Major findings |
| (239) | Immobilization/acoustic stress (2 h/day for 4 days) | Male Swiss ICR mice (WT and CB1–/– KO) |
CB1 agonist: arachidonyl-2′-chloroethylamide (ACEA) (2.5 mg/kg, daily before stress, intraperitoneally) |
WT: stress (1) ↑ CB1 mRNA and protein, (2) ↑ TNF-a mRNA (3) ↑ MCP-1, (4) ↑ NOS-2. ACEA pretreatment is protective against neuroinflammatory response to stress. KO: stress (1) dysregulates HPA axis, (2) ↑ TNF-a, (3) ↑ MCP-1. In sum: pretreatment with CB1 agonist is protective and CB1–/– KO aggravates neuroinflammation after stress. |
| (240) | Repeated social defeat (2 h/night for 6 nights) | Male C57BL/6 and aggressor CD-1 mice | CB1/2 agonist WIN55,212-2 (WIN) (1 mg/kg, daily 30 min before stress, intraperitoneally) | WIN (1) ↓ stress-induced anxiety, (2) ↓ IL-1 beta in CD68+ microglia (3) prevented stress-induced prolonged fear response and repaired fear extinction |
| (241) | Chronic unpredictable mild stress (several times a day for 7–8 weeks) | Male Swiss ICR mice (WT and CB2 overexpressors) | CB2 antagonist (6-iodo-2-methyl-1-(2-morpholinoethyl)-1H-indol-3-yl) (4-methoxyphenyl)methanone (AM630). (1 mg/kg, twice daily, post-stress, for 4 weeks, intraperitoneally) |
WT: stress (1) induced depression, (2) ↓ BDNF in hippocampus. CB2 overexpressor: (1) ↓ susceptibility to depression (2) no change in BDNF. Chronic AM630 treatment acted as an anti-depressant in stressed mice, protected against BDNF reduction. |
| (242) | Mild CSD stress (1–2 min/day for 10 days) | Male WT and B6.cg Cnr1tm1Zim
Cnr1–/– KO mice Male CD-1 aggressor mice |
– | KO: (1) ↑ susceptibility to mild CSD stress, (2) ↑ CD11b on microglia (3) ↑ percentage of CD11b+ microglia (4) ↓ microglial complexity. |
WT, wild-type; KO, knockout; CB1, cannabinoid receptor 1; CB2, cannabinoid receptor 2; ↑, increased; ↓, decreased/reduced; HPA, hypothalamic-pituitary-adrenal; MCP-1, monocyte chemoattractant protein-1; NOS-2, nitric oxide synthase-2; BDNF, brain derived neurotrophic factor.
Rescuing cellular function as a treatment for MDD has been mainly considered for neurons but not for glial cells. However, many investigations have demonstrated the functional impairment in glia cells as mentioned throughout this review. Microglial malfunctions have been studied in many different neuroinflammatory settings (244). Research has shown that different aspects of microglia such as phagocytic activity, secretory profile and metabolic pathways can be affected by neuroinflammation. Due to this complexity, selective modulators rather than general anti-inflammatory agents might be needed to rescue microglial functions following different types of inflammatory insults (10). One interesting and promising target could be endocannabinoid system. Fine tuning of this system in microglia can open a new avenue of research in pharmacotherapy of depression although several experimental and clinical studies should be performed long before being able to design cell-specific interventions for treating MDD.
Prostaglandin Signaling in Microglia
The PGs are a class of eicosanoids that are formed by the liberation of arachidonic acid from phospholipids and a 2-step conversion by COX, the rate-limiting enzymes (245). Two main isoforms of COX exist, COX-1 and COX-2. COX-1 is traditionally considered as a constitutive enzyme while COX-2 is inducible. However, such classification is not very precise especially in the brain where constitutive expression of COX-2 has been reported (245). One of the canonical PGs in the CNS is PGE2. It interacts with different G-protein-coupled receptors including EP1, EP2, EP3, and EP4 (Figure 3) (246). Intriguingly, elevated PGE2 in the saliva, serum and CSF of depressed patients has previously been reported (247–250). Clinical investigations also revealed that adjunctive therapy with non-steroidal anti-inflammatory drugs, known as COX inhibitors, might have therapeutic effects in a subset of MDD patients (251, 252).
Human studies have shown decreased dopamine metabolites in the CSF of MDD patients. Furthermore, acute treatment with antidepressants induces dopamine release in the medial PFC (253, 254). These findings imply that activation of the mesocortical dopaminergic pathway has anti-depressive properties. In this context, the role of PGE2 has been studied in several experimental paradigms. It has been shown that EP1-deficient mice showed hyperdopaminergic activity, leading to impulsive behaviors under acute social and environmental stress (255). It is noteworthy that EP1 is located on GABAergic terminals on midbrain dopamine neurons and electrophysiological recording indicates that EP1 stimulation potentiates inhibitory synaptic inputs to these neurons (256). These findings suggest that PGE2-EP1 signaling suppresses midbrain dopamine neurons and regulates impulsive behaviors under acute stress (257). However, the mechanisms underlying involvement of the mesocortical dopaminergic pathway in vulnerability to repeated stress is unknown. Indeed, PGE2-EP1 signaling attenuates mesocortical dopaminergic pathway, leading to susceptibility of mice to RSD (254). Analyses of c-Fos expression of ventral tegmental area dopamine neurons and dopamine turnover in medial PFC showed that the mesocortical dopaminergic pathway is activated upon social defeat and attenuated with repetition of social defeat in wild-type mice. EP1 deficiency abolished such repeated stress-induced attenuation of mesocortical dopaminergic pathway (254).
Intriguingly, PGE2 acting on striatal medium spiny neurons has been suggested to elicit a negative affective state in response to inflammatory or social stress (254, 258). Our knowledge about the source of PGE2 was limited until recently. Klawonn et al. revealed that microglial PG signaling as critical for inflammation-induced aversion and is a potential mechanism by which different types of stressors may converge to produce a negative affective state and potentially depression (259). Indeed, these findings demonstrated that striatal microglial activation induces negative affect and both IL-6 and PG dependent signaling in microglia is critical for inflammation induced aversion. Chemogenetic activation of striatal microglia induces an aversive affective state while chemogenetic inhibition of microglia blocks inflammation induced aversion. Microglial IL-6 signaling and PG synthesis regulate affective state and finally PGE2 from activated microglia reduces the excitability of striatal neurons (259). In agreement with these findings, different investigations indicate that low-dose aspirin, which primarily inhibits COX-1 and consequently PG production, reduces the risk of depression (260). The inducible form of COX, COX2, might also be involved in the MDD pathology, since the COX-2 selective inhibitor celecoxib has beneficial effects in subset of depressed patients (83, 261). Since strong microglial COX-1 expression is complemented by COX-2 in response to chronic inflammation and stress (262, 263), both enzymes could contribute to depressive symptoms at different stages of the disease or in distinct patient groups (259).
Mammalian Target of Rapamycin Signaling in Microglia
The mammalian target of rapamycin (mTOR), the evolutionarily conserved serine/threonine protein kinase, may be activated by phosphorylation in response to growth factors (such as BDNF), mitogens and stress (264, 265). The mTOR signaling pathway plays a fundamental role in the regulation of protein synthesis, energy metabolism, lipid metabolism, cell growth and autophagy (266). In the CNS, mTOR is also involved in axonal sprouting, axonal regeneration and myelination, ionic and receptor channel expression, dendritic spine growth, as well as astrocyte migration and proliferation. mTOR-regulated processes in the brain influence neuronal excitability, neuronal survival, synaptic and behavioral plasticity, cognition, feeding, and control of circadian rhythm (265). In recent years, special attention has been given to the role of mTOR signaling in MDD. Several investigations have reported decreased brain mTOR activation in animal models of depression (267). One of the most studied models is CUS, which mimics several behavioral and neurochemical alterations that occur in depressed individuals (268). Rodents exposed to CUS exhibit depressive-like behaviors associated with a reduction in phosphorylation levels of mTOR and its downstream signaling components, such as phosphor-p70S6K, in the PFC, hippocampus and amygdala (268, 269). Regarding the anti-depressive role of the mTOR pathway, an elegant study by Li et al. revealed a single dose of ketamine can activate mTOR, resulting in increased PFC synaptic protein expression within 2 h and increased dendritic spine density and synaptic activity within 24 h (267). Importantly, clinical evidence also confirms the role of mTOR signaling in MDD pathology (270). It has been shown that mTOR, p70S6K, eIF4B, and p-eIF4B protein expression in PFC of deceased MDD subjects were reduced when compared with controls, indicating a deficit in mTOR-dependent signaling leading to impairment in its downstream targets that control translation of synaptic proteins (270).
Intriguingly, mTOR signaling can regulate several aspects of microglial function such as phagocytosis and cell survival (271). For instance, inhibition of mTOR diminishes the viability of primary cultured microglia (272), whereas induction of mTOR activity by inhibiting its upstream suppressor, tuberous sclerosis 1, enhanced phagocytosis in microglia (271, 273). Furthermore, microglial-specific inhibition of mTOR pathway decreases proinflammatory cytokines and chemokines (274). More recently, it has been shown that mTOR-mediated metabolic reprogramming shapes distinct microglial functions in response to LPS and ATP (271). Hu et al. showed that both LPS and ATP induced rapid activation of mTOR and glycolysis in microglia. Blocking either glucose metabolism or mTOR activity inhibits glycolysis significantly and mitigates LPS-induced production of proinflammatory cytokines, indicating that mTOR-driven glycolysis is required for the proinflammatory responses of LPS-primed microglia (271). Additionally, blocking mTOR activity not only inhibits glycolysis but also suppresses BDNF and TNF-α production in ATP-activated microglia, suggesting the critical role of mTOR in tuning microglia function (271). Better understanding of the metabolic regulation of microglia help us to manipulate and control the activity of microglia following different neuroinflammatory insults. The distinct metabolic adaptation in microglia in response to different stressors may provide diverse approaches to target microglia at different states and restrain microglia-triggered neuroinflammation in neuropsychiatric disorders (271).
Our group provided the first evidence of increased microglial activation in dorsal ACC white matter of depressed suicides. Although total density of Iba1+ microglia remained unchanged between depressed suicides and matched controls, the ratio of primed to ramified microglia was significantly increased in depressed suicides (10, 38). The mechanisms underlying microglial priming are unknown. However, it has been shown that the mTOR signaling pathway plays a crucial role in microglial priming during aging. Keane et al. were the first to show that microglia from aged mice have upregulated mTOR complex 1 signaling controlling translation and protein levels of inflammatory mediators (275). Genetic ablation of mTOR signaling in mouse microglia caused an NF-κB–dependent upregulation of priming genes at the mRNA level. However, mice displayed reduced cytokine protein levels, lessened microglial activation and milder sickness behavior. Similar changes were present in aged human microglia revealing that upregulation of mTOR-dependent translation is an essential aspect of microglia priming in aging (275). It is possible that abnormalities in microglial mTOR signaling are involved in the emergence of the primed microglial phenotype in MDD.
Purinergic Signaling in Microglia
ATP, adenosine di-phosphate (ADP) and adenosine are molecules that are involved in purinergic signaling through P1, P2X, and P2Y receptors. G-protein-coupled P1 receptors are selective for adenosine and there are four subtypes A1, A2A, A2B, and A3. Meanwhile P2 receptors are activated by both ATP and ADP. There are two types of P2 receptors, namely P2X ionotropic channels and P2Y G-protein-coupled receptors (Figure 3). P2X receptors have seven subtypes while P2Y have eight subtypes. Microglia express several purinergic receptors (276) including: P2X1, P2X4, P2X7 (277), P2Y4, P2Y7, P2Y6, P2Y11 P2Y12, P2Y13, A1, A2A and A2B (278). Notably, adenosine binding to A2A has been shown to mediate microglial process retraction (279). Besides microglial motility, purinergic signaling is important in many other processes such as neurodevelopment and neuron-glial crosstalk and inflammation (280). Interestingly, many aspects of the purinergic signaling system have been implicated in depression (281) and here, we will focus on the receptors expressed by microglia (Table 4).
TABLE 4.
Summary of microglial purinergic signal transduction.
| Receptor | Type | Ligand | Role |
| A2A | G-protein coupled receptor | Adenosine | Process retraction (279) |
| P2Y1 | G protein coupled receptor | ADP | Migration and chemotaxis (285, 286) |
| P2Y6 | G-protein coupled receptor | Uridine | Mediates cytokine secretion (295) |
| P2Y12 | G-protein coupled receptor | ADP | Specifically expressed in microglia (288). Migration and chemotaxis (285, 286, 289). Neuron-microglia interactions (287, 288, 293). Regulates innate fear behaviors (293) |
| P2X4 | Ligand gated ion channel | ATP | Phenotype switch to pro-inflammatory (276) Drives release of BDNF (294) |
| P2X7 | Ligand gated ion channel | ATP | Mediates cytokine secretion (295) Oligomerization of NLR3P inflammasome (296) |
BDNF, brain derived neurotrophic factor; NLR3P, NLR family pyrin domain containing 3.
ATP is released by injured cells into the extracellular space acting as DAMPs and a chemoattractant for microglial processes (233, 282, 283). Experimentally injected ATP causes microglia chemotaxis and process extension toward the site of injection (284). This process occurs through the G-protein-coupled P2Y (P2Y1R and P2Y12R) receptors (285, 286). P2Y12R is involved in long-range communication between neurons and microglia (287, 288), as well as microglia chemotaxis preceding phagocytosis (288). Additionally, P2Y12R acts as a marker for healthy microglia and is downregulated in the active pro-inflammatory phenotype in mice (289). There seems to be a species difference in the expression of P2Y12R as human brain resection from epileptic temporal lobe shows PY212R in both ramified and amoeboid microglia (290). Furthermore, the same tissue shows ADP stimulating process retraction (291) conversely to process extension in mouse microglia (284, 289). Although similar methods of comparative investigation have not yet been applied to all purinergic signaling, these findings alone suggest that they may differ greatly between species, providing necessary context to rodent studies. Loss of P2Y12R is associated with alteration in recognition and social memory as well as anxiety-like behavior in adult mice (292). Also, microglial P2Y12R regulates neural excitability and fear behaviors in developing and adult mice (293). Intriguingly, a human postmortem study found increased levels of P2Y12R protein in MDD microglia from four different brain regions, suggesting more resting state microglia in MDD and contradicting the neuroinflammation hypothesis (98).
Activation of microglial P2X4R by ATP (Figure 3) leads to a phenotype switch to pro-inflammatory state (276). Interestingly, in an ischemic stroke model, deletion of P2X4R from myeloid cells was shown to be protective in mice by inhibition of excessive pro-inflammatory cytokine release. However, after 30 days, the mice developed depressive-like phenotypes possibly due to missing P2X4R’s crucial role in BDNF release (294).
Once microglia are activated, cytokine secretion is mediated by P2Y6R (295) and P2X7R. Activation of P2X7R by ATP acting as DAMPs signal leads to oligomerization of NLR3P inflammasomes (Figure 3) (296). NLR3P inflammasome cleavage of pro-caspase 1 into caspase 1 results in the production of IL1-β from these activated microglia in situations of neuroinflammation such as in depression (297–299). In fact, a 2017 study showed that a 3-week CUS paradigm induced a depressive phenotype, increased extracellular ATP leading to P2X7R activation; this was inhibited by chronic treatment with P2X7R antagonists Brilliant Blue G (BBG) and A438079. Furthermore, P2X7R null mice that underwent the CUS did not develop a depressive phenotype irrespective of sex (300). In fact, the antidepressant phenotype of P2X7R deletion (301, 302) might be mediated by increased neurogenesis and serotonin bioavailability in the hippocampus (303). In humans, studies have suggested that polymorphisms Gln460Arg (rs2230912) (304) and His155Tyr (rs208294) in the P2RX7 gene are associated with depression and other mood disorders (305, 306). However, a 2014 meta-analysis has contradicted these findings (307).
In the healthy brain, P2X7R is not activated by physiological ATP levels, in fact, P2X7R activation only occurs when ATP acts as DAMPs (308). Intriguingly, P2X7R antagonists that cross the BBB are in clinical trials with the hopes of using them as adjunct or monotherapy for depression caused by neuroinflammation (309, 310). Interestingly, cholesterol and other lipids like phosphatidylglycerol and sphingomyelin modulate the function of P2X7R (311). Our lab and others have found altered brain lipids in psychopathology (312) and depression (313–315) suggesting lipid control of P2X7R should be further investigated. Generally, lipids in the cell membrane organize position and function of proteins. They can be also released from the membrane acting as messengers transmitting signals (316). Lipid composition of the brain affects cognition, perception and mood most likely by influencing neuroinflammation and neurogenesis (317). Moreover, decreased polyunsaturated fatty acids seem to be the most frequently implicated lipid change in depression (313, 317) and they are known in regulating CB1 receptor (317) and P2X7R (311, 318). Interestingly, dietary supplementation with fish oil rich in polyunsaturated fats decreases depressive-like behavior in LPS treated mice and suppresses activation of the NLRP3 inflammasome and P2X7R (318). Meanwhile, sphingolipids have been implicated in both the etiology of MDD and the beneficial effects of antidepressants (316, 319). In addition to diet (320), lipid composition in the brain can be altered by environmental factors including exercise (321) and medications (322), making them promising targets for treatment (319).
To our knowledge, there is a paucity of studies on the role of microglial adenosine receptors in depression. However, A1 receptor activation generally inhibits serotonin release in the hippocampus, while A2 receptor activation promotes the release of this neurotransmitter (323). Moreover, A2 receptors have been found to be involved in the dysregulation of the HPA axis observed in depression (324). Furthermore, a 2021 study showed that 14 days of restraint stress induced depression and anxiety in the mice with a neuroinflammatory phenotype. The authors discovered increased expression of P2X7R and A2A receptor in the hippocampus and prefrontal cortex. Next, they showed that BBG a P2X7R-selective antagonist and caffeine an A2A receptor antagonist attenuated the depressive phenotype (325).
Iron Metabolism in Microglia
Iron metabolism is crucial for normal functioning of the brain, including myelination by oligodendrocytes and neurotransmitter synthesis (serotonin, dopamine, and norepinephrine) (326). Transferrin, a glycoprotein is mainly responsible for the movement of iron through the body and has high affinity to Fe3+ (ferric iron) (327). Movement of iron from the periphery into the brain occurs through the BBB. Transferrin receptor 1 (TfR1) on endothelial cells mediates the uptake of transferrin bound iron (Tf-Fe) into the brain, while also regulating the return of iron-depleted transferrin back to the blood (326, 328). Tf-Fe is packaged into endosomes and then released into the brain through ferroportin 1 (Fp1) as Fe2+ where it is oxidized to Fe3+ through ferroxidases hephaestin or ceruloplasmin (Cp) (326, 329). Fp1 levels are controlled by hepcidin (HepC) which binds to Fp1 leading to its internalization and subsequent degradation (330). The metabolism of iron in the brain is highly controlled as it is extremely important for proper cell functioning, however, iron overload can be extremely toxic and cause cell death through oxidative stress (329). In the brain, iron is distributed heterogeneously with concentration varying by region (331, 332) and cell type (333). Iron is mainly distributed in the hippocampus (331) and the basal ganglia stored in ferritin (334) or neuromelanin (335). Presumably all cell types can uptake iron through either Tf-Fe or non-transferrin bound pathways. Neurons and astrocytes uptake Tf-Fe through TfR1 and divalent metal transporter 1 (DMT1). Oligodendrocytes uptake iron through the TfR1/DMT1 pathway only in immature states; once they mature, iron enters through heavy (H-) chain ferritin binding to H-ferritin receptor on the cell membrane (336). Oligodendrocytes have very high iron necessity for their normal functioning and proper myelination (333, 336). Microglia have two different ways of regulating iron influx; the first being through Tf-Fe uptake via the TfR1/DMT1 pathway while the second is uptake of non-Tf-Fe through transferrin-independent mechanisms (326). In healthy brain, iron usually travels bound to transferrin, which is produced by oligodendrocytes and ChP, however, it is only secreted by the latter (337).
Pathogens such as bacteria utilize iron from the host to replicate. To protect the host, immune cells are programmed to reduce iron availability to prevent further infiltration by the invader (338). This happens through increased production of HepC in hepatocytes, stimulated by IL-6 release from immune cells (339) which reduces the export of iron into the blood by negative regulation of ferroportin (340). It has been shown that different activation status in microglia prefer one iron intake pathway over the other (341). In neuroinflammatory conditions, iron is sequestered in pro-inflammatory microglia and neurons through non-transferrin bound pathways (342). In vitro LPS stimulation of microglia results in downregulation of TfR1 (338, 343) and ferroportin (338) and upregulation of DMT1 and ferritin (344). Under this condition, iron accumulates intracellularly and secretion of inflammatory cytokines and metalloproteinases is increased (343). Conversely, when microglia are stimulated with IL-4, an anti-inflammatory phenotype of microglia prefers Tf-Fe uptake and increases cellular transferrin receptor levels (344).
The WHO estimates that 37% of females are iron deficient globally, while males are rarely diagnosed (345). Both iron deficiency and depression were in the top disorders for most years lived with disability globally (346). Interestingly, females are twice as more likely to be diagnosed with MDD. Recent literature has implicated iron dysregulation, specifically iron deficiency, in the pathology of depression (347–351). Indeed, a recent study found that depressed individuals are 3 times more likely than healthy individuals to have hypoferritinemia (low iron storage) and acquired hypotransferrinemia (decreased levels of protein transferrin) (351). The fact that iron is a co-factor for rate limiting steps of serotonin, dopamine and norepinephrine synthesis and neuronal uptake further implicates involvement of iron metabolism in the etiology of depression (351, 352). Whether iron deficiency detected in the periphery is adequately representative of iron deficiency in the brain is still under speculation due to the tight control of iron metabolism in the brain (352). However, a 2007 study by Vostrikov et al., reported significantly reduced numbers of oligodendrocytes in layer 3 of BA9 in the PFC of postmortem samples from MDD patients (353). Given that oligodendrocytes hold the most iron in the brain, it is reasonable to infer decreased iron levels in postmortem MDD brain. Moreover, research from our lab has implicated oligodendrocyte precursor cell dysregulation with MDD (354). Iron deficiency has been shown to lead to hypomyelination in both animals and humans (355). A 2018 study revealed that developmental iron deficiency kept not only oligodendrocyte lineage cells in immature states but also other glia cells. Interestingly, iron deficiency inhibits microglial inflammatory cytokine secretion after LPS stimulation (356). Additionally, it has been shown that microglia constitute the primary source of iron to oligodendrocyte precursor cells during myelination (357).
Considering that microglia are involved in oligodendrocyte differentiation and myelin repair (358), it is a logical next step to investigate the role of iron metabolism in microglia and the relationship to depression. Few studies have investigated the role of microglia iron metabolism and depression and the ones that examined this relationship in animals found a different trend than the aforementioned human studies. Gao et al. showed that CSD stress resulted in depressive phenotype, increased microglial activation, increased iron, decreased Fp1 and increased ferritin, DMT1 and HepC in the hippocampus (359). Similarly, Jiao et al. found that mice with CUMS-induced behavioral despair were found to have increased brain iron, increased inflammation and Fe2+ levels in the hippocampus. Genes involved in ferroptosis were differentially regulated; specifically, glutathione peroxidase (GPX4, necessary for repairing oxidative damage) was downregulated after CUMS suggesting a role of ferroptosis in the phenotype of depression. In addition, this study also showed that fluoxetine not only resolved depressive behaviors but also restored normal ferroptosis signaling (360). Experimental models also display an increase in iron load in the brain which results in anxiety symptoms. A study by Texel et al. shows that Cp knockout mice have iron overload in the periphery, but lower levels of iron in the hippocampus accompanied by decreased levels of 5-HT, norepinephrine and BDNF (361). In another study, Pellegrino et al. showed that knockout of transferrin receptor 2 leads to iron overload in the brain, dysregulation of microglia activation status and increased anxiety levels in the animals (362). Overall, regulation of iron metabolism by microglia is an important factor in brain physiology and pathophysiology. Future studies are needed to investigate the postmortem brain of depressed individuals to better understand the link between iron metabolism abnormalities in brain innate immune system and MDD pathology.
Sexual Dimorphism in Microglial Response in Healthy Brain and Following Stress
In recent years, special attention has also been given to the sexually dimorphic role of microglia in both healthy neurodevelopmental and diseased neurodegenerative brains (15). Sex differences have been reported in distribution, structure, function, transcriptomic and proteomic profiles of microglia in both physiological and neuroinflammatory conditions (10). Evidence demonstrates that microglia play an instrumental role in the sexually dimorphic differentiation of the developing brain (363). Interestingly, the number and phenotype of microglia differ in many regions of the brain when comparing male and female rodents (364). Brain areas such as the preoptic area, cerebral cortex and the amygdala and hippocampus have been reported to have microglial sexual dissimilarity (363–366). Importantly, the postnatal sexual dimorphism persists into the adult brain. These marked differences may lead to development of distinct, sex-dependent microglia inflammatory responses in pathological conditions. In fact, sex differences in microglial activation patterns following neuroinflammation have been reported by different groups (367, 368). However, whether the observed differences are exclusively hormone-dependent and/or stem from distinct developmental mechanisms remains to be elucidated. The analyses of the different anti- and pro-inflammatory signaling events reveal that they differ in males and females.
Several observations suggest marked sex differences in the microglial activation patterns following stress. In a pioneer study, Bollinger et al. revealed differential effects of stress on microglial activation in female and male medial PFC (369). Importantly, dysfunction of this brain area is implicated in MDD pathology. It has been shown that chronic stress affects the medial PFC in a sex-dependent manner and impairs prefrontal mediated behaviors in males and females (369). Unstressed female rats show a greater proportion of primed to ramified microglia relative to males, alongside increased CX3CL1-CX3CR1 levels. In addition, acute stress and CRS diminished the ratio of primed to ramified microglia and microglial CD40 expression in females but not in males (369). Another intriguing study revealed that the expression of genes related to cellular stress, neuroimmune state and neuron-microglia communication varied between unstressed male and female rats in a region-specific manner (370). Namely, in the dorsal hippocampus, chronic stress increased immune markers expression in males but not females. It is noteworthy that the type of stress can also mediate sex-specific innate immune response. For instance, acute stress increased microglia-associated transcripts in basolateral amygdala in males, whereas chronic stress altered immune factor expression in basolateral amygdala more broadly in females (370). It was recently shown that chronic stress induces different neurobiological adaptations in the PFC of male and female mice. CUS causes behavioral changes and microglia-mediated neuronal remodeling only in the frontal cortex of male mice (371). Fluorescence-activated cell sorting and gene expression analyses by Woodburn et al. indicate that CUS increased expression of markers of phagocytosis only in male PFC microglia (371). Overall, these findings suggest that sex differences might impact microglia pro- and anti-inflammatory signaling pathways, leading to different outcomes.
Conclusion
Cytokine research in MDD faces several difficulties, such as conflicting results and high variability of cytokine levels within samples. Several studies have only reported serum or plasma cytokine levels without taking into account potential confounding factors such as age, body weight, smoking, alcohol consumption and medication, which can all influence the plasma content of cytokines (372, 373). These limitations imply that serum levels of cytokines may not reflect their levels in the brain and, therefore, may not reflect pathology (373, 374). Direction of causality is another important factor that is not clear in the cytokine research in psychiatric disorders. Although there is strong evidence for the involvement of cytokines in the pathology of MDD, the direction of this relationship has not yet been clarified with certainty. For instance, the alteration of cytokine levels might also be the consequence of a psychiatric disorder. Factors such as antidepressants or body mass index (that could change as a result of the disorder) might cause fluctuation in cytokine levels (373, 375).
An association between neuroinflammation and MDD has been reported by multiple studies (10) and it is well-established that inflammatory markers are increased in a subset of MDD patients (84, 85, 376) but not all (10, 21). Although microglial abnormalities have been reported in MDD, no link has been made with specific diagnostic categories. It is noteworthy that recent attempts at targeting inflammation as a new therapy for MDD have not been very promising. In fact, it appears that anti-inflammatory treatment options may only be effective in patients who show signs of increased peripheral inflammation along with depressive behaviors (83, 84). Namely, it has been shown that antagonizing TNF-α as a therapy may only prove useful in patients with high baseline inflammatory biomarkers (85). With similar uncertainty, general immunosuppressants and anti-inflammatory agents such as minocycline and non-steroidal anti-inflammatory drugs that were promising in animal studies (10) have failed in treating neuropsychiatric disorders probably due to their non-selective dampening of the immune response. Targeting of pro- or anti-inflammatory brain cytokines for developing new pharmacotherapeutics is not easy. As discussed in detail in this review, these cytokines have essential physiological actions and blockade of their signaling might influence several crucial processes such as cell survival and neural plasticity. Furthermore, the diversity of brain cell types that produce cytokines makes it difficult to target their signaling in a neuroinflammatory context. Finally, different receptor subtypes have different roles following neuroinflammation (e.g., TNF-α and IL-6 receptors).
It is becoming increasingly obvious that microglia act as a double-edged sword in the etiopathology of MDD. On one hand, some experimental studies both in vitro and in vivo have linked neuronal damage with the inflammatory phenotype of microglia releasing neurotoxic mediators and ROS (377). On the other hand, other investigations have reported the beneficial roles of microglial anti-inflammatory phenotype in neuronal regeneration and neurogenesis. Whether microglia play a positive or negative role depends on several factors including type of neuroinflammatory insult, brain region, time, sex and age. Indeed, heterogeneity of microglia and their context-dependent properties warrant more research to gain a clearer understanding of this dynamic cell type before new therapeutics can be successfully developed for psychiatric disorders such as MDD.
Author Contributions
RR, CB, and NM conceived the review. RR, CB, and RC wrote the manuscript. NM supervised the project and revised the manuscript. All authors read and agreed with the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
- MDD
major depressive disorder
- TNF- α
tumor necrosis factor-alpha
- IL
interleukin
- RA
rheumatoid arthritis
- TGF- β
transforming growth factor β
- PPAR- γ
peroxisome proliferator-activated receptors-gamma
- CNS
central nervous system
- NF κ B
nuclear factor kappa-light-chain-enhancer of activated B cells
- BBB
blood–brain barrier
- CUS
chronic unpredictable stress
- TNF- α
tumor necrosis factor-alpha
- ACC
anterior cingulate cortex
- LPS
lipopolysaccharide
- CSD
chronic social defeat
- ROS
reactive oxygen species
- CUMS
chronic unpredictable mild stress
- TLR
toll-like receptor
- TNFR1
TNF- α Receptor 1
- TNFR2
TNF- α Receptor 2
- MAPK
mitogen-activated protein kinases
- CSF
cerebrospinal fluid
- HPA
hypothalamic-pituitary-adrenal
- gp130
glycoprotein 130
- RSD
repeated social defeat
- IL-6R
IL-6 receptor
- DAMPs
danger-associated molecular patterns
- MyD88
myeloid differentiation primary response 88
- AD
Alzheimer’s disease
- PFC
prefrontal cortex
- IFN- γ
interferon-gamma
- JAK
janus kinase
- STAT
signal transducer and activator of transcription
- ERK
extracellular-signal-regulated-kinase
- SSRIs
selective serotonin reuptake inhibitors
- ChP
choroid plexus
- α 7 nAChR
Alpha-7 nicotinic acetylcholine receptor
- Nrf2
nuclear factor-erythroid factor 2-related factor 2
- CRS
chronic restraint stress
- Gal-3
galectin-3
- PG
prostaglandin
- PPARs
peroxisome proliferator-activated receptors
- NMDA
N-methyl-D-aspartate
- IGF-1
insulin-like growth factor 1
- DAM
disease-associated microglia
- BDNF
brain-derived neurotrophic factor
- endocannabinoid
endogenous cannabinoid
- AEA
anandamide
- 2-AG
2-arachidonoylglycerol
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- GPR55
G-protein -coupled receptor 55
- FAAH
fatty acid amide hydrolase
- COX
cyclooxygenase
- MAGL
monoacylglycerol lipase
- ATP
adenosine triphosphate
- ACEA
arachidonyl-2 ′ -chloroethylamide
- mTOR
mammalian target of rapamycin
- ADP
adenosine di-phosphate
- BBG
Brilliant Blue G
- Tf-Fe
transferrin bound iron
- Fp1
ferroportin 1
- Cp
ceruloplasmin
- HepC
hepcidin.
Funding
This work was funded by a CIHR grant to NM (PJT-156346). RR is an FRQ-S fellow and CB the recipient of an FRQ-S doctoral scholarship.
References
- 1.Young JJ, Bruno D, Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J Affect Disord. (2014) 169:15–20. 10.1016/j.jad.2014.07.032 [DOI] [PubMed] [Google Scholar]
- 2.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. (2006) 27:24–31. 10.1016/j.it.2005.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. (2009) 66:287–92. 10.1016/j.biopsych.2009.01.030 [DOI] [PubMed] [Google Scholar]
- 4.Brundin L, Sellgren CM, Lim CK, Grit J, Pålsson E, Landén M, et al. An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl Psychiatry. (2016) 6:e865–865. 10.1038/tp.2016.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. (2016) 21:1358–65. 10.1038/mp.2015.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haroon E, Fleischer CC, Felger JC, Chen X, Woolwine BJ, Patel T, et al. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry. (2016) 21:1351–7. 10.1038/mp.2015.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haroon E, Chen X, Li Z, Patel T, Woolwine BJ, Hu XP, et al. Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia. Transl Psychiatry. (2018) 8:189. 10.1038/s41398-018-0241-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, et al. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. (1995) 34:301–9. 10.1016/0165-0327(95)00028-L [DOI] [PubMed] [Google Scholar]
- 9.Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum Il-6 and Il-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. (1997) 9:853–8. 10.1006/cyto.1997.0238 [DOI] [PubMed] [Google Scholar]
- 10.Rahimian R, Wakid M, O’Leary LA, Mechawar N. The emerging tale of microglia in psychiatric disorders. Neurosci Biobehav Rev. (2021) 131:1–29. 10.1016/j.neubiorev.2021.09.023 [DOI] [PubMed] [Google Scholar]
- 11.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. (2010) 67:446–57. 10.1016/j.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- 12.Marrie RA, Walld R, Bolton JM, Sareen J, Patten SB, Singer A, et al. Psychiatric comorbidity increases mortality in immune-mediated inflammatory diseases. Gen Hosp Psychiatry. (2018) 53:65–72. 10.1016/j.genhosppsych.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 13.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. (2017) 23:1018–27. 10.1038/nm.4397 [DOI] [PubMed] [Google Scholar]
- 14.Tan Y-L, Yuan Y, Tian L. Microglial regional heterogeneity and its role in the brain. Mol Psychiatry. (2020) 25:351–67. 10.1038/s41380-019-0609-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahimian R, Cordeau P, Jr, Kriz J. Brain response to injuries: when microglia go sexist. Neuroscience. (2019) 405:14–23. 10.1016/j.neuroscience.2018.02.048 [DOI] [PubMed] [Google Scholar]
- 16.Bordt EA, Ceasrine AM, Bilbo SD. Microglia and sexual differentiation of the developing brain: a focus on ontogeny and intrinsic factors. Glia. (2020) 68:1085–99. 10.1002/glia.23753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. (2017) 169:1276–90.e1217. 10.1016/j.cell.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 18.Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. (2019) 50:253–71.e256. 10.1016/j.immuni.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Sagar, et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. (2019) 566:388–92. 10.1038/s41586-019-0924-x [DOI] [PubMed] [Google Scholar]
- 20.Mechawar N, Savitz J. Neuropathology of mood disorders: do we see the stigmata of inflammation? Transl Psychiatry. (2016) 6:e946. 10.1038/tp.2016.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mondelli V, Vernon AC, Turkheimer F, Dazzan P, Pariante CM. Brain microglia in psychiatric disorders. Lancet Psychiatry. (2017) 4:563–72. 10.1016/s2215-0366(17)30101-3 [DOI] [PubMed] [Google Scholar]
- 22.Singhal G, Baune BT. Microglia: an interface between the loss of neuroplasticity and depression. Front Cell Neurosci. (2017) 11:270. 10.3389/fncel.2017.00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci. (2015) 38:637–58. 10.1016/j.tins.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 24.Tay TL, Béchade C, D’Andrea I, St-Pierre M-K, Henry MS, Roumier A, et al. Microglia gone rogue: impacts on psychiatric disorders across the lifespan. Front Mol Neurosci. (2018) 10:421. 10.3389/fnmol.2017.00421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng SL, Chen JG, Wang F. Microglia: a central player in depression. Curr Med Sci. (2020) 40:391–400. 10.1007/s11596-020-2193-1 [DOI] [PubMed] [Google Scholar]
- 26.Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, et al. Identification of a unique TGF-β–dependent molecular and functional signature in microglia. Nat Neurosci. (2014) 17:131–43. 10.1038/nn.3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahimian R, Lively S, Abdelhamid E, Lalancette-Hebert M, Schlichter L, Sato S, et al. Delayed galectin-3-mediated reprogramming of microglia after stroke is protective. Mol Neurobiol. (2019) 56:6371–85. 10.1007/s12035-019-1527-0 [DOI] [PubMed] [Google Scholar]
- 28.Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. (2020) 7:272–81. 10.1016/S2215-0366(19)30302-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Timberlake MA, II, Prall K, Dwivedi Y. The recent progress in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. (2017) 77:99–109. 10.1016/j.pnpbp.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enache D, Pariante CM, Mondelli V. Markers of central inflammation in major depressive disorder: a systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun. (2019) 81:24–40. 10.1016/j.bbi.2019.06.015 [DOI] [PubMed] [Google Scholar]
- 31.Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci. (2017) 20:1752–60. 10.1038/s41593-017-0010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudek KA, Dion-Albert L, Lebel M, LeClair K, Labrecque S, Tuck E, et al. Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc Natl Acad Sci U.S.A. (2020) 117:3326–36. 10.1073/pnas.1914655117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. (2010) 141:775–85. 10.1016/j.cell.2010.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schubert I, Ahlbrand R, Winter A, Vollmer L, Lewkowich I, Sah R. Enhanced fear and altered neuronal activation in forebrain limbic regions of CX3CR1-deficient mice. Brain Behav Immun. (2018) 68:34–43. 10.1016/j.bbi.2017.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milior G, Lecours C, Samson L, Bisht K, Poggini S, Pagani F, et al. Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain Behav Immun. (2016) 55:114–25. 10.1016/j.bbi.2015.07.024 [DOI] [PubMed] [Google Scholar]
- 36.Kana V, Desland FA, Casanova-Acebes M, Ayata P, Badimon A, Nabel E, et al. CSF-1 controls cerebellar microglia and is required for motor function and social interaction. J Exp Med. (2019) 216:2265–81. 10.1084/jem.20182037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia X, Gao Z, Hu H. Microglia in depression: current perspectives. Sci China Life Sci. (2021) 64:911–25. 10.1007/s11427-020-1815-6 [DOI] [PubMed] [Google Scholar]
- 38.Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun. (2014) 42:50–9. 10.1016/j.bbi.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 39.Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. (2011) 8:94. 10.1186/1742-2094-8-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boutej H, Rahimian R, Thammisetty SS, Béland LC, Lalancette-Hébert M, Kriz J. Diverging mRNA and protein networks in activated microglia reveal SRSF3 suppresses translation of highly upregulated innate immune transcripts. Cell Rep. (2017) 21:3220–33. 10.1016/j.celrep.2017.11.058 [DOI] [PubMed] [Google Scholar]
- 41.Dillingh MR, van Poelgeest EP, Malone KE, Kemper EM, Stroes ESG, Moerland M, et al. Characterization of inflammation and immune cell modulation induced by low-dose LPS administration to healthy volunteers. J Inflamm. (2014) 11:28. 10.1186/s12950-014-0028-1 [DOI] [Google Scholar]
- 42.Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. (2015) 12:114. 10.1186/s12974-015-0332-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan YF, Huang GB, Xu MD, Gao F, Lin S, Huang J, et al. Anti-depression effects of ketogenic diet are mediated via the restoration of microglial activation and neuronal excitability in the lateral habenula. Brain Behav Immun. (2020) 88:748–62. 10.1016/j.bbi.2020.05.032 [DOI] [PubMed] [Google Scholar]
- 44.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner L, Tonia M, et al. β-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. (2011) 31:6277. 10.1523/JNEUROSCI.0450-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. (2012) 37:1491–505. 10.1016/j.psyneuen.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, et al. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry. (2014) 75:970–81. 10.1016/j.biopsych.2013.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci. (2014) 34:2583. 10.1523/JNEUROSCI.3723-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, Godbout JP. Neuroinflammatory dynamics underlie memory impairments after repeated social defeat. J Neurosci. (2016) 36:2590. 10.1523/JNEUROSCI.2394-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramirez K, Sheridan JF. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive- like behaviors. Brain Behav Immunity. (2016) 57:293–303. 10.1016/j.bbi.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lehmann ML, Weigel TK, Poffenberger CN, Herkenham M. The behavioral sequelae of social defeat require microglia and are driven by oxidative stress in mice. J Neurosci. (2019) 39:5594. 10.1523/JNEUROSCI.0184-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. (2009) 24:27–53. 10.1007/s11011-008-9118-1 [DOI] [PubMed] [Google Scholar]
- 52.Vogt MA, Mallien AS, Pfeiffer N, Inta I, Gass P, Inta D. Minocycline does not evoke anxiolytic and antidepressant-like effects in C57BL/6 mice. Behav Brain Res. (2016) 301:96–101. 10.1016/j.bbr.2015.12.015 [DOI] [PubMed] [Google Scholar]
- 53.Zhang C, Zhang YP, Li YY, Liu BP, Wang HY, Li KW, et al. Minocycline ameliorates depressive behaviors and neuro-immune dysfunction induced by chronic unpredictable mild stress in the rat. Behav Brain Res. (2019) 356:348–57. 10.1016/j.bbr.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 54.Miyaoka T, Wake R, Furuya M, Liaury K, Ieda M, Kawakami K, et al. Minocycline as adjunctive therapy for patients with unipolar psychotic depression: an open-label study. Prog Neuropsychopharmacol Biol Psychiatry. (2012) 37:222–6. 10.1016/j.pnpbp.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 55.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. (1996) 334:1717–25. 10.1056/nejm199606273342607 [DOI] [PubMed] [Google Scholar]
- 56.Horiuchi T, Mitoma H, Harashima S-I, Tsukamoto H, Shimoda T. Transmembrane TNF-α: structure, function and interaction with anti-TNF agents. Rheumatology. (2010) 49:1215–28. 10.1093/rheumatology/keq031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. (2010) 20:87–103. 10.1615/critreveukargeneexpr.v20.i2.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Idriss HT, Naismith JH. TNFα and the TNF receptor superfamily: structure-function relationship(s). Microsc Res Tech. (2000) 50:184–95. 10.1002/1097-0029(20000801)50:33.0.CO;2-H [DOI] [PubMed] [Google Scholar]
- 59.Subedi L, Lee SE, Madiha S, Gaire BP, Jin M, Yumnam S, et al. Phytochemicals against TNFα-mediated neuroinflammatory diseases. Int J Mol Sci. (2020) 21:764. 10.3390/ijms21030764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guadagno J, Xu X, Karajgikar M, Brown A, Cregan SP. Microglia-derived TNFα induces apoptosis in neural precursor cells via transcriptional activation of the Bcl-2 family member Puma. Cell Death Dis. (2013) 4:e538. 10.1038/cddis.2013.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uzzan S, Azab AN. Anti-TNF-α compounds as a treatment for depression. Molecules. (2021) 26:2368. 10.3390/molecules26082368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spriggs DR, Deutsch S, Kufe DW. Genomic structure, induction, and production of TNF-alpha. Immunol Ser. (1992) 56:3–34. [PubMed] [Google Scholar]
- 63.Muhammad M. Tumor Necrosis Factor Alpha: A Major Cytokine of Brain Neuroinflammation. London: IntechOpen; (2020). [Google Scholar]
- 64.To SQ, Knower KC, Clyne CD. Origins and actions of tumor necrosis factor α in postmenopausal breast cancer. J Interferon Cytokine Res. (2013) 33:335–45. 10.1089/jir.2012.0155 [DOI] [PubMed] [Google Scholar]
- 65.Probert L. TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience. (2015) 302:2–22. 10.1016/j.neuroscience.2015.06.038 [DOI] [PubMed] [Google Scholar]
- 66.Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. (2010) 17:6–10. 10.1016/j.jocn.2009.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Syed MM, Phulwani NK, Kielian T. Tumor necrosis factor-alpha (TNF-α) regulates toll-like receptor 2 (TLR2) expression in microglia. J Neurochem. (2007) 103:1461–71. 10.1111/j.1471-4159.2007.04838.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodriguez M, Zoecklein L, Papke L, Gamez J, Denic A, Macura S, et al. Tumor necrosis factor alpha is reparative via TNFR2 [corrected] in the hippocampus and via TNFR1 [corrected] in the striatum after virus-induced encephalitis. Brain Pathol. (2009) 19:12–26. 10.1111/j.1750-3639.2008.00151.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stacey D, Redlich R, Büschel A, Opel N, Grotegerd D, Zaremba D, et al. TNF receptors 1 and 2 exert distinct region-specific effects on striatal and hippocampal grey matter volumes (VBM) in healthy adults. Genes Brain Behav. (2017) 16:352–60. 10.1111/gbb.12318 [DOI] [PubMed] [Google Scholar]
- 70.Jang D-I, Lee AH, Shin H-Y, Song H-R, Park J-H, Kang T-B, et al. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int J Mol Sci. (2021) 22:2719. 10.3390/ijms22052719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Medler J, Wajant H. Tumor necrosis factor receptor-2 (TNFR2): an overview of an emerging drug target. Exp Opin Therap Targets. (2019) 23:295–307. 10.1080/14728222.2019.1586886 [DOI] [PubMed] [Google Scholar]
- 72.Vasanthi P, Nalini G, Rajasekhar G. Role of tumor necrosis factor-alpha in rheumatoid arthritis: a review. APLAR J Rheumatol. (2007) 10:270–4. 10.1111/j.1479-8077.2007.00305.x [DOI] [Google Scholar]
- 73.Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin. (2008) 29:1275–88. 10.1111/j.1745-7254.2008.00889.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Troubat R, Barone P, Leman S, Desmidt T, Cressant A, Atanasova B, et al. Neuroinflammation and depression: a review. Eur J Neurosci. (2021) 53:151–71. [DOI] [PubMed] [Google Scholar]
- 75.Olmos G, Lladó J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Med Inflamm. (2014) 2014:1–12. 10.1155/2014/861231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karol DE, Criscione-Schreiber LG, Lin M, Clowse ME. Depressive symptoms and associated factors in systemic lupus erythematosus. Psychosomatics. (2013) 54:443–50. 10.1016/j.psym.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 77.Postal M, Lapa AT, Sinicato NA, de Oliveira Peliçari K, Peres FA, Costallat LTL, et al. Depressive symptoms are associated with tumor necrosis factor alpha in systemic lupus erythematosus. J Neuroinflammation. (2016) 13:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zou W, Feng R, Yang Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS One. (2018) 13:e0197267. 10.1371/journal.pone.0197267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. (2013) 246:199–229. 10.1016/j.neuroscience.2013.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res. (2014) 79:1–12. 10.1016/j.neures.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 81.Ohgidani M, Kato TA, Sagata N, Hayakawa K, Shimokawa N, Sato-Kasai M, et al. TNF-α from hippocampal microglia induces working memory deficits by acute stress in mice. Brain Behav Immun. (2016) 55:17–24. 10.1016/j.bbi.2015.08.022 [DOI] [PubMed] [Google Scholar]
- 82.Miller AH. Beyond depression: the expanding role of inflammation in psychiatric disorders. World Psychiatry. (2020) 19:108–9. 10.1002/wps.20723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. (2014) 71:1381–91. 10.1001/jamapsychiatry.2014.1611 [DOI] [PubMed] [Google Scholar]
- 84.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. (2016) 16:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. (2013) 70:31–41. 10.1001/2013.jamapsychiatry.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raffaele S, Lombardi M, Verderio C, Fumagalli M. TNF production and release from microglia via extracellular vesicles: impact on brain functions. Cells. (2020) 9:2145. 10.3390/cells9102145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dong Y, Fischer R, Naudé PJ, Maier O, Nyakas C, Duffey M, et al. Essential protective role of tumor necrosis factor receptor 2 in neurodegeneration. Proc Natl Acad Sci U.S.A. (2016) 113:12304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao H, Danzi MC, Choi CS, Taherian M, Dalby-Hansen C, Ellman DG, et al. Opposing functions of microglial and macrophagic TNFR2 in the pathogenesis of experimental autoimmune encephalomyelitis. Cell Rep. (2017) 18:198–212. 10.1016/j.celrep.2016.11.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Veroni C, Gabriele L, Canini I, Castiello L, Coccia E, Remoli ME, et al. Activation of TNF receptor 2 in microglia promotes induction of anti-inflammatory pathways. Mol Cell Neurosci. (2010) 45:234–44. 10.1016/j.mcn.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 90.Hodes GE, Ménard C, Russo SJ. Integrating interleukin-6 into depression diagnosis and treatment. Neurobiol Stress. (2016) 4:15–22. 10.1016/j.ynstr.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tanaka T, Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol Res. (2014) 2:288–94. 10.1158/2326-6066.Cir-14-0022 [DOI] [PubMed] [Google Scholar]
- 92.Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, Haegeman G, et al. Interleukin-6, a mental cytokine. Brain Res Rev. (2011) 67:157–83. 10.1016/j.brainresrev.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 93.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. (2015) 16:448–57. 10.1038/ni.3153 [DOI] [PubMed] [Google Scholar]
- 94.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. (2015) 49:206–15. 10.1016/j.bbi.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS, et al. IL-1β, IL-6, TNF- α and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci Rep. (2018) 8:12050. 10.1038/s41598-018-30487-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. (2000) 22:370–9. 10.1016/s0893-133x(99)00134-7 [DOI] [PubMed] [Google Scholar]
- 97.Sasayama D, Hattori K, Wakabayashi C, Teraishi T, Hori H, Ota M, et al. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J Psychiatr Res. (2013) 47:401–6. 10.1016/j.jpsychires.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 98.Böttcher C, Fernández-Zapata C, Snijders GJL, Schlickeiser S, Sneeboer MAM, Kunkel D, et al. Single-cell mass cytometry of microglia in major depressive disorder reveals a non-inflammatory phenotype with increased homeostatic marker expression. Transl Psychiatry. (2020) 10:310. 10.1038/s41398-020-00992-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramirez K, Shea DT, McKim DB, Reader BF, Sheridan JF. Imipramine attenuates neuroinflammatory signaling and reverses stress-induced social avoidance. Brain Behav Immun. (2015) 46:212–20. 10.1016/j.bbi.2015.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aniszewska A, Chłodzińska N, Bartkowska K, Winnicka MM, Turlejski K, Djavadian RL. The expression of interleukin-6 and its receptor in various brain regions and their roles in exploratory behavior and stress responses. J Neuroimmunol. (2015) 284:1–9. 10.1016/j.jneuroim.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 101.Maes M, Anderson G, Kubera M, Berk M. Targeting classical IL-6 signalling or IL-6 trans-signalling in depression? Expert Opin Ther Targets. (2014) 18:495–512. 10.1517/14728222.2014.888417 [DOI] [PubMed] [Google Scholar]
- 102.Sun Y, Wang D, Salvadore G, Hsu B, Curran M, Casper C, et al. The effects of interleukin-6 neutralizing antibodies on symptoms of depressed mood and anhedonia in patients with rheumatoid arthritis and multicentric Castleman’s disease. Brain Behav Immun. (2017) 66:156–64. 10.1016/j.bbi.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 103.Ting EY, Yang AC, Tsai SJ. Role of interleukin-6 in depressive disorder. Int J Mol Sci. (2020) 21:2194. 10.3390/ijms21062194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. (2003) 85:85–95. 10.1016/S0165-2478(02)00228-6 [DOI] [PubMed] [Google Scholar]
- 105.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. (2005) 3:36–46. 10.1038/nrmicro1068 [DOI] [PubMed] [Google Scholar]
- 106.Płóciennikowska A, Hromada-Judycka A, Borzęcka K, Kwiatkowska K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. (2015) 72:557–81. 10.1007/s00018-014-1762-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Figueroa-Hall LK, Paulus MP, Savitz J. Toll-like receptor signaling in depression. Psychoneuroendocrinology. (2020) 121:104843. 10.1016/j.psyneuen.2020.104843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rocha DM, Caldas AP, Oliveira LL, Bressan J, Hermsdorff HH. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis. (2016) 244:211–5. 10.1016/j.atherosclerosis.2015.11.015 [DOI] [PubMed] [Google Scholar]
- 109.Tripathi P, Aggarwal A. NF-kB transcription factor: a key player in the generation of immune response. Curr Sci. (2006) 90:519–31. [Google Scholar]
- 110.Kuriyan J, Thanos D. Structure of the NF-κB transcription factor: a holistic interaction with DNA. Structure. (1995) 3:135–41. 10.1016/S0969-2126(01)00143-5 [DOI] [PubMed] [Google Scholar]
- 111.Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. (2006) 25:6680–4. 10.1038/sj.onc.1209954 [DOI] [PubMed] [Google Scholar]
- 112.Kaltschmidt C, Kaltschmidt B, Lannes-Vieira J, Kreutzberg GW, Wekerle H, Baeuerle PA, et al. Transcription factor NF-κB is activated in microglia during experimental autoimmune encephalomyelitis. J Neuroimmunol. (1994) 55:99–106. 10.1016/0165-5728(94)90151-1 [DOI] [PubMed] [Google Scholar]
- 113.Lucas K, Maes M. Role of the toll like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol. (2013) 48:190–204. 10.1007/s12035-013-8425-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu Y-C, Yeh W-C, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. (2008) 42:145–51. 10.1016/j.cyto.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 115.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. (2004) 16:3–9. 10.1016/j.smim.2003.10.003 [DOI] [PubMed] [Google Scholar]
- 116.Young A, Campbell E, Lynch S, Suckling J, Powis S. Aberrant NF-kappaB expression in autism spectrum condition: a mechanism for neuroinflammation. Front Psychiatry. (2011) 2:27. 10.3389/fpsyt.2011.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bassani TB, Vital MA, Rauh LK. Neuroinflammation in the pathophysiology of Parkinson’s disease and therapeutic evidence of anti-inflammatory drugs. Arq Neuropsiquiatr. (2015) 73:616–23. 10.1590/0004-282x20150057 [DOI] [PubMed] [Google Scholar]
- 118.MacDowell KS, Pinacho R, Leza JC, Costa J, Ramos B, García-Bueno B. Differential regulation of the TLR4 signalling pathway in post-mortem prefrontal cortex and cerebellum in chronic schizophrenia: relationship with SP transcription factors. Prog Neuro Psychopharmacol Biol Psychiatry. (2017) 79:481–92. 10.1016/j.pnpbp.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 119.Song M, Jin J, Lim J-E, Kou J, Pattanayak A, Rehman JA, et al. TLR4 mutation reduces microglial activation, increases Aβ deposits and exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. J Neuroinflammation. (2011) 8:92. 10.1186/1742-2094-8-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou Y, Chen Y, Xu C, Zhang H, Lin C. TLR4 targeting as a promising therapeutic strategy for Alzheimer disease treatment. Front Neurosci. (2020) 14:602508. 10.3389/fnins.2020.602508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang J, Yang C, Liu Z, Li X, Liu M, Wang Y, et al. Association of the TLR4 gene with depressive symptoms and antidepressant efficacy in major depressive disorder. Neurosci Lett. (2020) 736:135292. 10.1016/j.neulet.2020.135292 [DOI] [PubMed] [Google Scholar]
- 122.Rasmusson AJ, Gallwitz M, Soltanabadi B, Ciuculete DM, Mengel-From J, Christensen K, et al. Toll-like receptor 4 methylation grade is linked to depressive symptom severity. Transl Psychiatry. (2021) 11:371. 10.1038/s41398-021-01481-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Salim S, Liu H, Atrooz F. P-395 - Early life stress, stress-resilience/susceptibility and oxidative stress. Free Radic Biol Med. (2018) 120:S165. 10.1016/j.freeradbiomed.2018.04.542 [DOI] [Google Scholar]
- 124.Olugbemide AS, Ben-Azu B, Bakre AG, Ajayi AM, Femi-Akinlosotu O, Umukoro S. Naringenin improves depressive- and anxiety-like behaviors in mice exposed to repeated hypoxic stress through modulation of oxido-inflammatory mediators and NF-kB/BDNF expressions. Brain Res Bull. (2021) 169:214–27. 10.1016/j.brainresbull.2020.12.003 [DOI] [PubMed] [Google Scholar]
- 125.Fu S, Wang J, Hao C, Dang H, Jiang S. Tetramethylpyrazine ameliorates depression by inhibiting TLR4-NLRP3 inflammasome signal pathway in mice. Psychopharmacology. (2019) 236:2173–85. 10.1007/s00213-019-05210-6 [DOI] [PubMed] [Google Scholar]
- 126.Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. (2012) 33:267–86. 10.1016/j.yfrne.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu J, Buisman-Pijlman F, Hutchinson MR. Toll-like receptor 4: innate immune regulator of neuroimmune and neuroendocrine interactions in stress and major depressive disorder. Front Neurosci. (2014) 8:309. 10.3389/fnins.2014.00309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rahimifard M, Maqbool F, Moeini-Nodeh S, Niaz K, Abdollahi M, Braidy N, et al. Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation. Ageing Res Rev. (2017) 36:11–9. [DOI] [PubMed] [Google Scholar]
- 129.Inserra A, Mastronardi CA, Rogers G, Licinio J, Wong ML. Neuroimmunomodulation in major depressive disorder: focus on caspase 1, inducible nitric oxide synthase, and interferon-gamma. Mol Neurobiol. (2019) 56:4288–305. 10.1007/s12035-018-1359-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kustova Y, Sei Y, Morse HC, Jr, Basile AS. The influence of a targeted deletion of the IFNgamma gene on emotional behaviors. Brain Behav Immun. (1998) 12:308–24. 10.1006/brbi.1998.0546 [DOI] [PubMed] [Google Scholar]
- 131.Litteljohn D, Cummings A, Brennan A, Gill A, Chunduri S, Anisman H, et al. Interferon-gamma deficiency modifies the effects of a chronic stressor in mice: implications for psychological pathology. Brain Behav Immun. (2010) 24:462–73. 10.1016/j.bbi.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 132.Campos AC, Vaz GN, Saito VM, Teixeira AL. Further evidence for the role of interferon-gamma on anxiety- and depressive-like behaviors: involvement of hippocampal neurogenesis and NGF production. Neurosci Lett. (2014) 578:100–5. 10.1016/j.neulet.2014.06.039 [DOI] [PubMed] [Google Scholar]
- 133.Zhang J, He H, Qiao Y, Zhou T, He H, Yi S, et al. Priming of microglia with IFN-γ impairs adult hippocampal neurogenesis and leads to depression-like behaviors and cognitive defects. Glia. (2020) 68:2674–92. 10.1002/glia.23878 [DOI] [PubMed] [Google Scholar]
- 134.Alboni S, Poggini S, Garofalo S, Milior G, El Hajj H, Lecours C, et al. Fluoxetine treatment affects the inflammatory response and microglial function according to the quality of the living environment. Brain Behav Immun. (2016) 58:261–71. 10.1016/j.bbi.2016.07.155 [DOI] [PubMed] [Google Scholar]
- 135.Horikawa H, Kato TA, Mizoguchi Y, Monji A, Seki Y, Ohkuri T, et al. Inhibitory effects of SSRIs on IFN-γ induced microglial activation through the regulation of intracellular calcium. Prog Neuropsychopharmacol Biol Psychiatry. (2010) 34:1306–16. 10.1016/j.pnpbp.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 136.Augusto-Oliveira M, Arrifano GP, Delage CI, Tremblay M, Crespo-Lopez ME, Verkhratsky A. Plasticity of microglia. Biol Rev Camb Philos Soc. (2022) 97:217–50. 10.1111/brv.12797 [DOI] [PubMed] [Google Scholar]
- 137.Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. (2016) 19:987–91. 10.1038/nn.4338 [DOI] [PubMed] [Google Scholar]
- 138.Devorak J, Torres-Platas SG, Davoli MA, Prud’homme J, Turecki G, Mechawar N. Cellular and molecular inflammatory profile of the choroid plexus in depression and suicide. Front Psychiatry. (2015) 6:138. 10.3389/fpsyt.2015.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kunis G, Baruch K, Rosenzweig N, Kertser A, Miller O, Berkutzki T, et al. IFN-γ-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair. Brain. (2013) 136(Pt 11):3427–40. 10.1093/brain/awt259 [DOI] [PubMed] [Google Scholar]
- 140.Fakhfouri G, Rahimian R, Ghia JE, Khan WI, Dehpour AR. Impact of 5-HT3 receptor antagonists on peripheral and central diseases. Drug Discov Today. (2012) 17:741–7. 10.1016/j.drudis.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 141.Seyedabadi M, Rahimian R, Ghia JE. The role of alpha7 nicotinic acetylcholine receptors in inflammatory bowel disease: involvement of different cellular pathways. Expert Opin Ther Targets. (2018) 22:161–76. 10.1080/14728222.2018.1420166 [DOI] [PubMed] [Google Scholar]
- 142.Fakhfouri G, Mousavizadeh K, Mehr SE, Dehpour AR, Zirak MR, Ghia JE, et al. From chemotherapy-induced emesis to neuroprotection: therapeutic opportunities for 5-HT3 receptor antagonists. Mol Neurobiol. (2015) 52:1670–9. 10.1007/s12035-014-8957-5 [DOI] [PubMed] [Google Scholar]
- 143.Khalifeh S, Fakhfouri G, Mehr SE, Mousavizadeh K, Dehpour AR, Khodagholi F, et al. Beyond the 5-HT3 receptors: a role for α7nACh receptors in neuroprotective aspects of tropisetron. Hum Exp Toxicol. (2015) 34:922–31. 10.1177/0960327114562034 [DOI] [PubMed] [Google Scholar]
- 144.Fakhfouri G, Rahimian R, Dyhrfjeld-Johnsen J, Zirak MR, Beaulieu JM. 5-HT receptor antagonists in neurologic and neuropsychiatric disorders: the iceberg still lies beneath the surface. Pharmacol Rev. (2019) 71:383–412. 10.1124/pr.118.015487 [DOI] [PubMed] [Google Scholar]
- 145.King JR, Gillevet TC, Kabbani N. A G protein-coupled α7 nicotinic receptor regulates signaling and TNF-α release in microglia. FEBS Open Bio. (2017) 7:1350–61. 10.1002/2211-5463.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Patel H, McIntire J, Ryan S, Dunah A, Loring R. Anti-inflammatory effects of astroglial α7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-κB pathway and activation of the Nrf2 pathway. J Neuroinflammation. (2017) 14:192. 10.1186/s12974-017-0967-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alzarea S, Rahman S. Effects of alpha-7 nicotinic allosteric modulator PNU 120596 on depressive-like behavior after lipopolysaccharide administration in mice. Prog Neuropsychopharmacol Biol Psychiatry. (2018) 86:218–28. 10.1016/j.pnpbp.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 148.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. (2006) 27:482–91. 10.1016/j.tips.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 149.Williams DK, Wang J, Papke RL. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem Pharmacol. (2011) 82:915–30. 10.1016/j.bcp.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. (2007) 151:915–29. 10.1038/sj.bjp.0707264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Papke RL, Kem WR, Soti F, López-Hernández GY, Horenstein NA. Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine. J Pharmacol Exp Ther. (2009) 329:791–807. 10.1124/jpet.108.150151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. (2004) 89:337–43. 10.1046/j.1471-4159.2004.02347.x [DOI] [PubMed] [Google Scholar]
- 153.Suzuki T, Hide I, Matsubara A, Hama C, Harada K, Miyano K, et al. Microglial alpha7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J Neurosci Res. (2006) 83:1461–70. 10.1002/jnr.20850 [DOI] [PubMed] [Google Scholar]
- 154.Abbas M, Alzarea S, Papke RL, Rahman S. The α7 nicotinic acetylcholine receptor positive allosteric modulator attenuates lipopolysaccharide-induced activation of hippocampal IκB and CD11b gene expression in mice. Drug Discov Ther. (2017) 11:206–11. 10.5582/ddt.2017.01038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Corradi J, Bouzat C. Understanding the bases of function and modulation of α7 nicotinic receptors: implications for drug discovery. Mol Pharmacol. (2016) 90:288–99. 10.1124/mol.116.104240 [DOI] [PubMed] [Google Scholar]
- 156.Alzarea S, Rahman S. Alpha-7 nicotinic receptor allosteric modulator PNU120596 prevents lipopolysaccharide-induced anxiety, cognitive deficit and depression-like behaviors in mice. Behav Brain Res. (2019) 366:19–28. 10.1016/j.bbr.2019.03.019 [DOI] [PubMed] [Google Scholar]
- 157.Rahman S, Alzarea S. Glial mechanisms underlying major depressive disorder: potential therapeutic opportunities. Prog Mol Biol Transl Sci. (2019) 167:159–78. 10.1016/bs.pmbts.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 158.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U.S.A. (2003) 100:1920–5. 10.1073/pnas.0438019100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao D, Xu X, Pan L, Zhu W, Fu X, Guo L, et al. Pharmacologic activation of cholinergic alpha7 nicotinic receptors mitigates depressive-like behavior in a mouse model of chronic stress. J Neuroinflammation. (2017) 14:234. 10.1186/s12974-017-1007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Martín-de-Saavedra MD, Budni J, Cunha MP, Gómez-Rangel V, Lorrio S, Del Barrio L, et al. Nrf2 participates in depressive disorders through an anti-inflammatory mechanism. Psychoneuroendocrinology. (2013) 38:2010–22. 10.1016/j.psyneuen.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 161.Parada E, Egea J, Buendia I, Negredo P, Cunha AC, Cardoso S, et al. The microglial α7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid Redox Signal. (2013) 19:1135–48. 10.1089/ars.2012.4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Egea J, Buendia I, Parada E, Navarro E, León R, Lopez MG. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem Pharmacol. (2015) 97:463–72. 10.1016/j.bcp.2015.07.032 [DOI] [PubMed] [Google Scholar]
- 163.Freitas AE, Egea J, Buendia I, Gómez-Rangel V, Parada E, Navarro E, et al. Agmatine, by improving neuroplasticity markers and inducing Nrf2, prevents corticosterone-induced depressive-like behavior in mice. Mol Neurobiol. (2016) 53:3030–45. 10.1007/s12035-015-9182-6 [DOI] [PubMed] [Google Scholar]
- 164.Navarro E, Gonzalez-Lafuente L, Pérez-Liébana I, Buendia I, López-Bernardo E, Sánchez-Ramos C, et al. Heme-oxygenase I and PCG-1α regulate mitochondrial biogenesis via microglial activation of alpha7 nicotinic acetylcholine receptors using PNU282987. Antioxid Redox Signal. (2017) 27:93–105. 10.1089/ars.2016.6698 [DOI] [PubMed] [Google Scholar]
- 165.Cuadrado A, Manda G, Hassan A, Alcaraz MJ, Barbas C, Daiber A, et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev. (2018) 70:348–83. 10.1124/pr.117.014753 [DOI] [PubMed] [Google Scholar]
- 166.Rahimian R, Fakhfouri G, Zirak MR. Pros and cons of 5-HT receptor antagonists in neuropsychiatric diseases. Biomed Pharmacother. (2019) 118:109301. 10.1016/j.biopha.2019.109301 [DOI] [PubMed] [Google Scholar]
- 167.Rezvani AH, Kholdebarin E, Brucato FH, Callahan PM, Lowe DA, Levin ED. Effect of R3487/MEM3454, a novel nicotinic alpha7 receptor partial agonist and 5-HT3 antagonist on sustained attention in rats. Prog Neuropsychopharmacol Biol Psychiatry. (2009) 33:269–75. 10.1016/j.pnpbp.2008.11.018 [DOI] [PubMed] [Google Scholar]
- 168.Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. J Immunol. (2012) 189:4213–9. 10.4049/jimmunol.1202246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu X, Liu J, Zhao S, Zhang H, Cai W, Cai M, et al. Interleukin-4 is essential for microglia/macrophage M2 polarization and long-term recovery after cerebral ischemia. Stroke. (2016) 47:498–504. 10.1161/strokeaha.115.012079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wachholz S, Knorr A, Mengert L, Plümper J, Sommer R, Juckel G, et al. Interleukin-4 is a participant in the regulation of depressive-like behavior. Behav Brain Res. (2017) 326:165–72. 10.1016/j.bbr.2017.03.020 [DOI] [PubMed] [Google Scholar]
- 171.Lee HJ, Park HJ, Starkweather A, An K, Shim I. Decreased interleukin-4 release from the neurons of the locus coeruleus in response to immobilization stress. Med Inflamm. (2016) 2016:3501905. 10.1155/2016/3501905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pu H, Ma C, Zhao Y, Wang Y, Zhang W, Miao W, et al. Intranasal delivery of interleukin-4 attenuates chronic cognitive deficits via beneficial microglial responses in experimental traumatic brain injury. J Cereb Blood Flow Metab. (2021) 41:2870–86. 10.1177/0271678x211028680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang J, Rong P, Zhang L, He H, Zhou T, Fan Y, et al. IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Sci Adv. (2021) 7:eabb9888. 10.1126/sciadv.abb9888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rahimian R, Lalancette-Hébert M, Weng YC, Sato S, Kriz J. Glucosamine-mediated immunomodulation after stroke is sexually dimorphic. Brain Behav Immun Health. (2020) 3:100041. 10.1016/j.bbih.2020.100041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vemuganti R. Therapeutic potential of PPARγ activation in stroke. PPAR Res. (2008) 2008:461981. 10.1155/2008/461981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. (2002) 53:409–35. 10.1146/annurev.med.53.082901.104018 [DOI] [PubMed] [Google Scholar]
- 177.Bernardo A, Minghetti L. PPAR-gamma agonists as regulators of microglial activation and brain inflammation. Curr Pharm Des. (2006) 12:93–109. 10.2174/138161206780574579 [DOI] [PubMed] [Google Scholar]
- 178.Landreth GE, Heneka MT. Anti-inflammatory actions of peroxisome proliferator-activated receptor gamma agonists in Alzheimer’s disease. Neurobiol Aging. (2001) 22:937–44. 10.1016/s0197-4580(01)00296-2 [DOI] [PubMed] [Google Scholar]
- 179.Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, et al. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. (1999) 400:378–82. 10.1038/22572 [DOI] [PubMed] [Google Scholar]
- 180.Zhou L, Yin J, Wang C, Liao J, Liu G, Chen L. Lack of seipin in neurons results in anxiety- and depression-like behaviors via down regulation of PPARγ. Hum Mol Genet. (2014) 23:4094–102. 10.1093/hmg/ddu126 [DOI] [PubMed] [Google Scholar]
- 181.Colle R, de Larminat D, Rotenberg S, Hozer F, Hardy P, Verstuyft C, et al. PPAR-γ agonists for the treatment of major depression: a review. Pharmacopsychiatry. (2017) 50:49–55. 10.1055/s-0042-120120 [DOI] [PubMed] [Google Scholar]
- 182.Youssef DA, El-Fayoumi HM, Mahmoud MF. Beta-caryophyllene alleviates diet-induced neurobehavioral changes in rats: the role of CB2 and PPAR-γ receptors. Biomed Pharmacother. (2019) 110:145–54. 10.1016/j.biopha.2018.11.039 [DOI] [PubMed] [Google Scholar]
- 183.Kurhe Y, Mahesh R. Pioglitazone, a PPARγ agonist rescues depression associated with obesity using chronic unpredictable mild stress model in experimental mice. Neurobiol Stress. (2016) 3:114–21. 10.1016/j.ynstr.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shelton RC, Miller AH. Inflammation in depression: is adiposity a cause? Dialog Clin Neurosci. (2011) 13:41–53. 10.31887/DCNS.2011.13.1/rshelton [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Capuron L, Lasselin J, Castanon N. Role of adiposity-driven inflammation in depressive morbidity. Neuropsychopharmacology. (2017) 42:115–28. 10.1038/npp.2016.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Alexaki VI. The impact of obesity on microglial function: immune, metabolic and endocrine perspectives. Cells. (2021) 10:1584. 10.3390/cells10071584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Qin X, Wang W, Wu H, Liu D, Wang R, Xu J, et al. PPARγ-mediated microglial activation phenotype is involved in depressive-like behaviors and neuroinflammation in stressed C57BL/6J and ob/ob mice. Psychoneuroendocrinology. (2020) 117:104674. 10.1016/j.psyneuen.2020.104674 [DOI] [PubMed] [Google Scholar]
- 188.Li J, Xu B, Chen Z, Zhou C, Liao L, Qin Y, et al. PI3K/AKT/JNK/p38 signalling pathway-mediated neural apoptosis in the prefrontal cortex of mice is involved in the antidepressant-like effect of pioglitazone. Clin Exp Pharmacol Physiol. (2018) 45:525–35. 10.1111/1440-1681.12918 [DOI] [PubMed] [Google Scholar]
- 189.Sadaghiani MS, Javadi-Paydar M, Gharedaghi MH, Fard YY, Dehpour AR. Antidepressant-like effect of pioglitazone in the forced swimming test in mice: the role of PPAR-gamma receptor and nitric oxide pathway. Behav Brain Res. (2011) 224:336–43. 10.1016/j.bbr.2011.06.011 [DOI] [PubMed] [Google Scholar]
- 190.Salehi-Sadaghiani M, Javadi-Paydar M, Gharedaghi MH, Zandieh A, Heydarpour P, Yousefzadeh-Fard Y, et al. NMDA receptor involvement in antidepressant-like effect of pioglitazone in the forced swimming test in mice. Psychopharmacology (Berl). (2012) 223:345–55. 10.1007/s00213-012-2722-0 [DOI] [PubMed] [Google Scholar]
- 191.Jahrling JB, Hernandez CM, Denner L, Dineley KT. PPARγ recruitment to active ERK during memory consolidation is required for Alzheimer’s disease-related cognitive enhancement. J Neurosci. (2014) 34:4054–63. 10.1523/jneurosci.4024-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Allami N, Javadi-Paydar M, Rayatnia F, Sehhat K, Rahimian R, Norouzi A, et al. Suppression of nitric oxide synthesis by L-NAME reverses the beneficial effects of pioglitazone on scopolamine-induced memory impairment in mice. Eur J Pharmacol. (2011) 650:240–8. 10.1016/j.ejphar.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 193.Almasi-Nasrabadi M, Javadi-Paydar M, Mahdavian S, Babaei R, Sharifian M, Norouzi A, et al. Involvement of NMDA receptors in the beneficial effects of pioglitazone on scopolamine-induced memory impairment in mice. Behav Brain Res. (2012) 231:138–45. 10.1016/j.bbr.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 194.Sepanjnia K, Modabbernia A, Ashrafi M, Modabbernia MJ, Akhondzadeh S. Pioglitazone adjunctive therapy for moderate-to-severe major depressive disorder: randomized double-blind placebo-controlled trial. Neuropsychopharmacology. (2012) 37:2093–100. 10.1038/npp.2012.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rahimian R, Béland LC, Sato S, Kriz J. Microglia-derived galectin-3 in neuroinflammation; a bittersweet ligand? Med Res Rev. (2021) 41:2582–9. 10.1002/med.21784 [DOI] [PubMed] [Google Scholar]
- 196.Sato S, St-Pierre C, Bhaumik P, Nieminen J. Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol Rev. (2009) 230:172–87. 10.1111/j.1600-065X.2009.00790.x [DOI] [PubMed] [Google Scholar]
- 197.Rahimian R, Béland LC, Kriz J. Galectin-3: mediator of microglia responses in injured brain. Drug Discov Today. (2018) 23:375–81. 10.1016/j.drudis.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 198.Nieminen J, Kuno A, Hirabayashi J, Sato S. Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J Biol Chem. (2007) 282:1374–83. 10.1074/jbc.M604506200 [DOI] [PubMed] [Google Scholar]
- 199.Elola MT, Blidner AG, Ferragut F, Bracalente C, Rabinovich GA. Assembly, organization and regulation of cell-surface receptors by lectin-glycan complexes. Biochem J. (2015) 469:1–16. 10.1042/bj20150461 [DOI] [PubMed] [Google Scholar]
- 200.Lalancette-Hébert M, Swarup V, Beaulieu JM, Bohacek I, Abdelhamid E, Weng YC, et al. Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury. J Neurosci. (2012) 32:10383–95. 10.1523/jneurosci.1498-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Baek JH, Kim SJ, Kang HG, Lee HW, Kim JH, Hwang KA, et al. Galectin-3 activates PPARγ and supports white adipose tissue formation and high-fat diet-induced obesity. Endocrinology. (2015) 156:147–56. 10.1210/en.2014-1374 [DOI] [PubMed] [Google Scholar]
- 202.MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, et al. Regulation of alternative macrophage activation by galectin-3. J Immunol. (2008) 180:2650–8. 10.4049/jimmunol.180.4.2650 [DOI] [PubMed] [Google Scholar]
- 203.Boza-Serrano A, Ruiz R, Sanchez-Varo R, García-Revilla J, Yang Y, Jimenez-Ferrer I, et al. Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer’s disease. Acta Neuropathol. (2019) 138:251–73. 10.1007/s00401-019-02013-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Burguillos MA, Svensson M, Schulte T, Boza-Serrano A, Garcia-Quintanilla A, Kavanagh E, et al. Microglia-secreted galectin-3 acts as a toll-like receptor 4 ligand and contributes to microglial activation. Cell Rep. (2015) 10:1626–38. 10.1016/j.celrep.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 205.Srejovic I, Selakovic D, Jovicic N, Jakovljević V, Lukic ML, Rosic G. Galectin-3: roles in neurodevelopment, neuroinflammation, and behavior. Biomolecules. (2020) 10:798. 10.3390/biom10050798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Stajic D, Selakovic D, Jovicic N, Joksimovic J, Arsenijevic N, Lukic ML, et al. The role of galectin-3 in modulation of anxiety state level in mice. Brain Behav Immun. (2019) 78:177–87. 10.1016/j.bbi.2019.01.019 [DOI] [PubMed] [Google Scholar]
- 207.King DR, Salako DC, Arthur-Bentil SK, Rubin AE, Italiya JB, Tan JS, et al. Relationship between novel inflammatory biomarker galectin-3 and depression symptom severity in a large community-based sample. J Affect Disord. (2021) 281:384–9. 10.1016/j.jad.2020.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Melin EO, Dereke J, Thunander M, Hillman M. Depression in type 1 diabetes was associated with high levels of circulating galectin-3. Endocr Connect. (2018) 7:819–28. 10.1530/ec-18-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Skaper SD, Di Marzo V. Endocannabinoids in nervous system health and disease: the big picture in a nutshell. Philos Trans R Soc Lond B Biol Sci. (2012) 367:3193–200. 10.1098/rstb.2012.0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lu HC, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. (2016) 79:516–25. 10.1016/j.biopsych.2015.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mecha M, Carrillo-Salinas FJ, Feliú A, Mestre L, Guaza C. Microglia activation states and cannabinoid system: therapeutic implications. Pharmacol Ther. (2016) 166:40–55. 10.1016/j.pharmthera.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 212.Roche M, Finn DP. Brain CB2 receptors: implications for neuropsychiatric disorders. Pharmaceuticals (Basel). (2010) 3:2517–53. 10.3390/ph3082517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J Neurosci. (2001) 21:823–33. 10.1523/jneurosci.21-03-00823.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Molina-Holgado E, Vela JM, Arévalo-Martín A, Almazán G, Molina-Holgado F, Borrell J, et al. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci. (2002) 22:9742–53. 10.1523/jneurosci.22-22-09742.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kendall DA, Yudowski GA. Cannabinoid receptors in the central nervous system: their signaling and roles in disease. Front Cell Neurosci. (2016) 10:294. 10.3389/fncel.2016.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Di Marzo V, De Petrocellis L, Sepe N, Buono A. Biosynthesis of anandamide and related acylethanolamides in mouse J774 macrophages and N18 neuroblastoma cells. Biochem J. (1996) 316(Pt 3):977–84. 10.1042/bj3160977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. (1997) 388:773–8. 10.1038/42015 [DOI] [PubMed] [Google Scholar]
- 218.Duffy SS, Hayes JP, Fiore NT, Moalem-Taylor G. The cannabinoid system and microglia in health and disease. Neuropharmacology. (2021) 190:108555. 10.1016/j.neuropharm.2021.108555 [DOI] [PubMed] [Google Scholar]
- 219.Ativie F, Komorowska JA, Beins E, Albayram Ö, Zimmer T, Zimmer A, et al. Cannabinoid 1 receptor signaling on hippocampal GABAergic neurons influences microglial activity. Front Mol Neurosci. (2018) 11:295. 10.3389/fnmol.2018.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Navarrete M, Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. (2010) 68:113–26. 10.1016/j.neuron.2010.08.043 [DOI] [PubMed] [Google Scholar]
- 221.Schmöle AC, Lundt R, Gennequin B, Schrage H, Beins E, Krämer A, et al. Expression analysis of CB2-GFP BAC transgenic mice. PLoS One. (2015) 10:e0138986. 10.1371/journal.pone.0138986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Stella N. Endocannabinoid signaling in microglial cells. Neuropharmacology. (2009) 56(Suppl. 1):244–53. 10.1016/j.neuropharm.2008.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Benito C, Romero JP, Tolón RM, Clemente D, Docagne F, Hillard CJ, et al. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J Neurosci. (2007) 27:2396–402. 10.1523/jneurosci.4814-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang H, Hilton DA, Hanemann CO, Zajicek J. Cannabinoid receptor and N-acyl phosphatidylethanolamine phospholipase D–evidence for altered expression in multiple sclerosis. Brain Pathol. (2011) 21:544–57. 10.1111/j.1750-3639.2011.00477.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Reusch N, Ravichandran KA, Olabiyi BF, Komorowska-Müller JA, Hansen JN, Ulas T, et al. Cannabinoid receptor 2 is necessary to induce toll-like receptor-mediated microglial activation. Glia. (2022) 70:71–88. 10.1002/glia.24089 [DOI] [PubMed] [Google Scholar]
- 226.Mecha M, Feliú A, Carrillo-Salinas FJ, Rueda-Zubiaurre A, Ortega-Gutiérrez S, de Sola RG, et al. Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav Immun. (2015) 49:233–45. 10.1016/j.bbi.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 227.Tao Y, Li L, Jiang B, Feng Z, Yang L, Tang J, et al. Cannabinoid receptor-2 stimulation suppresses neuroinflammation by regulating microglial M1/M2 polarization through the cAMP/PKA pathway in an experimental GMH rat model. Brain Behav Immun. (2016) 58:118–29. 10.1016/j.bbi.2016.05.020 [DOI] [PubMed] [Google Scholar]
- 228.O’Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. (2007) 152:576–82. 10.1038/sj.bjp.0707423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fakhfouri G, Ahmadiani A, Rahimian R, Grolla AA, Moradi F, Haeri A. WIN55212-2 attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-γ pathway. Neuropharmacology. (2012) 63:653–66. 10.1016/j.neuropharm.2012.05.013 [DOI] [PubMed] [Google Scholar]
- 230.Payandemehr B, Ebrahimi A, Gholizadeh R, Rahimian R, Varastehmoradi B, Gooshe M, et al. Involvement of PPAR receptors in the anticonvulsant effects of a cannabinoid agonist, WIN 55,212-2. Prog Neuropsychopharmacol Biol Psychiatry. (2015) 57:140–5. 10.1016/j.pnpbp.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 231.Benito C, Núñez E, Tolón RM, Carrier EJ, Rábano A, Hillard CJ, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci. (2003) 23:11136–41. 10.1523/jneurosci.23-35-11136.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. (2003) 23:1398–405. 10.1523/jneurosci.23-04-01398.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. (2005) 8:752–8. 10.1038/nn1472 [DOI] [PubMed] [Google Scholar]
- 234.Domschke K, Dannlowski U, Ohrmann P, Lawford B, Bauer J, Kugel H, et al. Cannabinoid receptor 1 (CNR1) gene: impact on antidepressant treatment response and emotion processing in major depression. Eur Neuropsychopharmacol. (2008) 18:751–9. 10.1016/j.euroneuro.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 235.Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, et al. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann N Y Acad Sci. (2008) 1139:434–49. 10.1196/annals.1432.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kong X, Miao Q, Lu X, Zhang Z, Chen M, Zhang J, et al. The association of endocannabinoid receptor genes (CNR1 and CNR2) polymorphisms with depression: a meta-analysis. Medicine (Baltimore). (2019) 98:e17403. 10.1097/md.0000000000017403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. (2009) 34:1257–62. 10.1016/j.psyneuen.2009.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hungund BL, Vinod KY, Kassir SA, Basavarajappa BS, Yalamanchili R, Cooper TB, et al. Upregulation of CB1 receptors and agonist-stimulated [35S]GTPgammaS binding in the prefrontal cortex of depressed suicide victims. Mol Psychiatry. (2004) 9:184–90. 10.1038/sj.mp.4001376 [DOI] [PubMed] [Google Scholar]
- 239.Zoppi S, Pérez Nievas BG, Madrigal JL, Manzanares J, Leza JC, García-Bueno B. Regulatory role of cannabinoid receptor 1 in stress-induced excitotoxicity and neuroinflammation. Neuropsychopharmacology. (2011) 36:805–18. 10.1038/npp.2010.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lisboa SF, Niraula A, Resstel LB, Guimaraes FS, Godbout JP, Sheridan JF. Repeated social defeat-induced neuroinflammation, anxiety-like behavior and resistance to fear extinction were attenuated by the cannabinoid receptor agonist WIN55,212-2. Neuropsychopharmacology. (2018) 43:1924–33. 10.1038/s41386-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.García-Gutiérrez MS, Pérez-Ortiz JM, Gutiérrez-Adán A, Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB receptors. Br J Pharmacol. (2010) 160:1773–84. 10.1111/j.1476-5381.2010.00819.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Beins EC, Beiert T, Jenniches I, Hansen JN, Leidmaa E, Schrickel JW, et al. Cannabinoid receptor 1 signalling modulates stress susceptibility and microglial responses to chronic social defeat stress. Transl Psychiatry. (2021) 11:164. 10.1038/s41398-021-01283-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. (2009) 34(Suppl. 1):S186–95. 10.1016/j.psyneuen.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 244.De Schepper S, Crowley G, Hong S. Understanding microglial diversity and implications for neuronal function in health and disease. Dev Neurobiol. (2021) 81:507–23. 10.1002/dneu.22777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hein AM, O’Banion MK. Neuroinflammation and memory: the role of prostaglandins. Mol Neurobiol. (2009) 40:15–32. 10.1007/s12035-009-8066-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Narumiya S. Physiology and pathophysiology of prostanoid receptors. Proc Jpn Acad Ser B Phys Biol Sci. (2007) 83:296–319. 10.2183/pjab/83.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Linnoila M, Whorton AR, Rubinow DR, Cowdry RW, Ninan PT, Waters RN. CSF prostaglandin levels in depressed and schizophrenic patients. Arch Gen Psychiatry. (1983) 40:405–6. 10.1001/archpsyc.1983.01790040059008 [DOI] [PubMed] [Google Scholar]
- 248.Ohishi K, Ueno R, Nishino S, Sakai T, Hayaishi O. Increased level of salivary prostaglandins in patients with major depression. Biol Psychiatry. (1988) 23:326–34. 10.1016/0006-3223(88)90283-1 [DOI] [PubMed] [Google Scholar]
- 249.Nishino S, Ueno R, Ohishi K, Sakai T, Hayaishi O. Salivary prostaglandin concentrations: possible state indicators for major depression. Am J Psychiatry. (1989) 146:365–8. 10.1176/ajp.146.3.365 [DOI] [PubMed] [Google Scholar]
- 250.Müller N. Immunology of major depression. Neuroimmunomodulation. (2014) 21:123–30. 10.1159/000356540 [DOI] [PubMed] [Google Scholar]
- 251.Mendlewicz J, Kriwin P, Oswald P, Souery D, Alboni S, Brunello N. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int Clin Psychopharmacol. (2006) 21:227–31. 10.1097/00004850-200607000-00005 [DOI] [PubMed] [Google Scholar]
- 252.Akhondzadeh S, Jafari S, Raisi F, Nasehi AA, Ghoreishi A, Salehi B, et al. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety. (2009) 26:607–11. 10.1002/da.20589 [DOI] [PubMed] [Google Scholar]
- 253.Tanda G, Carboni E, Frau R, Di Chiara G. Increase of extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential? Psychopharmacology (Berl). (1994) 115:285–8. 10.1007/bf02244785 [DOI] [PubMed] [Google Scholar]
- 254.Tanaka K, Furuyashiki T, Kitaoka S, Senzai Y, Imoto Y, Segi-Nishida E, et al. Prostaglandin E2-mediated attenuation of mesocortical dopaminergic pathway is critical for susceptibility to repeated social defeat stress in mice. J Neurosci. (2012) 32:4319–29. 10.1523/jneurosci.5952-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Matsuoka Y, Furuyashiki T, Yamada K, Nagai T, Bito H, Tanaka Y, et al. Prostaglandin E receptor EP1 controls impulsive behavior under stress. Proc Natl Acad Sci U.S.A. (2005) 102:16066–71. 10.1073/pnas.0504908102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tanaka Y, Furuyashiki T, Momiyama T, Namba H, Mizoguchi A, Mitsumori T, et al. Prostaglandin E receptor EP1 enhances GABA-mediated inhibition of dopaminergic neurons in the substantia nigra pars compacta and regulates dopamine level in the dorsal striatum. Eur J Neurosci. (2009) 30:2338–46. 10.1111/j.1460-9568.2009.07021.x [DOI] [PubMed] [Google Scholar]
- 257.Furuyashiki T, Narumiya S. Stress responses: the contribution of prostaglandin E and its receptors. Nat Rev Endocrinol. (2011) 7:163–75. 10.1038/nrendo.2010.194 [DOI] [PubMed] [Google Scholar]
- 258.Fritz M, Klawonn AM, Nilsson A, Singh AK, Zajdel J, Wilhelms DB, et al. Prostaglandin-dependent modulation of dopaminergic neurotransmission elicits inflammation-induced aversion in mice. J Clin Invest. (2016) 126:695–705. 10.1172/jci83844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Klawonn AM, Fritz M, Castany S, Pignatelli M, Canal C, Similä F, et al. Microglial activation elicits a negative affective state through prostaglandin-mediated modulation of striatal neurons. Immunity. (2021) 54:225–34.e226. 10.1016/j.immuni.2020.12.016 [DOI] [PubMed] [Google Scholar]
- 260.Kessing LV, Rytgaard HC, Gerds TA, Berk M, Ekstrøm CT, Andersen PK. New drug candidates for depression – a nationwide population-based study. Acta Psychiatr Scand. (2019) 139:68–77. 10.1111/acps.12957 [DOI] [PubMed] [Google Scholar]
- 261.Müller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Müller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. (2006) 11:680–4. 10.1038/sj.mp.4001805 [DOI] [PubMed] [Google Scholar]
- 262.Lehmann ML, Weigel TK, Cooper HA, Elkahloun AG, Kigar SL, Herkenham M. Decoding microglia responses to psychosocial stress reveals blood-brain barrier breakdown that may drive stress susceptibility. Sci Rep. (2018) 8:11240. 10.1038/s41598-018-28737-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zabala A, Vazquez-Villoldo N, Rissiek B, Gejo J, Martin A, Palomino A, et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol Med. (2018) 10:e8743. 10.15252/emmm.201708743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. (2012) 149:274–93. 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Réus GQJ, Rodrigues AL. mTOR signaling in the neuropathophysiology of depression: current evidence. J Recept Ligand Channel Res. (2015) 8:65–74. 10.2147/JRLCR.S70351 [DOI] [Google Scholar]
- 266.Bockaert J, Marin P. mTOR in brain physiology and pathologies. Physiol Rev. (2015) 95:1157–87. 10.1152/physrev.00038.2014 [DOI] [PubMed] [Google Scholar]
- 267.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. (2010) 329:959–64. 10.1126/science.1190287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zhong P, Wang W, Pan B, Liu X, Zhang Z, Long JZ, et al. Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling. Neuropsychopharmacology. (2014) 39:1763–76. 10.1038/npp.2014.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Chandran A, Iyo AH, Jernigan CS, Legutko B, Austin MC, Karolewicz B. Reduced phosphorylation of the mTOR signaling pathway components in the amygdala of rats exposed to chronic stress. Prog Neuropsychopharmacol Biol Psychiatry. (2013) 40:240–5. 10.1016/j.pnpbp.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. (2011) 35:1774–9. 10.1016/j.pnpbp.2011.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hu Y, Mai W, Chen L, Cao K, Zhang B, Zhang Z, et al. mTOR-mediated metabolic reprogramming shapes distinct microglia functions in response to lipopolysaccharide and ATP. Glia. (2020) 68:1031–45. 10.1002/glia.23760 [DOI] [PubMed] [Google Scholar]
- 272.Dello Russo C, Lisi L, Tringali G, Navarra P. Involvement of mTOR kinase in cytokine-dependent microglial activation and cell proliferation. Biochem Pharmacol. (2009) 78:1242–51. 10.1016/j.bcp.2009.06.097 [DOI] [PubMed] [Google Scholar]
- 273.Zhao X, Liao Y, Morgan S, Mathur R, Feustel P, Mazurkiewicz J, et al. Noninflammatory changes of microglia are sufficient to cause epilepsy. Cell Rep. (2018) 22:2080–93. 10.1016/j.celrep.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Li D, Wang C, Yao Y, Chen L, Liu G, Zhang R, et al. mTORC1 pathway disruption ameliorates brain inflammation following stroke via a shift in microglia phenotype from M1 type to M2 type. FASEB J. (2016) 30:3388–99. 10.1096/fj.201600495R [DOI] [PubMed] [Google Scholar]
- 275.Keane L, Antignano I, Riechers SP, Zollinger R, Dumas AA, Offermann N, et al. mTOR-dependent translation amplifies microglia priming in aging mice. J Clin Invest. (2021) 131:e132727. 10.1172/jci132727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Calovi S, Mut-Arbona P, Sperlágh B. Microglia and the purinergic signaling system. Neuroscience. (2019) 405:137–47. 10.1016/j.neuroscience.2018.12.021 [DOI] [PubMed] [Google Scholar]
- 277.Xiang Z, Burnstock G. Expression of P2X receptors on rat microglial cells during early development. Glia. (2005) 52:119–26. 10.1002/glia.20227 [DOI] [PubMed] [Google Scholar]
- 278.Burnstock G. Introduction to purinergic signaling. Methods Mol Biol. (2020) 2041:1–15. 10.1007/978-1-4939-9717-6_1 [DOI] [PubMed] [Google Scholar]
- 279.Orr AG, Orr AL, Li XJ, Gross RE, Traynelis SF. Adenosine A(2A) receptor mediates microglial process retraction. Nat Neurosci. (2009) 12:872–8. 10.1038/nn.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. (2005) 63:191–270. 10.1016/s0074-7742(05)63007-3 [DOI] [PubMed] [Google Scholar]
- 281.Cheffer A, Castillo ARG, Corrêa-Velloso J, Gonçalves MCB, Naaldijk Y, Nascimento IC, et al. Purinergic system in psychiatric diseases. Mol Psychiatry. (2018) 23:94–106. 10.1038/mp.2017.188 [DOI] [PubMed] [Google Scholar]
- 282.Tanaka K, Choi J, Cao Y, Stacey G. Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Front Plant Sci. (2014) 5:446. 10.3389/fpls.2014.00446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Vénéreau E, Ceriotti C, Bianchi ME. DAMPs from cell death to new life. Front Immunol. (2015) 6:422. 10.3389/fimmu.2015.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Iida T, Yoshikawa T, Kárpáti A, Matsuzawa T, Kitano H, Mogi A, et al. JNJ10181457, a histamine H3 receptor inverse agonist, regulates in vivo microglial functions and improves depression-like behaviours in mice. Biochem Biophys Res Commun. (2017) 488:534–40. 10.1016/j.bbrc.2017.05.081 [DOI] [PubMed] [Google Scholar]
- 285.De Simone R, Niturad CE, De Nuccio C, Ajmone-Cat MA, Visentin S, Minghetti L. TGF-β and LPS modulate ADP-induced migration of microglial cells through P2Y1 and P2Y12 receptor expression. J Neurochem. (2010) 115:450–9. 10.1111/j.1471-4159.2010.06937.x [DOI] [PubMed] [Google Scholar]
- 286.Vultaggio-Poma V, Sarti AC, Di Virgilio F. Extracellular ATP: a feasible target for cancer therapy. Cells. (2020) 9:2496. 10.3390/cells9112496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yu T, Zhang X, Shi H, Tian J, Sun L, Hu X, et al. P2Y12 regulates microglia activation and excitatory synaptic transmission in spinal lamina II neurons during neuropathic pain in rodents. Cell Death Dis. (2019) 10:165. 10.1038/s41419-019-1425-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lin SS, Tang Y, Illes P, Verkhratsky A. The safeguarding microglia: central role for P2Y(12) receptors. Front Pharmacol. (2020) 11:627760. 10.3389/fphar.2020.627760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. (2006) 9:1512–9. 10.1038/nn1805 [DOI] [PubMed] [Google Scholar]
- 290.Milior G, Morin-Brureau M, Chali F, Le Duigou C, Savary E, Huberfeld G, et al. Distinct P2Y receptors mediate extension and retraction of microglial processes in epileptic and peritumoral human tissue. J Neurosci. (2020) 40:1373–88. 10.1523/jneurosci.0218-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Morin-Brureau M, Milior G, Royer J, Chali F, Le Duigou C, Savary E, et al. Microglial phenotypes in the human epileptic temporal lobe. Brain. (2018) 141:3343–60. 10.1093/brain/awy276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lowery RL, Mendes MS, Sanders BT, Murphy AJ, Whitelaw BS, Lamantia CE, et al. Loss of P2Y12 has behavioral effects in the adult mouse. Int J Mol Sci. (2021) 22:1868. 10.3390/ijms22041868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Peng J, Liu Y, Umpierre AD, Xie M, Tian DS, Richardson JR, et al. Microglial P2Y12 receptor regulates ventral hippocampal CA1 neuronal excitability and innate fear in mice. Mol Brain. (2019) 12:71. 10.1186/s13041-019-0492-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Verma R, Cronin CG, Hudobenko J, Venna VR, McCullough LD, Liang BT. Deletion of the P2X4 receptor is neuroprotective acutely, but induces a depressive phenotype during recovery from ischemic stroke. Brain Behav Immun. (2017) 66:302–12. 10.1016/j.bbi.2017.07.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yang X, Lou Y, Liu G, Wang X, Qian Y, Ding J, et al. Microglia P2Y6 receptor is related to Parkinson’s disease through neuroinflammatory process. J Neuroinflammation. (2017) 14:38. 10.1186/s12974-017-0795-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Shao BZ, Xu ZQ, Han BZ, Su DF, Liu C. NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol. (2015) 6:262. 10.3389/fphar.2015.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S, et al. Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biol Psychiatry. (2016) 80:12–22. 10.1016/j.biopsych.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 298.He Y, Taylor N, Fourgeaud L, Bhattacharya A. The role of microglial P2X7: modulation of cell death and cytokine release. J Neuroinflammation. (2017) 14:135. 10.1186/s12974-017-0904-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kaufmann FN, Costa AP, Ghisleni G, Diaz AP, Rodrigues ALS, Peluffo H, et al. NLRP3 inflammasome-driven pathways in depression: clinical and preclinical findings. Brain Behav Immun. (2017) 64:367–83. 10.1016/j.bbi.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 300.Yue N, Huang H, Zhu X, Han Q, Wang Y, Li B, et al. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J Neuroinflammation. (2017) 14:102. 10.1186/s12974-017-0865-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Basso AM, Bratcher NA, Harris RR, Jarvis MF, Decker MW, Rueter LE. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behav Brain Res. (2009) 198:83–90. 10.1016/j.bbr.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 302.Boucher AA, Arnold JC, Hunt GE, Spiro A, Spencer J, Brown C, et al. Resilience and reduced c-Fos expression in P2X7 receptor knockout mice exposed to repeated forced swim test. Neuroscience. (2011) 189:170–7. 10.1016/j.neuroscience.2011.05.049 [DOI] [PubMed] [Google Scholar]
- 303.Csölle C, Baranyi M, Zsilla G, Kittel A, Gölöncsér F, Illes P, et al. Neurochemical changes in the mouse hippocampus underlying the antidepressant effect of genetic deletion of P2X7 receptors. PLoS One. (2013) 8:e66547. 10.1371/journal.pone.0066547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Nagy G, Ronai Z, Somogyi A, Sasvari-Szekely M, Rahman OA, Mate A, et al. P2RX7 Gln460Arg polymorphism is associated with depression among diabetic patients. Prog Neuropsychopharmacol Biol Psychiatry. (2008) 32:1884–8. 10.1016/j.pnpbp.2008.08.021 [DOI] [PubMed] [Google Scholar]
- 305.McQuillin A, Bass NJ, Choudhury K, Puri V, Kosmin M, Lawrence J, et al. Case-control studies show that a non-conservative amino-acid change from a glutamine to arginine in the P2RX7 purinergic receptor protein is associated with both bipolar- and unipolar-affective disorders. Mol Psychiatry. (2009) 14:614–20. 10.1038/mp.2008.6 [DOI] [PubMed] [Google Scholar]
- 306.Soronen P, Mantere O, Melartin T, Suominen K, Vuorilehto M, Rytsälä H, et al. P2RX7 gene is associated consistently with mood disorders and predicts clinical outcome in three clinical cohorts. Am J Med Genet B Neuropsychiatr Genet. (2011) 156b:435–47. 10.1002/ajmg.b.31179 [DOI] [PubMed] [Google Scholar]
- 307.Feng WP, Zhang B, Li W, Liu J. Lack of association of P2RX7 gene rs2230912 polymorphism with mood disorders: a meta-analysis. PLoS One. (2014) 9:e88575. 10.1371/journal.pone.0088575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Stokes L, Spencer SJ, Jenkins TA. Understanding the role of P2X7 in affective disorders-are glial cells the major players? Front Cell Neurosci. (2015) 9:258. 10.3389/fncel.2015.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Bhattacharya A, Drevets WC. Role of neuro-immunological factors in the pathophysiology of mood disorders: implications for novel therapeutics for treatment resistant depression. Curr Top Behav Neurosci. (2017) 31:339–56. 10.1007/7854_2016_43 [DOI] [PubMed] [Google Scholar]
- 310.Bhattacharya A. Recent advances in CNS P2X7 physiology and pharmacology: focus on neuropsychiatric disorders. Front Pharmacol. (2018) 9:30. 10.3389/fphar.2018.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Karasawa A, Michalski K, Mikhelzon P, Kawate T. The P2X7 receptor forms a dye-permeable pore independent of its intracellular domain but dependent on membrane lipid composition. Elife. (2017) 6:e31186. 10.7554/eLife.31186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Perlman K, Chouinard-Watkins R, Tanti A, Cisbani G, Orri M, Turecki G, et al. Fatty acid dysregulation in the anterior cingulate cortex of depressed suicides with a history of child abuse. Transl Psychiatry. (2021) 11:535. 10.1038/s41398-021-01657-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Yary T, Aazami S. The association between polyunsaturated fatty acids and depression among Iranian postgraduate students in Malaysia. Lipids Health Dis. (2011) 10:151. 10.1186/1476-511x-10-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Mühle C, Reichel M, Gulbins E, Kornhuber J. Sphingolipids in psychiatric disorders and pain syndromes. Handb Exp Pharmacol. (2013) 216:431–56. 10.1007/978-3-7091-1511-4_22 [DOI] [PubMed] [Google Scholar]
- 315.Walther A, Cannistraci CV, Simons K, Durán C, Gerl MJ, Wehrli S, et al. Lipidomics in major depressive disorder. Front Psychiatry. (2018) 9:459. 10.3389/fpsyt.2018.00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Müller CP, Reichel M, Mühle C, Rhein C, Gulbins E, Kornhuber J. Brain membrane lipids in major depression and anxiety disorders. Biochim Biophys Acta. (2015) 1851:1052–65. 10.1016/j.bbalip.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 317.Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. (2014) 15:771–85. 10.1038/nrn3820 [DOI] [PubMed] [Google Scholar]
- 318.Dang R, Zhou X, Tang M, Xu P, Gong X, Liu Y, et al. Fish oil supplementation attenuates neuroinflammation and alleviates depressive-like behavior in rats submitted to repeated lipopolysaccharide. Eur J Nutr. (2018) 57:893–906. 10.1007/s00394-016-1373-z [DOI] [PubMed] [Google Scholar]
- 319.Schneider M, Levant B, Reichel M, Gulbins E, Kornhuber J, Müller CP. Lipids in psychiatric disorders and preventive medicine. Neurosci Biobehav Rev. (2017) 76(Pt B):336–62. 10.1016/j.neubiorev.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 320.Chianese R, Coccurello R, Viggiano A, Scafuro M, Fiore M, Coppola G, et al. Impact of dietary fats on brain functions. Curr Neuropharmacol. (2018) 16:1059–85. 10.2174/1570159x15666171017102547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Liśkiewicz A, Przybyła M, Wojakowska A, Marczak Ł, Bogus K, Nowacka-Chmielewska M, et al. Physical activity reduces anxiety and regulates brain fatty acid synthesis. Mol Brain. (2020) 13:62. 10.1186/s13041-020-00592-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Tkachev A, Stekolshchikova E, Bobrovskiy DM, Anikanov N, Ogurtsova P, Park DI, et al. Long-term fluoxetine administration causes substantial lipidome alteration of the juvenile macaque brain. Int J Mol Sci. (2021) 22:8089. 10.3390/ijms22158089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Okada M, Nutt DJ, Murakami T, Zhu G, Kamata A, Kawata Y, et al. Adenosine receptor subtypes modulate two major functional pathways for hippocampal serotonin release. J Neurosci. (2001) 21:628–40. 10.1523/jneurosci.21-02-00628.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Batalha VL, Pego JM, Fontinha BM, Costenla AR, Valadas JS, Baqi Y, et al. Adenosine A(2A) receptor blockade reverts hippocampal stress-induced deficits and restores corticosterone circadian oscillation. Mol Psychiatry. (2013) 18:320–31. 10.1038/mp.2012.8 [DOI] [PubMed] [Google Scholar]
- 325.Dias L, Lopes CR, Gonçalves FQ, Nunes A, Pochmann D, Machado NJ, et al. Crosstalk between ATP-P(2X7) and adenosine A(2A) receptors controlling neuroinflammation in rats subject to repeated restraint stress. Front Cell Neurosci. (2021) 15:639322. 10.3389/fncel.2021.639322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Qian ZM, Ke Y. Brain iron transport. Biol Rev Camb Philos Soc. (2019) 94:1672–84. 10.1111/brv.12521 [DOI] [PubMed] [Google Scholar]
- 327.Ogun AS, Adeyinka A. Biochemistry, transferrin. In: StatPearls. Treasure Island, FL: StatPearls Publishing; (2022). [PubMed] [Google Scholar]
- 328.Moos T, Rosengren Nielsen T, Skjørringe T, Morgan EH. Iron trafficking inside the brain. J Neurochem. (2007) 103:1730–40. 10.1111/j.1471-4159.2007.04976.x [DOI] [PubMed] [Google Scholar]
- 329.Mills E, Dong XP, Wang F, Xu H. Mechanisms of brain iron transport: insight into neurodegeneration and CNS disorders. Future Med Chem. (2010) 2:51–64. 10.4155/fmc.09.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Ganz T, Nemeth E. The hepcidin-ferroportin system as a therapeutic target in anemias and iron overload disorders. Hematol Am Soc Hematol Educ Prog. (2011) 2011:538–42. 10.1182/asheducation-2011.1.538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Erikson KM, Pinero DJ, Connor JR, Beard JL. Regional brain iron, ferritin and transferrin concentrations during iron deficiency and iron repletion in developing rats. J Nutr. (1997) 127:2030–8. 10.1093/jn/127.10.2030 [DOI] [PubMed] [Google Scholar]
- 332.Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. (2014) 13:1045–60. 10.1016/s1474-4422(14)70117-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Reinert A, Morawski M, Seeger J, Arendt T, Reinert T. Iron concentrations in neurons and glial cells with estimates on ferritin concentrations. BMC Neurosci. (2019) 20:25. 10.1186/s12868-019-0507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Quintana C, Bellefqih S, Laval JY, Guerquin-Kern JL, Wu TD, Avila J, et al. Study of the localization of iron, ferritin, and hemosiderin in Alzheimer’s disease hippocampus by analytical microscopy at the subcellular level. J Struct Biol. (2006) 153:42–54. 10.1016/j.jsb.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 335.Haining RL, Achat-Mendes C. Neuromelanin, one of the most overlooked molecules in modern medicine, is not a spectator. Neural Regen Res. (2017) 12:372–5. 10.4103/1673-5374.202928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. (1996) 17:83–93. 10.1002/(sici)1098-1136(199606)17:23.0.Co;2-7 [DOI] [PubMed] [Google Scholar]
- 337.Leitner DF, Connor JR. Functional roles of transferrin in the brain. Biochim Biophys Acta. (2012) 1820:393–402. 10.1016/j.bbagen.2011.10.016 [DOI] [PubMed] [Google Scholar]
- 338.Ward RJ, Crichton RR, Taylor DL, Della Corte L, Srai SK, Dexter DT. Iron and the immune system. J Neural Transm (Vienna). (2011) 118:315–28. 10.1007/s00702-010-0479-3 [DOI] [PubMed] [Google Scholar]
- 339.Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr. (2010) 30:105–22. 10.1146/annurev.nutr.012809.104804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lokken KL, Tsolis RM, Bäumler AJ. Hypoferremia of infection: a double-edged sword? Nat Med. (2014) 20:335–7. 10.1038/nm.3526 [DOI] [PubMed] [Google Scholar]
- 341.Nnah IC, Wessling-Resnick M. Brain iron homeostasis: a focus on microglial iron. Pharmaceuticals (Basel). (2018) 11:129. 10.3390/ph11040129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Thomsen MS, Andersen MV, Christoffersen PR, Jensen MD, Lichota J, Moos T. Neurodegeneration with inflammation is accompanied by accumulation of iron and ferritin in microglia and neurons. Neurobiol Dis. (2015) 81:108–18. 10.1016/j.nbd.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 343.Mairuae N, Connor JR, Cheepsunthorn P. Increased cellular iron levels affect matrix metalloproteinase expression and phagocytosis in activated microglia. Neurosci Lett. (2011) 500:36–40. 10.1016/j.neulet.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 344.McCarthy RC, Sosa JC, Gardeck AM, Baez AS, Lee CH, Wessling-Resnick M. Inflammation-induced iron transport and metabolism by brain microglia. J Biol Chem. (2018) 293:7853–63. 10.1074/jbc.RA118.001949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Pasricha SR, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet. (2021) 397:233–48. 10.1016/s0140-6736(20)32594-0 [DOI] [PubMed] [Google Scholar]
- 346.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1789–858. 10.1016/s0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Hidese S, Saito K, Asano S, Kunugi H. Association between iron-deficiency anemia and depression: a web-based Japanese investigation. Psychiatry Clin Neurosci. (2018) 72:513–21. 10.1111/pcn.12656 [DOI] [PubMed] [Google Scholar]
- 348.Knyszyńska A, Radecka A, Zabielska P, Łuczak J, Karakiewicz B, Lubkowska A. The role of iron metabolism in fatigue, depression, and quality of life in multiple sclerosis patients. Int J Environ Res Public Health. (2020) 17:6818. 10.3390/ijerph17186818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Lee HS, Chao HH, Huang WT, Chen SC, Yang HY. Psychiatric disorders risk in patients with iron deficiency anemia and association with iron supplementation medications: a nationwide database analysis. BMC Psychiatry. (2020) 20:216. 10.1186/s12888-020-02621-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Shah HE, Bhawnani N, Ethirajulu A, Alkasabera A, Onyali CB, Anim-Koranteng C, et al. Iron deficiency-induced changes in the hippocampus, corpus striatum, and monoamines levels that lead to anxiety, depression, sleep disorders, and psychotic disorders. Cureus. (2021) 13:e18138. 10.7759/cureus.18138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Berthou C, Iliou JP, Barba D. Iron, neuro-bioavailability and depression. eJHaem. (2022) 3:263–75. 10.1002/jha2.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Hare D, Ayton S, Bush A, Lei P. A delicate balance: iron metabolism and diseases of the brain. Front Aging Neurosci. (2013) 5:34. 10.3389/fnagi.2013.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr Res. (2007) 94:273–80. 10.1016/j.schres.2007.04.014 [DOI] [PubMed] [Google Scholar]
- 354.Nagy C, Maitra M, Tanti A, Suderman M, Théroux JF, Davoli MA, et al. Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nat Neurosci. (2020) 23:771–81. 10.1038/s41593-020-0621-y [DOI] [PubMed] [Google Scholar]
- 355.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. (2003) 23:41–58. 10.1146/annurev.nutr.23.020102.075739 [DOI] [PubMed] [Google Scholar]
- 356.Rosato-Siri MV, Marziali L, Guitart ME, Badaracco ME, Puntel M, Pitossi F, et al. Iron availability compromises not only oligodendrocytes but also astrocytes and microglial cells. Mol Neurobiol. (2018) 55:1068–81. 10.1007/s12035-016-0369-2 [DOI] [PubMed] [Google Scholar]
- 357.Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia. (2009) 57:467–78. 10.1002/glia.20784 [DOI] [PubMed] [Google Scholar]
- 358.Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One. (2011) 6:e26317. 10.1371/journal.pone.0026317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Gao W, Wang W, Liu G, Zhang J, Yang J, Deng Z. Allicin attenuated chronic social defeat stress induced depressive-like behaviors through suppression of NLRP3 inflammasome. Metab Brain Dis. (2019) 34:319–29. 10.1007/s11011-018-0342-z [DOI] [PubMed] [Google Scholar]
- 360.Jiao H, Yang H, Yan Z, Chen J, Xu M, Jiang Y, et al. Traditional Chinese formula xiaoyaosan alleviates depressive-like behavior in CUMS mice by regulating PEBP1-GPX4-mediated ferroptosis in the hippocampus. Neuropsychiatr Dis Treat. (2021) 17:1001–19. 10.2147/ndt.S302443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Texel SJ, Camandola S, Ladenheim B, Rothman SM, Mughal MR, Unger EL, et al. Ceruloplasmin deficiency results in an anxiety phenotype involving deficits in hippocampal iron, serotonin, and BDNF. J Neurochem. (2012) 120:125–34. 10.1111/j.1471-4159.2011.07554.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Pellegrino RM, Boda E, Montarolo F, Boero M, Mezzanotte M, Saglio G, et al. Transferrin receptor 2 dependent alterations of brain iron metabolism affect anxiety circuits in the mouse. Sci Rep. (2016) 6:30725. 10.1038/srep30725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Lenz KM, McCarthy MM. A starring role for microglia in brain sex differences. Neuroscientist. (2015) 21:306–21. 10.1177/1073858414536468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Caplan HW, Cox CS, Bedi SS. Do microglia play a role in sex differences in TBI? J Neurosci Res. (2017) 95:509–17. 10.1002/jnr.23854 [DOI] [PubMed] [Google Scholar]
- 365.Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME, et al. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. (2002) 956:30–5. 10.1016/s0006-8993(02)03475-3 [DOI] [PubMed] [Google Scholar]
- 366.Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. (2012) 120:948–63. 10.1111/j.1471-4159.2011.07630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Villa A, Gelosa P, Castiglioni L, Cimino M, Rizzi N, Pepe G, et al. Sex-specific features of microglia from adult mice. Cell Rep. (2018) 23:3501–11. 10.1016/j.celrep.2018.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Hui CW, Vecchiarelli HA, Gervais É, Luo X, Michaud F, Scheefhals L, et al. Sex differences of microglia and synapses in the hippocampal dentate gyrus of adult mouse offspring exposed to maternal immune activation. Front Cell Neurosci. (2020) 14:558181. 10.3389/fncel.2020.558181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Bollinger JL, Bergeon Burns CM, Wellman CL. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav Immun. (2016) 52:88–97. 10.1016/j.bbi.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Bollinger JL, Collins KE, Patel R, Wellman CL. Behavioral stress alters corticolimbic microglia in a sex- and brain region-specific manner. PLoS One. (2017) 12:e0187631. 10.1371/journal.pone.0187631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Woodburn SC, Bollinger JL, Wohleb ES. Synaptic and behavioral effects of chronic stress are linked to dynamic and sex-specific changes in microglia function and astrocyte dystrophy. Neurobiol Stress. (2021) 14:100312. 10.1016/j.ynstr.2021.100312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Yamamoto I, Eyanagi R. Positive skin reaction induced by 4,4’-azoxybenzenedisulfonamide in relationship to the sulfanilamide allergy. Int Arch Allergy Appl Immunol. (1977) 54:538–41. 10.1159/000231874 [DOI] [PubMed] [Google Scholar]
- 373.Himmerich H, Patsalos O, Lichtblau N, Ibrahim MAA, Dalton B. Cytokine research in depression: principles, challenges, and open questions. Front Psychiatry. (2019) 10:30. 10.3389/fpsyt.2019.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. (2011) 130:226–38. 10.1016/j.pharmthera.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Himmerich H, Minkwitz J, Kirkby KC. Weight gain and metabolic changes during treatment with antipsychotics and antidepressants. Endocr Metab Immune Disord Drug Targets. (2015) 15:252–60. 10.2174/1871530315666150623092031 [DOI] [PubMed] [Google Scholar]
- 376.Miller AH, Raison CL. Are anti-inflammatory therapies viable treatments for psychiatric disorders?: where the rubber meets the road. JAMA Psychiatry. (2015) 72:527–8. 10.1001/jamapsychiatry.2015.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Luo XG, Chen SD. The changing phenotype of microglia from homeostasis to disease. Transl Neurodegen. (2012) 1:9. 10.1186/2047-9158-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]